Abstract
It is well known that in a given cell, at a particular time, only a fraction of the entire genome is expressed. Expression of a gene, nuclear, or organellar starts with the onset of transcription and ends in the synthesis of the functional protein. The regulation of gene expression is a complex process that requires the coordinated activity of different proteins and nucleic acids that ultimately determine whether a gene is transcribed, and if transcribed, whether it results in the production of a protein that develops a phenotype. The same also holds true for transgenic crops, which lie at the very core of insert design.
There are multiple checkpoints at which the expression of a gene can be regulated and controlled. Much of the emphasis of studies related to gene expression has been on regulation of gene transcription, and a number of methods are used to effect the control of gene expression. Controlling transgene expression for a commercially valuable trait is necessary to capture its value. Many gene functions are either lethal or produce severe deformity (resulting in loss of value) if over-expressed. Thus, expression of a transgene at a particular site or in response to a particular elicitor is always desirable.
Keywords: Transgenic Plant, Phytase Activity, Transgenic Crop, Alfalfa Mosaic Virus, Edible Vaccine
Introduction
It is well known that in a given cell, at a particular time, only a fraction of the entire genome is expressed. Expression of a gene, nuclear, or organellar starts with the onset of transcription and ends in the synthesis of the functional protein. The regulation of gene expression is a complex process that requires the coordinated activity of different proteins and nucleic acids that ultimately determine whether a gene is transcribed, and if transcribed, whether it results in the production of a protein that develops a phenotype. The same also holds true for transgenic crops, which lie at the very core of insert design.
There are multiple checkpoints at which the expression of a gene can be regulated and controlled. Much of the emphasis of studies related to gene expression has been on regulation of gene transcription, and a number of methods are used to effect the control of gene expression. Controlling transgene expression for a commercially valuable trait is necessary to capture its value. Many gene functions are either lethal or produce severe deformity (resulting in loss of value) if over-expressed. Thus, expression of a transgene at a particular site or in response to a particular elicitor is always desirable.
Usually, the regions responsible for the initiation of transcription lie within the 5′ region, upstream to the coding sequence of the gene. These are the promoter regions, defined as cis-acting (as they are on the same DNA strand that codes for the gene) nontranscribed elements, which provide sequences for the binding of various transcription initiation factors and RNA polymerase.
One can use a promoter that has known regulatory characteristics; for example, a promoter that is expressed throughout the plant tissue or only in vascular tissues, in the leaf epidermis, seed endosperm or embryo, and so on. One can mix and match fragments of DNA and transcription factors to develop chimeric promoters that have the desired patterns and levels of gene expression.
In this chapter, we will discuss the basic aspects of designing genes for insertion and quantifying transgene expression, followed by the different types of promoters and their use in transgenic crops. Also, several factors responsible for high-level expression and stability in transgenic plants/crops will be discussed.
Gene Design for Insertion
Once a gene of choice has been targeted and cloned, it has to undergo several modifications before it can be effectively inserted into a plant. A promoter sequence is added for the gene to be expressed. Most promoters used for transgenic crop varieties have been “constitutive,” i.e., causing gene expression throughout the life cycle of the plant in most of the tissues. The most commonly used constitutive promoter is CaMV 35S, from the cauliflower mosaic virus, which generally results in a high level of expression in most plants. Some promoters are more specific and are discussed in detail in Sect. 5.4.
Genes of interest are sometimes modified to achieve high level of expression. As plants prefer G-C rich regions, as compared to A-T rich bacterial genes, in order to overexpress bacterial genes in plants, A-T rich regions are to be substituted by G-C rich regions in such a way that the amino acid sequence of the protein remains unaltered (Evans et al. 2003). A selectable marker gene is inserted into the construct so as to identify the cells or tissues that have been successfully transformed (as discussed in Chap. 10.1007/978-3-642-04809-8_4). In some cases (e.g., resistance to pesticides), the transgene itself acts as a selectable marker. In other instances, a reporter gene is also inserted in the construct. A reporter gene is a coding sequence that upon expression in the transgenic plant provides conclusive evidence of genetic transformation. These reporter genes are very useful for transient expression experiments where the spatial and temporal activity of a promoter can be elucidated. The genes naturally exhibit an enzyme activity that does not exist in the host plant. Most common reporter genes are from bacteria, insects, or jellyfish as these organisms are so unrelated to angiosperms that their cis-regulatory elements are not functional in plants. Thus, when cloned into plant transformation vectors, a terminator sequence should also be fused downstream to the gene. Commonly used reporter genes are CAT (E. coli), βGUS (E. coli), luciferase (firefly), green fluorescence protein (jellyfish), etc.
Quantification of Transgene Expression
It is necessary to know how a transgene is expressing in order to evaluate its effectiveness and level of expression in transgenic plants. The transgene copy number can greatly influence the expression level and genetic stability in the plant, and therefore, estimation of the transgene number is of prime importance (Bhat and Srinivasan 2002). Previously, Southern and Northern analyses were used for this purpose (Sabelli and Shewry 1995a, b). But, with time, other methods such as comparative genomic hybridization, fluorescence in situ hybridization, multiplex amplifiable probe hybridization, and microarray had been employed to determine the transgene copy number. All these methods are time consuming, laborious, and require large quantities of DNA. Moreover, nucleic acid hybridization-based techniques often involve the application of hazardous radioisotopes.
Recently, quantitative real-time PCR (qPCR) has proved to be an efficient method for transgene expression studies in plants. In qPCR, expression level is monitored per cycle of the reaction, comparing the fluorescent signal generated by the DNA or mRNA sample proportional to its initial quantity (Page and Minocha 2004). It has proved to be a more sensitive and rapid method, providing stringent evaluation through the use of SYBR Green I Fluorescent intercalating dye, which has the ability to detect a single gene among a number of genes in combination with highly specific gene primers. Till now, qPCR has been extensively applied to several transgenic crops such as maize (Ingham et al. 2001; Song et al. 2002; Shou et al. 2004; Assem and Hassan 2008), wheat (Li et al. 2004), rice (Yang et al. 2005), potato (Toplak et al. 2004), rapeseed (Weng et al 2004), tomato (Mason et al. 2001), tobacco (Miyamoto et al. 2000), cassava (Beltrán et al. 2009), and strawberry (Schaart et al. 2002) for analyzing transgene expression.
Promoters
As discussed earlier, the 5′ upstream regions of a gene are not transcribed but provide sites for attachment of transcription initiation factors. The promoter itself contains many elements (short regions of a defined DNA sequence) for initiation factor attachment. The very basic of these elements is the TATA box, which is present about 25–30 bp upstream of the transcription start site and is primarily responsible for the correct positioning of RNA polymerase II. Many genes contain multiple operational TATA boxes, for example, three for inrpk1 gene in Ipomoea nil (Bassett et al. 2004) and three for phas gene in Phaseolus vulgaris (Grace et al. 2004). It was thought earlier that the absence of TATA box is associated with constitutively expressing housekeeping genes, but recently TATA box was found to be absent in some inducible genes as well. Apart from TATA box, CAAT and GC boxes are also found to be present upstream; they too enhance the activity of RNA polymerase. Sequence elements like TATA boxes are also referred to as minimal or core promoter elements.
Along with the core promoter elements, other sequence elements are also found that provide sites for attachment of specific transcription factors or enhancer binding protein that trigger transcription of the gene (Alberts et al. 2002). These sequence elements are also called regulatory elements, enhancer binding elements, or simply enhancers. Enhancers are consensus DNA sequence motifs and are associated with levels, place, and timing of expression in response to internal or external (biotic or abiotic) factors. Enhancers can be located upstream, downstream, within coding regions or even in the intron sequences. One of the chief factors responsible for control of gene expression at the transcription level is the activation of enhancer sequences. A few cis-elements have the ability to silence or repress expression of the gene; these are called silencers. The activities of some of the plant promoters are summarized in Table 5.1. The table was generated using TGP, PlantCARE, NCBI, and Plant-Promoter databases for different genes specifically expressed in plants under the influence of suitable reporters/inducers, resulting in higher expression.
Table 5.1.
Promoters used for transgene expression in plants
| Promoter | Gene | Specificity | Reporter/inducer | Comment | References |
|---|---|---|---|---|---|
| At:SAG12_Pl, P2 | sagl2, senescence-associated gene | transgenic Arabidopsis old leaves | mRNA, GUS activity/ auxin, cytokinin, sugar | Essential for senescence-specific regulation of sagl2 | Noh and Amasino (1999) |
| Ms:PEPC7_Pl, P2 | pepc7, pepe | transgenic alfalfa plants | GUS activity/Rhizobium | very high levels of GUS activity | Pathirana et al. (1997) |
| Nt:COMTII_Pl, P2, P3 | comtll | transgenic tobacco leaves | GUS activity/chitin, glucan, TMV, pectin, wounding, MeJa | two to seven-fold increase in GUS activity | Toquin et al. (2003) |
| Ib:BMYl_Pl, P2 | bmyl, amyb, beta-amy | transgenic tobacco plants | GUS activity/sucrose (10%) | 20–57-fold higher GUS activity | Maeo et al. (2001) |
| Dc:HCBT2_P1 P2,P3, P4 | hcbt2 | transgenic parsley protoplasts | GUS activity/elicitior | 3.7–5.5-fold higher GUS activity | Yang et al. (1998) |
| Le:E4_Pl, P2 | e4 | transgenic tomato plants | GUS activity/Ethylene | Increased 10–22-fold in unripe and 1,000-fold in ripened fruit | Montgomery et al. (1993a) |
| Zm:SBEl_Pl, P2 | sbel | transgenic maize suspension endosperm cells | LUC activity/sucrose (9%) | two-fold greater LUC activity | Kim and Guiltinan (1999) |
| Vf:GRP3_Pl,P2,P3 | grp3, glycine-rich early nodulin | transgenic Vicia hirsute (5 week-old) nodules | GUS activity/Rhizobium | Essential for full promoter activity | Kuster et al. (1995) |
| Nt:CHN48_Pl, P2 | chn48 | transgenic tobacco calli | LUC activity/elicitor | 10–40-fold higher activity | Yamamoto et al. (1999) |
| At:P5CSA_Pl, P2 | P5CSA_P1 | transgenic Arabidopsis plants | GUS activity/dehydration | five-fold increase in activity | Yoshiba et al. (1999) |
| Ib:SPOAl_Pl, P2 | spoal, gspo-al | transgenic tobacco plants | GUS activity/sucrose (3%) | tissue specific GUS staining | Ohta et al. (1991; |
| Pc:CMPGlb_Pl | cmpgl, elil7 | transgenic parsley suspension cells | GUS activity/elicitor | 44-fold higher activity | Kirsch et al. (2001) |
| Pc:PR2_Pl,P2 | pr2 | transgenic parsley protoplasts | GUS activity/elicitor | three- to eight-fold higher activity | van de Löcht et al. (1990) |
| Pc:PRl.l_Pl,P2, P3 | pr1.1 | transgenic parsley suspension cells | GUS activity/elicitor | 7.5–12.1-fold higher activity | Rushton et al. (1996) |
| Pc:PR1.2_Pl, P2 | prl.2 | transgenic parsley suspension cells | GUS activity/elicitor | 5.2–15.2-fold higher activity | Rushton et al. (1996) |
| Ps:PSL_Pl, P2, P3 | psl | transgenic tobacco seeds | GUS activity, protein | High level of expression | de Pater et al. (1996) |
| Lg:LHCB2_Pl, P2, P3, P4, P5 | lhcb2, cabAB19 | transgenic swollen duckweed seedlings | LUC activity/red light (2 min) | 2–14-fold higher activity | Kehoe et al. (1994) |
| Np:LHCB1.2_Pl,P2, P3, P4, P5 | lhcbl.2 | transgenic tobacco seedlings, transgenic Arabidopsis seedlings | GUS activity/far-red light (cont), red light (pulses 3 min) | Medium 8–20-fold higher expression level | Cerdan et al. (2000) |
| Os:AmylA_Pl, P2, P3, P4 | amylA | transgenic rice embryos | GUS activity/Glucose, gibberellin A3 | two- to six-fold lower expression with glucose, higher with gibberellin A3 | Morita et al. (1998) |
| Os:Amy3D_Pl | amy3D | transgenic rice embryos | GUS activity/glucose | six-fold lower Expression | Morita et al. (1998) |
| Zm:SHl_Pl | shl | transgenic tobacco plants | GUS activity/anaerobic conditions | High level of expression | Yang and Russell (1990) |
| At:CAB3_Pl, P2, P3, P4 | cab3 | transgenic tobacco shoots 3–4 week-old), suspension cells | CAT activity/white light | Medium to high level of expression | Mitra et al. (1989) |
| Pc:WRKYl_Pl, P2, P3 | wrkyl | transgenic parsley protoplasts | GUS activity/elicitor | 2–50-fold higher activity | Eulgem et al. (1999) |
| Hv:LOXA_Pl, P2, P3 | loxA | transgenic barley leaves | GUS activity/methyl jasmonate (MeJA) | 11.9–19.4-fold higher expression | Rouster et al. (1997) |
| Cr:CPR_Pl | crp | transgenic tobacco plants | GUS activity/elicitor | Essential for controlled expression | Cardoso et al. (1997) |
| Nt:BGLUCANASE_ P1,P2 | gln2, gglb50 | transgenic tobacco plants, transgenic tobacco plants (1 month-old) | GUS activity/tobacco mosaic virus, salicylic acid, ethephon (ethylene), water | 2–10-fold higher activity | van de Rhee et al. (1993), Livne et al. (1997) |
| Nt:PR2D_Pl, P2, P3 | pr2d | transgenic tobacco plants | GUS activity/tobacco mosaic virus, salicylic acid, water | 2–18-fold higher activity | Hennig et al. (1993) |
| Nt:PR2D_P2 | pr2b | transgenic tobacco plants | GUS activity/tobacco mosaic virus, salicylic acid, water | five- to nine-fold higher activity | van de Rhee et al. (1993) |
| Np:GNl_Pl | gul | transgenic tobacco plants | GUS activity/SA, ethylene, water, elicitor, wounding | 1.7–21-fold higher activity | Castresana et al. (1990) |
| At:CEL5_Pl | Atlg22880 | transgenic Arabidopsis seedlings | GUS activity/auxin, ABA | Effective GUS staining activity | del Campillo et al. (2004) |
| As:PHYA3_Pl, P2 | phyA3 | transgenic etiolated rice seedlings (2 day-old) | CAT activity/far-red light | five-fold higher activity | Bruce and Quail (1990) |
| St:GLUB_Pl | gluB8-l-3 | transgenic potato plants, transgenic tobacco plants | GUS activity/pathogen, tobacco mosaic virus, elicitor | 2–12-fold higher activity | Mac et al. (2004) |
| Hv:GIII_Pl | GIII | transgenic rice plants, transgenic rice calli | GUS activity/salicylic acid | Fragment length dependent GUS activity | Li et al. (2005) |
| Nt:PRlA_Pl, P2 | prlA | transgenic tobacco plants | GUS activity/salicylic acid, tobacco mosaic virus | 58–110-fold higher activity | Strompen et al. (1998), Uknes et al. (1993) |
| At:CYP85Al_Pl | cyp85Al | transgenic Arabidopsis seedlings | GUS activity/Brassinolide | weak activity | Castle et al. (2005) |
| At:APXl_Pl,P2,P3 | apxl | transgenic Arabidopsis seedlings (2 week-old), (10 day-old) | GUS mRNA, GUS activity/ heat shock, methyl viologen, iron | Effective GUS staining | Storozhenko et al. (1998), Fourcroy et al (2004) |
| At:APX2_Pl | apx2 | transgenic Arabidopsis plants | LUC activity/hydrogen peroxide (H202), high light | ten-fold higher activity | Kimura et al. (2001) |
| At:APX2_P2 | apxlb | transgenic tobacco mesophyll protoplasts | GUS activity/Heat Stress transcription Factor (HsfA2) | ten-fold higher activity | Schramm et al. (2006) |
| At:ATHB6_Pl | athb6 | transgenic Arabidopsis seedlings, transgenic Arabidopsis plants | GUS activity/drought, abscisic acid (ABA), salt | increased GUS staining with high level expression | Soderman et al. (1999) |
| At:CYP85A2_Pl | cyp85A2 | transgenic Arabidopsis seedlings | GUS activity/bras sinolide | promoter activity down-regulated by brassinolide | Castle et al. (2005) |
| At:ELIP2_Pl | elip2 | transgenic Arabidopsis seedlings (10—14 day-old) | LUC activity/high light | 100-fold increased expression | Kimura et al. (2001) |
| At:Ferl_Pl | ferl | transgenic Arabidopsis plants, transgenic Arabidopsis cells | GUS activity/iron, senescence | 17-fold derepression in response to 0.5 mM iron citrate | Tarantino et al. (2003) |
| At:Ferl_P2 | ferl | transgenic Arabidopsis cells | GUS activity/iron | six-fold higher activity in response to 0.5 mM iron citrate | Petit et al. (2001) |
| Ca:Chi2_Pl,P2,P3 | chi2 | transgenic tobacco | GUS activity/infection, mannitol, salt, NaCl, salicylic acid | 1.5–4.5-fold higher activity | Hong and Hwang (2006) |
| Gm:Fer_Pl, P2 | fer | transgenic soybean leaves | LUC activity, GUS activity/ iron | iron dependent GUS activity | Wei and Theil (2000) |
| Hv:BLT101.1_Pl, P2, P3 | bltlOl.l | transgenic barley | GUS activity/low temperature | 2.5-fold higher expression at low temperature | Brown et al. (2001) |
| Hv:BLT4.9_Pl,P2, P3 | blt4.9 | transgenic barley | GUS activity/low temperature | 2.5-6-fold higher expression at low temperature | Dunn et al. (1998) |
| POPLA:PALl_Pl | pall | transgenic tobacco | GUS activity | Differential Gus activity | Gray-Mitsumune et al. (1999) |
| POPLA:PAL2_Pl | pal2 | transgenic tobacco, transgenic poplar | GUS activity | Differential Gus activity | Gray-Mitsumune et al. (1999) |
| Ta:GERMIN_Pl | gf-2.8 | transgenic tobacco seedlings, transgenic tobacco plants | GUS activity/heavy metal, cadmium, copper, cobalt, wounding, TMV | Gus activity detected after induction | Berna and Benier F(1999) |
| Zm:Ferl_Pl | ferl | transgenic maize cells (BMS, Black Mexican Sweet) | GUS activity/iron | 2.5–8-fold increase in activity | Petit et al. (2001) |
| At:FPS2_Pl | fps2 | transgenic Arabidopsis plants, transgenic Arabidopsis protoplasts | GUS activity | Differential Gus activity | Cunillera et al. (2000) |
| At:PALl_Pl, P2 | pall | transgenic Arabidopsis plants (3 week-old), transgenic tobacco plants (6 leaf stage) | GUS activity, GUS mRNA, PAL mRNA/ wounding, HgC12, white light, elicitor, H2o2, mitomycin C (MMC) | 30% increase in GUS activity | Ohl et al. (1990) |
| At:PDF1.2_Pl | pdf 1.2 | transgenic Arabidopsis seedlings (10 day-old), transgenic Arabidopsis plants (4 week-old), transgenic tobacco seedlings (2 week-old), (6 week-old) | GUS activity/jasmonic acid (JA), pathogen, elicitor, methyl jasmonic acid (MeJA), paraquat, methyl viologen, rose bengal, TMV, ethylene | 9–25-fold higher activity | Manners et al. (1998) |
| At:RAD54_Pl | atrad54 | transgenic Arabidopsis seedlings (1 week-old) | GUS mRNA/gamma irradiation | GUS activity detected after gamma irradiation | Osakabe et al. (2006) |
| At:THIl_Pl, P2 | thil | transgenic Arabidopsis plants (14 day-old) | GUS activity/white light, flooding, salt, sugar deprivation | two to six-fold higher activity | Ribeiro et al. (2005) |
| Gm:SCAM4_Pl | cam-4 | transgenic Arabidopsis seedlings (2–4 day-old), transgenic Arabidopsis leaf protoplasts, transgenic Arabidopsis plants (4 week-old) | GUS mRNA, GUS activity/ salt, glycol chitin, Ca2+-ionophore A23187, elicitor, pathogen | 3–15-fold higher activity | Park et al. (2004) |
| Le:LAT52_Pl | lat52 | transgenic tobacco mature pollen | GUS activity, LUC activity | Essential for full promoter activity | Bate and Twell (1998) |
| Zm:GAPC4_Pl | gapc4, gpc4 | transgenic tobacco leaves | GUS activity/anaerobic conditions | seven-fold higher activity | Geffers et al. (2001) |
| Nt:SAR8.2B_Pl, P2, P3 | sar8.2b | transgenic Arabidopsis plants (3 week-old) | GUS activity/Salicylic acid | 4–31-fold higher GUS activity | Song et al. (2002) |
| Nt:PMTlA_Pl | pmtla | transgenic tobacco suspension cells | GUS activity/ (MeJA), ethephon | 10–15-fold higher activity | Xu and Timko (2004) |
| Nt:G10_Pl | g10, tobacco late pollen gene g10 | transgenic tobacco pollen and leaves | GUS activity | Essential for full promoter activity | Rogers et al. (2001) |
| Ps:DRR206D_Pl | drr206-d, pi206 | transgenic tobacco plants (6-laf stage) | GUS activity/Mitomycin C, actinomycin D, etoposide,H2O2, elicitor | Promoter only induced by natural tobacco Pathogen | Choi et al. (2001) |
| Nt:AP24_Pl, P2 | ap24 | transgenic tobacco seedlings, transgenic tobacco plants | GUS activity/ethylene, NaCl, absicisic acid | 2.5–13-fold higher Activity | Raghothama et al (1997) |
| At:OPRl_Pl | oprl | transgenic Arabidopsis seedlings (2-week old) | GUS activity/Methyl jasmonate (MeJ), senescence | 2–3.5-fold higher activity | He and Gan (2001) |
| Sc:OSML13_Pl Sc:OSML81_Pl | osmll3 osml81 | transgenic potato plants | GUS activity/ABA, NaCl, SA, wounding, fungal infection, cold | Differential GUS activity | Zhu et al. (1995) |
| Sc:CI21A_Pl, P2 | ci21A | transgenic potato plants | GUS activity/low temperature (cold) | 1.7–7-fold higher activity | Schneider et al. (1997) |
Types of Promoters and Their Applications in Transgenic Crops
Constitutive Promoters
Constitutive promoters maintain a constant level of activity. The cauliflower mosaic virus (CaMV) 35S promoter (derived from a DNA viral genome) is probably the most widely used plant promoter (Odell et al. 1985). Although “constitutive,” many show differences in the level of expression in different tissues. Apart from delivering very high levels of expression in virtually all regions of the transgenic plant, the CaMV 35S promoter is easily obtainable for research purposes as plant transformation vector cassettes that allow for easy subcloning of the insert transgene of interest.
High levels of transgene expression can be achieved by the CaMV 35S promoter in both monocot and dicot plants (Benfey et al. 1990; Battraw and Hall 1990). The original full-size promoter (−941 to +9) has no significant difference in activity when compared to a −243 bp fragment. Interaction between the cis -acting elements within 343 bp upstream of the promoter results in high constitutive expression (Fang et al. ). However, tissue-specific individual elements have also been found (Benfey and Chua 1989). For the control of expression in specific tissues, two domains “Domain A” (−90 to +8) and “Domain B” (−343 to −90) are very important. Domain A is involved in expression in roots (Lam et al. 1989), while Domain B contains a conserved GATA motif, very much similar to the light responsive cis elements of light inducible promoters (Potenza et al. 2004). Even though CaMV 35S is a very strong promoter, it is strongly down-regulated in plant parasitic nematode feeding sites (Urwin et al. 1997). With the success of CaMV 35S promoter, other viral promoters have also been developed. They include the cassava vein mosaic virus (CsVMV; Verdaguer et al. 1996, 1998; Li et al. 2001), Australian banana streak virus (BSV; Schenk et al. 2001), mirabilis mosaic virus (MMV; Dey and Maiti 1999), and figwort mosaic virus (FMV; Maiti et al. 1997) promoters.
There are a few limitations in the use of virus-derived promoters: first, the potential risk to human health from the genes of infective plant viruses (Hodgson 2000), and second, the ability of plant cells to recognize the inserted sequences of nonplant origin and inactivate them via “transcriptional gene silencing.” Silencing is less common in promoters of plant origins.
Strong constitutive promoters of plant origin have also been isolated and used for the development of transgenic plants. Actin, a fundamental cytoskeleton component of the cell, is expressed in almost all the cells of a plant. The Act2 promoter, developed from Arabidopsis showed strong expression in all other parts except the seed coat, hypocotyl, ovary, and pollen sac (An et al. 1996). Similarly, rice Act1 promoter has also been developed (Zhang et al. 1991). Ubiquitins, a highly conserved protein family, are linked to many important cellular functions like chroma-tin structure and DNA repair. Maize ubiquitin 1 promoter (pUbi) has been successfully used for plant transformation of monocots (Weeks et al. 1993; Gupta et al. 2001) and has shown high levels of expression in actively dividing cells. Transgenic plants developed using Ubi.U4 promoter from Nicotiana sylvestris showed a three-fold higher activity when compared to the CaMV-based promoters. The ubiquitin-derived promoters perform very well in metabolically and mitotically active cells.
The constitutive action of a promoter has many drawbacks. Expression (or overexpression) of the transgene at a place where it is not expressed or expressed at a wrong time can have severe consequences on the growth and development of the plant. It can lead to enhanced susceptibility to some pathogens (Berrocal-Lobo et al. 2002) or decreased growth (Bowling et al. 1997). Another concern is the development of resistance by target insects against overexpressed toxins like, e.g., Bt toxin (Huang et al. 1999). For these reasons, it is ideal to strategically develop promoters that are “switched on” precisely when they are needed.
Nonconstitutive (Tissue—Enhanced) Promoterss
Development of a new trait or value—addition of a previously existing one by genetic engineering requires the development of transgenes that are under control and expressed in a tissue-specific, developmental, or inducible manner. This will conserve energy and circumvent the drawbacks associated with constitutive expression to a large extent. It is more realistic to call these promoters tissue enhanced rather than tissue specific as their expression may not be confined to a specific tissue or plant part. Tissue-enhanced gene expression pattern is achieved as a result of several factors executed at various levels of gene control. Also, the more distant 50-cis acting enhancer element may be eliminated during isolation of the promoter, and there may not be effective functional interaction between the promoter cis-elements with the heterologous trans-acting factors present in the transgenic host plant. Thus, the development of such promoters can be very complex and difficult. Because of these complexities, it is preferable to use promoters from homologous or closely related plant taxa, and knowledge about the functionality of both homologous and heterologous promoters in target crop plants is essential.
Roots
These promoters are of high interest as they can be used for multiple applications. Expression of proteins responsible for resistance against drought and salt tolerance, resistance to bacterial (or fungal) pathogens or nematodes (Atkinson et al. 2003), and phytoremediation (Grichko et al. 2000) can improve crop yield. Although root-specific promoters have been isolated in plants (Yamamoto et al. 1991; Liu and Ekramoddoullah 2003), other stress-related studies have identified many candidate genes and their promoters. In maize, bacterial promoters are more popular (Qing et al. 2009). Agrobacterium rhizogenes causes hairy root disease in dicots. The promoters for rooting loci genes (rol) present in the root-inducing (Ri) plasmids are largely studied because of their root-mediated transformation and expression of the transgenes. Most important of the rol promoters is the rolD promoter, which has been much utilized (Stearns et al. 2005; Jayaraj et al. 2008) and extensively used in nitrogen assimilation studies (Fraisier et al. 2000; Fei et al. 2003). Very high levels of rolD promoter activity have been reported earlier (Elmayan and Tepfer 1995), but recently, in a comparative study, it was reported that Arabidopsis ubiquitin promoter (UBQ3) has the highest expression in roots (Wally et al. 2008). The domain A (−90 bp upstream) of CaMV 35S also shows root-specific activity (Benfey and Chua 1989; Benfey et al. 1990).
TobRB7, a putative membrane channel aquaporin, is another valuable plant based root-specific promoter isolated from tobacco (Yamamoto et al. 1991). Root-specific activity of this promoter was observed within 2 days of germination. Recently, a novel gene has been isolated from tomato, having very high expression levels in roots (SlREO); the 2.4-kb region representing the SlREO promoter sequence showed strict root specificity (Jones et al. 2008).
Root Nodules
Root nodules are formed as a result of an endosymbiotic association between Rhizobium and other species of bacteria with leguminous host plants. Within the nodule, the bacteroids fix atmospheric nitrogen that is used by the plant and in return, receive carbon substrates from the plant. This type of symbiosis is well studied. Leghemoglobin is an oxygen-binding protein synthesized in the nodule. The expression of leghemoglobin coincides with the nitrogen fixation in the nodule. Thus, this promoter can be used in nodules to increase nitrogen assimilation. The leghemoglobin promoter glb3 from Sesbania rostrata was expressed in Lotus corniculatus and tobacco-harboring chimeric glb3-uidA (gus) gene fusions (Szabados et al. 1990; Szczyglowski et al. 1996). A 1.9-kb fragment of the glb3 5′-upstream region was found to direct high level of nodule-specific (β-glucuronidase (GUS) activity in L. corniculatus that is restricted to the Rhizobium-infected cells of the nodules. In tobacco (a nonleguminous plant), the activity was restricted primarily to the roots and to phloem cells of the stem and petiole vascular system. A deletion analysis revealed that the region between −429 and −48 bp relative to the ATG was effective for nodule-specific expression.
Tubers
Tubers are storage organs in roots and are staple food source in many countries of the world. Improvement of tuber nutrition value, resistance toward infectious disease and pesticides can be manifested by using tuber-specific expression of transgenes. Patatins are glycoproteins that are one of the major products found in potato tuber. These are tuber- specific and can be induced by sucrose (Jefferson et al. 1990). The patatin promoters Pat1 and Pat2 were used to overexpress transgenes from the minipathway, of bacterial origin, to drive the synthesis of (3-carotene (Provitamin A) in vitamin-A-deficient tubers of potato (Diretto et al. 2007). To enhance the metabolism of the environmental contaminants in tubers the rat P450 monooxygenase gene (CYP1A1) was overexpressed in tubers of potato, also under the control of patatin promoter (Yamada et al. 2002). High transgene expression was seen in developing tubers, and the amount of residual herbicides was much lower than that in nontransgenic plants, indicating that the transgenic plant metabolized and detoxified the herbicides. The processing quality of potato products (fries and chips) was increased by overexpressing transgenes in potato tuber under the control of TSSR (tuber-specific and sucrose-responsive) sequence from potato class I patatin promoter (Zhu et al. 2008). It was also demonstrated that tuber-specific expression of the native and slightly modified MYB transcription factor gene StMtf1(M) activates the phenylpropanoid biosynthetic pathway. The transgenic potato tubers contained four-fold increased levels of caffeoylqui-nates, including chlorogenic acid while also accumulating various flavonols and anthocyanins (Rommens et al. 2008). An 800-bp 5′ upstream sequence of the granule-bound starch synthase (GBSS) gene from potato was highly expressed in stolons and tubers (Visser et al. 1991), where the activities of the transgene in these two organs were 3–25-fold higher than the expression of the CaMV-GUS gene. The GBSS gene promoter was also used to obtain tuber-specific high expression of AmA1, a nonallergenic seed albumin gene from Amaranthus hypo-chondriacus in potato (Chakraborty et al. 2000). Two promoters, sporamin and (β-amylase, have been well characterized in sweet potato (Maeo et al. 2001). The sweet potato sporamin promoter was found to control the expression of the E. coli appA gene in transgenic potato, which encoded a bifunctional enzyme exhibiting both acid phosphatase and phytase activities (Hong et al. 2008). Phytase expression levels in transgenic potato tubers were stable over several cycles of propagation. The study demonstrated that the sporamin promoter can effectively direct high-level recombinant protein expression in potato tubers. Moreover, overexpression of phytase in transgenic potato offers an ideal feed additive for improving phytate-Phosphorous digestibility in monogastric animals along with improvement of tuber yield, enhanced Phosphorous acquisition from organic fertilizers, and has a potential for phytoremediation.
Leaves
The light received in the environment can be roughly categorized as UV, visible, and far red. Three classes of photoreceptors have been identified in higher plants: red light and far-red light absorbing phytochromes (PHYs), blue-light receptors, and UV-light receptors. In Arabidopsis, five members compose the PHY family of photoreceptors (PHY A-E) and at least three different blue light photoreceptors have been identified [cryptochromes (CRYs), NPH1, and NPL1] (Martí?nez-Hernández et al. 2002). These photoreceptors, with association of other molecular systems (transcription factors), control the expression of many genes at the transcriptional and post-transcriptional level. Two important transcription factors are basic Leucine zipper factor HY5 (Oyama et al. 1997) and bHLH factor PIF3 (Martí?nez-Garcí?a*et al. 2000).
The photosynthesis-associated nuclear genes (PhANGS), like the chlorophyll a/b-binding proteins (Cab) and the small subunit of Rubisco (RbcS), contain a number of cis-acting elements, the transcription of which is controlled by light. Some of the motifs like G, I, and GTI boxes are found in the promoter regions of many light-regulated genes (Giuliano et al. 1988; Green et al. 1988; Menkens et al. 1995). The LS5-LS7 region from the Lemna gibba Cab19 gene (Kehoe et al. 1994) and the CGF-1 factor-binding site from the Arabidopsis CAB2 gene (Anderson and Kay 1995) contain the GATA and GT-1 sequences; still these two regions are unable to activate transcription, thus suggesting that additional regulatory elements are involved. This has led to the general hypothesis that light-responsive elements (LREs) are formed by the aggregation of different transcription factors. It has also been shown that artificial sequences composed of paired combinations of tetrameric repeats of G- and GATA boxes or GT1- and GATA-boxes, but not multimers of a single motif, function as LREs (Puente et al. 1996). Monocot rbcS promoters have different cis-acting elements and have different patterns of spatial expression than dicots. The C3 rbcS is specifically expressed in mesophyll cells, while the C4 rbcS is expressed in bundle sheath cells, and not in mesophyll cells (Nomura et al. 2000; Patel and Berry 2008). Overexpression of Arabidopsis phytocrome A (PHYA), under the control of rbcS promoter, in commercially important rice varieties produced an increased number of panicles per plant (Garg et al. 2006). In an attempt to obtain high-level production of intact Acidothermus cellulolyticus endoglucanase (E1) in transgenic tobacco plants using the constitutive (Mac) as well as light-inducible tomato Rubisco small subunit promoter (RbcS-3C), it was observed that RbcS-3 promoter was more favorable for E1 expression in transgenic plants than the Mac promoter (Dai et al. 2005). Moreover, by replacing RbcS-3C UTL with AMV RNA4 UTL, E1 production was enhanced more than two-fold. In a comparative study of the expression pattern of heterologous RbcS, RbcS3CP (0.8 kbp) from tomato, SRS1P (1.5 kbp) from soybean, and CaMV 35S in apple, it was found that the activity of SRS1P promoter was strictly dependent on light, whereas that of the RbcS-3C promoter appeared not to be so (Gittins et al. 2000). Later rolCP and CoYMVP were used for expression in vegetative tissues of apple; the CoYMV promoter was slightly more active than the rolC promoter, although expression was at a lower level than the CaMV 35S promoter (Gittins et al. 2003). The results indicated that both promoters could be suitable to drive the expression of transgenes to combat pests and diseases of apple that are dependent on interaction with the phloem.
The Cab proteins are highly expressed in green tissues and are often associated with other proteins to form the light-harvesting complex (Lhc). The expression pattern of the Cab gene in plants is different from that of the RbcS under certain physiological conditions as response to light quality and diurnal rhythm is different between these two genes (Ha and An 1988). Upon analysis of regulatory elements of Cab-E gene from Nicotiana plumbaginifolia, three positive and one negative cis-acting elements that influence photoregulation were found and of the three positive promoters two (PRE1 and PRE2) confer maximum level of photoregula-tion (Castresana et al. 1988). Tobacco plants when transformed with a chimeric gene encoding the A1 subunit of cholera toxin regulated by wheat Cab-1 promoter greatly reduced susceptibility to the bacterial pathogen Pseudomonas tabaci (Beffa et al. 1995).
Both RbcS and Cab are members of a multigene family and are expressed at very high level in green tissues (especially leaves), but many genes within the family contribute to the total protein content. Thus, the level of transgene expression is potentially dependent on the gene promoter used, so a strong green tissue-specific promoter from a single gene family will be most valuable.
Flowers
A substantial economic market has developed for cut flowers. Floral-specific promoters are therefore important for use in engineering transgenic flower varieties that may enhance vase life, visually appealing character of the flowers (reviewed by Mol et al. 1999) along with fragrance of interest and resistance to pests (Dolgov et al. 1995). The UEP1 promoter from Chrysanthemum when fused with a reporter gene (GUS) and transformed back into Chrysanthemum showed very high levels of expression in ray florets and three-fold lower expression in disk florets (Annadana et al. 2002). The activity of UEP1 promoter in ray florets is limited to petal tissues and does not extend into the tube of the petal or the sexual whorls of the floret. The promoter had 50-fold higher expression when compared with double CaMV-based promoters in petal tissues of ray florets (Annadana et al. 2002). This study also showed that CER6 promoter, associated with the wax biosynthesis pathway, had very high expression in ray florets, but the expression was much variable when compared to the UEP1 promoter.
Flavonoids are common color pigments in flowers and also perform many other functions including signaling and UV-protection. Engineering of the flavo-noid biosynthetic pathway has led to the development of blue carnations (Holton 1995) and blue roses (Katsumoto et al. 2007). Chalcone synthase (CHS) genes as well as promoters have been studied extensively. The French bean CHS15 promoter showed expression in flowers and root tips of transformed tobacco plants (Faktor et al. 1996). In flowers, expression was confined to the pigmented part of petals and was induced in a transient fashion. Floral and root-specific expression required two conserved motifs, G-box and H-box, located near the TATA box. To evaluate the tissue-specific role of these motifs, a 39-bp DNA fragment containing the two motifs was prepared and fused with minimal promoters of CHS15 and CaMV 35S along with a marker gene (GUS). Tobacco plants were transformed and it was observed that the 39-bp polymer confers, upon both minimal promoters, a high level of expression that follows the typical tissue-specific expression pattern (Faktor et al. 1997). A chromoplast-specific caroten-oid-associated gene (OgCHRC) and its promoter (Pchrc) was isolated from an orchid species (Oncidium), which showed very high and had flower-specific expression (Chiou et al. 2008).
Pistils
Pistil comprises the female part of the flower and includes stigma, style, and ovary. Identification of ovule-specific promoters is useful for the genetic engineering of crops with a variety of desirable traits, such as genetically engineered partheno-carpy, female sterility, or seedless fruits. The SK2 gene from Solanum tuberosum encodes a pistil-specific endochitinase; the promoter from this gene was fused with a reporter (GUS) and when transformed back into potato, high-level expression specific to pistil was observed (Ficker et al. 1997). The 2.4-kb 5′-flanking region of the pistil-specific thaumatin gene (PsTL1) from Japanese pear, when transformed in tobacco, showed high expression in pistil, low in anther, and no detectable expression in the floral organs or the leaves. The promoter for Arabidopsis AGL11 gene, when transformed back into Arabidopsis, showed high expression in the center of the young ovary, while expression was not seen in vegetative plant tissues, sepals, petals, or androecium (Nain et al. 2008).
Pollen/Anther
Anther as well as pollen-specific expression can be classified into “early” and “late” phases. The “early” phase comprises genes that are expressed during anther development and sporophytic tissue formation, while “late” phase involves expression during gametophyte generation and pollen formation/maturation. The 122-bp 50 region of a tapetum-specific gene (TA29) isolated from tobacco programmed tapetum-specific expression as seen by fusing this promoter with a reporter (Koltunow et al. 1990). The expression increased in the developing anther and decreased as the microspores began to mature into pollen. The TA29 promoter, fused with RNase (barnase), has been used to develop nuclear male-sterile plants (Mariani et al. 1990). In a comparative analysis, expression patterns of Bp4 promoter from rapeseed and the NTM19 promoter from tobacco were studied in transgenic tobacco (Custers et al. 1997). The Bp4 promoter became active only after the first pollen mitosis and not in the microspores, while the NTM19 promoter turned out to be highly microspore specific and directed very high level of GUS expression to the unicellular microspores; more importantly both the promoters were expressed only in the male germline (Custers et al. 1997). In indica rice, promoter of OSIPA was active during the late stages of pollen development and remained active till anthesis, whereas OSIPK promoter was active at a low level in developing anther till the pollen matured. OSIPK promoter activity diminished before anthesis. Both the promoters showed a potential to target expression of the genes of interest in developmental stage-specific manner and could help engineer pollen-specific traits in transgenic crops (Gupta et al. 2007). The anther- and tapetum-specific gene TomA108 was present in as single copy per haploid genome of tomato. The fusion of β-glucuronidase to the TomA108 promoter demonstrated that the promoter was highly active from early meiosis to free microspores production in tapetum of tobacco (Xu et al. 2006).
Recently, a gene from pea, PsEND1, showed very high and early expression in anther primordium cells. Later PsEND1 expression became restricted to the epidermis, connective, endothecium, and middle layer, but it was never observed in tapetal cells or microsporocytes. On fusion of the PsEND1 promoter region to the cytotoxic barnase gene to induce specific ablation of the cell layers, where the PsEND1 was expressed it produced male-sterile plants in tobacco and tomato (Roque et al. 2007). The PsEND1-barnase gene is quite different from other chimeric genes previously used to obtain male-sterile plants. The tapetum-specific promoter produces the ablation of specific cell lines during the initial steps of the anther development, but this chimeric construct (PsEND1-barnase) arrests the microsporogenesis before differentiation of the microspore mother cells and so, no viable pollen grains are produced. This strategy represents an excellent alternative to generate genetically engineered male-sterile plants. The PsEND1 promoter has high potential to prevent undesirable horizontal gene flow in many plant species (Roque et al. 2007). Two anther-specific cDNAs (designated GhACS1 and GhACS2) encoding acyl-CoA synthetases (ACSs) isolated from cotton flower cDNA library were seen to accumulate in developing anthers. GUS expression controlled under the GhACS1 promoter showed high and specific expression in primary sporogenous cells, pollen mother cells, microspores, and tapetal cells (Wang and Li 2009).
Compared to “early” phase genes a few “late” phase genes have also been characterized. The promoter of tomato Lat52 gene showed pollen-specific activity when transformed to tomato, tobacco, and Arabidopsis plants. Its expression was also correlated with the onset of microspore mitosis and increased progressively until anthesis (Twell et al. 1990). The elements necessary for expression in transgenics were present within 600 bp of the 5′ flanking region. The promoter sequence of BAN215-6 gene from Chinese cabbage (Brassica campestris) showed high similarity with the Lat52 gene (Kim et al. 1997). Expression studies, by Agrobacterium-mediated transformation of tobacco plants, revealed that 383 bp of the BAN215-6 promoter region was sufficient for the anther-specific expression. The expression level was increased during anther development, reaching highest levels in mature pollens (Kim et al. 1997). The promoter of a maize pectin methytransferase gene (ZmC5) was found to be expressed specifically in late pollen development when transformed to tobacco plants (Wakeley et al. 1998). By genome walking PCR, a novel b-mannase gene (LeMAN5) was discovered in tomato, which is involved in cell wall disassembly and degrading mannan polymers. The 5′-upstream region of this endo-b-mannanase gene contained four copies of the pollen-specific cisacting elements POLLEN1LELAT52 (AGAAA). The expression of the putative LeMAN5 promoter region (−543 to +38) in transgenic Arabidopsis was detected in mature pollen, sporangia, discharged pollen, and elongating pollen tubes (Filichkin et al. 2004).
Fruit
Fruits are one of the best delivery vehicles for value-added nutrients and other characters like increasing shelf-life, development of oral vaccines, etc. and there has always been a need for fruit-enhanced gene expression. The promoters of fruit-specific genes, especially fruit ripening genes, have been sought after. The ACC (1-aminocyclopropane-1-carboxylate) oxidase gene, the E8 gene, and polygalacturonase (PG) genes are all fruit-ripening-specific promoters and have been characterized from apple and tomato (Montgomery et al. 1993a,b; Nicholass et al. 1995; Atkinson et al. 1998). The ACC oxidase gene is induced by application of ethylene, and fragments of 1,966 and 1,159 bp of the 5′ region showed both fruit and ripening specificity, whereas for the PG gene promoter, fragments of 1,460 and 532 bp conferred ripening-specific expression in transgenic tomato fruit (Atkinson et al. 1998). The promoter of the E8 gene of tomato is by far the most important fruit-ripening-specific promoter. It has been successfully applied in a number of instances including enhancement of aroma of tomato by expressing Clarkia breweri S-linalool synthase gene (LIS) (Lewinsohn et al. 2001), fruit-specific expression of viral proteins (Sandhu et al. 2000), and cholera toxin gene (CTB) in an effort to make edible vaccines (He et al. 2008). The tomato PG gene is also associated with fruit ripening and its promoter was successfully employed to overexpress a bacterial phytoene synthase gene resulting in increased carotenoid content (Fraser et al. 2002) and a lemon basil a-zingiberene synthase gene (ZIS) in tomato fruit to increase both mono- and sesqui-terpene contents (Davidovich-Rikanati et al. 2008).
Seeds
Like fruits, seeds are also an excellent vehicle to pack transgenic products. Seed-specific transgenic technology can be used to enhance nutrient quality, production of pharmaceutical compounds, edible vaccines, etc. The genes expressed at very high level in the seeds are seed storage proteins and these have become the target of choice. Promoters for dicots as well as monocots have been extensively studied and several seed-specific elements have been characterized. The promoter region of soybean b-conglycinin was expressed in the embryo during the mid to late stages of seed development (Chen et al. 1989). The 2.4-kb upstream region of the sunflower Helianthinin gene (HaG3-A) also conferred high embryo-specific expression in transgenic Arabidopsis (Nunberg et al. 1994). The 0.8-kb fragment of the 5′ β-phaseolin gene of French bean (Phaseolus vulgaris) showed strong, temporally regulated, and embryo-specific expression in transgenic tobacco plants (Bustos et al. 1989). The expression pattern of the promoter fragment (1,108 bp) of the a-globulin gene in cotton was studied in transgenic cotton, Arabidopsis, and tobacco. Expression was initiated during the torpedo stage of seed development in tobacco, Arabidopsis, and during cotyledon expansion stage in cotton. The activity increased sharply until embryo maturation in all the three species. Expression was not detected in stem, leaf, root, pollen, or floral bud of transgenic cotton, thus confirming the high seed specificity of the promoter (Sunilkumar et al. 2002).
For monocots, several seed-specific promoters have been used successfully to incorporate many traits. The promoter region of the endosperm-specific protein hordein (D and B hordein) from barley has been well characterized in transgenic rice, barley, and wheat (Furtado et al. 2008, 2009). Six promoters (GluA-1, GluA-2, GluA-3, GluB-3, GluB-5, GluC) of seed storage glutenin genes were isolated from rice and their expression potential was checked in transgenic rice. The GluA-1, GluA-2, and GluA-3 promoters directed expression in the outer portion of the endosperm, while GluB-5 and GluC promoters directed expression in the whole endosperm. The GluB-3 promoter directed expression solely in aleurone and sub-aleurone layers, while maximum activity was pertained to the GluC promoter (Qu et al. 2008). Recently, edible vaccines are being made in transgenic rice against house dust mite allergy (Yang et al. 2008) and Japanese cedar pollen allergen (Yang et al. 2007) under the control of GluB-1 promoter and cholera toxin B subunit under the control of wheat Bx17 promoter containing an intron of the rice act1 (Oszvald et al. 2008). The zein promoters from maize have been used for many applications. Transgenic maize with enhanced provitamin A content in the kernel was developed by endosperm-specific expression of the bacterial genes (crtB and crtI) under the control of a “super g-zein promoter” (Aluru et al. 2008). Increase of total carote-noids was up to 34-fold with a preferential accumulation of b-carotene in the maize endosperm. The phyA2 from Aspergillus niger was successfully expressed in maize seeds using the maize embryo specific globulin-1 promoter. The transgenic seeds showed a 50-fold increase in phytase activity (Chen et al. 2008). The developed maize hybrids had improved phosphorus availability for pig and poultry feed.
Factors Affecting Stability and Level of Transgene Expression
SAR/MAR Effect on Transgene Expression
Transgenic plants often display the chromosomal position effect, which results because of transgene integration events taking place within euchromatin, producing irregular and mixed expression. The pre-existing chromatin structure at the site of integration ultimately determines the expression level, acting either as an enhancer or as a silencer (Taddei et al. 2004). The chromosomal position effect can be prevented if the transgene is flanked by matrix attachment regions (MARs) also known as scaffold attachment regions (SARs) which are DNA elements that bind to the nuclear matrix (Mirkovitch et al. 1984; Allen et al. 2000). The location of MARs within transcription regulatory elements suggests that MARs may serve to bring these DNA sequences in proximity to the scaffold, thereby promoting enhancer and promoter activity by facilitating interaction with transcription factors (Nardozza et al. 1996). This inference is supported by loop domain model studies in which different expression profiles were observed on comparative analysis of transgenes that were flanked by MARs and those lacking it. Transgenes lacking MARs are influenced much by the surrounding chromatin structure; their expression levels are also dependent on local chromatin state. Transgenes that are flanked by MARs act independent of local chromatin state; thus, multiple copies of MAR-flanked transgene insertion might proportionally increase expression (Gasser and Laemmli 1986; Stief et al. 1989).
During gene activation chromatin structure becomes relaxed and the DNA is more accessible to DNase I. MARs that flank the chromatin loop domain function as the boundaries to differentiate active from inactive chromatin (Martienssen 2003). Later, on the basis of this inference, comparative study of the higher order chromatin structure and their accessibility to DNase I was performed in Arabidopsis and maize nuclei resulting in 45-Kb and 25-Kb domains, respectively, (Paul and Ferl 1998). It was reported that transgenic plants containing the synthetic MAR (sMAR) sequences derived from the MAR 3′ end of the immunoglobulin heavy chain (IgH) enhancer, exhibited high levels of expression compared to transgenic plants that lacked the sMARs (Nowak et al. 2001). A diversity of promoters and MAR sequences has been used to analyze transgene expression. Mankin et al. (2003) analyzed the effects of a MAR, from the tobacco RB7 gene on transgene expression from six different promoters in stably transformed tobacco cell cultures. The presence of MARs flanking the transgene increased expression of constructs based on the constitutive CaMV 35S, NOS (nopaline synthase 50 region), and OCS (octopine synthase 50 region) promoters (Mankin et al. 2003). Expression from a heat-shock induced promoter also increased five- to nine-folds, and MARs did not cause expression in the absence of heat shock (Schöffl et al. 1993). The effect of MAR fragments from tobacco gene transformed to two hybrid poplar clones and in tobacco plants was analyzed and found that MARs increased expression approximately ten- and two-fold, respectively, 1 month after cocultivation with Agrobacterium. Apart from gene expression, increased frequency of kanamycin resistance was also reported in poplar shoots (Han et al. 1997).
Different studies on MAR function in plant transgene expression provided interesting conclusions. The effect of MAR on transgene expression is analyzed only after the integration of transgene construct within plant genome. Enhancement of transgene expression has been reported in MARs containing stably transformed plant cell lines of soybean Gmhsp 17.6.L (Schöffl et al. 1993), yeast ARS1 (Allen et al. 1993; Vain et al. 1999), tobacco and rice Rb7 (Allen et al. 1996), tomato HSC80 (Chinn and Comai 1996), bean phaseolin (van der Geest et al. 1994), maize Adh1, Mha1 (Brouwer et al. 2002), and Arabidopsis ARS (Liu and Tabe 1998).
In a nutshell, MARs are not highly conserved but possess AT-rich DNA motifs of 100–3,000 bp containing binding sites for DNA topoisomerase II, DNA helicase, and DNA polymerase and thus are involved in structural organization of the genome. The loops created by MARs are topologically independent units of gene regulation and were found to facilitate the transcription of genes by changing topology along with less-condensed chromatin structure. The transgene constructs containing MARS are observed to create its own chromatin domain favorable for transcription; thus, MARS can reduce variability of transgene expression and increase level of expression.
Effect of 5′ and 3′ UTR Regions
The use of a specific promoter, with or without one or more enhancers, does not necessarily guarantee the desired level of gene expression in plants. In addition to the desired transcription levels, other factors such as improper splicing, polyade-nylation, and nuclear export can affect accumulation of both mRNA and the protein of interest. Therefore, methods of increasing RNA stability and translational efficiency through mechanisms of post-transcriptional regulation are needed in the transgenic approach.
With regard to post-transcriptional regulation, it has been demonstrated that certain 50 and 30 untranslated regions (UTRs) of eukaryotic mRNAs play a major role in translational efficiency and RNA stability. For example, the 50 and 30 UTRs of tobacco mosaic virus (TMV) and alfalfa mosaic virus (AMV) coat protein mRNAs can enhance gene expression 5.4-fold and three-fold, respectively, in tobacco plants (Zeyenko et al. 1994). The 5′ and 3′ UTRs of the maize alcohol dehydrogenase-1 gene (adh1) are required for efficient translation in hypoxic protoplasts (Bailey-Serres and Dawe 1996; Hulzink et al. 2002).
Experiments with various 55′ UTR leader sequences demonstrate that various structural features of a 55′ UTR can be correlated with levels of translational efficiency. It was reported that 55′ UTR elements are required for the high-level expression of pollen ACT1 gene in Arabidopsis (Vitale et al. 2003). During the process of initiation of translation 40S ribosomal subunit enters at 5′ end of the mRNA and moves linearly until it reaches the first AUG codon, whereupon a 60S ribosomal subunit attaches and the first peptide bond is formed. Certain 55′ UTR contain AUG codons in mRNA, which interact with 40S ribosomal subunit resulting in a weak context in terms of initiation codon, thus decreasing the rate of translation (Kozak 1991; Lee et al. 2009; Luttermann and Meyers 2009). Additionally, the 55′ UTR nucleotide sequences flanking the AUG initiation site on the mRNA have an impact on translational efficiency. If the framework of the flanking 50 UTR is not favorable, part of the 40S ribosomal subunit fails to recognize the translation start site such that the rate of polypeptide synthesis will be slowed down (Kozak 1991; Pain 1996). Secondary structures of 55′ UTRs (e.g., hairpin formation) also obstruct the movement of 40S ribosomal subunits during their scanning process and therefore negatively impact the efficiency of translation (Kozak 1986; Sonenberg and Pelletier 1988). The relative GC content of a 55′ UTR sequence was shown to be the stability indicator of the potential secondary structure, high GC content indicated instability (Kozak 1991), and long UTRs exhibit a large number of inhibitory secondary structures. The translational efficiency of any given 55′ UTR is highly dependent upon its particular structure and optimization of the leader sequence, which has been shown to increase gene expression as a direct result of improved translation initiation efficiency. Furthermore, significant increase in gene expression has been produced by addition of leader sequences from plant viruses or heat-shock genes (Datla et al. 1993).
In addition to 55′ UTR sequences, 35′ UTR sequences of mRNAs also influence in gene expression and known to control nuclear export, polyadenylation status, subcellular targeting, and rates of translation and degradation of mRNA from RNases. In particular, 30 UTRs contain one or more inverted repeats that can fold into stem-loop structures, which act as a barrier to exoribonucleases, and interact with RNA-binding proteins known to promote RNA stability (Gutiérrez et al. 1999). However, certain elements found within 30 UTR were reported to be RNA destabilizing, one such example occurring in plants is the DST element which can be found in small auxin up RNAs (SAURs) (Gil and Green 1996). A further destabilizing feature of some 35′ UTRs is the presence of AUUUA pentamers (Ohme-Takagi et al. 1993).
The 3′ UTRs were demonstrated to play a significant role in gene expression of several maize genes. Specifically, a 200-bp 35′ sequence is responsible for suppression of light induction of maize small m3 subunit of the ribulose-1, 5-biphosphate carboxylase gene (rbc/m3) in mesophyll cells (Viret et al. 1994). Monde et al. (2000) observed that the pet D35′-UTR stem loop secondary structure was not able to form RNA-protein complex, essential for translational activity and thus acted as weak terminator required for RNA maturation. One 35′ UTR frequently used in genetic engineering of plants is derived from nopaline synthase gene (35′ nos) (Wyatt et al. 1993).
In certain plant viruses, such as alfalfa mosaic virus (AMV) and tobacco mosaic virus (TMV), the highly structured 30 UTRs are essential for replication and can be folded into either a linear array of stem-loop structures, which contain several high-affinity coat protein binding sites or a tRNA-like site recognized by RNA-dependent RNA polymerases (Olsthoorn et al. 1999).
Effect of Introns
Introns are the intragenic regions that are not translated into proteins. These noncoding portions are present in pre-mRNA and further removed by splicing to yield mature RNA. Introns contain acceptor and donor sites at either end as well as a branch point site, which is required for proper splicing by the spliceosome. The number and the length of introns vary widely among species and among genes within the same species. Introns with alternative splicing may introduce greater variability in protein sequences translated from a single gene. Introns also enhance the level of transgene expression in plants (Callis et al. 1987).
Recent studies provided several examples of introns, whose impact on expression is larger than that of the promoter from the same gene. Many genes with fully functional promoter are not essentially expressed at all but require an intron for their expression. A study in Arabidopsis showed that PRF2 intron is required for full expression of a PRF2 promoter and the β-glucoronidase (GUS) and also to convert PFR5:GUS fusion from a reproductive to vegetative pattern (Jeong et al. 2006). Introns can increase the expression level through their enhancer element, an alternative promoter activity, or it can be independent of their conventional enhancer elements, i.e., intron-mediated enhancement (IME). The second intron of Arabidopsis agamous gene (AG) is a well-characterized enhancer-containing intron that can function in both orientations to force the expression of a reporter from a minimal promoter. The Arabidopsis AG, STK, FLC introns and wheat VRN-1 intron act as enhancers. All these introns are large in size providing sufficient room for controlling elements and allow the establishment of stable chromatin conformation required for appropriate expression (Rose 2008).
The studies conducted by Morello et al. (2002, 2006) revealed the role of intron as an alternative promoter in rice. Presences of introns in promoterless genes drive weak expression; these introns are considered to contain promoters that are responsible for expression. The first intron acts as the alternative promoter as observed in Ostub16 and OsCDPK2 in rice, PpAct1 and PpAct5 in Physcomitrella patens and sesame, and FAD2 in Arabidopsis (Kim et al. 2006; Weise et al. 2006).
IME of gene expression in plants indicates that the insertion of one or more introns in a gene construct results in increased accumulation of mRNA and protein relative to similar fusions that lack introns (Mascarenhas et al. 1990). The deletion studies of different introns such as maize Adh1, Sh1 first intron, rice Ostub A1 first intron, Arabidopsis TRP1 first intron and PRF2 intron1 revealed that no specific sequences were absolutely required and no conserved motif was found between enhancing introns (Rose 2008). Sequence analog studies showed enhancement can be restored by substituting the U/GC-rich region of intron with similar sequence analog from another part of intron (Rose 2002). The mutation studies in Arabidopsis TRP1 intron1 and maize Sh1 intron1 revealed that IME is destroyed by simultaneous elimination of branch-points and the 55′ splice site, further indicating that splicing machinery is required for IME (Rose 2002). Additionally, the positions of introns also influence IME on gene expression. The most prominent is the location of the intron within the gene, i.e., the introns present in 55′ UTR of the rice rubi3 gene was shown to enhance expression (Lu et al. 2008). The other significant position of intron is near the starting of the gene (Rose 2004; Chung et al. 2006). Presently, there are several examples of introns (e.g., first intron of OsTua2, OsTua3, OsTub4, and OsTub6) that can greatly influence both the amount and the actual size of the expression, attributing different patterns of expression to the different intron iso-types, thus generating the intron-dependent spatial expression (IDSE) profile (Giané et al. 2009). The specificity of introns acts as enhancer, and alternative promoter, or mediates enhancement on the basis of their numbers and position in a gene construct. Some of the introns with defined specificity have been summarized in Table 5.2.
Table 5.2.
Introns affecting transgene expression in different plants
| Intron | Specificity | Remark | Reference |
|---|---|---|---|
| COX 5c-1 | Arabidopsis | Increased GUS expression level | Curi et al. (2005) |
| COX 5c-2 | |||
| Ubi7 | Potato | Ten-fold higher expression | Garbarino et al. (1995) |
| Adh1 | Maize | 40–100-fold increase in expression | Callis et al. (1987) |
| Act1 | Arabidopsis | High level of reproductive tissue expression | Vitale et al. (2003) |
| RBCS2 | Chlamydomonas reinhardtii | Stable high-level expression | Lumbreras et al. (1998) |
| reg A3 | Volvox carteri | Required for regA expression | Stark et al. (2001) |
| regA5 | |||
| STK | Arabidopsis | Intron-mediated promoter expression in ovules and septum | Kooiker et al. (2005) |
| VRN-1 | Wheat | Essential for promoter activity | Fu et al. (2005) |
| Ostub 16 | Rice | Required for maximum promoter activity | Morello et al. (2002) |
| OsCDPK 2 | Rice | Required for promoter activity | Morello et al. (2006) |
| PpAct 1 | Physcomitrella patens | 11–18-fold higher expression | Weise et al. (2006) |
| PpAct 5 | |||
| PpAct 7 | |||
| Sh1 | Maize | 10–1,000-fold enhanced expression | Maas et al. (1990, 1991) |
| Gap A1 | Maize | Required for full promoter activity | Donath et al. (1995) |
| Actin 3rd intron | Maize | IME | Luehrsen and Walbot (1991) |
| Hsp81 | Maize | IME | Sinibaldi and Mettler (1992) |
| Act 1 | Rice | IME | McElroy et al. (1990) |
| tpi | Rice | Required for promoter activity | Xu et al. (1994) |
Role of Transcription Factors
Transcription factors are sequence-specific DNA-binding proteins that interact with the promoter regions of the target genes and modulate the rate of initiation of mRNA synthesis by RNA polymerase II (Gantet and Memelink 2002). The role of transcription factors in transgene expression is studied by overexpression and antisense technology. For highly conserved transcription factors such as MADS-box or the Myb-like transcription factors, generation of antisense plant is difficult since the target requires an antisense RNA homology of over 50 bp, which is not preferred. Also, due to the presence of highly conserved regions, the specificity of antisense RNA is significantly reduced (Cannon et al. 1990). High-level expression of a transcription factor in a transgenic plant cell might favor the binding of the transcription factor to low affinity binding sites and result in activation of gene expression from noncognate promoters.
To study the effect of transcription factor on transgene, the steroid-binding domain of the glucocorticoid receptor is fused to a plant transcription factor. The absence of ligand represses nuclear localization and DNA-binding activities of transcription factor. After induction, repression is relieved and active protein can rapidly enter the nucleus and exert its transcription factor function. A glucocorticoid-responsive GAL4-VP16 fusion protein has been used to induce the activation of a luciferase reporter gene in transgenic Arabidopsis and tobacco plants, either by growing the plants on nutrient agar containing dexamethasone or by spraying the plants with the inducing compound (Aoyama and Chua 1997).
The Arabidopsis transparent testa glabra (ttg) mutant plants are not able to produce trichomes, anthocyanins, and seed coat pigment but generate excess root hairs. Production of trichomes and anthocyanins could be restored by overex-pression of the maize transcription factor R in a constitutive and inducible manner (Lloyd et al. 1994). An interaction study carried out between transcription factor (myb305) and its promoter-binding site in PAL2 in transgenic tobacco plant revealed that when leaves were inoculated with a PVX-construct expressing Myb305 reporter gene, expression increased (Sablowski et al. 1995). Thus, the ectopic expression of Myb305 in infected tissue incites the higher expression of GUS reporter gene in transgenic tobacco plant with nonmutant PAL2 promoter element.
Synthetic transcription factors are an assembly of multiple zinc finger domains designed to achieve better regulation of gene expression. It is estimated that Arabi-dopsis contains 85 genes that encode zinc finger transcription factors (Riechmann and Ratcliffe 2000). Such synthetic zinc finger transcription factors (TFsZF) can be custom designed for binding to any DNA sequence (Segal and Barbas 2001).
Furthermore, the addition of herpes simplex virus VP16 activation domain to the polydactyl six-zinc finger protein 2C7 increased the expression more than 450-fold in transgenic plants (Liu et al. 1997). Later, Van Eenennaam et al. (2004) constructed five, three-finger zinc finger protein (ZFP) DNA-binding domains which tightly bound to 9-bp DNA sequences located on either the promoter or the coding region of the Arabidopsis GMT gene. When these ZFPs were fused to a maize opaque-2 nuclear localization signal and the maize C1 activation domain, four out of the five resulting ZFP-TFs were able to up-regulate the expression of the GMT gene in leaf protoplast transient assays. The seed-specific expression of these ZFP-TFs was reported to produce heritable increase in seed a-tocopherol level in subsequent generations of transgenic Arabidopsis.
The transcription factors, R and C1, interact to regulate anthocyanin biosynthesis in the maize kernel (Grotewold et al. 2000). In a recent study, it was reported that ectopic expression of a conifer Abscisic Acid Insensitive 3 (ABI3) transcription factor induced high-level synthesis of recombinant human α-L-iduronidase gene in transgenic tobacco leaves (Kermode et al. 2007). Transgenic rice with DREB 1s/CBF or OsDREB 1A/1B transcription factor interact specifically with DRE/CRT or OsDRE cis-acting elements and control the expression of many stress-inducible genes (Ito et al. 2005). In continuation, Zhao et al. (2009) reported on the role of transcription factors on abiotic stress where the expression of yeast YAP1 gene in transgenic Arabidopsis resulted in increased salt tolerance. The YAP1 contains a basic leucine zipper domain similar to that of Jun (Moye-Rowley et al. 1989), which is a component of mammalian AP-1 transcription factor complexes. Nuclear YAP1 regulates the expression of up to 70 genes that are related to oxidative stress caused by high salinity (Zhao et al. 2009).
Effect of DNA Acetylation and Methylation
The interaction of histones with DNA plays an important role in chromatin remodeling and consequently the activation or repression of gene expression (Tian et al 2005). Intrinsic histone acetyltransferases (HATs) and histone deace-tylases (HDs, HDAs, HDACs) drive acetylation and deacetylation, respectively, thus providing a mechanism for reversibly modulating chromatin structure and transcriptional regulation (Jenuwein and Allis 2001). Hyperacetylation relaxes chromatin structure and activates gene expression, whereas hypoacetylation induces chromatin compaction and gene repression. Histone acetylation and deacetylation are reversible and therefore play a significant role in transcriptional regulation associated with developmental programs and environmental conditions. These include day-length (Tian et al. 2003), flowering (He et al. 2003), osmotic and oxidative stress (Brunet et al 2004, De Nadal et al 2004), and cell aging (Imai et al 2000).
Acetylation neutralizes the lysine residues on the amino terminal tails of the histones, thereby neutralizing the positive charges of histone tails and decreasing their affinity to bind DNA. HATs are often associated with proteins forming coactivator complexes, stabilizing the chromatin in an open conformation and transcriptionally active state. These complexes are targeted to promoters by specific transcription factors, allowing the RNApol II holoenzyme to access the promoter DNA sequence, which results in activation of transcription and increased gene expression. Histone deacetylases (HDAC) ameliorate the affinity of histones for DNA as deacetylation of histone tails result in stronger interaction between the basic histone tails and DNA. HDACs are often associated with other proteins that are associated with chromatin condensation and repression of transcription. These corepressor complexes promote heterochromatin formation, blocking access of RNApol II, thus resulting in repression of transcription.
Arabidopsis has 18 members of putative histone deacetylase family (Pandey et al. 2002). Among them AtHDA6 is responsible for silencing transgenes (Murfett et al. 2001), whereas AtHD1 is reported to be a global transcriptional regulator throughout the development of Arabidopsis (Tian et al. 2003). The analysis of microarray data revealed that gene activation is associated with increased levels of site-specific histone acetylation, whereas gene repression does not correlate with the changes in histone acetylation or histone methylation. Many of the HDACs found in plants are Rpd3, HD2, SIR2, and their homologs (Chen and Tian 2007).
DNA methylation is known to play a role in plant gene silencing (Ng and Bird 1999). Methyl CpG-binding protein (MeCP2) was reported to be involved in the recruitment of HDAC to methylated DNA through a corepressor complex, which results in gene silencing. Earlier studies showed that hemimethylation results in inhibition of transient gene expression, whereas nonmethylated gene expressed normally (Weber et al. 1990). In one of the studies, a mutation isolated via a transgene reactivation screen in Arabidopsis, mom1, was thought to act downstream of DNA methylation signals in controlling silencing because it did not confer obvious methylation changes (Amedeo et al. 2000). In recent studies, Shibuya et al. (2009) reported that the pMADS3 gene in petunia, specifically expressed in the stamen and carpels of developing flower, showed ectopic expression after introduction of intron 2. This is known as ect-pMADS3 phenomenon and is due to transcriptional activation based on RNA-directed DNA methylation (RdDM) occurring in a particular CG in a putative cis-element in pMADS3 intron 2. The CG methylation was maintained over generations, along with pMADS3 ectopic expression, even in the absence of RNA triggers. Transcriptional or post-transcriptional gene silencing was expected; instead, upregulated gene expression was observed (Shibuya et al. 2009).
Recently, the new Amplicon-plus targeting technology (APTT) has been developed to overcome the problems of post-transcriptional gene silencing and lower accumulation of transgenic protein. This technology uses a novel combination of techniques, i.e., expression of a mutated PTGS suppressor, P1/HC-Pro, with PVX (potato virus X vector) amplicon encoding a highly-labile L1 protein of canine oral papillomavirus (COPV L1). Appreciable amount of protein accumulation was achieved by targeting the L1 to various cellular compartments, by creating a fusion between the protein of interest and different targeting peptides. Additionally, a scalable “wound-and-agrospray” inoculation method has been developed that allows high-throughput Agrobacterium inoculation of Nicotiana tabacum to facilitate large-scale application of this technology (Azhakanandam et al. 2007).
Conclusions
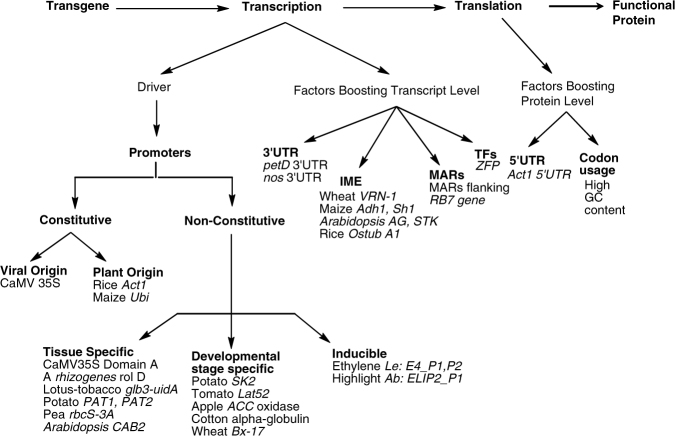
Genetic transformation of crops has opened a new dimension to increase production that benefits both producers and consumers. Its effect can be best utilized in less developed or developing countries where crop yield is severely affected by biotic and abiotic stress. Also, value addition of existing nutrients along with production of novel nutraceuticals will help alleviate nutrition-related deficiencies in famine-stricken countries. Apart from enhancing food value in crop species, transgenic technology can be used to develop visual marker systems to monitor crops and carry out fine scale studies of agricultural crops. Despite the hostility against genetically modified crops in Eastern Europe, many countries in Asia and North America have accepted transgenic crops. In the present scenario, some of the factors responsible for the control of transgene expression at different levels have been summarized in Fig. 5.1. The primary challenge lies with the detailed understanding of the underlying mechanism involved in gene expression, and there is a pressing need to study gene expression, especially its regulation.
Fig. 5.1.
Transgene expression and stability factors
Acknowledgments
RB is thankful to Prof. A.H. Paterson of PGML, UGA, for hosting as a BOYSCAST Fellow, and gratefully acknowledges the financial support from the Department of Science and Technology, GOI (SR/BY/L-08/2007).
Contributor Information
Chittaranjan Kole, Email: ckole@clemson.edu.
Charles H. Michler, Email: michler@purdue.edu
Albert G. Abbott, Email: aalbert@clemson.edu
Timothy C. Hall, Email: tim@idmb.tamu.edu
Rajib Bandopadhyay, Email: rajib-bandopadhyay@bitmesra.ac.in.
Inamul Haque, Email: inam.Hoque@yahoo.com.
Dharmendra Singh, Email: damfire@gmail.com.
Kunal Mukhopadhyay, Email: mkunalus@yahoo.com.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell. Garland Sci, New York, USA
- Allen GC, Hall G, Michalowski S, Newman W, Spiker S, Weissinger AK, Thompson WF. High-level transgene expression in plant cells: effects of a strong scaffold attachment region from tobacco. Plant Cell. 1996;8:899–913. doi: 10.1105/tpc.8.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GC, Hall GE, Jr, Childs LC, Weissinger AK, Spiker S, Thompson WF. Scaffold attachment regions increase reporter gene expression in stably transformed plant cells. Plant Cell. 1993;5:603–613. doi: 10.1105/tpc.5.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GC, Spiker S, Thompson WF. Use of matrix attachment regions (MARs) to minimize transgene silencing. Plant Mol Biol. 2000;43:361–376. doi: 10.1023/A:1006424621037. [DOI] [PubMed] [Google Scholar]
- Aluru M, Xu Y, Guo R, Wang Z, Li S, White W, Wang K, Rodermel S. Generation of transgenic maize with enhanced provitamin A content. J Exp Bot. 2008;59:3551–3562. doi: 10.1093/jxb/ern212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amedeo P, Habu Y, Afsar K, Mittelsten Scheid O, Paszkowski J. Disruption of the plant gene MOM releases transcriptional silencing of methylated genes. Nature. 2000;405:203–206. doi: 10.1038/35012108. [DOI] [PubMed] [Google Scholar]
- An YQ, McDowell JM, Huang S, McKinney EC, Chambliss S, Meagher RB. Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 1996;10:107–121. doi: 10.1046/j.1365-313X.1996.10010107.x. [DOI] [PubMed] [Google Scholar]
- Anderson SL, Kay SA. Functional dissection of circadian clock- and phytochrome-regulated transcription of the Arabidopsis CAB2 gene. Proc Natl Acad Sci USA. 1995;92:1500–1504. doi: 10.1073/pnas.92.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annadana S, Beekwilder MJ, Kuipers G, Visser PB, Outchkourov N, Pereira A, Udayakumar M, De Jong J, Jongsma MA. Cloning of the chrysanthemum UEP1 promoter and comparative expression in florets and leaves of Dendranthema grandiflora. Transgenic Res. 2002;11:437–445. doi: 10.1023/A:1016313924844. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Chua NH. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 1997;11:605–612. doi: 10.1046/j.1365-313X.1997.11030605.x. [DOI] [PubMed] [Google Scholar]
- Assem SK, Hassan OS. Real time quantitative PCR analysis of transgenic maize plants produced by Agrobacterium mediated transformation and particle bombardment. J Appl Sci Res. 2008;4:408–414. [Google Scholar]
- Atkinson HJ, Urwin PE, McPherson MJ. Engineering plants for nematode resistance. Annu Rev Phytopathol. 2003;41:615–639. doi: 10.1146/annurev.phyto.41.052002.095737. [DOI] [PubMed] [Google Scholar]
- Atkinson RG, Bolitho KM, Wright MA, Iturriagagoitia-Bueno T, Reid SJ, Ross GS. Apple ACC-oxidase and polygalacturonase: ripening-specific gene expression and promoter analysis in transgenic tomato. Plant Mol Biol. 1998;38:449–460. doi: 10.1023/A:1006065926397. [DOI] [PubMed] [Google Scholar]
- Azhakanandam K, Weissinger SM, Nicholson JS, Qu R, Weissinger AK. Amplicon-plus targeting technology (APTT) for rapid production of a highly unstable vaccine protein in tobacco plants. Plant Mol Biol. 2007;63:393–404. doi: 10.1007/s11103-006-9096-9. [DOI] [PubMed] [Google Scholar]
- Bailey-Serres J, Dawe RK. Both 5′ and 3′ sequences of maize adh1 mRNA are required for enhanced translation under low-oxygen conditions. Plant Physiol. 1996;112:685–695. doi: 10.1104/pp.112.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett CL, Nickerson ML, Farrell RE, Jr, Harrison M. Multiple transcripts of a gene for a leucine-rich repeat receptor kinase from morning glory (Ipomoea nil) originate from different TATA boxes in a tissue-specific manner. Mol Genet Genomics. 2004;271:752–760. doi: 10.1007/s00438-004-1031-7. [DOI] [PubMed] [Google Scholar]
- Bate N, Twell D. Functional architecture of a late pollen promoter: pollen-specific transcription is developmentally regulated by multiple stage-specific and co-dependent activator elements. Plant Mol Biol. 1998;37:859–869. doi: 10.1023/A:1006095023050. [DOI] [PubMed] [Google Scholar]
- Battraw MJ, Hall TC. Histochemical analysis of CaMV 35S promoter-β-glucuronidase gene expression in transgenic rice plants. Plant Mol Biol. 1990;15:527–538. doi: 10.1007/BF00017828. [DOI] [PubMed] [Google Scholar]
- Beffa R, Szell M, Meuwly P, Pay A, Vőgeli-Lange R, Métraux JP, Neuhaus G, Meins F, Jr, Nagy F. Cholera toxin elevates pathogen resistance and induces pathogenesis-related gene expression in tobacco. EMBO J. 1995;14:5753–5761. doi: 10.1002/j.1460-2075.1995.tb00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán J, Jaimes H, Echeverry M, Ladino Y, López D, Duque MC, Chavarriaga P, Tohme J. Quantitative analysis of transgenes in cassava plants using real-time PCR technology. In Vitro Cell Dev Biol Plant. 2009;45:48–56. doi: 10.1007/s11627-008-9159-5. [DOI] [Google Scholar]
- Benfey PN, Chua NH. Regulated genes in transgenic plants. Science. 1989;244:174–181. doi: 10.1126/science.244.4901.174. [DOI] [PubMed] [Google Scholar]
- Benfey PN, Ren L, Chua NH. Tissue-specific expression from CaMV 35S enhancer subdomains in early stages of plant development. EMBO J. 1990;9:1677–1684. doi: 10.1002/j.1460-2075.1990.tb08291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna A, Bernier F. Regulation by biotic and abiotic stress of a wheat germin gene encoding oxalate oxidase, a H2O2-producing enzyme. Plant Mol Biol. 1999;39:539–549. doi: 10.1023/A:1006123432157. [DOI] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Molina A, Solano R. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J. 2002;29:23–32. doi: 10.1046/j.1365-313x.2002.01191.x. [DOI] [PubMed] [Google Scholar]
- Bhat SR, Srinivasan S. Molecular and genetic analyses of transgenic plants: considerations and approaches. Plant Sci. 2002;163:673–681. doi: 10.1016/S0168-9452(02)00152-8. [DOI] [Google Scholar]
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell. 1997;9:1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer C, Bruce W, Maddock S, Avramova Z, Bowen B. Suppression of transgene silencing by matrix attachment regions in maize: A dual role for the maize 50ADH1 matrix attachment region. Plant Cell. 2002;14:2251–2264. doi: 10.1105/tpc.004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AP, Dunn MA, Goddard NJ, Hughes MA. Identification of a novel low-temperature-response element in the promoter of the barley (Hordeum vulgare L.) gene blt101.1. Planta. 2001;213:770–780. doi: 10.1007/s004250100549. [DOI] [PubMed] [Google Scholar]
- Bruce WB, Quail PH. cis-Acting elements involved in photoregulation of an oat phyto-chrome promoter in rice. Plant Cell. 1990;2:1081–1089. doi: 10.1105/tpc.2.11.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacety-lase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Bustos MM, Guiltinan MJ, Jordano J, Begum D, Kalkan FA, Hall TC. Regulation of β-glucuronidase expression in transgenic tobacco plants by an A/T-rich, cis-acting sequence found upstream of a French bean β-phaseolin gene. Plant Cell. 1989;1:839–853. doi: 10.1105/tpc.1.9.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis J, Fromm M, Walbot V. Introns increase gene expression in cultured maize cells. Genes Dev. 1987;1:1183–1200. doi: 10.1101/gad.1.10.1183. [DOI] [PubMed] [Google Scholar]
- Cannon M, Platz J, O'Leary M, Sookdeo C, Cannon F. Organ-specific modulation of gene expression in transgenic plants using antisense RNA. Plant Mol Biol. 1990;15:39–47. doi: 10.1007/BF00017722. [DOI] [PubMed] [Google Scholar]
- Cardoso MI, Meijer AH, Rueb S, Machado JA, Memelink J, Hoge J. A promoter region that controls basal and elicitor-inducible expression levels of the NADPH:cytochrome P450 reduc-tase gene (Cpr) from Catharanthus roseus binds nuclear factor GT-1. Mol Gen Genet. 1997;256:674–681. doi: 10.1007/pl00008617. [DOI] [PubMed] [Google Scholar]
- Castle J, Szekeres M, Jenkins G, Bishop GJ. Unique and overlapping expression patterns of Arabidopsis CYP85 genes involved in brassinosteroid C-6 oxidation. Plant Mol Biol. 2005;57:129–140. doi: 10.1007/s11103-004-6851-7. [DOI] [PubMed] [Google Scholar]
- Castresana C, de Carvalho F, Gheysen G, Habets M, Inzé D, Van Montagu M. Tissue-specific and pathogen-induced regulation of a Nicotiana plumbaginifolia β-1, 3-glucanase gene. Plant Cell. 1990;2:1131–1143. doi: 10.1105/tpc.2.12.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana C, Garcia-Luque I, Alonso E, Malik VS, Cashmore AR. Both positive and negative regulatory elements mediate expression of a photoregulated CAB gene from Nicoti-ana plumbaginifolia. EMBO J. 1988;7:1929–1936. doi: 10.1002/j.1460-2075.1988.tb03030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdan PD, Staneloni RJ, Ortega J, Bunge MM, Rodriguez-Batiller MJ, Sanchez RA, Casal JJ. Sustained but not transient phytochrome A signaling targets a region of an Lhcb1*2 promoter not necessary for phytochrome B action. Plant Cell. 2000;12:1203–1211. doi: 10.1105/tpc.12.7.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Chakraborty N, Datta A. Increased nutritive value of transgenic potato by expressing a nonallergenic seed albumin gene from Amaranthus hypochondriacus. Proc Natl Acad Sci USA. 2000;97:3724–3729. doi: 10.1073/pnas.050012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Xue G, Chen P, Yao B, Yang W, Ma Q, Fan Y, Zhao Z, Tarczynski MC, Shi J. Transgenic maize plants expressing a fungal phytase gene. Transgenic Res. 2008;17:633–643. doi: 10.1007/s11248-007-9138-3. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Tian L. Roles of dynamic and reversible histone acetylation in plant development and polyploidy. Biochim Biophys Acta. 2007;1769:295–307. doi: 10.1016/j.bbaexp.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Naito S, Nakamura I, Beachy RN. Regulated expression of genes encoding soybean beta-conglycinins in transgenic plants. Dev Genet. 1989;10:112–122. doi: 10.1002/dvg.1020100207. [DOI] [PubMed] [Google Scholar]
- Chinn AM, Comai L. The heat shock cognate 80 gene of tomato is flanked by matrix attachment regions. Plant Mol Biol. 1996;32:959–968. doi: 10.1007/BF00020492. [DOI] [PubMed] [Google Scholar]
- Chiou YC, Wu K, Yeh KW. Characterization and promoter activity of chromoplast specific carotenoid associated gene (CHRC) from Oncidium Gower Ramsey. Biotechnol Lett. 2008;30:1861–1866. doi: 10.1007/s10529-008-9767-5. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Klosterman SJ, Hadwiger LA. A comparison of the effects of DNA-damaging agents and biotic elicitors on the induction of plant defense genes, nuclear distortion, and cell death. Plant Physiol. 2001;125:752–762. doi: 10.1104/pp.125.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung BY, Simons C, Firth AE, Brown CM, Hellens RP. Effect of 50UTR introns on gene expression in Arabidopsis thaliana. BMC Genomics. 2006;7:120. doi: 10.1186/1471-2164-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunillera N, Boronat A, Ferrer A. Spatial and temporal patterns of GUS expression directed by 50 regions of the Arabidopsis thaliana farnesyl diphosphate synthase genes FPS1 and FPS2. Plant Mol Biol. 2000;44:747–758. doi: 10.1023/A:1026588708849. [DOI] [PubMed] [Google Scholar]
- Curi GC, Chan RL, Gonzalez DH. The leader intron of Arabidopsis thaliana genes encoding cytochrome c oxidase subunit 5c promotes high-level expression by increasing transcript abudance and translation efficiency. J Exp Bot. 2005;56:2563–2571. doi: 10.1093/jxb/eri250. [DOI] [PubMed] [Google Scholar]
- Custers JB, Oldenhof MT, Schrauwen JA, Cordewener JH, Wullems GJ, van Lookeren Campagne MM. Analysis of microspore-specific promoters in transgenic tobacco. Plant Mol Biol. 1997;35:689–699. doi: 10.1023/A:1005846527674. [DOI] [PubMed] [Google Scholar]
- Dai Z, Hooker BS, Quesenberry RD, Thomas SR. Optimization of Acidothermus cellulo-lyticus endoglucanase (E1) production in transgenic tobacco plants by transcriptional, post-transcription and post-translational modification. Transgenic Res. 2005;14:627–643. doi: 10.1007/s11248-005-5695-5. [DOI] [PubMed] [Google Scholar]
- Datla RSS, Bekkaoui F, Hammerlindl JK, Pilate G, Dunstan DI, Crosby WL. Improved high-level constitutive foreign gene expression in plants using an AMV RNA4 untranslated leader sequence. Plant Sci. 1993;94:139–149. doi: 10.1016/0168-9452(93)90015-R. [DOI] [Google Scholar]
- Davidovich-Rikanati R, Lewinsohn E, Bar E, Iijima Y, Pichersky E, Sitrit Y. Overexpres-sion of the lemon basil alpha-zingiberene synthase gene increases both mono- and sesquiter-pene contents in tomato fruit. Plant J. 2008;56:228–238. doi: 10.1111/j.1365-313X.2008.03599.x. [DOI] [PubMed] [Google Scholar]
- De Nadal E, Zapater M, Alepuz PM, Sumoy L, Mas G, Posas F. The MAPK Hog1 recruits Rpd3 histone deacetylase to activate osmoresponsive genes. Nature. 2004;427:370–374. doi: 10.1038/nature02258. [DOI] [PubMed] [Google Scholar]
- de Pater S, Pham K, Klitsie I, Kijne J. The 22 bp W1 element in the pea lectin promoter is necessary and, as a multimer, sufficient for high gene expression in tobacco seeds. Plant Mol Biol. 1996;32:515–523. doi: 10.1007/BF00019103. [DOI] [PubMed] [Google Scholar]
- del Campillo E, Abdel-Aziz A, Crawford D, Patterson SE. Root cap specific expression of an endo-b-1, 4-D-glucanase (cellulase): a new marker to study root development in Arabidop-sis. Plant Mol Biol. 2004;56:309–323. doi: 10.1007/s11103-004-3380-3. [DOI] [PubMed] [Google Scholar]
- Dey N, Maiti IB. Structure and promoter/leader deletion analysis of mirabilis mosaic virus (MMV) full-length transcript promoter in transgenic plants. Plant Mol Biol. 1999;40:771–782. doi: 10.1023/A:1006285426523. [DOI] [PubMed] [Google Scholar]
- Diretto G, Al-Babili S, Tavazza R, Papacchioli V, Beyer P, Giuliano G. Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. PLoS ONE. 2007;2:e350. doi: 10.1371/journal.pone.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgov SV, Mityshkina TU, Rukavtsova EB, Buryanov YI, Vainstein A, Weiss D. Production of transgenic plants of Chrysanthemum morifolium Ramat with the Bacillus thuringiensis delta endotoxin. Acta Hortic. 1995;420:46–47. [Google Scholar]
- Donath M, Mendel R, Cerff R, Martin W. Intron-dependent transient expression of the maize GapA1 gene. Plant Mol Biol. 1995;28:667–676. doi: 10.1007/BF00021192. [DOI] [PubMed] [Google Scholar]
- Dunn MA, White AJ, Vural S, Hughes MA. Identification of promoter elements in a low-temperature-responsive gene (blt4.9) from barley (Hordeum vulgare L.) Plant Mol Biol. 1998;38:551–564. doi: 10.1023/A:1006098132352. [DOI] [PubMed] [Google Scholar]
- Elmayan T, Tepfer M. Evaluation in tobacco of the organ specificity and strength of the rolD promoter, domain A of the 35S promoter and the 35S2 promoter. Transgenic Res. 1995;4:388–396. doi: 10.1007/BF01973757. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Schmelzer E, Hahlbrock K, Somssich IE. Early nuclear events in plant defence signalling: rapid gene activation by WRKY transcription factors. EMBO J. 1999;18:4689–4699. doi: 10.1093/emboj/18.17.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Coleman JOD, Kearns A. Plant cell culture. London, UK: BIOS Scientific Publ, Taylor and Francis; 2003. [Google Scholar]
- Faktor O, Kooter JM, Dixon RA, Lamb CJ. Functional dissection of a bean chalcone synthase gene promoter in transgenic tobacco plants reveals sequence motifs essential for floral expression. Plant Mol Biol. 1996;32:849–859. doi: 10.1007/BF00020482. [DOI] [PubMed] [Google Scholar]
- Faktor O, Loake G, Dixon RA, Lamb CJ. The G-box and H-box in a 39 bp region of a French bean chalcone synthase promoter constitute a tissue-specific regulatory element. Plant J. 1997;11:1105–1113. doi: 10.1046/j.1365-313X.1997.11051105.x. [DOI] [Google Scholar]
- Fang RX, Nagy F, Sivasubramaniam S, Chua NH. Multiple cis regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. Plant Cell. 1989;1:141–150. doi: 10.1105/tpc.1.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Chaillou S, Hirel B, Mahon JD, Vessey JK. Overexpression of a soybean cytosolic glutamine synthetase gene linked to organspecific promoters in pea plants grown in different concentrations of nitrate. Planta. 2003;216:467–474. doi: 10.1007/s00425-002-0873-7. [DOI] [PubMed] [Google Scholar]
- Ficker M, Wemmer T, Thompson RD. A promoter directing high level expression in pistils of transgenic plants. Plant Mol Biol. 1997;35:425–431. doi: 10.1023/A:1005898624425. [DOI] [PubMed] [Google Scholar]
- Filichkin SA, Leonard JM, Monteros A, Liu PP, Nonogaki H. A novel endo-β-mannanase gene in tomato LeMAN5 is associated with anther and pollen development. Plant Physiol. 2004;134:1080–1087. doi: 10.1104/pp.103.035998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcroy P, Vansuyt G, Kushnir S, Inzé D, Briat JF. Iron-regulated expression of a cytosolic ascorbate peroxidase encoded by the APX1 gene in Arabidopsis seedlings. Plant Physiol. 2004;34:605–613. doi: 10.1104/pp.103.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisier V, Gojon A, Tillard P, Daniel-Vedele F. Constitutive expression of a putative high-affinity nitrate transporter in Nicotiana plumbaginifolia: Evidence for post-transcriptional regulation by a reduced nitrogen source. Plant J. 2000;23:489–496. doi: 10.1046/j.1365-313x.2000.00813.x. [DOI] [PubMed] [Google Scholar]
- Fraser PD, Romer S, Shipton CA, Mills PB, Kiano JW, Misawa N, Drake RG, Schuch W, Bramley PM. Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc Natl Acad Sci USA. 2002;99:1092–1097. doi: 10.1073/pnas.241374598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Szucs P, Yan L, Helguera M, Skinner J, Hayes P, Dubcovsky J. Large deletions in the first intron of the VRN-1 vernalization gene are associated with spring growth habit in barley and polyploid wheat. Mol Genet Genomics. 2005;273:54–65. doi: 10.1007/s00438-004-1095-4. [DOI] [PubMed] [Google Scholar]
- Furtado A, Henry RJ, Pellegrineschi A. Analysis of promoters in transgenic barley and wheat. Plant Biotechnol J. 2009;7:240–253. doi: 10.1111/j.1467-7652.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- Furtado A, Henry RJ, Takaiwa F. Comparison of promoters in transgenic rice. Plant Biotechnol J. 2008;6:679–693. doi: 10.1111/j.1467-7652.2008.00352.x. [DOI] [PubMed] [Google Scholar]
- Gantet P, Memelink J. Transcription factors: tools to engineer the production of pharmacologically active plant metabolites. Trends Pharmacol Sci. 2002;23:563–569. doi: 10.1016/S0165-6147(02)02098-9. [DOI] [PubMed] [Google Scholar]
- Garbarino JE, Oosumi T, Belknap WR. Isolation of a polyubiquitin promoter and its expression in transgenic potato plants. Plant Physiol. 1995;109:1371–1378. doi: 10.1104/pp.109.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AK, Sawers RJ, Wang H, Kim JK, Walker JM, Brutnell TP, Parthasarathy MV, Vierstra RD, Wu RJ. Light-regulated overexpression of an Arabidopsis phytochrome A gene in rice alters plant architecture and increases grain yield. Planta. 2006;223:627–636. doi: 10.1007/s00425-005-0101-3. [DOI] [PubMed] [Google Scholar]
- Gasser SM, Laemmli UK. The organization of chromatin loops: characterization of a scaffold attachment site. EMBO J. 1986;5:511–518. doi: 10.1002/j.1460-2075.1986.tb04240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffers R, Sell S, Cerff R, Hehl R. The TATA box and a Myb binding site are essential for anaerobic expression of a maize GapC4 minimal promoter in tobacco. Biochim Biophys Acta. 2001;1521:120–125. doi: 10.1016/s0167-4781(01)00302-5. [DOI] [PubMed] [Google Scholar]
- Gianì S, Altana A, Camanoni P, Morello L, Breviario D. In trangenic rice, α- and β-tubulin regulatory sequences control GUS amount and distribution through intron mediated enhancement and intron dependent spatial expression. Transgenic Res. 2009;18:151–162. doi: 10.1007/s11248-008-9202-7. [DOI] [PubMed] [Google Scholar]
- Gil P, Green PJ. Multiple regions of the Arabidopsis SAUR-AC1 gene control transcript abundance: the 3′ untranslated region functions as an mRNA instability determinant. EMBO J. 1996;15:1678–1686. [PMC free article] [PubMed] [Google Scholar]
- Gittins JR, Pellny TK, Biricolti S, Hiles ER, Passey AJ, James DJ. Transgene expression in the vegetative tissues of apple driven by the vascular-specific rolC and CoYMV promoters. Transgenic Res. 2003;12:391–402. doi: 10.1023/A:1024286405310. [DOI] [PubMed] [Google Scholar]
- Gittins JR, Pellny TK, Hiles ER, Rosa C, Biricolti S, James DJ. Transgene expression driven by heterologous ribulose-1, 5-bisphosphate carboxylase/oxygenase small-subunit gene promoters in the vegetative tissues of apple (Malus pumila mill.) Planta. 2000;210:232–240. doi: 10.1007/PL00008130. [DOI] [PubMed] [Google Scholar]
- Giuliano G, Pichersky E, Malik VS, Timko MP, Scolnik PA, Cashmore AR. An evolution-arily conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci USA. 1988;85:7089–7093. doi: 10.1073/pnas.85.19.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace ML, Chandrasekharan MB, Hall TC, Crowe AJ. Sequence and spacing of TATA box elements are critical for accurate initiation from the β- phaseolin promoter. J Biol Chem. 2004;279:8102–8110. doi: 10.1074/jbc.M309376200. [DOI] [PubMed] [Google Scholar]
- Gray-Mitsumune M, Molitor EK, Cukovic D, Carlson JE, Douglas CJ. Developmentally regulated patterns of expression directed by poplar PAL promoters in transgenic tobacco and poplar. Plant Mol Biol. 1999;39:657–669. doi: 10.1023/A:1006148715050. [DOI] [PubMed] [Google Scholar]
- Green PJ, Yong MH, Cuozzo M, Kano-Murakami Y, Silverstein P, Chua NH. Binding site requirements for pea nuclear protein factor GT-1 correlate with sequences required for light-dependent transcriptional activation of the rbcS-3A gene. EMBO J. 1988;7:4035–4044. doi: 10.1002/j.1460-2075.1988.tb03297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichko VP, Filby B, Glick BR. Increased ability of transgenic plants expressing the bacterial enzyme ACC deaminase to accumulate Cd, Co, Cu, Ni, Pb, and Zn. J Biotechnol. 2000;81:45–53. doi: 10.1016/S0168-1656(00)00270-4. [DOI] [PubMed] [Google Scholar]
- Grotewold E, Sainz MB, Tagliani L, Hernandez JM, Bowen B, Chandler VL. Identification of the residues in the Myb domain of maize C1 that specify the interaction with the bHLH cofactor R. Proc Natl Acad Sci USA. 2000;97:13579–13584. doi: 10.1073/pnas.250379897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Raghuvanshi S, Tyagi AK. Assessment of the efficiency of various gene promoters via biolistics in leaf and regenerating seed callus of millets, Eleusine coracana and Echino-chloa crusgalli. Plant Biotechnol. 2001;18:275–282. [Google Scholar]
- Gupta V, Khurana R, Tyagi AK. Promoters of two anther-specific genes confer organ-specific gene expression in a stage-specific manner in transgenic systems. Plant Cell Rep. 2007;26:1919–1931. doi: 10.1007/s00299-007-0414-8. [DOI] [PubMed] [Google Scholar]
- Gutiérrez RA, MacIntosh GC, Green PJ. Current perspectives on mRNA stability in plants: multiple levels and mechanisms of control. Trends Plant Sci. 1999;4:429–438. doi: 10.1016/S1360-1385(99)01484-3. [DOI] [PubMed] [Google Scholar]
- Ha SB, An G. Identification of upstream regulatory elements involved in the developmental expression of the Arabidopsis thaliana cab1 gene. Proc Natl Acad Sci USA. 1988;85:8017–8021. doi: 10.1073/pnas.85.21.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KH, Ma C, Strauss SH. Matrix attachment regions (MARs) enhance transformation frequency and transgene expression in poplar. Transgenic Res. 1997;6:415–420. doi: 10.1023/A:1018439518649. [DOI] [Google Scholar]
- He Y, Gan S. Identical promoter elements are involved in regulation of the OPR1 gene by senescence and jasmonic acid in Arabidopsis. Plant Mol Biol. 2001;47:595–605. doi: 10.1023/A:1012211011538. [DOI] [PubMed] [Google Scholar]
- He Y, Michaels SD, Amasino RM. Regulation of flowering time by histone acetylation in Arabidopsis. Science. 2003;302:1751–1754. doi: 10.1126/science.1091109. [DOI] [PubMed] [Google Scholar]
- He ZM, Jiang XL, Qi Y, Luo DQ. Assessment of the utility of the tomato fruit-specific E8 promoter for driving vaccine antigen expression. Genetica. 2008;133:207–214. doi: 10.1007/s10709-007-9201-2. [DOI] [PubMed] [Google Scholar]
- Hennig J, Dewey RE, Cutt JR, Klessig DF. Pathogen, salicylic acid and developmental dependent expression of a β-1, 3-glucanase/GUS gene fusion in transgenic tobacco plants. Plant J. 1993;4:481–493. doi: 10.1046/j.1365-313X.1993.04030481.x. [DOI] [PubMed] [Google Scholar]
- Hodgson J. Scientists avert new GMO crisis. Nat Biotechnol. 2000;18:13. doi: 10.1038/71838. [DOI] [PubMed] [Google Scholar]
- Holton TA. Modification of flower colour via manipulation of P450 gene expression in transgenic plants. Drug Metabol Drug Interact. 1995;12:359–368. doi: 10.1515/dmdi.1995.12.3-4.359. [DOI] [PubMed] [Google Scholar]
- Hong JK, Hwang BK. Promoter activation of pepper class II basic chitinase gene, CAChi2, and enhanced bacterial disease resistance and osmotic stress tolerance in the CAChi2-over-expressing Arabidopsis. Planta. 2006;223:433–448. doi: 10.1007/s00425-005-0099-6. [DOI] [PubMed] [Google Scholar]
- Hong YF, Liu CY, Cheng KJ, Hour AL, Chan MT, Tseng TH, Chen KY, Shaw JF, Yu SM. The sweet potato sporamin promoter confers high-level phytase expression and improves organic phosphorus acquisition and tuber yield of transgenic potato. Plant Mol Biol. 2008;67:347–361. doi: 10.1007/s11103-008-9324-6. [DOI] [PubMed] [Google Scholar]
- Huang F, Buschman LL, Higgins RA, McGaughey WH. Inheritance to Bacillus thuringien-sis toxin (Dispel ES) in European corn borer. Science. 1999;284:965–967. doi: 10.1126/science.284.5416.965. [DOI] [PubMed] [Google Scholar]
- Hulzink RJM, de Groot PFM, Croes AF, Quaedvlieg W, Twell D, Wullems GJ, van Herpen MMA. The 50 -untranslated region of the ntp303 gene strongly enhances translation during pollen tube growth, but not during pollen maturation. Plant Physiol. 2002;129:342–353. doi: 10.1104/pp.001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Johnson FB, Marciniak RA, McVey M, Park PU, Guarente L. Sir2: an NAD-dependent histone deacetylase that connects chromatin silencing, metabolism, and aging. Cold Spring Harb Symp Quant Biol. 2000;65:297–302. doi: 10.1101/sqb.2000.65.297. [DOI] [PubMed] [Google Scholar]
- Ingham DJ, Beer S, Money S, Hansen G. Quantitative real-time PCR assay for determining transgene copy number in transformed plants. Biotechniques. 2001;31:132–140. doi: 10.2144/01311rr04. [DOI] [PubMed] [Google Scholar]
- Ito Y, Katsura K, Maruyama K, Taji T, Kobayashi M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol. 2005;47:141–153. doi: 10.1093/pcp/pci230. [DOI] [PubMed] [Google Scholar]
- Jayaraj J, Devlin R, Punja Z. Metabolic engineering of novel ketocarotenoid production in carrot plants. Transgenic Res. 2008;17:489–501. doi: 10.1007/s11248-007-9120-0. [DOI] [PubMed] [Google Scholar]
- Jefferson R, Goldsbrough A, Bevan M. Transcriptional regulation of a patatin-1 gene in potato. Plant Mol Biol. 1990;14:995–1006. doi: 10.1007/BF00019396. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jeong YM, Mun JH, Lee I, Woo JC, Hong CB, Kim SG. Distinct roles of the first introns on the expression of Arabidopsis profilin gene family members. Plant Physiol. 2006;140:196–209. doi: 10.1104/pp.105.071316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MO, Manning K, Andrews J, Wright C, Taylor IB, Thompson AJ. The promoter from SlREO, a highly-expressed, root-specific Solanum lycopersicum gene, directs expression to cortex of mature roots. Funct Plant Biol. 2008;35:1224–1233. doi: 10.1071/FP08139. [DOI] [PubMed] [Google Scholar]
- Katsumoto Y, Fukuchi-Mizutani M, Fukui Y, Brugliera F, Holton TA, Karan M, Nakamura N, Yonekura-Sakakibara K, Togami J, Pigeaire A, Tao GQ, Nehra NS, Lu CY, Dyson BK, Tsuda S, Ashikari T, Kusumi T, Mason JG, Tanaka Y. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 2007;48:1589–1600. doi: 10.1093/pcp/pcm131. [DOI] [PubMed] [Google Scholar]
- Kehoe DM, Degenhardt J, Winicov I, Tobin EM. Two 10-bp regions are critical for phytochrome regulation of a Lemna gibba Lhcb gene promoter. Plant Cell. 1994;6:1123–1134. doi: 10.1105/tpc.6.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermode AR, Zeng Y, Hu X, Lauson S, Abrams SR, He X. Ectopic expression of a conifer Abscisic Acid Insensitive 3 transcription factor induces high-level synthesis of recombinant human a-L-iduronidase in transgenic tobacco leaves. Plant Mol Biol. 2007;63:763–776. doi: 10.1007/s11103-006-9122-y. [DOI] [PubMed] [Google Scholar]
- Kim HU, Park BS, Jin YM, Chung TY. Promoter sequences of two homologous pectin esterase genes from Chinese cabbage (Brassica campestris L. ssp. pekinensis) and pollen-specific expression of the GUS gene driven by a promoter in tobacco plants. Mol Cell. 1997;7:21–27. [PubMed] [Google Scholar]
- Kim KN, Guiltinan MJ. Identification of cis-acting elements important for expression of the starch-branching enzyme I gene in maize endosperm. Plant Physiol. 1999;121:225–236. doi: 10.1104/pp.121.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Kim H, Shin JS, Chung CH, Ohlrogge JB, Suh MC. Seed-specific expression of sesame microsomal oleic acid desaturase is controlled by combinatorial properties between negative cis -regulatory elements in the SeFAD2 promoter and enhancers in the 5′-UTR intron. Mol Genet Genomics. 2006;276:351–368. doi: 10.1007/s00438-006-0148-2. [DOI] [PubMed] [Google Scholar]
- Kimura M, Yoshizumi T, Manabe K, Yamamoto YY, Matsui M. Arabidopsis transcrip-tional regulation by light stress via hydrogen peroxide-dependent and -independent pathways. Genes Cells. 2001;6:607–617. doi: 10.1046/j.1365-2443.2001.00446.x. [DOI] [PubMed] [Google Scholar]
- Kirsch C, Logemann E, Lippok B, Schmelzer E, Hahlbrock K. A highly specific pathogen-responsive promoter element from the immediate- early activated CMPG1 gene in Petroseli-num crispum. Plant J. 2001;26:217–227. doi: 10.1046/j.1365-313x.2001.01015.x. [DOI] [PubMed] [Google Scholar]
- Koltunow AM, Truettner J, Cox KH, Wallroth M, Goldberg RB. Different temporal and spatial gene expression patterns occur during anther development. Plant Cell. 1990;2:1201–1224. doi: 10.1105/tpc.2.12.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooiker M, Airoldi CA, Losa A, Manzotti PS, Finzi L, Kater MM, Colombo L. BASIC PENTACYSTEINE1, a GA binding protein that induces conformational changes in the regulatory region of the homeotic Arabidopsis gene SEEDSTICK. Plant Cell. 2005;17:722–729. doi: 10.1105/tpc.104.030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- Kuster H, Quandt HJ, Broer I, Perlick AM, Puhler A. The promoter of the Vicia faba L. VfENOD-GRP3 gene encoding a glycine-rich early nodulin mediates a predominant gene expression in the interzone II-III region of transgenic Vicia hirsuta root nodules. Plant Mol Biol. 1995;29:759–772. doi: 10.1007/BF00041166. [DOI] [PubMed] [Google Scholar]
- Lam E, Benfey PN, Gilmartin PM, Fang RX, Chua NH. Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci USA. 1989;86:7890–7894. doi: 10.1073/pnas.86.20.7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YY, Cevallos RC, Jan E. An upstream open reading frame regulates translation of gadd34 during cellular stresses that induce eif2α phosphorylation. J Biol Chem. 2009;284:6661–6673. doi: 10.1074/jbc.M806735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn E, Schalechet F, Wilkinson J, Matsui K, Tadmor Y, Nam KH, Amar O, Lastochkin E, Larkov O, Ravid U, Hiatt W, Gepstein S, Pichersky E. Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol. 2001;127:1256–1265. doi: 10.1104/pp.010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Zhu R, Xu P. Activation of the gene promoter of barley beta-1, 3-glucanase isoenzyme GIII is salicylic acid (SA)-dependent in transgenic rice plants. J Plant Res. 2005;118:215–221. doi: 10.1007/s10265-005-0213-7. [DOI] [PubMed] [Google Scholar]
- Li Z, Hansen JL, Liu Y, Zemetra RS, Berger PH. Using realtime PCR to determine copy number in wheat. Plant Mol Biol Rep. 2004;22:179–188. doi: 10.1007/BF02772725. [DOI] [Google Scholar]
- Li Z, Jayasankar S, Gray DJ. Expression of a bifunctional green fluorescent protein (GFP) fusion marker under the control of three constitutive promoters and enhanced derivatives in transgenic grape (Vitis vinifera) Plant Sci. 2001;160:877–887. doi: 10.1016/S0168-9452(01)00336-3. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Ekramoddoullah AK. Root-specific expression of a western white pine PR10 gene is mediated by different promoter regions in transgenic tobacco. Plant Mol Biol. 2003;52:103–120. doi: 10.1023/A:1023930326839. [DOI] [PubMed] [Google Scholar]
- Liu JW, Tabe LM. The influences of two plant nuclear matrix attachment regions (MARs) on gene expression in transgenic plants. Plant Cell Physiol. 1998;39:115–123. doi: 10.1093/oxfordjournals.pcp.a029282. [DOI] [PubMed] [Google Scholar]
- Liu Q, Segal DJ, Ghiara JB, Barbas CF. Design of polydactyl zinc-finger proteins for unique addressing within complex genomes. Proc Natl Acad Sci USA. 1997;94:5525–5530. doi: 10.1073/pnas.94.11.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livne B, Faktor O, Zeitoune S, Edelbaum O, Sela I. TMV-induced expression of tobacco β-glucanase promoter activity is mediated by a single, inverted, GCC motif. Plant Sci. 1997;130:159–169. doi: 10.1016/S0168-9452(97)00210-0. [DOI] [Google Scholar]
- Lloyd AM, Schena M, Walbot V, Davis RW. Epidermal cell fate determination in Arabidopsis: patterns defined by a steroid-inducible regulator. Science. 1994;266:436–439. doi: 10.1126/science.7939683. [DOI] [PubMed] [Google Scholar]
- Lu J, Sivamani E, Azhakanandam K, Samadder P, Li X, Qu R. Gene expression enhancement mediated by the 5′ UTR intron of the rice rubi3 gene varied remarkably among tissues in transgenic rice plants. Mol Genet Genomics. 2008;279:563–572. doi: 10.1007/s00438-008-0333-6. [DOI] [PubMed] [Google Scholar]
- Luehrsen KR, Walbot V. Intron enhancement of gene expression and the splicing efficiency of introns in maize cells. Mol Gen Genet. 1991;225:81–93. doi: 10.1007/BF00282645. [DOI] [PubMed] [Google Scholar]
- Lumbreras V, Stevens DR, Purton S. Efficient foreign gene expression in Chlamydomonas reinhardtii mediated by an endogenous intron. Plant J. 1998;14:441–447. doi: 10.1046/j.1365-313X.1998.00145.x. [DOI] [Google Scholar]
- Luttermann C, Meyers G. The importance of inter- and intramolecular base pairing for translation reinitiation on a eukaryotic bicistronic mRNA. Genes Dev. 2009;23:331–344. doi: 10.1101/gad.507609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas C, Laufs J, Grant S, Korfhage C, Werr W. The combination of a novel stimulatory element in the first exon of the maize Shrunken-1 gene with the following intron 1 enhances reporter gene expression up to 1000-fold. Plant Mol Biol. 1991;16:199–207. doi: 10.1007/BF00020552. [DOI] [PubMed] [Google Scholar]
- Maas C, Schaal S, Werr W. A feedback control element near the transcription start site of the maize shrunken gene determines promoter activity. EMBO J. 1990;9:3447–3452. doi: 10.1002/j.1460-2075.1990.tb07552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac A, Krzymowska M, Barabasz A, Hennig J. Transcriptional regulation of the gluB promoter during plant response to infection. Cell Mol Biol Lett. 2004;9:843–853. [PubMed] [Google Scholar]
- Maeo K, Tomiya T, Hayashi K, Akaike M, Morikami A, Ishiguro S, Nakamura K. Sugar-responsible elements in the promoter of a gene for beta-amylase of sweet potato. Plant Mol Biol. 2001;46:627–637. doi: 10.1023/A:1010684908364. [DOI] [PubMed] [Google Scholar]
- Maiti IB, Ghosh SK, Gowda S, Kiernan J, Shepherd RJ. Promoter/leader deletion analysis and plant expression vectors with the figwort mosaic virus (FMV) full length transcript (FLt) promoter containing single or double enhancer domains. Transgenic Res. 1997;6:143–156. doi: 10.1023/A:1018477705019. [DOI] [PubMed] [Google Scholar]
- Mankin SL, Allen GC, Phelan T, Spiker S, Thompson WF. Elevation of transgene expression level by flanking matrix attachment regions (MAR) is promoter dependent: a study of the interactions of six promoters with the RB7 3′ MAR. Transgenic Res. 2003;12:3–12. doi: 10.1023/A:1022194120518. [DOI] [PubMed] [Google Scholar]
- Manners MJ, Penninckx IAMA, Vermaere K, Kazan K, Brown RL, Morgan A, Maclean DJ, Curtis MD, Cammue BPA, Broekaert WF. The promoter of the plant defensin gene PDF1.2 from Arabidopsis is systemically activated by fungal pathogens and responds to methyl jasmonate but not to salicylic acid. Plant Mol Biol. 1998;38:1071–1080. doi: 10.1023/A:1006070413843. [DOI] [PubMed] [Google Scholar]
- Mariani C, De Beuckeleer M, Treuttner J, Goldberg RB. Induction of male sterility in plants by a chimaeric ribonuclease gene. Nature. 1990;347:737–741. doi: 10.1038/347737a0. [DOI] [Google Scholar]
- Martienssen RA. Maintenance of heterochromatin by RNA interference of tandem repeats. Nat Genet. 2003;35:213–214. doi: 10.1038/ng1252. [DOI] [PubMed] [Google Scholar]
- Martínez-García JF, Huq E, Quail PH. Direct targeting of light signals to a promoter element-bound transcription factor. Science. 2000;288:859–863. doi: 10.1126/science.288.5467.859. [DOI] [PubMed] [Google Scholar]
- Martínez-Hernández A, López-Ochoa L, Argüello-Astorga G, Herrera-Estrella L. Functional properties and regulatory complexity of a minimal RBCS light-responsive unit activated by phytochrome, cryptochrome, and plastid signals. Plant Physiol. 2002;128:1223–1233. doi: 10.1104/pp.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas D, Mettler IJ, Pierce DA, Lowe HW. Intron mediated enhancement of heterologous gene expression in maize. Plant Mol Biol. 1990;15:913–920. doi: 10.1007/BF00039430. [DOI] [PubMed] [Google Scholar]
- Mason G, Provero P, Vaira AM, Accotto GP. Estimating the number of integrations in transformed plants by quantitative real time PCR. BMC Biotechnol. 2001;2:20. doi: 10.1186/1472-6750-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy D, Zhang W, Cao J, Wu R. Isolation of an efficient actin promoter for use in rice transformation. Plant Cell. 1990;2:163–171. doi: 10.1105/tpc.2.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkens AE, Schindler U, Cashmore AR. The Gbox: a ubiquitous regulatory DNA element in plantsbound by the GBF family of bZIP proteins. Trends Biochem Sci. 1995;20:506–510. doi: 10.1016/S0968-0004(00)89118-5. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J, Mirault ME, Laemmli UK. Organisation of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984;39:223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Mitra A, Choi HK, An G. Structural and functional analyses of Arabidopsis thaliana chlorophyll a/b-binding protein (cab) promoters. Plant Mol Biol. 1989;12:169–179. doi: 10.1007/BF00020502. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Nakamura T, Nagao I, Obokata J. Quantitative analysis of transiently expressed mRNA in particle-bombarded tobacco seedlings. Plant Mol Biol Rep. 2000;18:101–107. doi: 10.1007/BF02824017. [DOI] [Google Scholar]
- Mol J, Cornish E, Mason J, Koes R. Novel coloured flowers. Curr Opin Biotechnol. 1999;10:198–201. doi: 10.1016/S0958-1669(99)80035-4. [DOI] [PubMed] [Google Scholar]
- Monde RA, Greene JC, Stern DB. The sequence and secondary structure of the 30UTR affect 30-end maturation, RNA accumulation, and translation in tobacco chloroplasts. Plant Mol Biol. 2000;44:529–542. doi: 10.1023/A:1026540310934. [DOI] [PubMed] [Google Scholar]
- Montgomery J, Goldman S, Deikman J, Margossian L, Fischer RL. Identification of an ethylene-responsive region in the promoter of a fruit ripening gene. Proc Natl Acad Sci USA. 1993;90:5939–5943. doi: 10.1073/pnas.90.13.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J, Pollard V, Deikman J, Fischer RL. Positive and negative regulatory regions control the spatial distribution of polygalacturonase transcription in tomato fruit pericarp. Plant Cell. 1993;5:1049–1062. doi: 10.1105/tpc.5.9.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello L, Bardini M, Cricri M, Sala F, Breviario D. Functional analysis of DNA sequences controlling the expression of the rice OsCDPK2 gene. Planta. 2006;223:479–491. doi: 10.1007/s00425-005-0105-z. [DOI] [PubMed] [Google Scholar]
- Morello L, Bardini M, Sala F, Breviario D. A long leader intron of the Ostub16 rice β-tubulin gene is required for high-level gene expression and can autonomously promote transcription both in vivo and in vitro. Plant J. 2002;29:33–44. doi: 10.1046/j.0960-7412.2001.01192.x. [DOI] [PubMed] [Google Scholar]
- Morita A, Umemura T, Kuroyanagi M, Futsuhara Y, Perata P, Yamaguchi J. Functional dissection of a sugar-repressed alpha-amylase gene (RAmy1A) promoter in rice embryos. FEBS Lett. 1998;13:81–85. doi: 10.1016/S0014-5793(98)00067-2. [DOI] [PubMed] [Google Scholar]
- Moye-Rowley WS, Harshman KD, Parker CS. Yeast YAP1 encodes a novel form of the jun family of transcriptional activator proteins. Genes Dev. 1989;3:283–292. doi: 10.1101/gad.3.3.283. [DOI] [PubMed] [Google Scholar]
- Murfett J, Wang XJ, Hagen G, Guilfoyle TJ. Identification of Arabidopsis histone deace-tylase HDA6 mutants that affect transgene expression. Plant Cell. 2001;13:1047–1061. doi: 10.1105/tpc.13.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nain V, Verma A, Kumar N, Sharma P, Ramesh B, Kumar PA. Cloning of an ovule specific promoter from Arabidopsis thaliana and expression of β-glucuronidase. Indian J Exp Biol. 2008;46:207–211. [PubMed] [Google Scholar]
- Nardozza TA, Quigley MM, Getzenberg RH. Association of transcription factors with the nuclear matrix. J Cell Biochem. 1996;61:467–477. doi: 10.1002/(SICI)1097-4644(19960601)61:3<467::AID-JCB14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ng HH, Bird A. DNA methylation and chromatin modification. Curr Opin Genet Dev. 1999;9:158–163. doi: 10.1016/S0959-437X(99)80024-0. [DOI] [PubMed] [Google Scholar]
- Nicholass FJ, Smith CJ, Schuch W, Bird CR, Grierson D. High levels of ripening-specific reporter gene expression directed by tomato fruit polygalacturonase gene-flanking regions. Plant Mol Biol. 1995;28:423–435. doi: 10.1007/BF00020391. [DOI] [PubMed] [Google Scholar]
- Noh YS, Amasino RM. Identification of a promoter region responsible for the senescence-specific expression of SAG12. Plant Mol Biol. 1999;41:181–194. doi: 10.1023/A:1006342412688. [DOI] [PubMed] [Google Scholar]
- Nomura M, Katayama K, Nishimura A, Ishida Y, Ohta S, Komari T, Miyao-Tokutomi M, Tajima S, Matsuoka M. The promoter of rbcS in a C3 plant (rice) directs organ-specific, light-dependent expression in a C4 plant (maize), but does not confer bundle sheath cell-specific expression. Plant Mol Biol. 2000;44:99–106. doi: 10.1023/A:1006461812053. [DOI] [PubMed] [Google Scholar]
- Nowak W, Gawlowska M, Jarmolowski A, Augustyniak J. Effect of nuclear matrix attachment regions on transgene expression in tobacco plant. Acta Biochim Pol. 2001;48:637–646. [PubMed] [Google Scholar]
- Nunberg AN, Li Z, Bogue MA, Vivekananda J, Reddy AS, Thomas TL. Developmental and hormonal regulation of sunflower helianthinin genes: proximal promoter sequences confer regionalized seed expression. Plant Cell. 1994;6:473–486. doi: 10.1105/tpc.6.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell JT, Nagy F, Chua NH. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature. 1985;313:810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Ohl S, Hedrick SA, Chory J, Lamb CJ. Functional properties of a phenylalanine ammonia-lyase promoter from Arabidopsis. Plant Cell. 1990;2:837–848. doi: 10.1105/tpc.2.9.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme-Takagi M, Taylor CB, Newman TC, Green PJ. The effect of sequences with high AU content on mRNA stability in tobacco. Proc Natl Acad Sci USA. 1993;90:11811–11815. doi: 10.1073/pnas.90.24.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S, Hattori T, Morikami A, Nakamura K. High-level expression of a sweet potato sporamin gene promoter: β-glucuronidase (GUS) fusion gene in the stems of transgenic tobacco plants is conferred by multiple cell type-specific regulatory elements. Mol Gen Genet. 1991;225:369–378. doi: 10.1007/BF00261676. [DOI] [PubMed] [Google Scholar]
- Olsthoorn RC, Mertens S, Brederode FT, Bol JF. A conformational switch at the 3′ end of a plant virus RNA regulates viral replication. EMBO J. 1999;18:4856–4864. doi: 10.1093/emboj/18.17.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe K, Abe K, Yoshioka T, Osakabe Y, Todoriki S, Ichikawa H, Hohn B, Toki S. Isolation and characterization of the RAD54 gene from Arabidopsis thaliana. Plant J. 2006;48:827–842. doi: 10.1111/j.1365-313X.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- Oszvald M, Kang TJ, Tomoskozi S, Jenes B, Kim TG, Cha YS, Tamas L, Yang MS. Expression of cholera toxin B subunit in transgenic rice endosperm. Mol Biotechnol. 2008;40:261–268. doi: 10.1007/s12033-008-9083-2. [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 1997;11:2983–2995. doi: 10.1101/gad.11.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AF, Minocha SC. Analysis of gene expression in transgenic plants. In: Pena L, editor. Methods in molecular biology. Transgenic plants: methods and protocols, vol 286. New Jersey, USA: Humana Press; 2004. pp. 291–311. [DOI] [PubMed] [Google Scholar]
- Pain VM. Initiation of protein synthesis in eukaryotic cells. Eur J Biochem. 1996;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x. [DOI] [PubMed] [Google Scholar]
- Pandey R, Muller A, Napoli CA, Selinger DA, Pikaard CS. Analysis of histone acetyl-transferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 2002;30:5036–5055. doi: 10.1093/nar/gkf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HC, Kim ML, Kang YH, Jeon JM, Yoo JH, Kim MC, Park CY, Jeong JC, Moon BC, Lee JH, Yoon HW, Lee SH, Chung WS, Lim CO, Lee SY, Hong JC, Cho MJ. Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 Box that interacts with a GT-1-like transcription factor. Plant Physiol. 2004;135:2150–2161. doi: 10.1104/pp.104.041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Berry JO. Rubisco gene expression in C4 plants. J Exp Bot. 2008;59:1625–1634. doi: 10.1093/jxb/erm368. [DOI] [PubMed] [Google Scholar]
- Pathirana MS, Samac DA, Roeven R, Yoshioka H, Vance CP, Gantt JS. Analyses of phosphoenolpyruvate carboxylase gene structure and expression in alfalfa nodules. Plant J. 1997;12:293–304. doi: 10.1046/j.1365-313X.1997.12020293.x. [DOI] [PubMed] [Google Scholar]
- Paul AL, Ferl RJ. Higher order chromatin structures in maize and Arabidopsis. Plant Cell. 1998;10:1349–1359. doi: 10.1105/tpc.10.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit JM, van Wuytswinkel O, Briat JF, Lobréaux S. Characterization of an iron-dependent regulatory sequence involved in the transcriptional control of AtFer1 and ZmFer1 plant ferritin genes by iron. J Biol Chem. 2001;276:5584–5590. doi: 10.1074/jbc.M005903200. [DOI] [PubMed] [Google Scholar]
- Potenza C, Aleman L, Sengupta-Gopalan C. Targeting transgene expression in research, agricultural, and environmental applications: Promoters used in plant transformation. In Vitro Cell Dev Biol Plant. 2004;40:1–22. [Google Scholar]
- Puente P, Wei N, Deng XW. Combinatorial interplay of promoter elements constitutes the minimal determinants for light and developmental control of gene expression in Arabidopsis. EMBO J. 1996;15:3732–3743. [PMC free article] [PubMed] [Google Scholar]
- Qing DJ, Lu HF, Li N, Dong HT, Dong DF, Li YZ. Comparative profiles of gene expression in leaves and roots of maize seedlings under the conditions of the salt stress and the removal of the salt stress. Plant Cell Physiol. 2009;50:889–903. doi: 10.1093/pcp/pcp038. [DOI] [PubMed] [Google Scholar]
- Qu LQ, Xing YP, Liu WX, Xu XP, Song YR. Expression pattern and activity of six glutelin gene promoters in transgenic rice. J Exp Bot. 2008;59:2417–2424. doi: 10.1093/jxb/ern110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG, Maggio A, Narasimhan ML, Kononowicz AK, Wang G, D'Urzo MP, Hasegawa PM, Bressan RA. Tissue-specific activation of the osmotin gene by ABA, C2H4 and NaCl involves the same promoter region. Plant Mol Biol. 1997;34:393–402. doi: 10.1023/A:1005812217945. [DOI] [PubMed] [Google Scholar]
- Ribeiro DT, Farias LP, de Almeida JD, Kashiwabara PM, Ribeiro AF, Silva-Filho MC, Menck CF, Van Sluys MA. Functional characterization of the thi1 promoter region from Arabidop-sis thaliana. J Exp Bot. 2005;56:1797–1804. doi: 10.1093/jxb/eri168. [DOI] [PubMed] [Google Scholar]
- Riechmann JL, Ratcliffe OJ. A genomic perspective on plant transcription factors. Curr Opin Plant Biol. 2000;3:423–434. doi: 10.1016/S1369-5266(00)00107-2. [DOI] [PubMed] [Google Scholar]
- Rogers HJ, Bate N, Combe J, Sullivan J, Sweetman J, Swan C, Lonsdale DM, Twell D. Functional analysis of cis-regulatory elements within the promoter of the tobacco late pollen gene g10. Plant Mol Biol. 2001;45:577–585. doi: 10.1023/A:1010695226241. [DOI] [PubMed] [Google Scholar]
- Rommens CM, Richael CM, Yan H, Navarre DA, Ye J, Krucker M, Swords K. Engineered native pathways for high kaempferol and caffeoylquinate production in potato. Plant Biotechnol J. 2008;6:870–886. doi: 10.1111/j.1467-7652.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- Roque E, Gómez MD, Ellul P, Wallbraun M, Madueño F, Beltrán JP, Canas LA. The PsEND1 promoter: a novel tool to produce genetically engineered male-sterile plants by early anther ablation. Plant Cell Rep. 2007;26:313–325. doi: 10.1007/s00299-006-0237-z. [DOI] [PubMed] [Google Scholar]
- Rose AB. Requirements for intron-mediated enhancement of gene expression in Arabidopsis. RNA. 2002;8:1444–1453. doi: 10.1017/S1355838202020551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AB. The effect of intron location on intron-mediated enhancement of gene expression in Arabidopsis. Plant J. 2004;40:744–751. doi: 10.1111/j.1365-313X.2004.02247.x. [DOI] [PubMed] [Google Scholar]
- Rose AB. Intron-mediated regulation of gene expression. In: Reddy ASN, Golovkin M, editors. Nuclear pre-mRNA processing in plants: current topics in microbiology and immunology, vol 326. Berlin: Springer; 2008. pp. 277–290. [DOI] [PubMed] [Google Scholar]
- Rouster J, Leah R, Mundy J, Cameron-Mills V. Identification of a methyl jasmonate-responsive region in the promoter of a lipoxygenase 1 gene expressed in barley grain. Plant J. 1997;11:513–523. doi: 10.1046/j.1365-313X.1997.11030513.x. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Torres JT, Parniske M, Wernert P, Hahlbrock K, Somssich IE. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 1996;15:5690–5700. [PMC free article] [PubMed] [Google Scholar]
- Sabelli PA, Shewry PR. Gene characterization by southern analysis. In: Jones H, editor. Methods in molecular biology. Plant gene transfer and expression protocols. New Jersey, USA: Humana Press; 1995. pp. 161–180. [DOI] [PubMed] [Google Scholar]
- Sabelli PA, Shewry PR. Northern analysis and nucleic acid probes. In: Jones H, editor. Methods in molecular biology. Plant gene transfer and expression protocols. New Jersey, USA: Humana Press; 1995. pp. 213–228. [DOI] [PubMed] [Google Scholar]
- Sablowski RWM, Baulcombe DC, Bevan M. Expression of a flower-specific Myb protein in leaf cells using a viral vector causes ectopic activation of a target promoter. Proc Natl Acad Sci USA. 1995;92:6901–6905. doi: 10.1073/pnas.92.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu JS, Krasnyanski SF, Domier LL, Korban SS, Osadjan MD, Buetow DE. Oral immunization of mice with transgenic tomato fruit expressing respiratory syncytial virus-F protein induces a systemic immune response. Transgenic Res. 2000;9:127–135. doi: 10.1023/A:1008979525909. [DOI] [PubMed] [Google Scholar]
- Schaart JG, Salentijn EMJ, Krens FA. Tissue-specific expression of the [3-glucuronidase reporter gene in transgenic strawberry (Fragaria ¼ananassa) plants. Plant Cell Rep. 2002;21:313–319. doi: 10.1007/s00299-002-0514-4. [DOI] [Google Scholar]
- Schenk PM, Remans T, Sagi L, Elliott AR, Dietzgen RG, Swennen R, Ebert PR, Grof CPL, Manners JM. Promoters for pregenomic RNA of banana streak badnavirus are active for transgene expression in monocot and dicot plants. Plant Mol Biol. 2001;47:399–412. doi: 10.1023/A:1011680008868. [DOI] [PubMed] [Google Scholar]
- Schneider A, Salamini F, Gebhardt C. Expression patterns and promoter activity of the cold-regulated gene ci21A of potato. Plant Physiol. 1997;113:335–345. doi: 10.1104/pp.113.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F, Schröder G, Kliem M, Rieping M. An SAR-sequence containing 395 bp-DNA fragment mediates enhanced, gene-dosage-correlated expression of a chimaeric heat shock gene in transgenic tobacco plants. Transgenic Res. 1993;2:93–100. doi: 10.1007/BF01969382. [DOI] [PubMed] [Google Scholar]
- Schramm F, Ganguli A, Kiehlmann E, Englich G, Walch D, von Koskull-Döring P. The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis. Plant Mol Biol. 2006;60:759–772. doi: 10.1007/s11103-005-5750-x. [DOI] [PubMed] [Google Scholar]
- Segal DJ, Barbas CF. Custom DNA-binding proteins come of age: Polydactyl zinc-finger proteins. Curr Opin Biotechnol. 2001;12:632–637. doi: 10.1016/S0958-1669(01)00272-5. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Fukushima S, Takatsuji H. RNA-directed DNA methylation induces transcriptional activation in plants. Proc Natl Acad Sci USA. 2009;106:1660–1665. doi: 10.1073/pnas.0809294106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou H, Frame BR, Whitham SA, Wang K. Assessment of transgenic maize events produced by particle bombardment or Agrobacterium-mediated transformation. Mol Breed. 2004;13:201–208. doi: 10.1023/B:MOLB.0000018767.64586.53. [DOI] [Google Scholar]
- Sinibaldi RM, Mettler IJ. Intron splicing and intronmediated enhanced expression in monocots. In: Cohn WE, Moldave K, editors. Progress in nucleic acid research and molecular biology. New York, USA: Academic Press; 1992. pp. 229–257. [DOI] [PubMed] [Google Scholar]
- Söderman E, Hjellstrom M, Fahleson J, Engstrom P. The HD-Zip gene ATHB6 in Arabi-dopsis is expressed in developing leaves, roots and carpels and up-regulated by water deficit conditions. Plant Mol Biol. 1999;40:1073–1083. doi: 10.1023/A:1006267013170. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Pelletier J. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Song P, Cai CQ, Skokut M, Kosegi BD, Petolino JF. Quantitative real-time PCR as a screening tool for estimating transgene copy number in WHISKERS-derived transgenic maize. Plant Cell Rep. 2002;20:948–954. doi: 10.1007/s00299-001-0432-x. [DOI] [Google Scholar]
- Stark K, Kirk DL, Schmitt R. Two enhancers and one silencer located in the introns of regA control somatic cell differentiation in Volvox carteri. Genes Dev. 2001;15:1449–1460. doi: 10.1101/gad.195101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns JC, Shah S, Greenberg BM, Dixon DG, Glick BR. Tolerance of transgenic canola expressing 1-aminocyclopropane-1-carboxylic acid deaminase to growth inhibition by nickel. Plant Physiol Biochem. 2005;43:701–708. doi: 10.1016/j.plaphy.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Stief A, Winter DM, Stratling WH, Sippel AE. A nuclear DNA attachment element mediates elevated and position-independent gene activity. Nature. 1989;341:343–345. doi: 10.1038/341343a0. [DOI] [PubMed] [Google Scholar]
- Storozhenko S, De Pauw P, Van Montagu M, Inzé D, Kushnir S. The heat-shock element is a functional component of the Arabidopsis APX1 gene promoter. Plant Physiol. 1998;118:1005–1014. doi: 10.1104/pp.118.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strompen G, Gruner R, Pfitzner UM. An as-1-like motif controls the level of expression of the gene for the pathogenesis-related protein 1a from tobacco. Plant Mol Biol. 1998;37:871–883. doi: 10.1023/A:1006003916284. [DOI] [PubMed] [Google Scholar]
- Sunilkumar G, Connell JP, Smith CW, Reddy AS, Rathore KS. Cotton alpha-globulin promoter: isolation and functional characterization in transgenic cotton, Arabidopsis, and tobacco. Transgenic Res. 2002;11:347–359. doi: 10.1023/A:1016322428517. [DOI] [PubMed] [Google Scholar]
- Szabados L, Ratet P, Grunenberg B, de Bruijn FJ. Functional analysis of the Sesbania rostrata leghemoglobin glb3 gene 5′-upstream region in transgenic Lotus corniculatus and Nicotiana tabacum plants. Plant Cell. 1990;2:973–986. doi: 10.1105/tpc.2.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczyglowski K, Potter T, Stoltzfus J, Fujimoto SY, de Bruijn FJ. Differential expression of the Sesbania rostrata leghemoglobin glb3 gene promoter in transgenic legume and non-legume plants. Plant Mol Biol. 1996;31:931–935. doi: 10.1007/BF00019482. [DOI] [PubMed] [Google Scholar]
- Taddei A, Hediger F, Neumann FR, Gasser SM. The function of nuclear architecture: a genetic approach. Annu Rev Genet. 2004;38:305–345. doi: 10.1146/annurev.genet.37.110801.142705. [DOI] [PubMed] [Google Scholar]
- Tarantino D, Petit JM, Lobreaux S, Briat JF, Soave C, Murgia I. Differential involvement of the IDRS cis-element in the developmental and environmental regulation of the AtFer1 ferritin gene from Arabidopsis. Planta. 2003;217:709–716. doi: 10.1007/s00425-003-1038-z. [DOI] [PubMed] [Google Scholar]
- Tian L, Fong PM, Wang JJ, Wei NE, Jiang H, Doerge RW, Chen ZJ. Reversible histone acetylation and deacetylation mediate genome-wide, promoter-dependent and locus-specific changes in gene expression during plant development. Genetics. 2005;169:337–345. doi: 10.1534/genetics.104.033142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wang J, Fong MP, Chen M, Cao H, Gelvin SB, Chen ZJ. Genetic control of developmental changes induced by disruption of Arabidopsis histone deacetylase 1 (AtHD1) expression. Genetics. 2003;165:399–409. doi: 10.1093/genetics/165.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak N, Okršlar V, Stanič-Racman D, Gruden K, Zĕl J. A high-throughput method for quantifying transgene expression in transformed plants with real-time PCR analysis. Plant Mol Biol Rep. 2004;22:237–250. doi: 10.1007/BF02773134. [DOI] [Google Scholar]
- Toquin V, Grausem B, Geoffroy P, Legrand M. Structure of the tobacco caffeic acid O-methyltransferase (COMT) II gene: identification of promoter sequences involved in gene inducibility by various stimuli. Plant Mol Biol. 2003;52:495–509. doi: 10.1023/A:1024810916909. [DOI] [PubMed] [Google Scholar]
- Twell D, Yamaguchi J, McCormick S. Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development. 1990;109:705–713. doi: 10.1242/dev.109.3.705. [DOI] [PubMed] [Google Scholar]
- Uknes S, Dincher S, Friedrich L, Negrotto D, Williams S, Thompson-Taylor H, Potter S, Ward E, Ryals J. Regulation of pathogenesis-related protein-1a gene expression in tobacco. Plant Cell. 1993;5:159–169. doi: 10.1105/tpc.5.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwin PE, Møller SG, Lilley CJ, McPherson MJ, Atkinson HJ. Continual green-fluorescent protein monitoring of cauliflower mosaic virus 35S promoter activity in nematode-induced feeding cells in Arabidopsis thaliana. Mol Plant Microbe Interact. 1997;10:394–400. doi: 10.1094/MPMI.1997.10.3.394. [DOI] [PubMed] [Google Scholar]
- Vain P, Worland B, Kohli A, Snape JW, Christou P, Allen GC, Thompson WF. Matrix attachment regions increase transgene expression levels and stability in transgenic rice plants and their progeny. Plant J. 1999;18:233–242. doi: 10.1046/j.1365-313X.1999.00446.x. [DOI] [Google Scholar]
- van de Löcht U, Meier I, Hahlbrock K, Somssich IE. A 125 bp promoter fragment is sufficient for strong elicitor-mediated gene activation in parsley. EMBO J. 1990;9:2945–2950. doi: 10.1002/j.1460-2075.1990.tb07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rhee MD, Lemmers R, Bol JF. Analysis of regulatory elements involved in stress-induced and organ-specific expression of tobacco acidic and basic β-1, 3-glucanase genes. Plant Mol Biol. 1993;21:451–461. doi: 10.1007/BF00028803. [DOI] [PubMed] [Google Scholar]
- van der Geest AHM, Hall GE, Jr, Spiker S, Hall TC. The beta-phaseolin gene is flanked by matrix attachment regions. Plant J. 1994;6:413–423. doi: 10.1046/j.1365-313X.1994.06030413.x. [DOI] [Google Scholar]
- Van Eenennaam AL, Li G, Venkatramesh M, Levering C, Gong X, Jamieson AC, Rebar EJ, Shewmaker CK, Case CC. Elevation of seed a-tocopherol levels using plant-based transcription factors targeted to an endogenous locus. Metab Eng. 2004;6:101–108. doi: 10.1016/j.ymben.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Verdaguer B, de Kochko A, Beachy RN, Fauquet C. Isolation and expression in transgenic tobacco and rice plants, of the cassava vein mosaic virus (CsVMV) promoter. Plant Mol Biol. 1996;31:1129–1139. doi: 10.1007/BF00040830. [DOI] [PubMed] [Google Scholar]
- Verdaguer B, de Kochko A, Fux CI, Beachy RN, Fauquet C. Functional organization of the cassava vein mosaic virus (CsVMV) promoter. Plant Mol Biol. 1998;37:1055–1067. doi: 10.1023/A:1006004819398. [DOI] [PubMed] [Google Scholar]
- Viret JF, Mabrouk Y, Bogorad L. Transcriptional photoregulation of cell-type-preferred expression of maize rbcS-m3: 3′ and 5′ sequences are involved. Proc Natl Acad Sci USA. 1994;91:8577–8581. doi: 10.1073/pnas.91.18.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser RGF, Stolte A, Jacobsen E. Expression of a chimaeric granule-bound starch synthase-GUS gene in transgenic potato plants. Plant Mol Biol. 1991;17:691–699. doi: 10.1007/BF00037054. [DOI] [PubMed] [Google Scholar]
- Vitale A, Wu RJ, Cheng Z, Meagher RB. Multiple conserved 5′ elements are required for high-level pollen expression of the Arabidopsis reproductive actin ACT1. Plant Mol Biol. 2003;52:1135–1151. doi: 10.1023/B:PLAN.0000004309.06973.16. [DOI] [PubMed] [Google Scholar]
- Wakeley PR, Rogers HJ, Rozycka M, Greenland AJ, Hussey PJ. A maize pectin methyles-terase-like gene, ZmC5, specifically expressed in pollen. Plant Mol Biol. 1998;37:187–192. doi: 10.1023/A:1005954621558. [DOI] [PubMed] [Google Scholar]
- Wally O, Jayaraj J, Punja ZK. Comparative expression of b-glucuronidase with five different promoters in transgenic carrot (Daucus carota L.) root and leaf tissues. Plant Cell Rep. 2008;27:279–287. doi: 10.1007/s00299-007-0461-1. [DOI] [PubMed] [Google Scholar]
- Wang XL, Li XB. The GhACS1 gene encodes an acyl-CoA synthetase which is essential for normal microsporogenesis in early anther development of cotton. Plant J. 2009;57:473–486. doi: 10.1111/j.1365-313X.2008.03700.x. [DOI] [PubMed] [Google Scholar]
- Weber H, Ziechmann C, Graessmann A. In vitro DNA methylation inhibits gene expression in transgenic tobacco. EMBO J. 1990;9:4409–4415. doi: 10.1002/j.1460-2075.1990.tb07891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JT, Anderson OD, Blechl AE. Rapid production of multiple independent lines of fertile transgenic wheat (Triticum aestivum) Plant Physiol. 1993;102:1077–1084. doi: 10.1104/pp.102.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Theil EC. Identification and characterization of the iron regulatory element in the ferritin gene of a plant (soybean) J Biol Chem. 2000;275:17488–17493. doi: 10.1074/jbc.M910334199. [DOI] [PubMed] [Google Scholar]
- Weise A, Rodriguez-Franco M, Timm B, Hermann M, Link S, Jost W, Gorr G. Use of Physcomitrella patens actin 5′ regions for high transgene expression: importance of 50 introns. Appl Microbiol Biotechnol. 2006;70:337–345. doi: 10.1007/s00253-005-0087-6. [DOI] [PubMed] [Google Scholar]
- Weng H, Pan A, Yang L, Zhang C, Liu Z, Zhang D. Estimating number of transgene copies in transgenic rapeseed by real-time PCR assay with HMG I/Y as an endogenous reference gene. Plant Mol Biol Rep. 2004;22:289–300. doi: 10.1007/BF02773139. [DOI] [Google Scholar]
- Wyatt RE, Ainley WM, Nagao RT, Conner TW, Key JL. Expression of the Arabidopsis AtAux2–11 auxin-responsive gene in transgenic plants. Plant Mol Biol. 1993;22:731–749. doi: 10.1007/BF00027361. [DOI] [PubMed] [Google Scholar]
- Xu B, Timko MP. Methyl jasmonate induced expression of the tobacco putrescine N-methyltransferase genes requires both G-box and GCC-motif elements. Plant Mol Biol. 2004;55:743–761. doi: 10.1007/s11103-004-1962-8. [DOI] [PubMed] [Google Scholar]
- Xu SX, Liu GS, Chen RD. Characterization of an anther- and tapetum-specific gene and its highly specific promoter isolated from tomato. Plant Cell Rep. 2006;25:231–240. doi: 10.1007/s00299-005-0056-7. [DOI] [PubMed] [Google Scholar]
- Xu Y, Yu H, Hall TC. Rice triosephosphate isomerase gene 59 sequence directs β-glucuronidase activity in transgenic tobacco but requires an intron for expression in rice. Plant Physiol. 1994;106:459–467. doi: 10.1104/pp.106.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Ishige T, Shiota N, Inui H, Ohkawa H, Ohkawa Y. Enhancement of metabolizing herbicides in young tubers of transgenic potato plants with the rat CYP1A1 gene. Theor Appl Genet. 2002;105:515–520. doi: 10.1007/s00122-002-0961-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Suzuki K, Shinshi H. Elicitor-responsive, ethylene- independent activation of GCC box-mediated transcription that is regulated by both protein phosphorylation and dephos-phorylation in cultured tobacco cells. Plant J. 1999;20:571–579. doi: 10.1046/j.1365-313X.1999.00634.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto YT, Taylor CG, Acedo GN, Cheng CL, Conkling MA. Characterization of cisacting sequences regulating root-specific gene expression in tobacco. Plant Cell. 1991;3:371–382. doi: 10.1105/tpc.3.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Ding J, Zhang C, Jia J, Weng H, Liu W, Zhang D. Estimating the copy number of transgenes in transformed rice by real-time quantitative PCR. Plant Cell Rep. 2005;23:759–763. doi: 10.1007/s00299-004-0881-0. [DOI] [PubMed] [Google Scholar]
- Yang L, Kajiura H, Suzuki K, Hirose S, Fujiyama K, Takaiwa F. Generation of a transgenic rice seed-based edible vaccine against house dust mite allergy. Biochem Biophys Res Comm. 2008;365:334–339. doi: 10.1016/j.bbrc.2007.10.186. [DOI] [PubMed] [Google Scholar]
- Yang L, Suzuki K, Hirose S, Wakasa Y, Takaiwa F. Development of transgenic rice seed accumulating a major Japanese cedar pollen allergen (Cry j 1) structurally disrupted for oral immunotherapy. Plant Biotechnol J. 2007;5:815–826. doi: 10.1111/j.1467-7652.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- Yang NS, Russell D. Maize sucrose synthase-1 promoter directs phloem cell-specific expression of Gus gene in transgenic tobacco plants. Proc Natl Acad Sci USA. 1990;87:4144–4148. doi: 10.1073/pnas.87.11.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Grimmig B, Matern U. Anthranilate N-hydroxycinnamoyl/benzoyltransferase gene from carnation: rapid elicitation of transcription and promoter analysis. Plant Mol Biol. 1998;38:1201–1214. doi: 10.1023/A:1006003731919. [DOI] [PubMed] [Google Scholar]
- Yoshiba Y, Nanjo T, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Stress-responsive and developmental regulation of Δ2(1)-pyrroline-5-carboxylate synthetase 1 (P5CS1) gene expression in Arabidopsis thaliana. Biochem Biophys Res Commun. 1999;11:766–772. doi: 10.1006/bbrc.1999.1112. [DOI] [PubMed] [Google Scholar]
- Zeyenko VV, Ryabova LA, Gallie DR, Spirin AS. Enhancing effect of the 30′-untranslated region of tobacco mosaic virus RNA on protein synthesis in vitro. FEBS Lett. 1994;354:271–273. doi: 10.1016/0014-5793(94)01126-5. [DOI] [PubMed] [Google Scholar]
- Zhang W, McElroy D, Wu R. Analysis of rice Act1 5′ region activity in transgenic rice plants. Plant Cell. 1991;3:1155–1165. doi: 10.1105/tpc.3.11.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Guo S, Chen S, Zhang H, Zhao Y. Expression of Yeast YAP1 in transgenic Arabidopsis results in increased salt tolerance. J Plant Biol. 2009;52:56–64. doi: 10.1007/s12374-008-9004-8. [DOI] [Google Scholar]
- Zhu B, Chen THH, Li PH. Activation of two osmotin-like protein genes by abiotic stimuli and fungal pathogen in transgenic potato plants. Plant Physiol. 1995;108:929–937. doi: 10.1104/pp.108.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Song B, Zhang C, Ou Y, Xie C, Liu J. Construction and functional characteristics of tuber-specific and cold-inducible chimeric promoters in potato. Plant Cell Rep. 2008;27:47–55. doi: 10.1007/s00299-007-0399-3. [DOI] [PubMed] [Google Scholar]