Abstract
Zika virus (ZIKV) infection is associated with adverse pregnancy outcomes in humans, and infection in the first trimester can lead to miscarriage and stillbirth. Vertical and sexual transmissions of ZIKV have been demonstrated, yet the impact of infection during the initial stages of pregnancy remains unexplored. Here we defined the impact of ZIKV on early embryonic and placental development with a rhesus macaque model. During in vitro fertilization (IVF), macaque gametes were inoculated with a physiologically relevant dose of 5.48log10 plaque-forming units (PFU) of Zika virus/H.sapiens-tc/PUR/2015/PRVABC59_v3c2. Exposure at fertilization did not alter blastocyst formation rates compared to controls. To determine the impact of ZIKV exposure at implantation, hatched blastocysts were incubated with 3.26log10, 4.26log10, or 5.26log10 PFU, or not exposed to ZIKV, followed by extended embryo culture for 10 days. ZIKV exposure negatively impacted attachment, growth, and survival in comparison to controls, with exposure to 5.26log10 PFU ZIKV resulting in embryonic degeneration by day 2. Embryonic secretion of pregnancy hormones was lower in ZIKV-exposed embryos. Increasing levels of infectious virus were detected in the culture media post-exposure, suggesting that the trophectoderm is susceptible to productive ZIKV infection. These results demonstrate that ZIKV exposure severely impacts the zona-free blastocyst, whereas exposure at the time of fertilization does not hinder blastocyst formation. Overall, early stages of pregnancy may be profoundly sensitive to infection and pregnancy loss, and the negative impact of ZIKV infection on pregnancy outcomes may be underestimated.
Keywords: Zika virus, blastocyst, trophoblast, in vitro fertilization (IVF), preimplantation embryo, peri-implantation embryo
Exposure to Zika virus at the peri-implantation stage of development reduced embryo attachment and altered trophoblast growth, survival, and function in a nonhuman primate in vitro implantation model.
Introduction
Zika virus (ZIKV) is a human pathogen with human-to-human transmissibility. It was originally isolated from a febrile rhesus macaque in Uganda in 1947 [1]. In the past decade, there have been numerous outbreaks of ZIKV [2], and the most recent outbreak, in Brazil in 2015, suggested congenital ZIKV infection is associated with fetal malformations, including microcephaly as a severe manifestation of disease [3, 4]. Although most infections in adults do not cause significant disease and are often asymptomatic, a ZIKV infection during the pregnancy can be catastrophic for the fetus and result in stillbirth and miscarriage [4–6].
Placental defects are noted in many cases of adverse pregnancy outcome [4, 7]. Consistent with these findings, ZIKV has been shown in vivo and in vitro to infect trophoblast cells, which have specialized niches in the placenta [8–16]. A study by Costa et al. [12] found that first-trimester human placental explants and primary trophoblasts were susceptible to infection with a ZIKV isolate obtained during the Brazil outbreak. Conversely, a study by Bayer et al. [13] reported that term primary human trophoblast cells were resistant to ZIKV. This increased resistance to ZIKV in later stages of development was also observed in nonhuman primate (NHP) studies, where maternal ZIKV infection in the third trimester resulted in less severe outcomes compared to infections in the first trimester [14].
Despite the grave repercussions of ZIKV infection during pregnancy, it is unknown how early in development ZIKV can infect the embryo and early trophoblast lineage. This gap in knowledge is largely due to a limited means of determining human pregnancy loss in the first month of gestation. Bridging this knowledge gap is important as epidemiological and experimental data suggest that an infection earlier in pregnancy may be more likely to result in birth defects or pregnancy loss than an infection in later stages of pregnancy [17, 18]. Sheridan et al. [19] demonstrated that human embryonic stem cells differentiated to form more primitive trophoblast cells were highly susceptible to African ZIKV strains (Nigeria, Senegal, and lab-adapted Uganda) [19]. Thus, the earliest placental lineage may have differential susceptibility to infection. This necessitates evaluation of how infection impacts embryonic trophoblasts.
Previous in vivo NHP pregnancy studies modeling ZIKV infection in our lab and others have reported similar findings regarding pregnancy outcomes and associated pathologies as those observed in humans [7, 14, 16, 20, 21]. Both macaques and humans are similar in placental development forming an interstitial implantation site, in which the trophoblast cells invade through the luminal epithelium of the uterus [22]. Thus, the NHP model recapitulates early human trophoblast development and is an essential and appropriate model to study the impact of ZIKV during pregnancy.
Sexual transmission of ZIKV may negatively impact an established pregnancy, but its impact on embryo development and the establishment of pregnancy is unknown. ZIKV RNA (vRNA) has been detected in human semen and cases of sexual transmission have been reported [23–27]. This is a concern as Zika vRNA has been sporadically detected in human semen for up to 370 days after infection [28]. Furthermore, Zika vRNA was detected in 25% of sperm cell fraction samples prepared by washing on a 90% gradient, the method of preparation recommended for HIV-infected men [23]. This may have implications in assisted reproductive technologies (ARTs), as it is unclear if infectious semen can impact fertilization and embryo development. NHP studies have also observed Zika vRNA shedding in semen and detectable vRNA in male reproductive tract tissues including the testes, epididymis, seminal vesicle, and prostate [29–33]. Hence, the NHP model presents an opportunity to evaluate how sexual transmission of ZIKV can impact the initial stages of embryo development to ensure ARTs, including in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), are safe and do not compromise developmental outcomes.
In order to study the direct impact of ZIKV on the earliest trophoblast cells, we implemented an in vitro implantation NHP model. Combining this model with IVF allowed us to study the effect of ZIKV on the earliest stages of pregnancy by monitoring implantation stage trophoblast/embryo outgrowths in a controlled environment, which are difficult to study in vivo. Our primary objective for this study was to determine the earliest stage that ZIKV could impact embryo development and the establishment of pregnancy, as well as how infection alters trophoblast function during implantation. Embryo implantation in the uterus is a critical event in establishing pregnancy, as disruption of this process is estimated to account for 60% of pregnancy loss [34]. Based on the adverse pregnancy outcomes from in utero ZIKV exposure, we hypothesized that ZIKV would have a detrimental impact on the development of the implanting embryo and on subsequent trophoblast growth. ARTs, such as IVF, are used to overcome infertility, and the risk of viral infection decreasing success rates would be devastating. The outcomes of this research directly define the impact of ZIKV infection on early embryo development and implantation, and address concerns regarding parental infection and the use of ARTs.
Methods
Rhesus macaque IVF and embryo culture
Rhesus monkeys (Macaca mulatta) were from the colony maintained at the Wisconsin National Primate Research Center. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and under the approval of the University of Wisconsin College of Letters and Sciences and Vice Chancellor Office for Research and Graduate Education Institutional Animal Care and Use Committee. A total of five semen donors and 22 oocyte donors were used for these studies, where oocytes from nine donors were designated to assess the impact of ZIKV at the time of fertilization, and hatched blastocysts from 13 oocyte donors were evaluated for the impact of ZIKV during the peri-implantation stage of development. Note that oocyte donors could undergo up to three ovarian stimulation events for oocyte collection.
Rhesus IVF embryos were generated as previously described [35–38] and methods are briefly outlined here. Female oocyte donors underwent ovarian hyperstimulation by administration of 30 international units (IU) recombinant human follicle-stimulating hormone (FSH; follitropin beta, Merck Sharp & Dohme Corp, Whitehouse Station, NJ, USA), twice daily for 8–9 days followed by one injection of 1000 IU recombinant human choriogonadotropin (hCG; Ovitrelle, Merck Serono Ltd, Middlesex, UK) the next day. Oocytes were retrieved by laparoscopic aspiration between 30 and 33 h after the hCG injection. Those that had resumed meiosis and were in metaphase I were placed into maturation medium, consisting of CMRL (Thermo Fisher Scientific, Cat #11530037, Waltham, MA, USA) medium supplemented with 0.5 mM sodium pyruvate (Sigma Aldrich, Cat #2256, St. Louis, MO, USA), 2 mM Alanyl-glutamine (Sigma, #G8541), and 20% fetal bovine serum (FBS; Peak Serum, Cat #PS-FB1, Wellington, CO, USA). Metaphase I (MI) oocytes were evaluated for progression to metaphase II (MII) at ∼36 h post-hCG injection and those that had extruded a polar body were transferred to fertilization medium composed of IVF-TL medium (Caisson Laboratories Inc, Smithfield, UT) supplemented with 0.5 mM sodium pyruvate, 2 mm Alanyl-glutamine, and 0.1 mg/mL Polyvinyl alcohol (PVA; Sigma, Cat #P8136). Oocytes that were MII at the time of recovery were placed directly into fertilization medium.
Semen was collected from male rhesus macaques by electroejaculation [39]. The coagulum was removed 30 min post-collection and sperm were washed twice in HEPES-TL (Caisson Laboratories, Cat #IVL01) supplemented with 0.1 mM sodium pyruvate and 3 mg/mL bovine serum albumin (Sigma, Cat #A8806). A total of 40 million sperm were added to 2 mL of capacitation medium consisting of IVF-TL (Caisson Laboratories, Cat #IVL02) supplemented with 0.1 mM sodium pyruvate and 0.1 mg/mL PVA. To facilitate capacitation, caffeine (Sigma, Cat #C0750), and dibutyryl-cAMP (Sigma, Cat #D0627) were added to the capacitation medium 30 min prior to fertilization at a final concentration of 1 mM and 0.5 mM, respectively [39]. At the time of fertilization, 2.5 μL sperm, 2 μL 100 mM caffeine, and 2 μL of 50 mM dibutyryl-cAMP were added to each fertilization drop (93.5 μL drops for gametes not exposed to ZIKV and 81 μL drops for gametes exposed to ZIKV). The difference in drop size it to account for the amount of ZIKV added.
Gametes were co-incubated for ~16 h and individual presumptive zygotes were transferred into microwells of CultureCoin Miri-TL dishes (Esco Medical, Denmark). A CultureCoin Miri-TL dish contains 14 microwells that contain 25 μL of media overlaid with 3 mL Ovoil (Vitrolife, Cat #10029, Englewood, CO, USA). Embryos were cultured in G-TL medium (Vitrolife, Cat #10145) supplemented with 5% FBS for eight to 11 days corresponding to the hatched blastocyst stage.
Inoculation of gametes and embryos with ZIKV
Zika virus/H.sapiens-tc/PUR/2015/PRVABC59_v3c2 (PRVABC59) (GenBank: KU501215) was originally isolated from a traveler to Puerto Rico and passaged three times on Vero cells (American Type Culture Collection (ATCC): CCL-81), was obtained from Brandy Russell (CDC, Ft. Collins, CO). Virus stocks were prepared by inoculation onto a confluent monolayer of C6/36 cells (Aedes albopictus larval cells; ATCC: CCL-1660) with two rounds of amplification and confirmed to be identical to the reference sequence at the consensus level. This isolate was chosen as it would complement other in vivo studies that utilized ZIKV-PRVABC59 and observed adverse pregnancy outcomes [7, 40].
To determine if ZIKV could impact oocyte fertilization, gametes were inoculated with 5.48log10 PFU (16 μL at 7.26log10 PFU/mL added to 81 μL fertilization medium) ZIKV-PRVABC59 at fertilization. To determine if ZIKV could impact trophoblast function and embryo implantation, blastocyst stage embryos were plated on Matrigel (BD Biosciences, Cat # 354253, Bedford, MA) [36] within 24 h of hatching, which occurred on ~day 8–10 of development. Embryos were then either exposed to ZIKV or were cultured as non-exposed controls. The day the blastocysts were plated on Matrigel, they were incubated with either 5.26log10 (high), 4.26log10 (medium), or 3.26log10 (low) PFU ZIKV for 5 h (Figure 1) or left as uninfected controls. Co-incubation of embryo and virus was accomplished by addition of 10 μL of ZIKV (or serially diluted ZIKV dependent on dose) to 90 μL culture medium. Mock infected embryos were exposed to C6/36 cell conditioned medium for 5 h they day they were plated on Matrigel to verify that the conditioned medium in which the ZIKV stock was produced was not embryotoxic.
Figure 1.

Schematic depiction of ZIKV exposure to gametes (A) and hatched blastocysts (B). (A) Rhesus gametes were co-incubated with ZIKV at the time of fertilization, followed by evaluation of embryo cleavage and blastocyst formation rates. (B) In vitro fertilized hatched blastocysts were plated on Matrigel and immediately exposed to one of three doses of ZIKV for 5 h or cultured as unexposed controls. On day (d) 2, d6 and d10 post-plating, media were collected, embryo outgrowths were imaged, and area and diameter were measured.
Extended embryo culture utilizing an in vitro peri-implantation model
Embryos were cultured as previously described [38] with modifications. Hatched blastocyst stage embryos were plated on Matrigel (Corning, Cat # 354230, Corning, NY, USA) with 200 μL of buffalo rat liver (BRL)-conditioned medium, which is equal parts EMEM (Quality Biologics, Cat #112018, Gaithersburg, MD, USA) and CMRL (Thermo Fisher Scientific, Cat #11530037) with 5% FBS. The medium was conditioned for 24-h, ultracentrifuged (SW41 rotor at 130 000 × g average) overnight to remove large particles and extracellular vesicles, sterile-filtered, and frozen back. Media were changed on days 2 and 6 post-plating (Figure 1B). Prior to media exchange, embryo outgrowths were imaged using a Nikon Eclipse TE300. Outgrowth area was calculated using ×4 images and ImageJ using a ratio of 1 mm = 620 pixels. Embryos that grew up the side of the well were excluded from the analysis (one control, two low dose, and two medium dose). Of note, four control embryos were removed from initial experiments to serve as age matched controls for transcriptome analysis (not shown) and no data from these four controls is present in the dataset.
Hormone secretion
Progesterone secretion was analyzed using an enzyme immunoassay (EIA) (Cat #582601, Cayman, Ann Arbor, Michigan, USA). Samples were diluted in enzyme-linked immunosorbent assay (ELISA) buffer, run in duplicate, the manufacturer’s protocol was followed as directed and the plate was read at 410 nm. The minimum level of detection was 0.01 ng/mL (Figure 3A). To control for baseline quantities of hormones in the conditioned media, blank media samples conditioned by BRL cells but not conditioned by an embryo, were collected. For the blank samples that could reliably be quantified, the amount of progesterone detected was 0.063 ± 0.016 ng/mL.
Figure 3.

Secretion of hormones and immunomodulators by embryo outgrowths. (A) Progesterone and mCG secretions normalized for outgrowth area (pg/mL/mm2) on days 2, 6, and 10 post-plating are shown as individual graphs as the y-axis is changed for ease of reading. Trophoblast cytokine and growth factor secretions normalized for outgrowth area (pg/mL/mm2) and are depicted over days post-plating. (B) MCP1, (C) VEGF-A, (D) IL-6 (E) IL-8, (F) IL-1RA, (G) bNGF, and (H) BDNF. The lower limit of detection (LLD) in picograms (pg) is depicted in the corner of each graph. Fisher’s exact test with Bonferroni’s correction was used to determine significance, which is indicated directly on the graphs. The following analytes were not detected in any sample: IL-2, IL-17A, IL-23, IP-10, GM-CSF, IFN-gamma, IL-1beta, IL-10, IL-12p70, IL-13, ITAC, MIG, MIP-1alpha, IL-7, and VEGF-D. The following analytes were detected in five samples or less: FGF-2, Eotaxin, IL-4, IL-18, IL-5, TNF-alpha, BLC, MIP-1beta, SDF-1alpha, CD-40ligand, G-CSF, IFN-alpha, IL-15, PDGF-BB, and SCF.
Monkey CG (mCG) secretion was analyzed by radioimmunoassay as previously published [41]. Samples were diluted in EMEM and run in duplicate. The amount of mCG detected in blank media samples was 13.202 ± 6.977 ng/mL. Interassay variation (CV) was 6.3%. For statistical purposes, samples that fell below the limit of detection (0.1 ng/mL) were assigned that value.
The Luminex assay for cytokine, chemokine, and growth factor secretion by embryo outgrowths
To analyze the secretion of cytokines, chemokines, and growth factors an NHP 37-plex Procarta Luminex assay (Thermo Fisher Scientific, Cat # EPX370-40045-901) was used and the manufacturer’s instructions were followed. Samples were run in duplicate on a Bioplex 200 (BioRad, Cat #171000201, Hercules, CA, USA) and analyzed with the Bioplex Manager Software. The lower level of detection was obtained for each analyte based on the manufacturer’s quantifications (Figure 3B–H).
Infectious virus detected by plaque assay
Plaque assays were used to assess embryo susceptibility to ZIKV by determining if infectious virus was produced in culture [7]. Three embryos were exposed to the medium dose of ZIKV for 5-h. Because of the different collection timepoints, the media used for this aspect of the study were not used for any other analysis. For each media change, all media were removed and fresh media were added. The quantity of virus detected was the total amount of new virus produced between media collections, which occurred at 12-h, 1, 2, 4, 6, and 10 days post-plating, respectively.
Statistical analysis
A Fisher’s exact test with a Bonferroni correction was used to determine significance (P < 0.05) for the ZIKV exposure at the time of fertilization dataset, as well as attachment and survival. For the growth and secretion data, analysis was completed using GraphPad Prism 8.0 (GraphPad Software) and a nonparametric Kruskal–Wallis test with Dunn’s corrections applied (P < 0.05). All graphs show the mean ± standard error of the mean (SEM).
Results
ZIKV exposure at the time of fertilization
Since ZIKV is present in semen and can be sexually transmitted, we exposed gametes to ZIKV at the time of fertilization to determine if exposure impacted in vitro embryo development. A total of 102 MII oocytes were obtained from nine ovarian stimulation events, in which 57 oocytes were inoculated with ZIKV and cultured in parallel to 45 control oocytes. Embryo development in terms of cleavage rate was significantly higher in ZIKV-exposed versus control embryos as shown in Table 1 (P < 0.01). Of note, the control cleavage rate was lower in experimental replicates toward the end of the IVF season. However, no significant difference for blastocyst rate was observed between the ZIKV-exposed gametes and control (P < 0.81). These results suggest that ZIKV exposure at the time of fertilization does not impact preimplantation embryo developmental rates.
Table 1.
In vitro embryo development rates following ZIKV exposure at fertilization.
| Embryo group | #Oocyte retrievals | #M2 | #Cleaved | Cleavage Rate | #Blastocysts | Blastocyst Rate |
|---|---|---|---|---|---|---|
| Controls | 9 | 45 | 26 | 57.78% | 8 | 30.77% |
| ZIKV | 9 | 57 | 47 | 82.46% | 14 | 29.79% |
| P-value | P < 0.01 | 0.81 |
The number (#) of MII oocytes for each group that subsequently underwent cleavage and blastocyst formation. Cleavage rate was determined by dividing the total number of embryos by the number of mature oocytes. Blastocyst rate was determined by dividing the total number of embryos that reached the blastocyst stage by those that cleaved. For the nine oocyte retrieval replicates, the ratio of MII oocytes for control/ZIKV-exposed are as follows: 2/7, 5/8, 11/5, 6/11, 5/6, 4/5, 5/7, 3/4, and 4/4.
ZIKV exposure decreased peri-implantation embryo survival, attachment, and growth
To determine the impact of ZIKV on development in the peri-implantation phase, we exposed 32 hatched blastocysts to ZIKV and cultured 18 embryos as unexposed controls. At this stage of development the embryo has shed its zona pellucida, thus leaving the trophectoderm in direct contact with its environment. Embryo survival was evaluated based on the following parameters: attachment to the Matrigel, trophoblast outgrowth dissociation, and overall appearance, e.g., dark or grainy appearance indicating cellular degeneration. 83% of control trophoblast outgrowths survived until day 10 post-plating on Matrigel while only 60, 33, and 0% of the low, medium, and high-dose survived, respectively (Table 2). There was a significant difference between control and high-dose embryo survival on days 2, 6, and 10 (P < 0.05) and between low- and high dose on day 2 as demonstrated in Table 2 (P < 0.05). The difference in survival between control and medium-dose embryos (83% and 33%, respectively) approached significance on day 10 (P = 0.052). Together, these results suggest a dose-related effect of ZIKV exposure on embryo survival.
Table 2.
ZIKV reduced embryo attachment and survival.
| Embryo group | d2 | d6 | d10 |
|---|---|---|---|
| % Attached | |||
| Control | 50 | 78a | 93 |
| Low | 70 | 70ab | 60 |
| Medium | 42 | 42ab | 33 |
| High | 0 | 0b | 0 |
| % Survival | |||
| Control | 95ac | 83a | 83a* |
| Low | 100ac | 60ab | 60ab |
| Medium | 83abc | 50ab | 33ab |
| High | 20b | 0b | 0b* |
The percent of embryos attached to the Matrigel and alive on each day post-plating is shown. A Fisher’s exact test with a Bonferroni correction on the number of embryos attached or alive was calculated to determine significance. Values that do not share superscripts are statistically different at P < 0.05 and a superscript asterisk indicates P < 0.001.
Embryo outgrowths were imaged on days 2, 6, and 10 post-plating to measure embryo growth and representative images are shown in Figure 2A. Supplementary Figure 1 shows a representative image of primitive syncytiotrophoblast and extravillous trophoblast cells. A decrease in outgrowth diameter was observed for embryos exposed to 5.26log10 PFU ZIKV (high dose) on day 2. Likewise, embryos that were exposed to 4.26log10 PFU ZIKV (medium dose) had significantly reduced diameter (P < 0.001) and area (P < 0.05) in comparison to control outgrowths at day 6 post-plating. The trend toward decreased size for embryos exposed to ZIKV continued through day 10 post-plating.
Figure 2.

Peri-implantation in vitro embryo outgrowths exposed to ZIKV reduces growth. (A) Representative images of day 2, 6, and 10 post-plating of a control, a low-dose exposed, and a medium-dose exposed trophoblast outgrowth (scale bars = 50 um). (B) Trophoblast outgrowth diameter. (C) Outgrowth area. Blastocyst experimental groups are denoted as follows: controls = black circles, low-dose ZIKV = light gray triangles, medium dose = medium gray upside-down triangles, and high dose = dark gray squares.
Establishment of pregnancy requires attachment of the embryo to the uterine epithelium. In this study, 50% of control embryos attached to the Matrigel culture surface by day 2 post-plating, and 83% were attached by day 10 (Table 2). Decreased attachment to the Matrigel was noted with exposure to increasing doses of ZIKV. There was a significant (P < 0.05) decrease in attachment for embryos exposed to the high dose of ZIKV compared to controls on days 6 and 10 post-plating. No attachment for high-dose blastocysts reflects the degeneration of these embryos by day 2. The difference in attachment between medium dose and control blastocysts (33% and 93%, respectively) approached significance on day 10. By day 2, 70% of the blastocysts exposed to low-dose ZIKV were attached and there was a slight decrease in attachment over time.
Trophoblast function assessed by secretion of pregnancy-related hormones, cytokines, chemokines and growth factors
To determine how ZIKV exposure impacted trophoblast function, we analyzed the media for the secretion of pregnancy-associated hormones, progesterone and mCG, that are produced by trophoblasts. Embryo conditioned and non-conditioned media were collected on days 2, 6, and 10 post-plating to quantify the amount of hormone secreted relative to the area of the embryo outgrowth (Figure 3). All high-dose exposed blastocysts had degenerated by day 2, hence no data could be collected for this group. No significant differences were observed between ZIKV and control embryos, although there was a trend toward decreased progesterone and mCG secretion on day 6 for ZIKV-exposed embryos. If progesterone and mCG secretion were not normalized to the area of the trophoblast outgrowth, hormone secretion decreased between control and ZIKV-exposed embryos with a significant difference between controls and medium-dose day 6 post-plating (P < 0.01; Supplemental Figure 2).
To assess if ZIKV triggers an embryonic immune response, a Luminex assay was performed to measure secretion of cytokines, chemokines, and growth factors. The amount of analyte secreted was normalized to the area of the outgrowth. Seven cytokines and growth factors were consistently detected in the conditioned media: monocyte chemoattractant protein 1 (MCP1), brain-derived neurotrophic factor (BDNF), interleukin-8 (IL-8), interleukin-6 (IL-6), interleukin 1 receptor antagonist (IL-1RA), vascular endothelial growth factor A (VEGF-A), and beta nerve growth factor (bNGF) (Figure 3). There was a significant increase in MCP1, VEGF-A, and IL-6 secretion by ZIKV-exposed embryos compared to controls on day 6 post-plating (P < 0.05, P < 0.002, and P < 0.05, respectively). There was also a trend toward increased IL-8 and IL-6 secretion on day 10, and increased MCP1, BDNF, VEGF-A, IL-1RA, and bNGF secretion on days 6 and 10 in ZIKV compared to controls.
Verification of embryonic ZIKV infection
To determine if the embryo outgrowths were productively infected by ZIKV, embryo-conditioned media were tested for infectious virus via plaque assay. The embryos were exposed to ZIKV immediately after being plated on Matrigel. The titer of the inoculum for the three embryos was 5.1log10 PFU ZIKV on average (data not shown). At each time point (12-h and 1, 2, 4, 6, and 10 days post-plating/post ZIKV exposure), all media were collected, and new media were added. Minimal virus was produced within the first 24 h post ZIKV exposure but enhanced viral production occurred 2 and 4 days post-plating (3.29 and 3.92 log10 PFU ZIKV on average, respectively) (Figure 4). ZIKV titers dropped off day 6 post-plating and remained low day 10 post-plating.
Figure 4.
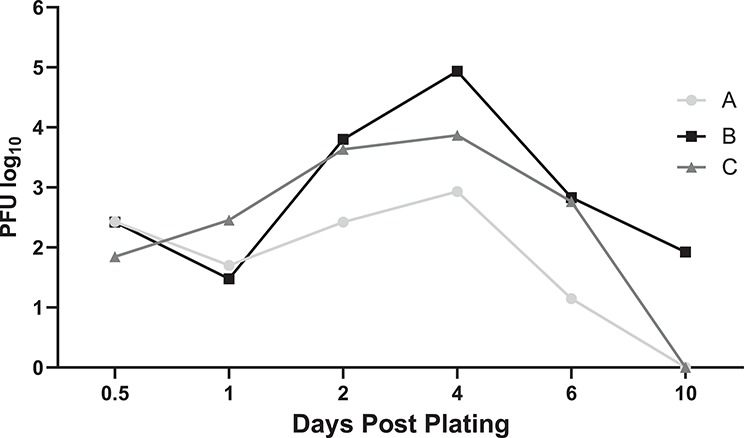
Infectious virus was detected in culture media following ZIKV exposure. Three embryos (A, B, and C) were exposed to medium-dose of ZIKV for 5 h. All media was removed from the well and collected at 12 h and 1, 2, 4, 6, and 10 days post plating/days post ZIKV exposure, respectively. The graph depicts the amount of virus detected (log10 PFU) over days post-plating.
Discussion
Embryo implantation is essential for the establishment of pregnancy and is difficult to directly study in humans, therefore the present study utilized rhesus macaque IVF-produced embryos an extended culture system to evaluate the impact of ZIKV exposure around the time of implantation. ZIKV-exposed oocytes had a higher cleavage rate than controls but an equivalent blastocyst rate, which suggests that ZIKV exposure at fertilization does not hinder preimplantation development rates. However, ZIKV exposure at the time of implantation is embryotoxic and impacts trophoblast function. ZIKV exposure in the zona-free stages of development severely impacted survival, growth, and attachment in a dose-related manner. Alterations in embryo secretions including mCG, MCP1, VEGF-A, and IL-6 support the hypothesis that ZIKV can impact the implanting blastocyst and impede trophoblast function as well as alter immune-related signaling by the trophoblasts. In addition, plaque assay data support the embryos were susceptible to productive ZIKV infection. These findings are supported by a recent study which demonstrated that the trophectoderm of both human and mouse embryos is susceptible to ZIKV infection [42]. Collectively, the observations that ZIKV impacts NHP, mouse, and human embryos should caution couples trying to naturally conceive or utilize ARTs for conception as the abundance of virus present in the semen of infected males may determine pregnancy success or failure.
Implantation encompasses the initial attachment and growth of the embryo as it invades into the endometrium. A decrease in the growth of embryo outgrowths was observed in response to ZIKV exposure, indicating that the trophoblast cells are either not proliferating or are undergoing cell death. High-dose ZIKV exposure was rapidly embryotoxic, which suggests cell death was the more likely scenario. However, it remains unclear whether exposure to low and medium doses of ZIKV were mainly impacting cellular proliferation or survival, both of which could be detrimental to the events of implantation. The negative impact of ZIKV infection near the time of implantation was recently demonstrated in an in vivo mouse pregnancy model. Tan et al. [42] reported ~50–70% reduction in pregnancy rates in a mouse model following subcutaneous ZIKV inoculation during the preimplantation stage of development at E2.5 and E3.5, whereas inoculation at the E4.5, during the peri-implantation stage, reduced the pregnancy rate by ~11%. Mating of male mice infected with ZIKV to wild-type females resulted in reduced pregnancy rates and fewer viable fetuses, although, sperm counts were also reduced [43]. Similarly, fetal demise was observed at day 10 and 12 post-mating in pregnancies where the virus was sexually transmitted to the female by mating with infected males 7 days post-inoculation [44]. These studies demonstrate that, in the presence of ZIKV, embryo survival is impeded near the time of implantation.
To better understand how ZIKV impacts trophoblast function, we measured the secretion of two trophoblast-secreted pregnancy hormones and several immune modulators. Embryos exposed to ZIKV had altered hormone secretion, which could impact the establishment of a pregnancy. Progesterone is a marker of trophoblast differentiation and important for maintaining pregnancy. Embryos exposed to ZIKV had decreased progesterone secretion compared to controls on day 6. However, on day 10, there was an increase in the amount of progesterone secreted. CG promotes trophoblast differentiation [35], cytotrophoblast growth [45], and the establishment of the pregnancy [46, 47], and decreased CG could impact the maintenance of the pregnancy. Based on morphology, the presence of multinucleated cells and the detection of mCG suggests syncytiotrophoblast are present in our model [38]. The decrease in mCG secretion by trophoblast outgrowths exposed to ZIKV suggests trophoblast differentiation and syncytiotrophoblast formation are impaired, an outcome that would be consistent with increased risk of early pregnancy loss.
Cytokines and growth factors are important signaling molecules between the blastocyst and uterus during implantation [48]. VEGF-A is an important growth factor during implantation as it promotes vascular remodeling of the maternal spiral arteries, while insufficient levels can lead to preeclampsia [49]. MCP1 is a mediator of angiogenesis, vascular remodeling, and plays a role in inflammation [50]. Flavivirus infection during pregnancy has been documented, and in one dengue virus-infected pregnancy that resulted in stillbirth, the placenta was inflamed, hemorrhagic, and necrotic and had increased levels of MCP1 and VEGF compared to control tissues [51]. This in vivo data is consistent with our observation that ZIKV exposure resulted in increased MCP1 and VEGF-A secretion. BDNF is involved in trophoblast growth and proliferation of the trophectoderm [52], and increased BDNF secretion was observed by embryos exposed to ZIKV, an observation that seems contrary to the decreased trophoblast outgrowth size of embryos exposed to ZIKV. Nonetheless, these cytokines and growth factors play important roles in trophoblast function, and their altered secretion supports the concept that ZIKV exposure can lead to aberrant placental development and function.
The plaque assay data demonstrated that the blastocysts were susceptible to ZIKV. In addition, these three embryos appeared dead around day 4 post-plating, which aligns with decreased ZIKV production detected days 6 and 10 post-plating, potentially due to a lack of live cells to produce virus. Several blastocysts were exposed to C6/36 conditioned medium to verify that C6/36 media itself is not embryonic lethal. These five embryos were exposed to the same duration and quantity of inoculating medium as the high-dose ZIKV embryos. Although the exact pathways and receptor mediating infection remain unclear, numerous in vitro and in vivo studies indicate ZIKV can infect cells of the placenta [4, 9, 13, 53]. The extent of trophoblast susceptibility may be variable throughout gestation [4, 53] and dependent upon the ZIKV strain [15, 19, 31].
Human and NHP studies show that first-trimester placentas are more vulnerable to pathogenic outcomes from a ZIKV infection [4, 40, 54]. Sheridan et al. evaluated primitive (i.e., embryonic stem cell-derived) trophoblast cell susceptibility and their conclusions further support our findings that the trophoblast cells present at the time of implantation are susceptible to ZIKV [19]. In another study, exposure of first-trimester placental explants to ZIKV showed that various placental cell types were susceptible to infection, and that infection of proliferating cytotrophoblasts resulted in cellular arrest [11]. This supports the current study’s finding that ZIKV exposure resulted in decreased trophoblast size and survival.
ZIKV has been detected in the semen of infected men [23] and NHPs [30, 33, 55]. The highest quantities of ZIKV detected were 8.5log10 [24], 8.6log10 [27], and 7.5log10 [26] copies/mL of semen. PRVABC59, the isolate used in these studies, has been shown to infect human male reproductive tract cells in vitro [28], which is relevant for this study in regards to ARTs and sexual transmission. ZIKV vRNA has been detected in both the seminal plasma and sperm cell fractions of human and NHPs isolated using a sperm wash method recommended for HIV-infected men [23, 55], which implies that sperm wash preparations prior to IVF may not completely remove ZIKV. Moreover, Mansuy et al. [56] detected ZIKV within the head of a human spermatozoon by immunostaining against ZIKV. It is uncertain whether transmission outcomes may differ when ZIKV is present and delivered to the oocyte as cargo within the sperm head or when bound to the exterior surface.
Although sexual transmission of ZIKV is known to occur [26, 28, 57, 58], its impact on the female reproductive tract, oocytes, and embryos remains unclear. Vaginal/endocervical swabs from non-pregnant humans were positive for ZIKV by qRT-PCR [59–62]. Comparatively, ZIKV infection during pregnancy in the mouse results in higher viral titers in female reproductive tract tissues than infection during the non-pregnant state [44, 63]. However, uterine viral loads in the non-pregnant state are detectable following sexual transmission [63] or intravaginal inoculation [44]. In vitro studies have shown that ZIKV can infect human endometrial stromal cells (HESC) and decidualized HESC [64]. In a clinical study of women undergoing oocyte vitrification cycles, no ZIKV was detected in 13 patient endometrial biopsies where five of the patients presented with positive vaginal vRNA at 19 days, 8, 14, 17 or 29 weeks prior to the endometrial biopsy [62]. It is plausible that ZIKV infection may have been cleared by endometrial cells at the time of biopsy collection, and biopsy collection closer to the time of infection, whether vector-born or sexual transmission, may be more informative. Mouse and NHP models of ZIKV infection in the non-pregnant state have demonstrated that intravaginal and subcutaneous inoculations result in positive ZIKV detection in female reproductive tract tissues as well as vaginal washes/swabs [20, 30, 44, 63, 65, 66], which supports potential embryo exposure to ZIKV in the female reproductive tract.
Conclusions
Following the ZIKV outbreak in Brazil in 2016, a significant decline in pregnancy rates was observed, which Castro, et al. hypothesized to be due to postponement and selected termination of pregnancy [67]. In the present study, ZIKV exposure at the time of in vitro implantation resulted in decreased embryo survival, attachment and growth, and altered the quantity of secreted products compared to controls. We speculate that the decline in pregnancy rates immediately following the ZIKV epidemic may in part be due to a decline in the successful establishment of pregnancies, via ZIKV impeding embryo implantation, sexual transmission of ZIKV, or potentially infectious follicular fluid, oocytes, or female reproductive tract tissues. However, further in vivo research into the impact of ZIKV infection on embryo implantation is required to elucidate how ZIKV infection may have impacted the pregnancy rates during the Brazil outbreak. The present study shows ZIKV can infect embryos and impact the earliest trophoblast cell function, and therefore suggests that ZIKV exposure could impact pregnancy sooner than previously demonstrated.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
LNB, TCF, TGG, and JKS contributed to the conception and design of the study. MTA and TCF provided the virus used in the experiments and consulted on the plaque assay. LNB, KDM, GJW, TGG and JKS collected and analyzed the data. LNB, TGG, and JKS drafted the manuscript. MLS is in the Wisconsin National Primate Research Center Scientific Protocol Implementation Unit who coordinated and performed the animal procedures. The publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Supplementary Material
Acknowledgements
The authors would like to extend our thanks to the animal care and veterinary staff at the Wisconsin National Primate Research Center, especially Dr. Kevin Brunner for his surgical assistance in collecting oocytes for IVF experiments, as well as Dr. Kristen Bernard and members of the Golos lab for thoughtful discussion of these experiments. We would like to thank the University of Wisconsin-Madison College of Agriculture and Life Sciences Statistical Consulting Lab, particularly Nicholas Keuler, for statistical consultation. We would also like to thank the NIH and the Wisconsin National Primate Center for funding support.
Conference Presentation: This work was presented in part at the 51st Annual Meeting of the Society for the Study of Reproduction (10–13 July 2018, New Orleans, Louisiana), the 38th Annual Meeting of the American Society for Virology (14–18 July 2018, College Park, Maryland), and the 66th Annual Meeting Society for Reproductive Investigation (12–16 March 2019, Paris, France).
Footnotes
† Grant Support: This research was funded by the National Institutes of Health (NIH), grant P51 OD011106-54 to the Wisconsin National Primate Research Center, grant R21 HD091163-01 to T.G.G, and grants F31 HD100057 and T32GM081061 to L.N.B. The content is solely responsibility of the authors and does not necessarily represent the official views of Office of Research Infrastructure Programs (ORIP) or the NIH.
References
- 1. Dick GW, Kitchen SF, Haddow AJ, Zikavirus I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–520. [DOI] [PubMed] [Google Scholar]
- 2. Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis 2015; 21:1885–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, Doriqui MJ, Neri JI, Neto JM, Wanderley HY, Cernach M, El-Husny AS et al. Possible association between Zika virus infection and microcephaly – Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:59–62. [DOI] [PubMed] [Google Scholar]
- 4. Bhatnagar J, Rabeneck DB, Martines RB, Reagan-Steiner S, Ermias Y, Estetter LB, Suzuki T, Ritter J, Keating MK, Hale G, Gary J, Muehlenbachs A et al. Zika virus RNA replication and persistence in brain and placental tissue. Emerg Infect Dis 2017; 23:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, Ribeiro EM, Ventura LO, Neto NN, Arena JF, Rasmussen SA. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017; 171:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agrawal R, Oo HH, Balne PK, Ng L, Tong L, Leo YS. Zika virus and the eye. Ocul Immunol Inflamm 2018; 26:654–659. [DOI] [PubMed] [Google Scholar]
- 7. Mohr EL, Block LN, Newman CM, Stewart LM, Koenig M, Semler M, Breitbach ME, Teixeira LBC, Zeng X, Weiler AM, Barry GL, Thoong TH et al. Ocular and uteroplacental pathology in a macaque pregnancy with congenital Zika virus infection. PLoS One 2018; e0190617:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, Goldsmith C, Hale G, Ritter J, Rollin D, Shieh WJ, Luz KG et al. Notes from the field: Evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses--Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:159–160. [DOI] [PubMed] [Google Scholar]
- 9. Aagaard KM, Lahon A, Suter MA, Arya RP, Seferovic MD, Vogt MB, Hu M, Stossi F, Mancini MA, Harris RA, Kahr M, Eppes C et al. Primary human placental Trophoblasts are permissive for Zika virus (ZIKV) replication. Sci Rep 2017; 7: 41389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, Armistead B, Tisoncik-Go J et al. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med 2016; 22:1256–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 2016; 20:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, Veas F, Al-Daccak R, Izopet J. Jabrane-Ferrat N. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep 2016; 6: 35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr, Cherry S, Sadovsky Y, Coyne CB, Type III. Interferons produced by human placental Trophoblasts confer protection against Zika virus infection. Cell Host Microbe 2016; 19:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nguyen SM, Antony KM, Dudley DM, Kohn S, Simmons HA, Wolfe B, Salamat MS, Teixeira LBC, Wiepz GJ, Thoong TH, Aliota MT, Weiler AM et al. Highly efficient maternal-fetal Zika virus transmission in pregnant rhesus macaques. PLoS Pathog 2017; 13:e1006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sheridan MA, Yunusov D, Balaraman V, Alexenko AP, Yabe S, Verjovski-Almeida S, Schust DJ, Franz AW, Sadovsky Y, Ezashi T, Roberts RM. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci U S A 2017; 114:E1587–E1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gurung S, Reuter N, Preno A, Dubaut J, Nadeau H, Hyatt K, Singleton K, Martin A, Parks WT, Papin JF, Myers DA. Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon. PLoS Pathog 2019; 15:e1007507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist 2007; 13:241–256. [DOI] [PubMed] [Google Scholar]
- 18. Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, Ahmad N, Macdonald J, Evert N, Bingham A, Ellington SR, Shapiro-Mendoza CK et al. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 2017; 317:59–68. [DOI] [PubMed] [Google Scholar]
- 19. Sheridan MA, Balaraman V, Schust DJ, Ezashi T, Roberts RM, African FAWE. Asian strains of Zika virus differ in their ability to infect and lyse primitive human placental trophoblast. PLoS One 2018; 13:e0200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirsch AJ, Roberts VHJ, Grigsby PL, Haese N, Schabel MC, Wang X, Lo JO, Liu Z, Kroenke CD, Smith JL, Kelleher M, Broeckel R et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat Commun 2018; 9:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dudley DM, Aliota MT, Mohr EL, Newman CM, Golos TG, Friedrich TC, O'Connor DH. Using macaques to address critical questions in Zika virus research. Annu Rev Virol 2019; 6:481–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee KY, DeMayo FJ. Animal models of implantation. Reproduction 2004; 128:679–695. [DOI] [PubMed] [Google Scholar]
- 23. Joguet G, Mansuy JM, Matusali G, Hamdi S, Walschaerts M, Pavili L, Guyomard S, Prisant N, Lamarre P, Dejucq-Rainsford N, Pasquier C, Bujan L. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis 2017; 17:1200–1208. [DOI] [PubMed] [Google Scholar]
- 24. D'Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Leparc-Goffart I. Evidence of sexual transmission of Zika virus. N Engl J Med 2016; 374:2195–2198. [DOI] [PubMed] [Google Scholar]
- 25. Atkinson B, Hearn P, Afrough B, Lumley S, Carter D, Aarons EJ, Simpson AJ, Brooks TJ, Hewson R. Detection of Zika virus in semen. Emerg Infect Dis 2016; 22:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015; 21:359–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, Izopet J, Martin-Blondel G. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis 2016; 16:405. [DOI] [PubMed] [Google Scholar]
- 28. Counotte MJ, Kim CR, Wang J, Bernstein K, Deal CD, Broutet NJN, Low N. Sexual transmission of Zika virus and other flaviviruses: a living systematic review. PLoS Med 2018; 15:e1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gurung S, Preno AN, Dubaut JP, Nadeau H, Hyatt K, Reuter N, Nehete B, Wolf RF, Nehete P, Dittmer DP, Myers DA, Papin JF. Translational model of Zika virus disease in baboons. J Virol 2018; 92. doi: 10.1128/JVI.00186-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange RW, Best K, Luo M, Hraber PT, Andersen-Elyard H, Ojeda EF et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med 2016; 22:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koide F, Goebel S, Snyder B, Walters KB, Gast A, Hagelin K, Kalkeri R, Rayner J. Development of a Zika virus infection model in Cynomolgus macaques. Front Microbiol 2016; 7:2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, DeFilippis VR, Denton M, Smith PP, Messer WB, Colgin LM, Ducore RM et al. Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog 2017; 13:e1006219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Silveira ELV, Rogers KA, Gumber S, Amancha P, Xiao P, Woollard SM, Byrareddy SN, Teixeira MM, Villinger F. Immune cell dynamics in rhesus macaques infected with a Brazilian strain of Zika virus. J Immunol 2017; 199:1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Emiliani S, Delbaere A, Devreker F, Englert Y. Embryo-maternal interactive factors regulating the implantation process: implications in assisted reproductive. Reprod Biomed Online 2005; 10:527–540. [DOI] [PubMed] [Google Scholar]
- 35. Chang TA, Bondarenko GI, Gerami-Naini B, Drenzek JG, Durning M, Garthwaite MA, Schmidt JK, Golos TG. Trophoblast differentiation, invasion and hormone secretion in a three-dimensional in vitro implantation model with rhesus monkey embryos. Reprod Biol Endocrinol 2018; 16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rozner AE, Dambaeva SV, Drenzek JG, Durning M, Golos TG. Modulation of cytokine and chemokine secretions in rhesus monkey trophoblast co-culture with decidual but not peripheral blood monocyte-derived macrophages. Am J Reprod Immunol 2011; 66:115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wolfgang MJ, Eisele SG, Knowles L, Browne MA, Schotzko ML, Golos TG. Pregnancy and live birth from nonsurgical transfer of in vivo- and in vitro-produced blastocysts in the rhesus monkey. J Med Primatol 2001; 30:148–155. [DOI] [PubMed] [Google Scholar]
- 38. Rozner AE, Durning M, Kropp J, Wiepz GJ, Golos TG. Macrophages modulate the growth and differentiation of rhesus monkey embryonic trophoblasts. Am J Reprod Immunol 2016; 76:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. VandeVoort CA, Tollner TL, Overstreet JW. Separate effects of caffeine and dbcAMP on macaque sperm motility and interaction with the zona pellucida. Mol Reprod Dev 1994; 37:299–304. [DOI] [PubMed] [Google Scholar]
- 40. Dudley DM, Van Rompay KK, Coffey LL, Ardeshir A, Keesler RI, Bliss-Moreau E, Grigsby PL, Steinbach RJ, Hirsch AJ, MacAllister RP, Pecoraro HL, Colgin LM et al. Miscarriage and stillbirth following maternal Zika virus infection in nonhuman primates. Nat Med 2018; 24:1104–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toni E, Ziegler RLM, Wegner FH. Detection of urinary gonadotropins in Callitrichid monkeys with a sensitive lmmunoassay based upon a unique monoclonal antibody. Am J Primatol 1993; 181–188. [DOI] [PubMed] [Google Scholar]
- 42. Tan L, Lacko LA, Zhou T, Tomoiaga D, Hurtado R, Zhang T, Sevilla A, Zhong A, Mason CE, Noggle S, Evans T, Stuhlmann H et al. Pre- and peri-implantation Zika virus infection impairs fetal development by targeting trophectoderm cells. Nat Commun 2019; 10:4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, Diamond MS. Zika virus infection damages the testes in mice. Nature 2016; 540:438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duggal NK, Ritter JM, Pestorius SE, Zaki SR, Davis BS, Chang GJ, Bowen RA, Brault AC. Frequent Zika virus sexual transmission and prolonged viral RNA shedding in an Immunodeficient mouse model. Cell Rep 2017; 18:1751–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yagel S, Casper RF, Powell W, Parhar RS, Lala PK. Characterization of pure human first-trimester cytotrophoblast cells in long-term culture: growth pattern, markers, and hormone production. Am J Obstet Gynecol 1989; 160:938–945. [DOI] [PubMed] [Google Scholar]
- 46. Seshagiri PB, Terasawa E, Hearn JP. The secretion of gonadotrophin-releasing hormone by peri-implantation embryos of the rhesus monkey: comparison with the secretion of chorionic gonadotrophin. Hum Reprod 1994; 9:1300–1307. [DOI] [PubMed] [Google Scholar]
- 47. Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci U S A 1999; 96:2543–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Simon C, Frances A, Piquette GN, el Danasouri I, Zurawski G, Dang W, Polan ML. Embryonic implantation in mice is blocked by interleukin-1 receptor antagonist. Endocrinology 1994; 134:521–528. [DOI] [PubMed] [Google Scholar]
- 49. Li Y, Zhu H, Klausen C, Peng B, Leung PC. Vascular endothelial growth factor-a (VEGF-A) mediates Activin A-induced human Trophoblast endothelial-like tube formation. Endocrinology 2015; 156:4257–4268. [DOI] [PubMed] [Google Scholar]
- 50. Yamada M, Kim S, Egashira K, Takeya M, Ikeda T, Mimura O, Iwao H. Molecular mechanism and role of endothelial monocyte chemoattractant protein-1 induction by vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 2003; 23:1996–2001. [DOI] [PubMed] [Google Scholar]
- 51. Nunes P, Nogueira R, Coelho J, Rodrigues F, Salomao N, Jose C, de Carvalho J, Rabelo K, de Azeredo E, Basilio-de-Oliveira R, Basilio-de-Oliveira C, Dos Santos F et al. A stillborn multiple organs' investigation from a maternal DENV-4 infection: Histopathological and inflammatory mediators characterization. Viruses 2019; 11:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kawamura K, Kawamura N, Sato W, Fukuda J, Kumagai J, Tanaka T. Brain-derived neurotrophic factor promotes implantation and subsequent placental development by stimulating trophoblast cell growth and survival. Endocrinology 2009; 150:3774–3782. [DOI] [PubMed] [Google Scholar]
- 53. Aldo P, You Y, Szigeti K, Horvath TL, Lindenbach B, Mor G. HSV-2 enhances ZIKV infection of the placenta and induces apoptosis in first-trimester trophoblast cells. Am J Reprod Immunol 2016; 76:348–357. [DOI] [PubMed] [Google Scholar]
- 54. Hoen B, Schaub B, Funk AL, Ardillon V, Boullard M, Cabie A, Callier C, Carles G, Cassadou S, Cesaire R, Douine M, Herrmann-Storck C et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med 2018; 378:985–994. [DOI] [PubMed] [Google Scholar]
- 55. Peregrine J, Gurung S, Lindgren MC, Husain S, Zavy MT, Myers DA, Papin JF. Zika virus infection, reproductive organ targeting, and semen transmission in the male olive baboon. J Virol 2019; 94. doi: 10.1128/JVI.01434-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mansuy JM, Suberbielle E, Chapuy-Regaud S, Mengelle C, Bujan L, Marchou B, Delobel P, Gonzalez-Dunia D, Malnou CE, Izopet J, Martin-Blondel G. Zika virus in semen and spermatozoa. Lancet Infect Dis 2016; 16:1106–1107. [DOI] [PubMed] [Google Scholar]
- 57. Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, Lanciotti RS, Tesh RB. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Magalhaes T, Foy BD, Marques ETA, Ebel GD, Weger-Lucarelli J. Mosquito-borne and sexual transmission of Zika virus: recent developments and future directions. Virus Res 2018; 254:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Visseaux B, Mortier E, Houhou-Fidouh N, Brichler S, Collin G, Larrouy L, Charpentier C, Descamps D. Zika virus in the female genital tract. Lancet Infect Dis 2016; 16:1220. [DOI] [PubMed] [Google Scholar]
- 60. Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, Herrmann C, Janky E, Joguet G. Zika virus in the female genital tract. Lancet Infect Dis 2016; 16:1000–1001. [DOI] [PubMed] [Google Scholar]
- 61. Nicastri E, Castilletti C, Balestra P, Galgani S, Ippolito G. Zika virus infection in the central nervous system and female genital tract. Emerg Infect Dis 2016; 22:2228–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Prisant N, Joguet G, Herrmann-Stock C, Moriniere C, Pavili L, Lurel S, Bujan L. Upper and lower genital tract Zika virus screening in a large cohort of reproductive-age women during the Americas epidemic. Reprod Biomed Online 2019; 39:624–632. [DOI] [PubMed] [Google Scholar]
- 63. Duggal NK, McDonald EM, Ritter JM, Brault AC. Sexual transmission of Zika virus enhances in utero transmission in a mouse model. Sci Rep 2018; 8:4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pagani I, Ghezzi S, Ulisse A, Rubio A, Turrini F, Garavaglia E, Candiani M, Castilletti C, Ippolito G, Poli G, Broccoli V, Panina-Bordignon P et al. Human endometrial stromal cells are highly permissive to productive infection by Zika virus. Sci Rep 2017; 7:44286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, Rourke T, Ma ZM, Fritts L, O'Connor S, Busch M, Miller CJ. Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog 2017; 13:e1006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, Iwasaki A. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell 2016; 166:1247–1256e1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Castro MC, Han QC, Carvalho LR, Victora CG, Franca GVA. Implications of Zika virus and congenital Zika syndrome for the number of live births in Brazil. Proc Natl Acad Sci U S A 2018; 115:6177–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


