Abstract
Purpose:
To characterize the population pharmacokinetics of cyclophosphamide, active 4-hydroxy-cyclophosphamide (4OH-CTX), and inactive carboxyethylphosphoramide mustard (CEPM), and their associations with hematological toxicities in infants and young children with brain tumors. To use this information to provide cyclophosphamide dosing recommendations in this population.
Experimental design:
Patients received four cycles of a 1-hour infusion of 1.5 g/m2 cyclophosphamide. Serial samples were collected to measure cyclophosphamide, 4OH-CTX, and CEPM plasma concentrations. Population pharmacokinetic modeling was performed to identify the patient characteristics influencing drug disposition. Associations between drug exposures and metrics reflecting drug-induced neutropenia, erythropenia, and thrombocytopenia were investigated. A Bayesian approach was developed to predict 4OH-CTX exposure using only cyclophosphamide and CEPM plasma concentrations.
Results:
Data from 171 patients (0.07–4.9 years) were adequately fitted by a two-compartment (cyclophosphamide) and one-compartment models (metabolites). Young infants (< 6 months) exhibited higher mean 4OH-CTX exposure than did young children (138.4 vs 107.2 µM·h p<0.0001). No genotypes exhibited clinically significant influence on drug exposures. Worse toxicity metrics were significantly associated with higher 4OH-CTX exposures. Dosing simulations suggested decreased cyclophosphamide dosage to 1.2 g/m2 for young infants vs 1.5 g/m2 for children to attain similar 4OH-CTX exposure. Bayesian-modeled 4OH-CTX exposure predictions were precise (mean absolute prediction error 14.8±4.2%) and had low bias (mean prediction error 4.9±5.1%).
Conclusion:
A 4OH-CTX exposure–toxicity association was established and a decreased cyclophosphamide dosage for young infants was suggested to reduce toxicity in this population. Bayesian modeling to predict 4OH-CTX exposure may reduce clinical processing-related costs and provide insights into further exposure–response associations.
INTRODUCTION
Central nervous system tumors are the leading cause of cancer-related deaths in children (1). For patients less than 3 years old, intensive chemotherapy is preferred over craniospinal irradiation to avoid both acute and long-term toxicities. However, these children still experience severe treatment-related toxicities and poor overall survival (2,3). A multi-institutional phase II trial (SJYC07; NCT00602667) was specifically designed for infants and young children with primary brain tumors and enrolled a large population of such children (4). Induction treatment in this trial comprised combination chemotherapy, including cyclophosphamide.
Cyclophosphamide, a well-known chemotherapeutic agent with demonstrated pediatric brain tumor efficacy (5–7), is associated with myelosuppression as a primary dose-limiting toxicity. Patients suffering from myelosuppression have an increased risk of infectious and hemorrhagic complications (8). Therapeutic interventions such as blood transfusion or growth factor injections are required to address this toxicity. However, these treatments remain highly expensive and may be of limited effectiveness (9,10). Thus, efforts should be made to explore ways to limit cyclophosphamide-induced myelosuppression. Identifying vulnerable groups of patients and determining dosage alterations for cyclophosphamide may help optimizing the extent of therapeutic interventions and the time to response to therapy.
The complex pharmacology of cyclophosphamide has been extensively investigated in adults (11–18) and to a lesser extent in children (19–21). However, the association among exposure, response, and toxicity is incompletely understood, and studies exploring this association have reported conflicting results (22–25). As a prodrug, cyclophosphamide may not be the best parameter in predicting toxicity. Furthermore, cyclophosphamide exposure is a poor correlate of its metabolite 4-hydroxy-cyclophosphamide (4OH-CTX), which is a widely considered surrogate for the active phosphoramide mustard (20,26–28). Although a few studies have related 4OH-CTX exposure to toxicities (29–31), none have defined a therapeutic 4OH-CTX systemic exposure target.
Research defining cyclophosphamide exposure is hampered by the challenge of analyzing 4OH-CTX, which is highly reactive and requires a specific procedure during sample collection and a separate bioanalytical method (32,33). To better characterize 4OH-CTX elimination, carboxyethylphosphoramide mustard (CEPM), a downstream inactive metabolite, can be analyzed simultaneously with cyclophosphamide (11). Pharmacokinetic modeling using cyclophosphamide and CEPM data alone may predict 4OH-CTX exposure, eliminating the need for direct 4OH-CTX measurements and establishing the 4OH-CTX exposure–response association.
Here, we first characterized the pharmacokinetics of cyclophosphamide, 4OH-CTX, and CEPM in patients enrolled in SJYC07 and investigated the influence of demographic, laboratory, and genetic covariates in these patients. We then determined the association between model-derived drug exposure and specific hematologic toxicities to determine potential dosing adjustments. Finally, we developed a Bayesian pharmacokinetic approach to evaluate the feasibility of predicting individual 4OH-CTX exposures on the basis of cyclophosphamide and CEPM data alone.
MATERIALS AND METHODS
Patient Population and Study Design
This study was carried out as part of a multi-institutional, phase II clinical trial (SJYC07; NCT00602667) at St. Jude Children’s Research Hospital (St. Jude), which evaluates a risk-adapted therapy for young children (≤ 5 years) with primary brain tumors (4). The study was approved by the St. Jude Institutional Review Board and followed ethical principles of the Declaration of Helsinki. Written informed consent was obtained by the patient’s parents or the legal guardians. Children younger than 3 years of age with newly diagnosed medulloblastoma, supratentorial primitive neuroectodermal tumor, atypical teratoid/rhabdoid tumor, high-grade glioma, choroid plexus carcinoma or ependymoma were eligible. Children between 3 and 5 years of age with new diagnosed non-metastatic medulloblastoma were also eligible. Briefly, patients underwent surgery and were then stratified by clinical and histological criteria into low-, intermediate- and high-risk treatment groups. As part of treatment, patients received induction, consolidation, and maintenance therapy. The induction therapy consisted of four 28-day cycles of high-dose methotrexate and conventional dosage vincristine, cisplatin, and cyclophosphamide. Specifically, cyclophosphamide was administrated on day 9 of each cycle as a 1-hour intravenous infusion of 1.5 g/m2.
After each infusion, patients received filgrastim (G-CSF) continuously until reaching a neutrophil count > 2,000/mm3 after nadir. Red blood cell (RBC) and platelet transfusions were administered as necessary in case of severe erythropenia and thrombocytopenia. Per protocol, each induction cycle was initiated according to the following criteria: (i) neutrophil count > 500/mm3 (after G-CSF discontinued), (ii) hemoglobin concentration > 8 g/dL (with or without transfusion support), (iii) platelet counts > 50,000/mm3 (without support), and (iv) total bilirubin concentration < 3-fold the institutional upper limit of normal.
Pharmacokinetic Sampling and Bioanalysis
Pharmacokinetic studies were performed in all patients during one course of induction therapy. Blood samples (1 mL) were drawn prior to the start of cyclophosphamide infusion, at the end of infusion (1 hour), and at 3, 6, and 24 hours after the end of infusion. At the bedside immediately after sample acquisition, 0.5 mL aliquots were placed into two tubes containing 1 mL of derivatizing solution of 277 mM phenylhydrazine (pH 6.0) for 4OH-CTX analysis or ethylenediaminetetraacetic acid (BD Microtainer K2-EDTA tube) for cyclophosphamide and CEPM analysis. Both tubes were immediately centrifuged for 2 minutes at 10,000 rpm to obtain plasma, which was immediately removed and stored at –80°C until further analysis.
Plasma samples were analyzed for cyclophosphamide and CEPM or for derivatized 4OH-CTX separately by liquid chromatography/mass spectrometry (LC-MS/MS) methods, which were modified from previous published methods (32,33). The limits of quantification for cyclophosphamide, CEPM, and derivatized 4OH-CTX assays in human plasma were 0.18, 0.2, and 0.05 µM, respectively. A detailed description of the bioanalytical methods is provided in Supplementary Material Section 1.
Genotyping Assays
In consenting patients, DNA was extracted from formalin-fixed paraffin-embedded tissues and blood with a Gentra Puregene Blood Kit (#158389, Qiagen, USA), according to the manufacturer instructions and quantified the DNA by using Nanodrop 2000 Spectrophotometer (Thermo Fisher Scientific). Genome-wide genotyping was performed in germline DNA with an Illumina Infinium Omni2.5Exome-8 BeadChip (Illumina Inc., San Diego, CA) to determine single nucleotide polymorphisms (SNPs) from multiple genes involved in cyclophosphamide metabolism and transport, selected according to those previously published. When available, patient data for these variants were extracted from the array. For missing SNPs on the array, imputations were conducted by using Minimac with reference data from the University of California Santa Cruz website (http://genome.ucsc.edu/cgi-bin/hgGateway). All imputed SNP makers had imputation quality scores of r2 > 0.85.
Population Pharmacokinetic Modeling and Covariate Analysis
Cyclophosphamide, 4OH-CTX, and CEPM plasma concentrations were simultaneously analyzed with a population pharmacokinetic approach, which permitted characterizing typical pharmacokinetics and interindividual variability within the population. Model parameters were estimated with the Stochastic Approximation Expectation Maximization algorithm in Monolix (version 2018R1. Antony, France: Lixoft SAS, 2018. http://lixoft.com/products/monolix/). Interindividual variability (IIV) terms were implemented on parameters using an exponential model and were assumed to follow a log-normal distribution. Additive and/or proportional error models were used to describe the residual unexplained variabilities. The residual error terms of the observation were assumed to be normally distributed with a mean 0 and variance σ2. Dose normalized to patient body surface area was used as model input, thus the pharmacokinetic parameters were normalized to body surface area. Data below the limit of quantification were handled as censored in Monolix according to the Beal method M3 (34). Different models were tested based on previously published cyclophosphamide models: one- and two-compartment models; linear or nonlinear absorption/formation; and linear, nonlinear, or time-dependent elimination clearance. Model selection was based on diagnostic plots (35), precision of parameter estimates, and changes in the objective function value. Internal validation was performed by using visual predictive checks (36). One thousand replicates of the analysis dataset were simulated using the final model pharmacokinetic parameters. For each compound, the observed concentration-time data were overlaid on the 5th, 50th, and 95th percentiles of the model simulations to visually assess concordance between the observations and the model-based simulated data. Shrinkage values based on the estimated individual random effect variances were considered as acceptable if less than 50% (37).
A covariate analysis was then performed to investigate potential associations between the model parameters and the following patient characteristics: age, BSA, actual bodyweight, height, gender, genotypic race, albumin, alkaline phosphatase, alanine amino-transferase, aspartate amino-transferase, total bilirubin, serum creatinine, concomitant treatment (i.e., phenobarbital or dexamethasone). A second analysis investigated the influence of SNPs on drug disposition obtained in consenting patients. Continuous covariates were implemented according to a power model scaled to the population median covariate value. Categorical covariates were modeled using an exponential change due to the covariate value. Significant covariates were selected by using a classic forward/backward stepwise approach, with criteria P values of 0.05 and 0.01 for the forward and backward steps, respectively (38). Wald tests were also used to test whether covariates should be kept in the model (criteria p-value<0.05). Predictive performance using VPC were re-evaluated after the covariate analysis.
Model-derived drug exposures were defined as the area under the curves (AUC0–24h) and were computed using the integrals of the concentration-time curve from 0 to 24 hours for each of the compound.
Assessment of Exposure–Toxicity Associations
Associations were explored between model-derived drug AUC0–24h and hematologic toxicities occurring post cyclophosphamide infusion. Toxicities included neutrophil counts, platelet counts, and hemoglobin concentrations, which were monitored routinely throughout the cycle. The frequency of the monitoring varied from daily to weekly among patients and within each individual treatment cycle. Several metrics were derived for each of these toxicities to assess exposure–toxicity associations, on the basis of the criteria defined to start the next cycle (See Patient population and study design). These metrics included: (i) the observed nadir before any transfusion, (ii) the day of observed nadir, and (iii) the time post-infusion to meet the criteria for starting the next induction cycle which was named “time to recovery (TR)”. TR was defined as the time after three consecutive measured values above the desired threshold after the observed nadir and/or after the last transfusion if any. If the third consecutive value was collected ≥ 4 days after the previous value, then TR was defined after two values above the desired threshold. A patient was considered as unevaluable if less than two values above the threshold were available. The metrics also included the number of red blood cell and platelet transfusions, and the duration below the desired threshold after nadir for neutrophil counts.
Spearman correlation coefficients were used to evaluate the associations between drug exposures and continuous metrics. Wilcoxon–Mann-Whitney (WMW) tests were used to assess differences in drug exposure distributions between two groups of patients (e.g., patients not receiving vs receiving transfusion support). A significance threshold of P = 0.05 was used without adjusting for multiplicity.
Model Simulations
Using the final population model including the covariate effects, simulations were conducted to further explore the age effect on cyclophosphamide, 4OH-CTX, and CEPM exposures and to potentially determine dosing recommendations to reduce hematologic toxicities. The simulated population was based on the study population dataset which was simulated n=1000 times to keep the same patient characteristics (i.e., patient age). Simulated AUC0–24h estimates were obtained for cyclophosphamide, 4OH-CTX, and CEPM. These AUC0–24h values were then plotted against the patients age range as a continuous or a categorical variable. The different age-based categories were first selected as defined by the National Institute of Child Health and Human Development: infancy (< 1 year), toddler (1–2 years), and early childhood (2–5 years) (39). The infancy group was further divided into young infants (<6 months) and infants between 6 months and 1 year.
Further model simulations were performed with de-escalated dosages from 1.5 to 1.0 g/m2 by 0.1 g/m2 increments, and age-based dosing adjustments were determined to reach similar drug exposures across all patients, if necessary.
Bayesian Pharmacokinetic Approach to Predict 4OH-CTX Plasma Exposure
This analysis aimed to evaluate if 4OH-CTX plasma exposure (AUC0–24h) could be accurately estimated based on cyclophosphamide and CEPM concentration-time data only. Individual 4OH-CTX AUC0–24h values previously generated by the population model were designated reference values (AUCref). The original dataset was randomly divided into an index set (2/3) and a validation set (1/3) to perform a cross-validation study. The index set included all of the data (i.e., cyclophosphamide, 4OH-CTX, and CEPM data), whereas the validation set included only cyclophosphamide and CEPM data. The index data were modeled using the same model, and all the population parameters were re-estimated. On a separate run, all the validation data were then fitted with the same model using the 4OH-CTX population and variability parameters estimated during the first step as fixed Bayesian priors, while the parameters describing cyclophosphamide and CEPM were re-estimated. Individual 4OH-CTX AUC0–24h values generated by the Bayesian model were designated predicted AUC (AUCpred).
This analysis was repeated twenty times, i.e., twenty index and validation sets were randomly created. The process described above was repeated for each of the index/validation sets. At the end of each analysis, the 4OH-CTX AUCpred and AUCref values were compared with measures of precision and bias, respectively, assessed by the mean absolute percentage prediction error (MAPE%) and percentage mean prediction error (MPE%) (40). MAPE% and MPE% values ≤ 15% were considered clinically acceptable to assess the predictive performance of the Bayesian approach (41).
RESULTS
Patient Characteristics and Data Summary
All 171 infants and children receiving cyclophosphamide during induction therapy consented to the pharmacokinetic studies. The study population included 42 infants (< 1 year), 57 toddlers (1–2 years), and 72 young children (2–5 years). 4OH-CTX was not quantified in the first 40 patients enrolled in the study for long-term sample stability issues. The characteristics of this patient group which didn’t include any infants are reported in Supplementary Table S1. A small number of concentrations were below the limit of quantification: five for cyclophosphamide, five for 4OH-CTX, and three for CEPM, all at 24 hours post infusion. A total of 29 SNPs from 12 genes were assayed in 142 consenting patients (Supplementary Table S2). The genotypic variants included genes involved in cyclophosphamide metabolism (cytochrome P450 2B6–2C9–2C19–3A5–3A4, aldehyde dehydrogenase ALDH-3A1, and glutathione-S-transferase GSTA1-P1), and transport (multidrug resistance proteins ABCB1-C2-C4). All the patient characteristics are summarized in Table 1.
Table 1.
Characteristics of patients included in the pharmacokinetic and pharmacogenetic analyses
| Patient Characteristics | Patients included in pharmacokinetic analysis | Patients included in pharmacogenetic analysis |
|---|---|---|
| Total number of patients | 171 | 142 |
| aInduction therapy course (%) | ||
| Course 1 | 131 (76.7) | 101 (71.1) |
| Course 2 | 29 (16.9) | 30 (21.1) |
| Course 3 | 10 (5.8) | 10 (7.0) |
| Course 4 | 1 (0.6) | 1 (0.7) |
| Sex | ||
| Male, n (%) | 95 (55.6) | 64 (45.0) |
| Female, n (%) | 76 (44.4) | 78 (55.0) |
| Age (months) | 21.5 (0.85–58.5) | 22.2 (0.85–58.5) |
| Total body weight (kg) | 11.3 (3.6–20.1) | 11.2 (3.6–20.1) |
| Body surface area (m²) | 0.53 (0.2–0.81) | 0.53 (0.24–0.81) |
| Height (cm) | 82 (48–113.3) | 83.6 (51–113.3) |
| Albumin (U/L) | 3.8 (2.8–4.7) | 3.8 (2.8–4.7) |
| Serum creatinine (mg/dL) | 0.2 (0.1–0.4) | 0.2 (0.1–0.4) |
| Phenobarbital use, n (%) | 7 (4.0) | 6 (4.2) |
| Dexamethasone use, n (%) | 26 (15.2) | 21 (14.8) |
| Genotypic race | ||
| European | 118 (83.1) | |
| African-American | 17 (12.0) | |
| Asian | 7.0 (4.9) |
Data represented as median (range) or frequency (%) for continuous or categorical characteristics.
Induction therapy cycle during which cyclophosphamide pharmacokinetic studies were performed for each patient.
Pharmacokinetic Modeling and Covariate Analysis
The selected model structure included a two-compartment model for cyclophosphamide, linked to a one-compartment model for 4OH-CTX, related to a one-compartment model for CEPM (Supplementary Fig. S1). Cyclophosphamide elimination was modeled with a linear clearance and a time-dependent metabolic clearance which described the non-linear formation of 4OH-CTX. The fraction of cyclophosphamide transformed into 4OH-CTX was fixed to 75%, while 25% of cyclophosphamide was assumed to be eliminated via other metabolic routes or renal excretion. These values were selected based on previous reports (17,26,27,42). 4OH-CTX elimination was also modeled using an apparent linear clearance and apparent time-dependent metabolic clearance which described the non-linear formation of CEPM. Both cyclophosphamide and 4OH-CTX metabolic clearances increased with time in an exponential manner. On average, cyclophosphamide and 4OH-CTX metabolic clearances were found to increase by 2.4 and 2.1-fold at 24 hours post dose and exhibited wide inter-patient variability (Supplementary Fig. S2). CEPM elimination was described using an apparent linear clearance. All of the model parameters were simultaneously estimated, except for the volumes of distribution of both 4OH-CTX and CEPM, which were fixed to previously reported values (i.e., 0.57 L/m2) to avoid identifiability issues (20). No correlations between IIV parameters were estimated. The central tendency and the variability of cyclophosphamide, 4OH-CTX, and CEPM concentrations were well predicted by this pharmacokinetic model, as depicted by the diagnostic plots and visual predictive checks (Supplementary Figs. S3 to S4).
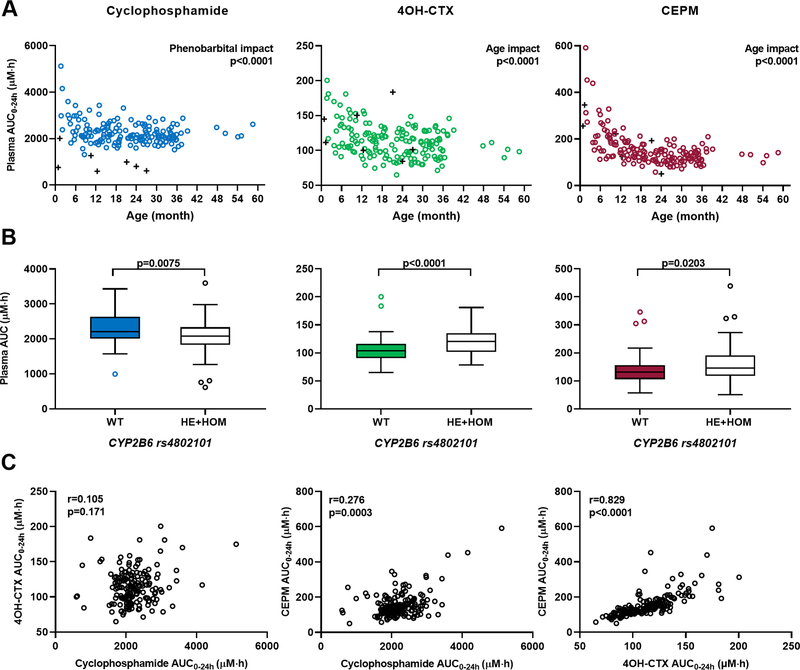
The covariate analysis revealed the significant influence of three patient characteristics on drug disposition: patient age as a continuous covariate, phenobarbital co-treatment, and genotypic variant CYP2B6 (rs4802101). Positive associations were found between age and both metabolite clearances and between age and cyclophosphamide central volume (Supplementary Fig. S5). Therefore, higher 4OH-CTX and CEPM systemic exposures were observed in younger children (Fig. 1A). Specifically, infants exhibited 1.2-fold and 1.85-fold higher mean 4OH-CTX and CEPM exposures, respectively, than did other children in the study cohort (p<0.0001). The inclusion of the age effect explained 38%, 11%, and 12% of the variability initially observed on cyclophosphamide volume, 4OH-CTX, and CEPM clearances, respectively. Higher cyclophosphamide clearance was observed in patients receiving phenobarbital cotreatment (Supplementary Fig. S5), resulting in lower cyclophosphamide exposure (Fig. 1A). The inclusion of the phenobarbital effect explained 20% of the variability observed on cyclophosphamide clearance. The CYP2B6 rs4802101 variant influenced several parameters: cyclophosphamide clearance and its time-dependent clearance coefficient, and 4OH-CTX clearance (Supplementary Fig. S5). Patients with at least one variant allele for this SNP exhibited lower cyclophosphamide exposure but higher 4OH-CTX exposure (Fig. 1B). The inclusion of CYP2B6 SNP explained 4%, 18%, and 6% of the cyclophosphamide clearance, time-dependent clearance coefficient, and 4OH-CTX clearance variabilities. The final model parameter estimates, and covariate effect coefficients are reported in Supplementary Table S3. All the parameters were estimated with good precision.
Figure 1.
Association of individual cyclophosphamide, 4OH-CTX, and CEPM exposures with patient covariates. (A) Association between cyclophosphamide, 4OH-CTX, and CEPM AUC0–24h and patient age or phenobarbital cotreatment. Dots and crosses represent individual drug AUC0–24h for patients with no cotreatment and patients receiving concomitant phenobarbital, respectively. For cyclophosphamide, the P value indicates the significant effect of phenobarbital cotreatment on drug AUC0–24h (Wilcoxon–Mann-Whitney). For 4OH-CTX and CEPM, the P values indicate the significant age effect on drug AUC0–24h (Spearman correlation). (B) Boxplots of cyclophosphamide, 4OH-CTX, and CEPM AUC0–24h association with the CYP2B6 (rs4802101) genotype, categorized as wild-type or variant (HE, heterozygous; HOM, homozygous mutant). P values indicate a significant genotypic effect (Wilcoxon–Mann-Whitney). (C) Correlation between individual cyclophosphamide and 4OH-CTX AUC0–24h, cyclophosphamide and CEPM AUC0–24h, and 4OH-CTX and CEPM AUC0–24h values. Spearman correlation coefficients and associated P values are indicated.
The plasma AUC0–24h were calculated for each of the three compounds using the final including the effects of age and phenobarbital co-treatment. Mean model-derived plasma AUC0–24h were 2,211, 114, and 155 µM*h for cyclophosphamide, 4OH-CTX, and CEPM, respectively. The 4OH-CTX AUC0–24h values did not correlate with cyclophosphamide AUC0–24h (spearman correlation coefficient r=0.105, p=0.17) but showed a strong correlation with CEPM AUC0–24h (r=0.83, p<0.0001) as shown in Fig. 1C. To note, the plasma AUC0–24h values for cyclophosphamide, 4OH-CTX, and CEPM represented 99.2% (±1.14), 98.6% (±1.14), and 96.8% (±1.14) of the total AUC0−∞ values, respectively, and were well correlated with their correspondant AUC0−∞ (Supplementary Fig. S6).
Exposure–Toxicity Associations
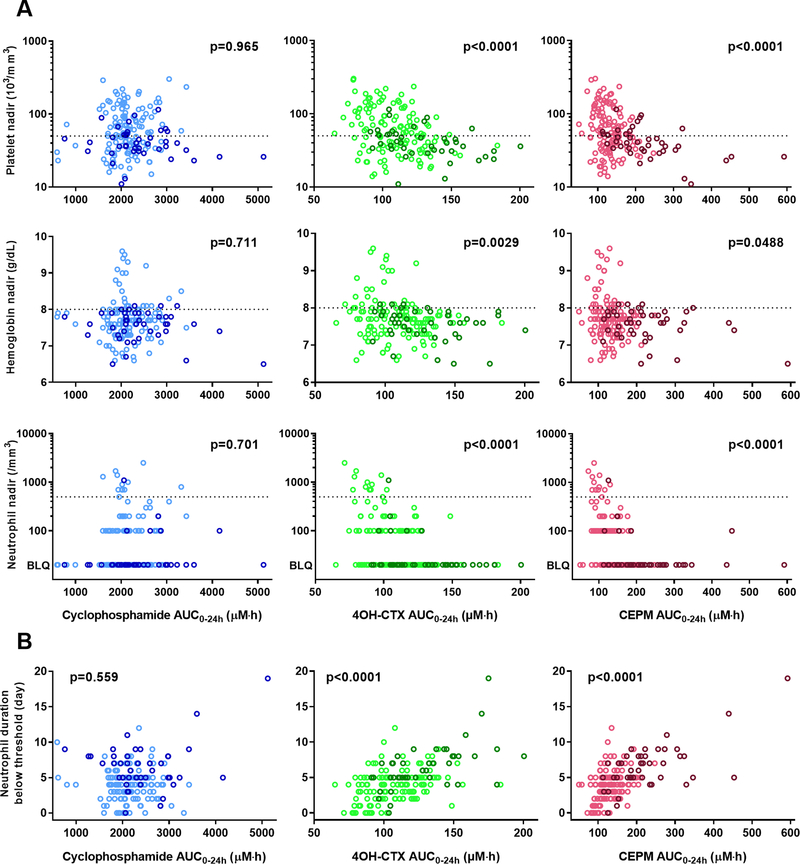
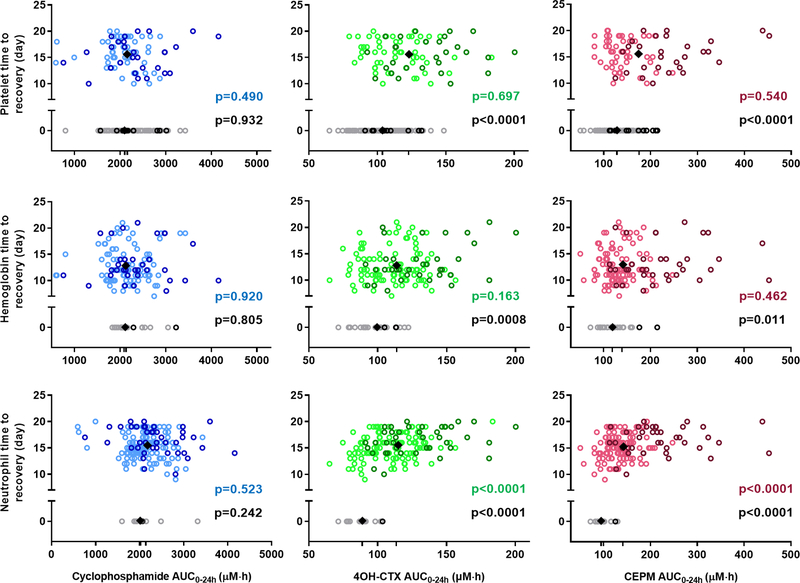
Descriptive statistics of the toxicity metrics derived based on the neutrophil and platelet counts, and hemoglobin concentrations are reported in Table 2. Associations between these toxicity metrics and individual model-derived drug AUC0–24h were explored. Significant correlations were found between the toxicity metrics and both 4OH-CTX and CEPM AUC0–24h, but not with cyclophosphamide exposure (Figs. 2 and 3). Higher 4OH-CTX and CEPM AUC0–24h were related to lower neutrophil, platelet, and hemoglobin nadirs (Fig. 2A), and to longer duration below the desired threshold for neutrophil counts (Fig 2B). Fig 3 depicts the TR values vs drug AUC0–24h. Patients that exhibited neutrophil, platelet, or hemoglobin values above the threshold throughout the cycle had a TR set to 0. Higher 4OH-CTX and CEPM AUC0–24h occurred in patients with TR > 0 than in those with TR = 0 for neutrophil, platelet, and hemoglobin variables. Furthermore, among patients with TR > 0, the TR for neutrophil counts and both 4OH-CTX and CEPM AUC0–24h were positively correlated. Significantly higher 4OH-CTX and CEPM AUC0–24h were also observed in patients who received red blood cell and platelet transfusions than in those who did not (Supplementary Fig. S7). No correlation between drug AUC0–24h and days of nadirs was found (data not shown).
Table 2.
Descriptive Statistics of Toxicity Metrics Generated for Neutrophil Counts, Platelet Counts, and Hemoglobin Concentrations
| Toxicity Metrics | All patients (N = 171) | Infant patients < 1 year (n = 42) | Young patients 1–5 years (n = 129) |
|---|---|---|---|
| Neutrophils | |||
| Observed nadir (per mm3) | 0 (0–2,500) | 0 (0–1,100) | 0 (0–2,500) |
| Post-dose day of nadir (days) | 7 (0–17) | 7 (5–10) | 7 (0–17) |
| aTR (days) | 15 (0–20) | 16 (0–20) | 15 (0–20) |
| Number of patients with TR = 0 | 12 | 1 | 11 |
| Duration below threshold (days) | 4 (0–19) | 6.5 (0–19) | 4 (0–12) |
| Platelets | |||
| Observed nadir (per mm3) | 50 (11–302) | 39.5 (11–115) | 55 (14–302) |
| Post dose day of nadir (days) | 9 (1–18) | 8.5 (4–12) | 9 (1–18) |
| bTR† (days) | 0 (0–20) | 13 (0–20) | 0 (0–20) |
| Number of patients with TR = 0 | 83 | 12 | 71 |
| Platelet transfusions | |||
| n = 0 | 87 | 13 | 74 |
| n = 1 | 48 | 15 | 33 |
| n = 2 | 23 | 6 | 17 |
| n ≥ 3 (max = 8) | 13 | 8 | 5 |
| Hemoglobin | |||
| Observed nadir (g/dL) | 7.7 (6.5–9.6) | 7.6 (6.5–8.1) | 7.7 (6.6–9.6) |
| Post dose day of nadir (days) | 7 (0–21) | 6 (0–13) | 7 (0–21) |
| cTR (days) | 12 (0–21) | 12 (0–21) | 11 (0–21) |
| Number of patients with TR = 0 | 22 | 2 | 20 |
| Red blood cells transfusions | |||
| n = 0 | 23 | 2 | 21 |
| n = 1 | 82 | 18 | 64 |
| n = 2 | 42 | 10 | 32 |
| n ≥ 3 (max = 7) | 24 | 12 | 12 |
Data are reported as median (range), except for platelet and red blood cells transfusions, for which the number of patients receiving a certain number of transfusions is reported. In addition, the number of patients with TR = 0 (i.e., patients meeting the defined criteria to start the next cycle throughout the study) is reported.
Abbreviation: TR, time to recovery.
For neutrophil TR: one infant and two young children were not evaluable.
For platelet TR: two infants and five young children were not evaluable.
For hemoglobin TR: four infants and fifteen young children were not evaluable.
Figure 2.
Observed nadirs for neutrophil counts, platelet counts, and hemoglobin concentrations in association with 4OH-CTX or CEPM AUC0–24h. (A) The first, second, and third rows depict the platelet, hemoglobin, and neutrophil observed nadir values, respectively, according to cyclophosphamide, 4OH-CTX, and CEPM AUC0–24h. Dashed lines represent the desired threshold to start the next cycle of therapy. (B) The duration below the threshold for absolute neutrophil counts (ANC) is represented based on cyclophosphamide, 4OH-CTX, or CEPM AUC0–24h (first, second, and third columns, respectively). P values indicate association between drug AUC0–24h and nadir values (A) and between the drug AUC0–24h and duration below the threshold for ANC (B), as determined by Spearman correlation analysis. Dark color dots represent data from infant patients (<1 year). Lighter color dots are data from other young children (1 to 5 years). BLQ, below limit of quantification.
Figure 3.
Time to recovery (TR) for neutrophil counts, platelet counts, and hemoglobin concentrations association with 4OH-CTX or CEPM exposure. Each dot represents an individual value. The first, second, and third rows depict the platelet, hemoglobin, and neutrophil time to recovery values, respectively, in association with cyclophosphamide, 4OH-CTX, or CEPM exposure. Black diamonds represent the mean drug exposure values for the two groups of patients with either TR = 0 or TR > 0. Black P values denote the statistical comparison of the drug exposure distribution between these two groups of patients (Wilcoxon–Mann-Whitney test). Color P values denote the Spearman correlation between the drug exposures and the TR > 0. Dark color dots represent data from infant patients (< 1 year), and light color dots are data from other children (1 to 5 years).
Dosing Adjustment Simulations Based on 4OH-CTX Exposure
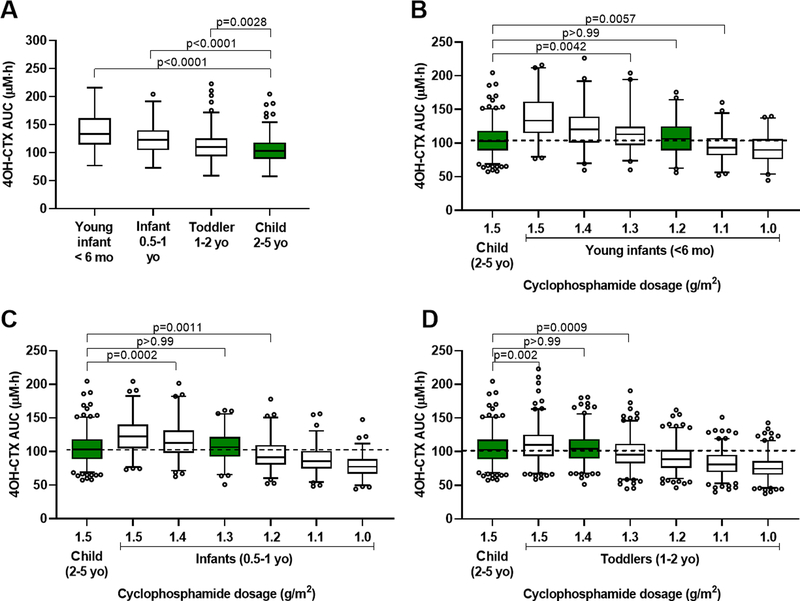
The simulation analysis was focused on 4OH-CTX exposure, since associations were found between 4OH-CTX exposure and the toxicity metrics, and 4OH-CTX was considered as the surrogate for the active phosphoramide mustard. Simulations were performed with the population pharmacokinetic model to further explore the influence of age on drug exposures.
When receiving the same 1.5 g/m2 cyclophosphamide dosage, young infants, infants and toddlers exhibited significantly higher 4OH-CTX AUC0–24h than did young children (p<0.0001 and p=0.0028) (Fig. 4A). The 4OH-CTX AUC0–24h range observed in young children following 1.5 g/m2 cyclophosphamide dosage was considered as our exposure of reference. To attain similar 4OH-CTX AUC0–24h in young infants to those of young children, the model simulations suggested a reduced dosage by 20% (i.e., 1.2 g/m2) for these patients (Fig. 4B). Similarly, the model simulations suggested a reduced dosage by 13% (i.e., 1.3 g/m2) for infants, and by 6.7% (i.e., 1.4 g/m2) for toddlers (Fig. 4C–D).
Figure 4.
Age-based cyclophosphamide dosing adjustment simulations to attain similar 4OH-CTX plasma exposure (AUC0–24h) across pediatrics. (A) 4OH-CTX exposure ranges (AUC0–24h) after a 1-hour infusion of cyclophosphamide (1.5 g/m2) in young infants (< 6 months), infants (0.5–1 year), toddlers (1–2 years), and young children (2–5 years). (B) 4OH-CTX AUC0–24h ranges after de-escalated cyclophosphamide dosages in young infants vs. 4OH-CTX AUC0–24h obtained in young children receiving 1.5 g/m2 cyclophosphamide. (C) 4OH-CTX AUC0–24h ranges after de-escalated cyclophosphamide dosages in infants vs. 4OH-CTX AUC0–24h obtained in young children receiving 1.5 g/m2 cyclophosphamide. (D) 4OH-CTX AUC0–24h ranges after de-escalated cyclophosphamide dosages in toddlers vs. 4OH-CTX AUC0–24h obtained in young children receiving 1.5 g/m2 cyclophosphamide. P values results from Dunnett’s multiple comparisons tests.
Prediction of 4OH-CTX Metabolite Plasma Exposure
The 40 patients for whom no 4OH-CTX concentrations were initially measured for bioanalytical reason were excluded from this analysis. Each index and validation set included 91 (2/3) and 40 (1/3) randomly selected patients, respectively. The characteristics of each validation set are reported in the Supplementary Table S4. The data from each index set were first modeled. Then the data from the corresponding validation sets were modeled using the 4OH-CTX elimination parameters (CL4OH, CLm4OH, and γ) previously estimated as fixed Bayesian priors. The analysis was first performed using the population model without any covariate. Bayesian modeling with cyclophosphamide and CEPM data alone predicted 4OH-CTX exposures with clinically acceptable precision (MAPE% ± standard deviation [SD], 12.5% ± 1.6%) and bias (MPE% ± SD, 2.8% ± 3.9%). One sampling set (S5) resulted in a MAPE% of 16.4%, slightly greater than the a priori defined clinical criteria of 15%. MPE% values showed a tendency for overprediction, but all values were well below 15%. The analysis was then repeated with the population model that included the age effects on 4OH-CTX and CEPM apparent clearances. Similar predictive performance was found with MAPE% ± SD of 12.1% ± 1.7%, and MPE% ± SD of 3.1% ± 3.1%. For these analyses (with and without covariates), the results for each sampling set are reported in Supplementary Table S5.
DISCUSSION
Population pharmacokinetic modeling was performed to describe cyclophosphamide, 4OH-CTX, and CEPM concentration-time data collected in a large population of infants and young children (0.07–5 years) with malignant brain tumors. Patient age had the greatest effect on drug disposition, with higher 4OH-CTX and CEPM exposure observed in younger children. Higher 4OH-CTX and CEPM exposures were associated with more severe neutropenia, thrombocytopenia, and erythropenia. On the basis of these findings, we suggested age-based dosages to minimize toxicities for young infants less than 6 months. We developed a Bayesian model to predict individual 4OH-CTX exposures according to our cyclophosphamide and CEPM findings, which may simplify future pharmacologic analyses.
Our model has the particularity to include exponential time-dependent coefficients of cyclophosphamide and 4OH-CTX metabolic clearances. Despite the non-extensive sampling time-points, these parameters were correctly estimated. To note, these parameters were sensitive to initial values and high shrinkage was observed for the parameter γ. However, with time-dependent coefficients, the model fits were considerably improved, and the objective function significantly decreased compared to all other tested models. These parameters suggested non-linear pharmacokinetics for both compounds. In previous reports, this phenomenon was observed after prolonged (e.g., 96-h infusion) or high cyclophosphamide dosages (e.g., 4000 mg/m2) administration, and was previously modeled using Michaelis-Menten or time-dependent elimination processes (27,42). The increased cyclophosphamide clearance and metabolite formation with time result from cyclophosphamide’s auto-induction capacity (11). Cyclophosphamide induces microsomal enzymes, and thus, its own metabolism. This mechanism was clearly observed after repeated doses, but was also reported to be detectable within 24 hours after the start of the treatment (11). The early start of the autoinduction process support what we observe with our data and the use of time-dependent clearances. This autoinduction process has also been integrated with more complex pharmacokinetic models in both adults and children receiving prolonged or repeated cyclophosphamide infusions (20,43,44).
Overall, our pharmacokinetic parameter estimates agreed with those previously reported in other pediatric studies (19–21). However, our study includes the largest sample size to date and focuses on infants as young as 1-month-old while the other pediatric studies included an age range between 1.3 and 18 years. The most important covariate revealed by our analysis was patient age which significantly influenced both 4OH-CTX and CEPM apparent clearances, as well as cyclophosphamide volume of distribution. Patient age was highly correlated with BSA; thus, the influence of age could be seen as the one of BSA as well. The impact of this covariate while the data were modeled using the BSA-normalized dosage as input, highlights the non-linear relationships that exists the drug exposure and the patient size. Both metabolite exposures (AUC0–24h) decreased with age with the sharpest drop seen between infants (<1 year) and toddlers (1–2 years). Only seven patients received phenobarbital concomitant treatment during the study; however, these patients exhibited approximately 2.5-fold lower cyclophosphamide AUC0–24h. The absence of a significant age effect on cyclophosphamide exposure and the role of phenobarbital cotreatment on cyclophosphamide, but not on metabolite exposure is not completely understood. Cyclophosphamide has a very complex metabolic profile, involving multiple enzymatic systems with potential ontogeny, a critical factor to account for when evaluating drug pharmacokinetics in pediatrics (45,46). These factors complicate the interpretation of our results, and may also explain the lack of findings from our genotypic covariate analysis. Only one SNP (i.e., CYP2B6 rs4802101) was associated with cyclophosphamide and 4OH-CTX clearances. However, its effect on drug exposure explained a small part of the variability associated to these parameters and had minimal impact on drug exposure. Thus, it may not be considered meaningful enough to warrant subsequent genotype-based dosage adjustments. We examined ontogeny and sex effects on genotype influence by evaluating the influence of the selected SNPs on the pharmacokinetics within specific groups of patients (e.g., within infants only, within patients younger than 2 years-old, within males or females only). However, no differences between different groups of age or between males and females were detected. Because our study focused on preselected SNP candidates, a global approach, such as genome-wide-association studies, may reveal genotypic variants of interest that were not interrogated in this analysis.
Once the individual cyclophosphamide, 4OH-CTX, and CEPM AUC0–24h were derived, we next determined their association with hematologic toxicities. This analysis focused on myelosuppression, which is often a dose-limiting toxicity for cyclophosphamide and clinically relevant metrics were defined on the basis of neutrophil and platelet counts and hemoglobin concentrations monitored clinically. We did not observe any correlation with cyclophosphamide AUC0–24h, confirming that cyclophosphamide exposure is not a good predictive parameter to study toxicity. Higher 4OH-CTX AUC0–24h was systematically related to lower neutrophil, platelet, and hemoglobin nadirs, greater time to reach the threshold required to start the next therapy cycle, and to the necessity of receiving transfusion support. Similar correlations with CEPM AUC0–24h were found; however, this may reflect the mathematical correlation between both metabolite exposures, rather than from a pharmacologic association, because CEPM is an inactive metabolite. To our knowledge, this is the first analysis showing associations between cyclophosphamide metabolite exposures and metrics reflecting hematological toxicities in infants and young children.
Higher toxicity metrics were related to higher metabolite exposures, which were more observed in infants <1 year and toddlers compared to young patients (2–5 years), based upon our pharmacokinetic analysis. These results indicate that age-based dosing adjustments may be recommended for cyclophosphamide to reduce toxicities in younger children. Therefore, model simulations were performed to determine the cyclophosphamide dosages for different categories of age, that would lead to similar 4OH-CTX exposures to those of young children (2–5 years) receiving 1.5 g/m2 cyclophosphamide. According our simulations, cyclophosphamide dosages of 1.2, 1.3 and 1.4 g/m2 for young infants (<6 months), infants (0.5–1 year) and toddlers are suggested, respectively. Overall, this age-based dosing adjustment required a 20% dose reduction for young infants but less than 15% reduction for children from 0.5–2 years, which may not be considered clinically relevant, taking the inter-individual variability into account. Therefore, we would suggest applying the suggested age-based dosage for patients <6 months only. Although a 20% change in the dosage for young infants may seem small, we believe that it will be meaningful as it will allow reducing the 4OH-CTX exposures and thus reducing the extent of myelosuppression in this group of vulnerable young patients. This also means that the extent of therapeutic interventions (e.g., blood and platelet transfusion) may be reduced which is not negligible. Using a similar reduced dosage for all young infants rather than individual dosages might lead to unnecessary lower exposures in some patients. However, our simulations suggest that among the young infants receiving 1.2 g/m2 cyclophosphamide, only 1.06% of them exhibit 4OH-CTX exposures lower than the minimum observed in young children. Using age-based categories defined by cut-off values rather than continuous dosages normalized by age may also raise interrogations for patients of age close to the cut-off value. However, this approach still remains the easiest to implement in the clinic. We envision continuous dosages normalized by age might be determined in the future once a targeted exposure will be better defined.
Although cyclophosphamide has been widely used in the last twenty years in various indications, no targeted exposure or therapeutic window has been defined for the parent drug or the metabolites to ensure both treatment efficacy and safety. Specifically, for infant and young patients with brain tumors who won’t receive craniospinal radiation therapy, the minimal cyclophosphamide or metabolite exposure to attain for antitumor efficacy remains unknown. Therefore, even if clear associations between 4OH-CTX exposure and our toxicity metrics were observed, it is still difficult to determine a targeted metabolite exposure to ensure adequate efficacy and avoid toxicity. Although myelosuppression can be managed through transfusion support and growth factor injections, efforts to minimize toxicities and to find to adequate drug exposure should continue. It is important to note that, in our study, cyclophosphamide was administered with other agents that may have also contributed to myelosuppression, such as cisplatin. However, no pharmacokinetic studies were performed for this agent; thus, the impact of cisplatin in the exposure-toxicity association could not be evaluated. More complex pharmacologic modeling will be performed to describe the complete time course of neutrophil and platelet counts with these different treatments, as well as the different transfusion and growth factor support.
The results of this study support the relevance of determining the concentrations of 4OH-CTX, as a surrogate for the ultimate active metabolite phosphoramide mustard. However, measuring 4OH-CTX is challenging and requires bedside processing and higher blood volumes. We successfully developed a Bayesian approach to predict individual 4OH-CTX exposures on the basis of cyclophosphamide and CEPM data only in pediatrics. Our model also demonstrated its robustness with good convergence and similar parameter estimates for each of the sampling sets. Future studies will be performed to further validate this model with external data that are currently collected in other St. Jude clinical trials. Implementing this approach in the clinic will remove the need for bedside sample processing and separate bioanalyses of 4OH-CTX. This approach is cost-effective and time-saving and will limit the blood collection volumes required from children. Therefore, similar pharmacokinetic studies to establish exposure–response associations and to specifically optimize cyclophosphamide dosing in other patient populations should be considered. Once this Bayesian approach will be fully validated, and a targeted exposure for 4OH-CTX well defined, the Bayesian model may also potentially be used to perform therapeutic drug monitoring, which would lead to more precise individual dosages.
Supplementary Material
TRANSLATIONAL RELEVANCE.
The clinical pharmacokinetics of cyclophosphamide and its metabolites 4-hydroxy-cyclophosphamide (4OH-CTX) and carboxyethylphosphoramide mustard (CEPM), and the drug-induced hematological toxicities were explored in infants and young children with brain tumors. Population-based analysis revealed significantly higher metabolite drug exposures in infants (<1 year). Higher 4OH-CTX exposures were associated with worse thrombocytopenia, erythropenia, and neutropenia events. To reduce toxicities in infants, a reduction in cyclophosphamide dosage by 18.5% was proposed to attain similar 4OH-CTX exposures to those observed in young children (≤ 5 years). Although 4OH-CTX is a metabolite of interest to study the exposure-response associations, measuring 4OH-CTX concentrations remains challenging because of its instability in biological fluids. Thus, a Bayesian pharmacokinetic model was developed to accurately predict 4OH-CTX exposure based on cyclophosphamide and CEPM data alone. Implementing this model in the clinic will reduce the sampling volume, simplify sample processing, and facilitate future studies investigating exposure-response associations to improve dosing optimization.
ACKNOWLEDGMENTS
We thank the clinical pharmacists, PK nurses, and nursing team at St. Jude for their assistance in this study; the Stewart laboratory for bedside collection and processing of the samples; and the Hartwell Center for performing Illumina Chip. We also thank Drs. Carl Panetta, Mike Tagen, Yogesh Patel, and Vinay Daryani for their contributions in the population pharmacokinetic and covariate analyses. This work was supported by grants from the National Cancer Institute (R01CA154619; C.F. Stewart), St. Jude Children’s Research Hospital Comprehensive Cancer Center Support (CORE; CA21765), and the American Lebanese Syrian Associated Charities (ALSAC) at St. Jude Children’s Research Hospital.
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018;124(13):2785–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gajjar A, Mulhern RK, Heideman RL, Sanford RA, Douglass EC, Kovnar EH, et al. Medulloblastoma in very young children: outcome of definitive craniospinal irradiation following incomplete response to chemotherapy. J Clin Oncol 1994;12(6):1212–6 [DOI] [PubMed] [Google Scholar]
- 3.Walter AW, Mulhern RK, Gajjar A, Heideman RL, Reardon D, Sanford RA, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children’s Research Hospital. J Clin Oncol 1999;17(12):3720–8 [DOI] [PubMed] [Google Scholar]
- 4.Robinson GW, Rudneva VA, Buchhalter I, Billups CA, Waszak SM, Smith KS, et al. Risk-adapted therapy for young children with medulloblastoma (SJYC07): therapeutic and molecular outcomes from a multicentre, phase 2 trial. Lancet Oncol 2018;19(6):768–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen JC, Helson L. High-dose cyclophosphamide chemotherapy for recurrent CNS tumors in children. J Neurosurg 1981;55(5):749–56 [DOI] [PubMed] [Google Scholar]
- 6.Yule SM, Foreman NK, Mitchell C, Gouldon N, May P, McDowell HP. High-dose cyclophosphamide for poor-prognosis and recurrent pediatric brain tumors: a dose-escalation study. Journal of Clinical Oncology 1997;15:3258–65 [DOI] [PubMed] [Google Scholar]
- 7.McCowage GB, Friedman HS, Moghrabi A, Kerby T, Ferrell L, Stewart E, et al. Activity of high-dose cyclophosphamide in the treatment of childhood malignant gliomas. Med Pediatr Oncol 1998;30(2):75–80 [DOI] [PubMed] [Google Scholar]
- 8.Maxwell MB, Maher KE. Chemotherapy-induced myelosuppression. Semin Oncol Nurs 1992;8(2):113–23 [DOI] [PubMed] [Google Scholar]
- 9.Dinan MA, Hirsch BR, Lyman GH. Management of chemotherapy-induced neutropenia: measuring quality, cost, and value. J Natl Compr Canc Netw 2015;13(1):e1–7 [DOI] [PubMed] [Google Scholar]
- 10.Taylor SJ, Duyvestyn JM, Dagger SA, Dishington EJ, Rinaldi CA, Dovey OM, et al. Preventing chemotherapy-induced myelosuppression by repurposing the FLT3 inhibitor quizartinib. Sci Transl Med 2017;9(402) [DOI] [PubMed] [Google Scholar]
- 11.de Jonge ME, Huitema AD, Rodenhuis S, Beijnen JH. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet 2005;44(11):1135–64 [DOI] [PubMed] [Google Scholar]
- 12.Chinnaswamy G, Errington J, Foot A, Boddy AV, Veal GJ, Cole M. Pharmacokinetics of cyclophosphamide and its metabolites in paediatric patients receiving high-dose myeloablative therapy. Eur J Cancer 2011;47(10):1556–63 [DOI] [PubMed] [Google Scholar]
- 13.Joerger M, Huitema AD, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, et al. Population pharmacokinetics and pharmacodynamics of doxorubicin and cyclophosphamide in breast cancer patients: a study by the EORTC-PAMM-NDDG. Clin Pharmacokinet 2007;46(12):1051–68 [DOI] [PubMed] [Google Scholar]
- 14.Nakajima M, Komagata S, Fujiki Y, Kanada Y, Ebi H, Itoh K, et al. Genetic polymorphisms of CYP2B6 affect the pharmacokinetics/pharmacodynamics of cyclophosphamide in Japanese cancer patients. Pharmacogenet Genomics 2007;17(6):431–45 [DOI] [PubMed] [Google Scholar]
- 15.Kim IW, Yun HY, Choi B, Han N, Kim MG, Park S, et al. Population pharmacokinetics analysis of cyclophosphamide with genetic effects in patients undergoing hematopoietic stem cell transplantation. Eur J Clin Pharmacol 2013;69(8):1543–51 [DOI] [PubMed] [Google Scholar]
- 16.Ekhart C, Doodeman VD, Rodenhuis S, Smits PH, Beijnen JH, Huitema AD. Influence of polymorphisms of drug metabolizing enzymes (CYP2B6, CYP2C9, CYP2C19, CYP3A4, CYP3A5, GSTA1, GSTP1, ALDH1A1 and ALDH3A1) on the pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide. Pharmacogenet Genomics 2008;18(6):515–23 [DOI] [PubMed] [Google Scholar]
- 17.de Jonge ME, Huitema AD, van Dam SM, Rodenhuis S, Beijnen JH. Population pharmacokinetics of cyclophosphamide and its metabolites 4-hydroxycyclophosphamide, 2-dechloroethylcyclophosphamide, and phosphoramide mustard in a high-dose combination with Thiotepa and Carboplatin. Ther Drug Monit 2005;27(6):756–65 [DOI] [PubMed] [Google Scholar]
- 18.Qiu R, Yao A, Vicini P, McDonald GB, Batchelder AL, Bouvier ME, et al. Diminishing the risk of nonrelapse mortality in hematopoietic stem cell transplantation: Prediction of exposure to the cyclophosphamide metabolite carboxyethylphosphoramide mustard. Clin Pharmacol Ther 2004;76(3):270–80 [DOI] [PubMed] [Google Scholar]
- 19.Balasubramanian P, Desire S, Panetta JC, Lakshmi KM, Mathews V, George B, et al. Population pharmacokinetics of cyclophosphamide in patients with thalassemia major undergoing HSCT. Bone Marrow Transplant 2012;47(9):1178–85 [DOI] [PubMed] [Google Scholar]
- 20.McCune JS, Salinger DH, Vicini P, Oglesby C, Blough DK, Park JR. Population pharmacokinetics of cyclophosphamide and metabolites in children with neuroblastoma: a report from the Children’s Oncology Group. J Clin Pharmacol 2009;49(1):88–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veal GJ, Cole M, Chinnaswamy G, Sludden J, Jamieson D, Errington J, et al. Cyclophosphamide pharmacokinetics and pharmacogenetics in children with B-cell non-Hodgkin’s lymphoma. Eur J Cancer 2016;55:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yule SM, Price L, McMahon AD, Pearson AD, Boddy AV. Cyclophosphamide metabolism in children with non-Hodgkin’s lymphoma. Clin Cancer Res 2004;10(2):455–60 [DOI] [PubMed] [Google Scholar]
- 23.Ayash LJ, Wright JE, Tretyakov O, Gonin R, Elias A, Wheeler C, et al. Cyclophosphamide pharmacokinetics: correlation with cardiac toxicity and tumor response. J Clin Oncol 1992;10(6):995–1000 [DOI] [PubMed] [Google Scholar]
- 24.Petros WP, Broadwater G, Berry D, Jones RB, Vredenburgh JJ, Gilbert CJ, et al. Association of high-dose cyclophosphamide, cisplatin, and carmustine pharmacokinetics with survival, toxicity, and dosing weight in patients with primary breast cancer. Clin Cancer Res 2002;8(3):698–705 [PubMed] [Google Scholar]
- 25.Nieto Y, Xu X, Cagnoni PJ, Matthes S, Shpall EJ, Bearman SI, et al. Nonpredictable pharmacokinetic behavior of high-dose cyclophosphamide in combination with cisplatin and 1,3-bis(2-chloroethyl)-1-nitrosourea. Clin Cancer Res 1999;5(4):747–51 [PubMed] [Google Scholar]
- 26.Ren S, Kalhorn TF, McDonald GB, Anasetti C, Appelbaum FR, Slattery JT. Pharmacokinetics of cyclophosphamide and its metabolites in bone marrow transplantation patients. Clin PharmacolTher 1998;64(3):289–301 [DOI] [PubMed] [Google Scholar]
- 27.Chen TL, Kennedy MJ, Anderson LW, Kiraly SB, Black KC, Colvin OM, et al. Nonlinear pharmacokinetics of cyclophosphamide and 4-hydroxycyclophosphamide/aldophosphamide in patients with metastatic breast cancer receiving high-dose chemotherapy followed by autologous bone marrow transplantation. Drug Metab Dispos 1997;25(5):544–51 [PubMed] [Google Scholar]
- 28.Sladek NE, Doeden D, Powers JF, Krivit W. Plasma concentrations of 4-hydroxycyclophosphamide and phosphoramide mustard in patients repeatedly given high doses of cyclophosphamide in preparation for bone marrow transplantation. Cancer Treat Rep 1984;68(10):1247–54 [PubMed] [Google Scholar]
- 29.Hassan M, Ljungman P, Ringden O, Hassan Z, Oberg G, Nilsson C, et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant 2000;25(9):915–24 [DOI] [PubMed] [Google Scholar]
- 30.Mouridsen HT, Witten J, Frederiksen PL, Hulsbaek I. Studies on the correlation between rate of biotransformation and haematological toxicity of cyclophosphamide. Acta Pharmacol Toxicol (Copenh) 1978;43(4):328–30 [DOI] [PubMed] [Google Scholar]
- 31.Huitema AD, Spaander M, Mathjt RA, Tibben MM, Holtkamp MJ, Beijnen JH, et al. Relationship between exposure and toxicity in high-dose chemotherapy with cyclophosphamide, thiotepa and carboplatin. Ann Oncol 2002;13(3):374–84 [DOI] [PubMed] [Google Scholar]
- 32.Kalhorn TF, Ren S, Howald WN, Lawrence RF, Slattery JT. Analysis of cyclophosphamide and five metabolites from human plasma using liquid chromatography-mass spectrometry and gas chromatography-nitrogen-phosphorus detection. J Chromatogr B Biomed Sci Appl 1999;732(2):287–98 [DOI] [PubMed] [Google Scholar]
- 33.Kalhorn TF, Howald WN, Cole S, Phillips B, Wang J, Slattery JT, et al. Rapid quantitation of cyclophosphamide metabolites in plasma by liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2006;835(1–2):105–13 [DOI] [PubMed] [Google Scholar]
- 34.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001;28(5):481–504 [DOI] [PubMed] [Google Scholar]
- 35.Nguyen TH, Mouksassi MS, Holford N, Al-Huniti N, Freedman I, Hooker AC, et al. Model Evaluation of Continuous Data Pharmacometric Models: Metrics and Graphics. CPT: pharmacometrics & systems pharmacology 2017;6(2):87–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holford NH. 2005. The Visual Predictive Check — Superiority to Standard Diagnostic (Rorschach) Plots. PAGE 14, Abstr 738 [http://www.page-meeting.org/?abstract=972].
- 37.Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. The AAPS journal 2009;11(3):558–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joerger M. Covariate pharmacokinetic model building in oncology and its potential clinical relevance. The AAPS journal 2012;14(1):119–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams K, Thomson D, Seto I, Contopoulos-Ioannidis DG, Ioannidis JP, Curtis S, et al. Standard 6: age groups for pediatric trials. Pediatrics 2012;129 Suppl 3:S153–60 [DOI] [PubMed] [Google Scholar]
- 40.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. JPharmacokinetBiopharm 1981;9(4):503–12 [DOI] [PubMed] [Google Scholar]
- 41.Ting LS, Villeneuve E, Ensom MH. Beyond cyclosporine: a systematic review of limited sampling strategies for other immunosuppressants. Ther Drug Monit 2006;28(3):419–30 [DOI] [PubMed] [Google Scholar]
- 42.Chen TL, Passos-Coelho JL, Noe DA, Kennedy MJ, Black KC, Colvin OM, et al. Nonlinear pharmacokinetics of cyclophosphamide in patients with metastatic breast cancer receiving high-dose chemotherapy followed by autologous bone marrow transplantation. Cancer Research 1995;55:810–6 [PubMed] [Google Scholar]
- 43.Hassan M, Svensson US, Ljungman P, Bjorkstrand B, Olsson H, Bielenstein M, et al. A mechanism-based pharmacokinetic-enzyme model for cyclophosphamide autoinduction in breast cancer patients. Br J Clin Pharmacol 1999;48(5):669–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huitema AD, Mathot RA, Tibben MM, Rodenhuis S, Beijnen JH. A mechanism-based pharmacokinetic model for the cytochrome P450 drug-drug interaction between cyclophosphamide and thioTEPA and the autoinduction of cyclophosphamide. J Pharmacokinet Pharmacodyn 2001;28(3):211–30 [DOI] [PubMed] [Google Scholar]
- 45.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med 2003;349(12):1157–67 [DOI] [PubMed] [Google Scholar]
- 46.Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther 2008;118(2):250–67 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.