Abstract
GZD824 is a novel third-generation BCR-ABL inhibitor. It entered Phase II clinical trials in China and Phase Ib clinical trials in USA in 2019 for treatment of patients with resistant chronic myeloid leukemia (CML). We found that at concentrations below 10 nM, GZD824 significantly suppresses FLT3, FGFR1 and PDGFRα kinase activities and inhibits their signal pathways in MV4-11Flt3-ITD, KG-1FGFR1OP2-FGFR1 and EOL-1FIP1L1-PDGFRa leukemia cells. It selectively inhibits the growth of MV4-11Flt3-ITD, KG-1FGFR1OP2-FGFR1 and EOL-1FIP1L1-PDGFRa cells, and also effectively suppresses the growth of Ba/F3-FLT3-ITD cells harboring F691I and other mutations with IC50 values <10 nM. GZD824 induces G0/G1 phase arrest and apoptosis in MV4-11, KG-1 and EOL-1 cells and activates cleavage of caspase-3 and PARP. In MV4-11, Ba/F3-ITD-F691I and KG-1 mouse xenograft models, GZD824 at 10 or 20 mg/kg, q2d, p.o. almost completely eradicates tumors. It also inhibits the viability of primary leukemic blasts from a FLT3-ITD positive AML patient but not those expressing native FLT3. Thus GZD824 suppresses leukemia cells of FLT3-ITD-driven AML and other hematologic malignancies driven by FGFR1 or PDGFRa, and it may be considered to be a novel agent for the treatment of leukemia.
Introduction
Mutation of the FLT3 gene is the most frequently encountered genetic alteration in acute myeloid leukemia (AML) and consists mainly of internal tandem duplication within the juxtamembrane domain (FLT3-ITD, 25%) and point mutations (5%) [5,6]. Mutation at the gatekeeper residue F691 and the tyrosine kinase domain (TKD) residue D835 are associated with the resistance to first generation FLT3 inhibitors [7]. Many agents have been used in clinical trials as FLT3 inhibitors [8], including type I inhibitors such as sunitinib, gilteritinib, crenolanib and midostaurin, and type II inhibitors including pexidartinib, ponatinib, quizartinib and sorafenib. Type I inhibitors inhibit FLT3 with ITD or TKD mutations in AML cells, but type II inhibitors inhibit FLT3 with ITD but not with TKD mutations although some D835 mutations preserve drug sensitivity [6]. Among the marketed drugs, only ponatinib has been reported [[9], [10], [11]] to overcome F691I and G697R mutations, but some unacceptable toxicities limit its usage.
Translocation rearrangements of FGFR1 and PDGFRα are found in a part of myeloproliferative neoplasms (MPN). According to these specific molecular abnormalities, a WHO classification in 2008 recognized the MPN with eosinophilia and abnormalities of PDGFR A/B or FGFR1 as a new subgroup of myeloid neoplasms, which is comprised of 7 rare specific diseases, including chronic eosinophilic leukemia (CEL) [12]. Several fusion partners of PDGFRA have been described, including FIP1L1, BCR, ETV6 and KIF5B, in which the FIP1L1-PDGFRa fusion protein is found in approximately 10% to 20% of CEL patients [13,14]. The 3 most common FGFR1 fusion partners are ZMYM2, CNTRL, and FGFR1OP [4]. Among these, the FGFR1OP2-FGFR1 fusion gene can rapidly transform to AML [15]. It has been reported that the patients with FGFR1 or PDGFR fusion proteins are sensitive to imatinib [16] and ponatinib [17].
GZD824 (HQP1351) is an oral third-generation BCR-ABL inhibitor designed and synthesized by our group [1] and targeting a broad spectrum of BCR-ABL mutants, including the T315I mutation. It was subsequently transferred to Ascentage Pharma for further development. Phase II clinical trials for patients with imatinib-resistant chronic myeloid leukemia (CML) have been initiated in China, and a Phase Ib clinical trial for Imatinib-resistant CML was approved by U.S. Food & Drug Administration (FDA) in July, 2019. Phase I results in China show that the complete hematologic response (CHR) rate was 96% in the chronic phase (CP, 86 cases), and 85% in the accelerated phase (AP, 14 cases) [2]. Unlike the marketed 3rd BCR-ABL inhibitor ponatinib, the side effects of blood clots or narrowing of blood vessels [3] with GZD824 were not detected in preclinical or phase 1 clinical data. Through a Kinomescan screening of 442 kinases, we have established that GZD824 is a multi-kinase inhibitor, which possesses binding activities with FLT3, PDGFRα and FGFR1.
Herein, we report the in vitro and in vivo activities of GZD824 against FLT3, FGFR1 and PDGFRa in leukemic cell lines harboring mutants our exploration of potential applications of GZD824 in leukemia beyond BCR-ABL-driven CML. GZD824 strongly suppresses FLT3-ITD, including F691I mutate resistance, FGFR1 and PDGFRa-driven leukemia cells in vitro and in vivo, and is a novel drug in clinical trials of leukemia therapy.
Materials and Methods
Agents
GZD824 was synthesized in our laboratory. Quizartinib (AC220), gilteritinib (ASP2215) and midostaurin (PKC412) were purchased from the Selleckchem Company (Houston, TX, USA). These compounds were dissolved in DMSO (Sigma-Aldrich, St. Louis, MO, USA) at a concentration of 10 mmol/L and the solution was stored at −20 °C. Primary antibodies against phosphor-FLT3 (4577), FLT3 (3462), Stat5 (25656S), phosphor-Stat5 (9359S), PLCγ (5690S), phosphor-PLCγ-1 (14008S), ERK1/2 (4695S), phosphor-ERK1/2 (4370S), FGFR1 (9740S), phosphor-FGFR1 (2544S), cleaved caspase-9 (9505S), cleaved caspase-3 (9664S), PARP (46D11) (9532S), Cyclin E1 (20808S), Cyclin D2 (2924), CDK2 (2546S), CDK4 (12790S), PDGFRα (3174), phosphor-PDGFRα (2992 T), Stat3 (9132S), phosphor-Stat3 (9145S), GAPDH- (2118) and anti-rabbit (7074S) or anti-mouse IgG horseradish peroxidase (HRP)-linked secondary antibodies were purchased from Cell Signaling Technology (Boston, MA, USA). Primary antibodies against AKT (SC8312), FRS2α (sc-17,841), were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
In Vitro Kinase Assays
FLT3, PDGFRA, FGFR1 and the Z′-Lyte Kinase Assay Kit were purchased from Invitrogen (Waltham, MA, USA), and the assays were performed according to the manufacturer's instructions. The concentrations of kinases were determined by optimization experiments. First, the solutions of the compounds were diluted to 10 mM in DMSO, and then were further diluted to 10 different concentrations by three times gradient dilution. Second, FLT3 kinase/peptide mixture containing 1× kinase and 2 μM Tyr2 peptide (PV3191; Invitrogen) was prepared immediately before use. Analogously, PDGFRA kinase/Tyr4 peptide (PV3193; Invitrogen) and FGFR1 kinase/Tyr4 peptide was prepared as described above. A kinase/peptide mixture was prepared by diluting peptide and kinase in 1× kinase buffer, and 2 μM of the corresponding peptide-phosphopeptide solutions were made by adding peptide-phosphopeptide to 1× kinase Buffer. The final 5 μL reaction solution consists of the appropriate concentration of FLT3/PDGFR/FGFR1, 2 μM Tyr peptide in 1× kinase buffer. Third, 5 nL of diluted compounds and ATP solution were added to 5 μL of kinase/peptide mixture by an Echo instrument. ATP final concentrations were 500 μM, 10 μM and 25 μM in FLT3 kinase/peptide, PDGFRA kinase/peptide and FGFR1 kinase/peptide mixture, respectively. The plate wells were mixed thoroughly and incubated for 1.5 h at room temperature (rt). Then 2.5 μL development solution was added to each well and the plate was incubated for 1 h at rt.; the phosphopeptides were cleaved at this time. Finally, 2.5 μL of stop reagent was added to terminate the reaction. For the control setting, 5 μL phosphopeptide solution instead of the kinase/peptide mixture was used as a 100% phosphorylation control. The kinase/peptide mixture containing no ATP solution was used as a 100% inhibition control, and DMSO instead of compound solution was used as the 0% inhibitor control. The plate was measured on an EnVision Multilabel Reader (Perkin-Elmer). Curve fitting and data presentation was performed using GraphPad Prism, version 5.0. Every experiment was repeated at least 2 twice.
Cell Cultures
The AML cell lines MV4-11, U937, KG-1, THP-1, and other leukemia cell lines HL60, NB4, MOLT4, MEG01 and EOL-1, were purchased from the American type culture collection (ATCC) or Shanghai Cell Bank (Type Culture Collection, Chinese Academy of Sciences). The base medium for MV4-11 and KG-1 cell line is gibco-formulated Iscove's Modified Dulbecco's Medium. All other cell lines were maintained in RPMI-1640 supplemented with 10% FBS, 100 U/mL penicillin, 50 mg/mL streptomycin, and 2 mmol/L glutamine in a humidified CO2 incubator at 37 °C. All cells were passaged for less than 3 months before renewal from frozen, early-passage stocks obtained from the indicated sources.
Construction of Ba/F3-FLT3 Stable Cells
The Ba/F3 cell lines stably expressing FLT3, FLT3-ITD or various mutants were self-established. Ba/F3 cells transfected the pCDNA3.1 plasmids using Amaxa Cell Line Nucleofector Kit V (Lonza, Cologne, Germany) by electroporation. Stable lines were selected by G418 (Merck, Whitehouse Station, NJ, USA) and withdrawal of Interleukin-3 (IL-3, R&D). All Ba/F3 stable cell lines were verified by monitoring both DNA sequences through DNA sequencing and protein expression levels of the corresponding FLT3 mutants through western blotting analysis. Parental Ba/F3 cells were cultured in RPMI 1640 supplemented with 10% Fetal Bovine Serum (FBS) and (Il-3, 10 ng/ml), while all FLT3-transformed Ba/F3 stable cell lines were cultured in in RPMI 1640 supplemented with 10% Fetal Bovine Serum (FBS) without Il-3.
Proliferation Assay
Cells were placed in 96-well plates (1500-3000/well) in complete medium. After incubation overnight, the cells were exposed to various concentrations (0.0015-30 μM) of compounds for a further 72 h. Cell proliferation was evaluated by Cell Counting Kit 8 (CCK8, CK04, Dojindo Laboratories, Kumamoto, Japan). IC50 values were calculated by concentration-response curve fitting using GraphPad Prism 5.0 software. Each IC50 value is expressed as mean ± SD.
Cell Cycle Analysis
After 48-hr treatment with GZD824 or DMSO, cells were harvested and washed twice with ice-cold PBS. Approximately 6 × 105 cells were re-suspended in 100 μL 1× BD Binding buffer solution (#556454, BD), and then incubated with Annexin V-PE (#556422, BD) and 7-ADD(#559925, BD) in the dark for 15 min. Finally, 400 μL 1× BD Binding buffer solution were replenished. The cells were then analyzed on a Guava easyCyte flow cytometer (Merck, Whitehouse Station, NJ, USA).
Western Blot Analysis
Cells were treated with various concentrations of each tested compound for a designated time. Then cells were lysed in using 1× SDS sample lysis buffer (CST recommended) with protease and phosphatase inhibitors. Cell lysates were loaded and electrophoresed onto 8% to 12% SDS-PAGE gel, then the separated proteins were transferred to a PVDF film. The film were blocked with 5% fat-free milk in TBS solution containing 0.5% Tween-20 for 4 h at rt., then incubated with the corresponding primary antibody (1:1000-1:200) overnight at 4 °C. After washing with TBST, HRP-conjugated secondary antibody was incubated for 2 h. The protein signals were visualized by ECL Western Blotting Detection Kit (Thermo Scientific, Waltham. MA, USA), and detected with Amersham Imager 600 system (GE, Boston, MA, USA).
Mouse Xenograft Tumor Models
Male CB17-SCID mice were purchased from Vital River Laboratory Animal Technology Inc. (Beijing, China). All animal experiments were carried out under protocols approved by the Institutional Animal Care and Use Committee of the Medical College of Jinan University. For Ba/F3-FLT3-ITD-F691I, 1x106 cells were injected subcutaneously in the right flank of SCID mice. For MV4-11 or KG-1, 5×106 cells combined with matrigel (v/v, 1:1) were injected subcutaneously in the right flank of SCID mice. Mice were randomly grouped when the mean tumor volume reached 100–200 mm3. The animals were treated for 16–20 consecutive days once every 2 days by oral gavage with GZD824 (10 or 20 mg/kg) or vehicle. Tumor volume and body weight were monitored once every 2 days. Tumor volume was calculated as L× W2/2, where L and W are the length and width of the tumor, respectively.
Patient Samples and Ethics Statement
The human primary AML cells were isolated from bone marrow (BM) of patients with their informed consent according to the Helsinki Declaration. All experiments were approved by the ethics committee of Zhujiang Hospital, Southern Medical University (Guangzhou, China). Samples were acquired during routine diagnostic assessments. Samples were enriched in leukemic cells by performing density-gradient separation to yield a mononuclear fraction according to the guidelines (P8610, Solarbio). Part of the primary cells were extracted RNA by the Trizol method, reverse transcripted to cDNA and the gene sequence of the PCR product was performed to identify gene fusions or mutations. Another portion of the primary cells was seeded in 96-well plates or 6-well plates for proliferation, apoptosis and signal pathway analysis.
Statistical Analysis
Data are expressed as the mean ± SD of three independent experiments. Comparisons between two groups involved two-tailed Student's t test, and comparisons among multiple groups involved one-way ANOVA with post hoc intergroup comparison using the Tukey test. Differences with P < .05 and P < .01 were considered as significant or very significant, and marked as * and **.
Results
GZD824 Displays FLT3, FGFR1 and PDGFRα Kinase Inhibitory Activities
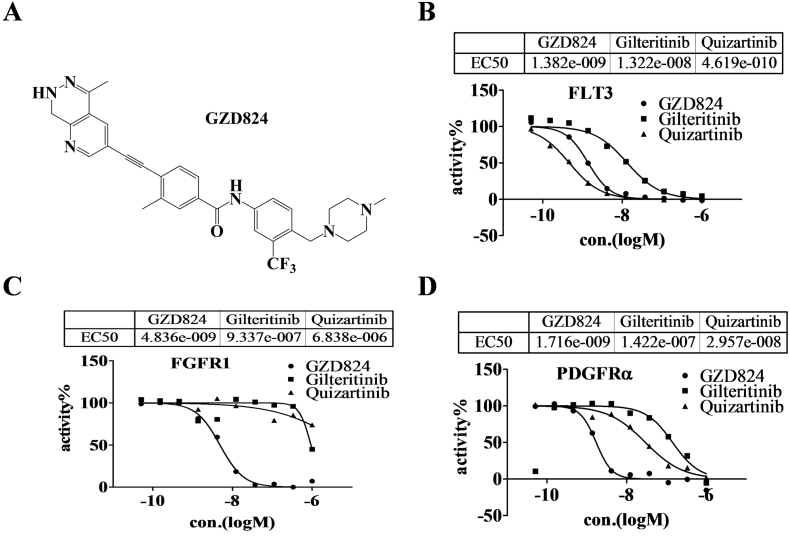
Our group has reported GZD824 (Figure 1A) as an ABL inhibitor, and has also shown its potential off-target kinases to be FLT3, PDGFRα with kinase inhibitory rates at 1 μM of 97.6% and 76% respectively. GZD824 has completed Phase I clinical trials approved by SFDA in China. To further explore the clinical applications of GZD824, the kinase inhibitory activities against FLT3, PDGFRα and FGFR of GZD824 were evaluated using a well-established FRET-based Z'-Lyte assay. Under the experimental conditions, GZD824 potently inhibits FLT3, PDGFRα and FGFR1 with IC50 values of 1.33 ± 0.074, 2.08 ± 0.51, 4.14 ± 0.96 nM respectively (Table S1), which are similar to that of ponatinib (1.70 ± 0.047, 1.85 ± 0.010, 8.37 ± 1.89 nM, respectively) and it is more potent than quizartinib (5.95 ± 1.89, 96.05 ± 4.11, >10,000 nM, respectively) and gilteribinib (13.32 ± 0.13, 147.9 ± 7.78, >1000 nM, respectively) in a parallel comparison.
Figure 1.
GZD824 possesses FLT3, FGFR1 and PDGFRα kinase inhibitory activity. (A) Structure of GZD824; (B) Kinase inhibitory activities against FLT3 by FRET-based Z′-Lyte assay; (C) Kinase inhibitory activities against FGFR1 by FRET-based Z′-Lyte assay; (D) Kinase inhibitory activities against PDGFRαby FRET-based Z′-Lyte assay.
GZD824 Inhibits the Proliferation of Leukemia Cells With Flt3-ITD, FGFR1OP2-FGFR1 or FIP1L1-PDGFRa Fusion Proteins
Previous studies have shown that ponatinib inhibits the proliferation of leukemic cells with activating mutations of FLT3, FGFR1 and PDGFRα in vitro and in vivo but intolerable side effects limit its clinical application. To validate the anti-proliferation effect of GZD824 in leukemia cells, we selected different single-gene-driven proliferation cells including MV4–11Flt3-ITD, MOLM-13Flt3-ITD, KG-1 FGFR1OP2-FGFR1 and EOL-1FIP1L1-PDGFRa as models. It was shown that GZD824 inhibits the viability of all 4 cell lines with IC50 values of 2.00 ± 1.10, 6.01 ± 6.10, 3.66 ± 1.93 and 7.56 ± 2.73 nM, respectively, but was more than 50-fold less potent against other leukemia cells such as NB4 and HL60, independent of FLT3-ITD, FGFR1OP2-FGFR1 or FIP1L1-PDGFRa mutations (Table 1).
Table 1.
Anti-proliferative activity of GZD824 in leukemia cells harboring gene fusions of FLT3, FGFR1 and PDGFRα
| IC50 (nM, AV ± SD) |
GZD824 (HQP1351) |
Quizaritinib (AC220) |
Gilteritinib (ASP2215) |
|---|---|---|---|
| MV411Flt3-ITD | 2.00 ± 1.10 | 1.07 ± 0.32 | 3.34 ± 0.91 |
| MOLM-13Flt3-ITD | 6.01 ± 6.10 | 5.67 ± 9.29 | 9.35 ± 2.23 |
| KG1FGFR1OP2-FGFR1 | 3.66 ± 1.93 | 1019.23 ± 571.53 | 820.58 ± 175.95 |
| EOL-1PDGFRα | 7.56 ± 2.73 | 1505.50 ± 450.77 | 719.08 ± 129.53 |
| NB4Flt3-WT | 1384.25 ± 1150.50 | 1990.00 ± 889.96 | 118.38 ± 674.43 |
| HL60Flt3-WT | 921.80 ± 209.65 | 1167.33 ± 72.13 | 688.75 ± 397.77 |
| U937Flt3-WT | 1167.00 ± 104.54 | 1430.60 ± 246.37 | 1134.62 ± 330.38 |
| THP1Flt3-WT | 2014.40 ± 414.56 | >1000.00 | >1000.00 |
| MEG01Flt-WT | 388.98 ± 151.74 | 3307.00 ± 1563.70 | 1137.64 ± 518.86 |
| MOLT4Flt3-WT | 245.69 ± 139.45 | 1946.78 ± 1749.86 | 891.85 ± 316.20 |
The anti-proliferative activities of the compounds were evaluated with a CCK-8 assay. The data presented are means from at least four independent experiments.
GZD824 Overcomes FLT3-ITD-F691I, G697R Mutation Resistance
We constructed Ba/F3 cells stably expressing different FLT3 or FLT3-ITD and their mutants. We investigated the anti-proliferation activities of GZD824 in Ba/F3 stable cells, and used three marketed FLT3 inhibitors, midostaurin, gilteritinib and quizartinib as controls. It was shown that these three FLT3 inhibitors were insensitive to FLT3-ITD-F691I and FLT3-ITD-F691R mutants, but GZD824 clearly inhibited cell growth with IC50 values of 1.4 and 9.4 nM, respectively (Table 2). GZD824 was also found to be sensitive to most cells harboring different FLT3 kinase domain mutants such as D835N//G/A and D842H/R but less sensitive to D835H/V/Y/I. Even for R834Q mutation, which is resistant to all marketed second generation FLT3 inhibitors, GZD824 possesses inhibitory activity with an IC50 value of 76.2 nM. Western blotting (WB) results showed that GZD824 clearly inhibits the activation of FLT3-ITD-F691I and its downstream signal proteins such as STAT5 (Figure 2B).
Table 2.
GZD824 inhibits proliferation in Ba/F3 cells expressing FLT3 and mutant isoforms
| IC50 (nM) | Midostaurin |
Gilteritinib (ASP2215) | Quizartinib |
GZD824 |
|---|---|---|---|---|
| (PKC412) | (AC220) | |||
| BaF3-Tel-FLT3-ITD | 21.8 | 5 | 2 | 1.8 |
| BaF3-tel-FLT3 | 14.4 | 57 | 3.1 | 2.3 |
| BaF3-FLT3-MOLM-ITD | 4 | 3.1 | 0.8 | 0.8 |
| BaF3-FLT3-ITD-D835N | 6.5 | 3.5 | 1.6 | 3 |
| BaF3-FLT3-ITD-Y842H | 1.3 | 1.5 | 5.3 | 3.2 |
| BaF3-FLT3-ITD-Y842R | 1.6 | 2.1 | 7.2 | 4.2 |
| BaF3-tel-FLT3-D835Y | 1 | 2.5 | 2.4 | 5.7 |
| BaF3-FLT3-ITD-D835G | 5.8 | 3.8 | 4.3 | 7.8 |
| BaF3-FLT3-ITD-D835A | 5.8 | 1.9 | 3.1 | 9.3 |
| BaF3-FLT3-ITD-N676D | 62.4 | 9.6 | 8.3 | 7.2 |
| BaF3-FLT3-ITD-F691I | 41.9 | 32.8 | 95 | 1.4 |
| BaF3-FLT3-ITD-G697R | 116.3 | 307 | 22.8 | 9.4 |
| BaF3-FLT3-D835H | 6.6 | 3.4 | 4 | 17.6 |
| BaF3-FLT3-D835V | 3.3 | 3.7 | 40.2 | 34.7 |
| BaF3-FLT3-ITD-D835Y | 6.4 | 2.9 | 35.5 | 35.7 |
| BaF3-FLT3-ITD-D835V | 3.8 | 1.6 | 125.6 | 49.9 |
| BaF3-FLT3-ITD-D835I | 3.5 | 1.6 | 182.8 | 58.5 |
| BaF3-tel-FLT3-R834Q | 63.1 | 252.1 | >1000 | 76.2 |
| BaF3-tel-FLT3-K663Q | 102.1 | 389.7 | >1000 | 853.3 |
The anti-proliferative activities of compounds evaluated with a CCK-8 assay.
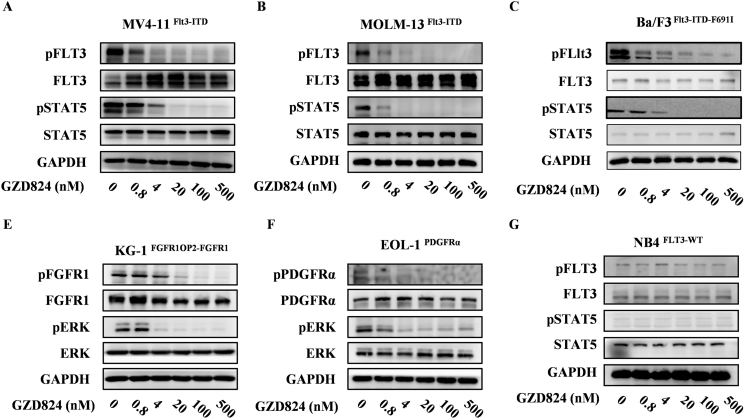
Figure 2.
GZD824 suppresses FLT3, FGFR1and PDGFRα downstream signaling pathways in leukemia cells. MV4–11, MOLM-13, Ba/F3-FLT3-ITD-F691I, KG-1, EOL-1 and NB4 cells were treated with or without GZD824 for 6 h at the indicated concentration. Cells were harvested and immunoblotted for phosphorylated FLT3, FGFR1, PDGFRα or ERK. Western blotting results show inhibition of phospho-FLT3 (A, B, C), phospho-FGFR1 (D), phospho-PDGFRα (E) and downstream signal molecules in cells.
GZD824 Dose-Dependently Inhibits the Phosphorylation of FLT3, FGFR1, PDGFRα and its Downstream Signaling in Leukemia Cells
It is well known that the mutation of FLT3-ITD causes aberrant activation of downstream kinases such as STAT5, AKT and ERK. We investigated the inhibitory effects of GZD824 on the FLT3, FGFR1, PDGFRα signaling pathways in MV4–11Flt3-ITD, Ba/F3-FLT3-ITD-F691I, KG-1FGFR1OP2-FGFR1 and EOL-1FIP1L1-PDGFRa and cells by WB analysis, respectively. As shown in Figure 2, GZD824, from 0.8 nM to 500 nM dose-dependently blocks the phosphorylation and the downstream signal proteins of Flt3, FGFR1 and PDGFRα in the model cells (Figure 2, A and B, D and E). In contrast, non-responsive results were observed in NB4 with native FLT3 (Figure 2C).
GZD824 Induces G0/G1 Phase Arrest and Apoptosis in Leukemia Cells
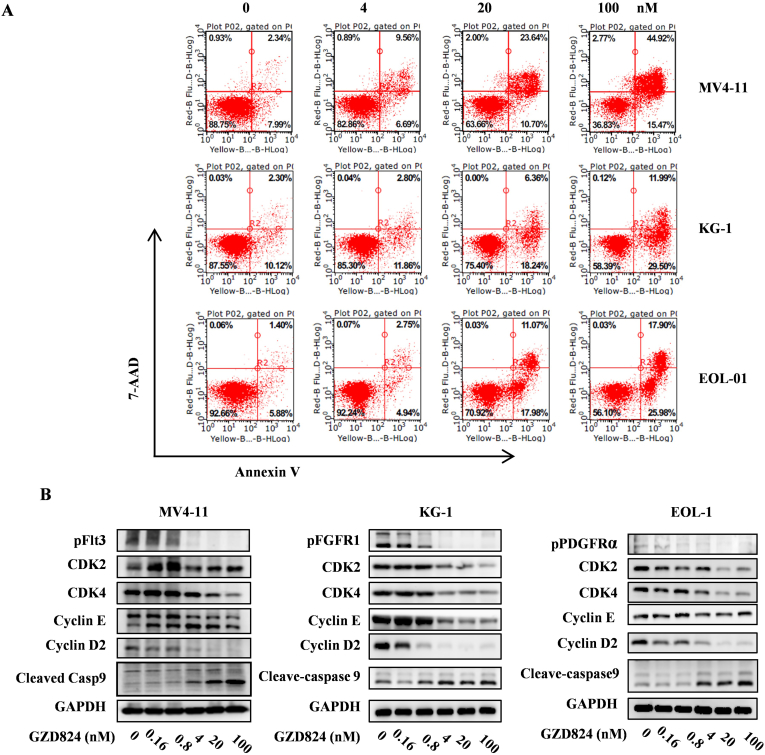
The ability of GZD824 to induce cell cycle arrest and apoptosis in MV4–11Flt3-ITD, KG-1 FGFR1OP2-FGFR1 and EOL-1FIP1L1-PDGFRa cells was investigated by flow cytometry analysis. It was shown that GZD824 dose-dependently induces G0/G1 phase arrest (Figure S1) and apoptosis (Figure 3B) in these model cells. Treatment with 100 nM of GZD824 for 48 h led to 60.39%, 41.49% and 43.88%% apoptosis in MV4–11Flt3-ITD, KG-1 FGFR1OP2-FGFR1 and EOL-1FIP1L1-PDGFRa cells, respectively (Figure 3A). Western blotting analysis showed that GZD824 dose-dependently decreases the protein levels of CDK2, CDK4, cyclin D2 and Cylin E and activates cleavage of Caspase- 9 in the model cells (Figure 3B).
Figure 3.
GZD824 induces G0/G1 cell cycle arrest and apoptosis in leukemia cells. (A) GZD824 induces apoptosis in MV4–11, KG-1 and EOL-1 cells. Cells were treated with 4, 20 or 100 nM for 24 h or 48 h before DNA labeling by Annexin V and 7-AAD and cell apoptosis analysis by flow cytometry. (B) The effect of GZD824 on the expression of CDK4, CDK2, Cyclin D2, Cyclin E and cleaved Caspase-9 was tested by western blotting. The cells were treated with GZD824 from 0.16 nM to 100 nM for 48 h. Representative results are shown and similar results were obtained in three other independent trials.
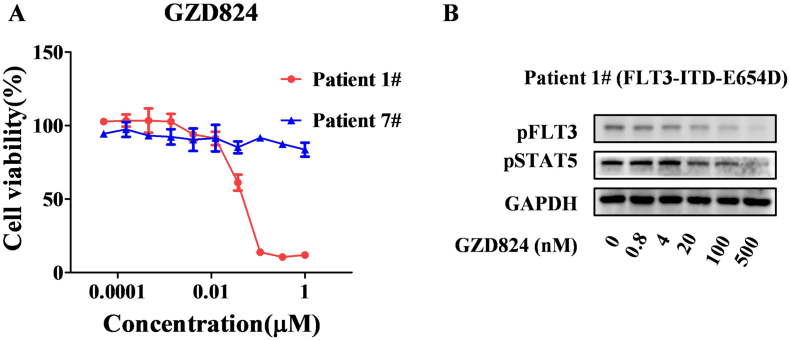
GZD824 Inhibits Proliferation of Primary AML Cells In Vitro
To examine the effect of GZD824 on primary cells isolated from the bone marrow of AML patients, we treated 8 patients, 7 of whom expressed native FLT3 and 1 harbored a FLT3-ITD-E654D confirmed by PCR and gene sequencing. The results showed that GZD824 at a dose of 20 nM or higher inhibits the proliferation of primary AML cells with FLT3-ITD-E654D (Figure 4A), but it is insensitive to the patients' cells with native FLT3 (IC50 >200 nM). WB results showed that GZD824 effectively suppresses the activation of FLT3-ITD-E654D in primary AML cells (Figure 4, C and D).
Figure 4.
GZD824 suppresses growth of primary AML cells. (A) GZD824 inhibits proliferation of primary AML cells isolated from bone marrow of patients. (B) GZD824 suppresses the activation of FLT3 and its down-stream signal proteins.
GZD824 Suppresses Growth in MV4–11, Ba/F3-FLT3-ITD-F691I and KG-1 Xenograft Model
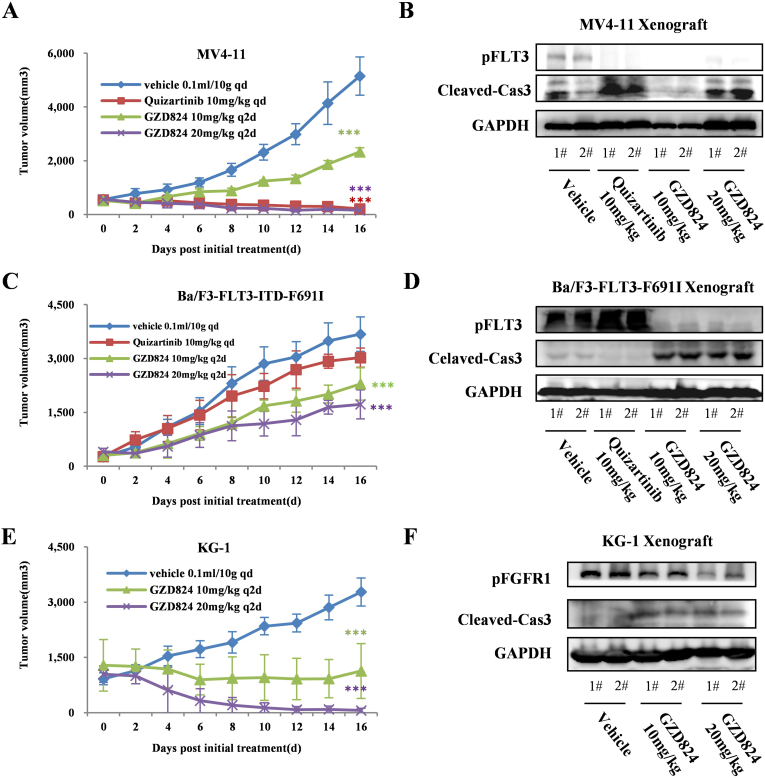
We further evaluated the antitumor efficacy in vivo of GZD824 in MV4–11Flt3-ITD, Ba/F3- FLT3-ITDF691I and KG-1 FGFR1OP2-FGFR1 cells using CB17-SCID mouse xenograft models. The animals were repeatedly administrated vehicle or GZD824 once every 2 days via oral gavage (10 and 20 mg/kg, q2d) for 16 consecutive days. GZD824 was well tolerated in all of the tested groups with no mortality or significant side effects observed during treatment (Tables S2, S3). It was shown that the GZD824 almost completely eradicates xenograft tumors of MV4–11 and KG-1 at a dose of 10 or 20 mg/kg respectively (Figure 5, A, C, E). WB assays in the tumor tissue showed that GZD824 suppresses the FLT3 and FGFR1 activation and induces apoptosis in MV4–11Flt3-ITD, Ba/F3- FLT3-ITDF691I and KG-1 FGFR1OP2-FGFR1 xenograft model (Figure 5, E and F).
Figure 5.
In vivo effects of GZD824 in MV4–11, Ba/F3-FLT3-F691I and KG-1 xenograft models. (A) Antitumor efficacy of GZD824 in a human AML MV4–11 xenograft mouse model. (B) Effect of GZD824 on signal pathway in MV4–11 xenograft. (C) Antitumor efficacy of GZD824 in a Ba/F3-FLT3-F691I xenograft mouse model. (D) Effect of GZD824 on signal pathway in Ba/F3-FLT3-F691I xenograft. (E) Antitumor efficacy of GZD824 in a human AML KG-1 xenograft mouse model. (F) Effect of GZD824 on signal pathway in KG-1 xenograft. Mice were orally dosed once every 2 days (q2d) for 16 days with vehicle or GZD824. Tumor volume and body weight were measured every 2 days. Tumors were harvested from the mice after dosing vehicle or GZD824 for 7 days (*P < .05, **P < .01).
Discussion
AML is the most common form of acute leukemia in adults and the second most common leukemia in children [4]. Although the cure rate of AML has improved remarkably during the past decade, the 5-year survival rate of patients is still only about 40% in young or middle-aged patients and less than 10% in elderly patients. Chemotherapy and hematopoietic stem cell transplantation (HSCT) can effectively control tumor progression, but high costs as well as side effects restrict their use. With the advances of sequencing technology, more driven gene and drug targets are being discovered and targeted therapy is becoming a new trend in leukemia therapy [18].
FLT3 is the most commonly mutated gene in AML and is a validated therapeutic target. Three drugs have been marketed as FLT3 inhibitors; midostaurin (PKC412), gilteritinib (ASP2215) and quizartinib (AC220), and many other multi-targeted drugs including FLT3 inhibitors such as sunitinib and sorafenib have been developed for AML treatment [25,26]. Midostaurin, the first marketed FLT3 inhibitor, approved in April 2017, is actually a multi-target drug [19], whose targets include FLT3, PKCα/β/γ, SYK, FLK-1, AKT and VEGFR1/2. It can effectively prolong the survival time for both FLT3-ITD-WT and D835/F697 mutant patients [20,21] but mostly in combination with other drugs, due to unfavorable safety profiles associated with the low selectivity against FLT3 [22]. Gilteritinib is a FLT3/AXL double-target inhibitor [23], which is ineffective in resistance to some drug resistant cells, such as F691L [7]. Quizartinib is currently the most selective listed FLT3 inhibitor including D835Y/V/H mutation [24], but it has no effect on F691L (>200 nM) [7] or R834Q. Therefore, it is still an urgent clinical problem to find effective and safe drugs with which to overcome resistance due to F697L and other mutations.
It has been reported that the 3rd ABL inhibitor, ponatinib exhibits inhibitory activities against FLT3-WT and FLT3-F691I in Ba/F3 stable cells with IC50 values of 3.0 and 4.2 nM respectively, but is insensitive to D835H/FY/V/C(IC50> 200 nM) [9]. AML patients may also fail to benefit from ponatinib treatment because of its well-known serious toxicity and side effects [3]. GZD824 is a promising 3rd ABL inhibitor developed by our group [1], and is now entering into Phase II clinical trials in China and Phase Ib trials in the USA. We found GZD824 displays an exciting effect against FLT3 with ITD and D835N/H/R/G/A (<10 nM), F691I (1.4 nM) and G697R (9.4 nM) in vitro and in vivo. In animal studies, no intolerable side effects or deaths were noted. On the other hand, we also reported that GZD824 exhibits FGFR1 and PDGFRα inhibitory activities in vitro and in vivo. Although fusion mutations of FGFR1 and PDGFRα in leukemia patients are rare, GZD824 has, with the progress of sequencing technology, the opportunity to provide effective treatment for suitable patients.
In previous pharmacokinetics research in SD rats (25 mg/kg, po), we found that GZD824 has a longer half-life of 10.6 h and a higher Cmax of 390.5 μg/L (733 nM), which are sufficient to support GZD824 as an effective treatment for leukemia when administered once every 2 or 3 days. In fact, unreported preclinical data in the animal model of CML confirm that is sufficient to completely eliminate xenografted tumors with oral administration of GZD824 once every 3 days.
In summary, we are reporting GZD824 as a multi-kinase inhibitor of FLT3, FGFR1and PDGFRα, which suppresses the signaling pathways of these kinases and proliferation of leukemia cells harboring mutant activating FLT3, FGFR1and PDGFRα in vitro and in vivo. It overcomes FLT3-D835N/H/R/G/A, F691I and G697R mutant resistance, and obviously suppresses the growth of F691I mutant xenograft tumors in animals. GZD824 may be considered to be an effective drug for anti-leukemia therapy in clinical trials.
Authors' Contributions
Yuting Wang performed in vitro and in vivo experiments. Lenghe Zhang performed experiments in vitro and clinically. Kaili Jiang performed partly in vitro experiments. Jinfeng Luo and Zhengchao Tu performed experiments in kinases. Xia Tang and Fang Xu constructed the Ba/F3 stable cells. Zhang Zhang and Yuting Wang performed statistical analysis. Shingpan Chan supplied the compound GZD824. Yuting Wang, Xiaomei Ren and Zhang Zhang analyzed and interpreted the data. Zhang Zhang and Ke Ding wrote this paper. Zhang Zhang, Ding Ke and Yuhua Li designed and coordinated the research study.
Acknowledgments
Acknowledgments
The authors appreciate the financial support from National Natural Science Foundation of China (81820108029, 21702075, 21572230, and 81973158), Guangdong Province (2016A050502041, 2017A030310253, 2018A030313685, 2019B020204002 and 2019A1515011235), Guangzhou city (201805010007), and Jinan University.
Conflict of Interest
No potential conflicts of interest are disclosed.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2020.100766.
Contributor Information
Yuhua Li, Email: liyuhua2011gz@163.com.
Zhang Zhang, Email: zhang_zhang@jnu.edu.cn.
Ke Ding, Email: dingke@jnu.edu.cn.
Appendix A. Supplementary Data
Supplementary material
References
- 1.Ren X., Pan X., Zhang Z., Wang D., Lu X., Li Y., Wen D., Long H., Luo J., Feng Y., Zhuang X., Zhang F., Liu J., Leng F., Lang X., Bai Y., She M., Tu Z., Pan J., Ding K. Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-Abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib. J Med Chem. 2013;56(3):879–894. doi: 10.1021/jm301581y. [DOI] [PubMed] [Google Scholar]
- 2.https://www.ascentagepharma.com/press-releases
- 3.Goodrich Daisy A. Ponatinib in the leukemia world: why a reevaluation is necessary for Philadelphia chromosome-positive patients with T315I mutation. Expert Rev Hematol. 2014;7(5):513–515. doi: 10.1586/17474086.2014.958465. [DOI] [PubMed] [Google Scholar]
- 4.Ling Y., Zhang Z., Zhang H., Huang Z. Protein kinase inhibitors as therapeutic drugs in AML: advances and challenges. Curr Pharm Des. 2017;23(29):4303–4310. doi: 10.2174/1381612823666170703164114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daver N., Schlenk R.F., Russell N.H., Levis M.J. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299–312. doi: 10.1038/s41375-018-0357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larrosa-Garcia M. Baer MR.FLT3 inhibitors in acute myeloid leukemia: current status and future directions. Mol Cancer Ther. 2017;16(6):991–1001. doi: 10.1158/1535-7163.MCT-16-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisberg E., Meng C., Case A.E., Sattler M., Tiv H.L., Gokhale P.C., Buhrlage S.J., Liu X., Yang J., Wang J., Gray N., Stone R.M., Adamia S., Dubreuil P., Letard S., Griffin J.D. Comparison of effects of midostaurin, crenolanib, quizartinib, gilteritinib, sorafenib and BLU-285 on oncogenic mutants of KIT, CBL and FLT3 in haematological malignancies. Br J Haematol. 2019 doi: 10.1111/bjh.16092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elshoury A., Przespolewski A., Baron J., Wang E.S. Advancing treatment of acute myeloid leukemia: the future of FLT3 inhibitors. Expert Rev Anticancer Ther. 2019;19(3):273–286. doi: 10.1080/14737140.2019.1573679. [DOI] [PubMed] [Google Scholar]
- 9.Smith C.C., Lasater E.A., Zhu X., Lin K.C., Stewart W.K., Damon L.E., Salerno S., Shah N.P. Activity of ponatinib against clinically-relevant AC220-resistant kinase domain mutants of FLT3-ITD. Blood. 2013;121(16):3165–3171. doi: 10.1182/blood-2012-07-442871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zirm E., Spies-Weisshart B., Heidel F., Schnetzke U., Böhmer F.D., Hochhaus A., Fischer T., Scholl S. Ponatinib may overcome resistance of FLT3-ITD harbouring additional point mutations, notably the previously refractory F691I mutation. Br J Haematol. 2012;157(4):483–492. doi: 10.1111/j.1365-2141.2012.09085.x. [DOI] [PubMed] [Google Scholar]
- 11.Barry E.V., Clark J.J., Cools J., Roesel J., Gilliland D.G. Uniform sensitivity of FLT3 activation loop mutants to the tyrosine kinase inhibitor midostaurin. Blood. 2007;110(13):4476–4479. doi: 10.1182/blood-2007-07-101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiter A., Gotlib J. Myeloid neoplasms with eosinophilia. Blood. 2017;129(6):704–714. doi: 10.1182/blood-2016-10-695973. [DOI] [PubMed] [Google Scholar]
- 13.Helbig G., Moskwa A., Hus M., Piszcz J., Swiderska A., Urbanowicz A., Całbecka M., Gajkowska J., Seferyńska I., Hałasz M., Woszczyk D., Markiewicz M., Krzemień S. Clinical characteristics of patients with chronic eosinophilic leukaemia (CEL) harbouring FIP1L1-PDGFRA fusion transcript--results of Polish multicentre study. Hematol Oncol. 2010;28(2):93–97. doi: 10.1002/hon.919. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler B., Peitsch W.K., Reiter A., Marx A., Goerdt S., Géraud C. Generalized eruptive histiocytosis associated with FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. JAMA Dermatol. 2015;151(7):766–769. doi: 10.1001/jamadermatol.2015.0154. [DOI] [PubMed] [Google Scholar]
- 15.Qin H., Wu Q., Cowell J.K. Ren M.FGFR1OP2-FGFR1 induced myeloid leukemia and T-cell lymphoma in a mouse model. Haematologica. 2016;101(3):e91–e94. doi: 10.3324/haematol.2015.137695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah S., Loghavi S., Garcia-Manero G., Khoury J.D. Discovery of imatinib-responsive FIP1L1-PDGFRA mutation during refractory acute myeloid leukemia transformation of chronic myelomonocytic leukemia. J Hematol Oncol. 2014;7:26. doi: 10.1186/1756-8722-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gozgit J.M., Wong M.J., Wardwell S., Tyner J.W., Loriaux M.M., Mohemmad Q.K., Narasimhan N.I., Shakespeare W.C., Wang F., Druker B.J., Clackson T., Rivera V.M. Potent activity of ponatinib (AP24534) in models of FLT3-driven acute myeloid leukemia and other hematologic malignancies. Mol Cancer Ther. 2011;10(6):1028–1035. doi: 10.1158/1535-7163.MCT-10-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shallis R.M., Wang R., Davidoff A., Ma X., Zeidan A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019;36:70–87. doi: 10.1016/j.blre.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Stone R.M., Manley P.W., Larson R.A., Capdeville R. Midostaurin: its odyssey from discovery to approval for treating acute myeloid leukemia and advanced systemic mastocytosis. Blood Adv. 2018;2(4):444–453. doi: 10.1182/bloodadvances.2017011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levis M.J. Midostaurin for patients with acute myeloid leukemia and FLT3 mutations. Clin Adv Hematol Oncol. 2019;17(6):323–325. [PubMed] [Google Scholar]
- 21.Levis M. Midostaurin approved for FLT3-mutated AML. Blood. 2017;129(26):3403–3406. doi: 10.1182/blood-2017-05-782292. [DOI] [PubMed] [Google Scholar]
- 22.Yamaura T., Nakatani T., Uda K., Ogura H., Shin W., Kurokawa N., Saito K., Fujikawa N., Date T., Takasaki M., Terada D., Hirai A., Akashi A., Chen F., Adachi Y., Ishikawa Y., Hayakawa F., Hagiwara S., Naoe T., Kiyoi H. A novel irreversible FLT3 inhibitor, FF-10101, shows excellent efficacy against AML cells with FLT3 mutations. Blood. 2018;131(4):426–438. doi: 10.1182/blood-2017-05-786657. [DOI] [PubMed] [Google Scholar]
- 23.Mori M., Kaneko N., Ueno Y., Yamada M., Tanaka R., Saito R., Shimada I., Mori K., Kuromitsu S. Gilteritinib, a FLT3/AXL inhibitor, shows antileukemic activity in mouse models of FLT3 mutated acute myeloid leukemia. Investig New Drugs. 2017;35(5):556–565. doi: 10.1007/s10637-017-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarrinkar P.P., Gunawardane R.N., Cramer M.D., Gardner M.F., Brigham D., Belli B., Karaman M.W., Pratz K.W., Pallares G., Chao Q., Sprankle K.G., Patel H.K., Levis M., Armstrong R.C., James J. Bhagwat SS.AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009;114(14):2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weis T.M., Marini B.L., Bixby D.L., Perissinotti A.J. Clinical considerations for the use of FLT3 inhibitors in acute myeloid leukemia. Crit Rev Oncol Hematol. 2019;141:125–138. doi: 10.1016/j.critrevonc.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 26.McMahon C.M., Canaani J., Rea B., Sargent R.L., Qualtieri J.N., Watt C.D., Morrissette J.J.D., Carroll M., Perl A.E. Gilteritinib induces differentiation in relapsed and refractory FLT3-mutated acute myeloid leukemia. Blood Adv. 2019;3(10):1581–1585. doi: 10.1182/bloodadvances.2018029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material