Abstract
Nuclear factor- (erythroid-derived 2-) like 2 (Nrf2) is a regulator of many processes of life, and it plays an important role in antioxidant, anti-inflammatory, and antifibrotic responses and in cancer. This review is focused on the potential mechanism of Nrf2 in the occurrence and development of ocular diseases. Also, several Nrf2 inducers, including noncoding RNAs and exogenous compounds, which control the expression of Nrf2 through different pathways, are discussed in ocular disease models and ocular cells, protecting them from dysfunctional changes. Therefore, Nrf2 might be a potential target of protecting ocular cells from various stresses and preventing ocular diseases.
1. Introduction
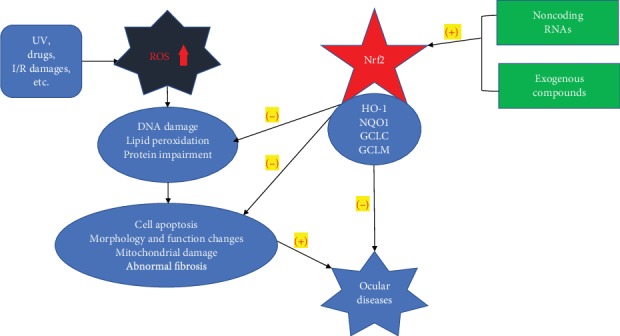
Oxidative stress (OS) usually comes after an imbalance between reactive oxygen species (ROS) production and elimination as a result of biological system defense mechanisms. In return, OS increases the production of ROS, which creates a vicious cycle. Damages caused by ROS are aimed at deoxyribonucleic acids (DNA), proteins, and lipids and have been observed and studied in corneal diseases [1], cataract [2], retinopathies [3], glaucoma [4], etc. One of the most inspiring discoveries about OS in recent decades has been the elucidation of nuclear factor- (erythroid-derived 2-) like 2 (Nrf2) signaling pathways that regulate OS responses (Figure 1).
Figure 1.
The effects of oxidative stress and the potential protective role of Nrf2 activation.
Nrf2 is a key regulator of protective antioxidant and anti-inflammatory responses that regulates the expression of hundreds of genes, including not only genes encoding antioxidant enzymes but also a series of genes involved in various processes, including inflammatory responses, cancer occurrence and metastasis, and tissue remodeling and fibrosis [5]. Due to its antioxidative capacity, Nrf2 has been found to mechanistically participate in various systemic diseases, including respiratory diseases [6], cardiovascular and cerebrovascular diseases [7], degenerative diseases, tumors [8], and especially ocular diseases. The Nrf2 signaling system, together with its regulatory molecules and interacting proteins, carries out critical antioxidant and anti-inflammatory functions in cells. Under normal conditions, Nrf2 is sequestered in the cytoplasm, where it mediates proteasomal degradation by binding Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1 (Keap1) to form a complex. Once cellular OS occurs, especially due to exposure to electrophiles including superoxide anion (O2−), hydrogen peroxide (H2O2), hydroxyl radical (-OH), and ROS, Keap1 undergoes conformational changes that allow Nrf2 to be transported to the nucleus, where it binds antioxidant response element (ARE) regions. Afterwards, Keap1 initiates transcription of antioxidant and phase II detoxification enzymes, such as NAD(P)H : quinone oxidoreductase 1 (NQO1), heme oxygenase-1 (HO-1), γ-glutamyl cysteine ligase catalytic subunit (GCLC), glutathione-S-transferase (GST), glutathione peroxidase (GPX), catalase (CAT), superoxide dismutase (SOD), and thioredoxin UDP-glucuronosyltransferase [9–12]. Alternatively, Nrf2 may be dissociated from the cytoplasmic Nrf2-Keap1-Cul3 complex by p62 (a marker associated with cell autophagy) [13]. Another mechanism is mediated by glycogen synthase kinase 3 (GSK-3) and the E3 ligase adaptor β-TrCP [14]. Under normal conditions, GSK-3α and β remain inactive. However, without receptor signaling, active GSK-3 phosphorylates Nrf2 in its Neh6 domain [15].
Some compounds, especially exogenous compounds including polyphenols, flavonoids, terpenoids, and noncoding ribonucleic acids (RNAs), were reported to be Nrf2 activators or inducers. These compounds may play critical roles in protecting ocular cells against oxidative damage, inflammation, and fibrosis.
2. Oxidative Stress and Nrf2 in Ocular Diseases
The eye is an organ subject to constant physical and chemical oxidation. Visible light, ultraviolet (UV) light, ionizing radiation, smog, fine particles in the atmosphere, and other types of pollutants can affect the cornea, lens, and the retina in particular. Correspondingly, OS is associated with many eye diseases [16].
2.1. Ocular Surface and Corneal Diseases
Due to its structure and function, the ocular surface and especially the cornea are constantly exposed to high oxygen tension, chemical burns, UV radiation (especially UVB), pathogenic microorganisms, or even urban air pollution [17, 18], which are the source of ROS and OS. The cornea is particularly susceptible to OS due to an imbalance between ROS and cellular antioxidant capacity. Increasing evidence shows that oxidative balance and mitochondrial function are abnormally altered under disease conditions. In addition, oxidative markers such as malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and nitrotyrosine showed significant changes [19–21]. Nrf2-mediated defense systems are aimed at upregulating the expression of antioxidant proteins and play a key role in protecting cells. Hayashi et al. found that the corneal epithelial wound healing time was much longer in Nrf2 knockout (KO) mice than in the wild-type (WT) mice and that Nrf2 contributed to the healing by accelerating cell migration [22]. Li et al. found that edaravone protected corneal epithelial cells against OS and apoptosis by activating Nrf2 [23]. Mutations in SLC4A11 can cause an increase in the generation of ROS and mitochondrial dysfunction due to oxidative stress [24]. Further study showed that the participation of antioxidative stress in corneal cells and SLC4A11 is necessary for Nrf2-mediated antioxidant gene expression [25].
2.1.1. Keratoconus (KC)
KC is a common degenerative disease of corneal dilatation that usually occurs in adolescence or early adulthood, and it is characterized by a progressive thinning and dilatation of the cornea on both sides, which can appear as a conical protrusion, accompanied by thinning of the central corneal stroma and changes in structural integrity, leading to irregular astigmatism, myopia, and, in severe cases, progressive blurred vision [26, 27]. Visual impairment in some patients with KC can be alleviated with spectacles, specialized contact lenses, or riboflavin-UVA-induced collagen crosslinking therapy; however, 10-20% of these patients need corneal transplantation [28, 29]. KC is a sporadic disease, but genetic factors were still found [30]. Genetic variations in antioxidant defense genes such as CAT and GPX can reduce antioxidant capacity or increase OS, altering the risk of KC [31]. In addition, KC has been reported to be the result of genetic and environmental factors in which OS is involved. In KC patients, CAT RNA and activity and the ratio of lactic acid to pyruvate [32] in the cornea were increased, while arginine and the glutathione/oxidized glutathione ratio were reduced [33]. An increase in oxidant status was also reported in the sera of KC patients [34]. Meanwhile, the accumulation of MDA, peroxide, and hydroxyl free radicals and a reduction in antioxidant defense levels also suggest that patients with KC are exposed to strong OS, shifting the redox balance toward oxidation [35, 36]. A study found that HO-2 enzyme levels were lower in KC corneal epithelial cells. Liu and Yan found that KC increased ROS and increased keratometry and decreased the central cornea thickness. These changes were neutralized or reversed by sulforaphane (SFN) treatment through the Nrf2/HO-1 pathway [37].
2.1.2. Fuchs' Endothelial Corneal Dystrophy (FECD)
FCED, an age-related cause of blindness with symptoms including poor vision, blurry cornea, poor night vision, and painful blinking, can eventually lead to full-layer corneal edema [38]. Corneal transplantation is the only way to restore vision loss in FECD patients. The main risk factors of FECD are family history, age (over 40), female sex, and smoking [39, 40]. FECD is a complex multifactorial inheritance disease with a variable expression rate and incomplete penetrance [41]. FECD-related genes include TCF4, COL8A2, ZEB1, AGBL1, SLC4A11, DMPK, LOXHD1, LAMC1, ATP1B1, and KANK4 [42–44], and environmental factors (especially UVA) also play an important role [45]. Due to its function and anatomical location, the corneal endothelial layer suffers from UVA exposure every day. The results of OS induced by UV usually include gene mutations, channelopathy, endoplasmic reticulum stress (ERS), and mitochondrial dysfunction [46]. The main characteristic changes due to FECD are collagen deposition in the Descemet membrane [47], cell morphological changes, apoptosis, and endothelial cell loss. Decreased antioxidant levels [48], deoxyribonucleic acid (DNA) damage, and apoptosis [49]; increased lipid peroxidation; excessive expression of cell senescence markers; and abnormal mitochondrial dynamics changes (decreasing mitochondrial DNA copy number, fragmented mitochondria, and increasing mitochondrial DNA damage) have been found in FECD [50]. Researchers found that Nrf2-mediated antioxidant defense and P53 play a key role in regulating FECD OS-induced apoptosis [49]. Cytoplasmic stability and the final translocation of Nrf2 are controlled by one of its stabilizers, DJ-1, which is decreased dramatically in FECD corneal endothelial cells (CECs) [51], accompanied by a decrease in Nrf2 and HO-1 [49] and impaired Nrf2 nuclear translocation [51]. These changes were attenuated to some extent by SFN, a Nrf2 activator. In both FECD CECs and an in vitro CEC OS model, SFN enhanced the nuclear transmigration of Nrf2, followed by the increasing expression of HO-1 and NQO1, decreasing expression of P53, and cell apoptosis reduction [52].
2.1.3. Pterygium
Pterygium is a kind of vascularized connective tissue from the conjunctiva that invades the cornea from the side of the nose, presenting an inflammatory, proliferative, and invasive growth. Excessive exposure to UVB (especially by way of OS) is one of the most important causes of pterygium [35]. UVB radiation may cause toxic light damage to DNA directly or by increasing ROS, which causes DNA damage [53]. In patients with pterygium, the serum total oxidant status (TOS), total antioxidant status (TAS), and nitric oxide (NO) and MDA content were significantly increased, and the antioxidant enzyme (SOD, CAT, and GPX) content was decreased [54, 55], indicating an increase in nonenzyme antioxidant activity. Meanwhile, the DNA damage parameters tail length (TL), tail intensity (TI), and tail moment were significantly increased [56]. Immunohistochemical staining for 8-OHDG in the nucleus showed more extensive and deeper staining than that observed in the normal conjunctiva. In ELISA, the average amount of 8-OHDG in the pterygium tissue was 4.7 times greater than that in the normal conjunctival tissue [57], while the p53 protein level changed and inflammatory mediators were increased [58]. Proteasome beta 5 (PSMB5), which can degrade many aberrant and denatured intracellular proteins as well as functional proteins, is mediated by the Nrf2-ARE pathway in many cell types. Recent research has found that in conjunctival fibroblasts, Nrf2/ARE mediated the downregulation of PSMB5 and that these changes could be reversed by the Nrf2 activator oltipraz [59].
2.1.4. Dry Eye
Dry eye, a multifactorial disease characterized by a loss of homeostasis of the tear film, appears together with ocular symptoms, ocular surface inflammation, and damage, and neurosensory abnormalities play etiological roles [60]. In recent years, air pollution has become increasingly serious (especially with the increase in fine particulate matter (PM2.5) and smoke) and has begun to be a factor that affects many diseases, including respiratory diseases [61] and hematological diseases [62]. Elementary carbon in PM2.5 produces a high concentration of ROS through the phagocytosis of macrophages, while organic carbon also produces ROS during its metabolism [63]. PM2.5 can inhibit SOD1 by promoting the expression of miRNA-206, leading to an increase in ROS and aggravating the pneumonia response and asthma symptoms [64]. Climate has also been shown to be associated with ocular surface integrity and tear film stability [65–67]. The tear film protects the ocular surface from many physical factors. Many antioxidants in the tear film, such as ascorbic acid, tyrosine, reduced glutathione, cysteine, and uric acid [68], serve as regulators in wound healing, corneal inflammatory response [69], and improving tear film stability. A study showed that the prevalence of dry eye is highest in the northern region of China, and it is lowest in the central region, suggesting that air pollution is associated with the onset of dry eye in which air pollutants including O3, PM2.5, and SO2 are potential risk factors [70]. The antioxidant enzymes SOD, CAT, and GPX were significantly less expressed in dry eye patients than in controls [71]. Furthermore, the expression of the lipid peroxidation markers 4-HNE and MDA was increased in dry eye patients with Sjøgren's syndrome and was closely related to tear film break-up time (BUT), Schirmer's tear value, tear clearance rate, keratoparaneliopathy score, conjunctival goblet cell density, and symptom score [72]. Some literature suggests that intervention with dietary supplements, vitamins, or omega-3 fatty acids can reduce OS [73]. Kojima et al. proposed that after exposure to sidestream cigarette smoke, Nrf2 KO mice had a significantly shortened BUT, significantly increased vital staining score, and reduced mucin 1 and Muc5ac staining compared to wild-type mice, suggesting that Nrf2 plays an important role in protecting eye surfaces from smoke exposure [74].
2.2. Cataract
Cataract is one of the most important causes of blindness worldwide, and age-related cataract is the most common type. Aging and OS are the main risk factors. Despite long-term exposure to UV, the lens has a well-established antioxidant system to combat OS and is rich in glutathione (GSH) [75]. Researchers used Lens Glutathione Synthesis KnockOut (LEGSKO) mice to develop a GSH defect model and confirmed that Nrf2 was the main upstream regulator of proteomic responses in LEGSKO lens fibroblasts [76]. However, with increasing age, GSH levels gradually decrease, and lens protein aggregation, DNA damage, lipid peroxidation, calcium homeostasis imbalance, and hydration occur, thus increasing lens turbidity [2, 77, 78]. In diabetic patients, the level of superoxide in the mitochondria is increased [79], and increased glucose utilization, insulin resistance, and OS lead to an increase in advanced glycation end products (AGEs) [80], which accelerates the formation of diabetic cataract.
As Nrf2 is a major antioxidant component, Nrf2 pathways regulate the expression of over 600 downstream antioxidant genes; imbalances in Nrf2 pathways have long been reported to be involved in the generation and development of cataract. With increasing age, the expression of Keap1 increases and that of Nrf2 decreases. Additionally, an increase in ROS also inhibits the antioxidant protective function of Nrf2 [81], which limits the transcription of downstream antioxidant enzymes, leading to a failure of the antioxidant system and accelerating the formation of cataract [82]. The protective mechanism of the ERS/unfolded protein response (UPR) is activated during the formation of most cataracts. Nrf2 pathways are activated under ERS to enhance the expression of multiple cellular protective enzymes that restore redox homeostasis [83]. The lens epithelial cells (LECs) of Nrf2 KO mice showed a higher cell death rate than those of wild-type mice treated with methylglyoxal at different concentrations. The above results indicated that normal Nrf2 levels are critical for lens survival under stress conditions [84].
Therefore, we have summarized some studies on the protection of LECs under stress through Nrf2 pathways. The use of Nrf2 activators (such as SFN pretreatment [85]) or the overexpression of Nrf2 [86] can reduce DNA fracture; upregulate Nrf2, NQO1, HO-1, etc. [87]; and protect LECs from OS damage. Puerarin [88], Rosa laevigata Michx. extract [89], hyperoside [90], acetyl-L-carnitine [91], morin [92], trimetazidine [93], rosmarinic acid [94], and DL-3-n-butylphthalide [95] have been shown to protect LECs from OS by activating the Nrf2 pathways. Besides, the inhibition of miRNA-4532 protected human LECs from UV-induced oxidative injury via activating SIRT6-Nrf2 signaling [96].
2.3. Glaucoma
Glaucoma is the second leading cause of irreversible human blindness worldwide, especially in elderly individuals, closely related to OS. Primary open-angle glaucoma (POAG) patients are susceptible to oxidative damage because their total reactive antioxidant capacity is reduced by 60%-70% [97]. Increasing evidence through clinical and experimental studies over the past decade has revealed that OS-induced dysfunction of trabecular meshwork cells (TMCs) can obstruct the outflow of the aqueous humor (AH), causing pathologically high intraocular pressure (IOP), which is consistent with the mechanical theory of glaucoma [98]. Pathologically high IOP can then cause retinal ganglion cell (RGC) mitochondrial dysfunction and apoptosis, therefore contributing to glaucoma vision loss [99].
The trabecular meshwork (TM) sustains OS due to the effects of the UV-ray-based oxidative byproducts of aqueous epithelial cells and CECs [100] and an imbalance between oxidants and antioxidants or excessive ROS accumulation [101]. TMCs under OS present typical changes observed in POAG, including extracellular matrix (ECM) accumulation, cell apoptosis, cell death, changes in the structure and function of the cytoplasm as well as lysosomes [102], and cytoskeletal disruption [103]. Cheng et al. found that Nrf2 expression was downregulated in glaucomatous TMCs compared to human TMCs and that in both cell types, the overexpression of Nrf2 could promote viability and reduce apoptosis [104].
Chronic hypertensive glaucoma and retinal ischemia caused by a sharp increase in IOP stimulate the production of ROS and dysregulate basic autophagy. The longer the injury lasts or the more dramatically increased the IOP is, the greater the extent of the immediate increase in autophagy is, inducing RGC death in a relatively short period of time [105]. Additionally, various proteins involved in cellular redox homeostasis and the OS response were upregulated in the retinas of ocular hypertensive humans [106]. In a retinal ischemia-reperfusion (I/R) model, the loss of neurons in the RGC layer was more severe in Nrf2 KO mice than in wild-type mice, and the RGC activity of Nrf2 KO mice was reduced, indicating that Nrf2 had an inherent protective effect in RGCs [107]. Repeated mild reperfusion led to chronic OS, especially in the mitochondria [108]. Virus-mediated delivery of Nrf2 effectively protected RGCs from oxidative damage after acute nerve damage [109].
Trabeculectomy, a classic glaucoma filtration surgery, destroys the structure of the conjunctiva and subconjunctival tissue and activates the immune system and the release of inflammatory cytokines. The activation of the vascular endothelial growth factor (VEGF-α) and the transforming growth factor (TGF-β) [110] leads to cell proliferation, migration, extracellular ECM formation, and collagen contraction, which promotes scar formation and is the key factor for surgical failure [111]. TGF-β1 causes cell apoptosis [112], fibrotic gene expression, and myofibroblast differentiation [113] due to ROS production and also inhibits the glutathione antioxidant system [114]. The miRNA-29 family is closely associated with TGF-β-mediated fibrosis [115, 116]. In patients with glaucoma, TGF-β2 was found to stimulate Tenon's capsule fibroblast proliferation via suppression of miR-29b expression regulated by Nrf2 [117], which indicates that Nrf2 may protect cells against TGF-β and even fibrosis by upregulating miR-29b.
2.4. Uveitis
Uveitis is a group of blindness-inducing autoimmune diseases. The mechanism of uveitis is not fully understood, but the imbalance between CD4+ CD25+ forkhead box protein+ T regulatory (Treg) cells and T helper 17 (Th17) cells is thought to be involved in the pathogenesis of autoimmune uveitis. The Nrf2 regulatory enzyme has been extensively studied in experimental autoimmune models because it plays an essential role in chemical reactions and provides a protective mechanism against autoimmune venereal diseases. In an encephalomyelitis mouse model, the absence of Nrf2 aggravated disease severity, which was reduced by treatment with SFN [118] or downregulation of the negative Nrf2 regulator Keap1 [119]. Nrf2 inhibited suppressive helper 1 (Th1) and Th17 cell responses and activated immunosuppressive Treg and Th2 cells [120], thus exerting protective effects.
In 2009, Nagai et al. confirmed the hypothesis that the Nrf2 pathway protects against injury in experimental uveitis by attenuating OS and modulating the innate immune response [121]. Chen et al. that found sodium butyrate (NaB) had great potential for inducing Treg cells in an experimental uveitis model. In vivo, NaB treatment reduced the number of Th17 cells in the spleens of mice with autoimmune uveitis and increased the number of Treg cells. In vitro, NaB treatment transformed original T cells from Th17 cells to Treg cells, and the inhibition of Th17 differentiation and the protective effect of NaB on uveitis may have been achieved by the Nrf2/HO-1 pathway [122].
2.5. Retinopathies
The anterior segment of the eye absorbs more than 99% of UV radiation, but the other 1%, especially UVA radiation, reaches the retina [123], leaving the retina continuously exposed to ROS and causing OS. OS is the main factor of retinal degeneration related to aging, such as age-related macular degeneration (AMD), and has also been linked to retinal inflammation and neuron degeneration. Furthermore, retinal I/R injury has been associated with the mechanisms of retinal vascular occlusion (RVO) and diabetic retinopathy.
2.5.1. Diabetic Retinopathy (DR)
DR is a common and progressive diabetic complication and the leading cause of blindness in the diabetic population. Historically, DR was described as an ocular microvascular disease caused by metabolic disorders (especially elevated glucose levels, oxidative phosphorylation, increased AGEs, and aldose reductase activity), increased ROS, and mitochondrial dysfunction, causing OS in only retinal cells and capillaries [124]. New evidence suggests that diabetes causes considerable damage to retinal neurons in the early stage of the disease [125, 126]. In the diabetic retina, neuronal apoptosis and the activation of neurogliocytes may also cause OS, thus creating a vicious cycle.
Nrf2 significantly contributes to protecting diabetic retinal cells from OS damage and inhibiting vascular inflammation. In animal experiments, Nrf2 KO mice showed significantly increased superoxide levels, glutathione was reduced, and early blood-retinal barrier dysfunction and the onset of neuron dysfunction were observed [127]. As for the anti-inflammatory protective effect of Nrf2, Nrf2 KO mice showed increased inflammation factors [128]. In endothelial cells exposed to high glucose or in the DR retinas, damage was observed which was prevented by the Nrf2 inducer t-BHQ and small interfering RNA (siRNA) against Keap1 [129]. C1q/TNF-related protein 3 (CTRP3) plays a role in the progression of diabetes and its complications, whose overexpression improved cell viability of high-glucose- (HG-) induced retinal pigment epithelium (RPE) cells by the activation of the Nrf2/HO-1 pathway [130]. In another study, the upregulation of casein kinase 2 interacting protein-1 (CKIP-1) inhibited HG-induced inflammation and OS in human retinal endothelial cells (RECs) and attenuated DR by modulating the Nrf2/ARE signaling pathway [131]. Forkhead box class O6 (FOXO6) is a member of the FOXO family that can regulate diabetes-induced OS, and the suppression of FOXO6 protects ARPE-19 cells from HG-induced OS and apoptosis, which is in part mediated by the activation of the Akt/Nrf2 pathway [132]. Activin receptor-like kinase 7 (ALK7), one of type I transforming growth factor β receptors, is involved in metabolic regulation, whose knockdown results in an increase in the expressions of Nrf2 and HO-1 in ARPE-19 cells in response to HG induction [132].
Recently, an increasing number of researchers have used Nrf2 inducers or activating agents, such as RS9 [133], CDDO-Im [126], dh404 [134], CKIP-1 [131], CTRP3 [130], curcumin [135], and SFN [136], to study the protective effect of Nrf2 against DR injury and made significant progress (see Table 1).
Table 1.
Nrf2 regulative function of exogenous compounds.
| Name | Experimental subjects | Functions and mechanisms |
|---|---|---|
| Edaravone | CECs | Augments the expression of Nrf2 and its target genes, such as HO-1, GPX-1, and GCLC [23] |
| SERPINA3K (SA3K) | CECs | Protects against oxidative stress by targeting the ROS generation/degradation system and modulating the Keap1-Nrf2 signaling pathway [189] |
| Cultured pterygial epithelial cells (PECs) | Inhibits NADPH oxidase 4 (NOX4) and elevates Nrf2, NQO1, and SOD2 [190] | |
| Rosmarinic acid (RA) | PECs | Reduces the cell viability of PECs. Increases Nrf2, HO-1, and NQO1 expression and activates SOD and CAT [191] |
| Chondrocyte-derived extracellular matrix (CDECM) | Human conjunctival epithelial cells (ConECs) and PECs | Inhibits NF-κB activation and improves Nrf2 induction by blocking the p38 MAPK and PKC signaling pathways [192] |
| 4-HNE | CECs | Elevates the ROS generation enzyme NOX4 and induces Nrf2 and its downstream effectors [193] |
| RPEs | Increases Nrf2 activity and GSH synthesis in a dose-dependent manner [194] | |
| Trichostatin A (TSA) | Corneal fibroblasts | Inhibits TGF-β-induced ROS accumulation and myofibroblast differentiation via enhanced Nrf2-ARE signaling [195] |
| DL-3-n-butylphthalide (NBP) | Rat diabetic cataract model | Delays the onset and progression of diabetic cataract by enhancing the expressions of Nrf2, TRX, and CAT [95] |
| Acetyl-L-carnitine | LECs | Prevents homocysteine-induced suppression of Nrf2/Keap1-mediated antioxidation [91] |
| Puerarin | Rat diabetic cataract model | Prevents cataract development and progression in diabetic rats through Nrf2/HO-1 signaling [88] |
| RPEs | Activates Nrf2/HO-1 antioxidant signaling pathway [196] | |
| Rosmarinic acid | Sprague-Dawley rat pup selenite-inducedcataractogenesis model | Increases the protein expressions of filensin, calpain 2, Nrf2, SOD, HO-1, and NQO1; the antioxidant enzyme activities; and the GSH level [94] |
| Morin | LECs | Induces HO-1 via the ERK-Nrf2 signaling pathway [92] |
| Calcium dobesilate | Rat D-galactose-induced cataract model | Increases Nrf2 and HO-1 levels and inhibits the Keap1 level [197] |
| Rosa laevigata Michx. | LECs | Inhibits ROS production and elevates mitochondrial membrane potential, through the induction of HO-1 expression mediated by the PI3K/Akt and Nrf2/ARE pathways [89] |
| Hyperoside | LECs | Increased Nrf2 level and the binding activity of its antioxidant components, increased the expression of HO-1, and restored cell vitality through ERK [90] |
| Quercetin | TMCs | Upregulates antioxidant peroxiredoxins through the activation of the Nrf2/Nrf1 transcription pathway [198] |
| RPEs | Protects RPEs from H2O2-induced cytotoxicity by activating the Nrf2 pathway [199] | |
| Sodium butyrate | Mouse experimental autoimmune uveitis (EAU) model | Regulates Th17/Treg cell balance to ameliorate uveitis via the Nrf2/HO-1 pathway [122] |
| Lutein | RPEs | Reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases [200] |
| Dh404 | Müller cells | Reduces Müller cell gliosis and vascular leakage as well as the hypoxia-induced increase in ROS and angiogenic factors with a concomitant increase in Nrf2-responsive antioxidants [134, 201] |
| Myricetin derivatives (F2) | RPEs | Protects RPE cells against OS by the activation of Nrf2 and SOD2 [202] |
| Fenofibrate | Mice diabetic retinas | Increases the expression of Nrf2, NQO1, and HO-1 [203] |
| Probucol | Müller cells | Activates the Keap1/Nrf2/ARE pathway [204] |
| Homocysteine | Müller cells | Increases the expression of Nrf2, NQO1, and HO-1 [205] |
| Ebselen | Müller cells | Reduces the ROS levels and increases the expression of Nrf2, HO-1, glutathione peroxidase-1, NQO1, and glutamate-cysteine ligase [206] |
| DL-3-n-butylphthalide | Müller cells | Increases the expression level of HO-1 in a time-dependent manner [207] |
| 3H-1,2-dithiole-3-thione | RPEs | Induces Nrf2 phosphorylation, causing Nrf2 disassociation with Keap1 and its subsequent nuclear accumulation [208] |
| Curcumin | RPEs | Prevents high glucose damage through ERK1/2-mediated activation of the Nrf2/HO-1 pathway [135] |
| Glycyrrhizin | RPEs | Protects against sodium iodate-induced RPE and retinal injury through activation of the Akt and Nrf2/HO-1 pathway [209] |
| Escin | RPEs | Activates Akt-Nrf2 signaling [210] |
| Ginsenoside Rh3 | RPEs and mice retinas | Rh3 induces miRNA-141 expression causing the downregulation of Keap1 and activating Nrf2 [211] |
| Hesperetin | RPEs | Upregulates the Keap1-Nrf2/HO-1 signal pathway [212] |
| Lycopene | RPEs | Inhibits ICAM-1 expression and NF-κB activation by Nrf2-regulated cell redox state [213] |
| 4-Acetoxyphenol | RPEs | Blocks the increase of cellular ROS and upregulates NQO1 and HO-1 genes by stabilizing and inducing the nuclear translocation of Nrf2 [214] |
| Taxifolin | RPEs | Enhances the nuclear accumulation of Nrf2 and increases the expression of HO-1, GCLC, GCLM, and NQO1 [215] |
| Genipin | RPEs | Reverses the inhibitory effects of H2O2 by promoting cell viability, attenuating ROS accumulation and cell apoptosis, and increasing the expression of Nrf2, HO-1, and NQO1 [216] |
| α-Tocopherol | RPEs | Activates the Keap1/Nrf2 pathway by increasing Nrf2 expression and inducing its translocation to the nucleus, and increases HO-1 as well as NQO1 [217] |
| Astaxanthin | RPEs | Activates the Nrf2-ARE pathway by inducing Nrf2 nuclear localization and increasing NQO1, HO-1, GCLM, and GCLC [218] |
| Salvianolic acid A | RPEs | Causes Nrf2 phosphorylation, accumulation, and nuclear translocation and increases the expression of HO-1 [219] |
| Salvianolic acid B | RPEs | Protects cells from OS-induced cell death by activating glutaredoxin 1 [220] |
| Lipoamide | RPEs | Induces the expression of Nrf2 and its translocation to the nucleus, leading to an increase in the expression or activity of NQO1, GST, GCL, catalase, and Cu/Zn SOD [221] |
| Thymoquinone | RPEs | Enhances the activation of the Nrf2/HO-1 signaling pathway [222] |
| Blueberry anthocyanins | Diabetes rat retinas | Increases the mRNA levels of Nrf2 and HO-1, and the nuclear location of Nrf2 and protein levels of HO-1 [223] |
| Grape seed proanthocyanidin extract | Diabetes rat retinas | Increases the expression of Nrf2 and HO-1 [224] |
| Carbamyl erythropoietin | Diabetes rat retinas | Increases the expression of Nrf2, HO-1, and NQO1 [225] |
| Ginsenoside Rb1 | Diabetes rat retinas | Increases the expression of Nrf2, GCLC, and GCLM [226] |
| Pyridoxamine | Retinal photoreceptor cells | Partly protects cells against light damage by Nrf2 expression [227] |
| Scutellarin (SC) | RECs and RPEs | Enhances nuclear Nrf2 accumulation and the SC-provided alleviation on BRB breakdown in STZ-induced diabetic mice was diminished in Nrf2 knockout mice [228] |
| Delphinidin (2-(3,4,5-trihydroxyphenyl) chromenylium-3,5,7-triol) | RPEs | Reverses the decreased activities of SOD, CAT, and GSH-PX and the elevated MDA level via increasing nuclear Nrf2 protein expression [229] |
| 4-Hydroxy-7-oxo-5-heptanoate (HOHA) lactone | RPEs | Induces upregulation of Nrf2, GCLM, HO-1, and NQO1 [230] |
| Melatonin | The retina of diabetic rats | Significantly upregulates glutamate-cysteine ligase by retaining Nrf2 in the nucleus and stimulating Akt phosphorylation [231] |
| Galangin | RECs and RPEs | Alleviates DR by reversing TNFα-induced blood-retinal barrier dysfunction by abrogating oxidative stress injury via activating Nrf2 [232] |
| Nonsteroidal anti-inflammatory drugs (NSAIDs) | Laser-induced CNV model in rat; RPEs | Inhibits neovascularization of choroid through the HO-1-dependent pathway [233] |
| RS9 | Retinal microvascular endothelial cells (RMECs) | Decreases retinal neovascularization through suppressing VEGF expression and increasing Nrf2, HO-1, and NQO1 [234] |
| 661W cells and Müller glia cells | Enhances the expression of Nrf2 and increases the expression of HO-1, GCLC, GCLM, and NQO1 [235] | |
| Acetaldehyde dehydrogenase 2 (ALDH2) | Diabetic rats' retinas | Increases the expression of Nrf2 [236] |
| Lipoic acid | RGCs | Induces the expression of HO-1 by promoting the translocation of Nrf2 to the nucleus [237, 238] |
| Sulforaphane | FECD corneal specimens | Enhances cell viability by decreasing ROS. Enhances nuclear translocation of Nrf2, DJ-1, HO-1, and NQO1 and decreases p53 [52] |
| Müller cells | Enhances the nuclear accumulation of Nrf2 and increases the expression of HO-1 and NQO1 [136] | |
| Rabbit KC corneas | Protects corneas against oxidative stress injury through activation of the Nrf2/HO-1 antioxidant pathway [37] | |
| LECs | Includes the expression of NQO1 and TXNRD1 and the Nrf2 translocation to the nucleus [87, 239] | |
| TMCs RGCs |
Attenuates H2O2-induced oxidative stress via PI3K/Akt-mediated Nrf2 signaling activation [240, 241] | |
| CDDO-Im | 661W | Inhibits ROS, increases OS, and increases neuronal cell survival after I/R injury [107] |
| Nrf2 KO mice | Increases expressions of Nrf2, HO-1, NQO1, and GCLM in the retina, reduces inflammatory mediator expression, and reduces leukocyte adherence to retinal vasculature [121] | |
| RTA 408 | RPEs | Activates Nrf2 and increases the expression of its downstream genes, such as HO-1, NQO1, SOD2, catalase, Grx1, and Trx1 [242] |
| Soluble P-selectin | RGCs | Increases NQO1 and HO-1 expression levels, along with increased transcription factor Nrf2 [243] |
| Eriodictyol | RGCs | Enhances the nuclear translocation of Nrf2 and elevates the expression of HO-1 [244] |
| RPEs | Induces the nuclear translocation of Nrf2, enhances the expression of HO-1 and NQO1, and increases the levels of intracellular glutathione [245] | |
| Neuroligin-3 | RGCs and RPEs | Activates Nrf2 signaling and enables Nrf2 protein stabilization, nuclear translocation, and expression of HO-1, NOQ1, and GCLC [246] |
| Long-acting (1R)-isopropyloxygenipin (IPRG001) | RGCs | The protective action depends on NO induction and the Nrf2/HO-1 antioxidant response element pathway by S-nitrosylation [247] |
| Resveratrol | RGCs | Upregulates the expression of Nrf2, HO-1, and NQO1 [248, 249] |
| L-carnitine (LC) | RGCs | Increases levels of Nrf2, HO-1, and γ-GCS and decreases expression of Keap1 protein [3] |
| SNJ-1945 (an exogenous calpain inhibitor) | RGCs | Protects RGCs against OS induced by high glucose [250] |
| Monomethyl fumarate | Ganglion cell layer | Fumaric acid esters exert a neuronal protective function in the retinal I/R model via Nrf2 modulation [251] |
| Trimetazidine | RGCs | Confers protection against RGC apoptosis via Nrf2/HO-1 signaling [252] |
| LECs | Reduces ROS production, inhibits Keap1 demethylation, and rescues Nrf2 expression level [93] | |
| Hydrogen sulfide gas (H2S) donor drugs | RGCs | Increases the levels of Nrf2 and HO-1 and inhibits OS-induced cell death [253] |
| 5α-Androst-3β, 5α, 6β-triol (TRIOL) | RGCs | Activates and upregulates Nrf2 and HO-1 by negative regulation of Keap1 [254] |
| Nipradilol | RGCs | Protects RGCs through S-nitrosylation of Keap1 and HO-1 induction [255] |
| Flavonoids | RGCs | Induces Nrf2 and HO-1 [256] |
| RPEs | Induces the expression of Nrf2 and HO-1 [257] | |
| Sulbutiamine | RGCs | Stimulates CAT and significantly increased Nrf2 and HO-1 levels [258] |
| Chalcone analog L2H17 | RGCs | Exhibits its antioxidative effects by activating the Nrf2 pathway [259] |
| Chlorogenic acid | RGCs | Relieves oxidative stress injury in retinal ganglion cells through lncRNA-TUG1/Nrf2 [260] |
2.5.2. Age-Related Macular Degeneration
Age-related macular degeneration (AMD), a kind of eye disease, mainly affects elderly people for which aging is the most severe risk factor. Complement factor H (CFH) mutation is a visible part of the genetic changes in AMD patients [137]. In addition to OS, there are environmental/lifestyle factors, including smoking, obesity, and a high-fat or low-antioxidant diet, and environmental factors such as exposure to UV radiation and blue light [138]. OS occurs in the above conditions and plays a vital role in the pathogenesis of AMD. AMD is associated with the progressive degeneration and death of RPEs, followed by adverse effects on rods and cones [139]. The ROS level was shown to be significantly higher in the RPEs of AMD patients than in those of a control group [140, 141]. RPEs are chronically affected by OS due to their exposure to the outer layer of photoreceptors, leading to a decrease in antioxidant enzyme levels [142] and an increase in the OS products MDA and 4-HNE, AGEs, and oxidation-specific epitopes in the macular area [143]. OS also accounts for the reduced number of mitochondria, lower mitochondrial matrix density, and mitochondrial DNA (mtDNA) mutation [144]. For the above reasons, researchers have performed much research on the antioxidant process in AMD, the most gratifying of which is that on the participation of Nrf2.
Aging can reduce Nrf2 mRNA or protein levels, thus weakening Nrf2 signal transduction. The retinas of Nrf2 KO mice [145] and si-Nrf2-transfected RPEs [146] were more susceptible to OS, which accelerated photoreceptor cell death; this damage could be alleviated by amplifying the endogenous Nrf2 pathway with electrophilic drugs or locally targeted antioxidant drugs [145, 147].
2.5.3. Retinitis Pigmentosa
Retinitis pigmentosa (RP) is a group of inherited retinal diseases characterized by rod and cone photoreceptor degeneration [148, 149]. As the rod cell pole accounts for approximately 95% of the total number of photoreceptors, it consumes most of the oxygen delivered to the retina; when a variety of genetic mutations cause rod cell death, the cone oxygen load increases and cone cells then die gradually [150, 151], eventually leading to tubular vision and blindness. A reduced GSH/GSSG ratio is a marker of OS in tissues. In patients with RP, the ratio of GSH/GSSG in the aqueous humor was significantly reduced, and the total antioxidant capacity and SOD3 activity and protein concentration were decreased [152], while the water-based protein carbonyl content was significantly increased. Also, the decreased activity of SOD3 in the peripheral blood, the increased formation of thiobarbituric acid-reactive substances, and the upregulated nitric oxide/ring GMP pathway were observed. These findings support the hypothesis that the antioxidant capacity of the eye is reduced in patients with RP [153].
Since Nrf2 is important for OS regulation, is Nrf2 involved in RP? The answer is yes. In 2009, Usui et al. proposed that increased expression of catalase and SOD 2 could reduce cone cell death in RP [154]. Xiong et al. delivered SOD2, CAT, and Nrf2 to the cones of an RP mouse model using adenoassociated virus carriers and found that the overexpression of Nrf2 was the most effective in saving cells, preserving the survival of RGCs after nerve compression and improving retinal morphology [109]. Wang et al. proposed that the absence of the Sigma 1 receptor (Sig1R) accelerated the death of photoreceptor cells in RP mice [155], and in their subsequent experiments, they concluded the protection of cones mediated by Sig1R required Nrf2 [156]. In a mouse model of retinal degeneration treated with SFN, significantly higher electroretinographic (ERG) a- and b-wave amplitudes and decreased photoreceptor cell death were observed [157]. These studies all provide a theoretical basis for the therapeutic potential of Nrf2 in RP.
2.6. Optic Neuritis
Optic neuritis, a disease defined by autoimmune demyelination of the optic nerve and the death of RGCs, is associated with visual impairment and multiple sclerosis (MS). In laboratory studies of optic neuritis, an experimental autoimmune encephalomyelitis (EAE) model of recurrent sclerosis is often used as a model of optic neuritis [158]. In 1998, Guy et al. tested the inhibition of oxidative damage in the optic nerves of EAE animals through virus-mediated CAT gene transfer and found that H2O2-related enzyme gene expression could decrease demyelination by 38%, swelling of the optic nerve head by 29%, and lymphocytes by 34% compared with the control [159]. Qi et al. also concluded in 2007 that with the inhibiting SOD2 expression, myelin fiber injury was increased by 27%. With the overexpression of the SOD2 level, myelin fiber damage was reduced by 51% and the RGC loss was saved by a factor of four [160]. When the role of Nrf2 was examined, Nrf2 KO EAE mice showed more severe visual impairment, optic nerve inflammation, and RGC degeneration, indicating that Nrf2 had a neuroprotective effect in EAE-related optic neuritis. The overexpression of Nrf2 increased RGC survival in an EAE model of optic neuritis [161]. Dimethyl fumarate (DMF) has been used in the treatment of MS [162], experimental Parkinson's diseases [163], long-term memory deficits [164], and other diseases of the nervous system. Recently, DMF was confirmed to reduce the severity of optic neuritis and retain vision and RGCs by the Nrf2 pathways. We believe these findings provide a useful perspective for the treatment of optic neuritis [165].
3. Novel Strategies for Activating Nrf2
3.1. Noncoding RNAs
MicroRNAs are a class of small noncoding RNAs (19–25 nucleotides) that regulate a wide range of cellular processes by repressing the transcription or translation of their target genes. lncRNAs are >200-nucleotide-long RNA molecules that lack or have limited protein-coding potential but can regulate transcription in cis or trans, the organization of nuclear domains, and RNA or protein formation through several different mechanisms [166, 167]. Recently, noncoding RNAs became a hot topic in scientific research, even in ocular research, as shown in Table 2. Noncoding RNAs provide an attractive opportunity to defend against OS for the diagnosis and prognosis of ocular diseases.
Table 2.
Nrf2 regulative function of noncoding RNAs.
| Name of miRNAs | Experimental subjects | Functions and mechanisms |
|---|---|---|
| miR-4532 | Human lens epithelial cells | Inhibition of miR-4532 protects HLECs from UVR-induced oxidative injury via activation of the SIRT6-Nrf2 pathway [96] |
| miR-27a | TMCs | Regulates Nrf2 expression at the posttranscriptional level [181] |
| miR-29b | Tenon's capsule fibroblast | TGF-β2 fibroblast proliferation via suppression of miR-29b expression regulated by Nrf2 [117] |
| miR-93 | TMCs | Inhibits viability and inducing apoptosis of the GTM cells by the suppression of Nrf2 [97, 182] |
| miR-141 | RGCs | Attenuates UV-induced oxidative stress via activating Keap1-Nrf2 signaling [183] |
| miR-144 | RPEs | Inhibiting microRNA-144 potentiates Nrf2-dependent antioxidant signaling and protects against OS-induced outer retinal degeneration [184] |
| miR-626 | RPEs | Ectopic overexpression of miR-626 targeting the 3′-UTR (3′-untranslated region) of Keap1 downregulates its expression, promoting Nrf2 protein stabilization and nuclear translocation, leading to the expression of HO-1, NOQ1, and GCLC [185] |
| miR-601 | RPEs | Overexpression of miR-601 inhibits Cul3 3′-UTR activity and downregulates Cul3 expression, leading to Nrf2 protein stabilization and its nuclear translocation as well as expression of HO-1, NQO1, and GCLC [186] |
| lncRNA-MEG3 | Tenon's capsule fibroblast | The functional interaction between lncRNA-MEG3 and Nrf2 constitutes the mechanism by which TGF-β2 induces Tenon's capsule fibroblast proliferation after glaucoma filtration surgery via the direct binding of MEG3 to Nrf2 [187] |
| lncRNA-Sox2OT | RGCs | Sox2OT knockdown plays an antioxidative role via regulating Nrf2/HO-1 signaling activity [188] |
3.2. Exogenous Compounds
Many types of compounds have anti-inflammatory, antioxidant, and antifibrotic properties by directly targeting Nrf2 and Nrf2 repressors (Keap1, Bach1, and c-Myc) [168–170] and have potential preventative functions in eye diseases. Interest in investigating exogenous compounds has revealed new treatment options against OS. Curcuminoids, cinnamic acid derivatives, coumarins, chalcones, flavonoids, and terpenoids are typical Nrf2 inducers. In addition, phenols and quinones (e.g., t-BHQ) [171], polyphenolic flavonoids (e.g., quercetin) [172], stilbenoid and nonflavonoid polyphenolic compounds (e.g., resveratrol) [173], and other compounds including SFN, sphaeropsidin A (SA), CDDO-Im, long-acting (1R)-isopropyloxygenipin (IPRG001), omaveloxolone, astaxanthin [174], and lycopene [175] activate Nrf2 and upregulate some downstream Nrf2 genes. Table 1 lists studies on Nrf2 activators connected to ocular diseases.
4. Nrf2: Negative Side
Although Nrf2 has many antistress functions, scientists have also revealed the dark side of Nrf2, which might be a driver role of cancer progression [176]. There are cancer-associated mutations that activate Nrf2 [177, 178]. Also, when ROS exceeds the critical threshold, Nrf2 binds to the ARE gene of Klf9 and upregulates Klf9 expression. Klf9, in turn, inhibits Trx reductase two expression, amplifying the ROS cascade and ultimately leading to cell death [179]. PI3K/AKT signaling and Nrf2 signaling are increased in cells with mutant PTEN, resulting in higher proliferation rates and increased tumorigenicity [180]. Therefore, it is essential to determine the boundary between the beneficial and potentially harmful effects of Nrf2 activation. Ongoing clinical trials will undoubtedly provide important progress in answering these questions in the coming years.
5. Conclusion and Prospect
More and more evidence show that Nrf2 plays a certain role in the occurrence and development of ocular diseases. Therefore, Nrf2 might be a potential target of protecting ocular cells from various stresses and preventing ocular diseases. We are looking forward to come across more clinical studies.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Cejka C., Cejkova J. Oxidative stress to the cornea, changes in corneal optical properties, and advances in treatment of corneal oxidative injuries. Oxidative Medicine and Cellular Longevity. 2015;2015:10. doi: 10.1155/2015/591530.591530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babizhayev M. A., Yegorov Y. E. Reactive oxygen species and the aging eye: specific role of metabolically active mitochondria in maintaining lens function and in the initiation of the oxidation-induced maturity onset cataract—a novel platform of mitochondria-targeted antioxidants with. American Journal of Therapeutics. 2016;23(1):e98–117. doi: 10.1097/MJT.0b013e3181ea31ff. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y., Li X., Wang C. J., et al. Role of NF-E2-related factor 2 in neuroprotective effect of L-carnitine against high glucose-induced oxidative stress in the retinal ganglion cells. Biomedicine & Pharmacotherapy. 2015;69:345–348. doi: 10.1016/j.biopha.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Kimura A., Namekata K., Guo X., Noro T., Harada C., Harada T. Targeting oxidative stress for treatment of glaucoma and optic neuritis. Oxidative Medicine and Cellular Longevity. 2017;2017:8. doi: 10.1155/2017/2817252.2817252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hybertson B. M., Gao B., Bose S. K., McCord J. M. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Molecular Aspects of Medicine. 2011;32(4-6):234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Tu W., Wang H., Li S., Liu Q., Sha H. The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases. Aging and Disease. 2019;10(3):637–651. doi: 10.14336/AD.2018.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q. M., Maltagliati A. J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiological Genomics. 2018;50(2):77–97. doi: 10.1152/physiolgenomics.00041.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geismann C., Arlt A., Sebens S., Schafer H. Cytoprotection “gone astray”: Nrf2 and its role in cancer. OncoTargets and Therapy. 2014;7:1497–1518. doi: 10.2147/OTT.S36624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X.-F., Zhou D.-D., Xie T., et al. The Nrf2 signaling in retinal ganglion cells under oxidative stress in ocular neurodegenerative diseases. International Journal of Biological Sciences. 2018;14(9):1090–1098. doi: 10.7150/ijbs.25996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H., Davies K. J. A., Forman H. J. Oxidative stress response and Nrf2 signaling in aging. Free Radical Biology & Medicine. 2015;88(Part B):314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki T., Yamamoto M. Molecular basis of the Keap1-Nrf2 system. Free Radical Biology & Medicine. 2015;88(Part B):93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz S., Pergola P. E., Zager R. A., Vaziri N. D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney International. 2013;83(6):1029–1041. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinisalo M., Karlund A., Koskela A., Kaarniranta K., Karjalainen R. O. Polyphenol stilbenes: molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxidative Medicine and Cellular Longevity. 2015;2015:24. doi: 10.1155/2015/340520.340520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hur E. M., Zhou F. Q. GSK3 signalling in neural development. Nature Reviews Neuroscience. 2010;11(8):539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robledinos-Anton N., Fernandez-Gines R., Manda G., Cuadrado A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxidative Medicine and Cellular Longevity. 2019;2019:20. doi: 10.1155/2019/9372182.9372182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pagano G., Aiello Talamanca A., Castello G., et al. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: toward mitochondria-targeted clinical strategies. Oxidative Medicine and Cellular Longevity. 2014;2014:27. doi: 10.1155/2014/541230.541230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallabh N. A., Romano V., Willoughby C. E. Mitochondrial dysfunction and oxidative stress in corneal disease. Mitochondrion. 2017;36:103–113. doi: 10.1016/j.mito.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Choi S. I., Dadakhujaev S., Ryu H., Im Kim T., Kim E. K. Melatonin protects against oxidative stress in granular corneal dystrophy type 2 corneal fibroblasts by mechanisms that involve membrane melatonin receptors. Journal of Pineal Research. 2011;51(1):94–103. doi: 10.1111/j.1600-079X.2011.00866.x. [DOI] [PubMed] [Google Scholar]
- 19.Cejkova J., Cejka C. The role of oxidative stress in corneal diseases and injuries. Histology and Histopathology. 2015;30(8):893–900. doi: 10.14670/HH-11-611. [DOI] [PubMed] [Google Scholar]
- 20.Lasagni Vitar R. M., Hvozda Arana A. G., Janezic N. S., et al. Urban air pollution induces redox imbalance and epithelium hyperplasia in mice cornea. Toxicology and Applied Pharmacology. 2019;384, article 114770 doi: 10.1016/j.taap.2019.114770. [DOI] [PubMed] [Google Scholar]
- 21.Arnal E., Peris-Martinez C., Menezo J. L., Johnsen-Soriano S., Romero F. J. Oxidative stress in keratoconus? Investigative Ophthalmology & Visual Science. 2011;52(12):8592–8597. doi: 10.1167/iovs.11-7732. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi R., Himori N., Taguchi K., et al. The role of the Nrf2-mediated defense system in corneal epithelial wound healing. Free Radical Biology & Medicine. 2013;61:333–342. doi: 10.1016/j.freeradbiomed.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Li Y., Liu H., Zeng W., Wei J. Edaravone protects against hyperosmolarity-induced oxidative stress and apoptosis in primary human corneal epithelial cells. PLoS One. 2017;12(3, article e0174437) doi: 10.1371/journal.pone.0174437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy S., Praneetha D. C., Vendra V. P. R. Mutations in the corneal endothelial dystrophy-associated gene SLC4A11 render the cells more vulnerable to oxidative insults. Cornea. 2015;34(6):668–674. doi: 10.1097/ICO.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 25.Guha S., Chaurasia S., Ramachandran C., Roy S. SLC4A11 depletion impairs NRF2 mediated antioxidant signaling and increases reactive oxygen species in human corneal endothelial cells during oxidative stress. Scientific Reports. 2017;7(1):p. 4074. doi: 10.1038/s41598-017-03654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson A. E., Hayes S., Hardcastle A. J., Tuft S. J. The pathogenesis of keratoconus. Eye. 2014;28(2):189–195. doi: 10.1038/eye.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soiberman U., Foster J. W., Jun A. S., Chakravarti S. Pathophysiology of keratoconus: what do we know today. The Open Ophthalmology Journal. 2017;11(Supplement 1, M9):252–261. doi: 10.2174/1874364101711010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dahl B. J., Spotts E., Truong J. Q. Corneal collagen cross-linking: an introduction and literature review. Optometry. 2012;83(1):33–42. doi: 10.1016/j.optm.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Colin J., Simonpoli S. Keratoconus: current surgical options. Journal Français d'Ophtalmologie. 2005;28(2):205–217. doi: 10.1016/S0181-5512(05)81045-9. [DOI] [PubMed] [Google Scholar]
- 30.Cristina Kenney M., Brown D. J. The cascade hypothesis of keratoconus. Contact Lens & Anterior Eye. 2003;26(3):139–146. doi: 10.1016/S1367-0484(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 31.Yari D., Saravani R., Saravani S., Ebrahimian K., Galavi H. R. Genetic polymorphisms of catalase and glutathione peroxidase-1 in keratoconus. Iranian Journal of Public Health. 2018;47(10):1567–1574. [PMC free article] [PubMed] [Google Scholar]
- 32.Karamichos D., Zieske J. D., Sejersen H., Sarker-Nag A., Asara J. M., Hjortdal J. Tear metabolite changes in keratoconus. Experimental Eye Research. 2015;132:1–8. doi: 10.1016/j.exer.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karamichos D., Hutcheon A. E. K., Rich C. B., Trinkaus-Randall V., Asara J. M., Zieske J. D. In vitro model suggests oxidative stress involved in keratoconus disease. Scientific Reports. 2015;4(1, article 4608) doi: 10.1038/srep04608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toprak I., Kucukatay V., Yildirim C., Kilic-Toprak E., Kilic-Erkek O. Increased systemic oxidative stress in patients with keratoconus. Eye. 2014;28(3):285–289. doi: 10.1038/eye.2013.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoham A., Hadziahmetovic M., Dunaief J. L., Mydlarski M. B., Schipper H. M. Oxidative stress in diseases of the human cornea. Free Radical Biology & Medicine. 2008;45(8):1047–1055. doi: 10.1016/j.freeradbiomed.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Behndig A., Karlsson K., Johansson B. O., Brannstrom T., Marklund S. L. Superoxide dismutase isoenzymes in the normal and diseased human cornea. Investigative Ophthalmology & Visual Science. 2001;42(10):2293–2296. [PubMed] [Google Scholar]
- 37.Liu R., Yan X. Sulforaphane protects rabbit corneas against oxidative stress injury in keratoconus through activation of the Nrf-2/HO-1 antioxidant pathway. International Journal of Molecular Medicine. 2018;42(5):2315–2328. doi: 10.3892/ijmm.2018.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magovern M., Beauchamp G. R., McTigue J. W., Fine B. S., Baumiller R. C. Inheritance of Fuchs' Combined Dystrophy. Ophthalmology. 1979;86(10):1897–1920. doi: 10.1016/S0161-6420(79)35340-4. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X., Igo RP Jr, Fondran J., et al. Association of smoking and other risk factors with Fuchs’ endothelial corneal dystrophy severity and corneal thickness. Investigative Ophthalmology & Visual Science. 2013;54(8):5829–5835. doi: 10.1167/iovs.13-11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlin S. E., Raber I. M., Eagle RC Jr, Scheie H. G. Keratoconus associated with corneal endothelial dystrophy. Cornea. 1990;9(4):299–304. [PubMed] [Google Scholar]
- 41.Weiss J. S., Møller H. U., Aldave A. J., et al. IC3D classification of corneal dystrophies—edition 2. Cornea. 2015;34(2):117–159. doi: 10.1097/ICO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 42.Biswas S., Munier F. L., Yardley J., et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Human Molecular Genetics. 2001;10(21):2415–2423. doi: 10.1093/hmg/10.21.2415. [DOI] [PubMed] [Google Scholar]
- 43.Chung D.-W. D., Frausto R. F., Ann L. B., Jang M. S., Aldave A. J. Functional impact of ZEB1 mutations associated with posterior polymorphous and Fuchs’ endothelial corneal dystrophies. Investigative Ophthalmology & Visual Science. 2014;55(10):6159–6166. doi: 10.1167/iovs.14-15247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riazuddin S. A., Parker D. S., McGlumphy E. J., et al. Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. American Journal of Human Genetics. 2012;90(3):533–539. doi: 10.1016/j.ajhg.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cadet J., Gentner N. E., Rozga B., Paterson M. C. Rapid quantitation of ultraviolet-induced thymine-containing dimers in human cell DNA by reversed-phase high-performance liquid chromatography. Journal of Chromatography. 1983;280(1):99–108. doi: 10.1016/S0021-9673(00)91543-7. [DOI] [PubMed] [Google Scholar]
- 46.Jurkunas U. V. Fuchs endothelial corneal dystrophy through the prism of oxidative stress. Cornea. 2018;37(Supplemen 1):S50–S54. doi: 10.1097/ICO.0000000000001775. [DOI] [PubMed] [Google Scholar]
- 47.Krachmer J. H., Purcell JJ Jr, Young C. W., Bucher K. D. Corneal endothelial dystrophy. A study of 64 families. Archives of Ophthalmology. 1978;96(11):2036–2039. doi: 10.1001/archopht.1978.03910060424004. [DOI] [PubMed] [Google Scholar]
- 48.Jurkunas U. V., Rawe I., Bitar M. S., et al. Decreased expression of peroxiredoxins in Fuchs’ endothelial dystrophy. Investigative Ophthalmology & Visual Science. 2008;49(7):2956–2963. doi: 10.1167/iovs.07-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jurkunas U. V., Bitar M. S., Funaki T., Azizi B. Evidence of oxidative stress in the pathogenesis of Fuchs endothelial corneal dystrophy. The American Journal of Pathology. 2010;177(5):2278–2289. doi: 10.2353/ajpath.2010.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halilovic A., Schmedt T., Benischke A. S., et al. Menadione-induced DNA damage leads to mitochondrial dysfunction and fragmentation during rosette formation in Fuchs endothelial corneal dystrophy. Antioxidants & Redox Signaling. 2016;24(18):1072–1083. doi: 10.1089/ars.2015.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bitar M. S., Liu C., Ziaei A., Chen Y., Schmedt T., Jurkunas U. V. Decline in DJ-1 and decreased nuclear translocation of Nrf2 in Fuchs endothelial corneal dystrophy. Investigative Ophthalmology & Visual Science. 2012;53(9):5806–5813. doi: 10.1167/iovs.12-10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ziaei A., Schmedt T., Chen Y., Jurkunas U. V. Sulforaphane decreases endothelial cell apoptosis in fuchs endothelial corneal dystrophy: a novel treatment. Investigative Ophthalmology & Visual Science. 2013;54(10):6724–6734. doi: 10.1167/iovs.13-12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wanzeler A. C. V., Barbosa I. A. F., Duarte B., et al. Mechanisms and biomarker candidates in pterygium development. Arquivos Brasileiros de Oftalmologia. 2019;82(6):528–536. doi: 10.5935/0004-2749.20190103. [DOI] [PubMed] [Google Scholar]
- 54.Balci M., Sahin S., Mutlu F. M., Yagci R., Karanci P., Yildiz M. Investigation of oxidative stress in pterygium tissue. Molecular Vision. 2011;17:443–447. [PMC free article] [PubMed] [Google Scholar]
- 55.Kormanovski A., Parra F., Jarillo-Luna A., Lara-Padilla E., Pacheco-Yepez J., Campos-Rodriguez R. Oxidant/antioxidant state in tissue of prymary and recurrent pterygium. BMC Ophthalmology. 2014;14(1):p. 149. doi: 10.1186/1471-2415-14-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kilic-Toprak E., Toprak I., Caliskan S., et al. Oxidative stress and genotoxicity in Pterygium. Eye & Contact Lens. 2019;45(6):399–404. doi: 10.1097/ICL.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 57.Kau H. C., Tsai C. C., Lee C. F., et al. Increased oxidative DNA damage, 8-hydroxydeoxy- guanosine, in human pterygium. Eye. 2006;20(7):826–831. doi: 10.1038/sj.eye.6702064. [DOI] [PubMed] [Google Scholar]
- 58.Chiang C. C., Tsai Y. Y., Bau D. T., et al. Pterygium and genetic polymorphisms of the DNA repair enzymes XRCC1, XPA, and XPD. Molecular Vision. 2010;16:698–704. [PMC free article] [PubMed] [Google Scholar]
- 59.Aletras A. J., Trilivas I., Christopoulou M. E., Drakouli S., Georgakopoulos C. D., Pharmakakis N. UVB-mediated down-regulation of proteasome in cultured human primary pterygium fibroblasts. BMC Ophthalmology. 2018;18(1):p. 328. doi: 10.1186/s12886-018-0987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Craig J. P., Nichols K. K., Akpek E. K., et al. TFOS DEWS II definition and classification report. The Ocular Surface. 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Jo E. J., Lee W. S., Jo H. Y., et al. Effects of particulate matter on respiratory disease and the impact of meteorological factors in Busan, Korea. Respiratory Medicine. 2017;124:79–87. doi: 10.1016/j.rmed.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 62.Janitz A. E., Campbell J. E., Magzamen S., Pate A., Stoner J. A., Peck J. D. Traffic-related air pollution and childhood acute leukemia in Oklahoma. Environmental Research. 2016;148:102–111. doi: 10.1016/j.envres.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sagai M. Toxic Components of PM2.5 and Their Toxicity Mechanisms—On the Toxicity of Sulfate and Carbon Components—. Nippon Eiseigaku Zasshi (Japanese Journal of Hygiene) 2019;74 doi: 10.1265/jjh.19004. [DOI] [PubMed] [Google Scholar]
- 64.Wang L., Xu J., Liu H., Li J., Hao H. PM2.5 inhibits SOD1 expression by up-regulating microRNA-206 and promotes ROS accumulation and disease progression in asthmatic mice. International Immunopharmacology. 2019;76:p. 105871. doi: 10.1016/j.intimp.2019.105871. [DOI] [PubMed] [Google Scholar]
- 65.Teson M., Lopez-Miguel A., Neves H., Calonge M., Gonzalez-Garcia M. J., Gonzalez-Meijome J. M. Influence of climate on clinical diagnostic dry eye tests: pilot study. Optometry and Vision Science. 2015;92(9):e284–e289. doi: 10.1097/OPX.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 66.Tan G., Li J., Yang Q., et al. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Scientific Reports. 2018;8(1, article 17828) doi: 10.1038/s41598-018-36181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mo Z., Fu Q., Lyu D., et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: a case-crossover study. Environmental Pollution. 2019;246:183–189. doi: 10.1016/j.envpol.2018.11.109. [DOI] [PubMed] [Google Scholar]
- 68.Gogia R., Richer S. P., Rose R. C. Tear fluid content of electrochemically active components including water soluble antioxidants. Current Eye Research. 1998;17(3):257–263. doi: 10.1076/ceyr.17.3.257.5213. [DOI] [PubMed] [Google Scholar]
- 69.Williams R. N., Paterson C. A. A protective role for ascorbic acid during inflammatory episodes in the eye. Experimental Eye Research. 1986;42(3):211–218. doi: 10.1016/0014-4835(86)90055-2. [DOI] [PubMed] [Google Scholar]
- 70.Yu D., Deng Q., Wang J., et al. Air pollutants are associated with dry eye disease in urban ophthalmic outpatients: a prevalence study in China. Journal of Translational Medicine. 2019;17(1):p. 46. doi: 10.1186/s12967-019-1794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cejkova J., Ardan T., Simonova Z., et al. Decreased expression of antioxidant enzymes in the conjunctival epithelium of dry eye (Sjögren's syndrome) and its possible contribution to the development of ocular surface oxidative injuries. Histology and Histopathology. 2008;23(12):1477–1483. doi: 10.14670/HH-23.1477. [DOI] [PubMed] [Google Scholar]
- 72.Choi W., Lian C., Ying L., et al. Expression of lipid peroxidation markers in the tear film and ocular surface of patients with non-Sjogren syndrome: potential biomarkers for dry eye disease. Current Eye Research. 2016;41(9):1143–1149. doi: 10.3109/02713683.2015.1098707. [DOI] [PubMed] [Google Scholar]
- 73.Hitoe S., Tanaka J., Shimoda H. MaquiBright standardized maqui berry extract significantly increases tear fluid production and ameliorates dry eye-related symptoms in a clinical pilot trial. Panminerva Medica. 2014;56(3, Supplement 1):1–6. [PubMed] [Google Scholar]
- 74.Kojima T., Dogru M., Higuchi A., et al. The Effect of Nrf2 Knockout on Ocular Surface Protection from Acute Tobacco Smoke Exposure : Evidence from. The American Journal of Pathology. 2015;185(3):776–785. doi: 10.1016/j.ajpath.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 75.Batliwala S., Xavier C., Liu Y., Wu H., Pang I. H. Involvement of Nrf2 in ocular diseases. Oxidative Medicine and Cellular Longevity. 2017;2017:18. doi: 10.1155/2017/1703810.1703810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitson J. A., Wilmarth P. A., Klimek J., Monnier V. M., David L., Fan X. Proteomic analysis of the glutathione-deficient LEGSKO mouse lens reveals activation of EMT signaling, loss of lens specific markers, and changes in stress response proteins. Free Radical Biology & Medicine. 2017;113:84–96. doi: 10.1016/j.freeradbiomed.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clark J. I. Self-assembly of protein aggregates in ageing disorders: the lens and cataract model. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368(1617, article 20120104) doi: 10.1098/rstb.2012.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lou M. F., Huang Q. L., Zigler JS Effect of opacification and pigmentation on human lens protein thiol/disulfide and solubility. Current Eye Research. 1989;8(9):883–890. [PubMed] [Google Scholar]
- 79.Vinson J. A. Oxidative stress in cataracts. Pathophysiology. 2006;13(3):151–162. doi: 10.1016/j.pathophys.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 80.Kandarakis S. A., Piperi C., Topouzis F., Papavassiliou A. G. Emerging role of advanced glycation-end products (AGEs) in the pathobiology of eye diseases. Progress in Retinal and Eye Research. 2014;42:85–102. doi: 10.1016/j.preteyeres.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Elanchezhian R., Palsamy P., Madson C. J., et al. Low glucose under hypoxic conditions induces unfolded protein response and produces reactive oxygen species in lens epithelial cells. Cell Death & Disease. 2012;3(4, article e301) doi: 10.1038/cddis.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao Y., Yan Y., Huang T. Human age-related cataracts: epigenetic suppression of the nuclear factor erythroid 2-related factor 2-mediated antioxidant system. Molecular Medicine Reports. 2015;11(2):1442–1447. doi: 10.3892/mmr.2014.2849. [DOI] [PubMed] [Google Scholar]
- 83.Periyasamy P., Shinohara T. Age-related cataracts: role of unfolded protein response, Ca2+ mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Progress in Retinal and Eye Research. 2017;60:1–19. doi: 10.1016/j.preteyeres.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palsamy P., Bidasee K. R., Ayaki M., Augusteyn R. C., Chan J. Y., Shinohara T. Methylglyoxal induces endoplasmic reticulum stress and DNA demethylation in the Keap1 promoter of human lens epithelial cells and age-related cataracts. Free Radical Biology & Medicine. 2014;72:134–148. doi: 10.1016/j.freeradbiomed.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chhunchha B., Kubo E., Singh D. P. Sulforaphane-induced Klf9/Prdx6 axis acts as a molecular switch to control redox signaling and determines fate of cells. Cell. 2019;8(10):p. 1159. doi: 10.3390/cells8101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma T. J., Lan D. H., He S. Z., et al. Nrf2 protects human lens epithelial cells against H2O2-induced oxidative and ER stress: the ATF4 may be involved. Experimental Eye Research. 2018;169:28–37. doi: 10.1016/j.exer.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 87.Liu H., Smith A. J. O., Lott M. C., et al. Sulforaphane can protect lens cells against oxidative stress: implications for cataract prevention. Investigative Ophthalmology & Visual Science. 2013;54(8):5236–5248. doi: 10.1167/iovs.13-11664. [DOI] [PubMed] [Google Scholar]
- 88.Zhang D., Li M. Puerarin prevents cataract development and progression in diabetic rats through Nrf2/HO‑1 signaling. Molecular Medicine Reports. 2019;20(2):1017–1024. doi: 10.3892/mmr.2019.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y., Luo W., Luo X., Yong Z., Zhong X. Effects of Rosa laevigata Michx. extract on reactive oxygen species production and mitochondrial membrane potential in lens epithelial cells cultured under high glucose. International Journal of Clinical and Experimental Medicine. 2015;8(9):15759–15765. [PMC free article] [PubMed] [Google Scholar]
- 90.Park J. Y., Han X., Piao M. J., et al. Hyperoside induces endogenous antioxidant system to alleviate oxidative stress. Journal of Cancer Prevention. 2016;21(1):41–47. doi: 10.15430/JCP.2016.21.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang S. P., Yang X. Z., Cao G. P. Acetyl-L-carnitine prevents homocysteine-induced suppression of Nrf2/Keap1 mediated antioxidation in human lens epithelial cells. Molecular Medicine Reports. 2015;12(1):1145–1150. doi: 10.3892/mmr.2015.3490. [DOI] [PubMed] [Google Scholar]
- 92.Park J. Y., Kang K. A., Kim K. C., Cha J. W., Kim E. H., Hyun J. W. Morin induces heme oxygenase-1 via ERK-Nrf2 signaling pathway. Journal of Cancer Prevention. 2013;18(3):249–256. doi: 10.15430/jcp.2013.18.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fang W., Ye Q., Yao Y., Xiu Y., Gu F., Zhu Y. Protective effects of trimetazidine in retarding selenite-induced lens opacification. Current Eye Research. 2019;44(12):1325–1336. doi: 10.1080/02713683.2019.1633359. [DOI] [PubMed] [Google Scholar]
- 94.Tsai C. F., Wu J. Y., Hsu Y. W. Protective effects of rosmarinic acid against selenite-induced cataract and oxidative damage in rats. International Journal of Medical Sciences. 2019;16(5):729–740. doi: 10.7150/ijms.32222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang F., Ma J., Han F., et al. DL-3-n-butylphthalide delays the onset and progression of diabetic cataract by inhibiting oxidative stress in rat diabetic model. Scientific Reports. 2016;6(1, article 19396) doi: 10.1038/srep19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun G. L., Huang D., Li K. R., Jiang Q. microRNA-4532 inhibition protects human lens epithelial cells from ultra- violet-induced oxidative injury via activating SIRT6-Nrf2 signaling. Biochemical and Biophysical Research Communications. 2019;514(3):777–784. doi: 10.1016/j.bbrc.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 97.Ferreira S. M., Lerner S. F. Á., Brunzini R., Evelson P. A., Llesuy S. F. Oxidative stress markers in aqueous humor of glaucoma patients. American Journal of Ophthalmology. 2004;137(1):62–69. doi: 10.1016/S0002-9394(03)00788-8. [DOI] [PubMed] [Google Scholar]
- 98.Grant W. M. Experimental aqueous perfusion in enucleated human eyes. Archives of Ophthalmology. 1963;69(6):783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- 99.Chrysostomou V., Rezania F., Trounce I. A., Crowston J. G. Oxidative stress and mitochondrial dysfunction in glaucoma. Current Opinion in Pharmacology. 2013;13(1):12–15. doi: 10.1016/j.coph.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 100.Stamer W. D., Clark A. F. The many faces of the trabecular meshwork cell. Experimental Eye Research. 2017;158:112–123. doi: 10.1016/j.exer.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yaz Y. A., Yıldırım N., Yaz Y., Tekin N., İnal M., Şahin F. M. Role of oxidative stress in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Turkish Journal of Ophthalmology. 2019;49(2):61–67. doi: 10.4274/tjo.galenos.2018.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gabelt B.’. A. T., Kaufman P. L. Changes in aqueous humor dynamics with age and glaucoma. Progress in Retinal and Eye Research. 2005;24(5):612–637. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 103.Babizhayev M. A., Yegorov Y. E. Senescent phenotype of trabecular meshwork cells displays biomarkers in primary open-angle glaucoma. Current Molecular Medicine. 2011;11(7):528–552. doi: 10.2174/156652411800615126. [DOI] [PubMed] [Google Scholar]
- 104.Cheng J., Liang J., Qi J. Role of nuclear factor (erythroid-derived 2)-like 2 in the age-resistant properties of the glaucoma trabecular meshwork. Experimental and Therapeutic Medicine. 2017;14(1):791–796. doi: 10.3892/etm.2017.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nita M., Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Medicine and Cellular Longevity. 2016;2016(12):23. doi: 10.1155/2016/3164734.3164734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang X., Hondur G., Li M., et al. Proteomics analysis of molecular risk factors in the ocular hypertensive human retina. Investigative Ophthalmology & Visual Science. 2015;56(10):5816–5830. doi: 10.1167/iovs.15-17294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu Z., Cho H., Hartsock M. J., et al. Neuroprotective role of Nrf2 for retinal ganglion cells in ischemia-reperfusion. Journal of Neurochemistry. 2015;133(2):233–241. doi: 10.1111/jnc.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lam T. T., Abler A. S., Tso M. O. Apoptosis and caspases after ischemia-reperfusion injury in rat retina. Investigative Ophthalmology & Visual Science. 1999;40(5):967–975. [PubMed] [Google Scholar]
- 109.Xiong W., MacColl Garfinkel A. E., Li Y., Benowitz L. I., Cepko C. L. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. Journal of Clinical Investigation. 2015;125(4):1433–1445. doi: 10.1172/jci79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eren K., Turgut B., Akin M. M., Demir T. The suppression of wound healing response with sirolimus and sunitinib following experimental trabeculectomy in a rabbit model. Current Eye Research. 2015;41(3):1–10. doi: 10.3109/02713683.2015.1023460. [DOI] [PubMed] [Google Scholar]
- 111.Liu Y., Kimura K., Orita T., Teranishi S., Suzuki K., Sonoda K.-H. Inhibition by all-trans-retinoic acid of transforming growth factor-β-induced collagen gel contraction mediated by human tenon fibroblasts. Investigative Opthalmology & Visual Science. 2014;55(7):4199–4205. doi: 10.1167/iovs.13-13572. [DOI] [PubMed] [Google Scholar]
- 112.Albright C. D., Salganik R. I., Craciunescu C. N., Mar M. H., Zeisel S. H. Mitochondrial and microsomal derived reactive oxygen species mediate apoptosis induced by transforming growth factor-β1 in immortalized rat hepatocytes. Journal of Cellular Biochemistry. 2003;89(2):254–261. doi: 10.1002/jcb.10498. [DOI] [PubMed] [Google Scholar]
- 113.Bracey N. A., Gershkovich B., Chun J., et al. Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome. Journal of Biological Chemistry. 2014;289(28):19571–19584. doi: 10.1074/jbc.M114.550624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu R.-M., Desai L. P. Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biology. 2015;6:565–577. doi: 10.1016/j.redox.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roderburg C., Urban G.-W., Bettermann K., et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53(1):209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 116.Cushing L., Kuang P. P., Qian J., et al. miR-29 is a major regulator of genes associated with pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2011;45(2):287–294. doi: 10.1165/rcmb.2010-0323oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ran W., Zhu D., Feng Q. TGF-β2 stimulates Tenon’s capsule fibroblast proliferation in patients with glaucoma via suppression of miR-29b expression regulated by Nrf2. International Journal of Clinical and Experimental Pathology. 2015;8(5):4799–4806. [PMC free article] [PubMed] [Google Scholar]
- 118.Li B., Cui W., Liu J., et al. Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Experimental Neurology. 2013;250:239–249. doi: 10.1016/j.expneurol.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 119.Kobayashi E. H., Suzuki T., Funayama R., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nature Communications. 2016;7(1, article 11624) doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Al-Huseini L. M. A., Yeang H. X. A., Sethu S., et al. Nuclear factor-erythroid 2 (NF-E2) p45-related factor-2 (Nrf2) modulates dendritic cell immune function through regulation of p38 MAPK-cAMP-responsive element binding protein/activating transcription factor 1 signaling. The Journal of Biological Chemistry. 2013;288(31):22281–22288. doi: 10.1074/jbc.M113.483420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nagai N., Thimmulappa R. K., Cano M., et al. Nrf2 is a critical modulator of the innate immune response in a model of uveitis. Free Radical Biology & Medicine. 2009;47(3):300–306. doi: 10.1016/j.freeradbiomed.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen X., Su W., Wan T., et al. Sodium butyrate regulates Th17/Treg cell balance to ameliorate uveitis via the Nrf2/HO-1 pathway. Biochemical Pharmacology. 2017;142:111–119. doi: 10.1016/j.bcp.2017.06.136. [DOI] [PubMed] [Google Scholar]
- 123.Glickman R. D. Ultraviolet phototoxicity to the retina. Eye & Contact Lens. 2011;37(4):196–205. doi: 10.1097/ICL.0b013e31821e45a9. [DOI] [PubMed] [Google Scholar]
- 124.Burgos-Morón E., Abad-Jiménez Z., Marañón A. M., et al. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. Journal of Clinical Medicine. 2019;8(9):p. 1385. doi: 10.3390/jcm8091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Antonetti D. A., Barber A. J., Bronson S. K., et al. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55(9):2401–2411. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 126.Jindal V. Neurodegeneration as a primary change and role of neuroprotection in diabetic retinopathy. Molecular Neurobiology. 2015;51(3):878–884. doi: 10.1007/s12035-014-8732-7. [DOI] [PubMed] [Google Scholar]
- 127.Xu Z., Wei Y., Gong J., et al. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia. 2014;57(1):204–213. doi: 10.1007/s00125-013-3093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Negi G., Kumar A., Joshi R. P., Sharma S. S. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: old perspective with a new angle. Biochemical and Biophysical Research Communications. 2011;408(1):1–5. doi: 10.1016/j.bbrc.2011.03.087. [DOI] [PubMed] [Google Scholar]
- 129.Zhong Q., Mishra M., Kowluru R. A. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Investigative Ophthalmology & Visual Science. 2013;54(6):3941–3948. doi: 10.1167/iovs.13-11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang J., He J. CTRP3 inhibits high glucose-induced oxidative stress and apoptosis in retinal pigment epithelial cells. Artificial Cells, Nanomedicine, and Biotechnology. 2019;47(1):3758–3764. doi: 10.1080/21691401.2019.1666864. [DOI] [PubMed] [Google Scholar]
- 131.Zhang L., Yu J., Ye M., Zhao H. Upregulation of CKIP-1 inhibits high-glucose induced inflammation and oxidative stress in HRECs and attenuates diabetic retinopathy by modulating Nrf2/ARE signaling pathway: an in vitro study. Cell & Bioscience. 2019;9(1):p. 67. doi: 10.1186/s13578-019-0331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou Z., Liu J., Bi C., Chen L., Jiao Y., Cui L. Knockdown of FOXO6 inhibits high glucose-induced oxidative stress and apoptosis in retinal pigment epithelial cells. Journal of Cellular Biochemistry. 2019;120(6):9716–9723. doi: 10.1002/jcb.28252. [DOI] [PubMed] [Google Scholar]
- 133.Nakagami Y., Hatano E., Inoue T., Yoshida K., Kondo M., Terasaki H. Cytoprotective effects of a novel Nrf2 activator, RS9, in rhodopsin Pro347Leu rabbits. Current Eye Research. 2016;41(8):1123–1126. doi: 10.3109/02713683.2015.1078362. [DOI] [PubMed] [Google Scholar]
- 134.Deliyanti D., Alrashdi S. F., Tan S. M., et al. Nrf2 activation is a potential therapeutic approach to attenuate diabetic retinopathy. Investigative Ophthalmology & Visual Science. 2018;59(2):815–825. doi: 10.1167/iovs.17-22920. [DOI] [PubMed] [Google Scholar]
- 135.Bucolo C., Drago F., Maisto R., et al. Curcumin prevents high glucose damage in retinal pigment epithelial cells through ERK1/2-mediated activation of the Nrf2/HO-1 pathway. Journal of Cellular Physiology. 2019;234(10):17295–17304. doi: 10.1002/jcp.28347. [DOI] [PubMed] [Google Scholar]
- 136.Li S., Yang H., Chen X. Protective effects of sulforaphane on diabetic retinopathy: activation of the Nrf2 pathway and inhibition of NLRP3 inflammasome formation. Experimental Animals. 2019;68(2):221–231. doi: 10.1538/expanim.18-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Warwick A., Lotery A. Genetics and genetic testing for age-related macular degeneration. Eye. 2018;32(5):849–857. doi: 10.1038/eye.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sobrin L., Seddon J. M. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Progress in Retinal and Eye Research. 2014;40:1–15. doi: 10.1016/j.preteyeres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hyttinen J. M. T., Viiri J., Kaarniranta K., Blasiak J. Mitochondrial quality control in AMD: does mitophagy play a pivotal role? Cellular and Molecular Life Sciences. 2018;75(16):2991–3008. doi: 10.1007/s00018-018-2843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Golestaneh N., Chu Y., Cheng S. K., Cao H., Poliakov E., Berinstein D. M. Repressed SIRT1/PGC-1α pathway and mitochondrial disintegration in iPSC-derived RPE disease model of age-related macular degeneration. Journal of Translational Medicine. 2016;14(1):p. 344. doi: 10.1186/s12967-016-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Golestaneh N., Chu Y., Xiao Y. Y., Stoleru G. L., Theos A. C. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death & Disease. 2017;8(1, article e2537) doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.McCord J. M., Edeas M. A. SOD, oxidative stress and human pathologies: a brief history and a future vision. Biomedicine & Pharmacotherapy. 2005;59(4):139–142. doi: 10.1016/j.biopha.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 143.Klettner A. Oxidative stress induced cellular signaling in RPE cells. Frontiers in Bioscience. 2012;S4(2):392–411. doi: 10.2741/s275. [DOI] [PubMed] [Google Scholar]
- 144.Karunadharma P. P., Nordgaard C. L., Olsen T. W., Ferrington D. A. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Investigative Ophthalmology & Visual Science. 2010;51(11):5470–5479. doi: 10.1167/iovs.10-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nagar S., Noveral S. M., Trudler D., et al. MEF2D haploinsufficiency downregulates the NRF2 pathway and renders photoreceptors susceptible to light-induced oxidative stress. Proceedings of the National Academy of Sciences. 2017;114(20):E4048–E4056. doi: 10.1073/pnas.1613067114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen W. J., Wu C., Xu Z., Kuse Y., Hara H., Duh E. J. Nrf2 protects photoreceptor cells from photo-oxidative stress induced by blue light. Experimental Eye Research. 2017;154:151–158. doi: 10.1016/j.exer.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang K., Zheng M., Lester K. L., Han Z. Light-induced Nrf2−/− mice as atrophic age-related macular degeneration model and treatment with nanoceria laden injectable hydrogel. Scientific Reports. 2019;9(1):p. 14573. doi: 10.1038/s41598-019-51151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bunker C. H., Berson E. L., Bromley W. C., Hayes R. P., Roderick T. H. Prevalence of retinitis pigmentosa in Maine. American Journal of Ophthalmology. 1984;97(3):357–365. doi: 10.1016/0002-9394(84)90636-6. [DOI] [PubMed] [Google Scholar]
- 149.Grondahl J. Estimation of prognosis and prevalence of retinitis pigmentosa and Usher syndrome in Norway. Clinical Genetics. 1987;31(4):255–264. doi: 10.1111/j.1399-0004.1987.tb02804.x. [DOI] [PubMed] [Google Scholar]
- 150.Yu D. Y., Cringle S., Valter K., Walsh N., Lee D., Stone J. Photoreceptor death, trophic factor expression, retinal oxygen status, and photoreceptor function in the P23H rat. Investigative Ophthalmology & Visual Science. 2004;45(6):2013–2019. doi: 10.1167/iovs.03-0845. [DOI] [PubMed] [Google Scholar]
- 151.Shen J., Yang X., Dong A., et al. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. Journal of Cellular Physiology. 2005;203(3):457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- 152.de la Cámara C. M.-F., Salom D., Sequedo M. D., et al. Altered antioxidant-oxidant status in the aqueous humor and peripheral blood of patients with retinitis pigmentosa. PLoS One. 2013;8(9, article e74223) doi: 10.1371/journal.pone.0074223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mochizuki H., Murphy C. J., Brandt J. D., Kiuchi Y., Russell P. Altered stability of mRNAs associated with glaucoma progression in human trabecular meshwork cells following oxidative stress. Investigative Ophthalmology & Visual Science. 2012;53(4):1734–1741. doi: 10.1167/iovs.12-7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Usui S., Komeima K., Lee S. Y., et al. Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Molecular Therapy. 2009;17(5):778–786. doi: 10.1038/mt.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang J., Saul A., Cui X., Roon P., Smith S. B. Absence of Sigma 1 receptor accelerates photoreceptor cell death in a murine model of retinitis pigmentosa. Investigative Ophthalmology & Visual Science. 2017;58(11):4545–4558. doi: 10.1167/iovs.17-21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang J., Zhao J., Cui X., et al. The molecular chaperone Sigma 1 receptor mediates rescue of retinal cone photoreceptor cells via modulation of NRF2. Free Radical Biology & Medicine. 2019;134:604–616. doi: 10.1016/j.freeradbiomed.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kang K., Yu M. Protective effect of sulforaphane against retinal degeneration in thePde6rd10mouse model of retinitis pigmentosa. Current Eye Research. 2017;42(12):1684–1688. doi: 10.1080/02713683.2017.1358371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gilgun-Sherki Y., Melamed E., Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. Journal of Neurology. 2004;251(3):261–268. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 159.Guy J., Qi X., Hauswirth W. W. Adeno-associated viral-mediated catalase expression suppresses optic neuritis in experimental allergic encephalomyelitis. Proceedings of the National Academy of Sciences. 1998;95(23):13847–13852. doi: 10.1073/pnas.95.23.13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Qi X., Lewin A. S., Sun L., Hauswirth W. W., Guy J. Suppression of mitochondrial oxidative stress provides long-term neuroprotection in experimental optic neuritis. Investigative Ophthalmology & Visual Science. 2007;48(2):681–691. doi: 10.1167/iovs.06-0553. [DOI] [PubMed] [Google Scholar]
- 161.McDougald D. S., Dine K. E., Zezulin A. U., Bennett J., Shindler K. S. SIRT1 and NRF2 gene transfer mediate distinct neuroprotective effects upon retinal ganglion cell survival and function in experimental optic neuritis. Investigative Ophthalmology & Visual Science. 2018;59(3):1212–1220. doi: 10.1167/iovs.17-22972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Narapureddy B., Dubey D. Clinical evaluation of dimethyl fumarate for the treatment of relapsing-remitting multiple sclerosis: efficacy, safety, patient experience and adherence. Patient Preference and Adherence. 2019;13:1655–1666. doi: 10.2147/PPA.S187529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ahuja M., Ammal Kaidery N., Yang L., et al. Distinct Nrf2 signaling mechanisms of fumaric acid esters and their role in neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced experimental Parkinson’s-like disease. The Journal of Neuroscience. 2016;36(23):6332–6351. doi: 10.1523/JNEUROSCI.0426-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Paraiso H. C., Kuo P. C., Curfman E. T., et al. Dimethyl fumarate attenuates reactive microglia and long-term memory deficits following systemic immune challenge. Journal of Neuroinflammation. 2018;15(1):p. 100. doi: 10.1186/s12974-018-1125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zyla K., Larabee C. M., Georgescu C., Berkley C., Reyna T., Plafker S. M. Dimethyl fumarate mitigates optic neuritis. Molecular Vision. 2019;25:446–461. [PMC free article] [PubMed] [Google Scholar]
- 166.Wawrzyniak O., Zarebska Z., Rolle K., Gotz-Wieckowska A. Circular and long non-coding RNAs and their role in ophthalmologic diseases. Acta Biochimica Polonica. 2018;65(4):497–508. doi: 10.18388/abp.2018_2639. [DOI] [PubMed] [Google Scholar]
- 167.Ulitsky I., Bartel D. . P. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Itoh K., Wakabayashi N., Katoh Y., et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes & Development. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shan Y., Lambrecht R. W., Donohue S. E., Bonkovsky H. L. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. The FASEB Journal. 2006;20(14):2651–2653. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- 170.Levy S., Forman H. J. C-Myc is a Nrf2-interacting protein that negatively regulates phase II genes through their electrophile responsive elements. IUBMB Life. 2010;62(3):237–246. doi: 10.1002/iub.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Prochaska H. J., Bregman H. S., de Long M. J., Talalay P. Specificity of induction of cancer protective enzymes by analogues of tert- butyl-4-hydroxyanisole (BHA) Biochemical Pharmacology. 1985;34(21):3909–3914. doi: 10.1016/0006-2952(85)90443-5. [DOI] [PubMed] [Google Scholar]
- 172.Tanigawa S., Fujii M., Hou D. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radical Biology & Medicine. 2007;42(11):1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 173.Kode A., Rajendrasozhan S., Caito S., Yang S.-R., Megson I. L., Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008;294(3):L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 174.Kim J.-H., Nam S.-W., Kim B.-W., et al. Astaxanthin improves stem cell potency via an increase in the proliferation of neural progenitor cells. International Journal of Molecular Sciences. 2010;11(12):5109–5119. doi: 10.3390/ijms11125109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lei X., Lei L., Zhang Z., Cheng Y. Neuroprotective effects of lycopene pretreatment on transient global cerebral ischemia-reperfusion in rats: the role of the Nrf2/HO1 signaling pathway. Molecular Medicine Reports. 2016;13(1):412–418. doi: 10.3892/mmr.2015.4534. [DOI] [PubMed] [Google Scholar]
- 176.Rojo de la Vega M., Chapman E., Zhang D. D. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34(1):21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nioi P., Nguyen T. A mutation of Keap1 found in breast cancer impairs its ability to repress Nrf2 activity. Biochemical and Biophysical Research Communications. 2007;362(4):816–821. doi: 10.1016/j.bbrc.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 178.Solis L. M., Behrens C., Dong W., et al. Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clinical Cancer Research. 2010;16(14):3743–3753. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zucker S. N., Fink E. E., Bagati A., et al. Nrf2 amplifies oxidative stress via induction of Klf9. Molecular Cell. 2014;53(6):916–928. doi: 10.1016/j.molcel.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rojo A. I., Rada P., Mendiola M., et al. The PTEN/NRF2 axis promotes human carcinogenesis. Antioxidants & Redox Signaling. 2014;21(18):2498–2514. doi: 10.1089/ars.2014.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhao J., Du X., Wang M., Yang P., Zhang J. Salidroside mitigates hydrogen peroxide-induced injury by enhancement of microRNA-27a in human trabecular meshwork cells. Artificial Cells, Nanomedicine, and Biotechnology. 2019;47(1):1758–1765. doi: 10.1080/21691401.2019.1608222. [DOI] [PubMed] [Google Scholar]
- 182.Wang Y., Li F., Wang S. MicroRNA93 is overexpressed and induces apoptosis in glaucoma trabecular meshwork cells. Molecular Medicine Reports. 2016;14(6):5746–5750. doi: 10.3892/mmr.2016.5938. [DOI] [PubMed] [Google Scholar]
- 183.Cheng L.-B., Li K.-r., Yi N., et al. miRNA-141 attenuates UV-induced oxidative stress via activating Keap1-Nrf2 signaling in human retinal pigment epithelium cells and retinal ganglion cells. Oncotarget. 2017;8(8, article 13186) doi: 10.18632/oncotarget.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jadeja R. N., Jones M. A., Abdelrahman A. A., et al. Inhibiting microRNA-144 potentiates Nrf2-dependent antioxidant signaling in RPE and protects against oxidative stress-induced outer retinal degeneration. Redox Biology. 2020;28, article 101336 doi: 10.1016/j.redox.2019.101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xu X. Z., Tang Y., Cheng L. B., et al. Targeting Keap1 by miR-626 protects retinal pigment epithelium cells from oxidative injury by activating Nrf2 signaling. Free Radical Biology & Medicine. 2019;143:387–396. doi: 10.1016/j.freeradbiomed.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 186.Chen Z. J., Rong L., Huang D., Jiang Q. Targeting cullin 3 by miR-601 activates Nrf2 signaling to protect retinal pigment epithelium cells from hydrogen peroxide. Biochemical and Biophysical Research Communications. 2019;515(4):679–687. doi: 10.1016/j.bbrc.2019.05.171. [DOI] [PubMed] [Google Scholar]
- 187.Wang Y., Wang J., Wei L.-J., Zhu D.-M., Zhang J.-S. Biological function and mechanism of lncRNA-MEG3 in Tenon's capsule fibroblasts proliferation: By MEG3-Nrf2 protein interaction. Biomedicine & Pharmacotherapy. 2017;87:548–554. doi: 10.1016/j.biopha.2016.12.040. [DOI] [PubMed] [Google Scholar]
- 188.Li C. P., Wang S. H., Wang W. Q., Song S. G., Liu X. M. Long noncoding RNA-Sox2OT knockdown alleviates diabetes mellitus-induced retinal ganglion cell (RGC) injury. Cellular and Molecular Neurobiology. 2017;37(2):361–369. doi: 10.1007/s10571-016-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhou T., Zong R., Zhang Z., et al. SERPINA3K protects against oxidative stress via modulating ROS generation/degradation and KEAP1-NRF2 pathway in the corneal epithelium. Investigative Ophthalmology & Visual Science. 2012;53(8):5033–5043. doi: 10.1167/iovs.12-9729. [DOI] [PubMed] [Google Scholar]
- 190.Zhu C., Pan F., Ge L., et al. SERPINA3K plays antioxidant roles in cultured pterygial epithelial cells through regulating ROS system. PLoS One. 2014;9(10, article e108859) doi: 10.1371/journal.pone.0108859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen Y. Y., Tsai C. F., Tsai M. C., Hsu Y. W., Lu F. J. Inhibitory effects of rosmarinic acid on pterygium epithelial cells through redox imbalance and induction of extrinsic and intrinsic apoptosis. Experimental Eye Research. 2017;160:96–105. doi: 10.1016/j.exer.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 192.Lee H., Lee M., Lee Y., Choi S., Yang J. Chondrocyte-derived extracellular matrix suppresses pathogenesis of human pterygium epithelial cells by blocking the NF-κB signaling pathways. Molecular Vision. 2016;22:1490–1502. [PMC free article] [PubMed] [Google Scholar]
- 193.Chen L., Zong R., Zhou J., et al. The oxidant role of 4-hydroxynonenal in corneal epithelium. Scientific Reports. 2015;5(1, article 10630) doi: 10.1038/srep10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen J., Wang L., Chen Y., Sternberg P., Cai J. Phosphatidylinositol 3 kinase pathway and 4-hydroxy-2-nonenal-induced oxidative injury in the RPE. Investigative Ophthalmology & Visual Science. 2009;50(2):936–942. doi: 10.1167/iovs.08-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang L., Qu M., Wang Y., et al. Trichostatin A inhibits transforming growth Factor-β–Induced reactive oxygen species accumulation and myofibroblast differentiation via enhanced NF-E2-related factor 2-antioxidant response element signaling. Molecular Pharmacology. 2013;83(3):671–680. doi: 10.1124/mol.112.081059. [DOI] [PubMed] [Google Scholar]
- 196.Wang K., Zhu X., Zhang K., et al. Puerarin inhibits amyloid β-induced NLRP3 inflammasome activation in retinal pigment epithelial cells via suppressing ROS-dependent oxidative and endoplasmic reticulum stresses. Experimental Cell Research. 2017;357(2):335–340. doi: 10.1016/j.yexcr.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 197.Sun J., Wang B., Hao Y., Yang X. Effects of calcium dobesilate on Nrf2, Keap1 and HO-1 in the lenses of D-galactose-induced cataracts in rats. Experimental and Therapeutic Medicine. 2018;15(1):719–722. doi: 10.3892/etm.2017.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Miyamoto N., Izumi H., Miyamoto R., et al. Quercetin induces the expression of peroxiredoxins 3 and 5 via the Nrf2/NRF1 transcription pathway. Investigative Ophthalmology & Visual Science. 2011;52(2, article 1055) doi: 10.1167/iovs.10-5777. [DOI] [PubMed] [Google Scholar]
- 199.Weng S., Mao L., Gong Y., Sun T., Gu Q. Role of quercetin in protecting ARPE19 cells against H2O2-induced injury via nuclear factor erythroid 2 like 2 pathway activation and endoplasmic reticulum stress inhibition. Molecular Medicine Reports. 2017;16(3):3461–3468. doi: 10.3892/mmr.2017.6964. [DOI] [PubMed] [Google Scholar]
- 200.Shivarudrappa A. H., Ponesakki G. Lutein reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases in retinal pigment epithelial (ARPE-19) cells. Journal of Cell Communication and Signaling. 2019 doi: 10.1007/s12079-019-00539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Deliyanti D., Lee J. Y., Petratos S., et al. A potent Nrf2 activator, dh404, bolsters antioxidant capacity in glial cells and attenuates ischaemic retinopathy. Clinical Science. 2016;130(15):1375–1387. doi: 10.1042/CS20160068. [DOI] [PubMed] [Google Scholar]
- 202.Arumugam B., Palanisamy U. D., Chua K. H., Kuppusamy U. R. Protective effect of myricetin derivatives from Syzygium malaccense against hydrogen peroxide-induced stress in ARPE-19 cells. Molecular Vision. 2019;25:47–59. [PMC free article] [PubMed] [Google Scholar]
- 203.Liu Q., Zhang F., Zhang X., et al. Fenofibrate ameliorates diabetic retinopathy by modulating Nrf2 signaling and NLRP3 inflammasome activation. Molecular and Cellular Biochemistry. 2018;445(1-2):105–115. doi: 10.1007/s11010-017-3256-x. [DOI] [PubMed] [Google Scholar]
- 204.Zhou X., Ai S., Chen Z., Li C. Probucol promotes high glucose-induced proliferation and inhibits apoptosis by reducing reactive oxygen species generation in Müller cells. International Ophthalmology. 2019;39(12):2833–2842. doi: 10.1007/s10792-019-01130-8. [DOI] [PubMed] [Google Scholar]
- 205.Navneet S., Cui X., Zhao J., et al. Excess homocysteine upregulates the NRF2-antioxidant pathway in retinal Muller glial cells. Experimental Eye Research. 2019;178:228–237. doi: 10.1016/j.exer.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tan S. M., Deliyanti D., Figgett W. A., Talia D. M., de Haan J. B., Wilkinson-Berka J. L. Ebselen by modulating oxidative stress improves hypoxia-induced macroglial Muller cell and vascular injury in the retina. Experimental Eye Research. 2015;136:1–8. doi: 10.1016/j.exer.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 207.Xing X., Huang L., Lv Y., et al. DL-3-n-butylphthalide protected retinal Müller cells dysfunction from oxidative stress. Current Eye Research. 2019;44(10):1112–1120. doi: 10.1080/02713683.2019.1624777. [DOI] [PubMed] [Google Scholar]
- 208.Li K.-r., Yang S.-q., Gong Y.-q., et al. 3H-1,2-Dithiole-3-thione protects retinal pigment epithelium cells against ultra-violet radiation via activation of Akt-mTORC1-dependent Nrf2-HO-1 signaling. Scientific Reports. 2016;6(1, article 25525) doi: 10.1038/srep25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.He H., Wei D., Liu H., et al. Glycyrrhizin protects against sodium iodate-induced RPE and retinal injury though activation of AKT and Nrf2/HO-1 pathway. Journal of Cellular and Molecular Medicine. 2019;23(5):3495–3504. doi: 10.1111/jcmm.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang K., Jiang Y., Wang W., Ma J., Chen M. Escin activates AKT-Nrf2 signaling to protect retinal pigment epithelium cells from oxidative stress. Biochemical and Biophysical Research Communications. 2015;468(4):541–547. doi: 10.1016/j.bbrc.2015.10.117. [DOI] [PubMed] [Google Scholar]
- 211.Tang C. Z., Li K. R., Yu Q., Jiang Q., Yao J., Cao C. Activation of Nrf2 by ginsenoside Rh3 protects retinal pigment epithelium cells and retinal ganglion cells from UV. Free Radical Biology & Medicine. 2018;117:238–246. doi: 10.1016/j.freeradbiomed.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 212.Zhu C., Dong Y., Liu H., Ren H., Cui Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomedicine & Pharmacotherapy. 2017;88:124–133. doi: 10.1016/j.biopha.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 213.Yang P. M., Wu Z. Z., Zhang Y. Q., Wung B. S. Lycopene inhibits ICAM-1 expression and NF-κB activation by Nrf2-regulated cell redox state in human retinal pigment epithelial cells. Life Sciences. 2016;155:94–101. doi: 10.1016/j.lfs.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 214.Hanus J., Kolkin A., Chimienti J., Botsay S., Wang S. 4-Acetoxyphenol prevents RPE oxidative stress-induced necrosis by functioning as an NRF2 stabilizer. Investigative Ophthalmology & Visual Science. 2015;56(9):5048–5059. doi: 10.1167/iovs.15-16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xie X., Feng J., Kang Z., et al. Taxifolin protects RPE cells against oxidative stress-induced apoptosis. Molecular Vision. 2017;23:520–528. [PMC free article] [PubMed] [Google Scholar]
- 216.Zhao H., Wang R., Ye M., Zhang L. Genipin protects against H2O2-induced oxidative damage in retinal pigment epithelial cells by promoting Nrf2 signaling. International Journal of Molecular Medicine. 2019;43(2):936–944. doi: 10.3892/ijmm.2018.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Feng Z., Liu Z., Li X., et al. α-Tocopherol is an effective Phase II enzyme inducer: protective effects on acrolein-induced oxidative stress and mitochondrial dysfunction in human retinal pigment epithelial cells. The Journal of Nutritional Biochemistry. 2010;21(12):1222–1231. doi: 10.1016/j.jnutbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 218.Li Z., Dong X., Liu H., et al. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Molecular Vision. 2013;19:1656–1666. [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang H., Liu Y. Y., Jiang Q., et al. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radical Biology & Medicine. 2014;69:219–228. doi: 10.1016/j.freeradbiomed.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 220.Liu X., Xavier C., Jann J., Wu H. Salvianolic acid B (Sal B) protects retinal pigment epithelial cells from oxidative stress-induced cell death by activating glutaredoxin 1 (Grx1) International Journal of Molecular Sciences. 2016;17(11, article 1835) doi: 10.3390/ijms17111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhao L., Liu Z., Jia H., Feng Z., Liu J., Li X. Lipoamide acts as an indirect antioxidant by simultaneously stimulating mitochondrial biogenesis and phase II antioxidant enzyme systems in ARPE-19 cells. PLoS One. 2015;10(6, article e0128502) doi: 10.1371/journal.pone.0128502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hu X., Liang Y., Zhao B., Wang Y. Thymoquinone protects human retinal pigment epithelial cells against hydrogen peroxide induced oxidative stress and apoptosis. Journal of Cellular Biochemistry. 2019;120(3):4514–4522. doi: 10.1002/jcb.27739. [DOI] [PubMed] [Google Scholar]
- 223.Song Y., Huang L., Yu J. Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. Journal of Neuroimmunology. 2016;301:1–6. doi: 10.1016/j.jneuroim.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 224.Sun Y., Xiu C., Liu W., Tao Y., Wang J., Qu Y. Grape seed proanthocyanidin extract protects the retina against early diabetic injury by activating the Nrf2 pathway. Experimental and Therapeutic Medicine. 2016;11(4):1253–1258. doi: 10.3892/etm.2016.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Xu X., Cai Y., Yu Y. Molecular mechanism of the role of carbamyl erythropoietin in treating diabetic retinopathy rats. Experimental and Therapeutic Medicine. 2018;16(1):305–309. doi: 10.3892/etm.2018.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dong C., Liu P., Wang H., Dong M., Li G., Li Y. Ginsenoside Rb1 attenuates diabetic retinopathy in streptozotocin-induced diabetic rats1. Acta Cirúrgica Brasileira. 2019;34(2, article e201900201) doi: 10.1590/s0102-8650201900201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ren X., Sun H., Zhang C., et al. Protective function of pyridoxamine on retinal photoreceptor cells via activation of the p-Erk1/2/Nrf2/Trx/ASK1 signalling pathway in diabetic mice. Molecular Medicine Reports. 2016;14(1):420–424. doi: 10.3892/mmr.2016.5270. [DOI] [PubMed] [Google Scholar]
- 228.Mei X., Zhang T., Ouyang H., Lu B., Wang Z., Ji L. Scutellarin alleviates blood-retina-barrier oxidative stress injury initiated by activated microglia cells during the development of diabetic retinopathy. Biochemical Pharmacology. 2019;159:82–95. doi: 10.1016/j.bcp.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 229.Ni T., Yang W., Xing Y. Protective effects of delphinidin against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. Bioscience Reports. 2019;39(8) doi: 10.1042/BSR20190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Linetsky M., Bondelid K. S., Losovskiy S., et al. 4-Hydroxy-7-oxo-5-heptenoic acid lactone is a potent inducer of the complement pathway in human retinal pigmented epithelial cells. Chemical Research in Toxicology. 2018;31(8):666–679. doi: 10.1021/acs.chemrestox.8b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jiang T., Chang Q., Cai J., Fan J., Zhang X., Xu G. Protective effects of melatonin on retinal inflammation and oxidative stress in experimental diabetic retinopathy. Oxidative Medicine and Cellular Longevity. 2016;2016:13. doi: 10.1155/2016/3528274.3528274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang T., Mei X., Ouyang H., et al. Natural flavonoid galangin alleviates microglia-trigged blood-retinal barrier dysfunction during the development of diabetic retinopathy. The Journal of Nutritional Biochemistry. 2019;65:1–14. doi: 10.1016/j.jnutbio.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 233.Yoshinaga N., Arimura N., Otsuka H., et al. NSAIDs inhibit neovascularization of choroid through HO-1-dependent pathway. Laboratory Investigation. 2011;91(9):1277–1290. doi: 10.1038/labinvest.2011.101. [DOI] [PubMed] [Google Scholar]
- 234.Nakamura S., Noguchi T., Inoue Y., et al. Nrf2 activator RS9 suppresses pathological ocular angiogenesis and hyperpermeability. Investigative Ophthalmology & Visual Science. 2019;60(6):1943–1952. doi: 10.1167/iovs.18-25745. [DOI] [PubMed] [Google Scholar]
- 235.Inoue Y., Shimazawa M., Noda Y., et al. RS9, a novel Nrf2 activator, attenuates light-induced death of cells of photoreceptor cells and Müller glia cells. Journal of Neurochemistry. 2017;141(5):750–765. doi: 10.1111/jnc.14029. [DOI] [PubMed] [Google Scholar]
- 236.He M., Long P., Yan W., et al. ALDH2 attenuates early-stage STZ-induced aged diabetic rats retinas damage _via_ Sirt1/Nrf2 pathway. Life Sciences. 2018;215:227–235. doi: 10.1016/j.lfs.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 237.Koriyama Y., Nakayama Y., Matsugo S., Kato S. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2-dependent heme oxygenase-1 induction in the RGC-5 cellline. Brain Research. 2013;1499(4):145–157. doi: 10.1016/j.brainres.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 238.Inman D. M., Lambert W. S., Calkins D. J., Horner P. J. α-Lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PLoS One. 2013;8(6, article e65389) doi: 10.1371/journal.pone.0065389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Varma S. D., Chandrasekaran K., Kovtun S. Sulforaphane-induced transcription of thioredoxin reductase in lens: possible significance against cataract formation. Clinical Ophthalmology. 2013;7:2091–2098. doi: 10.2147/OPTH.S52678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Liu Y., Liu P., Wang Q., Sun F., Liu F. Sulforaphane attenuates H2O2-induced oxidant stress in human trabecular meshwork cells (HTMCs) via the phosphatidylinositol 3-kinase (PI3K)/serine/threonine kinase (Akt)-mediated factor-E2-related factor 2 (Nrf2) signaling activation. Medical Science Monitor. 2019;25:811–818. doi: 10.12659/MSM.913849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pan H., He M., Liu R., Brecha N. C., Yu A. C. H., Pu M. Sulforaphane protects rodent retinas against ischemia-reperfusion injury through the activation of the Nrf2/HO-1 antioxidant pathway. PLoS One. 2014;9(12, article e114186) doi: 10.1371/journal.pone.0114186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu X., Ward K., Xavier C., et al. The novel triterpenoid RTA 408 protects human retinal pigment epithelial cells against H2O2-induced cell injury via NF-E2-related factor 2 (Nrf2) activation. Redox Biology. 2016;8:98–109. doi: 10.1016/j.redox.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kapupara K., Wen Y. T., Tsai R. K., Huang S. P. Soluble P-selectin promotes retinal ganglion cell survival through activation of Nrf2 signaling after ischemia injury. Cell Death & Disease. 2017;8(11, article e3172) doi: 10.1038/cddis.2017.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lv P., Yu J., Xu X., Lu T., Xu F. Eriodictyol inhibits high glucose-induced oxidative stress and inflammation in retinal ganglial cells. Journal of Cellular Biochemistry. 2019;120(4):5644–5651. doi: 10.1002/jcb.27848. [DOI] [PubMed] [Google Scholar]
- 245.Johnson J., Maher P., Hanneken A. The flavonoid, eriodictyol, induces long-term protection in ARPE-19 cells through its effects on Nrf2 activation and phase 2 gene expression. Investigative Ophthalmology & Visual Science. 2009;50(5):2398–2406. doi: 10.1167/iovs.08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Li X. M., Huang D., Yu Q., Yang J., Yao J. Neuroligin-3 protects retinal cells from H2O2-induced cell death via activation of Nrf2 signaling. Biochemical and Biophysical Research Communications. 2018;502(1):166–172. doi: 10.1016/j.bbrc.2018.05.141. [DOI] [PubMed] [Google Scholar]
- 247.Koriyama Y., Chiba K., Yamazaki M., Suzuki H., Ichiro Muramoto K., Kato S. Long-acting genipin derivative protects retinal ganglion cells from oxidative stress models in vitro and in vivo through the Nrf2/antioxidant response element signaling pathway. Journal of Neurochemistry. 2010;115(1):79–91. doi: 10.1111/j.1471-4159.2010.06903.x. [DOI] [PubMed] [Google Scholar]
- 248.Shen C., Cheng W., Yu P., et al. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Molecular Medicine Reports. 2016;14(4):3646–3654. doi: 10.3892/mmr.2016.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang G., Song X., Zhao L., Li Z., Liu B. Resveratrol prevents diabetic cardiomyopathy by increasing Nrf2 expression and transcriptional activity. BioMed Research International. 2018;2018(107):13. doi: 10.1155/2018/2150218.2150218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shanab A. Y., Nakazawa T., Ryu M., et al. Metabolic stress response implicated in diabetic retinopathy: the role of calpain, and the therapeutic impact of calpain inhibitor. Neurobiology of Disease. 2012;48(3):556–567. doi: 10.1016/j.nbd.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 251.Cho H., Hartsock M. J., Xu Z., He M., Duh E. J. Monomethyl fumarate promotes Nrf2-dependent neuroprotection in retinal ischemia-reperfusion. Journal of Neuroinflammation. 2015;12(1):p. 239. doi: 10.1186/s12974-015-0452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wan P., Su W., Zhang Y., Li Z., Deng C., Zhuo Y. Trimetazidine protects retinal ganglion cells from acute glaucoma via the Nrf2/Ho-1 pathway. Clinical Science. 2017;131(18):2363–2375. doi: 10.1042/CS20171182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Majid A. S. A., Majid A. M. S. A., Yin Z. Q., Ji D. Slow regulated release of H2S inhibits oxidative stress induced cell death by influencing certain key signaling molecules. Neurochemical Research. 2013;38(7):1375–1393. doi: 10.1007/s11064-013-1034-z. [DOI] [PubMed] [Google Scholar]
- 254.Sheng L., Lu B., Chen H., et al. Marine-steroid derivative 5α-Androst-3β, 5α, 6β-triol protects retinal ganglion cells from ischemia-reperfusion injury by activating Nrf2 pathway. Marine Drugs. 2019;17(5):p. 267. doi: 10.3390/md17050267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Koriyama Y., Kamiya M., Takadera T., et al. Protective action of nipradilol mediated through S -nitrosylation of Keap1 and HO-1 induction in retinal ganglion cells. Neurochemistry International. 2012;61(7):1242–1253. doi: 10.1016/j.neuint.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 256.Maher P., Hanneken A. Flavonoids protect retinal ganglion cells from oxidative stress-induced death. Investigative Ophthalmology & Visual Science. 2005;46(12):4796–4803. doi: 10.1167/iovs.05-0397. [DOI] [PubMed] [Google Scholar]
- 257.Hanneken A., Lin F. F., Johnson J., Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Investigative Ophthalmology & Visual Science. 2006;47(7):3164–3177. doi: 10.1167/iovs.04-1369. [DOI] [PubMed] [Google Scholar]
- 258.Majid A. S. A., Yin Z. Q., Ji D. Sulphur antioxidants inhibit oxidative stress induced retinal ganglion cell death by scavenging reactive oxygen species but influence nuclear factor (erythroid-derived 2)-like 2 signalling pathway differently. Biological and Pharmaceutical Bulletin. 2013;36(7):1095–1110. doi: 10.1248/bpb.b13-00023. [DOI] [PubMed] [Google Scholar]
- 259.Wang L., Chen H. C., Yang X., et al. The novel chalcone analog L2H17 protects retinal ganglion cells from oxidative stress-induced apoptosis. Neural Regeneration Research. 2018;13(9):1665–1672. doi: 10.4103/1673-5374.237140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gong W., Li J., Zhu G., Wang Y., Zheng G., Kan Q. Chlorogenic acid relieved oxidative stress injury in retinal ganglion cells through IncRNA-TUG1/Nrf2. Cell Cycle. 2019;18(14):1549–1559. doi: 10.1080/15384101.2019.1612697. [DOI] [PMC free article] [PubMed] [Google Scholar]


