Abstract
A nephropathogenic K2/01 strain of infectious bronchitis virus (IBV) was attenuated by 170 serial passages in embryonated chicken eggs for possible use as a future IBV vaccine strain. High-growth properties and narrow tissue tropisms (limited replication in respiratory tracts) were achieved by the adaptation process. Unlike the parent strain, the attenuated strain (K2p170) was safe in day-old specific-pathogen-free chicks since replication of the virus did not induce mortality and nephritis, and rarely induced histological changes in the trachea and kidney after intraocular administration. In day-old broilers, even though coarse spray administration of K2p170 induced clinical signs, ciliostasis, and histopathological lesions in the trachea and the kidney, they were all comparable to birds vaccinated with commercial H120 vaccine. Despite restriction of viral replication in the respiratory tract, K2p170 elicited the production of antiserum with a neutralization index of 4.5. K2p170 provided almost complete protection against both two distinct subgroups of Korean nephropathogenic strain (KM91-like and QX-like subgroup). Furthermore, K2p170 provided significantly greater cross-protection against two heterologous strains (Massachusetts and Korean respiratory strain) than those conferred by the commercial H120 vaccine. K2p170 also had no virulence reversion after five back passages in chickens. In conclusion, K2p170 exhibits a fine balance between attenuation and immunogenicity, possesses cross-protective efficacy, and merits further investigation as a potential live vaccine as an alternative means of protection against the recently emergent nephropathogenic IBV infection in many Eurasian countries.
Keywords: Nephropathogenic IBV, Live attenuated vaccine, K2
1. Introduction
Infectious bronchitis (IB) is a highly contagious disease of the respiratory and urogenital tract of chickens caused by infectious bronchitis virus (IBV). IB causes severe economic losses to the poultry industry because it causes poor weight gain and feed efficiency in broilers and suboptimal egg production and egg quality in laying birds. In addition, high mortality (<30%) sometimes occurs in young chickens due to kidney manifestations of some nephropathogenic strains. The disease process is often complicated by secondary bacterial infections that cause increased mortality of birds and condemnation at processing [1], [2].
The existence of many serotypes of IBV that do not confer cross-protection against each other has been observed [1], [2]. Cross-protection tends to decrease as the degree of amino acid identity between the spike (S) glycoprotein S1 subunit of two IBV strains decreases [3]. However, some strains occasionally confer broad protection against many heterologous serotypes. The concept of protectotypes [4] has been suggested as a valuable method that should be considered during the development of strategies to control IBV infections, and strains possessing cross-protective ability are generally considered to be useful vaccine candidates. To date, the Massachusetts (Mass) and 4/91 strains have been primarily used as live vaccines due to their epizootic distributions and cross-protective ability [2], [5].
Despite the widespread use of vaccines, economic losses caused by IBV infection have occurred continuously worldwide. Indeed, outbreaks related to nephropathogenic strains of different serotypes have increased in many countries [6], [7], [8], which have caused mortality related to nephritis and respiratory disease followed by airsaculitis. More recently, the QX-like nephropathogenic strain [9] appears to have become widespread in China [10], Korea [11], Russia [12], and many countries in Europe [13], [14], where flocks are commonly vaccinated with Mass or 4/91 vaccines. Therefore, it has been suggested that there is a need to develop new vaccines against these nephropathogenic strains.
Previous studies have shown that Korean nephropathogenic IBV strains have been genetically stable [15] and possessed a broad-spectrum ability to protect against some heterologous strains [8]. In the present study, we report the development of an attenuated IBV vaccine candidate using the Korean nephropathogenic IBV strain, K2/01. The K2/01 strain was highly attenuated to remove pathogenicity and then characterized for attenuated phenotypes, after which its protective efficacy against challenge with homologous and heterologous strains was examined. The results demonstrated that the newly developed vaccine candidate exhibits a desired level of immunogenicity and attenuation of virulence.
2. Materials and methods
2.1. Viruses
Twenty-nine Korean IBV isolates (Table 1 ) obtained from natural outbreak cases of IB and two different commercially available vaccines (H120 and Ma5 of the Mass serotype produced by Intervet, International) were used. All isolates were propagated using specific-pathogen-free (SPF) embryonated eggs (Hy-Vac.com, IA, USA) and kept at −70 °C until use.
Table 1.
Origin and accession number of IBV strains isolated from flocks in Korea.
| IBV isolates | Year | Tissue tropism | Accession number |
|---|---|---|---|
| Korean group I (respiratory type) | |||
| B4 | 1986 | Trachea | FJ807932 |
| EJ95 | 1995 | Trachea | FJ807933 |
| EY95 | 1995 | Trachea | FJ807935 |
| K620/97 | 1997 | Trachea | FJ807944 |
| K348/99 | 1997 | Trachea | FJ807940 |
| K571/99 | 1999 | Trachea | FJ807942 |
| Korean group II (nephropathogenic type) | |||
| Es90 | 1990 | Kidney | FJ807934 |
| KC90 | 1990 | Kidney | FJ807945 |
| KM91 | 1991 | Kidney | FJ807946 |
| K151/98 | 1998 | Kidney | FJ807937 |
| K152/98 | 1998 | Kidney | FJ807938 |
| K083/98 | 1998 | Kidney | FJ807936 |
| K242/99 | 1999 | Kidney | FJ807939 |
| K451/99 | 1999 | Kidney | FJ807941 |
| K576/99 | 1999 | Kidney | FJ807943 |
| K2/01 | 2001 | Kidney | NSa |
| K630/02 | 2002 | Kidney | FJ807925 |
| K1019/03 | 2003 | Kidney | FJ807927 |
| K1255/03 | 2003 | Kidney | FJ807928 |
| K1257/03 | 2003 | Kidney | FJ807929 |
| K1277/03 | 2003 | Kidney | FJ807930 |
| K035/04 | 2004 | Kidney | FJ807920 |
| K283/04 | 2004 | Kidney | FJ807923 |
| K463/04 | 2004 | Kidney | FJ807924 |
| K961/04 | 2004 | Kidney | FJ807926 |
| K1583/04 | 2004 | Kidney | FJ807931 |
| K154/05 | 2005 | Kidney | FJ807922 |
| Massachusetts group | |||
| RB86 | 1986 | Trachea | FJ807947 |
| K110/06 | 2006 | Trachea | FJ807921 |
Not submitted.
2.2. Chickens
SPF white leghorn chickens (Nam-Deog Sanitek Co., Korea) and commercial Ross broiler chickens (Sam Hwa Breeding Agri Inc., Korea) were maintained in positive pressure high-efficiency particulate air-filtered stainless steel isolation cabinets (Three Shine Inc., Korea) under constant illumination within a biosafety level 2 laboratory. All study procedures and animal care activities were conducted in accordance with the national and institutional guidelines for the care and use of laboratory animals.
2.3. Attenuation
The virulent nephropathogenic field strain K2/01 (K2parent) was passaged 190 times in the allantoic cavity of 9–11-day-old embryonated specific-pathogen-free (SPF) chicken eggs (0.1 ml/egg), and the 170th passage virus (K2p170) was evaluated as live attenuated IBV vaccine candidate. The allantoic fluid was harvested after incubation for 30 h at 37 °C. A dot-immunoblot assay was performed as previously described [16] to detect the virus, and the allantoic fluid was titrated at every 10 passages. For next inoculation, we prepared allantoic fluid with the titers adjusted to 105.5 to 106.5 EID50/ml, if the undiluted allantoic fluid induced early embryo mortality within 30 h post-inoculation.
2.4. Reverse transcription-polymerase chain reaction (RT-PCR) and sequencing
Viral RNA used in the RT-PCR was extracted from virus containing allantoic fluid using an RNeasy minikit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. The first strand was then synthesized following a previously described method using BeauS1R3, 5′-CAT AAC TAA CAT AAG GGC AA-3′ [8], [15] or IBV-212, 5′-ATA CAA AAT CTG CCA TAA-3′ [10]. For subsequent PCR amplification, primer BeauS1F3, 5′-TTT GAA AAC TGA ACA AAA GA-3′ was added and amplification was conducted using the GeneAmp PCR system 9600 (Perkin Elmer, Waltham, MA, USA) as previously described [8]. The PCR products were analyzed on a 1.5% agarose gel and sequenced after cloning into the pGEMT-easy vector (Promega, Madison, WI, USA). The DNA sequence was then determined using the Dye Terminator Cycle Sequencing method and analyzed using an ABI 377 autosequencer (Applied Biosystems, Foster City, CA, USA).
2.5. Comparison of S1 genes and phylogenetic analyses
The nucleotide sequences of the S1 gene of the 29 IBV isolates (Table 1) were assembled, aligned and compared with reference IBV strains (Table A1) using the CLUSTAL W method with the Bioedit software http://www.mbio.ncsu.edu/BioEdit/bioedit.html [17]. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 3.1 http://www.megasoftware.net/ [18]. The reliability of the resulting trees was evaluated by the bootstrap method with 1000 replications.
2.6. Safety study
2.6.1. In specific-pathogen-free (SPF) chickens
The safety of viruses was initially ascertained in 1-day-old SPF chickens. The birds in the experimental group were inoculated by the eye drop method with the IBV strains (K2parent, K2p170, H120 and Ma5) at 104.5EID50/bird, while those in the control group were inoculated with phosphate buffered saline (PBS) (Table 2 ). To determine the pathologic characteristics of IBV, we observed birds twice daily for clinical signs for 14 days. Then, chickens were sacrificed and their tracheas and kidneys were collected, fixed with 10% neutral buffered formalin, and routinely processed in paraffin, after which 5 μm sections were cut for hematoxylin and eosin staining for histological studies. The tracheal lesions scored included epithelium deciliation, proliferation, degeneration, exudate, congestion, and hemorrhage. The renal lesions scored included epithelial degeneration, tubulo nephrosis, interstitial nephritis, and regeneration. Lesions were scored as follows: 0 for normal, 1 for extensively focal lesions, 2 for multifocal lesions, and 3 for diffuse lesions. Additionally, we determined tissue tropisms of K2p170 in comparison with K2parent and respiratory strain K571/99 in 1-day-old SPF chickens (Table 3 ). At 7 days after intraocular challenge with IBV at 104.5EID50/bird, the trachea, lung, cecal tonsil, kidney, and bursa of Fabricius tissues were collected from the birds and then used for re-isolation of the virus by inoculating 9–11-day-old embryonated SPF chicken eggs to determine the tissue tropism of IBV.
Table 2.
Results of the pathology of K2p170 compared with the parent strain (K2parent) and commercial live attenuated vaccines (H120 and Ma5) in 1-day-old SPF chickens.
| Virusesa | Clinical signs |
Histopathologic lesion scores |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality (%) | Respiratory signs | Nephritis | Tracheab |
Kidneyb |
||||||||
| Loss of epithelial cilia | Epithelial proliferation | Epithelial degeneration | Exudate | Congestion/Hemorrhage | Epithelial degeneration | Tubulo nephrosis | Interstinal nephritis | Regeneration | ||||
| K2parent | 30 | ++c | ++d | 2.2e | 1.4 | 1.8 | 0.6 | 0.6 | 0.8 | 0 | 2.4 | 1.8 |
| K2p170 | 0 | – | – | 1.2 | 0.6 | 0.8 | 0.2 | 0.8 | 0.6 | 0 | 0.4 | 0.6 |
| H120 | 0 | – | – | 1.6 | 0 | 0.6 | 0.2 | 0.4 | 0.4 | 0 | 1.0 | 0 |
| Ma5 | 0 | – | – | 1.6 | 0.8 | 0.8 | 0 | 0.2 | 0.6 | 0 | 1.2 | 0.8 |
| None | 0 | – | – | 0.6 | 0 | 0 | 0 | 0.4 | 0.6 | 0 | 0.8 | 0.4 |
ND: not determined.
One-day-old SPF chicks were inoculated with IBV (104.5EID50/bird) via the intraocular route.
Two weeks after challenge, chicks were sacrificed and tissues were collected to observe the histological lesions.
++, coughing in more than 30% without dyspnea; –, no coughing.
Examined dead chickens only (++, moderate; –, mild).
Scores of histological lesions: 0, normal; 1, extensively focal; 2, multifocal; 3, diffuse.
Table 3.
Tissue tropisms of K2p170 compared with the parent strain (K2parent) and respiratory strain (K571/99) in 1-day-old SPF chickens.
| Virusesa (genogroup) | Virus isolationb |
||||
|---|---|---|---|---|---|
| Trachea | Lung | Cecal tonsil | Kidney | Bursa | |
| K2parent (Korean group II) | 10/10 | 9/10 | 8/10 | 8/10 | 10/10 |
| K2p170 (Korean group II) | 8/10 | 3/10 | 1/10 | 0/10 | 0/10 |
| K571/99 (Korean group I) | 6/10 | 0/10 | 1/10 | 0/10 | 1/10 |
| None | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
One-day-old SPF chicks were inoculated with IBV (104.5 EID50/bird) via the intraocular route.
Seven days after challenge chicks were sacrificed and tissues were collected for re-isolation of the challenge virus. Data are the number of chicks from which the virus was isolated/number of chicks inoculated with the virus.
2.6.2. In broiler chickens
To examine the incidence of adverse reactions in commercial broilers after spray administration, 120 one-day-old broiler chicks were divided into 3 groups with 40 chicks in each group (Table 4 ). Chicks were obtained from a parent flock vaccinated with commercially available IB inactivated combine vaccines comprising Mass and Korean nephropathogenic strain. These birds were housed in separate isolators, and 20 birds in each group were assigned for evaluation of safety, while another 20 birds were assigned for assessment of the weight gain over time. Birds in the experimental group were immunized by K2p170 or H120 at 104.5ID50/bird using a coarse sprayer (Desvac®, Desvac Inc., France, droplet size = 115 μm), while those in the control group were inoculated with PBS. During the observation of clinical signs for 14 days, 5 birds in each group was euthanized and used to score rale sound, ciliostasis, and histological lesions of the trachea, lung, and kidney at days 4, 8, 11, and 14 post-inoculation. Histopathologic lesion scoring was conducted as described in the safety study in SPF chickens, and lung lesions were assessed for lympho–histiocytic and intertitial pneumonia.
Table 4.
Responsiveness of 1-day-old broiler chickens to vaccination with the K2p170 vaccine by spray.
| Days post-exposurea | Rale soundb |
Mean ciliostasis scoresc |
Histopathologic lesion scoresd |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Inoculated |
Control | Inoculated |
Upper trachea |
Lower trachea |
Lung |
Kidney |
|||||||||||
| H120 | K2p170 | H120 | K2p170 | Control | Inoculated |
Control | Inoculated |
Control | Inoculated |
Control | Inoculated |
|||||||
| H120 | K2p170 | H120 | K2p170 | H120 | K2p170 | H120 | K2p170 | |||||||||||
| Day 4 | 0/5 | 3/5 | 3/5 | 0.15 | 0.04 | 0.00 | 0.05 | 0.31 | 0.95 | 0 | 0 | 0.10 | 0.40 | 0.40 | 0.80 | 0 | 0 | 0 |
| Day 8 | 0/5 | 3/5 | 3/5 | 0.06 | 3.04 | 2.66 | 0.05 | 2.30 | 2.65 | 0.10 | 2.05 | 0.90 | 0.40 | 0.40 | 0.90 | 0 | 0 | 0.10 |
| Day 11 | 0/5 | 1/5 | 1/5 | 0.14 | 2.02 | 1.78 | 0.20 | 2.35 | 1.85 | 0 | 1.10 | 1.10 | 0 | 0.20 | 0.20 | 0 | 0 | 0 |
| Day 14 | 0/5 | 0/5 | 0/5 | 0.12 | 1.82 | 1.14 | 0.10 | 1.80 | 1.65 | 0 | 1.75 | 0.70 | 0.30 | 0.50 | 0.10 | 0 | 0 | 0 |
One-day-old broiler chicks were inoculated with IBV (104.5EID50/bird) by coarse spray.
On days 4, 8, 11, and 14 post-vaccination, birds were checked individually for tracheal rales (a sound emanating from the bronchi, also detected by vibrations when holding a chick).
On days 4, 8, 11, and 14 post-vaccination, tracheas were removed from five chickens in each group (the same animals used for histological sampling). Ten trachea rings per chick were prepared (three upper, four middle, and three lower trachea rings). The rings were examined under low-power magnification and ciliary activity was scored as follows: 0, no ciliostasis; 1, 25% ciliostasis; 2, 50% ciliostasis; 3, 75% ciliostasis; 4, 100% ciliostasis.
Histopathology of the trachea, lung, and kidney at 4 days post-challenge with IBV was reported as histopathologic lesion scores.
2.6.3. Virulence reversion
To examine the in vivo reversion of virulence of K2p170, 1-day-old SPF chicks were divided into two groups of three chicks. The inoculated group received K2p170 intranasally at 104.5EID50/bird and were observed twice daily for clinical signs for 5 days. Birds in the control group were inoculated with PBS. At 5 days post-inoculation, the kidneys and tracheas were collected from all birds for virus detection by RT-PCR. After determination of the presence of the inoculated virus, the tissue homogenates were prepared in PBS with antibiotics and subsequently inoculated into the next group of chicks. This experiment was repeated five times. After the fifth chick-passage, 10 one-day-old SPF chicks were inoculated intranasally with 104.5EID50/bird of tissue homogenates and then observed twice daily for clinical signs for 14 days. Two weeks after challenge, birds were sacrificed, and histopathological scoring of the tracheas and kidneys was conducted as described in the safety study of SPF chickens.
2.7. Efficacy study
2.7.1. Protection from replication of the challenge virus in the trachea and kidney of SPF chickens
Total 180 three-week-old SPF chickens were divided into 18 groups of 10 chicks. Twelve groups were then immunized intraocularly with H120 or K2p170 at 103.0 EID50, while the other 6 groups were kept as non-immunized control groups. Three weeks after immunization, all birds were challenged intraocularly with 104.5 EID50 of three respiratory strains belonged to Mass group (Mass41) and Korean group I (K571/99, and K107/04) and three nephropathogenic strains belonged to Korean group II (K2parent, KM91, and K1277/03) (Table 5 and Fig. 1 ). Five days after challenge, we re-isolated the challenge virus from the trachea and kidney of birds by inoculating 9–11-day-old embryonated SPF chicken eggs to evaluate the protective efficacy of the vaccines.
Table 5.
Comparison of deduced amino acid sequences of the S1 gene of K2p170 and the parent strain (K2parent).
| Strain | Passage number | Positiona |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 117 | 130 | 133 | 299 | 360 | 384 | 388 | 531 | ||
| K2parent | 3 | I | M | H | F | Q | R | L | D | G |
| K2p170 | 170 | T | V | Y | I | P | Q | V | E | E |
The deduced amino acid positions in the S1 gene of IBV, starting at the AUG translation start codon.
Fig. 1.
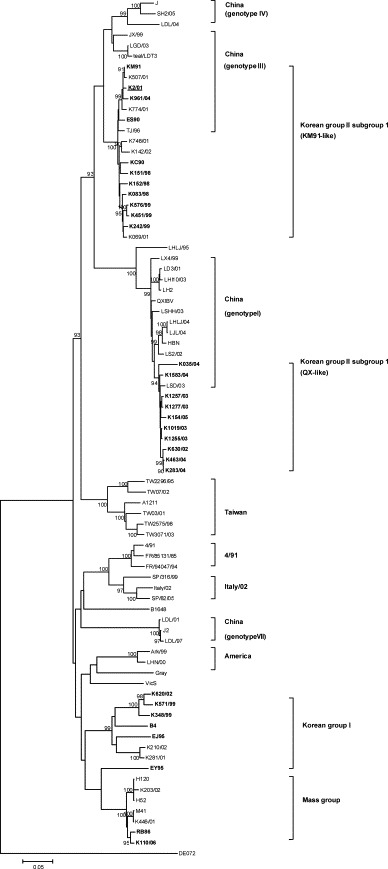
Phylogenetic tree showing partial S1 gene relationships between Korean IBV isolates and reference strains. ClustalW alignment method for S1 nucleotide positions 1–1611 corresponding to those of strain Massachusetts (GenBank accession number X04722). The unrooted phylogenetic trees were generated by the distance-based neighbor-joining method and the Kimura-2 parameter model using MEGA 3.1. Reliability of the trees was assessed by bootstrap analysis in 1000 replications. Bootstrap values >90% are displayed above the branch nodes. Viruses isolated in the present study are in bold, and the K2parent (K2/01) is underlined.
2.7.2. Neutralizing index
Sera from chicks of all groups in the efficacy studies were collected at 3 weeks after immunization and inactivated at 56 °C for 30 min prior to use in viral neutralization test [19]. Briefly, the viruses used for immunization were 10-fold serially diluted before mixing with an equal volume of inactivated serum samples. The virus–serum mixtures were placed for 1 h at 25 °C prior to inoculating 10-day-old embryonated SPF eggs (0.1 ml/egg, five eggs per diluents). The inoculated eggs were incubated for 7–8 days at 37 °C, and eggs that died within 24 h were discarded. At the end of the experimental period, the remaining live eggs were examined for IB lesions to determine EID50 of inoculated viruses and the neutralizing index (NI) was calculated.
2.8. Statistical analysis
A one-tailed Fisher's exact test was used to compare results among groups in the present study. A p-value of <0.05 was considered to be statistically significant.
3. Results
3.1. Phylogenetic analyses of Korean IBV strains
A phylogenetic tree was generated to describe the relationship between nucleotide sequences of the S1 gene of the K2parent and other field isolates in Korea (Fig. 1). The Korean IBV strains were separated into two distinct genetic groups (Korean group I and Korean group II), except RB86 and K110/06 formed a separate cluster with the Mass group (Table 1 and Fig. 1). Korean group I included all respiratory strains that split into a unique genetic group, while all nephropathogenic strains were included in Korean group II. These results were identical with previous classification of Korean IBV isolates [15] and suggested that tissue tropism of IBV correlated with genotype based on the S gene sequence. However, Korean group II was subsequently divided into two separate subgroups identified as KM91-like subgroup and QX-like subgroup. The IBV isolates detected in the 1990s to the early 2000s in Korea belonged to the KM91-like subgroup containing the K2parent, while IBV isolates obtained after 2003 belonged to the QX-like subgroup that included the QX strain, which is a representative Chinese strain related to nephritis [10]. The nucleotide identity of the QX-like subgroup strains with the KM91 was below 90% (84.6–85.2%) in spite of similar renal pathogenicity. The nucleotide sequences of the 28 isolates in the present study have been deposited in GenBank under the accession numbers listed in Table 1.
3.2. Safety of K2p170
3.2.1. Biological and molecular characterization of the vaccine candidate strain
With continuing passages, K2parent became adapted to embryonated eggs showing the typical embryonic changes such as the dwarfing, stunting, or curling of embryos since the 10th passages. The virus initially replicated in embryonated eggs to titers below 106.5 EID50/ml. After the 160th passages, however, the virus showed high-growth properties, reaching the titers over 107.5 EID50/ml, and the undiluted virus induced early embryo mortality within 30 h post-inoculation. Therefore, the virus titers were adjusted to 105.5 to 106.5 EID50/ml for next inoculation, and the diluted virus induced embryo mortality starting from 2 or 3 days post-inoculation. The deduced amino acid sequences of the S1 protein of K2p170 were compared with those of K2parent and nine point mutations caused by amino acid substitutions were found (Table 6 ).
Table 6.
Protective effects in 3-week-old SPF chickens immunized with H120 and K2p170 against challenge with IBV isolates representing the two major Korean IBV genogroups.
| IBV strain immunizeda | Genogroup of challenge virus | IBV strain of challenge virusb | No. of challenge virus isolated/no. of challengedc |
|||
|---|---|---|---|---|---|---|
| Trachea |
Kidney |
|||||
| Control | Vaccinated | Control | Vaccinated | |||
| H120 | Korean group I | Mass 41 | 10/10 | 0/10*** | 10/10 | 0/10*** |
| K571/99 | 9/10 | 5/10 | 1/10 | 0/10 | ||
| K107/04 | 7/10 | 4/9 | 0/10 | 0/9 | ||
| Korean group II | K2parent | 10/10 | 3/10** | 10/10 | 7/10 | |
| KM91 | 10/10 | 5/10 | 8/10 | 4/10 | ||
| K1277/03 | 10/10 | 8/10 | 9/10 | 8/10 | ||
| K2p170 | Korean group I | Mass 41 | 10/10 | 2/10*** | 10/10 | 0/10*** |
| K571/99 | 9/10 | 0/10***,† | 1/10 | 1/10 | ||
| K107/04 | 7/10 | 2/10 | 0/10 | 0/10 | ||
| Korean group II | K2parent | 10/10 | 1/10*** | 10/10 | 1/10***,† | |
| KM91 | 10/10 | 4/10* | 8/10 | 0/10*** | ||
| K1277/03 | 10/10 | 3/8** | 9/10 | 0/8***,†† | ||
Three-week-old chickens were immunized with IBV, H120 and K2p170 (103.0EID50/bird) via the intraocular route.
At 3 weeks post-immunization, all birds were challenged with 104.5EID50 of six Korean IBV strains via the intraocular route.
Five days after challenge, protection was evaluated by the absence of challenge virus in the kidney and trachea.
p < 0.01, by Fisher's exact test, compared to non-vaccinated control group.
p < 0.005, by Fisher's exact test, compared to non-vaccinated control group.
p < 0.001, by Fisher's exact test, compared to non-vaccinated control group.
p < 0.01, by Fisher's exact test, compared to the H120-vaccinated group.
p < 0.005, by Fisher's exact test, compared to the H120-vaccinated group.
3.2.2. Safety in SPF chickens
When administered intraocularly, K2parent was pathogenic to 1-day-old chicks, inducing 30% mortality as well as moderate nephritis; however, K2p170 was no longer pathogenic for 1-day-old chicks, as demonstrated by the absence of any clinical signs (Table 2). In addition, the histopathologic lesion scores of tracheas from birds inoculated with K2p170 did not differ significantly from those of birds inoculated with the commercial vaccines, H120 and Ma5. In the kidneys, K2p170 and H120 did not induce any histopathologic lesions when compared with the non-vaccinated control. To further examine the pathogenicity of K2p170, we compared the tissue tropism of K2p170 with K2parent and the respiratory strain K571/99. As shown in Table 3, K2parent replicated well in the various organs including the kidneys, whereas replication of K2p170 was confined to the respiratory tract, similar to respiratory strain K571/99.
3.2.3. Safety in broiler chickens
To determine if administration of K2p170 by coarse spray induced severe respiratory reactions, 1-day-old broiler chicks were given 104.5 EID50/bird of K2p170 or H120. Despite existence of serum maternal antibody specific both to Mass and Korean nephropathogenic strains in chicks, two IBV strains including H120 (Mass strain) and K2p170 (Korean nephropathogenic strain) induced ciliostasis in the tracheal epithelium in both inoculated groups during the period from 8 to 14 days post-inoculation. The mean ciliostasis scores in both inoculated groups peaked at 8 days and subsequently declined as the chicks grew, but there was no significant difference between two groups. Rale sound and histopathgologic lesion scores of respiratory organs changed in a similar pattern as ciliostasis, and focal histopathologic lesions were observed only in the upper part of the tracheas. In kidneys, no significant histopathological changes were observed in birds inoculated with K2p170. Indeed, no difference in average body weight was found between the experimental and control groups for 2 weeks after challenge (data not shown).
3.2.4. Virulence reversion
We detected IBV in tissue homogenates of inoculated groups at every passage, but not in control chickens. No clinical signs or deaths were observed upon five passages. Histological examination revealed the presence of mild tracheal lesions with loss of cilia and epithelial degeneration in chicks inoculated with viruses obtained following the fifth passage. However, the tracheal and renal lesion scores were not significantly different from those induced by K2p170 before passage in the chickens and H120 (data not shown). These results indicate that no virulence reversion of K2p170 occurred in the chickens.
3.3. Protective efficacy of K2p170
As shown in Table 6, chickens immunized with K2p170 were challenged with two nephropathogenic strains belonging to the KM91-like subgroup (K2parent and KM91), one nephropathogenic strain belonging to the QX-like subgroup (K1277/03), and three respiratory strains belonging to the Mass group (Mass41) and Korean group I (K571/99 and K107/04). In groups of chickens vaccinated with H120, complete protection of the respiratory tract against homologous strain Mass41 (p < 0.001) was observed, but partial protection against other challenge viruses was observed (p > 0.05). In the kidneys, H120 provided little protection against the nephropathogenic strain K2parent and KM91. Conversely, chickens immunized with K2p170 showed significantly higher levels of protection against challenge with all of the viruses except K107/04 at the kidney and trachea levels (p < 0.01 or better). However, K2p170 conferred better tracheal protection than H120 against challenge with K107/04. Sera from immunized birds were collected at 3 weeks post-immunization and the neutralization index (NI) of the experimental group was examined. IB vaccine is defined as being effective if the NI of immunized groups exceeds 2.0 and the NI of the non-immunized control group is <1.0. The NI of immunized chickens by K2p170 and H120 was 4.5 and 1.8, respectively, whereas that of the non-immunized control was 0.6 (data not shown).
4. Discussion
Although attenuation of viruses is usually achieved by passage of the virus in a foreign host such as embryonated eggs or tissue culture cells [20], it has been found that IBV, which is a member of the coronavirus family, was not easily attenuated using this method. In fact, long-term passage in embryonated eggs is necessary to reduce the virulence of IBV, and most commercially available IBV vaccines (e.g., H52, H120, 4/91 and GA98) are produced by more than 50 serial passages [5], [21]. In a previous study, the 120th passage virus of the Mass serotype (the H120 strain) did not induce any mortality in day-old chicks [5]. However, in this study, 120th passage virus of the K2parent did not induce mortality in day-old chicks by intraocular administration, but still induced mortality (<30%) in day-old chicks after coarse spray administration (data not shown). Therefore, we found that nephropathogenic strains required additional egg passages when compared to Mass strains for complete attenuation. On the other hand, the 170th passage virus (K2p170) was considered to be completely attenuated because it was non-pathogenic to day-old chicks. To our knowledge, the K2p170 seems to be the most highly egg passaged strain commercial IBV vaccine strains in the world [5].
The S protein of coronavirus has been shown to be related to the attenuation of virulence and to broadening host ranges or cell types [22], [23]. Casais et al. [24] demonstrated that the S protein is a determinant of cell tropism of IBV, and it has been suggested that some amino acid residues contributed to attenuation of IBV [25], [26]. However, amino acid changes in the S protein of K2p170 were not identical to these substitutions, even though K2p170 undergo distinct alteration of biological features including restriction in the tracheas and lack of renal pathogenicity. In agreement with previous studies conducted to determine a region that leads to the pathogenic phenotype of IBV [15], [27], our findings suggest that the renal tropism and pathogenicity of K2p170 are not related to the S1 gene, but to other genes [28]. Further genetic investigation of K2p170, such as comparison of the entire genome of attenuated IBV strains with its parent strains [29], may provide a better understanding of the pathogenicity related to renal tropism of IBV.
Despite a lack of renal tropism after attenuation, K2p170 was still able to replicate in the tracheas, which induced mild ciliostasis and histological lesions. It is likely that some damage to the tracheal mucosa is necessary for the development of local immunity against IBV [21]. The level of damage was found to be comparable to that of commercial vaccines (H120 and Ma5), and was in agreement with recent studies conducted to determine the attenuation phenotypes of the GA98 serotype vaccine via evaluation of tracheal lesion scores following challenge [21]. Moreover, K2p170 caused limited damage to the respiratory tract and did not affect the growth performance of broilers, even after coarse spray. In addition, no virulence reversion of the K2p170 occurred in SPF chickens, as demonstrated by the absence of clinical signs and mild histological lesions following in vivo back passage. These findings suggest that K2p170 is useful for hatchery spray vaccination, which is a method of mass vaccination for IBV that is used worldwide [2]. On the basis of its safety in day-old chicks demonstrated, delivery of K2p170 in ovo can be further attempted as a novel vaccination route of choice for IB vaccines [30].
Long-term passage may be beneficial for vaccine safety, but it is possible that over-attenuation of the virus leads to poor protection due to insufficient replication [31]. In the present study, the K2p170 induced strong local immunity following replication in the trachea, as evidenced by the complete protection of the tracheas against the homologous K2parent strain. Despite restricted replication in the trachea, neutralizing antibodies to the K2p170 were induced (NI of K2p170 was 4.5), and K2p170 induced complete protection of the kidneys. These findings indicate that K2p170 is highly immunogenic. As seen in a previous study describing the genetic stability of Korean nephropathogenic strains sharing 96% homology of the S1 gene for 10 years [15], we observed only eight amino acid changes in the S1 protein of K2p170. Interestingly, most altered residues were located outside of the hypervariable region (HVR), which is associated with the neutralizing epitope [1]. Because the S1 protein has been identified as a major inducer of protective immune responses, our findings lead to the conclusion that the genetic stability of K2p170 contributes to maintenance of constant immunogenicity, even after prolonged passage in embryonated eggs.
Although K2parent was chosen based on its cross-protective ability [8], it is not clear if the fully attenuated K2p170 still induced cross-protection. In efficacy studies, K2p170 provided almost complete protection against distinct QX-like subgroup strain of Korean group II (K1277/03) and significant protection against heterologous IBV strains belonged to Mass group (Mass41) and Korean group I (K107/04). It has been proposed that the use of a combination of two commercial vaccines, Mass and 4/91, could be partly effective against heterologous IBV strains, including QX-like strains [32]. However, the use of multiple strains of live vaccines should be practiced with caution due to concerns regarding the formation of variant viruses by recombination with field strains resulting from the spread of vaccine strains [33]. Conversely, the results presented here suggest that single administration of the K2p170 is markedly effective and economical due to its cross-protective ability. It will be important to determine the range of cross-protection conferred by K2p170 against other heterologous IBV strains.
Mass serotype vaccines have been used worldwide for almost 50 years due to their cross-protective ability. Nevertheless, nephropathogenic strains appear to be the 3rd epizootic strain with Mass and 4/91 strains in many parts of the world. Based on the data presented in this study, K2p170 has broad-spectrum protective ability. Furthermore, the attenuation and immunogenicity of the virus were comparable to currently available commercial vaccines, which indicate that K2p170 is suitable for field application to young chicks in hatcheries and farms. Therefore, K2p170 merits consideration as a novel live vaccine candidate for the reduction of economic losses caused by newly evolving nephropathogenic IBV strains and the many IBV variants that have been reported worldwide.
Acknowledgement
This work was supported by grants from Konkuk University (2001-A019-0130).
Appendix A. Appendix
See Table A1 .
Table A1.
Data for reference IBV strains and accession numbers.
| Strain |
Year | Country | Accession number | Remarks | |
|---|---|---|---|---|---|
| This study | Original | ||||
| K069/01 | K069-01 | 2001 | Korea | AY257061 | Korean genogroup II |
| K281/01 | K281-01 | 2001 | Korea | AY257062 | Korean genogroup I |
| K446/01 | K446-01 | 2001 | Korea | AY257063 | Korean genogroup I |
| K507/01 | K507-01 | 2001 | Korea | AY257063 | Korean genogroup II |
| K748/01 | K748-01 | 2001 | Korea | AY790358 | Korean genogroup II |
| K774/01 | K774-01 | 2001 | Korea | AY257065 | Korean genogroup II |
| K142/02 | K142-01 | 2002 | Korea | AY257060 | Korean genogroup II |
| K203/02 | K203-02 | 2002 | Korea | AY257067 | Korean genogroup I |
| K210/02 | K210-02 | 2002 | Korea | AY790350 | Korean genogroup I |
| LHLJ/95 | CK/CH/LHLJ/95I | 1995 | China | DQ167141 | Chinese genotype II |
| TJ/96 | TJ/96/02 | 1996 | China | AF257075 | Chinese genotype III |
| HBN | HBN | 1996–1998 | China | DQ070837 | Chinese genotype I |
| J2 | J2 | 1996–1998 | China | AF286303 | Chinese genotype VII |
| QXIBV | QXIBV | 1997 | China | AF193423 | Chinese genotype I |
| LDL/97 | CK/CH/LDL/97I | 1997 | China | DQ068701 | Chinese genotype VII |
| J | J | 1998 | China | AF352312 | Chinese genotype IV |
| JX/99 | JX/99/01 | 1999 | China | AF210735 | Chinese genotype III |
| LX4/99 | LX4 | 1999 | China | AY189157 | Chinese genotype I |
| LHN/00 | CK/CH/LHN/00I | 2000 | China | DQ167143 | Chinese genotype VI |
| LDL/01 | CK/CH/LDL/01I | 2001 | China | DQ167130 | Chinese genotype VII |
| LD3/01 | LD3 | 2001 | China | AY277632 | Chinese genotype I |
| LH2 | LH2 | 2001 | China | AY180958 | Chinese genotype I |
| LS2/02 | LS2 | 2002 | China | AY278246 | Chinese genotype I |
| LHI10/03 | LHI10 | 2003 | China | AY273193 | Chinese genotype I |
| LSHH/03 | CK/CH/LSHH/03II | 2003 | China | DQ167149 | Chinese genotype I |
| LSD/03 | CK/CH/LSD/03I | 2003 | China | DQ167148 | Chinese genotype I |
| LGD/03 | CK/CH/LGD/03I | 2003 | China | DQ167133 | Chinese genotype III |
| teal/LDT3 | tl/CH/LDT3/03 | 2003 | China | AY702975 | Chinese genotype III |
| LJL/04 | CK/CH/LJL/04I | 2004 | China | DQ167144 | Chinese genotype I |
| LHLJ/04 | CK/CH/LHLJ/04V | 2004 | China | DQ167139 | Chinese genotype I |
| LDL/04 | CK/CH/LDL/04II | 2004 | China | DQ167131 | Chinese genotype IV |
| SH2/05 | SH2 | 2005 | China | DQ075324 | Chinese genotype IV |
| A1171 | 1171/92 (A1171) | 1992 | Taiwan | AF250005 | Taiwan group I |
| A1211 | 1211/92 (A1211) | 1992 | Taiwan | AF250006 | Taiwan group I |
| TW2296/95 | 2296/95 | 1995 | Taiwan | AY606321 | Taiwan group II |
| TW2575/98 | 2575/98 | 1998 | Taiwan | AY606314 | Taiwan group I |
| TW03/01 | T03/01 | 2001 | Taiwan | AY606315 | Taiwan group I |
| TW07/02 | T07/02 | 2002 | Taiwan | AY606322 | Taiwan group II |
| TW2993/02 | 2993-02 | 2002 | Taiwan | AY606316 | Taiwan group I |
| TW3025/02 | 3025/02 | 2002 | Taiwan | AY606317 | Taiwan group I |
| TW3051/02 | 3051/02 | 2002 | Taiwan | AY606318 | Taiwan group I |
| TW3071/03 | 3071/03 | 2003 | Taiwan | AY606319 | Taiwan group I |
| M41 | Mass | 1941 | USA | X04722 | Mass group |
| Ark/99 | Ark-99 | 1973 | USA | L10384 | American group |
| Gray | Gray | – | USA | L18989 | American group |
| H52 | H52 | Vaccine strain | Netherlands | AF352315 | Mass group |
| H120 | H120 | Vaccine strain | Netherlands | EU822341 | Mass group |
| B1648 | B1648 | 1984 | Belgium | X87238 | B1648 group |
| SP316/99 | Spain/99/316 | 1999 | Spain | DQ064809 | Spanish genotype IV |
| SP5438/04 | Spain/5438/04 | 2004 | Spain | DQ386105 | Italy 02 group |
| SP82/05 | Spain/82/05 | 2005 | Spain | DQ386104 | Italy 02 group |
| Italy/02 | Italy-02 | 1999 | Italy | AF093794 | Italy/02 group |
| 4/91 | 4/91 | Vaccine strain | UK | AJ457137 | 4/91 group |
| FR85313/85 | FR-85313-85 | 2000 | France | AJ618985 | 4/91 group |
| FR94047/94 | FR-94047-94 | 2000 | France | AJ618987 | 4/91 group |
| VicS | VicS | Vaccine strain | Australia | U29519 | Australia group |
References
- 1.Cavanagh D., Naqi S. 11th ed. Iowa State Press; Ames: 2003. Infectious bronchitis virus. [Google Scholar]
- 2.Ignjatovic J., Sapats S. Avian infectious bronchitis virus. Rev Sci Tech. 2000;19(August (2)):493–508. doi: 10.20506/rst.19.2.1228. [DOI] [PubMed] [Google Scholar]
- 3.Cavanagh D., Ellis M.M., Cook J.K.A. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo. Avian Pathol. 1997;26:63–74. doi: 10.1080/03079459708419194. [DOI] [PubMed] [Google Scholar]
- 4.Dhinakar Raj G., Jones R.C. Protectotypic differentiation of avian infectious bronchitis viruses using an in vitro challenge model. Vet Microbiol. 1996;53(December (3–4)):239–252. doi: 10.1016/s0378-1135(96)01258-8. [DOI] [PubMed] [Google Scholar]
- 5.Bijlenga G., Cook J.K., Gelb J., Jr., de Wit J.J. Development and use of the H strain of avian infectious bronchitis virus from the Netherlands as a vaccine: a review. Avian Pathol. 2004;33(December (6)):550–557. doi: 10.1080/03079450400013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayry J., Goudar M.S., Nighot P.K., Kshirsagar S.G., Ladman B.S., Gelb J., Jr. Emergence of a nephropathogenic avian infectious bronchitis virus with a novel genotype in India. J Clin Microbiol. 2005;43(February (2)):916–918. doi: 10.1128/JCM.43.2.916-918.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meir R., Rosenblut E., Perl S., Kass N., Ayali G., Perk S. Identification of a novel nephropathogenic infectious bronchitis virus in Israel. Avian Dis. 2004;48(September (3)):635–641. doi: 10.1637/7107. [DOI] [PubMed] [Google Scholar]
- 8.Song C.S., Lee Y.J., Kim J.H., Sung H.W., Lee C.W., Izumiya Y. Epidemiological classification of infectious bronchitis virus isolated in Korea between 1986 and 1997. Avian Pathol. 1998;27:409–416. doi: 10.1080/03079459808419360. [DOI] [PubMed] [Google Scholar]
- 9.Liu S., Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian Pathol. 2004;33(June (3)):321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S.W., Zhang Q.X., Chen J.D., Han Z.X., Liu X., Feng L. Genetic diversity of avian infectious bronchitis coronavirus strains isolated in China between 1995 and 2004. Arch Virol. 2006;151(June (6)):1133–1148. doi: 10.1007/s00705-005-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee E.K., Jeon W.J., Lee Y.J., Jeong O.M., Choi J.G., Kwon J.H. Genetic diversity of avian infectious bronchitis virus isolates in Korea between 2003 and 2006. Avian Dis. 2008;52(June (2)):332–337. doi: 10.1637/8117-092707-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 12.Bochkov Y.A., Batchenko G.V., Shcherbakova L.O., Borisov A.V., Drygin V.V. Molecular epizootiology of avian infectious bronchitis in Russia. Avian Pathol. 2006;35(October (5)):379–393. doi: 10.1080/03079450600921008. [DOI] [PubMed] [Google Scholar]
- 13.Worthington K.J., Currie R.J., Jones R.C. A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathol. 2008;37(June (3)):247–257. doi: 10.1080/03079450801986529. [DOI] [PubMed] [Google Scholar]
- 14.Gough R.E., Cox W.J., De B., Welchman D., Worthington K.J., Jones R.C. Chinese QX strain of infectious bronchitis virus isolated in the UK. Vet Rec. 2008;162:99–100. doi: 10.1136/vr.162.3.99. [DOI] [PubMed] [Google Scholar]
- 15.Song C.S., Lee Y.J. Molecular and epidemiological characteristics of infectious bronchitis virus isolated in Korea. Korean J Poult Sci. 2000;27(2):91–98. [Google Scholar]
- 16.Song C.S., Kim J.H., Lee Y.J., Kim S.J., Izumiya Y., Tohya Y. Detection and classification of infectious bronchitis viruses isolated in Korea by dot-immunoblotting assay using monoclonal antibodies. Avian Dis. 1998;42(January–March (1)):92–100. [PubMed] [Google Scholar]
- 17.Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 18.Kumar S., Tamura K., Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5(June (2)):150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 19.Gelb J., Jr., Wolff J.B., Moran C.A. Variant serotypes of infectious bronchitis virus isolated from commercial layer and broiler chickens. Avian Dis. 1991;35(January–March (1)):82–87. [PubMed] [Google Scholar]
- 20.Condit R.C. Principles of virology. In: Knipe D.M., editor. Field's virology. 5th ed. Lippincott Williams & Wilkins; Philadelpia: 2007. pp. 25–58. [Google Scholar]
- 21.Jackwood M.W., Hilt D.A., Brown T.P. Attenuation, safety, and efficacy of an infectious bronchitis virus GA98 serotype vaccine. Avian Dis. 2003;47(July–September (3)):627–632. doi: 10.1637/6094. [DOI] [PubMed] [Google Scholar]
- 22.Schickli J.H., Zelus B.D., Wentworth D.E., Sawicki S.G., Holmes K.V. The murine coronavirus mouse hepatitis virus strain A59 from persistently infected murine cells exhibits an extended host range. J Virol. 1997;71(December (12)):9499–9507. doi: 10.1128/jvi.71.12.9499-9507.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baric R.S., Sullivan E., Hensley L., Yount B., Chen W. Persistent infection promotes cross-species transmissibility of mouse hepatitis virus. J Virol. 1999;73(January (1)):638–649. doi: 10.1128/jvi.73.1.638-649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casais R., Dove B., Cavanagh D., Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J Virol. 2003;77(August (16)):9084–9089. doi: 10.1128/JVI.77.16.9084-9089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Callison S.A., Jackwood M.W., Hilt D.A. Molecular characterization of infectious bronchitis virus isolates foreign to the United States and comparison with United States isolates. Avian Dis. 2001;45(2):492–499. [PubMed] [Google Scholar]
- 26.Liu S., Han Z., Chen J., Liu X., Shao Y.H., Kong X. S1 gene sequence heterogeneity of a pathogenic infectious bronchitis virus strain and its embryo-passaged, attenuated derivatives. Avian Pathol. 2007;36(3):231–234. doi: 10.1080/03079450701338730. [DOI] [PubMed] [Google Scholar]
- 27.Hodgson T., Casais R., Dove B., Britton P., Cavanagh D. Recombinant infectious bronchitis coronavirus Beaudette with the spike protein gene of the pathogenic M41 strain remains attenuated but induces protective immunity. J Virol. 2004;78(December (24)):13804–13811. doi: 10.1128/JVI.78.24.13804-13811.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavanagh D., Casais R., Armesto M., Hodgson T., Izadkhasti S., Davies M. Manipulation of the infectious bronchitis coronavirus genome for vaccine development and analysis of the accessory proteins. Vaccine. 2007;25(July (30)):5558–5562. doi: 10.1016/j.vaccine.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y.P., Wang C.H. Sequence changes of infectious bronchitis virus isolates in the 3′ 7.3 kb of the genome after attenuating passage in embryonated eggs. Avian Pathol. 2007;36(February (1)):59–67. doi: 10.1080/03079450601110015. [DOI] [PubMed] [Google Scholar]
- 30.Tarpey I., Orbell S.J., Britton P., Casais R., Hodgson T., Lin F. Safety and efficacy of an infectious bronchitis virus used for chicken embryo vaccination. Vaccine. 2006;24(November (47–48)):6830–6838. doi: 10.1016/j.vaccine.2006.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32(December (6)):567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terregino C., Toffan A., Beato M.S., Nardi R.D., Vascellari M., Meini A. Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathol. 2008;37(5):487–493. doi: 10.1080/03079450802356938. [DOI] [PubMed] [Google Scholar]
- 33.Farsang A., Ros C., Renstrom L.H., Baule C., Soos T., Belak S. Molecular epizootiology of infectious bronchitis virus in Sweden indicating the involvement of a vaccine strain. Avian Pathol. 2002;31(June (3)):229–236. doi: 10.1080/03079450220136530. [DOI] [PMC free article] [PubMed] [Google Scholar]


