Abstract
Following the anthrax attacks of 2001 and the recent SARS outbreak, concerns about emerging and re-emerging infectious diseases have catalyzed a renewed interest in developing new vaccination strategies that provide rapid and flexible response options to future threats. Because the probability of encountering one of these exotic agents is unknown, it is essential that new vaccine formulations employ methods that provide effective protection and extremely good safety profiles if they are to be used by either military or civilian populations. One approach, which potentially satisfies these criteria, is the use of live recombinant Gram-positive commensal bacteria as expression vectors. This review provides an overview of the system, its advantages and limitations, and details an example of how Gram-positive commensal bacteria are being developed as a fifth generation vaccine against a Class A biowarfare pathogen, namely smallpox.
Keywords: Mucosal immunity, Surface expression, Smallpox, Vaccinia antigens
1. Introduction
1.1. Biowarfare
One of the major biomedical concerns is the continued emergence of new infectious agents that cause disease in the human population. Recent years have provided a number of examples including HIV, West Nile Virus, SARS, and increasing frequency of antibiotic-resistant bacterial isolates. Although nature has been responsible for these problems, of equally great concern is the possibility that infectious agents will be introduced into our environment by a deliberate act of bioterrorism or biowarfare.
Biowarfare concerns used to be limited to battlefield interactions between armed combatants. However, with ready access to modern molecular biology and microbiological procedures, it is relatively easy for a person to obtain, grow, and disperse a biowarfare agent for the express purpose of causing morbidity and mortality in a target population. While chemicals and toxins are bona fide threats, infectious pathogens represent the biggest problem for several reasons. First, there is a wide diversity of pathogenic agents (bacteria, viruses, fungi, etc.) that are capable of causing a variety of diseases. Second, unlike chemicals or toxins, microorganisms have the ability to replicate and survive in the environment. And third, in some cases microorganisms have the ability to spread from individual to individual. Once a weapon like this is deployed, it cannot be recalled or controlled, nor can it distinguish between friend and foe.
1.2. Category A, B, C biopathogens
A list of viral or bacterial pathogens, rated according to their predicted risk to national security, was compiled by the Centers for Disease Control and Prevention (Table 1 ). Category A agents pose the most serious threat because they can easily be disseminated or spread from person to person, can result in high mortality rates, may cause public panic, and would require special action for public health preparedness. Those pathogens in Category B are second in priority for their moderate ease of dissemination and their moderate morbidity and low mortality rates. In addition, CDC's diagnostic capacity and disease surveillance capability for these agents is currently inadequate and would require vast improvements. Agents comprising Category C include those that could be easily engineered for mass dissemination in the future because of their availability and their potential to be easily produced and disseminated.
Table 1.
Biological threat agents of concern
| Category A |
|---|
| Variola major (smallpox) |
| Bacillus anthracis (anthrax) |
| Yersinia pestis (plague) |
| Clostridium botulinum (botulism) |
| Francisella tularensis (tularemia) |
| Filoviruses and arenaviruses |
| Category B |
| Coxiella burnetti (Q fever) |
| Brucella spp. (brucellosis) |
| Burkholderia mallei (glanders) |
| Alphaviruses |
| Ricinus communis (ricin) |
| Clostridium perfringens (ɛ toxin) |
| Staphylococcus aureas (enterotoxin B) |
| Various food and waterborne agents (e.g. Salmonella and Vibrio ssp.) |
| Category C |
| Nipah virus |
| Hantaviruses |
| Tickborne hemorrhagic fever viruses |
| Tickborne encephalitis viruses |
| Yellow fever virus |
| Multiple-drug-resistant Mycobacterium tuberculosis |
| Other |
| SARS virus |
| West Nile virus |
Of the pathogens listed in Table 1, those that cause smallpox, anthrax, and plague are particularly dangerous because they are easily spread from person to person via a respiratory route. An accidental, planned, or natural outbreak of one of these agents with respiratory mode of transmission, combined with frequent worldwide travel prevalent in today's society could lead to disastrous consequences. The potential for this to occur was evident during the recent SARS outbreak. Had the coronavirus responsible for causing SARS been more virulent, or more easily transmitted, the outcome of last year's outbreak could have been devastating.
In addition to the introduction of natural pathogens into the human population by bioterrorists, the capability exists for pathogens to be purposefully engineered to become even more infectious. The ease with which pathogens may be made more virulent was demonstrated in experiments performed by Jackson et al. [1]. In these experiments an immune-suppressing gene was spliced into a mousepox virus, creating a virus that caused significant mortality, even in immunized mice.
1.3. Components of biowarfare defense
In order to effectively protect the population against infectious biowarfare threats, a number of measures need to be deployed: Detection (or surveillance), development of methods for monitoring our environmental space and detecting when an unusual or unexpected infectious agent arrives; Diagnostics, rapid methods for determining what type of pathogen has arrived, be it a toxin, bacteria or virus, and perhaps to profile its genome for drug resistance; Treatment, development of drugs or therapeutics to treat individuals exposed or about to be exposed (first-responders or military personnel) to non-traditional pathogenic agents; Decontamination, neutralization and/or removal from the environment where it was introduced or shed by infected individuals; Epidemiology, to identify all of the individuals who were or are at risk, and finally; Prophylaxis, the development and deployment of effective and safe vaccines to either break an epidemic or prevent primary infections (Fig. 1 ). While all of these measures are important in the overall biodefense scheme, the development of a new generation of vaccines is the focus of the subsequent discussion.
Fig. 1.
Essential elements of an effective biowarfare defense strategy. Vaccines can be designed for both therapeutic and prophylactic uses.
1.4. Five generations of vaccines
Vaccines are widely considered to be one of the miracles of modern medicine. Their widespread implementation has greatly reduced the disease burden from once common diseases such as pertussis and measles, and in the case of smallpox, completely eliminated the disease from the human population. Historically, there have been four generations of vaccines. For example, with regard to viral vaccines, the first generation is represented by live viruses, such as vaccinia virus or rabies virus, that were grown in animals, harvested, and then chemically inactivated (in the case of rabies virus). The second generation of vaccines was grown on embryonated chicken eggs, as typified by the traditional influenza vaccine. The advent of cell culture technology heralded the third generation of vaccines, such as measles or mumps that were grown in tissue culture cells. The fourth generation of vaccines is currently the state-of-the-art, as typified by the Hepatitis B vaccine, in which a component of a virus is cloned, over-expressed, and purified from a recombinant microorganism and then delivered as a subunit vaccine in combination with an adjuvant.
There are some unique challenges associated with developing vaccines against biowarfare agents which make traditional vaccine development approaches difficult. In many cases there is a paucity of research data available about the replication and virulence mechanisms of exotic pathogens. Likewise, in some cases such as smallpox, there are no appropriate animal models in which to conduct challenge experiments. Moreover, since many of the disease agents of interest cause death they cannot be tested in humans directly.
Vaccine development for biowarfare agents represents a conundrum. On one hand, the diseases in question have high associated morbidity and mortality, yet on the other hand, the likelihood of encountering these pathogens is extremely low. Since all vaccines have some side effects associated with them, one needs to carefully consider the risk-to-benefit ratio for these types of products. In order for a vaccine against a biowarfare agent to be widely implemented, it will have to be shown to be effective in an animal model and extremely safe. It is unlikely that traditional vaccine approaches will meet these requirements. Therefore, fifth generation vaccine delivery technologies will need to be employed. While there are a number of new vaccine delivery technologies in development that show promise, including DNA vaccines [2], [3], transdermal patches [4], [5], and recombinant plants [6], the use of commensal bacteria as an antigen delivery platform holds particular promise for this utility.
2. Commensal bacteria as vaccine vectors
2.1. General concept
Commensal bacteria are those that are found in the natural flora of living organisms. These bacteria reside mainly within mucosal sites such as the mouth, the nose, the lungs, and the gastrointestinal and urogenital tracts. Although normally tolerant of commensal organisms, mucosal surfaces present physical barriers to invading pathogens. They also provide a primary line of defense because they possess specific local immune systems that provide an important component of protective immunity [7]. Antigen delivery directly to mucosal sites via non-pathogenic commensal bacterial vectors could provide a significant advantage over some of the more traditional vaccines in that live bacteria are capable of stimulating sustained systemic as well as local immune responses [8]. In addition, commensal bacteria are able to colonize the niche invaded by the pathogen and stimulate an immune response at their portal of entry to prevent infection. This ability, combined with the facts that bacteria are easily administered (i.e. topically), are cost efficient, and are well-tolerated [9], makes live commensal bacteria attractive vehicles for subunit vaccine delivery. One such promising commensal bacterium is Streptococcus gordonii, a normal inhabitant of the human oral cavity.
The secreted protein expression (SPEX) or the bacterial commensal vector (BCV) systems are novel protein expression vector systems that use S. gordonii to produce and export proteins (Fig. 2 ). These systems use the conserved pathway that all Gram-positive bacteria utilize to export and anchor proteins on the cell surface [10]. The foreign proteins are directed to the cell surface of the Gram-positive host by using portions of the M protein, a surface protein of S. pyogenes, as a fusion partner. The C-terminal end of the M protein consists of a conserved immunogenic C-repeat region (CRR), a hydrophilic region, the highly conserved enzymatic cleavage site (Pro/Gly), and hydrophobic amino acids that act to anchor the M protein to the cell surface (Fig. 3A). If secreted proteins are needed a stop codon is introduced at the carboxy terminus of the foreign protein. Without the membrane-anchoring regions to retain the protein on the surface of S. gordonii, the fusion protein is translocated through the cell membrane of the Gram-positive host cell, and secreted directly into the culture medium (Fig. 3B). This is called the SPEX vector system (for secreted protein expression).
Fig. 2.
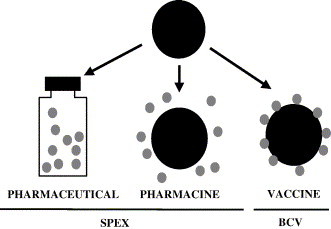
Uses of commensal bacteria in vaccine formulations.
Fig. 3.
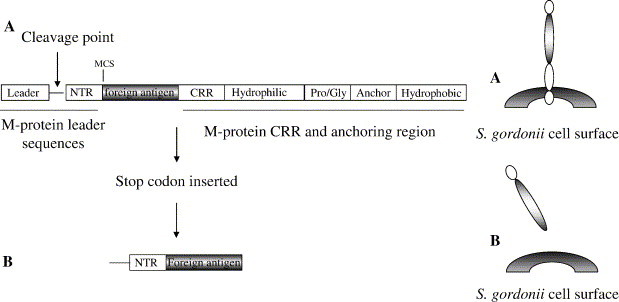
Construction of BCV recombinants. (A) Expression of antigen anchored to the bacterial surface using BCV. (B) Expression and secretion of antigen using SPEX.
2.2. Recombinant construction
After a candidate antigen has been identified, it is a relatively easy task to create the commensal BCV recombinant for its expression. Restriction sites are incorporated into primers homologous to the DNA sequence of interest, and the gene is synthesized by PCR. The DNA fragment is cloned into plasmid pSMB104. Because this plasmid cannot replicate in the Gram-positive host, it must integrate into the chromosome of the naturally competent S. gordonii in order to impart erythromycin resistance. Integration into the correct chromosomal location is confirmed by PCR, and expression of the encoded antigen is tested by Western blot using the appropriate antisera. Because the encoded antigen exists as an M-protein fusion, if no specific antiserum is available for the antigen, expression of the heterologous protein can be tested by reactivity with an anti-M protein monoclonal antibody [11].
A number of different promoter elements and insertion sites that enable the expression of multiple foreign inserts from the same recombinant have been identified in S. gordonii [12]. This could potentially allow for the simultaneous presentation of two or more antigens on a single bacterium. In addition, insertion sites containing inducible promoters have been characterized, thereby allowing the controlled expression of the heterologous gene [13]. Finally, it is possible to express the antigen from the chromosome or from a plasmid. The plasmid-based system can easily be adapted for expression in other Gram-positive species such as Lactococcus lactis. For example, we have successfully expressed portions of the S. pyogenes M6 protein on the surface of L. lactis from a plasmid (Hruby, unpublished).
2.3. Antigen expression
Proteins from virus, bacteria, protozoans, animals, and humans have been successfully expressed using S. gordonii. Table 2 lists the large number of heterologous proteins expressed by the BCV/SPEX system that have been shown to be reactive with the appropriate antisera. While this is an impressive list there are some limitations to the use of bacteria for the expression of eukaryotic proteins. Bacteria are not able to perform the post-translational processing, i.e. disulfide bonds, glycosylation, myristoylation, etc., important for the structure and function of many eukaryotic proteins. Hence, antibodies with specificities that include recognition of the modified proteins will not be generated in response to the BCV bacterial expression vector and alternative modes of antigen delivery would need to be explored.
Table 2.
Examples of foreign antigens expressed in the BCV and SPEX systems
| Protein | Source | Unfused mol. mass (kDa) | Form |
|---|---|---|---|
| Gp120 | HIV-1 | 1.7 | Anchored |
| Ag5.2 (hornet venom toxin) | Hornet | 23.5 | Anchored |
| E7 protein (transforming protein) | HPV-16 | 11.3 | Anchored |
| OVA (ovalbumin) | Chicken | 39.0 | Anchored |
| CRR (M6 protein) | S. pyogenes | 25.0 | Anchored |
| Other derivatives | 45.0 | Secreted | |
| gD2 | HSV | 36.6 | Anchored |
| Gp120 (envelope protein) | HIV | 55.1 | Anchored |
| Gp120 (V3 loop) | HIV | 12.9 | Anchored |
| FimA (N-terminal fimbrillin protein) | P. gingivalis | 22.3 | Anchored |
| Secreted | |||
| FimA (C-terminal fimbrillin protein) | P. gingivalis | 20.0 | Anchored |
| Secreted | |||
| IncA (inclusion membrane) | C. psittaci | 40.1 | Anchored |
| CWP-2 (cyst wall protein) | Giardia sp. | 40.0 | Anchored |
| L1 (early protein) | Canine oral papillomavirus | 10.5 | Intra |
| NY-ESO-1 (testicular tumor protein) | H. sapiens | 12.9 | Anchored |
| SEB (enterotoxin B) | S. aureus | 27.4 | Anchored |
2.4. Animal immunization
S. gordonii can readily colonize mice, rats, and monkeys as well as humans. Hence, the ability of recombinant BCV to induce an immune response can be examined in several animal models. The first demonstration that S. gordonii could successfully induce a mucosal and systemic immune response to foreign antigens was presented in experiments performed by Medaglini et al. [14]. Expression of the hornet venom allergen Ag5.2 as a fusion protein to the anchor region of the M6 protein of S. pyogenes induced a significant increase in Ag5.2-specific salivary and lung IgA as well as an increase in systemic IgG in mice.
Several additional studies have shown that a variety of antigens expressed on the surface of S. gordonii are immunogenic when delivered to mice or monkeys by either the systemic or mucosal routes [15], [16], [17], [18], [19], [20], [21], [22]. Immunization of rabbits with live or heat-killed S. gordonii expressing domains of the Porphyromonas gingivalis fimbrillin protein or the Bordetella S1 pertussis toxin induced antibodies specific for the encoded antigens [18], [23]. In addition to inducing potent humoral immune responses, recombinant S. gordonii can be internalized by human and mouse dendritic cells and its encoded antigens presented by MHC Class II molecules [24] as well as MHC class I molecules [25]. Most importantly, the potential for recombinant S. gordonii to be developed as a safe vaccine vector was supported by the finding that immunization of mice with S. gordonii expressing tetanus toxin fragment C (TTFC) induced protection from a lethal challenge with 50 LD50 of tetanus toxin [26]. Moreover, S. gordonii recombinants expressing FimA, the major subunit protein of P. gingivalis fimbriae, induced strong serum IgG and salivary IgA antibody responses, and protected against subsequent P. gingivalis-induced alveolar bone loss in germ free rats [27].
2.5. Human clinical trials
Two Phase I human clinical trials (total 150 subjects) were conducted at the Center for Vaccine Development, University of Maryland, School of Medicine, Baltimore, MD, to determine the time to eradication (spontaneous and antibiotic-induced) of a strain of S. gordonii that was experimentally implanted into the nose and mouth of healthy volunteers. These studies showed that antibiotic treatment cleared the implanted strain from subjects within a few days, and subjects receiving no antibiotic treatment were colonized for up to 28 days. Overall, the administration of S. gordonii to human volunteers was well tolerated (K. Kotloff, manuscript in preparation).
3. An example: BCV/smallpox vaccine
3.1. Smallpox as a BW pathogen
Smallpox was the most destructive disease in recorded history having killed, crippled, or disfigured nearly 1/10 of all humankind. In the 20th century alone, more than 300 million people succumbed to the disease. Variola virus, the causative agent of smallpox, is extremely infectious. It is usually acquired by inhalation of aerosol droplets spread by infected individuals. Variola major, a highly virulent form of smallpox, can cause death rates as high as 30%. The disease has a number of features that make it an “ideal” agent of bioterrorism (Table 3 ). Although smallpox is of paramount concern, one should not neglect other potential orthopoxvirus pathogens. For example, there have been several recent incursions of monkeypox virus into the human population. Such an event transpired in the Midwest in the summer of 2003 when prairie dogs sold as pets became infected with the monkeypox virus through contact with Gambian giant rats and other rodents. Over 70 people became ill during this outbreak [28]. Another more chilling scenario is the possibility that a laboratory strain of vaccinia virus or cowpox virus could be genetically engineered to produce a toxin to convert it into a potent pathogen [29]. Fortunately, orthopoxviruses are highly related at the DNA level (e.g. 90% between variola and vaccinia) making it likely that any vaccine developed would stimulate immunity to this entire group of viruses [30].
Table 3.
Attributes of smallpox as a biowarfare pathogen
| Easy to grow | Susceptible populace |
| No cold chain | Vaccine side-effects |
| Respiratory spread | No safe antiviral drug |
| Highly infectious | 10–14 day prodrome |
| Psychological effects | Potential global catastrophe |
| Environmental stability | Other poxviruses |
3.2. Existing vaccine/problems
Smallpox was eliminated from the US in the 1960s and subsequently, routine prophylactic immunization was discontinued. As a result, we now have a population that is highly susceptible to orthopoxvirus infection. However, mass immunization of the populace is not advisable as a response to the potential threat of bioterrorism because of the risk of serious complications from vaccination with the currently available vaccine. These potentially fatal complications include postvaccinial encephalitis, myopericarditis, progressive vaccinia, fetal vaccinia, and eczema vaccinatum (in addition to several other less serious complications). The need for a new, safe smallpox vaccine is significant.
A great deal of recent research has focused on the development of attenuated live vaccinia virus, mainly for use as recombinant vaccine delivery systems. One such virus is the modified vaccinia virus Ankara (MVA). Several studies show promising results as to the ability of MVA and other attenuated vaccinia to induce both humoral and cell-mediated immune responses against vaccinia and heterologous antigens [31], [32], [33], [34], [35], [36], [37]. It should be noted that although the MVA genome has deletions in many of the known poxvirus immunomodulatory genes, it still retains the ability to express a large number of viral gene products of unknown biological activity. Furthermore, MVA recombinants are being widely developed as therapeutic vaccines against cancers. Widespread vaccination of the populace could induce immunity against the MVA virus itself and thus limit its usability as an anti-cancer vector. Therefore, these attenuated vaccinia strains may not be as useful as hoped as a smallpox vaccine for the general population.
3.3. Candidate antigens
Unlike many other viruses, VV produces many virion forms, all of which appear to be infectious. The molecular details of poxvirus assembly and differentiation are thought to be as follows: After (or concurrent with) viral DNA replication, progeny DNA molecules, viral enzymes, and structural proteins coalesce to form pre-virion particles [38]. These particles acquire two membranes by budding through the intermediate compartment (between the endoplasmic reticulum and the Golgi) to become infectious intracellular mature virus (IMV). A portion of the IMV subsequently becomes enveloped by two additional membranes as it passes through the trans-Golgi network. This form of virus is called the intracellular enveloped virus (IEV). Following migration to the cell surface, the outermost IEV membrane fuses with the plasma membrane to give rise to enveloped virus (EV) [39]. The EV can either remain associated with the cell as a cell-associated enveloped virus, (CEV) or can be released into the external medium as extracellular enveloped virus (EEV) [40].
When developing a strategy for designing a vaccine against smallpox, it is important to consider the different forms of this family of poxvirus. The EEV form of the virus is important for the long range dissemination of the virus within the host while the IMV form is thought to be important for transmitting infection between hosts [40], [41]. Thus, a suitable smallpox vaccine must be effective against both forms of this virus. For this reason, several VV proteins that were chosen as BCV vaccine candidate proteins are associated with the IMV form of the virus while others are associated only with the EEV forms. Other criteria required for the candidate subunit VV proteins were one or more of the following: (1) Does the candidate elicit humoral or cell-mediated immune responses? (2) Have the antibodies specific for the proteins been shown to be protective in animal models? (3) Can the parenterally delivered purified VV protein elicit protection? and (4) Does the induction of a cell-mediated response by delivery of these genes in a DNA vaccine confer at least partial protection? Based on these criteria several vaccinia proteins were chosen as promising candidates for the commensal smallpox vaccine. Some of these proteins and their characteristics are listed in Table 4 .
Table 4.
Potential VV antigens for use in a subunit vaccine
| VV protein | Function | IMV or EEV | Immune response |
|---|---|---|---|
| L1R | IMV attachment and penetration | IMV | Strong humoral response; antibodies protective |
| D8L | Binds chondroitin sulfate on host cells | EEV | Strong humoral and cellular response |
| A27L | Virus-cell attachment and fusion: cell–cell spread | IMV | Protein protective; cellular immune response |
| A33R | Generation of actin-containing microvilli; cell–cell spread | EEV | Protein, DNA and antibodies protective |
Other possible alternatives include B5R, A4L, A10L and F13L.
3.4. BCV/VV recombinants
DNA encoding the VV Copenhagen proteins A27L, L1R, F13L, D8L, A33R, and A4L was cloned into the S. gordonii chromosome as described above. Expression of these proteins from S. gordonii was tested by streak blot Western analysis using antibodies specific for the M protein portion of the fusion protein [11]. Streak blot analysis tests for the surface expression of the heterologous proteins as whole bacteria are transferred to the nitrocellulose membrane. The VV A27L, F13L, and A4L fusion proteins were expressed at high levels (Fig. 4 ); however, expression of D8L, L1R, and A33R was very low (not shown). These three proteins contain large hydrophobic regions that could interfere with the processing and anchoring mechanism, or secretion through the membrane of S. gordonii. When these hydrophobic regions were deleted, S. gordonii was able to efficiently express the truncated proteins.
Fig. 4.
Expression of VV/M protein fusions in S. gordonii. Recombinant clones expressing the indicated full-length or deleted VV proteins, or the S. pyogenes M protein alone, were streaked onto a bacterial plate, lifted onto a nitrocellulose membrane and probed with monospecific antisera to the M protein to verify surface expression of fusion protein.
3.5. Animal immunization
Fig. 4 indicates that immunoreactive VV proteins can be expressed by S. gordonii. Preliminary experiments using the BCV∷A27L VV recombinants indicate that BALB/c mice inoculated either subcutaneously or intranasally had significant levels of A27L-specific serum IgG as well as salivary IgG and IgA antibodies. In addition, neutralizing antibodies (antibodies capable of inhibiting vaccinia virus-infection of tissue culture cells) were present in the serum of vaccinated animals. Additional studies using other BCV/VV recombinants are in progress and will include challenge studies to assess whether the recombinants are capable of inducing protective immunity against vaccinia virus infection.
The BALB/c mouse model for vaccinia virus challenge is an excellent system in which to study vaccines for their potential to prevent smallpox. However, because of the high degree of homology among the orthopoxvirus, several additional animal models using other orthopoxvirus can also provide important information. Smallpox is a respiratory pathogen acquired by aerosolized virus. Cowpox virus can efficiently infect BALB/c mice by aerosol administration and, as such, can be used to evaluate the efficacy of smallpox vaccines in preventing lethal respiratory infections. Perhaps the most rigorous model for testing candidate smallpox vaccines is the monkeypox model used in Rhesus macaques. Monkeypox is an orthopoxvirus that infects monkeys and sometimes humans and is over 85% homologous to the variola major genome [42]. This model has also been used to evaluate the efficacy of vaccines against smallpox.
4. Concluding remarks
4.1. Challenges facing BW vaccine development
Regardless of the technology employed, commercial development of vaccines against potential biowarfare pathogens will be a challenge for a number of reasons. First there will be the need to access sufficient federal funding to support the work. Given the political nature of the funding process, it is not known how long, or to what level funding will be available. Second, there is the difficulty of obtaining the pathogens themselves, given the recent legislation regulating their use. Third, there is the paucity of BSL-3 and BSL-4 laboratories available to use for the animal challenge experiments, as well as the experts to conduct the experiments. Fourth, there are the unanswered regulatory questions of what will be required in order to license a vaccine for human use. And finally, the potential market for a developed product must be considered. Who will be the consumer and how will they pay for the costs of the vaccine and its development? Regardless of the challenges, these are important goals in our defensive armature and it is hoped that the scientific, governmental and regulatory agencies will be able to effectively work together to make them a reality.
Acknowledgments
Over the years, development of the SPEX/BCV delivery system has been supported by DARPA, the National Institutes of Health, and SIGA Technologies. Special thanks to Kevin Jones and Elva Van Devender for critical reading of the manuscript, and to Lindsay Brown for technical assistance.
References
- 1.Jackson R.J., Ramsay A.J., Christensen C.D., Beaton S., Hall D.F., Ramshaw I.A. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J. Virol. 2001;75:1205–1210. doi: 10.1128/JVI.75.3.1205-1210.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly J.J., Ulmer J.B., Liu M.A. DNA vaccines. Dev. Biol. Stand. 1998;95:43–53. [PubMed] [Google Scholar]
- 3.Liu M.A. DNA vaccines: a review. J. Intern. Med. 2003;253:402–410. doi: 10.1046/j.1365-2796.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 4.Glenn G.M., Kenney R.T., Ellingsworth L.R., Frech S.A., Hammond S.A., Zoeteweij J.P. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev. Vaccines. 2003;2:253–267. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- 5.Hammond S.A., Guebre-Xabier M., Yu J., Glenn G.M. Transcutaneous immunization: an emerging route of immunization and potent immunostimulation strategy. Crit. Rev. Ther. Drug Carr. Syst. 2001;18:503–526. [PubMed] [Google Scholar]
- 6.Mason H.S., Warzecha H., Mor T., Arntzen C.J. Edible plant vaccines: applications for prophylactic and therapeutic molecular medicine. Trends Mol. Med. 2002;8:324–329. doi: 10.1016/s1471-4914(02)02360-2. [DOI] [PubMed] [Google Scholar]
- 7.McGhee J.R., Mestecky J., Dertzbaugh M.T., Eldridge J.H., Hirasawa M., Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 8.Liljeqvist S., Stahl S. Production of recombinant subunit vaccines: protein immunogens, live delivery systems and nucleic acid vaccines. J. Biotechnol. 1999;73:1–33. doi: 10.1016/s0168-1656(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 9.Medina E., Guzman C.A. Use of live bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine. 2001;19:1573–1580. doi: 10.1016/s0264-410x(00)00354-6. [DOI] [PubMed] [Google Scholar]
- 10.Schneewind O., Model P., Fischetti V.A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992;2:267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- 11.Jones K.F., Khan S.A., Erickson B.W., Hollingshead S.K., Scott J.R., Fischetti V.A. Immunochemical localization and amino acid sequences of crossreactive epitopes within the group A streptococcal M6 protein. J. Exp. Med. 1986;4:1226–1238. doi: 10.1084/jem.164.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrd C.M., Bolken T.C., Jones K.F., Warren T.K., Vella A.T., McDonald J., King D., Blackwood Z., Hruby D.E. Biological consequences of antigen and cytokine co-expression by recombinant Streptococcus gordonii vaccine vectors. Vaccine. 2002;20:2197–2205. doi: 10.1016/s0264-410x(02)00144-5. [DOI] [PubMed] [Google Scholar]
- 13.Bolken T.C., Franke C.A., Zeller G.O., Hruby D.E. Identification of an intragenic integration site for foreign gene expression in recombinant Streptococcus gordonii strains. Appl. Microbiol. Biotechnol. 2001;55:192–197. doi: 10.1007/s002530000519. [DOI] [PubMed] [Google Scholar]
- 14.Medaglini D., Pozzi G., King T.P., Fischetti V.A. Mucosal systemic immune responses to a recombinant protein expressed on the surface of the oral commensal bacterium Streptococcus gordonii after oral colonization. Proc. Natl. Acad. Sci. U. S. A. 1995;92:6868–6872. doi: 10.1073/pnas.92.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medaglini D., Rush C.M., Sestini P., Pozzi G. Commensal bacteria as vectors for mucosal vaccines against sexually transmitted diseases: vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine. 1997;15:1330–1337. doi: 10.1016/s0264-410x(97)00026-1. [DOI] [PubMed] [Google Scholar]
- 16.Di Fabio S., Medaglini D., Rush C.M., Corrias F., Panzini G.L., Pace M., Verani P., Pozzi G., Titti F. Vaginal immunization of Cynomolgus monkeys with Streptococcus gordonii expressing HIV-1 and HPV 16 antigens. Vaccine. 1998;16:485–492. doi: 10.1016/s0264-410x(97)80002-3. [DOI] [PubMed] [Google Scholar]
- 17.Pozzi G., Oggioni M.R., Manganelli R., Medaglini D., Fischetti V.A., Fenoglio D., Valle M.T., Kunkl A., Manca F. Human T-helper cell recognition of an immunodominant epitope of HIV-1 gp120 expressed on the surface of Streptococcus gordonii. Vaccine. 1994;12:1071–1077. doi: 10.1016/0264-410x(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.F., Halperin S.A., Wang H., MacArthur A. Oral colonization and immune responses to Streptococcus gordonii expressing a pertussis toxin S1 fragment in mice. FEMS Microbiol. Lett. 2002;208:175–178. doi: 10.1111/j.1574-6968.2002.tb11078.x. [DOI] [PubMed] [Google Scholar]
- 19.Maggi T., Oggioni M.R., Medaglini D., Bianchi Bandinelli M.L., Soldateschi D., Wiesmuller K.H., Muller C.P., Valensin P.E., Pozzi G. Expression of measles virus antigens in Streptococcus gordonii. New Microbiol. 2000;23:119–128. [PubMed] [Google Scholar]
- 20.Maggi T., Spinosa M., Ricci S., Medaglini D., Pozzi G., Oggioni M.R. Genetic engineering of Streptococcus gordonii for the simultaneous display of two heterologous proteins at the bacterial surface. FEMS Microbiol. Lett. 2002;210:135–141. doi: 10.1111/j.1574-6968.2002.tb11172.x. [DOI] [PubMed] [Google Scholar]
- 21.Oggioni M.R., Manganelli R., Contorni M., Tommasino M., Pozzi G. Immunization of mice by oral colonization with live recombinant commensal streptococci. Vaccine. 1995;13:775–779. doi: 10.1016/0264-410x(94)00060-z. [DOI] [PubMed] [Google Scholar]
- 22.Oggioni M.R., Medaglini D., Romano L., Peruzzi F., Maggi T., Lozzi L., Bracci L., Zazzi M., Manca F., Valensin P.E., Pozzi G. Antigenicity and immunogenicity of the V3 domain of HIV type 1 glycoprotein 120 expressed on the surface of Streptococcus gordonii. AIDS Res. Hum. Retrovir. 1999;15:451–459. doi: 10.1089/088922299311204. [DOI] [PubMed] [Google Scholar]
- 23.Sharma A., Nagata H., Hamada N., Sojar H.T., Hruby D.E., Kuramitsu H.K., Genco R.J. Expression of functional Porphyromonas gingivalis fimbrillin polypeptide domains on the surface of Streptococcus gordonii. Appl. Environ. Microbiol. 1996;62:3933–3938. doi: 10.1128/aem.62.11.3933-3938.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corinti S., Medaglini D., Cavani A., Rescigno M., Pozzi G., Ricciardi-Castagnoli P., Girolomoni G. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant Gram-positive bacteria to CD4+ T lymphocytes. J. Immunol. 1999;163:3029–3036. [PubMed] [Google Scholar]
- 25.Rescigno M., Citterio S., Thery C., Rittig M., Medaglini D., Pozzi G., Amigorena S., Ricciardi-Castagnoli P. Bacteria-induced neo-biosynthesis, stabilization, and surface expression of functional class I molecules in mouse dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5229–5234. doi: 10.1073/pnas.95.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medaglini D., Ciabattini A., Spinosa M.R., Maggi T., Marcotte H., Oggioni M.R., Pozzi G. Immunization with recombinant Streptococcus gordonii expressing tetanus toxin fragment C confers protection from lethal challenge in mice. Vaccine. 2001;19:1931–1939. doi: 10.1016/s0264-410x(00)00434-5. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A., Honma K., Evans R.T., Hruby D.E., Genco R.J. Oral immunization with recombinant Streptococcus gordonii expressing Porphyromonas gingivalis FimA domains. Infect. Immun. 2001;69:2928–2934. doi: 10.1128/IAI.69.5.2928-2934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDC Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin. Morb. Mortal. Wkly. Rep. 2003;52:642–646. [PubMed] [Google Scholar]
- 29.Hruby D.E. Vaccinia virus vectors: new strategies for producing recombinant vaccines. Clin. Microbiol. Rev. 1990;3:153–170. doi: 10.1128/cmr.3.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esposito J.J., Knight J.C. Orthopoxvirus DNA: a comparison of restriction profiles and maps. Virology. 1985;143:230–251. doi: 10.1016/0042-6822(85)90111-4. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez J.C., Gherardi M.M., Esteban M. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J. Virol. 2000;74:923–933. doi: 10.1128/jvi.74.2.923-933.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyatt L.S., Shors S.T., Murphy B.R., Moss B. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine. 1996;14:1451–1458. doi: 10.1016/s0264-410x(96)00072-2. [DOI] [PubMed] [Google Scholar]
- 33.Sutter G., Wyatt L.S., Foley P.L., Bennink J.R., Moss B. A recombinant vector derived from the host range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine. 1994;12:1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 34.Holzer G.W., Remp G., Antoine G., Pfeiderer M., Enzersberger O.M., Emsenhuber W., Hammerle T., Gruber F., Urban C., Falkner F.G., Dorner F. Highly efficient induction of protective immunity by a vaccinia virus vector defective in late gene expression. J. Virol. 1999;73:4536–4542. doi: 10.1128/jvi.73.6.4536-4542.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durbin A.P., Wyatt L.S., Siew J., Moss B., Murphy B.R. The immunogenicity and efficacy of intranasally or parenterally administered replication-deficient vaccinia-parainfluenza virus type 3 recombinants in rhesus monkeys. Vaccine. 1998;16:1324–1330. doi: 10.1016/s0264-410x(98)00010-3. [DOI] [PubMed] [Google Scholar]
- 36.Carroll M.W., Overwijk W.W., Chamberlain R.S., Rosenberg S.A., Moss B., Restifo N.P. Highly attenuated modified vaccinia virus Ankara (MVA) as an effective recombinant vector: a murine tumor model. Vaccine. 1997;15:387–394. doi: 10.1016/s0264-410x(96)00195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belyakov I.M., Moss B., Strober W., Berzofsky J.A. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4512–4517. doi: 10.1073/pnas.96.8.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss B. Poxviridae and their replication. In: Fields B., Knipe D., editors. vol. 2. Raven Press; New York: 1990. pp. 2079–2111. (Virology, 2nd edn). [Google Scholar]
- 39.Payne L.G., Kristenson K. Mechanism of vaccinia virus release and its specific inhibition by N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine. J. Virol. 1979;32:614–622. doi: 10.1128/jvi.32.2.614-622.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blasco R., Moss B. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 1992;66:4170–4179. doi: 10.1128/jvi.66.7.4170-4179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith G.L., Vanderplasschen A. Extracellular enveloped vaccinia virus. Entry, egress, and evasion. Adv. Exp. Med. Biol. 1998;440:395–414. [PubMed] [Google Scholar]
- 42.Shchelkunov S.N., Totmenin A.V., Babkin I.V., Safronov P.F., Ryazankina O.I., Petroy N.A., Gutorov V.V., Uvarova E.A., Mikheev M.V., Sisler J.R., Esposito J.J., Jahrling P.B., Moss B., Sandakhchiev L.S. Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett. 2001;509:66–70. doi: 10.1016/S0014-5793(01)03144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


