Highlights
-
•
IgM+ MBCs are early responders in malaria and may be vital in parasite clearance.
-
•
MBC heterogeneity may be expanded to tackle varying antigen in chronic infection.
-
•
Atypical MBCs, CD21neg and T-bet+CD11c+ B cells may share transcriptional programs.
-
•
In vivo studies will allow insight into intrinsic and extrinsic regulators of MBCs.
Abstract
Vaccine success relies on the formation of immunity. Humoral immunity is critical and is mediated by long-lived antibody-secreting cells and memory B cells (MBCs). Chronic infectious diseases cause a significant global burden of disease; pathogens that evade the immune system can cause phenotypical and functional changes to immune memory populations. Thus, recent studies have focused on MBC subset function. IgM+ MBCs have emerged as important early responders in malaria. Atypical MBCs have functional qualities associated with exhaustion in chronic infectious diseases, but the requirements for their formation and where they localize remains unknown. Similarly, the T-bet-driven transcriptional program drives formation of MBCs phenotypically similar to atypical MBCs. Identifying protective or detrimental roles of MBC subsets, and their regulators, will be important for clinical intervention.
Current Opinion in Immunology 2017, 45:89–96
This review comes from a themed issue on Lymphocyte development and activation
Edited by David Tarlinton and Gabriel D Victora
For a complete overview see the Issue and the Editorial
Available online 17th March 2017
http://dx.doi.org/10.1016/j.coi.2017.03.004
0952-7915/© 2017 Elsevier Ltd. All rights reserved.
Introduction
Infectious diseases are responsible for a significant global burden of disease [1, 2, 3, 4, 5]. These include human immunodeficiency virus (HIV) (which causes acquired immune deficiency syndrome (AIDs)), malaria, tuberculosis [3], neglected tropical diseases (caused by protozoan parasites, parasitic worms and animal-borne viruses) [4] and newly emerging infectious agents (including severe acute respiratory syndrome-coronavirus, Middle East respiratory syndrome-coronavirus, West Nile virus, Nipah virus, methicillin-resistant Staphylococcus aureus, novel influenza-A strains and the Ebola virus) [5]. Diseases such as HIV/AIDS, malaria and tuberculosis are particularly more pronounced in low socio-economic regions of developing countries [1, 2] and high rates of co-infection cause additional obstacles to successful clinical intervention [1].
Protective immunity depends on the longevity and rapid responsiveness to antigen of immune memory populations [6]. In the humoral arm of the immune system, immune memory consists of long-lived antibody-secreting plasma cells and memory B cells (MBCs). These high-affinity populations are generated during the initial immune response to infection and are mainly generated within the germinal center (GC), although they can also be generated in GC-independent pathways. Vaccines replicate this process without the threat replicating pathogens pose to the body. While vaccines have been the most effective intervention for many infectious diseases, there are still pathogens that have evaded successful vaccine design [7]. This can be the result of the genetic diversity of the pathogen, immune subversion and immunosuppression, and inconsistencies between local and systemic responses, for example, where a vaccine is not targeted to generating a mucosal immune response that may be critical for successful protection. In particular, persisting or recurrent pathogens such as HIV and Plasmodium are linked to an inadequate humoral response, in which the production of high-affinity neutralizing antibodies is delayed and the immune memory repertoire appears to be ineffective.
MBC population heterogeneity provides the functional diversity needed for the immune system to fight infection and disease [6]. MBC subsets can be segregated based on B cell receptor (BCR) isotype, phenotype, and distinct functional responses in health or immune disorders. Disease can drive alterations to MBC phenotype and function, which in turn influence antibody responses to vaccination [6]. The characteristics and regulators that shape the role of MBC subsets in infection and disease remain to be elucidated, and delineating these will provide insight into whether MBCs are beneficial or detrimental in different disease states. This review will focus on dissecting the diversity and roles of MBCs currently trending in the literature, including immunoglobulin (Ig) M+, atypical and T box transcription factor (T-bet)+ MBCs, and their roles during infectious disease.
Function of IgM+ MBC in protective responses and in disease
The MBC population is heterogenous in both phenotype and function [8]. This diversity allows the population to balance self-renewal with differentiation during a secondary response. Classically, MBCs have been distinguished by their Ig isotype: IgM+ or class switched (IgG+ IgA+ and IgE+) [6], but they can also be distinguished into subsets by surface markers such as CD73, CD80 and PD-L2 [9•]. MBC function had been defined by these subdivisions, for example, IgM+ MBCs proliferate and form secondary GCs for self-renewal and longevity of the MBC population, whilst IgG+ MBCs differentiate to become antibody-secreting cells [6] (Figure 1 , top panel). This distinction, however, is not absolute. It appears that the maturity of an MBC is the main predictor of whether it will reenter GCs or differentiate into an antibody-secreting cell during a secondary response, which correlates but is not absolutely dependent on Ig isotype. It is now evident that IgM+ MBCs can be both GC-derived and GC-independent, have unique phenotypes and functional properties compared to switched MBC, and persist to provide long-term memory [9•, 10, 11].
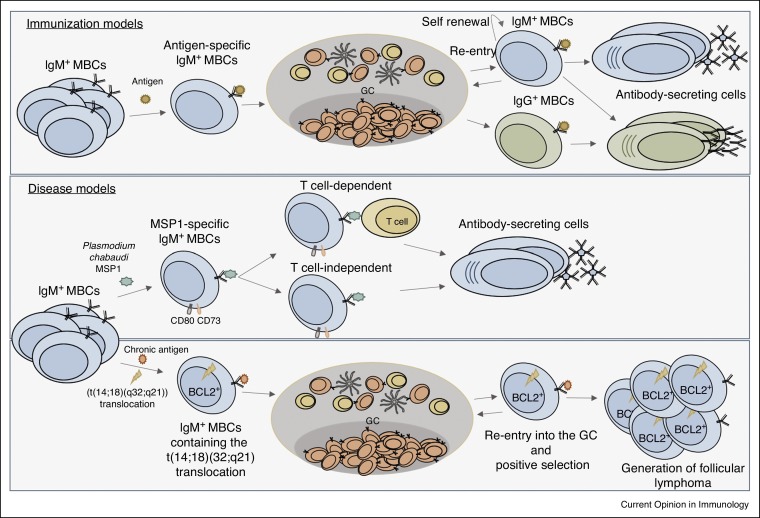
Figure 1.
Diversity of IgM+ MBCs in health and disease. (Top panel) Antigen-specific IgM+ MBCs can renew the MBC repertoire through GC formation and re-entry, form antibody-secreting cells and undergo isotype switching to form IgG+ MBCs that terminally differentiate into antibody-secreting cells. (Middle panel) MSP1-specific IgM+ MBCs encompass characteristics of switched MBC, including the phenotypic expression of CD80 and CD73, and the ability to differentiate into antibody-secreting cells in either a T-dependent or T-independent manner. (Bottom panel) IgM+ MBCs that constitutively express BCL2 and contain the t(14;18)(q32;q21) translocation, are able to re-enter the GC and undergo positive selection to drive the formation of follicular lymphoma.
While recent research has focused on defining subsets in vivo using immunization models, the role of IgM+ MBCs in chronic and recurrent disease remains ill defined. To this end, Pepper and colleagues produced B cell tetramers specific for blood stage Plasmodium chabaudi antigen, merozoite surface protein 1 (MSP1), to study antigen-specific MBCs in a model of malaria. MSP1-specific IgM+ MBCs were comparable to switched MBCs with respect to phenotypic expression of CD80 and CD73, their somatic hypermutation rates, affinity and function [12••]. Interestingly, MSP1-specific MBCs were early responders, persisted and were dominant producers of antibody-secreting cells during secondary responses [12••], suggesting that these cells play a protective role during reinfection (Figure 1, middle panel). Further, they were able to form antibody-secreting cells in the absence of T cell help. Genetic resistance to malaria has been correlated with enhanced IgM responses compared to IgG responses [8], also suggesting a possible role for IgM+ MBCs in long term protection from malaria. Given the propensity of IgM+ MBCs to generate new GCs [10, 11], IgM+ MBCs may also be important for expanding the MBC repertoire to target diversifying antigens.
By providing the ability of GC re-entry, the option to renew the repertoire allows for flexibility to contain fast evolving antigens. However, if a mutation disrupts this process it may lead to development of disease. In this regard, in a chronic infection model where MBCs constitutively express B cell lymphoma 2 (BCL2) and contain the follicular lymphoma hallmark, IgH/BCL2 (t(14;18)(q32;q21)) translocation, IgM+ MBCs are able to re-enter GCs repeatedly upon chronic immunization, leading to and driving follicular lymphomagenesis [13•] (Figure 1, lower panel). This finding revealed a novel role for IgM MBCs in cancer development and progression. A number of B cell leukemias and lymphomas express IgM and resemble MBCs in terms of phenotype and genetics [14]. It is unknown why IgM is commonly expressed, but considering IgM+ MBCs are poised to proliferate upon activation in comparison to switched MBCs, IgM+ MBCs may be at an increased risk to transform into constantly proliferating lymphomas [14].
Atypical MBCs are associated with exhaustion in chronic or recurrent infectious disease
MBC phenotype and function appears to be correlated with the nature of the infectious agent. In particular, the emergence of atypical or unresponsive MBCs has been associated with the presence of persistent antigen [15, 16]. Several studies have described the presence of an atypical MBC subset that is prominent in chronic infections including human immunodeficiency virus (HIV) [16, 17], malaria [15, 18•, 19, 20], HIV/malaria co-infected patients [21], hepatitis C [22] and cytomegalovirus infection [23]. These atypical MBCs are reportedly functionally impaired, often referred to as ‘exhausted’, due to the upregulation of inhibitory receptors, modulation of tissue trafficking receptors and poor proliferative abilities and antibody responses [15, 16, 18•, 19]. HIV-specific responses are enriched in functionally impaired atypical MBCs, suggesting that these cells are unable to produce antibody to target HIV antigens and explaining the poor HIV-specific antibody response in individuals [16]. This study was confirmed at a single cell level, using a trimeric HIV envelope probe, demonstrating that HIV-specific responses were enriched in an activated MBC subset prone to apoptosis [17]. Although a similar subset has been characterized in malaria patients, it is unclear whether atypical MBC are dysfunctional in malaria patients and thus whether they can be defined as the same subset as that found in HIV patients. A number of studies provide evidence of atypical dysfunctional MBCs in malaria [18•, 19, 20], although a conflicting study showed that atypical memory subsets could proliferate and secrete antibody [24••]. While the latter study suggested atypical MBCs might originate from different precursors due to differences in their somatic hypermutation loads and clonal relationship, others have shown a common developmental history [19, 25]. This discrepancy may be related to the large diversity of antigens in this disease. The majority of studies to date did not profile antigen specificity of atypical MBCs, making it difficult to fully elucidate their biological roles in disease. B cell tetramers targeted for different antigens will be useful in future studies of phenotypes and functions of antigen-specific MBCs in responses to persisting or recurrent pathogens that can lead to chronic disease.
MBC tissue localization and the importance of defining tissue-based memory
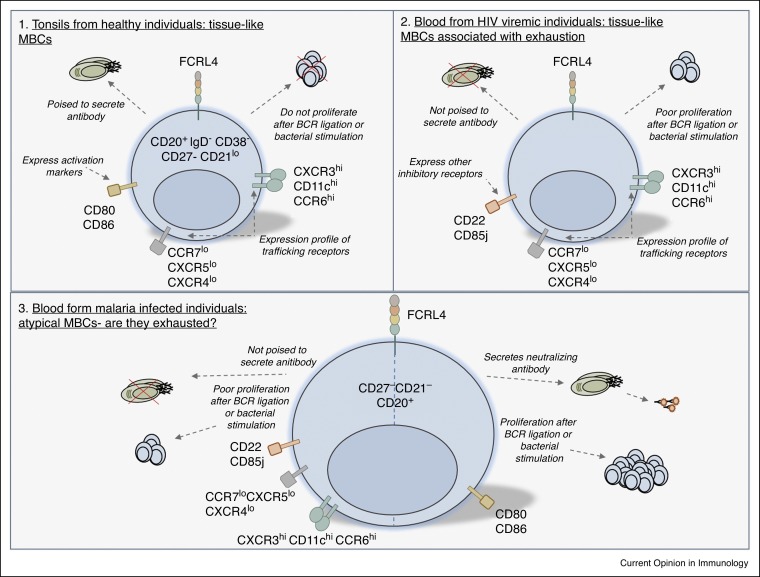
The markers that are used to distinguish atypical MBCs from other MBC subsets include downregulation of CD27 and CD21 [15, 16, 17, 18•, 19, 20, 21, 22, 23, 24••, 25, 26]. Atypical MBCs and other CD21neg B cell subsets [27, 28, 29, 30, 31, 32] are enriched in the peripheral blood of patients with diseases involving chronic immune stimulation [33]. While CD21neg subsets tend to differ in the expression of CD27 and BCRs in different diseases, atypical and CD21neg subsets exhibit common expression of many inhibitory and cell activation receptors that are associated with exhaustion and anergy (Table 1 ) [33]. Commonalities across subsets includes the increased expression of CD11c and CXCR3, which are molecules involved in homing to sites of inflammation and reduced expression of CCR7, CD62L, CXCR4 and CXCR5 which are required for migration to spleen or lymph nodes (Table 1). This suggests that atypical MBCs and CD21neg B cells migrate to sites of inflammation. Fc-receptor like (FCRL) receptors, in particular FCRL4, was also commonly expressed on these subsets (Table 1). FCRL4 MBCs were first described as tissue-localized memory cells in healthy individuals [34], and are speculated to define atypical MBCs in malaria [15, 24••] and HIV [16] (Figure 2 ). Other more recent studies suggest that functionally impaired atypical memory in malaria is delineated by the expression of FCRL5 rather than FCRL4 [18•, 19, 35], which may be due to cross reactivity of FCRL4 antibodies used in previous studies [18•], a factor that needs to be addressed in future studies.
Table 1.
Inhibitory and activation receptor/gene expression on atypical MBCs and CD21neg B cells. Inhibitory and activation receptor/gene expression (↑—upregulation and ↓—downregulation) is shown compared to classical MBCs, naive or CD21+ B cells
| Infection/disease | Receptor/gene expression | Reference |
|---|---|---|
| Malaria | CD11c ↑, CD22 ↑, CD72 ↑, CD85j ↑, CD200R1 ↑, CXCR3 ↑, FCGR2B ↑, FCRL3 ↑, FCRL4 ↑, FCRL5 ↑, LILRB1 ↑, LILRB2 ↑, SIGLEC-6 ↑. | [20, 23, 24••, 29] |
| CCR7 ↓,CD62L ↓, CXCR5 ↓. | ||
| Human immunodeficiency virus | CD11c ↑,CD22 ↑, CD72 ↑, CD85j ↑, CXCR3 ↑, CCR6 ↑, FCGR2B ↑, FCRL4 ↑, LILRB1 ↑, LILRB2 ↑, SIGLEC-6 ↑. | [20, 22] |
| CCR7 ↓, CD62L ↓, CXCR5 ↓. | ||
| Cytomegalovirus | CD11c ↑, CD22 ↑, CD72 ↑, CD85j ↑ CXCR3 ↑. | [23] |
| CCR7 ↓, CD62L ↓, CXCR4 ↓, CXCR5 ↓. | ||
| Primary Sjogren’s syndrome | CD11c ↑, CD22 ↑, CD72 ↑, FCRL2 ↑, FCRL3 ↑, SIGLEC ↑. | [31] |
| CD1c ↓. | ||
| Rheumatoid arthritis and common variable immune deficiency | CD11c ↑, CD72 ↑, CRL2 ↑, CXCR3 ↑, CXCR6 ↑, FCGR2B ↑, FCRL3 ↑, FCRL5 ↑, FCRLM1 ↑, FCRLM2 ↑ LILRB ↑, SIGLEC ↑ SOX5 ↑. | [32, 33, 34] |
| BCMA ↓, CCR7 ↓, CD40 ↓, CXCR5 ↓, IL4R ↓, IL13R ↓ OX40L ↓. | ||
| Hepatitis C associated-mixed cryoglobulinemia | CBLB ↑, CD22 ↑, CD72 ↑, CD200R1 ↑, EGR2 ↑, FCRL4 ↑, LAX1 ↑, LGALS1 ↑, Stra13 ↑, ZEB2 ↑. | [35, 36••, 37] |
| FOXP1 ↓, IL-4R ↓, TCL1 ↓. | ||
Figure 2.
Diversity of FCRL4+ MBCs in health and disease. (1) FCRL4+ tissue-like MBCs derived from tonsils of healthy individuals are poised to secrete antibody but do not proliferate after BCR ligation or bacterial stimulation. Tissue-like MBCs express high levels of CXCR3, CD11c and CCR6 and low levels of CCR7, CXCR5 and CXCR4. These cells also express activation markers CD80 and CD86. (2) FCRL4+ tissue-like MBCs found in the blood of patients with HIV are associated with exhaustion. Like tonsil-derived MBCs, these have a similar expression profile of trafficking receptors, but express additional inhibitory receptors and proliferate poorly after BCR ligation or bacterial stimulation. (3) Whether FCRL4+ atypical MBCs found in malaria are exhausted remains controversial. While some studies have shown factors associated with exhaustion including a lack of antibody secretion, poor proliferation, inhibitory receptor expression and similar trafficking expression profile to FCRL4+ MBCs in HIV, others have shown these cells secrete neutralizing antibody, proliferate and express activation markers.
The role of atypical MBCs in tissue localization is undefined in disease and warrants investigation, given that a recent study suggested tissue localization could provide broader protection from highly mutating viruses [36••]. MBCs residing at the site of influenza infection develop from persistent GCs in the lung, are highly mutated, and have cross-reactive antibody responses that neutralize flu escape variants [36••]. Defining the pathways that result in cross-reactivity may have significant implications for the creation of broadly protective vaccines against highly mutating pathogens and may help to shed light on discrepancies between systemic versus localized responses to vaccines. Furthermore, characterizing migration to and out of tissues will shed light on intrinsic and extrinsic regulators of MBCs during chronic infection. For example, IFN-I production in chronic lymphocytic choriomeningitis virus (LCMV) infection recruits T cells, monocytes and dendritic cells to directly act on antigen-specific B cells and thus inhibit LCMV-neutralizing antibody responses [37, 38, 39]. Currently there are no in vivo models of atypical memory, yet understanding migration and localization of both classical and atypical memory will be important in determining targets for clinical intervention.
Formation and regulation of T-bet+ MBCs after infection and in autoimmune conditions
Transcription factors are essential in regulating B cell differentiation [40]. B cell-specific T-bet expression is essential for IgG2a/c MBC survival [41], drives interferon γ (IFNγ)-mediated IgG2a/c class switching and upregulates CXCR3 expression on MBCs to allow migration to sites of inflammation [42]. In chronic LCMV infection, B cell-specific T-bet controls IgG2a/c production and is required to contain persistent infection [43•] (Figure 3 ), as well as a transcriptional program that affects cell migration, localization, differentiation, proliferation and antibody development compared to T-bet− cells [43•]. In particular, T-bet+ MBCs express high levels of CXCR3 (Figure 3), which is also expressed on atypical MBCs [15, 16, 23] and other CD21neg B cell subsets [33] (Table 1). Consistent with CXCR3 expression, T-bet MBCs have been found in small intestinal mucosa [43•], which is a site of inflammation induced by chronic LCMV infection [44]. This suggests that T-bet is involved in the control of multiple immune functions including tissue localization during chronic infection [43•]. In this regard, T-bet may be involved in the formation and function of MBCs that reside at the site of influenza infection and have cross-reactive antibody responses that neutralize flu escape variants [36••]. While there is a lack of studies examining the role of T-bet on atypical MBCs; one study has shown high expression of the T-bet gene, Tbx21 in FCRL5+ cells in healthy individuals [35]. Furthermore, T-bet is reported to regulate CD8+ T cell exhaustion in HIV infection [45]. Revealing a role for T-bet in formation and/or function of atypical MBCs may prove additional insight into dysfunctional humoral responses.
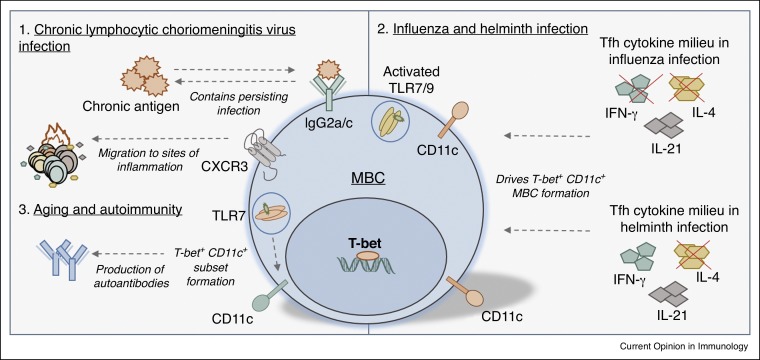
Figure 3.
Roles of T-bet+ MBCs in disease. (1) T-bet and IgG2a/c are required to contain persisting infection in a chronic model of LCMV. T-bet+ MBCs express high levels of CXCR3, a chemokine involved in the migration of cells to sites of inflammation. (2) In TLR7-activated B cells, the Tfh cytokine milieu regulates the formation of T-bet+ CD11c+ MBCs in infection. In influenza infection, the absence of IFN-γ and IL-4 drives T-bet+ CD11c+ subset formation, while in helminth infection the absence of IL-4 drives the formation of this subset. (3) TLR7 activation in MBCs associated with aging and autoimmunity also drives the formation of T-bet+ and CD11c+ subsets, contributing to the production of autoantibodies.
T-bet and CD11c expression on Toll-like receptor (TLR)-activated B cells is regulated by the T-follicular helper cell-associated cytokines, IL-21, IFN-γ and IL-4 [46•]. In particular, T-bet+ CD11c+ MBCs are promoted in IL-4-low environments. For example, after infection with influenza (which induces IL-21, IFN-γ and IL-4 microenvironments), T-bet+ CD11c+ MBCs are present in large quantities in IFN-γ and IL-4 double knockout mice, but not in IFN-γ knockout mice (Figure 3). In helminth infection (IL-21 and IL-4 present), T-bet+ CD11c+ MBCs are present in IL-4 knockout mice but not in wild-type mice (Figure 3). TLR7 activation also induces a CD11c+ subset in age-associated or autoimmune-associated B cells [28]. Interestingly, transcript profiling of this subset revealed high expression of T-bet [28] (Figure 3). This subset is CD21neg, has blunted BCR responses (similar to atypical MBCs), accumulates in aged female mice, forms early in female autoimmune prone mice and is present in patients with rheumatoid arthritis [28, 47]. Furthermore, this subset directly contributes to the production of autoantibodies and therefore may contribute to autoimmunity [28] (Figure 3). CD11c is also highly expressed on atypical MBCs, other CD21neg subsets (Table 1) [15, 16, 19, 20, 24••, 26, 28, 29], and in FCRL4+ MBCs that may play a role in mucosal defense [48]. Interestingly, many CD21neg subsets in disease also express IgM [33]. Determining the relationship between TLR engagement, T-bet activation, CD21, CXCR3, CD11c and IgM in MBCs may reveal mechanistic commonalities in dysfunctional humoral disorders that are characterized by aberrant immune stimulation.
Formation and regulation of MBC subsets
The common expression of inhibitory, migration and activation markers on atypical MBCs (Table 1) and in T-bet+ MBCs present in chronic infection, aging and autoimmunity suggests that a T-bet-driven transcriptional program may regulate the formation and/or function of atypical memory, in addition to age/autoimmune-associated subsets. Several questions arise: Is T-bet involved in tissue localization of atypical MBCs and other MBC subsets, if so how does this contribute to disease? In IL-4 low disease environments, such as T helper cell 1 dominant conditions, do T-bet+ CD11c+ MBCs possess the phenotypical and functional qualities of exhausted atypical MBCs and CD21neg cells? It will be critical to determine both intrinsic and extrinsic regulators of these cells to understand their contribution to immune responses in both health and disease. Although this population has been described in many studies, there is currently no in vivo model of atypical MBCs. Developing such a model would allow mechanistic studies into the formation, function and cellular interactions between different memory subsets, as well as with T cells, in an environment of persistent antigen. These studies may reveal if atypical MBCs and related subsets are protective or detrimental in acute or chronic infections.
Conclusions
In diseases involving highly diversifying antigen, MBC heterogeneity may be central in containing chronic infection. IgM+ MBCs have emerged as important early responders in malaria re-infection and therefore may be vital in early parasite clearance in humans. Whether IgM+ MBCs function as early responders in other chronic infections remains to be determined. Atypical MBCs and other CD21neg subsets are exhausted in several different chronic infectious diseases, but how these different diseases affect this subset and how they are formed and function within these diseases remains unknown. Targeting commonly expressed inhibitory and activation markers may provide a therapeutic avenue to ameliorate putative detrimental effects of atypical MBCs. Short inhibitory RNA knockdown of inhibitory receptors on atypical MBCs in HIV enhanced BCR-mediated proliferation, in particular the largest effect was observed when targeting FCRL4 and sialic acid-binding immunoglobulin-type lectin 6 (Siglec-6) [49]. These and other commonly expressed receptors may be potential targets for receptor antagonists to reactivate MBCs and induce protective antibody responses. Lastly, T-bet-driven transcriptional regulation may be involved in the formation and function of atypical MBCs and age/autoimmune-associated subsets. Identifying the characteristics and regulators of MBC heterogeneity in the context of chronic infection, and the impact on the generation of classical memory, will be important for innovative solutions to eradicate these diseases.
Conflict of interest
The authors declare no conflict of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was supported by a National Health and Medical Research Council (NHMRC) project grant (1057707) and an NHMRC Career Development Fellowship to KLG-J.
References
- 1.Bhutta Z.A., Sommerfeld J., Lassi Z.S., Salam R.A., Das J.K. Global burden, distribution, and interventions for infectious diseases of poverty. Infect Dis Poverty. 2014;3:21. doi: 10.1186/2049-9957-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heesterbeek H., Anderson R.M., Andreasen V., Bansal S., De Angelis D., Dye C., Eames K.T., Edmunds W.J., Frost S.D., Funk S. Modeling infectious disease dynamics in the complex landscape of global health. Science. 2015;347:aaa4339. doi: 10.1126/science.aaa4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray C.J., Ortblad K.F., Guinovart C., Lim S.S., Wolock T.M., Roberts D.A., Dansereau E.A., Graetz N., Barber R.M., Brown J.C. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Utzinger J., Becker S.L., Knopp S., Blum J., Neumayr A.L., Keiser J., Hatz C.F. Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly. 2012;142:w13727. doi: 10.4414/smw.2012.13727. [DOI] [PubMed] [Google Scholar]
- 5.Morens D.M., Fauci A.S. Emerging infectious diseases: threats to human health and global stability. PLoS Pathog. 2013;9:e1003467. doi: 10.1371/journal.ppat.1003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurosaki T., Kometani K., Ise W. Memory B cells. Nat Rev Immunol. 2015;15:149–159. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- 7.Li S., Plebanski M., Smooker P., Gowans E.J. Editorial: why vaccines to HIV HCV, and malaria have so far failed-challenges to developing vaccines against immunoregulating pathogens. Front Microbiol. 2015;6:1318. doi: 10.3389/fmicb.2015.01318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Good-Jacobson K.L., Tarlinton D.M. Multiple routes to B-cell memory. Int Immunol. 2012;24:403–408. doi: 10.1093/intimm/dxs050. [DOI] [PubMed] [Google Scholar]
- 9•.Zuccarino-Catania G.V., Sadanand S., Weisel F.J., Tomayko M.M., Meng H., Kleinstein S.H., Good-Jacobson K.L., Shlomchik M.J. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol. 2014;15:631–637. doi: 10.1038/ni.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a B cell transfer system, these authors showed that cell surface markers other than IgM and Ig class-switch could segregate memory B cell subsets and their function.
- 10.Pape K.A., Taylor J.J., Maul R.W., Gearhart P.J., Jenkins M.K. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dogan I., Bertocci B., Vilmont V., Delbos F., Megret J., Storck S., Reynaud C.A., Weill J.C. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 12••.Krishnamurty A.T., Thouvenel C.D., Portugal S., Keitany G.J., Kim K.S., Holder A., Crompton P.D., Rawlings D.J., Pepper M. Somatically hypermutated plasmodium-specific IgM+ memory B cells are rapid, plastic, early responders upon malaria rechallenge. Immunity. 2016;45:402–414. doi: 10.1016/j.immuni.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a tetramer, these authors demonstrated the functional role of IgM memory B cells in immune responses to malaria.
- 13•.Sungalee S., Mamessier E., Morgado E., Gregoire E., Brohawn P.Z., Morehouse C.A., Jouve N., Monvoisin C., Menard C., Debroas G. Germinal center reentries of BCL2-overexpressing B cells drive follicular lymphoma progression. J Clin Investig. 2014;124:5337–5351. doi: 10.1172/JCI72415. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that IgM memory B cells could play a detrimental role in lymphoma progression.
- 14.Seifert M., Kuppers R. Human memory B cells. Leukemia. 2016;30:2283–2292. doi: 10.1038/leu.2016.226. [DOI] [PubMed] [Google Scholar]
- 15.Weiss G.E., Crompton P.D., Li S., Walsh L.A., Moir S., Traore B., Kayentao K., Ongoiba A., Doumbo O.K., Pierce S.K. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183:2176–2182. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moir S., Ho J., Malaspina A., Wang W., DiPoto A.C., O’Shea M.A., Roby G., Kottilil S., Arthos J., Proschan M.A. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kardava L., Moir S., Shah N., Wang W., Wilson R., Buckner C.M., Santich B.H., Kim L.J., Spurlin E.E., Nelson A.K. Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J Clin Investig. 2014;124:3252–3262. doi: 10.1172/JCI74351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Sullivan R.T., Kim C.C., Fontana M.F., Feeney M.E., Jagannathan P., Boyle M.J., Drakeley C.J., Ssewanyana I., Nankya F., Mayanja-Kizza H. FCRL5 delineates functionally impaired memory B cells associated with Plasmodium falciparum exposure. PLoS Pathog. 2015;11:e1004894. doi: 10.1371/journal.ppat.1004894. [DOI] [PMC free article] [PubMed] [Google Scholar]; This authors demonstrate that FCRL5 was associated with dysregulated memory B cell s in malaria.
- 19.Portugal S., Tipton C.M., Sohn H., Kone Y., Wang J., Li S., Skinner J., Virtaneva K., Sturdevant D.E., Porcella S.F. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife. 2015;4 doi: 10.7554/eLife.07218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Illingworth J., Butler N.S., Roetynck S., Mwacharo J., Pierce S.K., Bejon P., Crompton P.D., Marsh K., Ndungu F.M. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol. 2013;190:1038–1047. doi: 10.4049/jimmunol.1202438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramaniam K.S., Skinner J., Ivan E., Mutimura E., Kim R.S., Feintuch C.M., Portugal S., Anastos K., Crompton P.D., Daily J.P. HIV malaria co-infection is associated with atypical memory B cell expansion and a reduced antibody response to a broad array of Plasmodium falciparum antigens in Rwandan adults. PLoS One. 2015;10:e0124412. doi: 10.1371/journal.pone.0124412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doi H., Tanoue S., Kaplan D.E. Peripheral CD27−CD21− B-cells represent an exhausted lymphocyte population in hepatitis C cirrhosis. Clin Immunol. 2014;150:184–191. doi: 10.1016/j.clim.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dauby N., Kummert C., Lecomte S., Liesnard C., Delforge M.L., Donner C., Marchant A. Primary human cytomegalovirus infection induces the expansion of virus-specific activated and atypical memory B cells. J Infect Dis. 2014;210:1275–1285. doi: 10.1093/infdis/jiu255. [DOI] [PubMed] [Google Scholar]
- 24••.Muellenbeck M.F., Ueberheide B., Amulic B., Epp A., Fenyo D., Busse C.E., Esen M., Theisen M., Mordmuller B., Wardemann H. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J Exp Med. 2013;210:389–399. doi: 10.1084/jem.20121970. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study concluded that atypical memory B cells were functionally capable to produce neutralizing antibodies, in contrast to other studies of malaria.
- 25.Zinocker S., Schindler C.E., Skinner J., Rogosch T., Waisberg M., Schickel J.N., Meffre E., Kayentao K., Ongoiba A., Traore B. The V gene repertoires of classical and atypical memory B cells in malaria-susceptible West African children. J Immunol. 2015;194:929–939. doi: 10.4049/jimmunol.1402168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saadoun D., Terrier B., Bannock J., Vazquez T., Massad C., Kang I., Joly F., Rosenzwajg M., Sene D., Benech P. Expansion of autoreactive unresponsive CD21−/low B cells in Sjogren’s syndrome-associated lymphoproliferation. Arthritis Rheum. 2013;65:1085–1096. doi: 10.1002/art.37828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isnardi I., Ng Y.S., Menard L., Meyers G., Saadoun D., Srdanovic I., Samuels J., Berman J., Buckner J.H., Cunningham-Rundles C. Complement receptor 2/CD21-human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115:5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubtsov A.V., Rubtsova K., Fischer A., Meehan R.T., Gillis J.Z., Kappler J.W., Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakhmanov M., Keller B., Gutenberger S., Foerster C., Hoenig M., Driessen G., van der Burg M., van Dongen J.J., Wiech E., Visentini M. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci U S A. 2009;106:13451–13456. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charles E.D., Brunetti C., Marukian S., Ritola K.D., Talal A.H., Marks K., Jacobson I.M., Rice C.M., Dustin L.B. Clonal B cells in patients with hepatitis C virus-associated mixed cryoglobulinemia contain an expanded anergic CD21low B-cell subset. Blood. 2011;117:5425–5437. doi: 10.1182/blood-2010-10-312942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terrier B., Joly F., Vazquez T., Benech P., Rosenzwajg M., Carpentier W., Garrido M., Ghillani-Dalbin P., Klatzmann D., Cacoub P. Expansion of functionally anergic CD21-/low marginal zone-like B cell clones in hepatitis C virus infection-related autoimmunity. J Immunol. 2011;187:6550–6563. doi: 10.4049/jimmunol.1102022. [DOI] [PubMed] [Google Scholar]
- 32.Visentini M., Cagliuso M., Conti V., Carbonari M., Cibati M., Siciliano G., Cristofoletti C., Russo G., Casato M., Fiorilli M. Clonal B cells of HCV-associated mixed cryoglobulinemia patients contain exhausted marginal zone-like and CD21 low cells overexpressing Stra13. Eur J Immunol. 2012;42:1468–1476. doi: 10.1002/eji.201142313. [DOI] [PubMed] [Google Scholar]
- 33.Thorarinsdottir K., Camponeschi A., Gjertsson I., Martensson I.L. CD21−/low B cells: a snapshot of a unique B cell subset in health and disease. Scand J Immunol. 2015;82:254–261. doi: 10.1111/sji.12339. [DOI] [PubMed] [Google Scholar]
- 34.Ehrhardt G.R., Hsu J.T., Gartland L., Leu C.M., Zhang S., Davis R.S., Cooper M.D. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med. 2005;202:783–791. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H., Borrego F., Nagata S., Tolnay M. Fc receptor-like 5 expression distinguishes two distinct subsets of human circulating tissue-like memory B cells. J Immunol. 2016;196:4064–4074. doi: 10.4049/jimmunol.1501027. [DOI] [PubMed] [Google Scholar]
- 36••.Adachi Y., Onodera T., Yamada Y., Daio R., Tsuiji M., Inoue T., Kobayashi K., Kurosaki T., Ato M., Takahashi Y. Distinct germinal center selection at local sites shapes memory B cell response to viral escape. J Exp Med. 2015;212:1709–1723. doi: 10.1084/jem.20142284. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated the importance of tissue localisation for memory B cells in responding effectively to influenza.
- 37.Moseman E.A., Wu T., de la Torre J.C., Schwartzberg P.L., McGavern D.B. Type I interferon suppresses virus-specific B cell responses by modulating CD8+ T cell differentiation. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aah3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sammicheli S., Kuka M., Di Lucia P., Jimenez de Oya N., De Giovanni M., Fioravanti J., Cristofani C., Maganuco C.G., Fallet B., Ganzer L. Inflammatory monocytes hinder antiviral B cell responses. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aah6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fallet B., Narr K., Ertuna Y.I., Remy M., Sommerstein R., Cornille K., Kreutzfeldt M., Page N., Zimmer G., Geier F. Interferon-driven deletion of antiviral B cells at the onset of chronic infection. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aah6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Good-Jacobson K.L. Regulation of germinal center B-cell memory, and plasma cell formation by histone modifiers. Front Immunol. 2014;5:596. doi: 10.3389/fimmu.2014.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang N.S., McHeyzer-Williams L.J., Okitsu S.L., Burris T.P., Reiner S.L., McHeyzer-Williams M.G. Divergent transcriptional programming of class-specific B cell memory by T-bet and RORα. Nat Immunol. 2012;13:604–611. doi: 10.1038/ni.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serre K., Cunningham A.F., Coughlan R.E., Lino A.C., Rot A., Hub E., Moser K., Manz R., Ferraro A., Bird R. CD8 T cells induce T-bet-dependent migration toward CXCR3 ligands by differentiated B cells produced during responses to alum-protein vaccines. Blood. 2012;120:4552–4559. doi: 10.1182/blood-2012-03-417733. [DOI] [PubMed] [Google Scholar]
- 43•.Barnett B.E., Staupe R.P., Odorizzi P.M., Palko O., Tomov V.T., Mahan A.E., Gunn B., Chen D., Paley M.A., Alter G. Cutting edge: B cell-intrinsic T-bet expression is required to control chronic viral infection. J Immunol. 2016;197:1017–1022. doi: 10.4049/jimmunol.1500368. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated the importance of B cell-intrinsic T-bet expression in clearing a chronic viral infection.
- 44.Sydora B.C., Jamieson B.D., Ahmed R., Kronenberg M. Intestinal intraepithelial lymphocytes respond to systemic lymphocytic choriomeningitis virus infection. Cell Immunol. 1996;167:161–169. doi: 10.1006/cimm.1996.0023. [DOI] [PubMed] [Google Scholar]
- 45.Buggert M., Tauriainen J., Yamamoto T., Frederiksen J., Ivarsson M.A., Michaelsson J., Lund O., Hejdeman B., Jansson M., Sonnerborg A. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 2014;10:e1004251. doi: 10.1371/journal.ppat.1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Naradikian M.S., Myles A., Beiting D.P., Roberts K.J., Dawson L., Herati R.S., Bengsch B., Linderman S.L., Stelekati E., Spolski R. Cutting edge: IL-4, IL-21, and IFN-γ interact to govern T-bet and CD11c expression in TLR-activated B cells. J Immunol. 2016;197:1023–1028. doi: 10.4049/jimmunol.1600522. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated the importance of balancing different cytokines in the microenvironment to drive appropriate B cell transcriptional programs.
- 47.Hao Y., O’Neill P., Naradikian M.S., Scholz J.L., Cancro M.P. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehrhardt G.R., Hijikata A., Kitamura H., Ohara O., Wang J.Y., Cooper M.D. Discriminating gene expression profiles of memory B cell subpopulations. J Exp Med. 2008;205:1807–1817. doi: 10.1084/jem.20072682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kardava L., Moir S., Wang W., Ho J., Buckner C.M., Posada J.G., O’Shea M.A., Roby G., Chen J., Sohn H.W. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Investig. 2011;121:2614–2624. doi: 10.1172/JCI45685. [DOI] [PMC free article] [PubMed] [Google Scholar]