Abstract
Background
Despite maximal ventilatory support, many patients die from hypoxia in the setting of potentially reversible pulmonary failure. There remains a pressing need for additional pulmonary supportive care measures, especially techniques that do not require systemic anticoagulation. The objective of our experiments was to determine whether systemic oxygenation could be increased in a large animal, with induced hypoxia, by perfusing the abdominal cavity with oxygenated perfluorocarbons.
Methods
Fifteen pigs with a mean (± SD) weight of 45 ± 5 kg were intubated and rendered hypoxic by ventilating them with a blend of nitrogen and oxygen to achieve subatmospheric concentrations of inspired oxygen ranging from 18 to 10%, resulting in baseline mean Pao2 range of 65.9 ± 9.7 to 26.6 ± 2.8 mm Hg, respectively. Peritoneal perfusion was performed in eight animals with oxygenated perfluorocarbon and in seven control animals with oxygenated saline solution.
Results
The average increase in Pao2 with oxygenated perfluorocarbon perfusion, compared to oxygenated saline solution perfusion, ranged from 8.1 to 18.2 mm Hg. A common treatment effect was estimated across all fraction of inspired oxygen (Fio2) values, representing the average mean difference in oxygen uptake between oxygenated perfluorocarbon and saline solution, irrespective of the level of Fio2. This average was 12.8 mm Hg (95% confidence interval, 7.4 to 18.2; p < 0.001). The most clinically relevant results occurred at an Fio2 of 14%, resulting in a baseline mean Pao2 of 39.4 ± 5.0 mm Hg with oxygenated saline solution perfusion, and a mean Pao2 of 55.3 ± 7.6 mm Hg with oxygenated perfluorocarbon perfusion. This corresponded to an increase in arterial oxygen saturation from 73 to 89%.
Conclusion
These results of our principle experiments demonstrate that the peritoneal cavity can be used for gas exchange and, in our model, yielded clinically relevant increases in systemic arterial oxygen levels. This technique may have the potential for the supportive care of patients dying from hypoxia in the setting of reversible lung injury.
Keywords: animals, ARDS, artificial respiration, extracorporeal membrane oxygenation, fluorocarbons, respiratory insufficiency
Abbreviations
- CI
confidence interval
- Fio2
fraction of inspired oxygen
- psi
pounds per square inch
- Sao2
arterial oxygen saturation
Potentially reversible pulmonary failure secondary to diseases such as avian flu, ARDS, severe acute respiratory syndrome, pulmonary embolism, and bacterial pneumonia claims many lives in the United States every year.1 In addition to this already formidable population, we now live in a world where the specter of bioterrorism with an aerosolized agent could multiply this number many fold at any time, literally overnight. Although multiorgan failure may result in the demise of a patient with any of these conditions, many patients die from isolated pulmonary insufficiency that is potentially reversible. The high mortality and increasing size of this population underscores the need for new and improved treatments for potentially reversible pulmonary failure.
Supportive care with mechanical ventilation is the cornerstone of all treatment strategies for patients with severe pulmonary failure. Numerous modes and parameters of mechanical ventilation can be adjusted in an effort to maximize gas exchange, but when these manipulations are no longer effective the clinician is left with very few options. Extracorporeal membrane oxygenation is a well-established and effective technique to supplement pulmonary function. This modality, however, is a scarce resource that requires special expertise to institute and trained personnel to manage, and, primarily due to the need for systemic anticoagulation, is accompanied by its own set of morbidities and limitations.2 3 4 5 6 7 8 9 10 Liquid ventilation, an attempt to improve the gas exchange capacity of diseased lungs by filling them with a gas-transporting fluid (perfluorocarbons), is an experimental modality that has yet to establish itself as a mainstream therapeutic option.11 12
Perfluorocarbons are inert, colorless, clear, nontoxic, fluorine-based organic compounds with extraordinary gas-dissolving properties. The solubility of O2 and CO2 in perfluorocarbons is typically in the range of 50 mL of gas per 100 mL of liquid and 200 mL of gas per 100 mL of liquid at 37°C, respectively. For comparison, the respective solubility of O2 and CO2 in saline solution at 37°C is 3 mL of gas per 100 mL of liquid and 57 mL of gas per 100 mL of liquid.13 14 15 16 17
The concept we wished to explore was whether it was possible to use the peritoneal membrane for gas exchange, which is analogous to the way in which this same surface can be used for solute and fluid exchange with peritoneal dialysis. It has been shown that significant oxygenation can be achieved in small rodents by filling the abdominal cavity with oxygenated saline solution or gaseous oxygen, but this effect has not been replicated in large-animal models.18 19 It is thought that the relative decrease in the peritoneal surface area, with increasing animal size, is responsible for this loss of effect.20 Our hypothesis was that using perfluorocarbons for gas exchange, in a perfusion circuit that maintains a high oxygen gradient, might overcome the limitation of a relatively smaller peritoneal surface area and permit significant levels of oxygen exchange in a large animal. To test this hypothesis, for proof of principle, we designed a large animal-induced hypoxia model.
MATERIALS AND METHODS
Animals
Fifteen Sus scrofa pigs with a mean (± SD) weight of 45 ± 5 kg were used for the experiments. All experiments were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee. The care and handling of all animals were in accord with US Department of Agriculture guidelines.
Surgical Procedure
Anesthesia was induced in the animals with tiletamine hydrochloride (Telazol; Fort Dodge Animal Health; Fort Dodge, IA), 5 mg/kg, injected IM, after which an IV catheter was placed in an ear vein and 30 mg of propofol (Baxter; Irvine, CA) was injected IV. The animals were then intubated with an endotracheal tube having a 7.5-mm internal diameter (Mallinckrodt Inc; St. Louis, MO) and maintained with isoflurane inhalation anesthesia, using intermittent propofol injections for any evidence of discomfort or awakening. The animals were placed on a mechanical ventilator (Dual Phase Control Respirator Pump; Harvard Apparatus; Holliston, MA) with a tidal volume of 10 to 15 mL/kg and a rate of 12 to 15 breaths/min. Prior to subjecting the animal to hypoxia, the respiratory rate was occasionally adjusted in an effort to maintain a neutral pH, as determined by arterial blood gas measurements. ECG leads were placed in the standard three-lead positions on each animal, starting at the time of induction, and were continuously monitored throughout the experiment. Oxygen saturation was also measured with a pulse oximeter (Vet/Ox Plus 4700; SDI; Waukesha, WI) with the probe placed on the tongue of the animal. Once under general anesthesia and in the supine position, a cervical cut down was performed through which a Swan-Ganz right heart catheter (Edwards Lifesciences; Irvine, CA) was placed via the internal jugular vein, and an 18-gauge angiocatheter was placed in the common carotid artery for access to the central arterial system for BP monitoring and arterial blood gas measurements. Both of these lines were transduced using a pressure-monitoring kit with a pressure transducer (TruWave; Edwards Lifesciences) that was connected to a monitor system (model 78834A; Hewlett-Packard; Palo Alto, CA) for monitoring the systemic and pulmonary arterial pressures and the central venous pressure.
After placement of the arterial and venous lines, a midline laparotomy was performed. A 28F venous return cannula catheter (Edwards Lifesciences) was placed in the left paracolic gutter, brought out through a separate site in the left lower quadrant, and sutured in place using a No. 1 polypropylene suture (Prolene suture; Ethicon; Sommerville, NJ). A 42F venous return cannula (Edwards Lifesciences) was placed in an identical manner on the right side, with the tip lodged above the dome of the liver. The abdomen was then closed in a single layer with a running No. 1 polypropylene suture. In the first two animals in each experimental group, an additional 14F catheter (Edwards Lifesciences) was placed near the midline for pressure-monitoring purposes and was maintained in a vertical attitude, attached to a ruler such that the height of the column of fluid could be easily monitored. Peritoneal pressure measurements (expressed in centimeters of H2O) were calculated by multiplying column height by the correction factor 1.67 (specific gravity of trans-bis-perfluorobutyl ethylene).
Hypoxia Circuit
A anesthesia machine (Matrx; Orchard Park, NY) was used for blending gases and isoflurane-inhaled anesthetic. Pure nitrogen, rather than filtered room air, was mixed with pure oxygen to achieve subatmospheric concentrations of oxygen. The animal was ventilated using a standard bifurcated anesthesia circuit to maintain a unidirectional flow of the gases. A machine (Datex; Puritan-Bennett Corporation; Overland Park, KS) was attached to the ventilation circuit that allowed real-time monitoring of the oxygen and anesthetic agent concentrations. The oxygen and nitrogen tanks were connected to the circuit through a flowmeter (Matrx). The probe of the flowmeter was placed at the origin of the endotracheal tube, allowing for accurate determination of the respective flows of oxygen and nitrogen required to achieve the desired Fio2.
Peritoneal Circulation Circuit
The 42F venous return cannula, which was used to recover the perfusate from the abdomen, was connected to a 127 cm length of three eighths inch (internal diameter) tubing (Gish Biomedical; Rancho Santa Margarita, CA). This tubing was connected to a No. 10 filter housing (Clear Cold Water Filter Housing; Filter Store, Inc.; Mendon, NY) containing a 50-μm pleated filter (Filter Store, Inc). After passing through the filter, the outflow drained by gravity into a custom built 10-L glass tank (Advanced Aquatanks; Los Angeles, CA). The outflow of the filter was placed 41 cm below the level of the operating table. A ceramic plate diffuser (Aquaculture Technology; Kitzbuhel, Austria) was placed at the bottom of the collection tank. This diffuser was designed for oxygenating ponds and gives off a steady stream of 10 to 200 μm bubbles when being infused with oxygen at 30 pounds per square inch (psi) [based on product information from the company]. This diffuser was connected to a medical oxygen tank via one quarter inch (internal diameter) tubing (Gish Biomedical). The gauge on the tank was able to measure the oxygen delivered to the diffuser in psi. (A standard cardiopulmonary bypass “lung” cannot be used to oxygenate perfluorocarbon as the liquid is able to pass through hollow fibers.)
Fluid was retrieved from the collecting tank with a 150-cm length of one quarter inch tubing that was connected to a piece of three eighths inch tubing that passed through a pump (Roller Blood Pump; Sarns, Inc; Ann Arbor, MI) that was used to control the flow rate. This outflow from the roller pump passed into a 43-cm length of one quarter inch tubing. This tubing was connected to the inflow end of a heat exchanger (ECMOtherm-II; Avecor; Minneapolis, MN). The heat exchanger was set to warm the abdominal perfusate to 39°C, which is normal body temperature for swine. This was accomplished using a circulatory heater (Haake; Berlin, Germany). After being warmed by the heat exchanger, the abdominal perfusate passed through a 63-cm length of one quarter inch tubing to the 28F inflow catheter. All tubing was standard cardiopulmonary bypass tubing. Thus, the circuit was as follows: abdomen, outflow catheter, filter, reservoir with oxygenator, pump, warmer, inflow catheter, and abdomen.
Establishing Flow Rate and Priming Circuit
After the cannulas were placed and the abdomen was closed, it was necessary to assure that the circuit was functional before initiating the progressive hypoxia sequence. Any perfusion circuit that is dependent on a siphon phenomenon for outflow may require manipulation of the outflow catheter to achieve optimal flow due to local geometric factors that can obstruct or occlude the catheter drainage ports. Through trial and error, we found that placing the outflow cannula above the liver yielded the fewest drainage problems. With the cannula in this location, it was never necessary to reopen the incision, but occasionally it was necessary to spin the catheter or move it several centimeters in or out to assure optimal flow.
The reservoir was initially primed with 8 L of perfusate. Once the circuit was flowing smoothly, the inflow pump was adjusted to match the maximum outflow rate for that animal. This rate was empirically determined and unique for each animal, falling between 1.2 and 4.2 L/min (Table 2 ). Once optimization of the circuit was complete and the maximum flow rate for that animal was identified, the outflow catheter was clamped and the abdomen was allowed to fill with 5 to 7 L of fluid. Once this volume was infused into the abdomen, the inflow catheter was then clamped, thereby trapping the perfusate in the abdomen. In designing this model, we found that this maneuver, which did not affect the intraabdominal pressure, was necessary in order to assure that the siphon would resume and that the circuit could be restarted after having been stopped for several hours during the stepwise hypoxia portion of the experiments. Thus, in every case there was a large volume of perfusate, saline solution, or perfluorocarbon harbored in the abdomen during the collection of the “circuit-off” hypoxia measurements.
Table 2.
Experimental Variables*
| Variables | Perfusion Medium | p Value | |
|---|---|---|---|
| Saline Solution (n = 7) | Perfluorocarbon (n = 8) | ||
| Gender | 1.0 | ||
| Male | 1 (14) | 1 (13) | |
| Female | 6 (86) | 7 (87) | |
| Weight, kg | 49.4 ± 2.8 | 48.6 ± 2.1 | 0.51 |
| Flow rate, L/min | 2.0 ± 0.9 | 2.6 ± 1.2 | 0.28 |
| Volume in peritoneal cavity, L | 2.5 ± 0.4 | 2.7 ± 0.3 | 0.34 |
| Total volume, L | 8.4 ± 1.7 | 9.1 ± 0.4 | 0.33 |
Values are given as No. (%) or mean ± SD.
Perfluorocarbon
Seventy liters of neat perfluorocarbon F44E (trans-bis-perfluorobutyl ethylene) were provided as a generous gift from Neuron Therapeutics, Inc (Malvern, PA). This perfluorocarbon is clear, colorless, odorless, and immiscible with water or blood, and has the physical characteristics listed in Table 1 .
Table 1.
Properties of Perfluorocarbon
| Properties | Values |
|---|---|
| Specific gravity | 1.668 g/mL |
| Oxygen capacity | 50 volume% |
| Carbon dioxide capacity | 200 volume% |
| Boiling point | 132°C |
Experiments
The experimental design is summarized in Figure 1 . The experimental groups included eight animals perfused with perfluorocarbon. The controls included seven animals in which saline solution was used as the perfusate (Table 2). In each case, the animals were stabilized at a fraction of inspired oxygen (Fio2) of 21% for 30 min, and then were sequentially dropped to Fio2 values of 18%, 16%, 14%, 12%, and 10% for 30 min at each level. Thirty minutes was an arbitrarily selected interval based on clinical observations that this interval would be sufficient to allow equilibration with a change in mechanical ventilation parameters. This time frame was also necessary due to the logistical constraints of the animal facility to keep the experiments to < 8 h. The desired Fio2 was achieved by empirically adjusting the blend of nitrogen and oxygen to achieve the desired Fio2, as indicated by the in-line gas monitor (Datex; Puritan-Bennett Corporation).
Figure 1.
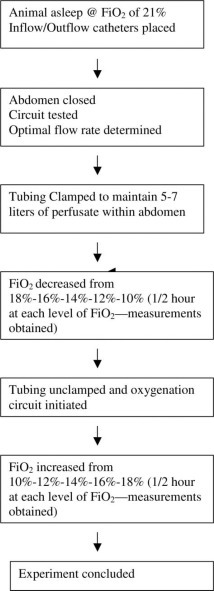
Experimental design.
During the progressive hypoxia phase, as the Fio2 descended from 18 to 10%, the circuit tubing remained clamped with 5 to 7 L of perfusate dwelling in the abdomen. Arterial and mixed venous blood gasses, along with heart rate and BP, central venous pressure, and pulmonary arterial pressures were obtained at each level. Blood gas measurements were performed (Gem Premier 3000; Instrumentation Laboratory; Lexington, MA). For the first two experiments in each group, the intraperitoneal pressure was also recorded, both before and during peritoneal perfusion.
At the conclusion of the descending-Fio2 phase (ie, after the animals had been at an Fio2 of 10% for 30 min with the circuit off and the perfusate dwelling in the abdomen), the tubing was unclamped, and the peritoneal oxygenation circuit was activated at the previously determined flow rate. The volume of perfusate that remained in the abdomen during perfusion was also idiosyncratic, remained relatively stable with a stable flow rate, and ranged from 2 to 3 L. The ascending Fio2 levels were achieved by resuming the same nitrogen and oxygen flow settings that had been used for the descending-Fio2 phase. The animals were again allowed to equilibrate for 30 min during the ascending phase prior to collecting blood gas measurements. The oxygen supply to the bubbler was always maintained at 30 psi.
Statistical Analysis
Mixed-effects linear regression was used to model oxygen uptake as a function of treatment group (ie, perfluorocarbon vs saline solution groups), oxygenation condition (ie, oxygenation circuit off vs oxygenation circuit on), and Fio2. Heuristically, this approach fits a “random” regression line for the Pao2 data of each animal as a function of Fio2. Then, it statistically averages these curves within each group and oxygenation condition to obtain the overall fixed (ie, average) effects. This method appropriately accounts for unbalanced data due to occasional missing measurements, as well as for the correlation of measurements obtained on the same animal. The interpretation of the fixed-effects estimates obtained from the linear regression model is similar to that of the usual linear regression (ie, in terms of mean Pao2 values and mean differences). The analyses were conducted using a statistical software package (SAS, version 8.2; SAS Institute; Cary, NC [from 1999 to 2001]).
RESULTS
Table 2 summarizes the groups of animals. The study included eight pigs in the experimental group (perfluorocarbon) and seven pigs in the control group (saline solution). All animals underwent the same experimental protocol with the exception that liquid was used as the perfusate. The measured intraperitoneal pressures for steady-state perfusion were < 15 cm H2O. The measured pulmonary artery pressures were 12 to 18 mm Hg and 4 to 8 mm Hg, respectively, for the two groups, and were unaffected by whether the perfusion circuit was running or the type of perfusate. No statistically significant difference was noted in the Paco2 or venous Pco2 between groups, irrespective of Fio2 or whether saline solution or perfluorocarbon was used as the perfusate.
During the experiments, the animals were observed at five different levels of Fio2 (18%, 16%, 14%, 12%, and 10%) under two different conditions (circuit off or circuit on). A total of 132 measurements were obtained, 69 when the oxygenation circuit was off and 63 when the oxygenation circuit was on. Table 3 presents the observed and fitted mean Pao2 values for all groups.
Table 3.
Observed and Fitted Pao2 Values for the Saline Solution and Perfluorocarbon Groups, by Oxygenation Circuit Condition and Fio2
| Fio2, % | O2 | Patients, No. | Saline Solution Group | Patients, No. | Perfluorocarbon Group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Fitted | Observed | Fitted | ||||||||
| Mean | SD | Mean | 95% CI | Mean | SD | Mean | 95% CI | ||||
| 10 | Off | 5 | 26.6 | 2.8 | 22.7 | 17.0–28.3 | 7 | 28.0 | 5.3 | 25.7 | 20.6–30.7 |
| 12 | 6 | 31.3 | 3.3 | 33.7 | 29.0–38.4 | 8 | 37.1 | 8.1 | 38.5 | 34.3–42.7 | |
| 14 | 6 | 41.2 | 4.5 | 44.7 | 40.4–49.1 | 8 | 48.8 | 11.4 | 51.3 | 47.3–55.3 | |
| 16 | 7 | 57.6 | 4.9 | 55.7 | 51.0–60.5 | 7 | 63.7 | 7.9 | 64.2 | 59.7–68.6 | |
| 18 | 7 | 65.9 | 9.7 | 66.7 | 61.0–72.5 | 8 | 79.8 | 7.7 | 77.0 | 71.6–82.4 | |
| 10 | On | 5 | 28.6 | 3.9 | 25.6 | 20.1–31.1 | 7 | 36.7 | 8.4 | 35.2 | 30.2–40.1 |
| 12 | 6 | 31.3 | 4.1 | 33.2 | 28.9–37.4 | 8 | 43.8 | 4.8 | 44.5 | 40.8–48.3 | |
| 14 | 5 | 39.4 | 5.0 | 40.7 | 36.6–44.8 | 7 | 55.3 | 7.6 | 53.9 | 50.2–57.6 | |
| 16 | 5 | 46.2 | 5.8 | 48.3 | 43.2–53.4 | 7 | 64.4 | 8.2 | 63.3 | 58.5–68.1 | |
| 18 | 6 | 60.5 | 11.6 | 55.9 | 49.0–62.7 | 7 | 68.7 | 10.4 | 72.7 | 66.2–79.2 | |
For the saline solution group, with the circuit off and saline solution dwelling in the abdomen, as the Fio2 was lowered from 18 to 10% the mean (± SD) Pao2 decreased from 65.9 ± 9.7 to 26.6 ± 2.8 mm Hg, respectively. This provided fitted means of 66.7 mm Hg (95% confidence interval [CI], 61.0 to 72.5 mm Hg) to 22.7 mm Hg (95% CI, 17.0 to 28.3 mm Hg), respectively. After obtaining data for the animals at an Fio2 of 10%, the oxygenation circuit was started. As the Fio2 of the animals was raised from 10 to 18%, the mean Pao2 increased from 28.6 ± 3.9 to 60.5 ± 11.6 mm Hg, respectively. This resulted in fitted mean values of 25.6 mm Hg (95% CI, 20.1 to 31.1 mm Hg) to 55.9 mm Hg (95% CI, 49.0 to 62.7 mm Hg), respectively. These results are depicted as fitted means in Figure 2 .
Figure 2.
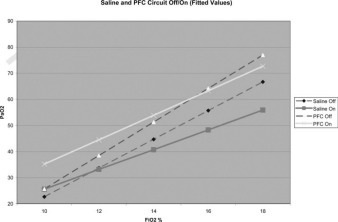
Perfluorocarbon vs saline solution with oxygenation circuit off and on. Data points represent observed measurements, while the lines are fitted.
Saline solution perfusion with the oxygenation circuit running was then compared to perfluorocarbon perfusion with the oxygenation circuit running (Fig 3 ). The data from animals that had oxygenated saline solution perfusion were compared directly to those of animals that underwent perfusion with oxygenated perfluorocarbon. As the Fio2 of the animals was raised from 10 to 18% with the circuit running with oxygenated perfluorocarbon perfused, the mean Pao2 increased from 36.7 ± 8.4 to 68.7 ± 10.4 mm Hg, respectively. This resulted in fitted mean Pao2 values of 35.2 mm Hg (95% CI, 30.2 to 40.1 mm Hg) to 72.7 mm Hg (95% CI, 66.2 to 79.2 mm Hg), respectively Fig 2. The average increase in fitted mean Pao2 with oxygenated perfluorocarbon perfusion compared to oxygenated saline solution perfusion ranged from 8.1 to 18.2 mm Hg. These differences represent the treatment effect comparing oxygenated perfluorocarbon perfusion to oxygenated saline solution perfusion. All five differences are statistically significant. A common treatment effect can be estimated across all Fio2 values, representing the average mean difference in oxygen uptake between perfluorocarbon and saline solution when the oxygenation circuit was running, irrespective of the level of Fio2 (Table 4 ). This average was 12.8 mm Hg (95% CI, 7.4 to 18.2 mm Hg; p < 0.001).
Figure 3.
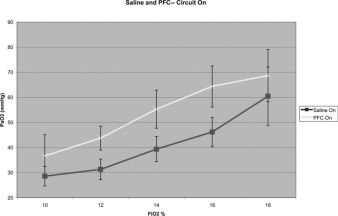
Perfluorocarbon vs saline solution with oxygenation circuit on.
Table 4.
Observed and Estimated Treatment Effect: Mean Pao2 Difference Between Perfluorocarbon vs Saline Solution, With Oxygenation Circuit on, for Different Fio2 Values
| Fio2, % | Observed Effect | Fitted | p Value | |
|---|---|---|---|---|
| Effect | 95% CI | |||
| 10 | 8.1 | 9.6 | 3.3–15.9 | 0.005 |
| 12 | 12.4 | 11.4 | 5.9–16.9 | 0.001 |
| 14 | 15.9 | 13.2 | 7.8–18.6 | 0.001 |
| 16 | 18.2 | 15.0 | 8.9–21.1 | 0.001 |
| 18 | 8.2 | 16.8 | 9.4–24.3 | 0.001 |
| Overall | 12.8 | 7.4–18.2 | 0.001 | |
The above difference between perfluorocarbon and saline solution perfusion, when the oxygenation circuit was running, should be put in the context of the same difference when the oxygenation circuit was off and the perfusate was dwelling in the abdomen (Fig 4 ). As mentioned above, when oxygenation was on, oxygen uptake was significantly higher in the perfluorocarbon group by 12.8 mm Hg on average (average difference of fitted mean Pao2 values between perfluorocarbon and saline solution perfusion at all Fio2 levels) [Fig 2]. When the oxygenation circuit was off and the perfusate was dwelling in the abdomen, oxygen uptake was also statistically significantly higher in the perfluorocarbon group than in the comparable saline solution group. The average mean difference in oxygen uptake between the perfluorocarbon and saline solution groups, when the oxygenation circuit was off and perfusate was dwelling in the abdomen, was 6.4 mm Hg (fitted mean 95% CI, 0.6 to 12.2 mm Hg; p = 0.034). When comparing the quantitative effects of the oxygenation circuit (ie, off vs on) between the groups (perfluorocarbon vs saline solution group) a positive group-by-oxygenation interaction effect was noted to be 6.4 mm Hg (95% CI, −1.2 to 14.0; p = 0.095).
Figure 4.
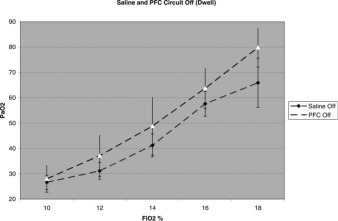
Perfluorocarbon vs saline solution with oxygenation circuit off.
The most clinically relevant increase in Pao2 came at an Fio2 of 14% Fig 2. The observed effect of perfluorocarbon compared to that of saline solution with the oxygenation circuit on demonstrated an observed mean increase of 15.9 mm Hg with a fitted mean increase of 13.2 mm Hg (95% CI, 7.8 to 18.6 mm Hg) [Table 4]. This resulted in a baseline mean Pao2 of 39.4 ± 5.0 mm Hg with oxygenated saline solution perfusion, and a mean Pao2 of 55.3 ± 7.6 mm Hg with oxygenated perfluorocarbon perfusion. For the observed means, this corresponds to an increase in arterial oxygen saturation (Sao2) from 73% with oxygenated saline solution perfusing the abdomen to 89% with oxygenated perfluorocarbon perfusing the abdomen.
DISCUSSION
These results of our principle experiments represent the first demonstration of the ability to perform peritoneal perfluorocarbon perfusion in a large animal resulting in the augmentation of systemic arterial oxygenation. Small-animal studies18 19 20 21 have previously demonstrated the ability to use the peritoneal surface to deliver oxygen using intraperitoneal gaseous oxygen, oxygenated saline solution, or oxygenated perfluorocarbon, but promising results have not previously been reported in any large-animal studies. The relative ease with which oxygenation can be increased in small-animal models, but not large-animal models, is likely a function of the significantly increased ratio of the peritoneal surface to body mass in small animals.22 Based on our results, we hypothesize that a perfusion circuit, with a medium like perfluorocarbon that is capable of carrying and unloading large quantities of oxygen, is necessary to achieve significant gas exchange through the peritoneal surface of a large animal.
The measured augmentation of systemic oxygen levels by perfusing the peritoneum with oxygenated perfluorocarbon was uniform and statistically significant for all five levels of induced hypoxia. It was the use of perfluorocarbon that resulted in the observed phenomenon, in our model, as saline solution failed to serve as an effective gas transport medium.
The animals that had perfluorocarbon dwelling in their abdomen, during the progressive hypoxia phase of the experiments Fig 1, also had higher oxygen levels than the corresponding animals that had saline solution dwelling in their abdomens during this portion of the experiments Fig 2. Our hypothesis to explain this consistent finding is that the volume of indwelling perfluorocarbon in the abdomen likely served as an oxygen reservoir and resulted in blunting of the induced hypoxia effect on arterial oxygen concentrations as the Fio2 was decreased from 18 to 10%. Although we were unable to directly measure the oxygen content of the perfluorocarbon, the perfluorocarbon would have initially equilibrated with atmospheric air, a Po2 of approximately 160 mm Hg, and could have held as much as 2.5 to 3.5 L of available oxygen.14 15 16 17 Given the rapidity with which gradient-driven gasses dissolve in perfluorocarbons and the fact that tissues extract essentially all oxygen from perfluorocarbons before extracting oxygen from hemoglobin, one would assume that the dwelling perfluorocarbon served as a renewable oxygen reservoir as the animals were rendered progressively hypoxic.23 That is, in addition to serving as an initial oxygen reservoir, it is likely that the reservoir was relatively “renewed” as the animals progressed from higher to lower levels of inspired oxygen. For example, if an animal was at an Fio2 level of 16%, the perfluorocarbon would equilibrate to the tissues at this level and would then be higher than the tissue levels when the Fio2 was decreased to 14%, thereby serving as a source of oxygen at the 14% Fio2 level.
It is interesting to note that saline solution, dwelling in the abdomen or with the oxygenation circuit running, and perfluorocarbon, dwelling in the abdomen but without progressing to a lower Fio2, yielded essentially the same arterial oxygen concentrations at the lowest Fio2 of 10% Fig 2. At this point, only activation of the oxygenation circuit, in the perfluorocarbon animals, resulted in increased arterial oxygen levels Fig 2. Examining the data at this lowest level of induced hypoxia is as close as we could come to eliminating the reservoir effect from our data. The necessity of maintaining a stagnant volume of perfusate in the abdomen during the progressive hypoxia measurements was a limitation of our experimental model.
Another consistent trend, likely resulting from the limitations of our model, is the fact that all animals demonstrated their highest arterial oxygen concentrations at the initiation of the experiments, with the oxygenation circuits not running. Our experiments were designed to ferret out any potential effect from peritoneal perfusion. To magnify the effect from a gradient-driven phenomenon, we elected to drop the animals to the profound level of hypoxia induced by ventilating them with an Fio2 of 10%. In retrospect, this degree of hypoxia is clinically irrelevant and likely placed the animals into a state of oxygen debt from which they could never recover during the ascending phase of the induced hypoxia measurements Fig 1. It is our hypothesis that this state of oxygen debt at the conclusion of the experiments is why all the animals demonstrated their highest levels of oxygenation at the beginning of the experiments, when they first entered a state of induced hypoxia Fig 2. We also feel that the parallel sequence of induced hypoxia increase and decrease, for both the saline solution and perfluorocarbon groups, is why the resulting data yielded parallel lines Fig 2 for perfusate that was dwelling in the abdomen and perfusate that was circulating. Although the results of our principle experiments demonstrate that arterial oxygenation can be supplemented by instilling perfluorocarbon into the abdomen, partially with dwelling and more so by circulating oxygenated perfluorocarbon, it is important to view these results within the context of the short duration of the experiments, the peculiarities of our induced hypoxia model, and the statistically significant, but relatively small, sample size of our study.
We designed this model to allow us to explore the effect of perfusion over a wide range of hypoxia. In order to specifically study the effects on oxygenation, we maintained the animals in a normocarbic state and, thus, cannot draw any conclusions regarding the impact of peritoneal perfluorocarbon perfusion to clear carbon dioxide. One could speculate, however, that the greater diffusivity of carbon dioxide compared to oxygen, and the very high solubility of carbon dioxide in perfluorocarbon would favor carbon dioxide clearance in a hypercarbic state.
In considering peritoneal perfusion as a potential treatment for patients with reversible pulmonary failure, arterial oxygen levels in the 40 mm Hg range might represent the point at which a clinician would entertain instituting heroic measures for appropriate patients. This degree of hypoxia was achieved in our model at an Fio2 of 14%, which resulted in a mean arterial oxygen concentration of 39.4 mm Hg with oxygenated saline solution perfusion, and an Sao2 of 73%. Perfusing the abdomen with oxygenated perfluorocarbon, compared to perfusing it with oxygenated saline solution, resulted in an average difference of 15.9 mm Hg of oxygen, which correlated with an average arterial oxygen level of 55.3 mm Hg. This corresponded to an average increase in the Sao2 from 73 to 89%. Although our experiments were short term, and strictly proof of principle, it is worth noting that this degree of augmentation in oxygenation could represent a potentially life-saving measure in a patient dying from reversible pulmonary failure.
The amount of oxygen delivery that we observed is significantly less than can be achieved with direct blood interface techniques, like extracorporeal membrane oxygenation. Peritoneal perfusion, however, would not require anticoagulation, and this is a potentially significant advantage. For example, a trauma patient with an intracranial hemorrhage and pulmonary contusions or ARDS may require extrapulmonary oxygen delivery but would not be a candidate for any modality requiring anticoagulation.
If further research reveals that this modality remains as innocuous for prolonged periods as we observed for these short-term proof-of-principle experiments, then there could be other roles for this modality beyond the salvage of a patient in terminal pulmonary failure. Another potential application would be to initiate this treatment in a prophylactic manner to prevent or limit ventilator-induced lung injury.24 As a nonpulmonary technique for gas exchange, it could allow the clinician to decrease the toxic ventilator settings that are sometimes required to support a patient who is dying from potentially reversible lung failure. Such a technique could short circuit the “catch-22” that results when high ventilator settings exacerbate the underlying lung dysfunction and mandate even higher ventilator settings, further worsening the dysfunction. From a technical surgical perspective, it would not be difficult to institute this type of treatment at the bedside.
CONCLUSION
The results from these proof-of-principle experiments demonstrate that systemic oxygen levels can be increased in a large-animal induced hypoxia model by perfusing the peritoneal surface with oxygenated perfluorocarbon. The gas-dissolving and gas-unloading properties of perfluorocarbon were necessary as the same effect was not obtained when saline solution was employed as the perfusate. Although supplemental oxygenation has been demonstrated in small animals (with their relatively larger peritoneal surface) by using various techniques to oxygenate the abdominal cavity, this is the first demonstration of investigators being able to consistently accomplish this objective in a large animal. Further studies are indicated and will take the form of longer duration peritoneal perfusion runs to look for toxic side effects of this treatment, the evaluation of the impact of this technique on carbon dioxide clearance, the performance of this technique in disease models that simulate human conditions of severe pulmonary failure, and the exploration of interventions that can enhance gas exchange across the peritoneal membrane. We speculate that this technique could potentially find a role in the supportive care of patients with profound, but reversible, pulmonary failure. As this is a pulmonary-independent technique for supplementing oxygenation, there could even be a role for treating patients with less severe, but worsening, pulmonary failure in order to prevent the compounding problem of ventilator-induced lung injury.
ACKNOWLEDGMENT
We gratefully acknowledge the perfluorocarbon gift from Neuron Therapeutics Inc., technical guidance and advice from Dr. Jewell Osterholm and technical assistance from Vanessa Paris and Tracey Sims.
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestjournal.org/misc/reprints.shtml)
Funding for this project was provided by the Department of Surgery at Thomas Jefferson University Hospital.
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
REFERENCES
- 1.Rubenfeld G. Epidemiology of acute lung injury. Crit Care Med. 2003;31:S276–S284. doi: 10.1097/01.CCM.0000057904.62683.2B. [DOI] [PubMed] [Google Scholar]
- 2.Bennett CC, Johnson A, Field DJ. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation: follow-up to age 4 years. Lancet. 2001;357:1094–1096. doi: 10.1016/S0140-6736(00)04310-5. [DOI] [PubMed] [Google Scholar]
- 3.Ko WJ, Lin CY, Chen RJ. Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann Thorac Surg. 2002;73:538–545. doi: 10.1016/s0003-4975(01)03330-6. [DOI] [PubMed] [Google Scholar]
- 4.Rich PB, Younger JG, Soldes OS. Use of extracorporeal life support for adult patients with respiratory failure and sepsis. ASAIO J. 1998;44:263–266. doi: 10.1097/00002480-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kolla S, Awad SS, Rich PB. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg. 1997;226:544–564. doi: 10.1097/00000658-199710000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett RH. Extracorporeal life support in the management of severe respiratory failure. Clin Chest Med. 2000;21:555–561. doi: 10.1016/s0272-5231(05)70166-0. [DOI] [PubMed] [Google Scholar]
- 7.Rich PB, Awad SS, Crotti S. A prospective comparison of atrio-femoral and femoro-atrial flow in adult venovenous extracorporeal life support. J Thorac Cardiovasc Surg. 1998;116:628–632. doi: 10.1016/S0022-5223(98)70170-9. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett RH, Roloff DW, Custer JR. Extracorporeal life support: the University of Michigan experience. JAMA. 2000;283:904–908. [PubMed] [Google Scholar]
- 9.Peek GJ, Firmin RK. Extracorporeal membrane oxygenation for cardiac support. Coron Artery Dis. 1997;8:371–388. doi: 10.1097/00019501-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Peek GJ, White S, Scott AD. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227:572–574. doi: 10.1097/00000658-199804000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kacmarek RM. Liquid ventilation. Respir Care Clin N Am. 2002;8:187–209. doi: 10.1016/s1078-5337(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 12.Hirschl RB, Croce M, Gore D. Prospective, randomized, controlled pilot study of partial liquid ventilation in adult acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;165:781–787. doi: 10.1164/ajrccm.165.6.2003052. [DOI] [PubMed] [Google Scholar]
- 13.Riess JG. Fluorocarbon-basedin vivooxygen transport and delivery systems. Vox Sang. 1991;61:225–239. doi: 10.1111/j.1423-0410.1991.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 14.Day SE, Gedeit RG. Liquid ventilation. Clin Perinatol. 1998;25:711–722. [PubMed] [Google Scholar]
- 15.Cox CA, Wolfson MR, Shaffer TH. Liquid ventilation: a comprehensive overview. Neonatal Netw. 1996;15:31–43. [PubMed] [Google Scholar]
- 16.Shaffer TH, Wolfson MR, Clark LC. Liquid ventilation. Pediatr Pulmonol. 1992;14:102–109. doi: 10.1002/ppul.1950140208. [DOI] [PubMed] [Google Scholar]
- 17.Clark LC, Jr, Gollan F. Survival of mammals breathing organic liquids equilibrated with oxygen at atmospheric pressure. Science. 1966;152:1755–1756. doi: 10.1126/science.152.3730.1755. [DOI] [PubMed] [Google Scholar]
- 18.Klein J, Faithfull NS, Salt PJ. Transperitoneal oxygenation with fluorocarbons. Anesth Analg. 1986;65:734–738. [PubMed] [Google Scholar]
- 19.Schmidt JA, Bilge FH, Colacino JM. Peritoneal oxygenation of normoxic and hypoxic dogs. ASAIO Trans. 1989;35:35–39. [PubMed] [Google Scholar]
- 20.Giffin DM, Gow KW, Warriner CB. Oxygen uptake during peritoneal ventilation in a porcine model of hypoxemia. Crit Care Med. 1998;26:1564–1568. doi: 10.1097/00003246-199809000-00025. [DOI] [PubMed] [Google Scholar]
- 21.Faithfull NS, Klein J, van der Zee HT. Whole body oxygenation using intraperitoneal perfusion of fluorocarbons. Br J Anaesth. 1984;56:867–872. doi: 10.1093/bja/56.8.867. [DOI] [PubMed] [Google Scholar]
- 22.Siriwardhana SA, Newfield AM, Lipton JM. Oxygen delivery by the peritoneal route. Can J Anaesth. 1990;37:S159. [PubMed] [Google Scholar]
- 23.Blood substitutes http://www.anaesthetist.com/anaes/drugs/bloodsubs.htm Available at: Accessed July 4, 2006.
- 24.Barr J, Livne A, Lushkov G. Peritoneal ventilation: an animal model of extrapulmonary ventilation in experimental adult respiratory distress syndrome. Pediatr Res. 1994;35:682–684. doi: 10.1203/00006450-199406000-00012. [DOI] [PubMed] [Google Scholar]


