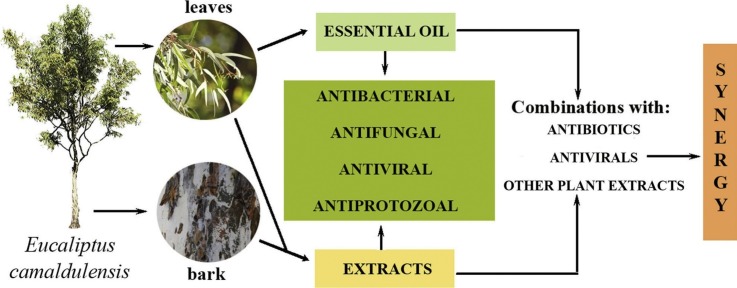
Graphical abstract
Keywords: Antimicrobial activity, Plant extracts, Essential oils, Eucalyptus camaldulensis
Highlights
-
•
Eucaliptus camaldulensis have been used in traditional medicine for various purposes.
-
•
Its plant extracts and essential oils have considerable antimicrobial effect.
-
•
Eucaliptus camaldulensis plant products interact synergistically with other antimicrobials.
-
•
It is a valuable source of pharmaceuticals against multidrug resistant microorganisms.
Abstract
Eucalyptus has become one of the world’s most widely planted genera and E. camaldulensis (The River Red Gum) is a plantation species in many parts of the world. The plant traditional medical application indicates great antimicrobial properties, so E. camaldulensis essential oils and plant extracts have been widely examined. Essential oil of E. camaldulensis is active against many Gram positive (0.07–1.1%) and Gram negative bacteria (0.01–3.2%). The antibacterial effect is confirmed for bark and leaf extracts (conc. from 0.08 μg/mL to 200 mg/mL), with significant variations depending on extraction procedure. Eucalyptus camaldulensis essential oil and extracts are among the most active against bacteria when compared with those from other species of genus Eucalyptus. The most fungal model organisms are sensitive to 0.125–1.0% of E. camaldulensis essential oil. The extracts are active against C. albicans (0.2–200 mg/mL leaf extracts and 0.5 mg/mL bark extracts), and against various dermatophytes. Of particular importance is considerable the extracts’ antiviral activity against animal and human viruses (0.1–50 μg/mL). Although the antiprotozoal activity of E. camaldulensis essential oil and extracts is in the order of magnitude of concentration several hundred mg/mL, it is considerable when taking into account current therapy cost, toxicity, and protozoal growing resistance. Some studies show that essential oils’ and extracts’ antimicrobial activity can be further potentiated in combinations with antibiotics (beta-lactams, fluorochinolones, aminoglycosides, polymyxins), antivirals (acyclovir), and extracts of other plants (e.g. Annona senegalensis; Psidium guajava). The present data confirm the river red gum considerable antimicrobial properties, which should be further examined with particular attention to the mechanisms of antimicrobial activity.
1. Introduction
The continuing increase in the degree of bacterial resistance to conventional antibiotics is a problem of global significance. The crisis of antimicrobial resistance has been ascribed to the misuse of these agents and today resistant strains are common, such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, drug resistant Streptococcus pneumoniae and Mycobacterium tuberculosis, carbapenem-resistant and extended-spectrum beta-lactamase producing enterobacteria, multidrug-resistant Pseudomonas aeruginosa and Acientobacter baumannii etc. It was estimated that the medical cost per patient with an antibiotic-resistant infection is up to $29,069 and infections are usually life treating (Ventola, 2018; Aslam et al., 2018). Similarly, fungi have become resistant to poliens, azoles and echinocandins, and the emergence of drug-resistant strains has been reported in all fungal species (Robbins et al., 2017). Beside the noticeable emergence of resistant protozoa and viruses, there is a problem with limited number of antiprotozoal and antiviral agents (El-Taweel, 2015; Irwin et al., 2016). This highlights the necessity for examination of new antimicrobial agents and treatment strategies of infections caused by the mentioned microorganisms.
The plant kingdom represents the source of various medicines. Indeed, since ancient times medicinal plants play an important role of health care population and could represent a significant source of new antimicrobial drugs for combating pan- and multi-drug resistant microorganisms. These new antimicrobial agents could be hidden in medicinal plant extracts and essential oils. One of the significant medicinal plants is Eucalyptus camaldulensis. Thus, this review represents the summary of previous researches data, regarding chemical composition, antimicrobial activity, and other significant effects of Eucalyptus camaldulensis.
1.1. Eucalyptus camaldulensis Dehn. (1832)
The River Red Gum (Eucalyptus camaldulensis Dehn.) is a tree belonging to the genus Eucalyptus from Myrtaceae family. This family includes 140 genera and about 3800 species distributed in tropical and subtropical regions of the world (Ali et al., 2011). The genus Eucalyptus was described and named in 1788 by the French botanist l’Héritier. The name is generic, from the Greek words ‘eu’ (well) and ‘kalyptos’ (covered), because the flowers of various Eucalyptus species are protected by an operculum (Taoubi et al., 1997). The genus is indigenous to Australia and Tasmania, consists of over 800 species, spreading worldwide and successfully introducing due to its easy adaptability and fast growth (Coppen, 2002). As a consequence, Eucalyptus has become one of the world’s most widely planted genera (Akin et al., 2010) and a plantation species in many parts of the world (Ames and Mathiews, 1968; Mubita et al., 2008).
Eucalyptus camaldulensis (formerly Eucalyptus rostrata Schl.), also known as long beak eucalyptus, murray red gum, red gum, river gum, and red river gum, is one of the most widely distributed Eucalyptus species. It is also considered one of the most widely planted trees in the world (ca. 5 000 000 ha planted) (N.A.S., 1980; Boland et al., 1984). Eucalyptus camaldulensis species is named for a private estate garden near the Camaldoli monastery near Naples (L'Hortus Camaldulensis di Napoli), from where the first specimen came to be described. Material from this tree was used by Frederick Dehnhardt, Chief Gardener at the Botanic Gardens in Naples, to describe this species in 1832 (Slee et al., 2006).
The habitus of the plant is very specific. Life form of Eucalyptus camaldulensis is a single-stemmed tree, with large trunk (Fig. 1 ). It is medium-sized to tall tree, average height 30 m (Bren and Gibbs, 1986), although some authors record trees to 45 m (Boland et al., 1984; Brooker et al., 2002). Leaves are grey-blue, alternate, drooping, 8–22 cm long, 1–2 cm wide, often curved or sickle shaped, tapering, and short pointed at base. Fruit is very small capsules at the end of thin stalks, 5–8 mm, valves 4, and containing minute seeds. Eucalyptus camaldulensis is an evergreen, perennial plant, and according to Jacobs (1955) it could reach ages of 500–1000 years. Commonly grows on riversides, whether of permanent or seasonal water (Brooker et al., 2002), generally dominate in the community, forming pure open forests or woodlands (Costermans, 1989).
Fig. 1.
Eucalyptus camaldulensis (river red gum) on the Murchison River in Western Australia (by courtesy of Prof. Stephen D. Hopper).
The species Eucalyptus camaldulensis consists of two variations: E. camaldulensis var. camaldulensis and E. camldulensis var. obtuse Blakely, and one subspecies Eucalyptus camaldulensis subsp. simulata Brooker & Kleinig (Table 1 ), found in North Queensland, which has been recognised as a hybrid of E. camldulensis var. obtusa and Eucalyptus tereticornis Smith (Brooker and Kleinig, 1994). According to the Centre for Plant Biodiversity Research (EUCLID, 2006), the operculum shape in E. camaldulensis is highly variable (Table 1). In the past, this character has been used to break up the group into different varieties or subspecies. The entire complex is currently under revision and new varieties or subspecies may be described or extant ones rationalised. Until this work is completed, EUCLID (2006) decided to adopt a conservative view of E. camaldulensis.
Table 1.
The main characters that distinguish Eucalyptus camaldulensis taxa.
| Taxon | Bark | Leaves | Operculum | |||
|---|---|---|---|---|---|---|
| E. camaldulensis var. camaldulensis | grey-white, rough at base bark |  |
non-glaucous, green, narrowly lanceolate juvenile leaves |  |
strongly beaked 0.3-0.7 cm long |  |
| E. camaldulensis var. obtusa | essentially white, smooth and seasonally powdery bark |  |
dull to slightly glossy green adult leaves with densely to very denselyreticulate venation |  |
rounded or obtusely conical operculum 0.4-0.7 cm long at maturity and glaucous juvenile growth |  |
| E. camadulensis subsp. simulata | grey-white, smooth to base bark |  |
ovate or lanceolate juvenile leaves, adult leaves with dense reticulation |  |
long horn-shaped operculum 0.9–1.6 cm long |  |
1.2. Eucalyptus camaldulensis traditional and contemporary application
Used for centuries as a traditional Aboriginal herbal remedy, eucalyptus leaves and their essential oils have found various applications in everyday life due to their antiseptic, anti-inflammatory and antipyretic properties (Jeane et al., 2003; Kumar et al., 1988). Ancient Aboriginal society in Australia used E. camaldulensis plant in medicines to treat gastrointestinal symptoms (including colic, diarrhea, and dysentery), respiratory disease (colds, coughs, asthma, laryngalgia, laryngitis, pharyngitis, sore throat, trachalgia), arrest bleeding, open wounds, and cuts, as well as its decoctions for the relief of spasms, aches, and pains in muscles, but also pains in joints and even tooth (Duke and Wain, 1981).
As previously stated, E. camaldulensis plant is also known as red gum eucalyptus, murray red gum, and river red gum, because it produces red kino i.e. red gum, in significant amount. Thus besides different plant parts Aborigines used its secondary products as folk remedies. They made incisions in the tree trunks for obtaining the red kino and applied it directly to abrasions and cuts. Except fresh kino, the dried, dehydrated kino was prepared and used in the same way as fresh, but after softening in water (Williams, 2011; Clarke, 2014). Another significant folk remedy is young leaves which were used for smoke bath, where burning leaves smoke surrounds patient. The smoking medicine was used for fevers, colds, flu and general sickness (Williams, 2011; Pennacchio et al., 2010; Duke and Wain, 1981). Being useful for treating various health conditions, E. camaldulensis and its folk remedies then were transferred and introduced to other parts of the world, such as Africa. In Sudan the red kino was used for sore throat and diarrhea, while the smoke of burnt leaves was inhaled in case of respiratory problems. In Senegal for stomach-ache decoctions from leaves were prepared with sugar, while in Zimbabwe for cough, flu, and fever a decoction of E. camaldulensis leaves were combined with Citrus limon (L.) Burm. f. fruits and Psidium guajava L. leaves (Doran and Wongkaev, 2008; Maroyi, 2013). Further more, to prevent tooth decay and periodontitis in Nigeria teeth cleaning sticks were made from tree (Bukar et al., 2004), and in traditional medicine for healing wound infections, poultice of leaves containing eucalyptus oil have been used (Adeniyi et al., 2006).
Nowdays, E. camaldulensis has been the subject of numerous studies, to confirm plant’s usefulness in traditional medicine for the treatment of various ailments (Coelho-de-Souza et al., 2005; Ghani, 2003; Ito et al., 2000). Its essential oils are reported to be anesthetic, antiseptic and astringent (Jeane et al., 2003; Kumar et al., 1988). In addition, a decoction of the leaves is reported to be a remedy for sore throat and other bacterial infections of the respiratory and urinary tracts (Bruneton, 1999). Due to numerous contemporary data regarding the antimicrobial activity of E. camaldulensis, this subject will be discussed in special section/chapter.
Except antimicrobial effects, E. camaldulensis plant extracts (PEx) and essential oils (EOs) and its constituents possess numerous other beneficial effects. One such effect is certainly gastrointestinal effect. In animal models, extracts of the leaves of E. camaldulensis and E. torelliana R. Muell are reported to decrease gastric acid production and thus appear useful for the treatment of gastric ulcers (Adeniyi et al., 2006). It has been proven that E. camaldulensis leaves methanol extracts possessed ulcer-healing promoting effect when investigated in acetic acid induced-ulcer in rat (Rattus norvegicus domesticus) (Lawal et al., 2014) (Table 2 ). Similarly, the poultice of the leaves is applied over wounds and ulcers (Gill, 1992).
Table 2.
Different significant effects of Eucalyptus camaldulensis.
| Different E. camaldulensis effects | Model organism and/or cell line | Plant part extract, oil or compound | Effect of dosage and/or application mode | Reference |
|---|---|---|---|---|
| Gastrointestinal effect | Acetic acid induced-ulcer in rat | Leaves methanol extracts | Reduction the size from day 5 in animals treated with 500 mg/kg body weight of reconstituted extracts at 24 h interval | Lawal et al. (2014) |
| Four ruminal fistulated buffaloes (Syncerus caffer), 4 years old with initial body weight 321 ± 20 kg | Leaves meal | Dietary treatments - different levels of leaf meal supplementation at 0, 40, 80, and 120 g/hd/d for 21 days. | Thao et al. (2015) | |
| Protozoa count and proteolytic bacteria population were reduced (p < 0.05). Fungal spores, amylolytic, cellulolytic and total viable bacteria were unchanged. | ||||
| Anti-inflammatory and analgesic effect | Healthy albino rats (200 ± 30 g) | Seed essential oils of E. camaldulensis var. nancy and E. camaldulensis var. petford | Carrageenan induced paw oedema test model in rats, which received 1000 μg kg−1 body weight; 43.75-87.5% of anti-inflammatory activity | Olawore and Ololade (2017) |
| Anti-nociceptive activities | Healthy albino rats (200 ± 30 g) | Seed essential oils of E. camaldulensis var. nancy and E. camaldulensis var. petford | Rats treated with 1000 μg kg−1 body weight, measured neurogenic and inflammatory pain responses, 41.03-99.09% of inhibition | Olawore and Ololade (2017) |
| Cytotoxic effect | Two human breast cancer cell lines (MCF 7 and MDA-MB-231) | Leaves methanol, ethyl acetate, n-buthanol, and water extracts | Significant cytotoxic potential with IC50 values ranging from 3 to 250 μg/mL after 72h | Hrubik et al. (2012) |
| L20B (a genetically engineered mouse cell line) and human rhabdomyo sarcoma cells | Crude methanol extracts | Moderate cytotoxicity | Adeniyi et al. (2015) | |
| Ehrlich's ascites carcinoma (EAC) in Swiss albino mice | Stem bark methanol extract | 25, 50 and 100 mg/kg/day for 5 days; High LD50 value (1120 mg/kg) | Islam et al. (2014) | |
| Anti-parasitic, insecticidal and repellent effects | Trypanosoma brucei infected mice | Leaves, stem and root barks extracts | 200-600 mg/kg body weight/day of the hexane, ethyl acetate, methanol and water extracts for 21consecutive days | Kabiru et al. (2013) |
| Promastigotes of Leishmania major in vitro | Methanol and aqueous extracts | IC50 values were 586.2 ± 47.6 and 1108.6 ± 51.9 μg/mL | Nosratabadi et al. (2015) | |
| Larvicidal activity against Anopheles stephenssi | Leaves extract and volatile oil | LC50 values of 89.85 and 397.75 ppm, respectively. Clear dose-response relationships, the highest dose of 320 ppm essential oil extract resulted almost in 100% mortality in the population after 24 h of exposure | Medhi et al. (2010) | |
| Mosquito larvicidal against two mosquito species, Aedes aegypti and Aedes albopictus | Leaves essential oils and their 12 constituents | 400, 200, 100, 50, and 25 μg/mL of essential oil were tested and each compound was tested at 50, 25, 12.5, and 6.25 μg/mL; Mortality was recorded after 24 h; LC50 values 31.0-55.3 lg/mL, LC90 values 71.8-192.4 lg/mL. | Cheng et al. (2009) | |
| Aphis gossypii Glover (Hem: Aphididae) | Essential oil | LC50values 2.28 μl L−1air | Ebrahimi et al. (2013) | |
| Repellency against the adult females of Culex pipiens | Dried fruits essential oil | Two different treatment levels (5 and 10 μl) in six exposure times (15, 75, 135, 195, 255 and 315 s) | Erler et al. (2006) | |
| Anti-diabetic effect | Albino rats | Leaves ethanol extract | Oral 500 mg/kg of body weight | Dawoud (2015) |
| Alloxan-induced diabetic rats | Leaves ethanol extract | 500 mg/kg of body weight in distilled water orally, E. camaldulensis leaves supplement incorporated into the diet 5 g/kg/day | Dawoud and Shayoub (2017) | |
The antioxidant effect represents one more useful E. camaldulensis characteristic. The free radical scavenging activities of the essential oils is assessed by measuring their scavenging abilities for 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals. The scavenging activity for the Eucalyptus camaldulensis was characterized as high (81.9%) (Ghaffar et al., 2015). The results of E. camaldulensis EO antioxidant effect evaluation indicated that the EO had high potent ferrous ions chelating and total antioxidant activities comparing to ascorbic acid and BHT (El-Baz et al., 2015). Also, the E. camaldulensis var. brevirostris leaves ethanol extract possessed antioxidant activity, where the prevailing antioxidants in the extract were gallic and ellagic acid (El-Ghorab et al., 2003). There are several more studies dealing with antioxidant activity of E. camaldulensis (Barra et al., 2010; Salem et al., 2015; Siramon and Ohtani, 2007; Olawore and Ololade, 2017), but it is interesting to mention here that E. camaldulensis flower EO inhibited melanogenesis through its antioxidant properties and by down-regulating both mitogen-activated protein kinases (MAPK) and protein kinase A (PKA) signaling pathways. This study indicated that the essential oil has the potential to be developed into a skin care product (Huang et al., 2015). Thus, except the medical application, E. camaldulensis extracts are also currently used in cosmetic formulations, and leaf extracts have been approved as food additives (Takahashi et al., 2004). Also, essential oils and their constituents have been used as flavoring agents in the formulation of different pharmaceutical products, cosmetics, and food industry (Cowan, 1999). Except this, E. camaldulensis PEx and EOs have the potential to be used as antibacterial and antifungal agents in cosmetic and pharmaceutical products. It is interesting to mention here the activity of E. camaldulensis EOs (2–8 mg/mL) on dental biofilm formation in vivo where detected inhibition was 14.5–39.2% after four weeks, comparing to chlorhexidine (2 mg/mL), which inhibited maximum 13.9% of dental biofilm formation (Rasooli et al., 2009). Extracts and essential oils of this aromatic plant can be used as food preservatives in order to reduce the dependency on synthetic chemicals in food preservation. In Australia, it is also used as sources of wild honey, providing bees with good quality pollens and heavy yields of nectar (Boland et al., 1984). Moreover, in industry, the wood of E. camaldulensis has been used for heavy construction, railway sleepers, flooring, framing, fencing, plywood, and veneer manufacture, wood turning, firewood, and charcoal production (Boland et al., 1984). Eucalyptus camaldulensis wood burns well and make a good fuel, also its dense wood and coppicing ability make it an excellent species for fuelwood production used in several countries such as Brazil (Jacobs, 1981; Eldridge et al., 1993). Furthermore, Eucalyptus biomass residues from agro-forest and pulping industries represent a valuable source of high-value compounds such as triterpenic compounds (Domingues et al., 2011; Ferreira et al., 2018).
Due to the diversity of E. camaldulensis beneficial effects, all effects reported in the literature are summarized in Table 2. Beside the beneficial effects, essential oils and plant extracts can exert potentially unfavorable effects as complex mixtures of different compounds. A risk assessment of their hazard is always necessary before commercialization, so the estimation of EOs toxicity has been already conducted resulting in human oral and dermal dose limit recommendations. For most EOs the recommended dose is in range 1–4%, but for cineole-rich Eucalyptus sp. EOs, including E. camaldulensis, the limit dose is 10%, indicating a generally low application risk (Lis-Balchin, 2006). The safe daily oral dose in human adults is 300–600 mg, while semisolid preparations for topical use may contain 5–20% of Eucalyptus oil (Blumenthal and Busse, 1998; Tisserand and Young, 2014). Similarly, E. camaldulensis bark methanolic extract LD50 value for Swiss albino mice (Mus musculus) is very high – 1120 mg/kg, indicating its low host toxic effects (Islam et al., 2014). The low toxicity and natural origin of E. camaldulensis essential oil and extracts, in contrast to synthetic antimicrobials, favor their application as antimicrobial agents.
2. Chemical composition
2.1. Eucalyptus camaldulensis plant chemical composition
Eucalyptus camaldulensis leaves contain 0.1–0.4% essential oil, of which 77% is 1,8-cineole. There is considerable amount of cuminal, phellandrene, aromadendren (or aromadendral), valerylaldehyde, geraniol, cymene, and phellandral (Council of Scientific and Industrial Research, 1948–1976Council of Scientific and Industrial Research (S.I.R, 1948Council of Scientific and Industrial Research, 1948–1976; Slee et al., 2006). Leaves contain 5–11% tannin. The kino (a class of wood exudates), contains 45% kinotannic acid as well as kino red, a glucoside, catechol, and pyrocatechol. Leaves and fruits test positive for flavonoids and sterols. The bark contains 2.5–16% tannin, the wood 2–14%, and the kino 46.2–76.7% (Watt and Breyer-Brandwijk, 1962). Some of the reported phytoconstituents of the tree included essential oils, sterols, alkaloids, glycosides, flavonoids, tannins, and phenols.
2.2. Eucalyptus camaldulensis essential oil chemical composition
A considerable variation in the yield of leaf essential oil from E. camaldulensis has been reported (Boland et al., 1984; Shieh, 1996; Moudachirou et al., 1999; Farah et al., 2002), depending on multiple biotope factors, and also genetic and/or epigenetic characteristics of the plant. The yields of E. camaldulensis leaves EO (0.90–0.98%) originating from Pakistan and Morocco were similar (Ashraf et al., 2010; Farah et al., 2002), while Moudachirou et al. (1999) reported a variable oil content of 0.6–1.4% from different locations of Benin. The oil yield of E. camaldulensis from Jerusalem was 0.5% (Chalchat et al., 2000) and significantly higher oil yield was reported for E. camaldulensis from Taiwan: 2.3–3.0% with respect to different seasons (Shieh, 1996). Similar EOs yield (0.77–2.53%) has been reported for E. globulus, as one of the economically important plants for essential oil production (Joshi, 2012; Selvakumar et al., 2012; Harkat-Madouri et al., 2015). The reported essential oil yield for other Eucalyptus species is slightly higher, ranging from 1.2% to 3% (w/w): the highest yield was obtained from E. cinerea F. Muell. ex Benth and E. sideroxylon A. Cunn. ex Woolls (3.0%), followed by E. lehmannii (Schauer) Benth. (2.8%), E. bicostata Maiden, Blakely & J.H.Simmonds (2.0%), E. leucoxylon F. Muell (1.6%), E. maidenii F.Muell. (1.5%), and E. astringens Maiden (1.2%) (Sebei et al., 2015).
All the E. camaldulensis essential oil single compounds belong to chemical class of hydrocarbons terpenes, further devided according to the number of isoprene units (C5H8) to monoterepenes (C10H16), sesquterepenes (C15H24), and longer chains of isoprene units. Oxygenated terpenes (oxygenated monoterepenes and oxygenated sesquterepenes) are called trepenoids. According to the literature, in E. camaldulensis EOs dominates 1,8-cineole (eucalyptol), trans-pinocarveol and terpinen-4-ol from chemical class of oxygenated monoterepenes. The oils also contain considerable amount of monoterpene hydrocarbons (β-pinene, α-thujene, γ-terpinene, p-cymene), while sesquterepene hydrocarbons and oxygenated sesquterepenes are detected in significantly lower amounts (Table 3 ).
Table 3.
Classes of major Euclyptus camaldulensis essential oils compounds and their content in different plant parts.
| Chemical classes | Chemical subclasses | Sublaclasses content in leaf and fruit (%)a | Major essential oils compounds | Major compounds content in oila, b |
||
|---|---|---|---|---|---|---|
| L | Fr | Fl | ||||
| Terpenes | Monoterpene hydrocarbons | 5.7-52.2 L | α-Pinene | 1.7-28.3 | 1.12-3 | 3.51 |
| 18.7 Fr | β-Pinene | 0.3-18.6 | 8.8 | 7.7-27.09 | ||
| 18.5 Fl | α-Thujene | 1.0-3.4 | 0.3 | 0.6-0.77 | ||
| β-Phellandrene | 0.5-7.5 | 0.3 | 2.1-2.2 | |||
| p-Cymene | tr. -6.5 | 4.8 | 9.32 | |||
| γ-Terpinene | 0.19-7.6 | 0.2 | 0.4-0.5 | |||
| Sesquiterpene hydrocarbons | 1.8-3.6 L | Aromadendrene | 0.1-11 | 0.2 | 0.21-0.24 | |
| 6.1 Fr | allo-Aromadendrene | 0.2-1 | 3 | 0.2 | ||
| 1.2 F•l | Bicyclogermacrene | 0.3-1.8 | 2 | 0.09-0.4 | ||
| α-Copaene | 0.9 | tr. | – | |||
| Terpenoids | Oxygenated monoterpenes | 40.8-87.42 L | 1,8-Cineole | 13.73-84.9 | 3.8 | 34.7-69.26 |
| 14.1 Fr | trans-Pinocarveol | 0.06-8.5 | 0.2 | 0.11-1.9 | ||
| 50 Fl | Terpinen-4-ol | 0.27-5.2 | 1.9 | 3.29-3.6 | ||
| Myrtenol | 1.4-9.75 | 2.1 | 0.12 | |||
| Cuminal | 0.09-3.2 | 1.3 | 0.94-1.01 | |||
| Oxygenated sesquiterpenes | 4.9-39.6 L | Spathulenol | 0.16-19.2 | 19 | 0.12-10.18 | |
| 23.2 Fr | Elemol | 0.6-3 | – | – | ||
| 12.7 Fl | β-Eudesmol | 0.13-4.4 | – | – | ||
| cis-Farnesol | 0.9-5 | – | 0.1 | |||
L- leaf, Fr-fruit, Fl-flower.
tr., traces (<0.05%).
When the composition of five Eucalyptus essential oils (Eucalyptus camaldulensis, Eucalyptus astringens Maiden, Eucalyptus leucoxylon, Eucalyptus lehmannii and Eucalyptus rudis Endl) are compared, a high percentage of monoterpenes, mainly oxygenated compounds, with lower quantities of sesquiterpenes were recorded throughout the four seasons (Ben Jemaa et al., 2012). In E. camaldulensis oil, monoterpenes were prevalent (34.6–56.3% for all seasons) while sesquiterpene hydrocarbons (6.6–16.5%) and oxygenated sesquiterpenes (2.1–11.1%) were present in less extent. Eucalyptus astringens oil had resembling chemical characteristic to E. camaldulensis oil, the abundant quantity of monoterpenes, which represented more than 50% of the total oil amounts for the all seasons (53.4–63.6 %). Similarlly, E. leucoxylon essential oil, contained mainly oxygenated monoterpenes with a prevalence of 1,8 cineole (13.1–17.6%), such as in E. camaldulensis oil (15.5–20.6%). The essential oil of E. lehmannii also was made up largely of monoterpenes (hydrocarbons 27.6–44.9% and oxygenated 30.9–62.8%), with smaller amounts of sesquiterpene hydrocarbons and absence of oxygenated sesquiterpenes, unlike the oils of other Eucalyptus species (Ben Jemaa et al., 2012).
The difference in the chemical composition of the E. camaldulensis EOs may be due to many reasons, which can generally be classified in five main groups: (1) the change in plant genes through generations and hybridizations (naturally and induced) may result in production a variety of volatile oils compared with those of different habitat; (2) nutrients of different soils and their accumulation in the leaves may result in different plant metabolism and consequently production of different bio-products and also EOs made of diverse compounds in variable amounts; (3) acclimation of species to the Australian environment in which it is growing in the past, compared with the introduced and/or worldwide planted trees on plantations; (4) different ecotypes of the E. camaldulensis and (5) differences may be due to plant part used for essential oils extraction and its stage of development (maturity). Knowing that these factors express significant effect on the percent compositions of some EO components for this species, E. camaldulensis can be grown in the corresponding areas and in specific conditions to enhance EO production.
The variations in the chemical composition of eucalyptus EOs with respect to seasons have also been reported (Tsiri et al., 2003), but the most commonly detected as major components in the eucalyptus essential oil are 1,8-cineole, β-pinene, γ-terpinene, and p-cymene. It is important to emphasize here that according to chemical composition the Eucalyptus camaldulensis EO generally can be divided in two different types. Type I is a cineole-rich essential oil containing 80–90% 1,8-cineole plus pinene, and Type II is a cineole-poor essential oil, containing significantly less cineol (Williams, 2011). Plant genotype is important factor influencing the final chemical composition of EO (Djilani and Dicko, 2012). Due to genetic and epigenetic factors same plant species can produce a similar EO, but with different chemical composition and therapeutic activities. Brophy and Southwell (2002) examined essential oils of two variations, Eucalyptus camaledulensis var. camaldulensis and Eucalyptus camaledulensis var. obtuse, and the main compounds of Eucalyptus camaledulensis var. camaldulensis were p-cymene (22%), cryptone (14%), and spathulenol (17%), while Eucalyptus camaledulensis var. obtusa had different main compounds: 1,8-cinole (52%), α-pinene (15%), and aromadendrene (3%), suggesting that these subspecies belong to different types of eucalyptus essential oils. The content of 1,8-cineole was also variable but in the same range for essential oils reported from Greece (25.3–44.2%) (Tsiri et al., 2003), Pakistan (34.4–40.0%) (Ashraf et al., 2010), Mozambique (37.1–40.0%) (Pegula et al., 2000), Nigeria (32.8–70.4%) (Oyedeji et al., 1999), and Taiwan (34.0–68.2%) (Shieh, 1996). Similarly, the major component of Eucalyptus camaldulensis oils originating form Burundi, Morocco, and Benin was 1,8-cineole ranging from 31.0 to 72.5% with no presence of cryptone (Dethier et al., 1994; Zrira and Benjilali, 1991; Zrira et al., 1992; Moudachirou et al., 1999). Cryptone has been shown to be present in low-cineole varieties of EOs from Australia (Bignell et al., 1996), Uruguay (Dellacassa et al., 1990) and South Florida (Pappas and Sheppard-Hanger, 2000). For example, the major constituents identified in the essential oil from South Florida included p-cymene (35.0%), cryptone (13.7%), terpinen-4-ol (5.7%), spathulenol (4.3%), and cuminaldehyde (3.7%), with a very low amount of 1,8-cineole (2.7%) (Pappas and Sheppard-Hanger, 2000).
It was proven that essential oils of different plant parts have different chemical composition (Table 3). Previous studies on the essential oil of E. camaldulensis flowers revealed the presence of 1,8-cineole, β-pinene, and spathulenol as the most abundant constituents (Giamakis et al., 2001). The essential oil of the leaves was found to contain p-cymene, γ-terpinene, α-pinene, 1,8-cineole, terpinen-4-ol, α-terpineol, carvacrol, and thymol as the major components (Siramon and Ohtani, 2007). The major components of the fruits essential oil were aromadendrene, α-pinene, drimenol, and cubenol (El-Ghorab et al., 2002).
Plant developmental stage and maturity of plant parts used for essential oil extraction also affect essential oil chemical composition. Giamakis et al. (2001) analyzed the immature flowers and calli grown in Athens (Greece), and they found that the main monoterpenes produced in E. camaldulensis calli, cultured in darkness and under light conditions, were 1,8-cineole, 62.70 and 69.26% as well as β-pinene, 27.09 and 25.31%, respectively. In lower amounts α-pinene (1.2 and 1.1%, respectively) and the terpenoids, camphene, myrcene, isocineole, myrtenol, bicyclogermacrene, spathulenol, and trans-pinocarveol were present (less than 1%). Apart from isocineole, these constituents were also determined in the essential oil from immature flowers. This is remarkable because Giamakis et al. (2001) showed that undifferentiated calli are capable of producing high amounts of monoterpenoid compounds (approx. one third of that produced by the explant). This makes immature flowers from E. camaldulensis an interesting candidate for the development of calli able to produce high percentages of these two important monoterpenes, 1,8-cineole and β-pinene (Giamakis et al., 2001). Also, the influence of light conditions on essential oil chemical composition showed that light conditions did not considerably affect the production of monoterpenes and sesquiterpenes compounds by calli developed from immature flowers (Giamakis et al., 2001).
Furthermore, plant culture conditions can influence the essential oil chemical composition. Soil salinity is a key ecological stress that severely influences plant productivity (Williams, 2011). Ashraf et al. (2010) showed that salinity had a considerable effect on the percent compositions of some components of E. camaldulensis leaves essential oil. The mean values of 1,8-cineole content of EO from saline and non-saline provenances of Pakistan were 34.42 and 40.05%, respectively (Ashraf et al., 2010). Therefore, they recommend stressing of this species by growing in the saline areas to enhance essential oil production for its various medicinal and pharmacological uses.
2.3. Eucalyptus camaldulensis extracts chemical composition
Extraction represents the primary step in obtaining the crude mixture of compounds from plants. Quality and quantity of the extracts dependent of the target compound structures, natural sources, and type of processes (Karacabey et al., 2013), explaining the different phenolic composition in the extracts obtained with different procedures. The most commonly plant extract have been obtained by conventional solvent extraction methods (infusion, decoction, digestion, maceration, and percolation) (Azwanida, 2015) using solvents such as water, ethanol, methanol, chloroform, dimethyl-sulfoxide etc. However, these techniques are demanding regarding the extraction process duration, organic solvent consumption, and lack of extraction automation. The potential and powerful alternative to conventional liquid solvent extraction methods are Ultrasonic-Assisted Extraction (UAE) and Microwave-Assisted Extraction (MAE), especially in the case of plant material (Hao et al., 2002; Eskilsson and Bjrklund, 2000). Interest in MAE has increased significantly over the past 5–10 years in particular medicinal plant research, as a result of its inherent advantages as special heating mechanism, moderate capital cost, and its good performance under atmospheric conditions (Ballard et al., 2010; Chan et al., 2011). In addition, MAE technique possesses many advantages compared with other methods for the extraction of compounds such as bioactive compounds (Sanchez-Aldana et al., 2013) saving in processing time and solvent, higher extraction rate, better products with lower cost, reduced energy consumption (up to 85-fold savings), and waste generation (Yan et al., 2010; Yemis and Mazza, 2012). This is confirmed for E. camaldulensis extraction of phenolic and flavonoid compounds, where the compounds were extracted with MAE for 5 min which was equivalent with UAE (60 min) and traditional extraction (24 h) methods (Gharekhani et al., 2012).
Many authors reported the chemical composition of E. camaldulensis extracts. The leaves of E. camaldulensis from the zoo-botanical garden in Giza (Egypt) yielded 4 major fractions (Singab et al., 2011). The major components of the first fraction (eluted with water) were identified as HHDP-glucopyranose, chlorogenic acid, and phloroglucinol derivatives. The second 30% methanol fraction was found to contain different galloyl-HHDP-glucopyranose positional isomers and pedunculagin as major components. The third 60% methanol fraction was predominantly composed of digalloyl-HHDP-glucopyranose (tellimagrandin I) α and β anomers, while the last 100% methanol fraction was composed of a mixture of ellagitannin dimers. The profiling of the obtained fractions by HPLC–PDA–ESI/MS/MS indicated that ellagitannins were the most predominant components of all three methanol fractions (Singab et al., 2011).
The secondary metabolites screening of E. camaldulensis leaf extracts from Nigeria confirmed presence of tannin, saponins, and cardiac glycosides (Ayepola and Adeniyi, 2008). Analyzed n-hexane, chloroform, and methanol extracts of E. camaldulensis stem bark and leaf also grown in Nigeria showed the presence of tannins and saponins in the stem bark and in the leaf of E. camaldulensis with absence of alkaloids in all extracts (Adeniyi et al., 2009). Furthermore, the crude methanol leaf extracts also from Nigeria contained in addition volatile oils and balsam (gum) (Babayi et al., 2004). Similarly, the phytochemical screening of ethanol, methanol, and petroleum ether leaf extracts from Nigeria contained in moderate to high amount secondary metabolites: alkaloids, saponins, tannins, flavonoids, steroid, carbohydrates, and cardiac glycosides, and not anthraquinones (Chuku et al., 2016). Crude methanol leaf extracts of E. camaldulensis from Iran had saponins, tannins, volatile oils, and balsam (gum), while the components such as anthraquinones, hydrolysable tannin, flavonoid, alkaloid, and glycosides were not detected (Jouki and Khazaei, 2010); crude methanolic leaves extract of E. camaldulensis from India contained anthraquinones, flavonoids, saponins, and terpenoids, while alkaloids, cardiac glycosides, and tannins were not detected (Singh and Thakur, 2016). Phytochemical screening of the crude stem barks methanol extract of E. camaldulensis from Bangladesh indicated presence of saponins, flavonoids, tannins, and also volatile oils, while anthraquinones, hydrolysable tannins, alkaloids, and glycosides were not present (Islam et al., 2014). The polyphenolic composition (flavonoids and phenolic acids and aldehydes) also was studied in the soluble fractions of the methanolic extracts of Eucalyptus camaldulensis originating from two Spain provinces, Huelva and Pontevedra: gallic, protocatechuic, vanillic and ellagic acids, and protocatechic aldehyde were identified, along with eriodictyol, quercetin, naringenin, vanillin, naringin, quercitrin, luteolin, and kaempferol (Cadahia et al., 1997). Eucalyptus camaldulensis extracts are generally rich in tannins which vary qualitatively and quantitatively according to the origin of the samples, and consequently protoanthocyanidin levels were influenced by the geographical origin (Cadahia et al., 1997).
3. Antimicrobial effect of Eucalyptus camaldulensis extracts and essential oils
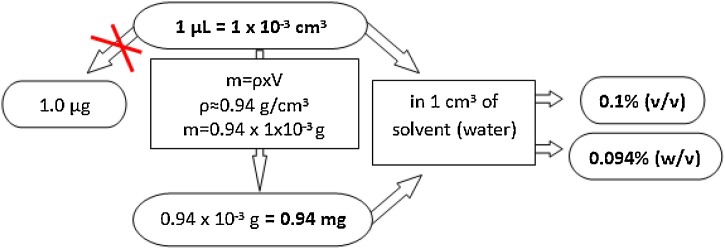
Antimicrobial activity of E. camaldulensis EO and extracts are well documented against many microorganisms listed in the Table 4 . For easier comparison, all data commented here for EOs were re-calculated from microliter per milliliter or microgram/milligram per milliliter to percentage (v/v or w/v), using the equitations presented in Fig. 2 . We considered here only minimal inhibitory and minimal bactericidal concentrations, while results obtained using disc or agar diffusion methods were not discussed. However, MIC/MBC results vary between several micrograms to several milligrams. Such high variation does not seem as real, even taking into account variation in oil composition, and are rather a consequence of erroneous equalization of one microliter and one microgram, or typographical errors. For instance, there are some nonsense MICs, such as 2000 μL/mL, that is practically impossible to obtain (Salem et al., 2015). In some manuscripts even a species was not precisely indicated (Harkenthal et al., 1999; Karpanen et al., 2008; Warnke et al., 2009; Tadtong et al., 2016) and these results were not considered in the present review.
Table 4.
Antimicrobial activity of E. camaldulensis essential oils and extracts.
|
E. camaldulensis |
Microorganism | Activitya |
Recalculated (%) |
References | |||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | ||||||
| Essential oil | Leaves essential oil (%, v/v) from Northern Cyprus | S. aureus | 0.5 | Akin et al. (2010) | |||||
| L. monocytogenes, E. durans, Salmonella Typhi, E. coli, B. subtilis, P. aeruginosa | >1 | ||||||||
| Leaves essential oil (μg/mL) from Iran | S. aureus, E. coli | 8 | 0.0008 | Lima et al. (2013) | |||||
| Leaves essential oil (μg/mL) from Iran | S. aureus | 3.9 | 3.9-7.8 | 0.00039 | 0.00039– 0.00078 | Panahi et al. (2011) | |||
| Leaves essential oil (μL/mL) from Ethiopia | Trichophyton sp. | 2.5–5.0 | 0.27–0.53 | Nasir et al. (2015) | |||||
| Microsporum, Candida Rhodotorula | 5.0 | 0.53 | |||||||
| A. niger | 2.5 | 0.27 | |||||||
| E. coli, Shigella sp, Bacillus sp, streptococci | 5.0 | 0.53 | |||||||
| Essential oil (μg/mL) from Iran | P. aeruginosa ATCC 27853 | 64 | 128 | 0.0064 | 0.0128 | Owlia et al. (2009) | |||
| Leaves essential oil (%) from Iran | S.aureus, A. baumannii | 0.1 | 0.2 | Ostad Asiaei et al. (2018) | |||||
| E.coli | 0.15 | 0.25 | |||||||
| P. vulgaris | 0.25 | 0.35 | |||||||
| S. sonnei | 0.30 | 0.45 | |||||||
| P. aeruginosa | 0.2 | 0.40 | |||||||
| K. pneumonia | 0.05 | 0.15 | |||||||
| S. eneterica serovar. Typhi | 0.4 | 0.6 | |||||||
| S. eneterica serovar. Paratyphi | 0.35 | 0.45 | |||||||
| S. eneterica serovar. Enteritidis, Infantis | 0.6 | 0.8 | |||||||
| Leaves essential oil (mg mL-1) from Iran | Streptococcus mutans, S. pyogenes | 1 | 2 | 0.1 | 0.2 | Rasooli et al. (2009) | |||
| Leaves essential oil (mg mL-1) from Thailand | T. cucumeris, F. oxysporum, C. globosum | 5 | 0.5 | Siramon et al. (2013) | |||||
| A. niger, C. cladosporioides, F. palustris, P. citrinum, T. versicolor | 10 | 1 | |||||||
| R. oryzae | >10 | >1 | |||||||
| Leaves essential oil (μl mL-1) from Montenegro | Reference Acinetobacter baumannii and MDR clinical strains | 0.5–1 | 0.7–4 | 0.05–0.1 | 0.07–0. 4 | Knezevic et al. (2016) | |||
| S. aureus ATCC 25923, E. coli ATCC 25922 | 1 | 1–2 | 0. 1 | 0.1–0.2 | |||||
| Leaves essential oil (μl/mL) from Kenya | Fusarium sp. | 7–8 | 8–10 | 0.7–0.85 | 0.85–1 | Gakuubi et al. (2017) | |||
| Leaves essential oil (μl/mL) from Algeria | F. graminearum | 2.5 | 0.27 | Mehani et al. (2014) | |||||
| F. sporotrichioide | 1.25 | 0.13 | |||||||
| Leaves essential oil (μg mL-1) from Brazil | S. aureus | 1000 | 0.1 | Chaves et al. (2018) | |||||
| E. coli | >1000 | >0.1 | |||||||
| Leaves essential oil from Egypt | % of reduction | El-Baz et al. (2015) | |||||||
| Rotavirus Wa strain | 50 | ||||||||
| Coxsackievirus B4 | 53.3 | ||||||||
| Herpes virus type 1 | 90 | ||||||||
| Adenovirus type 7 | 0 | ||||||||
| EOs in vegetable oil (mg/mL) | Trypanosoma brucei brucei and Trypanosoma evansi | 100 | 10 | Habila et al. (2010) | |||||
| Leaves essential oil (mg/mL) from Syria | S. aureus | 0.2 | 0.8 | 0.02 | 0.8 | Khubeiz et al. (2016) | |||
| B. subtilis | 1.6–3.2 | 1.6–3.2 | 0.16–0.32 | 0.16–0.32 | |||||
| M. luteus | 0.2–0.4 | 0.4–1.6 | 0.02–0.04 | 0.04–0.16 | |||||
| S. pyogenes | 0.4–0.8 | 0.8–1.6 | 0.04–0.08 | 0.08–0.16 | |||||
| K. pneumonia | 1.6–3.2 | 3.2 | 0.16–0.32 | 0.32 | |||||
| V. parahaemolyticus | 0.1 | 0.2 | 0.01 | 0.02 | |||||
| S. Typhimurium | 1.6–3.2 | 3.2 | 0.16–0.32 | 0.32 | |||||
| E. coli | 0.4–3.2 | 0.8–3.2 | 0.04–0.32 | 0.08–0.32 | |||||
| P. vulgaris, Ps. aeruginosa | >12.8 | >12.8 | >1.28 | >1.28 | |||||
| Leaves essential oil from Iran | GI | Katooli et al. (2011) | |||||||
| P. ultimum, R. solani, B. sorokiniana, C. gloeosporioides | 100% | ||||||||
| P. digitatum, A. flavus | 0% | ||||||||
| Plant extract | Leaves acetone extract (mg/l) from Nigeria | MDR Staphylococcus aureus | 20–50 | 0.002–0.005 | Ibrahim et al. (2014) | ||||
| Leaves methanol extract (mg/mL) from Egypt | MDR S. aureus | 0.78 | 0.078 | Reda et al. (2017) | |||||
| MDR P. aeruginosa | 3.12 | 0.312 | |||||||
| Bark butanol extract (μg mL-1) from Egypt | Pectobacterium cartovorum | 16 | 0.0016 | EL-Hefny et al. (2017) | |||||
| Ralstonia solanacearum | 125 | 0.0125 | |||||||
| Agrobacterium tumefaciens | 250 | 0.025 | |||||||
| Dickeya spp. | 500 | 0.05 | |||||||
| Leaves methanol extracts (mg/mL) from India | S. aureus, B. subtilis | 5 | 0.5 | Potdara et al. (2015) | |||||
| P. aeruginosa, S. eneterica serovar. Paratyphi | 10 | 1.0 | |||||||
| P. vulgaris, K. pneumonia, | 25 | 2.5 | |||||||
| S. eneterica serovar. Typhi | 50 | 5.0 | |||||||
| E. coli | 75 | 7.5 | |||||||
| Leaves crude methanolic extracts (μg mL-1) from Egypt | Dickeya solani, B. cereus | 0.08 | 0.16 | 0.000008 | 0.000016 | Elansary et al. (2017) | |||
| S. aureus | 0.22 | 0.40 | 0.000022 | 0.00004 | |||||
| Leaves methanol, ethanol, and petroleum ether extracts (mg mL-1) from Nigeria | P. aeruginosa | 100 | 200 | 10 | 20 | Chuku et al. (2016) | |||
| B. subtilis | 50–200 | 200–400 | 5–20 | 20-40 | |||||
| E. coli | 50–200 | 100–200 | 5–20 | 10–20 | |||||
| Penicillium expansum | 50–100 | 50–400 | 5–10 | 5–40 | |||||
| C. albicans | 50–200 | 50–200 | 5–20 | 5–20 | |||||
| Leaves methanol–dichloromethane extracts (mg mL-1) from Japan | S. aureus, methicillin-resistant S. aureus | 0.063 | 0.0063 | Takahashi et al. (2004) | |||||
| B. cereus, E. faecalis, Propionibacterium acnes, Trichophyton mentagrophytes | 0.125 | 0.0125 | |||||||
| E. coli, Pseudomonas putida, Alicyclobacillus acidoterrestris | >0.250 | >0.0250 | |||||||
| Leaves crude methanol extracts (μg mL-1) from Nigeria | B. subtilis, C. albicans, S. aureus (ATCC103207), S. aureus (clinical) | 200 | 0.02 | Babayi et al. (2004) | |||||
| E. coli (ATCC 10418), P. aeruginosa (ATCC 27853), S. Typhi | >200 | >0.02 | |||||||
| Leaves methanol extract (mg mL-1) from Nigeria | Klebsiella spp, Salmonella Typhi, P. aeruginosa | 10 | 1.0 | Ayepola and Adeniyi (2008) | |||||
| Leaves dichloromethane fraction (mg mL-1) from Nigeria | S. aureus | 1.25 | 0.125 | ||||||
| Yersinia enterocolitica | 0.157 | 0.0157 | |||||||
| B. subtilis | 0.04 | 0.004 | |||||||
| Klebsiella spp, Salmonella Typhi, P. aeruginosa | 10 | 1.0 | |||||||
| S. aureus, Yersinia enterocolitica | 0.625 | 0.0625 | |||||||
| B. subtilis | 0.79 | 0.079 | |||||||
| Leaves crude n-hexane extracts; crude chloroform leaves and steam extracts (μg mL-1) from Nigeria | Helicobacter pylori ATCC43504 and ATCC47619 | <25 | <0.0025 | Adeniyi et al. (2009) | |||||
| Steam crude n-hexane extracts (μg mL-1) from Nigeria | <12.5 | <0.00125 | |||||||
| Leaves and steam bark hexane, chloroform, methanol extracts (μg/mL) from Nigeria | Mycobacterium tuberculosis H37Rv | 4–52.2 | 0.0004–0.0064 | Lawal et al. (2012) | |||||
| Leaves methanol extracts 80% (μg/mL) from Ethiopia | M. tuberculosis | 6.25–50 | 0.000625–0.005 | Gemechu et al. (2013) | |||||
| M. bovis | 12.5–50 | 0.00125–0.005 | |||||||
| Leaves crude water extracts (mg/mL) from Nigeria | E. coli, Salmonella Typhi, S. aureus | 50 | 5.0 | Abubakar (2010) | |||||
| Leaves crude ethanol extracts (mg/mL) from Nigeria | Proteus mirabilis, Klebsiella pneumoniae | 25 | 2.5 | ||||||
| Leaves crude acetone extracts (mg/mL) from Nigeria | E. coli, | 50 | 5.0 | ||||||
| Salmonella Typhi, S. aureus, Klebsiella pneumoniae | 25 | 2.5 | |||||||
| Proteus mirabilis | 15 | 1.5 | |||||||
| E. coli, Klebsiella pneumoniae | 25 | 2.5 | |||||||
| Salmonella Typhi, S. aureus, Proteus mirabilis | 15 | 1.5 | |||||||
| Leaves essential oil (mg/mL) from Egipt | S. aureus | 0.7 | 0.07 | Reda et al. (2017) | |||||
| P. aeruginosa | 3.12 | 0.312 | |||||||
| Leaves DMSO extract (μg/mL) from Jordan | Newcastle Disease Virus (NDV) | 50-500 | 0.005-0.05 | Al-Hadid (2016) | |||||
| Leaves methanol extract (μg/mL) from Israel | IC50 | IC50 | Abu-Jafar and Huleihel (2017) | ||||||
| Herpes simplex virus -1 | 0.1±0.08 0.3±0.02 | 0.00001±0.000008 | |||||||
| Herpes simplex virus -2 | 1±0.03 | 0.00003±0.000002 | |||||||
| Varicella-Zoster Virus | 0.0001±0.000003 | ||||||||
| Leaves methanol extracts from Nigeria | Poliovirus type I | neutralization index of one log and above | Adeniyi et al. (2015) | ||||||
| Coxsackievirus B | |||||||||
| Echovirus 6 | |||||||||
| Bark n-butanol extract (μg /mL) from Egypt | P. carotovorum | 16 | 0.0016 | El-Hefny et al. (2017) | |||||
| R. solanacearum | 125 | 0.0125 | |||||||
| A. tumefaciens | 250 | 0.025 | |||||||
| Dickeya spp. | 500 | 0.05 | |||||||
| Leaves methanol extracts (mg/mL) from Iran | Microsporum canis | 0.8 | 6.4 | 0.08 | 0.64 | Falahati et al. (2005) | |||
| M. gypseum | 1.6 | 3.2 | 0.16 | 0.32 | |||||
| Tricophyton rubrum | 1.6 | 1.6 | 0.16 | 0.16 | |||||
| T. schoenleinii | 0.4 | 0.8 | 0.04 | 0.08 | |||||
| T. mentagrophytes | 0.2 | 0.8 | 0.02 | 0.08 | |||||
| Epedermophyton floccosum | 0.2 | 0.8 | 0.02 | 0.08 | |||||
| Bark dichloromethane: methanol extract; aqueous extract (mg/mL) from South Africa | MICD:M | MICAQ | MICD:M | MICAQ | Mabona et al. (2013) | ||||
| S. aureus (3 strains, including MRSA) | 0.25–1.0 | 0.5–0.63 | 0.025–0.1 | 0.05–0.063 | |||||
| S. epidermidis | 0.5 | 2.0 | 0.05 | 0.2 | |||||
| P. aeruginosa | 2.0 | 4.0 | 0.2 | 0.4 | |||||
| C. albicans | 0.5 | 2.0 | 0.05 | 0.2 | |||||
| Brevibacillus agri | 0.25 | 0.20 | 0.025 | 0.02 | |||||
| Propionobacterium acnes | 0.10 | 2.0 | 0.01 | 0.2 | |||||
| Trichophyton mentagrophytes | 1.0 | 1.0 | 0.1 | 0.1 | |||||
| M. canis | 4.0 | 2.0 | 0.4 | 0.2 | |||||
| Leaves methanol and aqueous extracts (μg/mL) from Iran | Leishmania major | IC50 | IC50 | Nosratabadi et al. (2015) | |||||
| 586.2-1108.6 | 0.05862–0.11086 | ||||||||
| Leaves crude, diethyl ether, ethyl acetate, aqueous (mg/mL) from Iran | Trichomonas vaginalis | 100% growth inhibition with 12.5–50; 24–72 h | 100% growth inhibition with 1.25–5%; 24–72 h | Hassani et al. (2013) | |||||
| Leaves wather and ethanolic extracts (μg/mL) from Iran | Trichomonas vaginalis | Dead for 72 h with 60-90 | Dead for 72 h with 0.006–0.009% | Youse et al. (2012) | |||||
| Leaves aqueous extract (mg/mL) from Iraq | Trichomonas vaginalis | Dead for 24 h with 500 | Dead for 24 h with 50% | Mahdi et al. (2006) | |||||
MIC – minimal inhibitory concentration, MBC – minimal bactericidal concentration, GI – growth inhibition, IC50 - 50% inhibitory concentration.
Fig. 2.
Schematic diagram for re-calculation from microliter or milligram per milliliter to percentage (v/v or w/v).
3.1. Antibacterial effect
Eucalyptus camaldulensis plant extracts and EOs were tested against wide range of bacteria. The most frequently included Gram positive bacterium in screening is S. aureus. Minimal inhibitory concentrations in most studies were in range 0.07–0.5% indicating moderately high activity against this bacterium. Beside S. aureus, antibacterial effect was confirmed against B. subtilis (0.17–0.34%), M. luteus (0.2–0.4%), and S. pyogenes (0.4–1.1%) (Rasooli et al., 2009; Akin et al., 2010; Knezevic et al., 2016; Khubeiz et al., 2016; Reda et al., 2017; Ostad Asiaei et al., 2018). Activity of EOs was examined aginst L. monocytogenes in only one study and MIC was not obtained with the highest used concentration of 1.0%, and the same was reported for Enterococcus durans (Akin et al., 2010).
Activity against Gram negative bacteria has been documented in a greater extent and MICs for the most frequently used model organism E. coli was in range 0.15–3.2%. Sensitivity of other enterobacteria is similar, with MICs in range from 0.05 to 0.32% for K. pneumoniae (Khubeiz et al., 2016; Ostad Asiaei et al., 2018), 0.16–0.32% for Salmonella enterica subsp. enterica serovar Typhimurium (Khubeiz et al., 2016); 0.35–0.4% for serovars Typhi, Paratyphi, and 0.6% for S. Enteritis (Ostad Asiaei et al., 2018). Other enterobacteria are similarily sensitive: Shigella soneii was inhibited with 0.3% of EO (Ostad Asiaei et al., 2018), while Proteus vilgaris was sensitive in a broad range from 0.25% (Ostad Asiaei et al., 2018) to more than 1.28% (Khubeiz et al., 2016). The published data for P. aeruginosa are in the same range as for P. vulgaris, which is not surprising, as both species are generally highly resistant to antimicrobial agents. On the contrary, A. baumannii which is also known to be multi-drug resistant, showed sensitivity to EO in a range 0.05–0.1% (Knezevic et al., 2016). The lowest MIC among Gram negative bacteria was recorded for a gastrointestinal pathogen V. parachaemoliticus (0.01%) (Khubeiz et al., 2016).
Although the Gram positive bacteria are consider more sensitive to EOs in comparison to Gram negative, it cannot be applied for essential oils of E. camaldulensis, since the most sensitive bacteria are Gram negative – A. baumannii and V. parahaemolyticus. The minimal bactericidal concentrations for most bacteria are equal to, or rarely up to 4 times greater than MICs. This low range in MIC/MBC < 4 indicates that E. camaldulensis EOs act as bactericidal agents (Pankey and Sabath, 2004).
Antibacterial effects of E. camaldulensis extracts have been examined in a wider extent than essential oils. Inhibitory concentrations varied depending on extraction method, plant properties, and model organism, being in broad range from 0.08 μg/mL to 200 mg/mL. Crude aqueous leaf extracts show lower activity against various bacteria (range 25–50 mg/mL) (Abubakar, 2010), while bark aqueous extracts were more effective, with MIC from 0.1 mg/mL for Propionobacterium acnes to 4.0 mg/mL for P. aeruginosa (Mabona et al., 2013). The antimicrobial activity of methanol, ethanol or petroleum leaf extracts of E. camaldulensis showed significant variation; for instance, MICs against B. subtilis varied from 0.04 up to 200 mg/mL or against S. aureus 1.25–25 mg/mL (Chuku et al., 2016; Ayepola and Adeniyi, 2008). For most examined Gram negative bacteria MICs were in range 10–200 mg/mL, while P. aeruginosa was even more susceptible (MICs 10–100 mg/mL). Similar or better antibacterial effect showed acetone leaf extract, being active against examined bacteria in range 15–50 mg/mL. The highest activity was obtained with dichloromethen extracts, with MICs for Staphylococcus spp. in range 0.25–1.0 mg/mL, and even lower for B. subtilis, P. acnes and B. agri (MICs 0.10–0.79 mg/mL). Interestingly, generally highly resistant M. tuberculosis was sensitive to methanol, n-hexane or chloroform extract with MICs in range 0.004–0.064 mg/mL, while M. bovis was slightly less sensitive with MICs 0.01–0.05 mg/mL (Lawal et al., 2012; Gemechu et al., 2013).
The progresses made on the investigation of essential oils mode of action, especially against bacterial cell targets, gave new perspectives in this combat. The knowledge about the essential oils and their target(s) on bacterial cell is crucial to understand which parts of bacterial cell are affected. The essential oils antibacterial action is linked to oil hydrophobicity that increases cell permeability and consequent leakage of cell constituents (Dorman and Deans, 2000; Lambert et al., 2001; Helander et al., 1998; Turgis et al., 2009; Ultee et al., 2002; Faleiro, 2011). It is important to perceive that a disturbed cell structures may affect stability of other cellular structures in a cascade type of action (Carson et al., 2002). Essential oils have several target sites on bacterial cells; however it seems that all are directly or indirectly connected to the primary effect of essential oils on the bacterial envelopes. First of all, EOs may cause cell wall and membrane disturbance (Lambert et al., 2001; Oussalah et al., 2006), which further can lead to the significant loss of intracellular ATP (Oussalah et al., 2006; Turgis et al., 2009), induction the synthesis of heat shock proteins (Burt et al., 2007), pH disturbance (Turgis et al., 2009; Oussalah et al., 2006), and intracytoplasmic changes (e.g. coagulation, periplasmic space enlargement) (Becerril et al., 2007). Although the number of studies dealing with the EO mechanisms of action is increasing, there are still many questions to be answered before the precise mechanism is revealed. This is at the same time one of the main limitations for EOs and extracts wide usage as antimicrobials. Thus, the so far knowledge must be further improved enabling the combat bacterial pathogens and its resistance.
Contemporary research of EOs and extracts are also focused on mechanism that allows bacteria to regulate some physiological activities, such as virulence, competition amongst populations, motility, sporulation, conjugation, antibiotic production, and biofilm formation (Rodriguez-Garcia et al., 2014). This system of intercellular communication, quorum sensing (QS), is based on production of the signal molecules, called autoinducers (Abraham et al., 2011). This communication system is relative to cell density and some compounds interfere with the QS communication system and attenuating the bacterial pathogenicity, in the phenomenon known as anti-QS compounds (Abraham et al., 2011). There are reports of anti-QS activity of various plants species EOs from genus Eucalyptus, such as Eucalyptus globulus L. (Luís et al., 2016; Cervantes-Ceballos et al., 2015), Eucalyptus radiata D. (Luís et al., 2016), Eucalyptus citriodora Hook., Eucalyptus smithii R. T. Baker and Eucalyptus staigeriana F. Muell. ex Bailey (Luís et al., 2016). Unfortunately, there is still no data regarding E. camaldulensis anti-QS activity.
In one study, antibacterial and in vivo biofilm preventive efficacies of E. camaldulensis oil were significantly higher than that of M. spicata oil and chlorhexidine, suggesting that E. camaldulensis EO is capable of affecting biofilm formation (Rasooli et al., 2009). Considering the anti-QS activity of above mentioned Eucalyptus species and also detected antimicrobial and anti-biofilm effect of E. camaldulensis it can be assumed that it possess similar or even higher anti-QS activity. However, future detailed studies of anti-QS and anti-biofilm E. camaldulensis activity are needed in order to confirm this assumption.
3.2. Antifungal effect
The E. camaldulensis leaves extracts and EOs have a potential as antifungal agents. They are able to act as a moderate antifungal agent against household molds, wood rot fungi (Siramon et al., 2013), and phytopathogenic fungi (Gakuubi et al., 2017; Mehani et al., 2014).
Eucalyptus camaldulensis EO has been studied for antifungal activity and was active in concentration 0.125–1.0% against most model organisms. The most sensitive seems to be F. sporotrichioides with MIC 0.125% (Mehani et al., 2014), while R. oryzae is the most resistant, as 1% of EO was ineffective against this fungus (Siramon et al., 2013). The best known human and animal pathogenic yeast Candida sp. showed considerable sensitivity to E. camaldulensis EO, with MIC approx. 0.5% (Siramon et al., 2013; Nasir et al., 2015). Most EOs from Sardinia inhibited growth of A. niger and B. cinerea in concentration of 20 μL/plate, while lower concentrations of the oils, such as 5 μL/plate were ineffective (Barra et al., 2010).
It is interesting to notice that liposomes containing E. camaldulensis EO were prepared (Moghimipour et al., 2012) for antifungal oil activity. The particle size varied from 40.5 to 298 nm for the different formulations, with approx. 95% of the essential oil entrapped. Inhibition of Microsporum canis, M. gypseum, Trichophyton rubrum, and T. verrucosum was achieved with 125 μL, and the liposomal gel formulation of the EO was proposed to improve antifungal activity.
Aqueous and organic extracts of E. camaldulensis have been reported to have antifungal activity (Table 4). Methanol leaves extracts showed inconsistent activity against C. albicans: in one study it was in range 50–200 mg/mL (Chuku et al., 2016), while in another it was 0.2 mg/mL (Babayi et al., 2004), indicating difference in active concentration of one thousand times. The bark methanole extract was active in concentration 0.5 mg/mL. Methanol leaf and bark extracts showed considerable activity against dermatophytes: 0.8–1.6 mg/mL against Microsporum spp, 0.125–1.6 mg/mL against Trichophyton spp., and 0.2 mg/mL against Epidemophyton flocossum (Takahashi et al., 2004; Falahati et al., 2005; Mabona et al., 2013).
Beside the antifungal activity of EOs and extracts of E. camaldulensis, it is worth noticing that this species, along with E. blakelyi M., E. gomphocephala A. DC, E. rudis Endl., and E. tereticornis Sm., is a reservoir of an emerging pathogenic fungus – Cryptococcus gattii. This fungus is usually related to tropic and subtropic regions, affecting the respiratory and nervous systems of the immunocompetent humans and domestic animals (Sorrell et al., 1996; Chakrabarti et al., 1997; Bielska and May, 2016; Roe et al., 2018). Similarily, C. neoformans var. grubii can be isolated from flowers and bark of E. camaldulensis (Gugnani et al., 2005). According to the literature, E. camaldulensis is natural reservoir of Cryptococcus and is considered responsible for occurring cryptococcosis worldwide. In this context, it is interesting to observe that C. neoformans is moderately sensitive to E. citriodora Hook EOs, with MIC90 0.5% (wt/v) (Pattnaik et al., 1996; Luqman et al., 2008) or E. globulus Labill. with MIC 0.13% (wt/v) (Suliman et al., 2010). Unfortunately, data on Cryptococcus sp. sensitivity to EOs and plant extracts of reservoir species of Eucalyptus, including E. camaldulensis, remains unknown.
Although there are no reports regarding E. camaldulensis mode of antifungal action, according to previous studies with other essential oils and fungi, the plasma membrane and the mitochondria are the probable antifungal targets of EOs. According to recent studies, the antifungal activity of EOs results from its ability to disrupt the permeability barrier of the plasma membrane and from the mitochondrial dysfunction-induced ROS accumulation in A. flavus and C. albicans ( Tian et al., 2012; Chen et al., 2013). The exact mechanism of E. camaldulensis antifungal activity is unknown and should be elucidated in the future.
3.3. Antiviral effect
Infections caused by viruses are very common and sometimes life threatening, especially in immunocompromised patients and neonates (Snoeck, 2000; Khan et al., 2005). Despite the recent significant progress in antiviral drug development, viral infections are considered as one of the major causes of death worldwide (Müller et al., 2007; Meyers et al., 1982). Thus, novel natural antiviral agents need to be found urgently. Many natural products possess antiviral activity and some of them are already in use for treatment of human viral infections with both RNA and DNA viruses (e.g. myricetin against coronavirus, linalool, urosolic acid, and apigenin against coxsackievirus, quercetin and narasin against denge virus, curcumin against hepatitis B and C virus) (Lin et al., 2014; Kitazato et al., 2007). Numerous secondary plant metabolites such as essential oils, flavonoids, saponins, tannins, alkaloids, lignans, terpenes, and phenolic acids express significant antiviral activity against different viruses (Jassim and Naji, 2003; Chiang et al., 2003; Sanchez Palomino et al., 2002). Recently few studies confirmed the E. camaldulensis EOs and plant extracts antiviral activity (Table 4).
Eucalyptus camaldulensis EOs reduce coxackie B4 and rotavirus Wa multiplication for 50%, herpes simplex virus 1 for 90%, but have no effect on adenovirus 7 multiplication (El-Baz et al., 2015). Similarly, methanolic extracts showed 50% inhibition of HSV 1 and 2 in concentration 0.1–0.3 μg/mL, and against Varicella zoster virus at concentration 1.0 μL/ml (Abu-Jafar and Huleihel, 2017). Dimethyl sulfoxide (DMSO) extracts inhbit multiplication of animal New Castle virus in concentration range 50–500 μg/mL (Al-Hadid, 2016). Antiviral activity has been observed for E. camaldulensis methanolic extracts against polio virus, coxsackie B, and echovirus 6 (Adeniyi et al., 2015). The data on antiviral activity of E. camaldulensis EO and extracts, although scarce, indicate their great potential, and necessity for further studies in this context.
Despite the fact that many plant extracts and essential oils were previously reported for their antiviral activities, the mechanism of action still remains poorly understood. There are many factors that influencing the EOs mode of action, which should be taken in consideration when antiviral activity of plant antimicrobials is examined. One of such factors is the difference between enveloped and non-enveloped viruses, because the observed antiviral effect has usually been greater for enveloped viruses (Yamada et al., 2009; Siddiqui et al., 1996). In majority of the studies dealing with antiviral mode of action, the focus has been on either the inhibition of viral adsorption to host cells or examination of the plant antimicrobials effectiveness against intracellular virus multiplication (Gilling et al., 2014). So, most commonly described modes of antiviral actions are virus inactivation and the impaired virus adsorption to host cells, which is often difficult to distinguish. Like in other antimicrobial modes of action, antiviral mechanism is also dependent on EOs or extracts compounds activity. This is one more factor that should be considered, because it affects the final antiviral mechanism of action. For some compounds the potential mechanism is reported, i.e. carvacrol acts directly upon the virus capsid and subsequently the nucleic acid (Gilling et al., 2014). When EOs antiviral mechanism of action is considered, EOs may cause the loss of the viral capsid integrity, ultimately leading to exposure of the viral genome. In addition, some EOs subsequently act directly upon the viral nucleic acid (DNA or RNA). According to one study, E. camaldulensis EOs may be promising antiviral agent against RNA viruses with no effect against DNA virus (El-Baz et al., 2015). Also, with shorter periods of exposure to the antimicrobial, the virus is able to adsorb specifically to host cells; however, it may or may not be able to cause successful infection depending upon the integrity of the viral genome. On the other hand, after exposure to some EOs virus capsid and genome remains intact. These antimicrobials appear to exert their antiviral effect by coating the capsid and thereby preventing specific adsorption of the virus to host cells (Gilling et al., 2014). Although, there are still no reports regarding E. camaldulensis EOs antiviral mode of action, there are some promising results about its antiviral effect (Table 4). These promising results represent foundation for further research and potential application of E. camalulensis EOs as potent antiviral agent.
3.4. Antiprotzoal effect
Among all deverse benefitial activities, E. camaldulensis expresess also an antiprotozoal effect as well. The reports regarding this effect are listed in Table 4. Eucalyptus camaldulensis methanolic and aqueous extracts were active against Leishmania major with IC50 values (50% inhibitory concentration) 586.2 ± 47.6 and 1108.6 ± 51.9 μg/mL, respectively (Nosratabadi et al., 2015). This was characterized as moderate leishmanicidal activity, but considering fact that present therapy consist antimony compounds which are expensive, toxic, and drug resistance is prevalent, E. camaldulensis plant extract derivatives represent safe, inexpensive, and promising alterantive solution.
There are also several reports regarding antiprotozoal activity against Trichomonas vaginalis, a causative agent of trichomoniasis which is the most prevalent nonviral sexual infection. The first report on E. camaldulensis aqueous extract anti-trichomonas activity showed that extract was active at concentration 500 mg/mL after 24 h (Mahdi et al., 2006). However, recent studies reported better effect of the E. camaladulensis extracts (Hassani et al., 2013; Youse et al., 2012). Five different E. camaldulensis leaves extracts including total extract, diethyl ether, chloroform, ethyl acetate, and water fractions were active in concentration range 12.5–50 mg/mL, with growth inhibiton achived after 24–72 h (Hassani et al., 2013). Ethyl acetate fraction showed the highest percentage of growth inhibition with the lowest concentration (12.5 mg/mL) after 24 and 48 h (Hassani et al., 2013). Even better anti-trichomonas activity was detedted for wather and ethanolic extracts, where concentration 60–90 μg/mL killed Trichomonas vaginalis for 72 h (Youse et al., 2012). The mainstay medication for trichomoniasis is metronidazole; however some resistant strains to this treatment have been detected making these results of E. camaldulensis anti-trichomonas activity very promising as antiprotozoal agent.
Except extracts, significant antiprotozoal activity was reported for E. camaldulensis EOs. The essential oils were found to possess antitrypanosomal activity in vitro in a dose-dependent manner in a short time. The decrease of Trypanosoma evansi number over time was achieved in doses of 400 mg/mL for 3 min, 200 mg/mL for 4 min, and 100 mg/mL for 15 min. Against Trypanosoma brucei brucei EOs was more potent in the concentration of 400 mg/mL, decreasing the number of parasite for 3 min, 200 mg/mL for 4 min, and 100 mg/mL for 11 min (Habila et al., 2010). Such prompt decrease in parasite number in the in vitro tests for both Trypanosoma brucei brucei and T. evansisuggests that the EOs kill the parasites efficiently, but by an unknown mechanism. There are suggestions of some potential mechanisms of antiprotozoal EOs action. The activity of EOs could be due to the hydrophobic nature of the cyclic hydrocarbons, which allow EOs to interact with the protozoans causing conformational changes in the parasite membrane structure, resulting in the loss of membrane stability (Calsamiglia et al., 2007). The essential oils also can act by inhibiting some key enzymes in the parasite glycolytic pathway (Smith-Palmer et al., 2004). Furthermore, some EOs components inhibit acetylcholinesterase activity and act on other vulnerable sites, such as cytochrome p450 (Maciel et al., 2010). This multicomponent nature of plant EOs is an advantage for several target sites on protozoans, which is of great importance.
3.5. Antimicrobial activity of E. camaldulensis Dehn. vs. other species of genus Eucalyptus
It was shown that EOs of E. camaldulensis are more potent against B. subtilis, S. aureus, E. coli, P. aeruginosa, S. lutea, and P. carotovorum in comparison to EOs from E. gomphocephala DC., but not against A. tumefaciens. Furthermore, the E. camaldulensis var. obtuse showed even better effect than E. camaldulensis (Salem et al., 2015). Among seven examine essential oils of various Eucalyptus species, EO of E. camaldulensis was among the best against B. subtilis, A. niger, and R. solani, but not against E. coli and S. aureus (Ghaffar et al., 2015). Similar activity of E. camaldulensis and E. torelliana F. Muell. was recorded for extracts against six strains of H. pylori (Adeniyi et al., 2009).
It is worth to notice that among 132 extracts from 42 plants growing in southern Africa, E. camaldulensis bark extract showed considerable activity against bacteria and fungi, with an exception against P. aeruginosa and M. canis (Mabona et al., 2013). Finaly, in many studies regarding antimicrobial activity of various Eucalyptus species, E. camaldulensis was not always included (Ashour, 2008; Safaei-Ghomi and Ahd, 2010; Mulyaningsih et al., 2010; Elaissi et al., 2012; Sebei et al., 2015).
4. Interaction of Eucalyptus camaldulensis Dehn. EOs/extracts and other antimicrobial agents
Due to rapid emerging of microbial resistance to conventional drugs, the necessity for efficient solution(s) is rising. The main strategy represents finding new antimicrobial agents; however another strategy goes in the direction of reducing degree of bacterial resistance and/or bacterial re-sensitization to conventional antibiotics. This can be achieved using combined therapies. The most of the tested combinations are dual combinations, but the combinations of three, four or more agents also can be efficient (Lesjak et al., 2016). This is in line with antimicrobial efficiency and potential of EOs and plant extracts which are complex mixtures of numerous compounds. Combination strategy could be very promising regarding the diversity of agents that could be tested: (1) conventional non-antimicrobial agent (e.g. anti-inflammatory or anti-psychotic drug) + conventional antimicrobial agent (antibiotic); (2) conventional antimicrobial agent (antibiotic) + natural antimicrobial agent (essential oil, plant extract, bacteriophage, antimicrobial peptide ect.); (3) conventional antimicrobial agent (antibiotic) + single compound isolated from natural antimicrobial agent (with previously confirmed antimicrobial activity); (4) combination of two or more single compounds isolated from natural antimicrobial agents (with previously confirmed antimicrobial activity).
Being a plant with already detected and evaluated antimicrobial activity, it can be assumed that E. camaldulensis also have a potential in combined therapy. This assumption is confirmed in some studies in vitro and in vivo (Table 5 ). Eucalyptus camaldulensis EOs and extracts in vitro reduced resistance of MDR A. baumannii in combination with conventional antibiotics: β-lactams, ciprofloxacin, gentamicin, polymyxin B (Knezevic et al., 2016). Similarily, the increase of β-lactamase produing MRSA and E. coli sensitivity to cephalexin, cefuroxime, amoxicillin, and ampicillin has been obtained through combination with E. camaldulensis EOs (Chaves et al., 2018). Synergistic activity has been recorded between E. camaldulensis plant extracts and gentamicin or ceftriaxone against MDR S. aureus and P. aeruginosa (Reda et al., 2017; Ibrahim et al., 2014), as well as in combination with ampicillin against S. aureus (Ibrahim et al., 2014).
Table 5.
Effects of Eucalyptus camaldulensis and other antimicrobial agents in combination.
| E. camaldulensis extract or essential oil | Antimicrobial agent(s) in combination | Test organism | Method | Effect of combination | Reference |
|---|---|---|---|---|---|
| Leaf essential oils from Montenegro | Ciprofloxacin, Gentamicin, Polymyxin B | Reference Acinetobacter baumannii and MDR clinical strains | Two-dimensional checkerboard method (Verma, 2007) | Synergism (FICI ≤ 0.5) | Knezevic et al. (2016) |
| Leaf essential oil from Brazil | Cephalexin | Staphylococcus aureus 29 | The MIC of the antibiotics were determined in the presence and absence of sub-inhibitory concentrations (125 μg mL−1) of the essential oil (Coutinho et al., 2010) | Combining the essential oil with β-lactams reduced the resistance of tested strains | Chaves et al. (2018) |
| Cefuroxime | S. aureus 55 (MRSA) E. coli (all strains resistant to β-lactam antibiotics) | ||||
| Amoxicillin | |||||
| Ampicillin | |||||
| Leaf methanol extract (0.01 μg/mL) from Israel | Acyclovir (0.01 μg/mL) | In vitro infection of Vero cells with Herpes simplex virus -1 Herpes simplex virus -2 Varicella-Zoster Virus | Treating the cells with different combinations of the extract and acyclovir at the time and post-infection with the virus | Significant inhibition (˜75%) of the viral infection, comparing to single agent (10-20%) | Abu-Jafar and Huleihel (2017) |
| Leaf methanol extract from Nigeria | Leaf methanol extracts of Annona senegalensis | In vivo albino mice infection with Trypanosoma brucei brucei (Lafia strain) | 200 mg/kg bodyweight/day (for 21 days) of crude methanol extracts of A. senegalensis and E. camaldulensis combinations in ratios 1:1, 1:2, and 2:1, respectively. | Synergism (only the combination 1:1 resulted in the complete clearance of parasites from the circulation of animal) | Kabiru et al. (2012) |
| Leaf methanol extract from Egypt | Gentamicin, Ceftriaxone | MDR S. aureus | Lajubutu et al. (1995) | Synergism | Reda et al. (2017) |
| MDR P. aeruginosa | |||||
| Leaf acetone extract from Nigeria | Ampicillin | MDR S. aureus | Collins et al. (1995) | Synergism | Ibrahim et al. (2014) |
| Ciprofloxacin | |||||
| Leaf ethanol extract from Nigeria | Psidium guajava ethanol extract | E. coli | Agar disc diffusion method was employed as described by Kirby and Bauer (1966), adopted by Yushau et al. (2009) and Bashir et al. (2011) | Synergism | Bala et al. (2014) |
| MDR S. aureus |
Furthermore, the combination of E. camaldulensis extract with another plant extract Psidium guajava L. was also efficient against MDR bacteria (Bala et al., 2014). Except antibacterial activity, other activities of E. camaldulensis extracts in combination were detected and characterized as efficient. Antiviral activity was confirmed for the combination of E. camaldulensis 80% methanol leaves extract and acyclovir against herpes simplex virus -1 and -2 and varicella-zoster virus (Abu-Jafar and Huleihel, 2017). Similaily, the combination of Annona senegalensis L. leaf methanol extract and E. camaldulensis extract efficiently cured in vivo albino mice infection with parasite Trypanosoma brucei brucei (Lafia strain) (Kabiru et al., 2012). All these data are very promising, but the methods used in different studies and results interpretation vary (Table 5). As a consequence, the data should be taken with precautions, as can not be compared and properly discussed. To avoid this problem, the testing combinations of different agents should be conducted using standardized methods, such as time-kill method (CLSI, 1999; Verma, 2007), checkerboard method (Verma, 2007; EUCAST, 2000), Chou-Talalay method (Chou, 2010) or Boik method (Boik, 2010). All these methods possess some shortfalls, such as time-consuming, labor-intensive, limitations regarding the number of the agents in combination, etc. Unfortunately, there is no one gold standard for synergy testing and prior the further application of different phytochemicals, this issue should be overcome.
5. Conclusion
Summarizing the available data on antimicrobial properties of Eucalyptus camaldulensis essential oil and extracts, it is obvious that this plant is a valuable source of phytotherapeutics. The essential oil, as well as leaf and bark extracts are particularly valuable as antibacterial, antiviral, and antifungal agents, and their antiprotozoal activity should not be neglected taking into account current therapy cost, toxicity, and protozoal growing resistance. Some E. camaldulensis plant characteristics such as easy cultivation, wide distribution by plantation, and rapid growth additionally support further examination of antimicrobial activity, in order to enhance the commercial production of E. camaldulensis based pharmaceuticals. The future studies should be focused on determination of the mechanisms of antimicrobial activity, paticularly potential anti-biofilm and anti-QS effects, as well as the activity enhancement in combination with other available agents.
Acknowledgments
This study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, grant OI 172058. The authors acknowledge Prof. Stephen D. Hopper (School of Agriculture and Environment, The University of Western Australia) for Eucalyptus camaldulensis image and Prof. Ljiljana Knezevic (Faculty of Sciences, University of Novi Sad) for the language revision.
References
- Abraham S.V.P., Palani A., Ramaswamy B.R., Shunmugiah K.P., Arumugam V.R. Antiquorum sensing and antibiofilm potential of Capparis spinosa. Arch. Med. Res. 2011;42:658–668. doi: 10.1016/j.arcmed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Abubakar E.M.M. Antibacterial potential of crude leaf extracts of Eucalyptus camaldulensis against some pathogenic bacteria. Afr. J. Plant Sci. 2010;4(6):202–209. [Google Scholar]
- Abu-Jafar A., Huleihel M. Antiviral activity of Eucalyptus camaldulensis leaves ethanolic extract on herpes viruses infection. Int. J. Clin. Virol. 2017;1:001–009. doi: 10.29328/journal.ijcv.1001001. [DOI] [Google Scholar]
- Adeniyi B.A., Odufowoke R.O., Olaleye S.B. Antibacterial and gastro-protective properties of Eucalyptus torelliana F. Muell crude extracts. Int. J. Pharmacol. 2006;2:362–365. [Google Scholar]
- Adeniyi C.B.A., Lawal T.O., Mahady G.B. In vitro susceptibility of Helicobacter pylori to extracts of Eucalyptus camaldulensis and Eucalyptus torelliana. Pharm. Biol. 2009;47(1):99–102. doi: 10.1080/13880200802397988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeniyi B.A., Ayepola O.O., Adu F.D. The antiviral activity of leaves of Eucalyptus camaldulensis (Dehn) and Eucalyptus torelliana (R. Muell) Pak. J. Pharm. Sci. 2015;28(5):1773–1776. [PubMed] [Google Scholar]
- Akin M., Aktumsek A., Nostro A. Antibacterial activity and composition of the essential oils of Eucalyptus camaldulensis Dehn and Myrtus communis L. growing in Northern Cyprus. Afr. J. Biotechnol. 2010;9:531–535. [Google Scholar]
- Al-Hadid K.J. Evaluation of antiviral activity of different medicinal plants against Newcastle Disease Virus. Am. J. Agric. Biol. Sci. 2016;11(4):157–163. doi: 10.3844/ajabssp.2016.157.163. [DOI] [Google Scholar]
- Ali N., Ahmed G., Ali Shah S., Shah I., Ghias M., Khan I. Acute toxicity, brine shrimp cytotoxicity and relaxant activity of fruits of callistemon citrinus Curtis. BMC Complement. Altern. Med. 2011;11:99. doi: 10.1186/1472-6882-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G.R., Mathiews V.S. The distillation of essential oils. Trop. Prod. Inst. 1968;10(1):136–149. [Google Scholar]
- Ashour H.M. Antibacterial, antifungal, and anticancer activities of volatile oils and extracts from stems, leaves, and flowers of Eucalyptus sideroxylonand Eucalyptus torquata. Cancer Biol. Ther. 2008;7(3):399–403. doi: 10.4161/cbt.7.3.5367. [DOI] [PubMed] [Google Scholar]
- Ashraf M., Ali O., Anwar F., Hussain A.I. Composition of leaf essential oil of Eucalyptus camaldulensis. Asian J. Chem. 2010;22(3):1779–1786. [Google Scholar]
- Aslam B., Wang W., Arshad M.I., Khurshid M., Muzammil S., Rasool M.H., Nisar M.A., Alvi R.F., Aslam M.A., Qamar M.U., Salamat M.K.F., Baloch Z. Antibiotic resistance: a rundown of a global crisis. Infect. Drug Resist. 2018;11:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayepola O.O., Adeniyi B.A. The antibacterial activity of leaf extracts of Eucalyptus camaldulensis (Myrtaceae) J. Appl. Sci. Res. 2008;4(11):1410–1413. [Google Scholar]
- Azwanida N.N. A Review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants. 2015;4:3. doi: 10.4172/2167-0412.1000196. [DOI] [Google Scholar]
- Babayi H., Kolo I., Okogun J.I., Ijah U.J.J. The antimicrobial activities of methanolic extracts of Eucalyptus camaldulensis and Terminalia catappa against some pathogenic microorganisms. Biokemistri. 2004;16(2):106–111. doi: 10.4314/biokem.v16i2.32578. [DOI] [Google Scholar]
- Bala I., Yushau M., Lawal D., Abubakar S. Synergistic effect of eucalyptus (Eucalyptus camaldulensis) and guava (Psidium guajava) ethanolic extracts on Escherichia coli and Staphylococcus aureus. Acad. Res. Int. 2014;5(6):35–41. [Google Scholar]
- Ballard T.S., Mallikarjunan P., Zhou K., O’Keefe S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010;120:1185–1192. doi: 10.1016/j.foodchem.2009.11.063. [DOI] [Google Scholar]
- Barra A., Coroneo V., Dessi S., Cabras P., Angioni A. Chemical variability, antifungal and antioxidant activity of Eucalyptus camaldulensis essential oil from Sardinia. Nat. Prod. Commun. 2010;5(2):329–335. 10.1016%2Fj.indcrop.2015.04.059. [PubMed] [Google Scholar]
- Bashir M., Yusuf I., Kutama A.S. In-vitro studies on the sensitivity of Staphylococcus aureus to some ethno-medicinal preparations sold around Kano, Nigeria. Bayero J. Pure Appl. Sci. 2011;4(1):22–25. doi: 10.4314/bajopas.v4i1.4. [DOI] [Google Scholar]
- Becerril R., Gómez-Lus R., Goñi P., López P. Combination of analytical and microbiological techniques to study the antimicrobial activity of a new active food packaging containing cinnamon or oregano against E. coli and S. aureus. Anal. Bioanal. Chem. 2007;388:1003–1011. doi: 10.1007/s00216-007-1332-x. [DOI] [PubMed] [Google Scholar]
- Ben Jemaa J.M., Haouel S., Bouaziz M., Khouja M.L. Seasonal variations in chemical composition and fumigant activity of five Eucalyptus essential oils against three moth pests of stored dates in Tunisia. J. Stored Prod. Res. 2012;48:61–67. doi: 10.1016/j.jspr.2011.10.001. [DOI] [Google Scholar]
- Bielska E., May R.C. What makes Cryptococcus gattii a pathogen? FEMS Yeast Res. 2016;16(1):fov106. doi: 10.1093/femsyr/fov106. [DOI] [PubMed] [Google Scholar]
- Bignell C.M., Dunlop P.J., Brophy J.J., Jackson J.F. Volatile leaf oils of some South-western and southern Australian species of the genus Eucalyptus part VII-subgenus symphyomyrtus, section exsertaria. Flav. Fragr. J. 1996;11:35–41. doi: 10.1002/ffj.2730100206. [DOI] [Google Scholar]
- Blumenthal M., Busse W.R. The Complete German Commission E Monographs. American Botanical Council; Austin, Tex: 1998. Bundesinstitut für Arzneimittel und Medizinprodukte (Germany) [Google Scholar]
- Boik J.C. An R package for assessing drug synergism/antagonism. J. Stat. Softw. 2010;34(6):1–18. [Google Scholar]
- Boland D.J., Brooker M.I.H., Chippendale G.M., Hall N., Hyland B.P.M., Johnston R.D., Kleinig D.A., Turner J.D. Nelson and CSIRO; Melbourne: 1984. Forest Trees of Australia. [Google Scholar]
- Bren L.J., Gibbs N.L. Relationships between flood frequency, vegetation and topography in a river red gum forest. Aust. For. Res. 1986;16:357–370. [Google Scholar]
- Brooker M.I.H., Kleinig D.A. Inkata Press; Sydney: 1994. Field Guide to Eucalypts: Volume 3 Northern Australia. [Google Scholar]
- Brooker M.I.H., Connors J.R., Slee A.V., Duffy S. CSIRO Publishing; Collingwood: 2002. EUCLID: Eucalypts of Southern Australia (CD Rom) [Google Scholar]
- Brophy J.J., Southwell I.A. Eucalyptus chemistry. In: Coppen J.J.W., editor. Eucalyptus: The Genus Eucalyptus. Taylor & Francis; London and New York: 2002. pp. 102–160. [Google Scholar]
- Bruneton J. 2nd ed. Intercept Ltd.; London: 1999. Pharmacognosy: Phytochemistry, Medicinal Plants. [Google Scholar]
- Bukar A., Danfillo I.S., Adeleke O.A., Ogunbodede E.O. Traditional oral health practices among Kanuri women of Borno State, Nigeria. Odontostomatol. Trop. 2004;27(107):25–31. [PubMed] [Google Scholar]
- Burt S.A., van der Zee R., Koets A.P., De Graaff A.M., van Knapen, Gaastra W., Haagsmann H.P., Veldhuizen E.J.A. Carvacrol induces heat shock protein 60 and inhibit synthesis of flagellin in Escherichia coli O157:H7. Appl. Environ. Microb. 2007;73:4484–4490. doi: 10.1128/AEM.00340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadahia E., Cadahia E., Díez-Barra R., García-Vallejo M.C. Tannin composition of Eucalyptus camaldulensis, E. globulus and E. rudis. Part I. Wood. Holzforschung. 1997;51(2):119–124. doi: 10.1515/hfsg.1997.51.2.125. [DOI] [Google Scholar]
- Calsamiglia S., Busquet M., Cardozo P.W., Castillejos L., Ferret A. Invited review: essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007;90(6):2580–2595. doi: 10.3168/jds.2006-644. [DOI] [PubMed] [Google Scholar]
- Carson C.F., Mee B.J., Riley T.V. Mechanism of action of Melaleuca alternifolia (tea tree) on Staphylococcus aureus determined by time-kill, leakage and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002;46:1914–1920. doi: 10.1128/AAC.46.6.1914-1920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Ceballos L., Caballero-Gallardo K., Olivero-Verbel J. Repellent and anti-quorum sensing activity of six aromatic plants occurring in Colombia. Nat. Prod. Commun. 2015;10(10):1753–1757. [PubMed] [Google Scholar]
- Chakrabarti A., Jatana M., Kumar P., Chatha L., Kaushal A., Padhye A.A. Isolation of Cryptococcus neoformans var. Gattii from Eucalyptus camaldulensis in India. J. Clin. Microbiol. 1997;35(12):3340–3342. doi: 10.1128/jcm.35.12.3340-3342.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalchat J.C., Gary R.P., Sidibe L., Harama M. Aromatic plants of Mali (V): chemical composition of four Eucalyptus species implanted in Mali, Eucalyptus camaldulensis, E. torelliana, E. citriodora, E. Tereticornis. J. Essent. Oil Res. 2000;12:695–701. doi: 10.1080/10412905.2000.9712194. [DOI] [Google Scholar]
- Chan C.H., Yusoff R., Ngoh G., Kung F.W. Microwave-assisted extraction of active ingredients from plants – a review. J. Chromatogr. A. 2011;1218:6213–6225. doi: 10.1016/j.chroma.2011.07.040. [DOI] [PubMed] [Google Scholar]
- Chaves T.P., Pinheiro R.E.E., Melo E.S., Soares M.J.S., Souza J.S.N., de Andrade T.B., de Lemos T.L.G., Coutinho H.D.M. Essential oil of Eucalyptus camaldulensis Dehn potentiates β-lactam activity against Staphylococcus aureus and Escherichia coli resistant strains. Ind. Crop Prod. 2018;112:70–74. doi: 10.1016/j.indcrop.2017.10.048. [DOI] [Google Scholar]
- Chen Y., Zeng H., Tian J., Ban X., Ma B., Wang Y. Antifungal mechanism of essential oil from Anethum graveolens seeds against Candida albicans. J. Med. Microbiol. 2013;62:1175–1183. doi: 10.1099/jmm.0.055467-0. [DOI] [PubMed] [Google Scholar]
- Cheng S.S., Huang C.G., Chen Y.J., Yu J.J., Chen W.J., Chang S.T. Chemical compositions and larvicidal activities of leaf essential oils from two Eucalyptus species. Bioresour. Technol. 2009;100(1):452–456. doi: 10.1016/j.biortech.2008.02.038. [DOI] [PubMed] [Google Scholar]
- Chiang L.C., Cheng H.Y., Liu M.C., Chiang W., Lin C.C. In Vitro anti-herpes simplex viruses and antiadenoviruses activity of twelve traditionally used medicinal plants in Taiwan. Biol. Pharm. Bull. 2003;26:1600–1604. doi: 10.1248/bpb.26.1600. [DOI] [PubMed] [Google Scholar]
- Chou T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- Chuku A., Ogbonna A.I., Obande G.A., Namang M., Ahmad I.R. Antimicrobial effects of leaves of Eucalyptus camaldulensis on some microbial pathogens. Eur. J. Med. Plants. 2016;14(2):1–8. doi: 10.9734/EJMP/2016/25759. [DOI] [Google Scholar]
- Clarke P. Rosenberg Publishing Pty Ltd.; Australia, A: 2014. Discovering Aboriginal Plant Use: The Journeys of an Australian Anthropologist. [Google Scholar]
- CLSI . Clinical and Laboratory Standards Institute, USA; Wayne, PA: 1999. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline. CLSI Document M26-A. [Google Scholar]
- Coelho-de-Souza L.N., Leal-Cardoso J.H., de Abreu Matos F.J. Relaxant effects of the essential oil of Eucalyptus tereticornis and its main constituent 1,8-cineole on guinea-pig tracheal smooth muscle. Planta Med. 2005;71:1173–1175. doi: 10.1055/s-2005-873173. [DOI] [PubMed] [Google Scholar]
- Collins C.H., Lynes P.M., Grange J.M. 7th ed. Butterwort-Heinemann Ltd.; Britain: 1995. Microbioloical Methods; pp. 175–190. [Google Scholar]
- Coppen J.W. Taylor and Francis Inc.; London and New York: 2002. Eucalyptus the Genus Eucalyptus. [Google Scholar]
- Costermans L.F. 1989. Native Trees and Shrubs of South-Eastern Australia. Weldon, Sydney. [Google Scholar]
- Council of Scientific and Industrial Research (S.I.R.) vol. 11. 1948. (The Wealth of India). New Delhi, 1948–1976. [Google Scholar]
- Coutinho H.D., Costa J.G., Falcão-Silva V.S., Siqueira-Júnior J.P., Lima E.O. In vitro additive effect of Hyptis martiusii in the resistance to aminoglycosides of methicillin-resistant Staphylococcus aureus. Pharm. Biol. 2010;48:1002–1006. doi: 10.3109/13880200903382686. [DOI] [PubMed] [Google Scholar]
- Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawoud A.D.H. Effects of ethanolic leaf extract of Eucalyptus camaldulensis on oral glucose tolerance test in type-2 model diabetic rats. J. Netw. Commun. Emerg. Technol. (JNCET) 2015;2(2):6–8. [Google Scholar]
- Dawoud A.D.H., Shayoub M.H. Antidiabetic activity of Eucalyptus camaldulensis in alloxan-induced diabetic rats. Int. J. Adv. Innov. Res. 2017;4(4):1–9. [Google Scholar]
- Dellacassa E., Soler E., Menéndez P., Moyna P. Essential oils from Lippia alba (Mill.) N.E. Brown and Aloysia chamaedrifolia Cham. (Verbenaceae) from Uruguay. Flavour Fragr. J. 1990;5:107–108. doi: 10.1002/ffj.2730050210. [DOI] [Google Scholar]
- Dethier M., Nduwimana A., Cordier Y., Menut C., Lamaty G. Aromatic plants of tropical Central Africa. XVI. Studies on essential oils of five Eucalyptus species grown in Burundi. J. Essent. Oil Res. 1994;6:469–473. doi: 10.1080/10412905.1994.9698428. [DOI] [Google Scholar]
- Djilani A., Dicko A. The therapeutic benefits of essential oils. In: Bouayed J., editor. Nutrition, Well-Being and Health. InTech.; 2012. pp. 155–178. [Google Scholar]
- Domingues R.M.A., Patinha D.J.S., Sousa G.D.A., Villaverde J.J., Silva C.M., Freire C.S.R., Silvestre A.J.D., Pascoal Neto C. Eucalyptus biomass residues from agro-forest and pulping industries as sources of high-value triterpenic compounds. Cellulose Chem. Technol. 2011;45(7-8):475–481. [Google Scholar]
- Doran J.C., Wongkaev W. Eucaliptus camaldulensis Dehnh. In: Louppe D., Oteng Amoako A.A., Brink M., editors. Plant Resources of Tropical Africa 7(1): Timbers 1. PROTA Foundation. Bachkuys Publisher; Wageningen, Netherlands: 2008. Leiden Netherlands; CTA, Wageningen, Netherlands. [Google Scholar]
- Dorman H.J.D., Deans S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Duke J.A., Wain K.K. vol. 3. 1981. (The Medicinal Plants of the World, Computer index with More than 85,000 Enteries). [Google Scholar]
- Ebrahimi M., Safaralizade M.H., Valizadegan O., Amin B.H.H. Efficacy of three plant essential oils, Azadirachta indica (Adr. Juss.), Eucalyptus camaldulensis (Dehn.) and Laurus nobilis (L.) on mortality cotton aphids, Aphis gossypii Glover (Hem: Aphididae) Arch. Phytopathol. Plant Prot. 2013;46(9):1093–1101. doi: 10.1080/03235408.2012.758347. [DOI] [Google Scholar]
- Elaissi A., Rouis Z., Salem N.A., Mabrouk S., ben Salem Y., Salah K.B., Aouni M., Farhat F., Chemli R., Harzallah-Skhiri F., Khouja M.L. Chemical composition of 8 eucalyptus species’ essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Complement. Altern. Med. 2012;12:81. doi: 10.1186/1472-6882-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elansary H.O., Salem M.Z.M., Ashmawy N.A., Yessoufou K., El-Settawy A.A.A. In vitro antibacterial, antifungal and antioxidant activities of Eucalyptus spp. leaf extracts related to phenolic composition. Nat. Prod. Res. 2017;31(24):2927–2930. doi: 10.1080/14786419.2017.1303698. [DOI] [PubMed] [Google Scholar]
- El-Baz F.K., Mahmoud K., El-Senousy W.M., Darwesh O.M., ElGohary A.E. Antiviral – antimicrobial and schistosomicidal activities of Eucalyptus camaldulensis essential oils. Int. J. Pharm. Sci.: Rev. Res. 2015;31(1):262–268. [Google Scholar]
- Eldridge K.G., Davidson J., Harwood C.E., Van Wyk G. Clarendon Press; Oxford, UK: 1993. Eucalypt Domestication and Breeding. UK. [Google Scholar]
- El-Ghorab A.H., El-Massry K.F., Fadel H.M. The Egyptian Eucalyptus camaldulensis var. brevirostris: chemical compositions of the fruit volatile oil and antioxidant activity. Flavour Frag. J. 2002;17:306–312. doi: 10.1002/ffj.1085. [DOI] [Google Scholar]
- El-Ghorab A.H., El-Massry K.F., Marx F., Fadel H.M. Antioxidant activity of Egyptian Eucalyptus camaldulensis var. brevirostris leaf extracts. Nahrung. 2003;47(1):41–45. doi: 10.1002/food.200390009. [DOI] [PubMed] [Google Scholar]
- EL-Hefny M., Ashmawy N.A., Salem M.Z.M., Salem A.Z.M. Antibacterial activities of the phytochemicals-characterized extracts of Callistemon viminalis, Eucalyptus camaldulensis and Conyza dioscoridis against the growth of some phytopathogenic bacteria. Microb. Pathog. 2017;113:348–356. doi: 10.1016/j.micpath.2017.11.004. [DOI] [PubMed] [Google Scholar]
- El-Taweel H.A. Understanding drug resistance in human intestinal protozoa. Parasitol. Res. 2015;114:1647–1659. doi: 10.1007/s00436-015-4423-1. [DOI] [PubMed] [Google Scholar]
- Erler F., Ulug I., Yalcinkaya B. Repellent activity of five essential oils against Culex pipiens. Fitoterapia. 2006;77(7–8):491–494. doi: 10.1016/j.fitote.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Eskilsson C.S., Bjrklund E. Analytical-scale microwave-assisted extraction. J. Chromatogr. 2000;902(1):227–250. doi: 10.1016/S0021-9673(00)00921-3. [DOI] [PubMed] [Google Scholar]
- EUCAST . Vol. 6. CMI; 2000. (Terminology Relating to Methods for the Determination of Susceptibility of Bacteria to Antimicrobial Agents. European Committee for Antimicrobial Susceptibility Testing of the European Society of Clinical Microbiology and Infectious Diseases (EUCAST) Definitive Document E. Def 1.2, 503-508). [DOI] [PubMed] [Google Scholar]
- EUCLID . third edition. Centre for Plant Biodiversity Research CSIRO Publishing; 2006. Eucalypts of Australia. Collingwood October 2006 ISBN: 0643093354 (DVD-ROM) AU. [Google Scholar]
- Falahati M., Tabrizib N.O., Jahaniani A. Anti dermatophyte activities of Eucalyptus camaldulensis in comparison with griseofulvin. Iran. J. Pharmacol. Ther. 2005;4(2):80–83. [Google Scholar]
- Faleiro M.L. The mode of antibacterial action of essential oils. In: Méndez-Vilas A., editor. Science Against Microbial Pathogens: Communicating Current Research and Technological Advances ©FORMATEX 2011. 2011. [Google Scholar]
- Farah A., Fechtal M., Chaouch A., Zrira S. The essential oils of Eucalyptus camaldulensis and its natural hybrid (clone 583) from Morocco. Flavour Fragr. J. 2002;17:395–397. doi: 10.1002/ffj.1114. [DOI] [Google Scholar]
- Ferreira J.P.A., Miranda I., Pereira H. Chemical composition of lipophilic extractives from six Eucalyptus barks. Wood Sci. Technol. 2018;52:1685. doi: 10.1007/s00226-018-1054-6. [DOI] [Google Scholar]
- Gakuubi M.M., Maina A.W., Wagacha J.M. Antifungal activity of essential oil of Eucalyptus camaldulensis Dehnh. against selected Fusarium spp. Int. J. Microbiol. 2017:1–7. doi: 10.1155/2017/8761610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemechu A., Giday M., Worku A., Ameni G. In vitro anti-mycobacterial activity of selected medicinal plants against Mycobacterium tuberculosis and Mycobacterium bovis strains. BMC Complement. Altern. Med. 2013;13:291. doi: 10.1186/1472-6882-13-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffar A., Yameen M., Kiran S., Kamal S., Jalal F., Munir B., Saleem S., Rafiq N., Ahmad A., Saba I., Jabbar A. Chemical composition and in vitro evaluation of the antimicrobial and antioxidant activities of essential oils extracted from seven Eucalyptus species. Molecules. 2015;20:20487–20498. doi: 10.3390/molecules201119706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani A. Asiatic Society of Bangladesh; Dhaka, Bangladesh: 2003. Medicinal Plants of Bangladesh. [Google Scholar]
- Gharekhani M., Ghorbani M., Rasoulnejad N. Microwave-assisted extraction of phenolic and flavonoid compounds from Eucalyptus camaldulensis Dehn. leaves as compared with ultrasound-assisted extraction. Lat. Am. Appl. Res. 2012;42:305–310. [Google Scholar]
- Giamakis A., Kretsi O., Chinou I., Spyropoulos C.G. Eucalyptus camaldulensis: volatiles from immature flowers and high production of 1,8-cineole and β-pinene by in vitro cultures. Phytochemistry. 2001;58:351–355. doi: 10.1016/S0031-9422(01)00193-5. [DOI] [PubMed] [Google Scholar]
- Gill L.S. Uniben Press; Benin City: Nigeria: 1992. Ethnomedical Uses of Plants in Nigeria; pp. 15–35. [Google Scholar]
- Gilling D.H., Kitajima M., Torrey J.R., Bright K.R. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J. Appl. Microbiol. 2014;116(5):1149–1163. doi: 10.1111/jam.12453. [DOI] [PubMed] [Google Scholar]
- Gugnani H.C., Mitchell T.G., Litvintseva A.P., Lengeler K.B., Heitman J., Kumar A., Basu S., Paliwal-Joshi A. Isolation of Cryptococcus gattii and Cryptococcus neoformans var. grubii from the flowers and bark of Eucalyptus trees in India. Med. Mycol. 2005;43(6):565–569. doi: 10.1080/13693780500160785. [DOI] [PubMed] [Google Scholar]
- Habila N., Agbaji A.S., Ladan Z., Bello I.A., Haruna E., Dakare M.A., Atolagbe T.O. Evaluation of in vitro activity of essential oils against trypanosoma brucei brucei and Trypanosoma evansi. J. Parasitol. Res. 2010 doi: 10.1155/2010/534601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J., Han W., Huang S., Xue B., Deng X. Microwave assisted extraction of artemisinin from Artemisia annua L. Sep. Purif. Technol. 2002;28:191. doi: 10.1016/S1383-5866(02)00043-6. e196. [DOI] [Google Scholar]
- Harkat-Madouri L., Asma B., Madani K., Said Z.B.O.S., Rigou P., Grenier D., Allalou H., Remini H., Adjaoud A., Boulekbache-Makhlouf L. Chemical composition, antibacterial and antioxidant activities of essential oil of Eucalyptus globulus from Algeria. Ind. Crop. Prod. 2015;78:148–153. [Google Scholar]
- Harkenthal M., Reichling J., Geiss H.K., Saller R. Comparative study on the in vitro antibacterial activity of Australian tea tree oil, cajuput oil, niaouli oil, manuka oil, kanuka oil, and eucalyptus oil. Pharmazie. 1999;54(6):460–463. [PubMed] [Google Scholar]
- Hassani S., Asghari G., Yousefi H., Kazemian A., Rafieiean M., Darani H.Y. Effects of different extracts of Eucalyptus camaldulensis on Trichomonas vaginalis parasite in culture medium. Adv. Biomed. Res. 2013;2:47. doi: 10.4103/2277-9175.114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander I.M., Alakomi H.L., Latva-Kala K., Mattila-Sandholm T., Pol I., Smid E.J., Gorris L.G.M. Characterization of the action of selected essential oil components on gram-negative bacteria. J. Agricult. Food Chem. 1998;46:3590–3595. doi: 10.1021/jf980154m. [DOI] [Google Scholar]
- Hrubik J.D., Kaisarevic S.N., Glisic B.D., Jovin E.D., Mimica Dukic N.M., Kovacevic R. Myrtus communis and Eucalyptus camaldulensis cytotoxicity on breast cancer cells. Proc. Nat. Sci. Matica Srpska Novi Sad. 2012;123:65–73. doi: 10.2298/ZMSPN1223065H. [DOI] [Google Scholar]
- Huang H.C., Ho Y.C., Lim J.M., Chang T.Y., Ho C.L., Chang T.M. Investigation of the anti-melanogenic and antioxidant characteristics of Eucalyptus camaldulensis flower essential oil and determination of its chemical composition. Int. J. Mol. Sci. 2015;16:10470–10490. doi: 10.3390/ijms160510470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim T.A., Fagbohun E.D., Olalumade B.B. Study of the synergistic effect of antibiotics and plant extracts against clinical Staphylococcus aureus strains. Res. Rev. Pharm. Pharm. Sci. 2014;3(1):1–4. [Google Scholar]
- Irwin K.K., Renzette N., Kowalik T.F., Jensen J.D. Antiviral drug resistance as an adaptive process. Virus Evol. 2016;2(1):vew014. doi: 10.1093/ve/vew014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam F., Khatun H., Khatun M., Ali S.M., Khanam J.A. Growth inhibition and apoptosis of Ehrlich ascites carcinoma cells by the methanol extract of Eucalyptus camaldulensis. Pharm. Biol. 2014;52(3):281–290. doi: 10.3109/13880209.2013.834365. [DOI] [PubMed] [Google Scholar]
- Ito H., Koreishi M., Tokuda H., Nishino H., Yoshida T. Cypellocarpins A-C, phenol glycosides esterified with oleuropeic acid, from Eucalyptus cypellocarpa. J. Nat. Prod. 2000;63:1253–1257. doi: 10.1021/np0001981. [DOI] [PubMed] [Google Scholar]
- Jacobs M.R. Forestry and Timber Bureau; Canberra: 1955. Growth Habits of the Eucalypts. [Google Scholar]
- Jacobs M.R. 2nd ed. FAO Forestry Series No 11. Food and Agriculture Organization of the United Nations; Rome, Italy: 1981. Eucalypts for Planting. Eucalypts for Planting. [Google Scholar]
- Jassim S.A., Naji M.A. Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- Jeane S., Worku A., Sousa S.M., Duarte V.G., Machado M.I.L., Matos F.J.A. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003;89:277–283. doi: 10.1016/j.jep.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Joshi R.K. Aroma profile of Eucalyptus globulus: collected from north west Karnataka, India. Sci. World. 2012;10:89–90. doi: 10.3126/sw.v10i10.6870. [DOI] [Google Scholar]
- Jouki M., Khazaei N. The antimicrobial activities of methanolic extracts of Eucalyptus camaldulensis against Bacillus subtilis, Staphylococcus aureus and Escherichia coli. J. Res. Agric. Sci. 2010;6:63–67. doi: 10.4314/biokem.v16i2.32578. [DOI] [Google Scholar]
- Kabiru Y.A., Okogun J.I., Gbodi T.A., Makun H.A., Ogbadoyi E.O. Evaluation of the efficacy of combination therapy in T. b. brucei – infected mice using extracts of Annona senegalensis and Eucalyptus camaldulensis. IOSR J. Pharm. 2012;2(4):32–37. doi: 10.9790/3013-24403237. [DOI] [Google Scholar]
- Kabiru Y.A., Ogbadoyi E.O., Okogun J.I., Gbodi T.A., Makun H.A. Anti-trypanosomal potential of Eucalyptus camaldulensis. Br. J. Pharmacol. Toxicol. 2013;4(2):25–32. doi: 10.19026/bjpt.4.5374. [DOI] [Google Scholar]
- Karacabey E., Bayindirli L., Artik N., Mazza G. Modeling solid-liquid extraction kinetics of trans-resveratrol and trans-e-viniferin from grape cane. J. Food Process. Eng. 2013;36(1):103–112. doi: 10.1111/j.1745-4530.2011.00660.x. [DOI] [Google Scholar]
- Karpanen T.J., Worthington T., Hendry E.R., Conway B.R., Lambert P.A. Antimicrobial efficacy of chlorhexidine digluconate alone and in combination with eucalyptus oil, tea tree oil and thymol against planktonic and biofilm cultures of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2008;62(5):1031–1036. doi: 10.1093/jac/dkn325. [DOI] [PubMed] [Google Scholar]
- Katooli N., Maghsodlo R., Razavi S.E. Evaluation of eucalyptus essential oil against some plant pathogenic fungi. J. Plant Breed. Crop Sci. 2011;3(2):41–43. [Google Scholar]
- Khan M.T., Atherb A., Thompson K.D., Gambari R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antivir. Res. 2005;67:107–119. doi: 10.1016/j.antiviral.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Khubeiz M.J., Mansour G., Zahraa B. Chemical compositions and antimicrobial activity of leaves Eucalyptus camaldulensis essential oils from four syrian samples. Int. J. Curr. Pharm. Res. 2016;7:251–257. [Google Scholar]
- Kirby W.M., Bauer A.W. Antibiotc susceptibility testing; a standard single disc method. Am. J. Clin. Pathol. 1966;45:493–494. [PubMed] [Google Scholar]
- Kitazato K., Wang Y., Kobayashi N. Viral infectious disease and natural products with antiviral activity. Drug Discov. Ther. 2007;1(1):14–22. [PubMed] [Google Scholar]
- Knezevic P., Aleksic V., Simin N., Svircev E., Petrovic A., Mimica-Dukic N. Antimicrobial activity of Eucalyptus camaldulensis essential oils and their interactions with conventional antimicrobial agents against multi-drug resistant Acinetobacter baumannii. J. Ethnopharmacol. 2016;178:125–136. doi: 10.1016/j.jep.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Kumar A., Sarma V.D., Sing A.K., Kamla S. Antimicrobial properties of different Eucalyptus oils. Fitoterapia. 1988;59:141–144. doi: 10.1016/S2221-1691(12)60220-2. [DOI] [Google Scholar]
- Lajubutu B.A., Pinney R.J., Robert M.F., Odelola H.A., Oso B.A. Antimicrobial activity of diosquinone and plumbagin from Diospyros mespiliformis (Hostch) (Ebenaceae) Phytother. Res. 1995;9:346–350. doi: 10.1002/ptr.2650090508. [DOI] [Google Scholar]
- Lambert R.J.W., Skandamis P.N., Coote P.J., Nychas G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001;91:453–462. doi: 10.1046/j.1365-2672.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Lawal T.O., Adeniyi B.A., Adegoke A.O., Franzblau S.G., Mahady G.B. In vitro susceptibility of Mycobacterium tuberculosis to extracts of Eucalyptus camaldulensis and Eucalyptus torelliana and isolated compounds. Pharm. Biol. 2012;50(1):92–98. doi: 10.3109/13880209.2011.625953. [DOI] [PubMed] [Google Scholar]
- Lawal T.O., Adeniyi B.A., Olaleye S.B. Ulcer-healing promoting activities of methanol extracts of Eucalyptus camaldulensis Dehnh. and Eucalyptus torelliana F. Muell in rat. Arch. Basic Appl. Med. 2014;2:147–152. [Google Scholar]
- Lesjak M., Simin N., Orcic D., Franciskovic M., Knezevic P., Beara I., Aleksic V., Svircev E., Buzas K., Mimica-Dukic N. Binary and tertiary mixtures of Satureja hortensis and Origanum vulgare essential oils as potent antimicrobial agents against Helicobacter pylori. Phytother. Res. 2016;30(3):476–484. doi: 10.1002/ptr.5552. [DOI] [PubMed] [Google Scholar]
- Lima L.M., Babakhani B., Boldaji S.A.H., Asadi M., Boldaji R.M. Essential oils composition and antibacterial activities of Eucalyptus camaldulensis Dehn. Int. J. Med. Aromatic Plants. 2013;3(2):214–219. [Google Scholar]
- Lin L.T., Hsu W.C., Lin C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014;4(1):24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis-Balchin M. Pharmaceutical Press; Great Britain: 2006. Aromatherapy Science: A Guide for Healthcare Professionals. [Google Scholar]
- Luís Â., Duarte A., Gominho J., Domingues F., Duarte A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crop Prod. 2016;79:274–282. doi: 10.1016/j.indcrop.2015.10.055. [DOI] [Google Scholar]
- Luqman S., Dwivedi G.R., Darokar M.P., Kalra A., Khanuja S.P.S. Antimicrobial activity of Eucalyptus citriodora essential oil. Int. J. Essent. Oil Ther. 2008;2:69–75. [Google Scholar]
- Mabona U., Viljoen A., Shikanga E., Marston A., Van Vuuren S. Antimicrobial activity of South African medicinal plants with dermatological relevance: from a screening approach, to combination studies and bioactive isolation. J. Ethnopharmacol. 2013;148:45–55. doi: 10.1016/j.jep.2013.03.056. [DOI] [PubMed] [Google Scholar]
- Maciel M.V., Morais S.M., Bevilaqua C.M.L. Chemical composition of Eucalyptus spp. essential oils and their insecticidal effects on Lutzomyia longipalpi. Vet. Parasitol. 2010;167(1):1–7. doi: 10.1016/j.vetpar.2009.09.053. [DOI] [PubMed] [Google Scholar]
- Mahdi N.K., Gany Z.H., Sharief M. Alternative drugs against Trichomonas vaginalis. E. Mediterr. Health J. 2006;12(5):679. http://www.who.int/iris/handle/10665/117136 [PubMed] [Google Scholar]
- Maroyi A. Traditional use of medicinal plants in south-central Zimbabwe: review and perspectives. J. Ethnobiol. Ethnomed. 2013;9:31. doi: 10.1186/1746-4269-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhi S.M., Reza S.A., Mahnaz K., Reza A.M., Abbas H., Fatemeh M., Hassan V. Phytochemistry and larvicidal activity of Eucalyptus camaldulensis against malaria vector, Anopheles stephensi. Asian Pac. J. Trop. Med. 2010;3(1):841–845. doi: 10.1016/S1995-7645(10)60203-9. [DOI] [Google Scholar]
- Mehani M., Salhi N., Valeria T., Ladjel S. Antifungal effect of essential oil Eucalyptus camaldulensis plant on Fusarium graminearum and Fusarium sporotrichioide. Int. J. Curr. Res. 2014;6(12):10795–10797. [Google Scholar]
- Meyers J.D., Wade J.C., Mitchell C.D., Saral R., Lietman P.S. Multicenter collaborative trial of intravenous acyclovir for treatment of mucocutaneous herpes simplex virus infection in the immunocompromised host. Am. J. Med. 1982;73:229–235. doi: 10.1016/0002-9343(82)90097-3. [DOI] [PubMed] [Google Scholar]
- Moghimipour E., Aghel N., Mahmoudabadi A.Z., Ramezani Z., Handali S. Preparation and characterization of liposomes containing essential oil of Eucalyptus camaldulensis leaf. Jundishapur J. Nat. Pharm. Prod. 2012;7(3):117–122. doi: 10.17795/jjnpp-5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudachirou M., Gbenou J.D., Chalchat J.C., Chabard J.L., Lartigue C. Chemical composition of essential oils of Eucalyptus from Benin: Eucalyptus citriodora and E. camaldulensis. Influence of location, harvest time, storage of plants and time of steam distillation. J. Essent. Oil Res. 1999;11:109–118. doi: 10.1080/10412905.1999.9701085. [DOI] [Google Scholar]
- Mubita C., Syakalima M., Chisenga C., Munyeme M., Bwalya M., Chifumpa G. Antibiograms of faecal Escherichia coli and Enterococci species isolated from pastoralist cattle in the interface areas of the Kafue basin in Zambia. Vet. Arhiv. 2008;78(2):179–185. [Google Scholar]
- Müller V., Chávez J.H., Reginatto F.H., Zucolotto S.M., Niero R. Evaluation of antiviral activity of South American plant extracts against herpes simplex virus type 1 and rabies virus. Phytother. Res. 2007;10:970–974. doi: 10.1002/ptr.2198. [DOI] [PubMed] [Google Scholar]
- Mulyaningsih S., Sporer F., Zimmermann S., Reichling J., Wink M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine. 2010;17:1061–1066. doi: 10.1016/j.phymed.2010.06.018. [DOI] [PubMed] [Google Scholar]
- N.A.S . National Academy of Sciences; Washington, D.C: 1980. Firewood Crops. Shrub and Tree Species for Energy Production. [Google Scholar]
- Nasir M., Tafess K., Abate D. Antimicrobial potential of the Ethiopian Thymus schimperi essential oil in comparison with others against certain fungal and bacterial species. BMC Complem. Altern. M. 2015;15:260–265. doi: 10.1186/s12906-015-0784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosratabadi S.J., Sharifi I., Sharififar F., Bamorovat M., Daneshvar H., Mirzaie M. In vitro antileishmanial activity of methanolic and aqueous extracts of Eucalyptus camaldulensis against Leishmania major. J. Parasitic Dis. 2015;39(1):18–21. doi: 10.1007/s12639-013-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olawore N.O., Ololade Z.S. Eucalyptus camaldulensis var. nancy and Eucalyptus camaldulensis var. petford seed essential oils: phytochemicals and therapeutic potentials. Chem. Sci. J. 2017;8:148. doi: 10.4172/2150-3494.1000148. [DOI] [Google Scholar]
- Ostad Asiaei E., Moghimipour E., Fakoor M.H. Evaluation of antimicrobial activity of Eucalyptus camaldulensis essential oil against the growth of drug-resistant bacteria. Jundishapur J. Nat. Pharm. Prod. 2018;13(4) doi: 10.5812/jjnpp.65050. e65050. [DOI] [Google Scholar]
- Oussalah M., Caillet S., Saucier L., Lacroix M. Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 2006;73:236–244. doi: 10.1016/j.meatsci.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Owlia P., Saderi H., Rasooli I., Sefidkon F. Antimicrobial characteristics of some herbal oils on Pseudomonas aeruginosa with special reference to their chemical compositions. Iran. J. Pharm. Res. 2009;8(2):107–114. [Google Scholar]
- Oyedeji A.O., Ekundayo O., Olawoye O.N., Adeniyi B.A., Koenig W.A. Antimicrobial activity of essential oils of five Eucalyptus species growing in Nigeria. Fitoterapia. 1999;70:526–528. doi: 10.1016/S0367-326X(99)00083-0. [DOI] [Google Scholar]
- Pankey G.A., Sabath L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin. Infect. Dis. 2004;38(6):864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- Pappas R.S., Sheppard-Hanger S. The essential oil of Eucalyptus camaldulensis Dehn. from South Florida: a high cryptone/low cineole Eucalyptus. J. Essent. Oil Res. 2000;12:383–384. doi: 10.1080/10412905.2000.9699541. [DOI] [Google Scholar]
- Pattnaik S., Vemulpad S., Kole C. Antibacterial and antifungal activity of tan essential oils in vitro. Microbios. 1996;86:237–246. [PubMed] [Google Scholar]
- Pegula F.P., Bastailer K.H.C., Kurkctailouglu M. Essential oil composition of Eucalyptus camalddunensis. Dehn. from Mozambique. J. Essen. Oil Res. 2000;12:333–335. doi: 10.1080/10412905.2000.9699530. [DOI] [Google Scholar]
- Pennacchio M., Jefferson L., Havens K. Oxford University Press Inc.; United Kingdom: 2010. Uses and Abuses of Plant-Derived Smoke: Its Ethnobotany as Hallucinogen, Perfume, Incense and Medicine. [Google Scholar]
- Potdara K., Gharpureb M., Wadher B. Antimicrobial activity of Eucalyptus camaldulensis and Calotropis gigantea against various pathogens. Int. J. Appl. Biol. Pharmaceut. Technol. 2015;6(3):104–110. [Google Scholar]
- Rasooli I., Shayegh S., Astaneh S.D.A. The effect of Mentha spicata and Eucalyptus camaldulensis essential oils on dental biofilm. Int. J. Dent. Hyg. 2009;7:196–203. doi: 10.1111/j.1601-5037.2009.00389.x. [DOI] [PubMed] [Google Scholar]
- Reda F.M., El-Zawahry Y.A., Omar A.R. Synergistic effect of combined antibiotic and methanol extract of Eucalyptus camaldulensis leaf against Staphylococcus aureus and Pseudomonas aeruginosa. Int. J. Appl. Sci. Biotechnol. 2017;5(4):486–497. doi: 10.3126/ijasbt.v5i4.18620. [DOI] [Google Scholar]
- Robbins N., Caplan T., Cowen L.E. Molecular evolution of antifungal drug resistance. Annu. Rev. Microbiol. 2017;71(1):753–1775. doi: 10.1146/annurev-micro-030117-020345. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Garcia A., Rojo-Ruiz J., Navas-Navarro P., Aulestia F.J., Gallego-Sandin S., Garcia-Sancho J., Alonso M.T. GAP, an aequorin-based fluorescent indicator for imaging Ca2+ in organelles. Proc. Natl. Acad. Sci. U. S. A. 2014;111(7):2584–2589. doi: 10.1073/pnas.1316539111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe C.C., Bowers J., Oltean H., DeBess E., Dufresne P.J., McBurney S., Overy D.P., Wanke B., Lysen C., Chiller T., Meyer W., Thompson G.R., Lockhart S.R., Hepp C.M., Engelthaler D.M. Dating the Cryptococcus gattii dispersal to the North American Pacific Northwest. mSphere. 2018;3 doi: 10.1128/mSphere.00499-17. e00499-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaei-Ghomi J., Ahd A.A. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn. Mag. 2010;6(23):172–175. doi: 10.4103/0973-1296.66930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem M.Z., Ashmawy N.A., Elansary H.O., El-Settawy A.A. Chemotyping of diverse Eucalyptus species grown in Egypt and antioxidant and antibacterial activities of its respective essential oils. Nat. Prod. Res. 2015;29(7):681–685. doi: 10.1080/14786419.2014.981539. [DOI] [PubMed] [Google Scholar]
- Sanchez Palomino S., Abad M.J., Bedoya L.M., García J., Gonzales E. Screening of South American plants against Human Immunodeficiency Virus: preliminary fractionation of aqueous extract from Baccharis trinervis. Biol. Pharm. Bull. 2002;25:1147–1150. doi: 10.1248/bpb.25.1147. [DOI] [PubMed] [Google Scholar]
- Sanchez-Aldana D., Aguilar C.N., Nevarez-Moorillon G.V., Contreras Esquivel J.C. Comparative extraction of pectic and polyphenols from Mexican lime pomace and bagasse. Am. J. Agric. Biol. Sci. 2013;8:309–322. doi: 10.3844/ajabssp.2013.309.322. [DOI] [Google Scholar]
- Sebei K., Sakouhi F., Herchi W., Khouja M.L., Boukhchina S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015;48:7. doi: 10.1186/0717-6287-48-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumar P., Edhayanaveena B., Dprakash S. Studies on the antidandruff activity of the essential oil of Coleus amboinicus and Eucalyptus globulus. Asian Pac. J. Trop. Biomed. 2012;2:715–719. doi: 10.1016/S2222-1808(12)60250-3. [DOI] [Google Scholar]
- Shieh J.C. Yields and chemical components of essential oils in Eucalyptus camaldulensis leaves. Taiwan J. For. Sci. 1996;11:149–157. [Google Scholar]
- Siddiqui Y.M., Ettayebi M., Haddad A.M., Al-Ahdal M.N. Effect of essential oils on the enveloped viruses: antiviral activity of oregano and clove oils on herpes simplex virus type 1 and Newcastle disease virus. Med. Sci. Res. 1996;24:185–186. [Google Scholar]
- Singab A.N., Ayoub N., Al-Sayed E., Martiskainen O., Sinkkonen J., Pihlaja K. Phenolic constituents of Eucalyptus camaldulensis Dehnh, with potential antioxidant and cytotoxic activities. Rec. Nat. Prod. 2011;5(4):271–280. [Google Scholar]
- Singh R., Thakur R. Antibacterial activity of Eucalyptus camaldulensis against bacteria causing food-borne illness. Ind. Med. Gaz. 2016:195–199. [Google Scholar]
- Siramon P., Ohtani Y. Antioxidative and antiradical activities of Eucalyptus camaldulensis leaf oils from Thailand. J. Wood Sci. 2007;53:498–504. doi: 10.1007/s10086-007-0887-7. [DOI] [Google Scholar]
- Siramon P., Ohtani Y., Ichiura H. Chemical composition and antifungal property of Eucalyptus camaldulensis leaf oils from Thailand. Rec. Nat. Prod. 2013;7(1):49–53. [Google Scholar]
- Slee A., Brooker M.I.H., Duffy S.M., West J.G. Centre for Plant Biodiversity Research; 2006. River Red Gum. Eucalyptus camaldulensis var. obtusa. Retrieved 2012-06-16. [Google Scholar]
- Smith-Palmer A., Stewart J., Fyfe L. Influence of subinhibitory concentrations of plant essential oils on the production of enterotoxins A and B and α-toxin by Staphylococcus aureus. J. Med. Microbiol. 2004;53(10):1023–1027. doi: 10.1099/jmm.0.45567-0. [DOI] [PubMed] [Google Scholar]
- Snoeck R. Antiviral therapy of herpes simplex. Int. J. Antimicrob. Agents. 2000;16:157–159. doi: 10.1016/S0924-8579(00)00233-8. [DOI] [PubMed] [Google Scholar]
- Sorrell T.C., Brownlee A.G., Ruma P., Malik R., Pfeiffer T.J., Ellis D.H. Natural environmental sources of Cryptococcus neoformans var. gattii. J. Clin. Microbiol. 1996;34(5):1261–1263. doi: 10.1128/jcm.34.5.1261-1263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliman S., Van Vuuren S.F., Viljoen A.M. Validating the in vitro antimicrobial activity of Artemisia afra in polyherbal combinations to treat respiratory infections. S. Afr. J. Bot. 2010;76:655–661. doi: 10.1016/j.sajb.2010.07.003. [DOI] [Google Scholar]
- Tadtong S., Puengseangdee C., Prasertthanawut S., Hongratanaworakit T. Antimicrobial constituents and effects of blnded Eucalyptus, rosemary, patchouli, pine, and cajuput essential oils. Nat. Prod. Commun. 2016;11(2):267–270. [PubMed] [Google Scholar]
- Takahashi T., Kokubo R., Sakaino M. Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Lett. Appl. Microbiol. 2004;39:60–64. doi: 10.1111/j.1472-765X.2004.01538.x. [DOI] [PubMed] [Google Scholar]
- Taoubi K., Fauvel M.T., Gleye J., Moulis C. Phenylpropanoid glycosides from Lantana camara and Lippia multiflora. Planta Med. 1997;63:192–193. doi: 10.1055/s-2006-957647. [DOI] [PubMed] [Google Scholar]
- Thao N.T., Wanapat M., Kang S., Cherdthong A. Effects of supplementation of Eucalyptus (E. camaldulensis) leaf meal on feed intake and rumen fermentation efficiency in swamp buffaloes. Asian Australas. J. Anim. Sci. 2015;28(7):951–957. doi: 10.5713/ajas.14.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Ban X., Zeng H., He J., Chen Y., Wang Y. The Mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS One. 2012;7(1):e30147. doi: 10.1371/journal.pone.0030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand R., Young R. Elsevier Health Sciences; USA: 2014. Essential Oil Safety: A Guide for Health Care Professionals. [Google Scholar]
- Tsiri D., Kretsi O., Chinou I.B., Spyropoulos C.G. Composition of fruit volatiles and annual changes in the volatiles of leaves of Eucalyptus camaldulensis Dehn. growing in Greece. Flavour Frag. J. 2003;18(3):244–247. doi: 10.1002/ffj.1220. [DOI] [Google Scholar]
- Turgis M., Han J., Caillet S., Lacroix M. Antimicrobial activity of mustard essential oil against Escherichia coli O157:H7 and Salmonella typhi. Food Control. 2009;20:1073–1079. doi: 10.1016/j.foodcont.2009.02.001. [DOI] [Google Scholar]
- Ultee A., Bennink M.H.J., Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002;68:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola C.L. The antibiotic resistance crisis: part 1: causes and threats. P T. 2018;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- Verma P. Methods for determining bactericidal activity and antimicrobial interactions: synergy testing, time-kill curves, and population analysis. In: Schwalbe R., Steele-Moore L., Goodwin A.C., editors. Antimicrobial Susceptibility Testing Protocols. CRC Press; NewYork: 2007. pp. 275–299. [Google Scholar]
- Warnke P.H., Becker S.T., Podschun R., Sivananthan S., Springer I.N., Russo P.A., Wiltfang J., Fickenscher H., Sherry E. The battle against multi-resistant strains: renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. J. Craniomaxillofac. Surg. 2009;37(7):392–397. doi: 10.1016/j.jcms.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Watt J.M., Breyer-Brandwijk M.G. 2nd edition. E. and S. Livingstone Ltd.; Edinburgh: 1962. The Medicinal and Poisonous Plants of Southern and Eastern Africa. [Google Scholar]
- Williams C. Rosenberg Publishing Pty Ltd.; Australia: 2011. Medicinal Plants in Australia Volume 2: Gums, Resins, Tannin and Essential Oils. 2; pp. 77–79. [Google Scholar]
- Yamada K., Ogawa H., Hara A., Yoshida Y., Yonezawa Y., Karibe K., Nghia V.B., Yoshimura H., Yamamoto Y., Yamada M., Nakamura K., Imai K. Mechanism of the antiviral effect of hydroxytyrosol on influenza virus appears to involve morphological change of the virus. Antiviral Res. 2009;83:35–44. doi: 10.1016/j.antiviral.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Yan M.M., Liu W., Fu Y.J., Zu Y.G., Chen C.Y. Optimisation of the microwave-assisted extraction process for four main astragalosides in Radix astragali. Food Chem. 2010;119:1663–1670. doi: 10.1016/j.foodchem.2009.09.021. [DOI] [Google Scholar]
- Yemis O., Mazza G. Optimization of furfural and 5-hydroxymethylfurfural production from wheat straw by a microwave-assisted process. Bioresour. Technol. 2012;109:215–223. doi: 10.1016/j.biortech.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Youse H.A., Kazemian A., Sereshti M., Rahmanikhoh E., Ahmadinia E., Rafaian M., Maghsoodi R., Darani H.Y. Effect of Echinophora platyloba, Stachys lavandulifolia, and Eucalyptus camaldulensis plants on Trichomonas vaginalis growth in vitro. Adv. Biomed. Res. 2012;1:79. doi: 10.4103/2277-9175.102987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushau M., Onuorah F.C., Murtala Y. In-vitro sensitivity pattern of some urinary tract isolates to Carica papaya extracts. Bayero J. Pure Appl. Sci. 2009;2(2):75–78. doi: 10.4314/bajopas.v2i2.63786. [DOI] [Google Scholar]
- Zrira S.S., Benjilali B.B. The essential oil of the leaves and the fruits of E. camaldulensis. J. Essent. Oil Res. 1991;3:443–444. doi: 10.1080/10412905.1991.9697981. [DOI] [Google Scholar]
- Zrira S.S., Benjilali B.B., Fechtal M.M., Richard H.H. Essential oils of twenty- seven Eucalyptus species grown in Morocco. J. Essent. Oil Res. 1992;4:259–264. doi: 10.1080/10412905.1992.9698059. [DOI] [Google Scholar]