Abstract
Type I interferons (IFN-α/β) were originally discovered by their strong and direct antiviral activity [A. Isaacs, J. Lindenmann, Virus interference. I. The interferon, Proc. R. Soc. Lond. B Biol. Sci. 147 (1957) 258–267]. (see review by J. Lindenmann on p. 719, in this issue). Nevertheless, only very recently it was entirely realized that viruses would not succeed without efficient tools to undermine this potent host defense system. Current investigations are revealing an astonishing variety of viral IFN antagonistic strategies targeting virtually all parts of the IFN system, often in a highly specific manner. Viruses were found to interfere with induction of IFN synthesis, IFN-induced signaling events, the antiviral effector proteins, or simply shut off the host cell macromolecule synthesis machinery to avoid booting of the antiviral host defense. Here, we will describe a few well-characterized examples to illustrate the sophisticated and often multi-layered anti-IFN mechanisms employed by viruses.
Keywords: Virus, Interferon, Interferon escape mechanism, Interferon antagonism
1. Interference with interferon induction
In most nucleated body cells, viral infections activate transcription of the “classic” IFN-β gene [1] by a signaling chain which is initiated by the RNA sensors RIG-I and MDA-5, which in turn act trough the adaptor IPS-1 and the kinases TBK-1 and IKK-ɛ to activate the transcription factor IRF-3 (see reviews by P. Pitha and by T. Fujita on pages 744 and 754 this issue respectively). A parallel pathway involves the dsRNA-binding kinase PKR, the TRAF adaptor molecules and the NF-κB kinase IKKα/β (see review by García et al., on p. 799 this issue). Most viruses investigated so far interfere with one or several steps in these important signaling chains [2], [3], [4], [5], [6]. Fig. 1 provides a schematic overview over the IFN induction pathway and some selected viral counterparts.
Fig. 1.
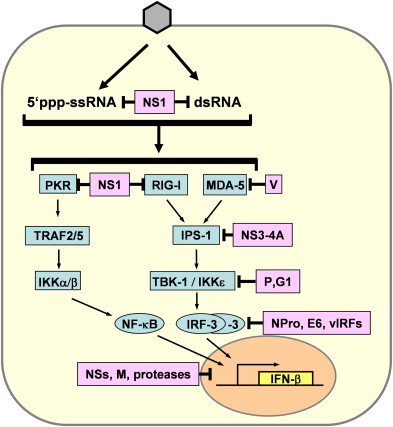
Viral inhibition of IFN induction. Intracellular recognition of 5′-triphosphorylated ssRNA and dsRNA by the intracellular receptors PKR, RIG-I and MDA-5 leads to activation of the transcription factors NF-κB and IRF-3 via several intermediate signaling factors. IRF-3 is phosphorylated by the kinases TBK-1 and IKKɛ which in turn are activated by RIG-I and MDA5 via IPS-1. NF-κB is mainly activated by the PKR pathway. Examples of viral IFN antagonists interfering with different steps in the IFN induction pathways are the NS1 of influenza viruses, the V protein of paramyxoviruses, the NS3-4A protein of hepatitis C virus, the P protein of Rabies virus, the G1 protein of hantavirus NY-1, the NPro protein of classical swine fever virus and bovine viral diarrhea virus, the E6 protein of human papilloma virus 16, the viral IRF homologs (vIRFs) of human herpes virus 8, the NSs proteins of bunyaviruses, the M protein of vesicular stomatitis virus, and the proteases of Picornaviruses.
Until very recently, it was thought that the only IFN-inducing molecule which clearly distinguishes viruses from their host (i.e. self vs. non-self) is double-stranded RNA (dsRNA). Many RNA and DNA viruses therefore express proteins which bind this key molecule to avoid both IFN induction and activation of dsRNA-dependent antiviral enzymes [7], [8]. Well-investigated examples are the NS1 protein of influenza A virus [9], [10], [11], [12], the E3L protein of poxviruses [13], [14], the VP35 protein of Ebola virus [15], [16], the sigma3 protein of reoviruses [17], and the US11 protein of herpes simplex virus [18], [19]. The murine cytomegalovirus encodes two proteins, m142 and m143 which together block dsRNA-mediated signaling pathways [20], [21]. However, in the case of the influenza virus NS1 and the Ebola virus VP35 dsRNA-binding appears only to contribute to the IFN antagonism without being essential [15], [22], [23]. In addition, we have recently shown that some viruses do not produce detectable amounts of dsRNA at all [24], indicating that in these cases other molecules IFN-eliciting molecules are important. Indeed, viral ssRNAs bearing a 5′triphosphate group are a potent trigger of IFN induction, acting through RIG-I [25], [26]. In line with this, it was shown that the NS1 of influenza A virus can bind ssRNA as well, and is able to form complexes with RIG-I [26], [27]. Similarly, a dsRNA-binding defective VP35 mutant can still block IFN induction [15], suggesting a similar mode of action. Thus, RNA binding by these viral IFN antagonists appears to be contributing to their IFN antagonism without being sufficient. An IFN induction antagonist devoid of any RNA-binding activity is the V protein of the paramyxovirus SV5. This small protein inhibits IFN induction by sequestering the RIG-I-related RNA sensor MDA-5 [28], [29], raising the question how SV5 deals with the parallel RIG-I pathway. This paramyxovirus-specific problem can be avoided by blocking components of the signaling pathway which are situated further downstream and therefore needed by both RIG-I and MDA-5. The next in line, the adaptor protein IPS-1, connects the RNA sensors with the IRF-3 kinases TBK-1/IKK-ɛ and is specifically cleaved by the NS3-4A protease of hepatitis C virus [30], [31]. The activation of IRF-3 by TBK-1 is prevented by the phosphoprotein P of Rabies virus [32] and the G1 glycoprotein of the hantavirus NY-1 [33]. IRF-3 itself is degraded by the NPro proteins of classical swine fever virus and of bovine viral diarrhea virus [34], [35], [36], [37]. Also, the E6 protein of human papilloma virus 16 binds and inactivates IRF-3 [38], and human herpes virus 8 (HHV-8) expresses several IRF homologues, termed vIRFs, which exert a dominant-negative effect [39], [40], [41], [42], [43], [44], [45].
Target-specific IFN-escape strategies are often pursued by viruses causing persistent infections, e.g. herpes viruses. By contrast, many viruses which lytically infect the host cell simply impose a general block on host cell transcription and translation. For example, the non-structural NSs proteins of the Rift Valley Fever virus and Bunyamwera virus interfere with the basic cellular transcription machinery [46], [47], [48]. Although this strategy appears to be unspecific, in vivo experiments clearly demonstrated that the biological purpose of this broad-band shut-off is to inhibit IFN synthesis [49], [50]. The matrix (M) protein of vesicular stomatitis virus (VSV) is also a potent host cell shutoff factor which inhibits basal transcription [51], impairs nuclear-cytoplasmic transport of RNAs and proteins [52], and inactivates translation factors [53]. As is the case with bunyavirus NSs, the biological significance of VSV M-mediated shutoff is to suppress IFN induction [54], [55]. Also, proteinases of Picornaviruses (e.g. Foot and Mouth disease virus, Theiler's virus, Polio virus) and Pestiviruses (e.g. Classical Swine fever virus) cause a shutoff-of the host cell metabolism to interfere with the IFN response [37], [56], [57], [58], [59], [60].
Interestingly, the non-structural protein NS1 of influenza A virus also impairs the post-transcriptional processing and nuclear export of cellular pre-mRNAs [61], [62], [63] in order to counteract the antiviral host response [64], [65]. Thus, NS1 is a versatile protein with the ability to prevent IFN induction both by IFN pathway-specific and by less specific means, and recent studies suggest that there is a surprisingly great strain-specific variation in these activities [66].
2. Interference with interferon-activated signaling
IFN-β and the multiple IFN-α subspecies activate a common type I IFN receptor (IFNAR) which signals to the nucleus through the so-called JAK-STAT pathway ( Fig. 2). The STAT proteins are latent cytoplasmic transcription factors which become phosphorylated by the Janus kinases JAK-1 and TYK-2 [67]. Phosphorylated STAT-1 and STAT-2 recruit a third factor, IRF-9, to form a complex known as IFN-stimulated gene factor 3 (ISGF-3) which translocates to the nucleus and binds to the IFN-stimulated response element (ISRE) in the promoter region of interferon-stimulated genes (ISGs).
Fig. 2.
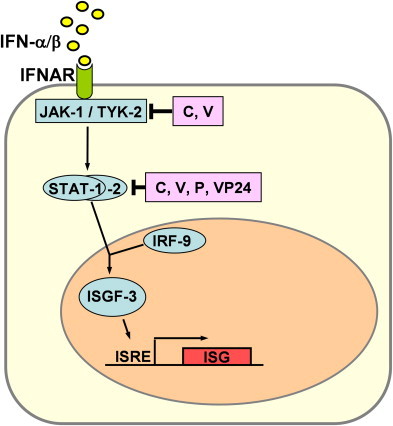
Viral inhibition of IFN signaling. IFN-α and IFN-β binds to the type I IFN receptor (IFNAR) and activate the expression of numerous IFN-stimulated genes (ISGs) via the JAK/STAT pathway. Most viral signaling antagonists described so far interfere on the level of either the JAK/TYK kinases or the STATs. Prominent examples are the C and V proteins of paramyxoviruses, the P protein of Rabies virus, and the VP24 protein of Ebola virus.
The IFN signal transduction pathway represents another important target of viruses (Fig. 2). Members of the paramyxovirus family, which contains mainly important pathogens, encode two different (but genetically related) proteins named C and V which interfere with STAT function. Depending on the virus species, these IFN antagonists act either by binding the STAT proteins, by inducing their degradation, or by inhibiting the JAK kinases [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82]. The P protein of Rabies virus binds to activated STAT1 and STAT2 and retains them in the cytoplasm [83]. Thus, the paramyxoviral V protein as well as the rabies virus P protein have a dual anti-IFN function as they block both IFN induction (see above) and STAT signaling. Ebola virus, by contrast uses a different protein, VP24, to block nuclear import of STAT by interacting with the transporter protein karyopherin alpha1 [84]. STAT signaling is also disturbed by viruses causing persistent infections, such as Hepatitis C virus [85], [86], herpes simplex virus [87], [88], HHV-8 [41], or cytomegalovirus [89], [90]. Poxviruses inhibit IFN-stimulated gene expression by a different strategy. They express soluble IFN-binding proteins to neutralize secreted IFN molecules [91], [92], [93], [94].
3. Inhibition of with interferon effector proteins
The dsRNA-binding proteins mentioned above also serve a second purpose, namely the inhibition of the dsRNA-activated antiviral enzymes. This has been demonstrated for the influenza virus NS1 [11], [12], [95], [96], [97], [98], [99], the poxvirus E3L [97], the reovirus sigma3 [100], the herpesvirus US11 [19], [101], and the dsRNA-binding proteins of human and murine cytomegaloviruses [20], [21], [102]. Importantly, also for this anti-IFN effector function more that just dsRNA binding appears to be necessary, since in many cases a direct interaction with e.g. PKR has been demonstrated (reviewed in Ref. [7]). Sequestering dsRNA may also inhibit the 2–5OAS pathway and ADAR, although this has only been shown in a few cases [12], [14]. dsRNA-independent inhibition of the RNaseL system is achieved by the ICP0 protein of herpes simplex virus [103] and by upregulation of RLI, a cellular inhibitor of RNaseL, in HIV- and Picornavirus-infected cells [104], [105].
PKR is also attacked by other means. The γ34.5 protein of Herpes simplex virus triggers the dephosphorylation of eIF-2α, thus reverting the translational block established by PKR [106]. The E2 protein of Hepatitis C virus [107], the Tat protein of HIV [108] and the K3L protein of Vaccinia virus [109] act as pseudosubstrates for PKR. Another strategy is to encode small, highly structured RNAs which compete with dsRNA and inactivate PKR. This was demonstrated for adenoviruses [110], Hepatitis C virus [111], Epstein-Barr virus [112], and HIV [113]. However, for Epstein-Barr virus it was shown that the PKR inhibition by the so-called EBER RNAs observed in vitro does not occur in vivo [114], suggesting that EBERs are important for other activities such as inhibition of apoptosis.
It is obvious from the listings above that viruses have evolved multiple means to disrupt the IFN response. In some cases, there are specialized anti-IFN factors such the non-structural proteins of influenza viruses. In many other cases, however, viral gene products with a defined function in virus replication cycle can additionally acquire the ability to block the IFN system. Important examples include the V, W and C proteins of paramyxoviruses [79], [115], the P protein of rabies virus [32], [83] and the VP35 protein of Ebola virus [116], which are regulators of the viral polymerase. Also, the matrix proteins of Thogoto virus [117] and vesicular stomatitis virus [58], the nucleoprotein of arenaviruses [118], and the glycoprotein of hantaviruses [33] not only have structural functions, but are IFN antagonists as well. Some viruses such as Dengue virus or SARS-coronavirus encode a multitude of anti-IFN factors which together may strongly contribute to an enhanced virulence [119], [120], [121]. Apparently, modulating the IFN system can be achieved either by “inventing” one or several specialized factors or by expanding the function of existant gene products.
4. Outlook
Understanding the interplay between viruses and the IFN response can help to design new strategies for prevention and therapy. Viruses unable to counteract the IFN response are excellent candidates for live virus vaccines. They can be grown to high titers in IFN-deficient cell cultures but are attenuated in vivo since they elicit a robust innate and adaptive immune responses. This concept has been proven for influenza viruses [122], [123], [124], [125], human parainfluenza virus type 1 [126], human and bovine respiratory syncytial viruses [127], [128], [129], and may likewise apply to other viruses.
Oncolytic viruses designed for the targeted destruction of tumors is another promising application. Tumor cells often eliminate one or several parts of the IFN system during the transformation process [130], [131], [132], [133]. For example, tumor cells were shown to acquire specific mutations leading to resistance of cellular translation to inhibition by PKR [134]. The payoff is an increased susceptibility to infection [131], [134], [135], and the tumor selectivity of viruses can be further increased by using mutants with defective IFN antagonists. The inability of these mutant viruses to fight the IFN response is complemented by the IFN-deficiency of the tumor cells. At the same time, these viruses are unable to infect the IFN-competent body cells. This concept is proven by an IFN-inducing VSV mutant [55] and a herpes simplex virus lacking the anti-PKR gene γ34.5 [136], [137] which specifically destroyed tumors in immunocompetent hosts.
Thus, unravelling the strategies by which viruses counteract the IFN system not only helps to better understand viral pathogenesis but can also result in novel vaccination strategies and therapies.
Acknowledgments
Our own work described in the text was supported by grants from the Deutsche Forschungsgemeinschaft.
References
- 1.Isaacs A., Lindenmann J. Virus interference. I., The interferon, Proc. R. Soc. Lond. B Biol. Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 2.Basler C.F., Garcia-Sastre A. Viruses and the type I interferon antiviral system: induction and evasion. Int. Rev. Immunol. 2002;21:305–337. doi: 10.1080/08830180213277. [DOI] [PubMed] [Google Scholar]
- 3.Conzelmann K.K. Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. J. Virol. 2005;79:5241–5248. doi: 10.1128/JVI.79.9.5241-5248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Sastre A., Biron C.A. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 5.Haller O., Kochs G., Weber F. The interferon response circuit: Induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber F., Kochs G., Haller O. Inverse interference: how viruses fight the interferon system. Viral Immunol. 2004;17:498–515. doi: 10.1089/vim.2004.17.498. [DOI] [PubMed] [Google Scholar]
- 7.Langland J.O., Cameron J.M., Heck M.C., Jancovich J.K., Jacobs B.L. Inhibition of PKR by RNA and DNA viruses. Virus Res. 2006;119:100–110. doi: 10.1016/j.virusres.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs B.L., Langland J.O., Brandt T. Characterization of viral double-stranded RNA-binding proteins. Methods. 1998;15:225–232. doi: 10.1006/meth.1998.0626. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Sastre A., Egorov A., Matassov D., Brandt S., Levy D.E., Durbin J.E., Palese P., Muster T. Influenza A virus lacking the NS1 gene replicates in interferon- deficient systems. Virology. 1998;252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Sastre A. Inhibition of interferon-mediated antiviral responses by Influenza A viruses and other negative-strand RNA viruses. Virology. 2001;279:375–384. doi: 10.1006/viro.2000.0756. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y., Wambach M., Katze M.G., Krug R.M. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology. 1995;214:222–228. doi: 10.1006/viro.1995.9937. [DOI] [PubMed] [Google Scholar]
- 12.Min J.Y., Krug R.M. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: Inhibiting the 2′-5′ oligo (A) synthetase/RNase L pathway. Proc. Natl. Acad. Sci. USA. 2006;103:7100–7105. doi: 10.1073/pnas.0602184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornemann S., Harlin O., Staib C., Kisling S., Erfle V., Kaspers B., Hacker G., Sutter G. Replication of modified vaccinia virus Ankara in primary chicken embryo fibroblasts requires expression of the interferon resistance gene E3L. J. Virol. 2003;77:8394–8407. doi: 10.1128/JVI.77.15.8394-8407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiang Y., Condit R.C., Vijaysri S., Jacobs B., Williams B.R., Silverman R.H. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J. Virol. 2002;76:5251–5259. doi: 10.1128/JVI.76.10.5251-5259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardenas W.B., Loo Y.M., Gale M., Jr., Hartman A.L., Kimberlin C.R., Martinez-Sobrido L., Saphire E.O., Basler C.F. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J. Virol. 2006;80:5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartman A.L., Dover J.E., Towner J.S., Nichol S.T. Reverse genetic generation of recombinant Zaire Ebola viruses containing disrupted IRF-3 inhibitory domains results in attenuated virus growth in vitro and higher levels of IRF-3 activation without inhibiting viral transcription or replication. J. Virol. 2006;80:6430–6440. doi: 10.1128/JVI.00044-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs B.L., Langland J.O. Reovirus sigma 3 protein: dsRNA binding and inhibition of RNA-activated protein kinase. Curr. Top. Microbiol. Immunol. 1998;233:185–196. doi: 10.1007/978-3-642-72092-5_9. [DOI] [PubMed] [Google Scholar]
- 18.Mohr I. Neutralizing innate host defenses to control viral translation in HSV-1 infected cells. Int. Rev. Immunol. 2004;23:199–220. doi: 10.1080/08830180490265600. [DOI] [PubMed] [Google Scholar]
- 19.Poppers J., Mulvey M., Khoo D., Mohr I. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J. Virol. 2000;74:11215–11221. doi: 10.1128/jvi.74.23.11215-11221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Child S.J., Hanson L.K., Brown C.E., Janzen D.M., Geballe A.P. Double-stranded RNA binding by a heterodimeric complex of murine cytomegalovirus m142 and m143 proteins. J. Virol. 2006;80:10173–10180. doi: 10.1128/JVI.00905-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valchanova R.S., Picard-Maureau M., Budt M., Brune W. Murine cytomegalovirus m142 and m143 are both required to block protein kinase R-mediated shutdown of protein synthesis. J. Virol. 2006;80:10181–10190. doi: 10.1128/JVI.00908-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donelan N.R., Dauber B., Wang X., Basler C.F., Wolff T., Garcia-Sastre A. The N- and C-terminal domains of the NS1 protein of influenza B virus can independently inhibit IRF-3 and beta interferon promoter activation. J. Virol. 2004;78:11574–11582. doi: 10.1128/JVI.78.21.11574-11582.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donelan N.R., Basler C.F., Garcia-Sastre A. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 2003;77:13257–13266. doi: 10.1128/JVI.77.24.13257-13266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber F., Wagner V., Rasmussen S.B., Hartmann R., Paludan S.R. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung V., Ellegast J., Kim S., Brzozka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K.K., Schlee M. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 26.Pichlmair A., Schulz O., Tan C.P., Naslund T.I., Liljestrom P., Weber F., Reis E.S.C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′ phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 27.Mibayashi M., Martinez-Sobrido L., Loo Y.M., Cardenas W.B., Gale M., Jr., Garcia-Sastre A. Inhibition of retinoic acid-inducible gene-I-mediated induction of interferon-{beta} by the NS1 protein of influenza A virus. J. Virol. 2007;81:514–524. doi: 10.1128/JVI.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrejeva J., Childs K.S., Young D.F., Carlos T.S., Stock N., Goodbourn S., Randall R.E. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. USA. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Childs K., Stock N., Ross C., Andrejeva J., Hilton L., Skinner M., Randall R., Goodbourn S. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology. 2007;359:190–200. doi: 10.1016/j.virol.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Lin R., Lacoste J., Nakhaei P., Sun Q., Yang L., Paz S., Wilkinson P., Julkunen I., Vitour D., Meurs E., Hiscott J. Dissociation of a MAVS/IPS-1/VISA/Cardif-IKKepsilon molecular complex from the mitochondrial outer membrane by hepatitis C virus NS3-4A proteolytic cleavage. J. Virol. 2006;80:6072–6083. doi: 10.1128/JVI.02495-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meylan E., Curran J., Hofmann K., Moradpour D., Binder M., Bartenschlager R., Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 32.Brzozka K., Finke S., Conzelmann K.K. Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J. Virol. 2005;79:7673–7681. doi: 10.1128/JVI.79.12.7673-7681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alff P.J., Gavrilovskaya I.N., Gorbunova E., Endriss K., Chong Y., Geimonen E., Sen N., Reich N.C., Mackow E.R. The pathogenic NY-1 hantavirus G1 cytoplasmic tail inhibits RIG-I- and TBK-1-directed interferon responses. J. Virol. 2006;80:9676–9686. doi: 10.1128/JVI.00508-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauhofer O., Summerfield A., McCullough K.C., Ruggli N. Role of double-stranded RNA and Npro of classical swine fever virus in the activation of monocyte-derived dendritic cells. Virology. 2005;343:93–105. doi: 10.1016/j.virol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Hilton L., Moganeradj K., Zhang G., Chen Y.H., Randall R.E., McCauley J.W., Goodbourn S. The NPro product of bovine viral diarrhea virus inhibits DNA binding by interferon regulatory factor 3 and targets it for proteasomal degradation. J. Virol. 2006;80:11723–11732. doi: 10.1128/JVI.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.La Rocca S.A., Herbert R.J., Crooke H., Drew T.W., Wileman T.E., Powell P.P. Loss of interferon regulatory factor 3 in cells infected with classical swine fever virus involves the N-terminal protease, Npro. J. Virol. 2005;79:7239–7247. doi: 10.1128/JVI.79.11.7239-7247.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruggli N., Bird B.H., Liu L., Bauhofer O., Tratschin J.D., Hofmann M.A. N(pro) of classical swine fever virus is an antagonist of double-stranded RNA-mediated apoptosis and IFN-alpha/beta induction. Virology. 2005;340:265–276. doi: 10.1016/j.virol.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 38.Ronco L.V., Karpova A.Y., Vidal M., Howley P.M. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burysek L., Yeow W.S., Lubyova B., Kellum M., Schafer S.L., Huang Y.Q., Pitha P.M. Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300. J. Virol. 1999;73:7334–7342. doi: 10.1128/jvi.73.9.7334-7342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burysek L., Yeow W.S., Pitha P.M. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2) J. Hum. Virol. 1999;2:19–32. [PubMed] [Google Scholar]
- 41.Fuld S., Cunningham C., Klucher K., Davison A.J., Blackbourn D.J. Inhibition of interferon signaling by the Kaposi's sarcoma-associated herpesvirus full-length viral interferon regulatory factor 2 protein. J. Virol. 2006;80:3092–3097. doi: 10.1128/JVI.80.6.3092-3097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M., Lee H., Guo J., Neipel F., Fleckenstein B., Ozato K., Jung J.U. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor. J. Virol. 1998;72:5433–5440. doi: 10.1128/jvi.72.7.5433-5440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lubyova B., Pitha P.M. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 2000;74:8194–8201. doi: 10.1128/jvi.74.17.8194-8201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lubyova B., Kellum M.J., Frisancho A.J., Pitha P.M. Kaposi's sarcoma-associated herpesvirus-encoded vIRF-3 stimulates the transcriptional activity of cellular IRF-3 and IRF-7. J. Biol. Chem. 2004;279:7643–7654. doi: 10.1074/jbc.M309485200. [DOI] [PubMed] [Google Scholar]
- 45.Zimring J.C., Goodbourn S., Offermann M.K. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1-mediated transcription. J. Virol. 1998;72:701–707. doi: 10.1128/jvi.72.1.701-707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Billecocq A., Spiegel M., Vialat P., Kohl A., Weber F., Bouloy M., Haller O. NSs protein of Rift Valley Fever Virus blocks interferon production by inhibiting host gene transcription. J. Virol. 2004;78:9798–9806. doi: 10.1128/JVI.78.18.9798-9806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le May N., Dubaele S., De Santis L.P., Billecocq A., Bouloy M., Egly J.M. TFIIH transcription factor, a target for the Rift Valley hemorrhagic fever virus. Cell. 2004;116:541–550. doi: 10.1016/s0092-8674(04)00132-1. [DOI] [PubMed] [Google Scholar]
- 48.Thomas D., Blakqori G., Wagner V., Banholzer M., Kessler N., Elliott R.M., Haller O., Weber F. Inhibition of RNA polymerase II phosphorylation by a viral interferon antagonist. J. Biol. Chem. 2004;279:31471–31477. doi: 10.1074/jbc.M400938200. [DOI] [PubMed] [Google Scholar]
- 49.Bouloy M., Janzen C., Vialat P., Khun H., Pavlovic J., Huerre M., Haller O. Genetic evidence for an interferon-antagonistic function of rift valley fever virus nonstructural protein NSs. J. Virol. 2001;75:1371–1377. doi: 10.1128/JVI.75.3.1371-1377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber F., Bridgen A., Fazakerley J.K., Streitenfeld H., Randall R.E., Elliott R.M. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 2002;76:7949–7955. doi: 10.1128/JVI.76.16.7949-7955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan H., Yoza B.K., Lyles D.S. Inhibition of host RNA polymerase II-dependent transcription by vesicular stomatitis virus results from inactivation of TFIID. Virology. 1998;251:383–392. doi: 10.1006/viro.1998.9413. [DOI] [PubMed] [Google Scholar]
- 52.Her L.S., Lund E., Dahlberg J.E. Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science. 1997;276:1845–1848. doi: 10.1126/science.276.5320.1845. [DOI] [PubMed] [Google Scholar]
- 53.Connor J.H., Lyles D.S. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J. Virol. 2002;76:10177–10187. doi: 10.1128/JVI.76.20.10177-10187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferran M.C., Lucas-Lenard J.M. The vesicular stomatitis virus matrix protein inhibits transcription from the human beta interferon promoter. J. Virol. 1997;71:371–377. doi: 10.1128/jvi.71.1.371-377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stojdl D.F., Lichty B.D., tenOever B.R., Paterson J.M., Power A.T., Knowles S., Marius R., Reynard J., Poliquin L., Atkins H. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 56.Delhaye S., van Pesch V., Michiels T. The leader protein of Theiler's virus interferes with nucleocytoplasmic trafficking of cellular proteins. J. Virol. 2004;78:4357–4362. doi: 10.1128/JVI.78.8.4357-4362.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Los Santos T., de Avila Botton S., Weiblen R., Grubman M.J. The leader proteinase of foot-and-mouth disease virus inhibits the induction of beta interferon mRNA and blocks the host innate immune response. J. Virol. 2006;80:1906–1914. doi: 10.1128/JVI.80.4.1906-1914.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyles D.S. Cytopathogenesis and inhibition of host gene expression by RNA viruses. Microbiol. Mol. Biol. Rev. 2000;64:709–724. doi: 10.1128/mmbr.64.4.709-724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruggli N., Tratschin J.D., Schweizer M., McCullough K.C., Hofmann M.A., Summerfield A. Classical swine fever virus interferes with cellular antiviral defense: evidence for a novel function of N(pro) J. Virol. 2003;77:7645–7654. doi: 10.1128/JVI.77.13.7645-7654.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Pesch V., van Eyll O., Michiels T. The leader protein of Theiler's virus inhibits immediate-early alpha/beta interferon production. J. Virol. 2001;75:7811–7817. doi: 10.1128/JVI.75.17.7811-7817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Z., Li Y., Krug R.M. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. Embo J. 1999;18:2273–2283. doi: 10.1093/emboj/18.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fortes P., Beloso A., Ortin J. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks mRNA nucleocytoplasmic transport. Embo J. 1994;13:704–712. doi: 10.1002/j.1460-2075.1994.tb06310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y., Chen Z.Y., Wang W., Baker C.C., Krug R.M. The 3′-end-processing factor CPSF is required for the splicing of single-intron pre-mRNAs in vivo. Rna. 2001;7:920–931. doi: 10.1017/s1355838201010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim M.J., Latham A.G., Krug R.M. Human influenza viruses activate an interferon-independent transcription of cellular antiviral genes: outcome with influenza A virus is unique. Proc. Natl. Acad. Sci. USA. 2002;99:10096–10101. doi: 10.1073/pnas.152327499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noah D.L., Twu K.Y., Krug R.M. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3′ end processing of cellular pre-mRNAS. Virology. 2003;307:386–395. doi: 10.1016/s0042-6822(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 66.Hayman A., Comely S., Lackenby A., Murphy S., McCauley J., Goodbourn S., Barclay W. Variation in the ability of human influenza A viruses to induce and inhibit the IFN-beta pathway. Virology. 2006;347:52–64. doi: 10.1016/j.virol.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 67.Levy D.E., Darnell J.E., Jr. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 68.Andrejeva J., Young D.F., Goodbourn S., Randall R.E. Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 2002;76:2159–2167. doi: 10.1128/jvi.76.5.2159-2167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Didcock L., Young D.F., Goodbourn S., Randall R.E. The V protein of simian virus 5 inhibits interferon signalling by targeting STAT1 for proteasome-mediated degradation. J. Virol. 1999;73:9928–9933. doi: 10.1128/jvi.73.12.9928-9933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcin D., Marq J.B., Strahle L., le Mercier P., Kolakofsky D. All four Sendai Virus C proteins bind Stat1, but only the larger forms also induce its mono-ubiquitination and degradation. Virology. 2002;295:256–265. doi: 10.1006/viro.2001.1342. [DOI] [PubMed] [Google Scholar]
- 71.Parisien J.P., Lau J.F., Rodriguez J.J., Sullivan B.M., Moscona A., Parks G.D., Lamb R.A., Horvath C.M. The V protein of human parainfluenza virus 2 antagonizes type I interferon responses by destabilizing signal transducer and activator of transcription 2. Virology. 2001;283:230–239. doi: 10.1006/viro.2001.0856. [DOI] [PubMed] [Google Scholar]
- 72.Ulane C.M., Rodriguez J.J., Parisien J.P., Horvath C.M. STAT3 ubiquitylation and degradation by mumps virus suppress cytokine and oncogene signaling. J. Virol. 2003;77:6385–6393. doi: 10.1128/JVI.77.11.6385-6393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gotoh B., Takeuchi K., Komatsu T., Yokoo J. The STAT2 activation process is a crucial target of Sendai virus C protein for the blockade of alpha interferon signaling. J. Virol. 2003;77:3360–3370. doi: 10.1128/JVI.77.6.3360-3370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nanda S.K., Baron M.D. Rinderpest virus blocks type I and type II interferon action: role of structural and nonstructural proteins. J. Virol. 2006;80:7555–7568. doi: 10.1128/JVI.02720-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palosaari H., Parisien J.P., Rodriguez J.J., Ulane C.M., Horvath C.M. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 2003;77:7635–7644. doi: 10.1128/JVI.77.13.7635-7644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park M.S., Shaw M.L., Munoz-Jordan J., Cros J.F., Nakaya T., Bouvier N., Palese P., Garcia-Sastre A., Basler C.F. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 2003;77:1501–1511. doi: 10.1128/JVI.77.2.1501-1511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rodriguez J.J., Parisien J.P., Horvath C.M. Nipah virus V protein evades alpha and gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. J. Virol. 2002;76:11476–11483. doi: 10.1128/JVI.76.22.11476-11483.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez J.J., Wang L.F., Horvath C.M. Hendra virus V protein inhibits interferon signaling by preventing STAT1 and STAT2 nuclear accumulation. J. Virol. 2003;77:11842–11845. doi: 10.1128/JVI.77.21.11842-11845.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shaw M.L., Garcia-Sastre A., Palese P., Basler C.F. Nipah virus V and W proteins have a common STAT1-binding domain yet inhibit STAT1 activation from the cytoplasmic and nuclear compartments, respectively. J. Virol. 2004;78:5633–5641. doi: 10.1128/JVI.78.11.5633-5641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shaw M.L., Cardenas W.B., Zamarin D., Palese P., Basler C.F. Nuclear localization of the Nipah virus W protein allows for inhibition of both virus- and toll-like receptor 3-triggered signaling pathways. J. Virol. 2005;79:6078–6088. doi: 10.1128/JVI.79.10.6078-6088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takeuchi K., Komatsu T., Yokoo J., Kato A., Shioda T., Nagai Y., Gotoh B. Sendai virus C protein physically associates with Stat1. Genes Cells. 2001;6:545–557. doi: 10.1046/j.1365-2443.2001.00442.x. [DOI] [PubMed] [Google Scholar]
- 82.Yokota S., Saito H., Kubota T., Yokosawa N., Amano K., Fujii N. Measles virus suppresses interferon-alpha signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-alpha receptor complex. Virology. 2003;306:135–146. doi: 10.1016/s0042-6822(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 83.Brzozka K., Finke S., Conzelmann K.K. Inhibition of interferon signaling by rabies virus phosphoprotein P: activation-dependent binding of STAT1 and STAT2. J. Virol. 2006;80:2675–2683. doi: 10.1128/JVI.80.6.2675-2683.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reid S.P., Leung L.W., Hartman A.L., Martinez O., Shaw M.L., Carbonnelle C., Volchkov V.E., Nichol S.T., Basler C.F. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J. Virol. 2006;80:5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Francois C., Duverlie G., Rebouillat D., Khorsi H., Castelain S., Blum H.E., Gatignol A., Wychowski C., Moradpour D., Meurs E.F. Expression of hepatitis C virus proteins interferes with the antiviral action of interferon independently of PKR-mediated control of protein synthesis. J. Virol. 2000;74:5587–5596. doi: 10.1128/jvi.74.12.5587-5596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heim M.H., Moradpour D., Blum H.E. Expression of hepatitis C virus proteins inhibits signal transduction through the Jak-STAT pathway. J. Virol. 1999;73:8469–8475. doi: 10.1128/jvi.73.10.8469-8475.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chee A.V., Roizman B. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites. J. Virol. 2004;78:4185–4196. doi: 10.1128/JVI.78.8.4185-4196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yokota S., Yokosawa N., Okabayashi T., Suzutani T., Miura S., Jimbow K., Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J. Virol. 2004;78:6282–6286. doi: 10.1128/JVI.78.12.6282-6286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khan S., Zimmermann A., Basler M., Groettrup M., Hengel H. A cytomegalovirus inhibitor of gamma interferon signaling controls immunoproteasome induction. J. Virol. 2004;78:1831–1842. doi: 10.1128/JVI.78.4.1831-1842.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zimmermann A., Trilling M., Wagner M., Wilborn M., Bubic I., Jonjic S., Koszinowski U., Hengel H. A cytomegaloviral protein reveals a dual role for STAT2 in IFN-{gamma} signaling and antiviral responses. J. Exp. Med. 2005;201:1543–1553. doi: 10.1084/jem.20041401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alcami A., Smith G.L. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J. Virol. 1995;69:4633–4639. doi: 10.1128/jvi.69.8.4633-4639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alcami A., Symons J.A., Smith G.L. The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN. J. Virol. 2000;74:11230–11239. doi: 10.1128/jvi.74.23.11230-11239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Puehler F., Weining K.C., Symons J.A., Smith G.L., Staeheli P. Vaccinia virus-encoded cytokine receptor binds and neutralizes chicken interferon-gamma. Virology. 1998;248:231–240. doi: 10.1006/viro.1998.9278. [DOI] [PubMed] [Google Scholar]
- 94.Symons J.A., Alcami A., Smith G.L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 95.Bergmann M., Garcia-Sastre A., Carnero E., Pehamberger H., Wolff K., Palese P., Muster T. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 2000;74:6203–6206. doi: 10.1128/jvi.74.13.6203-6206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hatada E., Saito S., Fukuda R. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J. Virol. 1999;73:2425–2433. doi: 10.1128/jvi.73.3.2425-2433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Langland J.O., Jacobs B.L. Inhibition of PKR by vaccinia virus: role of the N- and C-terminal domains of E3L. Virology. 2004;324:419–429. doi: 10.1016/j.virol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 98.Li S., Min J.Y., Krug R.M., Sen G.C. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology. 2006;349:13–21. doi: 10.1016/j.virol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 99.Dauber B., Schneider J., Wolff T. Double-stranded RNA binding of influenza B virus nonstructural NS1 protein inhibits protein kinase R but is not essential to antagonize production of alpha/beta interferon. J. Virol. 2006;80:11667–11677. doi: 10.1128/JVI.01142-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Imani F., Jacobs B.L. Inhibitory activity for the interferon-induced protein kinase is associated with the reovirus serotype 1 sigma 3 protein. Proc. Natl. Acad. Sci. USA. 1988;85:7887–7891. doi: 10.1073/pnas.85.21.7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peters G.A., Khoo D., Mohr I., Sen G.C. Inhibition of PACT-mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. J. Virol. 2002;76:11054–11064. doi: 10.1128/JVI.76.21.11054-11064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hakki M., Geballe A.P. Double-stranded RNA binding by human cytomegalovirus pTRS1. J Virol. 2005;79:7311–7318. doi: 10.1128/JVI.79.12.7311-7318.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sobol P.T., Mossman K.L. ICP0 prevents RNase L-independent rRNA cleavage in herpes simplex virus type 1-infected cells. J. Virol. 2006;80:218–225. doi: 10.1128/JVI.80.1.218-225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martinand C., Montavon C., Salehzada T., Silhol M., Lebleu B., Bisbal C. RNase L inhibitor is induced during human immunodeficiency virus type 1 infection and down regulates the 2–5A/RNase L pathway in human T cells. J. Virol. 1999;73:290–296. doi: 10.1128/jvi.73.1.290-296.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinand C., Salehzada T., Silhol M., Lebleu B., Bisbal C. RNase L inhibitor (RLI) antisense constructions block partially the down regulation of the 2–5A/RNase L pathway in encephalomyocarditis-virus-(EMCV)-infected cells. Eur. J. Biochem. 1998;254:248–255. doi: 10.1046/j.1432-1327.1998.2540248.x. [DOI] [PubMed] [Google Scholar]
- 106.He B., Gross M., Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taylor D.R., Shi S.T., Romano P.R., Barber G.N., Lai M.M. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science. 1999;285:107–110. doi: 10.1126/science.285.5424.107. [DOI] [PubMed] [Google Scholar]
- 108.Roy S., Katze M.G., Parkin N.T., Edery I., Hovanessian A.G., Sonenberg N. Control of the interferon-induced 68-kilodalton protein kinase by the HIV-1 tat gene product. Science. 1990;247:1216–1219. doi: 10.1126/science.2180064. [DOI] [PubMed] [Google Scholar]
- 109.Davies M.V., Elroy-Stein O., Jagus R., Moss B., Kaufman R.J. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J. Virol. 1992;66:1943–1950. doi: 10.1128/jvi.66.4.1943-1950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mathews M.B., Shenk T. Adenovirus virus-associated RNA and translation control. J. Virol. 1991;65:5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vyas J., Elia A., Clemens M.J. Inhibition of the protein kinase PKR by the internal ribosome entry site of hepatitis C virus genomic RNA. Rna. 2003;9:858–870. doi: 10.1261/rna.5330503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elia A., Laing K.G., Schofield A., Tilleray V.J., Clemens M.J. Regulation of the double-stranded RNA-dependent protein kinase PKR by RNAs encoded by a repeated sequence in the Epstein–Barr virus genome. Nucleic Acids Res. 1996;24:4471–4478. doi: 10.1093/nar/24.22.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gunnery S., Rice A.P., Robertson H.D., Mathews M.B. Tat-responsive region RNA of human immunodeficiency virus 1 can prevent activation of the double-stranded-RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA. 1990;87:8687–8691. doi: 10.1073/pnas.87.22.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ruf I.K., Lackey K.A., Warudkar S., Sample J.T. Protection from interferon-induced apoptosis by Epstein–Barr virus small RNAs is not mediated by inhibition of PKR. J. Virol. 2005;79:14562–14569. doi: 10.1128/JVI.79.23.14562-14569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goodbourn S., Didcock L., Randall R.E. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- 116.Basler C.F., Mikulasova A., Martinez-Sobrido L., Paragas J., Muhlberger E., Bray M., Klenk H.D., Palese P., Garcia-Sastre A. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 2003;77:7945–7956. doi: 10.1128/JVI.77.14.7945-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pichlmair A., Buse J., Jennings S., Haller O., Kochs G., Staeheli P. Thogoto virus lacking interferon-antagonistic protein ML is strongly attenuated in newborn Mx1-positive but not Mx1-negative mice. J. Virol. 2004;78:11422–11424. doi: 10.1128/JVI.78.20.11422-11424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martinez-Sobrido L., Zuniga E.I., Rosario D., Garcia-Sastre A. de la Torre JC. Inhibition of the type I interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 2006;80:9192–9199. doi: 10.1128/JVI.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kopecky-Bromberg S.A., Martinez-Sobrido L., Frieman M., Baric R.A., Palese P. Sars coronavirus proteins Orf 3b, Orf 6, and nucleocapsid function as interferon antagonists. J. Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Munoz-Jordan J.L., Sanchez-Burgos G.G., Laurent-Rolle M., Garcia-Sastre A. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA. 2003;100:14333–14338. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spiegel M., Pichlmair A., Martinez-Sobrido L., Cros J., Garcia-Sastre A., Haller O., Weber F. Inhibition of Beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 2005;79:2079–2086. doi: 10.1128/JVI.79.4.2079-2086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferko B., Stasakova J., Romanova J., Kittel C., Sereinig S., Katinger H., Egorov A. Immunogenicity and protection efficacy of replication-deficient influenza A viruses with altered NS1 genes. J. Virol. 2004;78:13037–13045. doi: 10.1128/JVI.78.23.13037-13045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fernandez-Sesma A., Marukian S., Ebersole B.J., Kaminski D., Park M.S., Yuen T., Sealfon S.C., Garcia-Sastre A., Moran T.M. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol. 2006;80:6295–6304. doi: 10.1128/JVI.02381-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Richt J.A., Lekcharoensuk P., Lager K.M., Vincent A.L., Loiacono C.M., Janke B.H., Wu W.H., Yoon K.J., Webby R.J., Solorzano A., Garcia-Sastre A. Vaccination of pigs against swine influenza viruses by using an NS1-truncated modified live-virus vaccine. J. Virol. 2006;80:11009–11018. doi: 10.1128/JVI.00787-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Talon J., Salvatore M., O'Neill R.E., Nakaya Y., Zheng H., Muster T., Garcia-Sastre A., Palese P. Influenza A and B viruses expressing altered NS1 proteins: A vaccine approach. Proc. Natl. Acad. Sci. USA. 2000;97:4309–4314. doi: 10.1073/pnas.070525997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Cleve W., Amaro-Carambot E., Surman S.R., Bekisz J., Collins P.L., Zoon K.C., Murphy B.R., Skiadopoulos M.H., Bartlett E.J. Attenuating mutations in the P/C gene of human parainfluenza virus type 1 (HPIV1) vaccine candidates abrogate the inhibition of both induction and signaling of type I interferon (IFN) by wild-type HPIV1. Virology. 2006;352:61–73. doi: 10.1016/j.virol.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 127.Valarcher J.F., Furze J., Wyld S., Cook R., Conzelmann K.K., Taylor G. Role of alpha/beta interferons in the attenuation and immunogenicity of recombinant bovine respiratory syncytial viruses lacking NS proteins. J. Virol. 2003;77:8426–8439. doi: 10.1128/JVI.77.15.8426-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wright P.F., Karron R.A., Madhi S.A., Treanor J.J., King J.C., O'Shea A., Ikizler M.R., Zhu Y., Collins P.L., Cutland C. The interferon antagonist NS2 protein of respiratory syncytial virus is an important virulence determinant for humans. J. Infect. Dis. 2006;193:573–581. doi: 10.1086/499600. [DOI] [PubMed] [Google Scholar]
- 129.Teng M.N., Whitehead S.S., Bermingham A., St Claire M., Elkins W.R., Murphy B.R., Collins P.L. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J. Virol. 2000;74:9317–9321. doi: 10.1128/jvi.74.19.9317-9321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Battcock S.M., Collier T.W., Zu D., Hirasawa K. Negative regulation of the alpha interferon-induced antiviral response by the Ras/Raf/MEK pathway. J. Virol. 2006;80:4422–4430. doi: 10.1128/JVI.80.9.4422-4430.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krishnamurthy S., Takimoto T., Scroggs R.A., Portner A. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J. Virol. 2006;80:5145–5155. doi: 10.1128/JVI.02618-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stojdl D.F., Lichty B., Knowles S., Marius R., Atkins H., Sonenberg N., Bell J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 133.Strong J.E., Coffey M.C., Tang D., Sabinin P., Lee P.W. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. Embo J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Balachandran S., Barber G.N. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5:51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 135.Coffey M.C., Strong J.E., Forsyth P.A., Lee P.W. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 136.Hunter W.D., Martuza R.L., Feigenbaum F., Todo T., Mineta T., Yazaki T., Toda M., Newsome J.T., Platenberg R.C., Manz H.J., Rabkin S.D. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J. Virol. 1999;73:6319–6326. doi: 10.1128/jvi.73.8.6319-6326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mineta T., Rabkin S.D., Yazaki T., Hunter W.D., Martuza R.L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]


