Abstract
The cell surface has various functions: communicating with other cells, integrating into the tissue, and interacting with the extracellular matrix. Proteases play a key role in these processes. This review focuses on cell-surface peptidases (ectopeptidases, oligopeptidases) that are involved in the inactivation or activation of extracellular regulatory peptides, hormones, paracrine peptides, cytokines, and neuropeptides. The nomenclature of cell-surface peptidases is explained in relation to other proteases, and information is provided on membrane anchoring, catalytic sites, regulation, and, in particular, on their physiological and pharmacological importance. Furthermore, nonenzymatic (binding) functions and participation in intracellular signal transduction of cell surfaces peptidases are described. An overview on the different cell-surface peptidases is given, and their divergent functions are explained in detail. An example of actual pharmacological importance, dipeptidyl-peptidase IV (CD26), is discussed.
Keywords: Plasma membrane, Protease, Peptide hormones, Neuropeptides
I. Introduction
The cell surface is the site for communication of cells with each other or with the whole body, integration of cells into their tissue, and modeling of the tissue matrix. Some of these diverse functions are regulated by proteases that cleave and thereby may activate, inactivate, or release other proteins or peptides. These regulatory proteases are either located on the cell surface or are secreted by the cells. Both types of proteases may fulfill similar functions, but cell-surface proteases appear to have in general more regulatory and specialized functions than secreted ones. In particular, they are involved in the regulation of the activity of peptides that communicate between cells or tissues (oligopeptidases, cell-surface peptidases in the original sense), in the degradation of extracellular components (matrix proteases), or in the shedding of cell-surface proteins (secretases, sheddases).
Since this comprises a broad spectrum of different types of proteases this review focuses on cell-surface (oligo)peptidases (Table I ), a special group of cell-surface proteases that is involved in the regulation of the biological activity of small bioactive peptides that serve in cell communication, namely peptide hormones, neuropeptides, growth factors, or cytokines. These important physiological roles have focused attention on cell-surface peptidases in recent pharmacological research. Inhibitors are widely used as drugs or are under development. This review will provide an overview of the general properties and physiological and pharmacological importance of mammalian cell-surface (oligo)peptidases. A closer view will be given to a selected peptidase of actual pharmacological interest, the dipeptidyl-peptidase IV.
TABLE I.
Overview of Cell-Surface Peptidases
| Peptidase | Abbreviation | EC | Catalytic type | Alternative names (examples) |
|---|---|---|---|---|
| Aminopeptidase N | APN (CD13) | 3.4.11.2 | Metallo | Alanine aminopeptidase, aminopeptidase M, microsomal aminopeptidase, Cys-Gly dipeptidase |
| Aminopeptidase A | APA | 3.4.11.7 | Metallo | Angiotensinase A, gp160, glutamyl aminopeptidase, membrane aminopeptidase II, mouse BP-1⧸6C3 antigen |
| Aminopeptidase P | APP | 3.4.11.9 | Metallo | Aminopeptidase P2, membrane aminopeptidase P, proline aminopeptidase, X-Pro aminopeptidase |
| X-Trp aminopeptidase | 3.4.11.16 | Metallo | Aminopeptidase W | |
| Pyroglutamyl-peptidase II | 3.4.19.6 | Metallo | TRH-degrading ectoenzyme, thyroliberinase | |
| Dipeptidyl-peptidase IV | DPP IV (CD26) | 3.4.14.5 | Serine | Gly-Pro naphthylamidase, Xaa-Pro-dipeptidyl-aminopeptidase, adenosine deaminase binding protein, glycoprotein GP110, thymocyte-activating molecule |
| Angiotensin-converting enzyme | ACE (CD143) | 3.4.15.1 | Metallo | Peptidyl-dipeptidase A, dipeptidyl carboxypeptidase I, kininase II |
| Angiotensin-converting enzyme-2 | ACE2, ACEH | Metallo | ||
| Carboxypeptidase M | CPM | 3.4.17.12 | Metallo | Max.1 |
| Carboxypeptidase P | CPP | 3.4.17.16 | Metallo | Membrane Pro-X carboxypeptidase |
| γ-Glutamyl transpeptidase | γ-GT (CD224) | 2.3.2.2 | γ-Glutamyltransferase | |
| Membrane dipeptidase | MDB | 3.4.13.19 | Metallo | Leukotriene D4 hydrolase, renal dipeptidase |
| Neprilysin | NEP (CD10) | 3.4.24.11 | Metallo | Neutral endopeptidase, endopeptidase 24.11, enkephalinase, atriopeptidase, CALLAa |
| Endothelin-converting enyzme | ECE-1 | 3.4.24.71 | Metallo |
CALLA, common acute lymphoblastic leukemia antigen.
Other types of cell-surface proteases act mainly on larger protein⧸proteoglycan substrates. Membrane-type matrix metalloproteinases (MT-MMPs) belong to the group of matrix metalloproteinases (MMPs) that is primarily involved in the degradation of extracellular matrix components. MMPs are mostly secreted, and cell-surface MT-MMPs either act like the secreted MMPs or activate them. Secretases or sheddases are proteases that release soluble forms from transmembrane proteins or peptides, e.g., cell-surface receptors, membrane anchored cytokines or chemokines, other membrane proteases, or the amyloid precursor protein. They belong to the group of ADAMs (a disintegrin and metalloproteinase). ADAMs may cleave and release other cell-surface peptidases to yield soluble forms. Since MT-MMPs and ADAMs both act on larger proteins and belong either to a broader group of secreted proteases (MMPs) or represent a distinct group from the (oligo)peptidases, they will be excluded from this review (the reader is referred to specialized reviews for more information: Itoh 2002, Moss 2002, Visse 2003, Zucker 2003).
Interestingly, many cell-surface (oligo)peptidases—though specialized on short peptides—belong to families of proteins that have a broader spectrum of activities, not only as peptidases for regulatory peptides, but also as proteases for larger proteins, e.g., extracellular matrix proteins or proteoglycans, or even as binding proteins for these molecules without any proteolytic activity. Therefore it is not surprising that some cell-surface peptidases may have multiple functions, as a regulatory peptidase, extracellular protease, or a binding protein. These diverse functions will be discussed in this review in terms of the cell-surface peptidase dipeptidyl-peptidase IV (CD26) and the members of its family, since this cell-surface peptidase is of actual pharmacological importance.
Today, the properties, structures, distribution, and physiological and pharmacological importance of most cell-surface peptidases are well described, but less is known about their role in cell biology. For example, when a cell-surface peptidase is a multimeric enzyme, it is rarely explored whether it can form heteromers with another member of its gene family resulting in further functions. Also relatively little is known about the dynamics of the peptidases on the cell surface, their association with other proteases, receptors, transporters, or binding proteins, and their fate during endocytotic processes.
In this review I will provide a general overview of cell-surface (oligo)peptidases (Table I), in particular their nomenclature, structure, cell anchoring, and physiological and pharmacological importance. These properties will then be discussed in terms of a selected surface peptidase with actual pharmacological interest.
II. Classification and General Properties of Cell-Surface Peptidases
A. Nomenclature and Classification
Enzymes hydrolyzing peptide bonds are commonly termed proteases, proteinases, peptidases, or proteolytic enzymes. Historically, these terms have had slightly different meanings, namely peptidases act on small peptides, proteases degrade larger proteins, and proteinases (endopeptidases) cleave peptide chains internally (Barrett 1980, McDonald 1986), but these terms are now often used synonymously. The Enzyme Nomenclature EC list (1992) (http:⧸⧸www.chem.qmw.ac.uk⧸iubmb⧸enzyme⧸index.html) recommends the term peptidase as the general form. Here, the term cell-surface peptidase is used in the former sense for a proteolytic enzyme acting on small peptides (now oligopeptidase) that is located at the cell surface with an extracellular active site (for which the general term ectoenzyme is used).
Exopeptidases cleave only one or a few residues from the N- or C-terminus of a peptide, whereas endopeptidases (formerly identical to proteinases) act internally in a peptide chain. Aminopeptidases liberate either a single amino acid residue from the N-terminus (aminopeptidases, EC 3.4.11) or a dipeptide or tripeptide from the N-terminus (dipeptidyl-peptidases and tripeptidyl-peptidases, EC 3.4.14). Those cleaving a single residue from the C-terminus are termed carboxypeptidases (EC 3.4.16–18) and those liberating a dipeptide are termed peptidyl-peptidases (EC 3.4.15). Other exopeptidases are specific for dipeptides (dipeptidases, EC 3.4.13), or remove terminal residues that are substituted, cyclized, or linked to isopeptide bonds (peptide linkage at amino acid side chains); the later are termed omega peptidases (EC 3.4.19). The term oligopeptidase is also used for endopeptidases that act optimally on smaller peptides, not on proteins. Cell-surface peptidases acting on small peptides belong to all groups (endopeptidases, exopeptidases, and specialized peptidases); those acting on larger proteins are endopeptidases.
Proteases are further classified according to their catalytic center into serine, cysteine, aspartic, or metalloproteases (Barrett, 1994). Cell-surface peptidases are either serine or (more often) metalloproteases. Based on molecular structure and evolutionary relationship they can be divided into families and clans. This system is explained in detail by Barrett et al. (1998) and is used in the MEROPS database (http:⧸⧸merops.sanger.ac.uk⧸). This database gives excellent, precise, and detailed information about proteases of many species. In this scheme, a family of peptidases is a group in which each member shows a statistically significant relationship in amino acid sequence to at least another member of the family. The term clan is used to describe a group of families the members of which have evolved from a single ancestral protein, but have diverged so far that their relationship cannot be proved by direct comparison of their primary structures.
Apart from the excellent MEROPS database (http:⧸⧸merops.sanger.ac.uk⧸), information on the protein structures of proteases can be obtained from the SwissProt protein sequence database (http:⧸⧸www.expasy.ch⧸) or especially for enzymes from http:⧸⧸www.expasy.org⧸enzyme⧸, or the PIR database (http:⧸⧸pir.georgetown.edu⧸). Genetic information can be obtained from http:⧸⧸www.ncbi.nlm.nih.gov⧸ or http:⧸⧸bioinformatics.weizmann.ac.il⧸cards⧸ or from http:⧸⧸www.dsi.univ-paris5.fr⧸genatlas⧸. The catalytic properties of proteases are complied in the Brenda enzyme information system (http:⧸⧸brenda.bc.uni-koeln.de⧸); further information and especially animations are given at http:⧸⧸delphi.phys.univ-tours.fr⧸Prolysis⧸. An excellent print compilation of proteases is given in the “Handbook of Proteolytic Enzymes” (Barrett et al., 1998). For older references, the compilations of Barrett 1980, McDonald 1986, Kenny 1977, Kenny 1991, and Kenny et al. (1987) are still valuable sources.
B. Membrane Anchoring of Cell-Surface Peptidases and Soluble Forms
In contrast to intracellular or extracellular secreted proteases, cell-surface peptidases have to be anchored into the plasma membranes. They are nearly exclusively integral membrane proteins, but there are a few examples that soluble, secreted proteases can also be bound to the cell surface via receptors. For example, the secreted, soluble urokinase (urokinase-type plasminogen activator, EC 3.4.21.73), a protease involved in the activation of other proteases, blot-clot lysis, and cell invasion, is bound to the cell surface via a specific membrane receptor. As integral membrane proteins, cell-surface peptidases either possess a transmembrane region with the N- (type I) or C-terminus (type II) extracellular, or they are linked to the membrane via a glycosyl-phosphatidylinositol (GPI) anchor (Fig. 1 ). As cell-surface proteins they are glycoproteins with a high degree (e.g., of about 30% of their molecular mass) of carbohydrates that are found at the extracellular site.
FIG. 1.
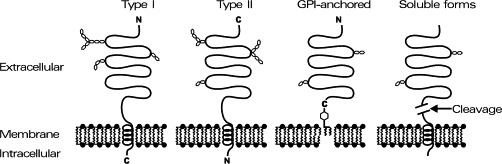
Topology of cell-surface peptidases. Cell-surface peptidases are anchored in the plasma membrane either with a transmembrane region (schematic: lipid bilayer with helical lipophilic amino acid sequences; type I = N-terminus extracellular; type II = C-terminus extracellular), or they are linked to the membrane via a glycosyl-phosphatidylinositol (GPI) anchor. The extracellular region is highly glycosylated (schematic: hexoses). Secretases may liberate the extracellular parts of cell-surface peptidases to yield soluble variants.
Some cell-surface peptidases also exist in soluble forms. These soluble forms are apparently generated by proteolytic shedding from the cell surface (Fig. 1) rather than being different splice forms. So far, the conditions for their shedding and the secretases involved have rarely been investigated. An exception is angiotensin-converting enzyme (ACE; EC 3.4.15.1) from which a soluble form is known to be liberated by members from the ADAM family (Hooper 1997, Parkin 2002). Soluble ACE (as some other soluble forms of cell-surface peptidases) is present under normal physiological conditions in blood plasma, amniotic fluid, seminal plasma, and other body fluids. The levels of soluble ACE are altered in certain diseases such as sarcidosis, diabetes mellitus, Gaucher's disease, leprosy, and hyperthyroidism.
However, soluble equivalents to cell-surface peptidases also exist that are encoded by different genes. Aminopeptidase P (APP, X-Pro aminopeptidase, EC 3.4.11.9) has two forms: a membrane-bound form (AP-P2) encoded by a gene on the human X chromosome (Sprinkle et al., 1998) and a cytosolic form (AP-P1) encoded by a gene on human chromosome 10 (Sprinkle et al., 2000), both with nearly identical substrate specificities. The membrane-bound form is located as an ectoenzyme (via a glycosyl-phosphatidylinositol anchor) on the plasma membrane of endothelial and epithelial cells whereas the soluble form is a cytosolic enzyme found in leukocytes, platelets, and brain (Harbeck 1991, Hooper 1990, Simmons 1992). The cytosolic form displays 43% amino acid identity and 63% similarity to the membrane-bound from (Cottrell et al., 2000) and both are classified as members of the peptidase clan MG on the basis of sequence and structural similarities (Hyde et al., 1996) with other exopeptidases, which include the soluble peptidases such as methionyl aminopeptidases type I and II (EC 3.4.11.18) and the X-Pro dipeptidases (prolidases, EC 3.4.13.9).
C. Catalytic Sites and Kinetics of Cell-Surface Peptidases
Since cell-surface peptidases act on extracellular regulatory peptides, the active catalytic site is located in their large extracellular domain. Therefore, cell surface peptidases are also termed ectopeptidases. According to catalytic type, cell-surface peptidases possess either a catalytic triad of His, Asp, and Ser (serine peptidases) or a metallo-binding motif for a divalent cation, normally zinc (metalloproteases). The known metal ligands in metalloproteases are His, Glu, Asp, or Lys. In addition to the metal ligands, at least one other residue is required for catalysis, mostly Glu, but also Arg and Lys. Normally, the catalytic site is found only in one catalytic center, but, e.g., angiotensin-converting enzyme (EC 3.4.15.1) can have two catalytic HEXXH metalloprotease motifs (Barrett 1998, Hooper 1994). The three-dimensional structure of some cell-surface peptidases (metallo- and serine peptidases) has been clarified in some examples of pharmacological importance, and the reader is referred to original and more specialized review articles (Engel 2003, Lowther 2002, Natesh 2003, Rasmussen 2003).
The specificity of the cleavage for peptide bonds by cell-surface peptidases can be described with the terminology of Berger and Schechter (1970). The residues to the peptide substrate are numbered according to (N-terminus) … P3–P2–P1 + P1′–P2′–P3′ … (C-terminus; + marks the cleavage site). Some cell-surface peptidases show exceptionally high cleavage selectivity, e.g., APP liberates only P1 residues from the N-terminus of peptides with a proline in the P1′ position; others show a much broader selectivity.
The Michaelis–Menten kinetics of enzymes is normally characterized by K m and V max (respectively k cat = V max/mol enzyme) values. However, K m values for oligopeptidases are in the micomolar range whereas the physiological concentrations of regulatory peptides (hormones, neuropeptides, cytokines) are in the nanomolar or picomolar range (as the K d values of their receptors). At these low concentrations of substrates S (in comparison to K m values) the reaction rates of the enzymes E are given by v=[E] [S]k cat/K m, i.e., the linear curve of dependence of v from S (Fersht, 1985). Therefore, the rate (specificity) constant k cat⧸K m is the best basis for comparing the potency of a peptidase with different peptides at physiological concentrations. High rate constants correspond to high cleavage rates at low (physiological) concentrations.
D. Cell-Surface Proteases and CD Nomenclature
Certain cell-surface molecules, including some cell-surface peptidases, have been identified on immune cells by the use of monoclonal antibodies (initially without knowing their broader tissue distribution). Based on this differentiation by monoclonal antibodies, cluster of differentiation (CD) antigens have been defined that have later been shown to be identical to some well-studied cell-surface peptidases (Table II ). For example, CD10 was originally described as CALLA (common acute lymphoblastic leukemia antigen), because of its association with ALL (acute lymphoblastic leukemia) and its usefulness in diagnosis, and has been identified later as the already known neutral endopeptidase 24.11 (neprilysin, enkephalinase, EC 3.4.24.11). Further information on CD antigens, their nomenclature, distribution, and function can be found at http:⧸⧸www.ncbi.nlm.nih.gov⧸PROW⧸.
TABLE II.
Cell-Surface Peptidases in the CD Nomenclature
| CD | Alternative names | Peptidase | EC |
|---|---|---|---|
| CD10 | CALLAa | Neutral endopeptidase (NEP), neprilysin, enkephalinase | 3.4.24.11 |
| CD13 | Aminopeptidase N (APN) | 3.4.11.2 | |
| CD26 | Adenosine deaminase- binding protein, THAMb | Dipeptidyl-peptidase IV (DPP IV) | 3.4.14.5 |
| CD143 | Angiotensin-converting enzyme (ACE), peptidyl dipeptidase A | 3.4.15.1 | |
| CD224 | γ-Glutamyl transpeptidase (γ-GT) | 2.3.2.2 | |
| Max.1 | Carboxypeptidase M (CPM) | 3.4.17.12 | |
| BP-1⧸6C3 antigen | Aminopeptidase A (APA) | 3.4.11.7 |
CALLA, common acute lymphoblastic leukemia antigen.
THAM, thymocyte activation molecule.
III. Physiological Functions and Pharmacological Importance of Cell-Surface Peptidases
Initially, cell-surface proteases were isolated from kidney or intestinal brush border membranes, both rich sources of membrane-bound proteases (Kenny 1977, Kenny 1987). These locations pointed to a physiological role in the final degradation of peptides or peptide fragments produced by other proteases. In fact, cell-surface proteases act mostly on substrates with less than 80 amino acid residues and some exhibit broader substrate specificities (Table III ). Therefore, it can be anticipated that cell-surface peptidases on the intestinal, luminal surface are involved in the digestion of nutritional proteins. This digestive role has been shown for cell-surface proteases of broad specificity such as aminopeptidase N as well as for proline-specific peptidases such as dipeptidyl-peptidase IV or carboxypeptidase P (Brandsch 1995, Erickson 1989, Heymann 1986, Yoshioka 1988). Since peptide bonds involving proline residues are resistant to the attack of nonspecific proteases such as trpysin, chymotrypsin, aminopeptidases, or carboxypeptidases, the proline-specific exopeptidases appear to be necessary for the final digestion of peptides. However, so far knockout or deficient animals show no clinical abnormalities in protein digestion (see below, Table IV ); however, a case of (probable) human aminopeptidase P deficiency showed peptidurea of Xaa-Pro-peptides (Blau et al., 1988).
TABLE III.
Physiological Substrates of Cell-Surface Peptidases
| Peptidase | Substrate specificity | Endogenous substrates | Characteristic inhibitors |
|---|---|---|---|
| APN (CD13) EC 3.4.11.2 | Xaa-↓-Xaa- | Enkephalins, neurokinin A, somatostatin | Bestatin, actinonin, probestin, amastatin |
| APA EC 3.4.11.7 | Glu⧸Asp-↓-Xaa… | Angiotensin II | Amastatin |
| APP EC 3.4.11.9 | Xaa-↓-Pro- | Bradykinin, neuropeptide Y | Apstatin |
| Pyroglutamyl- peptidase II EC 3.4.19.6 | Glp-↓-His-Pro-NH2 | TRH | 1,10-Phenanthroline (nonselective) |
| DPP IV (CD26) EC 3.4.14.5 | Xaa-Pro⧸Ala-↓-Yaa | See Table IV | Diprotin A, Xaa-thiazolides, Xaa-pyrrolidides, Xaa-boroPro |
| CPM EC 3.4.17.12 | …Xaa-↓-Arg⧸Lys | Bradykinin, anaphylatoxins, EGF | MGTA, GEMSAa |
| CPP EC 3.4.17.16 | …Pro-↓-Xaa | Angiotensin II, enterostatin | Phenanthroline (nonselective) |
| ACE (CD143) EC 3.4.15.1 | …Xaa-↓-Yaa-Zaa | Angiotensin II, bradykinin, enkephalins | Captopril, enalapril, lisinopril |
| ACE-2 | …Xaa-↓-Yaa-Zaa | Angiotensin II | Metal chelators |
| MDB(−1) EC 3.4.13.19 | Dipeptides | Peptidyl leukotriene D4 β-lactam antibiotics | Cilastatin |
| NEP (CD10) EC 3.4.24.11 | …Xaa-↓-Yaa | Enkephalins, bradykinin, substance P, neurotensin, atrial natriuretic factor, gonadoliberin, calcitonin | Phosphoramidon, thiorphan, omapratilat (mixed inhibitor) |
| ECE-1 EC 3.4.24.71 | Big endothelin | Phosphoramidon, PD 069185 |
MGTA, 2-mercaptomethyl-3-guanidinoethylthiopropanoic acid; GEMSA, guanidinoethylmercaptosuccinic acid.
TABLE IV.
Phenotype of Knockout or Deficient Animals for Cell-Surface Peptidases
| Peptidase | Phenotype | References |
|---|---|---|
| APN (CD13) | Dogs with deficiency in small intestine were clinically healthy | Pemberton et al., 1997 |
| APA | Mice with deficiency developed normally, normal numbers of T and B cells, normal antibody responses and IgG | Lin et al., 1998 |
| APP | Candidate gene for premature ovarian failure | Prueitt et al., 2000 |
| DPP IV (CD26) | Knockout mice are refractory to obesity and hyperinsulinemia after high-fat diet, have higher glucose tolerance and GLP-1 levels | Conarello et al., 2003 |
| Higher glucose tolerance in knockout mice or rats with mutated enzyme | Marguet 2000, Mitani 2002, Nagakura 2001 | |
| Reduced tumor metastasis in rats with mutated enzyme | Shingu et al., 2003 | |
| ACE (CD143) | Knockout mice: reduced blood pressure, renal pathology, and reduced male fertility | Esther et al., 1996 |
| NEP (CD10) | Knockout mice: sensitivity to endotoxin shock, opioid-related increase in thermonociceptive threshold, modified ventilatory response to hypoxia, higher response to experimentally induced colitis. Overexpression: reduced deposition of β-amyloid | Lu 1995, Marr 2003, Saria 1997, Sturiale 1999 |
| ECE-1 | Null mutation in mice results in embryonic craniofacial and cardiac abnormalities | Yanagisawa et al., 1998 |
| Mutations in humans are associated with Hirschsprung disease | Hofstra et al., 1999 | |
| MDB-1 | Knockout mice: no phenotype, redundant enzymes exist | Habib et al., 1998 |
Later, cell-surface peptidases with very narrow specificity such as pyroglutamyl-peptidase II or endothelin-converting enzyme were detected. Furthermore, it was discovered that all cell-surface peptidases acted on regulatory peptides, and even those with an intestinal location turned out to be located on more discrete sites where they have contact with endogenous hormones or neuropeptides. As a consequence, the degradation of bioactive peptides or other cellular (binding) functions is regarded as the main function of cell-surface peptidases.
A. Inactivation and Activation of Regulatory Peptides and their Pharmacological Importance
Proteolytic processing is involved in the inactivation, partial inactivation, or activation of regulatory peptides such as hormones, neuropeptides, or cytokines⧸chemokines. Activation of intracellular stored peptides is normally accomplished by signal peptidases and prohormone convertases within the secretory vesicles. Extracellular proteolytic activation occurs for some secreted hormones (e.g., angiotensinogen⧸angiotensin) or for the conversion of membrane-bound forms to active soluble forms (e.g., tumor necrosis factor-α, fractalkine). Whereas in the later case the soluble forms are generated mostly by members of the ADAM family from the integral membrane proforms, secreted prohormones are cleaved by cell-surface proteases (or their shedded soluble forms) or proteases of broader specificity.
For activation of regulatory peptides by cell-surface proteases the prominent examples are ACE, which activates angiotensin I to the potent vasopressor octapeptide angiotensin II by release of a C-terminal dipeptide, and endothelin-converting enzyme (ECE), which cleaves big endothelin to endothelins (Table III). Whereas ACE displays a relatively broad specificity (in addition to angiotensin I bradykinin, neurotensin, substance P, and gonadoliberin are also cleaved) and is implicated in blood pressure regulation as well as a range of further biological processes such as immunity, reproduction, and neuropeptide regulation, ECE is a more specific enzyme that activates (inactive) big endothelin to the active vasoconstrictor endothelin(s). Consequently, inhibitors of ACE or ECE reduce the formation of the active, hypertensive hormones angiotensin II or endothelins and are therefore used as antihypertensive drugs (for details on ACE or ECEs see below, Barrett et al., 1998; or the MERPOS database at http:⧸⧸merops.sanger.ac.uk⧸).
Partial inactivation⧸activation is a process by which regulatory peptides are processed to truncated forms that retain only physiological activity to one receptor subtype or create activity to a new one. Since this differential inactivation often depends on N- or C-terminal residues, exopeptidases are mostly involved in this process. Detailed examples are discussed below for peptide truncation by dipeptidyl-peptidase IV (DPP IV), but similar partial inactivation of regulatory peptides is also observed for aminopeptidase P, carboxypeptidase M (bradykinin), and aminopeptidase A (forming angiotensin III).
Sometimes truncated peptides still bind to their receptors but do not transduce signals into their target cells. Consequently, cell-surface peptidases can generate antagonists. Their affinity is normally less or equal to the native peptide, and, therefore, it is doubtful that production of antagonists is of high physiological relevance in vivo. However, these proteolytically generated antagonists can down-regulate the surface expression of their receptors. This is of crucial importance for receptors with high internalization rates, e.g., chemokine receptors. Here, cell surface peptidases (DPP IV, see below) create antagonists with reduced affinity, but still important receptor regulation properties (Ludwig et al., 2002).
Inactivation of regulatory peptides is regarded as the main physiological function of cell-surface peptidases. This role concerns peptide hormones circulating in the blood stream, paracrine⧸autocrine hormones in tissues, and neuropeptides in the brain (or peripheral sites). Inactivation is a necessary event for regulated signal transmission, otherwise only permanent stimuli would result. The half-lives of signal molecules for extracellular communication differ considerably: from milliseconds to seconds for “classical” neurotransmitters (acetylcholine, amino acids, catecholamines), seconds to minutes for neuropeptides and peptide hormones, and minutes to hours or even days for growth factors and cytokines. Proteolytic degradation or other chemical modifications (for peptides oxidation or conjugation reactions occur in rare examples, but with questionable physiological relevance) are not the only inactivation mechanism; elimination by diffusion or filtration is also important (high-affinity cellular uptake of peptides is not observed). For example, a 30-residue peptide hormone such as glucagon-like peptide-1 (GLP-1, see below) circulates in the blood for 30–60 min after postprandial release from the intestine; during this time it is removed by filtration into the urine by the kidney. However, inactivation by proteolytic truncation (in this case by DPP IV, see below) considerably reduces the active form during its physiological activity as an insulin releaser. Consequently, cell-surface peptidase inhibitors are potential drugs that prolong the activity of endogenous regulatory peptides (or, in case of activation, hinder their generation).
Table III lists some important physiological substrates for cell-surface peptidases. Common for all peptidases is their restriction to peptide substrates up to about 80 residues (therefore the term oligopeptidases is adequate). Some of them exhibit a rather broad specificity, e.g., aminopeptidase N (APN) and neprilysin (NEP), others are restricted to certain types of bonds that are cleaved, e.g., APP and DPP IV, and still others are substrate specific, namely pyroglutamyl-peptidase II and (not strictly) ECE. If a certain regulatory peptide is cleaved by different cell-surface peptidases, e.g., substance P, bradykinin, and enkephalins, it depends on the tissue⧸cellular environment, which for the cell-surface proteases are of crucial importance. Methods to determine the degradation of regulatory peptides often include cell culture systems or purified systems, and the degradation is monitored now by high-performance liquid chromatography (HPLC) with reversed-phase separation columns using gradients of acetonitrile in 0.1% trifluoroacetic acid, capillary electrophoresis, or mass spectroscopy (Mentlein and Lucius, 1997).
Interestingly, although a peptidase may cleave a variety of peptides, inhibitors can target mainly one system in a certain disease. Inhibitors of ACE (inhibition of angiotensin II formation), ECE (inhibition of endothelin formation), and NEP (prolongation of the actions of atrial natriuretic peptides) are used to reduce blood pressure, DPP IV inhibitors (prolongation of GLP-1 action, see below) are under development for the treatment of type 2 diabetes, and most of the targets (ACE, NEP, and DPP IV) also cleave various other regulatory peptides.
The following sections contain a general overview of different cell-surface peptidases and their roles in the inactivation or activation of regulatory peptides. This is restricted to important examples. In one example of actual pharmacological importance (DPP IV) a detailed description is given at the end that summarizes not only its diverse functions as a regulatory protease, but also provides information on further members of its protein family and illustrates additional, nonproteolytic functions of cell-surface peptidases.
1. Aminopeptidases
Aminopeptidase N can attack a variety of substrates (up to 40 residues), although hydrophobic and neutral residues are preferred. Enkephalins⧸dynorphins appear to be the most important substrates. It should be noted that truncation of some regulatory peptides (e.g., somatostatin) does not inactivate them. The enzyme (homodimer of highly glycosylated, type II integral membrane 140–150 kDa subunits) is found in the brush border membranes of kidney, intestinal mucosal cells, and liver. Important localizations for the catabolism of regulatory peptides are endothelial cells (apical domain), smooth muscle cells, monocytes⧸macrophages (or related cells, e.g., microglial cells), and granulocytes (Barrett 1998, Lucius 1995, Mentlein 1996, Mizutani 1993, Safavi 1995).
Aminopeptidase A truncates angiotensin II to brain angiotensin III, which exerts a tonic stimulatory action on the central control of blood pressure. Thus, central inhibitors of APA constitute putative central antihypertensive agents. APA is expressed in many tissues; among hematopoietic cells (B cell stages) it is lineage and differentiation stage specific (BP-1⧸6C3 antigen). With respect to angiotensin II catabolism endothelial cells are an important localization (Bausback 1988, Barrett 1998, Mentlein 1996, Rozenfeld 2002).
Aminopeptidase P (the cell surface form is termed aminopeptidase P2 to distinguish it from the soluble form P1 that is coded by a different, related gene, see above) liberates N-terminal Arg from bradykinin; the following Pro-Pro residues may be further liberated by DPP IV (Orawski et al., 1987). APP is anchored via a GPI anchor on the cell surface. The inactivation of bradykinin appears to be important in the lung, especially in rodents, where high APP activity is observed (Prechel et al., 1995). Inactivation of neuropeptide Y appears to be of less importance (Mentlein 1993a, Orawski 1995). Interestingly, some ACE inhibitors also inhibit APP (Hooper 1992, Kim 2000); this effect may be additionally responsible for some side effects of ACE inhibitors. Membrane-bound APP is found in kidney and small intestine brush border membranes and endothelial and lymphoid cells (Barrett et al., 1998). There is one report of (probable) human APP deficiency showing peptiduria of Xaa-Pro peptides, but with no other clincal symptoms (Blau et al., 1988).
Pyroglutamyl-peptidase II is a strict substrate-specific enzyme: It liberates the terminal pyroglutamic acid from thyrotropin-releasing hormone (TRH); other pyroglutaminyl peptides such as gonadoliberin or neurotensin are not attacked (Bauer 1987, Bauer 1994). The enzyme is found in particular in the brain (neurons), anterior pituitary (mammotrops), retina, lung, and liver (Bauer et al., 1990); also a soluble serum, obviously shedded form exists (Schmitmeier et al., 2002). The extraordinarily high degree of substrate specificity and the restricted localization indicate that pyroglutamyl-peptidase II has a very specialized function as an inactivation enzyme for TRH as a neurotransmitter in the brain and as a hypothalamic releasing hormone in the circulation (Bauer 1994, Schomburg 1999). Dipeptidyl-peptidase IV and its physiological roles are discussed in detail below.
2. Carboxypeptidases and Dipeptidases
Carboxypeptidase M (CPM) cleaves the basic amino acids Arg or Lys from a variety of peptide substrates. Bradykinin, Arg-6-⧸Lys-6-enkephalins, and dynorphin A1–13 appear to be natural substrates. It is a widely distributed enzyme and is anchored to the cell surface by a GPI anchor. The highest levels are found in lung, placenta, kidney, intestine, blood vessels, brain, and peripheral nerves. On hematopoietic, cells CPM is found on discrete B lymphocyte developmental stages and on mature (mostly activated) T cells (de Saint-Vis et al., 1995). Also, soluble forms exist in various body fluids. CPM is supposed to inactivate bradykinin partially: CPM-truncated bradykinin is inactive at the B2 receptor, but binds to the B1 receptor. Furthermore, CPM removal of the C-terminal Arg of human anaphylatoxin C5a abolishes its spasmogenic and histamine-releasing activity, whereas about 10% of the chemotactic activity is retained. Also, several growth factors (epidermal growth factor, hepatocyte growth factor, erythropoietin, brain-derived neurotrophic factors, vascular endothelial growth factor, oncostatin M, and others) are truncated by CPM, but the physiological importance remains to be established (Barrett 1998, Mentlein 1996, Skidgel 1998).
Carboxypeptidase P liberates C-terminal amino acids from peptides with a penultimate Pro. So far, a role in the intestinal digestion of proline-containing peptides⧸fragments has been recognized, although some regulatory peptides (angiotensin II, enterostatin) have been also identified as substrates (Hedeager-Sørensen 1985, Mentlein 1996, Yoshioka 1988).
Angiotensin-converting enzyme (ACE) liberates dipeptides from small peptides provided that the ultimate and penultimate residues are not Asp⧸Glu or Pro. The main physiological substrates are angiotensin I and bradykinin. However, the substrate specificity is relatively broad and enkephalins, dynorphins, neurotensin, and other are also cleaved. Furthermore, ACE also displays endopeptidase activities toward des-Arg-9-bradykinin, dynorphin, substance P, and gonadoliberin. ACE exists in two isoforms, a somatic form of 150–180 kDa in endothelial, epithelial, and neuronal cells, and a smaller isoform (90–110 kDa) in germinal cells. Furthermore, a soluble (shedded) form exists. Based on the conversion of angiotensin I to (active) angiotensin II by ACE, the control of blood pressure and salt metabolism is regarded as its main physiological role. Consequently, highly specific ACE inhibitors have been developed that are widely used as antihypertensive drugs. The main side effects of ACE inhibitors are attributed the prolongation of the lifetime⧸effects of bradykinin. However, ACE may also have another function as a regulatory peptidase, especially in the brain. For details on this well-investigated cell-surface peptidase and its physiological and pharmacological importance the reader is referred to detailed reviews (Barrett 1998, Corvol 1995, Menard 2001, Skidgel 1987). In addition to the long known ACE, a further similar cell-surface enzyme has been recently discovered, angiotensin-converting enzyme-2 (ACE2, ACEH), which also converts angiotensin I to angiotensin II, but not bradykinin. ACE2 is not inhibited by lisinopril or captopril. The organ- and cell-specific expression of ACE2 and its unique cleavage of key vasoactive peptides suggest an essential role for ACE2 in the local renin–angiotensin system of the heart and kidney (Danilczyk 2003, Turner 2002).
Membrane dipeptidase (MBD, MDB-1) is a GPI-anchored enzyme in various tissues, e.g., kidney and lung. It acts on dipeptides, peptidyl leucotrienes, and some β-lactam antibiotics. In the kidney it is implicated in the metabolism of glutathione (after removal of the glutamate residue by γ-glutamyl transpeptidase). In the lung it converts the peptidyl leukotriene D4, a major component of the slow-reacting substances of anaphylaxis (broncho- and vasocontrictors), to the inactive leukotriene E4. Furthermore, some β-lactam antibiotics are cleaved and inactivated (Barrett et al., 1998). Recently, additional members of this dipeptidase family have been identified (Habib et al., 2003).
3. Endopeptidases
Neprilysin or neutral endopeptidase (NEP) preferentially cleaves peptide bonds with bulky, hydrophobic residues in the P1 position as an endopeptidase. With some substrates it also displays a peptidyl-dipeptidase character (enkephalin, dynorphin). Peptides up to 40 residues are cleaved, although the efficiency of hydrolysis declines with peptide length. The principal substrates are enkephalins, atrial natriuretic peptides, substance P, and other tachykinins, but various other regulatory peptides (e.g., somatostatin, neurokinin, cholecystokinin-8, bradykinin, gastrin-releasing peptide, calcitonin, calcitonin gene-related peptide, and the chemotactic bacterial peptide fMet-Leu-Phe) are cleaved (Kenny 1977, Kenny 1991). A recently discovered target is the amyloid β-peptide, whose accumulation causes Alzheimers's disease in the brain (Shirotani et al., 2001). Endopeptidase is widely distributed in various tissues (e.g., kidney, intestinal and placental brush border membranes, immune cells, choroids plexus, some neurons in the brain, and Schwann cells). Thus, NEP inhibitors may have potential in the treatment of hypertension (already used in clinical trials), pain, and inflammation. For detailed information the reader is referred to special reviews (Barrett et al., 1998, or the MERPOS database at http:⧸⧸merops.sanger.ac.uk⧸).
Endothelin-converting enzyme 1 is structurally related to NEP and is physiologically involved in the activation of endothelins, potent vasconstrictors generated from the intermediate proform big endothelin. Big endothelins are biologically inactive 38–41 amino acid residue intermediates that are generated from the endothelin peptide precursors of approximately 200 residues by prohormone processing enzymes (furines) in the secretory vesicles. Activation of the big endothelins to the active, 21-residue vasoconstrictors endothelin-1, -2, or -3 (ET-1 to -3) is accomplished by cleavage of the Trp-21-↓-Val-22 ⧸Ile-22 bonds by ECE. ECE-1 has been shown to be located either at the cell surface or in the Golgi depending on the tissue. The related ECE-2 appears to be Golgi protein and, therefore, in a strict sense is not a cell-surface protease (see above). Consequently, these enzymes are potential drug targets for the treatment of hypertension (Barrett et al., 1998).
B. Further Roles of Cell-Surface Peptidases (CSP)
1. CSP as Binding Proteins⧸Receptors
Cell-surface peptidases are rather large, highly glycosylated proteins. Compared with simple proteases such as trypsin with molecular masses between 20 and 30 kDa, huge portions of their peptide chains appear not to be necessary for enzymatic activity. Therefore, it is not surprising that the extracellular domain of some cell-surface peptidases fulfills other functions as binding protein or as a receptor. In fact, for certain cell-surface peptidases structure homologues without peptidase activity exist that appear to have binding functions.
DPP IV is an excellent example of a cell-surface peptidase with a dual function as an enzyme and a binding protein (see below): (human) DPP IV acts as a binding molecule for adenosine deaminase, an important enzyme for cellular and humoral immunity, as well as for extracellular matrix substances. Furthermore, DPP IV belongs to a gene family with proteins specialized in peptidase function (DPP IV) and binding functions: DPP6 and DPP10 have a transmembrane structure similar to DPP IV, are supposed to have arisen by gene duplication, but lack peptidase activity and appear to be binding proteins. Details are given below.
Specific expression of cell-surface peptidases might also contribute to the specialization of certain tissues. The specialization of the breast vasculature has been attributed to the expression of APP in this tissue (Essler and Ruoslahti, 2002). APP is able to bind certain synthetic peptide sequences that mimic structures of cancer cells responsible for metastasis into distinct tissues.
Viruses use cell-surface molecules for infection after endocytosis. Apart from receptors and carbohydrates, peptidases also might serve this function. In fact, APN acts as a receptor for the coronavirus 229E, which infects epithelial cells of the respiratory tract and mediates human cytomegalovirus infection (responsible for Herpes) by enhancing its binding to the surface of infected cells (Delmas et al., 1992).
In summary, the main function of cell-surface peptidases appears to be their enzymatic activity, but they may have additional binding activities. Structure homologues can have evolved that specialize in this function (or, less likely, vice versa, the cell surface may have evolved from cell-surface-binding proteins). Furthermore, as plasma membrane proteins they can function for binding⧸entry of viruses or homing of other cells⧸cancer cells.
2. CSP and Intracellular Signal Transduction
The intracellular sequence of cell-surface peptidases is normally very short (less than 20 amino acids) and contains no obvious motifs. Therefore, direct signal transduction effects without association with other functional molecules cannot be expected. However, in connection with other receptors or cell-membrane-associated enzymes signal transducing effects have been observed, in particular for APN, DPP IV, and γ-glutamyl transpeptidase (γ-GT). Induction of the signals may be triggered by inhibitors, substrates, or binding proteins.
Inhibition of either APN gene expression or of APN enzymatic activity has profound effects on proliferation and function in T cells (Lendeckel et al., 2003). The most prominent changes observed after application of inhibitors such as actinonin or probestin include the activation of mitogen-activated protein kinases (MAPK) Erk 1⧸2 (extracellular-regulated kinases) or the Wnt pathway, a decrease in the production of proinflammatory cytokines (interleukin-2 and -12), and the induction of the immunosuppressive cytokine transforming growth factor-β (TGF-β). However, the precise mechanism, whether direct or indirect, remains speculative.
DPP IV is regarded as a costimulator of T cells (Kähne et al., 1999). Association with antigens, binding proteins, especially adenosine deaminase and extracellular matrix proteins, or inhibitors induces cytokine production and proliferation. The protein tyrosine phosphatase CD45 as well as MAPK appears to be involved in this process (see below for details).
Since γ-GT is a key enzyme for the homeostasis of reduced glutathione (GSH), it is implicated in the regulation of the cellular redox state. Furthermore, in B cells γ-GT is associated with CD19 and members of the tetraspan 4 family including CD53, CD81, and CD82 (Nichols et al., 1998). It remains unclear whether antigen activation or the modulation of oxidative signals could be responsible for the observed effects on intracellular signal transduction systems (Paolicchi et al., 2002).
Taken together, signal transduction effects of cell-surface peptidases have been reported despite their short cytoplasmic domain (Antczak et al., 2001a). It appears, however, that these effects result mainly from their association with other cell-surface molecules.
3. CSP in Cell Invasion
Cell-surface peptidases might be implicated in cell invasion by their possible ability to adhere to extracellular matrix proteins, to degrade them, or to induce genes involved in this process. At present, there is some experimental evidence for such roles of cell-surface peptidases, especially for DPP IV and APN, but the exact mechanisms remain uncertain (Atherton 1992, Shingu 2003). DPP IV has been shown to bind to collagens I and III (see below). Furthermore, close structure homologues of DPP IV are devoid of enzymatic activity and appear to act only as binding proteins (see below). So far, the function of cell-surface peptidases (and their inactive homologues) in cell invasion and tissue integration is relatively unexplored, but is an interesting emerging field of research.
4. CSP in Diseases
Many investigations have been conducted on the aberrant expression of cell-surface peptidases in various diseases. To be distinguished is whether the activity of soluble forms in body fluids was measured, or whether the expression of membrane-bound forms was determined by activity measurements, enzyme⧸immunohistochemical strainings, or flow cytometric (FACS) analysis. For soluble activity, these studies are hampered by little knowledge of their shedding from the cell surface and its regulation. For the membrane-bound forms little is known about their induction by growth factors and their corresponding transcriptional elements, which are often tissue⧸cell-type specific. These aspects are covered by two recent reviews (Antczak 2001a, Antczak 2001b).
Generally, high expression (in body fluids or at the cell surface) is correlated with hyperplasia of the tissue normally expressing the cell-surface peptidase, e.g., in cancer and chronic inflammatory diseases. Some cell-surface peptidases are also excellent markers for tissue differentiation, especially those expressed on hematopoietic cells (see cell-surface peptidases and CD nomenclature); therefore, they can be used to monitor this process or identify neoplastic transformations, in particular of leukemias and lymphomas. Also, cell activation can sometimes be detected by their elevated expression (see previous examples), although the precise regulation by growth factors⧸cytokines or a developmental program is less known. Today, there is also little knowledge of whether tissue-dependent regulation of their expression and consequently their peptidase activity influences their main physiological function, namely the inactivation of regulatory peptides.
5. Elucidation of the Physiological Functions by Knockout Animals
Although caution should be exercised in extrapolating results of animal⧸mice models to humans, targeted inactivation or inherited defects of the genes for cell-surface peptidases will provide important hints concerning their physiological functions (Table IV). Whereas in a few examples (ACE and ECE-1) abnormalities are directly evident, in most other examples investigated so far defects or physiological advantages are visible only under certain experimental conditions. This points to a nonredundant, intrinsic role only for certain surface peptidases, whereas most others are of importance in certain environmental or stressor situations (or are redundant genes). Nevertheless, NEP or DPP IV knockout mice⧸defective rats demonstrate that these enzymes function as regulatory peptidases not only in vitro, but that they are important regulators of neuropeptides and peptide hormones also in vivo. Interestingly, beside this main function they also appear to participate in more complicated diseases such as tumor metastasis or Alzheimer's disease.
Interestingly, human deficiencies or mutations of cell-surface peptidase genes are not well explored (there is a rare, not clear documented example for APP: Blau et al., 1988). However, increasing information about genetic variations will certainly reveal their role as susceptibility or disease genes. This can be anticipated in particular for genes of cell-surface peptidases for which inhibitors are used as drugs.
C. Dipeptidyl Peptidase IV as an Example of the Diverse Functions of Cell-Surface Peptidases
Dipeptidyl-(amino)peptidases (DPP, EC 3.4.14) liberate dipeptides from the N-terminus of peptides. With the exception of DPP IV, they are lysosomal or cytosolic proteases. Two types of specificities can be distinguished: DPPs with relative broad specificity (DPP I, lysosomal Cys-protease; DPP III, cytosolic metalloprotease), but that are restricted by a proline in the second, the N-terminal penultimate position, and DDPs with complementary specificity (DPP II, DPP IV, and DPP8) liberating Xaa-Pro or Xaa-Ala dipeptides from the N-terminus. Generally, all DPPs act mainly on short or medium chain peptides (up to 80 residues) and less on larger proteins. Both types of DPPs could be regarded as merely digestive or as regulatory proteases. As digestive enzymes they are involved in the final degradation of peptides, either intra- or extracellularly. As regulatory enzymes they activate or inactivate bioactive peptides. Since these are extracellular communication molecules, only cell-surface dipeptidyl peptidase IV (DPP IV)⧸CD26 is of physiological importance.
In recent years, DPP IV has attracted considerable interest as a drug target, in particular for the treatment of type 2 diabetes. Therefore, this enzyme will be described in detail as an example of the importance of cell-surface peptidases (De Meester 1999, Gorrell 2001, Kähne 1999, Mentlein 1988, Mentlein 1999). Despite no surface oligopeptidases, brief information will be given on DPP IV-related molecules.
1. Molecular Properties
Human DPP IV is a dimeric 240-kDa glycoprotein composed of two 120-kDa subunits (Table V ). The mRNA codes for a type II integral membrane protein of 766 amino acids (Misumi et al., 1992) that is anchored in the plasma membrane by a 22 amino residue hydrophobic membrane-spanning domain (VLLG LLGAAALVTI ITVPVVLL) preceded by a short, intracellular hydrophilic sequence (MKTPWK) at the N-terminus. The extracellular sequence contains nine potential N-linked glycosylation sites. Due to the presence of sialic acids in the carbohydrate structure, DPP IV has an acidic isoelectric point. The rat sequence exhibits about 85% identity to the human sequence, lacking one residue and one glycosylation site (Misumi et al., 1992). The C-terminal regions (residues 625–752) of the human and rat sequences are completely conserved.
TABLE V.
Dipeptidyl Peptidase IV (DPP IV, DP4) and Fibroblast Activation Protein (FAP) α (FAP, Seprase)
| Abbrevations | DPP IV, DP4 | FAP |
|---|---|---|
| EC number | 3.4.14.5 | 3.4.21.– |
| Alternative names | Dipeptidyl aminopeptidase IV | Seprase |
| Xaa-Pro-dipeptidylaminopeptidase | ||
| Gly-Pro naphthylamidase | ||
| Postproline dipeptidyl aminopeptidase IV | ||
| CD26 | ||
| Adenosine deaminase binding protein-2 | ||
| Glycoprotein gp110 | ||
| Genes | 2q24.3 (81.8 kb, 26 exons) | 2q24.2 (72.9 kb) |
| Transcription elements | SP-1, AP2, NFκB, BRE, HNF-1, GAS | |
| mRNA | 4.2 and 2.8 kb (tissue dependent) | |
| Protein | 766 aa, glycosylated 120 kDa | 760 aa, glycosylated 97 kDa |
| Native protein dimeric 240 kDa | Native protein dimeric 170 kDa | |
| Active site | Ser-… -Asp-…-His | Ser-… -Asp-…-His |
| GWSYG | GWSYG | |
| Distribution | Endothelial cells | Reactive fibroblasts (development, tissue repair, cancer) |
| Epithelial cells (intestine, pancreatic and bile duct, renal proximal tubular cells, spleen, sinus lining cells) | Cultivated melanocytes | |
| Mature thymocytes | Melanoma cells | |
| Activated T cells | ||
| Hepatocytes | ||
| Specialized fibroblasts (myofibroblasts, synovial fibroblasts) | ||
| Reaction catalyzed | Xaa-Pro⧸Ala-↓-Yaa…, Yaa≠Pro | |
| Assay substrate | Gly-Pro-↓-NA (or AMC) | Gelatin, denatured collagen I, IV, also Gly-Pro-?-AMC |
| pH optimum | 7.8–8.0 | Neutral |
The crystal structure of DPP IV homodimers has been published recently (Engel 2003, Rasmussen 2003). Each subunit consists of two domains: an α⧸β-hydrolase domain and an eight-bladed β-propeller domain. Between these two domains, a large cavity of ∼30–45 Å is found that is accessible via tow openings. Substrates and products pass either through a funnel in the center of the propeller domain or through a much larger opening in the side between the domains.
The human DPP IV gene is located on the long arm of chromosome 2 (2q24.3) and spans approximately 82 kb. It contains 26 exons, ranging in size from 45 bp to 1.4 kb (Abbott et al., 1994). The 5′-flanking domain contains neither a TATA box nor a CAAT box, but a 300-bp region extremely rich in C and G (72%) has potential binding sites for several transcription factors, such as NFκB, AP2, or Sp1 (Table V, Abbott 1994, Böhm 1995). The DPP IV gene encodes two mRNAs sized about 4.2 and 2.8 kb that are expressed at different relative rates in individual tissues.
2. Localization
DPP IV has a wide tissue distribution; it is found on many epithelial and certain specialized mesodermal cells (Table V). It is often present at sites of physiological borders (e.g., the blood–brain barrier). Most important for its physiological functions (see below) is its location on endothelial cells of blood vessels, on activated T cells, on renal proximal tubular cells, and on intestinal epithelial cells (Fig. 2 ). Electron microscopy clearly shows its presence at the cell surface, but little is known about the dynamics of DPP IV from the plasma membrane to intracellular vesicles during endocytotic processes (Fig. 2C). It appears that DPP IV is associated with glycolipid- and cholesterol-rich membrane microdomains known as “rafts” rather than coated pits (Riemann et al., 2001; Fig. 2C).
FIG. 2.
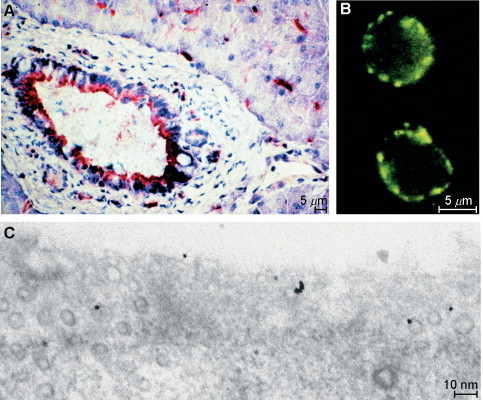
Localization of DPP IV. (A) Histochemical staining of DPP IV activity with Gly-Pro-4- methoxy-2-naphthylamide and a diazonium salt on a fresh section of porcine pancreas. Epithelial cells of the pancreatic duct and endothelial cells of blood vessels are stained red; blue: nuclear counterstaining with hemalum. (B) Immunostaining of human T lymphocytes with rabbit antihuman DPP IV followed by a fluoresceine-labeled secondary antibody. The green immunofluorescence covers the cell surface. (C) Preembedding immunostaining of cultivated endothelial cells (human umbilical vein endothelial cells) with rabbit antihuman DPP IV followed by a 20-nm gold-labeled secondary antibody. Staining is found on the cell surface, in endosomal pits, and in vesicles (probably caveolae). A coated pit (at the left) is immunonegative.
In addition to the membrane-bound form, soluble DPP IVs exist. These have no transmembrane domain: the human serum DPP IV lacks the first 39 residues (Durinx 2000, Iwaki-Egawa 1998) and the seminal plasma form lacks the first 29 or 32 residues (Durinx 2000, Ohkubo 1994). These soluble forms are considered to be produced by proteolytic shedding from the cell surface of endothelial or epithelial cells. However, the proteases responsible for their release and the conditions activating this process have not yet been identified.
3. Catalytic Properties
DPP IV was discovered as an enzyme liberating naphthylamine from Gly-Pro-2-naphthylamide and was initially termed glycylproline naphthylamidase (Hopsu-Havu and Glenner, 1966). In fact, mammalian DPP IV exhibits restricted substrate specificity (Fig. 3 ).
FIG. 3.
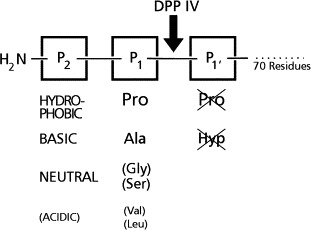
Schematic representation of substrates cleaved by DPP IV. Dipeptides are liberated from the N-terminus of peptides with Pro or Ala in the P1 position. Certain peptides with other small amino acids in the P1 position are cleaved with low rates. In the P2 position bulky, hydrophobic, or basic amino acids with an obligate free amino group are preferred. Peptides with Pro or Hyp in the P1′ position are resistant to DPP IV.
In the P1 position proline and alanine are accepted almost exclusively. Peptides or chromogenic substrates with Pro are better hydrolyzed than corresponding ones with Ala. However, important natural peptide substrates have both, penultimate Pro or Ala (Table VI ). Apart from these, few peptides with other small P1 residues (e.g., Ser, Val, Gly, Leu) are cleaved, although with considerably lower rates, e.g., glucagon (Hinke 2002, Martin 1993, Pospisilik 2001), analogues of growth hormone–releasing hormone (GRH) (Bongers et al., 1992), and macrophage-derived chemokine (MDC) from which Gly-Pro and Tyr-Gly are sequentially released (Proost et al., 1999).
TABLE VI.
Regulatory Peptides as Substrates for Dipeptidyl Peptidase IV
| Peptide | N-terminus | aa | Significance | References |
|---|---|---|---|---|
| Inactivation | ||||
| Neuropeptides | ||||
| Endomorphin-2 | YP-FF-NH2 | 4 | Inactivation (in vivo) | Shane et al., 1999 |
| Hormones | ||||
| GRH | YA-D… | 29⧸44 | Inactivation (in vivo, in vitro) | Kubiak 1998, Mentlein 1993b |
| GLP-1 | HA-E… | 30 | Inactivation (in vivo, in vitro) | Deacon et al., 1995; Kiefer et al., 1995; Mentlein et al., 1993b |
| GLP-2 | HA-E… | 34 | Inactivation (in vivo, in vitro) | Drucker et al., 1997a |
| GIP | YA-E… | 42 | Inactivation (in vivo, in vitro) | Deacon 2001, Mentlein 1993b |
| Glucagon | HS-QG-T… | 29 | Inactivation (in vitro) | Hinke et al., 2000; Popsilik et al., 2001 |
| Chemokines | ||||
| SDF-1 (CXCL12) | KP-V… | 68⧸72 | Inactivation (in vitro) | Proost 1998, Shioda 1998 |
| MDC (CCL22) | GP-YG-A… | 69 | Inactivation (in vitro) | Proost et al., 1999 |
| I-TAC (CXCL11) | FP-M… | 73 | Inactivation (in vitro) | Ludwig 2002, Proost 2001 |
| IP-10 (CXCL10) | VP-L… | 77 | Inactivation (in vitro) | Proost et al., 2001 |
| Mig (CXCL9) | TP-V… | 103 | Inactivation (in vitro) | Proost et al., 2001 |
| Eotaxin (CCL11) | GP-A… | 74 | Inactivation (in vitro) | Oravecz et al., 1997 |
| LD78β (CCL3) | AP-L… | 69 | Inactivation (in vitro) | Proost et al., 2000 |
| Partial inactivation | ||||
| Neuropeptides | ||||
| Neuropeptide Y | YP-S… | 36 | At Y1 receptors in vitro | Mentlein et al., 1993a |
| Substance P | RP-KP-Q… | 11 | Lower binding NK1 receptor | Heymann and Mentlein, 1978 |
| Neurogenic inflammation | Grouzmann et al., 2002 | |||
| Hormones | ||||
| Peptide YY | YP-I… | 36 | At Y1 receptors in vitro | Mentlein et al., 1993a |
| Chemokines | ||||
| RANTES (CCL5) | SP-Y… | 68 | At CCR1 and CCR3 in vitro | Oravecz et al., 1997 |
| Cleavage with no or unknown physiological effects (examples) | ||||
| Enterostatin | VP-DP-R | 5 | Inactivation? | Bouras et al., 1995 |
| Corticotrophin-like | RP-V… | 22 | Probably no effect | Nausch et al., 1990 |
| intermediate | ||||
| lobe peptide | ||||
| Gastrin-releasing peptide | VP-LP-A… | 27 | Probably no effect | Nausch et al., 1990 |
| GCP-2 (CXCL6) | GP-V… | 77 | No effect | Proost et al., 1998 |
In the P2 position any unsubstitued proteinogenic amino acid is accepted (including proline). Substrates with hydrophobic or basic residues are better hydrolyzed than those with acidic ones. The peptide bond between the P1 and P2 residues must be in the trans position. Peptides with Pro or Hyp in the P1′ position are not cleaved, e.g., bradykinin (Arg-Pro-Pro- …). The chain length of the peptide substrates has not been systematically investigated. Peptides up to 70 residues are degraded; sequestration of larger proteins may depend on their tertiary structures (Nausch et al., 1990). This restriction can be explained by the three-dimensional structure of DPP IV (Rasmussen et al., 2003). Optimal cleavage rates are generally observed at pH values between 7.5 and 8.5.
Apart from this action as a strict exopeptidase, endopeptidase activities on collagen or other extracellular matrix proteins have been postulated occasionally for DPP IV (Bermpohl et al., 1998). At present it can, however, not be excluded that these endopeptidase activities result from proteases closely related to or associated with DPP IV, e.g., fibroblast activation protein α (seprase) that can form heterodimers with DPP IV.
DPP IV belongs to the serine class of proteases, clan SC, family S9 (compare Barrett et al., 1998, or http:⧸⧸merops.sanger.ac.uk⧸). The catalytic triad, Ser-630, Asp-708, and His-740, is invertedly arranged as compared to the trypsin-like serine proteases (His-Asp-Ser); therefore, DPP IV is classified as a nonclassical serine protease. The active site sequence GWSYG around the catalytic serine residue (David 1993, Ogata 1992) corresponds to the consensus sequence GXSXG of serine proteases and is found in one copy at positions 628–632 (human sequence). A single substitution in this sequence in a substrain of Fisher-344 rats (G to R; active site GWSYR) results in loss of enzymatic activity. Due to this deficiency for active DPP IV, this Fisher-344 rat strain has become a valuable model to study physiological functions (Fujiwara 1992, Watanabe 1987).
In accordance with its catalytic structure, DPP IV is inhibited more or less powerfully by serine enzyme inhibitors that covalently modify their active site. Diisopropylfluorophosphate is a good inhibitor, whereas phenylmethylsulfonylfluoride is relatively ineffective (McDonald 1986, Püschel 1982). Metal ions such as Pb2+, Hg2+, and Zn2+ are strongly inhibitory. Inhibitors of other classes of proteases or inhibitors of aminopeptidases (e.g., bestatin) are ineffective.
Specific DPP IV inhibitors acting at low concentrations are of special interest for physiological investigations and for potential clinical applications. The microbial peptides diprotin A (Ile-Pro-Ile) and diprotin B (Val-Pro-Leu) are competitive substrates that are slowly hydrolyzed and act as selective competitive inhibitors for DPP IV at micromolar concentrations (Rahfeld et al., 1991). In addition, several types of substrate analogues have been synthesized that are powerful and specific inhibitors at micromolar to picomolar concentrations, e.g., proline boronic acid dipeptide inhibitors (reversible), dipeptide phosphonates (irreversible), dipeptide 2-cyanopyrrolidides, or aminoacyl-pyrrolidides (both reversible; Villhauer et al., 2001). Natural peptides with an N-terminal Xaa-Xaa-Pro sequence are more or less strong inhibitors of DPP IV, e.g., peptide YY(3–36) or the Tat protein of the human immunodeficiency virus type-1 (HIV-1) (Wrenger et al., 1996).
4. Physiological Substrates and the Importance of DPP IV Cleavage
Mammalian DPP IV cleaves a variety of regulatory peptides—neuropeptides, peptide hormones, and chemokines (Table VI). This N-terminal truncation can inactivate, partially inactivate (to certain receptor subtypes⧸effects), or have no (or an unknown) effect on the biological activity of the peptide. Regulatory peptides with an Xaa-Pro-Pro … sequence, e.g., bradykinin (RPP…, 9 residues) and longer peptides, e.g., interleukin-2 (APT…, 133 residues) or interleukin-1β (APV…, 153 residues), are not cleaved (Mentlein, 1999). Activation of peptide precursors by DPP IV cleavage (or DPP IV-like enzymes) has only been observed in lower vertebrates and invertebrates, e.g., processing of the prohormone for caerulein in the frog Xenopus laevis, of an antifreeze protein in the winter flounder, proceropins (antibacterial peptides) in moth, promelittin (bee venom) in the honeybee, or preprohormones in sea anemones (Martensen et al., 1998).
The relative degradation rates of the different peptides can vary considerably, and some of the reported substrates are cleaved only after prolonged incubation times in vitro (Lambeir 2001b, Mentlein 1999, Zhu 2003). Therefore, the physiological significance of DPP IV truncation remains questionable for some substrates, e.g., glucagon and most chemokines. However, a pivotal role of DPP IV in the final or partial inactivation of some peptide hormones (GLP-1, GLP-2, GIP, and peptide YY) and neuropeptides (neuropeptide Y, substance P, PACAP-38, and endomorphin-2) in vivo is evident. For chemokines a function of DPP IV truncation in vivo has not yet been proven, but may be anticipated for some (SDF-1, MDC, and I-TAC), at least under certain physiological conditions.
Circulating peptide hormones are exposed to large quantities of DPP IV on the surface of endothelial cells. In comparison, soluble serum DPP IV or CD26 on leukocytes has less total activity (Mentlein, 1999), but contributes further to hormone degradation. The most important physiological and pharmacological DPP IV substrates to date are the incretin hormones GLP-1 and GIP. They belong to a family of 27–44 residue peptides, the glucagon peptide family. Those with a penultimate Ala, namely GRH, GLP-1, GLP-2, GIP, and PHM, are good DPP IV substrates (Lambeir 2002, Mentlein 1993b); those with a penultimate Ser, namely glucagon, secretin, VIP, and PACAP-27, are relatively poor substrates (Hinke 2000, Lambeir 2001a) and will not be discussed here.
Glucagon-like peptide-1 [GLP-1; formerly GLP-1(7–36)NH2] and glucagon-like peptide-2 (GLP-2) are, respectively, 30- and 33-residue gastrointestinal peptide hormones released postprandially from enteroglucagon cells (L cells) in the small and large intestine, and are generated by an alternative posttranslational processing of proglucagon (positions 78–108 and 126–159 of the human proglucagon precursor). GLP-1 stimulates insulin secretion in vitro and in vivo. It plays an important role in the regulation of postprandial insulin release and is the most important incretin (insulinotropic hormone) known so far (Fig. 4 ). In patients with type 2 diabetes, exogenous GLP-1 lowers postprandial plasma glucose excursions by stimulating insulin release and by inhibiting glucagon secretion. Since the biological half-life of GLP-1 is very short, the use of exogenous GLP-1 or prolongation of its action is considered as a new possibility in the treatment of patients with type 2 diabetes. GLP-1 is also produced in the brain, and intracerebroventricular GLP-1 in rodents is a potent inhibitor of food and water intake. GLP-2 displays an intestinal growth factor activity in rodents, raising the possibility that GLP-2 may be therapeutically useful for enhancement of mucosal regeneration in patients with intestinal diseases (Drucker 1998, Drucker 2003a). Understanding the inactivation of GLP-1 and GLP-2 is therefore of potential therapeutic interest.
FIG. 4.
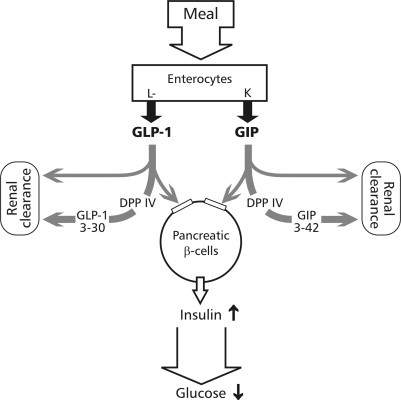
Schematic drawing of the role for DPP IV in the inactivation of incretins. GLP-1 and GIP are released postprandially from intestinal L or K cells, transported in the blood, and stimulate insulin secretion from pancreatic β-cells. DPP IV at the surface of endothelial cells or soluble in the blood degrades both peptides at the N-terminus resulting in a rapid loss of hormonal activity.
Several studies in vitro and in vivo have shown that GLP-1 is primarily degraded at the N-terminus by the action of DPP IV resulting in biologically inactive des(His-Ala)-GLP-1 (Deacon 1995a, Deacon 1995b, Deacon 1998a, Deacon 1998b, Kieffer 1995, Mentlein 1993b). After subcutaneous or intravenous administration of GLP-1 in healthy or type 2 diabetic humans more than 75% was recovered from the plasma as des(His-Ala)-GLP-1 (Deacon et al., 1995b). After intravenous injection of 125I-radiolabeled GLP-1 at picomolar concentrations into rats more than 50% was metabolized to des(His-Ala)-GLP-1 within 2 mins, but not in DPP IV-negative rats (Kieffer et al., 1995). This conversion could be blocked in vitro and in vivo by specific DPP IV inhibitors such as diprotin a or Lys- or Val-pyrrolidide. Pharmacological inhibition of DPP IV with valine-pyrrolidide in anesthetized pigs, at a dose that reduced plasma DPP IV activity by more than 90%, increased the amount of intact GLP-1 both in the basal state and after infusion (Deacon et al., 1995b). Moreover, inhibition of DPP IV by Ile-thiazolidide in Zucker rats potentiated the insulinotropic effect of GLP-1 after intravenous infusion alone or with glucose (Pederson et al., 1998). Furthermore, glucose tolerance and insulin secretion are enhanced in DPP IV knockout mice (Marguet et al., 2000) and in DPP IV activity-deficient Fischer 344 rats (Nagakura et al., 2001) in comparison to normal mice or Fischer rats. However, inhibition of DPP IV in these normal animals showed similar effects. Meanwhile, improved glucose tolerance under DPP IV inhibition has also been proven in humans (Ahrén et al., 2002). Therefore, inhibition of DPP IV is regarded as a validated target for treatment of type 2 diabetes.
Like GLP-1, GLP-2 is also easily degraded to des(His-Ala)-GLP-2 by purified human DPP IV or with serum containing DPP IV activity (Drucker et al., 1997a) as well as in rats and in humans in vivo (Hartmann 2000, Tavares 2000). When injected into normal rats, GLP-2 induces an increase in intestinal villus height and has a small trophic effect on small bowel weight. Administration of GLP-2 into DPP IV-deficient rats markedly increased the bioactivity of rat GLP-2 resulting in a significant increase in small bowel weight (Drucker et al., 1997a). Again, DPP IV-resistant GLP-2 analogues show higher effects than GLP-2 in wild-type animals, e.g., a statistically significant increase in small bowel mass was produced by the analogue r[Gly(2)]-GLP-2 in rats and by the human analogue h[Gly(2)]-GLP-2 in mice (Drucker 1997a, Drucker 1997b). These results show that inactivation of GLP-2 by DPP IV is of critical importance for its growth factor properties in the intestine and that DPP IV-resistant analogues may be useful therapeutics to improve mucosal regeneration.
Gastric inhibitory polypeptide or glucose-dependent insulinotropic polypeptide (GIP), a 42-amino acid polypeptide, is secreted by duodenal K cells after ingestion of fat and glucose. It inhibits the secretion of gastric acid and stimulates insulin release from pancreatic β cells in the presence of elevated glucose levels. Thus, in addition to GLP-1, GIP also contributes to incretin activity of postprandial serum (Fig. 4), more in rodents and less in humans (Meier et al., 2002). As the other members of the GRH-glucagon family with a penultimate Ala, GIP is also rapidly degraded to GIP(3–42) by purified human DPP IV or serum with DPP IV activity in vitro (Mentlein et al., 1993b) and in vivo (Deacon et al., 2000). This truncation of GIP in serum could be successfully inhibited by coincubation with specific DPP IV inhibitors in vitro and in vivo (Deacon 2001, Kieffer 1995, Mentlein 1993b). GIP(3–42) was also detected after purification of GIP from pig intestine (Jörnvall et al., 1981), and later it was shown that it lacked any biological activity and is even a weak GIP receptor antagonist in vivo (Gault 2002, Schmidt 1987). As reported for GLP-1, GIP was also rapidly degraded in vivo to the truncated form (50% after 2 min) after injection of radioiodinated GIP into wild-type rats, but not in DPP IV-deficient ones (Kieffer et al., 1995). In accordance, after oral administration of a DPP IV inhibitor the half-life of GIP was prolonged in wild-type rats or pigs (Deacon 2001, Kieffer 1995) or DPP IV-resistant analogues are more potent in normal or obese rats (Hinke et al., 2002). Thus, as for GLP-1 and GLP-2, DPP IV truncation is also the main degradation route for GIP in vivo.
Growth hormone-releasing hormone (GRH) is a 44-residue peptide of which biologically active, C-terminal truncated fragments are known. It is released from the hypothalamus and stimulates the release and the synthesis of growth hormone from and in the pituitary. In human plasma GRH(1–44)-NH2 (or fragments) is rapidly metabolized to GRH(3–44)-NH2; further minor degradation products derive from endoproteolytic cleavage of the Arg(11)-Lys(12) or Lys(12)-Val(13) bonds (Frohman et al., 1989). All these proteolytic fragments are biologically inactive. By use of specific inhibitors, DPP IV and a trypsin-like endoprotease were identified as the proteases responsible for this processing. Following this initial report, DPP IV-mediated proteolysis was established as a major route of GRH degradation and inactivation in rat (Boulanger et al., 1992), porcine, and bovine plasma in vitro and in cattle in vivo (Kubiak 1998, Kubiak 1989). Again, DPP IV-resistant peptide analogues of GRH(1–29) showed similar biological activity to induce growth hormone release in vitro (cultivated pituitary cells), but higher potency to increase serum growth hormone levels in vivo up to 60 min after injection into cattle due to longer stability in vivo (Kubiak and Friedman, 1998).
Peptide YY (PYY) is produced by endocrine cells of the small intestine and the colon and is released after a meal as a peptide hormone circulating in the blood that inhibits several gastrointestinal functions, e.g., gastric acid release. The amino terminal sequence Tyr-Pro is conserved in neuropeptide Y and PYY among all vertebrate species and both appear to be the best peptide substrates for DPP IV. Although PYY is rapidly cleaved by human serum and purified DPP IV in vitro (Mentlein et al., 1993a) to PYY(3–36) and this fragmentation product is the major molecular form of PYY extracted from human or rabbit intestine (Eberlein et al., 1989) or circulating in the blood (Grandt et al., 1994), the physiological consequences have not been explored.
Among the neuropeptides degraded by DPP IV (endomorphin-2, substance P, neuropeptide Y; Table VI), neuropeptide Y (NPY) is probably the most interesting substrate. Together with PYY and pancreatic polypeptide (PP), it belongs to a peptide family of 36-residue peptides with high homology in primary and tertiary structure. NPY is an abundant neuropeptide in the central and peripheral nervous system; it is involved in the control of feeding, energy homeostasis, and blood pressure. As with PYY, NPY is also rapidly degraded by DPP IV in vitro to des(Tyr-Pro)NPY (Mentlein et al., 1993a), and this truncated form has been identified as a considerable part (35%) of the total NPY extracted from porcine brain (Grandt et al., 1996) and in the human cerebrospinal fluid (Nilsson et al., 1998). Full-length NPY, PYY, and PP bind with different affinities to at least five receptor subtypes (Y1 and Y2—NPY and PYY, Y3—NPY > PYY, Y4—PP, and Y5—all three), but N-terminally truncated NPY (and PYY) lacks affinity to the Y1 receptor (Gerald et al., 1996), which is located in the cerebral cortex and on vascular smooth muscle cells where it promotes vasoconstriction and cell proliferation. Due to the receptor subtype-specific regulation of NPY activity, DPP IV has already been termed an “NPY-converting enzyme.” In this respect, DPP IV appears to be involved in the regulation of vascular smooth muscle contraction, angiogenesis (Zukowska-Grojec et al., 1998), and, in particular, endothelial cell migration (Ghersi et al., 2001) and edema (Dimitrijevic et al., 2002).
Substance P is a widespread neuropeptide that acts, e.g., as a transmitter of sensory information including noxious stimuli, as a potent contractor of smooth muscles, and as an immunoregulator. Three different G-protein-coupled receptor subtypes, NK1, NK2, and NK3, are known that are also stimulated by neurokinins A and B, which share a common C-terminal pentapeptide sequence (Phe-Xaa-Gly-Leu-Met-NH2) with substance P. Therefore, the sequential liberation of the dipeptides Arg-Pro and Lys-Pro from the N-terminus of the undecapeptide substance P (Heymann 1978, Kato 1978) does not abolish its biological activity at the tachykinin receptors. However, the fragment substance P-(5–11) has a lower binding activity for the preferred substance P receptor, the NK1 receptor (Mussap et al., 1993). Only effects for which the first four residues in substance P are essential, e.g., the degranulation of mast cells (probably non-receptor-mediated by pertubation of the membrane), may be completely abolished by DPP IV cleavage. However, substance P truncated by DPP IV can be further attacked by aminopeptidase N, which is an important degradation pathway in human plasma, at the vascular endothelium, at fibroblasts, and muscle myocytes (Wang 1991, Wang 1994). This may explain the potentiation of neurogenic inflammation and enhanced vasodilation by DPP IV inhibitors in nasal mucosa (Grouzmann et al., 2002).
Endomorphin-1 (YPWF-NH2) and -2 (YPFF-NH2) are endogenous opioid peptides with high affinity at μ opioid receptors that produce potent analgesia in mice. DPP IV liberated the terminal Tyr-Pro with high activity from endomorphin-2, whereas an analogue with d-Pro in the penultimate position was resistant. This analogue was more potent and longer lasting to induce analgesia in vivo; moreover the DPP IV inhibitor Ala-pyrrolidonyl-2-nitrile itself elicited analgesia and potentiated the actions of endomorphin-2 (Shane et al., 1999). These results suggest a role for DPP IV in endomorphin inactivation in vivo.
Among the chemokines cleaved by DPP IV (CCL3, CCL5, CCL11, CCL22, CXCL9, CXCL10, CXCL11, and CXCL12, Table VI), SDF-1 (CXCL12), I-TAC (CXCL11), and MDC (CCL22) appear to be most important, since they are cleaved with the highest rates (Lambeir et al., 2001b). Chemokines constitute a large group of small (8–10 kDa) secreted proteins that act as cell-type-selective chemoattractants. They can be divided into four subfamilies, based on structural, functional, and genetic criteria. The two most important subfamilies are the CXC and CC chemokines (α- and β-family), which differ in the spacing of the first two cysteine residues that form disulfide links with two other cysteines. In CXC chemokines these first cysteine residues are separated by one amino acid; in CC chemokines they are directly neighbored. Generally, DPP IV cleavage of chemokines abolishes their chemotactic activity. In some cases the truncated forms act as weak antagonists. However, up to now no experimental evidence has been given on the importance of this process in vivo. DPP IV inhibitors often show an antiinflammatory effect (Steinbrecher et al., 2001), not a potentiation of the inflammatory response as expected for prolongation of chemokine action. In accordance, DPP IV inhibitors did not enhance the chemotaxis of I-TAC (CXCL11) on activated human T cells in vitro that express both DPP IV⧸CD26 and the chemokine receptor CXCR3 (Mentlein et al., 2003). However, different results have been found for the chemotactic effect of SDF-1α (CXCL12) on human cord blood CD34+ progenitors cells that also coexpress DPP IV⧸CD26 and the receptor CXCR4 (Christopherson et al., 2002).
In conclusion, for several circulating hormones (GLP-1, GLP-2, GIP, GRH, and PYY), neuropeptides (NPY, substance P, and endomorphin), and chemokines (SDF-1α, others) the role of DPP IV in their complete or receptor subtype-specific inactivation has been well documented. Pharmacologically most important is the action on GLP-1.
5. Pharmacological Importance of DPP IV
Despite its multiple functions in the degradation of several regulatory peptides and the invasiveness of tumors, DPP IV is at present mainly considered a new, validated target for treatment of type 2 diabetes (Augustyns 2003, Drucker 2003b, Holst 2002, Holst 1998). Prolongation of GLP-1 action by inhibition of its degradation or application of DPP IV-resistant GLP-1 analogues has been shown to enhance insulin secretion and glucose tolerance in vivo. GLP-1 analogues modified at the N-terminal His(1), Ala(2), and Glu(3) by the corresponding d- or different amino acids, e.g., d-Ala(2)-GLP-1, have longer half-lives and consequently higher insulinotropic potencies in vitro and in vivo (Burcelin 1999, Deacon 1998b, Siegel 1999a, Siegel 1999b). Though this modification is a rather specific approach for type 2 diabetes with low side effects, it has the drawback that GLP-1 peptide analogues cannot be given orally, in contrast to small DPP IV inhibitors (Augustyns et al., 2003), which have more potential side effects. However, recent clinical studies with an orally active DPP IV inhibitor in type 2 diabetic patients show a considerably better insulin tolerance and improved HBA1c levels after 4 weeks of treatment (Ahrén et al., 2002). Side effects were surprisingly low (partly nasopharyngitis, pruritus, and transient nephritic syndrome). Thus, DPP IV inhibitors appear to be promising drugs for treatment of some stages of type 2 diabetes.
6. DPP IV as a Binding Protein
Apart from its function as a regulatory protease, other important roles for DPP IV have been reported. Intestinal and renal DDP IV is involved in the final digestion of proline-containing oligopeptides and in the absorption of their fragments (Brandsch et al., 1995). DPP IV also acts as a costimulatory and a binding protein.
DPP IV has been shown to be identical to the cell-surface marker CD26 (thymocyte-activating molecule THAM is the mouse homologue; 2B9 antigen) that is expressed on a fraction of resting T cells at low density but strongly up-regulated following T cell activation (Dang 2002, Gorrell 2001, Morimoto 1998, Reinhold 2002, von Bonin 1998). CD26⧸DPP IV can deliver a potent costimulatory activation signal into T cells despite its small cytoplasmic region of only six amino acids. Signaling is dependent on its interaction with other molecules. It associates, presumably through its extracellular domain, with CD45, a protein tyrosine phosphatase, and with adenosine deaminase (ADA, EC 3.5.4.4), an enzyme involved in the salvage of purine nucleosides, which catalyzes the deamination of adenosine and 2′-deoxyadenosine to inosine and 2′-deoxyinosine and whose deficiency causes severe combined immunodeficiency disease (SCID) in humans (DPP IV = adenosine deaminase complexing protein = ADAbp). Moreover, CD26 signaling is dependent on the expression of the T cell receptor (TCR) complex.
For tumor cells, endothelial cells, and trophoblasts, DPP IV expression is associated with a lower invasive or metastatic potential (Cheng 1998, Kajiyama 2002, Sato 2002, Shingu 2003, Wesley 1999). It could be that this effect is attributable to interaction with extracellular matrix substances, e.g., collagens I and III to which DPP IV binds via its cysteine-rich domain, not by its catalytic site, or fibronectin (Löster et al., 1995). The dual function of DPP IV as a protease and a binding⧸stimulatory molecule is sustained by structural homologous proteins for which either one or the other function has been reported (see below).
7. DPP IV-Related Proteins
In the past years several DPP IV activity and⧸or structure homologues (DASH proteins) have been discovered (Sedo and Malik, 2001). Some of them appear to be rediscovered proteins with new names.
The following activity homologues also cleave chromogenic⧸fluorogenic DPP IV substrates such as Gly-Pro-4-nitroaniline, but are soluble enzymes.
Dipeptidyl peptidase II (DPP II, EC 3.4.14.2) appears to be identical (Leiting et al., 2003) to quiescent cell proline peptidase (QPP) and dipeptidyl peptidase 7 (DPP 7⧸DPP VII). DPP II is a soluble lysosomal protein of 110 kDa consisting of two 53-kDa subunits and cleaves at acidic pH only short Xaa-Pro⧸Ala peptides (Mentlein and Struckhoff, 1989).
Dipeptidyl peptidase 8 (DPP8) is a cytosolic 82-kDa protein active at neutral pH with DPP IV activity (Abbott et al., 2000). It might be identical to dipeptidyl peptidase IV-β.
Dipeptidyl peptidase 9 (DPP9) is related to DPP 8, a 98-kDa cytosolic protein with DPP IV activity (Olsen and Wagtmann, 2002).
The following structure homologues are cell-surface proteins, but they either lack enzymatic activity or exhibit a different spectrum. Attractin (mahogany homolog, DPPT-L) is a 175-kDa type I transmembrane protein from which four secreted isoforms are produced by alternative splicing (Gunn et al., 1999). It is expressed and secreted by activated T cells and at low levels by other tissues. Attractin has probably low DPP IV activity, but whether it also cleaves the same peptides has not been thoroughly investigated. The protein is supposed to be involved in initial immune cell clustering during the inflammatory response and may regulate the chemotactic activity of chemokines. Furthermore, it may play a role in melanocortin signaling pathways that regulate energy homeostasis and hair color.
Fibroblast activation protein α (FAP, seprase, 3.4.21–) is a type II transmembrane protein of 97-kDa subunits that has about 52% homology (human sequences) to DPP IV but acts mainly as an endopeptidase on extracellular matrix protein (Table V; Barrett 1998, Goldstein 1997, Scanlan 1994). In normal tissues, FAP is found only in fetal mesenchymal cells and a subset of glucagon-producing cells (A cells) in pancreatic islets. However, activated fibroblasts, either cultivated, in wounds or in tumors, cultivated melanocytes, and sarcoma cells express FAP. The biological function has not been clarified, but it is suspected that FAP is involved in wound healing. Due to its selective occurrence, FAP is a good target for tumor immunodetection and destructive tumor therapy. DPP IV and FAP reside on a neighbor gene locus (Table V) and may therefore originate by gene duplication. They can form homodimers, but usually do not occur together.
Glutamate carboxypeptidase II (GCPII, EC 3.4.17.21) or N-acetylated α-linked acidic dipeptidase (NAALADase I, also folate hydrolase, pteroylpoly-γ-glutamate carboxypeptidase) is a type II transmembrane protein of 750 amino acids and about 84 kDa (Barrett et al., 1998). It releases unsubstituted, C-terminal glutamyl residues from Ac-Asp-Glu or pteroyl-γ; in particular it hydrolyzes γ-peptide bonds in Ac-Asp-Glu, Asp-Glu, and Glu-Glu, but also γ-glutamyl bonds in γ-Glu-Glu and folylpoly-γ-glutamates. According to more recent investigations it has no DPP IV activty (Barinka et al., 2002). The enzyme requires zink (2 per subunit), is activated by cobalt, and is inhibited by quisqualic acid, 2-(phosphonomethyl) pentanedioic acid (PMPA), and EDTA. Primary sources are prostate epithelium, as well as the small intestine (specifically in jejunum brush border membranes), brain (ventral striatum and brain stem), kidney, liver, spleen, colon, and the capillary endothelium of a variety of tumors. In the prostate, a cytosolic isoform termed PSMA' (produced by alternative splicing) is abundant in normal tissue; the membrane-bound PSMA-1 (prostate-specific membrane antigen, different from prostate-specific antigen PSA!) is found in primary prostate tumors. As physiological functions, GPPII⧸NAALADase I is required for the uptake of folate in the intestine, and in the brain it modulates excitatory neurotransmission through the hydrolysis of the neuropeptide N-acetylaspartylglutamate (NAAG), thereby releasing glutamate. PSMA is used as a diagnostic and prognostic indicator of prostate cancer and as a possible marker for various neurological disorders such as schizophrenia, Alzheimer's disease, and Huntington's disease. GPPII⧸NAALDase I inhibitors have analgetic (Yamamoto et al., 2001) and neuroprotective effects (Williams et al., 2001). NAALADase II and III are similar to the former enzyme (Pangalos et al., 1999).
Dipeptidylpeptidase-like protein (DPP6) exists in two alternative splice forms, DPPX-L and DPPX-S, and is a type II transmembrane protein (Wada et al., 1992). Due to the substitution of an aspartate residue for the serine residue in the proposed catalytic triad, it has no dipeptidyl aminopeptidase activity. DPP6 is expressed predominantly in the brain and may be involved in brain function.
Dipeptidyl peptidase 10 (DPP10) is another type II transmembrane protein of 796 amino acids with high (32%) homology to DPP IV (Qi et al., 2003). However, the active serine is replaced by a serine and therefore DPP10 lacks dipeptidyl peptidase activity. The protein is mainly expressed in brain and pancreas.
IV. Conclusions and Perspectives
Most cell-surface (oligo)peptidases have been initially characterized as digestive or clearing enzymes although they cleave only peptides upto 70–80 residues. They are now regarded as regulatory proteases that control the activation or inactivation of circulating hormones and neuropeptides. Consequently, some of them (ACE, NEP, ECE, DPP IV, and in the future probably APP, CPM, and others) are validated drug targets. Inhibitors (or peptidase-resistant analogues) are already widely used as drugs or are under development, mostly for hypertension and diabetes type 2. Future areas appear to be the treatment of pain or inflammatory diseases. Despite the widespread or proposed clinical use of inhibitors, little is known about defective mutations of cell-surface peptidase genes or about the regulation of their expression in disease.
Cell-surface peptidases have also attracted attention in diagnosis, especially for the differentiation of hematopoietic cells and leukemias, although their distribution is more widespread. Endothelial cells are frequent localizations corresponding to their role of inactivation enzymes for hormones. Here, more work is needed to explore their expression in different types of vessels and their probable functional or diagnostic role in angiogenesis.
Research in the past years has made it evident that the integral membrane-bound peptidases belong to gene families that include soluble, often similar peptidases, as well as further integral membrane proteins that are either proteases of different specificity or act as binding proteins. Similar, nonenzymatic functions as binding proteins have also been discovered for some cell-surface peptidases, and there is some evidence that they participate in cell invasion, adherence, and even in signal transduction of other receptor proteins. It appears that these nonenzymatic functions are mostly not related to the catalytic site and other domains of the molecules are involved. Future research is needed to identify further binding targets and especially the physiological relevance of these interactions.
In conclusion, cell-surface peptidases are the key regulators of the inactivation or activation of hormones and neuropeptides. Although most are peptide bond specific, not peptide specific, inhibitors for some of them have become or will become drugs. Also, knowledge concerning their specificity and action is important for the design of peptidase-resistant regulatory peptides. An emerging field of research is their role as binding proteins, especially in cell adherence or invasion.
Footnotes
Dedicated to Professor Dr. Eberhard Heymann on the occasion of his 65th birthday.
References
References
- Abbott C.A., Baker E., Sutherland G.R., McCaughan G.W. Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics. 1994;40:331–338. doi: 10.1007/BF01246674. [DOI] [PubMed] [Google Scholar]
- Abbott C.A., Yu D.M., Woollatt E., Sutherland G.R., McCaughan G.W., Gorrell M.D. Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8. Eur. J. Biochem. 2000;267:6140–6150. doi: 10.1046/j.1432-1327.2000.01617.x. [DOI] [PubMed] [Google Scholar]
- Ahrén B., Simonsson E., Larsson H., Landin-Olsson M., Torgeirsson H., Jansson P.A., Sandqvist M., Bavenholm P., Efendic S., Eriksson J.W., Dickinson S., Holmes D. Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4-week study period in type 2 diabetes. Diabetes Care. 2002;25:869–875. doi: 10.2337/diacare.25.5.869. [DOI] [PubMed] [Google Scholar]
- Antczak C., De Meester I., Bauvois B. Ectopeptidases in pathophysiology. BioEssays. 2001;23:251–260. doi: 10.1002/1521-1878(200103)23:3<251::AID-BIES1035>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antczak C., De Meester I., Bauvois B. Transmembrane proteases as disease markers and targets for therapy. J. Biol. Regul. Homeost. Agents. 2001;15:130–139. [PubMed] [Google Scholar]
- Atherton A.J., Monaghan P., Warburton M.J., Robertson D., Kenny A.J., Gusterson B.A. Dipeptidyl peptidase-IV expression identifies a functional sub-population of breast fibroblasts. Int. J. Cancer. 1992;50:15–19. doi: 10.1002/ijc.2910500105. [DOI] [PubMed] [Google Scholar]
- Augustyns K., Van der Veken P., Senten K., Haemers A. Dipeptidyl peptidase IV inhibitors as new therapeutic agents for the treatment of type 2 diabetes. Expert Opin. Ther. Patents. 2003;13:499–510. [Google Scholar]
- Barinka C., Rinnova M., Sacha P., Rojas C., Majer P., Slusher B.S., Konvalinka J. Substrate specificity, inhibition and enzymological analysis of recombinant human glutamate carboxypeptidase II. J. Neurochem. 2002;80:477–487. doi: 10.1046/j.0022-3042.2001.00715.x. [DOI] [PubMed] [Google Scholar]
- Barrett A.J. Classification of peptidases. Methods Enzymol. 1994;244:1–15. doi: 10.1016/0076-6879(94)44003-4. [DOI] [PubMed] [Google Scholar]
- Barrett A.J., McDonald J.K. “Mammalian Proteases—A Glossary and Bibliography, Vol. 1 Endopeptidases.”. Academic Press; London: 1980. [Google Scholar]
- Barrett A.J., Rawlings N.D., Woessner J.F. “Handbook of Proteolytic Enzymes.”. Academic Press; Amsterdam: 1998. [Google Scholar]
- Bauer K. Adenohypophyseal degradation of thyrotropin releasing hormone regulated by thyroid hormones. Nature. 1987;330:375–377. doi: 10.1038/330375a0. [DOI] [PubMed] [Google Scholar]
- Bauer K. Purification and characterization of the thyrotropin-releasing-hormone-degrading ectoenzyme. Eur. J. Biochem. 1994;224:387–396. doi: 10.1111/j.1432-1033.1994.00387.x. [DOI] [PubMed] [Google Scholar]
- Bauer K., Carmeliet P., Schulz M., Baes M., Denef C. Regulation and cellular localization of the membrane-bound thyrotropin-releasing hormone-degrading enzyme in primary cultures of neuronal, glial and adenohypophyseal cells. Endocrinology. 1990;127:1224–1233. doi: 10.1210/endo-127-3-1224. [DOI] [PubMed] [Google Scholar]
- Bausback H.H., Churchill L., Ward P.E. Angiotensin metabolism by cerebral microvascular aminopeptidase A. Biochem. Pharmacol. 1988;37:155–160. doi: 10.1016/0006-2952(88)90712-5. [DOI] [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping of the active site of papain with the aid of peptide substrates and inhibitors. Philos. Trans. R. Soc. Lond. B. 1970;257:249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Bermpohl F., Löster K., Reutter W., Braun O. Rat dipeptidyl peptidase IV (DPP IV) exhibits endopeptidase activity with specificity for denatured fibrillar collagens. FEBS Lett. 1998;428:152–156. doi: 10.1016/s0014-5793(98)00515-8. [DOI] [PubMed] [Google Scholar]
- Blau N., Niederwieser A., Shmerling D.H. Peptiduria presumably caused by aminopeptidase-P deficiency. A new inborn error of metabolism. J. Inherit. Metab. Dis. 1988;11(Suppl. 2):240–242. doi: 10.1007/BF01804246. [DOI] [PubMed] [Google Scholar]
- Böhm S.K., Gum J.R., Erickson R.H., Hicks J.W., Kim Y.S. Human dipeptidyl peptidase IV gene promoter: Tissue-specific regulation from a TATA-less GC-rich sequence characteristic of a housekeeping gene promoter. Biochem. J. 1995;311:835–843. doi: 10.1042/bj3110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongers J., Lambros T., Ahmad M., Heimer E.P. Kinetics of dipeptidyl peptidase IV proteolysis of growth hormone-releasing factor and analogues. Biochim. Biophys. Acta. 1992;1122:147–153. doi: 10.1016/0167-4838(92)90317-7. [DOI] [PubMed] [Google Scholar]
- Boulanger L., Roughly P., Gaudreau P. Catabolism of rat growth hormone-releasing factor(1-29) amide in rat serum and liver. Peptides. 1992;13:681–689. doi: 10.1016/0196-9781(92)90173-z. [DOI] [PubMed] [Google Scholar]
- Bouras M., Huneau J.F., Luengo C., Erlanson-Albertsson C., Tomé D. Metabolism of enterostatin in rat intestine, brain membranes, and serum: Differential involvement of proline-specific peptidases. Peptides. 1995;16:399–405. doi: 10.1016/0196-9781(94)00213-p. [DOI] [PubMed] [Google Scholar]
- Brandsch M., Ganpathy V., Leibach F.H. Role of dipeptidyl peptidase IV (DP IV) in intestinal and renal absorption of peptides. In: Fleischer B., editor. “Dipeptidyl Peptidase IV (CD26) in Metabolism and the Immune Response”. R. G. Landes Co; Austin, TX: 1995. pp. 111–130. [Google Scholar]
- Burcelin R., Dolci W., Thorens B. Long-lasting antidiabetic effect of a dipeptidyl peptidase IV-resistant analog of glucagon-like peptide-1. Metabolism. 1999;48:252–258. doi: 10.1016/s0026-0495(99)90043-4. [DOI] [PubMed] [Google Scholar]
- Cheng H.C., Abdel-Ghany M., Elble R.C., Pauli B.U. Lung endothelial dipeptidyl peptidase IV promotes adhesion and metastasis of rat breast cancer cells via tumor cell surface-associated fibronectin. J. Biol. Chem. 1998;273:24207–24215. doi: 10.1074/jbc.273.37.24207. [DOI] [PubMed] [Google Scholar]
- Christopherson K.W., II, Hangoc G., Broxmeyer H.E. Cell surface peptidase CD26⧸dipeptidylpeptidase IV regulates CXCL12⧸stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J. Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- Conarello S.L., Li Z., Ronan J., Roy R.S., Zhu L., Jiang G., Liu F., Woods J., Zycband E., Moller D.E., Thornberry N.A., Zhang B.B. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc. Natl. Acad. Sci. USA. 2003;100:6825–6830. doi: 10.1073/pnas.0631828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvol P., Williams T.A., Soubrier F. Peptidyl dipeptidase A: Angiotensin I-converting enzyme. Methods Enzymol. 1995;248:283–305. doi: 10.1016/0076-6879(95)48020-x. [DOI] [PubMed] [Google Scholar]
- Cottrell G.S., Hooper N.M., Turner A.J. Cloning, expression, and characterization of human cytosolic aminopeptidase P: A single manganese(II)-dependent enzyme. Biochemistry. 2000;39:15121–15128. doi: 10.1021/bi001585c. [DOI] [PubMed] [Google Scholar]
- Danilczyk U., Eriksson U., Crackower M.A., Penninger J.M. A story of two ACEs. J. Mol. Med. 2003;81:227–234. doi: 10.1007/s00109-003-0419-x. [DOI] [PubMed] [Google Scholar]
- Dang N.H., Morimoto C. CD26: An expanding role in immune regulation and cancer. Histol. Histopathol. 2002;17:1213–1226. doi: 10.14670/HH-17.1213. [DOI] [PubMed] [Google Scholar]
- David F., Bernard A.M., Pierres M., Marguet D. Identification of serine624, aspartic acid702, and histidine734 as the catalytic triade residues of mouse dipeptidyl peptidase IV (CD26). A member of a novel family of nonclassical serine hydrolases. J. Biol. Chem. 1993;268:17247–17252. [PubMed] [Google Scholar]
- Deacon C.F., Johnson A.H., Holst J.J. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J. Clin. Endocrinol. Metab. 1995;80:952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- Deacon C.F., Nauck M.A., Toft-Nielsen M., Pridal L., Willms B., Holst J.J. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- Deacon C.F., Hughes T.E., Holst J.J. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes. 1998;47:764–769. doi: 10.2337/diabetes.47.5.764. [DOI] [PubMed] [Google Scholar]
- Deacon C.F., Knudsen L.B., Madsen K., Wiberg F.C., Jacobsen O., Holst J.J. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologica. 1998;41:271–278. doi: 10.1007/s001250050903. [DOI] [PubMed] [Google Scholar]
- Deacon C.F., Nauck M.A., Meier J., Hucking K., Holst J.J. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J. Clin. Endocrinol. Metab. 2000;85:3575–3581. doi: 10.1210/jcem.85.10.6855. [DOI] [PubMed] [Google Scholar]
- Deacon C.F., Danielsen P., Klarskov L., Olesen M., Holst J.J. Dipeptidyl peptidase IV inhibition reduces the degradation and clearance of GIP and potentiates its insulinotropic and antihyperglycemic effects in anesthetized pigs. Diabetes. 2001;50:1588–1597. doi: 10.2337/diabetes.50.7.1588. [DOI] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L'Haridon R., Vogel L.K., Sjostrom H., Noren O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meester I., Korom S., Van Damme J., Scharpe S. CD26, let it cut or cut it down. Immunol. Today. 1999;20:367–375. doi: 10.1016/s0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- de Saint-Vis B., Cupillard L., Pandrau-Garcia D., Ho S., Renard N., Grouard G., Duvert V., Thomas X., Galizzi J.P., Banchereau J. Distribution of carboxypeptidase M on lymphoid and myeloid cells parallels the other zinc-dependent proteases CD10 and CD13. Blood. 1995;86:1098–1105. [PubMed] [Google Scholar]
- Dimitrijevic M., Stanojevic S., Vujic V., Kovacevic-Jovanovic V., Beck-Sickinger A., Demuth H., von Hörsten S. Effect of neuropeptide Y on inflammatory paw edema in the rat: Involvement of peripheral NPY Y1 and Y5 receptors and interaction with dipeptidyl-peptidase IV (CD26) J. Neuroimmunol. 2002;129:35–42. doi: 10.1016/s0165-5728(02)00173-x. [DOI] [PubMed] [Google Scholar]
- Drucker D.J. Glucagon-like peptides. Diabetes. 1998;47:159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- Drucker D.J. Glucagon-like peptides: Regulators of cell proliferation, differentiation, and apoptosis. Mol. Endocrinol. 2003;17:161–171. doi: 10.1210/me.2002-0306. [DOI] [PubMed] [Google Scholar]
- Drucker D.J. Therapeutic potential of dipeptidyl peptidase IV inhibitors for the treatment of type 2 diabetes. Expert Opin. Investig. Drugs. 2003;12:87–100. doi: 10.1517/13543784.12.1.87. [DOI] [PubMed] [Google Scholar]
- Drucker D.J., DeForest L., Brubaker P.L. Intestinal response to growth factors administered alone or in combination with human [Gly2]glucagon-like peptide 2. Am. J. Physiol. 1997;273:G1252–G1262. doi: 10.1152/ajpgi.1997.273.6.G1252. [DOI] [PubMed] [Google Scholar]
- Drucker D.J., Shi Q., Crivici A., Sumner-Smith M., Tavers W., Hill M., DeForest L., Cooper S., Brubaker P.L. Regulation of the biological activity of glucagon-like peptide 2 in vivo by dipeptidyl peptidase IV. Nature Biotechnol. 1997;15:673–677. doi: 10.1038/nbt0797-673. [DOI] [PubMed] [Google Scholar]
- Durinx C., Lambeir A.M., Bosmans E., Falmagne J.B., Berghmans R., Haemers A., Scharpe S., De Meester I. Molecular characterization of dipeptidyl peptidase activity in serum: Soluble CD26⧸dipeptidyl peptidase IV is responsible for the release of X-Pro dipeptides. Eur. J. Biochem. 2000;267:5608–5613. doi: 10.1046/j.1432-1327.2000.01634.x. [DOI] [PubMed] [Google Scholar]
- Eberlein G.A., Eysselein V.E., Schaeffer M., Layer P., Grandt D., Goebell H., Niebel W., Davis M., Lee T.D., Shively J.E., Reeve J.R. A new molecular form of PYY: Structural characterization of human PYY(3-36) and PYY(1-36) Peptides. 1989;10:797–803. doi: 10.1016/0196-9781(89)90116-2. [DOI] [PubMed] [Google Scholar]
- Engel M., Hoffmann T., Wagner L., Wermann M., Heiser U., Kiefersauer R., Huber R., Bode W., Demuth H.U., Brandstetter H. The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc. Natl. Acad. Sci. USA. 2003;100:5063–5068. doi: 10.1073/pnas.0230620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson R.H., Song I.S., Yoshioka M., Gulli R., Miura S., Kim Y.S. Identification of proline-specific carboxypeptidase localized to brush border membrane of rat small intestine and its possible role in protein digestion. Dig. Dis. Sci. 1989;34:400–406. doi: 10.1007/BF01536262. [DOI] [PubMed] [Google Scholar]
- Essler M., Ruoslahti E. Molecular specialization of breast vasculature: A breast-homing phage-displayed peptide binds to aminopeptidase P in breast vasculature. Proc. Natl. Acad. Sci. USA. 2002;99:2252–2257. doi: 10.1073/pnas.251687998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esther C.R., Jr., Howard T.E., Marino E.M., Goddard J.M., Capecchi M.R., Bernstein K.E. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab. Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- Fersht A. “Enzyme Structure and Mechanism,”. 2nd ed. W. H. Freeman and Company; New York: 1985. pp. 105–106. [Google Scholar]
- Frohman L.A., Downs T.R., Heimer E.P., Felix A.M. Dipeptidyl peptidase IV and trypsin-like enzymatic degradation of human growth hormone-releasing hormone in plasma. J. Clin. Invest. 1989;83:1533–1540. doi: 10.1172/JCI114049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Tsuji E., Misumii Y., Takami N., Ikehara Y. Selective cell-surface expression of dipeptidyl peptidase IV with mutations at the active site sequence. Biochem. Biophys. Res. Commun. 1992;185:776–784. doi: 10.1016/0006-291x(92)91693-k. [DOI] [PubMed] [Google Scholar]
- Gault V.A., Parker J.C., Harriott P., Flatt P.R., O'Harte F.P. Evidence that the major degradation product of glucose-dependent insulinotropic polypeptide, GIP(3-42), is a GIP receptor antagonist in vivo. J. Endocrinol. 2002;175:525–533. doi: 10.1677/joe.0.1750525. [DOI] [PubMed] [Google Scholar]
- Gerald C., Walker M.W., Criscione L., Gustafson E.L., Batzl-Hartmann C., Smith K.E., Vaysse P., Durkin M.M., Laz T.M., Linemeyer D.L., Schaffhauser A.O., Whitebread S., Hofbauer K.G., Taber R.I., Branchek T.A., Weinshank R.L. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168–171. doi: 10.1038/382168a0. [DOI] [PubMed] [Google Scholar]
- Ghersi G., Chen W., Lee E.W., Zukowska Z. Critical role of dipeptidyl peptidase IV in neuropeptide Y-mediated endothelial cell migration in response to wounding. Peptides. 2001;22:453–458. doi: 10.1016/s0196-9781(01)00340-0. [DOI] [PubMed] [Google Scholar]
- Goldstein L.A., Ghersi G., Pineiro-Sanchez M.L., Salamone M., Yeh Y., Flessate D., Chen W.T. Molecular cloning of seprase: A serine integral membrane protease from human melanoma. Biochim. Biophys. Acta. 1997;1361:11–19. doi: 10.1016/s0925-4439(97)00032-x. [DOI] [PubMed] [Google Scholar]
- Gorrell M.D., Gysbers V., McCaughan G.W. CD26: A multifunctional integral membrane and secreted protein of activated lymphocytes. Scand. J. Immunol. 2001;54:249–264. doi: 10.1046/j.1365-3083.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- Grandt D., Schimiczek M., Beglinger C., Layer P., Goebell H., Eysselein V.E., Reeve J.R. Two molecular forms of peptide YY (PYY) are abundant in human blood: Characterization of a radioimmunoassay recognizing PYY 1-36 and PYY 3-36. Regul. Peptides. 1994;51:151–159. doi: 10.1016/0167-0115(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Grandt D., Schimiczek M., Rascher W., Feth F., Shively J., Lee T.D., Davis M.T., Reeve J.R., Michel M.C. Neuropeptide Y 3-36 is an endogenous ligand selective for Y2 receptors. Regul. Peptides. 1996;67:33–37. doi: 10.1016/s0167-0115(96)00104-8. [DOI] [PubMed] [Google Scholar]
- Grouzmann E., Monod M., Landis B., Wilk S., Brakch N., Nicoucar K., Giger R., Malis D., Szalay-Quinodoz I., Cavadas C., Morel D.R., Lacroix J.S. Loss of dipeptidylpeptidase IV activity in chronic rhinosinusitis contributes to the neurogenic inflammation induced by substance P in the nasal mucosa. FASEB J. 2002;16:1132–1134. doi: 10.1096/fj.01-0939fje. [DOI] [PubMed] [Google Scholar]
- Gunn T.M., Miller K.A., He L., Hyman R.W., Davis R.W., Azarani A., Schlossman S.F., Duke-Cohan J.S., Barsh G.S. The mouse mahogany locus encodes a transmembrane form of human attractin. Nature. 1999;398:152–156. doi: 10.1038/18217. [DOI] [PubMed] [Google Scholar]
- Habib G.M., Shi Z.Z., Cuevas A.A., Guo Q., Matzuk M.M., Lieberman M.W. Leukotriene D4 and cystinyl-bis-glycine metabolism in membrane-bound dipeptidase-deficient mice. Proc. Natl. Acad. Sci. USA. 1998;95:4859–4863. doi: 10.1073/pnas.95.9.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib G.M., Shi Z.Z., Cuevas A.A., Lieberman M.W. Identification of two additional members of the membrane-bound dipeptidase family. FASEB J. 2003;17:1313–1315. doi: 10.1096/fj.02-0899fje. [DOI] [PubMed] [Google Scholar]
- Harbeck H.T., Mentlein R. Aminopeptidase P from rat brain. Purification and action on bioactive peptides. Eur. J. Biochem. 1991;198:451–458. doi: 10.1111/j.1432-1033.1991.tb16035.x. [DOI] [PubMed] [Google Scholar]
- Hartmann B., Thulesen J., Kissow H., Thulesen S., Orskov C., Ropke C., Poulsen S.S., Holst J.J. Dipeptidyl peptidase IV inhibition enhances the intestinotrophic effect of glucagon-like peptide-2 in rats and mice. Endocrinology. 2000;141:4013–4020. doi: 10.1210/endo.141.11.7752. [DOI] [PubMed] [Google Scholar]
- Hedeager-Sørensen S., Kenny A.J. Proteins of the kidney microvillar membrane. Purification and properties of carboxypeptidase P from pig kidneys. Biochem. J. 1985;229:251–257. doi: 10.1042/bj2290251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann E., Mentlein R. Liver dipeptidyl aminopeptidase IV hydrolyzes substance P. FEBS Lett. 1978;91:360–364. doi: 10.1016/0014-5793(78)81210-1. [DOI] [PubMed] [Google Scholar]
- Heymann E., Mentlein R. Complementary action of dipeptidyl peptidase IV and aminopeptidase M in the digestion of beta-casein. J. Dairy Res. 1986;53:229–236. doi: 10.1017/s0022029900024833. [DOI] [PubMed] [Google Scholar]
- Hinke S.A., Pospisilik J.A., Demuth H.U., Mannhart S., Kühn-Wache K., Hoffmann T., Nishimura E., Pederson R.A., McIntosh C.H. Dipeptidyl peptidase IV (DPIV⧸CD26) degradation of glucagon. Characterization of glucagon degradation products and DPIV-resistant analogs. J. Biol. Chem. 2000;275:3827–3834. doi: 10.1074/jbc.275.6.3827. [DOI] [PubMed] [Google Scholar]
- Hinke S.A., Gelling R.W., Pederson R.A., Manhart S., Nian C., Demuth H.U., McIntosh C.H. Dipeptidyl peptidase IV-resistant [D-Ala(2)]glucose-dependent insulinotropic polypeptide (GIP) improves glucose tolerance in normal and obese diabetic rats. Diabetes. 2002;51:652–661. doi: 10.2337/diabetes.51.3.652. [DOI] [PubMed] [Google Scholar]
- Hofstra R.M., Valdenaire O., Arch E., Osinga J., Kroes H., Loffler B.M., Hamosh A., Meijers C., Buys C.H. A loss-of-function mutation in the endothelin-converting enzyme 1 (ECE-1) associated with Hirschsprung disease, cardiac defects, and autonomic dysfunction. Am. J. Hum. Genet. 1999;64:304–308. doi: 10.1086/302184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J.J. Therapy of type 2 diabetes mellitus based on the actions of glucagon-like peptide-1. Diabetes Metab. Res. Rev. 2002;18:430–441. doi: 10.1002/dmrr.328. [DOI] [PubMed] [Google Scholar]
- Holst J.J., Deacon C.F. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998;47:1663–1670. doi: 10.2337/diabetes.47.11.1663. [DOI] [PubMed] [Google Scholar]
- Hopsu-Havu V.K., Glenner G.G. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-β-naphthylamide. Histochemie. 1966;7:197–201. doi: 10.1007/BF00577838. [DOI] [PubMed] [Google Scholar]
- Hooper N. Families of zinc metalloproteases. FEBS Lett. 1994;354:1–6. doi: 10.1016/0014-5793(94)01079-x. [DOI] [PubMed] [Google Scholar]
- Hooper N.M., Hryszko J., Turner A.J. Purification and characterization of pig kidney aminopeptidase P. A glycosyl-phosphatidylinositol-anchored ectoenzyme. Biochem. J. 1990;267:509–515. doi: 10.1042/bj2670509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper N.M., Hryszko J., Oppong S.Y., Turner A.J. Inhibition by converting enzyme inhibitors of pig kidney aminopeptidase P. Hypertension. 1992;19:281–285. doi: 10.1161/01.hyp.19.3.281. [DOI] [PubMed] [Google Scholar]
- Hooper N.M., Karran E.H., Turner A.J. Membrane protein secretases. Biochem. J. 1997;321:265–279. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde R., Hooper N.M., Turner A.J. Molecular cloning and expression in COS-1 cells of pig kidney aminopeptidase P. Biochem. J. 1996;319:197–201. doi: 10.1042/bj3190197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y., Nagase H. Matrix metalloproteinases in cancer. Essays Biochem. 2002;38:21–36. doi: 10.1042/bse0380021. [DOI] [PubMed] [Google Scholar]
- Iwaki-Egawa S., Watanabe Y., Kikuya Y., Fujimoto Y. Dipeptidyl peptidase IV from human serum: Purification, characterization, and N-terminal amino acid sequence. J. Biochem. (Tokyo) 1998;124:428–433. doi: 10.1093/oxfordjournals.jbchem.a022130. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Carlquist M., Kwauk S., Otte S.C., McIntosh C.H.S., Brown J.C., Mutt V. Amino acid sequence and heterogeneity of the gastric inhibitory polypeptide (GIP) FEBS Lett. 1981;123:205–210. doi: 10.1016/0014-5793(81)80288-8. [DOI] [PubMed] [Google Scholar]
- Kähne T., Lendeckel U., Wrenger S., Neubert K., Ansorge S., Reinhold D. Dipeptidyl peptidase IV: A cell surface peptidase involved in regulating T cell growth. Int. J. Mol. Med. 1999;4:3–15. doi: 10.3892/ijmm.4.1.3. [DOI] [PubMed] [Google Scholar]
- Kajiyama H., Kikkawa F., Suzuki T., Shibata K., Ino K., Mizutani S. Prolonged survival and decreased invasive activity attributable to dipeptidyl peptidase IV overexpression in ovarian carcinoma. Cancer Res. 2002;62:2753–2757. [PubMed] [Google Scholar]
- Kato T., Nagatsu T., Fukasawa K., Harada M., Nagatsu I., Sakakibara S. Successive cleavage of N-terminal Arg1-Pro2 and Lys3-Pro4 from substance P but no release of Arg1-Pro2 from bradykinin, by X-Pro dipeptidyl aminopeptidase. Biochim. Biophys. Acta. 1978;525:417–422. doi: 10.1016/0005-2744(78)90237-1. [DOI] [PubMed] [Google Scholar]
- Kenny A.J. Proteinases associated with cell membranes. In: Barrett A.J., editor. “Proteinases in Mammalian Cells and Tissues”. Elsevier; Amsterdam: 1977. pp. 393–444. [Google Scholar]
- Kenny A.J., Hooper N.M. Peptidases involved in the metabolism of bioactive peptides. In: Henrickson J.H., editor. “Degradation of Bioactive Substances: Physiology and Pathophysiology”. CRC Press; Boca Raton, FL: 1991. pp. 47–79. [Google Scholar]
- Kenny A.J., Stephenson S.L., Turner A.J. Cell surface peptidases. In: Kenny A.J., Turner A.J., editors. “Mammalian Ectoenzymes”. Elsevier; Amsterdam: 1987. pp. 169–210. [Google Scholar]
- Kieffer T.J., McIntosh C.H.S., Pederson R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Kumar S., Simmons W.H., Brown N.J. Inhibition of aminopeptidase P potentiates wheal response to bradykinin in angiotensin-converting enzyme inhibitor-treated humans. J. Pharmacol. Exp. Ther. 2000;292:295–298. [PubMed] [Google Scholar]
- Kubiak T.M., Friedman A.R. 1998. Stabilized potent GRF analogs. United States Patent 5,756,458. [Google Scholar]
- Kubiak T.M., Kelly C.R., Kabrill L.F. In vitro metabolic degradation of a bovine growth-hormone-releasing factor analog Leu27-bGRF(1-29)NH2 in bovine and porcine plasma. Drug. Metab. Dispos. 1989;17:393–397. [PubMed] [Google Scholar]
- Lambeir A.M., Durinx C., Proost P., Van Damme J., Scharpé S., De Meester I. Kinetic study of the processing by dipeptidyl-peptidase IV⧸CD26 of neuropeptides involved in pancreatic insulin secretion. FEBS Lett. 2001;507:327–330. doi: 10.1016/s0014-5793(01)02982-9. [DOI] [PubMed] [Google Scholar]
- Lambeir A.M., Proost P., Durinx C., Bal G., Senten K., Augustyns K., Scharpé S., Van Damme J., De Meester I. Kinetic investigation of chemokine truncation by CD26⧸dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J. Biol. Chem. 2001;276:29839–29845. doi: 10.1074/jbc.M103106200. [DOI] [PubMed] [Google Scholar]
- Lambeir A.M., Proost P., Scharpé S., De Meester I. A kinetic study of glucagon-like peptide-1 and glucagon-like peptide-2 truncation by dipeptidyl peptidase IV, in vitro. Biochem. Pharmacol. 2002;64:1753–1756. doi: 10.1016/s0006-2952(02)01415-6. [DOI] [PubMed] [Google Scholar]
- Leiting B., Pryor K.D., Wu J.K., Marsilio F., Patel R.A., Craik C.S., Ellman J.A., Cummings R.T., Thornberry N. Catalytic properties and inhibition of proline-specific dipeptidyl peptidases II, IV and VII. Biochem. J. 2003;371:525–532. doi: 10.1042/BJ20021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendeckel U., Arndt M., Bukowska A., Tadje J., Wolke C., Kähne T., Neubert K., Faust J., Ittenson A., Ansorge S., Reinhold D. Synergistic action of DPIV and APN in the regulation of T cell function. Adv. Exp. Med. Biol. 2003;524:123–131. doi: 10.1007/0-306-47920-6_16. [DOI] [PubMed] [Google Scholar]
- Lin Q., Taniuchi I., Kitamura D., Wang J.Y., Kearney J.F., Watanabe T., Cooper M.D. T and B cell development in BP-1⧸6C3⧸aminopeptidase A-deficient mice. J. Immunol. 1998;160:4681–4687. [PubMed] [Google Scholar]
- Löster K., Zeilinger K., Reutter W. The cysteine-rich region of dipeptidyl peptidase IV (CD26) is the collagen-binding site. Biochem. Biophys. Res. Commun. 1995;217:341–348. doi: 10.1006/bbrc.1995.2782. [DOI] [PubMed] [Google Scholar]
- Lowther W.T., Matthews B.W. Metalloaminopeptidases: Common functional themes in disparate structural surroundings. Chem. Rev. 2002;102:4581–4608. doi: 10.1021/cr0101757. [DOI] [PubMed] [Google Scholar]
- Lu B., Gerard N.P., Kolakowski L.F., Jr., Bozza M., Zurakowski D., Finco O., Carroll M.C., Gerard C. Neutral endopeptidase modulation of septic shock. J. Exp. Med. 1995;181:2271–2275. doi: 10.1084/jem.181.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucius R., Sievers J., Mentlein R. Enkephalin metabolism by microglial aminopeptidase N (CD13) J. Neurochem. 1995;64:1841–1847. doi: 10.1046/j.1471-4159.1995.64041841.x. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Schiemann F., Mentlein R., Lindner B., Brandt E. Dipeptidyl peptidase IV (CD26) on T cells cleaves the CXC chemokine CXCL11 (I-TAC) and abolishes the stimulating but not the desensitizing potential of the chemokine. J. Leukoc. Biol. 2002;72:183–191. [PubMed] [Google Scholar]
- Marguet D., Baggio L., Kobayashi T., Bernard A.M., Pierres M., Nielsen P.F., Ribel U., Watanabe T., Drucker D.J., Wagtmann N. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc. Natl. Acad. Sci. USA. 2000;97:6874–6879. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr R.A., Rockenstein E., Mukherjee A., Kindy M.S., Hersh L.B., Gage F.H., Verma I.M., Masliah E. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J. Neurosci. 2003;23:1992–1996. doi: 10.1523/JNEUROSCI.23-06-01992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensen I., Koolman J., Mentlein R. A proline-specific dipeptidyl peptidase from flies hydrolyzes the ecdysiostatic peptide Neb-TMOF (trypsin-modulating oostatic factor) Arch. Insect Biochem. Physiol. 1998;37:146–157. doi: 10.1002/(SICI)1520-6327(1998)37:2<146::AID-ARCH3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Martin R.A., Cleary D.L., Guido D.M., Zurcher-Neely H.A., Kubiak T.M. Dipeptidyl peptidase IV (DPP-IV) from pig kidney cleaves analogs of bovine growth hormone-releasing factor (bGRF) modified at position 2 with Ser, Thr or Val. Extended DPP-IV substrate specificity? Biochim. Biophys. Acta. 1993;1164:252–260. doi: 10.1016/0167-4838(93)90256-q. [DOI] [PubMed] [Google Scholar]
- McDonald J.K., Barrett A.J. “Mammalian Proteases: A Glossary and Bibliography, Vol. 2, Exopeptidases.”. Academic Press; London: 1986. [Google Scholar]
- Meier J.J., Nauck M.A., Schmidt W.E., Gallwitz B. Gastric inhibitory polypeptide: The neglected incretin revisited. Regul. Peptides. 2002;107:1–13. doi: 10.1016/s0167-0115(02)00039-3. [DOI] [PubMed] [Google Scholar]
- Menard J., Patchett A.A. Angiotensin-converting enzyme inhibitors. Adv. Protein Chem. 2001;56:13–75. doi: 10.1016/s0065-3233(01)56002-7. [DOI] [PubMed] [Google Scholar]
- Mentlein R. Proline residues in the maturation and degradation of peptide hormones and neuropeptides. FEBS Lett. 1988;234:251–256. doi: 10.1016/0014-5793(88)80092-9. [DOI] [PubMed] [Google Scholar]
- Mentlein R. Dipeptidyl-peptidase IV (CD26)—role in the inactivation of regulatory peptides. Regul. Peptides. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- Mentlein R., Lucius R. Methods for the investigation of neuropeptide catabolism and stability in vitro. Brain Res. Brain Res. Protoc. 1997;1:237–246. doi: 10.1016/s1385-299x(96)00035-9. [DOI] [PubMed] [Google Scholar]
- Mentlein R., Roos T. Proteases involved in the metabolism of angiotensin II, bradykinin, calcitonin gene-related peptide (CGRP), and neuropeptide Y by vascular smooth muscle cells. Peptides. 1996;17:709–720. doi: 10.1016/0196-9781(96)00066-6. [DOI] [PubMed] [Google Scholar]
- Mentlein R., Struckhoff G. Purification of two dipeptidyl aminopeptidases II from rat brain and their action on proline-containing neuropeptides. J. Neurochem. 1989;52:1284–1293. doi: 10.1111/j.1471-4159.1989.tb01877.x. [DOI] [PubMed] [Google Scholar]
- Mentlein R., Dahms P., Grandt D., Krüger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul. Peptides. 1993;49:133–144. doi: 10.1016/0167-0115(93)90435-b. [DOI] [PubMed] [Google Scholar]
- Mentlein R., Gallwitz B., Schmidt W.E. Dipeptidyl peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36) amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- Mentlein R., Schiemann F., Ludwig A., Brandt E. Modification of the biological activty of chemokines by dipeptidyl peptidase IV—a side effect in the use of inhibitors? Adv. Exp. Med. Biol. 2003;524:37–47. doi: 10.1007/0-306-47920-6_4. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Hayashi Y., Arakawa F., Ikehara Y. Molecular cloning and sequence analysis of human dipeptidyl peptidase-IV, a serine proteinase on the cell surface. Biochim. Biophys. Acta. 1992;1131:333–336. doi: 10.1016/0167-4781(92)90036-y. [DOI] [PubMed] [Google Scholar]
- Mitani H., Takimoto M., Kimura M. Dipeptidyl peptidase IV inhibitor NVP-DPP728 ameliorates early insulin response and glucose tolerance in aged rats but not in aged Fischer 344 rats lacking its enzyme activity. Jpn. J. Pharmacol. 2002;88:451–458. doi: 10.1254/jjp.88.451. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Goto K., Nomura S., Ino K., Goto S., Kikkawa F., Kurauchi O., Goldstein G., Tomoda Y. Possible action of human placental aminopeptidase N in feto-placental unit. Res. Commun. Chem. Pathol. Pharmacol. 1993;82:65–80. [PubMed] [Google Scholar]
- Morimoto C., Schlossman S.F. The structure and function of CD26 in the T-cell immune response. Immunol. Rev. 1998;161:55–70. doi: 10.1111/j.1600-065x.1998.tb01571.x. [DOI] [PubMed] [Google Scholar]
- Moss M.L., Lambert M.H. Shedding of membrane proteins by ADAM family proteases. Essays Biochem. 2002;38:141–153. doi: 10.1042/bse0380141. [DOI] [PubMed] [Google Scholar]
- Mussap C.J., Geraghty D.P., Burcher E. Tachykinin receptors: A radioligand binding perspective. J. Neurochem. 1993;60:1987–2009. doi: 10.1111/j.1471-4159.1993.tb03484.x. [DOI] [PubMed] [Google Scholar]
- Nagakura T., Yasuda N., Yamazaki K., Ikuta H., Yoshikawa S., Asano O., Tanaka I. Improved glucose tolerance via enhanced glucose-dependent insulin secretion in dipeptidyl peptidase IV-deficient Fischer rats. Biochem. Biophys. Res. Commun. 2001;284:501–506. doi: 10.1006/bbrc.2001.4999. [DOI] [PubMed] [Google Scholar]
- Natesh R., Schwager S.L., Sturrock E.D., Acharya K.R. Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature. 2003;421:551–554. doi: 10.1038/nature01370. [DOI] [PubMed] [Google Scholar]
- Nausch I., Mentlein R., Heymann E. The degradation of bioactive peptides and proteins by dipeptidyl peptidase IV from human placenta. Biol. Chem. Hoppe-Seyler. 1990;371:1113–1118. doi: 10.1515/bchm3.1990.371.2.1113. [DOI] [PubMed] [Google Scholar]
- Nichols T.C., Guthridge J.M., Karp D.R., Molina H., Fletcher D.R., Holers V.M. Gamma-glutamyl transpeptidase, an ecto-enzyme regulator of intracellular redox potential, is a component of TM4 signal transduction complexes. Eur. J. Immunol. 1998;28:4123–4129. doi: 10.1002/(SICI)1521-4141(199812)28:12<4123::AID-IMMU4123>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Nilsson C., Westman A., Blennow K., Ekman R. Processing of neuropeptide Y and somatostatin in human cerebrospinal fluid as monitored by radioimmunoassay and mass spectrometry. Peptides. 1998;19:1137–1146. doi: 10.1016/s0196-9781(98)00071-0. [DOI] [PubMed] [Google Scholar]
- Ogata S., Misumi Y., Tsuji E., Takami N., Oda K., Ikehara Y. Identification of the active site residues in dipeptidyl peptidase IV by affinity labelling and site-directed mutagenesis. Biochemistry. 1992;31:2582–2587. doi: 10.1021/bi00124a019. [DOI] [PubMed] [Google Scholar]
- Ohkubo I., Huang K., Ochiai Y., Takagaki M., Kani K. Dipeptidyl peptidase IV from porcine seminal plasma: Purification, characterization, and N-terminal amino acid sequence. J. Biochem. (Tokyo) 1994;116:1182–1186. doi: 10.1093/oxfordjournals.jbchem.a124647. [DOI] [PubMed] [Google Scholar]
- Olsen C., Wagtmann N. Identification and characterization of human DPP9, a novel homologue of dipeptidyl peptidase IV. Gene. 2002;299:185–193. doi: 10.1016/s0378-1119(02)01059-4. [DOI] [PubMed] [Google Scholar]
- Oravecz T., Pall M., Rodriquez G., Gorrell M.D., Ditto M., Nguyen N.Y., Boykins R., Unsworth E., Norcross M.A. Regulation of the receptor specificty and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J. Exp. Med. 1997;186:1865–1872. doi: 10.1084/jem.186.11.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orawski A.T., Simmons W.H. Purification and properties of membrane-bound aminopeptidase P from rat lung. Biochemistry. 1995;34:11227–11236. doi: 10.1021/bi00035a032. [DOI] [PubMed] [Google Scholar]
- Orawski A.T., Susz J.P., Simmons W.H. Aminopeptidase P from bovine lung: Solubilization, properties, and potential role in bradykinin degradation. Mol. Cell. Biochem. 1987;75:123–132. doi: 10.1007/BF00229900. [DOI] [PubMed] [Google Scholar]
- Pangalos M.N., Neefs J.M., Somers M., Verhasselt P., Bekkers M., van der Helm L., Fraiponts E., Ashton D., Gordon R.D. Isolation and expression of novel human glutamate carboxypeptidases with N-acetylated alpha-linked acidic dipeptidase and dipeptidyl peptidase IV activity. J. Biol. Chem. 1999;274:8470–8483. doi: 10.1074/jbc.274.13.8470. [DOI] [PubMed] [Google Scholar]
- Paolicchi A., Dominici S., Pieri L., Maellaro E., Pompella A. Glutathione catabolism as a signaling mechanism. Biochem. Pharmacol. 2002;64:1027–1035. doi: 10.1016/s0006-2952(02)01173-5. [DOI] [PubMed] [Google Scholar]
- Parkin E.T., Trew A., Christie G., Faller A., Mayer R., Turner A.J., Hooper N.M. Structure-activity relationship of hydroxamate-based inhibitors on the secretases that cleave the amyloid precursor protein, angiotensin converting enzyme, CD23, and pro-tumor necrosis factor-alpha. Biochemistry. 2002;41:4972–4981. doi: 10.1021/bi015936e. [DOI] [PubMed] [Google Scholar]
- Pederson R.A., White H.A., Schlenzig D., Pauly R.P., McIntosh C.H.S., Demuth H.-U. Improved tolerance in Zucker fatty rats by oral administration of the dipeptidyl peptidase IV inhibitor isoleucine thiazolidide. Diabetes. 1998;47:1253–1258. doi: 10.2337/diab.47.8.1253. [DOI] [PubMed] [Google Scholar]
- Pemberton P.W., Lobley R.W., Sorensen S.H., Batt R.M. An aminopeptidase N deficiency in dog small intestine. Res. Vet. Sci. 1997;63:133–138. doi: 10.1016/s0034-5288(97)90006-0. [DOI] [PubMed] [Google Scholar]
- Pospisilik J.A., Hinke S.A., Pederson R.A., Hoffmann T., Rosche F., Schlenzig D., Glund K., Heiser U., McIntosh C.H., Demuth H. Metabolism of glucagon by dipeptidyl peptidase IV (CD26) Regul. Peptides. 2001;96:133–141. doi: 10.1016/s0167-0115(00)00170-1. [DOI] [PubMed] [Google Scholar]
- Prechel M.M., Orawski A.T., Maggiora L.L., Simmons W.H. Effect of a new aminopeptidase P inhibitor, apstatin, on bradykinin degradation in the rat lung. J. Pharmacol. Exp. Ther. 1995;275:1136–1142. [PubMed] [Google Scholar]
- Proost P., Struyf S., Schols D., Durinx C., Wuyts A., Lenaerts J.P., De Clerq E., De Meester I., Van Damme J. Processing by CD26⧸dipeptidyl-peptidase IV reduces the chemotactic and anti-HIV-1 activity of stromal-cell-derived factor-1alpha. FEBS Lett. 1998;432:73–76. doi: 10.1016/s0014-5793(98)00830-8. [DOI] [PubMed] [Google Scholar]
- Proost P., Struyf S., Schols D., Opdenakker G., Sozzani S., Allavena P., Mantovani A., Augustyns K., Bal G., Haemers A., Lambeir A.M., Scharpé S., Van Damme J., De Meester I. Truncation of macrophage-derived chemokine by CD26⧸dipeptidyl peptidase IV beyond its predicted cleavage site affects chemotactic activity and CC chemokine receptor 4 interaction. J. Biol. Chem. 1999;274:3988–3993. doi: 10.1074/jbc.274.7.3988. [DOI] [PubMed] [Google Scholar]
- Proost P., Menten P., Struyf S., Schutyser E., De Meester I., Van Damme J. Cleavage by CD26⧸dipeptidyl peptidase IV converts the chemokine LD78beta into a most efficient monocyte attractant and CCR1 agonist. Blood. 2000;96:1674–1680. [PubMed] [Google Scholar]
- Proost P., Schutyser E., Menten P., Struyf S., Wuyts A., Opdenakker G., Detheux M., Parmentier M., Durinx C., Lambeir A.M., Neyts J., Liekens S., Maudgal P.C., Billiau A., Van Damme J. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood. 2001;98:3554–3561. doi: 10.1182/blood.v98.13.3554. [DOI] [PubMed] [Google Scholar]
- Prueitt R.L., Ross J.L., Zinn A.R. Physical mapping of nine Xq translocation breakpoints and identification of XPNPEP2 as a premature ovarian failure candidate gene. Cytogenet. Cell Genet. 2000;89:44–50. doi: 10.1159/000015560. [DOI] [PubMed] [Google Scholar]
- Püschel G., Mentlein R., Heymann E. Isolation and characterization of dipeptidyl peptidase IV from human placenta. Eur. J. Biochem. 1982;126:359–365. doi: 10.1111/j.1432-1033.1982.tb06788.x. [DOI] [PubMed] [Google Scholar]
- Qi S.Y., Riviere P.J., Trojnar J., Junien J.L., Akinsanya K.O. Cloning and characterization of dipeptidyl peptidase 10 (DPP10), a new member of an emerging subgroup of serine proteases. Biochem. J. 2003;373:179–189. doi: 10.1042/BJ20021914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahfeld J., Schierhorn M., Hartrodt B., Neubert K., Heins J. Are diprotin A (Ile-Pro-Ile) and diprotin B (Val-Pro-Leu) inhibitors or substrates of dipeptidyl peptidase IV? Biochim. Biophys. Acta. 1991;1076:314–316. doi: 10.1016/0167-4838(91)90284-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen H.B., Branner S., Wiberg F.C., Wagtmann N. Crystal structure of human dipeptidyl peptidase IV⧸CD26 in complex with a substrate analog. Nat. Struct. Biol. 2003;10:19–25. doi: 10.1038/nsb882. [DOI] [PubMed] [Google Scholar]
- Reinhold D., Kähne T., Steinbrecher A., Wrenger S., Neubert K., Ansorge S., Brocke S. The role of dipeptidyl peptidase IV (DP IV) enzymatic activity in T cell activation and autoimmunity. Biol. Chem. 2002;383:1133–1138. doi: 10.1515/BC.2002.123. [DOI] [PubMed] [Google Scholar]
- Riemann D., Hansen G.H., Niels-Christiansen L., Thorsen E., Immerdal L., Santos A.N., Kehlen A., Langner J., Danielsen E.M. Caveolae⧸lipid rafts in fibroblast-like synoviocytes: Ectopeptidase-rich membrane microdomains. Biochem. J. 2001;354:47–55. doi: 10.1042/0264-6021:3540047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R., Iturrioz X., Maigret B., Llorens-Cortes C. Contribution of molecular modeling and site-directed mutagenesis to the identification of two structural residues, Arg-220 and Asp-227, in aminopeptidase A. J. Biol. Chem. 2002;277:29242–29252. doi: 10.1074/jbc.M204406200. [DOI] [PubMed] [Google Scholar]
- Safavi A., Hersh L.B. Degradation of dynorphin-related peptides by the puromycin-sensitive aminopeptidase and aminopeptidase M. J. Neurochem. 1995;65:389–395. doi: 10.1046/j.1471-4159.1995.65010389.x. [DOI] [PubMed] [Google Scholar]
- Saria A., Hauser K.F., Traurig H.H., Turbek C.S., Hersh L., Gerard C. Opioid-related changes in nociceptive threshold and in tissue levels of enkephalins after target disruption of the gene for neutral endopeptidase (EC 3.4.24.11) in mice. Neurosci. Lett. 1997;234:27–30. doi: 10.1016/s0304-3940(97)00660-5. [DOI] [PubMed] [Google Scholar]
- Sato Y., Fujiwara H., Higuchi T., Yoshioka S., Tatsumi K., Maeda M., Fujii S. Involvement of dipeptidyl peptidase IV in extravillous trophoblast invasion and differentiation. J. Clin. Endocrinol. Metab. 2002;87:4287–4296. doi: 10.1210/jc.2002-020038. [DOI] [PubMed] [Google Scholar]
- Scanlan M.J., Raj B.K., Calvo B., Garin-Chesa P., Sanz-Moncasi M.P., Healey J.H., Old L.J., Rettig W.J. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc. Natl. Acad. Sci. USA. 1994;91:5657–5661. doi: 10.1073/pnas.91.12.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W.E., Siegel E.G., Kümmel H., Gallwitz B., Creutzfeld W. Commercially available preparations of porcine glucose-dependent insulinotropic polypeptide (GIP) contain a biologically inactive GIP-fragment and cholecystokinin-33⧸-39. Endocrinology. 1987;120:835–837. doi: 10.1210/endo-120-2-835. [DOI] [PubMed] [Google Scholar]
- Schomburg L., Turwitt S., Prescher G., Lohmann D., Horsthemke B., Bauer K. Human TRH-degrading ectoenzyme—cDNA cloning, functional expression, genomic structure and chromosomal assignment. Eur. J. Biochem. 1999;265:415–422. doi: 10.1046/j.1432-1327.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- Schmitmeier S., Thole H., Bader A., Bauer K. Purification and characterization of the thyrotropin-releasing hormone (TRH)-degrading serum enzyme and its identification as a product of liver origin. Eur. J. Biochem. 2002;269:1278–1286. doi: 10.1046/j.1432-1033.2002.02768.x. [DOI] [PubMed] [Google Scholar]
- Sedo A., Malik R. Dipeptidyl peptidase IV-like molecules: Homologous proteins or homologous activities? Biochim. Biophys. Acta. 2001;1550:107–116. doi: 10.1016/s0167-4838(01)00278-3. [DOI] [PubMed] [Google Scholar]
- Shane R., Wilk S., Bodnar R.J. Modulation of endomorphin-2-induced analgesia by dipeptidyl peptidase IV. Brain Res. 1999;815:278–286. doi: 10.1016/s0006-8993(98)01121-4. [DOI] [PubMed] [Google Scholar]
- Shioda T., Kato H., Ohnishi Y., Tashiro K., Ikegawa M., Nakayama E.E., Hu H., Kato A., Sakai Y., Liu H., Honjo T., Nomot A., Iwamoto A., Morimoto C., Nagai Y. Anti-HIV-1 and chemotactic activities of human stromal cell-derived factor 1alpha (SDF-1alpha) and SDF-beta are abolished by CD26⧸dipeptidyl peptidase IV-mediated cleavage. Proc. Natl. Acad. Sci. USA. 1998;95:6331–6336. doi: 10.1073/pnas.95.11.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingu K., Helfritz A., Kuhlmann S., Zielinska M., Meyer D., Jacobs R., Schmidt R.E., Mentlein R., Papst R., von Hörsten S. CD26 (dipeptidyl-peptidase IV) expression determines lung metastasis in Fischer 344 rats: Involvement of natural killer (NK) cell functions and soluble CD26. Cancer Immunol. Immunother. 2003;52:546–554. doi: 10.1007/s00262-003-0392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirotani K., Tsubuki S., Iwata N., Takaki Y., Harigaya W., Maruyama K., Kiryu-Seo S., Kiyama H., Iwata H., Tomita T., Iwatsubo T., Saido T.C. Neprilysin degrades both amyloid beta peptides 1-40 and 1-42 most rapidly and efficiently among thiorphan- and phosphoramidon-sensitive endopeptidases. J. Biol. Chem. 2001;276:21895–21901. doi: 10.1074/jbc.M008511200. [DOI] [PubMed] [Google Scholar]
- Siegel E.G., Gallwitz B., Scharf G., Mentlein R., Morys-Wortmann C., Fölsch U.R., Schrezenmeir J., Drescher K., Schmidt W.E. Biological activity of GLP-1-analogues with N-terminal modifications. Regul. Peptides. 1999;79:93–102. doi: 10.1016/s0167-0115(98)00155-4. [DOI] [PubMed] [Google Scholar]
- Siegel E.G., Scharf G., Gallwitz B., Mentlein R., Morys-Wortmann C., Fölsch U.R., Schmidt W.E. Comparison of the effect of native glucagon-like peptide 1 and dipeptidyl peptidase IV-resistant analogues on insulin release from rat pancreatic islets. Eur. J. Clin. Invest. 1999;29:610–614. doi: 10.1046/j.1365-2362.1999.00440.x. [DOI] [PubMed] [Google Scholar]
- Simmons W.H., Orawski A.T. Membrane-bound aminopeptidase P from bovine lung. Its purification, properties, and degradation of bradykinin. J. Biol. Chem. 1992;267:4897–4903. [PubMed] [Google Scholar]
- Skidgel R.A., Erdös E.G. Cellular carboxypeptidases. Immunol. Rev. 1998;161:129–141. doi: 10.1111/j.1600-065x.1998.tb01577.x. [DOI] [PubMed] [Google Scholar]
- Skidgel R.A., Defendi R., Erdös E.G. Angiotensin I converting enzyme and its role in neuropeptide metabolism. In: Turner A.J., editor. “Neuropeptides and Their Peptidases”. VCH-Ellis Horwood; Chichester, UK: 1987. pp. 165–182. [Google Scholar]
- Sprinkle T.J., Stone A.A., Venema R.C., Denslow N.D., Caldwell C., Ryan J.W. Assignment of the membrane-bound human aminopeptidase P gene (XPNPEP2) to chromosome Xq25. Genomics. 1998;50:114–116. doi: 10.1006/geno.1998.5302. [DOI] [PubMed] [Google Scholar]
- Sprinkle T.J., Caldwell C., Ryan J.W. Cloning, chromosomal sublocalization of the human soluble aminopeptidase P gene (XPNPEP1) to 10q25.3 and conservation of the putative proton shuttle and metal ligand binding sites with XPNPEP2. Arch. Biochem. Biophys. 2000;378:51–56. doi: 10.1006/abbi.2000.1792. [DOI] [PubMed] [Google Scholar]
- Steinbrecher A., Reinhold D., Quigley L., Gado A., Tresser N., Izikson L., Born I., Faust J., Neubert K., Martin R., Ansorge S., Brocke S. Targeting dipeptidyl peptidase IV (CD26) suppresses autoimmune encephalomyelitis and up-regulates TGF-beta 1 secretion in vivo. J. Immunol. 2001;166:2041–2048. doi: 10.4049/jimmunol.166.3.2041. [DOI] [PubMed] [Google Scholar]
- Sturiale S., Barbara G., Qiu B., Figini M., Geppetti P., Gerard N., Gerard C., Grady E.F., Bunnett N.W., Collins S.M. Neutral endopeptidase (EC 3.4.24.11) terminates colitis by degrading substance P. Proc. Natl. Acad. Sci. USA. 1999;96:11653–11658. doi: 10.1073/pnas.96.20.11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares W., Drucker D.J., Brubaker P.L. Enzymatic- and renal-dependent catabolism of the intestinotropic hormone glucagon-like peptide-2 in rats. Am. J. Physiol. Endocrinol. Metab. 2000;278:E134–E139. doi: 10.1152/ajpendo.2000.278.1.E134. [DOI] [PubMed] [Google Scholar]
- Turner A.J., Tipnis S.R., Guy J.L., Rice G., Hooper N.M. ACEH⧸ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can. J. Physiol. Pharmacol. 2002;80:346–353. doi: 10.1139/y02-021. [DOI] [PubMed] [Google Scholar]
- Villhauer E.B., Coppola M., Hughes T.E. Vol. 36. Academic Press; London: 2001. DPP-IV inhibition and therapeutical potential; pp. 191–200. (“Annual Reports in Medicinal Chemistry”). [Google Scholar]
- Visse R., Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- von Bonin A., Huhn J., Fleischer B. Dipeptidyl-peptidase IV⧸CD26 on T cells: Analysis of an alternative T-cell activation pathway. Immunol. Rev. 1998;161:43–53. doi: 10.1111/j.1600-065x.1998.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Wada K., Yokotani N., Hunter C., Doi K., Wenthold R.J., Shimasaki S. Differential expression of two distinct forms of mRNA encoding members of a dipeptidyl aminopeptidase family. Proc. Natl. Acad. Sci. USA. 1992;89:197–201. doi: 10.1073/pnas.89.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Ahmad S., Benter I.F., Chow A., Mizutani S., Ward P.E. Differential processing of substance P and neurokinin A by plasma dipeptidyl(amino)peptidase IV, aminopeptidase M and angiotensin-converting enzyme. Peptides. 1991;12:1357–1364. doi: 10.1016/0196-9781(91)90220-j. [DOI] [PubMed] [Google Scholar]
- Wang L., Sadhoun E., Stephens R.E., Ward P.E. Metabolism of substance P and neurokinin a by human vascular endothelium and smooth muscle. Peptides. 1994;15:497–503. doi: 10.1016/0196-9781(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Kojima T., Fuijmoto Y. Deficiency of membrane-bound dipeptidyl aminopeptidase in a certain rat strain. Experientia. 1987;43:400–401. doi: 10.1007/BF01940426. [DOI] [PubMed] [Google Scholar]
- Wesley U.V., Albino A.P., Tiwari S., Houghton A.N. A role for dipeptidyl peptidase IV in suppressing the malignant phenotype of melanocytic cells. J. Exp. Med. 1999;190:311–322. doi: 10.1084/jem.190.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A.J., Lu X.M., Slusher B., Tortella F.C. Electroencephalogram analysis and neuroprotective profile of the N-acetylated-alpha-linked acidic dipeptidase inhibitor, GPI5232, in normal and brain-injured rats. J. Pharmacol. Exp. Ther. 2001;299:48–57. [PubMed] [Google Scholar]
- Wrenger S., Reinhold D., Hoffmann T., Kraft M., Frank R., Faust J., Neubert K., Ansorge S. The N-terminal X-X-Pro sequence of the HIV-1 Tat protein is important for the inhibition of dipeptidyl peptidase IV (DP IV⧸CD26) and the suppression of mitogen-induced proliferation of human T cells. FEBS Lett. 1996;383:145–149. doi: 10.1016/0014-5793(96)00221-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nozaki-Taguchi N., Sakashita Y., Inagaki T. Inhibition of spinal N-acetylated-alpha-linked acidic dipeptidase produces an antinociceptive effect in the rat formalin test. Neuroscience. 2001;102:473–479. doi: 10.1016/s0306-4522(00)00502-9. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H., Yanagisawa M., Kapur R.P., Richardson J.A., Williams S.C., Clouthier D.E., de Wit D., Emoto N., Hammer R.E. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development. 1998;125:825–836. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]
- Yoshioka M., Erickson R.H., Kim Y.S. Digestion and assimilation of proline-containing peptides by rat intestinal brush border membrane carboxypeptidases. Role of the combined action of angiotensin-converting enzyme and carboxypeptidase P. J. Clin. Invest. 1988;81:1090–1095. doi: 10.1172/JCI113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Tamvakopoulos C., Xie D., Dragovic J., Shen X., Fenyk-Melody J.E., Schmidt K., Bagchi A., Griffin P.R., Thornberry N.A., Sinha Roy R. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: In vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1–38) J. Biol. Chem. 2003;278:22418–22423. doi: 10.1074/jbc.M212355200. [DOI] [PubMed] [Google Scholar]
- Zucker S., Chen W.-T. “Cell Surface Proteases.”. Elsevier; St. Louis, MO: 2003. [Google Scholar]
- Zukowska-Grojec Z., Karwatowska-Prokopczuk E., Rose W., Rone J., Movafagh S., Ji H., Yeh Y., Chen W.T., Kleinman H.K., Grouzmann E., Grant D.S. Neuropeptide Y: A novel angiogenic factor from the sympathetic nerves and endothelium. Circ. Res. 1998;83:187–195. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]


