Abstract
Shiga toxin–producing Escherichia coli (STEC) are important enteric pathogens worldwide, causing diarrhea with or without blood visibly present and hemolytic uremic syndrome. STEC are unique among diarrheogenic E coli in producing Shiga toxin type 1 and type 2, the virulence factors responsible for bloody diarrhea and hemolytic uremic syndrome. Cattle and other ruminants are the natural reservoir of STEC as their normal intestinal flora. Humans become infected by consumption of foods contaminated with cattle feces. Early diagnosis of STEC infection is important because of the contraindication for treating STEC using antimicrobial agents, and the intense supportive care needed if renal failure occurs.
Keywords: Escherichia coli, STEC, Enterohemorrhagic, Verotoxigenic, Diarrhea, Hemolytic uremic syndrome
Shiga toxin–producing Escherichia coli (STEC) comprise one of four generally recognized groups of E coli causing diarrhea in humans who acquire infections by ingestion of contaminated food or water or another fecal-oral route. The natural reservoir for STEC is ruminant animals, notably cattle, in which STEC can occur as normal intestinal flora (Fig. 1 ).1, 2 STEC is unique among these E coli by virtue of harboring and expressing the genes for Shiga toxins type 1 (Stx1) and 2 (Stx2). Shiga toxin is named for the Japanese microbiologist Kiyoshi Shiga (1870–1957), for whom the genus Shigella is named, inasmuch as the toxin produced by Shigella dysenteriae type 1 is very similar to the Stx1 and Stx2 produced by STEC. Table 1 provides a brief summary of these four well-characterized diarrheogenic E coli. They are distinct from the commensal E coli strains that inhabit the human gut and cause urinary tract infections, bacteremia, meningitis, and pneumonia in susceptible patients.3
Fig. 1.

Cattle and other ruminants are natural reservoirs of STEC.
Courtesy of Sarah Hunt, St Paul, MN.
Table 1.
Four major groups of diarrheogenic E coli
| Diarrheogenic E coli | Toxins | Other Virulence Factors | Worldwide Disease Burden | Clinical and Public Health Aspects |
|---|---|---|---|---|
| STEC (EHEC, VTEC) | Produces Shiga toxins Stx1, Stx2 (Verotoxins) | Survival in undercooked beef, on raw vegetables, in milk, and in water and fruit juices; adhesins for adherence to intestinal epithelium | Significant cause of HUS following bloody or nonbloody diarrhea; O157:H7 most prevalent, but STEC non-O157 important | A zoonotic disease acquired from foods and water contaminated with feces of cattle and other ruminants; secondary cases likely; low infectious dose |
| EIEC | None | Low infectious dose; invades intestinal epithelium | Rare; endemic in some countries; localized outbreaks in nurseries | Similar to Shigella, with fever, pain, dysentery; food-borne and person-to-person transmission |
| EPEC | None | Protein factors for attachment to and effacement of enterocyte microvilli; distinct pili for attachment to enterocytes | Significant cause of infant (<1 y) diarrhea; associated with weaning in infants; dehydration may be severe and fatal | Watery diarrhea with mucus, fever, with nausea and vomiting; foodborne transmission |
| ETEC | Heat-labile toxin (LT), heat-stable toxin (ST) | Colonization factors (proteins) expressed in intestinal lumen | Many fatal cases in children <5 y; associated with weaning in infants; dehydration may be severe | Profuse watery diarrhea; usually self-limiting as a common complaint of adult travelers |
Abbreviations: STEC (EHEC, VTEC), Shiga toxin–producing E coli (enterohemorrhagic E coli, verocytotoxin- or verotoxin-producing E coli); EIEC, enteroinvasive E coli; EPEC, enteropathogenic E coli; ETEC, enterotoxigenic E coli; HUS, hemolytic-uremic syndrome.
Data from Nataro JP, Bopp CS, Fields PI, et al. Escherichia, Shigella, and Salmonella. In: Murray PR, Baron EJ, Jorgensen JH, et al, editors. Manual of clinical microbiology. 9th edition. Washington, DC: American Society for Microbiology; 2007. p. 670–87; Fontaine O, Griffin P, Henao O, et al. Diarrhea, acute. In: Heymann DL, editor. Control of communicable diseases manual. 19th edition. Washington, DC: American Public Health Association; 2008. p. 179–95; Centers for Disease Control and Prevention. Diagnosis and management of foodborne illnesses: a primer for physicians and other health care professionals. MMWR Morb Mortal Wkly Res 2004;53:1–33.
Transmission of STEC to humans occurs through consumption of undercooked ground (minced) beef, foods eaten raw (eg, lettuce, sprouts, or spinach from manured gardens), water, or unpasteurized milk or juices, contaminated with STEC originating from cattle feces.4 Direct animal-to-human and human-to-human transmission have occurred.4, 5 Worldwide, STEC are often referred to as “verotoxin-producing” or “verocytotoxin-producing” E coli (VTEC), making reference to their cytotoxic effect on the Vero monkey kidney cell line (Fig. 2 ).6 In older literature, STEC was referred to as “enterohemorrhagic E coli” (EHEC), referring to bloody stools that are often a part of the clinical presentation,4, 7 and use of the EHEC acronym continues.
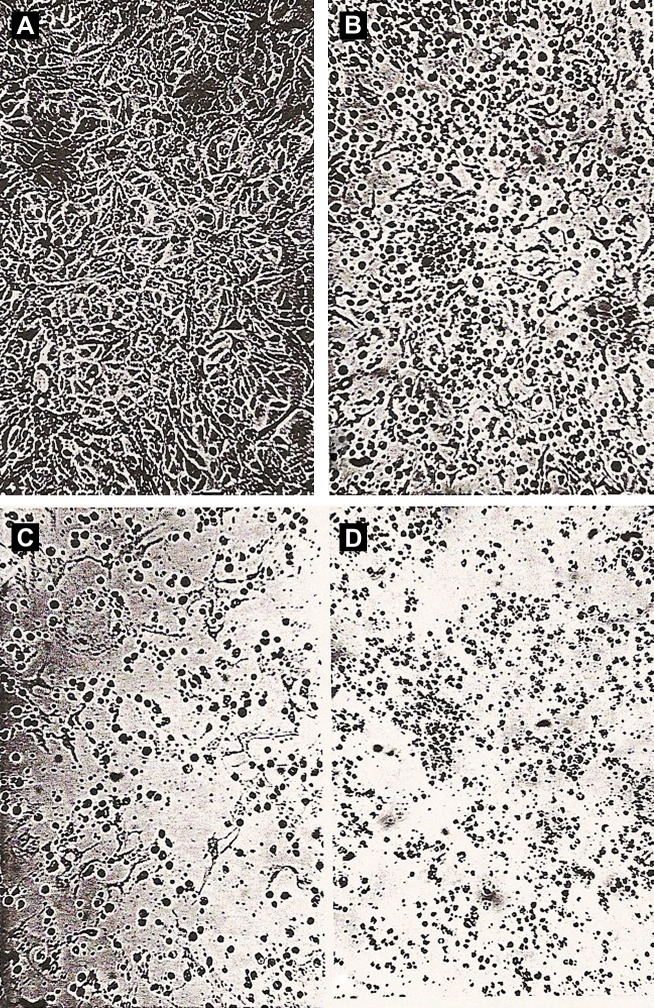
Fig. 2.
Shiga toxin (Verotoxin) effect on Vero cell monolayers. (A) Medium control, 1 day, cells in continuous monolayer. With STEC filtrate, (B) 1 day, (C ) 2 days, (D) 5 days; monolayer being destroyed. Phase-contrast microscopy. Magnification ×237.
From Konowalchuk J, Speirs JI, Stavrik S. Vero response to a cytotoxin of Escherichia coli. Infect Immun 1977;18:775–9; with permission.
Historical emergence of STEC as a diarrheal pathogen
Reported as E coli strains having distinct O-antigenic serotypes associated with bloody diarrhea and postdiarrheal hemorrhagic colitis in children in Canada6 and in the United States,8 STEC has emerged as a frequent cause of food-borne gastroenteritis, both sporadically and in outbreaks, creating substantial risk of hemolytic uremic syndrome (HUS) and life-threatening renal failure in children.9 Over the past 25 years, most attention has been focused on one particular STEC O-antigen serotype, O157, of flagellar serotype H7 (E coli O157:H7, or STEC O157), which predominates in reported outbreaks and sporadic cases in the United States and elsewhere worldwide, although not universally.4 In Germany, for example, STEC O91 is the predominant serotype isolated from adult patients.10 STEC O157 is notable for having caused an outbreak of diarrhea in the United States in 1982 traced to contaminated beef served by a fast-food restaurant chain that resulted in four deaths8; and a 1996 Japanese outbreak affecting an estimated 6000 children, three of whom died.11
The two toxins that can be produced by STEC have been referred to historically as “Shiga-like toxins,” and “verotoxins” or “verocytotoxins.” The acronyms STEC, EHEC, and VTEC are used interchangeably. Stx1 differs in one amino acid from Shiga toxin of Shigella dysenteriae serotype 1, whereas Stx2 shares only about 60% amino acid similarity with Stx1. Sequence variants of both Stx1 and Stx2 are known, and multiple variants may be produced by one STEC bacterium.12
With heightened awareness of the importance of STEC O157, and improvements in its laboratory detection in stool specimens,3 sporadic infections and outbreaks of varying magnitude continue to be documented. Laboratory testing has improved to the point that a standard of care is to always suspect and test for this particular pathogen in stool specimens from patients with diarrhea. Importantly, it is the clinician's responsibility to communicate with the clinical microbiology laboratory to ensure that appropriate specimen collection and transport occurs, and that cultures are ordered and performed to recover and isolate STEC O157 from stool specimens submitted for bacterial cultures.3, 13 Isolation of a pure culture of STEC from patients is the gold standard for confirming the diagnosis. In addition, pure cultures provide organisms for molecular epidemiology and outbreak investigations by public health laboratories working to prevent transmission in the community.14, 15 With the emerging awareness of STEC non-O157 as diarrheal pathogens, many clinical laboratories now offer immunoassay tests for Shiga toxins or for several non-O157 STEC O-antigens in parallel with stool cultures.3, 7 Immunoassay results can often be provided to the clinician before a final culture result is available. Culture confirmation of STEC should always be attempted by the clinical laboratory, however, or by a reference laboratory to which suspected STEC isolates are sent.16
Emergence of STEC non-O157 disease
Early recognition that STEC serotypes other than O157 were associated with diarrhea and hemorrhagic colitis prompted development of laboratory testing methods for the detection of now over 150 known STEC non-O157 strains.3, 6 In North America, STEC serotypes O26, O45, O103, O111, and O121 are the most common3, 4; and in Europe, these serotypes, and O91 and O145, are most frequently isolated from ill patients.3, 4, 10 Serotypes of STEC isolated from ruminant reservoir animals and from infected patients vary in prevalence worldwide, indicating a need for clinical laboratories to determine common endemic serotypes while being on the alert for less common or “imported” serotypes in sporadic infections.1, 2, 4, 17, 18 The non-O157 STEC also harbor and express one or both of the Shiga toxin Stx genes present on temperate bacteriophages in the STEC genome and, like STEC O157, have caused diarrhea, hemorrhagic colitis, and HUS.3, 4, 6, 10 Detection of non-O157 STEC in stool cultures is problematic because unlike most O157 STEC, they do ferment sorbitol, and do not grow as sorbitol-negative (nonfermenting) colonies on sorbitol-MacConkey (SMAC) agar that has been so useful for recovery of sorbitol-nonfermenting STEC O157.3, 9
Emerging trends in STEC disease
The severity and long-term sequelae associated with STEC disease warrant a careful consideration of how to improve patient outcome.17, 19 Awareness that STEC non-O157 serotypes are capable of causing postdiarrheal HUS is well established,3, 4, 7 but the recovery of diverse serotypes by most clinical laboratories is difficult and can result in delays in providing useful information to clinicians. DNA amplification methods can be used to identify STEC independent of serotype, if genes encoding Stx1 or Stx2 are present.20 This can be done from isolated colonies, from mixed growth on agar plates, or directly from stool specimens. Commercial kits for real-time amplification tests for Shiga toxin genes, using instrument platforms currently in use in clinical laboratories, may eventually be available.21
Sorbitol-fermenting STEC O157, the so-called “SF STEC,” are typically nonmotile (H-) STEC O157 first identified in a 1988 outbreak of HUS in Germany.22 These pathogens present identification challenges analogous to those of non-O157 STEC. The epidemiology of SF STEC O157:H- is interesting because of its geographic clustering in Europe.20, 22
Loss of the genes that encode Shiga toxins during infection has recently been observed for patients with HUS following STEC diarrhea. Strains of E coli having typical STEC serotypes (eg, O26, O111, O103, O121, O145, and O157) but which lack genes for Stx1 and Stx2 have been isolated from stool specimens of HUS patients.23 Assuming that these strains of E coli were the cause of HUS for these patients, the Shiga toxin genes were apparently lost during the course of the infection. This situation potentially renders ineffectual current Shiga toxin immunoassays and Stx gene amplification testing of bacterial isolates from diarrheal stool specimens in search of STEC. Culturing early in the course of clinical illness,13 and successive culturing, may allow recovery of toxin-producing STEC before the Shiga toxin genes are lost.13
Microbiology
Bacterial Physiology and Genetics of STEC
Escherichia coli is the predominant gram-negative facultative anaerobe found as usual intestinal flora in warm-blooded animals, including humans, although it is outnumbered in the intestine by obligate anaerobes, such as Bacteroides spp.24 E coli is typically motile by peritrichous flagella, the location of the E coli H-antigen, of which over 50 serotypes are known. Some commensal E coli may possess a capsule, the site of the E coli K-antigen, which serves as a virulence factor for extraintestinal colonization, urinary tract infections, and invasive disease. Over 80 serologically distinct K-antigen specificities are known.24
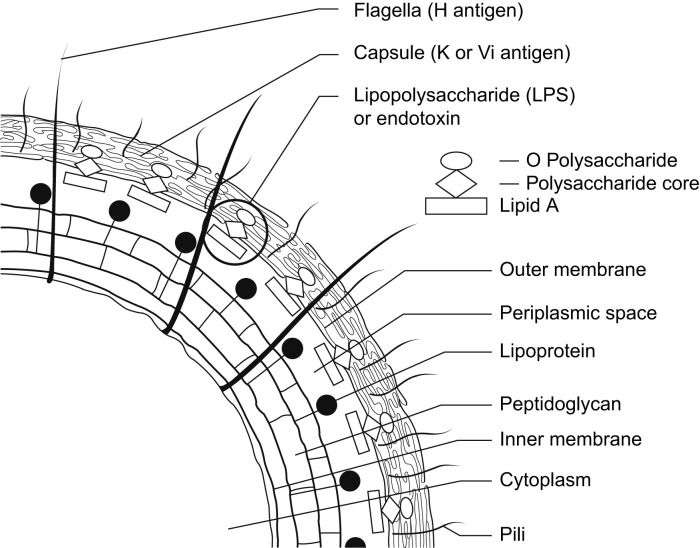
Enterobacteriaceae, the taxonomic family to which E coli belongs, includes opportunistic commensal genera, such as Citrobacter, Enterobacter, Klebsiella, and Proteus, and noncommensal human pathogens, such as Salmonella, Shigella, and Yersinia.3, 24 The gram-negative cell wall of the Enterobacteriaceae is characterized by the presence of a lipopolysaccharide that is the location of the O-antigen. The O-antigen is a polysaccharide composed of repeating monosaccharide trimers in diverse combinations and sequences. The O-antigen is anchored in the cell wall's outer membrane by a lipid moiety, Lipid A, by an oligosaccharide core (Fig. 3 ). The complex structure of these lipopolysaccharide polysaccharides generates the 100 to 200 distinct E coli O-antigen serotypes. Diarrheogenic E coli (STEC, ETEC, EIEC, and EPEC) (see Table 1) have a relatively restricted number of O-antigen serotypes,3 and STEC is unique among these in its ability to produce Shiga toxins.
Fig. 3.
Antigenic structure of Enterobacteriaceae.
From Murray PR, Rosenthal KS, Kabayashi S, et al. Enterobacteriaceae. In: Medical microbiology. 4th edition. Philadelphia: Elsevier Mosby; 2002. p. 267; with permission.
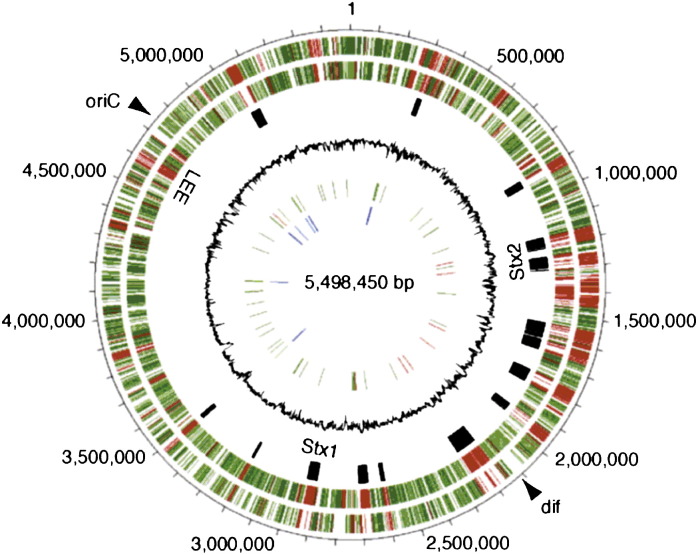
The STEC O157 chromosome is a closed circular, double-stranded DNA molecule about 5.5 megabases in size.11, 25 Fig. 4 provides a graphic representation of the STEC O157 chromosome from the strain responsible for the 1996 outbreak in Sakai City, Japan, derived from genome sequencing. The fourth circle of the figure indicates in black the locations of the temperate bacteriophage genomes integrated into the E coli chromosome that harbor the genes encoding Stx1 and Stx2. These bacteriophages account for some of the differences evident between the Sakai STEC O157 genome and the genome of a nonpathogenic laboratory strain of E coli. Other areas of difference illustrated in Fig. 4 reflect virulence genes present in STEC O157 but not in a nonpathogenic E coli.11, 25
Fig. 4.
Circular representation of the E coli O157 chromosome. The outermost circle indicates locations on the 5.5-MB genome. The second and third circles show in red the predicted genes in O157 that differ from those of a nonpathogenic laboratory strain of E coli. The fourth circle indicates in black the locations of the integrated temperate bacteriophage genomes encoding Stx1 and Stx2 in the O157 chromosome.
From Hayashi T, Makino K, Ohnishi M, et al. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res 2001;8:13; with permission.
The integrated, temperate bacteriophage genomes carrying the Shiga toxin genes can be induced to replicate lytically and generate bacteriophage progeny by exposure of STEC to chemical agents or ultraviolet light. These bacteriophages are lambdoid in morphology, with hexagonal heads and long tails.26 They may be responsible for transmission of Shiga toxin genes between different strains of E coli in the gastrointestinal tract, and even toxin gene transfer to other genera and species of bacteria, such as Citrobacter spp, Aeromonas spp, and Enterobacter spp, in which Shiga toxin genes have been reported to occur.12
Diagnostic Microbiology of STEC
In the clinical microbiology laboratory, recovery of STEC O157 from stool specimens has been facilitated by the inability of most STEC O157 to ferment sorbitol.3, 9 Because of the worldwide occurrence of this pathogen and its well-documented ability to cause serious disease, media chosen for bacterial cultures of all stools from patients with diarrhea or HUS should include SMAC agar for recovery and isolation of STEC O157. SMAC agar containing cefixime and potassium tellurite has been used to suppress interfering gram-negative bacterial growth when isolating STEC O157, but these agents prevent growth of some STEC and are not recommended for routine stool cultures.3
Chromogenic agars (eg, CHROMAgar, Becton Dickinson, Sparks, MD, USA) are selective differential media for the visual identification of specific pathogens based on their biochemical phenotypes. Chromogenic O157 agar, like SMAC agar, can provide the laboratory with isolated colonies after overnight incubation for presumptive visual identification as STEC O157, and facilitate prompt confirmation of suspect colonies as STEC O157:H7 by subculture and latex agglutination for O157 and H7 antigens, or for Shiga toxins.3 Alternatively, “sweeps” of confluent growth from a plate with suspect STEC colonies can be tested for Shiga toxin or O-antigens using a commercial immunoassay for which this specimen source is approved according to the package insert.
Recovery of STEC non-O157 from stool specimens of patients with diarrhea is challenging. These pathogens ferment sorbitol and are not detected on SMAC or current chromogenic agars. The importance of testing for non-O157 STEC cannot be overemphasized, however, because at least one third of STEC isolated from ill patients are non-O157 serotypes.3, 4, 27, 28 Immunoassays from several manufacturers are available to test patient specimens or cultures for STEC.29
Direct testing of fresh stool specimens for Shiga toxins by immunoassay before culture can provide the clinician with a qualitative result in 20 minutes to 2 hours, depending on the type of test used. Only immunoassays specifying fresh stool as an acceptable specimen in the package insert should be used for direct “point-of-care” Shiga toxin testing. An optical immunoassay (eg, Biostar OIA SHIGATOX, Inverness Medical Professional Diagnostics, Princeton, NJ, USA) is available for direct testing of stool specimens, providing a toxin result in 20 minutes, without differentiation of Stx1 and Stx2.28 A microwell plate immunoassay (eg, Premier EHEC, Meridian Bioscience, Cincinnati, OH, USA) for direct testing of stool specimens provides toxin results in about 2 hours. The sensitivity of these direct tests is good, between 80% and 100%, and specificity is 99%.28 A greater variety of immunoassays is available for detection of Shiga toxins and O-antigens in growth from overnight (18–24 hours) cultures, either in broth or on agar media. If no toxins are detected in direct testing of stool specimen, a Shiga toxin immunoassay can be repeated from overnight growth, usually with greater sensitivity than can be obtained from direct testing of stool specimens.27, 28
One approach is to sample “sweeps” of mixed colonial growth from SMAC or other agar media after overnight incubation. Individual colonies of potential STEC growing on SMAC or other enteric culture plates may be picked for identification by serotyping or for Shiga toxin immunoassay.3
Suspensions of the sweeps or colonies can be tested for Shiga toxins using an optical immunoassay (eg, Biostar OIA SHIGATOX),28 a lateral flow immunoassay (eg, Meridian Immunocard STAT!EHEC, Meridian Bioscience, Cincinnati, OH, USA) (Fig. 5 ), or an enzyme immunoassay (eg, ProSpect Shiga toxin Escherichia coli Microplate Assay, Alexon-Trend, Ramsey, MI, USA).27 Rapid tests (optical and lateral flow immunoassays) provide a qualitative Shiga toxin result in 10 to 15 minutes and microplate assays in about 2 hours. The sensitivity and specificity of immunoassays should not be assumed to be 100%, so results must be interpreted cautiously until STEC colonies are isolated and identified. Real-time polymerase chain reaction testing of stool specimens directly, or of overnight cultures, can also provide Stx1 or Stx2 gene results to clinicians in 30 to 60 minutes when available for clinical laboratories.20, 21 As with immunoassay results, molecular testing results must be confirmed by isolation of STEC from stool and culture.
Fig. 5.
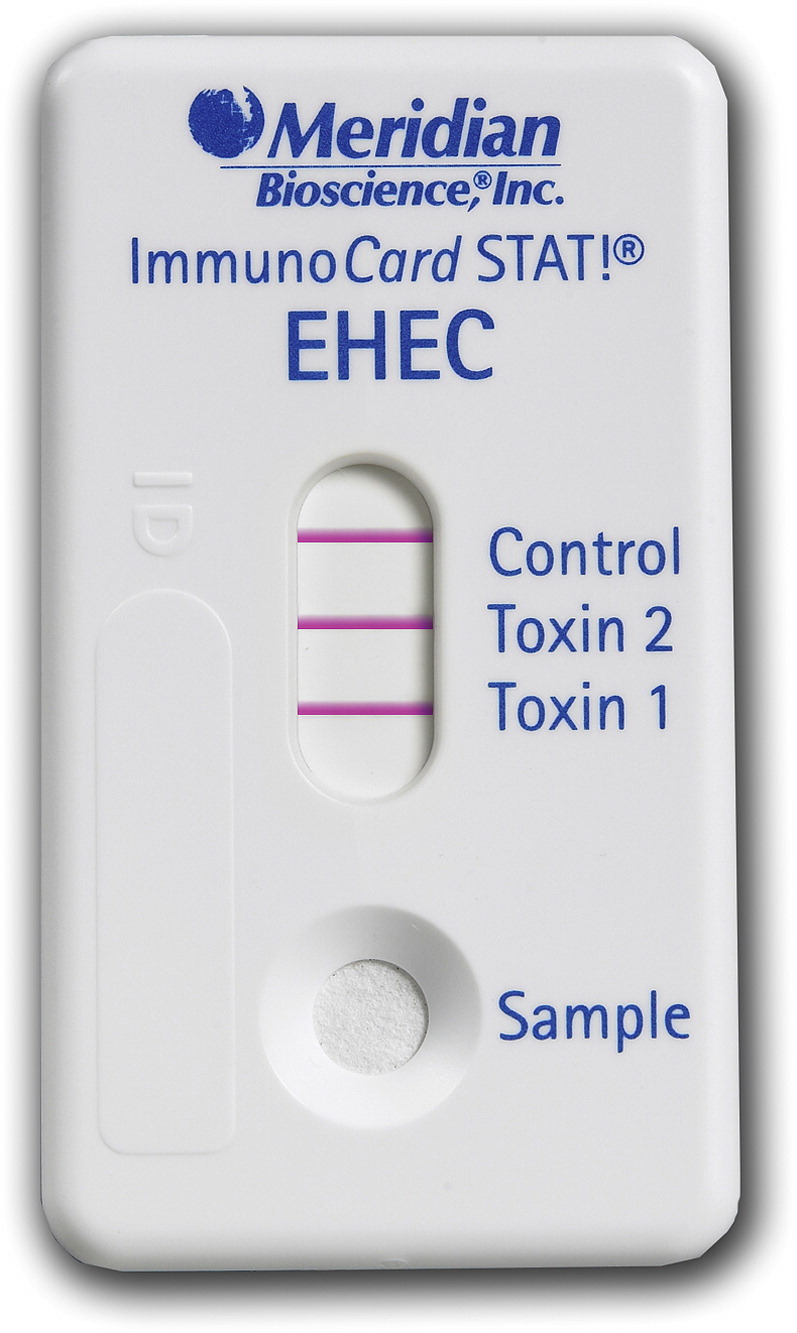
Shiga toxin lateral-flow immunoassay device for testing broth and agar cultures. This device distinguishes Stx1 from Stx2.
Courtesy of Meridian Bioscience, Cincinnati, OH.
Many STEC express a distinctive hemolytic phenotype on enterohemolysin (Ehly) agar (eg, Sifin, Berlin, Germany).3, 10, 30 If this agar is available, individual colonies from Ehly agar can be picked and tested by latex agglutination for common STEC O-antigen serotypes, such as O157, O26, and O111, or for Shiga toxins by immunoassay.3, 6
Immunomagnetic separation is used in the food industry for enhancing the recovery of diverse STEC serotypes.3 It could be used on a research use only basis for the enrichment of stool specimens or broth cultures for STEC by incubation with sterile magnetic beads to which selected STEC O-antigen–specific antibody molecules have been attached. Following incubation the beads are aseptically rinsed and cultured, and colonies or mixed growth tested as described previously. Reagents for immunomagnetic separation are commercially available for several STEC (eg, O157, O111, O26, from Denka Seiken, Japan; O157, from Invitrogen Dynal, Oslo, Norway). Beads for immunomagnetic separation could be custom-requested from manufacturers by laboratories based on local STEC serotype prevalence in patients and animal reservoirs.1, 2, 3, 4
Epidemiology
STEC infection is a zoonotic disease, for which small and large ruminant animals, notably cattle, are the natural reservoir, harboring STEC as normal gastrointestinal flora.1, 2 Transmission to humans commonly occurs through consumption of STEC-contaminated raw or undercooked meat, or of produce contaminated with cattle feces through farming or production practices. The infectious dose of STEC is low, about 100 bacteria,3 so person-to-person transmission can occur, leading to secondary cases in contacts of infected persons. Clinically recognized STEC diarrhea, and probably many subclinical STEC infections, occurs through direct animal-to-human contact.5 Risk of transmission to humans from food sources can be substantially reduced by careful washing of produce; pasteurization of juices and milk; and cooking to allow the internal temperature of meat, especially ground (minced) beef, to reach 70°C (160°F).4
Approximately 75% of cases of HUS occur in children, following diarrhea caused by an STEC infection. HUS is a significant cause of acute renal failure in children.9, 19 In the United States, approximately 90% of HUS cases are caused by STEC O157:H7, but only 50% or less in other countries.3, 4, 9, 13 Children under 10 years of age are at greatest risk for serious STEC infections. Approximately 15% of children with STEC diarrhea develop HUS.9, 31 Half of these require dialysis for renal failure, and the HUS case-fatality rate is approximately 5%.4, 9, 19 The incidence of HUS worldwide varies widely. In Argentina, the incidence is 12 per 100,000 children under 5 years of age,32 but can be 10-fold lower elsewhere in the world.9
The worldwide distribution and diversity of STEC serotypes in recent outbreaks is evident from Table 2 . Several interactive sources for tracking STEC disease and serotypes worldwide are accessible electronically. Data for STEC from 36 reporting countries are available from Enter-Net, funded by the European Center for Disease Control (http://ecdpc.europa.eu/documents/ENTER_NET/vtec07q2.pdf; accessed August 5, 2009).
Table 2.
Recent STEC outbreaks, 2006–2009
| Country and Year of Outbreak | STEC Serotype | Transmission Source or Vehicle | Cases/Hosp/HUS/Deathsa | Action Taken | Text References |
|---|---|---|---|---|---|
| United Kingdom, Wales, 2009 | O157:H7 | Fast food outlet | 4/2/2/0 | Analysis of isolates by PT, PFGE, VNTR; active case finding by local practitioners | 33 |
| United Kingdom, Wales, 2009 | O157:H7 | Dance camp; campsites on farms with animals; unchlorinated water being investigated | 2/--/--/-- | Contacts sought by social network Web sites, telephone, e-mail | 34 |
| United States, 2009 | O157:H7 | Refrigerated cookie dough, uncooked | 72/34/10/0 | Recall of product by manufacturer | 35 |
| Netherlands, 2008–2009 | O157:H- (nonmotile) | Raw minced beef | 20/7/0/0 | Traceback investigation | 36 |
| United States, 2008 | O157:H7 | Commercial ground (minced) beef | 49/27/1/0 | Beef product recall (5.3 million pounds) | 37 |
| Canada, 2008 | O157:H7 | Raw onions | 235/26/1/0 | Traceback investigation | 38 |
| Netherlands and Iceland, 2007 | O157:H- | Lettuce processed at Dutch plant | Netherlands: 41/0/0/0 Iceland: 9/0/0/0 |
Traceback investigation | 39 |
| Scotland, 2007 | O157:H7 | Cold meat salad | 9/2/0/0 | Public notified; hotel kitchen closed and cleaned; disinfectant washing procedure instituted | 40 |
| United States, 2007 | O157:H- | Petting zoo (goats, sheep, llama) | 7/2/0/0 | Case finding among staff, visitors; zoo closed; animals tested for colonization | 5 |
| United States, 2006 | O157:H7 | Spinach | 199/102/31/3 | Public warning; product recall; PFGE analysis of outbreak strains | 41 |
| Norway, 2006 | O103:H25 (notable for loss of Stx2 gene during infection) | Cured mutton sausage | 17/–/10/1 | Public warning; product recall; sheep slaughter changes implemented | 42 |
| Japan, 2006 | O26 | Nursery school; person-to-person spread from index patient inferred | 26/0/0/0 | Case finding; PFGE analysis of isolates | 43 |
| Japan, 2006 | O103 | Nursery school; person-to-person spread from index patient inferred | 8/0/0/0 | Case finding; PFGE analysis of isolates | 44 |
Similar data, both in tabular and map format, are available for the United States in publications from the Centers for Disease Control and Prevention.45 The World Health Organization Weekly Epidemiologic Record indexes “VTEC,” with fewer entries for “STEC,” at www.who.int/wer/en (accessed June 10, 2009). The World Health Organization provides interactive map building capability for the World Health Organization European Region through its Computerized Information System for Infectious Disease, accessible through www.who.int. For STEC, the Computerized Information System for Infectious Disease map query-builder uses “6080” as the reference number for generating distribution maps of “enterohemorrhagic E coli” (accessed June 10, 2009).
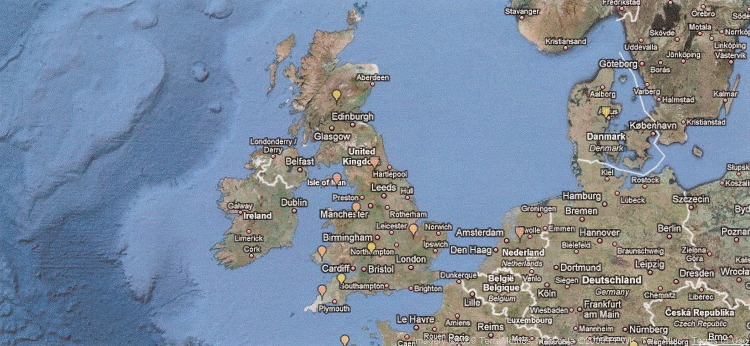
International interactive maps of disease occurrence are generated by ProMED, a global reporting system of emerging infectious diseases, at http://www.promedmail.org/. The maps are accessible at www.healthmap.org. A healthmap.org map locating current STEC outbreaks in the United Kingdom and Wales33, 34 is shown in Fig. 6 .
Fig. 6.
Interactive map showing location in United Kingdom and Wales of two STEC O157:H7 outbreaks, July, 2009.
From ProMED. PRO/AH/EDR>E. COLI O157-UK: Wales dance camp, alert 17-Aug-2009. Archive Number 20090817.2915. Available at: http://www.promedmail.org and www.healthmap.org. Accessed August 18, 2009; Brownstein JS, Freifeld CC, Reis BY, et al. Surveillance sans frontières: internet-based emerging infectious disease intelligence and the healthmap project. PLoS Med 2008;5:e151; with permission.
Statutory reporting of STEC infections has evolved with the increasing recognition of serious disease from non-O157 STEC infections. Currently in the United States, STEC infections of all serotypes are reportable to the National Notifiable Diseases Surveillance System of the Centers for Disease Control and Prevention.45 Molecular techniques, such as phage typing and pulsed-field gel electrophoresis, are useful for comparing STEC isolates from outbreaks to link ill patients with contacts and with potential sources of infection. Databases are available for using these techniques to compare newly isolated outbreak strains for tracking the sources of outbreaks.14, 15
Clinical presentation
The clinical presentation of STEC infection is diarrhea, consisting of more than three unformed stools in a 24-hour period with or without blood.5 The diarrhea may be intermittent, watery, or nonwatery, and may be associated with dehydration consistent with the fluid loss. Other symptoms often associated with STEC diarrhea include abdominal cramping, nausea, headache, vomiting, and fever.3, 4, 7, 9, 19
Based on experience with STEC O157 in children, diarrhea, abdominal pain, painful defecation, vomiting, and fever occur about 3 days after ingestion of an infectious dose of bacteria. Bloody diarrhea develops in 90% of children after another 3 days. The diarrhea symptoms abate about 7 days after onset. About 85% of children recover spontaneously, with 15% developing HUS and at risk of death (5% mortality).9
The clinical presentation of HUS in a patient with prior gastrointestinal or influenza-like symptoms is usually evidence of bleeding, either in vomitus or stool; severe oliguria; hematuria; microangiopathic hemolytic anemia; hypotension; and perhaps neurologic changes. Because of the risk of HUS, renal failure, and death in patients with intestinal STEC infections, especially in children, it is extremely important to assess renal function of the patient at presentation, which along with dehydration, may necessitate emergent care. The question of whether or not to treat a diarrheal infection caused by STEC must be considered promptly because of the generally accepted risk of increasing the severity of diarrhea and HUS caused by STEC if antimicrobial agents are given to STEC-infected patients.4, 9, 19, 31
Pathogenesis
Ingested STEC reach the small intestine and the colon and multiply there in competition with the normal bacterial flora. A number of bacterial structures (fimbriae, pili) and adhesion molecules (adhesins) are thought to mediate adherence of STEC to the intestinal epithelial cells, allowing the Shiga toxins secreted by STEC to interact with the enterocyte plasma membrane surface.23, 46, 47, 48
The STEC secrete Shiga toxins Stx 1 and Stx2, which bind to the enterocytes, the absorptive epithelial cells present on the luminal surface of the small and large intestines (Fig. 7 ). Stx1 and Stx2 are exotoxins of the AB5 class of toxins.12 Fig. 8 is a “ribbon diagram” illustrating the three-dimensional structure of the A and B polypeptides of Stx2 based on radiograph crystallography.49, 50 The pentameric B portion of the toxin, consisting of five identical B polypeptides, binds to the cellular glycolipid receptor globotriaosylceramide (Gb3) present on the plasma membrane of enterocytes and other cells. The monomeric A subunit of the Shiga toxin then enters the enterocyte by endocytosis and is transported to the rough endoplasmic reticulum by the Golgi apparatus.12 The A subunit is proteolytically cleaved in the cell cytoplasm, liberating the N-terminal (A1) portion, a glycosidase that hydrolyzes a specific adenine-ribose bond in the ribosomal 28S RNA. This cleavage prevents aminoacyl-tRNA binding, and irreversibly inhibits protein synthesis, resulting in cell death (see Fig. 2).48
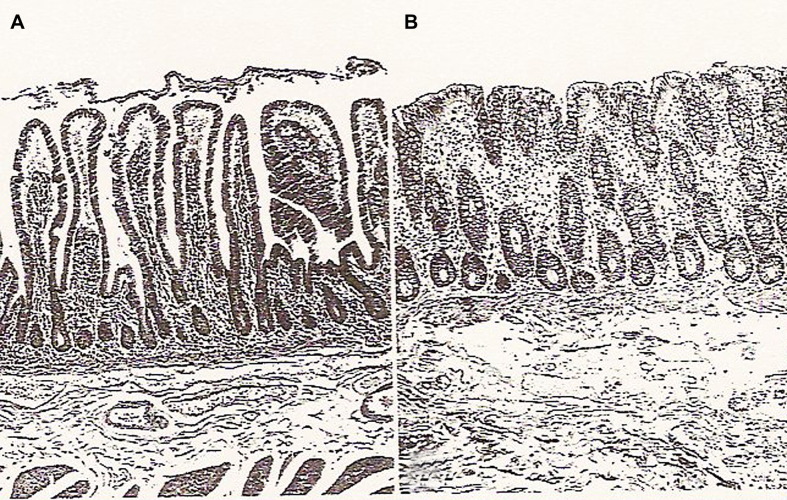
Fig. 7.
Normal intestinal mucosa. (A) Luminal surface of the normal small intestine, with enterocytes lining the villi and intestinal crypts. (B) Normal colon histology showing colonic crypts and a flat mucosal surface lined with enterocytes. The enterocytes are the target of STEC Shiga toxins. H&E staining. Magnification ×70 approximately.
From Chen L, Crawford JM. The gastrointestinal tract. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran pathologic basis of disease. 7th edition. Philadelphia: Elsevier Saunders; 2005. p. 828; with permission.
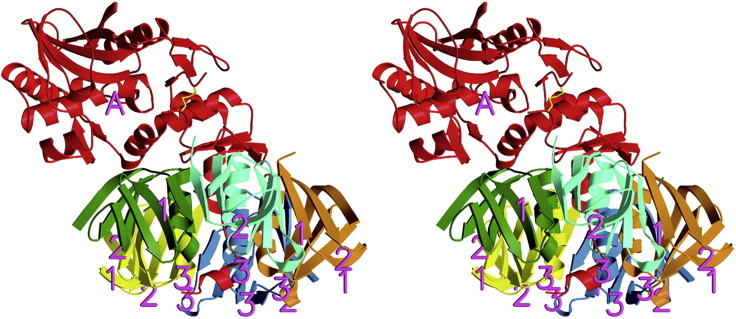
Fig. 8.
Ribbon diagram of Stx2 from E coli O157:H7. Shiga toxin is a class AB5 toxin. The monomeric A polypeptide is red. The five B polypeptides are orange, cyan, green, yellow, and blue. Binding of the pentameric B portion of the toxin to the cell surface allows entry of the A subunit into the cell, where it functions enzymatically to stop protein synthesis and kill enterocytes and other cells to which the Shiga toxin binds.
From Fraser ME, Fujinaga M, Cherney MM, et al. Structure of Shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J Biol Chem 2004;279:27513; with permission.
Once the Shiga toxins enter the bloodstream by the damaged intestinal epithelium, the precise mechanism of which is still unclear, capillary endothelial cells are exposed to the Shiga toxin and killed by the same mechanism. The endothelial cell lysis is accompanied by platelet activation and aggregation, leukocyte adherence, cytokine secretion, and vasoconstriction, contributing to fibrin deposition and clot formation within the capillary lumen and in the subendothelial tissue.17, 46, 47 This thrombotic microangiopathy then occurs distally as the Shiga toxins are carried by the bloodstream to the kidneys, resulting in fibrin deposition in glomerular capillaries, hematuria, and renal failure (Fig. 9 ).9, 31, 47
Fig. 9.
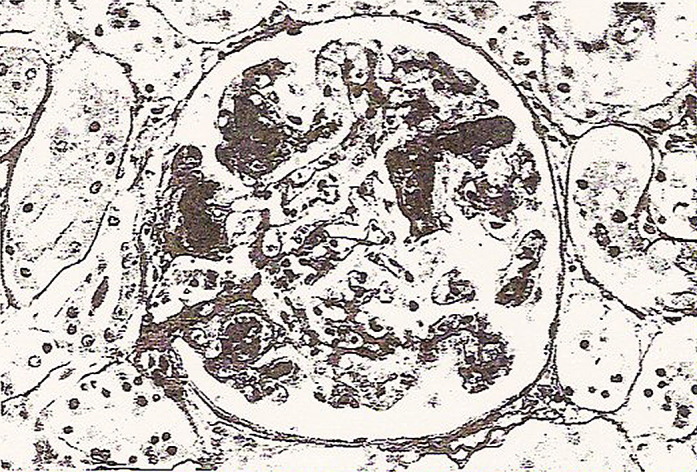
Fibrin stain showing platelet-fibrin thrombi (dark areas) in the glomerular capillaries. Glomerular injury is characteristic of microangiopathic disorders, such as hemolytic uremic syndrome (HUS). Fibrin stain. Magnification ×240 approximately.
From Alpers CE. The kidney. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran pathologic basis of disease. 7th edition. Philadelphia: Elsevier Saunders; 2005. p. 1010; with permission.
Diagnosis
The clinical presentation of STEC infection is diarrhea, with or without blood present, which may be intermittent, watery, or nonwatery, often accompanied with abdominal cramping, nausea, headache, vomiting, and fever.4, 7, 9, 51
Laboratory diagnosis of STEC infection in a patient with diarrhea is based on microscopic examination of stool when appropriate (eg, to exclude parasites and to visualize Vibrio and Campylobacter spp)7; bacterial stool cultures; detection of Shiga toxin by immunoassay; and detection of genes encoding Shiga toxin by DNA amplification. Confirmation of STEC as the etiologic agent of diarrhea requires the recovery and isolation in pure culture of STEC from a patient's stool specimen.3
Stool specimens should be obtained for culture as early as possible from the patient presenting with diarrhea, and specimens for culture collected successively if no pathogen is initially identified. In patients who develop HUS, STEC may be unrecoverable by the time HUS develops.13 Ideally, specimens for toxin or O-antigen immunoassay, or DNA amplification, should be the same ones collected for cultures. These tests may provide preliminary information useful to clinicians in advance of a confirmatory culture result that requires 1 or more days to obtain. Any report from the laboratory stating that STEC O157 specifically was not recovered or detected should include a comment that STEC non-O157, such as STEC O26 and O111, can also cause diarrhea and HUS and should be considered as potential etiologies.3, 4 This reporting by the laboratory allows the clinician to request additional testing if it seems indicated.
STEC isolates recovered by the clinical laboratory, and cultures of any presumptive STEC identified by toxin immunoassay or other test, should be submitted to a local or national laboratory for confirmatory testing and outbreak investigation if appropriate.3, 14, 15, 45
Differential diagnosis
A patient presenting with diarrhea must be assessed not only for infection with gastrointestinal pathogens, but also for other illnesses and conditions with associated diarrhea, such as Legionnaire's disease, severe acute respiratory syndrome, influenza, and chemical and physical injury.7, 51
Table 3 summarizes 23 causes of diarrhea, including STEC, and provides information useful in formulating a broad differential diagnosis, which can then be refined based on the patient's current illness course and exposure history (food consumption, contact with other ill persons, animals, fomites, social settings). An appreciation of this broad differential is essential to identify the most immediately life-threatening etiologies in a patient and begin a treatment plan without delay.
Table 3.
Differential diagnosis of diarrhea in patients with suspected STEC
| Pathogen | Incubation Period | Blood in Stool | Abdominal Cramping and Pain | Nausea or Vomiting | Fever | Other Signs and Diagnostic Aids | Diagnostic Laboratory Testing |
|---|---|---|---|---|---|---|---|
| Bacillus anthracis (gastrointestinal anthrax) | 2 d to wk | + | + | + | + | Patient history: exposure may be accidental from consumption of infected meat | Culture of food, any lesions, blood; CDC select agent; culture hazard |
| Brucella spp | 7–21 d | + | − | − | + | Patient history: bacteremia and fever; unpasteurized dairy product consumption; travel to endemic area; muscle and joint pain; headache | Blood cultures with special incubation request; CDC select agent; culture hazard |
| Campylobacter spp | 2–5 d | +/− | + | + | + | Consumption of or contact with raw or undercooked poultry | Stool culture (special request) |
| Clostridium difficile (C difficile associated disease); Community-associated C difficile associated disease | Variable (d to mo) | +/− | + | +/− | + | May occur in patients with or without prior antibiotic use | Stool toxin EIA; anaerobic stool culture and toxin EIA of isolates |
| Cryptosporidium spp | 2–28 d | − | + | + | + | May be chronic in ICH | Microscopic stool examination; DFA; EIA |
| Cyclospora spp | 1–11 d | − | + | + | − | Fatigue; may be chronic if unrecognized | Microscopic stool examination |
| EIEC | 10–18 h | − early; <10% + late | + | − | + | Fecal leukocytes late, as seen with Shigella spp | Stool culture for other pathogens |
| Entamoeba histolytica | 2 d–4 wk | + | + | − | + | Invasive; liver abscess if chronic; may be confused with STEC; patient food and exposure history very important | Microscopic stool examination; serology for chronic or invasive disease; stool EIA or PCR |
| EPEC | 9–12 h | +/− | + | + | + | Infant diarrhea; mucus in stool; dehydration severe; prolonged infections | Stool culture for other pathogens |
| ETEC | 1–3 d | − | + | − | − | Patient history useful (travel, infant weaning) | Stool culture for other pathogens |
| Giardia spp | 1–4 wk | − | + | − | − | Flatulence, bloating; may be chronic if unrecognized | O&P stool examination; stool DFA or EIA |
| Norovirus | 24–48 h | − | − | + | − | Outbreak settings common; most common viral cause of gastroenteritis | PCR testing of stool |
| Rotavirus | 1–3 d | − | − | + | +/− | Common in children; outbreak settings | Stool EIA for rotavirus |
| Salmonella enterica subsp. enterica; 2500 serovars; nontyphoidal | 1–3 d | − | +/− | + | + | May be chronic if not recognized or untreated | Stool culture |
| Shigella spp | 1–2 d | + | + | + | + | S dysenteriae serotype 1 especially severe due to Shiga toxin; all Shigella are invasive of intestinal epithelium | Stool culture (special request for S dysenteriae serotype 1) |
| STEC, O157 or non-O157 (EHEC, VTEC) | 1–8 d | +/− | + | + | +/− | Nonbloody diarrhea may precede blood in stool; oliguria, renal failure, hemolytic uremic syndrome; history of undercooked beef consumption | Stool culture to include SMAC agar for O157 STEC (special request); Shiga toxin EIA; O157 EIA; PCR testing of stool for Stx genes |
| Toxins, bacterial, preformed: Bacillus cereus, Clostridium botulinum, Clostridium perfringens, Staphylococcus aureus | 1–16 h Bc; 12–72 h Cb; 8–16 h Cp; 1–6 h Sa | − | + | + | −/+ | Sudden onset vomiting with Sa; diplopia and muscle paralysis with Cb; Cb is life-threatening | Toxin testing of food |
| Toxins: fish, shellfish, and mushrooms | <30 min to 8 h | − | + | + | +/− | Visual disturbance, confusion, numbness, altered sensations; may be life-threatening | Toxin testing of food |
| Toxins, chemical: organic compounds, metals (As, Sn, Tl, Zn), nitrite, fluoride | 5 min to 8 h | − | + | + | − | Headache, nervousness, twitching movements, visual disturbance | Toxin testing of food |
| Trichinella spp | 1 d–8 wk | − | + | + | + | Myalgias, periorbital edema; cardiac and neurological involvement possible | Larval cysts detectable in muscle tissue by microscopy |
| Vibrio cholerae, serogroup 01 or 0139 | 1–3 d | − | − | + | − | Profuse watery diarrhea; dehydration life-threatening | Stool culture (special request) |
| Vibrio para-hemolyticus, V mimicus, V fluvialis, V furnissii, V hollisae | 2–48 h | +/− | + | + | + | Patient history of seafood consumption | Stool culture (special request) |
| Yersinia enterocolitica and pseudotuberculosis | 24–48 h | +/− | +/− | + | + | Mesenteric lymphadenitis mimicking appendicitis | Stool culture (special request) |
Symbols used: + usually present; +/− may be present; − rarely present.
Abbreviations: DFA, direct fluorescent antibody staining; EIA, enzyme immunoassay; ICH, immunocompromised host; Bc Cb Cp Sa (for toxins, bacterial, preformed) are Bacillus cereus, Clostridium botulinum, Clostridium perfringens, and Staphylococcus aureus, respectively; O&P, ova and parasite; PCR, polymerase chain reaction; SMAC, sorbitol-Mac Conkey.
Treatment, prognosis, and long-term outcome
The treatment of patients with STEC diarrhea consists of fluid replacement, supportive care, and careful monitoring of kidney function, without antibiotic therapy.4, 31 Close attention must be given to the possible development of HUS or other associated thrombotic microangiopathy-associated conditions, such as hemorrhagic colitis or thrombotic thrombocytopenic purpura.9, 13, 47 HUS can develop in patients of all ages, so vigilance must extend across the age spectrum.13, 47 Renal failure can usually be managed by dialysis and patients usually recover in several weeks.13, 31 Treatment of STEC infections with antimicrobial agents is not recommended because of studies demonstrating ineffectiveness and even potential harm to patients who receive antibiotics for STEC diarrhea.19, 31 Long-term follow-up studies to determine the outcome of patients who have experienced STEC diarrhea-associated HUS indicate that there is some increase in risk of renal impairment and hypertension in these patients.19, 31, 47, 48, 52
Summary
STEC (VTEC, EHEC) are important enteric pathogens worldwide, causing diarrhea with or without blood visibly present, and HUS. Children under the age of 10 years are at greatest risk. In children with STEC diarrhea, 15% develop HUS, which has 5% mortality rate. The STEC are unique among diarrheogenic E coli in producing Shiga toxin type 1 and type 2, the virulence factors responsible for bloody diarrhea and HUS. Cattle and other ruminants are the natural reservoir of STEC as their normal intestinal flora. Humans become infected by consumption of foods contaminated with cattle feces, notably undercooked ground (minced) beef, nonpasteurized products, and leafy vegetables that are consumed without cooking. The O157:H7 serotype of STEC predominates in human infections, and has been associated with outbreaks of diarrhea and HUS, but non-O157 STEC currently cause at least one third of STEC diarrhea and HUS. Diagnosis of STEC infection and of HUS is based on clinical signs; patient history; monitoring of renal function (especially in children); rapid testing of stool specimens for Shiga toxins; and isolation of STEC from stool cultures. Early diagnosis of STEC infection is important because of the contraindication for treating STEC using antimicrobial agents, and the intense supportive care needed if renal failure occurs.
Footnotes
The author is employed as an independent contractor by Children's Hospitals and Clinics of Minnesota to provide consulting for laboratory test development.
References
- 1.Hussein H.S., Bollinger L.M. Prevalence of Shiga toxin-producing Escherichia coli in beef cattle. J Food Prot. 2005;68(10):2224–2241. doi: 10.4315/0362-028x-68.10.2224. [DOI] [PubMed] [Google Scholar]
- 2.Oporto B., Esteban J.I., Aduriz G. Escherichia coli O157:H7 and non-O157 Shiga toxin-producing E. coli in healthy cattle, sheep and swine herds in Northern Spain. Zoonoses Public Health. 2008;55(2):73–81. doi: 10.1111/j.1863-2378.2007.01080.x. [DOI] [PubMed] [Google Scholar]
- 3.Nataro J.P., Bopp C.S., Fields P.I. Escherichia, Shigella, and Salmonella. In: Murray P.R., Baron E.J., Jorgensen J.H., editors. Manual of clinical microbiology. 9th edition. American Society for Microbiology; Washington, DC: 2007. pp. 670–687. [Google Scholar]
- 4.Fontaine O., Griffin P., Henao O. Diarrhea, acute. In: Heymann D.L., editor. Control of communicable diseases manual. 19th edition. American Public Health Association; Washington, DC: 2008. pp. 179–195. [Google Scholar]
- 5.CDC Outbreak of Shiga toxin-producing Escherichia coli O157 infection associated with a day camp petting zoo—Pinellas County, Florida, May-June 2007. MMWR Morb Mortal Wkly Rep. 2009;58(16):426–428. [PubMed] [Google Scholar]
- 6.Konowalchuk J., Speirs J.I., Stavrik S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977;18(3):775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC Diagnosis and management of foodborne illnesses: a primer for physicians and other health care professionals. MMWR Recomm Rep. 2004;53(RR04):1–33. [PubMed] [Google Scholar]
- 8.Riley L.W., Remis R.S., Helgerson S.D. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308(12):681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 9.Tarr P.I., Gordon C.A., Chandler W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365(9464):1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 10.Bielaszewska M., Stoewe F., Fruth A. Shiga toxin, cytolethal distending toxin, and hemolysin repertoires in clinical Escherichia coli O91 isolates. J Clin Microbiol. 2009;47(7):2061–2066. doi: 10.1128/JCM.00201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi T., Makino K., Ohnishi M. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 2001;8(1):11–22. doi: 10.1093/dnares/8.1.11. [DOI] [PubMed] [Google Scholar]
- 12.Sandvig K. The Shiga toxins: properties and action on cells. In: Alouf J.E., Popoff M.R., editors. The comprehensive sourcebook of bacterial protein toxins. 3rd edition. Elsevier Academic Press; Philadelphia: 2006. pp. 310–322. [Google Scholar]
- 13.Tarr P.I. Shiga toxin-associated hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: distinct mechanisms of pathogenesis. Kidney Int Suppl. 2009;(112):S29–S32. doi: 10.1038/ki.2008.615. [DOI] [PubMed] [Google Scholar]
- 14.Enter_Net. European Centre for Disease Prevention and Control (ECDC) http://ecdpc.europa.eu/Activities/surveillance/ENTER_NET/reports.html Available at: Accessed July 30, 2009.
- 15.Gerner-Smidt P., Hise K., Kincaid J. PulseNet USA: a five-year update. Foodborne Pathog Dis. 2006;3(1):9–19. doi: 10.1089/fpd.2006.3.9. [DOI] [PubMed] [Google Scholar]
- 16.CDC Importance of culture confirmation of Shiga toxin-producing Escherichia coli infection as illustrated by outbreaks of gastroenteritis—New York and North Carolina, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(38):1042–1045. [PubMed] [Google Scholar]
- 17.Karmali M. The way forward: what should we be doing? VTEC 2009, Buenos Aires, May 10th–13th, 2009. Meeting presentation. Available at: http://www.vtec2009.com.ar/index.cfm?fuseaction=main.home&seccion=1. Accessed August 18, 2009.
- 18.Leotta G.A., Miliwebsky E.S., Chinen I. Characterization of Shiga toxin-producing Escherichia coli O157 strains isolated from humans in Argentina, Australia and New Zealand. BMC Microbiol. 2008;9:46. doi: 10.1186/1471-2180-8-46. http://www.biomedcentral.com/1471-2180/8/46 Available at: Accessed July 28, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaine P.H., Rowe P.C., Orrbine E. Experiences with HUS in Canada: what have we learned about childhood HUS in Canada? Kidney Int Suppl. 2009;75(Suppl 112):S25–S28. doi: 10.1038/ki.2008.614. [DOI] [PubMed] [Google Scholar]
- 20.Orth D., Grif K., Zimmerhackl L.B. Sorbitol-fermenting Shiga toxin-producing Escherichia coli O157 in Austria. Wien Klin Wochenschr. 2009;121(3-4):108–112. doi: 10.1007/s00508-008-1133-z. [DOI] [PubMed] [Google Scholar]
- 21.Grys T.E., Sloan L.M., Rosenblatt J.E. Rapid and sensitive detection of Shiga toxin-producing Escherichia coli from non-enriched stool specimens by real-time PCR in comparison to enzyme immunoassay and culture. J Clin Microbiol. 2009;47(7):2008–2012. doi: 10.1128/JCM.02013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karch H., Bielaszewska M. Sorbitol-fermenting Shiga toxin-producing Escherichia coli O157:H- strains: epidemiology, phenotypic and molecular characteristics, and microbiological diagnosis. J Clin Microbiol. 2001;39(6):2043–2049. doi: 10.1128/JCM.39.6.2043-2049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bielaszewska M., Middendorf B., Koeck R. Shiga toxin-negative attaching and effacing Escherichia coli: distinct clinical associations with bacterial phylogeny and virulence traits and inferred in-host evolution. Clin Infect Dis. 2008;47(2):208–217. doi: 10.1086/589245. [DOI] [PubMed] [Google Scholar]
- 24.Welch R.A. The genus Escherichia. In: Dworkin M., Falkow S., Rosenberg E., editors. 3rd edition. vol. 6. Springer Science, Business Media LLC; New York: 2006. pp. 60–71. (The prokaryotes). Chapter 3.3.3. [Google Scholar]
- 25.Ogura Y., Ooka T., Asadulghani T.J. Extensive genomic diversity and selective conservation of virulence-determinants in enterohemorrhagic Escherichia coli strains O157 and non-O157 serotypes. Genome Biol. 2007;8(7):R138. doi: 10.1186/gb-2007-8-7-r138. http://genomebiology.com/2007/8/7/R138 Available at: Accessed July 22, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karama M., Gyles C.L. Characterization of verotoxin-encoding phages from Escherichia coli O103:H2 strains of bovine and human origins. Appl Environ Microbiol. 2008;74(16):5153–5158. doi: 10.1128/AEM.00723-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavin P.J., Peterson L.R., Pasquariello A.C. Evaluation of performance and potential clinical impact of Prospect Shiga toxin Escherichia coli microplate assay for detection of Shiga toxin-producing E. coli in stool samples. J Clin Microbiol. 2004;42(4):1652–1656. doi: 10.1128/JCM.42.4.1652-1656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teel L.D., Daly J.A., Jerris R.C. Rapid detection of Shiga toxin-producing Escherichia coli by optical immunoassay. J Clin Microbiol. 2007;45(10):3377–3380. doi: 10.1128/JCM.00837-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CDC Recommendations for diagnosis of Shiga toxin-producing Escherichia coli infections by clinical laboratories. MMWR Morb Mortal Wkly Rep. 2009;58(RR12):1–18. [PubMed] [Google Scholar]
- 30.Beutin L., Zimmermann S., Gleier K. Rapid detection and isolation of Shiga-like toxin (verocytotoxin)-producing Escherichia coli by direct testing of individual enterohemolytic colonies from washed sheep blood agar plates in the VTEC-RPLA assay. J Clin Microbiol. 1996;34(11):2812–2814. doi: 10.1128/jcm.34.11.2812-2814.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bitzan M. Treatment options for HUS secondary to Escherichia coli O157:H7. Kidney Int Suppl. 2009;75(Suppl 112):S62–S66. doi: 10.1038/ki.2008.624. [DOI] [PubMed] [Google Scholar]
- 32.Rivero M.A., Padola N.L., Etcheverría A.I. Enterohemorrhagic Escherichia coli and hemolytic-uremic syndrome in Argentina. Medicina (B Aires) 2004;64(4):352–356. [in Spanish] [PubMed] [Google Scholar]
- 33.Hart J., Smith G. Verocytotoxin-producing Escherichia coli O157 outbreak in Wrexham, North Wales, July 2009. Euro Surveill. 2009;14(32) http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19300 Article 5. Available at: Accessed August 18, 2009. [PubMed] [Google Scholar]
- 34.ProMED PRO/AH/EDR>E. COLI O157-UK: Wales dance camp, alert 17-Aug-2009. http://www.promedmail.org Archive Number 20090817.2915. Available at: Accessed August 18, 2009.
- 35.CDC Multistate outbreak of E. coli O157:H7 infections linked to eating raw refrigerated, prepackaged cookie dough. http://www.cdc.gov/ecoli/2009/0630.html Updated June 30, 2009. Available at: Accessed August 18, 2009.
- 36.Greenland K., de Jager C., Heuvelink A. Nationwide outbreak of STEC O157 infection in the Netherlands, December 2008-January 2009: continuous risk of consuming raw beef products. Euro Surveill. 2009;14(8):19129. [PubMed] [Google Scholar]
- 37.CDC Investigation of multistate outbreak of E. coli O157:H7 infections. http://www.cdc.gov/ecoli/june2008outbreak/ Updated July 18, 2008. Available at: Accessed August 19, 2009.
- 38.ProMED PRO/AH/EDR>E. coli O157, restaurant - Canada, 2008: (ON), onions. http://www.promedmail.org Post June 23, 2009. Available at: Accessed August 20, 2009.
- 39.Friesma I., Sigmundsdottir G., van der Zwaluw K. An international outbreak of Shiga toxin-producing Escherichia coli O157 infection due to lettuce, September - October 2007. Euro Surveill. 2008;13(50) doi: 10.2807/ese.13.50.19065-en. http://www.eurosurveillance.org/ViewArticle.aspx?Articleid=19065 Article 6. Available at: Accessed August 19, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Webster D., Cowden J., Locking M. An outbreak of Escherichia coli O157 in Aberdeen Scotland, September, 2007. Euro Surveill. 2007;12(39) doi: 10.2807/esw.12.39.03273-en. http://www.eurosurveillance.org/ViewArticle.aspx?Articleid=3273 Article 1. Available at: Accessed August 19, 2009. [DOI] [PubMed] [Google Scholar]
- 41.CDC Update on multi-state outbreak of E. coli O157:H7 infections from fresh spinach, October 6, 2006. http://www.cdc.gov/ecoli/2006/september/updates/100606.htm Available at: Accessed August 18, 2009.
- 42.Schimmer B., Nygard K., Eriksen H.-M. Outbreak of haemolytic uraemic syndrome in Norway caused by stx2-positive Escherichia coli O103:H25 traced to cured mutton sausages. BMC Infect Dis. 2008;8:41. doi: 10.1186/1471-2334-8-41. http://ncbi.nlm.nih.gov/pubmed/18387178?ordinalpos=1&itool=En…_Discovery_PMC&linkpos=1&logpos$=citedinpmarticles&logdbfrom=pubmed Available at: Accessed August 19, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonoda C., Tagami A., Nagatomo D. An enterohemorrhagic Escherichia coli O26 outbreak at a nursery school in Miyazaki, Japan. Jpn J Infect Dis. 2008;61(1):92–93. [PubMed] [Google Scholar]
- 44.Muraoka R., Okazaki O., Fugimoto Y. An enterohemorrhagic Escherichia coli O103 outbreak at a nursery school in Miyazaki Prefecture, Japan. Jpn J Infect Dis. 2007;60(6):410–411. [PubMed] [Google Scholar]
- 45.CDC Summary of notifiable diseases—United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;56(53):1–94. [PubMed] [Google Scholar]
- 46.Bardiau M., Labrozzo S., Mainil J.G. Putative adhesins of enteropathogenic and enterohemorrhagic Escherichia coli of serogroup 026 isolated from humans and cattle. J Clin Microbiol. 2009;47(7):2090–2096. doi: 10.1128/JCM.02048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alpers C.E. The kidney. In: Kumar V., Abbas A.K., Fausto N., editors. Robbins and Cotran pathologic basis of disease. 7th edition. Elsevier Saunders; Philadelphia: 2005. pp. 955–1021. [Google Scholar]
- 48.Karmali M.A. Host and pathogen determinants of verocytotoxin-producing Escherichia coli-associated hemolytic uremic syndrome. Kidney Int Suppl. 2009;75(Suppl 112):S4–S7. doi: 10.1038/ki.2008.608. [DOI] [PubMed] [Google Scholar]
- 49.Fraser M.E., Chernaia M.M., Kozlov Y.V. Shiga toxin. In: Parker M.W., editor. Protein toxin structure. Chapman and Hall; New York: 1996. pp. 173–190. [Google Scholar]
- 50.Fraser M.E., Fujinaga M., Cherney M.M. Structure of Shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J Biol Chem. 2004;279(26):27511–27517. doi: 10.1074/jbc.M401939200. [DOI] [PubMed] [Google Scholar]
- 51.Chen L., Crawford J.M. The gastrointestinal tract. In: Kumar V., Abbas A.K., Fausto N., editors. Robbins and Cotran pathologic basis of disease. 7th edition. Elsevier Saunders; Philadelphia: 2005. pp. 797–875. [Google Scholar]
- 52.Garg A.X., Suri S., Barrowman N. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA. 2003;290(10):1360–1370. doi: 10.1001/jama.290.10.1360. [DOI] [PubMed] [Google Scholar]