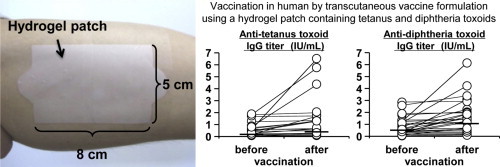
Graphical abstract
Highlights
► We developed a transcutaneous vaccine formulation using a hydrogel patch. ► We performed clinical trial for vaccination against tetanus and diphtheria in human. ► The vaccine formulation did not induce local or systemic severe adverse events. ► Toxoid-specific serum antibody titers increased by application of the formulation. ► This formulation will enable mass treatment and increase of vaccination rates.
Keywords: Transcutaneous immunization, Clinical study, Hydrogel patch, Tetanus, Diphtheria
Abbreviations: DT, diphtheria toxoid; ICDRG, International Contact Dermatitis Research Group; IU, International Unit; LC, Langerhans cell; TCI, transcutaneous immunization; TT, tetanus toxoid
Abstract
Transcutaneous immunization (TCI) is a non-invasive and easy-to-use vaccination method. We demonstrated the efficacy and safety of a transcutaneous vaccine formulation using a hydrogel patch in animal experiments. In the present study, we performed a clinical study to apply our TCI formulation for vaccination against tetanus and diphtheria in human. The TCI device was a hydrogel patch (antigen-free) applied to the left brachial medial skin of 22 healthy volunteers for 48 h. Next, the hydrogel patch, containing 2 mg tetanus toxoid (TT) and 2 mg diphtheria toxoid (DT) as the TCI formulation, was applied to 27 healthy volunteers for 24 h and some volunteers were vaccinated again by TCI formulation. For safety assessment, the patch application site was observed to assess local adverse events, and systemic adverse events were determined by a blood test. The antigen-free hydrogel patch and TCI formulation containing TT and DT did not induce local or systemic severe adverse events. For vaccine efficacy estimation, toxoid-specific serum antibody titers were determined by ELISA and the toxin-neutralizing activity of the induced antibody was evaluated in a passive-challenge experiment. The anti-TT IgG titer and the anti-DT IgG titer increased, and a significant effect was detected by paired t-test. The antibody titers were maintained at higher level than that before vaccination for at least 1 year. Moreover, toxoid-specific antibodies were produced by the second vaccination in some subjects. Antibodies induced by application of the TCI formulation neutralized the toxin and prevented toxic death in mice. In addition, changes in the skin condition due to application of the TCI formulation were observed under in vivo confocal Raman spectroscopy. The amount of water and patch components in the stratum corneum increased after application of the TCI formulation, suggesting that the change in the skin condition was related to antigen penetration. These data indicate that this easy-to-use TCI system induces an immune response without severe adverse reactions in humans. This easy-to-use and safe TCI formulation enables mass treatment in an outbreak setting and increased vaccination rates in developing countries, and will greatly contribute to worldwide countermeasures against infectious diseases.
1. Introduction
Vaccination is the only fundamental prophylaxis against illness and death from infectious disease. The epidemic of emerging infectious diseases such as severe acute respiratory syndrome [1] and highly pathogenic avian influenza (H5N1) [2] and the threat of reemergence infectious diseases such as tuberculosis [3] and malaria [4] emphasize the importance of vaccination. One example of the global threat of infectious disease is the recent outbreak of new influenza A (H1N1), which was declared to have a phase 6 pandemic status by the World Health Organization in June 2009 [5]. The availability of novel vaccination methods is urgently needed at the outset of a pandemic or bioterrorism attack, because conventional injectable vaccination is associated with several problems such as pain, requirement for medical personnel or techniques, needle-related disease or injuries, and storage and transportation issues such as the need for a cold chain [6], [7]. Transcutaneous immunization (TCI) is an attractive vaccination method that eliminates these problems of injectable vaccination [8], [9].
The skin as a target organ for TCI contains various immunocompetent cells such as Langerhans cells (LCs), keratinocytes, dermal dendritic cells, and mast cells [10], [11], [12], [13]. In particular, LCs have a critical role as potent antigen-presenting cells against external antigens. TCI must deliver antigenic proteins to the LCs in the epidermal layer to induce antigen-specific immune responses. This is difficult, however, because the stratum corneum provides a physical barrier to substance penetration [14], [15], [16]. Other transcutaneous vaccine delivery techniques like electroporation [17], [18] and iontophoresis [19], [20] include risks of inflammation and infection resulting from damage to the stratum corneum. In addition, these methods require large-size and specific equipment to use for vaccination. Therefore, an easy-to-use and safe TCI system must be developed for cost-effective and widely available vaccination.
We previously reported the development of a TCI system using a hydrogel patch that delivered antigenic proteins into the skin without destroying or removing the stratum corneum [21]. We demonstrated that LCs captured antigen proteins in the epidermal layer, and migrated to lymph nodes. We reported that the TCI formulation effectively induced the production of antigen-specific antibodies to several model antigens. In addition, a TCI formulation containing tetanus toxoid (TT) and diphtheria toxoid (DT) as practical antigens induced an immune response [22], and the toxoid-specific antibodies produced by this TCI formulation neutralized the tetanus and diphtheria toxins. Moreover, side effects were not observed in animal experiments, based on Draize scoring, pathologic examination, and blood tests. Thus, vaccination using our TCI formulation is not only effective but also safe, and we believe that this TCI system can be applied to humans.
Based on our previous results, we conducted a clinical study of our TCI system using a hydrogel patch. Here we show that our TCI formulation is a practical and novel vaccination system, by evaluation of the safety and efficacy of the vaccine against tetanus and diphtheria in humans.
2. Materials and methods
2.1. Mice
Female ICR mice (7 weeks old) were purchased from SLC Inc. (Hamamatsu, Japan). Mice were maintained in the experimental animal facility at the Osaka University and experiments were conducted in accordance with the guidelines provided by the Animal Care and Use Committee of Osaka University.
2.2. Hydrogel patch formulation
The hydrogel patch formulation, comprising cross-linked HiPAS™ acrylate medical adhesives (CosMED Pharmaceutical Co. Ltd., Kyoto, Japan): octyldodecyl lactate:glycerin:sodium hyaluronan = 100:45:30: 0.2 as weight ratio of composition, was prepared as described previously [21], [22], [23].
2.3. Patch test of a hydrogel patch
Twenty-two healthy volunteers (22–74 years old; Asian people (Japanese); Male 17, Female 5) were enrolled in the study. Informed consent was obtained from all volunteers before enrollment. All clinical procedures were approved by the ethics committee of Nara Medical University.
A TCI device comprising an antigen-free hydrogel patch (1 cm × 1 cm) was applied to the left brachial medial skin of the volunteers for 48 h and covered with wound protection film (Libatape Pharmaceutical Co. Ltd., Kumamoto, Japan) to allow for better skin adherence (Fig. 1 ). Forty-eight hours after patch application, the patch was removed from the investigational sites. Skin irritation reactions were scored according to the classification of the International Contact Dermatitis Research Group (ICDRG) [24], [25] to assess local adverse events at 48 h and 72 h after applying the patch. Blood samples were collected at 0 h and 48 h. A general peripheral blood test and biochemical tests of liver and renal function were performed to evaluate the presence of systemic adverse reactions. In addition, the volunteers were interviewed regarding the presence of subjective symptoms. During this period, the volunteers were instructed to avoid any activity that might influence the investigational sites.
Fig. 1.
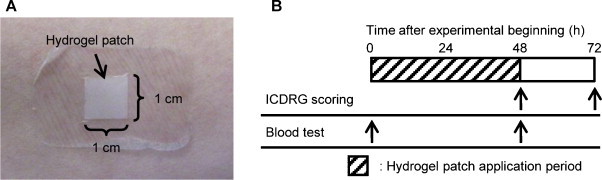
Experimental design of patch test. (A) A hydrogel patch (1 cm × 1 cm) was applied to the left brachial medial skin. (B) Each experiment was conducted at the indicated points.
2.4. Safety and efficacy of the TCI formulation
Twenty-seven healthy volunteers (22–74 years old; Asian people (Japanese); Male 16, Female 11) were enrolled in the study. Informed consent was obtained from all volunteers before enrollment. All clinical procedures were approved by the ethics committee of Nara Medical University.
Toxoid solution, containing 2 mg TT and 2 mg DT (Lot number: TT, FTC0604; DT, DC0408. The Research Foundation for Microbial Diseases of Osaka University, Suita, Japan) in phosphate-buffered saline was dropped on the surface of a hydrogel patch (5 cm × 8 cm), and the water was absorbed under aseptic conditions. The hydrogel patch containing both TT and DT as a TCI formulation was applied to the left brachial medial skin of volunteers for 24 h and covered with wound protection film to allow for better skin adherence (Fig. 2A). Fig. 2B summarizes the vaccination protocol using the TCI formulation. Twenty-four hours after application, the TCI formulation was removed from the investigational sites. Skin irritation reactions were scored according to the classification of the ICDRG to assess local adverse responses at 24 h and 48 h. A general peripheral blood test and biochemical tests of liver and renal function were performed at 0 h and 48 h to evaluate the presence of systemic adverse responses. In addition, the volunteers were interviewed to assess the presence of subjective symptoms. During this period, the volunteers were instructed to avoid any activity that might influence the investigational sites. Sixty days after application of the TCI formulation, serum was collected from all subjects to measure toxoid-specific antibody titers. In addition, a second vaccination in 5 subjects was administered 140 days after first vaccination using the same procedures. ICDRG scoring was performed on day 141 and 142, and a blood test was performed on day 140 and 142 to evaluate possible side effects induced by the second vaccination. To measure antibody production induced by the second vaccination, serum was collected on day 140 and 200. Moreover, to assess long-term vaccine effects, serum was collected from 6 subjects 365 days after the first vaccination.
Fig. 2.
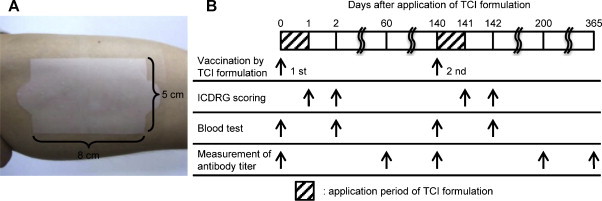
Experimental design of TCI formulation. (A) A hydrogel patch (5 cm × 8 cm) containing TT and DT (2 mg each) was applied on the left brachial medial skin. (B) Each experiment was conducted at the indicated points.
2.5. ELISA for TT and DT
TT- or DT-specific serum IgG titers were measured using an ELISA kit (Tetanus IgG ELISA or diphtheria IgG ELISA; IBL International GmbH, Hamburg, Germany) according to the manufacturer's instructions. Serum IgG titers were expressed as International Units per milliliter (IU/ml).
2.6. Passive-challenge experiment with tetanus toxin
Test sera were collected from 5 subjects on day 0 and 60. ICR mice were injected subcutaneously with a 50-μl mixture of 25-μl test serum dilution (dilution factor; 25, 50, 100, 200, 400, and 800) and 25 μl of a solution containing a lethal amount (20 ng) of tetanus toxin (Sigma–Aldrich Inc., St. Louis, MO) after incubation at 37 °C for 1 h. Mice were monitored for survival for 1 week.
2.7. Analysis of skin condition after application of the TCI formulation
Raman spectra at a range of depth (skin surface down to 20 or 25 μm) were recorded using in vivo confocal Raman spectroscopy (the Model 3510 Skin Composition Analyzer; River Diagnostics, Rotterdam, the Netherlands) in 5 subjects. The left brachial medial skin was measured immediately and 1 h after removing the hydrogel patch containing TT and DT. As a control, the Raman spectra of the right brachial medial skin (untreated skin) were collected.
The water content (mass-%) in the stratum corneum was determined from the water: protein ratio obtained by Raman signal intensity. Raman spectra were measured for skin components (natural moisturizing factor, lactate acid, and ceramide) and the components of the hydrogel patch (glycerin). Their amounts were expressed as relative to the keratin concentration. These procedures were conducted as reported by Caspers et al. [26].
2.8. Statistical analysis
The antibody analysis was performed using the natural logarithm of the antibody values for the paired t-test analysis because the raw data did not follow a Gaussian distribution. For the skin condition analysis, the values at each time point were compared using a Steel–Dwass test at every 2 μm.
3. Results
3.1. Safety of a hydrogel patch
For effective development of TCI, the TCI device should be safe for application in humans. We previously confirmed that our TCI formulation induces few side effects in animal experiments. Accordingly, we estimated that the hydrogel patch was a safe TCI device for humans based on degree of skin irritation and systemic adverse effects. Immediately after patch removal, the scores of most subjects were classified as negative (Table 1A). Although patch application caused faint erythema in 8 subjects (36.4%), these reactions disappeared completely within 24 h of patch removal. In addition, patch application did not have systemic adverse effects as determined by a general peripheral blood test and biochemical tests of liver and renal function (Table S1). None of the subjects described particularly adverse symptoms. Thus, we concluded that our hydrogel patch was safe in humans and TCI using a hydrogel patch is promising for application without local or systemic adverse effects.
Table 1.
Local adverse event according to ICDRG.
| Time after removal | ICDRG score |
||||
|---|---|---|---|---|---|
| −a | ?+b | +c | ++d | ||
| (A) Application of a hydrogel patch | 0 h | 14/22 (63.6%) | 8/22 (36.4%) | 0/22 (0.0%) | 0/22 (0.0%) |
| 24 h | 22/22 (100.0%) | 0/22 (0.0%) | 0/22 (0.0%) | 0/22 (0.0%) | |
| (B) Application of TCI formulation | 0 h | 17/27 (63.0%) | 4/27 (14.8%) | 6/27 (22.2%) | 0/27 (0.0%) |
| 24 h | 15/26 (57.7%) | 2/26 (7.7%) | 9/26 (34.6%) | 0/26 (0.0%) | |
Negative reaction.
Doubtful reaction (faint erythema only).
Weak (non-vesicular) positive reaction (erythema, infiltration and possibly papules).
Strong (vesicular) positive reaction (erythema, infiltration, papules, vesicles).
3.2. Adverse responses due to application of TCI formulation
We prepared a novel TCI formulation containing TT and DT using a hydrogel patch, which was verified to be a safe vaccine device in humans. TT and DT are used practically all over the world as antigen proteins for injectable vaccine. We examined the safety of our TCI formulation in humans. More than half of the subjects were scored as having a negative reaction 24 h after application (Table 1B). Faint erythema was elicited in 4 subjects (14.8%) and a weak positive reaction was observed in 6 subjects (22.2%). Although the number of positive subjects increased (34.6%) within 24 h after removal of the TCI formulation and some subjects described an itching sensation, those irritant responses disappeared several days later. Additionally, systemic adverse responses were not recognized by a general peripheral blood test or biochemical tests of liver and renal function (Table S2). The second vaccination tended to induce similar local and systemic adverse responses (Tables S3 and S4). Taken together, these findings indicate that the TCI formulation using our hydrogel patch can be applied to humans without inducing severe side reactions.
3.3. Immune response to vaccination using TCI formulation
Because we confirmed the safety of a hydrogel patch containing antigen proteins in humans, we next evaluated the efficacy of the TCI formulation against tetanus and diphtheria. As shown in Table 2 and Fig. 3 , anti-TT IgG and anti-DT IgG increased (paired t-test; p < 0.01) following the first vaccination using the TCI formulation, indicating that a single application of our TCI formulation could induce an immune response in humans. We also administered a second vaccination to 5 subjects in whom neither antibody titer was significantly increased by the first vaccination. The IgG titers increased in a part of subjects following the second vaccination, suggesting that an additional application increases the efficacy of the TCI formulation (Table 3 and Fig. 4A and B). Antibody titers on day 365 after application of the TCI formulation were maintained at a higher level than those on day 0 in all subjects examined, although antibody titers tended to be lower on day 365 than on day 60 (Table 4 and Fig. 4C and D). Moreover, to evaluate the toxin-neutralizing effects of the induced antibodies, we performed a passive-challenge experiment with tetanus toxin using the sera of 5 subjects whose antibody levels were increased by the first vaccination. The maximum dilution factor of sera that neutralized toxin on day 60 was higher than that on day 0 in all subjects examined (Table 5 ). Thus, the TCI formulation induced a protective immune response against bacterial toxin. Therefore, our TCI formulation appears to be applicable in humans based on the antibody production by single or multiple vaccinations, the long-term vaccine effect, and the toxin-neutralizing effect.
Table 2.
Distribution of anti-TT and anti-DT antibody titers before and 60 days after application of TCI formulation.
| Anti-TT antibody titer |
Anti-DT antibody titer |
||||
|---|---|---|---|---|---|
| IU/mL | Day 0* | Day 60* | IU/mL | Day 0* | Day 60* |
| <0.1 | 8 | 7 | <0.1 | 1 | 1 |
| 0.1–1.0 | 15 | 9 | 0.1–1.0 | 17 | 8 |
| 1.0–5.0 | 4 | 9 | 1.0–2.0 | 7 | 12 |
| 5.0< | 0 | 2 | 2.0< | 2 | 6 |
| Total | 27 | 27 | Total | 27 | 27 |
| Range | <0.10–1.83 | <0.10–6.47 | Range | <0.10–2.87 | <0.10–6.15 |
| Geometric mean | 0.22 | 0.39 | Geometric mean | 0.56 | 1.06 |
Day 0 vs. 60, p < 0.01 in both anti-toxoids IgG titer by paired t-test.
Number of subjects.
Fig. 3.
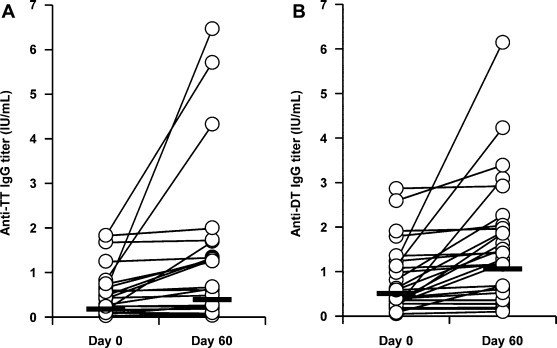
Toxoid-specific IgG titer before and 60 days after application of TCI formulation. The hydrogel patch containing TT and DT (2 mg each/5 cm × 8 cm) was applied on the left brachial medial skin for 24 h. Sixty days later, the serum were collected and anti-TT (A) or DT (B) IgG titer was determined by ELISA. Open circle indicated each sample, and bar indicated geometric mean value.
Table 3.
Distribution of anti-TT and anti-DT antibody titers before (Day 140) and 60 days (Day 200) after second application of TCI formulation.
| Anti-TT antibody titer |
Anti-DT antibody titer |
||||
|---|---|---|---|---|---|
| IU/mL | Day 140* | Day 200* | IU/mL | Day 140* | Day 200* |
| <0.1 | 2 | 2 | <0.1 | 1 | 0 |
| 0.1–1.0 | 3 | 3 | 0.1–1.0 | 3 | 4 |
| 1.0–5.0 | 0 | 0 | 1.0–2.0 | 1 | 1 |
| 5.0< | 0 | 0 | 2.0< | 0 | 0 |
| Total | 5 | 5 | Total | 5 | 5 |
| Range | <0.10–0.46 | <0.10–0.63 | Range | <0.10–1.09 | <0.10–1.33 |
| Geometric mean | 0.09 | 0.12 | Geometric mean | 0.24 | 0.31 |
Number of subjects.
Fig. 4.
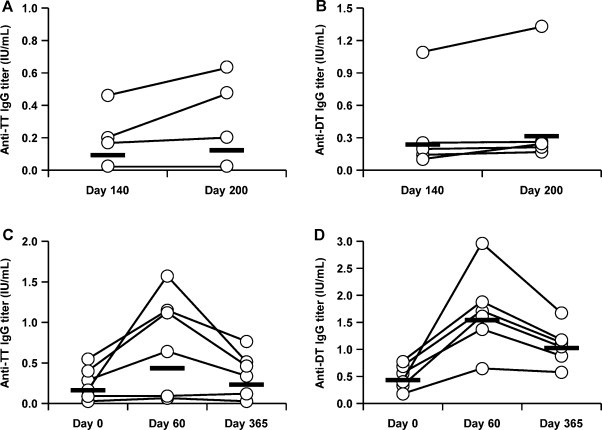
Toxoid-specific IgG titer in second application or 365 days after first application. The hydrogel patch containing TT and DT (2 mg each/5 cm × 8 cm) was applied on the left brachial medial skin for 24 h. The second vaccination in 5 subjects was administered 140 days after first vaccination using the same procedures. Serum was collected on days 140 and 200 and anti-TT (A) or DT (B) IgG titer was determined by ELISA. Additionally, serum was collected from 6 subjects 365 days after the first vaccination and anti-TT (C) or DT (D) IgG titer was determined by ELISA. Open circle indicated each sample, and bar indicated geometric mean value.
Table 4.
Distribution of anti-TT and anti-DT antibody titers before, 60 days, and 365 days after application of TCI formulation.
| Anti-TT antibody titer |
Anti-DT antibody titer |
||||||
|---|---|---|---|---|---|---|---|
| IU/mL | Day 0 | Day 60* | Day 365* | IU/mL | Day 0* | Day 60* | Day 365* |
| <0.1 | 2 | 2 | 1 | <0.1 | 0 | 0 | 0 |
| 0.1–1.0 | 4 | 1 | 5 | 0.1–1.0 | 6 | 1 | 2 |
| 1.0–5.0 | 0 | 3 | 0 | 1.0–2.0 | 0 | 4 | 4 |
| 5.0< | 0 | 0 | 0 | 2.0< | 0 | 1 | 0 |
| Total | 6 | 6 | 6 | Total | 6 | 6 | 6 |
| Range | <0.10–0.54 | <0.10–1.57 | <0.10–0.76 | Range | 0.17–0.77 | 0.64–2.96 | 0.57–1.67 |
| Geometric mean | 0.16 | 0.43 | 0.23 | Geometric mean | 0.43 | 1.54 | 1.02 |
Number of subjects.
Table 5.
Passive-challenge experiment of mice with tetanus toxin.
| ID | Maximum dilution factor of sera for neutralizing toxin |
|
|---|---|---|
| Day 0 | Day 60 | |
| 06 | 400 | 800< |
| 07 | 100 | 800< |
| 09 | 100 | 200 |
| 20 | 25 | 200 |
| 22 | 50 | 200 |
800<; survival of mice injected with the mixture of serum (dilution factor; 800) and tetanus toxin.
3.4. Analysis of skin condition after application of TCI formulation
We used in vivo confocal Raman spectroscopy to analyze the condition of the TCI application site on the skin at the molecular level. Immediately after removing the TCI formulation, the water content in the stratum corneum near the skin surface (0–6 μm) was increased (Fig. 5A), suggesting that corneum hydration is associated with water-soluble antigen penetration of the skin. The water content returned to the level of untreated skin within 1 h after removing the TCI formulation. Natural moisturizing factor in the stratum corneum near the epidermal layer (6–10 μm) also slightly increased (Fig. 5B), although the reason for the increase is unclear. There were no critical changes on other skin components, such as cholesterol, t-urocanic acid (pH 4.0), t-urocanic acid (pH 8.0), urea, pyrrolidone-5-carboxylic acid, serine, or proline (data not shown), indicating that application of the TCI formulation had little adverse effect on skin. Moreover, the amount of glycerin, lactate acid, and ceramide increased in the stratum corneum near the skin surface following application of the TCI formulation (Fig. 5C–E), suggesting that glycerin and octyldodecyl lactate which are components of the hydrogel patch penetrated the stratum corneum. The amount of change in the octyldodecyl lactate can be inferred from the lactate acid and ceramide levels, because octyldodecyl lactate consists of lactate acid and carbon chains, which are ceramide structures. Glycerin is a humectant, holding water in the skin and softening the skin [27]. Octyldodecyl lactate is an absorption enhancer that disrupts the lipid bilayer of the corneum and promotes the transmission or absorption of a material [28]. It is thus assumed that these components penetrating the stratum corneum act to enhance the delivery of antigen proteins into the skin. These data demonstrate that corneum swelling and disruption by application of the TCI formulation contribute to enhance the permeation of antigens into the stratum corneum.
Fig. 5.
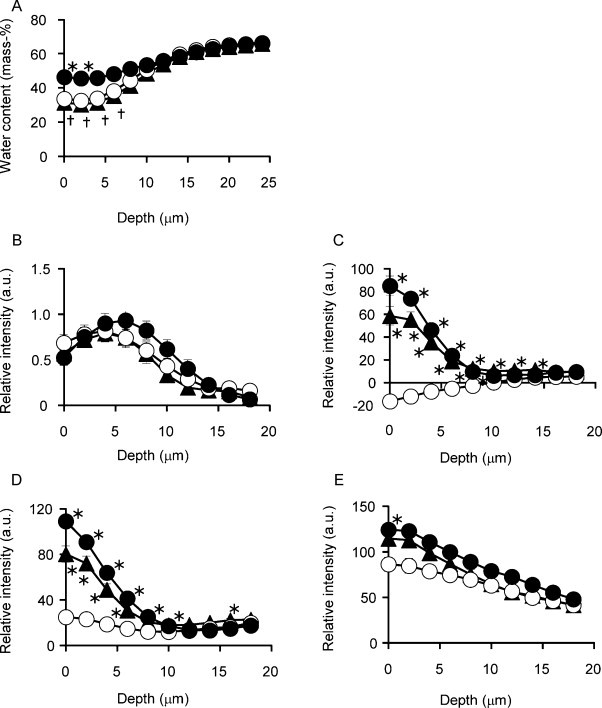
Profile change of skin components or patch compositions in the stratum corneum. The hydrogel patch containing TT and DT (2 mg each/5 cm × 8 cm) was applied on the left brachial medial skin for 24 h. Raman spectra were recorded from the left brachial medial skin immediately (●) and 1 h (▴) after patch removal, and from the right brachial medial skin as untreated skin (○). (A) Water content profiles are expressed in mass-% (grams of water per 100 g of wet tissue). (B–E) The amount profiles are expressed as relative intensity (a. u.) to keratin, (B) natural moisturizing factor, (C) glycerin, (D) lactate acid, (E) ceramide. Data are expressed as mean ± SEM of results from 5 subjects. *p<0.05 vs. untreated skin and †p<0.05 vs. immediately after patch removal by a Steel–Dwass test between groups at every 2 μm.
4. Discussion
In this clinical study, we confirmed that our hydrogel patch was safe for use as a TCI device and observed that a hydrogel patch containing TT and DT as a TCI formulation induced an immune response in humans without a severe adverse reaction. Although other groups have reported developing TCI systems for practical use [29], [30], these systems comprised a gauze patch as the TCI device. TCI systems using a gauze patch are inconvenient and require a cold storage and transportation for the antigen solution as well as a conventional injectable vaccination system, because these systems require that the gauze patch was saturated with antigen solution just before application to the skin. Additionally, safety is an issue in a TCI system using the gauze patch, because the stratum corneum must be disrupted for transcutaneous delivery of enough antigens to induce an immune response. On the other hand, in our system, we can prepare a manageable TCI formulation like general fomentations with a concentrated antigen layer on the surface of the hydrogel patch [21]. Our hydrogel patch is made of safe materials that are already applied to humans. In fact, the TCI formulation caused few severe side effects, and the toxoid-specific antibodies were produced in some subjects by a single application without removing the stratum corneum. Moreover, antibody titers were higher at 1 year after the first vaccination than that on day 0. The immune response is expected to be enhanced by several applications, because a second application of the TCI formulation increased the antibody titers in a part of subjects. Fifth vaccinations are recommended as standard for vaccine against tetanus and diphtheria toxoids [31], [32]. In several applications, this easy-to-use TCI system might be a better formulation than an injectable vaccination with regard to administration. We previously reported that the hydrogel patch promotes antigen penetration through the stratum corneum by causing corneum hydration in an animal experiment [23]. To clarify the mechanism of antigen permeation in humans, we analyzed the skin condition after applying the TCI formulation by using in vivo confocal Raman spectroscopy, and demonstrated that the water content in the stratum corneum increased immediately after patch application. In addition to hydration of the stratum corneum, some of the patch components (glycerin and octyldodecyl lactate) penetrated the stratum corneum. The humectation of the stratum corneum induced by the glycerin [27] and the disruption of the lipid bilayer induced by the octyldodecyl lactate [28] were likely associated with the antigen permeation. Unfortunately, although we planned to examine the antigen permeation of the stratum corneum, we were unable to obtain specific Raman spectra of TT or DT. We speculated that the TCI formulation delivered antigens to the LCs by changing the skin properties without destroying the stratum corneum as a physical barrier, because the immune response was actually induced by application of the TCI formulation. Therefore, we developed an easy-to-use TCI formulation to induce an immune response without severe side effects in humans.
The number of subjects classified as having a doubtful reaction or non-vesicular positive reaction following TCI application was higher than that following a patch test. We assumed that the erythema induced by antigen-free hydrogel patch resulted from the application of adhesive to the skin. On the other hand, when a hydrogel patch containing antigens was applied, local irritation was observed not only at 0 h but also at 24 h after removal of the TCI formulation. We thought that physical stimulation and an inflammatory reaction occurred. In other words, antigens penetrated the skin and induced an immune response to protect the body. One potential reason is that the immunocompetent cells were stimulated by the antigens penetrating the skin. In 10 subjects of the 13 subjects who reported skin irritation, either or both of the anti-TT and DT antibody titers on day 60 increased more than 0.1 IU/mL than that on day 0. Another potential reason is that the subjects already had antibodies against the antigens. Of these 13 subjects, 2 subjects had a high antibody titer against DT on day 0 (1.9 or 2.8 IU/mL). Thus, we inferred that the irritant responses to application of the TCI formulation are not simply side effects, but rather are indicative of immune responses or protective reactions against antigens penetrating the skin. Fever as a systemic symptom and flare or swelling as a local adverse event are observed with injectable vaccine [31], [32]. Because there was no group that was vaccinated by injection in this study, we did not directly compare adverse effects between injectable and transcutaneous vaccination. Based on the results of this study, we do not think that there are likely to be large differences between injectable and transcutaneous vaccination. Therefore, we believe that this TCI system will be able to overcome several of the problems in injectable vaccination and achieve safe and easy-to-use vaccination.
Antibody titers increased and a significant effect was detected by paired t-test. On the other hand, antibody titers in some subjects did not increase even after one or two applications. Almost subjects had already been vaccinated against tetanus and diphtheria as infants. We assumed that in these subjects an increase of the antibody titers was not induced by application of the TCI formulation because the antibody titers were already at their maximum level. Antibody titers greater than 0.1 IU/ml are reported to be necessary for prophylaxis for tetanus [31] or diphtheritic neuropathy [32]. On day 0, the anti-TT antibody titer was higher than 0.1 IU/ml in 19 subjects, and the anti-DT antibody titer was higher than 0.1 IU/ml in 26 subjects. Unfortunately, this explanation could not be confirmed because the vaccination histories of the subjects in this study were not collected. Antibody titers did not increase in 7 of 8 subjects in whom antibody titers were lower than 0.1 IU/ml before TCI application, although antibody titer of 1 subject increased more than 0.15 IU/ml. This suggests that TCI using a hydrogel patch induced little immune response as a primary vaccination. For this reason, we thought that antigens penetrating the skin were insufficient to induce an immune response. We have analyzed the antigen permeation in animal experiments, although we could not in human skin. A hydrogel patch containing 125I-labeled TT or DT was applied to rats, and the amount of antigen distributed to the stratum corneum and antigen penetrating the stratum corneum was calculated from the radioactivity using a gamma counter. The data indicated that the amount of antigen penetrating the skin was a few percent of the antigen applied to the skin [unpublished data], suggesting that the antigen-delivery amount by TCI formulation was small in humans. Additionally, there might be individual differences in the antigen penetration of the stratum corneum. Probably, the potency of the immune response likely differs individually. The current injectable vaccination contains aluminum salts as an adjuvant with few dozens of micrograms of the antigen. Adjuvants would enhance the immune response against antigens in the TCI system as well as in injection. Increasing antigen penetration into the skin or developing a transcutaneous adjuvant might improve the response rate. Moreover, costs may be cut by decreasing the antigen quantity used for the TCI. Studies are in progress to improve the antigen penetration by modifying the hydrogel patch and to develop a transcutaneous adjuvant to activate the immune system like toll-like receptor ligands.
A previous study revealed that the TCI system, which delivers antigens through the stratum corneum, seems to enhance Th2-dominant immune responses, likely in relation to actions of LCs [21]. To analyze the role of LCs in detail, we are now investigating the characteristics of the immune response in transgenic mice, whose LCs or CD11c-positive cells can be depleted. Evaluation of how the TCI formulation impacts the Th1/Th2 balance in humans will help to define the characteristics of the TCI system, and to determine additional applications of the hydrogel patch. For example, our TCI system might be applicable for treating Th1-type autoimmune diseases by correcting the Th1/Th2 balance. Moreover, the hydrogel patch formulation has potential for a wide variety of applications, not only for vaccine/immunotherapy but also for drug delivery therapy in diseases such as atopic dermatitis.
5. Conclusions
We developed a hydrogel patch as a TCI device and demonstrated that our TCI formulation using a hydrogel patch could induce an immune response without causing local and systemic severe adverse reactions in humans. For practical application of our TCI system, the hydrogel patch must be improved and manufacturing methods for the TCI formulation must be established. This easy-to-use TCI formulation might be applicable for mass treatment in the event of an outbreak and for increasing vaccination rates in developing countries. We expect that our TCI system as an innovative vaccination method will greatly contribute to decrease the mortality and morbidity by infectious diseases.
Acknowledgments
We are grateful to The Research Foundation for Microbial Diseases of Osaka University (Suita, Japan) for providing the tetanus and diphtheria toxoids. We thank Dr. Toshimitsu Hamasaki for his advice regarding the statistical analysis. This work was supported by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO) and in part by Health and Labour Sciences Research Grants in Research on New Drug Development from the Ministry of Health, Labour and Welfare.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vaccine.2011.12.130.
Appendix A. Supplementary data
References
- 1.Leppin A., Aro A.R. Risk perceptions related to SARS and avian influenza: theoretical foundations of current empirical research. Int J Behav Med. 2009;16(1):7–29. doi: 10.1007/s12529-008-9002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haque A., Lucas B., Hober D. Influenza A/H5N1 virus outbreaks and preparedness to avert flu pandemic. Ann Biol Clin (Paris) 2007;65(2):125–133. [PubMed] [Google Scholar]
- 3.Valadas E., Antunes F. Tuberculosis, a re-emergent disease. Eur J Radiol. 2005;55(2):154–157. doi: 10.1016/j.ejrad.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Campbell C.C. Malaria: an emerging and re-emerging global plague. FEMS Immunol Med Microbiol. 1997;18(4):325–331. doi: 10.1111/j.1574-695X.1997.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 5.Jones J.H., Salathe M. Early assessment of anxiety and behavioral response to novel swine-origin influenza A(H1N1) PLoS One. 2009;4(12):e8032. doi: 10.1371/journal.pone.0008032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azad N., Rojanasakul Y. Vaccine delivery – current trends and future. Curr Drug Deliv. 2006;3(2):137–146. doi: 10.2174/156720106776359249. [DOI] [PubMed] [Google Scholar]
- 7.Kersten G., Hirschberg H. Needle-free vaccine delivery. Expert Opin Drug Deliv. 2007;4(5):459–474. doi: 10.1517/17425247.4.5.459. [DOI] [PubMed] [Google Scholar]
- 8.Glenn G.M., Scharton-Kersten T., Alving C.R. Advances in vaccine delivery: transcutaneous immunisation. Expert Opin Investig Drugs. 1999;8(6):797–805. doi: 10.1517/13543784.8.6.797. [DOI] [PubMed] [Google Scholar]
- 9.Levine M.M. Can needle-free administration of vaccines become the norm in global immunization? Nat Med. 2003;9(1):99–103. doi: 10.1038/nm0103-99. [DOI] [PubMed] [Google Scholar]
- 10.Mathers A.R., Larregina A.T. Professional antigen-presenting cells of the skin. Immunol Res. 2006;36(1–3):127–136. doi: 10.1385/IR:36:1:127. [DOI] [PubMed] [Google Scholar]
- 11.Metz M., Siebenhaar F., Maurer M. Mast cell functions in the innate skin immune system. Immunobiology. 2008;213(3–4):251–260. doi: 10.1016/j.imbio.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Sugita K., Kabashima K., Atarashi K., Shimauchi T., Kobayashi M., Tokura Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clin Exp Immunol. 2007;147(1):176–183. doi: 10.1111/j.1365-2249.2006.03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutyambizi K., Berger C.L., Edelson R.L. The balance between immunity and tolerance: the role of Langerhans cells. Cell Mol Life Sci. 2009;66(5):831–840. doi: 10.1007/s00018-008-8470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rougier A., Rallis M., Krien P., Lotte C. In vivo percutaneous absorption: a key role for stratum corneum/vehicle partitioning. Arch Dermatol Res. 1990;282(8):498–505. doi: 10.1007/BF00371943. [DOI] [PubMed] [Google Scholar]
- 15.Bos J.D., Meinardi M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–169. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 16.Barry B.W. Breaching the skin's barrier to drugs. Nat Biotechnol. 2004;22(2):165–167. doi: 10.1038/nbt0204-165. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y.L., Murthy S.N., Manjili M.H., Guan L.J., Sen A., Hui S.W. Induction of cytotoxic T-lymphocytes by electroporation-enhanced needle-free skin immunization. Vaccine. 2006;24(9):1282–1290. doi: 10.1016/j.vaccine.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Laddy D.J., Yan J., Khan A.S., Andersen H., Cohn A., Greenhouse J. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J Virol. 2009;83(9):4624–4630. doi: 10.1128/JVI.02335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batheja P., Thakur R., Michniak B. Transdermal iontophoresis. Expert Opin Drug Deliv. 2006;3(1):127–138. doi: 10.1517/17425247.3.1.127. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Thakur R., Fan Q., Michniak B. Transdermal iontophoresis: combination strategies to improve transdermal iontophoretic drug delivery. Eur J Pharm Biopharm. 2005;60(2):179–191. doi: 10.1016/j.ejpb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Ishii Y., Nakae T., Sakamoto F., Matsuo K., Quan Y.S., Kamiyama F. A transcutaneous vaccination system using a hydrogel patch for viral and bacterial infection. J Control Release. 2008;131(2):113–120. doi: 10.1016/j.jconrel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo K., Ishii Y., Quan Y.S., Kamiyama F., Mukai Y., Yoshioka Y. Transcutaneous vaccination using a hydrogel patch induces effective immune responses to tetanus and diphtheria toxoid in hairless rat. J Control Release. 2011;149(1):15–20. doi: 10.1016/j.jconrel.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo K., Ishii Y., Quan Y.S., Kamiyama F., Mukai Y., Okada N. Characterization of transcutaneous protein delivery by a hydrogel patch in animal, human, and tissue-engineered skin models. Biol Pharm Bull. 2011;34(4):586–589. doi: 10.1248/bpb.34.586. [DOI] [PubMed] [Google Scholar]
- 24.Fregert S. Munksgaard; Copenhagen: 1981. Manual of contact dermatitis. pp. 71–76. [Google Scholar]
- 25.Ivens U., Serup J., O’Goshi K. Allergy patch test reading from photographic images: disagreement on ICDRG grading but agreement on simplified tripartite reading. Skin Res Technol. 2007;13(1):110–113. doi: 10.1111/j.1600-0846.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 26.Caspers P.J., Lucassen G.W., Carter E.A., Bruining H.A., Puppels G.J. In vivo confocal Raman microspectroscopy of the skin: noninvasive determination of molecular concentration profiles. J Invest Dermatol. 2001;116(3):434–442. doi: 10.1046/j.1523-1747.2001.01258.x. [DOI] [PubMed] [Google Scholar]
- 27.De Paepe K., Wibaux A., Ward C., Rogiers V. Skin efficacy and biophysical assessment of glycerol-containing hydrocolloid patches. Skin Pharmacol Physiol. 2009;22(5):258–265. doi: 10.1159/000235553. [DOI] [PubMed] [Google Scholar]
- 28.Hood H.L., Kraeling M.E., Robl M.G., Bronaugh R.L. The effects of an alpha hydroxy acid (glycolic acid) on hairless guinea pig skin permeability. Food Chem Toxicol. 1999;37(11):1105–1111. doi: 10.1016/s0278-6915(99)00100-3. [DOI] [PubMed] [Google Scholar]
- 29.Etchart N., Hennino A., Friede M., Dahel K., Dupouy M., Goujon-Henry C. Safety and efficacy of transcutaneous vaccination using a patch with the live-attenuated measles vaccine in humans. Vaccine. 2007;25(39–40):6891–6899. doi: 10.1016/j.vaccine.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Glenn G.M., Taylor D.N., Li X., Frankel S., Montemarano A., Alving C.R. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat Med. 2000;6(12):1403–1406. doi: 10.1038/82225. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. The immunological basis for immunization series, Module 3 Tetanus; 2007.
- 32.World Health Organization. The immunological basis for immunization series, Module 2 Diphtheria; 2009.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


