Highlights
-
•
Drug-resistant influenza viruses present a major public health problem.
-
•
We present an assay for the identification of resistance-conferring mutations.
-
•
We tested 26 clinical samples of an infectious H7N9 influenza virus for mutations.
-
•
The assay successfully identified a mixed population of E120/E120V associated with drug-resistant mutant.
-
•
Parallel testing of clinical specimens and cultured samples may avoid misinterpreting culture-induced mutations.
Keywords: Avian influenza A (H7N9) virus, Pyrosequencing, NAIs resistance, E120V mutation
Abstract
A novel reassortant avian influenza A virus (H7N9) emerged in humans in Eastern China in late February 2013. All virus strains were resistant to adamantanes (amantadine and rimantadine), but susceptible to neuraminidase inhibitors (NAIs) (oseltamivir and zanamivir). One strain (A/shanghai/1/2013) contained the R294K substitution in the neuraminidase (NA) gene, indicating resistance to oseltamivir. Pyrosequencing has proven to be a useful tool in the surveillance of drug resistance in influenza A viruses. Here, we describe a reverse transcription (RT)-PCR assay coupled with pyrosequencing to identify the NA residues E120, H276, and R294 (N9 numbering) of H7N9 viruses. A total of 43 specimens (26 clinical samples and 17 isolates) were tested. Only one isolate containing the E120V heterogenic mutation was detected by pyrosequencing and confirmed by Sanger sequencing. However, this mutation was not detected in the original clinical specimen. Since virus isolation might lead to the selection of variants that might not fully represent the virus population in the clinical specimens, we suggest that using pyrosequencing to detect NAI resistance in H7N9 viruses directly from clinical specimens rather than from cultured isolates. No cross-reactions with other types of influenza virus and respiratory tract viruses were found, and this assay has a sensitivity of 100 copies of synthetic RNA for all three codons. The high sensitivity and specificity of the assay should be sufficient for the detection of positive clinical specimens. In this study, we provide a rapid and reliable method for the characterization of NAI resistance in H7N9 viruses.
1. Introduction
A new type of avian influenza A (H7N9) emerged at the beginning of 2013 in several provinces of Eastern China. Since the first reported case in Shanghai, there have been a total of 131 confirmed avian influenza A (H7N9) infections in humans reported to the WHO, including 36 deaths from February 19 until May 17, 2013 (World Health Organization, 2013a). Most cases progressed rapidly, accompanied by acute respiratory distress syndrome, mediastinal emphysema, and septic shock, resulting in disturbance of consciousness and acute kidney injury. H7N9 viruses have not been previously reported to infect humans. Infections with other pathogenic avian influenza viruses including H5N1, H7N2, H7N3, H7N7, and H9N2 have previously been reported in China and other parts of the world (Chan, 2009, Cheng et al., 2011, Fouchier et al., 2004, Ostrowsky et al., 2012, Tweed et al., 2004). Since the H7N9 virus is a novel, avian-origin reassortant influenza A (H7N9) virus (Gao et al., 2013) without an effective vaccine, antiviral drugs provide a valuable option for the early containment of highly virulent and/or novel strains.
Currently, two classes of drugs, adamantanes (M2 channel blockers) and neuraminidase inhibitors (NAIs) such as zanamivir and oseltamivir are available for the treatment of influenza infections (Nguyen et al., 2012). However, drug-resistant influenza viruses have been reported, such as the emergence and global spread of adamantine-resistant H3N2 viruses in 2003–2004 (Bright et al., 2005) and oseltamivir-resistant seasonal H1N1 viruses in 2007–2009 (Dharan et al., 2009). Since the 2009 H1N1 pandemic, all influenza viruses have been found to be generally resistant to adamantines (Nguyen et al., 2012). Fortunately, NAI resistance was rarely detected in H1N1pdm09 viruses (Graitcer et al., 2011). Oseltamivir-resistant mutants are more common due to the rapid development of resistance compared to zanamivir (Okomo-Adhiambo et al., 2010). Recently, a Q136K mutation which reduced sensitivity to zanamivir, but not oseltamivir, was detected in H3N2 viruses isolated from patients not previously treated with NAIs (Dapat et al., 2010). Although several other classes of anti-influenza compounds exist, NAIs are currently the only option in most clinical settings (Moscona, 2008). The Centers for Disease Control and Prevention (Atlanta, GA, USA) have recommended that all confirmed or probable cases of H7N9 infection under investigation should be treated with NAIs (oseltamivir and zanamivir) as early as possible due to the potential severity of illness associated with H7N9 virus infection (Centers for Disease Control and Prevention (CDC), 2013). Among oseltamivir-resistant viruses, the most commonly detected substitutions (N2 numbering) are H274Y in N1 subtypes (Dharan et al., 2009, Graitcer et al., 2011), E119V and R292K in viruses of N2 subtypes (Ferraris and Lina, 2008, Hurt et al., 2009, Sheu et al., 2008), and D198N/F in type B viruses (Ferraris and Lina, 2008). Recently, one cultured virus isolate (A/shanghai/1/2013) was found to contain the R294K substitution (position 292 with N2 numbering; position 294 with N9 numbering) in the NA protein (Gao et al., 2013). Of 14 H7N9-infected patients treated with oseltamivir, the R294K mutation was subsequently detected in two patients (Hu et al., 2013), indicating that there may be a low barrier to the development of drug resistance in H7N9-infected patients treated with oseltamivir. However, increased surveillance of oseltamivir susceptibility is urgently required.
Recently, nucleotide-based assays such as pyrosequencing have been developed for the detection of NAI-resistance-conferring mutations. Compared with conventional Sanger sequencing methods, pyrosequencing can identify established resistance-conferring substitutions much more rapidly. In addition, the assay can be used directly on clinical specimens (such as nasal or oropharyngeal swabs). In this study, we describe an assay using reverse transcription (RT)-PCR coupled with a pyrosequencing procedure (RT-PCR-Pyroseq) to detect the NA residues E120, H276, and R294 (N9 numbering) of H7N9 viruses. Advantages of this assay include a rapid analysis, simplicity and ease of use, and it provides direct results for characterizing oseltamivir resistance in H7N9 viruses.
2. Materials and methods
2.1. Patient information
The E120V heterogenic mutant isolate is from a 32-year-old female resident of Wuxi City in Jiangsu Province. She was admitted to Wuxi City People's Hospital due to cough and the highest temperature reached was 39.6 °C on March 21, 2013. Chest radiography showed pneumonia in an upper lobe of the left lung. On March 27 and March 31, a throat swab and sputum were sent to the laboratory of Jiangsu Centers for Disease Control and Prevention (JSCDC) for testing by RT-PCR and the results revealed the presence of avian influenza A (H7N9) virus. Meanwhile laboratory tests for other pathogens, including influenza A (H1N1, H3N2, H5N1, H9N2) virus, influenza B virus, and coronavirus, were all negative. Specimens in viral-transport medium were inoculated into Madin–Darby canine kidney (MDCK) cells for virus isolation on March 31, including two or three blind passages. Oral oseltamivir (75 mg) was administered twice daily beginning on March 24, 2013. On March 28th, the patient was transferred to the intensive care unit (ICU). Unfortunately, the patient died of acute respiratory distress syndrome on April 24, 2013.
2.2. Clinical specimens and virus isolates
Twenty-six original clinical samples (throat swabs or sputum) in Jiangsu, China were collected by the JSCDC from February to April 2013. Most of them were collected from patients after treatment with oseltamivir. All specimens were first propagated in the allantoic cavities of 9- to 11-day-old specific pathogen-free embryonated chicken eggs. Viruses that were not successfully propagated in eggs were cultured in MDCK cells at the JSCDC. A total of 10 viral isolates from embryonated chicken eggs and seven isolates from MDCK cells were successfully collected. This project was approved by the Ethics Committee of Jiangsu Provincial Center for Diseases Prevention and Control and written informed consent was obtained from all participants.
2.3. RNA extraction and virus identification
Viral RNA from the isolates and clinical samples were extracted with a nucleic acid extraction kit (QIAsymphony virus/bacteria mini kit, Qiagen Germany) on a MagNA Pure™ system (Roche Applied Science, Germany) according to the manufacturer's instructions. RT-PCR was performed on all specimens using the SuperScript III Platinum One-Step Quantitative RT-PCR kit (Invitrogen, USA) on an ABI 7900 real-time PCR system (ABI, USA) with WHO issued primers and probes according to the WHO recommended protocol (World Health Organization, 2013b). All samples had positive PCR results with cycle threshold (Ct) values ≤38 for influenza A, H7, and N9 targets.
2.4. Primer design
The NA sequences of H7N9 viruses were used as templates in this study and were obtained from the GenBank database (A/Hangzhou/1/2013(H7N9), Accession Number: KC853765). Primers were designed using the Pyrosequening Assay Design Software (Qiagen, Sweden), and were then aligned with 377 N9 sequences from human and avian influenza A virus isolates acquired from GenBank at the Influenza Sequence Database. The primers were synthesized by Sangon Biotech Co. (China). Table 1 shows the list of RT-PCR and pyrosequencing primers used in this study.
Table 1.
The RT-PCR and sequencing primers used in this study.
| Primers | Sequence (5′-3′) | NA target residue |
|---|---|---|
| RT-PCR | ||
| H7N9-NA-F764a | AAGAGGGRAAAATATTGAAATGG | |
| H7N9-NA-R919-biota | TTGCYACTGGRTCTATCTGAATCA | |
| H7N9-NA-F215 | TGGAAGAGAGARCAARCAGGAATT | |
| H7N9-NA-R386-biot | GCATAGAAYCTGCATTCATCTGG | |
| Pyrosequencing | ||
| H7N9-NA-S785 | GGGARTCTCTGACTGGA | H276 |
| H7N9-NA-S844 | ACAGGAATTACYTGCAC | R294 |
| H7N9-NA-S323 | CGGATGTTTTAGTCACAAG | E120 |
The same primer was used for NA residue 294.
2.5. RT-PCR and pyrosequencing
RT-PCR amplifications were performed using a One-Step RT-PCR Kit (Qiagen, Germany). Primers (Table 1) were added at a concentration of 0.2 μM in a standard 50 μL reaction volume. The RT-PCR protocol was as follows: 50 °C for 30 min, then 95 °C for 15 min, followed by 45 cycles of 30 s at 94 °C, 30 s at 55 °C and 30 s at 72 °C. To confirm the results of the RT-PCR, the products were visualized using the QIAxcel System (Qiagen, Germany) for DNA fragment analysis. One of the PCR primers was biotinylated and was captured on streptavidin-coated Sepharose beads (GE Healthcare, USA) by incubation at room temperature for 10–15 minutes with agitation at 1400 rpm. Single-stranded DNA was obtained by washing the immobilized PCR product with 70% EtOH, and was then denatured with 0.1 M NaOH and washed with washing buffer using a Vacuum Prep Tool and Vacuum Prep Worktable (Qiagen, Germany). The beads were then suspended in 45 μL annealing buffer containing 0.3 μM of the sequencing primer. Single-stranded DNA was hybridized to the sequencing primer by incubation at 80 °C for 2 min and then at room temperature for 5 min. Primed single-stranded PCR products were sequenced using a PSQTM HS96A System (Qiagen, Germany). All of the sequencing reactions were performed with the PSQ 96 Reagent Kit (Qiagen, Germany). Consensus pre-programmed dispensation orders were determined by integrating sequence information for each strain at a given sequencing site. We detected the antiviral susceptibility at NA positions 120, 276, and 294 of H7N9 viruses according to the manufacturer's protocol. Each sample was run in duplicate by RT-PCR-Pyroseq and positive samples which contained resistant codons were confirmed by Sanger sequencing.
2.6. Sanger sequencing for the NA E120 residue
Sanger sequencing was employed to verify the results of one isolate containing the E120V mutation and the matching clinical sample. The RT-PCR amplicons for pyrosequencing were cycle sequenced using the BigDye Terminators Reaction Kit (ABI, USA) on an ABI automated DNA sequencer (3130 DNA Analyzer).
2.7. Detection sensitivity and specificity of the RT-PCR-pyroseq method
To determine the sensitivity of the assay for detecting mutated oseltamivir-resistant codons – 120V (GTA), 276Y (TAT), and 294K (AAG) and particularly when they appear in a mixed population of drug-sensitive and drug-resistant quasispecies, five NA plasmids (Genescript, China), one wild-type containing both H276 (CAT) and R294 (AGG) codons, one wild-type containing E120 (GAA) codon, three mutations containing 276Y (TAT), 294K (AAG) and 120V (GTA) codons were constructed, respectively. The H7N9 viral RNA transcripts of NA genes were amplified from these plasmids with primers containing T7 promoter sequence in the reverse sides and were in vitro transcribed with T7 RNA polymerase (TaKaRa, China) according to the manufacturer's instructions. The synthetic RNA transcripts were then purified, quantified, mixed in equal-molar amounts, and diluted serially to final concentrations from 107 to 101 RNA copies per assay. The synthetic RNA transcripts were also adjusted to defined 120E/V, 294R/K, and 276H/Y mixtures (100/0, 90/10, 80/20, 50/50, 20/80, 10/90, 0/100), respectively, and were amplified and pyrosequenced.
To evaluate the specificity of the RT-PCR-Pyroseq procedure, the isolates of seasonal influenza H1N1, H1N1pdm09, H3N2, H5N1, H9N2, influenza type B, parainfluenza viruses (types 1, 2, and 4), human coronaviruses (229E, OC43, HKU1, and NL63), respiratory syncytial viruses and rhinoviruses were tested using the RT-PCR-Pyroseq method.
3. Results
3.1. Detection of NA residues H276 and R294
We amplified the targeted 156 bp NA gene fragment containing the H276 and R294 codons from all of the H7N9-positive specimens by RT-PCR. Both H276 and R294 residues were detected in all 26 clinical samples and 17 isolates by the RT-PCR-Pyroseq method. A histidine to tyrosine mutation at NA residue 276 (CAT to TAT) of H5N1 viruses was reported to confer resistance to oseltamivir (Deyde et al., 2009, Le et al., 2005). Pyrosequencing results show a histidine (CAT) residue at position 276 in all samples (Fig. 1A). The amplicon generated from the primer set H7N9-NA-F764 and H7N9-NA-R919-biot (where biot represents biotin) was also used as a DNA template to analyze changes at residue R294, which is known to confer resistance to oseltamivir. Previous studies reported an arginine (AGG) to lysine (AAG) mutation in one isolate (A/shanghai/1/2013) (Gao et al., 2013) and in two H7N9-infected patients after oseltamivir therapy (Hu et al., 2013). However, we did not find the R294K mutation in any samples collected from Jiangsu province. Pyrosequencing results show the wild-type sequence with AGG (arginine) at codon 294 (Fig. 1B).
Fig. 1.
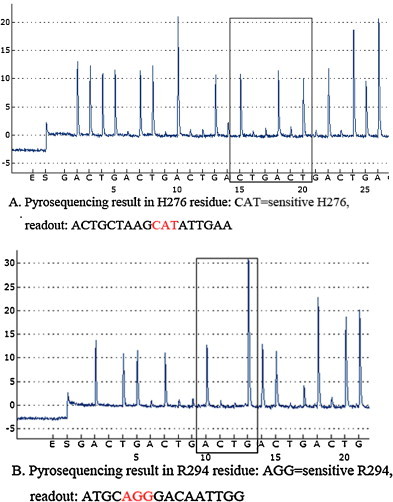
Detection of H276 and R294 codons using the cyclic dispensation order (GACT)10. Pyrogram sequence results represent the wild-type NA-H276 (CAT, red color) using the primer S785 and (GACT)10 (A). Pyrogram sequence results represent the wild-type NA-R294 (AGG, red color) using the primer S844 and (GACT)10 (B). The letters listed below the peaks of each panel represent the nucleotides dispensed during pyrosequencing on the PyroMark ID system. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Detection of the NA residue E120
It is a challenge to design a universal set of pyrosequencing primers for all three codons of interest, namely H276, R294, and E120 in H7N9 viruses since E120 is distant from the others. As such, the amplicon generated from the primer set H7N9-NA-F215 and H7N9-NA-R386-biot (Table 1) was used as a DNA template to analyze changes at residue E120, which is known to confer resistance to oseltamivir. RT-PCR successfully amplified the targeted 172 bp NA gene fragment containing the E120 codon from all the H7N9-positive specimens. An E120V (position 119 with N2 numbering; position 120 with N9 numbering) substitution (GAA to GTA) in the NA gene of H3N2 viruses has been reported to confer resistance to oseltamivir (Okomo-Adhiambo et al., 2010). In our study, the results of the pyrosequencing assay showed that only one viral isolate from the patient described above (which was propagated one generation in MDCK cells) had the sequence GT/AA (Fig. 2A). To further investigate this isolate, its RT-PCR products were tested by Sanger sequencing. The results revealed a mix of nucleotides with T/A coding for the amino acid at position 120 (Fig. 2B), indicating that glutamic acid (GAA) was partially replaced by valine (GTA). High-throughput Illumina Miseq sequencing results also confirmed and differentiated the mixed population of GAA and GTA at position 120 from the NA gene, which showed a 54:46 Glu/Val ratio in the cultured virus population (data not shown). Interestingly, the heterogenic mutant E120V was not detected in the matching clinical specimen using either pyrosequencing (Fig. 2C) or Sanger sequencing (Fig. 2D). All of the other H7N9 clinical samples were detected as wild-type E120 (GAA), suggesting that this variant was present in very low proportions (<20%) in clinical specimens.
Fig. 2.
Detection of E120 using the primer S323 and the cyclic dispensation order (GTAC)10. Pyrogram sequence results at residue E120 (highlighted) had the sequence (GT/AA, red color), indicating that glutamic acid (GAA) was partly replaced by valine (GTA) in the specimen isolated from MDCK cells (A) while Sanger sequencing results showed a mix of nucleotides with T/A coding for the amino acid at position 120 (highlighted) in the same specimen (B). Pyrogram sequence results at residue E120 (highlighted) representing the oseltamivir-susceptible codon (GAA, red color) (C) and Sanger sequencing results showed GAA coding for the amino acid at position 120 (D) in its original clinical specimen. The letters listed below the peaks of each panel are the nucleotides dispensed during pyrosequencing on the PyroMark ID system. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Sensitivity and specificity
The analytical sensitivity of the RT-PCR-Pyroseq assay was assessed using 10-fold serial dilutions of synthetic H7N9 viral RNA transcripts. The results demonstrated that the assay has a detection limit of 100 copies of synthetic RNA for all six codons (Table 2 ). The evaluation of the pyrosequencing revealed that the NA-120, NA-294 and NA-276 variants in mixtures (100/0, 90/10, 80/20, 50/50, 20/80, 10/90, 0/100) were detectable from 107 to 102 RNA copies. In this range, the results demonstrated that the detection limit of minor variants was about 20% (20/80 or 80/20) in a mixed population of drug-sensitive and -resistant quasispecies. A cyclic dispensation order (GTCA)10, (GACT)10, and (GTAC)10 was used for detection of NA variants. Fig. 3 showed the representative Pyrosequencing results of 276H/Y, 294R/K, and 120E/V polymorphism in differential mixtures (50/50, 100/0, and 0/100).
Table 2.
Sensitivity of the RT-PCR-pyroseq assay.
| Copies | 276H/Y codon | 294R/K codon | 120E/V codon |
|---|---|---|---|
| 107–102 | CAT/TAT | AGG/AAG | GAA/GTA |
| 101 | ND | ND | ND |
ND: not detectable.
Fig. 3.
Pyrosequecing analysis of NA-276, NA-294, and NA-120 polymorphisms in synthetic H7N9 viral RNA mixtures (50/50, 100/0, and 0/100, respectively). The pyrogram represents a NA-276 H/Y variants with codon (C/TAT) (A), wild-type 276H (CAT) (B), and mutant 276Y (TAT) (C) using the cyclic entry (GTCA)10. The pyrogram represents a NA-294 R/K variants with codon (AG/AG) (D), wild-type 294R (AGG) (E), and mutant 294K (AAG) (F) using the cyclic entry (GACT)10. The pyrogram represents a NA-120 E/V variants with codon (GA/TA) (G), wild-type 120E (GAA) (H), and mutant 120V (GTA) (I) using the cyclic entry (GTAC)10.
To assess the specificity of the assay, seasonal influenza H1N1, H1N1pdm09, H3N2, H5N1, H9N2, influenza type B, parainfluenza viruses (types 1, 2, and 4), human coronaviruses (229E, OC43, HKU1, and NL63), respiratory syncytial viruses and rhinoviruses were tested. There were no positive results obtained with the viral RNA extracts described above, indicating that the assays has a high specificity.
4. Discussion
Monitoring the susceptibilities of influenza A (H7N9) viruses to anti-influenza drugs, especially NAIs, is critical, since these viruses are resistant to adamantanes (Gao et al., 2013). NAIs, most commonly oseltamivir, have been advocated by many countries for use during influenza season. The widespread use of NAIs has caused the emergence of drug-resistant viruses and has become a serious public health problem. For example, Hu et al. (2013) reported that an R294K mutation in the NA gene that conferred resistance to NAIs was identified in two patients infected with H7N9 viruses after antiviral treatment (Hu et al., 2013). In this study, we demonstrated that a rapid and high-throughput pyrosequencing method could be used for the reliable detection of sequences associated with antiviral susceptibility at positions E120, H276, and R294 of H7N9 viruses. Importantly, neither the H276Y nor the R294K mutations were detected in 26 clinical specimens. Previous studies have observed the emergence of two NAI-resistant variants of an influenza A (H3N2) virus, with either valine (E120V) or isoleucine (E120I) at position 120 (N9 numbering), in an oseltamivir-treated, immunocompromised child (Okomo-Adhiambo et al., 2010, Simon et al., 2011). In our study, only one cultured virus isolate from the patient described above with the heterogenic E120V mutation was detected by our pyrosequencing method. The high-throughput sequencing data showed a 54:46 Glu/Val ratio in the virus population (unpublished data). Unfortunately, we did not receive the clinical sample prior to the commencement of oseltamivir therapy. The heterogenic mutation was detected in the isolate after 8 days of oseltamivir treatment. However, this mutation was not detected in the original clinical specimen using either Sanger sequencing or pyrosequencing. Our study provides evidence that although the heterogenic E120V mutation has been shown to emerge following oseltamivir treatment, the detection could be limited to the virus isolate propagated in MDCK cells, where this virus variant may has an apparent growth advantage over wild-type viruses. This phenomenon, though not clearly understood, could be a consequence of different requirements in the NA activity when virus replication occurs in cell culture versus the human host (Okomo-Adhiambo et al., 2010). The pyrosequencing method developed in this study has a detection limit of minor variants about 20%. If virus carried mutations in proportions below the level of detection, false negative result may be occurred by this assay. A more sensitive technique such as molecular cloning may needed to detect very minor virus variants in clinical specimens. Since virus isolation might lead to the selection of mutant viruses that might not fully represent the virus population in the clinical specimen, we should detect NAI resistance among clinical specimens of avian H7N9 influenza virus and avoid culture-induced heterogenic mutations.
Mutations in viruses with RNA genomes and error-prone polymerases such as influenza viruses have been identified more frequently among isolates obtained after virus propagation in cell culture compared with primary clinical specimens, suggesting that virus replication in tissue culture can lead to the selection of variants with altered receptor specificity (DeVries et al., 2012). For example, the E120V mutation in the NA gene was detected in cultured viruses but not in matching clinical specimens of influenza A (H3N2) viruses (Okomo-Adhiambo et al., 2010). However, it is not definitively clear whether the mutations arise during passaging, and would irrespective of cell culture, or whether mutations are selectively driven by the specific cell system used. The generation of quasispecies also occurs in infected individuals, suggesting that mutations arise as the virus replicates (Lauring and Andino, 2010). Therefore, the importance of performing molecular testing for influenza viruses on matching clinical and virus isolates obtained on the same sampling date is emphasized.
Although clinical resistance to NAIs has not been conclusively established, the pyrosequencing assay presented in this study provides a reliable tool for the rapid and high-throughput detection of viral variants in an epidemic or pandemic outbreak of influenza. When compared to Sanger sequencing, pyrosequencing is less expensive and time-consuming for determining the sequences associated with NAI resistance in H7N9 viruses. In addition, the pyrosequencing system has built-in alignment capabilities, making sequence analysis simpler. This assay has a sensitivity of 100 copies of synthetic RNA for all three codons (Table 2). The sensitivity and specificity of the assay should be sufficient for the detected of positive clinical specimens.
In conclusion, the timely determination of drug susceptibility is critical in order to make informed decisions about antiviral usage in the control of influenza A (H7N9) virus infections. Pyrosequencing provides a high-throughput and rapid platform to directly detect NAI resistance in H7N9 viruses without extensive sample preparation.
Conflict of interest
We declare that we have no conflict of interest.
Acknowledgments
This study was supported in part by the National Major Science & Technology Projects for Infectious Disease Control and Prevention (2013ZX10004103-006 and 2012ZX10004401), the Jiangsu Province National Science Foundation (BK20131451), the Jiangsu Province Health Development Project with Science and Education (ZX201109), the Jiangsu Province Key Medical Talent Foundation (RC2011191, RC2011084, JKRC2011001), and the Science and Technology Pillar Program of Jiangsu Province (BE2011796).
References
- Bright R.A., Medina M.J., Xu X., Perez-Oronoz G., Wallis T.R., Davis X.M., Povinelli L., Cox N.J., Klimov A.I. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet. 2005;366(9492):1175–1181. doi: 10.1016/S0140-6736(05)67338-2. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention (CDC) Centers for Disease Control and Prevention (CDC); 2013. Interim Guidance on the Use of Antiviral Agents for Treatment of Human Infections with Avian Influenza A (H7N9)http://www.cdc.gov/flu/avianflu/h7n9-antiviral-treatment.htm [Google Scholar]
- Chan P.K. A review on human influenza A H5N1 infections in Hong Kong. Sci. China C: Life Sci. 2009;52(5):412–418. doi: 10.1007/s11427-009-0063-y. [DOI] [PubMed] [Google Scholar]
- Cheng V.C., Chan J.F., Wen X., Wu W.L., Que T.L., Chen H., Chan K.H., Yuen K.Y. Infection of immunocompromised patients by avian H9N2 influenza A virus. J. Infect. 2011;62(5):394–399. doi: 10.1016/j.jinf.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Dapat C., Suzuki Y., Saito R., Kyaw Y., Myint Y.Y., Lin N., Oo H.N., Oo K.Y., Win N., Naito M., Hasegawa G., Dapat I.C., Zaraket H., Baranovich T., Nishikawa M., Saito T., Suzuki H. Rare influenza A (H3N2) variants with reduced sensitivity to antiviral drugs. Emerg. Infect. Dis. 2010;16(3):493–496. doi: 10.3201/eid1603.091321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries A., Wotton J., Lees C., Boxrud D., Uyeki T., Lynfield R. Neuraminidase H275Y and hemagglutinin D222G mutations in a fatal case of 2009 pandemic influenza A (H1N1) virus infection. Influenza Other Respi. Viruses. 2012;6(6):e85–e88. doi: 10.1111/j.1750-2659.2011.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyde V.M., Nguyen T., Bright R.A., Balish A., Shu B., Lindstrom S., Klimov A.I., Gubareva L.V. Detection of molecular markers of antiviral resistance in influenza A (H5N1) viruses using a pyrosequencing method. Antimicrob. Agents Chemother. 2009;53(3):1039–1047. doi: 10.1128/AAC.01446-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharan N.J., Gubareva L.V., Meyer J.J., Okomo-Adhiambo M., McClinton R.C., Marshall S.A., St George K., Epperson S., Brammer L., Klimov A.I., Bresee J.S., Fry A.M. Infections with oseltamivir-resistant influenza A (H1N1) virus in the United States. JAMA. 2009;301(10):1034–1041. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- Ferraris O., Lina B. Mutations of neuraminidase implicated in neuraminidase inhibitors resistance. J. Clin. Virol. 2008;41(1):13–19. doi: 10.1016/j.jcv.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Fouchier R.A., Schneeberger P.M., Rozendaal F.W., Broekman J.M., Kemink S.A., Munster V., Kuiken T., Rimmelzwaan G.F., Schutten M., Van Doornum G.J., Koch G., Bosman A., Koopmans M., Osterhaus A.D. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. U.S.A. 2004;101(5):1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Cao B., Hu Y., Feng Z., Wang D., Hu W., Chen J., Jie Z., Qiu H., Xu K., Xu X., Lu H., Zhu W., Gao Z., Xiang N., Shen Y., He Z., Gu Y., Zhang Z., Yang Y., Zhao X., Zhou L., Li X., Zou S., Zhang Y., Yang L., Guo J., Dong J., Li Q., Dong L., Zhu Y., Bai T., Wang S., Hao P., Yang W., Han J., Yu H., Li D., Gao G.F., Wu G., Wang Y., Yuan Z., Shu Y. Human Infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013;368(20):1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- Graitcer S.B., Gubareva L., Kamimoto L., Doshi S., Vandermeer M., Louie J., Waters C., Moore Z., Sleeman K., Okomo-Adhiambo M., Marshall S.A., St George K., Pan C.Y., LaPlante J.M., Klimov A., Fry A.M. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg. Infect. Dis. 2011;17(2):255–257. doi: 10.3201/eid1702.101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Lu S., Song Z., Wang W., Hao P., Li J., Zhang X., Yen H.L., Shi B., Li T., Guan W., Xu L., Liu Y., Wang S., Tian D., Zhu Z., He J., Huang K., Chen H., Zheng L., Li X., Ping J., Kang B., Xi X., Zha L., Li Y., Zhang Z., Peiris M., Yuan Z. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet. 2013;381(9885):2273–2279. doi: 10.1016/S0140-6736(13)61125-3. [DOI] [PubMed] [Google Scholar]
- Hurt A.C., Holien J.K., Parker M.W., Barr I.G. Oseltamivir resistance and the H274Y neuraminidase mutation in seasonal, pandemic and highly pathogenic influenza viruses. Drugs. 2009;69(18):2523–2531. doi: 10.2165/11531450-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lauring A.S., Andino R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 2010;6(7):e1001005. doi: 10.1371/journal.ppat.1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q.M., Kiso M., Someya K., Sakai Y.T., Nguyen T.H., Nguyen K.H., Pham N.D., Ngyen H.H., Yamada S., Muramoto Y., Horimoto T., Takada A., Goto H., Suzuki T., Suzuki Y., Kawaoka Y. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437(7062):1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- Moscona A. Medical management of influenza infection. Annu. Rev. Med. 2008;59:397–413. doi: 10.1146/annurev.med.59.061506.213121. [DOI] [PubMed] [Google Scholar]
- Nguyen H.T., Fry A.M., Gubareva L.V. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir. Ther. 2012;17(1 Pt B):159–173. doi: 10.3851/IMP2067. [DOI] [PubMed] [Google Scholar]
- Okomo-Adhiambo M., Demmler-Harrison G.J., Deyde V.M., Sheu T.G., Xu X., Klimov A.I., Gubareva L.V. Detection of E119V and E119I mutations in influenza A (H3N2) viruses isolated from an immunocompromised patient: challenges in diagnosis of oseltamivir resistance. Antimicrob. Agents Chemother. 2010;54(5):1834–1841. doi: 10.1128/AAC.01608-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowsky B., Huang A., Terry W., Anton D., Brunagel B., Traynor L., Abid S., Johnson G., Kacica M., Katz J., Edwards L., Lindstrom S., Klimov A., Uyeki T.M. Low pathogenic avian influenza A (H7N2) virus infection in immunocompromised adult, New York, USA, 2003. Emerg. Infect. Dis. 2012;18(7):1128–1131. doi: 10.3201/eid1807.111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu T.G., Deyde V.M., Okomo-Adhiambo M., Garten R.J., Xu X., Bright R.A., Butler E.N., Wallis T.R., Klimov A.I., Gubareva L.V. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob. Agents Chemother. 2008;52(9):3284–3292. doi: 10.1128/AAC.00555-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P., Holder B.P., Bouhy X., Abed Y., Beauchemin C.A., Boivin G. The I222V neuraminidase mutation has a compensatory role in replication of an oseltamivir-resistant influenza virus A/H3N2 E119V mutant. J. Clin. Microbiol. 2011;49(2):715–717. doi: 10.1128/JCM.01732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweed S.A., Skowronski D.M., David S.T., Larder A., Petric M., Lees W., Li Y., Katz J., Krajden M., Tellier R., Halpert C., Hirst M., Astell C., Lawrence D., Mak A. Human illness from avian influenza H7N3, British Columbia. Emerg. Infect. Dis. 2004;10(12):2196–2199. doi: 10.3201/eid1012.040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2013. Number of Confirmed Human Cases for Avian Influenza A (H7N9) Reported to WHO-Update.http://www.who.int/influenza/human_animal_interface/influenza_h7n9/06_ReportWebH7N9Number.pdf [Google Scholar]
- World Health Organization . World Health Organization; 2013. Real-time RT-PCR Protocol for the Detection of Avian Influenza A (H7N9) Virus.http://www.who.int/influenza/gisrs_laboratory/cnic_realtime_rt_pcr_protocol_a_h7n9.pdf [Google Scholar]


