Abstract
Bovine Neonatal Pancytopenia (BNP) is a new emerging disease observed since 2007 in Germany and neighbouring countries. The syndrome affects newborn calves and is characterized by pancytopenia, severe bleeding and high lethality. So far, a causative role of infectious or toxic agents has been ruled out. Instead, the syndrome is induced after ingestion of colostrum, the first milk that supplies the calf with maternal antibodies. In analogy to similar diseases in humans it has therefore been postulated that BNP is caused by alloreactive, maternal antibodies. There is a striking association between BNP and a previous vaccination of the respective dams with a particular vaccine against Bovine Virus Diarrhoea (BVD). This association has led to a suspension of the marketing authorisation for the vaccine, by the European Commission. The current study investigates the role of this vaccine in the pathogenesis of BNP. By flow cytometry we were able to demonstrate that sera of BNP dams (dams that gave birth to a BNP calf) harbour alloreactive antibodies binding to surface antigens on bovine leukocytes. A significantly weaker alloreactivity was observed with sera of non-BNP dams that have been vaccinated with the same vaccine but delivered healthy calves. No binding was seen with non-BVD-vaccinated control cows and animals that were vaccinated with other inactivated BVD vaccines so far not associated with BNP. The binding is functionally relevant, because opsonization of bovine leukocytes with alloantibodies led to an elevated cytophagocytosis by bovine macrophages. To test whether the vaccine induces alloreactive antibodies two strategies were employed: Guinea pigs were vaccinated with a panel of commercially available BVD-vaccines. Only the incriminated vaccine induced antibodies binding surface antigens on bovine leukocytes. Additionally, two calves were repeatedly vaccinated with the suspected vaccine and the development of alloreactivity was monitored. In dependence of the number of booster immunizations the induction of alloreactive antibodies could be observed. Finally, by affinity purification we were able to directly demonstrate that BNP associated alloantibodies cross react with the bovine kidney cell line used for vaccine production. Together this provides strong evidence that this particular BVD vaccine has the potential to induce BNP associated alloantibodies.
Abbreviations: BNP, Bovine Neonatal Pancytopenia; BVD, Bovine Virus Diarrhoea; MFI, Median fluorescence intensity; PEI, Paul-Ehrlich-Institute; SNT, Sero-Neutralization-Test
Keywords: Veterinary vaccines, Bovine Neonatal Pancytopenia, Alloantibodies, Bovine Virus Diarrhoea
1. Introduction
Thrombocytopenia in calves is a well-known but rare disease which can have a variety of different causes [1]. In 2007 an accumulation of cases with severe haemorrhagic presentation was noted in Bavaria but consecutively also in other parts of Germany and in neighbouring countries. A Satellite Symposium of the European Buiatric Congress was dedicated to this new syndrome, henceforward termed Bovine Neonatal Pancytopenia (BNP). By end of December 2010 more than 4000 cases were reported for the EU with nearly 3000 cases in Germany (Cussler, unpublished observation). Clinical symptoms usually develop with the age of 10–20 days and comprise cutaneous bleeding, petechiae, and melena. In general 5 days after onset of clinical symptoms the patients succumb to blood loss and secondary infections [2]. The case fatality rate reaches up to 90% [3]. Haematologically, the syndrome is characterized by a marked pancytopenia, including thrombo- and leukocytopenia, and the pathognomonic finding at post-mortem is an aplasia of the bone marrow (panmyelophthisis) accompanied by extensive internal and/or external bleeding [3]. So far, most of the known causes of haemorrhagic symptoms in bovines such as toxins [1] or infectious agents [4], [5] in particular Bovine Viral Diarrhoea Virus (BVDV) and bluetongue virus have been ruled out [2], [3]. Kappe et al. discuss the involvement of Porcine Circovirus 2 (PCV-2) [6] but this has not been confirmed by others [7]. In addition, a small-scale study that we performed in collaboration with the Friedrich-Loeffler-Institute, the German Federal Institute for Animal Health, on samples from BNP calves and non-BNP calves from different regions of Germany provided no evidence for a causative role of PCV-2 (Schirrmeier, personal communication). The syndrome develops upon ingestion of colostrum and it can experimentally be reproduced when newborn calves are fed with colostrum of BNP dams, i.e. cows that gave birth to a BNP calf [8]. This is not strictly dependent on the genetic relationship between colostrum donor and calf, i.e. calves from other dams can develop symptoms upon ingestion of colostrum from a BNP dam and – vice versa – calves from BNP dams remain healthy if they receive foreign colostrum [9]. As a first measure against BNP it has therefore been suggested to discard the colostrum of BNP dams [10].
In cattle maternal antibodies are not transferred in utero due to the particular anatomy of the bovine placenta. Instead the supply with maternal antibodies is achieved via colostrum during the first hours of life. It has therefore been hypothesized that maternal antibodies “toxic” for blood and bone marrow cells of the calf are the decisive component that induces BNP. Alloimmune phenomena are well described in humans and other species: one example is Neonatal Alloimmune Thrombocytopenia (NAIT). Women homozygous for a certain single nucleotide polymorphism may develop anti Human Platelet Antigen 1 antibodies when they are pregnant with a heterozygous foetus. During a subsequent gravidity these antibodies traverse the placental barrier and cause thrombocytopenia and subsequent intracranial haemorrhage in the foetus. The syndrome occurs with a frequency of about 1 in 1000 pregnancies [11]. In contrast to NAIT, which is known since decades [12], the first cases retrospectively recognised as BNP occurred in 2005 in Germany and in Belgium the first cases were traced back to 2006 (Cussler, unpublished observation). It is therefore unlikely, that BNP is due to natural sensitisation, i.e. through contact with foetal blood cells as is the case for NAIT, because then the syndrome should have been observed much earlier. The occurrence of BNP after 2005 and the spreading to some, but not all neighbouring states was a remarkable feature of the disease.
First epidemiological investigations revealed that the farms affected by BNP regularly performed vaccinations against BVD [2]. This was confirmed by pharmacovigilance investigations in Germany and also at the EU level. These investigations demonstrated that the vast majority of BNP cases is associated with one particular BVD vaccine, PregSure®BVD, which was further corroborated by the observation, that the occurrence of BNP is restricted to EU member states where the vaccine was marketed. Taking the growing evidence for an involvement of the vaccine into account, the Marketing Authorisation Holder announced a marketing stop for PregSure®BVD in April, 2010 for Germany [13]. In July 2010 the European Medicines Agency (EMA) recommended to suspend the marketing authorisation for PregSure®BVD “until scientific evidence is available to demonstrate that the administration of the vaccine … does not lead to an increased risk of Bovine Neonatal Pancytopenia or that risk mitigation measures … can be implemented” [14]. As the National Competent Authority of the Reference Member State that issued the first marketing authorisation [15] the Paul-Ehrlich-Institute (PEI) set out to investigate the role of PregSure®BVD in the pathogenesis of BNP. In particular, we wanted to address: (i) whether alloreactive antibodies could account for the induction of BNP, (ii) whether such alloantibodies could be functionally relevant, and (iii) whether PregSure®BVD could be involved in the induction of such antibodies.
2. Materials and methods
2.1. Field cases and clinical material
The majority of BNP cases were identified by M.H. of the Tiergesundheitsdienst (Cattle Health Service), North Rhine Westfalia. BNP diagnosis based on clinical findings was confirmed by haematology and bone marrow biopsy or post-mortem. All cases have been reported to and reviewed by the national pharmacovigilance system. Sera from BNP dams were obtained by venipuncture and transferred within 24 h for further investigation to the PEI. All BNP dams had received at least two vaccinations with PregSure®BVD according to the recommended vaccination scheme and most of them received booster vaccinations. Additional sera from BNP dams were provided by a veterinary practice in the northern part of Hesse and by the Clinics of Internal Medicine and Surgery of Ruminants of the Veterinary Faculty in Munich. The same case definitions as described above apply to these samples. Control sera were sampled from a dairy herd that had not been vaccinated against BVD, from a herd that had been vaccinated with a different inactivated BVD vaccine and from a herd that had been vaccinated with PregSure®BVD but had so far no history of BNP cases. The complete vaccination history of all animals included in this study is available to the authors. For leukocyte preparations whole blood samples were taken from healthy calves up to the age of 10 weeks. For individual experiments blood was obtained from adult cows. Where relevant the age of the individual animal is stated in the figures legend.
2.2. Cell preparation and cell culture
Leukocytes were prepared from whole blood by ammonium-chloride lysis. Briefly, 20 ml EDTA blood from calves were centrifuged for 10 min at 400 × g. Erythrocytes were lysed for 10 min by the addition of a buffer containing 0.15 M NH4Cl, 10 mM NaHCO3, 0.1 mM EDTA. The resulting leukocyte pellet was then washed with PBS. In many instances it was additionally purified by Ficoll Paque (1.077 g/ml; GE Healthcare) gradient centrifugation: Leukocytes were re-suspended in 5 ml PBS containing 0.5% FCS, carefully layered on 5 ml Ficoll Paque and centrifuged for 20 min at 400 × g with low deceleration rate. The interphase was recovered and the resulting PBMC pellet was washed twice with PBS containing 0.5% FCS. For individual experiments short-term T cell lines were obtained by Phytohaemagglutinin (PHA) stimulation. To this end PBMCs were re-suspended in complete medium, i.e. RPMI 1640 (Gibco) supplemented with l-Glutamine, Penicillin-Streptomycin and 10% FCS and stimulated at 1 × 106 cells/ml with 0.1 μg/ml purified PHA (Oxoid) and a 1:20 dilution of a hybridoma supernatant containing human IL-2. The resulting polyclonal T cell lines are referred to as PHA blasts.
For individual experiments permanent cell lines such as MDBK, a bovine kidney cell line, BHK-21, a hamster kidney cell line or RK13, a rabbit kidney cell line from the cell culture stock of the PEI were used. Additionally, three cell lines used for BVD vaccine production kindly provided by manufacturers were tested. All permanent cell lines were maintained according to manufacturer's instruction. The bovine kidney cell lines used in our experiments were tested to be free of BVDV.
2.3. Sero-Neutralization-Test (SNT)
SNT was performed according to OIE guidelines [16]: briefly, serial dilutions of bovine sera were incubated for 2 h with 100 CCID50 of cytopathogenic BVD virus strain, NADL. Preincubated virus was transferred to microtiter-plates that had been seeded over night with 4 × 104 MDBK cells per well. After 3–4 days the development of a cytopathic effect (CPE) was assessed by microscopy. CombiStats software (distributed by the European Directorate for the Quality of Medicines) was used to calculate individual SN titres.
2.4. Flow cytometry
To test for the presence of alloreactive antibodies up to 1 × 105 bovine leukocytes were distributed in 1 ml tubes and re-suspended in PBS containing 0.5% FCS. Sera of BNP dams or control sera were added to a final dilution of 1:10. After 1 h incubation at 4 °C, cells were washed with PBS containing 0.5% FCS. Bound bovine IgG was subsequently detected using a FITC conjugated polyclonal sheep-α-bovine IgG antibody (AbD serotec) and analyzed using a flow cytometer (BD LSR II). In general, the median fluorescence intensity (MFI) of the FITC signal was determined for the respective cell population and used for further analysis. This protocol was used accordingly to stain bovine PBMCs, PHA blasts and permanent cell lines. The respective cell type is stated in the figures legend. For individual experiments murine monoclonal antibodies against bovine CD3 and bovine CD14 (VMDR) and an anti murine IgG conjugated to PE Cy5.5 (Invitrogen) were used accordingly.
2.5. Western blot analysis
To test for the specificity of alloimmune sera western blots were performed using standard protocols: briefly, whole cell lysates of PHA blasts were separated by preparative SDS-PAGE under non-reducing conditions. Proteins were blotted onto nitrocellulose membranes (Whatman) at 0.8 mA/cm2. The membrane was blocked with a blocking solution containing PBS, 0.05% Tween 20 (Sigma–Aldrich) and 1% (w/v) Vegetable Extract (BD-DIFCO), cut into strips and incubated with 1:10 dilutions of individual test sera. The strips were washed with blocking solution and subsequently incubated with 1:1000 dilutions of anti-bovine IgG or anti-guinea pig IgG conjugated to horse radish peroxidase (Dianova). After repeated washing peroxidase activity was detected using the chromogenic substrate 9-amino-3-ethyl-carbazole (Sigma–Aldrich).
2.6. Phagocytosis assay
The functional relevance of alloreactive antibodies was investigated using a phagocytosis assay (adapted from [17]): plastic-adherent, bovine monocytes were cultivated for 10–14 days in complete medium (see above). Macrophages were then detached by 10 min incubation at 37 °C using an in-house prepared detachment solution containing 0.8% sodium chloride, 0.04% potassium chloride, 0.1% glucose, 0.058% sodium hydrogencarbonate, 0.02% EDTA and 0.5 mg/ml Trypsin (BD-DIFCO), harvested, washed two times with PBS/0.5% FCS and stained with a 2 μM solution of PKH-26 red fluorescent dye (Sigma–Aldrich) according to manufacturer's instruction. Bovine PHA blasts were used as target cells and stained with green fluorescent dye, CFSE (Alexis Biochemicals) at a working concentration of 0.5 μM. CFSE labelled cells were incubated in complete medium at 37 °C to allow for a complete detachment of non-covalently bound dye. After overnight incubation, target cells were washed and cultured for 1 h at 4 °C with 1/10 dilutions of test sera. Since heat-inactivation of test sera had no effect on cell viability under these experimental conditions as determined by propidium iodide incorpororation (data not shown), we left the sera non-heat-inactivated. Cells were washed and seeded into 96 well plates together with 2 × 104 red fluorescent macrophages at an effector:target ratio of 1:4. After overnight incubation cells were again harvested by trypsin detachment and analyzed by flow cytometry. The percentage of green fluorescent macrophages within the macrophage gate was enumerated as a measure for phagocytosis of opsonized target cells.
2.7. Immunization of animals
Five guinea pigs were immunized subcutaneously with 100 μl PregSure®BVD. After 3 weeks the animals received a booster immunization. Six weeks later blood samples were collected. As a control, other, commercially available inactivated BVD vaccines were tested in parallel. All animal experiments were announced to the responsible authority according to animal welfare legislation.
In addition, two calves were vaccinated subcutaneously four times with one dose of PregSure®BVD at an interval of 3 weeks. Serum samples were taken at weekly intervals.
2.8. Affinity purification of alloreactive antibodies
Alloreactive antibodies binding to cell surface antigens on bovine leukocytes were affinity purified from alloreactive sera by detachment from the cell surface of intact cells: For this purpose we diluted sera of PregSure®BVD vaccinated dams and non-immunized controls 1:10 in PBS. PHA blasts were then incubated for 1 h in serum dilution and repeatedly washed with 0.9% sodium chloride. Subsequently, cell-bound antibodies were detached by incubating the cells for 15 min in ice-cold citrate buffer (0.12 M sodium citrate, pH 3.5). After centrifugation the supernatant was harvested and immediately neutralized with buffer (1 M Tris–HCl, pH 9.0).
3. Results
3.1. PregSure®BVD induces high neutralizing antibody titres
PregSure®BVD has been assigned to induce a very strong and long-lasting immune response against BVDV [18], [19]. This was confirmed with sera from four different groups of cattle: (a) PregSure®BVD immunized BNP dams, (b) PregSure®BVD immunized non-BNP dams, (c) dams that were immunized with another commercially available BVD vaccine, and (d) non-BVD-immunized control dams. Eight sera per group were arbitrarily chosen and tested by SNT as described. Neutralizing antibody titres of PregSure®BVD immunized animals exceeded the titres of non-immunized or animals immunized with other vaccines by several log scales (Fig. 1 ). BNP dams showed a ten times higher neutralizing antibody titre on average compared to PregSure®BVD immunized non-BNP dams. This observation corroborates the notion that PregSure®BVD induces very high neutralizing antibody titres that – also in our hands– even exceeded titres of naturally infected animals (Bastian, unpublished observation).
Fig. 1.
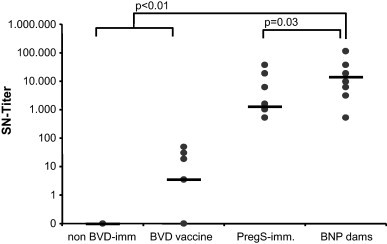
Sera of BNP dams contain high BVDV-neutralizing antibody titres. Sera were obtained from non-BVD-immunized controls (non-BVD-imm.), cows that had been vaccinated with an alternative BVD vaccine (BVD-vaccine), PregSure®BVD immunized non-BNP dams (PregS-imm.) and PregSure®BVD immunized BNP dams (BNP dams). Eight sera per group were tested by SNT for their BVDV-neutralizing activity. Black dots represent individual serum titres, black bars indicate the median value for each group. P-Values were calculated by simple Student's t-test.
3.2. Antibodies from PregSure®BVD immunized animals bind bovine leukocytes
In a second set of experiments we tested the hypothesis of an alloreactive antibody-based aetiology of BNP. To this end, we incubated calf leukocytes with sera from BNP dams and respective control groups and assessed binding of a FITC conjugated secondary antibody by flow cytometry. Fig. 2 shows that sera from BNP dams contain antibodies, which bind extracellular antigens on bovine leukocytes. The gating strategy, based on forward- and sideward-scatter (see Fig. 2A, upper left panel) allows to differentiate between lymphocytes, monocytes and granulocytes. This differentiation was confirmed by a predominant staining (80%) for the T lymphocyte marker CD3 in the lymphocyte gate (L¢) and a predominant staining for CD14 (90%) – a characteristic marker of monocytes and macrophages – in the monocyte gate (M¢) (Fig. 2A, upper middle and right panel). The alloreactive binding is strongest in lymphoid cells. Monocytes and granulocytes are bound to a lesser extent (Fig. 2A, lower panel). This observation is well in accordance with previous publications [20], [21]. Within the different cell populations the respective alloantigen(s) are evenly distributed as seen by the general shift of the fluorescence intensity. Since alloreactive binding was predominantly observed in lymphocytes and monocytes, from here on we concentrated on the analysis of these populations. When comparing alloreactive binding between the different groups it is of note that only sera of animals that received a PregSure®BVD immunization show alloreactivity (Fig. 2B) and the binding is significantly higher in BNP dams compared to non-BNP-dams. Still, there are large differences when sera of individual BNP dams are compared (Fig. 2B, right scatter plot). In part this is due to differences in alloantibody titres, but it also reflects heterogeneity in the specificity of alloantibodies. This becomes obvious when sera of BNP dams are tested against lymphocytes from different donors. In Fig. 2C, we tested a panel of seven BNP sera with PHA blasts from three different donors. Lymphocytes from donor A are bound by all BNP sera, one BNP serum does not react with donor B, and cells from donor C are only recognised by sera of three BNP dams, indicating that alloantibodies from individual BNP dams recognize different epitopes or antigens. Furthermore, this shows that the expression of alloantigens differs between individuals. In this context it is remarkable, that donor C is a calf that survived a challenge with colostrum from a non-related BNP dam. The serum of the corresponding BNP dam did not bind leukocytes of donor C (data not shown). To test for their specificity we investigated BNP dam sera together with the respective controls by western blot (Fig. 2D). Whole cell lysates of PHA blasts were separated by SDS-PAGE and blotted on nitrocellulose membranes: With sera from five of eight BNP dams and with three of eight PregSure®BVD immunized Non-BNP dams a reactive band at about 50 kDa was observed. Although we did not observe this reactivity in animals that had not been vaccinated with PregSure®BVD, the western blot analysis remains somewhat inconclusive, because there was no correlation with the flow cytometry signal obtained with the respective serum (data not shown).
Fig. 2.
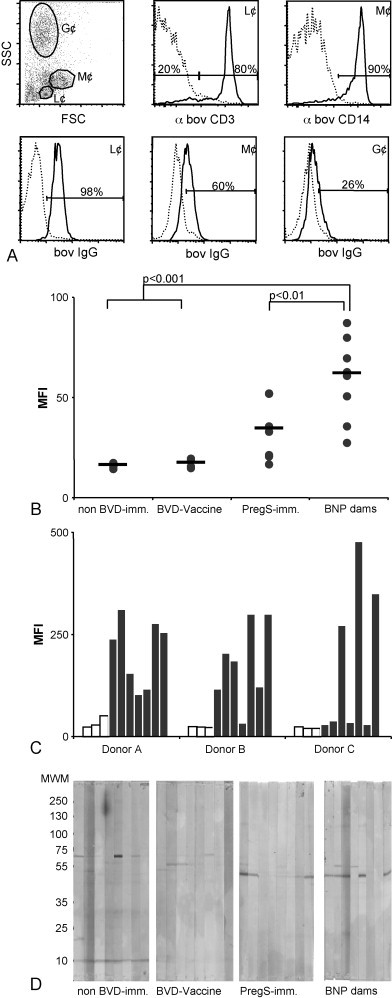
Sera of BNP dams contain alloreactive antibodies that bind surface antigens on bovine leukocytes. (A) Bovine leukocytes were isolated from whole blood of a 17 days old calf and analyzed by flow cytometry. An exemplary FACS analysis is shown: the upper left panel shows the gating strategy. According to forward and sideward scatter lymphocytes (L¢), monocytes (M¢) and granulocytes (G¢) are differentiated. This is confirmed by additional staining: the upper middle panel shows the expression of CD3 for the lymphocyte gate, the upper right panel shows the expression of CD14 for the monocyte gate. Dotted lines represent isotype controls. The lower panel depict fluorescence intensity histograms obtained with serum from a BNP dam (bold line) or a non-immunized control (dotted line) for the respective leukocyte populations. Numbers indicate the percentage of the population that does not overlap with the control. (B) The same serum panel as in Fig. 1 was tested by flow cytometry. Results are shown for PBMCs from one representative leukocyte donor, a 2-week-old calf. Black dots represent the MFI within the lymphocyte gate for individual sera, black bars indicate the median value for each group. P-Values were calculated by simple Student's t-test. (C) A panel of sera from seven BNP dams (filled columns) and three non-immunized controls (empty columns) was tested for alloreactivity against PHA blasts from three different leukocyte donors. Donor A and B were adult cows, Donor C was a young bull of 7 months age. Sera are always shown in the same order. (D) The same serum panel as in Fig. 1 was tested by Western blot. Whole cell lysates of PHA blasts from a 1-year-old heifer were separated by non-denaturing SDS-PAGE and blotted onto nitrocellulose membranes. For each group eight sera were investigated and the membrane strips are grouped accordingly.
3.3. Alloreactive antibodies accelerate cytophagocytosis
To test whether Fc-receptor mediated phagocytosis is involved in the pathogenesis of BNP we investigated the effect of alloreactive BNP sera in a phagocytosis assay. To this end we cocultured red fluorescent bovine macrophages with green fluorescent target cells that had been preincubated with serum of BNP dams or sera of non-immunized controls. After over night incubation cells were analyzed by flow cytometry. As seen in Fig. 3 A, the two cell populations can easily be distinguished (left panel). The uptake of cellular material from target cells leads to an increase of green fluorescence in the upper macrophage gate. At the same time the population of green target cells becomes smaller (right panel). This indicates that binding of alloreactive antibodies enhances the uptake of green fluorescent target cells. There is some green background staining in the macrophage gate after preincubation of target cells with control sera. However, after preincubation with alloreactive sera of BNP dams cytophagocytosis is enhanced by 20–30% (Fig. 3B).
Fig. 3.
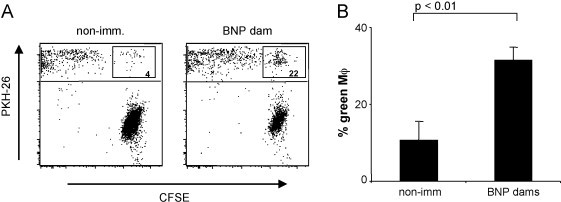
Sera of BNP dams induce cytophagocytosis. Green fluorescent target cells were preincubated with alloreactive serum of BNP dams or from non-immunized controls. Subsequently, target cells were cocultured with red fluorescent bovine macrophages. The uptake of green target cells was measured by flow cytometry. (A) A typical dot blot is shown: the macrophage gate includes all red fluorescent cells, double fluorescent cells are considered as macrophages after phagocytosis of green target cells. For the left panel target cells were incubated with serum from a non-immunized control, for the right panel target cells were incubated with serum from a BNP dam. (B) The quantitative analysis for one representative experiment of four is shown: columns represent the mean percentage of green macrophages for three control sera and three BNP sera, resp. Error bars depict the standard deviation. P-Values were calculated by simple Student's t-test.
3.4. PregSure®BVD immunization induces alloreactive antibodies
The observation that only sera of PregSure®BVD immunized animals showed alloreactive surface binding prompted us to directly test whether the vaccine induces alloreactivity. To this end we first immunized guinea pigs with four different commercially available BVD vaccines. Again, two experimental approaches were chosen to test for alloreactive antibodies: Western blotting revealed that the three inactivated BVD vaccines tested induced an almost identical pattern of reactive bands when sera of immunized guinea pigs were tested against whole cell lysates of bovine PHA blasts, whereas the one live attenuated BVD vaccine induced no significant alloreactivity (Fig. 4 A). Using this technique the reactivity to the total of cellular proteins is detected. By contrast, flow cytometry allows for the analysis of antibodies that bind native antigens on the cell surface. Fig. 4B reveals that only sera of PregSure®BVD immunized guinea pigs showed surface staining of bovine lymphocytes, which is in accordance with the missing correlation between Western blot and flow cytometry observed with bovine sera (see Fig. 2). To investigate the induction of alloreactive antibodies in the target species two calves were vaccinated four times with PregSure®BVD in 3 weeks intervals. At the indicated time points serum was collected and tested by flow cytometry for alloreactivity (Fig. 4C): the bovine kidney cell line used for the production of PregSure®BVD and bovine lymphocytes were tested in parallel. The upper panel shows the reactivity to the cell line. In dependence of the number of immunizations, both animals develop cell line specific antibodies. The lower panel shows the alloreactivity to heterologous, bovine PHA blasts. The reactivity follows a very similar pattern as observed with the kidney cell line, but in this case, only one of two calves is responding. Together these data show that PregSure®BVD is able to induce alloreactive antibodies specific for surface antigens both on the cell line used for virus production and on bovine lymphocytes.
Fig. 4.
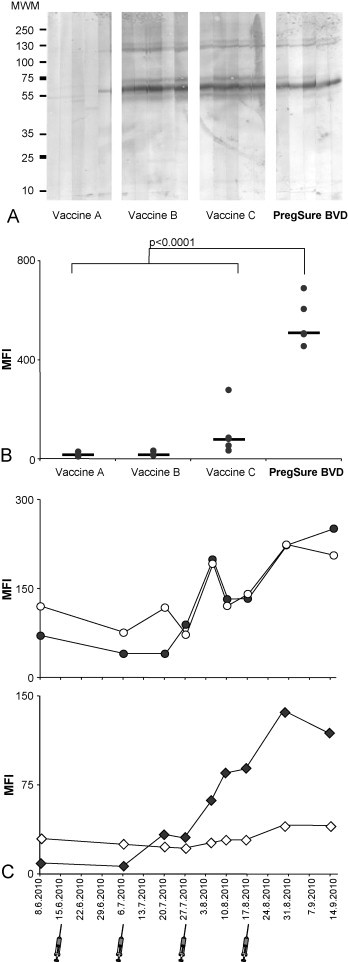
PregSure®BVD induces alloreactive, surface binding antibodies. (A) Five guinea pigs per group were immunized twice with PregSure®BVD or three alternative, commercially available BVD vaccines. After 6 weeks sera were analyzed by western blot as described for Fig. 2D. Five sera per group were tested and the strips are grouped accordingly. (B) The same sera were tested by flow cytometry for the presence of antibodies binding to the cell surface of bovine leukocytes. Results are shown for one representative experiment. Black dots represent the MFI of PHA blasts from a 1-year-old heifer after staining with individual immune sera, black bars indicate the median value for each group. P-Values were calculated by simple Student's t-test. (B) Two calves were vaccinated four times with PregSure®BVD. At the indicated time points serum was obtained and tested for alloreactive binding to the bovine kidney cell line used for vaccine production (upper panel) or PHA blasts from a 1-year-old heifer (lower panel). Open symbols represent the MFI for one calf 1, filled symbols represent the other calf 2.
3.5. BNP associated alloantibodies cross-react with the cell line used for PregSure®BVD production
Finally, we investigated the specificity of BNP associated leukocyte-specific antibodies. For this purpose we affinity-purified alloantibodies by eluting cell-bound antibodies from the surface of bovine lymphocytes as it has previously been described [22]. The eluates were retested for alloreactive binding on bovine lymphocytes and showed a tenfold increase in alloreactive binding when compared to the corresponding sera. This effect was only observed with sera from BNP dams (Fig. 5 A) and PregSure®BVD immunized non-BNP dams (Supplemental Fig. 3). It was not observed with non-immunized controls (Fig. 5A) or animals vaccinated with another BVD vaccine (Supplemental Fig. 3). To exclude the possibility that the vaccine-induced alloreactivity is due to cross-reactive viral antigens we tested the same panel of sera and affinity purified alloantibodies by SNT. Again, sera from BNP dams showed very high BVDV-neutralizing antibody titres. With affinity-purified alloantibodies this neutralizing effect was completely abolished (Fig. 5B) indicating that PregSure®BVD-induced alloantibodies are different from BVDV-specific antibodies detected in the SNT. Finally, we tested BNP associated alloantibodies eluted from bovine lymphocytes for their capacity to bind a panel of cell lines commonly used in vaccine production. No cross-reactivity of affinity-eluates was observed with hamster or rabbit kidney cell lines. Some reactivity was observed with a bovine lung cell line used for the production of a BVD vaccine other than PregSure®BVD, but it must be emphasized that this vaccine did not induce leukocyte-binding antibodies in guinea pigs (see also Fig. 4B). The strongest binding was observed with the bovine kidney cell used for PregSure®BVD production (Fig. 5C) showing that BNP associated alloantibodies cross-react with cell surface antigens both on bovine leukocytes and on the cell line.
Fig. 5.
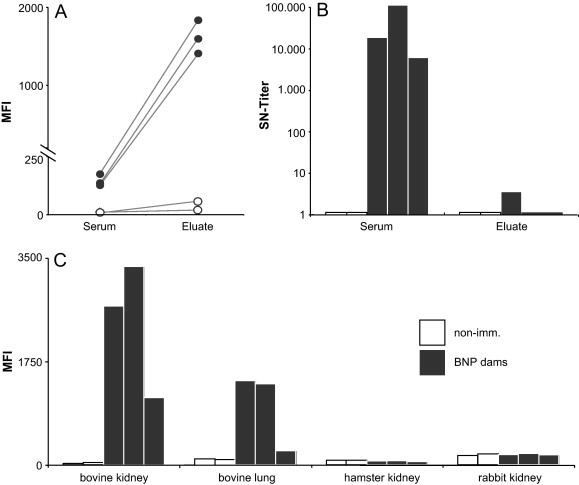
BNP associated alloantibodies cross react with the bovine kidney cell line used for PregSure®BVD production. Bovine lymphocytes were incubated with sera from BNP dams or non-immunized controls. Bound alloantibodies were eluted from the cell surface and further investigated. (A) Affinity purified alloantibodies and the corresponding sera (filled symbols) were analyzed for alloreactive binding to bovine PHA blasts obtained from a 1-year-old heifer. Open symbols represent two pairs of identically treated control sera. Circles show individual MFI values, the grey line indicates corresponding pairs. (B) The same panel of sera and affinity-purified alloantibodies was tested for anti-BVDV-neutralizing activity as described above. Filled columns represent sera of BNP dams and affinity purified antibodies, open columns represent non-immunized controls. (C) The same panel of affinity-purified alloantibodies was tested for binding to a selection of cell lines that are used for vaccine production. The bovine kidney cell line shown is used for the production of PregSure®BVD.
4. Discussion
In comparison to live attenuated vaccines, inactivated vaccines are usually considered safe but less effective. Therefore, a lot of effort is invested into research on and development of new adjuvants to improve the efficacy of inactivated vaccines. Adverse effects of inactivated vaccines in veterinary medicine are usually restricted to local reactions mainly due to irritating properties of the adjuvant and sometimes mild systemic reactions such as fever or milk reduction. Autoimmune reactions induced by licensed vaccines have been described for humans [23] and are discussed for pet animals [23] but have very rarely been reported for livestock animals [24]. Nevertheless, there are reports on alloimmune haemolytic anaemia in calves that ingested colostrum of cows vaccinated with experimental vaccines against anaplasmosis [25] demonstrating that vaccine-induced maternal antibodies can lead to alloimmune-pathology in bovine neonates [26]. The data presented here provide strong evidence that a similar immune-mediated mechanism may account for the pathogenesis of BNP. The observed association between BNP in the calf and the presence of alloreactive antibodies in the serum of the dam is an important hint and well in accordance with findings of other research groups [27]. These alloantibodies are transferred to the calf through colostrum-uptake, because it has recently been reported that calf leukocytes are coated with alloantibodies shortly after ingestion of colostrum from BNP dams [20]. Little is known about the pathomechanism that causes the typical cytopenia and panmyelophthisis leading to the clinical symptoms of BNP. However, in the current manuscript we demonstrate in vitro that alloreactive BNP sera induce cytophagocytosis by bovine macrophages. In addition, activated macrophages and haemophagocytosis have repeatedly been reported in bone marrow aspirates of BNP calves [3], [20]. It is therefore very likely that – similar to human alloimmune thrombocytopenia [28] – activated phagocytes of the Reticulo-Endothelial-System eliminate alloantibody-opsonized cells through Fc-receptor mediated phagocytosis. Nevertheless, it is conceivable that other immune-mechanisms such as Antibody Dependent Cellular Cytotoxicity (ADCC) or complement activation also contribute to the pathogenesis of BNP. Further investigations are required to clarify this issue.
Another important question is how BNP associated alloantibodies are elicited. Epidemiological observations revealed that almost all BNP dams share a common vaccination history [2]. However, to the best of our knowledge this is the first report directly addressing the potential role of the vaccine, PregSure®BVD, in the pathophysiology of BNP. The vaccine is manufactured like other conventional, inactivated BVD vaccines: virus is produced on a bovine kidney cell line, harvested and inactivated. A particular feature is the microfluidized oil-in-water adjuvant system named Procision-A®, which is composed of Quil-A, Cholesterol, Amphigen Base and Drakeol 5 [29]. Due to the unique adjuvant the vaccine induces very robust, protective antibody titres against BVDV type 1 and type 2 [18], [19], [30]. This is well confirmed by the data presented in the current manuscript. However, in addition to protective immunity the vaccine also induces alloreactive antibodies and the source of alloantigens – as seen from the cross-reactivity experiments – is the cell line used for vaccine production. Using two different techniques – western blotting and flow cytometry – we were able to discriminate an overall alloreactivity to whole cell lysates of bovine leukocytes from alloantibodies that are specific for cell surface molecules. With sera from BVD immunized cows only a few reactive bands were observed by western blot (Fig. 2D). By contrast, all sera from guinea pigs that had been vaccinated with inactivated BVD vaccines displayed strong and almost identical reactivity pattern (Fig. 4A). This indicates that all inactivated BVD vaccines tested in our experiments have the potential to induce alloreactivity, because they all contain a similar amount of residual bovine antigens originating from the manufacturing process. The difference in the western blot reactivity between cattle and guinea pigs is probably due to the fact that for guinea pigs the cellular antigens in the vaccine represent xenoantigens that readily elicit an immune response, whereas cattle recognize the majority of these bovine antigens as self and therefore mount no or only a weak immune response. The relevance of western blot reactive alloantibodies is unclear at the moment. Since there is no correlation of western blot reactivity with the flow cytometry signal western blot reactive antibodies are presumably directed to cytoplasmic proteins or hidden epitopes not accessible on living cells. Therefore they are probably clinically irrelevant. Nevertheless, it is conceivable that Western blot detectable alloantibodies are paralleled by alloreactive T cells of the same specificity, which could recognize their respective epitope in the context of MHC molecules and therefore could play a role in vivo, but so far there is no evidence that this has clinical implications. It is therefore assumed that only surface binding alloantibodies are clinically relevant, also because the pathomechanisms discussed above depend on a surface opsonization of cells. In view of the strong western blot reactivity observed with the different groups of guinea pig sera it is therefore remarkable that only PregSure®BVD immunized animals – guinea pigs and cattle – developed surface binding antibodies detectable by flow cytometry. So far, the reason for this difference is not clear. One possible explanation is that the surface exposed, BNP associated alloantigens are poorly immunogenic and require a highly efficient adjuvant and/or repeated booster immunizations before an alloimmune response is mounted. Alternatively, it could be that the immunogenicity of these alloantigens depends on a conformational stabilization by the microfluidized oil-in-water adjuvant, as it has been similarly described for conformational epitopes of influenza virus [31]. This clearly awaits further investigation, but according to these two non-mutually exclusive possibilities, a key problem could be the combination of the highly potent adjuvant with inadequately purified virus antigens. Since SDS-PAGE inevitably leads to a partial protein denaturation the importance of a native epitope conformation for alloantibody recognition would also explain, why there is no correlation between the reactivity in the western blot and flow cytometry as discussed for Fig. 2D and also why affinity purified alloantibodies eluted from the surface of bovine leukocytes have found to be non-reactive when tested by western blot (Bastian, unpublished observation). So far, this technical problem has impeded our attempts to identify individual BNP associated alloantigens. However, ongoing efforts – at the PEI but also in other institutions – will eventually succeed and it may be awaited that the identification of individual alloantigens will provide an explanation for the differences in alloantibody binding between individual leukocyte subsets. Similarly, the identification of alloantigens will also be important for the interpretation of the heterogeneous recognition pattern shown in Fig. 2C when sera of BNP-dams were tested against lymphocytes from different donors. The result is well in accordance with previous observations using freshly isolated leukocytes from newborn calves [20]. The authors of this previous publication discuss the possibility that the observed heterogeneity could be age related or due to temporary differences in the expression level of alloantigens. Here, we have used uniformly cultivated lymphocytes of adult or adolescent animals to exclude an influence of such variables. In addition, the experiments shown in Supplemental Fig. 1 provide no evidence for age related differences. Together with the findings shown in Supplemental Fig. 2 our observation therefore allows for the conclusion that individual BNP dams respond to different alloantigens (or different alleles of the same polymorphic antigen) and also that the expressed alloantigens vary from individual to individual. In light of this observation it is likely that more than one alloantigen can serve as a target for BNP inducing alloantibodies. So far, our assay system focused on the analysis of leukocytes, but it could well be that other important alloantigens are expressed by different cell types, e.g. thrombocytes or bone marrow cells (see below). To avoid this experimental bias, we have now started to focus on the bovine kidney cell line used for vaccine production. Since the cell line is presumably the origin of BNP associated alloreactivity, this strategy will allow us to cover the whole spectrum of BNP associated alloantigens. This is illustrated by the example given in Supplemental Fig. 3, where BNP-associated alloantibodies have been purified by desorbing them from the cell surface of the cell line.
The Western blot experiments with immune sera from guinea pigs showed that the vaccine (among others) contains a whole array of bovine cell antigens that have the potential to sensitise vaccinated animals. The repertoire of autoantigens expressed by the vaccinated animal and the respective auto-tolerance mechanisms – i.e. negative T- and B-cell selection in the thymus and bone marrow as well as peripheral tolerance induction – probably dictate which of these antigens are ignored and which elicit an alloreactive immune response. In other words, from the alloantigen cocktail in the vaccine the animals will only “pick up” non-self antigens. For the development of BNP it depends whether the cell surface antigens of the calf – inherited from the father – match those that have been “picked up” as non-self by the mother. This concept of alloreactivity and self-tolerance is supported by the observation that sera of BNP dams do not bind autologous leukocytes (Supplemental Fig. 2). It is conceivable that the self-tolerance may occasionally be broken, but in the majority of cases it will prevent the development of autoimmunity. This also provides a plausible explanation why vaccinated animals do not show clinical symptoms despite high bovine specific antibody titres. In fact, PregSure®BVD has been considered very safe during the licensing procedure and is still recommended as being “safe to administer at any stage of pregnancy” according to the summary of product characteristics (SPC). However, a recent report casts doubt on the latter statement: The author describes an outbreak of BNP in a large dairy herd after the BVD management had been changed. To obtain a rapid onset of protection against BVD infection of dams it was decided to vaccinate all cows and heifers only once (instead of two injections recommended for basic vaccination) but to administer all dams a booster immunization with PregSure®BVD in high pregnancy when they were dried off. This change in the vaccination regimen led to a marked increase in calf mortality rate and in eight cases BNP could be confirmed by post-mortem [32]. The vaccination scheme used in this report is comparable to dam vaccinations intended to maximise antibody transfer to the offspring, e.g. to prevent neonatal scours caused by Escherichia coli or rota and corona virus infections. Taking this into account, the report suggests that a maximum alloantibody titre at delivery increases the risk to induce BNP. Together this corroborates the notion that the development of BNP depends on titre and specificity of ingested alloantibodies and the respective alloantigen expression in the calf.
However, this view may be too simplistic because as shown in Fig. 2B also PregSure®BVD immunized non-BNP dams have considerable titres of leukocyte binding alloantibodies and the data shown in Fig. 4C insinuates that 50% of PregSure®BVD immunized cows should induce BNP. By contrast, even for Germany – the country with the highest number of BNP cases so far – the incidence of BNP after PregSure®BVD immunization has been estimated to be not higher than 0.3% (Cussler, unpublished observation) and on farms affected by BNP usually not more than 5–10% of the dams actually induce BNP (Holsteg, unpublished observation). Additional factors may therefore influence the clinical outcome of alloantibody ingestion. For example, there could be differences in the expression pattern of BNP associated alloantigens between relevant cell populations that are not detectable with the flow cytometric analysis of leukocytes alone. Subclinical cases of BNP have been reported that exhibited only a temporary depression in thrombocyte or leukocyte counts (Holsteg, unpublished observation, [3]). It was concluded that clinical symptoms only occur after prolonged cytopenia and that the prognosis quoad vitam depends on the degree of bone marrow destruction [2]. In this line we were able to demonstrate significant cross-reactivity of affinity-purified alloantibodies from BNP dams with cells from calf bone marrow aspirates (see Supplemental Fig. 3B). So far we have not investigated this systematically, but it is well conceivable that the key event in the immunopathology of BNP is the binding of PregSure®BVD induced alloantibodies and as a consequence the destruction of haematopoietic progenitor cells. Apart from these immunological aspects, there are of course additional variables such as colostrum management (e.g. mixed colostrum), the amount of colostrum intake, the timepoint of colostrum feeding, etc. that may affect the extent of pancytopenia and bone marrow damage which could explain the broad spectrum of reactions ranging from lethal haemorrhage to subclinical disease or even no obvious effect on blood cells.
In summary, our data show that sera of PregSure®BVD vaccinated BNP dams contain high titres of BVDV neutralizing but also alloreactive leukocyte binding antibodies. This is in accordance with the hypothesis that BNP is induced by alloreactive, maternal antibodies transferred by colostrum intake. Comparing the antibody binding to leukocyte preparations from different animals we find that alloantibodies from individual BNP dams may recognize different (sets of) antigens. Alloantibody binding is functionally relevant because it induces cytophagocytosis by bovine macrophages. With two different immunization approaches we demonstrate that PregSure®BVD – in contrast to other commercially available BVD vaccines – induces alloantibodies specific for surface antigens on bovine leukocytes. Finally, we show that the same alloantibodies that bind bovine leukocytes cross-react with the bovine kidney cell line used for PregSure®BVD production. By contrast, no cross-reactivity is observed with BVDV or with cell lines that are used for the manufacturing of other vaccines, e.g. vaccines used for mass vaccinations during the recent Bluetongue epidemic in Central Europe. Together our data provide strong evidence that repeated vaccination with PregSure®BVD is involved in the induction of BNP associated alloantibodies. Yet, there are still a number of issues that need to be addressed. This comprises the role of the adjuvant, the identity of alloantigens, and last but not least the difference between alloreactive BNP and non-BNP dams has to be elucidated. Ongoing efforts are aiming to resolve these open questions. The answers should help to prevent similar incidents in the future.
Acknowledgements
We appreciate the support by Pfizer Animal Health through open discussions and courteous supply of research material. We acknowledge the good collaboration with and the contribution of H. Schirrmeier from the Friedrich-Loeffler-Institute, A. Schwarzmaier and G. Seemann of the Cattle Health Service in Baden Wurttemberg, and A. Friedrich and W. Klee of the Ludwig-Maximilians-University Munich. We are grateful to P. Schröter of the University of Veterinary Medicine Hannover and P. Bridger and K. Doll of the Veterinary faculty of Giessen University for helpful and inspiring discussions. We pay tribute to E. Schwedinger and the staff from the PEI pharmacovigilance unit. Without their dedication this work would never have been accomplished.Conflict of interest statement: The authors have no financial conflict of interest. The work was done in fulfilment of the institute's legal commitment and financed by own resources.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vaccine.2011.05.012.
Appendix A. Supplementary data
References
- 1.Stober M. Hämorrhagische Diathesen. In: Dirksen G., Gründer H., Stöber M., editors. Innere Medizin und Chirurgie der Rindes. Paul Parey; Berlin: 2002. pp. 247–253. [Google Scholar]
- 2.Friedrich A., Klee W. Gehäuftes Auftreten von hämorrhagischer Diathese infolge Knochenmarkschädigung bei jungen Kälbern. Tierärztl Umschau. 2009;64:423–431. [Google Scholar]
- 3.Pardon B., Steukers L., Dierick J., Ducatelle R., Saey V., Maes S. Haemorrhagic diathesis in neonatal calves: an emerging syndrome in Europe. Transbound Emerg Dis. 2010;57(3):135–146. doi: 10.1111/j.1865-1682.2010.01098.x. [DOI] [PubMed] [Google Scholar]
- 4.DeMaula C.D., Leutenegger C.M., Bonneau K.R., MacLachlan N.J. The role of endothelial cell-derived inflammatory and vasoactive mediators in the pathogenesis of bluetongue. Virology. 2002;296(2):330–337. doi: 10.1006/viro.2002.1476. [DOI] [PubMed] [Google Scholar]
- 5.Corapi W.V., French T.W., Dubovi E.J. Severe thrombocytopenia in young calves experimentally infected with noncytopathic bovine viral diarrhea virus. J Virol. 1989;63(9):3934–3943. doi: 10.1128/jvi.63.9.3934-3943.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kappe E.C., Halami M.Y., Schade B., Alex M., Hoffmann D., Gangl A. Bone marrow depletion with haemorrhagic diathesis in calves in Germany: characterization of the disease and preliminary investigations on its aetiology. Berl Munch Tierarztl Wochenschr. 2010;123(1–2):31–41. [PubMed] [Google Scholar]
- 7.Willoughby K., Gilray J., Maley M., Dastjerdi A., Steinbach F., Banks M. Lack of evidence for circovirus involvement in bovine neonatal pancytopenia. Vet Rec. 2010;166(14):436–437. doi: 10.1136/vr.c1685. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich A., Carlin A., Assad A., Büttner M., Rademacher G., Sauter-Louis C. Experimental production of the syndrome. In: Klee W., editor. Satellite symposium on haemorrhagic diathesis in calves; Societe Française de Buiatrie, Marseille; 2009. p. 27. [Google Scholar]
- 9.Schroeter P., Kuiper H., Holsteg M., Puff C., Haas L., Kaske M. Reproduktion der Bovinen Neonatalen Panzytopenie mittels Kolostrum-Versuch. In: Bonn D.G.f.Z.e.V., editor. vol. 83. Kiel; 2010. (DGfZ-Jahrestagung und DGfZ-/GfT-Vortragstagung). [Google Scholar]
- 10.Bell C.R., Scott P.R., Kerr M.G., Willoughby K. Possible preventive strategy for bovine neonatal pancytopenia. Vet Rec. 2011;167(19):758. doi: 10.1136/vr.c6209. [DOI] [PubMed] [Google Scholar]
- 11.Skogen B., Husebekk A., Killie M.K., Kjeldsen-Kragh J. Neonatal alloimmune thrombocytopenia is not what it was: a lesson learned from a large prospective screening and intervention program. Scand J Immunol. 2009;70(6):531–534. doi: 10.1111/j.1365-3083.2009.02339.x. [DOI] [PubMed] [Google Scholar]
- 12.Pearson H.A., Shulman N.R., Marder V.J., Cone T.E., Jr. Isoimmune neonatal thrombocytopenic purpura. Clinical and therapeutic considerations. Blood. 1964;23:154–177. [PubMed] [Google Scholar]
- 13.Pfizer (PAH). Pfizer animal health stops sale of cattle BVD vaccine in the EU; 2010. http://www.pfizerah.co.uk/sites/sante-animale/UK-English/News/Pages/Pregsure_BVD_press_release.aspx.
- 14.EMA. Opinion following an Article 78 procedure for Pregsure BVD and associated names; 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Pregsure_BVD_78/WC500095959.pdf.
- 15.Bekanntmachung Nr. 273 über die Zulassung von Sera und Impfstoffen sowie andere Amtshandlungen (vom 05.05.2004). In: Bundesanzeiger, vol. 104; 2004. p. 11946.
- 16.Drew T. Bovine Viral Diarrhea. In: Commission O.B.S., editor. vol. 2. Office international des épizooties; Paris: 2008. pp. 698–711. (Manual of diagnostic tests and vaccines for terrestrial animals). [Google Scholar]
- 17.Fendel R., Mordmuller B., Kreidenweiss A., Rudat A., Steuer C., Ambrosch C. New method to quantify erythrophagocytosis by autologous monocytes. Cytometry A. 2007;71(4):258–264. doi: 10.1002/cyto.a.20360. [DOI] [PubMed] [Google Scholar]
- 18.Raue R., Harmeyer S.S., Nanjiani I.A. Antibody responses to inactivated vaccines and natural infection in cattle using bovine viral diarrhoea virus ELISA kits: assessment of potential to differentiate infected and vaccinated animals. Vet J. 2010;187(3):330–334. doi: 10.1016/j.tvjl.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Harmeyer S.S., Antonis A.F.G., Salt J.S., Bruschke C.J.M. The comparative efficacy of commercially available BVDV vaccines in cattle challenged with European BVDV type 1. Res Vet Sci. 2005;78(Suppl 1):15. [Google Scholar]
- 20.Bridger P.S., Bauerfeind R., Wenzel L., Bauer N., Menge C., Thiel H.-J. Detection of colostrum-derived alloantibodies in calves with bovine neonatal pancytopenia. Vet Immunol Immunopathol. 2011;141(1–2):1–10. doi: 10.1016/j.vetimm.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Pardon B., Stuyven E., Stuyvaert S., Hostens M., Dewulf J., Goddeeris B. Flow cytometric and immunofluorescence staining studies on bovine neonatal pancytopenia in calves. Vet Immunol Immunopathol. 2011;141(3–4):293–300. doi: 10.1016/j.vetimm.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Ehrlich R., Witz I.P. The elution of antibodies from viable murine tumor cells. J Immunol Methods. 1979;26(4):345–353. doi: 10.1016/0022-1759(79)90180-7. [DOI] [PubMed] [Google Scholar]
- 23.Salemi S., D’Amelio R. Could autoimmunity be induced by vaccination? Int Rev Immunol. 2010;29(3):247–269. doi: 10.3109/08830181003746304. [DOI] [PubMed] [Google Scholar]
- 24.Yeruham I., Avidar Y., Harrus S., Fishman L., Aroch I. Immune-mediated thrombocytopenia and putative haemolytic anaemia associated with a polyvalent botulism vaccination in a cow. Vet Rec. 2003;153(16):502–504. doi: 10.1136/vr.153.16.502. [DOI] [PubMed] [Google Scholar]
- 25.Dennis R.A., O’Hara P.J., Young M.F., Dorris K.D. Neonatal immunohemolytic anemia and icterus of calves. J Am Vet Med Assoc. 1970;156(12):1861–1869. [PubMed] [Google Scholar]
- 26.Kocan K.M., de la Fuente J., Guglielmone A.A., Melendez R.D. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin Microbiol Rev. 2003;16(4):698–712. doi: 10.1128/CMR.16.4.698-712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridger P.S., Bauerfeind R., Menge C., Wenzel L., Bauer N., Reinacher M. Bovine Neonatal Pancytopenia (BNP): detection of leukocyte-binding alloantibodies. Herbstagung der Deutschen Gesellschaft für Immunologie; Leipzig; 2010. [Google Scholar]
- 28.Ghevaert C., Wilcox D.A., Fang J., Armour K., Clark M., Ouwehand W. Developing recombinant HPA-1a-specific antibodies with abrogated Fcgamma receptor binding for the treatment of fetomaternal alloimmune thrombocytopenia. J Clin Invest. 2008;118(8):2929–2938. doi: 10.1172/JCI34708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summary of Product Characteristics, PregSure BVD. http://www.vmd.gov.uk/ProductInformationDatabase/.
- 30.Salt S., Antonis A.F.G., Peters A.R., Brune A., Jahnecke S., Traeder W. PregSure®BVD – eine neue inaktivierte BVD-Vakzine Breite Kreuzneutralisation von europäischen BVDV-Typ-1- und -Typ-2-Stämmen und signifikante Verbesserung der Fertilität nach Testinfektionen. Tierärztliche Praxis (Großtiere) 2004;32(4):191–195. [Google Scholar]
- 31.Khurana S., Chearwae W., Castellino F., Manischewitz J., King L., Honorkiewicz A. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med. 2010;2(15):15ra15. doi: 10.1126/scitranslmed.3000624. [DOI] [PubMed] [Google Scholar]
- 32.Klemt A. The occurrence of bovine neonatal pancytopenia and haematological examinations of calves in a dairy farm with 1500 cows. Tierärztl Umschau. 2010;65:257–270. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


