Abstract
The purpose of this study was to explore the turnaround times, section and image quality of a number of more “difficult” specimens destined for rapid diagnostic electron microscopy (EM) after microwave-assisted processing. The results were assessed and compared with those of conventionally processed samples.
A variety of infectious agents, some with a potential for bioterrorism, and liver biopsies serving as an example for routine histopathology samples were studied. The samples represented virus-producing cell cultures (such as SARS-coronavirus, West Nile virus, Orthopox virus), bacteria suspensions (cultures of Escherichia coli and genetically knockout apathogenic Bacillus anthracis), suspensions of parasites (malaria Plasmodium falciparum, Leishmania major, Microsporidia cuniculi, Caenorhabditis elegans), and whole Drosophila melanogaster flies infected with microsporidia. Fresh liver samples and infected flies were fixed in Karnovsky-fixative by microwaving (20 min), all other samples were fixed in buffered glutaraldehyde or Karnovsky-fixative overnight or longer. Subsequently, all samples were divided to evaluate alternative processing protocols: one part of the sample was OsO4-postfixed, ethanol-dehydrated, Epon-infiltrated (overnight) in an automated tissue processor (LYNX, Leica), and polymerized at 60 °C for 48 h; in parallel the other part was microwave-assisted processed in the bench microwave device (REM, Milestone), including post-osmication and the resin block polymerization.
The microwave-assisted processing protocol required at minimum 3 h 20 min: the respective epon resin blocks were uniformly polymerized allowing an easy sectioning of semi- and ultrathin sections. Sections collected on non-coated 200 mesh grids were stable in the electron beam and showed an excellent preservation of the ultrastructure and high contrast, thus allowing an easy, unequivocal and rapid assessment of specimens.
Compared with conventional routine methods, microwave technology facilitates a significant reduction in sample processing time from days to hours without any loss in ultrastructural details. Microwave-assisted processing could, therefore, be a substantial benefit for the routine electron microscopic diagnostic workload. Due to its speed and robust performance it could be applied wherever a rapid electron microscopy diagnosis is required, e.g., if bioterrorism or emerging agents are suspected. Combining microwave technology with digital image acquisition, the 1-day diagnosis based on ultrathin section electron microscopy will become possible, with crucial or interesting findings being consulted or shared worldwide with experts using modern telemicroscopy tools via Internet.
Keywords: Electron microscopy, Same-day diagnosis, Microwave-assisted processing, Rapid processing, Tissue embedding, Clinical urgent samples, Emerging infectious diseases, Bioterrorism
1. Introduction
Diagnostic electron microscopy (EM) can contribute decisively to the laboratory diagnosis of infectious diseases and will be of particular importance if a rapid diagnosis is required, as in a potential bioterror scenario or if emerging infectious agents are to be characterized (O’Toole, 1999, Hazelton and Gelderblom, 2003, Madeley, 2003, Miller, 2003, Ng et al., 2003, Goldsmith et al., 2004, Morens et al., 2004). Likewise EM should be applied in critical clinical setting of controversial or urgent pathological cases (Kamondi et al., 2000, Nordhausen and Barr, 2001, Turbat-Herrera et al., 2004). In many instances, in infectious diseases a rapid and accurate diagnosis is achieved after negative staining of virus or bacterial suspensions (Madeley and Field, 1988, Biel and Madeley, 2001, Gelderblom, 2001, Gelderblom, 2003, Gentile and Gelderblom, 2005; Robert Koch Institute http://www.rki.de). However, in other cases, if infected cell cultures or tissues (e.g., biopsies taken to complement material for the negative staining approach) have to be examined, the need for rapid embedding methods enabling a same-day diagnosis from ultrathin sections is evident. In the past a number of simplified tissue embedding procedures using reduced dehydration times and increased polymerization temperatures were proposed (Bencosme and Tsutsumi, 1970, Johannessen, 1973, Doane et al., 1974, Rowden and Lewis, 1974, Miller, 1982, Bozzola and Russell, 1992).
Rapid diagnostic EM could probably benefit from the microwave employment, a technology that has been successfully applied in organic chemistry (De la Hoz et al., 2005), industrial food processing (Vaid and Bishop, 1998), sterilization of hospital waste (Celandroni et al., 2004), and also in the clinical laboratory. Here a great number of microwave accelerated procedures for light microscopy and EM tissue processing, staining reactions, and immunolabelling was developed (Mayers, 1970, Leong et al., 1985, Login and Dvorak, 1988, Login and Dvorak, 1994, Kok and Boon, 1990, Kok and Boon, 2003, Shi et al., 1991, Leong and Sormunen, 1998, Cavusoglu et al., 2001, Giberson and Demaree, 2001, Giberson et al., 2003, Leria et al., 2004, Munoz et al., 2004), all of them resulting in a significant time reduction. The effect of microwave irradiation on polar substances is mainly well understood. It is attributed to dielectric heating, also called “thermal effect” (Leonard and Shepardson, 1994, Giberson and Demaree, 1995, Kok and Boon, 2003) causing a temperature rise in the whole sample (“internal heating”; in contrast to conventional heating which starts at the specimen surface). The existence of an additional “non-thermal” effect of microwave energy (Marani and Feirabend, 1993, De la Hoz et al., 2005), which may be particularly effective in biological hydrated material in influencing the fixation of cells (Ruijgrok et al., 1993, Leria et al., 2004, Wendt et al., 2004), is still unproven.
A number of efforts has been undertaken to standardize the microwave-assisted procedures (Login, 1998). Different microwave bench “ovens” designed for the laboratory requirements are now commercially available. Assuming the rise in internal temperature as the primary mechanism responsible for the enhanced diffusion of reagents and protein cross-linking during fixation, microwave device manufacturers continually add features into their equipment to provide the end user with more control over the irradiated reaction process.
We report here on the application of a microwave device (REM, Milestone, Sorisole, Italy) recently developed for EM rapid tissue processing equipped with a customized software and a sample basket handling system compatible to our routinely used tissue processor (LYNX, Leica, Bensheim, Germany). Using this system, various specimens representing critical infectious or potential bioterrorism agents and liver biopsies which substitute pathological samples were processed in parallel following both a conventional and a microwave-assisted protocol. Ultrathin sections were evaluated, images were documented digitally and the results of both techniques compared.
2. Materials and methods
2.1. Sample collection and primary fixation
The following specimens in the primary fixative were sent by post to the EM laboratory in Regensburg for subsequent comparative processing and evaluation: cell suspensions infected with SARS-coronavirus, West Nile virus, an Orthopox virus isolated from an elephant (fixed in 2.5% phosphate buffered glutaraldehyde (GA), pH 7.2: Robert Koch Institute, Berlin), suspensions of Escherichia coli and Acanthamoeba castellanii (fixed in 4% cacodylate buffered GA, pH 7.3: Medical Service of Federal Armed Forces, Koblenz), suspensions containing Plasmodium falciparum, Leishmania major, Microsporidia cuniculi and the worm Caenorhabditis elegans (fixed in 2% cacodylate buffered GA, pH 7.2: Bernhard-Nocht-Institute for Tropical Medicine, Hamburg). A part of each sample kept at the collaborative site was processed following the respective in-house protocols. Grids with stained ultrathin sections were sent to the EM laboratory in Regensburg for evaluation and documentation.
Agar culture (6-day-old) of apathogenic genetically knockout pX-02 Bacillus anthracis samples (courtesy of Dr. H.-J. Linde, Microbiology Department, University Regensburg), Drosophila melanogaster flies infected with microsporidia Tubulinosema ratisbonensis (courtesy of Dr. C. Franzen, Internal Medical Department, University Regensburg), and murine and human surgical liver samples were fixed in modified Karnovsky-fixative (1% paraformaldehdyde/2.5% GA in cacodylate buffer, pH 7.3) overnight as well as 20 min under microwave irradiation (Central EM Laboratory, Regensburg).
Subsequently, cells and microorganisms were sedimented by centrifugation (800 × g for 5 min), enclosed in low-melting-point agar at 45 °C and chilled on ice. The agar blocks with the agents as well as the solid tissue samples were dissected with a razor blade into cubes of 1-mm edge length. Each sample was processed according to a conventional and a microwave-assisted protocol.
2.2. Conventional processing
One part of each sample was processed in one batch exclusively in the automated tissue processor (LYNX) as a control, involving 1% OsO4 post-fixation, dehydration in graded ethanols, infiltration (overnight) and embedding in the EMbed-812 epoxy resin (all reagents from Science Services, Munich, Germany).
After 48 h polymerization at 60 °C, semithin sections were cut, stained with toluidine blue and basic fuchsin, and after selection of areas of interest the Epon blocks were trimmed for ultrathin sectioning. Ultrathin sections 70 nm in thickness were cut with a diamond knife (45°, Diatome, Biel, Switzerland) on a Reichert Ultracut-S ultramicrotome, mounted on non-coated 200 mesh copper grids, and double stained with aqueous 2% uranyl acetate and lead citrate solutions for 10 min each.
2.3. Microwave processing
In parallel, the other part of each sample was processed using the Rapid Electron Microscope microwave device (REM, Fig. 1 ) utilizing the same specimen baskets (Fig. 2 ) as for the LYNX-processor. This microwave bench unit is claimed by the manufacturer to have an even microwave distribution within the device resonant “cavity” (Visinoni et al., 1998) during the processing. Each vial, containing the samples immersed in the process solutions, is placed in a special designed safety carrier, which locates the vial in a defined position in the microwave “cavity”. A non-contact infra-red temperature sensor measures the current solution temperature in the vial, while a magnetic stirrer is ensuring uniform heat distribution in the vial solution. The solution temperature is the critical parameter to monitor the magnetron wattage (maximum 700 W) power output, which is controlled via a feedback loop during the continuous microwave irradiation of the sample. The graphical display of the slope of temperature rise, irradiation temperature stabilization and time for each processing step, could be easily defined on a dedicated touch screen monitor. The whole microwave-assisted processing is controlled by a microprocessor and dedicated software package. This software also allows routine documentation of the entire microwave process for Quality Assurance and Quality Control.
Fig. 1.

Milestone rapid electron microscopy (REM) histoprocessor. Note the vial with processed samples placed in a predefined position in the microwave “oven”.
Fig. 2.

Sample preparation tools for the REM. The baskets are the same as for the automated LYNX-processor.
As outlined in Fig. 3 , also the primary fixation of the “fresh” samples (Drosophila flies infected with microsporidia and liver tissue samples) with an adjusted temperature of 50 °C, not merely the OsO4-post-fixation, ethanol-dehydration, Epon-infiltration and polymerization (BEEM-capsules, under water) was carried out with microwave irradiation of the samples. This completely microwave-assisted sample processing required 4 h 25 min, except handling time, e.g., for solution exchange. In order to shorten the process further, we tested also an abbreviated microwave-assisted protocol with time reduction in all steps with the SARS-coronavirus infected cells, the B. anthracis, and human surgical liver tissue samples; here the microwave processing time was 3 h 20 min (Table 1 ).
Fig. 3.
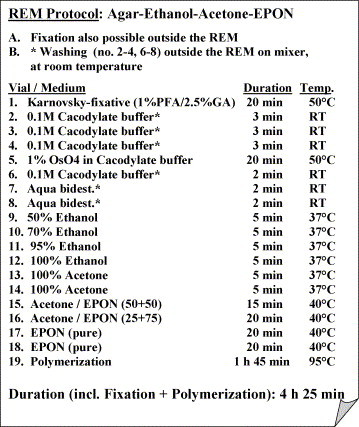
Protocol outline of the microwave-assisted specimen processing.
Table 1.
Comparison of processing times for conventional and microwave-assisted protocols
| Processing step | Conventional (LYNX) | Microwave | Microwave shortened |
|---|---|---|---|
| Primary fixation | ≥2 h | 20 min | 15 min |
| Buffer washes | 1 h | 9 min | 3 min |
| Osmium post-fixation | 2 h | 20 min | 10 min |
| Water washes | 40 min | 6 min | 3 min |
| Dehydration | 1 h 45 min | 20 min | 10 min |
| Infiltration | 19 h | 1 h 25 min | 59 min |
| Polymerization | 48 h | 1 h 45 min | 1 h 40 min |
| Total | ≥74 h 25 min | 4 h 25 min | 3 h 20 min |
The polymerized Epon blocks were sectioned and stained in the same manner as described for the conventionally processed samples.
2.4. Evaluation of the sections and images
The sections were examined in a LEO 912AB transmission electron microscope (Zeiss, Oberkochen, Germany) operating at 80 kV in zero-loss mode and equipped with a side-entry and a bottom-mounted fiber-optic coupled CCD-camera (Proscan, Lagerlechfeld, Germany) capable to record images with 1024 × 1024 pixels. Efforts were taken to standardize the conditions of image acquisition (constant beam brightness, exposure time and magnification settings) during the EM examination. Imaging and measuring were done with the analySIS software, version 3.2 (Soft Imaging System GmbH, Muenster, Germany), without any digital image post-processing manipulations. Notes of the section quality and beam stability during the examination were made, and the quality of the images obtained by the conventional versus microwave-assisted procedures was evaluated visually on a calibrated CRT monitor and by high-resolution laser prints.
3. Results
A significant processing time reduction was realized between the conventional (3 days) and microwave-assisted protocol (4 h 25 min or 3 h 20 min, Table 1). As the solution exchange in the microwave “oven” is not automated, it had to be performed manually as well as the transfer of the samples into the BEEM-capsules for polymerization (including probe identity tags mounting), which required additional time depending on the number of samples processed in parallel (approximately 1 h).
The microwave processed resin blocks were polymerized uniformly and revealed good sectioning properties for semi- and ultrathin sections. The sections examined in the EM were stable in the electron beam. The images were crisp displaying a high and uniform contrast.
After microwave-assisted processing the fine structure of virus-infected cells and infectious agents was well preserved (Fig. 4, Fig. 5, Fig. 6, Fig. 7, Figs. 8 and 9, Fig. 10, Fig. 11 ). The SARS-coronaviruses can be clearly recognized in the dilated perinuclear space and RER-cisternae, also the microtubules of the cytoskeleton and mitochondria of the host cells are visible in detail (Fig. 4b). In cells infected with the elephant Orthopox virus artificial extraction of the core material was much less common (Fig. 5). This is a phenomenon often experienced in conventional embedding preparations. In the apathogenic B. anthracis and E. coli culture similar good preservation of the vegetative organisms as well as of mature Anthrax spores were achieved in both conventional LYNX and microwave-assisted embedding (Fig. 6, Fig. 7); each mature Anthrax spore displayed a clearly delineated endo- and exosporium covered with tiny surface microfilaments (Fig. 6b and d).
Fig. 4.
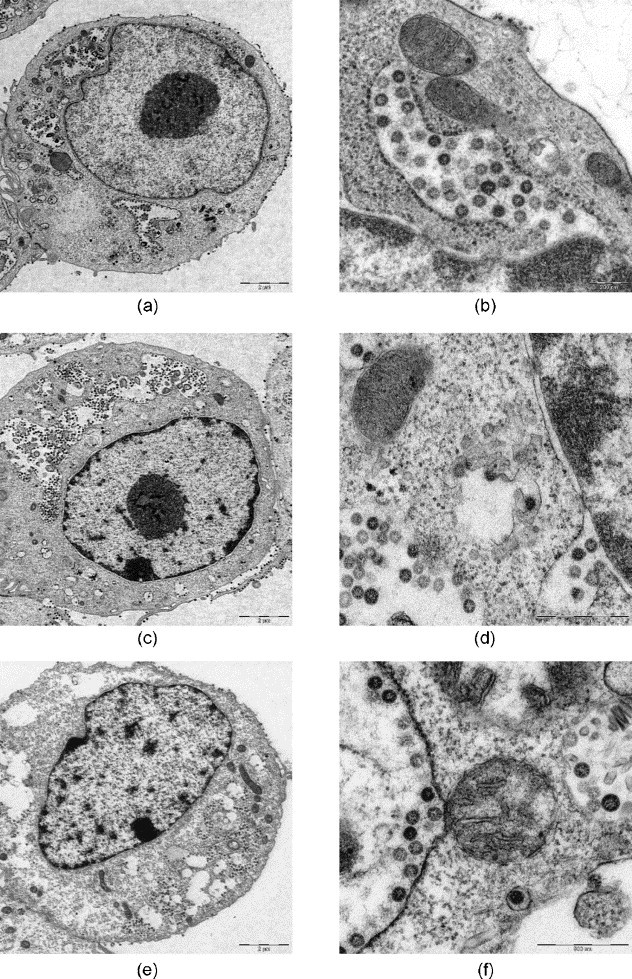
SARS-coronaviruses produced in Vero. 6 cell culture, conventional fixation (2.5% GA): (a), (b) REM-microwave embedding; (c), (d) LYNX-embedding; (e), (f) external embedding (Robert Koch Institute, Berlin). Original magnification: (a), (c), (e) 1250×; (b), (d), (f) 10,000×.
Fig. 5.
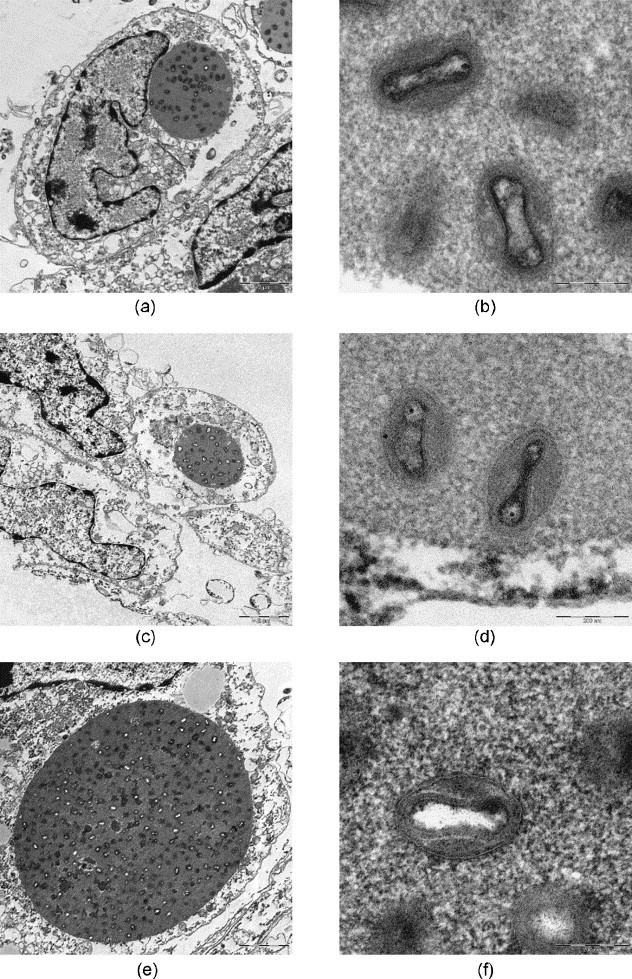
Orthopox virus isolated from an elephant (cell culture), conventional fixation (2.5% GA): (a), (b) REM-microwave embedding; (c), (d) LYNX-embedding; (e), (f) external embedding (Robert Koch Institute, Berlin). Original magnification: (a), (c), (e) 1250×; (b), (d), (f) 20,000×.
Fig. 6.
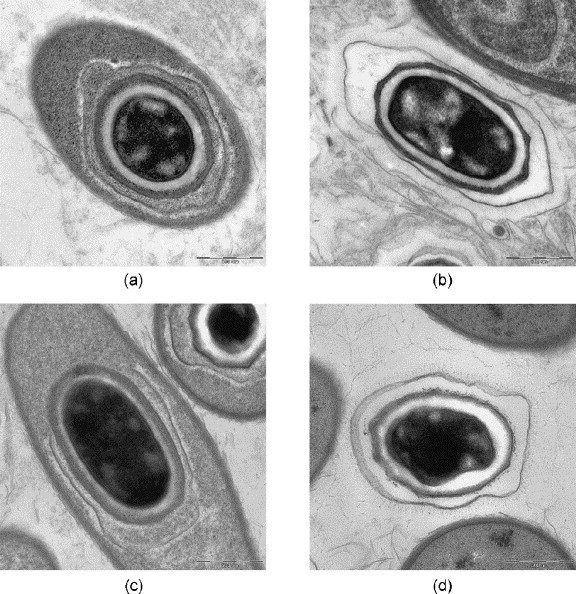
Bacillus anthracis, conventional fixation (4% GA). (a), (b) REM-microwave embedding; (c), (d) LYNX-embedding. Original magnification: (a), (b), (c) 8000×; (d) 10,000×.
Fig. 7.
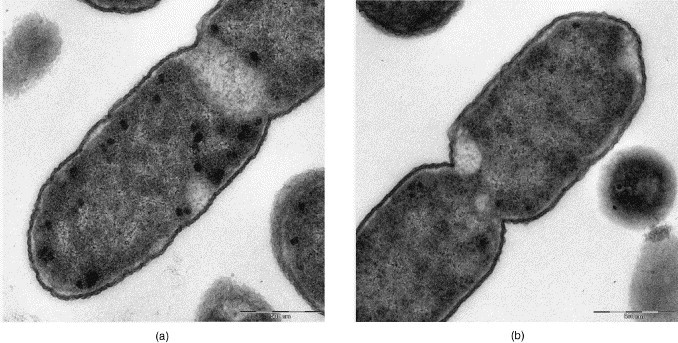
Escherichia coli, conventional fixation (4% GA), Medical Service of Federal Armed Forces, Koblenz: (a) REM-microwave embedding; (b) LYNX-embedding. Original magnification: 8000×.
Figs. 8 and 9.
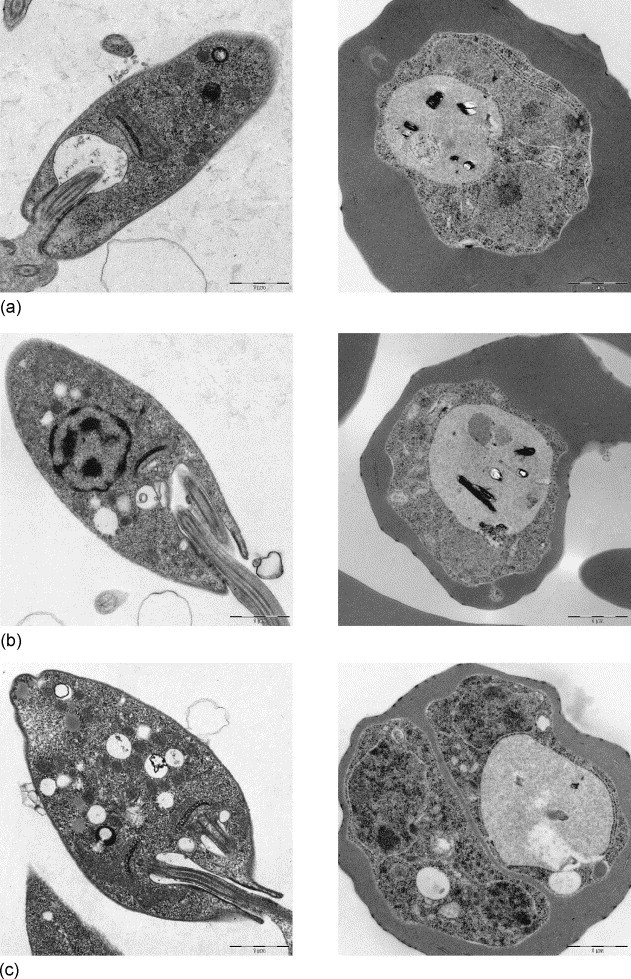
(8) Leishmania major, conventional fixation (2% GA). (a) REM-microwave embedding; (b) LYNX-embedding; (c) external embedding (Bernhard-Nocht-Institute for Tropical Medicine, Hamburg). Original magnification: 3150×. (9) Malaria Plasmodium falciparum, conventional fixation (2% GA): (a) REM-microwave embedding; (b) LYNX-embedding; (c) external embedding (Bernhard-Nocht-Institute for Tropical Medicine, Hamburg). Original magnification: 3150×.
Fig. 10.
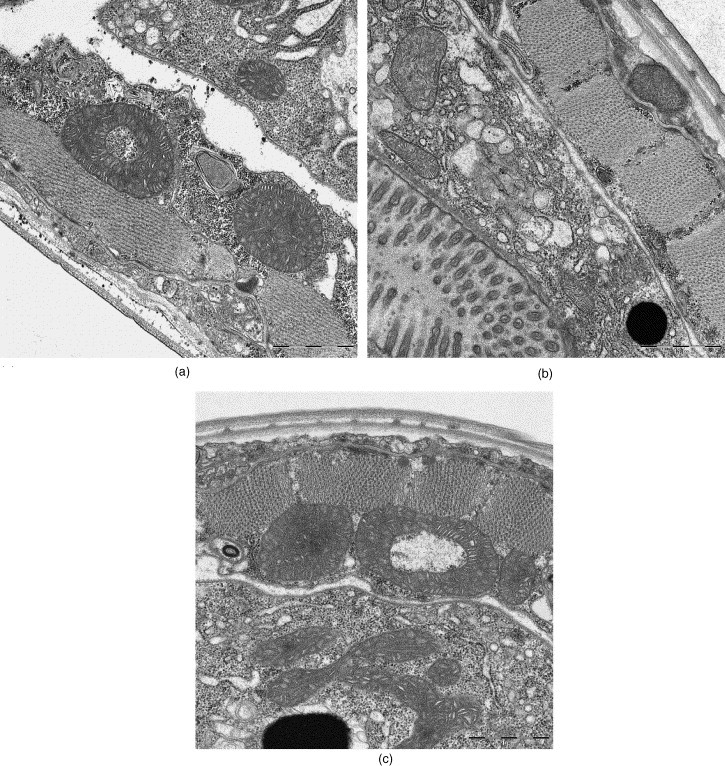
Caenorhabditis elegans, conventional fixation (2% GA); (a) REM-microwave embedding; (b) LYNX-embedding; (c) external embedding (Bernhard-Nocht-Institute for Tropical Medicine, Hamburg). Original magnification (side-entry camera): 10,000×.
Fig. 11.
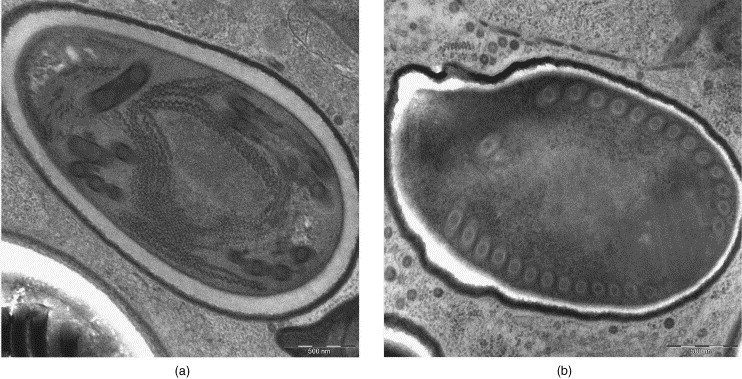
Microsporidia Tubulinosema ratisbonensis from infected Drosophila melanogaster flies (modified Karnovsky-fixative): (a) REM-microwave fixation and embedding; (b) conventional fixation, LYNX-embedding. Original magnification: 6300×.
In the protozoon L. major, the causative agent for tropical sore, the kinetochore–mitochondrium complex – which is the ultrastructural hallmark of the flagellated promastigote form – was preserved excellently in all procedures used (Fig. 8). The malaria merozoites of Pl. falciparum had well discernible nuclei and vacuoles with typical pigment inclusions (Fig. 9).
The cross-sections through the adult worm C. elegans body displayed very well-preserved layers of the cuticula with underlying muscle cells and the internal organs in all preparations (Fig. 10). The very good ultrastructural preservation of the mitochondria was a notable feature of this specimen (Fig. 10a).
In the infected Drosophila flies, microsporidia were observed in all body compartments enclosed by the chitine external skeleton (lateral incised for better solution infusion). Well-preserved mature spores were abundant in the completely microwave-assisted fixed and embedded samples (Fig. 11) often showing an uniformly delineated space between the endo- and exosporium (Fig. 11a).
The mouse liver samples, which were microwave-assisted fixed and thereafter alternatively conventional or microwave-assisted processed and embedded, showed excellently preserved ultrastructural criteria of the hepatocytes as well as the glycogen and lipid inclusions (Fig. 12 ). At higher magnification fine structural details, e.g., the double membrane profiles of the nucleus and mitochondria as well as the cytoplasmic and mitochondrial matrix, did not appear to differ between the two procedures (Fig. 12b and d). This high quality ultrastructure preservation was obtained in samples not exceeding 1-mm edge length. In larger samples, the centres of the tissue blocks showed in semithin sections a reduced staining intensity, in ultrathin sections cell shrinkage (dilated intercellular and Disse space), cytoplasmic matrix disruptions and artificial cavity formation as well as loss of mitochondrial fine structure (Fig. 13 ).
Fig. 12.
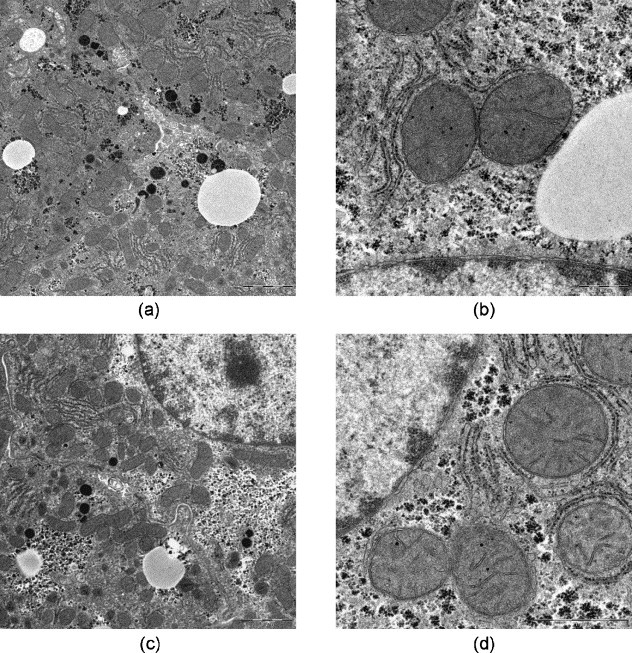
Liver (mouse), REM-microwave fixation (modified Karnovsky-fixative): (a), (b) REM-microwave embedding; (c), (d) LYNX-embedding. Original magnification: (a), (c) 1250×; (b), (d) 5000×.
Fig. 13.
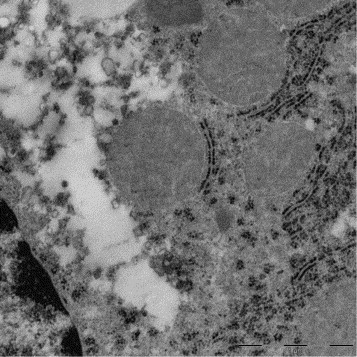
Liver (mouse), REM-microwave fixation (modified Karnovsky-fixative). A hepatocyte selected from the centre of a large (2 mm × 2 mm × 3 mm) tissue block displaying artificial cavity formation in the perinuclear cytoplasm and loss of mitochondrial fine structure. Original magnification: 5000×.
Noteworthy, also with the application of the shortened (3 h 20 min) microwave-assisted protocol convincing results were achieved: a SARS-virus producing cell (Fig. 14a), a mature B. anthracis spore (Fig. 14b), and the human surgical liver sample (Fig. 14c) displayed good quality of the examined ultrastructure. The cytoplasm and organelles of cells and the pathogenic agents, however, clearly differed in densities (Fig. 6, Fig. 12), as there was more “compactness” and also the membranes appeared coarser, in comparison with the 4.5 h protocol preparation.
Fig. 14.
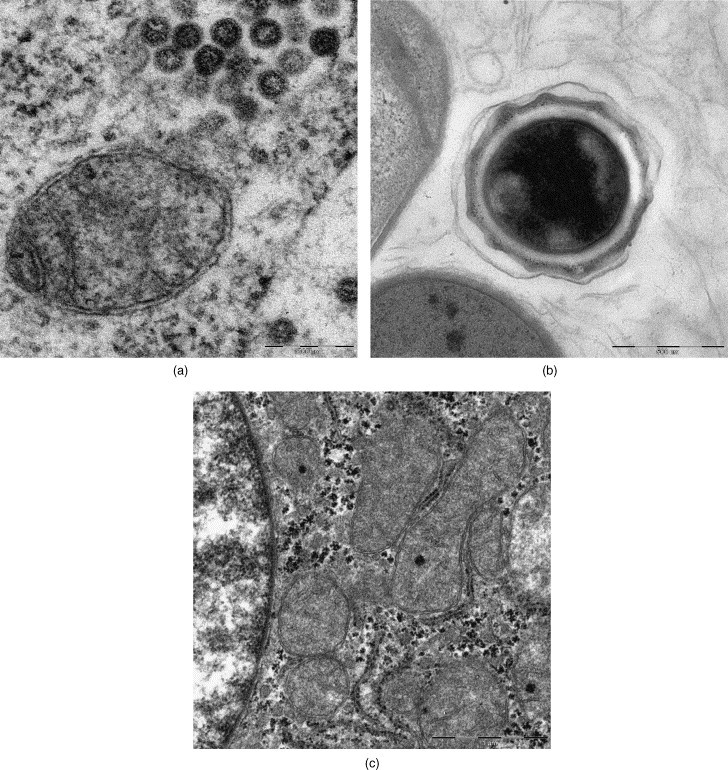
Reduced microwave-assisted processing protocol: 3 h 20 min: (a) SARS-coronavirus; (b) Bacillus anthracis spore; (c) liver (human), REM-microwave fixation. Original magnification: (a) 20,000×; (b) 10,000×; (c) 5000×.
4. Discussion
Short turnaround times as well as reliable good section and image quality are important criteria when rapid embedding protocols are considered. Comparing the samples processed according to a microwave-assisted versus a conventional protocol, we obtained very satisfying results with the microwave bench “oven”. The experienced reduction in sample processing time from days to hours with no compromised ultrastructural preservation of selected “difficult” specimens is in agreement with other workers using this technology. Microwave processing time of 3–4 h (Nordhausen and Barr, 2001, Gerrity and Forbes, 2003, Laboux et al., 2004) and short as 2 h (Giberson et al., 2003) were reported from EM laboratories with similar sample spectrum and workload as those of the authors.
The microwave-assisted processed samples were comparable or superior in section and image quality to the conventionally processed samples and compliant to published standards (Morioka et al., 1992, Miller, 2003, Peiris et al., 2003, Buchanan, 2004, Celandroni et al., 2004, Franzen et al., 2005, Larsson, 2005). The completely microwave-assisted processed parenchymal tissue of liver, used in our comparative approach as a substitute for other pathological solid tissues, revealed good results as shown in Fig. 12 in samples not exceeding 1-mm edge length. In the centre of the block where larger samples had been taken, there was a loss of ultrastructural preservation, and artefacts were seen such as have been described previously by Wild et al. (1989).
This phenomenon reflects the continuing discussion about the probably “dichotomous” nature of the non-ionizing microwave radiation in respect to the “heat” and the “non-thermal” or direct microwave effect (Galvez et al., 2004). Despite the complex aspects influencing the aldehyde fixation process of tissues (Fox et al., 1985, Leong and Sormunen, 1998, Izumi et al., 2000, Werner et al., 2000, Giberson and Elliott, 2001), recent observations tend to support the existence of the direct mechanism of microwave energy action (Kok and Boon, 2003, Leria et al., 2004, Wendt et al., 2004, De la Hoz et al., 2005, Gaber et al., 2005). This needs to be considered in the microwave-assisted tissue processing because also our results suggest, that an “overheating” inside the solid tissue samples can adversely affect tissue ultrastructure. Therefore, further development work should include efforts to reduce the critical “thermal” effect and increase direct “non-thermal” microwave action during tissue fixation (Galvez et al., 2004, Leria et al., 2004).
The REM Milestone microwave device is run with the sample baskets that are used also in the widely applied automated LYNX-processor for routine conventional tissue embedding. We observed that this shared equipment is a great benefit for the EM laboratory, e.g., a sample received late afternoon can be rapidly fixed in the microwave (e.g., 10–20 min in place of at least 2 h at room temperature) and added (without basket change) to other tissues which are to be processed automatically in the LYNX overnight anyway, and if urgent, rapidly polymerized in the microwave in 1 h 45 min the next morning (instead of 48 h). This option generates more flexibility of the laboratory workflow in handling clinical urgent samples and improves the economic employment of the available manpower. Thus, a “same-day” electron microscopy diagnosis for cells and tissues becomes reality.
The results obtained with the shortened protocol appear especially promising. As judged from sectioning properties and section quality, the samples were uniformly polymerized, i.e., comparable to the results with the longer microwave protocol. The observed denser appearance of cell cytoplasm could be explained by less extraction of cell material during the shorter course of the complete procedure. Therefore, in view of the acceptable results with the approximately 3.5 h protocol, attempts to cut down further the preparation schedule appear worthwhile.
Clearly, urgent samples reaching the diagnostic laboratory at unpredictable daytime need to be examined immediately in potential bioterrorism scenario or in case of an emerging agent disease (Lane et al., 2001, Morens et al., 2004). The microwave-assisted sample preparation enables EM to evaluate also thin sections for rapid diagnosis. Basing on our experience, the subsequent section ultramicrotomy, staining and examination in the EM can be completed in about 2 h. In consequence, using the potential of accelerated section preparation, EM-diagnosis from biopsy material can be rendered on the same workday. Thus, both negative staining of small particulate suspensions – from the smallest viruses up to bacteria, spores and parasites – as well as tissues and diagnostic cell cultures can be scrutinized by the “open view” of EM (Hazelton and Gelderblom, 2003) as part of the “frontline” diagnostic methods guiding further measures, e.g., containment and therapeutic activities. In combination with digital image acquisition and telemicroscopy networking tools (Schroeder et al., 2001) crucial or interesting findings could be consulted or shared worldwide in very short time.
Acknowledgements
The authors would like to thank Beate Voll and Heiko Ingo Siegmund (Central EM Laboratory, Regensburg) for excellent technical support and help during the manuscript preparation.
References
- Bencosme S.A., Tsutsumi V. A fast method for processing biological material for electron microscopy. Lab. Invest. 1970;23:447–451. [PubMed] [Google Scholar]
- Biel S.S., Madeley D. Diagnostic virology—the need for electron microscopy: a discussion paper. J. Clin. Virol. 2001;22:1–9. doi: 10.1016/s1386-6532(01)00151-2. [DOI] [PubMed] [Google Scholar]
- Bozzola J.J., Russell L.D. second ed. Jones and Bartlett Publishers; Sudbury, Massachusetts: 1992. Electron Microscopy: Principles and Techniques for Biologists. [Google Scholar]
- Buchanan J. Microwave processing of Drosophila tissues for electron microscopy. Microsc. Today. 2004;12(6):42. [Google Scholar]
- Cavusoglu I., Minbay F.Z., Temel S.G., Noyan S. Rapid polymerisation with microwave irradiation for transmission electron microscopy. Eur. J. Morphol. 2001;39(5):313–317. doi: 10.1076/ejom.39.5.313.7376. [DOI] [PubMed] [Google Scholar]
- Celandroni F., Longo I., Tosoratti N., Giannessi F., Ghelardi E., Salvetti S., Baggiani A., Senesi S. Effect of microwave radiation on Bacillus subtilis spores. J. Appl. Microbiol. 2004;97(6):1220–1227. doi: 10.1111/j.1365-2672.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- De la Hoz A., Diaz-Ortiz A., Moreno A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005;34(2):164–178. doi: 10.1039/b411438h. [DOI] [PubMed] [Google Scholar]
- Doane F.A., Anderson N., Chao J., Nooman A. Two-hour embedding procedure for intracellular detection of viruses by electron microscopy. Appl. Microbiol. 1974;27(2):407–410. doi: 10.1128/am.27.2.407-410.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C.H., Johnson F.B., Whiting J., Roller P.P. Formaldehyde fixation. J. Histochen. Cytochem. 1985;33:845–853. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- Franzen C., Fischer S., Schroeder J., Schölmerich J., Schneuwly S. Morphological and molecular investigations of Tubulinosema ratisbonensis gen. nov., sp. nov. (microsporidia: Tubulinosematidae fam. nov.), a parasite infecting a laboratory colony of Drosophila melanogaster (diptera: Drosophilidae) J. Eukaryot. Microbiol. 2005;52(2):141–152. doi: 10.1111/j.1550-7408.2005.04-3324.x. [DOI] [PubMed] [Google Scholar]
- Gaber M.H., Abd El Halim N., Khalil W.A. Effect of microwave radiation on the biophysical properties of liposomes. Bioelectromagnetics. 2005;26(3):194–200. doi: 10.1002/bem.20064. [DOI] [PubMed] [Google Scholar]
- Galvez J.J., Giberson R.T., Cardiff R.D. Microwave mechanisms—the energy/heat dichotomy. Microsc. Today. 2004;12(2):18–23. [Google Scholar]
- Gelderblom H.R. Electron microscopy in diagnostic virology. BIOforum Int. 2001;5:64–67. [Google Scholar]
- Gelderblom H.R. Elektronenmikroskopie im Methodenspektrum der Bioterrorismus-Diagnostik. Bundesgesundheitsbl-Gesundheitsforsch-Gesundheitsschutz. 2003;46:984–988. [Google Scholar]
- Gentile M., Gelderblom H.R. Rapid viral diagnosis: role of electron microscopy. New Microbiol. 2005;28(1):1–12. [PubMed] [Google Scholar]
- Gerrity R.G., Forbes G.W. Microwave processing in diagnostic electron microscopy. Microsc. Today. 2003;11(6):38–41. [Google Scholar]
- Giberson R.T., Demaree R.S., Jr. Microwave fixation: understanding the variables to achieve rapid reproducible results. Microsc. Res. Technol. 1995;32(3):246–254. doi: 10.1002/jemt.1070320307. [DOI] [PubMed] [Google Scholar]
- Giberson R.T., Demaree R.S. Humana Press Inc.; Totowa, NJ: 2001. Microwave Techniques and Protocols. [Google Scholar]
- Giberson R.T., Elliott D.E. Microwave-assisted formalin fixation of fresh tissue: a comparative study. In: Giberson R.T., Demaree R.S. Jr., editors. Microwave Techniques and Protocols. Humana Press Inc.; Totowa, NJ: 2001. pp. 191–208. [Google Scholar]
- Giberson R.T., Austin R.L., Charlesworth J., Adamson G., Herrera G.A. Microwave and digital imaging technology reduce turnaround times for diagnostic electron microscopy. Ultrastruct. Pathol. 2003;27(3):187–196. doi: 10.1080/01913120309937. [DOI] [PubMed] [Google Scholar]
- Goldsmith C.S., Tatti K.M., Ksiazek T.G., Rollin P.E., Comer J.A., Lee W.W., Rota P.A., Bankamp B., Bellini W.J., Zaki S.R. Ultrastructural characterization of SARS coronavirus. Emerg. Infect. Dis. 2004;10:320–326. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelton P.R., Gelderblom H.R. Electron microscopy for rapid diagnosis of infectious agents in emergent situations. Emerg. Infect. Dis. 2003;9:294–303. doi: 10.3201/eid0903.020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y., Hammerman S.B., Benz A.M., Labruyere J., Zorumski C.F., Olney J.W. Comparison of rat retinal fixation techniques: chemical fixation and microwave irradiation. Exp. Eye Res. 2000;70(2):191–198. doi: 10.1006/exer.1999.0779. [DOI] [PubMed] [Google Scholar]
- Johannessen J.V. Rapid processing of kidney biopsies for electron microscopy. Kidney Int. 1973;3:46–52. doi: 10.1038/ki.1973.7. [DOI] [PubMed] [Google Scholar]
- Kamondi A., Szegedi A., Papp A., Seres A., Szirmai I. Vogt-Koyanagi-Harada disease presenting initially as aseptic meningoencephalitis. Eur. J. Neurol. 2000;7(6):719–722. doi: 10.1046/j.1468-1331.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- Kok L.P., Boon M.E. Microwaves for microscopy. J. Microsc. 1990;158:291–322. doi: 10.1111/j.1365-2818.1990.tb03003.x. [DOI] [PubMed] [Google Scholar]
- Kok L.P., Boon M.E. Coulomb Press Leyden; Leiden: 2003. Microwaves for the Art of Microscopy. [Google Scholar]
- Laboux O., Dion N., Arana-Chavez V., Ste-Marie L.G., Nanci A. Microwave irradiation of ethanol-fixed bone improves preservation, reduces processing time, and allows both light and electron microscopy on the same sample. J. Histochem. Cytochem. 2004;52(10):1267–1275. doi: 10.1177/002215540405201003. [DOI] [PubMed] [Google Scholar]
- Lane H.C., Montagne J.L., Fauci A.S. Bioterrorism: a clear and present danger. Nat. Med. 2001;7(12):1271–1273. doi: 10.1038/nm1201-1271. [DOI] [PubMed] [Google Scholar]
- Larsson J.I. Fixation of microsporidian spores for electron microscopy. J. Invertebr. Pathol. 2005;90(1):47–50. doi: 10.1016/j.jip.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Leonard J.B., Shepardson S.P. A comparison of heating modes in rapid fixation techniques for electron microscopy. J. Histochem. Cytochem. 1994;42(3):383–391. doi: 10.1177/42.3.8308256. [DOI] [PubMed] [Google Scholar]
- Leong A.S., Daymon M.E., Milios J. Microwave irradiation as a form of fixation for light and electron microscopy. J. Pathol. 1985;146(4):313–321. doi: 10.1002/path.1711460404. [DOI] [PubMed] [Google Scholar]
- Leong A.S., Sormunen R.T. Microwave procedures for electron microscopy and resin-embedded sections. Micron. 1998;29:397–409. doi: 10.1016/s0968-4328(98)00018-3. [DOI] [PubMed] [Google Scholar]
- Leria F., Marco R., Medina F.J. Structural and antigenic preservation of plant samples by microwave-enhanced fixation, using dedicated hardware, minimizing heat-related effects. Microsc. Res. Technol. 2004;65(1–2):86–100. doi: 10.1002/jemt.20109. [DOI] [PubMed] [Google Scholar]
- Login G.R., Dvorak A.M. Microwave fixation provides excellent preservation of tissue, cells and antigens for light and electron microscopy. Histochem. J. 1988;20(6–7):373–387. doi: 10.1007/BF01002732. [DOI] [PubMed] [Google Scholar]
- Login G.R., Dvorak A.M. Methods of microwave fixation for microscopy. A review of research and clinical applications: 1970–1992. Prog. Histochem. Cytochem. 1994;27(4):1–127. [PubMed] [Google Scholar]
- Login G.R. The need for clinical laboratory standards for microwave-accelerated procedures. J. Histotechnol. 1998;21(1):7–9. [Google Scholar]
- Madeley C.R., Field A.M. second ed. Churchill Livingstone; London: 1988. Virus Morphology. [Google Scholar]
- Madeley C.R. Diagnosing smallpox in possible bioterrorist attack. Lancet. 2003;361:97–98. doi: 10.1016/s0140-6736(03)12241-6. [DOI] [PubMed] [Google Scholar]
- Marani E., Feirabend H.K. A nonthermal microwave effect does not exist. Eur. J. Morphol. 1993;31:141–144. [PubMed] [Google Scholar]
- Mayers C.P. Histological fixation by microwave heating. J. Clin. Pathol. 1970;2:273–275. doi: 10.1136/jcp.23.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.A. Practical rapid embedding procedure for transmission electron microscopy. Lab. Med. 1982;13:752–756. [Google Scholar]
- Miller S. Bioterrorism and electron microscopic differentiation of poxviruses from herpesviruses: does and don’ts. Ultrastruct. Pathol. 2003;27:133–140. doi: 10.1080/01913120309932. [DOI] [PubMed] [Google Scholar]
- Morens D.M., Folkers G.K., Fauci A.S. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka H., Suganuma A., Tachibana M. Ultrastructure of Staphylococcus aureus as revealed by microwave fixation. J. Electron Microsc. 1992;41(1):1–6. [PubMed] [Google Scholar]
- Munoz T.E., Giberson R.T., Demaree R., Day J.R. Microwave-assisted immunostaining: a new approach yields fast and consistent results. J. Neurosci. Methods. 2004;137(2):133–139. doi: 10.1016/j.jneumeth.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Ng M.L., Tan S.H., See E.E., Ooi E.E., Ling A.E. Entry and early events of severe acute respiratory syndrome coronavirus. J. Med. Virol. 2003;71:323–331. doi: 10.1002/jmv.10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordhausen R.W., Barr B.C. Specimen preparation for thin-section electron microscopy utilizing microwave-assisted rapid processing in a veterinary diagnostic laboratory. In: Giberson R.T., Demaree R.S. Jr., editors. Microwave Techniques and Protocols. Humana Press Inc.; Totowa, NJ: 2001. pp. 49–66. [Google Scholar]
- O’Toole T. Smallpox: an attack scenario. Emerg. Infect. Dis. 1999;5:540–546. doi: 10.3201/eid0504.990416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., Nicholls J., Yee W.K., Yan W.W., Cheung M.T., Cheng V.C., Chan K.H., Tsang D.N., Yung R.W., Ng T.K., Yuen K.Y., SARS study group Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert Koch Institute, Consultant Lab for Diagnostic EM in Infectious Diseases: http://www.rki.de/EN/Content/Institute/DepartmentsUnits/NRC/CONSULAB/consulab__node.html.
- Rowden G., Lewis M.G. Experience with a three-hour electron microscopy service. J. Clin. Pathol. 1974;27(6):505–510. doi: 10.1136/jcp.27.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijgrok J.M., Boon M.E., Feirabend H.K., Ploeger S. Does microwave irradiation have other than thermal effects on glutaraldehyde crosslinking of collagen? Eur. J. Morphol. 1993;31(4):290–297. [PubMed] [Google Scholar]
- Schroeder J.A., Voelkl E., Hofstaedter F. Ultrastructural Telepathology—remote EM-diagnostic via Internet. Ultrastruct. Pathol. 2001;25(4):307–310. doi: 10.1080/019131201753136322. [DOI] [PubMed] [Google Scholar]
- Shi S.R., Key M.E., Kalra K.L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. 1991;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Turbat-Herrera E.A., D’Agostino H., Herrera G.A. The use of electron microscopy to refine diagnoses in the daily practice of cytopathology. Ultrastruct. Pathol. 2004;28(2):55–66. doi: 10.1080/01913120490430418. [DOI] [PubMed] [Google Scholar]
- Vaid A., Bishop A.H. The destruction by microwave radiation of bacterial endospores and amplification of the released DNA. J. Appl. Microbiol. 1998;85:115–122. [Google Scholar]
- Visinoni F., Milios J., Leong A.S., Boon M.E., Kok L.P., Malcangi F. Ultra-rapid microwave/variable pressure-induced histoprocessing: description of a new tissue processor. J. Histotechnol. 1998;21:219–224. [Google Scholar]
- Wendt K.D., Jensen C.A., Tindall R., Katz M.L. Comparison of conventional and microwave-assisted processing of mouse retinas for transmission electron microscopy. J. Microsc. 2004;214(1):80–88. doi: 10.1111/j.0022-2720.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- Werner M., Chott A., Fabiano A., Battifora H. Effect of formalin tissue fixation and processing on immunohistochemistry. Am. J. Surg. Pathol. 2000;24(7):1016–1019. doi: 10.1097/00000478-200007000-00014. [DOI] [PubMed] [Google Scholar]
- Wild P., Krähenbühl M., Schraner E.M. Potency of microwave irradiation during fixation for electron microscopy. Histochemistry. 1989;91(3):213–220. doi: 10.1007/BF00490135. [DOI] [PubMed] [Google Scholar]


