Abstract
Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) belong to a group of mammalian immunoglobulin-related glycoproteins. They are involved in cell–cell recognition and modulate cellular processes that range from the shaping of tissue architecture and neovascularization to the regulation of insulin homeostasis and T-cell proliferation. CEACAMs have also been identified as receptors for host-specific viruses and bacteria in mice and humans, respectively, making these proteins an interesting example of pathogen–host co-evolution. Forward and reverse genetics in the mouse now provide powerful novel models to elucidate the action of CEACAM family members in vivo.
Introduction
In multicellular organisms, cell–cell adhesion is vital to guide cells to their proper location during embryonic development and to mediate the integration of single cells into functional tissues and organs. Members of the immunoglobulin superfamily of cell adhesion molecules (IgCAMs) constitute a large group of cell surface glycoproteins with ancient roots in the animal kingdom that specialize in cell–cell adhesion. All IgCAMs posses at least one immunoglobulin (Ig)-like domain, a compact structure ∼85–110 amino acids long characterized by two β-sheets packed against each other [1]. The Ig fold comes in minor variations that allow subdivision into Ig variable (IgV) and Ig constant (IgC)-1 and -2 domains. In evolutionary terms, the Ig-fold is a success story that has made Ig-domain-encoding sequences the most abundant genes in the human genome. This does not come as a surprise, as the Ig domain seems to be perfectly suited to provide proteins with a universal interface that can be fine-tuned for almost every binding task. In the case of IgCAMs, the binding specificity is often directed towards molecules of their own class or towards themselves, a feature that has been well studied on the genetic, biochemical and structural levels for several neuronal IgCAMs, in particular N-CAM [2, 3].
Another prominent member of the IgCAM superfamily is the carcinoembryonic antigen (CEA), which is involved in homotypic and heterotypic interactions with other closely related IgCAMs and which constitutes a clinically relevant diagnostic marker in the surveillance of colon tumors. Together with its paralogues, CEA has been grouped in the CEA-related cell adhesion molecule (CEACAM) family, a subdivision of IgCAMs so far only known from mammals [4]. The overall domain organization of human CEACAMs, the number of known splice variants and the distribution of orthologues in other mammalian species is presented in Figure 1 . It is important to note that over the years members of the CEACAM family have been going through a number of name changes; for example, the original biliary glycoprotein (Bgp), later classified as the CD66a antigen, has now become CEACAM1 (for current and historic nomenclature of the CEACAM family see also Figure 1). Though CEACAMs have been studied for decades with regard to their tumor-associated features [5], it appears from recent studies that members of this family are critical modulators of several physiological processes. Moreover, these receptors have been found to be targets for bacterial and viral pathogens that employ CEACAM-binding adhesins to successfully colonize their host [6]. This review aims to summarize recent findings with regard to the physiological functions of CEACAMs and, by discussing individual members of the family, will also try to highlight the specific features of these closely related molecules.
Figure 1.
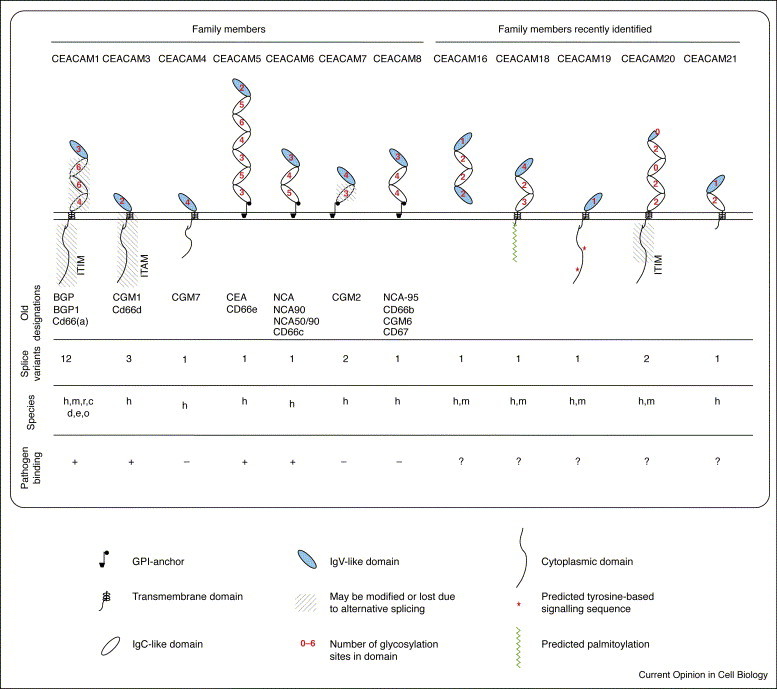
Overview of the human CEACAM family. Schematic representation of major isoforms of the different CEACAM family members are depicted. Further information with regard to alternative splice variants can be found at http://cea.klinikum.uni-muenchen.de/. Species abbreviations: c, cow; d, dog; e, elephant; h, human; m, mouse; o, opossum; r, rat.
CEA
In the mid-1960s, CEA was identified as a prominent tumor-associated antigen in human colon cancer [7]. CEA is the product of the CEACAM5 gene and is characterized by having seven extracellular Ig domains and a glycosyl-phosphatidylinositol (GPI) anchor. This kind of semi-penetrating membrane anchorage is also found in several other human (but not rodent) CEACAM family members, such as CEACAM6 or CEACAM8, and might be one of the reasons why soluble CEA can be detected in serum. Normal expression of this family member is restricted to epithelial cells and CEA is most abundantly found on the apical surface of the gastrointestinal epithelium, but also on other mucosal epithelia such as the nasopharynx, the lung and the urogenital tract as well as in sweat glands [8]. Importantly, to date no orthologues of this molecule have been identified in non-primate species, making this protein a unique primate invention. CEA can mediate homotypic binding to itself or heterotypic binding to one of several other CEACAM family members and thereby mediate cell–cell adhesion [9]. These interactions are predominantly mediated by the IgV-like N-terminal domain and appear to involve one of the two β-sheets (the CFG-face) of the Ig-fold [10]. 10–30-fold overexpression of CEA (a level similar to what is observed in human tumors) or overexpression of CEACAM6, another GPI-linked CEACAM family member, have been shown to disturb the ordered tissue architecture that is seen in 3D cultures of several colon carcinoma cell lines [11] and transgenic mice that overexpress several human CEACAMs display increased colon tumor formation after azoxymethane treatment [12•]. When myoblasts expressing CEA or CEACAM6 were grown in the absence of matrix attachment, apoptosis was reduced, again arguing for a role of these CEACAM family members in promoting aberrant growth of adherent cells [13]. As shown for CEA, the inhibition of apoptosis required the IgV-like N-terminal domain, suggesting that adhesive interactions mediated by this part of CEA are involved [13]. However, transgenic mice overexpressing CEA under its native control elements (CEAtg) do not show an increased tumor incidence, even in the absence of a functional adenomatous polyposis coli (APC) protein [14, 15]. Therefore, the physiological function of CEA and its potential involvement in tumor formation and progression still await clear-cut demonstration.
CEACAM1
CEACAM1 (formerly designated Bgp or CD66a) has the widest tissue distribution of all characterized family members. This receptor is not only found on epithelial cells, but also on various leukocytes and its expression can be induced on other cells, such as endothelial cells or T cells. CEACAM1 comes in a number of splice variants (see the CEA homepage at http://cea.klinikum.uni-muenchen.de/) with most isoforms harboring a transmembrane domain and either a long (L) or a short (S) cytoplasmic domain. The ‘L’ isoforms encompass ∼70 amino acids (71 in humans) in the cytoplasm including several serine, threonine and tyrosine residues that can be phosphorylation targets and can participate in signal transduction and protein–protein interactions. In contrast, the ‘S’-isoforms only encode 10 cytoplasmic residues lacking any phosphorylation sites. Both isoforms are co-expressed in most CEACAM1-expressing tissues, and the ratio between the two isoforms determines the signaling outcome [16, 17]. Importantly, two tyrosine residues within the long cytoplasmic domain constitute a functional immunoreceptor tyrosine-based inhibitory motif (ITIM) [18] that appears to be the basis for the inhibitory activity of CEACAM1 in different contexts (discussed later). Homologues of CEACAM1 have been identified in rodents and a number of other mammals [19], making this family member accessible to in vivo experimentation. Indeed, the genetic deletion of CEACAM1 from the mouse genome and the generation of transgenic mice expressing defined mutants have recently demonstrated a modulatory role for CEACAM1 in neovascularization, insulin metabolism, T-cell regulation and tumorigenesis [20••, 21, 22••, 23••].
CEACAM1 in angiogenesis
A number of previous studies have implicated CEACAM1 in angiogenesis. For example, soluble CEACAM1 exhibits pro-angiogenic effects by stimulating the proliferation, chemotaxis and capillary-like tube formation of human microvascular endothelial cells in vitro, as well as increasing the vascularization of the chorioallantoic membrane of chicken embryos in vivo [24]. The finding that vascular endothelial growth factor (VEGF) increases CEACAM1 expression in endothelial cells and that VEGF-induced endothelial tube formation is blocked by a monoclonal CEACAM1 antibody leads to the conclusion that CEACAM1 might be a major effector of VEGF in early microvessel formation [24]. Consistent with the results of Ergun and colleagues, Chen et al. [25] found an increase in the expression of CEACAM1 in H9c2 cells after incubation with VEGF.
Analyses with cDNA arrays revealed that CEACAM1 is part of the hypoxia-induced genetic program, as it is prominently induced on the microvessels of the left ventricle of chronically hypoxic rats as well as upon myocardial infarction of mice [25, 26]. It is therefore not surprising that CEACAM1 appears to be involved in tumor angiogenesis, as it is expressed on tumor-associated small endothelia but not in large and quiescent blood vessels [27, 28]. In agreement with the idea that CEACAM1 helps to promote neovascularization, CEACAM1-deficient mice poorly vascularize implanted extracellular matrix gels that contain pro-angiogenic factors such as angiopoietin-1, basic fibroblast growth factor or VEGF [22••]. In addition, neovascularization and vascular remodeling after ischemia in the adult animals is severely impaired [22••]. In contrast, overexpression of CEACAM1-4L in endothelial cells promotes vessel growth and vessel integrity in vitro and in vivo, further demonstrating that CEACAM1 is a critical modulator of neovascularization [22••]. The molecular mechanism behind CEACAM1's angiogenic properties is currently not known and it is unclear if CEACAM1-mediated adhesion is critical for this process. However, it has been reported that CEACAM1 overexpression alters the adhesive and migratory properties of rat endothelial cell lines [29]. In addition, stimulation of CEACAM1 in human epithelial cells has been shown to result in the expression of CD105 (endoglin), a member of the TGFβ1 receptor family that is critical for angiogenesis, and a similar mechanism could operate in endothelial cells [30••].
CEACAM1 helps to limit the action of insulin
Besides its function in angiogenesis, CEACAM1 plays a role in the regulation of insulin action. Insulin binding to the insulin receptor (IR) promotes the phosphorylation of specific cellular target molecules, including members of the insulin receptor substrate (IRS) family, Shc, and CEACAM1. Indeed, the ‘L’ isoform of CEACAM1 is a direct substrate of the IR, which phosphorylates CEACAM1 at Tyr-488, and this modification requires an intact Ser-503 residue in the cytoplasmic tail of CEACAM1 (reviewed in [31]). Expression of wild-type CEACAM1, but not of its phosphorylation-defective isoforms, decreases growth of hepatocytes in response to insulin, suggesting that CEACAM1 phosphorylation by the IR negatively regulates the mitogenic action of insulin. This down-regulation of insulin signaling can be attributed to the fact that CEACAM1 promotes endocytosis of the IR–insulin complex, leading to insulin degradation in lysosomes. The N-terminal domain of CEACAM1 is dispensable for enhancing insulin endocytosis, suggesting that the adhesive properties of CEACAM1 are not required in this context [32]. Recent studies from Poy et al. [33] demonstrate that CEACAM1 directly associates with Shc, another substrate of the IR. Binding of the SH2 domain of Shc to phosphorylated Tyr-488 of CEACAM1 competes with Shc binding to the IR and not only down-regulates the Ras/MAP kinase pathway but also interferes with IRS-1 phosphorylation, leading to reduced PI-3 kinase/Akt stimulation in response to insulin.
To investigate the role of CEACAM1 on hepatic insulin metabolism in vivo, transgenic mice (L-SACC1) over-expressing a dominant-negative, phosphorylation-defective mutant of CEACAM1 (CEACAM1 S503A) in hepatocytes were generated [21]. In contrast to normal mice, L-SACC1 mice developed hyperinsulinemia resulting from impaired insulin clearance in the liver. The elevated insulin levels in L-SACC1 mice caused secondary insulin resistance in these mice that was linked to hyperglycemia, increased serum free fatty acids and visceral obesity [21, 34]. Therefore, hepatic CEACAM1 is likely to regulate systemic insulin concentration by associating with the insulin receptor and regulating insulin-induced signalling events as well as insulin uptake and degradation. In addition to being a substrate for the insulin receptor, CEACAM1 serves as a substrate for the epidermal growth factor receptor (EGFR) [35]. Similar to insulin-receptor-induced signalling, EGFR phosphorylates CEACAM1 at Tyr-488, leading to association with the adaptor molecule Shc and resulting in reduced EGF-stimulated Ras/MAPK pathway activation.
CEACAM1 knock-out mice and the role of CEACAM1 in tumorigenesis
Surprisingly, CEACAM1 knock-out mice (CEACAM1−/− mice) do not show dramatic phenotypic alterations; they are viable and fertile, are not overweight and do not exhibit any vascular abnormalities or form spontaneous tumors [20••]. A more detailed analysis has yet to be published, but it has already been noted that these mice are hyperinsulinemic and insulin resistant as a result of defects in hepatic insulin clearance, confirming the data obtained by liver-specific expression of a dominant-negative CEACAM1. Furthermore, CEACAM1−/− mice show reduced neovascularization and vascular remodelling in adult animals [22••], and T-lymphocytes isolated from CEACAM1−/− mice display altered proliferation and cytokine secretion (mentioned in [20••]). The latter report highlights previous findings regarding the inhibitory role of the ‘L’-isoform of CEACAM1 in lymphocyte activation [36, 37], a topic that has been reviewed recently [38].
CEACAM1 expression has also been linked to tumorigenesis. In contrast to CEA or CEACAM6, CEACAM1 appears to function as a tumor suppressor and this property depends on the long cytoplasmic isoform (CEACAM1-4L), in particular on the integrity of Tyr-488 and Ser-503, as well as on its expression levels in relation to the ‘S’-isoform (reviewed [39, 40]). Surprisingly, the tumor inhibitory capacity of CEACAM1 does not rely on homotypic binding, as a mutant of CEACAM1-4L that lacks the N-terminal IgV-like domain necessary for CEACAM1-mediated cell–cell aggregation is still able to interfere with tumor formation in nude mice [41]. Nevertheless, in human tumor samples and carcinoma cell lines a negative correlation between CEACAM1 expression and tumor progression has been observed [42, 43]. Accordingly, one would expect an increase in the incidence of tumor formation in CEACAM1−/− mice. Though spontaneous tumors are not formed in different organs in the absence of CEACAM1 expression, CEACAM1−/− mice do show an increase in the number and size of azoxymethane-induced colon tumors in comparison to wild-type littermates [23••]. The tumor-suppressing activity of CEACAM1 can be partially explained by the fact that cell-cycle inhibitors such as the Cdk inhibitors p21Cip1 and p27Kip1 show reduced expression in colon tissue of CEACAM1-deficient animals. Furthermore, the rate of apoptosis is reduced in colon cells from CEACAM1−/− mice, demonstrating that CEACAM1 operates in vivo as a suppressor of cell proliferation and a promoter of apoptosis [23••].
CEACAM1 as a pathogen receptor
In mice, CEACAM1 is encoded by two alternative alleles, CEACAM1a and CEACAM1b. Interestingly, CEACAM1a serves as a receptor for mouse hepatitis virus (MHV), a murine coronavirus, and inbred mice strains carrying the CEACAM1a allele are sensitive to MHV [44]. Importantly, CEACAM1−/− mice are completely resistant to MHV [21]. In humans, carbohydrate moieties in CEACAM1 appear to be recognized by enterobacteria such as E. coli and Salmonella strains [45, 46], and the N-terminal domain of CEACAM1 is the main target of several bacterial pathogens specialized to colonize the human mucosa (for review see [6]). In addition to Neisseria gonorrhoeae and N. meningitidis, Haemophilus influenzae and Moraxella catarrhalis also express specific surface proteins that bind to CEACAM1 and several other CEACAM family members expressed on the apical membrane compartment of polarized epithelial cells. CEACAM engagement by these microbes not only results in tight association between the bacterium and the cell surface, but also stimulates the internalization of the micro-organisms and can trigger CEACAM-initiated gene expression events. A limited set of host genes is specifically induced upon infection of CEACAM-expressing cells with CEACAM-binding bacteria, and the increased expression of CD105, a member of the TGFβ1 receptor family, has been found to be necessary and sufficient to enhance matrix adhesion of the infected cells [30••]. Importantly, the enhanced adhesiveness counteracts the innate exfoliation response of the epithelium, prohibiting the detachment and sloughing of infected cells [30••]. Therefore, CEACAM recognition might be a specific adaptation of these microbes to facilitate the colonization of the human mucosa.
CEACAM3
In addition to CEACAM1, CEA and CEACAM6, which are found on epithelial cells, several bacterial adhesins recognize CEACAM3, a member of the family exclusively expressed on human granulocytes [47, 48]. CEACAM3 has a single extracellular IgV-like domain, but does not engage in homo- or heterophilic interactions with other family members. The most distinctive feature of CEACAM3 resides in the 71-amino-acid cytoplasmic domain, and comprises an amino acid sequence reminiscent of an immunoreceptor tyrosine-based activation motif (ITAM). Several studies have demonstrated that CEACAM3 is phosphorylated by protein tyrosine kinases of the Src family, in particular Hck and Fgr, which are activated upon bacterial infection of human phagocytes [49, 50]. CEACAM3-bound bacteria are efficiently engulfed by an actin-cytoskeleton-dependent process by CEACAM3-transfected cell lines as well as human phagocytes in an opsonin-independent manner [49, 50]. The rapid uptake mediated by CEACAM3 requires an intact ITAM as well as the stimulation of the small GTPase Rac, a critical regulator of actin cytoskeleton dynamics and the oxidative burst [51•]. Uptake and killing of CEACAM-binding bacteria by primary human granulocytes can be prevented by CEACAM3-blocking antibodies or by transducing the phagocytes with dominant-negative versions of Rac, but not dominant-negative forms of the closely related GTPase Cdc42 [51•]. Together, these findings suggest that CEACAM3, for which no endogenous ligand has yet been identified and for which no homologue has been found in other mammalian species, is a specialized member of the CEACAM family that has specifically evolved to allow innate immune control of human-specific bacterial pathogens.
Conclusions
Considerable progress in understanding the molecular interactions and the functional role of cell–cell adhesion molecules of the CEACAM family has been made. From a number of in vitro and in vivo studies, it appears that GPI-linked human CEACAMs have a role in shaping the architecture of epithelia, whereas CEACAM1 has a modulatory role in multiple cell types such as epithelial cells, endothelial cells, T-cells and hepatocytes and also contributes to tumor suppression. Several family members serve as receptors for viruses and bacteria and deletion of these family members can render the host resistant against particular pathogens. Recently, novel CEACAM family members have been detected in the human genome, but also in other mammalian species, further expanding the range of tissues with CEACAM expression as well as adding to the repertoire of potential physiological functions of these molecules [52••]. It will be interesting to observe the further elucidation of CEACAM function and the phylogenic diversification of these glycoproteins in light of their exploitation as pathogen receptors in multiple mammalian species.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
The authors would like to thank the Deutsche Forschungsgemeinschaft (Ha2568/3-2) for financial support and W. Zimmermann (LMU München, Germany) for discussions and suggestions on this manuscript. We also would like to apologize to our colleagues whose interesting contributions have not been cited due to space constraints.
References
- 1.Vaughn D.E., Bjorkman P.J. The (Greek) key to structures of neural adhesion molecules. Neuron. 1996;16:261–273. doi: 10.1016/s0896-6273(00)80045-8. [DOI] [PubMed] [Google Scholar]
- 2.Crossin K.L., Krushel L.A. Cellular signaling by neural cell adhesion molecules of the immunoglobulin superfamily. Dev Dyn. 2000;218:260–279. doi: 10.1002/(SICI)1097-0177(200006)218:2<260::AID-DVDY3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Walsh F.S., Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu Rev Cell Dev Biol. 1997;13:425–456. doi: 10.1146/annurev.cellbio.13.1.425. [DOI] [PubMed] [Google Scholar]
- 4.Beauchemin N., Draber P., Dveksler G., Gold P., Gray-Owen S., Grunert F., Hammarstrom S., Holmes K.V., Karlsson A., Kuroki M. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–249. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 5.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 6.Hauck C.R., Agerer F., Muenzner P., Schmitter T. Cellular adhesion molecules as targets for bacterial infection. Eur J Cell Biol. 2006;85:235–242. doi: 10.1016/j.ejcb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Gold P., Freedman S.O. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122:467–481. doi: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson J.A. Molecular cloning and expression of carcinoembryonic antigen gene family members. Tumour Biol. 1995;16:10–16. doi: 10.1159/000217923. [DOI] [PubMed] [Google Scholar]
- 9.Benchimol S., Fuks A., Jothy S., Beauchemin N., Shirota K., Stanners C.P. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57:327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- 10.Taheri M., Saragovi U., Fuks A., Makkerh J., Mort J., Stanners C.P. Self recognition in the Ig superfamily. Identification of precise subdomains in carcinoembryonic antigen required for intercellular adhesion. J Biol Chem. 2000;275:26935–26943. doi: 10.1074/jbc.M909242199. [DOI] [PubMed] [Google Scholar]
- 11.Ilantzis C., DeMarte L., Screaton R.A., Stanners C.P. Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia. 2002;4:151–163. doi: 10.1038/sj.neo.7900201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Chan CH, Cook D, Stanners CP: Increased colon tumor susceptibility in azoxymethane treated CEABAC transgenic mice. Carcinogenesis 2006, in press. [DOI] [PubMed]; This study makes use of transgenic mice encoding several human CEACAMs, which show increased incidence of chemically induced colon cancer.
- 13.Ordonez C., Screaton R.A., Ilantzis C., Stanners C.P. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 2000;60:3419–3424. [PubMed] [Google Scholar]
- 14.Eades-Perner A.M., van der Putten H., Hirth A., Thompson J., Neumaier M., von Kleist S., Zimmermann W. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res. 1994;54:4169–4176. [PubMed] [Google Scholar]
- 15.Thompson J.A., Eades-Perner A.M., Ditter M., Muller W.J., Zimmermann W. Expression of transgenic carcinoembryonic antigen (CEA) in tumor-prone mice: an animal model for CEA-directed tumor immunotherapy. Int J Cancer. 1997;72:197–202. doi: 10.1002/(sici)1097-0215(19970703)72:1<197::aid-ijc28>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Turbide C., Kunath T., Daniels E., Beauchemin N. Optimal ratios of biliary glycoprotein isoforms required for inhibition of colonic tumor cell growth. Cancer Res. 1997;57:2781–2788. [PubMed] [Google Scholar]
- 17.Singer B.B., Scheffrahn I., Obrink B. The tumor growth-inhibiting cell adhesion molecule CEACAM1 (C-CAM) is differently expressed in proliferating and quiescent epithelial cells and regulates cell proliferation. Cancer Res. 2000;60:1236–1244. [PubMed] [Google Scholar]
- 18.Chen T., Zimmermann W., Parker J., Chen I., Maeda A., Bolland S. Biliary glycoprotein (BGPa, CD66a, CEACAM1) mediates inhibitory signals. J Leukocyte Biol. 2001;70:335–340. [PubMed] [Google Scholar]
- 19.Kammerer R., Popp T., Singer B.B., Schlender J., Zimmermann W. Identification of allelic variants of the bovine immune regulatory molecule CEACAM1 implies a pathogen-driven evolution. Gene. 2004;339:99–109. doi: 10.1016/j.gene.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 20••.Hemmila E., Turbide C., Olson M., Jothy S., Holmes K.V., Beauchemin N. Ceacam1α−/− mice are completely resistant to infection by murine coronavirus mouse hepatitis virus A59. J Virol. 2004;78:10156–10165. doi: 10.1128/JVI.78.18.10156-10165.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports the complete deletion of CEACAM1 from the mouse genome and its effects on disease susceptibility with regard to MHV infection, and briefly introduces the phenotype of the CEACAM1-deficient mice.
- 21.Poy M.N., Yang Y., Rezaei K., Fernstrom M.A., Lee A.D., Kido Y., Erickson S.K., Najjar S.M. CEACAM1 regulates insulin clearance in liver. Nat Genet. 2002;30:270–276. doi: 10.1038/ng840. [DOI] [PubMed] [Google Scholar]
- 22••.Horst A.K., Ito W.D., Dabelstein J., Schumacher U., Sander H., Turbide C., Brummer J., Meinertz T., Beauchemin N., Wagener C. Carcinoembryonic antigen-related cell adhesion molecule 1 modulates vascular remodeling in vitro and in vivo. J Clin Invest. 2006;116:1596–1605. doi: 10.1172/JCI24340. [DOI] [PMC free article] [PubMed] [Google Scholar]; A forward and a reverse genetic approach provide strong evidence that CEACAM1 enhances neovascularization in adult mice.
- 23••.Leung N, Turbide C, Olson M, Marcus V, Jothy S, Beauchemin N: Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene 2006, in press. [DOI] [PubMed]; This study confirms previous findings that CEACAM1 suppresses the progression of colon tumors in mice.
- 24.Ergun S., Kilic N., Ziegeler G., Hansen A., Nollau P., Gotze J., Wurmbach J.H., Horst A., Weil J., Fernando M. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell. 2000;5:311–320. doi: 10.1016/s1097-2765(00)80426-8. [DOI] [PubMed] [Google Scholar]
- 25.Chen W.J., Chen H.W., Yu S.L., Huang C.H., Wang T.D., Chen J.J., Chien C.T., Chen H.Y., Yang P.C., Lee Y.T. Gene expression profiles in hypoxic preconditioning using cDNA microarray analysis: altered expression of an angiogenic factor, carcinoembryonic antigen-related cell adhesion molecule 1. Shock. 2005;24:124–131. doi: 10.1097/01.shk.0000170352.72694.36. [DOI] [PubMed] [Google Scholar]
- 26.Wu R.X., Laser M., Han H., Varadarajulu J., Schuh K., Hallhuber M., Hu K., Ertl G., Hauck C.R., Ritter O. Fibroblast migration after myocardial infarction is regulated by transient SPARC expression. J Mol Med. 2006;84:241–252. doi: 10.1007/s00109-005-0026-0. [DOI] [PubMed] [Google Scholar]
- 27.Oh P., Li Y., Yu J., Durr E., Krasinska K.M., Carver L.A., Testa J.E., Schnitzer J.E. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 28.Kilic N., Oliveira-Ferrer L., Wurmbach J.H., Loges S., Chalajour F., Neshat-Vahid S., Weil J., Fernando M., Ergun S. Pro-angiogenic signaling by the endothelial presence of CEACAM1. J Biol Chem. 2005;280:2361–2369. doi: 10.1074/jbc.M409407200. [DOI] [PubMed] [Google Scholar]
- 29.Muller M.M., Singer B.B., Klaile E., Obrink B., Lucka L. Transmembrane CEACAM1 affects integrin-dependent signaling and regulates extracellular matrix protein-specific morphology and migration of endothelial cells. Blood. 2005;105:3925–3934. doi: 10.1182/blood-2004-09-3618. [DOI] [PubMed] [Google Scholar]
- 30••.Muenzner P., Rohde M., Kneitz S., Hauck C.R. CEACAM engagement by human pathogens enhances cell adhesion and counteracts bacteria-induced detachment of epithelial cells. J Cell Biol. 2005;170:825–836. doi: 10.1083/jcb.200412151. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides a fresh look on the role of epithelial CEACAM recognition by human pathogens and argues for a role of CEACAM stimulation in the prevention of the innate exfoliation response of mucosal tissues.
- 31.Najjar S.M. Regulation of insulin action by CEACAM1. Trends Endocrinol Metab. 2002;13:240–245. doi: 10.1016/s1043-2760(02)00608-2. [DOI] [PubMed] [Google Scholar]
- 32.Soni P., Al-Hosaini K.A., Fernstrom M.A., Najjar S.M. Cell adhesion properties and effects on receptor-mediated insulin endocytosis are independent properties of pp120, a substrate of the insulin receptor tyrosine kinase. Mol Cell Biol Res Commun. 1999;1:102–108. doi: 10.1006/mcbr.1999.0116. [DOI] [PubMed] [Google Scholar]
- 33.Poy M.N., Ruch R.J., Fernstrom M.A., Okabayashi Y., Najjar S.M. Shc and CEACAM1 interact to regulate the mitogenic action of insulin. J Biol Chem. 2002;277:1076–1084. doi: 10.1074/jbc.M108415200. [DOI] [PubMed] [Google Scholar]
- 34.Dai T., Abou-Rjaily G.A., Al-Share Q.Y., Yang Y., Fernstrom M.A., Deangelis A.M., Lee A.D., Sweetman L., Amato A., Pasquali M. Interaction between altered insulin and lipid metabolism in CEACAM1-inactive transgenic mice. J Biol Chem. 2004;279:45155–45161. doi: 10.1074/jbc.M404764200. [DOI] [PubMed] [Google Scholar]
- 35.Abou-Rjaily G.A., Lee S.J., May D., Al-Share Q.Y., Deangelis A.M., Ruch R.J., Neumaier M., Kalthoff H., Lin S.H., Najjar S.M. CEACAM1 modulates epidermal growth factor receptor-mediated cell proliferation. J Clin Invest. 2004;114:944–952. doi: 10.1172/JCI21786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boulton I.C., Gray-Owen S.D. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nature Immunol. 2002;3:229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- 37.Chen D., Iijima H., Nagaishi T., Nakajima A., Russell S., Raychowdhury R., Morales V., Rudd C.E., Utku N., Blumberg R.S. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J Immunol. 2004;172:3535–3543. doi: 10.4049/jimmunol.172.6.3535. [DOI] [PubMed] [Google Scholar]
- 38.Gray-Owen S.D., Blumberg R.S. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–446. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- 39.Obrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol. 1997;9:616–626. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obrink B., Sawa H., Scheffrahn I., Singer B.B., Sigmundsson K., Sundberg U., Heymann R., Beauchemin N., Weng G., Ram P. Computational analysis of isoform-specific signal regulation by CEACAM1-A cell adhesion molecule expressed in PC12 cells. Ann N Y Acad Sci. 2002;971:597–607. doi: 10.1111/j.1749-6632.2002.tb04536.x. [DOI] [PubMed] [Google Scholar]
- 41.Izzi L., Turbide C., Houde C., Kunath T., Beauchemin N. cis-Determinants in the cytoplasmic domain of CEACAM1 responsible for its tumor inhibitory function. Oncogene. 1999;18:5563–5572. doi: 10.1038/sj.onc.1202935. [DOI] [PubMed] [Google Scholar]
- 42.Neumaier M., Paululat S., Chan A., Matthaes P., Wagener C. Biliary glycoprotein, a potential human cell adhesion molecule, is down- regulated in colorectal carcinomas. Proc Natl Acad Sci USA. 1993;90:10744–10748. doi: 10.1073/pnas.90.22.10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh J.T., Luo W., Song W., Wang Y., Kleinerman D.I., Van N.T., Lin S.H. Tumor suppressive role of an androgen-regulated epithelial cell adhesion molecule (C-CAM) in prostate carcinoma cell revealed by sense and antisense approaches. Cancer Res. 1995;55:190–197. [PubMed] [Google Scholar]
- 44.Dveksler G.S., Dieffenbach C.W., Cardellichio C.B., McCuaig K., Pensiero M.N., Jiang G.S., Beauchemin N., Holmes K.V. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J Virol. 1993;67:1–8. doi: 10.1128/jvi.67.1.1-8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leusch H.G., Drzeniek Z., Markos-Puztai Z., Wagener C. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic glycosides of mannose. Infect Immun. 1991;59:2051–2057. doi: 10.1128/iai.59.6.2051-2057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauter S.L., Rutherfurd S.M., Wagener C., Shively J.E., Hefta S.A. Identification of the specific oligosaccharide sites recognized by type 1 fimbriae from Escherichia coli on non-specific cross-reacting antigen, a CD66 cluster granulocyte glycoprotein. J Biol Chem. 1993;268:15510–15516. [PubMed] [Google Scholar]
- 47.Nagel G., Grunert F., Kuijpers T.W., Watt S.M., Thompson J., Zimmermann W. Genomic organization, splice variants and expression of CGM1, a CD66-related member of the carcinoembryonic antigen gene family. Europ J Biochem. 1993;214:27–35. doi: 10.1111/j.1432-1033.1993.tb17892.x. [DOI] [PubMed] [Google Scholar]
- 48.Chen T., Gotschlich E.C. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hauck C.R., Meyer T.F., Lang F., Gulbins E. CD66-mediated phagocytosis of Opa52Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 1998;17:443–454. doi: 10.1093/emboj/17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCaw S.E., Schneider J., Liao E.H., Zimmermann W., Gray-Owen S.D. Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol Microbiol. 2003;49:623–637. doi: 10.1046/j.1365-2958.2003.03591.x. [DOI] [PubMed] [Google Scholar]
- 51•.Schmitter T., Agerer F., Peterson L., Muenzner P., Hauck C.R. Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J Exp Med. 2004;199:35–46. doi: 10.1084/jem.20030204. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides experimental evidence that CEACAM3 is the critical family member triggering the opsonin-independent phagocytosis of CEACAM-binding bacteria by human granulocytes.
- 52••.Zebhauser R., Kammerer R., Eisenried A., McLellan A., Moore T., Zimmermann W. Identification of a novel group of evolutionarily conserved members within the rapidly diverging murine Cea family. Genomics. 2005;86:566–580. doi: 10.1016/j.ygeno.2005.07.008. [DOI] [PubMed] [Google Scholar]; This paper reports new CEACAM family members and provides hints about their putative function and evolution.


