Abstract
Thymic atrophy or thymus absence causes depressed thyroid–thymus axis (TTA) efficiency in old, young propyl-thiouracil (PTU) (experimental hypothyroidism) and in young-adult thymectomised (Tx) mice, respectively. Altered zinc turnover may be also involved in depressed TTA efficiency. Zinc turnover is under the control of zinc-bound metallothioneins (Zn-MTs) synthesis. Thyroid hormones, corticosterone and nutritional zinc affect Zn-MT induction. Zn-MT releases zinc in young-adult age during transient oxidative stress for prompt immune response. In constant oxidative stress (ageing and liver regeneration after partial hepatectomy), high liver Zn-MTs, low zinc ion bioavailability and depressed TTA efficiency appear. This last finding suggested that MT might not release zinc during constant oxidative stress leading to impaired TTA efficiency. The aim of this work/study is to clarify the role of Zn-MTs (I+II) in TTA efficiency during development and ageing. The main results are (1) Old and PTU mice display high corticosterone, enhanced liver MTmRNA, low zinc and depressed TTA efficiency restored by zinc supply. Increased survival and no significant increments in basal liver Zn-MTs proteins occur in old and PTU mice after zinc supply. (2) Lot of zinc ions bound with MT in the liver from old mice than young (HPLC). (3) Young-adult Tx mice, evaluated at 15 days from thymectomy, display high MTmRNA and nutritional–endocrine–immune damage restored by zinc supply or by thymus grafts from old zinc-treated mice. (4) Young-adult Tx mice, but evaluated at 40 days from thymectomy, display natural normalisation in MTmRNA and nutritional–endocrine–immune profile with survival similar to normal mice. (5) Stressed (constant dark for 10 days) mice overexpressing MT display low zinc, depressed immunity, reduced thymic cortex, high corticosterone, altered thyroid hormones turnover showing a likeness with old mice. These findings, taken altogether, show that corticosterone is pivotal in MTs induction under stress. MTs bind preferentially zinc ions in constant oxidative stress, but with no release of zinc from MT leading to impaired TTA efficiency. Zinc supply restores the defect because zinc has no interference in affecting pre-existing Zn-MTs protein concentrations in old and PTU mice. Therefore, free zinc ions are available for TTA efficiency after zinc supply. Thymus from old zinc-treated mice induces the same restoring effect when transplanted in Tx recipients. However, Tx mice display natural normalisation in MTmRNA and in nutritional–endocrine–immune profile in the long run. Therefore, Zn-MTs (I+II) are crucial in zinc homeostasis for endocrine–immune efficiency during the entire life assuming a role of potential and novel ‘biological clock of ageing’.
Keywords: Zinc, Metallothioneins, Ageing, Inflammation, Thyroid–thymus axis, Corticosterone, Thymus grafts, PTU, Stress, MTs transgenic mice, Biological clock, Survival
Abbreviations: AT, active thymulin; MTs, metallothioneins; MT-I*, metallothioneins overexpressing mice; PTU, propyl-thiouracil; TECs, thymic epithelial cells; TSH, thyroid stimulating hormone; TTA, thyroid–thymus axis; TT, total thymulin; Tx, thymectomy
1. Introduction
Thymic atrophy leads to depressed thyroid–thymus axis (TTA) efficiency in old and in young propyl-thiouracil (PTU) treated mice (exp. hypothyroidism) (Fabris, 1992, Abou-Rabia and Kendall, 1994). Young-adult thymectomised (Tx) mice display impaired immune and thyroid functions. Neonatal thymus grafts or triiodothyronine (T3) treatments restore TTA efficiency (Fabris and Mocchegiani, 1985). In this context, the bioavailability of free zinc ions may be pivotal. Indeed, zinc supply restores TTA efficiency in old mice with prolonged survival (Mocchegiani et al., 1998b) as well as in clinical hypothyroidism (Down's syndrome) (DS) with reduction of infectious episodes (Licastro et al., 1992). The reasons are two. First, zinc confers biological activity to thymulin (Zn-FTS) (AT) (Dardenne et al., 1982), which decreases in ageing and DS (Fabris et al., 1984). The zinc-unbound form (FTS) of thymulin is inactive and increases in the plasma of both conditions. The in vitro zinc addition up to plasma samples unmasks FTS showing the total amount of thymulin produced (active Zn-FTS+inactive FTS) (TT). Therefore, the low zinc ion bioavailability allows a lack in saturating all thymulin molecules produced in ageing and DS (Fabris et al., 1984). Second, zinc finger home domains are required in activating T3 receptors (Darling et al., 1998) on T-cells (Arpin et al., 2000) and on thymic epithelial cells (TECs) believed to secrete thymulin (Villa-Verde et al., 1992). Zinc turnover is affected by metallothioneins (MTs) synthesis (Kagi and Shaffer, 1988) which is, in turn, induced by thyroid hormones (Yeiser et al., 1999), corticosterone and nutritional zinc (Cousins and Lee-Ambrose, 1992). MTs bind preferentially zinc than copper in ageing (Hamer, 1986) and hypothyroidism (Yeiser et al., 1999). Zn-MTs (I+II) are zinc donors during transient oxidative stress in young-adult age (Jacob et al., 1999) and in young TECs to activate thymulin (Coto et al., 1992). However, enhanced liver Zn-MTs, low zinc, depressed AT, reduced natural killer (NK) activity and hypothyroidism occur in constant oxidative stress, such as in ageing and liver regeneration after partial hepatectomy (Mocchegiani et al., 1997, Maliekal et al., 1997) as well as in constant dark regarding NK cells distribution (Dhabhar et al., 1995). Thus, while, on one hand, MTs capture zinc in zinc deficiency because zinc ions must not be lost (Kelly et al., 1996), on the other hand, MTs might not be zinc donors in ageing. This latter may cause low zinc ion bioavailability for TTA efficiency and NK activity (Mocchegiani et al., 1998a). The aim of this work/study is to clarify the role of Zn-MTs (I+II) in TTA efficiency during development and ageing. NK cells activity is included because it is affected by zinc and TTA efficiency, via Zn-FTS saturation (Muzzioli et al., 1992), and by stress (Dhabhar et al., 1995).
2. Materials and methods
2.1. Animal models used and treatments
Young, adult, old Balb/c inbreed H-2 compatible male mice as well as young Tx mice (thymectomised at 2 months of age after puberty) (adult thymectomy) (Fabris and Mocchegiani, 1985) and PTU mice (0.17 mg/g bw/day of propyl-thiouracil for 30 days in drinking water) (Mocchegiani et al., 1990) were used. Mice were divided in various groups (ten mice/group) according to treatments (zinc sulphate or thymus grafts) (see Table 1 ). Tx mice were called: Tx1=evaluated 15 days after thymectomy and Tx2=evaluated 40 days after thymectomy (Table 1, groups f, g). Tx1 mice were used for treatments (zinc sulphate or thymus grafts) at 15th day from thymectomy (Piantanelli et al., 1978). No treatment in Tx2 mice.
Table 1.
Animal models used and treatments
| Animal model | Age (months) | Number | Treatment |
|---|---|---|---|
| (a) Young | 2 | 10 | No treatment (used as controls). |
| (b) Adult | 12 | 10 | No treatment (used as controls). |
| (c) Old | 22 | 10 | No treatment (used as controls). |
| (d) Old | 22 | 10 | Treated with zinc sulphate in drinking water (18 μg/ml Zn++) for 30 days. |
| (e) sham-Tx | 2 | 10 | No treatment (used as controls of thymectomised mice). |
| (f) Tx1 | 2 | 10 | Thymectomised at 2 months of age and evaluated 15 days after thymectomy. |
| (g) Tx2 | 2 | 10 | Thymectomised at 2 months of age and evaluated 40 days after thymectomy. |
| (h) Tx1 | 2 | 10 | Treated with zinc sulphate in drinking water (18 μg/ml Zn++) for 30 days. |
| (i) Tx1 | 2 | 10 | Grafted with thymus fragment (2–3 mg) from young mice of 2 months of age. |
| (j) Tx1 | 2 | 10 | Grafted with thymus fragment (2–3 mg) from adult mice of 12 months of age. |
| (k) Tx1 | 2 | 10 | Grafted with thymus fragment (2–3 mg) from old mice of 22 months of age. |
| (l) Tx1 | 2 | 10 | Grafted with thymus fragment (2–3 mg) from old zinc-treated mice of 22 months of age. |
| (m) sham-grafted Tx | 2 | 10 | No treatment (used as control of Tx1 thymus grafted mice). |
| (n) PTU | 2 | 10 | Treated with PTU (0.17 mg/g bw/day) in drinking water for 30 days. Successively, these mice received tap water. |
| (o) PTU | 2 | 10 | After PTU treatment (see point n), these mice were treated with zinc-sulphate in drinking water (18 μg/ml Zn++) for 30 days. |
| (p) MT-I* mice | 2 | 10 | Used as controls of stressed MT-I* mice (group q). |
| (q) MT-I* mice | 2 | 10 | Underwent to constant dark for 10 days (stressed). |
| (r) C57BL/6J | 2 | 10 | Used as controls of stressed C57 BL/6J mice (group s). |
| (s) C57BL/6J | 2 | 10 | Underwent to constant dark for 10 days (stressed). |
| (t) PTU mice | 2 | 50 | Treated with PTU for 30 days and used for analysis survival. |
| (u) Tx mice | 2 | 50 | Thymectomised at 2 months of age and used for analysis survival. |
| (v) PTU mice | 2 | 50 | After 30 days of PTU treatment, mice were constantly treated with zinc sulphate in drinking water (18 μg/ml Zn++) and used for analysis survival. |
In particular, old, Tx1 and PTU mice were treated with zinc sulphate in drinking water (18 μg/ml Zn++) (physiological dose) (Mocchegiani et al., 1998a) for 30 days (Table 1, groups d, h, o). Controls were treated with tap water (Table 1, groups a, b, c, e, n). Other Tx1 mice were grafted with thymus fragments from young, adult, old and old zinc-treated mice [Table 1, groups i, j, k, l; sham-grafted Tx is respective control (group m)]. The thymus graft was performed after 15 days from thymectomy (Piantanelli et al., 1978).
Young male transgenic MT mice (MT-I*) [C57BL/6J-TgN(Mt1)174Bri] (Jackson Laboratory, Bar Harbor, ME, USA), in which 56 copies of the MT-I gene (Mt1) were transferred into the genome (Palmiter et al., 1993) and controls C57BL/6J were also used (Table 1, groups p, r). Another group of MT-I* and C57BL/6J mice underwent to stress by constant dark for 10 days (Table 1, groups q, s). This model of stress induces high corticosterone in young animals with major peaks post-meridiem during the circadian cycle (Fischman et al., 1988), as it occurs in old ones (Mocchegiani et al., 1998b). MT-I* mice were stressed because of no difference in basal liver MT between MT-I* and controls (Deng et al., 1999). Other mice (PTU, Tx and PTU+zinc) (50/group) (Table 1, groups t, u, v) were exclusively used for survival analysis (see below).
2.2. Housing
Inbreed Balb/c, C57BL/6J and MT-I* mice used were bred in our ‘conventional barrier’ (Mocchegiani et al., 1998b), in plastic non-galvanised cages, five mice per cage (36×20×14 cm) (Technoplast, Italy), and fed with standard pellet food (Nossan, Italy, containing 185 ppm of zinc) and tap water in sterilised bottles ad libitum. The animals were maintained on a 12-h light/12-h dark cycle from 7:00 a.m. to 7:00 p.m. with fixed timer governing two standard fluorescent fixtures (Philips TLD 36 W/84), at constant temperature (20±1 °C) and humidity (50±5%). Check-up of environmental factors and bacteriological and serological analysis were carried out monthly following FELASA (1994) suggestions. The maximum life span of inbreed Balb/c mice in our housing breeding condition was of 30 months (Mocchegiani et al., 1998b).
2.3. Surgical procedures
2.3.1. Thymectomy
Thymectomy was performed in young mice by suction under ether anaesthesia. At sacrifice, mice showing thymic remnants were discarded. The successful of thymectomy was of 90% (Fabris and Mocchegiani, 1985). Therefore, thymic remnants occurred in one mouse/ten Tx mice. Following that, additional mice (40) were thymectomised in order to substitute discarded Tx mice (1–2/group considered). Thus, ten successful Tx mice/group were guaranteed (Table 1, groups f, g, h, i, j, k, l).
2.3.2. Thymus graft
Grafts of thymuses from mice of different age was performed using a little lobe of the thymus (2–3 mg) in order to avoid differences in total thymus weight between young and old mice (Piantanelli et al., 1978). Such fragments were transplanted in Tx1 mice under the kidney capsule with ether anaesthesia (see Table 1, groups i, j, k, l) (Piantanelli et al., 1978). At sacrifice, the thymus grafted was analysed by histological analysis of the kidney capsule. Grafted mice with no acceptance of the thymus were discarded. The success of the thymus grafted under the kidney capsule was 90% (Piantanelli et al., 1978). Therefore, success of the thymus grafted occurred in nine Tx1/ten Tx1 mice that underwent to transplantation. Following that, an additional number of Tx1 mice (20) grafted with thymus fragments from young, adult, old and old zinc-treated mice was taken into account in order to substitute the discarded Tx1 thymus grafted mice (1–2/group considered). Thus, ten successful Tx1 thymus grafted mice/group were guaranteed (see Table 1, groups i, j, k, l).
2.4. Sacrifice
Thymus grafted mice were killed after 30 days from transplantation, because the effects of thymus-grafted were evident after 1 month from transplantation (Piantanelli et al., 1978). All other mice were sacrificed following protocols of the treatments (30 days after treatments with zinc sulphate or PTU) (see Table 1). Animals were sacrificed under ether anaesthesia (law n.86/609 by CEE) at 10–12 h p.m. using a red lamp (Philips) in a separate room to avoid aspecific stress to other animals. The choice of 10–12 h p.m. is because corticosterone displays higher peaks during these hours in the circadian cycle in young and old animals (Fischman et al., 1988, Mocchegiani et al., 1998b). Heparined blood samples (1 ml) were collected by cardiac puncture. Plasma samples stored at −70 °C until used. Tests were performed in blind.
Taking into account the animal models as well as the lot of mice used (Table 1), in vivo treatments were carried out once a time (Fabris and Mocchegiani, 1985), but with ten mice/group. This number is optimal to achieve statistically significant means of the experimental data (Matthews and Farewell, 1988).
2.5. Active thymulin (ZnFTS) (AT) and total thymulin (ZnFTS+FTS) (TT) determination
Plasma active zinc-bound thymulin (ZnFTS), as extensively described elsewhere (Fabris et al., 1984), was measured using a bioassay based on the ability to restore the inhibitory effect of azathioprine on rosette formation in spleen cells from young Tx mice. Results were expressed as the log−2 of the reciprocal maximal dilution of tested plasma able to induce this phenomenon (Fabris et al., 1984). This bioassay is still required because questions raised over the specificity of the radioimmunoassay (RIA) (Mocchegiani et al., 1998b). In order to avoid interference due to zinc turnover, the thymulin bioassay was also performed by in vitro addition of zinc sulphate at final concentration of 200 nM up to plasma samples. This fact shows the total amount of thymulin produced (active ZnFTS+inactive FTS) (TT) (Fabris et al., 1984).
2.6. Histological and immunocytochemistry studies
2.6.1. Histological studies
Four micrometer cryosections of frozen thymus were fixed in 80% cold acetone for 15 min and stained with haematoxylin–eosin.
2.6.2. Immunocytochemistry studies
Immunocytochemical characterisation of thymic epithelial cells (TECs) was detected in situ from thymic sections using anti-thymulin MoAb (kindly supplied by M. Dardenne) which can be revealed by the GAM/IgG2a/FITC diluted 1/20 (Dardenne et al., 1989). Anti pan-cytokeratin IgG1/FITC MoAb (Sigma, USA) diluted 1/25 and anti-keratin MoAb (Sigma, USA) diluted 1/20 were also used. For this latter, guinea pig IgG/FITC (Sigma, USA) diluted 1/60 was used as second antibody. These MoAbs are specific to detect TECs (cortical and medullary) (Itoh et al., 1982, Dardenne et al., 1989, Kurz et al., 1996). In situ TECs were assessed at fluorescence microscope by counting 100 microscope fields of 135 000 μm2 from three or four frozen 2 μm sections obtained at different level of the organ (Savino and Dardenne, 1984, Dardenne et al., 1989). For in vitro analysis, percentages of separated TECs (see below) from PTU mice and after stimulating agents were counted in 1000 cells under fluorescence microscope. In situ and in vitro tests were performed after pre-fixation with cold methanol in the slides. Controls were performed without the primary antibodies.
2.6.3. Whole thymuses in vitro cultures
Whole thymuses (n.2) from young and PTU mice were put in plate wells containing M10 zinc-free chelated (Chelex 100) medium, as previously described (Mocchegiani et al., 1990, Mocchegiani et al., 1998b). Two whole thymuses were optimal to test in vitro kinetic thymulin (Mocchegiani et al., 1990). Supernatant samples (50 μl) were taken at two different times of culture (30 min and 6 h). Maximum thymulin production occurs after 6 h of culture with a plateau maintenance in thymulin production up to 12 h of culture with a progressive decline thereafter (Mocchegiani et al., 1990). Such a production is de novo synthesis because completely prevented by preincubation with cycloheximide (Mocchegiani et al., 1990). Zinc sulphate was added at final concentration of 1 μM in the culture medium alone or with T3 (1 ng/ml) (Mocchegiani et al., 1990, Mocchegiani et al., 1998b).
2.6.4. TEC separation
TECs were separated following methods described by Itoh (1979) and Kurz et al. (1996). Briefly, the thymus from young and PTU mice after 6 h of culture was minced into small fragments and incubated with collagenase (1 mg/ml, Sigma, USA) in PBS for 1 h at 37 °C (1 ml of collagenase solution/thymus). The suspension was then centrifuged (2 min, 400 g) and the pellet suspended in 1 ml of Dulbecco's modified Eagle medium/Ham's F12 medium (1:1) (DMEM/F12, Gibco, Germany). The cells were subjected to two-steps trypsin (0.1 and 0.25%, respectively) and 0.001% DNase treatment in order to avoid fibroblasts (Kurz et al., 1996). After three washes in PBS, the cells were dissociated by cautious triturating through Eppendorf tips and incubated in 3 ml of DMEM/F12 medium for 2–3 h at 37 °C in humidified 5% CO2-atmosphere in order to make to adhere the cells. The supernatant containing unattached TEC was seeded into another plastic flask containing DMEM/F12 medium supplemented with 10% horse serum and put in culture in humidified 5% CO2-atmosphere. The cultures were inspected for morphologically visible fibroblasts (spindle shaped cells). In cases of significant contamination, the cells were washed with PBS and underwent again to trypsinisation (Kurz et al., 1996). Separated TECs were washed three times in PBS. An aliquota (103) was resuspended in 1 ml of medium and underwent to TEC percentage analysis as described by Dardenne et al. (1989).
2.6.5. TEC proliferation
After TEC separation, another aliquota (40×103) was resuspended in 4 ml of zinc-free chelated (Chelex 100) DMEM/F12 medium for TEC proliferation analysis, which was approached using [3H] thymidine incorporation using 96 microtiter plates (Nunc, Denmark). 40×103 TECs were put in 40 wells (100 μl/well=103 TECs/well). Ten wells were used as controls; ten were added zinc (1 μM); ten were added T3 (1 ng/ml); ten were added zinc+T3. Concomitantly, 1 μCi [3H]-timidine/well (Amersham, UK) was also added. The plates were incubated in humidified 5%-CO2 atmosphere for 6 h. Automatic harvester collected the samples and the amount of incorporated radioactivity was determined in a liquid scintillation beta-counter (Perkin–Elmer, USA).
2.6.6. TEC assessment and proliferation in pure rat TEC cell line (IT-45R1)
The same TEC proliferation radioactivity procedure as well as TECs percentage and thymulin production (described above) were tested in 103 pure rat TEC cell line (IT-45R1) (HSRRB, Japan), suggested to produce thymulin (Itoh et al., 1982), in standard culture condition and after zinc (1 μM) and/or T3 (1 ng/ml) addition for 6 h in the culture chelated medium (DMEM/F12) at 37 °C in CO2-atmosphere. In this last contest, corticosterone (0.5 μg/ml) (Kurz et al., 1996) was also added to IT-45R1 cells for 6 h of culture.
2.6.7. Crude zinc balance and tissue zinc content
Crude zinc balance was determined during a metabolic period (7 days before sacrifice). Food, water intake, urinary and faecal excretion were measured every day for each mouse in metabolic non-galvanised cages (Technoplast, Italy). The faecal weight was determined in humid faeces. The measure of zinc in urine, faeces, water and food was performed with methods extensively described elsewhere (Mocchegiani et al., 1997). The crude zinc balance is the difference between zinc intake (food and water) and zinc excretion (urine and faeces). The negativity implies a zinc loss from the body (Mocchegiani et al., 1998a). Plasma and tissue zinc content were determined in Atomic Absorption Spectrophotometry (AAS) against zinc references standard (Sigma, USA), as extensively described elsewhere (Mocchegiani et al., 1997, Mocchegiani et al., 1998b).
2.6.8. NK cells assay
The target cells for NK activity were murine lymphoma cell line YAC-1. NK activity, as described elsewhere (Muzzioli et al., 1992), was tested in splenocytes using 2×106 cells as target and 100 μCi of 51Cr (Na2CrO4 Amersham, UK). Splenocytes were used at the final concentrations of 10×106 cells. Results are expressed in Lytic Unity (L.U. 20/107 cells) (Mocchegiani et al., 1997).
2.6.9. Thyroid hormones and corticosterone determination
T3, thyroxine (T4) and thyroid stimulating hormone (TSH) were detected in the plasma using RIA commercial kits (Byk-Mallinckrodt, Germany). Plasma corticosterone level (ng/ml) was determined by RIA rat-corticosterone-3H kit (ICN Biomedicals, CA, USA) and referred against a standard curve. The percentage of cross-reaction with other steroids was <0.01. The sensitivity was of 0.05 ng/ml of corticosterone.
2.6.10. RNA isolation and RT-PCR analysis
Total RNA was extracted from frozen liver using Tri-Reagent according manufacture's instructions (Sigma, USA). Total RNA (0.1 μg) was reverse transcribed using a reaction mixture containing 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 150 ng oligo dT, 20 units of Rnase inhibitor, 0.5 mM deoxynucleotide triphosphates and 200 units M-MLV Reverse Transcriptase (Sigma, USA). PCRs were performed using sense and antisense primers as follows:
MT-I 5′-ATGGACCCCAACTGCTCCTGCTCCACC-3′, 5′-GGGTGGAACTGTATAGGAAGACGCTGG-3' (259 bp), β actin 5′-GGACTCCTATGTGGGTGACGAGG-3′
5′-GGGAGAGCATAGCCCTCGTAGAT-3′(366 bp). Conditions for amplification were as follows: for MT-I each cycle consisted of 94 °C 0.30 min, 50 °C 0.30 min, 72 °C 0.30 min with 30 cycles; for β-actin each cycle consisted of 94 °C 1 min, 61 °C 1 min, 72 °C 1 min, with 28 cycles. The products of the RT-PCR reactions were size-fractionated by 2% agarose gel electrophoresis and visualised by staining with ethidium bromide. Semi-quantitative analysis of the amplified products was performed by an image analyser (Gel-doc 2000 instrument, BIO-RAD, USA). The result was evaluated as a relative unit determined by normalisation of the density of each band to that of the β-actin band.
2.6.11. Tissue MTs (I+II) protein concentrations detection
Liver MTs concentrations were detected by silver-saturation method as extensively described elsewhere (Mocchegiani et al., 1997). Ag bases this technique in the saturation of MTs. Ag+ concentration was measured in AAS using Ag+ standard solution (Sigma, USA). MTs (I+II) amount was calculated from the obtained Ag+ concentrations assuming those 17 mol of Ag+ bind 1 mol of MT (Mocchegiani et al., 1997). This methodological procedure has the same sensitivity of radioimmunoassay MTs (I+II) protein detection as previously demonstrated (Leibrant et al., 1991).
2.6.12. Liver MT (I+II) isolation
The peritoneal cavity was opened and the excised livers were kept frozen at −70 °C until analysed. Suzuki's method (1980) was performed. Briefly, livers (0.4–0.7 g) from young and old mice were homogenised in three volumes (w/v) of 20 mM Tris–HCl buffer (pH 8.6 at 25 °C) in presence or in absence of 10 mM mercaptoethanol. The homogenate was centrifuged at 100 000g for 1 h at 4 °C. The resulting supernatants (0.5 ml) were used for MT isolation on a Sephadex G-75 column (0.8 cm×30). This was equilibrated with 1 mM Tris–HCl buffer solution in presence or in absence of the reducing agent mercaptoethanol (Suzuki, 1980). The flow rate was 0.4 ml/min. Fractions of 0.5 ml were collected and rabbit MT was used as a calibration marker. Rabbit MT peak eluted in correspondence of fractions 13–17, which were recognised as containing MT (I+II) (Suzuki, 1980). Zinc and copper concentrations were determined by AAS in each fraction.
2.6.13. Survival analysis
Fifty mice are sufficient for survival analysis (Mocchegiani et al., 1998b). Fifty PTU mice were continuously treated with zinc sulphate (18 μg/ml Zn++) in drinking water (Table 1, group v). 50 PTU mice treated with tap water served as controls (Table 1, group t). Fifty Tx mice (adult thymectomy) were also used for survival analysis (Table 1, group u). Mice were censored for 2 days and individually weighted at time intervals. Health status of mice was monitored with bacteriological analysis at bronchoalveolar and gastrointestinal levels using standard laboratory methods. Serological analysis (Sendai virus, mouse hepatitis virus, mycoplasma pulmonis and corona virus) (ELISA kits) following FELASA suggestions (1994), was carried out (INRCA Veterinary Service).
2.6.14. Statistical methods
Two-tailed Student's t-test, ANOVA test (one-way) and Bonferroni test evaluated differences between means. In particular, Bonferroni test was also used to simultaneously compare all experimental groups before and after treatments. Correlations were determined by linear regression analysis by the least square method. Differences were evaluated by analysis of covariance. Survival analysis was performed using Kaplan–Meier method. Differences were significant when P<0.05.
3. Results
3.1. Nutritional–endocrine–immune profile in Tx, PTU and old mice: effect of zinc supply
Taking into account the neuroendocrine–immune damage in Tx, PTU and old mice (Fabris, 1992, Abou-Rabia and Kendall, 1994) and the role of zinc in affecting neuroendocrine–immune network (Fabris, 1994), some endocrine–immune parameters are tested and related to zinc pool.
Crude zinc balance is negative and AT, NK cells activity, T3, T4 levels are decreased in Tx1, PTU and old mice as compared to sham-Tx and young-adult controls (P<0.01) (Table 2 ). TT is within the adult normal range in old and PTU mice (Table 2). TSH and corticosterone increase in Tx1, PTU, old mice as compared to young-adult controls (P<0.05 and <0.01, respectively). By contrast, Tx2 mice display crude zinc balance, NK cells activity, thyroid hormones and corticosterone levels similar to those ones found in adult controls (Table 2). Therefore, zinc supply is carried out in Tx1, old and PTU mice. Zinc supply restores crude zinc balance and endocrine–immune parameters in Tx1, PTU and old mice (Table 2). No difference exists between AT and TT in old and in PTU zinc-treated mice (Table 2). Significant correlation exists between crude zinc balance and NK activity or T3 or T4 or TSH or corticosterone before and after zinc supply in Tx1, PTU and old mice (data not shown).
Table 2.
Parameters studied in Tx1, PTU and old mice before and after zinc supply
| Parameters | Animal models |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Young (a) | sham-Tx (e) | Tx1 (f) | Tx2 (g) | Adult (b) | PTU (n) | Old (c) | Tx1+Zn (h) | PTU+Zn (o) | Old+Zn (d) | |
| Crude Zn balance (μg/day/mice) | +1.5±0.5 | +1.2±0.4 | −0.8±0.4* | +0.5±0.2 | +0.8±0.5 | −1.6±0.5* | −1.3±0.3* | +1.0±0.4 | +1.0±0.3 | +1.2±0.4 |
| Plasma Zn (μg/dl) | 110±11 | 98±8 | 80±6* | 90.1±3.2 | 90±7 | 82±7* | 81±6.3* | 90±4.5 | 95±6 | 91.3±3.1 |
| A.T. (log−2) | 5.2±0.3 | 5.0±0.4 | 1.0±0.4* | 1.5±0.2* | 4.2±0.4 | 1.0±0.2* | 1.0±0.2* | 2.0±0.3* | 4.0±0.3 | 4.5±0.4 |
| T.T. (log−2) | 5.2±0.2 | 5.1±0.3 | 1.0±0.4* | 1.5±0.2* | 4.5±0.3 | 4.1±0.3 | 4.0±0.3 | 2.0±0.3* | 4.2±0.3 | 4.6±0.3 |
| NK (L.U. 20/107) | 31±4 | 28±7 | 12±5* | 21±2 | 20±6 | 10±2* | 7±5* | 26±6 | 20±4 | 19±5 |
| T3 (ng/dl) | 103±6.4 | 102±7.1 | 60±4.1* | 87±4.5 | 85±3.4 | 60±10* | 45±5.3* | 92±6.1 | 90±9 | 90±3.7 |
| T4 (μg/dl) | 6.2±2.2 | 6.0±1.7 | 4.0±1.0* | 5.6±1.3 | 5.3±1.4 | 4.0±1.3 | 4.3±1.4* | 5.0±1.1 | 5.0±1.0 | 5.0±1.1 |
| TSH (μU/ml) | 2.3±0.7 | 2.1±0.8 | 3.7±1.4** | 3.3±0.7 | 3.4±1.0 | 6.0±3.1* | 3.8±1.6** | 2.5±0.8 | 2.3±1.0 | 2.4±0.6 |
| Corticost. (ng/ml) | 153±18.3 | 161±16.4 | 277±22.4* | 155±16 | 177±14.4 | 225±17.1* | 265±23.2* | 166±13.3 | 144±15 | 152±12.2 |
Ten mice/group. Mean±SD.
P<0.01.
P<0.05 when compared PTU, Old and Tx1 mice with respective controls (young-adult for old and PTU mice and sham-Tx for Tx1 mice) (two-tailed Student's t-test, ANOVA test, Bonferroni test). A restoration in nutritional–endocrine–immune profile occurs after zinc supply in Tx1, PTU and old mice when compared simultaneously with young, sham-Tx and adult mice (P>0.05) (Bonferroni test).
3.2. Nutritional–endocrine–immune profile in Tx1 mice: effect of thymus grafts
Taking into account: (i) the relevance of zinc supply in recovering endocrine–immune response (Table 2) and thymic efficiency in old mice (Fabris et al., 1997); (ii) the importance of young thymus grafts in restoring endocrine–immune response in Tx mice (Piantanelli et al., 1978, Fabris and Mocchegiani, 1985), a relevant question arises: is thymus from old zinc-treated mice also able to restore endocrine–immune response when transplanted in Tx mice? Thus, thymus grafts from old zinc-treated mice are also carried out in Tx1 mice.
The endocrine–immune profile is altered and the crude zinc balance is still negative in Tx1 mice grafted with thymus from old mice as compared to Tx1 and old mice (Table 3 ). Grafts with thymus from old zinc-treated mice restore zinc pool and the endocrine–immune profile in Tx1 mice, as it occurs with thymus grafts from young and adult mice (Table 3). No significant difference exists between AT and TT in Tx1 mice grafted with thymus from old zinc-treated mice (Table 3). Significant correlation exists between crude zinc balance and AT or NK activity or T3 or T4 or TSH or corticosterone in Tx1 mice before and after thymus grafts from old zinc-treated mice (data not shown).
Table 3.
Parameters studied after thymus grafts in Tx1 recipients
| Parameters | Animal models |
||||||
|---|---|---|---|---|---|---|---|
| sham-Tx graft (m) | Tx1 (f) | Tx2 (g) | Tx1+old thymus (k) | Tx1+old thymus+Zn (l) | Tx1+young thymus (i) | Tx1+adult thymus (j) | |
| Crude Zn bal. (μg/day/mice) | +1.1±0.3 | −0.8±0.4* | +0.5±0.2 | −0.9±0.3* | +1.0±0.3 | +1.3±0.4 | +0.7±0.2 |
| Plasma Zn (μg/dl) | 95±7 | 80±6* | 90±3.2 | 80±3.4* | 89±2.7 | 91±4.5 | 84±4.0 |
| A.T. (log−2) | 5.2±0.4 | 1.0±0.4* | 1.5±0.2 | 1.0±0.3* | 4.6±0.3 | 5.0±0.4 | 4.5±0.3 |
| T.T. (log−2) | 5.3±0.3 | 1.5±0.2* | 4.0±0.3 | 2.7±0.2* | 5.3±0.4 | 5.0±0.4 | 4.7±0.4 |
| NK (L.U. 20/107) | 26±5 | 12±5* | 21±2 | 11.7±2.2* | 24±3 | 26±3.5 | 22±4.0 |
| T3 (ng/dl) | 100±6.7 | 60±4.1* | 87±4.5 | 61±3.8* | 95±4.6 | 103±5.8 | 91±4.8 |
| T4 (μg/ml) | 6.1±1.9 | 4.0±1.0* | 4.6±1.3 | 4.5±1.0* | 5.7±1.3 | 5.8±1.4 | 5.5±1.3 |
| TSH (μU/ml) | 2.3±0.9 | 3.7±1.4** | 2.8±0.7 | 3.5±1.3** | 2.8±0.8 | 2.6±0.9 | 3.5±1.3 |
| Corticosterone (ng/ml) | 163±15.4 | 277±22.4 | 155±16 | 267±16* | 168±15 | 165±13.7 | 171±16.2 |
Ten mice/group. Mean±SD.
P<0.01.
P<0.05 when compared to Tx1 thymus grafted mice (groups l, i, j), sham-Tx graft and Tx2 mice (Bonferroni test).
3.3. TECs assessment and proliferation: effect of in vitro zinc
In order to confirm in vivo data (Table 2), in vitro experiments on thymic epithelial cells (TECs) assessment and proliferation by adding zinc and/or T3 in the culture medium are carried out.
AT production, TEC proliferation, as well as TEC assessment (percent and number) are reduced after 6 h of culture in PTU mice as compared to young (P<0.01) (Fig. 1 A). In vitro zinc restores AT production, TEC proliferation and assessment (after 6 h of culture) (Fig. 1A). No synergetic increment occurs with in vitro zinc+T3 (Fig. 1A). A quota of zinc is present in thymic tissue of PTU mice after 6 h of culture (93.7±6.4 μg/g vs. 153.3±9.7 μg/g in young controls; P<0.05). It justifies the presence of TECs in PTU because zinc affects TECs assessment and proliferation, as shown after BrdUrd incorporation in the thymus of old zinc-treated mice (Fabris et al., 1997). But, this quota of zinc is not sufficient to induce the activation of all thymulin molecules produced after 6 h of culture, as it occurs in old thymic tissue (Mocchegiani et al., 1998b).
Fig. 1.

(A) In vitro effect of zinc and zinc+T3 on active thymulin production from thymic explant of PTU mice during two times of culture (30 min and 6 h). TECs number detected after 6 h of culture. Photos of fluorescent TECs (white arrow) are reported. Lecture (40×); photos (magnitude 10×). [Note: yellow spots are aspecific fluorescence impossible to avoid due to fraying of the thymus in the medium after 6 h of culture with new perivascular spaces imbued in the medium (Mocchegiani et al., 1998b)]. *P<0.01 vs. young (ANOVA, Bonferroni test). TECs proliferation and percentages are also reported. Significant decrements in PTU mice (β) are observed as compared to young (α) (P<0.01). Zinc restores them (γ) with no synergism after zinc+T3 addition (δ) as compared to zinc alone (γ). Mean±SD of four cultures with two thymuses each. Number of TECs and photos were performed using anti-thymulin MoAb. Anti-keratin MoAb for testing TEC percentage. No differences in in situ TECs number were observed between anti-thymulin and anti-keratin MoAbs (data not shown). (B) Active thymulin production, TEC proliferation and percentage in pure murine TEC cell line 9IT-45R1) and after the addition of zinc, T3, zinc+T3 and corticosterone. Mean±SD of four experiments each on triplicate cultures. Active thymulin was evaluated by the rosette assay, TEC percentage by using anti pan-cytocheratin FITC MoAb. *P<0.05 as compared to none (control culture) or T3. **P<0.01 as compared to none (ANOVA, Bonferroni test).
Taking into account 6 h of culture plus the essential times in TECs separation technical procedure, TEC percentage is performed after 16–18 h from the beginning of whole thymuses cultures. Thymulin production progressively declines after 13–24 h of culture (Mocchegiani et al., 1990). Therefore, these percentages are in agreement with in vitro TEC percentages after 1 day of murine culture (Dardenne et al., 1989), suggesting that a quota of TECs are in resting status.
Zinc also significantly increases active thymulin production, TEC percentage and proliferation in pure rat TEC cell line (IT-45R1) as compared to control culture (P<0.05) (Fig. 1B). T3 has no effect with any synergism in TECs assessment and proliferation by in vitro addition of T3+zinc (Fig. 1B). Corticosterone decreases IT-45R1 cell assessment and proliferation as compared to control culture (P<0.01) (Fig. 1B).
3.4. Zinc and copper bound to MT in the liver of young and old mice
Zinc ions affect immune efficiency (Table 2) and MTs bind zinc and copper (Kagi and Shaffer, 1988). Thus, it is interesting to explore the quota of zinc or copper ions bound with MTs in ageing. We have used the liver for two reasons. First, because MTs are mainly produced in the liver (Kagi and Shaffer, 1988); second, because the liver is a site of extrathymic T-cell pathway prominent in ageing (Abo et al., 2000), and zinc is also required for extrathymic T-cell pathway (Mocchegiani et al., 1998a). Thus, separation of MTs is carried out from the liver of young and old mice using Sephadex G-75 columns (see Section 2).
Gelfiltration chromatography of all liver cytosol reveals the presence of two main peaks of zinc. Typical chromatograms of liver preparations from a young and old mouse are reported in Fig. 2 . A major peak of zinc, bound to high molecular weight proteins (HP), elutes between fractions 8 and 12. A minor one, bound to MT, is present in fractions 13–17. As regard to copper only one peak, in correspondence of MT elution volume, is observed. The amount of zinc bound to MT is significantly higher (P<0.05) in old mice (0.79±0.1 μg; n=3) respect to young ones (0.65±0.01 μg; n=3). On the contrary zinc sequestrated by HP is higher in young mice (1.5±0.011 μg) respect to old mice (1.05±0.013 μg). However, this differences is not significant (P>0.05) (Fig. 2). As regard to copper, more reduced peak exists as compared to zinc, with no significant differences in Cu-MTs between young and old mice (0.26±0.013 μg in young vs. 0.29±0.011 μg in old; P>0.05) (Fig. 2). Similar results are obtained in presence of mercaptoethanol (data not shown).
Fig. 2.
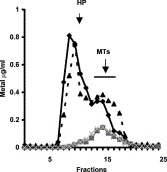
Sephadex G-75 elution profile of liver cytosol from young (black line) and old (hatched line) mice. The column was equilibrated with 20 mM Tris–HCl, pH 8.6. (Black triangle and rhombus for zinc; grey square and symbol X for copper.) Peak (fractions 7–12) for high molecular weight proteins (HP). Peak (fractions 13–17) for MT.
3.5. MTmRNA expression, Zn-MTs levels and zinc content in the liver of old, Tx, PTU mice
Following data obtained from Table 2 and Fig. 1, Fig. 2 and taking into account that zinc affects MTmRNA expression (Cousins and Lee-Ambrose, 1992), MTmRNA and Zn-MTs protein concentrations are tested before and after zinc supply in old and PTU mice.
Liver MTmRNA expression (RT-PCR) and its quantification are reported in Fig. 3 A and B, respectively. The semi-quantitative analysis shows that MTmRNA is increased in old, PTU and Tx1 mice as compared to young, sham-Tx and Tx2 mice (P<0.01) (Fig. 3B). Zn-MTs protein concentrations and zinc content are increased in the liver from old, Tx1 and PTU mice as compared to young, sham-Tx and Tx2 mice (P<0.01 and P<0.05, respectively) (Table 4 ) because AAS tests zinc-bound and zinc-unbound (Mocchegiani et al., 1998a). Zinc supply increases liver MTmRNA expression in old and PTU mice (Fig. 3A and B), with no significant modifications in Zn-MTs protein levels as compared to old and PTU controls (P>0.05) (Table 4). Zinc content is normal in the liver of Tx2, PTU and old mice after zinc supply (Table 4). Significant inverse correlation exists between Zn-MTs proteins and corticosterone concentrations before and after zinc supply in old and PTU mice (r=−0.61, P<0.05 and r=−0.54, P<0.05, respectively).
Fig. 3.
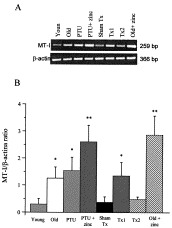
(A) RT-PCR analysis of liver mRNA using specific primers for murine MT-I and β-actin under conditions described in Materials and Methods. (B) After mRNA isolation and cDNA synthesis, the amount of mRNA for MT-I was determined by semi-quantitative PCR. Results of densitometry analysis are show in the histograms and are expressed as MT-I/β-actin ratio. Results are the mean±SD of 10 mice/group. *P<0.01 as compared to young, sham-Tx and Tx2 mice; **P<0.01 as compared to PTU and old mice (ANOVA, Bonferroni test).
Table 4.
Zn-MTs (I+II) protein concentrations and zinc content in the liver of mice
| Parameters | Animal models |
|||||||
|---|---|---|---|---|---|---|---|---|
| Young | Old | PTU | PTU+zinc | Sham-Tx | Tx1 | Tx2 | Old+zinc | |
| Zn-MTs (I+II) (μg/g ww) | 4.8±1.4 | 8.5±1.8* | 7.3±2.1* | 11.3±3.5 | 5.7±1.5 | 9.3±2.6* | 4.5±1.9 | 11.1±4.1 |
| Zinc tissue (μg/g) | 17.4±2.7 | 21.3±4.7** | 22.7±4.4** | 15.6±4.3 | 16.2±2.0 | 19.4±2.9** | 13.8±1.2 | 18.0±4.1 |
Ten mice/group. Mean±SD.
P<0.01.
P<0.05 when compared to young or sham-Tx control (ANOVA, Bonferroni test).
3.6. Nutritional–endocrine–immune profile in MT-I* mice in absence/presence of stress
In order to confirm that high constant corticosterone may induce abnormal MTs induction with no subsequent release of zinc from MT and endocrine–immune damage, stressed mice overexpressing MT (MT-I*) are good models because MTmRNA is similar between MT-I* and controls in absence of stress (Deng et al., 1999). This fact suggests that MTs are in quiescent status in absence of stress.
No stressed MT-I* mice show quite normal nutritional–endocrine–immune parameters with, however, a trend to alterations in comparison to respective controls (C57BL/6J) (Table 5 ). Thymic cortex is quite similar to young controls (Fig. 4 a and c). Such a trend becomes significant in stressed MT-I* mice. Indeed, AT levels and NK cells activity are reduced in stressed MT-I* as compared to respective controls (P<0.01), despite plasma zinc concentrations are within the normal range (Table 5). TT levels are within the normal range in stressed MT-I* mice as compared to respective controls (Table 5). The thickness of the thymic cortex is reduced in stressed MT-I* and old mice as compared to MT-I* and young controls (P<0.001) (Table 6 ). Thyroid hormones turnover is altered. Plasma corticosterone and liver Zn-MTs concentrations are higher in stressed MT-I* mice in comparison with MT-I* and young controls (P<0.01) (Table 5). With regard to stressed C57BL/6J mice, the nutritional–endocrine–immune profile is altered and the thickness of the thymic cortex is reduced as compared to no stressed C57BL/6J (P<0.01) (Table 6). However, stressed MT-I* mice display a more significant damage in nutritional–endocrine–immune profile and in thymic cortex than stressed C57BL/6J mice (Table 5, Table 6 and Fig. 4). No differences exist among C57BL/6J and inbreed Balb/c (Table 5).
Table 5.
Parameters studied in stressed MT-I* and C57BL/6J
| Parameters | Animal models |
||||
|---|---|---|---|---|---|
| str. MT-I* | MT-I* | str.C57BL/6J | C57BL/6J | Inbr. Balb/c | |
| Plasma Zn (μg/dl) | 95±8.7 | 108±6.7 | 100±6.4 | 116±9 | 110±11 |
| A.T. (log−2) | 1.3±0.2** | 3.2±0.2 | 2.5±0.2 | 5.7±0.3 | 5.2±0.3 |
| T.T. (log−2) | 5.0±0.3 | 5.1±0.3 | 4.8±0.3 | 5.6±0.2 | 5.2±0.2 |
| NK (L.U. 20/107) | 15.7±2.0* | 30.6±1.8 | 24.6±2.8 | 31.8±4.2 | 31±4 |
| T3 (ng/dl) | 75.4±4.2** | 95.2±5.7 | 85.6±4.7 | 108±6.4 | 103±6.1 |
| T4 (μg/dl) | 4.4±1.3** | 5.3±1.0 | 5.0±1.1 | 6.3±2.3 | 6.2±2.2 |
| TSH (μU/dl) | 4.0±0.9** | 3.0±0.6 | 2.9±0.5 | 2.2±0.5 | 2.3±0.7 |
| Corticosterone (ng/ml) | 290±15.2* | 180±12.4 | 240±16.6 | 145±21.2 | 153±18.3 |
| Liv. Zn-MTs (μg/g ww) | 13.5±1.3** | 6.2±1.6 | 8.5±1.5 | 5.0±1.2 | 4.8±1.4 |
Ten mice/group. Mean±SD.
P<0.01.
P<0.05 as compared to MT-I*, str. C57BL/6J and controls (ANOVA and Bonferroni test).
Fig. 4.
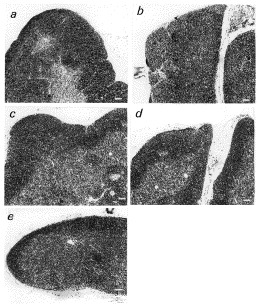
Thymic histology in young (a), old (b), MT-I*(c), young stressed C57BL/6J (d) and stressed MT-I* (e) mice. Young (2 months of age) and old (22 months of age) are C57BL/6J controls (magnitude 10×). The thickness of the thymic cortex (more dark area of thymic cellularity) [measured in various fields of thymic sections by computer assisted image analyser (KS 300, Kontron, USA)], is reported in Table 5. Thymic cortex is absent in old mice (b) and very limited in stressed MT-I* mice (e). Scale bar in white=125 μm.
Table 6.
Thickness of thymic cortex in MT-I* mice and controls exposed to stress
| Animals | Thickness (μm) |
|---|---|
| Young controls (C57BL/6J) | 450.3±19.6 |
| Old controls (C57BL/6J) | 78.2±13.4* |
| Young-stressed controls (C57BL/6J) | 253.2±21.1** |
| MT-I* | 315.8±28.6 |
| Stressed MT-I* | 138.7±17.6* |
Ten mice/group. Mean±SD.
P<0.001 as compared to MT-I* and young controls.
P<0.01 as compared to young controls (ANOVA and Bonferroni test).
3.7. Survival in PTU and Tx mice
PTU and old mice display nutritional–endocrine–immune damage (Table 2) and increased MTmRNA (Fig. 3A and B). Zinc supply prolongs the survival in old mice (Mocchegiani et al., 1998b) with no interference in basal MT levels (Table 4). Tx2 mice display natural normalisation in nutritional–endocrine–immune profile and in MTmRNA (Table 2, Table 4). Thus, the survival in PTU mice during zinc supply and in Tx ones is assessed.
Before PTU treatment, the health status is within the FELASA ‘conventional housing’ normal range (INRCA Veterinary Service). After PTU treatment, PTU mice show shorter survival (15 months) as compared to normal mice (30 months) (P<0.001, Log-rank test) (Fig. 5 ). Degenerative diseases and lung infections with frequency of 42 and 58%, respectively, are the main causes of death in PTU mice. Zinc supply increases the survival in PTU mice up to 28 months (Fig. 5) with decrements from 58 to 32% in infections and from 42 to 20% in degenerative diseases. The survival is similar between Tx and normal mice (27 vs. 30 months) (P>0.05, Log-rank test) (Fig. 5). No variations in food intake and body weight are observed during treatments (data not shown).
Fig. 5.
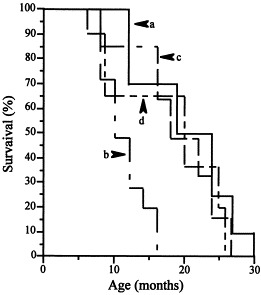
Rate of survival (Kaplan–Meir) in normal (a), PTU (b), PTU zinc-treated (c) and Tx mice (d).
4. Discussion
Zinc-bound MTs I+II (Zn-MTs), via corticosterone turnover, is crucial in zinc homeostasis for TTA efficiency during development and ageing. Negative crude zinc balance, depressed thymic and NK activities, high liver MTs, enhanced corticosterone and altered thyroid hormones turnover occur in old and PTU mice (Table 2, Table 4). The likeness between old and PTU mice is largely due to the thymic atrophy in both strains of mice (Abou-Rabia and Kendall, 1994, Fabris et al., 1997). As such, degenerative diseases rise in PTU mice (Rittenhouse and Redei, 1997) affecting the survival, despite good ‘health status’ observed before PTU treatment (F. Orlando, unpublished observation). Physiological zinc supply restores thymic and NK activities as well as the survival in old (Mocchegiani et al., 1998a) and PTU mice with no modifications in basal liver MT concentration (Table 4). Young-adult Tx1 mice (evaluated 15 days after thymectomy) display high liver MTs, increased corticosterone, negative crude balance, altered thyroid hormones turnover and impaired NK cells activity, which are restored by zinc supply or by thymus grafts from old zinc-treated mice (Table 3). In contrast, young-adult Tx2 mice (evaluated 40 days after thymectomy) display positive crude zinc balance, normal plasma corticosterone and liver MT level, satisfactory thyroid–immune profile and survival similar to normal mice (Table 2, Table 4 and Fig. 5). In vitro zinc restores TEC assessment and proliferation in PTU mice (Fig. 1A), as it occurs in old ones (Mocchegiani et al., 1998b). Significant inverse correlation exists between corticosterone and Zn-MTs before and after zinc supply in old and PTU mice. High corticosterone decreases TEC proliferation in pure TEC cell line (IT-45R1) (Fig. 1B).
Immune damage (Khansari et al., 1990) and zinc loss occur during stress (King 1990). A synchrony exists between high TSH and enhanced adrenocorticotropin (ACTH) within the anterior pituitary in stress condition (Childs, 1992). Therefore, our findings confirm that high corticosterone is associated with low zinc ion biovailabilty and thyroid–immune alteration in old and PTU mice. But, at the same time, they pin-point that high constant corticosterone affects abnormal MTs induction with no release of zinc from MT. Zinc is relevant for endocrine–immune response (Mocchegiani et al., 2000b) and MT binds preferentially zinc ions in ageing (Fig. 2). Therefore, the no release of zinc by MT leads to few free zinc ions for TTA efficiency with subsequent TTA impairment. Zinc supply restores nutritional–endocrine–immune profile and prolongs survival in old (Mocchegiani et al., 1998a) and PTU mice (Fig. 5). Thus, MTs synthesis, via corticosterone, is crucial in zinc homeostasis for TTA efficiency during development and ageing. Such an interpretation is much more relevant and, at the same time, very intriguing because zinc is also required for liver extrathymic T-cell pathway (Mocchegiani et al., 1998a), which is gradually prominent in Tx and old mice in order to compensate thymus absence or thymic atrophy, respectively (Abo et al., 2000). Indeed, Tx2 mice display natural normalisation in MTmRNA, in nutritional–endocrine–immune profile and in survival. Therefore, the thymus gland may be more useful in TTA efficiency during development than in ageing in which, on the contrary, zinc turnover may become prominent. The restoration in nutritional–endocrine–immune profile by thymus grafts from old zinc-treated mice or by zinc supply in Tx1 recipients is on line with this interpretation. The discovery showing multiple neonatal thymus grafts ineffective on survival in old mice (Hirokawa, 1997), in comparison with zinc supply (Mocchegiani et al., 1998b), supports this interpretation.
Zn-MTs release zinc in young TECs to activate thymulin (Coto et al., 1992) and in the cytosol of endothelial cells for antioxidant enzyme activity, via glutatione reductase or NO nitrosylation (Jacob et al., 1998, Maret et al., 1999, Zangger et al., 2001). Such a release occurs in young-adult age during transient oxidative stress (Jacob et al., 1999). Oxidative stress and zinc deficiency are persistent in ageing (Fabris, 1994) and hypothyroidism (Licastro et al., 1992, Thoei et al., 1997). MTs capture zinc in zinc deficiency because zinc ions must not be lost (Kelly et al., 1996). But, the task of Zn-MTs as zinc donors (Coto et al., 1992, Kelly et al., 1996, Jacob et al., 1999) may be questioned in constant oxidative stress. Indeed: (i) exogenous zinc is necessary in the plasma samples from old (Mocchegiani et al. 1998a) and PTU mice as well as in the plasma from Down's syndrome subjects to activate thymulin, despite plasma zinc values are within the normal range for age (Fabris et al., 1984); (ii) in vitro zinc, rather than T3, restores TECs assessment and proliferation in PTU thymic cultures and increases TEC proliferation in pure rat TEC cell line (IT-45R1) (Fig. 1A and B). In this context, direct mechanisms of zinc involving protein-kinase-C (PKC) activation (Coto et al., 1992, Saha et al., 1995) or phase S stimulation of the cell cycle (Dreosti, 2001) have been proposed; (iii) high liver Zn-MTs (I+II), low zinc and depressed TTA efficiency are observed in young partial hepatectomised mice during liver regeneration (model of persistent oxidative stress and great inflammation) (Mocchegiani et al., 1997); (iv) lot of zinc ions bound with MT in the liver from old mice than young (Fig. 2); (v) mice overexpressing MTs (MT-I*) underwent to stress (constant dark for 10 days) display altered nutritional–endocrine–immune profile looking like old mice. The likeness is especially in reduced thymic cortex and in adding exogenous zinc in the plasma to reactivate thymulin, despite plasma zinc levels are within the normal range (Table 5, Table 6). All these points suggest no or very limited release of zinc from MT during high constant corticosterone production leading to low zinc ion bioavailability for endocrine–immune response.
Some direct and indirect evidences support this interpretation. The most important direct evidences are: (i) the presence of high Zn-MTs concentrations and low zinc ion bioavaliability in the atrophic thymus of old mice (Mocchegiani et al., 1998a), and high corticosterone decreases TEC proliferation (Fig. 1B) and increases thymocytes apoptosis in old (Fabris et al., 1997) and stressed MT-I* mice (Deng et al., 1999); (ii) the persistence of high liver MTmRNA, enhanced corticosterone, low zinc and depressed liver NK cells activity during the whole circadian cycle in old mice (Mocchegiani et al., 2000a); (iii) the presence of high MTmRNA in lymphocytes from Down's syndrome subjects and old people in comparison to young (Mocchegiani et al., 2001). The most relevant indirect evidence is the failure of zinc-finger protein (A20) in protecting liver cells by apoptosis induced by TNF-α (Lee et al., 2000), which is in turn augmented in ageing (Fagiolo et al., 1993) and involved in MT induction (Andrews, 2000).
Zinc supply may provoke the release of zinc because zinc supply induces a faster degradation of Zn-MTs in lysosomes with complete release of zinc from MT (Klaassen et al., 1994). In agreement with Cousins and Lee-Ambrose (1992), zinc supply increases liver MTmRNA in old and PTU mice (Fig. 3A and B) with, however, no significant modifications in basal liver Zn-MTs (I+II) concentrations after zinc supply (Table 4). These findings confirm that Zn-MTs (I+II) are saturated by pre-existing zinc ions during constant oxidative stress, as shown in old hepatectomised mice during liver regeneration (Mocchegiani et al., 1997). But, at the same time, they also suggest that increased MTmRNA by zinc may be related to faster Zn-MTs degradation with a balance between Zn-MTs production and degradation. As a consequence, a plateau in Zn-MTs protein production is maintained. Therefore, zinc supply preserves this balance and, as such, free zinc ions are always available for TTA efficiency. The presence of significant inverse correlation between Zn-MTs and corticosterone before and after zinc supply in PTU and old mice is on line with this assumption, which is reinforced by low MTmRNA in lymphocytes from very old mice (30 months of age) and human exceptional individuals (centenarians) (Mocchegiani et al., 2001).
In conclusion, abnormal Zn-MTs (I+II) production, via high corticosterone, are not of benefit in TTA efficiency in ageing because of the inability to release zinc. Zinc supply, rather than thymus grafts, restores the defect. Thus, zinc-bound MTs (I+II) may be pivotal in maintaining zinc homeostasis and, consequently, TTA efficiency during the entire life with a possible role of potential and novel ‘biological clock of ageing’.
The trafficking of Zn-MTs within the cytosol and the nucleus involving chaperones activity (Cherian and Apostolova, 2001) may be related to no release of zinc from MT in ageing. Chaperones activity is required in the correct folding of proteins, is zinc-dependent (Jakob et al., 2000) and decreases in old age (Cuervo and Dice, 2000). Therefore, the different task of Zn-MTs as zinc donors between young and old age may be related to modified chaperones activity, representing, as such, an interesting future tool to understand the role of Zn-MTs in immune–neuroendocrine senescence.
Acknowledgements
Supported by INRCA, Italian Health Ministry (R.F. No. 99/107 to E.M.) and CEE (ImAginE project: No. QLK6-CT-1999-02031). We are grateful to Mr G. Bernardini, Mr G. Gardoni and Mr M. Solazzi for excellent technical assistance and graphic support and to Dr P. Fattoretti (Lab. of Neurobiology, INRCA, Ancona, Italy) for thymic thickness analysis. We thank Mrs Monica Glebocki for revising the test.
References
- Abo T., Kawamura T., Watanabe H. Physiological responses of extrathymic T-cells in the liver. Immunol. Rev. 2000;174:135–149. doi: 10.1034/j.1600-0528.2002.017415.x. [DOI] [PubMed] [Google Scholar]
- Abou-Rabia N., Kendall M.D. Involution of the rat thymus in experimentally induced hypothyroidism. Cell Tissue Res. 1994;277:447–455. doi: 10.1007/BF00300217. [DOI] [PubMed] [Google Scholar]
- Andrews G.K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- Arpin C., Pihlgren M., Fraichard A., Aubert D., Samarut J., Chassande O., Marvel J. Effects of T3R alpha 1 and T3R alpha 2 gene deletion on T and B lymphocyte development. J. Immunol. 2000;164:152–160. doi: 10.4049/jimmunol.164.1.152. [DOI] [PubMed] [Google Scholar]
- Childs G.V. Structure–function correlates in the corticotropes of the anterior pituitary. Front. Neuroendocrinol. 1992;13:271–317. [PubMed] [Google Scholar]
- Cherian M.G., Apostolova N.D. Nuclear localization of metallothionein during cell proliferation and differentiation. Cell. Mol. Biol. 2001;46:347–356. [PubMed] [Google Scholar]
- Coto J.A., Hadden E.M., Sauro M., Zorn N., Hadden J.W. Interleukin-1 regulates secretion of zinc-thymulin by human thymic epithelial cells and its action on T-lymphocyte proliferation and nuclear protein kinase C. Proc. Natl. Acad. Sci. USA. 1992;89:7752–7756. doi: 10.1073/pnas.89.16.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R.J., Lee-Ambrose L.M. Nuclear zinc uptake and interactions and metallothionein gene expression are influenced by dietary zinc in rats. J. Nutr. 1992;122:56–64. doi: 10.1093/jn/122.1.56. [DOI] [PubMed] [Google Scholar]
- Cuervo A.M., Dice J.F. Age-related decline in chaperone-mediated autophagy. J. Biol. Chem. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- Dardenne M., Pleau J., Nabama B., Lefancier P., Denien M., Choay J., Bach J.F. Contribution of zinc and other metals to the biological activity of the serum thymic factor. Proc. Natl. Acad. Sci. USA. 1982;79:5370–5373. doi: 10.1073/pnas.79.17.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne M., Savino W., Gagnerault M.C., Itoh T., Bach J.F. Neuroendocrine control of thymic hormome production: I. Prolactin stimulates in vivo and in vitro the production of thymulin by human and murine thymic epithelial cells. Endocrinology. 1989;125:3–12. doi: 10.1210/endo-125-1-3. [DOI] [PubMed] [Google Scholar]
- Darling D.S., Gaur N.K., Zhu B. A zinc finger home domain transcription factor binds specific thyroid hormone response elements. Mol. Cell. Endocrinol. 1998;139:25–35. doi: 10.1016/s0303-7207(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Deng D.X., Cai L., Chakrabarti S., Cherian M.G. Increased radiation-induced apoptosis in mouse thymus in the absence of metallothioneins. Toxicology. 1999;134:39–49. doi: 10.1016/s0300-483x(99)00026-8. [DOI] [PubMed] [Google Scholar]
- Dhabhar F.S., Miller A.H., McEwen B.S., Spencer R.L. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J. Immunol. 1995;154:5511–5527. [PubMed] [Google Scholar]
- Dreosti I.E. Zinc and the gene. Mutat. Res. 2001;475:161–167. doi: 10.1016/s0027-5107(01)00067-7. [DOI] [PubMed] [Google Scholar]
- Fabris N. Biomarkers of ageing in neuroendocrine–immune domain. Time for a new theory of ageing? Ann. N. Y. Acad. Sci. USA. 1992;663:335–348. doi: 10.1111/j.1749-6632.1992.tb38677.x. [DOI] [PubMed] [Google Scholar]
- Fabris N. Neuroendocrine–immune ageing: an integrative view on the role of zinc. Ann. N. Y. Acad. Sci. USA. 1994;719:353–368. doi: 10.1111/j.1749-6632.1994.tb56842.x. [DOI] [PubMed] [Google Scholar]
- Fabris N., Mocchegiani E. Endocrine control of thymic serum factor production in young-adult and old mice. Cell. Immunol. 1985;91:325–335. doi: 10.1016/0008-8749(85)90230-8. [DOI] [PubMed] [Google Scholar]
- Fabris N., Mocchegiani E., Amadio L., Licastro F., Zannotti M., Franceschi C. Thymic hormone deficiency in normal ageing and Down's syndrome: is there a primary failure of the thymus? Lancet. 1984;1:615–618. doi: 10.1016/s0140-6736(84)92325-0. [DOI] [PubMed] [Google Scholar]
- Fabris N., Mocchegiani E., Provinciali M. Plasticity of neuroendocrine–immune interactions during ageing. Exp. Gerontol. 1997;32:415–429. doi: 10.1016/s0531-5565(96)00166-0. [DOI] [PubMed] [Google Scholar]
- Fagiolo U., Cossarizza A., Sala E., Fanales-Belasio E., Ortolani C., Cozzi D., Monti D., Franceschi C., Paganelli R. Increased cytokines production in mononuclear cells of healthy and elderly people. Eur. J. Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- FELASA (Working Group on Animal Health), 1994. Recommendations for the health monitoring of mouse, rat, hamster, guinea pig, and rabbit breeding colonies. Lab. Anim. 28, 1–30. [DOI] [PubMed]
- Fischman A.J., Kastin A.J., Graf M.V., Moldov R.L. Constant light and dark affect the circadian rhythm of the hypothalamic-pituitary-adrenal axis. Neuroendocrinology. 1988;47:309–316. doi: 10.1159/000124930. [DOI] [PubMed] [Google Scholar]
- Hamer D.H. Metallothionein review. Annu. Rev. Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hirokawa K. Reversing and restoring immune functions. Mech. Ageing Dev. 1997;93:119–124. doi: 10.1016/s0047-6374(96)01828-3. [DOI] [PubMed] [Google Scholar]
- Itoh T. Establishment of an epithelial cell line from rat thymus. Am. J. Anat. 1979;156:99–104. doi: 10.1002/aja.1001560110. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kasahara S., Mori T. A thymic epithelial cell line, IT-45R1, induces the differentiation of prethymic progenitor cells into postthymic cells through direct contact. Thymus. 1982;4:69–75. [PubMed] [Google Scholar]
- Jacob C., Maret W., Vallee B.L. Control of zinc transfer between thionein, metallothionein and zinc proteins. Proc. Natl. Acad. Sci. USA. 1998;95:3489–3494. doi: 10.1073/pnas.95.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S.T., Ghoshal K., Sheridan J.F. Induction of metallothioneins by stress and its molecular mechanisms. Gene Expr. 1999;7:301–310. [PMC free article] [PubMed] [Google Scholar]
- Jakob U., Eser M., Bardwell J.C. Redox switch of hps33 has a novel zinc-binding motif. J. Biol. Chem. 2000;275:38302–38310. doi: 10.1074/jbc.M005957200. [DOI] [PubMed] [Google Scholar]
- Kagi J.H.R., Shaffer A. Biochemistry of metallothionein. Biochemistry. 1988;27:8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- Kelly E.J., Quaife C.F., Froelik G.J., Palmiter R.D. Metallothionein I and II against zinc deficiency and zinc toxicity. J. Nutr. 1996;126:1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- Khansari D.N., Murgo A.J., Faith A.J. Effect of stress on the immune system. Immunol. Today. 1990;11:170–175. doi: 10.1016/0167-5699(90)90069-l. [DOI] [PubMed] [Google Scholar]
- King J.C. Assessment of zinc status. J. Nutr. 1990;120(Suppl. 11):1474–1479. doi: 10.1093/jn/120.suppl_11.1474. [DOI] [PubMed] [Google Scholar]
- Klaassen C.D., Choudhuri S., Mckim J.M., Jr., Lehman-Mckeeman L.D., Kershaw W.C. In vitro and in vivo studies on the degradation of metallothionein. Environ. Health Perspect. 1994;102:141–146. doi: 10.1289/ehp.94102s3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz B., von Gaudecker B., Krisch B., Mentlein R. Rat thymic epithelial cells in vitro and in situ: characterization by immunocytochemistry and morphology. Cell Tissue Res. 1996;283:221–229. doi: 10.1007/s004410050533. [DOI] [PubMed] [Google Scholar]
- Lee E.G., Boone D.L., Chai S., Libby S.L., Chien M., Lodolce J.P., Ma A. Failure to regulate TNF-induced NF-kB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrant M.E., Koropatnick J., Harris J.F., Cherian M.G. Radioimmunoassay of metallothionein in rabbit, rat, mouse, chinese hamster and human cells. Biol. Trace Elem. Res. 1991;30:245–256. doi: 10.1007/BF02991419. [DOI] [PubMed] [Google Scholar]
- Licastro F., Mocchegiani E., Zannotti M., Arena G., Masi M., Fabris N. Zinc affects the metabolism of thyroid hormones in children with Down's syndrome: normalisation of thyroid stimulating hormone and of reversal triiodothyronine plasmic levels by dietary zinc supplementation. Int. J. Neurosci. 1992;65:259–268. doi: 10.3109/00207459209003299. [DOI] [PubMed] [Google Scholar]
- Maliekal T.T., Sudah B., Paulose C.S. Kinetic parameters of thymidine kinase and DNA synthesis during liver regeneration: role of thyroid hormones. Life Sci. 1997;60:1867–1874. doi: 10.1016/s0024-3205(97)00147-1. [DOI] [PubMed] [Google Scholar]
- Maret W., Jacob C., Vallee B.L., Fisher E.H. Inhibitory sites in enzymes: zinc removal and reactivation by thionein. Proc. Natl. Acad. Sci. USA. 1999;96:1936–1940. doi: 10.1073/pnas.96.5.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.E., Farewell V.T. Using and Understanding Medical Statistic. Karger; Basel: 1988. [Google Scholar]
- Mocchegiani E., Amadio L., Fabris N. Neuroendocrine–immune interactions. I. In vitro modulation of thymic factor by thyroid hormones. J. Endocrinol. Invest. 1990;13:139–147. doi: 10.1007/BF03349524. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Verbanac D., Santarelli L., Tibaldi A., Muzzioli M., Radosevic-Stasic B., Milin C. Zinc and metallothioneins effectiveness during liver regeneration in young and old mice. Life Sci. 1997;61:1125–1145. doi: 10.1016/s0024-3205(97)00646-2. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Muzzioli M., Cipriano C., Giacconi R. Zinc, T-cell pathways, ageing: role of metallothioneins. Mech. Ageing Dev. 1998;106:183–204. doi: 10.1016/s0047-6374(98)00115-8. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Santarelli L., Tibaldi A., Muzzioli M., Bulian D., Cipriano C., Olivieri F., Fabris N. Presence of links between zinc and melatonin during the circadian cycle in old mice: effects on thymic endocrine activity and on survival. J. Neuroimmunol. 1998;86:111–122. doi: 10.1016/s0165-5728(97)00253-1. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Muzzioli M., Cipriano C., Giacconi R. Metallothionein and extrathymic functions (liver natural killer activity) during the circadian cycle in young and old mice. In: Centeno J.A., Collery P., Vernet G., Finkelman R.B., Gibb H., Etienne J.C., editors. Vol. 6. John Libbey Eurotext; Paris: 2000. pp. 150–153. (Metal Ions in Biology and Medicine). [Google Scholar]
- Mocchegiani E., Muzzioli M., Giacconi R. Zinc and immunoresistance to infections in ageing: new biological tools. Trends Pharmacol. Sci. 2000;21:205–208. doi: 10.1016/s0165-6147(00)01476-0. [DOI] [PubMed] [Google Scholar]
- Mocchegiani E., Giacconi R., Cipriano C., Muzzioli M., Fattoretti P., Bertoni-Freddari C., Isani G., Zambenedetti P, Zatta P. Zinc-bound metallothioneins as potential biological markers of ageing. Brain Res. Bull. 2001;55:147–153. doi: 10.1016/s0361-9230(01)00468-3. [DOI] [PubMed] [Google Scholar]
- Muzzioli M., Mocchegiani E, Bressani L., Bevilacqua P., Fabris N. In vitro restoration by thymulin of the NK activity of cells from old mice. Int. J. Immunopharmacol. 1992;14:57–61. doi: 10.1016/0192-0561(92)90105-t. [DOI] [PubMed] [Google Scholar]
- Palmiter R.D., Sandgren E.R., Koeller D.M., Bristner R.L. Distal regulatory elements from the mouse metallothionein locus stimulate gene expression in transgenic mice. Mol. Cell. Biol. 1993;13:5266–5275. doi: 10.1128/mcb.13.9.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantanelli L., Basso A., Muzzioli M., Fabris N. Thymus-dependent reversibility of physiological and isoproterenol evoked age-related parameters in athymic (nude) and old normal mice. Mech. Ageing Dev. 1978;7:171–182. doi: 10.1016/0047-6374(78)90063-5. [DOI] [PubMed] [Google Scholar]
- Rittenhouse P.A., Redei E. Thyroxine administration prevents streptococcal cell wall-induced inflammatory response. Endocrinology. 1997;138:1434–1439. doi: 10.1210/endo.138.4.5045. [DOI] [PubMed] [Google Scholar]
- Saha A.R., Hadden E.M., Hadden J.W. Zinc induces thymulin secretion from human thymic epithelial cells in vitro and augments splenocytes and thymocytes response in vivo. Int. J. Immunopharmacol. 1995;17:729–733. doi: 10.1016/0192-0561(95)00061-6. [DOI] [PubMed] [Google Scholar]
- Savino W., Dardenne M. Thymic hormone-containing cells VI. Immunohistologic evidence for the simultaneous presence of thymulin, thymopoietin and thymosin alpha 1 in normal and pathological human thymuses. Eur. J. Immunol. 1984;14:987–991. doi: 10.1002/eji.1830141105. [DOI] [PubMed] [Google Scholar]
- Suzuki K.T. Direct connection of high-speed liquid chromatograph (equipped with gel permeation column) to atomic absorption spectrophotometry for metalloprotein analysis: metallothionein. Anal. Biochem. 1980;102:31–34. doi: 10.1016/0003-2697(80)90312-7. [DOI] [PubMed] [Google Scholar]
- Thoei A., Akai M., Tomabechi T., Mamada M., Taya K. Adrenal and gonadal function in hypothyroid adult male rats. J. Endocrinol. 1997;152:147–154. doi: 10.1677/joe.0.1520147. [DOI] [PubMed] [Google Scholar]
- Villa-Verde D.M., Defresne M.P, Vannier-Dos-Santos M.A., Dussault J.H., Boniver J., Savino W. Identification of nuclear triiodothyronine receptors in the thymic epithelium. Endocrinology. 1992;131:1313–1320. doi: 10.1210/endo.131.3.1505466. [DOI] [PubMed] [Google Scholar]
- Yeiser E.C., Fitch C.A., Horning M.S., Rutkoski M., Levenson C.W. Regulation of metallothionein-3 mRNA by thyroid hormone in developing rat brain and primary cultures of rat astrocytes and neurons. Brain Res. Dev. Brain Res. 1999;115:195–200. doi: 10.1016/s0165-3806(99)00063-2. [DOI] [PubMed] [Google Scholar]
- Zangger K., Oz G., Haslinger E., Kunert O., Armitage I.M. Nitric oxide selectively releases metals from the amino-terminal domain of metallothioneins: potential role at inflammatory sites. FASEB J. 2001;15:1303–1305. doi: 10.1096/fj.00-0641fje. [DOI] [PubMed] [Google Scholar]


