Abstract
The globalization of the world's economies, accompanied by increasing international travel, changing climates, altered human behaviour and demographics is leading to the emergence of different viral diseases, many of which are highly pathogenic and hence are considered of great public and animal health importance. To undertake basic research and therapeutic development, many of these viruses require handling by highly trained staff in BSL-3/4 facilities not readily available to the majority of the global R&D community. In order to circumvent the enhanced biosafety requirement, the development of non-pathogenic, replication-defective pseudotyped viruses is an effective and established solution to permit the study of many aspects of virus biology in a low containment biosafety level (BSL)-1/2 laboratory. Under the spectre of the unfolding Ebola crisis, this timely conference (the second to be organised by the Viral Pseudotype Unit, www.viralpseudotypeunit.info*) discusses the recent advances in pseudotype technology and how it is revolutionizing the study of important human and animal pathogens (human and avian influenza viruses, rabies/lyssaviruses, HIV, Marburg and Ebola viruses). Key topics addressed in this conference include the exploitation of pseudotypes for serology and serosurveillance, immunogenicity testing of current and next-generation vaccines and new pseudotype assay formats (multiplexing, kit development).
*The first pseudotype-focused Euroscicon conference organised by the Viral Pseudotype Unit was recently reviewed [1].
Keywords: Pseudotyped virus, Vaccine, Serology, Zoonotic virus
1. Introduction
Alternatives to wild-type viruses are now widely used tools, offering a range of benefits in research, diagnostics and as therapeutic treatment options. Pseudotyped viruses (PVs), replication-defective viral particles formed with a structural and enzymatic core from one virus and the envelope glycoprotein of another, are a flexible option with little associated risk. Professor Robin Weiss (University College London, UK) reviewed the field of “Retrovirus pseudotypes: From oncology to vaccinology” in his keynote address. Retroviruses and vesicular stomatitis virus (VSV) have the ability to assemble each other's envelopes, leading to the development of retroviral vectors bearing the VSV envelope glycoprotein. Retroviral vectors are commonly used, delivering a gene of choice within a PV for gene transfer and gene therapy applications. In addition to VSV, the envelopes of highly pathogenic RNA viruses can be pseudotyped onto retroviral vectors with the benefit that high-containment is not necessary. Important example applications of this technology were presented by Prof. Weiss, including the use of rabies virus pseudotypes for the study of neutralising antibodies in sera from vaccinated subjects [2] and the use of retroviral pseudotypes with H5N1 haemagglutinin (HA) for the selection of cross-neutralising influenza virus mAbs directed against the HA2 stalk region [3].
For his chair's address, Dr. Nigel Temperton (Joint Director, Viral Pseudotype Unit, University of Kent, UK) introduced “The influenza virus pseudotype resource” that his group has been establishing over the last few years. The remit of this is to establish a pandemic preparedness resource consisting of retroviral vectors pseudotyped with the HAs of all 16 influenza virus subtypes (with a wild bird reservoir), in combination with a wide array of reporters for deployment in a multitude of resource settings, from field to industry (Table 1 ). These HA pseudotypes can be employed for serosurveillance, immunogenicity testing and as basic tools for the study of virus entry. They are produced via transfection of 293T cells with 3 (for HPAI strains belonging to the H5 and H7 subtypes) or 4 (for seasonal and LPAI strains) plasmids: HIV or MLV gag-pol, an envelope glycoprotein and a reporter gene (Fig. 1 ). Additionally, for HAs containing a single basic amino acid at the cleavage site, a protease-expressing plasmid is required [4]. 48 h and 72 h post-transfection, supernatant is harvested, filtered and titrated onto susceptible target cell lines (293T, MDCK or A549).
Table 1.
Details of influenza A and B strains that have been pseudotyped by the Viral Pseudotype Unit (University of Kent). Strains are presented in subtype order by year of isolation. Strains highlighted in red are included in the WHO document “Antigenic and genetic characteristics of zoonotic influenza viruses and development of candidate vaccine viruses for pandemic preparedness” (September 2014).
 |
Fig. 1.
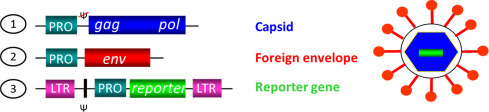
Schematic representation of the three plasmid platform used to produce pseudovirus. (1) The structural gag and pol enzymatic proteins are expressed from a single plasmid lacking a packaging signal. (2) The envelope (env) gene is expressed from a plasmid with a promoter (PRO) specific to the envelope. (3) The reporter gene is expressed from a plasmid incorporating a packaging signal (Ψ) and flanking long tandem repeats (LTR), along with an upstream promoter.
Upon virus emergence, the pseudotype platform offers the ability to rapidly commence serosurveillance and antigenic characterisation studies at a low containment level, thus assisting vaccine and antiviral development. This is particularly advantageous in the study of highly pathogenic zoonotic viruses, which pose a significant public health threat. As a main research interest, Dr. Edward Wright (University of Westminster, UK) spoke of pseudotyping emerging zoonotic viruses from the Lyssavirus, Filovirus and Henipavirus genera (Table 2 ). Providing the viral candidate buds from the host cell plasma membrane, has no more than two envelope glycoproteins, which are not toxic to producer cells, and does not require additional proteases or processes for expression, the pseudotyping platform described by Temperton and Wright (2009) proves a highly successful approach. All five Ebolavirus species have been generated by Dr. Wright's group within a short timeframe, including the Makona variant (Guinea 2014) implicated in the current outbreak. These Ebolavirus pseudotypes are currently being implemented in a pseudotyped virus neutralisation assay (PVNA) (Fig. 2 ) to assist clinical trials of the vaccine ChAd3 EBOZ by collaborators at the Jenner Institute, University of Oxford.
Table 2.
Details of Filoviridae and Lyssavirus isolates that have been pseudotyped by the Viral Pseudotype Unit (Fitzrovia).
|
Filoviridae | ||
|---|---|---|
| Genus | Species | Strain/Isolate |
| Ebolavirus | Tai Forest ebolavirus | Tai Forest isolate |
| Zaire ebolavirus | Mayinga isolate | |
| Makona isolate | ||
| Sudan ebolavirus | Boniface's isolate | |
| Reston ebolavirus | Pennsylvania isolate | |
| Bundibugyo ebolavirus | 2008 isolate | |
| Cuevavirus | Lloviu cuevavirus | Spain 2003 isolate |
|
Marburgvirus |
Marburg marburvirus |
Lake Victoria isolate |
|
Lyssavirus | |
|---|---|
| Species | Isolate |
| Rabies virus | Challenge virus standard 11 (CVS-11) |
| Evelyn Rokitniki Abelseth | |
| Arctic-like isolates (India.dog.88.RV61, Pakistan.dog.89.RV193, Russia.rodent.RV250 and Pakistan.goat.RV277) | |
| Lagos bat virus | LBV.SA2004 |
| LBV.NIG56-RV1 | |
| Shimoni bat virus | Kenya.bat.09 |
| Mokola virus | MOKV.98/071-RA361 |
| MOKV.NIG68-RV4 | |
| Duvenhage virus | DUVV.RSA2006 |
| DUVV.ZIM86-RV131 | |
| European bat lyssavirus type-1 | Germany.bat.68.9395GER.RV9 |
| Denmark.bat.86.RA552.RV20 | |
| European bat lyssavirus type-2 | UK.bat.96.96/18.RV628 |
| UK.bat.04.603/04.RV1787 | |
| Australian bat lyssavirus | Australia.bat.96.960648.RV634 |
| West Caucasian bat virus | Russia.bat.02 |
| Aravan virus | ARAV.KGZ1991 |
| Irkut virus | IRKV.RUS2002 |
| Khujand virus | KHUV.TJK2001 |
Fig. 2.
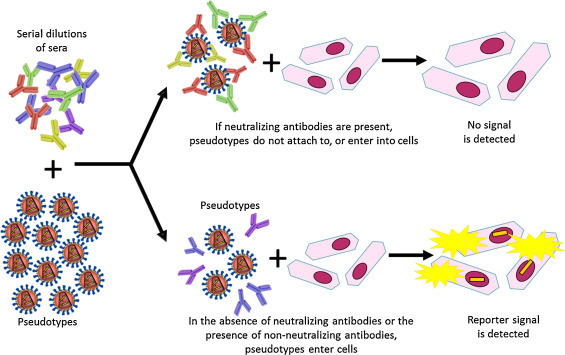
A schematic representation depicting the use of pseudotyped virus as antigen within a neutralisation assay set up.
2. Serology
Serological studies are vital to assess the neutralising ability of antibodies targeted to the envelope glycoprotein and can assist tracking of virus spread, providing important public health and epidemiological data. PVs form a robust wild-type virus alternative for serological assays [5] and Dr. Wright discussed a proven flexibility and sensitivity in studies undertaking neutralisation assays. The range of reporter genes which can be incorporated within the PV core offer a variety of cost and time benefits with a choice of quantitative or qualitative readouts. Work to generate Lyssavirus PVs and test the anti-rabies virus neutralising response found those results correlated with, or were more sensitive than, those from validated wild-type virus assays [6], [7]. Further, the reported stability of PVs, which maintain high viral titres during cold-chain storage and following freeze-thaw cycles, offers a significant advantage over wild-type virus, where titres drop rapidly, and permits use in countries with less reliable infrastructure [8].
More recently, this flexibility has further been substantiated by studies producing PV expressing chimeric envelope glycoproteins. Pseudotyping with a VSV envelope glycoprotein (VSV-G) is highly efficient – the relatively low titres achieved for a rabies CVS strain (challenge virus standard; B2c) PV were shown to be rescued by constructing a chimeric glycoprotein where the cytoplasmic domain of the rabies virus G protein was switched for that of VSV-G [9]. Dr. Wright's group has followed this approach to engineer the envelope glycoprotein of Arctic-like rabies virus, successfully increasing a previously unworkable titre. This has allowed serology studies to be performed, assessing the previously unknown efficacy of current vaccines and antivirals against this rabies virus sub-set.
The value of focused meetings and intra-disciplinary collaboration and communication is highlighted by a project derived from the 2013 Euroscicon PV meeting. Dr. Giada Mattiuzzo (NIBSC, UK) has been working to validate an alternative neutralisation assay to test the biological potency of post-exposure prophylaxis (PEP) rabies immunoglobulins (RIG). Rabies is a significant public health burden, resulting in almost 60,000 deaths per year with over 20 million people receiving PEP worldwide [10]. Administration of RIG is an important component of PEP, providing passive immunisation and inhibiting viral spread before sufficient active immunity is achieved via vaccination. Currently, RIG derived from immunised human plasma donors must undergo potency testing, as established in the European Pharmacopoeia monograph (v8.3 01/2015:0723), before release into the European market. The Rapid Fluorescent Focus Inhibition Test (RFFIT) is the biological potency assay outlined within the monograph. This cell-based neutralisation assay must be undertaken at BSL 4 due to the use of wild-type rabies virus, namely the laboratory strain challenge virus standard 11 (CVS-11). Dr. Mattiuzzo is evaluating a PVNA which alleviates the need for high containment, requiring BSL 2, using a PV expressing CVS-11 glycoprotein initially constructed by Dr. Wright [2].
Further to the increased safety and accessibility offered by the PVNA, Dr. Mattiuzzo outlined how the expression of a luciferase reporter gene offers more objective result readouts. The current RFFIT requires immunostaining of cells and counting fluorescence via microscopy of 20 fields per dilution for each preparation – a significant disadvantage in comparison to automated luminescence measurements when using a luciferase reporter gene. Similar to the current RFFIT, the PVNA evaluates biological potency of RIG based on its ability to inhibit target cell infection. The potency test assesses RIG in parallel with the World Health Organisation 2nd International Standard (IS) for anti-rabies immunoglobulins. Dr. Mattiuzzo has evaluated 5 commercial RIG samples using the PVNA and shown they calibrate with IS and produce similar potencies to those obtained via RFFIT from the manufacturer and an independent Institute which produces RIG for batch release. While intellectual property issues surrounding commercial use of the reporter plasmid construct pCSFLW need to be resolved, the PVNA represents a promising alternative system for potency testing of RIG. After further collaborative validation studies, Dr. Mattiuzzo will present the PVNA for inclusion into the European Pharmacopoeia monograph for RIG, to increase safety and accessibility of potency testing.
Dr. Temperton spoke on the advantages of using PV for seasonal influenza virus serology [11]. PVNAs are ideal for influenza virus strains that are difficult to propagate in culture, like some of the recent H3 viruses. Virus culture is not necessary as the serological assay is entirely synthetic. Also, PVNAs may prove easier to standardise as they do not rely on erythrocytes, which have exhibited significant variability in haemagglutination-inhibition (HI) assays. As HA is the only influenza virus antigen incorporated into the pseudotype particle, only antibody solely directed against this protein is measured. These assays can thus be used as a tool for dissecting out responses observed using wild-type virus microneutralisation (MN) assays in the short assay format (where essentially anti-HA responses are measured) and in the long assay format (where both anti-HA and anti-NA responses are measured due to viruses undertaking more than one cycle of replication: neutralisation of infection and release of virus). Dr. Temperton proposed that the pseudotype assay is useful as an adjunct to the EMA/FDA approved serology assays, HI and single radial hemolysis (SRH) [12], [13]. Compared to the routinely run HI, this assay is “serum-sparing”, “antigen-sparing” and biosafe. It is straightforward to scale-up for large sample sizes and in a multiplex format, the inter-assay variability is likely to be reduced [8]. The multiplexing format can be adapted for different subtype pairs (H5/H7, H1/B, H3/B) and for the measurement of antibody responses against two viruses belonging to different respiratory virus groups (such as influenza viruses in conjunction with SARS or MERS coronaviruses; [14]). Table 1 shows the current influenza virus strains that constitute the VPU influenza resource.
The use of PV as an alternative to current gold standards was further demonstrated by Professor Emanuele Montomoli (VisMederi, Siena, Italy), presenting a comparison of “Pseudotypes versus traditional neutralisation, advantages and future perspectives” from the standpoint of a Small and Medium Enterprise (SME) involved in the immunogenicity testing of influenza virus vaccines for the pharmaceutical industry. MN assays titrate functional antibodies and are the current gold standard for confirmation. However, they are difficult to employ for the screening of large sample sets and high-containment facilities are required for pandemic strains such as H5N1. MN detects antibodies that bind around the globular head and block virus attachment and entry. It additionally detects cross-reactive antibodies that bind to the stem region of HA. In contrast to HI and SRH, no correlate of protection has been established for the PVNA, yet a titre of 1:20-80 is used to indicate a seropositive for H5N1 and seasonal strains. The PVNA is suitable for screening large sample sets and exhibits good correlation with MN for both seasonal and pandemic strains of influenza virus [15]. Currently PVNA is not widely established and remains un-validated for clinical usage. The CDC MN protocol involves pre-treating sera with receptor-destroying enzyme and results are obtained by measuring viral infection via a nucleoprotein (NP) ELISA. Other readouts performed for MN include the evaluation of cytopathic effect in each well (a subjective interpretation) or performing an HA assay on the supernatant using either turkey or guinea pig erythrocytes (possible influence of erythrocyte type on the MN titre). MN exhibits poor reproducibility between laboratories using different protocols (31–724 fold) [16] and this assay would benefit from further validation and standardisation (virus input and growth kinetics, differences in serum treatment and dilution). PVNA does not require treatment of sera, and the readout is performed by the simple measurement of transduced cells using GFP or luciferase. An extrapolated PVNA titre of 1:357 (corresponding to an MN titre of 1:80) has been calculated as a seroprotection endpoint in a Novartis monovalent subunit H5N1 vaccine study [12]. Further studies have shown that PVNA can be used for both serum and plasma and that NA pseudotypes with mismatched HA can be used to measure NA antibodies in an enzyme-linked lectin assay.
Stuart Mather (Viral Pseudotype Unit, University of Kent, UK) described the feasibility of lyophilising pseudotyped lentiviruses bearing an array of heterologous glycoproteins, which could realistically be included in a serological kit. The flexibility of reporter genes that can be encapsulated by retroviral cores, as well as the ability to multiplex virus neutralisation assays with pseudotypes bearing different envelopes and reporter genes, significantly increases the utility and broadens the scope of employing these assays worldwide, especially in resource limited regions [7], [8]. However, a current major obstacle to disseminating PVs is the necessity for maintaining cold-chain conditions during their transport and long-term storage. Mr Mather presented that candidate H5 influenza, rabies and Marburg pseudotyped lentiviruses could withstand lyophilisation (in the presence of a 0.5 M sucrose-PBS cryoprotectant), medium-term storage (at temperatures up to 37 °C) and reconstitution (in either water or DMEM culture medium), whilst retaining high-titre pseudotype infectivity, enabling their exploitation in downstream serological assays [17]. The durability of pseudotyped lentiviruses confirmed by Mr Mather's research alludes to the possibility of their incorporation in serological assay kits, which could be globally distributed at a fraction of the current cost of dry ice shipments, and stored for a number of weeks, regardless of ambient temperature and climate.
3. Vaccination
In his keynote address, Prof. Weiss spoke on how PVs have been employed in the search for an HIV-1 vaccine. For HIV-1, currently more than 250 cloned envelope genes from all clades and circulating recombinant forms (CRFs) are available as PVs, an invaluable reagent developed for the vaccine community. Neutralising antibodies to HIV-1 target the envelope glycoproteins gp41 and gp120. Whilst potent and broadly neutralising human mAbs have been isolated and mapped using PV immunogen screening, gp140 complexes elicit only weak and narrow neutralising polyclonal antibodies. The Weiss lab sought to address this issue by using llamas to elicit broadly neutralising mAbs. Camelidae have classical and non-classical IgG and produce heavy chain antibodies which can be re-formatted as VHH single chain antibodies. These VHH have been exploited for HIV [18], rabies virus, influenza virus and RSV [19] exhibiting potent neutralisation profiles (90/100 HIV-1 PV strains neutralised by llama VHH J3, [20]). If two VHH are combined (J3 and 3e3) then 100% of strains are neutralised. Studying the binding of VHH 3e3 and J3 to the CD4 pocket, it was found that this depends on CD4 binding site integrity in gp120. An exciting translational application of VHH is their usage in vaginal immunoprophylaxis against HIV transmission. They lend themselves to this application as they are stable in buffered gels (pH3-7) and refold after heating to 80 °C. VHH can be formulated in gel and silicon, and additionally can be secreted by commensal Lactobacilli in the vagina. Currently, a macaque vaginal gel trial is in progress. When 2 VHH are joined via a 25aa Gly/Ser (GS) linker peptide to form a bivalent VHH, potency can be dramatically increased: Hetero-bihead VHH (CD4bs-gp41) are 350x more potent; Bivalent VHH 2H10 (MPER in gp41) are >200x more potent.
Dr. Simon Scott (Joint Director, Viral Pseudotype Unit, University of Kent, UK) outlined the definition and application of PV from both serological and gene therapy perspectives, emphasising the versatility of PV due to the array of cores and envelope proteins that can be employed into the platform. Dr. Scott presented his research on dissecting the vaccinology of equine influenza virus using lentiviral pseudotypes – firstly, PVNAs correlate well with the established SRH assay to quantify the neutralising antibody responses of a panel of vaccinated equine sera [21]; also, equine influenza virus pseudotypes are being utilised to study vaccine breakdown in horses, by introducing specific point mutations to putative antigenic epitopes of HA representing pre and post-outbreak influenza virus strains, before gauging their contribution to vaccine escape via reduction of neutralising antibody recognition in PVNA assays. Dr. Scott's ongoing projects were also discussed, which include the production of PV displaying the envelopes of seal and canine influenza virus isolates. Further, the inherent complexities in generating PVs for Japanese encephalitis and West Nile flaviviruses were discussed, which bud internally through the endoplasmic reticulum (ER), meaning that their prM and E envelope proteins assemble and embed into the ER membrane, as opposed to the plasma membrane. In this case, an alternative core for pseudotyping, such as foamy virus vectors [22], could offer a feasible solution.
Influenza associated illness is a significant global healthcare burden, with annual epidemics resulting in 3–5 million cases of severe illness and approximately 250,000–500,000 associated deaths [23]. Licensed influenza virus vaccines are suboptimal in protecting the population cohorts most at risk of influenza associated disease – older adults and young children [13]. A protective role for heterosubtypic influenza virus-specific T-cells has been demonstrated in human challenge studies and the internal influenza virus antigens, NP and matrix protein (M1), induce strong T-cell specific responses. Building on this, Dr. Teresa Lambe (Jenner Institute, Oxford, UK) spoke of her group's work to rationally design a vaccine, named MVA-NP + M1 (modified vaccinia virus Ankara vector expressing the influenza virus antigens NP and M1) that boosts T-cell directed influenza virus responses in both younger and older adults. In a Phase IIa challenge, symptoms were less pronounced in the vaccinees when compared to controls [24]. Adjuvant properties for poxviruses have previously been reported and Dr. Lambe's group has demonstrated that MVA-NP + M1 can augment humoral immunity following seasonal influenza virus vaccination, in both pre-clinical and clinical studies [25], [26]. Pre-clinical studies used a PVNA and luciferase expressing lentiviral pseudotypes (produced at the Viral Pseudotype Unit, Kent) to evaluate the functional capacity of antibodies, allowing assessment of antibodies directed to HA head and stem regions. An additional study looking at the safety and immunogenicity of a second viral vector boosting influenza virus-specific T-cell responses, ChAdOx1-NP + M1 (replication-deficient chimpanzee adenovirus vector expressing influenza virus antigens NP and M1), has been established [27].
Dr. Anne Moore (University College Cork, Ireland) presented findings on how the immune response induced by dissolvable microneedle (DMN) patches placed on the skin differs to those induced by needle-and-syringe based parenteral immunisation. The UCC research team is developing a transcutaneous immunisation system based on microneedle arrays, termed ImmuPatch. Microneedles are micron-scale needles that penetrate into the skin creating conduits for vaccine administration. Dr. Moore previously demonstrated that delivery of liquid or coated vaccine with silicon microneedles induces potent and protective immune responses in mice [28], [29], [30]. In this meeting, Dr. Moore exhibited a novel DMN patch technology, where vaccine is embedded into a microneedle that dissolves upon contact with moisture in the skin, thereby eliminating the requirement for vaccine reconstitution while retaining vaccine potency. Long-term stability of a seasonal influenza virus vaccine in ImmuPatch, which was stored at 40 °C and 75% relative humidity was presented; thus demonstrating the potential of this technology to overcome cold-chain obstacles. There is a strong focus on developing an influenza virus vaccine that induces broadly neutralising antibody responses. In this presentation, the immunogenicity of influenza virus vaccines delivered by DMN patches or by the intramuscular route in mice and pigs was discussed. Dr. Moore's group has investigated the breadth of the humoral response induced by intramuscular or patch-based immunisation, using a PVNA. They demonstrated significant differences when different administration routes were used, with respect to antibody recognition of heterosubtypic influenza virus HA proteins that were not present in the 2011/2012 seasonal influenza virus vaccine. These pre-clinical results therefore suggest that, in addition to enhanced stability, ImmuPatch may also have clinical utility with respect to broadening humoral immunity, a critical feature of reducing the potential of pathogen escape.
4. Vectorology
Common factors required for successful gene therapy and genetic vaccination applications are not dissimilar and can include uptake of vector by appropriate cells, vector entry into the host and either evading the host immune defences (gene therapy) or resulting in immuno-stimulation (vaccines). Among the viral vector systems for gene therapy applications, adenovirus, lentivirus and adeno-associated virus (AAV) are common. Dr. Takis Athanasopoulos (University of Wolverhampton, UK) spoke of his work in using these vector systems as pseudotyped gene therapeutics or genetic vaccines targeting HIV/AIDS. Adenovirus has >50 natural isolates plus species-specificities, such as ChAd capsid base modification variants, and has been widely tested as a vaccine vehicle. In previous studies, his group has used the common serotype 5 and/or the alternative serotype 11 as effective skin vaccination vehicles [31], [32], [33], [34]. Adenovirus vectors expressing Ub-gag–CN54 effectively targeted Langerhans cells as genetic vaccines. A cohort of lentiviral pseudotypes were also tested in Professor Dickson's lab (Vandermuelen, unpublished, UK) to generate multi-epitope VLP-enveloped vaccines [34]. Lastly, AAV vector pseudotyping, a tool widely used in the field of gene therapy, could be applicable to target HIV via secretion of immunoadhesins [35], using muscle as a platform for expression, in immuno-prophylaxis protecting humanised mice from mucosal HIV transmission [36] or in studies targeting the CCR5 chemokine co-receptor, using gene editing technologies.
Lessons learned from the field of gene therapy, such as against Duchenne Muscular Dystrophy (DMD), can be applied to design effective immunotherapeutics, such as against HIV, and vice versa. A Phase I clinical trial using a poorly designed constitutively expressing mini-dystrophin cassette packaged in an AAV2.5 pseudotype, characterised by high sero-prevalence in the human population, resulted in dystrophin immunity/vaccine type outcome [37]. In contrast, other AAV pseudotypes (AAV8 or AAV9) or capsid mutants expressing tissue-species specific genes of interest, such as a canine microdystrophin administered in the dog model of DMD [38], may be far more effective as a gene therapeutic approach, yet poor if directly administered as a vaccine vehicle, since these may escape immune-surveillance. Nevertheless, Dr. Athanasopoulos discussed how such ‘poor vaccine’ candidates could be used as effective gene editing tools. This includes expressing TALENs, ZNFs, or as in Dr. Athanasopoulos's group's own project, a CRISP-R/cas9 system vectorised into AAV or lentivirus which can target in vivo or ex vivo cell receptors (CCR5, CXCR4) or other pathways against HIV/AIDS and other infectious diseases.
Dr. Yasuhiro Takeuchi (University College London, UK) exhibited his research on HIV-based pseudotype particles from two distinct approaches: their stable, continuous production, and sugar antigen display on the pseudotype surface. The utilisation of lentiviral vectors for gene therapy purposes is held back by the relative paucity of packaging cell lines (PCLs) that can consistently produce safe, effective lentiviral vector (LV) batches, whilst adhering to strict Current Good Manufacturing Practice (CGMP) guidelines. Dr. Takeuchi provided details of his group's work to construct a CGMP-compliant PCL that can stably produce self-inactivating LVs – WinPac, based upon their original constitutive LV PCL named STAR [39], but with preferable safety characteristics. To generate WinPac cells, active genomic loci in 293FT cells were initially loxP-tagged by transduction of a gammaretroviral vector, before isolation of a stable, highly-expressing clone and introduction of a codon-optimised HIV gag-pol cassette via Cre recombinase-mediated cassette exchange (RMCE) (Fig. 3 ). Plasmids encoding HIV rev, RDpro – the RD114 endogenous retrovirus envelope protein – and a GFP-expressing LV were then stably transfected into the WinPac cells. The RDpro-pseudotyped lentiviruses stably produced from the WinPac PCL were able to efficiently transduce human T-cells and CD34+ cells, thus offering promise for WinPac to be utilised for the production of LVs that could deliver therapeutic genes in the treatment of X-SCID and B-cell malignancies.
Fig. 3.
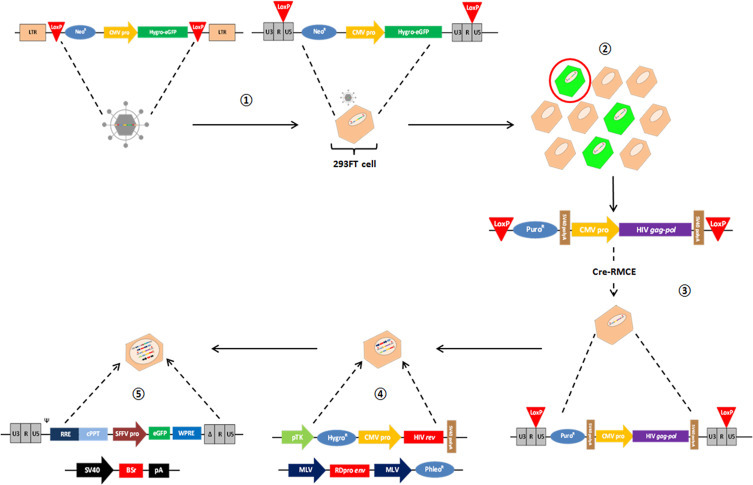
Development of the WinPac lentiviral vector packaging cell line.  Transduction of a gammaretroviral vector into 293FT cells and integration of a hygro-eGFP gene into active genomic loci tagged by mutant LoxP sites.
Transduction of a gammaretroviral vector into 293FT cells and integration of a hygro-eGFP gene into active genomic loci tagged by mutant LoxP sites.  Isolation of a cell clone with a single, stable, highly-expressing genomic locus.
Isolation of a cell clone with a single, stable, highly-expressing genomic locus.  Site-directed insertion of a codon-optimised HIV gag-pol construct using Cre-LoxP recombinase-mediated cassette exchange.
Site-directed insertion of a codon-optimised HIV gag-pol construct using Cre-LoxP recombinase-mediated cassette exchange.  and
and  Stable transfection of plasmids encoding HIV rev, RDpro and a GFP-expressing, self-inactivating lentiviral vector (in tandem with pSELECT antibiotic resistance plasmid), prior to multi-antibiotic selection of cell clones actively expressing lentiviral vector constructs. LTR = long terminal repeat; NeoR = neomycin resistance gene; CMV pro = cytomegalovirus promoter; Hygro-eGFP = hygromycin-resistant enhanced green fluorescent protein; PuroR = puromycin resistance gene; SV40 polyA = simian virus 40 polyadenylation sequence; HIV gag-pol = human immunodeficiency virus gag-pol genes; Cre-RMCE = Cre-recombinase mediated cassette exchange; pTK = thymidine kinase promoter; HygroR = hygromycin resistance gene; HIV rev = human immunodeficiency virus rev gene; MLV = murine leukaemia virus; RDpro env = RD114-HIV chimeric virus envelope glycoprotein; PhleoR = phleomycin resistance gene; Ψ = encapsidation signal; RRE = HIV rev response element; cPPT = HIV central polypurine tract; SFFV pro = spleen focus forming virus promoter; WPRE = Woodchuck hepatitis virus post-transcriptional regulatory element; BSr = blasticidin S-resistance gene; pA = polyadenylation sequence.
Stable transfection of plasmids encoding HIV rev, RDpro and a GFP-expressing, self-inactivating lentiviral vector (in tandem with pSELECT antibiotic resistance plasmid), prior to multi-antibiotic selection of cell clones actively expressing lentiviral vector constructs. LTR = long terminal repeat; NeoR = neomycin resistance gene; CMV pro = cytomegalovirus promoter; Hygro-eGFP = hygromycin-resistant enhanced green fluorescent protein; PuroR = puromycin resistance gene; SV40 polyA = simian virus 40 polyadenylation sequence; HIV gag-pol = human immunodeficiency virus gag-pol genes; Cre-RMCE = Cre-recombinase mediated cassette exchange; pTK = thymidine kinase promoter; HygroR = hygromycin resistance gene; HIV rev = human immunodeficiency virus rev gene; MLV = murine leukaemia virus; RDpro env = RD114-HIV chimeric virus envelope glycoprotein; PhleoR = phleomycin resistance gene; Ψ = encapsidation signal; RRE = HIV rev response element; cPPT = HIV central polypurine tract; SFFV pro = spleen focus forming virus promoter; WPRE = Woodchuck hepatitis virus post-transcriptional regulatory element; BSr = blasticidin S-resistance gene; pA = polyadenylation sequence.
Additionally, Dr. Takeuchi outlined the display of a number of clinically-relevant carbohydrate antigens on HIV pseudotypes for use in virus-based assays. For instance, Gal(α1-3)Gal terminal carbohydrates (αGal) are expressed in most mammals but not in humans, as they lack a functional α1,3 galactosyltransferase (αGT) gene. Therefore, humans raise a strong antibody response against αGal which causes acute rejection during xenotransplantation [40]. Similarly, humans cannot produce N-glycolylneuraminic acid (Neu5Gc) due to inactivity of a required hydroxylase, meaning burns victims receiving pigskin elicit high anti-Neu5GC antibody levels [41]. Production of GFP-harbouring HIV PVs displaying saccharide antigens such as those at the virus envelope can be utilised in assays such as the virus pull down assay, which works by monitoring the amount of virus ‘pulled down’ by targeted antibodies on magnetic beads, using a SYBR Green real-time PCR-enhanced reverse transcriptase assay [42]. Quantification of antibody responses raised against these oligosaccharides using this novel approach could significantly improve the understanding of rejection mediated by such antigens during organ and tissue xenotransplantation.
5. Conclusion
This meeting has demonstrated that PVs represent a highly-flexible platform that has applications in many domains including serology, serosurveillance, antibody standard development, vaccine immunogenicity testing and gene therapy/vaccination amongst others. Many of the viral pathogens of public and animal health importance wreaking havoc across the globe today are high-containment zoonotics and thus access to the wild-type virus is severely constrained for biosecurity reasons. The synthetic nature of the pseudotype system renders it invaluable for the study of such viruses. It is apparent from this meeting that PVs are now becoming more widely used and entrenched within the R&D landscape of multiple sectors. The benefits of PVs were highlighted throughout the meeting, together with recommendations towards future development (Table 3 ). It is with heightened anticipation that we wait to see what further uses they can be put to during the next instalment of this conference: Vaccines 2015 – Pseudotypes and VLPs: production, standardisation and vaccination (Greenwich, 15th October 2015): https://www.regonline.co.uk/builder/site/Default.aspx?EventID=1629228.
Table 3.
Highlights of the presentations delivered by the chair of the meeting and keynote speaker.
| Chairs’ summary & recommendations | |
|---|---|
| Pseudotyped viruses | Recommendation |
| • Entirely synthetic so no virus culture is required • Highly stable at different storage temperatures and when subjected to freeze-thawing • Amenable to lyophilisation thus facilitating shipping and deployment in low-resource areas Pseudotyped virus neutralisation assay • Useful as an adjunct to the EMA/FDA approved HI and SRH (influenza virus) • Serum-sparing, antigen-sparing and biosafe • Measures anti-HA head and stalk responses (influenza virus- functional assay) • Can be used to measure anti-HA stalk responses using a hybrid HA with a mismatched unreactive head (influenza virus) • Readily scaled up, and in a multiplex format the inter-assay variability is likely to be reduced |
• Multiplex assay should be adapted to measure antibody responses against two different respiratory virus groups (influenza virus/coronaviruses) • Pseudotype kit development should be progressed in line with WHO/OIE/SME standards • Pseudotype coverage of all influenza virus A&B HA subtypes as a pandemic preparedness resource |
| Keynote speaker summary & recommendations | |
|---|---|
| Pseudotyped viruses | Recommendation |
| • Have ‘real’ uses in virology and vaccinology • Do not require high level containment (Ebola virus, SARS coronavirus, rabies virus) • Can detect viral cell surface receptors and receptor-blocking drugs: HIV: CD4 + CCR5; rabies: nAchR + NCAM; SARS coronavirus: ACE-2R • Useful for viruses difficult to propagate in vitro (HCV, HTLV) • Simple reverse genetics of envelope genes (HIV, HCV) can be undertaken • Exhibit high sensitivity and specificity (all enveloped viruses) in neutralisation assays • For use in neutralisation assays, very small volumes of sera are required (1 μl samples from bats) • Can be used to monitor sera from candidate vaccines (Ebola virus, HIV, influenza virus: H5N1) |
• Expand serological surveillance using pseudotypes (human, veterinary and wild life) • Development of a triple assay: firefly + renilla luciferases + GFP (Ebola + Lyssa + SARS) • Expand the exploitation of empty PVs as immunogens |
Footnotes
A Euroscicon conference, 22nd October 2014, Cineworld, Greenwich, London.
References
- 1.King B., Daly J. Pseudotypes: your flexible friends. Future Microbiol. 2014;9:135–137. doi: 10.2217/fmb.13.156. [DOI] [PubMed] [Google Scholar]
- 2.Wright E., Temperton N.J., Marston D.A., McElhinney L.M., Fooks A.R., Weiss R.A. Investigating antibody neutralization of lyssaviruses using lentiviral pseudotypes: a cross-species comparison. J Gen Virol. 2008;89:2204–2213. doi: 10.1099/vir.0.2008/000349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corti D., Suguitan A.L., Pinna D., Silacci C., Fernandez-Rodriguez B.M., Vanzetta F. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120:1663–1673. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara F., Molesti E., Böttcher-Friebertshäuser E., Cattoli G., Corti D., Scott S.D. The human Transmembrane Protease Serine 2 is necessary for the production of Group 2 influenza A virus pseudotypes. J Mol Genet Med. 2012;7:309–314. [PMC free article] [PubMed] [Google Scholar]
- 5.Mather S., Scott S., Temperton N., Wright E., King B., Daly J. Current progress with serological assays for exotic emerging/re-emerging viruses. Future Virol. 2013;8:745–755. [Google Scholar]
- 6.Wright E., McNabb S., Goddard T., Horton D.L., Lembo T., Nel L.H. A robust lentiviral pseudotype neutralisation assay for in-field serosurveillance of rabies and lyssaviruses in Africa. Vaccine. 2009;27:7178–7186. doi: 10.1016/j.vaccine.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright E., Hayman D.T.S., Vaughan A., Temperton N.J., Wood J.L.N., Cunningham A.A. Virus neutralising activity of African fruit bat (Eidolon helvum) sera against emerging lyssaviruses. Virology. 2010;408:183–189. doi: 10.1016/j.virol.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molesti E., Wright E., Terregino C., Rahman R., Cattoli G., Temperton N.J. Multiplex evaluation of influenza neutralizing antibodies with potential applicability to in-field serological studies. J Immunol Res. 2014;201:4. doi: 10.1155/2014/457932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpentier D.C.J., Vevis K., Trabalza A., Georgiadis C., Ellison S.M., Asfahani R.I. Enhanced pseudotyping efficiency of HIV-1 lentiviral vectors by a rabies/vesicular stomatitis virus chimeric envelope glycoprotein. Gene Ther. 2011;19:761–774. doi: 10.1038/gt.2011.124. [DOI] [PubMed] [Google Scholar]
- 10.Fooks A.R., Banyard A.C., Horton D.L., Johnson N., McElhinney L.M., Jackson A.C. Current status of rabies and prospects for elimination. Lancet. 2014;6736:1–11. doi: 10.1016/S0140-6736(13)62707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carnell G.W., Ferrara F., Grehan K., Thompson C.P., Temperton N.J. Pseudotype-based neutralization assays for influenza: a systematic analysis. Front Immunol. 2015;6 doi: 10.3389/fimmu.2015.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alberini I., Del Tordello E., Fasolo A., Temperton N.J., Galli G., Gentile C. Pseudoparticle neutralization is a reliable assay to measure immunity and cross-reactivity to H5N1 influenza viruses. Vaccine. 2009;27:5998–6003. doi: 10.1016/j.vaccine.2009.07.079. [DOI] [PubMed] [Google Scholar]
- 13.Trombetta C., Perini D., Mather S., Temperton N., Montomoli E. Overview of serological techniques for influenza vaccine evaluation: past, present and future. Vaccines. 2014;2:707–734. doi: 10.3390/vaccines2040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temperton N.J., Chan P.K., Simmons G., Zambon M.C., Tedder R.S., Takeuchi Y. Longitudinally profiling neutralizing antibody response to SARS coronavirus with pseudotypes. Emerg Infect Dis. 2005;11:411–416. doi: 10.3201/eid1103.040906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molesti E., Milani A., Terregino C., Cattoli G., Temperton N.J. Comparative serological assays for the study of h5 and h7 avian influenza viruses. Influenza Res Treat. 2013;2013:286158. doi: 10.1155/2013/286158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephenson I., Das R.G., Wood J.M., Katz J.M. Comparison of neutralising antibody assays for detection of antibody to influenza A/H3N2 viruses: an international collaborative study. Vaccine. 2007;25:4056–4063. doi: 10.1016/j.vaccine.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 17.Mather S., Wright E., Scott S., Temperton N. Lyophilisation of influenza, rabies and Marburg lentiviral pseudotype viruses for the development and distribution of a neutralisation-assay-based diagnostic kit. J Virol Methods. 2014;210:51–58. doi: 10.1016/j.jviromet.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Forsman A., Beirnaert E., Aasa-Chapman M.M.I., Hoorelbeke B., Hijazi K., Koh W. Llama antibody fragments with cross-subtype human immunodeficiency virus type 1 (HIV-1)-neutralizing properties and high affinity for HIV-1 gp120. J Virol. 2008;82:12069–12081. doi: 10.1128/JVI.01379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hultberg A., Temperton N.J., Rosseels V., Koenders M., Gonzalez-Pajuelo M., Schepens B. Llama-derived single domain antibodies to build multivalent, superpotent and broadened neutralizing anti-viral molecules. PLoS ONE. 2011;6:e17665. doi: 10.1371/journal.pone.0017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCoy L.E., Quigley A.F., Strokappe N.M., Bulmer-Thomas B., Seaman M.S., Mortier D. Potent and broad neutralization of HIV-1 by a llama antibody elicited by immunization. J Exp Med. 2012;209:1091–1103. doi: 10.1084/jem.20112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott S., Molesti E., Temperton N., Ferrara F., Böttcher-Friebertshäuser E., Daly J. The use of equine influenza pseudotypes for serological screening. J Mol Genet Med. 2012;6:304–308. [PMC free article] [PubMed] [Google Scholar]
- 22.Ho Y.-P., Schnabel V., Swiersy A., Stirnnagel K., Lindemann D. A small-molecule-controlled system for efficient pseudotyping of prototype foamy virus vectors. Mol Ther. 2012;20:1167–1176. doi: 10.1038/mt.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert L.C., Fauci A.S. Influenza vaccines for the future. N Engl J Med. 2010;363:2036–2044. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]
- 24.Lillie P.J., Berthoud T.K., Powell T.J., Lambe T., Mullarkey C., Spencer A.J. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP + M1, in humans. Clin Infect Dis. 2012;55:19–25. doi: 10.1093/cid/cis327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullarkey C.E., Boyd A., van Laarhoven A., Lefevre E.A., Veronica Carr B., Baratelli M. Improved adjuvanting of seasonal influenza vaccines: preclinical studies of MVA-NP + M1 coadministration with inactivated influenza vaccine. Eur J Immunol. 2013;43:1940–1952. doi: 10.1002/eji.201242922. [DOI] [PubMed] [Google Scholar]
- 26.Powell T.J., Peng Y., Berthoud T.K., Blais M.-E., Lillie P.J., Hill A.V.S. Examination of influenza specific T cell responses after influenza virus challenge in individuals vaccinated with MVA-NP + M1 vaccine. PLOS ONE. 2013;8:e62778. doi: 10.1371/journal.pone.0062778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antrobus R.D., Coughlan L., Berthoud T.K., Dicks M.D., Hill A.V., Lambe T. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved Influenza A antigens. Mol Ther. 2014;22:668–674. doi: 10.1038/mt.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey J.B., Vrdoljak A., O’Mahony C., Hill A.V.S., Draper S.J., Moore A.C. Microneedle-mediated immunization of an adenovirus-based malaria vaccine enhances antigen-specific antibody immunity and reduces anti-vector responses compared to the intradermal route. Sci Rep. 2014;4:6154. doi: 10.1038/srep06154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vrdoljak A., McGrath M.G., Carey J.B., Draper S.J., Hill A.V.S., O’Mahony C. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J Control Release. 2012;159:34–42. doi: 10.1016/j.jconrel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carey J.B., Pearson F.E., Vrdoljak A., McGrath M.G., Crean A.M., Walsh P.T. Microneedle array design determines the induction of protective memory CD8+ T cell responses induced by a recombinant live malaria vaccine in mice. PLoS ONE. 2011;6:e22442. doi: 10.1371/journal.pone.0022442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachy V., Hervouet C., Becker P.D., Chorro L., Carlin L.M., Herath S. Langerin negative dendritic cells promote potent CD8+ T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proc Natl Acad Sci U S A. 2013;110:3041–3046. doi: 10.1073/pnas.1214449110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benlahrech A., Harris J., Meiser A., Papagatsias T., Hornig J., Hayes P. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc Natl Acad Sci U S A. 2009;106:19940–19945. doi: 10.1073/pnas.0907898106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benlahrech A., Meiser A., Herath S., Papagatsias T., Athanasopoulos T., Li F. Fragmentation of SIV-gag vaccine induces broader T cell responses. PLOS ONE. 2012;7:e48038. doi: 10.1371/journal.pone.0048038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herath S., Benlahrech A., Papagatsias T., Athanasopoulos T., Bouzeboudjen Z., Hervouet C. Fusion of ubiquitin to HIV gag impairs human monocyte-derived dendritic cell maturation and reduces ability to induce gag T cell responses. PLOS ONE. 2014;9:e88327. doi: 10.1371/journal.pone.0088327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson P.R., Schnepp B.C., Zhang J., Connell M.J., Greene S.M., Yuste E. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15:901–906. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balazs A.B., Ouyang Y., Hong C.M., Chen J., Nguyen S.M., Rao D.S. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med. 2014;20:296–300. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendell J.R., Campbell K., Rodino-Klapac L., Sahenk Z., Shilling C., Lewis S. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koo T., Okada T., Athanasopoulos T., Foster H., Takeda S., Dickson G. Long-term functional adeno-associated virus-microdystrophin expression in the dystrophic CXMDj dog. J Gene Med. 2011;13:497–506. doi: 10.1002/jgm.1602. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda Y., Takeuchi Y., Martin F., Cosset F.-L., Mitrophanous K., Collins M. Continuous high-titer HIV-1 vector production. Nat Biotechnol. 2003;21:569–572. doi: 10.1038/nbt815. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi Y., Porter C.D., Strahan K.M., Preece A.F., Gustafsson K., Cosset F.L. Sensitization of cells and retroviruses to human serum by (alpha 1-3) galactosyltransferase. Nature. 1996;379:85–88. doi: 10.1038/379085a0. [DOI] [PubMed] [Google Scholar]
- 41.Scobie L., Padler-Karavani V., Le Bas-Bernardet S., Crossan C., Blaha J., Matouskova M. Long-term IgG response to porcine Neu5Gc antigens without transmission of PERV in burn patients treated with porcine skin xenografts. J Immunol. 2013;191:2907–2915. doi: 10.4049/jimmunol.1301195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai R.P.J., Yan J., Heeney J., McClure M.O., Göttlinger H., Luban J. Nef decreases HIV-1 sensitivity to neutralizing antibodies that target the membrane-proximal external region of TMgp41. PLoS Pathog. 2011;7:e1002442. doi: 10.1371/journal.ppat.1002442. [DOI] [PMC free article] [PubMed] [Google Scholar]


