Abstract
The use of recombinant gene technologies by the vaccine industry has revolutionized the way antigens are generated, and has provided safer, more effective means of protecting animals and humans against bacterial and viral pathogens. Viral and bacterial antigens for recombinant subunit vaccines have been produced in a variety of organisms. Transgenic plants are now recognized as legitimate sources for these proteins, especially in the developing area of oral vaccines, because antigens have been shown to be correctly processed in plants into forms that elicit immune responses when fed to animals or humans. Antigens expressed in maize (Zea mays) are particularly attractive since they can be deposited in the natural storage vessel, the corn seed, and can be conveniently delivered to any organism that consumes grain. We have previously demonstrated high level expression of the B-subunit of Escherichia coli heat-labile enterotoxin and the spike protein of swine transmissible gastroenteritis in corn, and have demonstrated that these antigens delivered in the seed elicit protective immune responses. Here we provide additional data to support the potency, efficacy, and stability of recombinant subunit vaccines delivered in maize seed.
Keywords: Transgenic corn, Seed-derived vaccine, Antigen stability, Transmissible gastroenteritis virus, Escherichia coli heat-labile enterotoxin
1. Introduction
There are a number of elements that are critical to successful development of a vaccine (reviewed in [1], [2], [3], [4]). A vaccine must be effective at protecting against infection by a target pathogen without bringing harm to the host. In addition, a vaccine must be potent enough to limit dosage to a practical and affordable size. Further, a vaccine must remain potent, efficacious, and safe throughout transport and storage to the time of administration. These considerations are of high concern to the commercial animal farming industry where large numbers of animals raised in close proximity need to be immunized in a timely and cost-effective manner in order to preserve livestock health. These concerns also translate to human populations where vaccine cost, storage condition, and method of administration can limit a country’s ability to effectively immunize its population, especially in developing and tropical countries. Therefore, there is need to further pursue development of new animal and human vaccines that are less expensive, more robust, and easier to deliver, yet are just as safe, potent, and efficacious as existing ones.
The use of recombinant gene technologies by the vaccine industry has revolutionized the way antigens are generated and has provided safer, more effective means of protecting host organisms against bacterial, viral, and parasite pathogens [5], [6]. Recombinant subunit vaccines have become especially popular because they can be produced without using materials derived from infected animal or human hosts, thus reducing the potential for infectious contaminants. In addition, subunit vaccines can be formulated to include only essential antigens, thus limiting undesirable secondary effects associated with unnecessary components. Despite these enhancements, concerns still exist that recombinant vaccines can be cost prohibitive and, when derived from mammalian cell culture, still have the potential to be contaminated with animal pathogens. Furthermore, recombinant subunit vaccines are usually administered through parenteral injection of purified protein components thus requiring the need for controlled storage conditions to ensure stability and sterility, and equipment to administer injections.
Development of a broadly applicable oral delivery system could allow efficient widespread administration of vaccines without the need for needles, syringes, and trained personnel. This has proven to be a challenging goal since the hurdle of protein digestion must be overcome to allow effective oral delivery. Furthermore, even if an oral system could be developed where significant quantities of antigen survive the gut, there is no certainty that the antigen could be absorbed in sufficient quantities to elicit a protective immune response.
Plants are increasingly being recognized as legitimate systems for production of recombinant proteins and antigens (reviewed in [7], [8], [9]). As alternative eukaryotic expression systems, plants have been shown to synthesize and process a variety of mammalian proteins to yield high level expression of active, properly folded proteins. Plant expression systems hold the advantage over animal cell systems in that animal viruses do not infect plants. The utility of plant systems is further supported by a growing number of studies which illustrate that plant species can be used to express foreign antigens for subunit vaccines (reviewed in [7], [9], [10]). When administered orally, such antigens can induce immune responses that provide protection against a subsequent challenge with a pathogen [11], [12], [13].
Maize is an established eukaryotic expression system for high-level expression and commercial production of recombinant proteins and antigens (reviewed in [14]). Recent commercialization of recombinant proteins (avidin and GUS) purified from maize seed has demonstrated the potential of this system for large-scale production of proteins that retain structural integrity and biological activity [15], [16]. Protein expression as high as 0.1% of dry weight of seed has been obtained for several proteins [unpublished results]. Thus, recombinant biopharmaceutical proteins delivered in maize seed can be produced at amounts exceeding 2 kg per acre for a cost of just pennies per milligram [17]. The existing infrastructure for harvesting grain coupled with established processes for fractionation and handling of grain products provides further economical advantages to maize seed for production of valuable protein products. Furthermore, maize seed is a natural protein storage site that can be harnessed as a powerful vehicle for oral delivery of antigens. The natural bioencapsulation of proteins in maize seed may enhance antigen survival in the gut and promote antigen delivery to mucosal surfaces [11], [13], [18], [19], [20]. We are exploring the use of corn grain as a delivery system for edible vaccines against enterotoxigenic strains of Escherichia coli (ETEC) and swine transmissible gastroenteritis virus (TGEV).
Among children under 5 in developing countries, ETEC are responsible for over 650 million cases of diarrhea resulting in about 800 000 deaths each year [21]. About 20% of visitors to developing countries also contract travelers’ diarrhea from ETEC [22]. The major disease agent of ETEC is the heat-labile toxin (Lt). This toxin has a multisubunit structure very similar to cholera toxin and consists of a pentamer of receptor binding (B) subunits linked to a single enzymatic (A) subunit [23]. Approximately 66% of ETEC strains harbor Lt, and in about half of these strains Lt is the only toxin present [24]. These pathogens can infect nonhuman hosts as well with enteric disease due to strains of ETEC being the most commonly occurring form of colibacillosis in pigs and calves [25]. Thus, development of an oral vaccine against ETEC can have a broad impact on animal as well as human populations.
Swine transmissible gastroenteritis (TGE) is recognized as a major cause of illness and death in piglets, particularly under conditions of intensive farming [26]. It is a highly contagious enteric disease that is characterized by vomiting, severe diarrhea and high mortality in piglets less than 2 weeks of age. The causal agent of TGE is a multisubunit, enveloped, single-stranded RNA virus, TGEV, belonging to the genus Coronavirus of the family Coronaviridae [26]. It contains three structural proteins designated M, N and S. The M protein is an integral membrane protein, N is a phosphoprotein that encapsulates the viral RNA genome, and S (or spike) is a large surface glycoprotein [26]. Pigs that survive a first infection are immune to subsequent infections of the virus, probably due to local mucosal immunity in the intestine through the production of S-IgA [27]. Thus, vaccines that target the activation of lymphoid tissues on the mucosal surface of the intestine are particularly attractive in the control of TGE and similar diseases. Attempts to generate a subunit vaccine that protects against TGEV using more conventional expression systems have been limited largely due to poor expression of the S protein, an important target for generation of protective (virus neutralizing) antibody, whereas plant systems have shown much promise [11], [28], [29], [30].
Previously, we reported generation of transgenic maize engineered to express the Lt-B and TGEV-S antigens, and demonstrated that oral delivery of transgenic maize grain containing these antigens elicits protective immune responses in mice and piglets, respectively [11], [18]. Here we report data further supporting the potency, stability, and flexibility of transgenic maize seed as a delivery system for oral subunit vaccines.
2. Materials and methods
2.1. Transgenic maize lines
The development of maize lines expressing Lt-B and TGEV-S proteins in the seed has been previously reported [11], [18]. Briefly, synthetic maize codon optimized versions of Lt-B (based on GenBank accession M17874) and TGEV-S (based on Miller strain of TGEV) were cloned in frame of a maize codon optimized version of the barley α-amylase signal sequence to provide a cell secretion signal at the N-terminus of Lt-B and TGEV-S for protein accumulation in the cell wall. They were placed in a maize expression cassette within a transformation vector that included right and left border sequences of an Agrobacterium tumefaciens Ti plasmid and the pat gene of Streptomyces viridiochromogenes conferring resistance to the herbicide Basta. Agrobacterium-mediated transformation of maize embryos and selection of transformants was described [11]. Subsequent propagation of seed was performed through crossing plants derived from the original T1 seed with various maize lines, with identification of high-expressing lines performed through analysis of each subsequent generation of seed by protein-specific ELISAs. Soluble extracts were prepared as described previously [11], and soluble protein measured by the Bradford assay [31]. Quantitation of recombinant Lt-B and TGEV-S proteins was performed as described previously [11].
2.2. Fractionation of grain
Control grain as well as grain carrying the Lt-B (Lt-B corn) and the TGEV-S (TGEV corn) genes were either coarsely ground as described previously [11] or fractionated into component parts using standard milling practices. For fractionation, up to 250-lb samples of transgenic corn or control corn were cleaned using an aspirator (Kice, Wichita, KS, USA) and screens of two different mesh sizes to remove large and small impurities. Following the cleaning, water was then added to the samples to approximately 21% moisture and the kernels were left to temper for about 2 h. The tempered kernels were cracked in a Ripple mill with an impact rotor. The cracked kernels were then dried at 38–41 °C for 40 min. Using a screen with a 0.275-cm mesh size, bran or hull (pericarp tissue), germ (embryo tissue) and large coarse grits (large pieces of endosperm tissue) were separated from medium and fine grits (small pieces of endosperm tissue) and smaller particles of meal and flour. The germ fraction was dried in the forced air oven at 38–41 °C until the moisture level was reduced to less than 12%. The recovered germ samples were flaked using a flaking roll (Ferrell-Ross, Amarillo, TX, USA) with a roll gap setting of 0.018 cm. Oil, or fat, in the germ flakes was removed by conducting repeated extractions with hexane in a stainless steel batch extractor. After solvent had been drained the samples were allowed to air dry to evaporate any residual hexane. The defatted germ samples were sifted using a 0.061-cm screen, with all material passing through the screen from sequential rounds of sifting were pooled. These samples were stored at 4 °C.
2.3. Immunization of mice
BALB/c mice were housed individually and fed a basic diet of mouse chow with water allowed ad libitum. The mouse chow was removed overnight prior to administering test samples on days 0, 7 and 21 of the study. The mice were divided into four groups, with ten individuals in each group. Test samples consisted of wild type defatted corn germ, or transgenic defatted corn germ expressing 0.33, 3.3 or 33 μg Lt-B. Blood samples were collected by conducting tail bleeds prior to the first feeding of test samples (on day 0), and on days 7, 14, 21, 28, 35 and 42 of the study. Fecal samples corresponding to material excreted over the previous 24 h were collected prior to the feeding of test samples (on day 0), and on days 3, 7, 10, 14, 17, 21, 24, 28, 31, 35, 38 and 42 of the study.
2.4. Detection of anti-Lt-B antibodies in mouse serum and fecal samples
For detection of serum anti-Lt-B IgG, 96-well plates were coated with Lt-B protein, repeatedly washed with phosphate buffered saline (PBS), and blocked with PBS containing 3% BSA for 1 h at 37 °C. The blocking solution was replaced with serum recovered from mouse blood diluted in the blocking solution. Samples were incubated for 2 h at 37 °C and then repeatedly washed with PBS containing 0.05% Tween 20 (PBS–T). For detection of anti-Lt-B IgG, anti-mouse IgG conjugated to alkaline phosphatase (diluted 1000-fold) was loaded onto plates in blocking solution for 2 h at 37 °C. Plates were repeatedly washed with PBS–T and 1 mg ml−1 p-nitrophenylphosphate was added to each well. Following 30 min of incubation at 37 °C the absorbance at 405 nm was determined. Detection of fecal anti-Lt-B IgA was as described previously [11].
2.5. Immunization of swine
Two swine feeding trials were performed. The first trial was to examine serum from animals fed TGEV corn to determine if they had produced neutralizing anti-TGE virus antibodies. Briefly, 10–12 day-old piglets that were TGEV sero-negative and were from a herd with a low incidence of disease were used. Twelve piglets were divided into three treatment groups; a control group receiving control corn meal, a second group receiving TGEV corn meal, and the third group receiving normal rations. On days 0–7 of the study, and again on days 15–21, all piglets in the first and second group were fasted overnight, prior to administering corn rations. Corn rations consisted of either 100 g of wild type corn or 50 g of transgenic corn (corresponding to approximately 2 mg of the S protein of TGEV) mixed with 50 g of wild type corn. The corn was mixed with medicated milk replacer to give a thick oatmeal-like consistency. Piglets in the third group were maintained on normal rations (Frostcoat, Moorman’s) throughout the course of the study. Upon completion of the feeding regimen, piglets were returned to regular water and normal rations. On day 29 of the study all animals were administered a 1-ml oral dose of virulent TGEV [Purdue strain, 50% fluorescent antibody infectious dose (FAID50) of 107.6 FAID50s per dose]. Serum samples collected on day 0, 8, 16, 23, 30 and 40, were analyzed for their ability to neutralize TGEV.
The second trial was designed to measure protection of piglets after a challenge with TGEV. Briefly, subjects were 10–12-day-old specific pathogen-free piglets that were TGEV sero-negative and were from a herd with a low incidence of disease. Fifty piglets were divided into five groups; one control group receiving control corn for 16 consecutive days, three groups fed TGEV corn for either 4, 8 or 16 consecutive days, and one group receiving modified live virus vaccine as a positive control group. The corn ration for each piglet consisted of either 50 g of wild type corn or 50 g of transgenic corn (corresponding to approximately 2 mg of the S protein of TGEV). The corn was mixed with medicated milk replacer to give a thick oatmeal-like consistency. For the four groups of piglets receiving corn, a line of prepared meal sufficient for the whole group was placed on a clean dry floor and attempts were made to ensure that each piglet received an adequate portion. The piglets were then returned to regular water and medicated weaning rations. Piglets in the fifth group were orally vaccinated with a commercially available modified live TGEV vaccine (MLV, Intervet) on days 0 and 7 of the study according to label directions. On day 18 of the study all animals were orally challenged with a 2-ml dose of virulent TGEV [Purdue strain, 50% fluorescent antibody infectious dose (FAID50) of 107.6 FAID50s per dose]. This dose when administered on day 12 in a similarly designed study produced a clinically typical watery diarrhea in 21–28-day-old piglets that would persist for 7–10 days, but would not be lethal [11]. By delaying administration of virus for 6 additional days (day 18) in the study described here, it was expected that clinical symptoms would decrease in intensity slightly but would still be apparent by day 27. Following challenge, piglets were scored twice daily for signs of diarrhea (normal=0, creamy=1, watery=2) and other symptoms (dehydration and depression, or anorexia=1, vomitus=3, moribund or death=10) to give a total clinical score. Clinical symptoms scored for each study group are presented as follows: either percent morbidity incidence [(number of animals with clinical signs scoring ≥2 divided by total number of animals)×100], percent morbidity incidence and duration [(total number of clinical observations ≥2 divided by total number of pig days)×100], or clinical severity index (total clinical score divided by total number of pig days). To confirm viral challenge, fecal samples were collected from randomly selected animals within any group that produced watery diarrhea. These samples were tested for TGEV activity by inoculating confluent swine testicular (ST) cells in culture and staining by specific immunofluorescence.
3. Results
3.1. Fractionation of transgenic maize seed
We previously reported generation of transgenic maize lines expressing recombinant Lt-B and TGEV-S antigens in corn seed [11], [18]. Lt-B protein levels as high as 9% of total soluble protein have been expressed in seed, and the protein was shown to assemble into the active pentameric form in planta [18]. Expression of TGEV-S protein at levels as high as 2% of total soluble protein has been observed in transgenic corn seed, and expression correlates with the presence of a protein which is recognized by anti-TGE virus antiserum (data not shown). In the present study we started with corn seed containing Lt-B and TGEV-S proteins present at approximately 0.8 and 0.1% of total soluble protein, respectively. Corn seed is composed of several distinct compartments where proteins are known to accumulate differentially and which can be fractionated by established commercial processing methods. In order to determine whether antigens intended for oral vaccine formulation could survive the fractionation process, and to examine the distribution of these antigens within seed-derived fractions, we fractionated grain expressing the Lt-B and TGEV-S antigens using a standard milling process designed to divide the seed into component parts. The major fractions resulting from this process include germ (the plant embryo), grits (starch-rich compartment corresponding to the seed endosperm), and bran (the seed coat or ‘pericarp’). A constitutive promoter was used to drive expression of Lt-B and TGEV-S gene products in these corn lines and expression is expected in all seed compartments. Analysis of proteins present in extracts derived from each of these fractions was performed using protein-specific ELISAs. Results of fractionating Lt-B corn seed are shown in Fig. 1 . Lt-B protein was found in all compartments of the seed. However, on a per-weight-of-tissue basis, Lt-B antigen was greatly enriched in the germ fraction. Germ is also a rich source for oil that can be extracted by hexane treatment, leaving behind a defatted germ fraction. When the Lt-B germ meal was treated with hexane to remove the fat portion of the germ, the resulting defatted corn germ meal fraction was also analyzed for Lt-B content. The results indicate that Lt-B is stable to the process of defatting the germ fraction since the measurable level of Lt-B protein was not diminished as compared to the full-fat germ fraction and actually increased, likely reflecting the increase in dry weight as a result of the loss of oil. Fractionation of TGEV corn yielded similar results (data not shown).
Fig. 1.
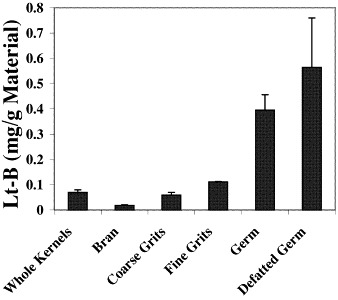
Levels of Lt-B Protein in fractions of milled grain. Grain fractions were separated and analyzed for Lt-B as described in Materials and methods. Values represent mean±one standard deviation.
3.2. Stability of Lt-B and TGEV-S antigens in maize seed-derived fractions
To determine whether Lt-B expressed in maize seed and present in the germ meal fraction is stable to storage at different temperatures, aliquots (3 g) of defatted Lt-B corn germ meal were placed at either 4 or at 23 °C. At defined times, aliquots were removed and extracts were prepared. Lt-B content in extracts was analyzed by ELISA, and the results are shown in Fig. 2A . Storage at either 4 or 23 °C for longer than a year had negligible affects on measurable Lt-B levels. The ELISA used to measure the Lt-B levels has a substantial bias toward Lt-B pentamer (the active form of Lt-B). Denaturation of an Lt-B standard from pentamer to monomer, through boiling of the protein, reduces ELISA signal by at least 20-fold without degrading the Lt-B subunits themselves (data not shown). Therefore this analysis indicates that not only does the Lt-B antigen persist over time, but that it persists in the active, pentameric form.
Fig. 2.
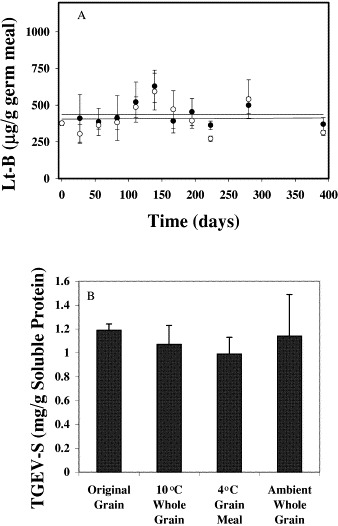
Antigen stability in tissues from transgenic corn seed. (A) Measurement of Lt-B levels in defatted corn germ meal stored at either 4 °C (○) or 23 °C (●) over a 392-day period; (B) measurement of extracted TGEV-S antigen as mg antigen per g extracted soluble protein from grain or grain meal stored for 10 months at either 4 °C, 10 °C, or at ambient temperature in a grain storage facility in Iowa. Values represent mean±one standard deviation.
Persistence of TGEV-S antigens in corn grain over time was also assessed. TGEV corn grain was harvested and stored under a variety of conditions as either whole grain or as coarsely ground whole grain meal. Grain meal was placed in storage at 4 °C. Whole grain was stored at either 10 °C in a seed storage facility where humidity was maintained at 50% (conditions routinely used to preserve seed germination potential) or at ambient temperature in a grain storage facility in Iowa where neither temperature nor humidity were controlled. Initially, TGEV corn was analyzed for TGEV-S levels in extracts prepared from a small aliquot of ground grain. After 10 months, extracts were prepared from the whole grain stored at both ambient conditions and at 10 °C as well as from grain meal stored at 4 °C. Fig. 2B shows the levels of TGEV-S antigen measured in extracts derived from samples stored at the conditions indicated. These results show that TGEV-S antigen levels persist over time either in grain meal stored at 4 °C, or in whole seed stored at higher temperatures. Importantly, persistence of the TGEV-S antigen occurred even under uncontrolled grain storage conditions indicating that temperature-controlled storage facilities are not necessary for stability of this antigen in the seed.
3.3. Potency of a corn germ-derived Lt vaccine
A previous study demonstrated that Lt-B expressed in whole corn grain and delivered orally induces both serum and secretory immune responses [11]. We further investigated the immunogenicity of Lt-B transgenic corn by examining a defatted germ fraction, in which the concentration of antigen is increased over whole kernels, and by delivering the antigen over a wider dose range, including very small doses. Mice were fed defatted Lt-B corn germ meal or defatted wild type corn germ meal, and serum and fecal samples were collected and analyzed. For all groups except the negative control (receiving wild-type corn germ meal) the serum IgG responses were evident after the first dose, or second dose for the lowest dose level, and increased throughout the study (Fig. 3A). All mice within groups fed 3.3 and 33 μg of Lt-B and 8/10 mice within the group fed 0.33 μg of Lt-B responded with Lt-B specific serum IgG assay values exceeding 2× the average value for preimmune serum (data not shown). Thus, even 0.33 μg of the Lt-B antigen is sufficient to give a serum IgG response. This is approximately 15-fold lower than any dose previously administered in plant material [11], [12]. Given the expression level of Lt-B in the defatted germ, the amount of corn material fed in this case is only 0.7 mg. Thus, clearly very small doses of Lt-B delivered in corn material can induce a response, and the amount of corn material that is administered is a very manageable dose size.
Fig. 3.
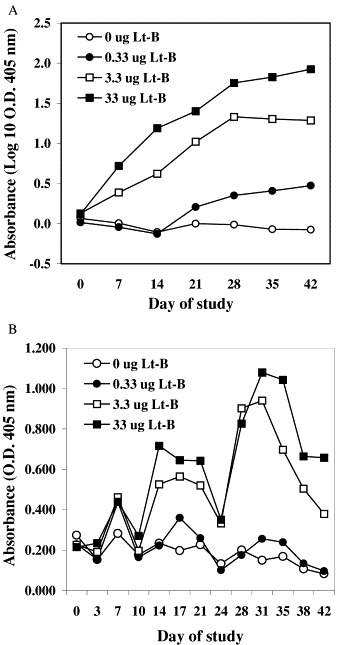
Immune responses of mice fed defatted Lt-B corn germ meal. (A) Anti-Lt-B specific IgG in serum. (B) Anti-Lt-B specific IgA in fecal material. The mean responses for the ten mice in each group are shown at defined times throughout the feeding study for animals fed defatted Lt-B corn germ meal containing the amount of Lt-B indicated.
All groups except the negative control also have mucosal IgA responses (Fig. 3B ). Again, a 0.33 μg dose of Lt-B antigen in defatted germ material is sufficient to induce a response. The responses are evident after the first dose and tend to cycle through the study, peaking approximately 1 week after each dose administration. The responses are greater with increasing doses of defatted Lt-B germ meal. Thus, as with a previous study using whole corn kernels, the response when using an edible plant vaccine appears at mucosal surfaces as well as in serum [11]. This indicates that edible vaccines may be particularly suited toward combating disease agents that infect or gain access to the body through mucosal surfaces.
3.4. Efficacy of a corn-derived TGEV vaccine
Previously, we demonstrated that TGEV corn, when fed to piglets, could induce partial protection from a subsequent challenge using TGE whole virus [11]. This suggested that oral administration (feeding) of TGEV corn could result in generation of a protective immune response. Therefore to test whether administration of TGEV corn leads to generation of virus neutralizing antibody, we examined serum from piglets that had been fed TGEV corn and then exposed to TGE whole virus. Piglets were fed TGEV corn for 7 consecutive days followed by normal feed for 7 days, and then finally boosted with a second 7-consecutive-day feeding of TGEV corn. On day 29 piglets were exposed to an amount of TGE whole virus that would result in a subclinical infection. Serum was collected over the experimental time course and assayed for its ability to interfere with TGE virus infection of a swine testicular cell line in vitro. Results of analysis of the neutralization titers are shown in Fig. 4 . Although neutralizing antibodies were not detected in the serum of any of the piglets prior to virus exposure, administration of whole virus resulted in rapid induction of high levels of neutralizing antibody in serum from piglets that had previously eaten TGEV corn. Three out of the four animals in the TGEV corn-fed group responded with titers ranging from 512 to 2048 yielding a geometric mean titer of the four animal group of 768.5. This was in contrast to piglets that had eaten control corn meal prior to exposure to whole virus, which developed low levels of neutralizing antibody suggestive of a subclinical infection with the highest titer from any single animal in either of the control groups of only 64. Therefore, a clear memory response leading to elevated levels of neutralizing antibody was obtained in animals fed transgenic corn containing recombinant TGEV-S antigen.
Fig. 4.
Induction of TGEV neutralizing antibodies in serum from piglets fed transgenic corn seed expressing the S protein of TGEV. Mean responses are shown for the four piglets in each group that were fed normal rations, control corn, or TGEV-S corn.
Our previously reported viral challenge study employed a feeding regimen where piglets were administered TGEV corn for 10 consecutive days prior to challenge with virus on day 12 [11]. Piglets fed TGEV corn showed fewer overall symptoms of infection as compared to those fed control corn or a modified live virus vaccine, but recovery of affected piglets was slower in the TGEV corn fed group than in the whole virus administered group. Since dose amount is a critical factor contributing to the protective potential of a vaccine, we examined several doses of TGEV corn for effectiveness at protecting piglets from a subsequent viral challenge. We compared the effect of feeding TGEV corn for 4, 8 or 16 consecutive days with administration of a commercial modified live TGEV vaccine for their ability to protect piglets from a viral challenge on day 18. A negative control group fed nontransgenic corn for 16 days was also included. The percent morbidity incidence shows that 50% of the piglets fed wild type corn developed TGE clinical symptoms (Fig. 5A ). In contrast, none of the piglets that received transgenic corn for 4 days exhibited symptoms. Interestingly, the 8- and 16-day feedings were less effective than the 4-day feeding of TGEV corn with 20 and 36%, respectively, showing symptoms. A total of 9% of the piglets receiving the commercial modified live vaccine still developed symptoms. The percent morbidity incidence and duration as well as the clinical severity index data (Fig. 5B and C) further reflected the protection of the piglets by TGEV corn, with the greatest level of protection with a 4-day administration. Analysis (χ 2) was performed using Yates correction for continuity in calculating the statistical test, which indicated that animals fed control corn had a significantly higher rate of morbidity incidence and duration (P<0.001) and severity of infection (P<0.001) compared to animals that received TGEV corn for 4 days. These significant differences were comparable to the differences observed between the control corn and the modified live virus (morbidity incidence and duration (P=0.004) and severity of infection (P<0.001)). Taken together, these results indicate that feeding TGEV corn vaccine is effective at reducing, and under certain circumstances even eliminating, clinical disease symptoms associated with exposure to TGEV.
Fig. 5.
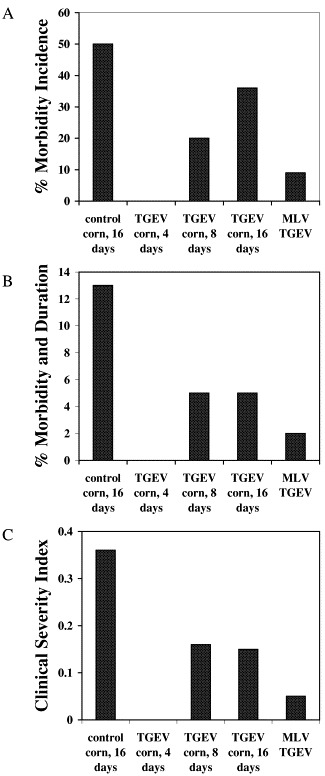
Protection against TGEV of piglets fed transgenic corn expressing the S protein or a modified live vaccine (MLV). (A) Percent morbidity incidence; (B) percent morbidity incidence and duration; (C) clinical severity index. See text for definitions of clinical indices.
4. Discussion
Previously, we demonstrated expression of TGEV-S and Lt-B antigens at high levels in corn, and showed that these proteins delivered in the seed elicit protective responses [11]. Here we report data further supporting maize corn seed, and fractions thereof, as a system for delivery of potent, effective recombinant subunit vaccines that are stable, easy to deliver, and inexpensive. Two different vaccines, derived from either whole seed or a fraction thereof, were shown to be effective at eliciting appropriate immune responses.
TGEV whole corn meal was effective at protecting piglets from a subsequent virus challenge. Our results indicate that administration of TGEV corn vaccine leads to establishment of immune memory that can be recalled as neutralizing antibodies in the serum (Fig. 4). This serum response may play a role in protection of the piglets from virus, but it is also possible that TGEV corn induces a mucosal response similar to that observed for the Lt vaccine which may also contribute to viral protection. Interestingly, a 4-day dose of the TGEV corn was shown to be more effective at preventing disease symptoms than administration of TGEV corn for either 8 or 16 days. This shows that efficacy of this oral vaccine does not require long, continuous administration of antigen and supports the case for ease of administration. In fact, in this study, extended exposure of antigen by the oral route may have limited effectiveness. However, there is no evidence that extended administration of TGEV corn had a toleragenic effect on the piglets since even in the 16-day TGEV corn group, piglets still recovered quicker, and had fewer clinical symptoms, than piglets fed control corn (Fig. 5B and C). Further, piglets fed TGEV corn for 14 days still responded with generation of significant levels of neutralizing anti-TGEV antibody when subsequently exposed to virus (Fig. 4).
Lt-B corn germ meal was also shown to be an effective source of immunogen when administered to mice, based on induction of serum anti-Lt-B IgG and fecal anti-Lt-B IgA. This is consistent with the induction of serum and mucosal immune responses observed previously using Lt whole grain [11]. Measurable serum and mucosal anti-Lt-B observed with a 0.33 μg dose of Lt-B contained in 0.7 mg corn germ meal demonstrates the utility of fractionation to enhance vaccine potency. To our knowledge, this is the lowest level of Lt-B expressed in plant material ever reported to show a measurable anti-Lt-B response. This effectiveness at eliciting a response to Lt-B is likely due to the natural bioencapsulation of plant-expressed antigens enhancing antigen survival in the gut [7], [9], [13], [20], [32]. Furthermore, it is likely that the demonstrated serum and mucosal immunogenic responses to defatted Lt-B corn germ reported here could translate to protection of mice from a subsequent challenge with Lt holotoxin as was observed previously with the Lt whole corn vaccine [11].
The observation that antigens can survive standard grain processing and be enriched in particular fractions provides a significant degree of flexibility to vaccine formulation using transgenic corn seed without adding appreciable cost to the final product. The defatted germ fraction of Lt-B corn is highly enriched for Lt-B protein, eight-fold over whole grain. This corresponds to a significant reduction in volume of material necessary to achieve the same dose of vaccine. With an antigen level of 500 μg per gram of germ, an enormous dose of subunit vaccine could easily be incorporated into animal feed, or pelletized separately to deliver an antigen in convenient compact form, thus maximizing vaccine potency. Therefore, strategies for delivery of oral vaccines using maize seed can use existing processing/fractionation options producing a number of seed fractions that can serve as sources for antigen delivery enhancing flexibility to accommodate each subunit vaccine (Fig. 6 ). Here we demonstrated the effectiveness of antigen delivery in coarsely ground whole seed (TGEV vaccine) and in a germ fraction (Lt-B vaccine), but it is conceivable that grit or other fractions could also serve as sources for antigens that preferentially accumulate in the endosperm or pericarp, respectively. Furthermore, the demonstration that antigens present in either whole grain or in the germ fraction are stable without refrigeration illustrates that vaccines produced in this fashion are amenable to low-cost transport and storage conditions.
Fig. 6.
Processing of corn seed for vaccine production.
Although the maize system has shown quite promising as a vaccine candidate that could protect against strains of ETEC which harbor the Lt holotoxin, only approximately 66% of ETEC strains harbor Lt, and in only half of these strains Lt is the only toxin present [24]. Therefore an effective anti-diarrheal vaccine suitable for broad protection of a population would likely require targeting multiple pathogens. The maize expression system is flexible enough to address such a concern. Multivalent vaccines could be designed by using transgenic lines in which multiple antigens have been co-expressed within the same seed, or formulated using corn materials from separate lines blended to include multiple antigens.
This study further illustrates the potential of corn-based edible vaccines for veterinary applications. Here we show effective protection of a common animal species, raised for human consumption. It is likely that this approach will find broad application in a number of economically significant animal species. We have preliminary data where chickens were fed Lt-B corn meal and responded with induction of anti-Lt-B antibodies (unpublished results) indicating that this technology may have application in avian systems as well. Of course this technology need not be limited to veterinary vaccine delivery. Recent human vaccine clinical trials have demonstrated that plant-based delivery systems have the potential to impact human health [33], [34]. Plants systems are also potentially amenable to delivery of other proteinacious biopharmaceuticals such as antibodies, growth factors, and other medically relevant agents. The strength of the maize system for production of large quantities of stable, potent, and efficacious recombinant subunit vaccines that can be administered easily without the need for specialized equipment will likely impact both animal and human health care.
5. Conclusion
We demonstrate that seed from transgenic corn is an effective system for oral delivery of potent, stable, and inexpensive recombinant subunit vaccines. The spike protein of TGEV and the Lt-B subunit of heat labile enterotoxin were expressed in corn seed to serve as sources of antigen for candidate vaccines to protect against two pathogenic agents, ETEC and TGEV. Lt-B antigen enriched in the germ fraction of corn seed by standard grain milling processes was shown to be potent for eliciting both serum and mucosal immune responses when fed to mice, and remained stable for at least a year when stored at either 4 or 23 °C. TGEV-S antigen in whole corn seed was shown to be stable for at least 10 months at 4 °C, 10 °C, or at ambient grain storage conditions in Iowa, and effective when fed to piglets at eliciting neutralizing anti-TGEV antibodies in the serum and protecting against a subsequent viral challenge.
Acknowledgements
The feeding trial to measure protection of piglets after a challenge with TGE virus was conducted at Ames (IA, USA) by Mark Welter (Oragen Technologies) and David Carter, DVM (Veterinary Resources). Grain fractionation was conducted at the Food Protein Research and Development Center at Texas A&M University, College Station, TX, USA. We also thank Kathy Beifuss, Jocelyne Mayor and Liz Wilfong for assistance with supportive unpublished results, as well as Michele Bailey for helpful discussion and critical review of the manuscript.
References
- 1.Van Kampen K.R. Recombinant vaccine technology in veterinary medicine. Vet. Clin. North Am. Small Anim. Pract. 2001;31:535–538. doi: 10.1016/s0195-5616(01)50607-5. [DOI] [PubMed] [Google Scholar]
- 2.Davenport L.W. Regulatory considerations in vaccine design. Pharm. Biotechnol. 1995;6:81–96. doi: 10.1007/978-1-4615-1823-5_4. [DOI] [PubMed] [Google Scholar]
- 3.Melnick J.L. Virus vaccines: principles and prospects. Bull. World Health Organ. 1989;67:105–112. [PMC free article] [PubMed] [Google Scholar]
- 4.Melnick J.L. Viral vaccines: achievements and challenges. Acta Virol. 1989;33:482–493. [PubMed] [Google Scholar]
- 5.Del Giudice G., Pizza M., Rappuoli R. Molecular basis of vaccination. Mol. Aspects Med. 1998;19:1–70. doi: 10.1016/s0098-2997(98)00002-8. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence D.N., Goldenthal K.L., Boslego J.W., Chandler D.K., La Montagne J.R. Public health implications of emerging vaccine technologies. Pharm. Biotechnol. 1995;6:43–60. doi: 10.1007/978-1-4615-1823-5_2. [DOI] [PubMed] [Google Scholar]
- 7.Daniell H., Streatfield S.J., Wycoff K. Medical molecular farming: production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci. 2001;6:219–226. doi: 10.1016/S1360-1385(01)01922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cramer C.L., Boothe J.G., Oishi K.K. Transgenic plants for therapeutic proteins: linking upstream and downstream strategies. Curr. Top. Microbiol. Immunol. 1999;240:95–118. doi: 10.1007/978-3-642-60234-4_5. [DOI] [PubMed] [Google Scholar]
- 9.Giddings G., Allison G., Brooks D., Carter A. Transgenic plants as factories for biopharmaceuticals. Nature Biotechnol. 2000;18:1151–1155. doi: 10.1038/81132. [DOI] [PubMed] [Google Scholar]
- 10.Mason H.S., Arntzen C.J. Transgenic plants as vaccine production systems. Trends Biotechnol. 1995;13:388–392. doi: 10.1016/S0167-7799(00)88986-6. [DOI] [PubMed] [Google Scholar]
- 11.Streatfield S.J., Jilka J.M., Hood E.E., Turner D.D., Bailey M.R., Mayor J.M., Woodard S.L., Beifuss K.K., Horn M.E., Delaney D.E., Tizard I.R., Howard J.A. Plant-based vaccines: unique advantages. Vaccine. 2001;19:2742–2748. doi: 10.1016/S0264-410X(00)00512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason H.S., Haq T.A., Clements J.D., Arntzen C.J. Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): potatoes expressing a synthetic LT-B gene. Vaccine. 1998;16:1336–1343. doi: 10.1016/s0264-410x(98)80020-0. [DOI] [PubMed] [Google Scholar]
- 13.Modelska A., Dietzschold B., Sleysh N., Fu Z.F., Steplewski K., Hooper D.C., Koprowski H., Yusibov V. Immunization against rabies with plant-derived antigen. Proc. Natl. Acad. Sci. USA. 1998;95:2481–2485. doi: 10.1073/pnas.95.5.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood E.E., Jilka J.M. Plant-based production of xenogenic proteins. Curr. Opin. Biotechnol. 1999;10:382–386. doi: 10.1016/S0958-1669(99)80069-X. [DOI] [PubMed] [Google Scholar]
- 15.Hood E.E., Witcher D.R., Maddock S., Meyer T., Baszczynski C., Bailey M.R., Flynn P., Register J., Marshall L., Bond D., Kulisek E., Kusnadi A., Evangelista R., Nikolov Z., Wooge C., Mehigh R.J., Hernan R., Kappel W.K., Ritland D., Li C.-P., Howard J.A. Commercial production of avidin from transgenic maize: Characterization of transformant, production, processing, extraction and purification. Molec. Breed. 1997;3:291–306. [Google Scholar]
- 16.Witcher D.R., Hood E.E., Perterson D., Bailey M., Bond D., Kusnadi A., Evangelista R., Nikolov Z., Wooge C., Mehigh R., Kappel W., Register J., Howard J.A. Commercial production of β-glucosidase (GUS): a model system for the production of proteins in plants. Molec. Breed. 1998;4:301–312. [Google Scholar]
- 17.Hood E.E., Kusnadi A., Nikolov Z., Howard J.A. Molecular farming of industrial proteins from transgenic maize. Adv. Exp. Med. Biol. 1999;464:127–147. doi: 10.1007/978-1-4615-4729-7_11. [DOI] [PubMed] [Google Scholar]
- 18.Streatfield S.J., Mayor J.M., Barker D.K., Brooks C., Lamphear B.J., Woodard S.L., Beifuss K.K., Vicuna D.V., Massey L.A., Horn M.E., Delaney D.E., Nikolov Z.L., Hood E.E., Jilka J.M., Howard J.A. Development of an edible subunit vaccine in corn against enterotoxigenic strains of Escherichia coli. In Vitro Dev. Biol.-Plant. 2002;38:11–17. [Google Scholar]
- 19.Haq T.A., Mason H.S., Clements J.D., Arntzen C.J. Oral immunization with a recombinant bacterial antigen produced in transgenic plants. Science. 1995;268:714–716. doi: 10.1126/science.7732379. [DOI] [PubMed] [Google Scholar]
- 20.M.R. Bailey. A model system for edible vaccination using recombinant avidin produced in corn seed. Master of Science thesis. Texas A&M University, 2000.
- 21.Black R.E. The epidemiology of cholera and enterotoxigenic E. coli diarrheal disease. In: Holmgren J., Lindberg A., Mollby R., editors. Development of Vaccines and Drugs Against Diarrhea; 11th Nobel Conference, Stockholm; 1986. pp. 23–32. [Google Scholar]
- 22.Black R.E. Epidemiology of travelers’ diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 1990;12(Suppl. 1):S73–S79. doi: 10.1093/clinids/12.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- 23.Sixma T.K., Pronk S.E., Kalk K.H., Wartna E.S., van Zanten B.A., Witholt B., Hol W.G. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991;351:371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- 24.Svennerholm A.-M., Holmgren J. Oral B-subunit whole-cell vaccines against cholera and enterotoxigenic Escherichia coli diarrhea. In: Ala’Aldeen D.A.A., Hormaeche C.E., editors. Molecular and Chemical Aspects of Bacterial Vaccine Development. Wiley; Chichester: 1995. pp. 205–232. [Google Scholar]
- 25.Nagy B., Fekete P.Z. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet. Res. 1999;30:259–284. [PubMed] [Google Scholar]
- 26.Laude H., Rasschaert D., Delmas B., Godet M., Gelfi J., Charley B. Molecular biology of transmissible gastroenteritis virus. Vet. Microbiol. 1990;23:147–154. doi: 10.1016/0378-1135(90)90144-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saif L.J., van Cott J.L., Brim T.A. Immunity to transmissible gastroenteritis virus and porcine respiratory coronavirus infections in swine. Vet. Immunol. Immunopathol. 1994;43:89–97. doi: 10.1016/0165-2427(94)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez N., Carrillo C., Salinas J., Parra F., Borca M.V., Escribano J.M. Expression of immunogenic glycoprotein S polypeptides from transmissible gastroenteritis coronavirus in transgenic plants. Virology. 1998;249:352–358. doi: 10.1006/viro.1998.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez N., Wigdorovitz A., Castanon S., Gil F., Ordas R., Borca M.V., Escribano J.M. Oral immunogenicity of the plant derived spike protein from swine-transmissible gastroenteritis coronavirus. Arch. Virol. 2000;145:1725–1732. doi: 10.1007/s007050070087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuboly T., Yu W., Bailey A., Degrandis S., Du S., Erickson L., Nagy E. Immunogenicity of porcine transmissible gastroenteritis virus spike protein expressed in plants. Vaccine. 2000;18:2023–2028. doi: 10.1016/s0264-410x(99)00525-3. [DOI] [PubMed] [Google Scholar]
- 31.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Kong Q., Richter L., Yang Y.F., Arntzen C.J., Mason H.S., Thanavala Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc. Natl. Acad. Sci. USA. 2001;98:11539–11544. doi: 10.1073/pnas.191617598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tacket C.O., Mason H.S., Losonsky G., Clements J.D., Levine M.M., Arntzen C.J. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat. Med. 1998;4:607–609. doi: 10.1038/nm0598-607. [DOI] [PubMed] [Google Scholar]
- 34.Tacket C.O., Mason H.S., Losonsky G., Estes M.K., Levine M.M., Arntzen C.J. Human immune responses to a novel Norwalk virus vaccine delivered in transgenic potatoes. J. Infect. Dis. 2000;182:302–305. doi: 10.1086/315653. [DOI] [PubMed] [Google Scholar]


