Abstract
Hypoxic-ischemic (HI) brain injury has a high occurrence rate of 1–4 per 1000 live births and is the leading cause of neurological disabilities. Despite the improvement in neonatal care, the effectiveness of current therapeutic strategies is limited, and thus, additional therapies with better results are of much needed. Pterostilbene is a stilbenoid possessing numerous preventive and therapeutic properties. The current study aimed to assess whether pterostilbene exerted protective effects in neonatal rats against experimentally induced ischemic brain injury. Pterostilbene was administered via oral gavage from postnatal day 3 to day 8. Rat pups that were seven-day-old were exposed to hypoxic-ischemic insult via ligation of the common carotid artery and hypoxic environment exposure. Pterostilbene treatment reduced neuronal loss and infarct volume. Pterostilbene administration regulated the NF-κB pathway, and the levels of inflammatory mediators (Nitric oxide, TNF-α, IL-1β, and IL-6) were reduced. HI-induced oxidative stress was significantly reduced by pterostilbene, as presented by decreased production of malondialdehyde and reactive oxygen species. Levels of glutathione were enhanced by pterostilbene. Pterostilbene regulated Nrf2/HO-1 and JNK expression and activated the PI3K/Akt-mTOR signals. These findings suggest that pterostilbene is a candidate compound for the treatment of neonatal HI.
Keywords: Heme oxygenase-1, Ischemic brain injury, Mammalian target of rapamycin, Nuclear factor erythroid-2-related factor 2, Pterostilbene
Introduction
Perinatal hypoxia–ischemia (HI), synonymous with hypoxic-ischemic encephalopathy (HIE), is one of the major causes of perinatal cerebral injury leading to death and neurologic sequelae such as cerebral palsy, epilepsy, visual and hearing impairments, motor disabilities, and learning deficits (Grow and Barks 2002; Ferriero 2004). HI occurs in 1–4 per 1000 live births (Azzopardi 2014; Rocha-Ferreira and Hristova 2016) and is caused by partial or complete anoxia and decreased cerebral blood flow as a result of perinatal asphyxia (Shankaran et al. 2014). Various mechanisms, including excitotoxicity, cellular apoptosis, metabolic acidosis, inflammatory, and immune responses, have been reported to be associated with HI-induced cerebral injury (Martin et al. 1998; Saito et al. 2005; Zhang et al. 2006; Wang et al. 2007).
Mitochondrial dysfunction, Ca2+ overload, and inflammatory processes lead to the production of raised production of reactive oxygen species/reactive nitrogen species (ROS/RNS) that contribute to oxidative stress, which consequently leads to ischemic cell death (Coyle and Puttfarcken 1993; Lewen et al. 2000). Nuclear factor erythroid 2-related factor 2(Nrf2) is the prime factor of transcription, which involves the regulation of an extensive set of enzymes involved in antioxidant defense and detoxification (Ishii et al. 2000; Shih et al. 2003). Enzymes, NAD(P)H quinone oxidoreductase, heme oxygenase-1 (HO-1), and glutathione S-transferases (GSTs), regulated by Nrf2 constitute chief cellular defense mechanisms that work against ROS/RNS and also detoxify electrophiles and xenobiotics (Lee et al. 2003; Satoh et al. 2006). HO-1 is a redox-sensitive and stress-induced enzyme that converts heme to biliverdin (Motterlini et al. 2002). The Nrf2 pathway also regulates inflammatory responses and is associated with the process involved in relief from calcium overload (Wu et al. 2015).
PI3K-Akt-mTOR/JNK is one of the major signaling pathways that regulate several vital processes, such as cell survival, cell proliferation, and apoptosis (Nijboer et al. 2010; Liu et al. 2017). The pathway is known to be implicated in the pathogenesis of HI brain injury (Endo et al. 2006; Xu et al. 2015). PI3K/Akt signaling was reported to be involved in the protection against cerebral injury (Lu et al., 2011). Akt regulates—JNK, a mitogen-activated protein kinase that is associated with cell survival, apoptosis, and inflammatory responses (Zhao et al. 2006; Kamada et al. 2007). Activation of Akt signaling following transient cerebral ischemia has been reported to help the existence of neurons and inhibit neuronal cell loss (Noshita et al. 2001). The mammalian target of rapamycin (mTOR), a crucial downstream target of Akt, is implicated in promoting neuronal cell survival and axon regeneration (Park et al. 2008; Sun et al. 2011).
Accumulating evidence suggests that cerebral inflammation, characterized by microglial activation, leukocyte infiltration, and raised levels of inflammatory mediators such as cytokines (Barone and Feuerstein 1999; del Zoppo et al. 2000), substantially contribute to HI brain injury (Benjelloun et al. 1999). Nuclear factor-kappa beta (NF-κB) is well documented in the pathology of several conditions, traumatic and ischemic brain injury (Williams et al. 2006). NF-κB is a major transcription factor associated with gene expression that is involved in inflammatory responses (Gao et al. 2009), including interleukins (ILs)—IL-1α and IL-1β, tumor necrosis factor α (TNF-α), cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) (Saliba and Henrot 2001; Karin et al. 2002). These inflammatory factors are considered the chief contributors to ischemic brain injury (Williams et al. 2006; Barakat et al. 2014). Thus, strategies targeting multiple molecular pathways are extensively useful for reducing HI-induced neuronal damage.
Recent studies are much focussed on the protective effects of natural compounds derived from plants in brain injury (Arteaga et al. 2015; Lv et al. 2015). A number of antioxidant compounds such as curcumin, thioperamide, and ropinirole have been reported to potentially protect neurons from ROS-induced cell damage in animal models of HI injury (Iida et al. 1999; Badary et al. 2003; Jayaprakasha et al. 2006; Akhtar et al. 2008).
Pterostilbene (trans-3,5-dimethoxy-4-hydroxystilbene) is a stilbenoid detected mainly in blueberries and heartwood of Pterocarpus marsupium, and its structure is similar to that of resveratrol (Roupe et al. 2006; Lin et al. 2009). Pterostilbene possesses multiple health benefits as an antioxidant, lowers blood sugar levels, and exerts numerous bioactive effects like anti-inflammatory, cardioprotective, and anticarcinogenic properties (Satheesh and Pari 2006; Remsberg et al. 2008; Chakraborty et al. 2010; McCormack et al. 2012). Pterostilbene is more lipophilic than resveratrol due to the presence of two methoxy groups and thus exhibits higher bioavailability (80%) than resveratrol (20%) (McCormack and McFadden 2013). The aim of this study was to explore the effects of pterostilbene in perinatal HI-induced brain injury rodent model.
Materials and methods
Ethics approval
The Putian University Animal Ethics Committee approved (Ethical approval Number: TXY/20160703) all the study design and protocols of this study. The protocols were also carried out by the guidelines for the care and use of laboratory animals of the National Institutes of Health (NIH) (NIH publication no. 85-23, revised 1996) (Garber 2011).
Study design and hypoxia–ischemia induction
Timed-pregnant female rats (Sprague–Dawley) were procured from the Animal Laboratory of Shandong University (Jinan, China). The animals were held under controlled temperature at 22–23 ℃ with a 12:12-h light/dark cycle and 55–60% relative humidity. Sprague–Dawley rats were provided with unrestricted access to water and pelleted rat chow. The rats were monitored carefully for the time of delivery. Healthy male pups (n = 72) at postnatal day three were used for the study.
Hypoxia–ischemia was induced in rats on postnatal day 7, as mentioned by Rice et al. (1981) with minor alterations. The P7 pups (weighing 52 ± 1 g) were exposed to isoflurane (3.5%) anesthesia (Sigma-Aldrich, St.Louis, MO, USA) in oxygen (1.5% isoflurane for maintenance), and the left common carotid artery (CCA) was isolated and ligated using 6–0 surgical silk. To ensure that the blood flow through the ipsilateral carotid circulation was cut off through the total period of study, the CCA was transfected between the ligatures. The surgery site was sutured, and the rat pups were permitted to recover from anesthesia for 2 h and were placed at 36 °C in a humidified chamber. HI was induced by perfusion of 8% oxygen in nitrogen at 5 L/min for 135 min. The study animals were sent to their dams following hypoxic exposure. Pterostilbene (Sigma-Aldrich, St. Louis, MO, USA) in saline and was doled via oral gavage at a dose of 12.5, 25, or 50 mg/ kg b.wt starting P3 to P8 days. On the day of HI insult, pterostilbene was administered 1 h prior to insult. Control rats were not subjected to insult or given pterostilbene. The HI-control group was subjected to HI insult but not administered pterostilbene. A separate group of rat pups were given pterostilbene at 50 mg/kg dose for P3 to P8 days but were not subjected to HI.
The rat pups were sacrificed at 24 h post-HI induction by cervical decapitation under isoflurane anesthesia. Brains were removed immediately after sacrifice and used for analysis.
Tissue preparation for histological analysis
The excised tissues of the brain were post-fixed in paraformaldehyde and embedded in paraffin after dehydration. Tissue sections (5 µm thickness, sliced coronally) were hematoxylin and eosin (HE) stained and observed using a confocal microscope (magnification, 20 ×; Zeiss, LSM510; Zeiss AG, Oberkochen, Germany).
Brain water content detection
Immediately after excision, the brain was weighed and noted as wet weighed. The brain was then kept at 105 °C for 24 h in an oven, and the dry weight was measured (Chen et al. 2011). The brain water percentage was determined using the formula [(wet weight − dry weight)/wet weight] × 100%.
TTC staining
2,3,5-triphenyltetrazoliumchloride (TTC) (Sigma-Aldrich, St.Louis, MO, USA) staining was done to measure the infarction volume. The excised brains frozen at − 20 °C for 15–20 min were sliced into 2 mm thick sections. The sections were incubated for 30 min (37 °C) with TTC and were immersed overnight in 4% paraformaldehyde. Normal regions in the brain stained deep red with TTC, while the infarcted tissues remained unstained. The infarct area was detected using NIH Image J software (Version 1.61; National Institutes of Health, Bethesda, MD). The intensity of staining was measured in the right hemisphere (ipsilateral side) and at the contralateral side on the left hemisphere. The magnitude of tissue loss was calculated using the formula ([C − I]/C) × 100, where C = mean of the contralateral area; I = mean value of the ipsilateral area. The results were expressed as percentage infarction/ipsilateral hemisphere.
TUNEL analysis
Terminal transferase-mediated dUTP nick end-labeling (TUNEL) staining was done to measure the extent of cellular apoptosis following HI injury. The TUNEL assay kit (DeadEnd TM fluorometric TUNEL system kit) from Promega (Madison, WI, USA) was used according to the directions specified by the manufacturer. Positive TUNEL cells in the brain tissue sections were observed and examined using NIS-Elements BR imaging processing and analysis software (Nikon Corporation, Japan).
Determination of ROS, lipid peroxidation, and glutathione levels
Brain tissues were homogenized in ice-cold PBS and subjected to centrifugation (3000 rpm; 15 min). The supernatant collected was used for the assay of ROS, lipid peroxidation, and glutathione levels. In the supernatant, the total protein content was detected by BCA method with protein assay kit from BioRad (Hercules, CA, USA). Malondialdehyde (MDA) and glutathione (GSH) contents in the brain tissues were detected using kits from Sigma-Aldrich, by following instructions given by the manufacturer.
The OxiSelect™ ROS/RNS assay kit (Cell Bio Labs Inc.) was used to determine ROS levels. A fluorogenic probe dichlorodihydrofluorescein DiOxyQ (DCFH-DiOxyQ), which is precise to free radicals—ROS/RNS, was used. DCFH-DiOxyQ is converted to DCFH, which is extremely reactive and which reacts with RNS and ROS in the sample and reacts to fluorescent DCF. The intensity of fluorescence reflects the amount of ROS/RNS in the sample. Using a Synergy™ 2 Multi-function Microplate Reader, the fluorescence was measured (480 nm excitation and 530 nm emission).
Determination of levels of cytokines
Serum was separated from whole blood samples and used for analysis. The TNF-α, IL-1β, and IL-6 serum concentrations were determined using ELISA kits according to the kit protocol (Biolegend).
Determination of serum nitric oxide levels
Levels of serum nitric oxide (NO) were determined using a NO assay kit (Abcam). Accumulation of nitrite reflecting NO levels was determined based on the reaction involving enzyme nitrate reductase, which converts nitrate to nitrite. Griess reagent (1% sulfanilamide, 2.5% phosphoric acid and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride) converts the nitrite formed to a deep purple azo compound. The amount of the chromophore formed precisely indicates the levels of nitric oxide. The absorbance of the purple compound was read at 540 nm in a 96-well microplate reader (Spectra MAX 340PC, Molecular Devices). The amount of NO in the samples was calculated using standard sodium nitrite at 0–150 µM concentration.
Real-time PCR (RT-PCR)
A complete set of RNA from samples of the brain tissue (cortical) was isolated according to instructions specified by the manufacturer using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Isolated RNA 5 μg was used for the synthesis of the first strand of cDNA employing random primers using the Superscript First-Strand Synthesis System for RT-PCR from Invitrogen (Carlsbad, CA, USA). PCR was executed using SYBR Green PCR Master Mix from Applied Biosystems (Foster City, CA, USA). The following primers were used for amplification as follows:
IL-1β forward-CACCTCTCAAGCAGAGCACAG, reverse-GGGTTCCATGGTGAAGTCAAC; IL-6 forward-TCCTACCCCAACTTCCAATGCTC, reverse- and TTGGATGGTCTTGGTCCTTAGCC; iNOS forwardGTGCTAATGCGGAAGGTCATG reverse-GCTTCCGACTTTCCTGTCTCAGTA; TNF-α forward-AAATGGGCTCCCTCTCATCAGTTC, reverse-TCTGCTTGGTGGTTTGCTACGAC; GAPDH forward-CCAGCCTCGTCTCATAGACA, reverse-GTAACCAGGCGTCCGATACG, respectively.
GAPDH has been used as an internal control to evaluate test gene expression.
Western blotting
In ice-cold RIPA cell-lysis buffer (Santa Cruz Biotechnology, Inc., TX, USA), brain tissues were homogenized, and whole-cell lysates were centrifuged at 14,000×g for 30 min at 4 °C. Also, for determination of NF-κB (p65) expression in the nuclear and cytosol fractions, the homogenate of equal volumes from the different groups was separated into nuclear fractions using NE-PER nuclear and cytoplasmic extraction reagent kit (Pierce Biotechnology, Rockford, IL, USA). The total protein content in the supernatant and in nuclear and cytosol fractions was determined (BCA protein assay kit, Thermo Fischer Scientific). Equal amounts (30 µg) of protein samples from different experimental groups (for NF-κB (p65) from both the fractions/ group; Nrf2 in the nuclear fraction and HO-1 in the cytosolic fraction) were separated electrophoretically on SDS-PAGE (8–12%). The protein bands were blot transferred onto a nitrocellulose membrane (0.2 μm, Sigma-Aldrich, St. Louis, MO, USA) after separation. The membranes were blocked for any endogenous peroxidase activity with 5% non-fat blocking grade milk (Bio-Rad, Hercules, CA, USA) following which the membranes were incubated overnight at 4 °C with primary antibodies against—Nrf2, HO-1, TNF-α, NF-κB p65, IκBα, IKKβ, IKKα, p-IκBα, p-IKKβ, p-IKKα, β-actin (1:1000, Santa Cruz Biotechnology, USA), JNK, c-JUN, p-JNK, p-cJUN, mTOR, p-mTOR, Akt and p-AKT (1:1000, Cell Signaling Technology, USA). The membranes were washed well with TBST and then incubated for 1 h at room temperature with secondary antibodies combined with HRP (1:2000, Santa Cruz Biotechnology, USA). Positive bands were then visualized and analyzed by chemiluminescence method (Millipore, USA) and using a ChemiDoc XRS imaging system (Bio-Rad, USA). Test protein’s expression was standardized with that of β-actin expression, which was used as an internal control.
Statistical study
The results of the analysis were statistically analyzed using SPSS software (version 21.0) (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) and Duncan’s Multiple Range Test (DMRT) was performed for comparing data from multiple groups. Values were identified as statistically significant at P < 0.05.
Results
Pterostilbene improved the histology and reduced neuronal cell loss in the brain tissues of pups subjected to HI
The brain tissues of the HI-induced animals were assessed for histological changes. HE staining of the brain sections revealed marked neuronal degeneration with larger areas of neuronal loss (Fig. 1). The neurons of the cerebral cortex were shrunken with pyknotic nuclei. Neuronal cell density was markedly reduced.
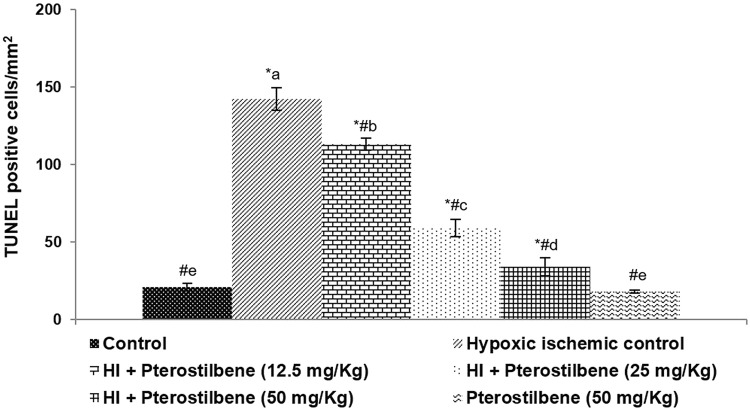
Fig. 1.
Pterostilbene decreased neuronal apoptosis. Values are represented as mean ± SD, n = 6; P < 0.05 as determined by one-way ANOVA followed by DMRT analysis. *P < 0.05 vs. control; #P < 0.05 vs. HI control; a–e mean values from different experimental groups that differ from each other at P < 0.05
Further, observations of the TUNEL assay presented a significant (P < 0.05) increase in TUNEL positive cell counts indicating raised neuronal loss following HI. Pterostilbene administration considerably improved the architecture of damaged brain tissues and decreased TUNEL positive cells dose-dependently. A 50 mg dose of pterostilbene-treated HI-induced animals presented brain tissues with near-normal architecture. Also, pterostilbene alone (50 mg) did not cause any changes in the tissue morphology and was more comparable to the healthy control animals.
Pterostilbene significantly reduced brain edema
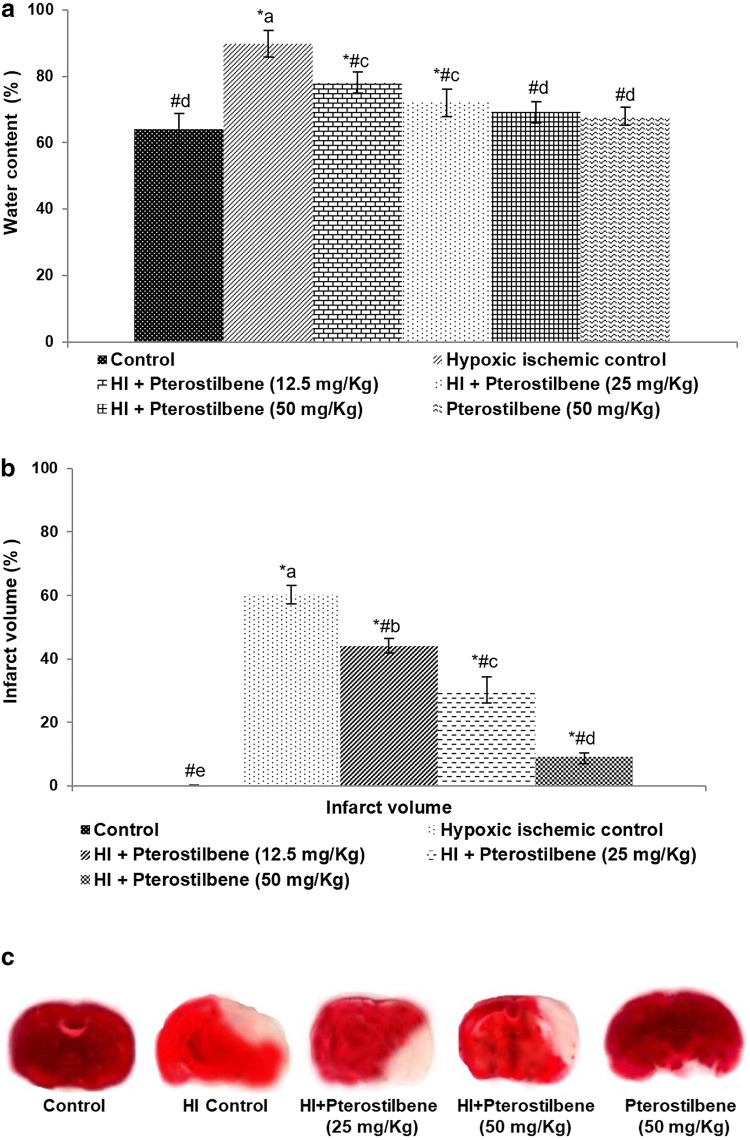
Brain edema was assessed following HI induction (Fig. 2a). HI-induced animals presented with increased brain water content as compared to healthy control pups. The brain water content in the HI control animals was 89.80 ± 4.02%. Pterostilbene caused a significant reduction in the water content in a dose-dependent manner. 50 mg pterostilbene treated HI-induced rats presented with 69.20 ± 3.10% water content, indicating an effective reduction in brain edema.
Fig. 2.
Pterostilbene reduced brain edema and infarct volume following HI injury a Brain water content, b infarct volume and c TTC staining of the infract. Values are represented as mean ± SD, n = 6; P < 0.05 as determined by one-way ANOVA followed by DMRT analysis. *P < 0.05 vs. control; #P < 0.05 vs. HI control; a–e mean values from different experimental groups that differ from each other at P < 0.05
Pterostilbene reduced brain infarction
The brain tissues were stained with TTC stain to assess the magnitude of infarction after HI. These observations indicated severe brain infarction in HI-induced animals (Fig. 2b, c). HI resulted in significantly (P < 0.05) increased the volume of infarction (60.2 ± 2.92%). Administration of pterostilbene at 12.5, 25.0 and 50.0 mg/kg to the pups brought a significant (P < 0.05) decrease in infarct volume (44.10 ± 2.23%, 30.25 ± 4.10% and 9.16 ± 1.08%, respectively) vs. HI control animals.
Pterostilbene decreased ROS levels following HI
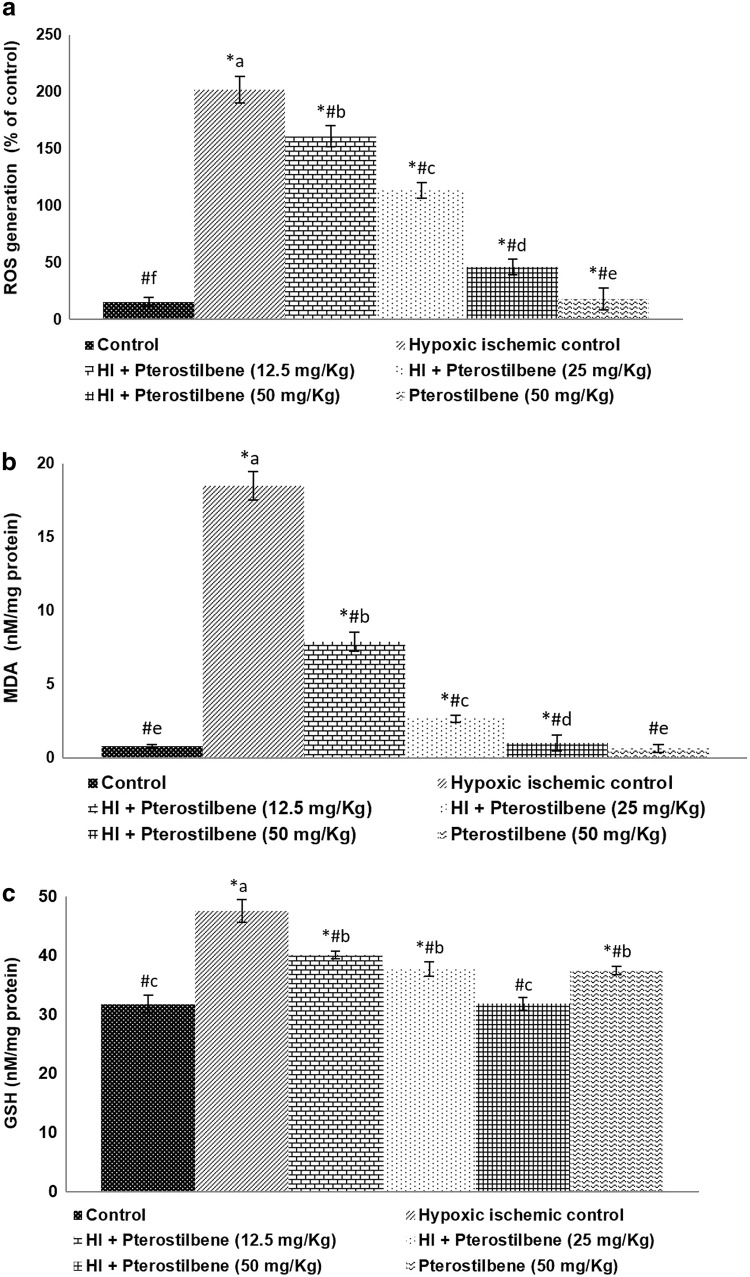
Oxidative stress is well documented in HI brain injury. The results of the study showed significant (P < 0.05) increase in ROS production at 24 h following HI (Fig. 3a). ROS generation increased to 206.10 ± 11.5% in HI control animals vs. 15.18 ± 3.76% normal control. However, ROS generation decreased to 160.91 ± 9.25%, 113.20 ± 7.10% and 45.81 ± 6.75% after treatment with pterostilbene at 12.5, 50, and 50 mg, illustrating the antioxidant potential of pterostilbene. Furthermore, the levels of ROS in the pterostilbene alone treated group were noticeably lower than the normal control group. Along with ROS levels, MDA content was detected to be significant (P < 0.05) in the HI control group compared to the normal control (Fig. 3b). Furthermore, elevated GSH levels (47.5 ± 1.95 nM/mg protein) seen in HI-induced pups vs. 31.76 ± 1.50 nM/mg protein in normal control (Fig. 3c) could be a defense measure to neutralize the overproduction of ROS. Pterostilbene treatment caused a significant (P < 0.05) decrease in MDA in a dose-dependent manner. Pterostilbene (50 mg) exerted the highest protective effects at 12.5 and 25 mg doses. MDA content reduced from 18.5 ± 0.96 nM/mg protein to 1.01 ± 0.54 nM/mg protein on the administration of 50 mg pterostilbene. GSH content also noticed to be raised strikingly in pterostilbene supplementation at all three doses. Furthermore, administration of pterostilbene alone at 50 mg caused a noticeable increase in GSH levels vs. the normal control. Pterostilbene is reported to possess potent antioxidant capacity more efficiently than resveratrol (Tsai et al. 2017). Thus, the antioxidant properties of pterostilbene could have caused the improvement in the antioxidant status by reducing MDA and ROS levels.
Fig. 3.
Pterostilbene reduced oxidative stress following HI injury. Pterostilbene reduced a ROS generation, b MDA levels and c regulated GSH levels. Values are represented as mean ± SD, n = 6; P < 0.05 as determined by one-way ANOVA followed by DMRT analysis. *P < 0.05 vs. control; #P < 0.05 vs. HI control; a–e represents mean values from different experimental groups that differ from each other at P < 0.05
Pterostilbene promoted the expression of Nrf2 and HO-1
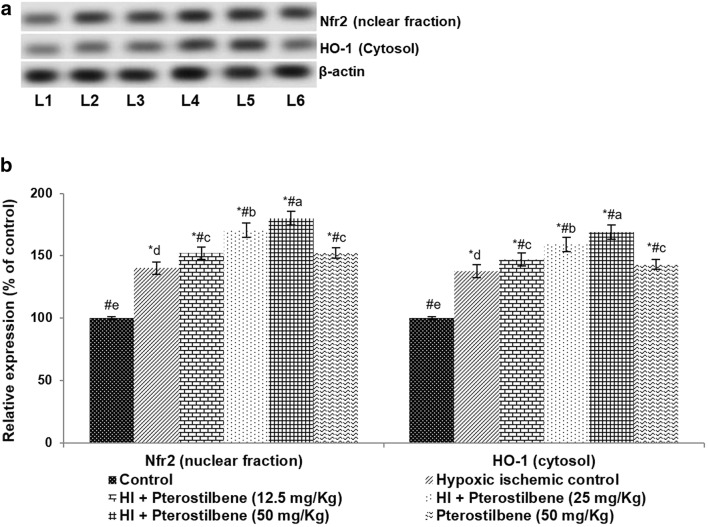
After 24 h of HI induction, Nrf2 and HO-1 expression were evaluated using western blot analysis. The observed data indicated that systemic administration of pterostilbene caused a substantial (P < 0.05) upregulation of Nrf2 and HO-1 expression (Fig. 4a, b). The expression of Nrf2 increased to 140 ± 5.12% vs. the normal control. HO-1 expression increased to 168.92% upon treatment with 50 mg of pterostilbene vs. 137.7% in HI control pups. The enhanced nuclear expression of Nrf2 along with elevated HO-1 in the cytosol suggests that pterostilbene up-regulated the Nrf2 signaling pathway.
Fig. 4.
Pterostilbene regulates the antioxidant regulators. a Representative immunoblot, b relative protein expressions. Values are represented as mean ± SD, n = 6; P < 0.05 as determined by one-way ANOVA followed by DMRT analysis. *P < 0.05 vs. control; #P < 0.05 vs. HI control; a–e represents mean values from different experimental groups that differ from each other at P < 0.05. L1-Control; L2-Hypoxic Ischemic Control; L3-HI + Pterostilbene (12.5 mg/kg); L4-HI + Pterostilbene (25 mg/kg); L5-HI + Pterostilbene (50 mg/kg); L6-Pterostilbene (50 mg/kg)
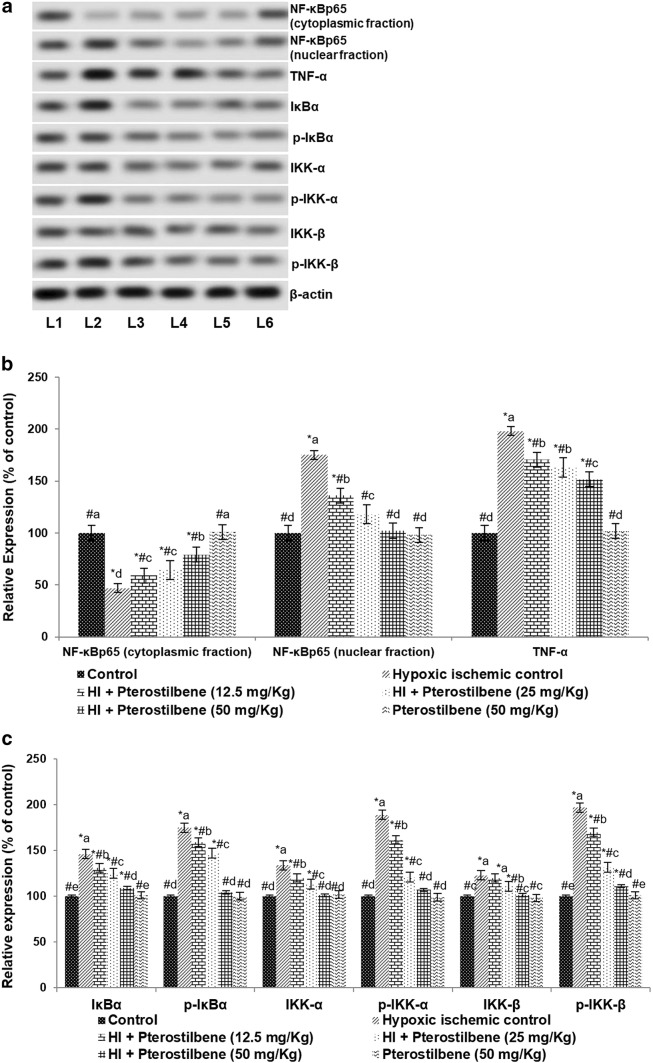
Pterostilbene downregulated NF-κB signaling cascade
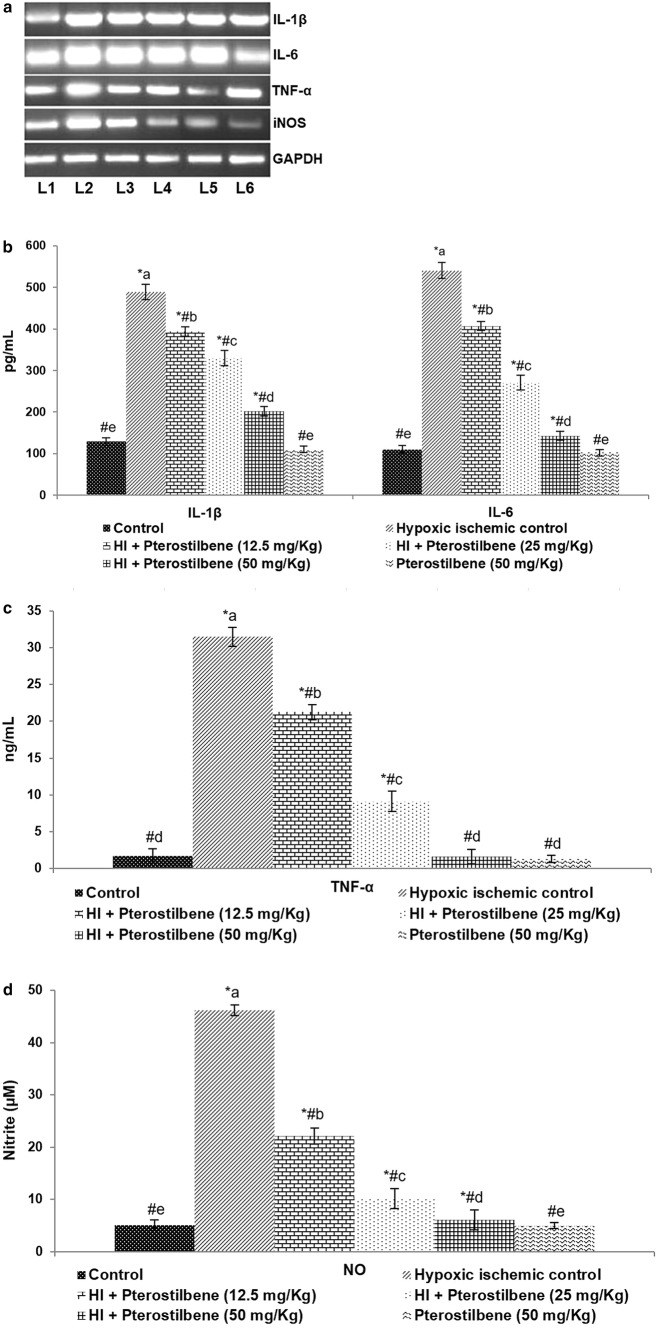
After 24 h of HI induction, enhanced expression of NF-κB (p65) with considerably (P < 0.05) decreased cytosolic levels of NF-κB (p65) were observed. The observations indicated stimulation of the NF-κB pathway. Further raised expression of TNF-α and the regulatory kinases—IKKα, p-IKKα, IKKβ, p-IKKβ, IκBα, and p-IκBα following HI insult were observed (Fig. 5a–c). Pterostilbene suggestively suppressed NF-κB p65 (nuclear fraction) expression compared to HI control. A 50 mg dose of pterostilbene reduced NF-κB p65 expression in the nuclear fraction from 175.15 to 102.3%. Also, pterostilbene significantly (P < 0.05) decreased the levels of p-IKKα, p-IKKβ and p-IκBα compared to the HI control group. The expression of total IKKα, IKKβ, and IκBα was brought down to near normal values, indicating down-regulation. Furthermore, the increase in the levels of serum NO along with enhanced iNOS mRNA levels (Fig. 6a, b) observed following HI induction were significantly downregulated in pterostilbene administration. The enhanced mRNA and serum levels of IL-1β, IL-, and TNF-α (Fig. 6c, d) in HI were found to be decreased in pups that were administered with pterostilbene. These observations suggest the anti-inflammatory effects of pterostilbene. Previous in vitro studies with pterostilbene have shown that pterostilbene inhibits NF-κB signaling and suppresses the production of inflammatory cytokines (Pan et al. 2008; Hou et al. 2015).
Fig. 5.
Pterostilbene regulates NF-κB signaling following HI. a Representative immunoblot, b, c relative protein expressions. Values are represented as mean ± SD, n = 6; P < 0.05 as determined by one-way ANOVA followed by DMRT analysis. *P < 0.05 vs. control; #P < 0.05 vs. HI control; a–e represents mean values from different experimental groups that differ from each other at P < 0.05. L1-Control; L2-Hypoxic Ischemic Control; L3-HI + Pterostilbene (12.5 mg/Kg); L4-HI + Pterostilbene (25 mg/kg); L5-HI + Pterostilbene (50 mg/Kg); L6-Pterostilbene (50 mg/kg)
Fig. 6.
Pterostilbene reduced the levels of inflammatory cytokines. a mRNA expression levels—representative gel image, b–d serum levels of inflammatory mediators—IL-1β, IL-6, TNF-α, and NO. Values are represented as mean ± SD, n = 6; P < 0.05 as determined by one-way ANOVA followed by DMRT analysis. *P < 0.05 vs. control; #P < 0.05 vs. HI control; a–e represents mean values from different experimental groups that differ from each other at P < 0.05. L1-Control; L2-Hypoxic Ischemic Control; L3-HI + Pterostilbene (12.5 mg/Kg); L4-HI + Pterostilbene (25 mg/Kg); L5-HI + Pterostilbene (50 mg/Kg); L6-Pterostilbene (50 mg/Kg)
Pterostilbene regulated PI3K/mTOR/JNK signaling
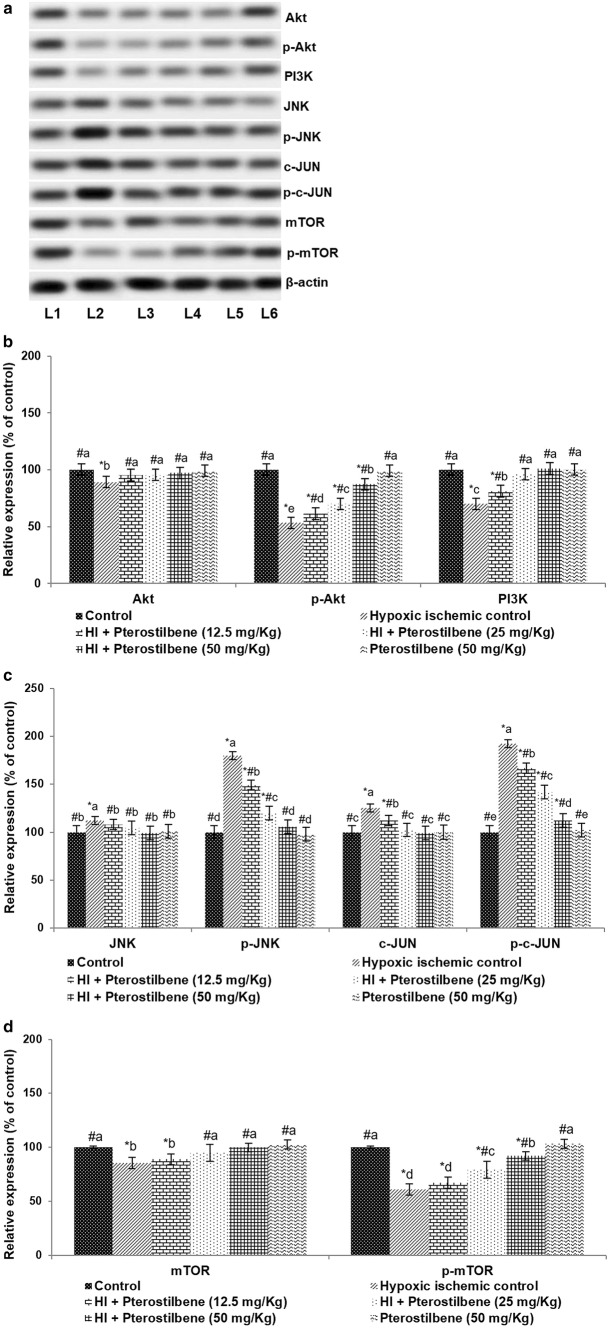
The PI3K/Akt/mTOR axis is well documented in cerebral HI injury. Immunoblotting analysis was performed to evaluate the expression of PI3K, Akt, and mTOR following HI. The expression of PI3K decreased to 70.10% 24 h following HI vs. normal control (Fig. 7a–c). The levels of p-Akt and p-mTOR expression decreased to 53.15% and 60.7% respectively, indicating down-regulation of the pathway. Pterostilbene improved the expression of PI3K along with the phosphorylated forms of Akt and mTOR in a dose-dependent manner. 50 mg pterostilbene improved the expressions of p-Akt to 87.30% and p-mTOR levels to 92.08%.
Fig. 7.
Pterostilbene up-regulated Akt activation following HI. a Representative immunoblot, b, c Relative expressions of proteins. Values are represented as mean ± SD, n = 6; P < 0.05 as determined by one-way ANOVA followed by DMRT analysis. *P < 0.05 vs. control; #P < 0.05 vs. HI control; a–e represents mean values from different experimental groups that differ from each other at P < 0.05. L1-Control; L2-Hypoxic Ischemic Control; L3-HI + Pterostilbene (12.5 mg/Kg); L4-HI + Pterostilbene (25 mg/Kg); L5-HI + Pterostilbene (50 mg/Kg); L6-Pterostilbene (50 mg/Kg)
Further, the phosphorylation intensities of JNK and c-JUN were also analyzed following HI. The results revealed significantly increased both p-JNK and p-c-JUN expression levels in HI control animals vs. normal control (Fig. 7a, d). Interestingly, down-regulated p-c-Jun and p-JNK levels were observed after the pterostilbene administration. p-JNK expression was reduced to 105.73% from 179.75% and p-c-JUN levels decreased to 112.16% from 192.05% following systemic supplementation of 50 mg pterostilbene. These observations suggest that pterostilbene inhibited JNK/c-JUN signaling.
Discussion
Neonatal hypoxic-ischemic (HI) brain injury is an important cause of death and also morbidity in neonates and infants. Survivors of HI experience long-term neurological impairments such as cognitive, sensorimotor deficits, epilepsy, and cerebral palsy (Bryce et al. 2005; Cooper 2011). The pathology of HI is complex and involves many factors such as neuronal apoptosis, excitotoxicity, aberrant inflammatory responses, and oxidative stress (Ferriero and Bonifacio 2014; Moskowitz et al. 2010). Currently, available therapies have limited effects, thus making identification of newer strategies inevitable.
The present study aimed to assess the effects of systemic supplementation of pterostilbene in a rodent model of neonatal HI brain injury. It is known that HI brain injury causes significant neuronal loss. Thus, reducing neuronal cell loss and stimulating neuronal cell survival is pivotal for the prevention of incidence of long-term neurological deficits (Nijboer et al. 2010). The study results indicated that administration of pterostilbene significantly improved brain tissue architecture and prevented neuronal loss. Brain edema was also considerably reduced. TTC staining is widely employed to assess neuronal damage and subsequent neurological impairment (Liszczak et al. 1984; Bederson et al. 1986). Pterostilbene significantly reduced the infarct area as determined by TTC staining.
Oxidative stress is known to be a major contributor to ischemic brain injury (Warner et al. 2004). Oxidative stress is shown to result in mitochondrial dysfunction and the generation of more ROS (Ferriero 2001; Revuelta et al. 2015). The increased levels of free radicals generated following HI lead to oxidative stress which causes neuronal damage (Burchell et al. 2013). Low levels of antioxidant defences, along with high metabolic rate and abundant lipids, make brain cells highly sensitive to lipid peroxidation and oxidative damage (Chang et al. 2011; Perrone et al. 2015). Compounds with antioxidant activities have been found to exert beneficial effects against ROS-induced neuronal damage in HI (Jayaprakasha et al. 2006; Huang et al. 2014). Rutin-encapsulated chitosan nanoparticles that were targeted to the brain were found to effectively reduce cerebral infarct size and neuronal loss (Ahmad et al. 2016).
The study data demonstrated that pterostilbene treatment efficiently decreased ROS production and levels of MDA in pups following HI injury. Nrf2 and HO-1 protein expression were found to be significantly (p < 0.05) raised 24 h following HI injury, along with increased (p < 0.05) levels of GSH observed following HI, indicating the stimulation of innate defense mechanisms under oxidative stress. Nrf2, a major transcription factor, is a chief regulator of innate antioxidative responses in the brain (Shah et al. 2007; Vargas et al. 2008). Nrf2 also regulates inflammatory responses and protects cells from calcium overloading (Rzepecka et al. 2015). In the absence of stress and under typical physiological conditions, Nrf2 that is in the cytoplasm remains bound to Keap1 protein (Li et al. 2014) while oxidative stress condition stimulates the phosphorylation of Nrf2. The phosphorylated Nrf2 separates from Keap1 and moves to the nucleus, thereby regulating its downstream target genes (Yang et al. 2015). HO-1, alongside phase II detoxification enzymes, exerts antioxidant effects against ROS-induced oxidative stress. In neuronal cells, the transcription of HO-1 is stimulated by Nrf2. Increased HO-1 and Nrf2 expression as noticed following HI, is indicative of activated Nrf2 signaling. These observations 24 h after induction of HI injury reflect the innate defense mechanism against HI-induced oxidative stress. Elevated HO-1 expression significantly reduces cell membrane damage and prevents neuronal cell death (Li et al. 2014). Also elevated HO-1 levels decrease ROS production (Wu et al. 2015). Pterostilbene administration was also observed to significantly increased Nrf2 and HO-1 expression at all tested doses. The increased Nrf2 and HO-1expressions was found to be in line with decreased ROS and MDA levels. The results illustrate that the pterostilbene-mediated decrease in ROS levels could be due to its direct antioxidant effects or an increase in Nrf2 /HO-1 signaling. These observations indicate the efficacy of pterostilbene.
The inflammatory process has been recognized as one of the significant contributors to neonatal (Benjelloun et al. 1999; Cuartero et al. 2013). NF-κB is a pivotal transcription factor that controls and regulates the expression of proteins of the inflammatory process, including iNOS, Cox-2, TNF-α, IL-6, IL-1α, and IL-1β (Saliba and Henrot 2001). The effects of pterostilbene administration on NF-κB activation and signaling following HI were evaluated where serum levels of IL-1β, IL-6, and TNF-α were determined. RT-PCR analysis revealed markedly elevated mRNA levels of iNOS, TNF-α, IL-1β, and IL-6 following HI brain injury. The mRNA levels were enhanced in line with serum levels of TNF-α, IL-1β, and IL-6. The serum NO levels were also raised, as reflected by raised mRNA levels of iNOS. NO is well documented as a crucial player in immune and inflammatory responses (Lv et al. 2015). Under regular physiological conditions, NF-κB (consisting of subunits p50 and p65) remains localized in the cytoplasm in its inactive state bound to inhibitory proteins—IκBs. Upon stimulation, IκB, gets phosphorylated and activated by the IkB kinase (IKK) complex, and is rapidly degraded (Scheidereit 2006; Hansberger et al. 2007). The IKK complex comprises kinases, IKKα and β (Yamamoto and Gaynor 2004; Hayden and Ghosh 2008). This phosphorylation causes the dissociation of the NF-kBp65 subunit from IκBα, an inhibitory protein. NF-kBp65 then translocates to the nucleus and initiates transcription of the target genes including- TNF-α, IL-1β, and IL-6 (Hayden and Ghosh 2008). Upregulated NF-kBp65 expression in the nuclear fraction following HI injury indicates activation of NF-κB signaling. Prior investigations have also revealed the activation of NF-κB signaling in HI brain injury (Stephenson et al. 2000; Nurmi et al. 2004). The significantly elevated mRNA levels of TNF-α, IL-1β and IL-6 and the levels in the serum also indicate marked activation of NF-kB signaling. Pterostilbene administration leads to significant down-regulation in the phosphorylation of IκBα, IKKα, and IKK-β. This suppression by pterostilbene could have contributed to the inhibition of NF-kB activation as also indicated by reduced nuclear levels of NF-κBp65. Studies have shown that suppression of NF-κB signaling could be protective against HI-induced neuronal injury (Verma 2004; Wang et al. 2009). Pterostilbene-mediated reduced levels of cytokines and NO levels further revealed anti-inflammatory efficacy.
PI3K-Akt-mTOR/JNK signaling has also been described to be associated with neuronal death following HI injury and stroke (Kamada et al. 2007; Xu et al. 2015). Akt, a main downstream target of the PI3K pathway is a crucial protein involved in multiple pathways in cellular homeostasis. Akt promotes cell survival and inhibits cellular apoptosis through its downstream molecules. As one of the vital down-stream target molecules for Akt, mTOR plays a central role in cell survival and differentiation (Park et al. 2008). Activation of the PI3K/Akt pathway is known to induce neovascularization that aids in the reduction of infarct volume following ischemia (Zhang and Ren et al. 2010). Here, a marked decrease in the expression of PI3K, Akt, p-Akt, and p-mTOR was observed indicating downregulation of Akt activation following HI-induced brain injury. These observations suggest that neuronal death could be related to the down-regulation of PI3K/Akt signal.
Interestingly, the expression levels of JNK, another target protein of Akt was observed to be enhanced. Elevated expression of p-JNK indicates activation of JNK. Activated JNK then phosphorylates c-Jun, a nuclear substrate. c-Jun increases activator protein-1 transcription activity eventually leading to transcription of genes associated with apoptosis. JNK also regulates non-nuclear substrates such as Bcl-2 family proteins (Guan et al. 2005, 2008). Studies have also reported activation of JNK signaling, enhanced p–c-Jun levels and downregulated PI3K/Akt/mTOR signaling pathways in HI injury (Nakajima et al. 2004; Aubert et al. 2006). Significantly (P < 0.05) elevated PI3K, p-Akt and p-mTOR expression along with downregulated p-JNK and p-c-JUN levels on pterostilbene supplementation illustrate the neuroprotective effects of pterostilbene. Huang et al. (2014) demonstrated that Rhyncophylline exerted neuroprotective effects via activation of the PI3K/Akt pathway following HI-induced brain injury. The results of our study suggest that pterostilbene possibly exerts neuroprotective effects by regulating the PI3K/Akt/mTOR pathway. The higher bioavailability and lipophilic nature of pterostilbene could also contribute to the neuroprotective efficiency (McCormack and McFadden 2012; Chen et al. 2017).
These observations suggest that pterostilbene could be employed in the treatment of HI. However, more studies have to be conducted in terms of standardisation of dosage for treatment and other effects if any. Nevertheless, pterostilbene has been shown to possess several bioactive properties including anti-inflammatory and anti-cancer effects (McCormack and McFadden 2012; Ma et al. 2019). Structural methoxylation at the 3 and 5 positions renders pterostilbene more lipophilic which aids in efficient intestinal absorption. This contributes to a higher potential for biological uptake (Lin et al. 2009; Kapetanovic et al. 2011). Furthermore, pterostilbene was found to possess metabolic stability and thus a better pharmacokinetic profile (Wang and Sang 2018). Also, pterostilbene has been found to have negligible side effects and is classified as low risk. Human clinical trials have shown that pterostilbene is safe for use at doses of up to 250 mg/day (Ruiz et al. 2009; Richie et al. 2013). The safety margin and higher bioavailability and metabolic stability make pterostilbene a potent candidate that could be further investigated in HI therapy.
Conclusion
The study demonstrated that pterostilbene reduced neuronal cell death, brain edema, improved brain architecture, and exerted anti-oxidant effects by reducing ROS and regulating Nrf2 and HO-1 signals. Furthermore, pterostilbene regulated NF-κB signaling and the PI3K/Akt/mTOR-JNK pathway. These observations propose pterostilbene as a potential therapeutic compound that could be explored further in the treatment of neonatal HI brain injury.
Acknowledgements
This study was supported by Fujian Province Young Family Talent Training Program (No:2018-ZQN-61) and Fujian Science and Technology Innovation Joint Fund Project (No: 2017Y9120).
Author contributions
All authors had equally contributed to this work and performed all activities relating to this study.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
References
- Ahmad N, Ahmad R, Naqvi AA, Alam MA, Ashafaq M, Samim M, Iqbal Z, Ahmad FJ. Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of Cerebral Ischemia. Int J Biol Macromol. 2016;91:640–655. doi: 10.1016/j.ijbiomac.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Akhtar M, Pillai K, Vohora D. Effect of thioperamide on oxidative stress markers in middle cerebral artery occlusion model of focal cerebral ischemia in rats. Hum Exp Toxicol. 2008;27:761–767. doi: 10.1177/0960327108094608. [DOI] [PubMed] [Google Scholar]
- Arteaga O, Revuelta M, Urigüen L, Álvarez A, Montalvo H, Hilario E. Pre-treatment with resveratrol prevents neuronal injury and cognitive deficits induced by perinatal hypoxia-ischemia in rats. PLoS ONE. 2015;10:e0142424. doi: 10.1371/journal.pone.0142424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert N, Falluel-Morel A, Vaudry D, Xifro X, Rodriguez-Alvarez J, Fisch C, de Jouffrey S, Lebigot JF, Fournier A, Vaudry H, Gonzalez BJ. PACAP and C2-ceramide generate different AP-1 complexes through a MAP-kinase-dependent pathway: involvement of c-Fos in PACAP-induced Bcl-2 expression. J Neurochem. 2006;99:1237–1250. doi: 10.1111/j.1471-4159.2006.04148.x. [DOI] [PubMed] [Google Scholar]
- Azzopardi D. Predictive value of the amplitude integrated EEG in infants with hypoxic ischaemic encephalopathy: data from a randomised trial of therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2014;99:F80–F82. doi: 10.1136/archdischild-2013-303710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat WN, Safwet NN, El-Maraghy ZMN. Candesartan and glycyrrhizin ameliorate ischemic brain damage through downregulation of the TLR signaling cascade. Eur J Pharmacol. 2014;724:43–50. doi: 10.1016/j.ejphar.2013.12.032. [DOI] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Benjelloun N, Renolleau S, Represa A, Ben-Ari Y, Charriaut-Marlangue C. Inflammatory responses in the cerebral cortex after ischemia in the P7 neonatal rat. Stroke. 1999;30:1916–1923. doi: 10.1161/01.str.30.9.1916. [DOI] [PubMed] [Google Scholar]
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- Burchell SR, Dixon BJ, Tang J, Zhang JH. Isoflurane provides neuroprotection in neonatal hypoxic ischemic brain injury. J Investig Med. 2013;61:1078–1083. doi: 10.231/JIM.0b013e3182a07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Gupta N, Ghosh K, Roy P. In vitro evaluation of the cytotoxic, anti-proliferative and anti-oxidant properties of pterostilbene isolated from Pterocarpus marsupium. Toxicol In Vitro. 2010;24:1215–1228. doi: 10.1016/j.tiv.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Chang CC, Wang YH, Chern CM, Liou KT, Hou YC, Peng YT, Shen YC. Prodigiosin inhibits gp91(phox) and iNOS expression to protect mice against the oxidative/nitrosative brain injury induced by hypoxia-ischemia. Toxicol Appl Pharmacol. 2011;257:137–147. doi: 10.1016/j.taap.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Chen RJ, Lee YH, Yeh YL, Wu WS, Ho CT, Li CY, Wang BJ, Wang YJ. Autophagy-inducing effect of pterostilbene: A prospective therapeutic/preventive option for skin diseases. J Food Drug Anal. 2017;25:125–133. doi: 10.1016/j.jfda.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Tang J, Khatibi NH, Zhu M, Li Y, Wang C, Jiang R, Tu L, Wang S. Treatment with Z-ligustilide, a component of Angelica sinensis, reduces brain injury after a subarachnoid haemorrhage in rats. J Pharmacol Exp Ther. 2011;337:663–672. doi: 10.1124/jpet.110.177055. [DOI] [PubMed] [Google Scholar]
- Cooper DJ. Induced hypothermia for neonatal hypoxic–ischemic encephalopathy: pathophysiology, current treatment, and nursing considerations. Neonatal Netw. 2011;30:29–35. doi: 10.1891/0730-0832.30.1.29. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, Corbí ÁL, Lizasoain I, Moro MA. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARγ agonist rosiglitazone. Stroke. 2013;44:3498–3508. doi: 10.1161/STROKEAHA.113.002470. [DOI] [PubMed] [Google Scholar]
- del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H, Nito C, Kamada H, Yu F, Chan PH. Akt/GSK3beta survival signaling is involved in acute brain injury after subarachnoid hemorrhage in rats. Stroke. 2006;37:2140–2146. doi: 10.1161/01.STR.0000229888.55078.72. [DOI] [PubMed] [Google Scholar]
- Ferriero DM, Bonifacio SL. The search continues for the elusive biomarkers of neonatal brain injury. J Pediatr. 2014;164:438–440. doi: 10.1016/j.jpeds.2013.11.042. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Oxidant mechanisms in neonatal hypoxia-ischemia. Dev Neurosci. 2001;23:198–202. doi: 10.1159/000046143. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Gao Y, Fang X, Tong Y, Liu Y, Zhang B. TLR4-mediated MyD88-dependent signaling pathway is activated by cerebral ischemia-reperfusion in cortex in mice. Biomed Pharmacother. 2009;63:442–450. doi: 10.1016/j.biopha.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Garber JC. Guide for the care and use of laboratory animals. 8. New York: National Academy of Sciences; 2011. Committee for the update of the guide for the care and use of laboratory animals. [Google Scholar]
- Grow J, Barks JD. Pathogenesis of hypoxic-ischemic cerebral injury in the term infant: current concepts. Clin Perinatol. 2002;29:585–602. doi: 10.1016/s0095-5108(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Guan QH, Pei DS, Zhang QG, Hao ZB, Xu TL, Zhang GY. The neuroprotective action of SP600125, a new inhibitor of JNK, on transient brain ischemia/reperfusion-induced neuronal death in rat hippocampal CA1 via nuclear and non-nuclear pathways. Brain Res. 2005;1035:51–59. doi: 10.1016/j.brainres.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Guan QH, Pei DS, Zong YY, Xu TL, Zhang GY. Neuroprotection against ischemic brain injury by a small peptide inhibitor of c-Jun N-terminal kinase (JNK) via nuclear and non-nuclear pathways. Neuroscience. 2008;39:609–627. doi: 10.1016/j.neuroscience.2005.11.067. [DOI] [PubMed] [Google Scholar]
- Hansberger MW, Campbell JA, Danthi P, Arrate P, Pennington KN, Marcu KB, Ballard DW, Dermody TS. I kappa B kinase subunits alpha and gamma are required for activation of NF-kappa B and induction of apoptosis by mammalian reovirus. J Virol. 2007;81:1360–1371. doi: 10.1128/JVI.01860-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappa B signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hou Y, Li N, Xie G, Wang J, Yuan Q, Jia C, Liu X, Li G, Tang Y, Wang B. Pterostilbene exerts anti-neuroinflammatory effect on lipopolysaccharide-activated microglia via inhibition of MAPK signaling pathways. J Funct Foods. 2015;19:676–687. [Google Scholar]
- Huang H, Zhong R, Xia Z, Song J, Feng L. Neuroprotective effects of rhynchophylline against ischemic brain injury via regulation of the Akt/mTOR and TLRs signaling pathways. Molecules. 2014;19:11196–11210. doi: 10.3390/molecules190811196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida M, Miyazaki I, Tanaka K, Kabuto H, Iwata-Ichikawa E, Ogawa N. Dopamine D2 receptor mediated antioxidant and neuroprotective effects of ropinirole, a dopamine agonist. Brain Res. 1999;838:51–59. doi: 10.1016/s0006-8993(99)01688-1. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 co-ordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha GK, Jaganmohan Rao L, Sakariah KK. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006;98:720–724. [Google Scholar]
- Kamada H, Nito C, Endo H, Chan PH. Bad as a converging signaling molecule between survival PI3-K/Akt and death JNK in neurons after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2007;27:521–533. doi: 10.1038/sj.jcbfm.9600367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2011;68:593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappa B in cancer: from innocent by stander to major culprit. Nature Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Li B, Choi HJ, Lee DS, Oh H, Kim YC, Moon JY, Park WH, Park SD, Kim JE. Amomum tsao-ko suppresses lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages via Nrf2-dependent heme oxygenase-1 expression. Am J Chin Med. 2014;42:1229–1244. doi: 10.1142/S0192415X14500773. [DOI] [PubMed] [Google Scholar]
- Lin HS, Yue BD, Ho PC. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed Chromatogr. 2009;23:1308–1315. doi: 10.1002/bmc.1254. [DOI] [PubMed] [Google Scholar]
- Liszczak TM, Hedley-Whyte ET, Adams JF, Han DH, Kolluri VS, Vacanti FX, Heros RC, Zervas NT. Limitations of tetrazolium salts in delineating infarcted brain. Acta Neuropathol. 1984;65:150–157. doi: 10.1007/BF00690469. [DOI] [PubMed] [Google Scholar]
- Liu M, Bamodu OA, Huang WC, Zucha MA, Lin YK, Wu ATH, Huang CC, Lee WH, Yuan CC, Hsiao M, Deng L, Tzeng YM, Yeh CT. 4-Acetylantroquinonol B suppresses autophagic flux and improves cisplatin sensitivity in highly aggressive epithelial cancer through the PI3K/Akt/mTOR/p70S6K signaling pathway. Toxicol Appl Pharmacol. 2017;325:48–60. doi: 10.1016/j.taap.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Lu C, Liu L, Chen Y, Ha T, Kelley J, Schweitzer J, Kalbfleisch JH, Kao RL, Williams DL, Li C. TLR2 ligand induces protection against cerebral ischemia/reperfusion injury via activation of phosphoinositide 3-kinase/Akt signaling. J Immunol. 2011;187:1458–1466. doi: 10.4049/jimmunol.1003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y, Qian Y, Fu L, Chen X, Zhong H, Wei X. Hydroxy safflor yellow A exerts neuroprotective effects in cerebral ischemia reperfusion-injured mice by suppressing the innate immune TLR4-inducing pathway. Eur J Pharmacol. 2015;769:324–332. doi: 10.1016/j.ejphar.2015.11.036. [DOI] [PubMed] [Google Scholar]
- Ma Z, Zhang X, Xu L, Liu D, Di S, Li W, Zhang J, Zhang H, Li X, Han J, Yan X. Pterostilbene: Mechanisms of its action as oncostatic agent in cell models and in vivo studies. Pharmacol Res. 2019;145:104265. doi: 10.1016/j.phrs.2019.104265. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- McCormack DE, Mannal P, McDonald D, Tighe S, Hanson J, McFadden D. Genomic analysis of pterostilbene predicts its antiproliferative effects against pancreatic cancer in vitro and in vivo. J Gastrointest Surg. 2012;16:1136–1143. doi: 10.1007/s11605-012-1869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack D, McFadden D. Pterostilbene and cancer: current review. J Surg Res. 2012;173:e53–61. doi: 10.1016/j.jss.2011.09.054. [DOI] [PubMed] [Google Scholar]
- McCormack D, McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev. 2013;2013:575482. doi: 10.1155/2013/575482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R, Green CJ, Foresti R. Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antioxid Redox Signal. 2002;4:615–624. doi: 10.1089/15230860260220111. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Iwabuchi S, Miyazaki H, Okuma Y, Kuwabara M, Nomura Y, Kawahara K. Preconditioning prevents ischemia-induced neuronal death through persistent Akt activation in the penumbra region of the rat brain. J Vet Med Sci. 2004;66:521–527. doi: 10.1292/jvms.66.521. [DOI] [PubMed] [Google Scholar]
- Nijboer CH, van der Kooij MA, van Bel F, Ohl F, Heijnen CJ, Kavelaars A. Inhibition of the jnk/ap-1 pathway reduces neuronal death and improves behavioral outcome after neonatal hypoxic-ischemic brain injury. Brain Behav Immun. 2010;24:812–821. doi: 10.1016/j.bbi.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Noshita N, Lewén A, Sugawara T, Chan PH. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:1442–1450. doi: 10.1097/00004647-200112000-00009. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J. Nuclear factor-kappa B contributes to infarction after permanent focal ischemia. Stroke. 2004;35:987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- Pan MH, Chang YH, Tsai ML, Lai CS, Ho SY, Badmaev V, Ho CT. Pterostilbene suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. J Agric Food Chem. 2008;56:7502–7509. doi: 10.1021/jf800820y. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone S, Tataranno LM, Stazzoni G, Ramenghi L, Buonocore G. Brain susceptibility to oxidative stress in the perinatal period. J Matern Fetal Neonatal Med. 2015;28:S2291–2295. doi: 10.3109/14767058.2013.796170. [DOI] [PubMed] [Google Scholar]
- Remsberg CM, Yanez JA, Ohgami Y, Vega-Villa KR, Rimando AM, Davies NM. Pharmacometrics of pterostilbene: preclinical pharmacokinetics andmetabolism, anticancer, anti-inflammatory, antioxidant and analgesic activity. Phytother Res. 2008;22:169–179. doi: 10.1002/ptr.2277. [DOI] [PubMed] [Google Scholar]
- Revuelta M, Arteaga O, Montalvo H, Alvarez A, Hilario E, Martinez-Ibargüen A. Antioxidant treatments recover the alteration of auditory evoked potentials and reduce morphological damage in the inferior colliculus after perinatal asphyxia in rat. Brain Pathol. 2015;26:186–198. doi: 10.1111/bpa.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Riche DM, McEwen CL, Riche KD, Sherman JJ, Wofford MR, Deschamp D, Griswold M. Analysis of safety from a human clinical trial with pterostilbene. J Toxicol. 2013;2013:463595. doi: 10.1155/2013/463595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Ferreira E, Hristova M. Plasticity in the neonatal brain following hypoxic-ischaemic injury. Neural Plast. 2016;2016:4901014. doi: 10.1155/2016/4901014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roupe KA, Remsberg CM, Yanez JA, Davies NM. Pharmacometrics of stilbenes: seguing towards the clinic. Curr Clin Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- Ruiz MJ, Fernández M, Picó Y, Mañes J, Asensi M, Carda C, Asensio G, Estrela JM. Dietary administration of high doses of pterostilbene and quercetin to mice is not toxic. J Agric Food Chem. 2009;57:3180–3186. doi: 10.1021/jf803579e. [DOI] [PubMed] [Google Scholar]
- Rzepecka J, Pineda MA, Al-Riyami L, Rodgers DT, Huggan JK, Lumb FE, Khalaf AI, Meakin PJ, Corbet M, Ashford ML, Suckling CJ, Harnett MM, Harnett W. Prophylactic and therapeutic treatment with a synthetic analogue of a parasitic worm product prevents experimental arthritis and inhibits IL-1β production via NRF2-mediated counter-regulation of the inflammasome. J Autoimmun. 2015;60:59–73. doi: 10.1016/j.jaut.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Maier CM, Narasimhan P, Nishi T, Song YS, Yu F, Liu J, Lee YS, Nito C, Kamada H, Dodd RL, Hsieh LB, Hassid B, Kim EE, González M, Chan PH. Oxidative stress and neuronal death/ survival signaling in cerebral ischemia. Mol Neurobiol. 2005;31:105–116. doi: 10.1385/MN:31:1-3:105. [DOI] [PubMed] [Google Scholar]
- Saliba E, Henrot A. Inflammatory mediators and neonatal brain damage. Biol Neonate. 2001;79:224–227. doi: 10.1159/000047096. [DOI] [PubMed] [Google Scholar]
- Satheesh MA, Pari L. The antioxidant role of pterostilbene in streptozotocin-nicotinamide-induced type 2 diabetes mellitus in Wistar rats. J Pharm Pharmacol. 2006;58:1483–1490. doi: 10.1211/jpp.58.11.0009. [DOI] [PubMed] [Google Scholar]
- Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II inducers. Proc Natl Acad Sci. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C. I kappa B kinase complexes: gateways to NF-kappa B activation and transcription. Oncogene. 2006;25:6685–6705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Dore S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience. 2007;147:53–59. doi: 10.1016/j.neuroscience.2007.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Pappas A, McDonald SA, Das A, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA. 2014;312:2629–2639. doi: 10.1001/jama.2014.16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D, Yin T, Smalstig EB, Hsu MA, Panetta J, Little S, Clemens J. Transcription factor nuclear factor-kappa B is activated in neurons after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:592–603. doi: 10.1097/00004647-200003000-00017. [DOI] [PubMed] [Google Scholar]
- Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HY, Ho CT, Chen YK. Biological actions and molecular effects of resveratrol, pterostilbene, and 3'-hydroxypterostilbene. J Food Drug Anal. 2017;25:134–147. doi: 10.1016/j.jfda.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma IM. Nuclear factor (NF) - kappa B proteins: therapeutic targets. Ann Rheum Dis. 2004;63:ii57–ii61. doi: 10.1136/ard.2004.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang YJ, Wu WN, Dong LD, Chen JG. Protection by tetra hydroxy stilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-kappa B pathways and inhibition of intracellular ROS/RNS generation. Free Rad Biol Med. 2009;47:229–240. doi: 10.1016/j.freeradbiomed.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Wang P, Sang S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors. 2018;44:16–25. doi: 10.1002/biof.1410. [DOI] [PubMed] [Google Scholar]
- Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Dave JR, Tortella FC. Neuroprotection with the proteasome inhibitor MLN519 in focal ischemic brain injury: relation to nuclear factor kappa B (NF-kappa B), inflammatory gene expression, and leukocyte infiltration. Neurochem Int. 2006;49:106–112. doi: 10.1016/j.neuint.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Wu Z, Uchi H, Morino-Koga S, Shi W, Furue M. Z-ligustilide ameliorated ultraviolet B-induced oxidative stress and inflammatory cytokine production in human keratinocytes through upregulation of Nrf2/HO-1 and suppression of NF-κB pathway. Exp Dermatol. 2015;24:703–708. doi: 10.1111/exd.12758. [DOI] [PubMed] [Google Scholar]
- Xu XH, Li GL, Wang BA, Qin Y, Bai SR, Rong J, Deng T, Li Q. Diallyl trisufide protects against oxygen glucose deprivation- induced apoptosis by scavenging free radicals via the PI3K/Akt -mediated Nrf2/HO-1 signaling pathway in B35 neural cells. Brain Res. 2015;1614:38–50. doi: 10.1016/j.brainres.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. I kappa B kinases: key regulators of the NF-kappa B pathway. Trends Biochem Sci. 2004;29:72–79. doi: 10.1016/j.tibs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Yang H, Xu W, Zhou Z, Liu J, Li X, Chen L, Weng J, Yu Z. Curcumin attenuates urinary excretion of albumin in type II diabetic patients with enhancing nuclear factor erythroid-derived 2-like 2 (Nrf2) system and repressing inflammatory signaling efficacies. Exp Clin Endocrinol Diabetes. 2015;123:360–367. doi: 10.1055/s-0035-1545345. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li H, Hu S, Zhang L, Liu C, Zhu C, Liu R, Li C. Brain edema after intracerebral hemorrhage in rats: the role of inflammation. Neurol India. 2006;54:402–407. doi: 10.4103/0028-3886.28115. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ren J. Autophagy in ALDH2-elicited cardioprotection against ischemic heart disease: Slayer or savior? Autophagy. 2010;6:1212–1213. doi: 10.4161/auto.6.8.13652. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]