Abstract
Patients with severe burn injuries are extremely susceptible to infection, and the host's antibacterial responses are frequently suppressed by alternatively activated macrophages (M2Mϕ), commonly demonstrated in these patients. An immunosuppressive subset of neutrophils (PMN-II), demonstrated in the peripheral blood of thermally injured patients, has been described as an inducer of M2Mϕ. In the present studies, the inhibitory effect of glycyrrhizin (GL) on M2Mϕ generation stimulated by PMN-II was examined. M2Mϕ were generated from resident Mϕ (R-Mϕ, lower chamber) after cultivation with PMN-II (upper chamber) in a dual-chamber transwell. However, M2Mϕ were not generated from R-Mϕ when the same transwell cultures were performed in the presence of GL. M2Mϕ were not generated from R-Mϕ after cultivation with PMN-II previously treated with GL, while R-Mϕ previously treated with GL converted to M2Mϕ after they were cultured with PMN-II in transwells. Interleukin-10 and CCL2 released from PMN-II were shown to be effector molecules responsible for the generation of M2Mϕ. However, these soluble factors were not produced by PMN-II treated with GL. These results indicate that GL inhibits PMN-II-stimulated M2Mϕ generation through the inhibition of CCL2/interleukin-10 production by PMN-II.
Keywords: Glycyrrhizin, Alternatively activated macrophages, Neutrophils, CCL2, Interleukin-10
1. Introduction
The majority of deaths in thermally injured patients are associated with infection rather than the physical damage to the skin or the resulting abnormal metabolism [1], [2], [3], [4]. As compared with healthy individuals, thermally injured patients have an increased susceptibility to infection because of burn-associated defects in their antibacterial responses [5]. Macrophages (Mϕ) are one of the critical effector cells in antibacterial innate immune responses [6]. After certain stimulations, resident Mϕ (R-Mϕ, Mϕ isolated from healthy individuals) convert into two different populations: classically activated Mϕ (M1Mϕ) and alternatively activated Mϕ (M2Mϕ) [7], [8]. M1Mϕ exhibit the ability to (i) kill infected cells, (ii) express inducible nitric oxide synthase, and (iii) secrete nitric oxide, pro-inflammatory cytokines and Th1 response-associated cytokines [8]. In contrast, M2Mϕ are commonly found in patients with severe infections, malignancies, thermal injuries, or after major surgery [7], [9], [10]. In a mouse model of thermal injury, M2Mϕ have been demonstrated 3–5 days after thermal injury [11], [12]. These Mϕ preferentially express receptors for foreign antigens (mannose receptor, β-glucan receptors and scavenger receptors) and produce interleukin (IL)-1 receptor antagonist, IL-10, CCL17, CCL22 and arginase [7], [9], [10]. In addition, IL-10 and CCL17 released from M2Mϕ inhibit M2Mϕ generation [13]. Therefore, M1Mϕ do not appear in hosts whose M2Mϕ predominate [13].
A specific population of neutrophils (PMNs) has been described as an inducer of M2Mϕ [14]. Recently, we have reported three different subsets of PMNs [14]. These PMNs are distinguished from each other in the following ways: PMN-I (Gr-1+CD11b−CD49d+) produce IL-12/CCL3 and express Toll-like receptor 2 (TLR2)/TLR4/TLR5/TLR8; PMN-II (Gr-1+CD11b+CD49d−) produce IL-10/CCL2 and express TLR2/TLR4/TLR7/TLR9; normal PMN (PMN-N, Gr-1+CD11b−CD49d−), lacking the ability to produce these cytokines, express TLR2/TLR4/TLR9 [14]. PMN-II isolated from MRSA-susceptible mice functioned to generate M2Mϕ from R-Mϕ, while PMN-I isolated from MRSA-resistant mice stimulated R-Mϕ conversion to M1Mϕ. PMN-N did not show any activities on the modification of Mϕ. CCL2 and IL-10 released from PMN-II were shown as effector molecules for M2Mϕ generation [14].
Glycyrrhizin (GL), an extract from licorice roots with a structure of 20β-carboxy-11-oxo-30-norolean-12-en-3β-yl-2-O-β-d-glucopyranuronosyl-α-d-glucopyranosiduronic acid, has been reported for inhibiting the production of IL-10 and CCL2 by Mϕ infected with HIV [15]. Therefore, in this study, the inhibitory effect of GL on M2Mϕ generation induced by PMN-II was investigated. In Japan, GL has been used clinically for more than 20 years in patients with chronic hepatitis [16], [17], [18], [19]. The antiviral activities of GL against human cytomegalovirus, herpes simplex virus type 2, influenza virus, and coronavirus that related to severe acute respiratory syndrome have been reported [20], [21], [22], [23]. This compound has also been described to inhibit inflammation, augment natural killer cell activity and induce interferon (IFN)-γ, CCL4 and CCL5 production [24], [25], [26].
2. Materials and methods
2.1. Animals
Eight- to 11-week-old BALB/c mice purchased from The Jackson Laboratory (Bar Harbor, ME) were used in this study. All procedures utilizing animal experiments were approved by the Institutional Animal Care and Use Committee of The University of Texas Medical Branch at Galveston (IACUC approval number: 01-04-010).
2.2. Reagents and media
Recombinant murine IL-10 and CCL2 were purchased from PeproTech (Rocky Hill, NJ). Monoclonal antibodies (mAbs) for IL-10 and CCL2 were obtained from BD PharMingen (San Diego, CA). Recombinant murine CCL17 and anti-CCL17 mAb were purchased from R&D Systems (Minneapolis, MN). SAC was purchased from Calbiochem–Behring Corp. (La Jolla, CA). RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine and antibiotics (complete medium) was utilized for the cultivation of various cell preparations.
2.3. GL
GL was supplied by Minophagen Pharmaceutical Co. Ltd., Tokyo, Japan. As described previously [15], 1 mg of GL was dissolved in 1 ml of culture medium at room temperature, and diluted further to appropriate concentrations when it was used in experiments.
2.4. Thermal injury
According to our previous reports [13], [27], thermally injured mice were prepared, as follows: Mice, anesthetized with pentobarbital (40 mg/kg, i.p.), were shaved of the hair on their backs from groin to axilla. Thermal injury was produced by pressing a custom-made insulated mold (with a 2.5 × 3.5-cm window) firmly against the shaved back of each mouse and subsequently exposing the area to a gas flame for 9 s using a Bunsen burner equipped with a flame-dispersing cap. This procedure produced a third-degree burn, on approximately 15% of the total body surface area for a 26 g mouse. Immediately after thermal injury, physiologic saline (2 ml/mouse, i.p.) was administered for fluid resuscitation. Animals were then housed until used for experiments. Control mice, not exposed to the gas flame, had their back hair shaved and received physiologic saline (2 ml/mouse, i.p.).
2.5. Preparation of Mϕ and PMN
Without any stimulation, Mϕ were freshly isolated from the peritoneal exudates of normal mice using fibronectin-coated dishes, as previously described [14], [27]. The purity of the final Mϕ preparation was routinely more than 92% when it was analyzed by a FACScan flow cytometer (Becton Dickinson, San Jose, CA). These Mϕ were utilized as R-Mϕ. As previously described [14], PMN were isolated from the whole peripheral blood of normal and thermally injured mice, using Ficoll-Hypaque and dextran sedimentations. PMN isolated from normal mice were designated as PMN-N, and PMN derived from mice 18 h after burn injury were utilized as PMN-II, as previously described [14]. Briefly, peripheral blood was withdrawn from the heart of each mouse with a heparinized syringe. The peripheral blood was centrifuged with Ficoll-Hypaque, and precipitates were obtained as a PMN rich fraction. The precipitates were suspended in 1% dextran (T-500, Pharmacia, Piscataway, NJ) and kept for 1 h at room temperature to allow for the sedimentation of residual erythrocytes. The PMN fraction was further treated with erythrocyte-lysing kits (R&D Systems) to eliminate the remaining erythrocytes. The purity of PMN from the final preparation was routinely more than 93%, when analyzed by flow cytometry with FITC-conjugated anti-Gr-1 mAb and Wright–Giemsa/alkaline phosphatase stainings. In the PMN preparation from thermally injured mice, CCL2-producing cells (PMN-II) were consistently measured as 99%, and CCL3-producing cells (PMN-I) were not contained in the remaining cell population [14].
2.6. The effect of GL on M2Mϕ generation
In the presence of GL (1–100 μg/ml), R-Mϕ (6 × 105 cells/well) placed into the lower chamber of dual-chamber transwells (0.4 μm pore, Corning Costar, Corning, NY) were cultured with PMN-II (0.08–2 × 105 cells/well, upper camber) in the presence of SAC (0.0075%) [14]. Eighteen hours after the cultivation, cells in the lower chamber were recultured for 6–30 h without any stimulation. In some experiments, R-Mϕ previously treated with GL (100 μg/ml) for 3–12 h were cultured with PMN-II in dual-chamber transwells. R-Mϕ were cultured with PMN-II previously treated with GL (100 μg/ml) for 3–12 h in dual-chamber transwells. Culture fluids, harvested 24 h after the cultivation of Mϕ were assayed for CCL17, as a typical chemokine produced by M2Mϕ. Amounts of CCL17 produced into the culture fluids were measured using ELISA. The detection limits of CCL17 were 16 pg/ml. Each assay was performed three times.
2.7. The effect of GL on the production of CCL2 and IL-10 from PMN-II
PMN-II (2 × 106 cells/ml) were treated with 100 μg/ml of GL in the presence of SAC [14]. Culture fluids, harvested various hours after SAC stimulation, were assayed for CCL2 and IL-10 using ELISA. The detection limits for IL-10 and CCL2 were 16 and 8 pg/ml, respectively. Each assay was performed three times.
2.8. Statistical analysis
Data are presented as mean ± S.D. All results were statistically analyzed by ANOVA using Statview 4.5 (Brain Power, Calabasas, CA). If a p value was lower than 0.05, the result was considered to be significant.
3. Results
3.1. GL inhibits the generation of M2Mϕ stimulated with PMN-II
The inhibitory effect of GL on PMN-II-associated M2Mϕ generation was examined in vitro using transwell cultures. R-Mϕ (6 × 105 cells/ml, lower chamber) were cultured in a dual-chamber transwell with PMN-II (2 × 105 cells/ml, upper chamber) supplemented with Staphylococcus aureus Cowan I (SAC). Eighteen hours after cultivation, the upper chamber was removed and cells in the lower chamber were recultured for 6–30 h. Culture fluids harvested were assayed for CCL17, a typical parameter of M2Mϕ. Twelve hours after cultivation, CCL17 production by these Mϕ was first demonstrated, and it peaked 24 h after cultivation. However, the chemokine was not produced by Mϕ cultured with PMN-II in transwells supplemented with 100 μg/ml of GL ( Fig. 1A). Mϕ harvested from control cultures (R-Mϕ only cultured with or without PMN-N) did not produce CCL17 into their culture fluids. As shown in Fig. 1B, R-Mϕ converted to M2Mϕ in association with the number of PMN-II added to the upper chamber of the transwells, because the total amounts of CCL17 produced by R-Mϕ were increased after transwell cultures in response to the numbers of PMN-II added to the cultures. However, the production of CCL17 from Mϕ stimulated with PMN-II was markedly inhibited (95%) when transwell cultures of 6 × 105 cells/well of R-Mϕ and 2 × 105 cells/well of PMN-II were performed in the presence of GL (Fig. 1B). When various doses of GL were added to the transwell cultures, a 10 μg/ml dose was shown to be 50% inhibitory on the production of CCL17. The maximum inhibition of CCL17 production was shown when transwell cultures were performed with 100 μg/ml of GL (Fig. 1C). These results indicate that GL inhibits M2Mϕ generation from R-Mϕ that were stimulated with PMN-II.
Fig. 1.
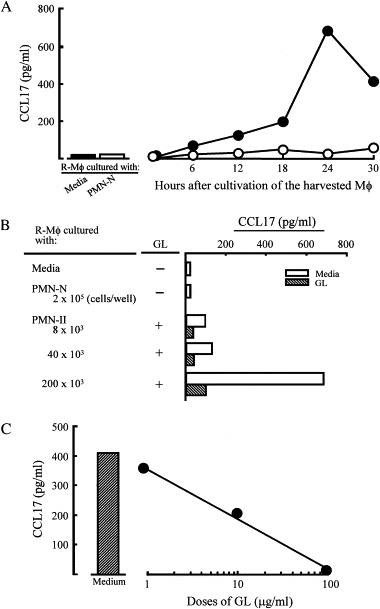
Inhibitory effect of GL on M2Mϕ generation stimulated with PMN-II. (A) Time course. R-Mϕ (6 × 105 cells/well, lower chamber) were cultured with PMN-II (2 × 105 cells/well, upper chamber) supplemented with SAC (0.0075%) in a dual-chamber transwell. The transwell cultures were performed in the presence (open circles) or absence (filled circles) of a 100 μg/ml dose of GL. Culture fluids harvested were assayed for CCL17, as a parameter of M2Mϕ. (B) M2Mϕ generation stimulated with PMN-II. R-Mϕ were cultured with PMN-II (0.08–2 × 105 cells/well, upper chamber) previously supplemented with SAC in a dual-chamber transwell. The transwell cultures were performed in the presence or absence of GL (100 μg/ml). Cells were harvested from the lower chamber 18 h after cultivation and recultured for 24 h without any stimulation. Culture fluids harvested were assayed for CCL17. (C) Dose–response inhibitory effects of GL. The transwell cultures were performed with various doses of GL. Culture fluids harvested were assayed for CCL17. The data are displayed as the mean CCL17 production ± S.D. and are representative of three experiments.
3.2. M2Mϕ were not induced by PMN-II previously treated with GL
M2Mϕ generation from R-Mϕ was inhibited when transwell cultures of R-Mϕ and PMN-II were performed in the presence of GL. Therefore, we next examined the effect of GL on M2Mϕ generation in cultures of R-Mϕ previously treated with GL. Also, transwell cultures of R-Mϕ were performed with PMN-II previously treated with GL. Thirty-seven percent of CCL17 production was inhibited when R-Mϕ were cultured with 1 μg/ml of GL. M2Mϕ were not generated from R-Mϕ in transwells after cultivation with PMN-II previously treated with a 100 μg/ml dose of GL for 12 h ( Fig. 2). In contrast, R-Mϕ previously treated with a 100 μg/ml dose of GL for 3–18 h converted to M2Mϕ when they were cultured with PMN-II in transwells ( Fig. 3). These results indicate that GL inhibits M2Mϕ generation through the alteration of the PMN-II function.
Fig. 2.
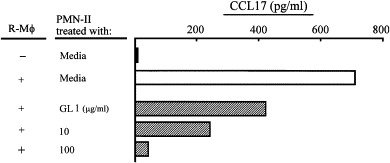
M2Mϕ were not generated in cultures of R-Mϕ and GL-treated PMN-II. R-Mϕ (6 × 105 cells/well, lower chamber) were cultured with PMN-II (2 × 105 cells/well, upper chamber) previously treated with GL (1–100 μg/ml) in dual-chamber transwells supplemented with SAC. Cells were harvested from the lower chamber 18 h after cultivation and recultured for 24 h. Culture fluids harvested were assayed for CCL17. The data are displayed as the mean ± S.D. and are representative of three experiments.
Fig. 3.
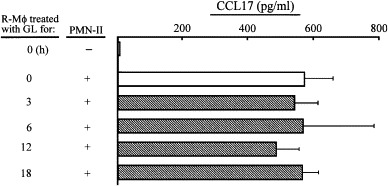
M2Mϕ were generated in cultures of GL-treated R-Mϕ and PMN-II. R-Mϕ (6 × 105 cells/well, lower chamber) previously treated with GL (100 μg/ml) for 3–12 h were cultured with PMN-II (2 × 105 cells/well, upper chamber) in dual-chamber transwells supplemented with SAC. Cells were harvested from the lower chamber 18 h after cultivation and recultured for 24 h. Culture fluids harvested were assayed for CCL17. The data are displayed as the mean ± S.D. and are representative of three experiments.
3.3. GL inhibits the production of CCL2 and IL-10 by PMN-II
In our previous studies, M2Mϕ were shown to be generated from R-Mϕ after cultivation with PMN-II, and CCL2 and IL-10 released from PMN-II were shown to be effector molecules for M2Mϕ generation from R-Mϕ [14]. Therefore, the inhibitory effect of GL on the production of CCL2 and IL-10 by PMN-II was examined. In the presence of a 100 μg/ml dose of GL, PMN-II were examined for their abilities to produce CCL2 and IL-10. This experiment was performed with a PMN-II preparation obtained from mice 18 h after thermal injury, based on the time course of the appearance of PMN-II in thermally injured mice [14]. PMN-II produced CCL2 into their culture fluids 6–18 h after cultivation. However, CCL2 production was significantly inhibited 12–18 h after GL treatment. Similarly, PMN-II produced IL-10 into their culture fluids 6–18 h after cultivation, while PMN-II treated with GL did not produce IL-10 into their culture fluids ( Fig. 4). These results suggest that GL inhibits M2Mϕ generation through the inhibition of CCL2 and IL-10 production by PMN-II.
Fig. 4.
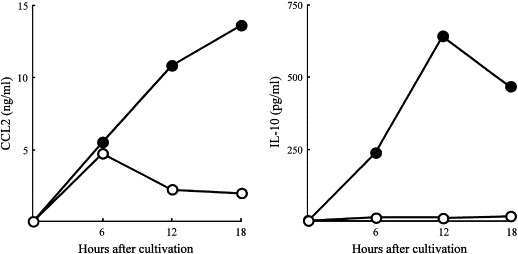
Inhibitory effect of GL on the production of CCL2 and IL-10 by PMN-II. PMN-II (2 × 106 cells/ml) were stimulated with SAC in the presence (open circles) or absence (filled circles) of GL (100 μg/ml). Culture fluids harvested 6–18 h after the cultivation were assayed for CCL2 and IL-10. The data are displayed as the mean production of CCL2 and IL-10 ± S.D., and are representative of three experiments.
4. Discussion
In the present study, the inhibitory effect of GL on M2Mϕ generation stimulated with PMN-II was investigated in vitro. M2Mϕ were generated from R-Mϕ after cultivation with PMN-II in dual-chamber transwells. However, M2Mϕ were not generated from R-Mϕ cultured with PMN-II in transwell cultures supplemented with GL. In our previous study, CCL2 and IL-10 released from PMN-II were shown as effector molecules for M2Mϕ generation, because a mixture of mAbs directed against CCL2 and IL-10 completely inhibited the generation of M2Mϕ from R-Mϕ stimulated with the PMN-II culture fluid [14]. In addition, M2Mϕ generation from R-Mϕ was demonstrated after treatment with a mixture of rCCL2 and rIL-10. When GL at a dose of 100 μg/ml was added to these cultures, M2Mϕ generation from R-Mϕ was not influenced. This suggests that GL does not prevent Mϕ conversion from R-Mϕ to M2Mϕ directly stimulated with CCL2 and IL-10. In contrast, GL showed an ability to inhibit the production of IL-10 and CCL2 from PMN-II. These results suggest that GL inhibits PMN-II-associated Mϕ conversion from R-Mϕ to M2Mϕ through the inhibition of CCL2 and IL-10 production by PMN-II. According to our recent studies [13], [14], in this experiment, peritoneal Mϕ were used. Because the repression mechanism of M2 skewing in bone marrow-derived Mϕ was shown to be different from that of tissue-derived Mϕ [28], further studies will be needed to clarify the effect of GL on PMN-II-stimulated M2Mϕ generation from various Mϕ populations. As reported in many papers [29], [30], [31], the non-cytotoxic properties of GL have been shown. Cytotoxicities of GL (1–200 μg/ml) against various murine and human cells were not demonstrated when the assay was performed by (i) trypan blue dye-exclusion test, (ii) the proliferative responses of cells stimulated with anti-CD3 mAb or Con A, (iii) H-2 class II antigen expression, and (iv) interferon production. These results strongly suggest that GL at concentrations of 1–100 μg/ml is not cytotoxic to Mϕ and PMN-II.
Recently, we have reported that mice with systemic inflammatory response syndrome (SIRS) are susceptible to Enterococcus faecalis, MRSA and infectious complications induced by cecal ligation and puncture [32]. M2Mϕ generated in association with SIRS manifestations have been determined as effector cells on the increased susceptibility of SIRS mice to various infections. Also, CCL2, a product of the SIRS development, was determined as an effector factor on M2Mϕ generation. On the other hand, the ability of Th2 cytokines (IL-10, IL-4 and/or IL-13) to induce M2Mϕ has been well documented [7], [8], [33]. In this paper, GL was shown to be an inhibitor of CCL2 and IL-10 production by PMN-II. The in vitro effect of GL on inhibiting CCL2 production has been reported in cultures of human peripheral blood monocytes infected with HIV or treated with an inducer of M2Mϕ generation (1-methyladenosine) [15], murine peritoneal Mϕ from SIRS mice [34] and R-Mϕ treated with norepinephrine [35]. Recently, several studies have indicated that the enhancer region of the CCL2 gene is inhibited by glucocorticoids, progesterone and estrogen in cells stimulated with LPS, IL-1 or TNF-α, after binding to their receptors [36], [37]. 18β-Glycyrrhetinic acid (GA), a final metabolite of GL in a living body, is similar to deoxycorticosterone and this metabolite binds to glucocorticoid receptors [38]. In fact, this metabolite has been shown to inhibit CCL2 production by peritoneal Mϕ stimulated with IL-1. GL may suppress CCL2 production through the inhibition of CCL2 gene activation after binding to glucocorticoid receptors. Further studies are required to clarify the molecular mechanism by which GL inhibits CCL2 and IL-10 production.
In our recent study, Gr-1+CD11b+ immature myeloid cells were demonstrated in the spleens of mice 6 h after burn injury. These burn-associated immature myeloid cells may be a precursor cell of M2Mϕ, or a stimulator cell of R-Mϕ to generate M2Mϕ. In addition, it has been described in recent papers that TLR reactivity of Mϕ increases in mice 7 days after thermal injury, but not early after the injury [39], [40]. GL may play a role on the myeloid cell generation or TLR reaction of Mϕ. Further studies are required.
In the present study, PMN-II obtained from the peripheral blood of thermally injured mice were utilized. PMN with properties similar to PMN-II have recently been demonstrated in the peripheral blood of mice with severe pancreatitis, progressive tumors and severe infections. These mice have also been recognized as hosts with M2Mϕ. In normal individuals, M1Mϕ are generated in response to bacterial products or cytokines released in the early stages of host innate responses against bacterial invasion, and contribute to eradicate early bacterial growth [7], [8]. Because M2Mϕ suppress the generation of M1Mϕ [13], M1Mϕ generation will be minimal even if hosts with a predominance of M2Mϕ generation are exposed to pathogens. For this reason, carriers of M2Mϕ would be susceptible to various infections. Utilizing the in vitro assay system, here we showed that GL inhibits M2Mϕ generation stimulated with PMN-II. The regulation of PMN-II-associated M2Mϕ generation by GL may provide a new therapeutic strategy, which could influence the outcome of certain severe infections in hosts with M2Mϕ generation.
Acknowledgments
This work was partially supported by grants (#8690 and #8840) from the Shriners of North America. The authors would like to thank Minophagen Pharmaceutical Co. Ltd. (Tokyo, Japan) for their generous donation of GL.
References
- 1.McManus A.T., Mason A.D., Jr., McManus W.F., Jr., Pruitt B.A. Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur J Clin Microbiol. 1985;4:219–223. doi: 10.1007/BF02013601. [DOI] [PubMed] [Google Scholar]
- 2.Mason A.D., Jr., McManus A.T., Pruitt B.A., Jr. Association of burn mortality and bacteremia. A 25-year review. Arch Surg. 1986;121:1027–1031. doi: 10.1001/archsurg.1986.01400090057009. [DOI] [PubMed] [Google Scholar]
- 3.Sittig K., Deitch E.A. Effect of bacteremia on mortality after thermal injury. Arch Surg. 1988;123:1367–1370. doi: 10.1001/archsurg.1988.01400350081012. [DOI] [PubMed] [Google Scholar]
- 4.Manson W.L., Coenen J.M., Klasen H.J., Horwitz E.H. Intestinal bacterial translocation in experimentally burned mice with wounds colonized by Pseudomonas aeruginosa. J Trauma. 1992;33:654–658. doi: 10.1097/00005373-199211000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Schwacha M.G. Macrophages and post-burn immune dysfunction. Burns. 2003;29:1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 6.Aderem A., Underhill D.M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 7.Goerdt S., Orfanos C.E. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–142. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 9.Elgert K.D., Alleva D.G., Mullins D.W. Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol. 1998;64:275–290. doi: 10.1002/jlb.64.3.275. [DOI] [PubMed] [Google Scholar]
- 10.Hickman S.P., Chan J., Salgame P. Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naive T cell polarization. J Immunol. 2002;168:4636–4642. doi: 10.4049/jimmunol.168.9.4636. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki F., Pollard R.B. Mechanism for the suppression of gamma-interferon responsiveness in mice after thermal injury. J Immunol. 1982;129:1811–1815. [PubMed] [Google Scholar]
- 12.Takagi K., Suzuki F., Barrow R.E., Wolf S.E., Kobayashi M., Herndon D.N. Growth hormone improves immune function and survival in burned mice infected with herpes simplex virus type 1. J Surg Res. 1997;69:166–170. doi: 10.1006/jsre.1997.5066. [DOI] [PubMed] [Google Scholar]
- 13.Katakura T., Miyazaki M., Kobayashi M., Herndon D.N., Suzuki F. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol. 2004;172:1407–1413. doi: 10.4049/jimmunol.172.3.1407. [DOI] [PubMed] [Google Scholar]
- 14.Tsuda Y., Takahashi H., Kobayashi M., Hanafusa T., Herndon D.N., Suzuki F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21:215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Takei M., Kobayashi M., Li X.D., Pollard R.B., Suzuki F. Glycyrrhizin inhibits R5 HIV replication in peripheral blood monocytes treated with 1-methyladenosine. Pathobiology. 2005;72:117–123. doi: 10.1159/000084114. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H., Ohta Y., Takino T., Fujisawa K., Hirayama C. Effects of glycyrrhizin on biomedical tests in patients with chronic hepatitis-double blind trial. Asian Med J. 1983;26:423–438. [Google Scholar]
- 17.van Rossum T.G., Vulto A.G., de Man R.A., Brouwer J.T., Schalm S.W. Glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment Pharmacol Ther. 1998;12:199–205. doi: 10.1046/j.1365-2036.1998.00309.x. [DOI] [PubMed] [Google Scholar]
- 18.Arase Y., Ikeda K., Murashima N., Chayama K., Tsubota A., Koida I. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494–1500. doi: 10.1002/(sici)1097-0142(19970415)79:8<1494::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Kumada H. Long-term treatment of chronic hepatitis C with glycyrrhizin (stronger neo-minophagen C (SNMC)) for preventing liver cirrhosis and hepatocellular carcinoma. Oncology. 2002;62(Suppl 1):94–100. doi: 10.1159/000048283. [DOI] [PubMed] [Google Scholar]
- 20.Numazaki K., Nagata N., Sato T., Chiba S. Effect of glycyrrhizin, cyclosporin A, and tumor necrosis factor α on infection of U-937 and MRC-5 cells by human cytomegalovirus. J Leukoc Biol. 1994;55:24–28. doi: 10.1002/jlb.55.1.24. [DOI] [PubMed] [Google Scholar]
- 21.Pompei R., Flore O., Marccialis M.A., Pani A., Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature. 1979;281:689–690. doi: 10.1038/281689a0. [DOI] [PubMed] [Google Scholar]
- 22.Utsunomiya T., Kobayashi M., Pollard R.B., Suzuki F. Glycyrrhizin, an active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus. Antimicrob Agents Chemother. 1997;41:551–556. doi: 10.1128/aac.41.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki H., Takei M., Kobayashi M., Pollard R.B., Suzuki F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV sero(+) patients. Pathobiology. 2002;70:229–236. doi: 10.1159/000069334. [DOI] [PubMed] [Google Scholar]
- 25.Abe N., Ebina T., Ishida N. Interferon induction by glycyrrhizin and glycyrrhetinic acid in mice. Microbiol Immunol. 1982;26:535–539. doi: 10.1111/j.1348-0421.1982.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 26.Itoh K., Kumagai K. Augmentation of NK activity by several anti-inflammatory agents. Excerpta Medica. 1983;641:460–464. [Google Scholar]
- 27.Furukawa K., Kobayashi M., Herndon D.N., Pollard R.B., Suzuki F. Appearance of monocyte chemoattractant protein 1 (MCP-1) early after thermal injury: role in the subsequent development of burn-associated type 2 T-cell responses. Ann Surg. 2002;236:112–119. doi: 10.1097/00000658-200207000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauh M.J., Ho V., Pereira C., Sham A., Sly L.M., Lam V. SHIP represses the generation of alternatively activated macrophages. Immunity. 2005;23:361–374. doi: 10.1016/j.immuni.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Shinada M., Azuma M., Kawai H., Sazaki K., Yoshida I., Yoshida T. Enhancement of interferon-γ production in glycyrrhizin-treated human peritoneal lymphocytes in response to concanavalin A and to surface antigen of hepatitis B virus. Proc Soc Exp Biol Med. 1986;181:205–210. doi: 10.3181/00379727-181-42241. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y.H., Yoshida T., Isobe K., Rahman S.M.J., Nagase F., Ding L. Modulation by glycyrrhizin of the cell-surface expression of H-2 class I antigens on murine tumor cell lines and normal cell populations. Immunology. 1990;70:405–410. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y.H., Isobe K., Nagase F., Lwin T., Kata M., Hamaguchi M. Glycyrrhizin as a promoter of the late signal transduction for interkeukin-2 production by splenic lymphocytes. Immunology. 1993;79:528–534. [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi H., Tsuda Y., Takeuchi D., Kobayashi M., Herndon D.N., Suzuki F. Influence of systemic inflammatory response syndrome on host resistance against bacterial infections. Crit Care Med. 2004;32:1879–1885. doi: 10.1097/01.ccm.0000139606.34631.61. [DOI] [PubMed] [Google Scholar]
- 33.Stein M., Keshav S., Harris N., Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takei M., Takahashi H., Tsuda Y., Kobayashi M., Herndon D.N., Suzuki F. GL inhibits manifestations of compensatory anti-inflammatory response syndrome appeared in association with systemic inflammatory response syndrome. FASEB J. 2003;17:C48. [Google Scholar]
- 35.Kobayashi M., Takei M., Takahashi H., Herndon D.N., Pollard R.B., Suzuki F. Glycyrrhizin inhibits norepinephrine-associated CCL2 production in mice with systemic inflammatory response syndrome. FASEB J. 2004;18:A444. [Google Scholar]
- 36.Mukaida N., Zachariae C.C., Gusella G.L., Matsushima K. Dexamethasone inhibits the induction of monocyte chemotactic-activating factor production by IL-1 or tumor necrosis factor. J Immunol. 1991;146:1212–1215. [PubMed] [Google Scholar]
- 37.Frazier-Jessen M.R., Kovacs E.J. Estrogen modulation of JE/monocyte chemoattractant protein-1 mRNA expression in murine macrophages. J Immunol. 1995;154:1838–1845. [PubMed] [Google Scholar]
- 38.Menard U.J., Corvol P. Binding of glycyrrhetinic acid to kidney mineralocorticoid and glucocorticoid receptors. Endocriol. 1975;97:46–51. doi: 10.1210/endo-97-1-46. [DOI] [PubMed] [Google Scholar]
- 39.Paterson H.M., Murphy T.J., Purcell E.J., Shelley O., Kriynovich S.J., Lien E. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 40.Maung A.A., Fujimi S., Miller M.L., MacConmara M.P., Mannick J.A., Lederer J.A. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:565–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]


