Abstract
Rotavirus diarrhea is a major cause of infantile gastroenteritis worldwide. This review is mainly devoted to the effects of Rotavirus on intestinal epithelial transport and to the pathophysiological mechanisms proposed to underlie the intestinal fluid secretion caused by the virus.
Keywords: Rotavirus, intestine, diarrhea
1. Introduction
Acute diarrheal diseases are still a major health problem throughout the world, causing 25–30% of all deaths among children younger than 5 years of age in developing countries 〚1〛. Diarrheal diseases are also of public health importance in developed countries and are associated with considerable morbidity and a substantial number of hospitalizations among children and the elderly. Acute diarrhea can be caused by many different agents including parasites, bacteria and viruses; the latter of which have been given significant attention in recent years. This review is devoted to Rotavirus-induced enteric infection, and in particular, to the pathophysiological mechanisms proposed to underlie the intestinal fluid secretion caused by the virus.
2. General characteristics of Rotavirus and Rotavirus enteric infections
Rotavirus is the leading cause of infantile gastroenteritis worldwide and is responsible for approximately 20% of diarrhea-associated deaths in children under 5 years of age 〚2〛. Rotaviruses were first identified in humans in1973 when characteristic particles were observed in the cytoplasm of duodenal epithelial cells obtained from young children admitted to the hospital for treatment of acute diarrhea 〚3〛. While Rotavirus infections are universal and occur regardless of socioeconomic status or environmental conditions, the outcome and consequences of the disease differ significantly between developed and developing countries.
2.1. General features of Rotavirus
Rotaviruses are members of the Reoviridae family and are characterized by their segmented (11 segments) double-stranded RNA genome. Each of the 11 genes codes for a single gene product. Six of the proteins are found in the virus particle (vp1, vp2, vp3, vp4, vp6 and vp7), whereas the remaining five proteins are non-structural (NDP1–NSP5). Rotavirus is classified into serogroups A–E based on antigenic properties. Only groups A–C have been shown to infect humans and most human Rotavirus disease is caused by group A Rotavirus. The group A Rotavirus are further classified into G (serotypes) and P types based on identification of antigens on the outer capsid proteins. Of the 14 serotypes (G-types) that have been identified in various species, nine have been recognized in humans, and of these, four serotypes (1–4) are dominant globally.
Several of the Rotavirus genes have been associated with their ability to cause disease 〚4〛. The non-structural proteins NSP1, NSP2 and NSP4 are believed to be involved in virulence in mice 〚5〛, 〚6〛 and the structural proteins vp3 and vp7 in pigs 〚7〛, 〚8〛. Finally, the structural protein vp4 is involved in a number of in vitro properties, including restriction of growth in cell culture, protease-enhanced infectivity and plaque formation and binding to cells. In addition, vp4 has been shown to be involved in virulence and pathogenicity in mice.
2.2. Rotavirus binding and entry into intestinal epithelial cells
Rotavirus infects the mature enterocytes in the mid and upper part of the villi of the small intestine, which ultimately leads to diarrhea. Current information indicates that Rotavirus attachment and entry into cells constitute a multistep process. Recent models suggest that rotaviruses interact first with a sialic acid receptor and then with a sialic acid-independent receptor 〚9〛. It has been proposed that the receptors might be part of lipid microdomains 〚10〛 and recognize integrins 〚11〛. In view of this and other information, two opposing hypotheses have been proposed as to how Rotavirus enters the target cell: through direct entry or fusion 〚12〛 and through Ca2+-dependent endocytosis 〚13〛. The Ca2+-dependent endocytosis process represents the most attractive mode of entry and is based on the fact that infectious Rotavirus is endocytosed into the cytoplasm with a very low Ca2+ concentration that leads to a Ca2+ afflux from the vesicles to the cytoplasm. Once the endosome Ca2+ concentration equilibrates with the cytoplasm, below the critical level for stability of the outer capsid, the virus loses its outer proteins and then lyses with the vesicle membrane, permitting the virus to escape to the cytoplasm 〚14〛.
2.3. Humoral and cell-mediated immune responses to Rotavirus
A symptomatic infection with Rotavirus stimulates a strong humoral IgG immune response which lasts for the lifetime. While the IgG responses are easily recorded, it is generally thought that protection from Rotavirus disease is mediated by local IgA antibodies. Several studies have shown that local IgA is important or at least a good predictor of protection 〚15〛. Two proteins on Rotavirus, vp4 and vp7, are thought to play a critical role in mounting a protective immune response. Both proteins are constituents of the outer capsid and have been found, independently of each other, to stimulate production of neutralizing antibodies. Children infected with a particular serotype of Rotavirus are more likely to be protected against challenge with the same serotype than against re-infection with a different serotype. However, heterotypic protection is operating, as has been found in several studies. There are several possible explanations for this. One is that local IgA antibodies recognize cross-reacting epitopes on vp4 and vp7, another is that antibodies to the inner capsid protein vp6 can modulate protection, and a third possibility is that cross-reacting virus-specific cytotoxic T lymphocytes (CTLs) participate in protection. The role of CTLs in Rotavirus infections is, however, not yet established.
2.4. Age range of human infections
Rotavirus infections may occur repeatedly in humans from birth to old age. Neonatal infections appear to be nosocomial in origin, because they are rarely seen in babies born at home or at village health centers. Serological surveys imply that most children have experienced a Rotavirus infection by 24 months of age. Contrasting with the capacity of Rotavirus to cause infection at any age, the clinical consequences of infection appear to be strongly influenced by age. For example most neonatal infections are asymptomatic or produce only mild symptoms. The relative resistance to clinical symptoms that is observed in older children and adults is not likely to be due to age-altered physiological status, as in certain small animals, but more likely due to active immunity, reinforced by repeated infections throughout life. Rotavirus gastroenteritis in adults has been well documented from several countries and even deaths have been reported 〚16〛.
3. Pathophysiology of Rotavirus-induced diarrhea
3.1. Histological changes evoked by Rotavirus infection
A large number of investigators have studied the histological changes that take place in the intestine following Rotavirus infection. In most cases these studies have involved naturally and experimentally infected animals. Here a brief survey of the literature will be given. For more detailed reviews of the literature, the reader is referred to reference 〚17〛.
In colostrum-deprived calves, Rotavirus infection leads to a change in the villus epithelium from columnar to cuboidal, and villi became stunted and shortened. Changes occur within 24 h of infection and are most pronounced in the proximal small intestine. Inflammation is virtually absent. Strains causing less illness were characterized by milder histological changes 〚18〛. These histological changes are not limited to calves with symptomatic response; in fact asymptomatically infected calves show villus blunting too 〚19〛. In pigs, the macroscopic changes include thinning of the intestinal wall, and microscopic changes include villus atrophy, villus blunting and conversion to a cuboidal epithelium. As with bovine Rotavirus, porcine rotaviruses have been shown to differ somewhat in their ability to cause histologic lesions 〚20〛. The SDSV strain of porcine Rotavirus causes more severe villus atrophy than the OSU strain, but the two strains produce similar clinical symptoms. Finally, rabbits infected experimentally with Rotavirus show normal histology in the face of marked changes in glucose and leucine uptake 〚21〛. These observations once again underline that there is no absolute correlation between histological lesions and clinical symptoms.
While Rotavirus infections have been studied more extensively in mice than in any other species, the model has one disadvantage: the restriction of illness to the first 2 weeks of life. The pathology of murine Rotavirus is generally similar to that of lambs, pigs and calves, but differs in certain aspects. Within 24 h after infection with murine Rotavirus, mouse intestinal enterocytes appear swollen and vacuolated. Infection and histologic changes are concentrated to the upper small intestine. Vacuolation of enterocytes is most prominent on the villus tip, but can occur in enterocytes throughout the villus. The vacuolation is more extensive in mice than in other species. Unlike other species, the villus blunting is limited and very transient in mice, despite clinical signs of diarrhea. The lack of extensive pathologic changes found in the mouse intestine and the observation that many enterocytes appear vacuolated but do not show evidence of replication led Osborne and coworkers 〚22〛 to propose that in mice, fluid loss in diarrhea is secondary to a local villus ischemia, as discussed in more detail below.
In contrast to animal studies, there are few pathology studies of the jejunal mucosa of infants infected with Rotavirus. Studies of biopsies have revealed shortening and atrophy of villi, distended endoplasmic reticulum, mononuclear cell infiltration, mitochondrial swelling and denudation of microvilli 〚23〛, 〚21〛.
3.2. Fluid and electrolyte transport in virus enteritis
Most of the studies of the effects of viral enteritis on fluid, sodium and chloride transport across the intestinal epithelium have been performed using viruses other than Rotavirus, not seldom a Coronavirus. It should be underlined that Coronavirus often causes a much more pronounced villus blunting than Rotavirus strains 〚17〛. Furthermore, one type of Coronavirus used in one and the same laboratory may, for unknown reasons, apparently evoke varying morphological and pathophysiological responses in different experimental series (compare, e.g., references 〚24〛 and 〚25〛). This variation in response makes it difficult to draw general conclusions from the reported observations.
There are surprisingly few in vivo studies of virus diarrhea in anesthetized animals. When the fluid perfusing the intestine contained glucose, Coronavirus inoculation turned a net uptake of sodium, chloride and water into net secretion. Unidirectional determinations of sodium using 22Na revealed that the altered sodium transport was, as expected, caused both by a decreased lumen-to-tissue flux and by an increased tissue-to-lumen flux 〚25〛. When no glucose was present in the intestinal lumen of newborn pigs, a net secretion of water and sodium ions was observed in control animals. Infecting these pigs with Coronavirus did not significantly change net water and sodium transport compared with animals not inoculated with virus 〚26〛.
A time sequence study of Rotavirus enteritis in rats was performed by Salim et al. 〚27〛 in vivo. In contrast to the pig experiments summarized above, a net fluid and sodium uptake was observed in the control rats when perfusing the intestinal lumen with an isotonic electrolyte solution devoid of glucose. Net fluid secretion and a significantly decreased net sodium uptake peaked 24 h after virus inoculation. Concomitantly, villus height had decreased to one-third of control, whereas crypt depth was unaltered. Similarly, Starkey et al. 〚28〛 investigated epithelial transport of murine intestines in vitro at different times after inoculation with Rotavirus. The intestinal segments were perfused with an isotonic electrolyte solution containing mannitol in an organ bath. A significant secretion of fluid, sodium and chloride ions was only seen at 72 h postinoculation, a time at which the clinical signs of diarrhea in awake mice was most pronounced 〚29〛. In line with this, Lundgren et al. 〚30〛 showed with a similar technique that 48–60 h after infecting newborn mice with Rotavirus a net fluid secretion was observed and indirect evidence was also obtained for an increased electrolyte secretion.
Most of the studies of electrolyte transport in virus-infected intestinal segments have been performed in vitro with the so-called Ussing chamber technique. The intestinal segment is then almost always stripped of its muscle layer to improve oxygenation of the tissue. Changes in net electrolyte transport are often monitored by recording the potential difference (PD) across the epithelium and/or by electrically short-circuiting the epithelium, measuring short circuit current (SCC). The Ussing chamber technique can also be combined with measurements of unidirectional fluxes of electrolytes.
When performing Ussing chamber experiments on intestines devoid of their muscle layers, the results reported are in some respects different from those recorded in vivo. In the presence of luminal glucose net sodium uptake is turned into secretion, whereas virus evokes no effect in the absence of glucose 〚24〛, 〚31〛, 〚32〛, 〚33〛. The induced change in sodium transport (glucose present) is in part explained by an attenuated mucosa-to-serosa flux. Surprisingly, the serosa-to-mucosa flux of sodium or chloride ions is larger in the control intestines than in the coronavirus-infected intestines 〚24〛, 〚31〛, 〚32〛, 〚33〛, 〚34〛, 〚35〛 despite the fact that morphological measurements often show that the crypts are larger in infected than in control intestines. In line with this, SCCs in control intestines were often found to be greater than in infected intestines in the face of a lower tissue conductance of the infected intestines 〚32〛, 〚34〛. In other studies SCC was similar in infected and non-infected intestinal segments 〚24〛, 〚32〛, 〚35〛, 〚36〛.
One possible explanation for observed differences between in vivo and in vitro experiments reviewed above is that stripping an intestinal segment of its muscle layers markedly alters the functions of the enteric nervous system, as will be discussed later.
3.3. Glucose and amino acid transport
Apical symports on villus enterocytes transport sodium ions together with glucose or amino acids. It has been repeatedly reported that the co-transport of glucose and sodium in some way is impaired in intestinal segments exposed to Rotavirus or Coronavirus. In Ussing chamber experiments the increases in PD and SCC upon adding glucose to the mucosal incubation medium were significantly smaller in the infected intestinal segments 〚32〛. Unidirectional measurements of sodium fluxes indicated that this difference in response was mainly explained by a blunted glucose effect on the lumen-to-tissue flux of sodium 〚32〛. In line with this, the glucose effect on intestinal net sodium and water flux, measured in infected piglets in vivo, was absent or considerably smaller than that seen in normal intestines 〚26〛. The influence of the virus infection on glucose transport has also been investigated using the more sophisticated technique of brush border membrane vesicles. Keljo et al. 〚37〛 were unable to demonstrate a high-affinity sodium-dependent glucose transporter in virus-infected intestines. In agreement with this, Halaihel et al. 〚38〛 showed that the Na-D-glucose symport (SGLT1) was strongly inhibited by Rotavirus. However, it should be pointed out that in vivo experiments show that in the presence of glucose Coronavirus-infected intestines show a net uptake of fluid and sodium.
Another factor of importance for the absorption of glucose and other sugars is the disaccharidases localized to the brush border region of enterocytes. In several reports it has been demonstrated that in viral enteritis the activity of all mucosal disaccharidases studied (sucrase, lactase, maltase) 〚24〛, 〚31〛, 〚34〛, 〚35〛, 〚39〛, 〚40〛, 〚41〛 is markedly attenuated. Lowered levels of disaccharidase activity have also been measured in biopsies from patients 〚23〛. Hence, the activity of the enzymes necessary for providing the sodium-monosaccharide symport with monosaccharides is in some way decreased by the virus.
The apical membrane of enterocytes is not only provided with symports for sodium/glucose but also for sodium/amino acids. The response of sodium absorption to L-alanine (20 mM) was significantly blunted in intestinal segments exposed to Coronavirus 〚33〛. This conclusion was further strengthened by studies of sodium transport into brush border vesicles, demonstrating a marked inhibition of Na-L-alanine symport in virus enteritis in piglets (Coronavirus) 〚42〛 and young rabbits (Rotavirus) 〚38〛. On the other hand, Rhoads et al. 〚43〛 could not record any blunted effects of L-glutamine or L-alanine on sodium transport in Rotavirus-infected pig intestines.
To summarize, virus enteritis in animals caused by Coronavirus or Rotavirus evokes in vivo a net secretion of fluid and an attenuated uptake/secretion of sodium and chloride. Most studies suggest that one mechanism that at least in part may explain the virus-induced attenuation of electrolyte and fluid absorption is an inhibition of the symports for sodium and glucose/amino acids. A lowered activity of the brush border enzymes probably also contributes to a diminished absorptive capacity. Finally, the presence of blunted villi, decreasing the surface area for fluid and solute uptake, may be of importance.
3.4. Sodium-potassium ATPase
The transport across the polar epithelium of the gut is to a large extent driven by the electro-chemical gradient across the apical border established by the sodium-potassium pump situated on the basolateral membrane of the enterocyte. There are many studies that report that the activity of Na, K ATPase is attenuated in virus-infected intestines 〚34〛, 〚35〛, 〚38〛, 〚40〛, 〚42〛 . This may reflect a true decrease of ATPase activity but may also be explained by the blunting of the intestinal villi reducing the number of enterocytes. Quantitatively, the relative decrease in Na, K ATPase activity is much smaller than observed, for example, in sucrase or lactase 〚33〛, 〚34〛, 〚37〛, 〚39〛, 〚41〛.
3.5. Epithelial permeability
Another variable of importance for intestinal epithelial transport is epithelial permeability. This parameter has been determined in the intestinal epithelium at various levels of integration after exposing the intestinal mucosa to virus. Cultured immortal intestinal epithelial cell lines represent the most reductionistic level. Three investigations have used Caco-2 cells originally derived from a colonic adenocarcinoma 〚44〛, 〚45〛, 〚46〛. All authors report that transepithelial electrical resistance decreased after Rotavirus exposure to the apical or basolateral plasma membrane. Molecular probes of various sizes demonstrated that intestinal permeability to molecules with masses ranging from 182 (mannitol) to 70 000 Da (dextran) increased in cultured cells exposed to Rotavirus 〚45〛, 〚46〛, reflecting an increased paracellular permeability, possibly caused by a disorganization of tight junction associated proteins claudin, occludin and ZO-1.
Ussing chamber experiments represent the next level of integration when studying epithelial permeability. Keljo et al. 〚37〛 and Heyman et al. 〚47〛 observed a transient increase in absorption of horse radish peroxidase (HRP; 40 000 Da) on the second day after infection. Electrical tissue conductance has been reported to be increased in Rotavirus-infected intestines 〚30〛, 〚48〛, whereas it was consistently reported to be decreased in studies using Coronavirus 〚31〛, 〚32〛, 〚33〛, 〚34〛, 〚35〛, 〚36〛, 〚37〛. The reason for this difference between virus strains is not known.
The results of the investigations of intestinal permeability in vivo, performed on newborn piglets exposed to Coronavirus 〚49〛, 〚50〛, 〚51〛, 〚52〛, 〚53〛, vary even within the same research group 〚49〛, 〚50〛, 〚51〛, 〚52〛. The varying results makes it impossible to draw any certain conclusions.
Intestinal permeability has also been investigated in young children with Rotavirus diarrhea using polyethylene glycols (PEGs) of varying molecular masses as probes of intestinal permeability. The PEG absorption was significantly lower during the acute phase of virus enteritis than 3–5 weeks postinfection. This was primarily ascribed to a decreased surface area for absorption, whereas the relationship between the rates of absorption of large and small molecules suggested an increased permeability for the PEGs studied 〚54〛.
To summarize, the permeability studies performed at different levels of integration using Rotavirus clearly suggest that the infection increases paracellular permeability via an effect on tight junctions. To what extent this is true for Coronavirus infection remains to be established. Increased permeability may be critical to the organism since it allows epithelial passage of potentially toxic and inflammatory substances.
4. Hypotheses
The discussion below regarding the pathophysiological mechanisms underlying the virus-evoked intestinal fluid losses is based on the assumption that in the normal small intestine villi absorb and crypts secrete electrolytes and fluid. There are several observations to support this view. In particular, a hyperosmolar compartment, mainly accounted for by sodium chloride, is present in the lamina propria of the upper third of the villus 〚55〛, 〚56〛. This tissue hyperosmolality has been shown to be well maintained after exposing the intestinal mucosa to cholera toxin in the face of an intestinal net fluid secretion 〚57〛. To what extent this holds true in intestinal secretory states caused by virus has never been directly determined. Indirect observations reported by Osborne et al. 〚22〛, 〚58〛 suggest that it may be the case. They observed that red cells in the upper part of normal and Rotavirus-infected villi were crenated, which they ascribed to the presence of a hyperosmolar compartment.
The pathophysiological mechanisms underlying the fluid losses seen in different types of diarrhea have been debated for decades. The dominating hypothesis up to the 1970s was that most diarrheas, including the infectious ones, were caused by motility disturbances. However, during the last three decades it has become increasingly evident that disturbances in the epithelial transport are often one of the major causes of intestinal fluid loss, although motility may contribute, at least in some types of diarrhea.
The demonstration that cholera toxin evoked an increase in intracellular cAMP concentration represented a major breakthrough at the time 〚59〛. Subsequently, it was shown that increasing the intracellular concentrations of several second messengers such as cAMP, cGMP and calcium ions also caused fluid losses from the intestine. It was believed that the secretory agent interacted with the secreting crypts to evoke a fluid secretion via one of the second messengers mentioned. This view is too simplistic since most luminal secretagogues, including Rotavirus, do not reach the crypts of Lieberkühn 〚60〛, 〚61〛. Furthermore, in the case of virus enteritis several studies have failed to demonstrate any intracellular increases in cAMP or cGMP in human or animal enterocytes exposed to virus 〚25〛, 〚39〛, 〚41〛, 〚62〛.
We will now discuss the four hypotheses that have been proposed with regard to virus-evoked intestinal secretion of fluid and electrolytes.
4.1. Diminished absorptive capacity of the intestinal epithelium
The experimental evidence for a diminished absorptive capacity of fluid, electrolytes, glucose and amino acids in Rotavirus-infected intestines was reviewed above when describing the morphological and functional effects of the virus. Under the influence of virus the rate of epithelial absorption is no doubt attenuated. However, one may ask the question whether an inhibited uptake of fluid as such may explain the large fluid losses occurring in Rotavirus diarrhea or if secretion has to be stimulated.
Hamilton and coworkers have proposed that the increased secretion may be explained by the secretory epithelium of the crypts ‘invading’ the villi, creating an imbalance between the absorptive villous and secretory crypt epithelium. In this context it should be pointed out that Hamiltonˈs studies were performed on neonatal pigs using Coronavirus, which may not mimic Rotavirus in all its effects, as pointed out earlier when discussing epithelial permeability. As support for their hypothesis Hamilton and collaborators reported cell biological changes that indicated that the villus cells were more crypt-like in infected intestines. Thus, Hamilton et al. explain the lowered glucose uptake (see above) in infected intestines as an indication of an increased number of crypt-like cells in the intestinal mucosa 〚39〛. Furthermore, as reviewed earlier, enzymatic activity measured in mucosal scrapings of control and virus-infected intestines differs markedly. Whereas mucosal sucrase, lactase and Na, K ATPase activity were found to be higher in controls than in infected intestines, the opposite was true for thymidine kinase 〚24〛, 〚31〛, 〚34〛, 〚35〛, 〚39〛, 〚40〛. Again, the lowered disaccharidase activity is taken as evidence for the enterocytes being crypt-like. The increased thymidine kinase activity may explain the findings reported by Shepherd et al. 〚40〛 that the migration of 3H-thymidine-labelled epithelial cells along the crypt-villus axis was faster during the acute phase of virus diarrhea than in control animals. Finally, in the Coronavirus-infected intestines, the inhibitory effect of theophylline on mucosa-to-serosa flux of sodium ions could not be demonstrated. All these observations were taken to indicate that the enterocytes of infected animals exhibit the functional characteristics of crypt cells.
The reviewed studies of enzyme activities were performed on mucosal scrapings from control and Coronavirus-infected animals. Collins et al. 〚63〛 investigated the distribution of lactase activity with a histochemical technique in Rotavirus-infected mice allowing a more precise cellular localization of the enzyme. In the control situation lactase activity was observed exclusively in the villus cells. This was true also when fluid secretion was most pronounced (72 h postinfection) although lactase activity in the brush border of enterocytes was low. The latter observation argues against the view that crypt cells are populating the villi at the peak of virus diarrhea. Furthermore, a clinical study failed to demonstrate any clear correlation between decreased enzyme activity and histological changes caused by Rotavirus 〚24〛. Also, based on in vitro experiments on Caco-2 cells Jourdan et al. 〚64〛 proposed that sucrase-isomaltase activity in Rotavirus-infected cells was specifically and selectively decreased by Rotavirus without any apparent cell destruction, caused by a block of sucrase-isomaltase transport from the trans-Golgi network to the brush border membrane. Finally, Halaihel et al. 〚38〛 failed to demonstrate any decrease in the total mass of SGLT1 in the intestinal epithelium of Rotavirus-infected young rabbits, which would have been expected if the enterocytes were crypt-like.
To summarize, the reviewed studies clearly suggest that exposing the enterocytes to Rotavirus and other virus leads to a decreased rate of absorption of water, electrolytes, glucose and amino acids, which at least in part may explain the intestinal secretory response to virus. To what extent this is explained by crypts cells invading the villi remains to be established. The latter mechanism may be more important in Coronavirus than in Rotavirus enteritis.
4.2. Rotavirus enterotoxin
One of the non-structural proteins of Rotavirus, NSP4, is a transmembrane, endoplasmatic reticulum-specific glycoprotein. The early studies of this protein demonstrated that NSP4 mimicked some of the cell biological effects of Rotavirus. Hence, NSP4 increases intracellular Ca concentration mainly by mobilizing Ca from the endoplasmic reticulum 〚65〛. It was, furthermore, shown that NSP4 destabilizes plasma membrane permeability to allow leakage of, e.g., lactate dehydrogenase out of the cells 〚66〛.
While making an antiserum against NSP4, Ball et al. 〚67〛 noted that giving the protein intraperitoneally induced diarrhea in newborn mice. This observation initiated a series of studies to elucidate if NSP4 might represent a virus enterotoxin. Several observations in mice suggest that a part of NSP4 may function as an enterotoxin. Thus, it was shown that a cleavage product of NSP4 with enterotoxin activity, having an apparent molecular mass of 7 kDa, was secreted into the incubation media of cells infected with the virus. This protein constituted amino acids 112–175 of NSP4 and it induced diarrhea in neonatal mice. Furthermore, like Rotavirus and full-length NSP4, NSP4 112–175 increased intracellular calcium levels when tested in different cell culture systems 〚68〛. Finally, mutating NSP4 demonstrated that the virus’ virulence could be markedly attenuated. Deletions and substitutions in the region of amino acids 131 and 140 seemed to be particularly important for virulence, as judged by the ability of mutated NSP4 to influence intracellular calcium concentration in vitro or cause diarrhea in newborn mice 〚69〛.
Subsequently, Estes et al. have also demonstrated other functional similarities between NSP4 114–135 and Rotavirus. Hence, NSP4 114–135 was demonstrated to be a specific inhibitor of SGLT1 〚70〛, and NSP4 increased paracellular permeability of MDCK cells 〚71〛. Taken together, the functional similarities between NSP4 and Rotavirus strengthen the proposal that NSP4 or a part of it may function as a viral enterotoxin.
One characteristic property of Rotavirus enteritis in mice is its age dependency. In this species Rotavirus fails to produce diarrhea from age 15 days on. In the first report on NSP4 as an enterotoxin Estes and collaborators reported that the secretory effect of NSP4 given intraperitoneally or intraileally was age dependent, evoking a dose-dependent response in young animals (6–9 days old) but no effect in mice 17–18 days old. Similar observations were made with NSP4 residues 114–135 〚67〛. In another investigation Morris et al. 〚5〛 exposed isolated murine colonic crypts to NSP4, recording the intracellular Ca response. It was shown that NSP4 increased Ca concentration in colonic crypt cells regardless of the age of the mice from which the crypts had been taken. It was concluded that age dependency was explained by events distal to the NSP4-elicited Ca mobilization. This conclusion is based on the implicit assumption that the virus enterotoxin directly influences the Ca concentration of the secreting crypt cells.
There are reports in the literature that are not consistent with NSP4 being an important factor in the pathophysiology of Rotavirus diarrhea. It was pointed out by Angel et al. 〚72〛 that the amino acid sequence of the 131–140 sequence of NSP4 is hypervariable both in human and murine Rotavirus and that there was no correlation between amino acid sequence and virulence in mice. Similar observations have been made in human studies 〚73〛, 〚74〛.
To summarize, the main body of experiments with NSP4 residues 114–135 suggest that it may function as a virus enterotoxin at least in mice. It remains to be established that it is of importance for the virus strains infecting humans.
4.3. Nervous mechanisms
The gastrointestinal tract is provided with an extensive nervous system, the enteric nervous system (ENS), which functions rather independently from the central nervous system as suggested by both morphological and functional studies. The morphological evidence for such an independent function is rather straightforward. It has been estimated that the ENS contains some 108 neurons in man, a number almost as great as that of the spinal cord 〚75〛. This enormous number of neurons in the gut wall is controlled by 1/300 as many efferent fibers from the central nervous system 〚76〛. The neurons of the ENS can apparently function without much control from the central nervous system.
The ENS is composed of two major nerve plexuses, the myenteric (between the two muscle layers) and the submucosal ones, and of their interconnections. Most of the neurons of the ENS are confined to the gastrointestinal wall, but extrinsic (afferent or efferent) neurons are also found in the ENS. The anatomical arrangement of the neurons of the ENS is, generally speaking, rather simple. Most myenteric neurons send projections to other myenteric nerve cells or to the smooth muscles, while most submucous neurons project to other submucous neurons and the mucosa/submucosa. There is, however, both morphological and functional evidence that the two plexuses are interconnected, myenteric neurons making contact with neurons of the submucous plexus and vice versa.
During the last 30 years an increasing number of polypeptides have been isolated from the gastrointestinal tract and have been characterized biochemically. These peptides have been localized to the ENS and/or to certain epithelial cells of the gastrointestinal mucosa (see e.g., 〚75〛). The peptides located in nerves have been ascribed a putative neurotransmitter function, implying that the number of transmitters in the ENS is at least 20. Most, if not all neurons contain more than one putative neurotransmitter. Presently, we know little of the functional implications of the colocalization of neurotransmitters in the ENS.
In the early 1980s Cassuto et al. published a series of reports (for a review, see 〚77〛) in which they showed that fluid secretion evoked by cholera toxin in extrinsically denervated intestines of anesthetized rats or cats could be markedly attenuated by three compounds influencing the functions of the ENS: tetrodotoxin (a blocker of sodium gates in excitable membranes), lidocaine (a local anesthetic) and hexamethonium (a nicotinic cholinergic receptor blocker). In line with this cholera toxin induced secretion was accompanied by an augmented release of vasoactive intestinal polypeptide, a neurotransmitter, into the venous effluent from feline intestinal segments. Taken together these observations strongly suggested that ENS in some way was activated by cholera toxin. This conclusion was further substantiated by Kirchgessener et al. 〚78〛, who monitored the activation of ENS neurons with a histochemical method using an antibody to the fos oncogene product, the expression of which has been shown to be a marker of nervous activity. Exposing the intestinal mucosa to cholera toxin activated neurons in both the myenteric and submucosal plexuses. Finally, cholera toxin could not elicit a secretory response in intestinal segments in which the myenteric plexus had been destroyed by exposing the serosal surface to benzalkonium chloride 〚79〛.
In subsequent investigations a nervous involvement was also demonstrated for several other intestinal secretagogues including bile acid, an invasive strain of Salmonella typhimurium and the enterotoxins produced by Escherichia coli. In fact, all luminal secretagogues tested in our laboratory have been shown to activate ENS in such a way that at least 60% of the fluid secretory response can be explained by a stimulation of the enteric nerves 〚77〛.
The enterotoxins produced by various bacteria have been investigated with regard to their effects on intestinal motility. For example, according to Mathias and Clench 〚80〛 cholera toxin induces a particular motility pattern that they named migrating action potential complexes. Functionally this pattern is very efficiently propelling the intestinal contents in an aboral direction. Interestingly, this motility effect of the toxin also seems to be mediated via ENS. Thus, the motility pattern can be attenuated by lidocaine and by nicotinic receptor blockade.
To summarize, bacterial enterotoxins produce an intestinal secretion and a propulsive motility response via an activation of ENS. This response may be regarded as a defense mechanism to potentially harmful mucosal influence, the fluid secreted diluting the noxious stimulus and the increased motility propelling the intestinal contents in an aboral direction. The involvement of ENS may also explain how enterotoxins, which apparently do not reach the intestinal crypts 〚60〛, 〚61〛 can influence the secretory cells of the crypts.
The experiments briefly summarized above prompted a study to elucidate whether Rotavirus-evoked fluid secretion in mice also was caused, at least in part, via an activation of ENS. To test this, three types of experiments were performed 〚30〛. In Ussing chamber experiments it was demonstrated that tetrodotoxin, lidocaine and mecamylamide (a nicotinic receptor blocker more lipophilic than hexamethonium) in a dose-dependent way attenuated the increased PD observed in intestines exposed to virus. Similarly, in experiments in which the lumen of intact intestinal segments were perfused in an organ bath, tetrodotoxin, lidocaine and hexamethonium significantly lowered the monitored PD and often turned fluid secretion into fluid absorption in virus-infected intestines. Finally, giving lidocaine repeatedly intraperitoneally to awake mice inoculated with Rotavirus significantly prevented the fecal losses of fluid. From the results obtained in vitro it was calculated that at least two thirds of the fluid and electrolyte secretion caused by the virus could be ascribed to an activation of the ENS.
It was pointed out above that experimental evidence existed showing that enterotoxins influenced intestinal motility via the ENS. There are few studies of motility during Rotavirus diarrhea and none of them has investigated the possible involvement of the ENS in the motility response. Molla et al. followed transit time (TT) of charcoal in patients with various types of diarrhea, including those caused by virus. When clinical signs of diarrhea were present, TT was shown to be about one-third to half of that seen 2 weeks after recovery from diarrhea 〚81〛. Burrows and Merritt 〚82〛 studied the myoelectrical activity of the jejunum of neonatal pigs before and after inoculating the animals with Coronavirus. When clinical signs of diarrhea were seen the normal motility pattern was disrupted. Hence, a prolonged migrating motility complex was observed as well as an increasing number of activity fronts. It seems likely that this motility pattern led to a shortened transit time, although this was not measured.
To summarize, several observations made during Rotavirus enteritis in neonatal mice suggest that the secretory response is in part explained by an activation of the ENS. The involvement of the ENS may explain how the comparatively few cells at the villus tips infected by the virus can influence the intestinal crypts to augment its secretion of electrolytes and water. The few studies of the effect of Rotavirus enteritis on intestinal motility indicate that intestinal transit time is shortened. The possible involvement of the ENS in this response is not known.
Many details regarding the hypothesis involving the ENS in Rotavirus-induced fluid secretion remain to be elucidated. One major question is how virus can activate enteric nerves. In the case of bacterial enterotoxin-evoked fluid secretion it has been proposed that enterotoxins via their effects on intracellular second messengers induce the release of amines/peptides from the endocrine cells of the intestinal epithelium. To exemplify, several lines of evidence indicate that cholera toxin causes the release of 5-hydroxytryptamine from the enterochromaffin cells 〚77〛. The secreted amines/peptides activate, alone or together, nervous dendrites located just underneath the intestinal epithelium. It seems possible that the proposed Rotavirus enterotoxin NSP4 may function in a similar manner by increasing the intracellular calcium concentration.
Other mechanisms may also explain how Rotavirus activates the ENS. It is now recognized that normal epithelial cells function as ˈsensorsˈ for microorganisms. When exposed, for example, to bacteria or virus the cells release a wide range of biologically active compounds such as cytokines, prostaglandins and nitrous oxide 〚83〛, 〚84〛. These compounds also participate in the inflammatory response. It is established that receptors located on neurons exist for some of those substances and that they, alone or together, may cause a membrane depolarization of dendrites to induce action potentials 〚85〛. In fact, an activation of enteric neurons by chemokines may explain the fact that Rotavirus only evokes diarrhea in mice younger than 15 days, since it has been demonstrated that chemokines are only released from murine epithelial cells during the first 2 weeks of life 〚84〛.
4.4. Deranged intestinal microcirculation
The research group around John Stephen published in the late 1980s a series of studies of various aspects of Rotavirus-induced intestinal secretion in newborn mice. On the basis of these studies they proposed a rather elaborate hypothesis regarding the pathophysiology of virus-induced intestinal secretion. Briefly summarized, the hypothesis infers that the invasion of the villus tip cells by Rotavirus triggers the release of ˈneuroactive/hormonal substancesˈ which cause a villus ischemia and subsequent shortening of the villi and, hence, a decreased absorptive capacity. The villus ischemia also leads to an increased rate of cell division, expanding the proliferative zone. In this zone, cells with a hypertonic intracellular compartment are present. When extruding their excess ions they create the osmotic force for fluid secretion 〚86〛, 〚87〛.
Villous ischemia plays a very important role in Stephenˈs hypothesis. However, there are no quantitative measurements of intestinal blood flow during Rotavirus-induced enteritis. Osborne et al. 〚22〛 made attempts to estimate villus blood flow in a qualitative manner using a morphological technique. The method involved the study of red cell distribution in villi by staining the cells with a peroxidase histochemical method. The number of red cells was assumed to reflect blood flow, although red cell content by definition reflects red cell volume rather than red cell flow. A time course study suggested that the villi were ischemic (fewer red cells seen) 24–48 h after exposing the mice to virus. Concomitantly, villi exhibited a reduced length (about 60% of control). The microcirculation showed varying degrees of recovery 72 and 96 h after infecting the animals. Fluid and electrolyte transport in the same experimental model was reported in another study 〚29〛. It demonstrated that fluid and electrolyte secretion was apparent only at 72 h after virus inoculation when the intestine seemed to be perfused a normal rate of blood flow.
It should be underlined that Osborne et al. 〚22〛 do not report any morphological observations indicating a villous ischemia apart from the red cell content in the villi. The characteristic sign of ischemia in villi is the detachment of the villus tip cells from the basement membrane 〚88〛, 〚89〛. None of the histological sections published by Osborne et al. exhibit this cardinal sign. The low number of red cells in some sections may reflect the fact that intestinal mucosal blood flow exhibits vasomotion (〚90〛; Lundgren, unpublished observations), i.e. blood flow in one and the same villus oscillates and may sometimes be very low. Moreover, Stephen 〚86〛 reports that mucosal ATP concentrations remained rather constant throughout the infection, which argues against a situation of decreased oxygen delivery. Stephen et al. propose that ischemia may be mediated by nerves. However, there are no reports to suggest that there exist any intramural vasoconstrictor nerve fibers in the gut. The known vasoconstrictor fibers are all extrinsic. Finally, in one review article Stephen and Osborne 〚87〛 propose that a decreased intestinal blood flow is of great importance also for the pathophysiology of cholera toxin-evoked intestinal secretion. In the case of cholera, intestinal mucosal blood flow has been studied with a quantitative method. It was shown that mucosal blood flow was doubled after exposing the intestinal mucosa to the toxin 〚91〛.
In another study from the same laboratory Spencer et al. 〚92〛 investigated the intracellular concentrations of electrolytes in enterocytes of villi and crypts using the sophisticated microprobe technique. After exposing the intestine to virus, the most pronounced changes were apparent in the cells at the villus base, where both sodium and chloride concentrations were significantly increased in infected intestines. The authors propose that these changes are caused by villus ischemia and/or nervously mediated. Furthermore, the intracellular ion hyperosmolarity produces the forces for secretion of electrolytes and fluid. However, the authors do not provide any experimental evidence for these suggestions.
The elaborate hypothesis proposed by Stephen et al., part of which is summarized above, has been described in detail in two review articles 〚86〛, 〚87〛 to which the reader is referred. Several steps in Stephenˈs hypothesis remain to be experimentally verified and the proposals can at present only be considered to be tentative.
5. Speculative hypothesis for Rotavirus-induced diarrhea
It is likely that the fluid and electrolyte secretion caused by Rotavirus is not explained by one single mechanism (figure 1 ). The experimental evidence for a decreased rate of absorption of electrolytes and glucose/amino acids is convincing and may reflect both an attenuated absorptive area (possibly more pronounced in Coronavirus than in Rotavirus infection) and a decreased transport capacity of epithelial cell symports. Furthermore, the enzymatic activity in the brush border region is markedly decreased, indirectly lowering the rate of transport of the symports for glucose and amino acids. It is more difficult to evaluate the importance of the Rotavirus-evoked increase in tissue conductance (most likely reflecting an augmented paracellular permeability) for the transport of fluid and electrolytes. The conductance increase may partly dissipate the hyperosmolar compartment in the upper parts of villi, lowering the osmotic forces moving water across the epithelial layer.
Figure 1.
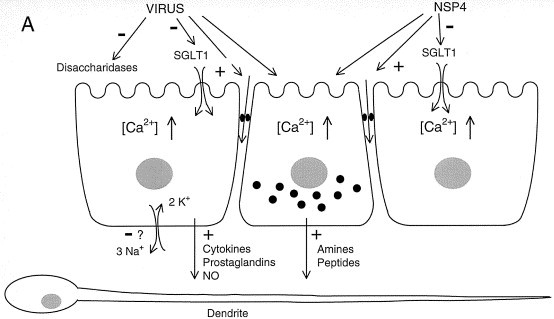
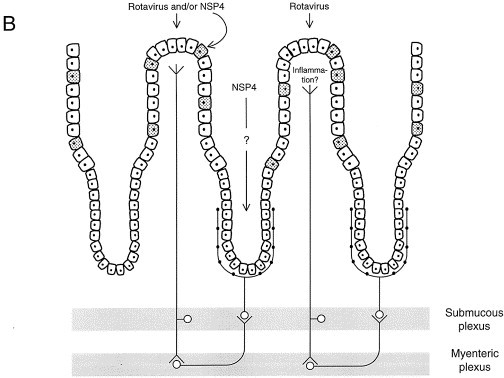
A speculative hypothesis for Rotavirus-induced diarrhea. Panel A. Cellular events leading to diarrhea. Rotavirus and/or its enterotoxin NSP4 inhibits fluid and electrolyte transport of the villus epithelium by attenuating the Na-glucose symport SGLT1 and possibly also Na-amino acid symports (not shown). Concomitantly, disaccharidase activity is inhibited. It is possible that the Na, K pump in the basolateral membrane is also attenuated, although quantitatively this effect is much less pronounced than for the disaccharidases. Taken together, these events will lower the rates of fluid, electrolyte and glucose absorption. The paracellular epithelial permeability is increased by Rotavirus and NSP4. The importance of this effect for epithelial transport is difficult to judge. Intracellular calcium concentration is increased in the intestinal epithelium in response to virus/NSP4. This may evoke the release of amines/peptides from intestinal endocrine cells (middle cell). Furthermore, cytokines, prostaglandins and NO are known to be released from the enterocytes in response to microorganisms. All these biologically active compounds may, alone or together, activate neuronal dendrites located just underneath the intestinal epithelium and hence stimulate secretory reflexes in the ENS. For a more detailed account of the intracellular mechanisms leading to an increased calcium concentration, the reader is referred to reference 〚14〛. Panel B. Integrative events leading to diarrhea. NSP4 may diffuse into the crypts to directly influence the intestinal secretory epithelium, possibly via the NSP4 effect on intracellular calcium. Furthermore, Rotavirus and/or NSP4 may activate secretory reflexes in the ENS in the way depicted in panel A. Inflammatory mediators, if present, may also stimulate nervous reflexes. The nervous reflex shown in the figure represents the simplest model that can be constructed from the observations made with cholera toxin. No pharmacological analysis has been made of the nervous reflex activated by Rotavirus. Minus sign indicates an attenuation; plus sign an increase. Shaded cells in panel B depict endocrine cells.
The magnitude of Rotavirus-evoked fluid secretion is such that it is probably not explained solely by a decreased fluid and electrolyte absorption. Secretory mechanism(s) are also at work. The question then arises how fluid secretion, mainly produced in the intestinal crypts, is stimulated. The Rotavirus enterotoxin, NSP4, is one possible candidate. However, it remains to be demonstrated that NSP4 reaches the intestinal crypts. It seems less likely that this occurs via the luminal route in vivo since a ˈphysiologicalˈ secretion from the crypts produces a convective flow against which the toxin molecule has to diffuse.
Stimulation of the ENS is another way in which a luminal ˈnoxiousˈ agent may influence the crypts. The mechanisms underlying the stimulation of the ENS are unknown. NSP4 may indirectly participate in the stimulation of ENS by causing the release of amines/peptides from villous endocrine cells via the effect of NSP4 on intracellular calcium. This, in turn, may activate ENS in a way similar to that demonstrated for cholera toxin. There are also many other biologically active molecules produced by epithelial cells and/or immunological cells that may participate in activating secretory reflexes in the ENS. The possible involvement of motility in Rotavirus diarrhea remains to be established.
Acknowledgements
The research on Rotavirus performed in our laboratories was supported by the Swedish Medical Research Council (grants 2877 and 10392). The secretarial help of Ms. Eva Magnusson is greatly appreciated.
References
- Martines J., Philips M., Feachem R. Diarreheal diseases. In: Jamison D, Mosley W, editors. Evolving Health Sector Priorities in Developing Countries. World Bank; Washington DC: 1991. pp. 1–49. [Google Scholar]
- de Zoysa I., Feachem R. Interventions for the control of diarrhoeal diseases among young children: rotavirus and cholera immunization. Bull. World Health Organ. 1985;63:569–583. [PMC free article] [PubMed] [Google Scholar]
- Bishop R.F., Davidson G.P., Holmes I.H., Ruck B.J. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973;8:1281–1283. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- Burke B., Desselberger U. Rotavirus pathogenecity. Virology. 1996;218:299–305. doi: 10.1006/viro.1996.0198. [DOI] [PubMed] [Google Scholar]
- Morris A.P., Scott J.K., Ball J.M., Zeng C.Q., O'Neal W.K., Estes M.K. NSP4 elicits age-dependent diarrhea and Ca(+)mediated I(–) influx into intestinal crypts of CF mice. Am. J. Physiol. 1999;277:G431–G444. doi: 10.1152/ajpgi.1999.277.2.G431. [DOI] [PubMed] [Google Scholar]
- Broome R.L., Vo P.T., Ward R.L., Clark H.F., Greenberg H.B. Murine rotavirus genes encoding outer capsid proteins VP4 and VP7 are not major determinants of host range restriction and virulence. J. Virol. 1993;67:2448–2455. doi: 10.1128/jvi.67.5.2448-2455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M., Kapikian A., Saif L.J., Kang S.Y. Genetic determinants of rotavirus virulence studied in gnotobiotic piglets. In: Ginsberg F.H.S, Brown, Chanock R.M, Lerner R.A, editors. Vaccines 93: Modern Approaches to New Vaccines, Including Prevention of AIDS. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. pp. 277–282. [Google Scholar]
- Hoshino Y., Sereno M., Kapikian A., Saif L.J., Kang S.Y. Identification of group A rotavirus genes associated with virulence of a porcine rotavirus and host range restriction of a human rotavirus in the gnotobiotic piglet model. Virology. 1995;209:274–280. doi: 10.1006/viro.1995.1255. [DOI] [PubMed] [Google Scholar]
- Jolly C.L., Beisner B.M., Holmes I.H. Rotavirus infection of MA-104 cells is inhibited by Ricinus lectin and separately expressed single binding domains. Virology. 2000;275:89–97. doi: 10.1006/viro.2000.0470. [DOI] [PubMed] [Google Scholar]
- Guerrero C.A., Zarate S., Corkidi G., Lopez S., Arias C. Biochemical characterization of rotavirus receptors in MA-104 cells. J. Virol. 2000;74:9362–9371. doi: 10.1128/jvi.74.20.9362-9371.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B.S., Londrigan S.L., Lee D.J. Rotavirus contains integrin ligand sequence and a disintegrin-like domain that are implicated in virus entry into cells. Proc. Natl. Acad. Sci. USA. 1997;94:5389–5394. doi: 10.1073/pnas.94.10.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaljot K.T., Shaw R.D., Rubin D.H., Greenberg H.B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J. Virol. 1999;62:1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M.C., Abad M., Charpilienne A., Cohen J., Michelangeli F. Cell lines susceptible to infection are permeabilized by cleaved and solubilized outer layer proteins of rotavirus. J. Gen. Virol. 1997;78:2883–2893. doi: 10.1099/0022-1317-78-11-2883. [DOI] [PubMed] [Google Scholar]
- Ruiz M.C., Cohen J., Michelangeli F. Role of Ca2+ in the replication and pathogenesis of rotavirus and other viral infections. Cell Calcium. 2000;28:137–149. doi: 10.1054/ceca.2000.0142. [DOI] [PubMed] [Google Scholar]
- Ward L.A., Rosen B.I., Yuan L., Saif L.J. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J. Gen. Virol. 1996;77:1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- Ryan M.J., Wall P.G., Adak G.K., Evans H.S., Cowden J.M. Outbreaks of infectious intestinal disease in residential institutions in England and Wales. J. Infect. 1994;34:49–54. doi: 10.1016/s0163-4453(97)80009-6. [DOI] [PubMed] [Google Scholar]
- Moon H.W. Pathophysiology of viral diarrhea. In: Kapikian A.L, editor. Viral Infections of the Gastrointestinal Tract. 1994. pp. 27–52. [Google Scholar]
- Carpio M., Bellamy J., Babiuk L. Comparative virulence of different bovine rotavirus isolates. Can. J. Comp. M. 1981;45:38–42. [PMC free article] [PubMed] [Google Scholar]
- Reynolds D., Hall G., Debney T., Parsons K. Pathology of natural rotavirus infection in clinically normal calves. Res. Vet. Sci. 1985;38:264–269. doi: 10.1016/S0034-5288(18)31791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Benfield D., Duimstra J. Comparative virulence of two porcine group A rotavirus isolates in gnotobiotic pigs. Am. J. Vet. Res. 1989;50:827–835. [PubMed] [Google Scholar]
- Holmes I.H., Ruck B., Bishop R., Davidson G. Infantile enteritis; morphogenesis and morphology. J. Virol. 1975;16:937–943. doi: 10.1128/jvi.16.4.937-943.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne M.P., Haddon S.J., Worton K.J., Spencer A.J., Starkey W.G., Thornber D., Stephen J. Rotavirus-induced changes in microcirculation of intestinal villi of neonatal mice in relation to the induction and persistence of diarrhea. J. Pediatr. Gastroenterol. Nutr. 1991;12:111–120. doi: 10.1097/00005176-199101000-00021. [DOI] [PubMed] [Google Scholar]
- Davidson G.P., Barnes G.L. Structural and functional abnormalities of the small intestine in infants and young children with rotavirus enteritis. Acta. Paediatr. Scand. 1979;68:181–186. doi: 10.1111/j.1651-2227.1979.tb04986.x. [DOI] [PubMed] [Google Scholar]
- Davidson G.P., Gall D.G., Petric M., Butler D.G., Hamilton J.R. Human rotavirus enteritis induced in conventional piglets. Intestinal structure and transport. J. Clin. Invest. 1977;60:1402–1409. doi: 10.1172/JCI108901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D.G., Gall D.G., Kelly M.H., Hamilton J.R. Transmissible gastroenteritis: mechanisms responsible for diarrhea in an acute enteritis in piglets. J. Clin. Invest. 1974;53:1335–1342. doi: 10.1172/JCI107681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telch J., Shepherd R.W., Butler D.G., Perdue M.H., Hamilton J.R., Gall D.G. Intestinal glucose transport in acute viral enteritis in piglets. Clin. Sci. 1981;61:29–34. doi: 10.1042/cs0610029. [DOI] [PubMed] [Google Scholar]
- Salim A.F., Phillips A.D., Walker-Smith J.A., Farthing M.J. Sequential changes in small intestinal structure and function during rotavirus infection in neonatal rats. Gut. 1995;36:231–238. doi: 10.1136/gut.36.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey W.G., Collins J., Candy D.C.A., Spencer A.J., Osborne M.P., Stephen J. Transport of water and electrolytes by rotavirus-infected mouse intestine: a time course study. J. Pediatr. Gastroenterol. Nutr. 1990;11:254–260. doi: 10.1097/00005176-199008000-00016. [DOI] [PubMed] [Google Scholar]
- Osborne M.P., Haddon S.J., Spencer A.J., Collins J., Starkey W.G., Wallis T.S., Clarke G.J., Worton K.J., Candy D.C.A., Stephen J. An electron microscopic investigation of time-related changes in the intestine of neonatal mice infected with murine rotavirus. J. Pediatr. Gastroenterol. Nutr. 1988;7:236–248. doi: 10.1097/00005176-198803000-00014. [DOI] [PubMed] [Google Scholar]
- Lundgren O., Timar Peregrin A., Persson K., Kordasti S., Uhnoo I., Svensson L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science. 2000;287:491–495. doi: 10.1126/science.287.5452.491. [DOI] [PubMed] [Google Scholar]
- Kerzner B., Kelly M.H., Gall D.G., Butler T., Hamilton J.R. Transmissible gastroenteritis: sodium transport and the intestinal epithelium during the course of viral enteritis. Gastroenterology. 1977;72:457–461. [PubMed] [Google Scholar]
- McClung H.J., Butler D.G., Kerzner B., Gall D.G., Hamilton J.R. Transmissible gastroenteritis: mucosal ion transport in acute viral enteritis. Gastroenterology. 1976;70:1091–1095. doi: 10.1016/S0016-5085(76)80317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads J.M., MacLeod R.J., Hamilton J.R. Alanine enhances jejunal sodium absorption in the presence of glucose: studies in piglet viral diarrhea. Pediatr. Res. 1986;20:879–883. doi: 10.1203/00006450-198609000-00015. [DOI] [PubMed] [Google Scholar]
- Shepherd R.W., Gall D.G., Butler D.G., Hamilton J.R. Determinants of diarrhea in viral enteritis. The role of ion transport and epithelial changes in the ileum in transmissible gastroenteritis in piglets. Gastroenterology. 1979;76:20–24. doi: 10.1016/S0016-5085(79)80122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homaidan F.R., Torres A., Donowitz M., Sharp G.W.G. Electrolyte transport in piglets infected with transmissible gastroenteritis virus. Gastroenterology. 1991;101:895–901. doi: 10.1016/0016-5085(91)90713-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keljo D.J., Butler D.G., Hamilton J.R. Altered jejunal permeability to macromolecules during viral enteritis in the piglet. Gastroenterology. 1985;88:998–1004. doi: 10.1016/S0016-5085(85)80020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keljo D.J., MacLeod R.J., Perdue M.H., Butler D.G., Hamilton J.R. D-glucose transport in piglet jejunal brush-border membranes: insights from a disease model. Am. J. Physiol. 1985;249:G751–G760. doi: 10.1152/ajpgi.1985.249.6.G751. [DOI] [PubMed] [Google Scholar]
- Halaihel N., Liévin V., Alvarado F., Vasseur M. Rotavirus infection impairs intestinal brush-border membrane Na+-solute cotransport activities in young rabbits. Am. J. Physiol. 2000;279:G587–G596. doi: 10.1152/ajpgi.2000.279.3.G587. [DOI] [PubMed] [Google Scholar]
- MacLeod R.J., Hamilton J.R. Absence of a cAMP-mediated antiabsorptive effect in an undifferentiated jejunal epithelium. Am. J. Physiol. 1987;252:G776–G782. doi: 10.1152/ajpgi.1987.252.6.G776. [DOI] [PubMed] [Google Scholar]
- Shepherd R.W., Butler D.G., Cutz E., Gall D.G., Hamilton J.R. The mucosal lesion in viral entritis. Extent and dynamics of the epithelial response to virus invasion in transmissible gastroenteritis of piglets. Gastroenterology. 1979;76:770–777. doi: 10.1016/S0016-5085(79)80177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Starkey W.G., Wallis T.S., Clarke G.J., Worton K.J., Spencer A.J., Haddon S.J., Osborne M.P., Candy D.C.A., Stephen J. Intestinal enzyme profiles in normal and rotavirus-infected mice. J. Pediatr. Gastroenterol. Nutr. 1988;7:264–272. doi: 10.1097/00005176-198803000-00017. [DOI] [PubMed] [Google Scholar]
- Rhoads J.M., MacLeod R.J., Hamilton J.R. Diminished brush border membrane Na-dependent L-alanine transport in acute viral enteritis in piglets. J. Pediatr. Gastroenterol. Nutr. 1989;9:225–231. doi: 10.1097/00005176-198908000-00016. [DOI] [PubMed] [Google Scholar]
- Rhoads J.M., Keku E.O., Quinn J., Woosely J., Lecce J.G. L-glutamine stimulates jejunal sodium and chloride absorption in pig rotavirus enteritis. Gastroenterology. 1991;100:683–691. doi: 10.1016/0016-5085(91)80012-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L., Finlay B.B., Bass D., von Bonsdorff C.H., Greenberg H.B. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J. Virol. 1991;65:4190–4197. doi: 10.1128/jvi.65.8.4190-4197.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obert G., Peiffer I., Servin A.L. Rotavirus-induced structural and functional alterations in tight junctions of polarized intestinal Caco-2 cell monolayers. J. Virol. 2000;74:4645–4651. doi: 10.1128/jvi.74.10.4645-4651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman K.G., Hempson S.J., Anderson J., Lippe S., Zhao L., Burakoff R., Shaw R.D. Rotavirus alters paracellular permeablity and energy metabolism in Caco-2 cells. Am. J. Physiol. 2000;279:G757–G766. doi: 10.1152/ajpgi.2000.279.4.G757. [DOI] [PubMed] [Google Scholar]
- Heyman M., Corthier G., Petit A., Meslin J.C., Moreau C., Desjeux J.F. Intestinal absorption of macromolecules during viral enteritis: an experimental study on rotavirus-infected conventional and germ-free mice. Pediatr. Res. 1987;22:72–78. doi: 10.1203/00006450-198707000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolauri E., Kaila M., Arvola T., Majamaa H., Rantala I., Virtanen E., Arvilommi H. Diet during rotavirus enteritis affects jejunal permeability to macromolecules in suckling rats. Pediatr. Res. 1993;33:548–553. doi: 10.1203/00006450-199306000-00002. [DOI] [PubMed] [Google Scholar]
- Vellenga L., Wensing T., Egberts H.J.A., Van Dijk J.E., Mouwen J.M.V.M., Breukink H.J. Intestinal permeability to macromolecules in piglets infected with transmissible gastroenteritis virus. Vet. Res. Commun. 1988;12:481–489. doi: 10.1007/BF01075478. [DOI] [PubMed] [Google Scholar]
- Vellenga L., Wensing T., Egberts H.J.A., Van Dijk J.E., Mouwen J.M.V.M., Breukink H.J. Intestinal permeability to polyethylene glycol 4000 and porcine albumin in piglets infected with transmissible gastroenteritis virus. Vet. Res. Commun. 1989;13:467–474. doi: 10.1007/BF00402570. [DOI] [PubMed] [Google Scholar]
- Egberts H.J.A., Vellenga L., Van Dijk J.E., Mouwen J.M.V.M. Intestinal permeability in piglets during transmissible gastroenteritis. J. Vet. Med. A. 1991;38:157–164. doi: 10.1111/j.1439-0442.1991.tb00997.x. [DOI] [PubMed] [Google Scholar]
- Vellenga L., Egberts H.J.A., Wensing T., Van Dijk J.E., Mouwen J.M.V.M., Breukink H.J. Intestinal permeability in pigs during rotavirus infection. Am. J. Vet. Res. 1992;53:1180–1183. [PubMed] [Google Scholar]
- Keljo D.J., Bloch K.J., Bloch M., Arighi M., Hamilton J.R. In vivo intestinal uptake of immunoreactive bovine albumin in piglet enteritis. J. Pediatr. Gastroenterol. Nutr. 1987;6:135–140. doi: 10.1097/00005176-198701000-00023. [DOI] [PubMed] [Google Scholar]
- Stinzing G., Johansen K., Magnusson K.E., Svensson L., Sundqvist T. Intestinal permeability in small children during and after rotavirus diarrhoea assessed with different-size polyethyleneglycols (PEG 400 and PEG 1000) Acta Pediatr. Scand. 1986;75:1005–1009. doi: 10.1111/j.1651-2227.1986.tb10331.x. [DOI] [PubMed] [Google Scholar]
- Jodal M., Lundgren O. Countercurrent mechanisms in the mammalian gastrointestinal tract. Gastroenterology. 1986;91:225–241. doi: 10.1016/0016-5085(86)90463-4. [DOI] [PubMed] [Google Scholar]
- Lundgren O. Factors controlling absorption and secretion in the small intestine. In: Donachie W, Griffiths E, Stephen J, editors. vol. 24. IRL Press; Oxford: 1988. pp. 97–112. (Bacterial infections of respiratory and gastroinestinal mucosae. Special publications of the Society for General Microbiology). [Google Scholar]
- Hallbäck D.A., Jodal M., Lundgren O. Effects of cholera toxin on villous tissue osmolality and fluid and electrolyte transport in the small intestine of the cat. Acta. Physiol. Scand. 1979;107:239–249. doi: 10.1111/j.1748-1716.1979.tb06469.x. [DOI] [PubMed] [Google Scholar]
- Osborne M.P., Haddon S.J., Worton K.J., Spencer A.J., Starkey W.G., Thornber D., Stephen J. A study of the microcirculation in whole villi of neonatal mice using a peroxidase histochemical method. J. Pediatr. Gastroenterol. Nutr. 1991;12:111–120. doi: 10.1097/00005176-199101000-00020. [DOI] [PubMed] [Google Scholar]
- Sharp G.W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971;229:266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Weiser M.M., Quill H. Intestinal fluid and crypt cell responses to cholera toxin. Gastroenterology. 1975;69:479–482. [PubMed] [Google Scholar]
- Hansson H.A., Lange S., Lonnroth I. Internalization in vivo of cholera toxin in the small intestinal epithelium of the rat. Acta. Pathol. Microbiol. Immunol. Scand. A. 1984;92:15–21. doi: 10.1111/j.1699-0463.1984.tb04372.x. [DOI] [PubMed] [Google Scholar]
- Levy A.G., Widerlite L., Schwartz C.J., Dolin R., Blacklow N.R., Gardner J.D., Kimber D.V., Trier J.S. Jejunal adenylate cyclase acitivity in human subjects during viral gastroenteritis. Gastroenterology. 1976;70:321–325. [PubMed] [Google Scholar]
- Collins J., Candy D.C.A., Starkey W.G., Spencer A.J., Osborne M.P., Stephen J. Disaccharides activities in small intestine of rotavirus-infected suckling mice: a histochemical study. J. Pediatr. Gastroenterol. Nutr. 1990;11:395–403. doi: 10.1097/00005176-199010000-00020. [DOI] [PubMed] [Google Scholar]
- Jourdan N., Brunet J.P., Sapin C., Blais A., Cotte-Lafitte J., Forestier F., Quero A.M., Trugnan G., Servin A.L. Rotavirus infection reduces sucrase-isomaltase expression in human intestinal epithelial cells by perturbing protein targeting and organization of microvillar cytoskeleton. J. Virol. 1998;72:7228–7236. doi: 10.1128/jvi.72.9.7228-7236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P., Estes M.K., Hu Y., Ball J.M., Zeng C.Q., Schilling W.P. The rotavirus nonstructural glycoprotein NSP4 moblizes Ca2+ from the endoplasmic reticulum. J. Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P., Ball J.M., Zeng C.Q., Estes M.K. The rotavirus nonstructural glycoprotein NSP4 possesses membrane destabilization activity. J. Virol. 1996;70:6973–6981. doi: 10.1128/jvi.70.10.6973-6981.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball J.M., Tian P., Zeng C.Q.Y., Morris A.P., Estes M.K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- Zhang M., Zeng C.Q., Morris A.P., Estes M.K. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells. J. Virol. 2000;74:11663–11670. doi: 10.1128/jvi.74.24.11663-11670.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Zeng C.Q., Dong Y., Ball J.M., Saif L.J., Morris A.P., Estes M.K. Mutations in rotavirus nonstructural glycoprotein NSP4 are associated with altered virus virulence. J. Virol. 1998;72:3666–3672. doi: 10.1128/jvi.72.5.3666-3672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaihel N., Liévin V., Ball J.M., Estes M.K., Alvarado F., Vasseur M. Direct inhibitory effect of rotavirus NSP4(114-135) peptide on the Na(+)-D-glucose symporter of rabbit intestinal brush border membrane. J. Virol. 2000;74:9464–9470. doi: 10.1128/jvi.74.20.9464-9470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafazoli F., Zeng C.Q., Estes M.K., Magnusson K.E., Svensson L. NSP4 enterotoxin of rotavirus induces paracellular leakage in polarized epithelial cells. J. Virol. 2001;75:1540–1546. doi: 10.1128/JVI.75.3.1540-1546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel J., Tang B., Feng N., Greenberg H.B., Bass D. Studies of the role for NSP4 in the pathogenesis of homologous murine rotavirus diarrhea. J. Infect. Dis. 1998;177:455–458. doi: 10.1086/517374. [DOI] [PubMed] [Google Scholar]
- Horie Y., Masamune O., Nakagomi O. Three major alleles of rotavirus NSP4 proteins identified by sequence analysis. J. Gen. Virol. 1997;78:2341–2346. doi: 10.1099/0022-1317-78-9-2341. [DOI] [PubMed] [Google Scholar]
- Lee C.N., Wang Y.L., Kao C.L., Zao C.L., Lee C.Y., Chen H.N. NSP4 gene analysis of rotaviruses recovered from infected children with and without diarrhea. J. Clin. Microbiol. 2000;38:4471–4477. doi: 10.1128/jcm.38.12.4471-4477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J.B., Costa M. Churchill Livingstone; New York: 1987. The Enteric Nervous System. [Google Scholar]
- Agostoni E., Chinnok J.E., De Burg Daly M., Murray J.G. Functional and histological studies to the heart, lungs and abdominal viscera in the cat. J. Physiol. (London) 1956;135:182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodal M., Lundgren O. Neural reflex modulation of intestinal epithelial transport. In: Gaginella T.S, editor. Regulatory Mechanisms in Gastrointestinal Function. CRC Press; Boca Raton, FL: 1995. pp. 99–144. [Google Scholar]
- Kirchgessner A.L., Tamir H., Gershon M.D. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: Activity-induced expression of fos immunoreactivity. J. Neurosci. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodal M., Holmgren S., Lundgren O., Sjöqvist A. Involvement of the myenteric plexus in the cholera toxin-induced net fluid secretion in the rat small intestine. Gastroenterology. 1993;105:1286–1293. doi: 10.1016/0016-5085(93)90130-5. [DOI] [PubMed] [Google Scholar]
- Mathias J.R., Clench M.H. Alterations of small intestine motility by bacteria and their enterotoxins. In: Wood J.D, editor. volume 1. American Physiology Society; Bethesda, MD: 1989. pp. 1301–1334. (Handbook of Physiology, Sect. 6. The Gastrointestinal System). [Google Scholar]
- Molla A., Molla A.M., Sarker S.A., Khatun M. Whole-gut transit time and its relationship to absorption of macronutrients during diarrhoea and after recovery. Scand. J. Gastroenterol. 1983;18:537–543. doi: 10.3109/00365528309181634. [DOI] [PubMed] [Google Scholar]
- Burrows C.F., Merritt A.M. Influence of coronavirus (transmissible gastroenteritis) infection on jejunal myoelectrical activity of the neonatal pig. Gastroenterology. 1984;87:386–391. doi: 10.1016/0016-5085(84)90717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff M.F., Eckmann L. Epithelial cells as sensors for microbial infection. J. Clin. Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollo E.E., Kumar K.P., Reich N.C., Chohen J., Angel J., Greenberg H.B., Sheth R., Anderson J., Oh B., Hempson S.J., Mckow E.R., Shaw R.D. The epithelial cell response to rotavirus infection. J. Immunol. 1999;163:4442–4452. [PubMed] [Google Scholar]
- Kirkup A.J., Brundsen A.M., Grundy D. Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am. J. Physiol. 2001;280:G787–G794. doi: 10.1152/ajpgi.2001.280.5.G787. [DOI] [PubMed] [Google Scholar]
- Stephen J. Viruses and the Gut. Swan Press; London: 1989. pp. 19–44. [Google Scholar]
- Stephen J., Osborne M.P. Pathophysiological mechanisms in diarrhoeal disease. In: Donachie W, Griffiths E, Stephen J, editors. vol. 24. IRL Press; Oxford: 1988. pp. 149–172. (Bacterial Infections of Respiratory and Gastrointestinal Mucosae, Special Publications of the Society for General Microbiology). [Google Scholar]
- Chiu C.J., McArdle A.H., Brown R., Scott H.J., Gurd F.N. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic and metabolic reappraisalArch. Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- Robinson J.W.L., Mirkovitch V., Winistörfer B., Saegesser F. Response of the intestinal mucosa to ischaemia. Gut. 1981;22:512–527. doi: 10.1136/gut.22.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorén A., Ricksten S.E., Lundin S., Gazelius B., Elam M. Baroreceptor-mediated reduction of jejunal mucosal perfusion, evaluated with endoluminal laser Doppler flowmetry in conscious humans. J. Auton. Nerv. Syst. 1998;68:157–163. doi: 10.1016/s0165-1838(97)00130-6. [DOI] [PubMed] [Google Scholar]
- Cedgård S., Hallbäck D.A., Jodal M., Lundgren O., Redfors S. The effect of cholera toxin on intramural blood flow distribution and capillary hydraulic conductivity in the cat small intestine. Acta. Physiol. Scand. 1978;102:148–158. doi: 10.1111/j.1748-1716.1978.tb06058.x. [DOI] [PubMed] [Google Scholar]
- Spencer A.J., Osborne M.P., Haddon S.J., Collins J., Starkey W.G., Candy D.C.A., Stephen J. X-ray microanalysis of rotavirus-infected mouse intestine: a new concept of diarrhoeal secretion. J. Pediatr. Gastroenterol. Nutr. 1990;10:516–529. doi: 10.1097/00005176-199005000-00016. [DOI] [PubMed] [Google Scholar]


