Abstract
Treatment of avian renal disease relies on supportive care, such as fluid therapy and nutritional support. Analgesia and adaptations of the environment are indicated in cases of renal disease associated with painful joints. Other treatments vary with the underlying etiology and may include systemic antibiotics, antifungal therapy, vitamin A supplementation, or chelation therapy.
Keywords: Avian, Fluid therapy, Nutrition, Supportive care, Allopurinol, Antifungal drugs, Chelation therapy, Chemotherapy
Key points
-
•
Fluid therapy is one of the most important treatments in cases of kidney disorders in birds. The choice between oral, subcutaneous, intravenous, and intraosseous routes depends on the patient and its needs.
-
•
Elevated dietary protein alone does not seem to be the underlying etiology of gout in all avian species because diets as high as 70% protein failed to induce gout in adult cockatiels.
-
•
The efficacy of allopurinol remains controversial in avian medicine and its use has not been reported in many avian species.
-
•
Surgical procedures, such as nephrectomy or renal transplantation, are not advisable in birds owing to the anatomic constraints of the avian kidney.
-
•
No effective therapy is recognized in birds with renal neoplasia.
Introduction
As in mammals, avian renal disease may be classified as acute or chronic. Acute renal failure results from an abrupt decrease in renal function, often caused by an ischemic or toxic insult.1 Chronic kidney disease is characterized by loss of functional renal tissue owing to a prolonged and usually progressive disease process.2
Causes of kidney disease may be classified as prerenal, renal, postrenal, or of mixed origin. A prerenal origin is characterized by hypoperfusion of the kidney. Conditions that commonly lead to the development of prerenal hyperuricemia include dehydration, hypovolemia, and congestive heart failure. Renal origin of kidney disease refers to an intrarenal process, leading to a dramatic decrease in the glomerular filtration rate. In birds, a decrease in glomerular filtration rate can either be a sign of renal disease or an appropriate physiologic response to water restriction.3 Causes of renal disease in the avian patient include infectious nephritis, hypovitaminosis A, heavy metal intoxication, and renal neoplasia. Postrenal hyperuricemia occurs when there is a disruption of the integrity of the urinary tract or an obstruction of urine outflow (eg, urolithiasis).2
The treatment of avian renal disease relies on supportive care such as fluid therapy and nutritional support. Analgesia and adaptations of the environment are indicated in cases of renal disease associated with painful joints or spinal nerve compression. Other treatments vary with the underlying etiology and may include systemic antibiotics, antifungal therapy, vitamin A supplementation, chelation therapy, and agents to lower uric acid levels such as allopurinol. Potentially nephrotoxic drugs should be used with extreme caution in patients with renal disease. Additionally, drugs that are excreted through the kidney may fail to reach therapeutic plasma levels in polyuric birds or reach toxic levels if drug excretion and elimination are impaired.4
General therapy for renal disease
Fluid Therapy
As in mammals,5 fluid therapy constitutes one of the most important treatments in cases of kidney disorders in birds. Uric acid is eliminated by active tubular secretion6 , 7 and water is needed to flush the suspension through the renal tubules.4 Without regular removal by diuresis, urates can accumulate within the kidneys.8
Fluid type is selected based on results of biochemical analyses, evaluation of blood electrolytes, glucose, and acid–base status. When these values are not known, a balanced isotonic crystalloid solution, such as lactated Ringer’s solution may be used for rehydration and hemodynamic support.9 Caution is recommended when using colloid fluids in patients with renal disease by extrapolation from mammals.
Route of administration
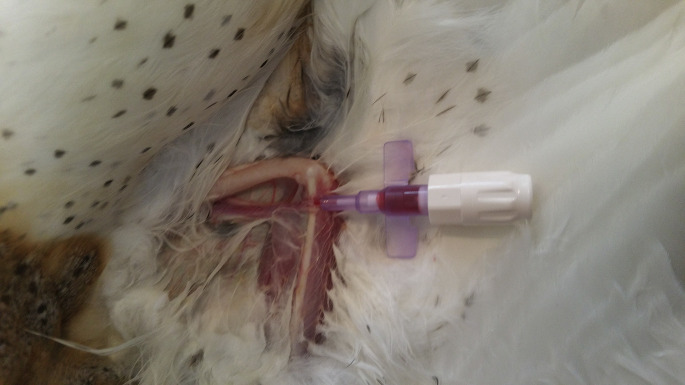
Depending on the clinical circumstance, fluids can be administered by oral, subcutaneous, intravenous (IV), and/or intraosseous (IO) routes. Fluids are often administered by mouth by gavage with liquid oral nutrition. This route is generally safe and adequate for avian patients that are not in shock or debilitated.10 In birds with mild dehydration, fluids can also be provided subcutaneously. Subcutaneous fluids can be administered in the inguinal (Fig. 1 ), interscapular, or axillary regions. Volumes as great as 20 mL/kg may be administered in 1 location.9 Subcutaneous fluids are easily delivered using a butterfly needle, which allows the animal to move without the needle being pulled out. Practitioners unfamiliar with avian anatomy should beware of the thin skin and the presence of abdominal air sacs close to the inguinal region. Thus, it is key to remain steady during the procedure and to firmly hold the leg in extension to avoid inadvertent coelomic puncture. Fluids given subcutaneously and by mouth are poorly absorbed if hypovolemic shock is present.
Fig. 1.
Subcutaneous injection of fluids in the inguinal region of a Fisher’s lovebird (Agapornis fisheri). An operator manually restrains the bird. The bird’s leg is gently pulled forward, revealing a translucent cutaneous fold between the body and the leg, proximally and medially to the quadriceps muscle.
(Courtesy of O. Cojean, méd vét, IPSAV, Saint-Hyacinthe, Canada).
The vasculature in birds can be accessed via IV or intraosseous (IO) routes.11 The choice between these routes depends on patient size, patient temperament, and the volume of fluids needed. IV catheters may be used for initial fluid therapy, but do not have the stability of an IO catheter. Permanent supervision of birds with IV catheters is also required to prevent fatal hemorrhage in case of accidental removal of the catheter.11 IO catheters can be placed quickly, are stable and reliable, and are relatively easy to maintain, but placement is more painful. Fluids can also be provided in a larger bolus by the IO route than the IV route.10 Unlike IV catheter placement, IO catheterization can also be performed even in very small birds.
IV catheters may be placed in ulnar (Fig. 2 ) and medial metatarsal veins (Fig. 3 ), or more rarely in the jugular vein.11 IV catheterization often requires sedation or general anesthesia to avoid stressful physical restraint. Jugular and ulnar catheters must be sutured in place.11 Medial metatarsal catheters can be secured using tape only.11 All catheters should then be covered with a nonadhesive bandage. Wing catheters are protected with a figure-of-eight bandage (Fig. 4 ).11
Fig. 2.
Placement of a 26G catheter in the ulnar vein of a barn owl (Tyto alba).
(Courtesy of O. Cojean, méd vét, IPSAV, Saint-Hyacinthe, Canada.)
Fig. 3.
Placement of a 24G catheter in the medial metatarsal vein of an Amazon parrot. The vein is manually occluded at the level of the proximal tibiotarsus.
(Courtesy of I. Langlois, DVM, DABVP, Knoxville, TN.)
Fig. 4.
A cockatiel (Nymphicus hollandicus) receiving fluid therapy via an IO catheter in the right ulna. The wing has been taped to the body. The patient is weak and thus does not need an Elizabeth collar.
(Courtesy of C. Grosset, méd vét, CES, IPSAV, DACZM, Saint-Hyacinthe, Canada.)
IO catheter sites include the proximal tibiotarsus and the distal ulna (Box 1 ).11 Pneumatized bones, such as the humerus and femur, should be avoided.9 Rarely, in large birds such as pelicans, California condors (Gymnogyps californianus),12 and turkey vultures (Cathartes aura),13 the ulna is also pneumatized.
Fig. 5.
Placement of an IO catheter in the ulna of an avian patient (see Box 1). (A) Computed tomography scan of an African gray parrot (Psittacus erithacus). Dorsal view of the right wing. The white arrow indicates the site and axis of insertion of the catheter in the ulna. (B) After aseptic preparation of the site and appropriate analgesic protocol administration, the ulna is grasped between the fingers. Palpate the styloid process of the distal ulna on the dorsal aspect of the wing. The needle is inserted in the distal ulna and directed proximally. (C) Check the patency of the catheter by using a small amount of heparinized saline flush. Visualize the flow in the ulnar vein as the fluid is injected.
(Courtesy of [A] S. Larrat, méd vét, MSc, DES, DACZM, Brech, France; and [C] M. Desmarchelier, DVM, MSc, DACVB, DACZM, DECZM, Saint-Hyacinthe, Canada.)
Box 1. IO catheter placement in the bird.
Placement of an IO catheter in the distal ulna
-
•
Palpate the styloid process of the distal ulna on the dorsal aspect of the wing.
-
•
Pluck the feathers over the surrounding site and prepare aseptically.
-
•
Ideally use a 20- to 25-gauge short spinal needle.
-
•
Flex the distal wing tip and grasp the ulna between the fingers of one hand. With the other hand, the spinal needle is inserted just ventral to the condyle and directed proximally toward the elbow along the ulnar shaft (Fig. 5A, 5B).
-
•
Apply gentle pressure as the bevel of the needle is rotated, allowing the needle to cut through the cortex of the bone and enter the medullary cavity.
-
•
If the lumen of the needle becomes plugged, the needle may be removed and replaced.
-
•
Check the patency of the catheter with a small amount of heparinized saline. Visualize flow in the ulnar vein as the fluid is injected (Fig. 5C).
-
•
Two orthogonal radiographic views may also be obtained to confirm correct placement.
-
•
Secure the catheter with butterfly taping and by suturing this tape to the skin, if necessary.
-
•
Place a figure-of-eight wing bandage to minimize wing movement.
Placement of an IO catheter in the proximal tibiotarsus
-
•
Flex the stifle, and palpate the cnemial crest at the proximal anterior surface of the tibiotarsus, just distal to the knee joint.
-
•
Insert the needle at the cnemial crest at, or to either side of, the insertion of the patellar tendon, to avoid penetration of the stifle joint.
-
•
Secure the catheter in place with tape.
Some birds may benefit from an Elizabethan or cervical restraint collar; however, these devices can be extremely stressful to some birds and may adversely affect patient condition. The ability to tolerate a collar should be assessed in each patient11 (see Box 1).
Fluid requirements
Daily maintenance fluid requirements have not been determined in birds; however, the recommendations of different authors range from 50 to 150 mL/kg/d, with the higher end of the range expected in smaller species.14 , 15 Maintenance plus one-half of the estimated fluid deficit is generally administered over the first 12 to 24 hours, with the remainder of the deficit replaced over the following 48 hours.9 Fluids for maintenance and correction of dehydration are given as a constant infusion, using a pediatric infusion pump or syringe pump.11 Fluids should be warmed to body temperature. Depending on the patient’s condition and species, the author will typically give 50 to 100 mL/kg of fluid twice a day subcutaneously, IV, IO, or via a combination of routes.16
Outpatient measures to maintain or improve proper hydration
Various tips may be given to clients to promote adequate hydration at home for avian patients with renal disease.17 Owners may offer fruit juice without added sugar or infant electrolyte replacement solution (Pedialyte, Abbott, Saint-Laurent, Quebec, Canada) full strength or diluted with water. Owners can also increase the proportion of fruits and vegetables in the diet or offer moistened seeds or other foods like warm, unsalted vegetable soup. Caretakers may float seeds in the water bowl to encourage drinking behavior. Regular access to a shower or bath can also promote drinking, acknowledging that individual birds vary greatly in the ways they choose to bathe. Some birds love the feeling of a trickling shower, some enjoy daily misting with a spray bottle, and some like to dunk themselves in a pool of water.18 If none of these measures prove adequate and the bird is still not drinking in sufficient amounts, the owner can use a plastic eyedropper, syringe, or straw with finger kept over 1 end to slowly offer fluids directly into the beak, followed by positive reinforcement like verbal praise. Reserve this method as a last resort and inform owners of the risk of fluid aspiration.
Nutritional Supportive Care
Patients with renal disease should be monitored for weight loss and appropriate nutritional support should be offered as needed.16
Dietary protein
Clinical studies in dogs and cats have demonstrated that dietary protein restriction can slow chronic kidney disease progression and improve survival.19 By extrapolation, few commercial diets low in proteins have been formulated for birds with renal insufficiency (eg, Roudybush AK formula; Woodland, CA), although evidence-based data on whether protein restriction is beneficial in birds are lacking. Precise protein content and composition is also not disclosed for this diet.
Renal lesions, such as gout, have been associated with excess dietary protein in birds, but only under specific conditions.16 In 1 study, a 42.28% protein diet fed to 18-day-old broiler chicks for 15 weeks induced multiple renal abnormalities, primarily nephrosis and visceral gout.16 In another study, diets high in urea were linked to outbreaks of nephritis in poultry16, however, cockatiels (Nymphicus hollandicus) fed high dietary protein (up to 70%) for 11 months did not develop renal lesions. The cockatiels were able to upregulate enzymes associated with amino acid catabolism and uric acid synthesis.6 Of note, these cockatiels received a gradually increasing protein concentration in their diet over 3 weeks. Uric acid increased linearly with dietary protein levels, but remained within normal limits in these birds, indicating that hyperuricemia is specific of renal disease or severe dehydration in granivorous avian species. Because the nutritional requirements vary among avian species, it is unknown if these conclusions can be extrapolated to other birds. Unlike carnivorous birds, granivorous species have low requirements for dietary amino acids and seem to be able to conserve amino acids by tight regulation of amino acid catabolism.6 Frugivorous birds have lower rates of nitrogen loss compared with granivorous birds and, thus, even lower dietary protein requirements.20 A safe recommendation is that birds with hyperuricemia should not consume diets with protein levels greater than what is considered normal for the given species.16
Fatty acid supplementation
Omega-3 fatty acid supplementation has been shown to decrease the risk of chronic renal disease and delay the progression of disease in dogs and humans.16 , 20 Cytokines derived from membrane-bound omega-6 fatty acids like arachidonic acid include prostaglandins, thromboxanes, and leukotrienes.19 These cytokines are proinflammatory and vasoactive, which promotes chronic kidney disease progression, owing to renal free radical production and antioxidant depletion.19
In humans, the positive effects of the polyunsaturated fatty acid eicosapentaenoic acid (EPA) are more pronounced than those of α-linolenic acid, another omega-3 fatty acid.21 Concentrated sources of longer chain omega-3 fatty acids (such as EPA and docosahexanoic acid) are limited to fish and other marine products and algal-based sources.21 Flaxseed is also a source of EPA, but is less commonly used in feed manufacturing because of susceptibility to oxidative rancidity.
In avian medicine, only anecdotal information exists regarding the use of omega-3 fatty acids in renal disease.16 Most psittacine diets are highly enriched in omega-6 fatty acids (primarily linoleic acid) and limited in omega-3 fatty acids (Table 1 ). Of note, diets high in polyunsaturated fatty acids require additional antioxidants to prevent lipid peroxidation during storage.14
Table 1.
Omega-3 concentration and omega 3:omega 6 ratio of selected food items frequently offered as supplements to birds
| Food Items | Omega 3 Concentration (g) |
Omega 3/Ω 6 Ratio |
|---|---|---|
| Value per 100 g | Value per 100 g | |
| Flax seeds | 22.8 | 3.86 |
| Chia seeds | 17.6 | 3.03 |
| Walnut | 9.1 | 0.24 |
| Soybean oil | 6.8 | 0.13 |
| Lafeber Senior Bird Nutri-Berries® | 0.48 | 0.16 |
| Edamame (green soybean) | 0.3 | 0.16 |
Data from United States Department of Agriculture Agricultural Research Service Database (USDA). Available at: https://ndb.nal.usda.gov/ndb/.
Incorporation of omega-3 into cockatiel red blood cells was greater after supplementation with fish oil22 , 23; however, the palatability of fish oil may be an issue when supplementing psittacine birds at home. Being carnivorous, some birds of prey are more likely to accept fish oil in their diet. In cases of concomitant gout, ensure the patient receives a plant-based source of EPA or docosahexanoic acid rather than a fish oil source, which may have higher purine levels17 (see Table 1).
Management of Hyperuricemia
Severe dehydration and many forms of renal disease, including obstructed ureters, can result in decreased uric acid elimination thus causing hyperuricemia.16 Fluid therapy (combined with medications for hyperuricemia if needed) is generally continued until uric acid decreases to either normal or mildly increased levels (10–20 mg/dL) and the bird demonstrates signs of improvement, such as eating or increased activity.16
The use of medications for hyperuricemia is extrapolated from human medicine, and the safety and efficacy of these treatments are often lacking in birds. These drugs have been poorly studied in psittacine birds and should only be used with close monitoring of uric acid levels.
Xanthine oxidase inhibitors
Xanthine oxidase inhibitors, such as allopurinol and febuxostat, decrease uric acid synthesis. The efficacy of allopurinol in avian medicine is controversial; information is available for only a limited number of species. In broilers, uricemia was reduced as well as xanthine oxidase and xanthine dehydrogenase activity in the kidney in birds treated with allopurinol (25 mg/kg by mouth).24 , 25 Allopurinol was unable to completely inhibit xanthine oxidoreductase activity.24
Toxicity has been reported following administration of allopurinol in red-tailed hawks (Buteo jamaicensis). Vomiting developed at 50 mg/kg by mouth every 24 hours and was attributed to the accumulation of oxypurinol, a metabolite that worsens renal gout.26 Allopurinol given at 25 mg/kg by mouth every 24 hours to red-tailed hawks was shown to be safe, but had no significant effect on plasma uric acid concentrations. Based on the lack of response at a dose of 25 mg/kg and the toxic effects at 50 mg/kg, allopurinol is not recommended in the red-tailed hawk.27 It is unknown whether this finding should be extrapolated to psittacine birds.
Uricase
Uricase oxidizes uric acid to allantoin in humans. Little information is available in veterinary medicine. Poultry on a high-protein diet developed hyperuricemia, which was reversed with uricase injection.28 In a more recent study, the uricolytic properties of uricase were studied in a granivorous bird (pigeon, Columba livia domestica) and a carnivorous avian species (red-tailed hawk). Plasma concentrations of allantoin and uric acid were determined in experimental groups before and after receiving 100, 200, and 600 UI/kg uricase intramuscularly once daily. All regimens caused a significant decrease in plasma uric acid concentrations within 2 days after the first administration, when compared with controls. Plasma allantoin concentrations were also significantly higher when compared with controls, suggesting a similar mechanism of action in these species.29
Xanthine dehydrogenase inhibitor
The xanthine dehydrogenase inhibitor, colchicine, is used for its antigout activity in humans.16 Colchicine has also been used to treat amyloidosis and renal fibrosis in small animals.16 In turkeys diagnosed with articular gout, colchicine administered at 0.18 mg/kg by mouth every 24 hours for 7 days failed to influence uric acid concentrations.30 No controlled study on colchicine has been published in birds.
Uricosuric drugs
Uricosuric drugs, such as probenecid, promote uric acid excretion by the kidneys. Uricosuric drugs are contraindicated when tubular urate crystals are present, which is frequently seen in birds.17 Probenecid has been shown to inhibit uric acid tubular secretion in chicken proximal tubule epithelium in vitro. 31 It has also been studied in vivo in chicken; depending on the dose given intravenously, the drug increased or decreased uric acid clearance.7 Probenecid use has been reported anecdotally in psittacine birds and reptiles with gout,32 but no efficacious dose is currently published.
Pain Management
Birds with renal disorders may suffer from pain caused by articular gout or nerve compression secondary to renal masses (Fig. 6 ). Affected birds are likely to spend more time on the cage floor and suffer from impaired locomotion. Husbandry adaptations and pain control are required to improve their quality of life.
Fig. 6.
Articular gout secondary to renal disease in a budgerigar parakeet (Melopsittacus undulatus).
(Courtesy of C. Grosset, Dr méd vét, CES, IPSAV, DACZM, Saint-Hyacinthe, Canada.)
Enclosure modifications
Water and food dishes can be placed as close to the bird as possible. Containers of different shapes and depths can stimulate consumption. Replace standard perches with perches of a larger diameter and ladders or ramps that allow the bird to use its beak. Once the bird is unable to perch normally, the claws may need to be trimmed and shaped more frequently than in a healthy bird. Patients with gout should not be restricted in their movements, and instead should be housed in as large a cage as possible. The minimum size considered adequate allows the bird enough space to spread its wings without hitting either the sides of the cage or other perches.18
Analgesia
Pain management is paramount in birds with articular gout or nerve compression by renal masses (Table 2 ). Long-term treatment with opioids may be considered. Intra-articular injections of corticosteroids are administered to humans with only 1 joint affected by gout,17 but this treatment modality has not been investigated in birds. The effectiveness of intra-articular bupivacaine injections in the suppression of osteoarthritic pain has also been demonstrated in humans.33 In an avian model of acute gouty arthritis, local anesthesia was effective in suppressing pain-associated behavior.34 It was concluded that the optimum intra-articular dose of bupivacaine for the treatment of musculoskeletal pain in the domestic fowl was 3 mg bupivacaine in 0.3 mL saline.34 Physical modalities such as thermotherapy and laser may also be used to diminish pain. Low-level laser therapy (660 nm, 9 J/cm2) has been shown to decrease neuropathic pain.35
Table 2.
Analgesic agents evaluated in Hispaniolan Amazon parrots (Amazona ventralis) by pharmacokinetic studies
| Agent | Dosage | Route | Frequency | Comment |
|---|---|---|---|---|
| Tramadol hydrochloride36 | 30 mg/kg | PO | q6-12h | – |
| Butorphanol tartrate (long-acting poloxamer 407 gel formulation)37 | 12.5 mg/kg | SQ | q4-6h | – |
| Gabapentin38 | 15 mg/kg | PO | q8 h | Neuropathic pain, effects takes days to weeks |
Abbreviations: PO, by mouth; SQ, subcutaneous; q, every.
Alternatively, after discussion with owners of the safety versus quality of life balance, the use of nonsteroidal anti-inflammatory drugs may be considered as a palliative treatment. A study in Hispaniolan Amazon parrots (Amazona ventralis) indicated 1.3 mg/kg by mouth every 12 hours of meloxicam to be a therapeutic dosage for relief of arthritic pain.39
Both severe gout and renal tumors carry a poor prognosis; therefore, euthanasia must be considered when analgesia and husbandry modifications fail to ensure an appropriate quality of life for the patient.17
Miscellaneous Conditions Associated with Chronic Renal Disease
Hyperphosphatemia is poorly documented in birds in association with renal failure, but it has been reported in some instances.40 If this condition develops, phosphate binders may be administered at doses extrapolated from small animals.
Although rarely reported with renal disease in birds, gastric ulcers may be treated with omeprazole41 (1–10 mg/kg by mouth every 12 hours) and sucralfate (25 mg/kg by mouth every 8 hours) staggered 2 hours apart from other oral treatments.
Chronic anemia owing to decreased erythropoietin secretion is challenging to manage because avian erythropoietin is structurally different from that of mammals.42 The effect of epoetin alfa in birds has not been documented but it is likely to cause antibody production.
Colchicine may be administered long term to treat amyloidosis and limit renal fibrosis.16 No controlled studies have been published in birds about this drug.
In mammals, peritoneal dialysis or hemodialysis is ideal for cases of renal disease not treatable with other medical options.43 The use of dialysis has not been described in birds and coelomic dialysis is not possible in the avian patient owing to the presence of abdominal air sacs. Renal transplantation has also never been described in avian medicine and is unrealistic given the position of the kidneys immediately ventral to the synsacrum, adjacent to air sacs, and in close relation with pelvic nerves.
Specific therapy for renal disease
In avian species, renal diseases are caused by various etiologies, including infectious nephritis (bacterial, viral, parasitic, fungal), renal neoplasms, toxic exposure, and nutritional disorders.44 Specific treatment options vary depending on the cause.
Bacterial Nephritis
Many bacteria have been reported to cause nephritis in birds, including Enterobacteriaceae, Pasteurella spp., Pseudomonas spp., Streptococcus spp., Staphylococcus spp., Listeria monocytogenes, Erysipelothrix rhusiopathiae, and chlamydial organisms.43 , 45 Mycobacterium spp. have also been rarely reported in the avian kidney.43 , 46 , 47
Antibiotics are indicated in suspected or confirmed cases of bacterial nephritis.16 Drug choice should ideally be based on a susceptibility panel from blood or histopathologic samples.16 Cloacal samples may also be used owing to the possibility of ascending infection but may not be reliable. In cats and dogs, bacterial nephritis is treated for at least 4 to 6 weeks.5 This recommendation may be extrapolated to birds in the absence of controlled studies regarding duration of treatment in avian medicine.16 Pending culture and sensitivity results, empirical broad-spectrum antibiotics that provide excellent therapeutic levels within renal tissue should be initiated such as β-lactams, trimethoprim-sulfamethoxazole, or fluoroquinolones.44 Avoid potentially nephrotoxic antibiotics, such as aminoglycosides.48 , 49
Viral Nephritis
Among viral infections, polyomavirus often results in clinically relevant renal disease.44 Polyomavirus is the most important cause of viral nephritis in the companion psittacine bird.43 Many other viruses can cause renal lesions in psittaciformes including, but not limited to, paramyxoviruses,43 , 44 bornavirus,50 , 51 and West Nile virus.52 In backyard chickens, infectious bronchitis virus is the most important cause of renal disease.43 Treatment of viral nephritis usually relies on nonspecific supportive care.
Parasitic Nephritis
Renal coccidiosis is the most common cause of parasitic nephritis. Renal diseases caused by the coccidian Eimeria spp. have been reported in several species, including juvenile waterfowl,53 domestic goose (Anser anser domesticus), and less commonly raptors.43 Although rare, renal cryptosporidiosis has also been reported in birds.44 , 54 , 55 Schizonts of Leukocytozoon spp., Plasmodium spp., and Haemoproteus spp. have been identified in avian renal tissue and associated with lymphoplasmacytic inflammation.44 , 56 Renal trematodes and cestodes have also been reported in multiple species of bird housed outdoors, including order Columbiformes, Passeriformes, Anseriformes, Psittaciformes, and Galliformes.44
Parasitic diseases associated with the kidneys are typically diagnosed from a fecal parasite examination or renal biopsy.44 Antiparasitic treatments vary greatly depending on the species and life cycle of the parasite, with ponazuril (20 mg/kg by mouth every 24 hours for 7 days) or toltrazuril (25 mg/kg by mouth once a week) being used for coccidia, and praziquantel (10 mg/kg subcutaneously 2 times 10 days apart) for trematodes and cestodes.44 , 57, 58, 59 Although toltrazuril has been shown to successfully control coccidiosis in broilers with a single 2-day treatment course,60 its use is not approved in food animal species in many countries. Practitioners should consult local regulations for approved anticoccidial agents. Monensin has been used for the treatment of renal coccidiosis, but is toxic in turkey and guinea fowl.16 Reports on resistance of Eimeria isolates to anticoccidial drugs are increasing,61, 62, 63, 64 and rotation of anticoccidial drugs is recommended to minimize the risk of resistance. Natural products, such as cider vinegar, are also emerging as alternative strategies to control avian coccidiosis.65, 66, 67, 68
Fungal Nephritis
Aspergillosis
Although predominantly a disease of the respiratory tract, systemic aspergillosis can occur.69 Renal aspergillosis has been reported in several avian species, including chickens66 and a black palm cockatoo (Probosciger aterrimus).70 Fungal culture from a biopsy is recommended because treatment options can vary depending on the fungal organism involved.44 Most systemic fungal infections require long-term therapy over a period of months.71 , 72 Initial IV administration of antifungal drugs followed by oral therapy is recommended.72
Amphotericin B is a polyene macrolide that acts by binding to ergosterol, the principal sterol in the fungal cell membrane.71 Amphotericin B has a broad antifungal spectrum, including Aspergillus spp. and Cryptococcus spp., although resistance has been reported.73 IV administration quickly establishes fungicidal concentrations, making amphotericin B a frequent choice for initial therapy. The use of amphotericin B has been associated with nephrotoxicity in mammals72; however no evidence of nephrotoxicity has been documented in birds. This difference may be associated with the shorter elimination half-life in birds compared with mammals after IV administration of amphotericin B.74
In combination with early, systemic antifungal therapy, topical amphotericin B can be administered through a polypropylene tube during endoscopic or surgical procedures.71 Topical therapy is recommended when renal lesions can be easily debrided to maximize drug concentrations in tissues; however, in many patients granulomas cannot be reached endoscopically.71
Itraconazole, fluconazole, and voriconazole are the most studied azoles in birds. The relative toxicity of an azole depends on the affinity to fungal cytochrome P450 enzyme, compared with its affinity to the avian cytochrome P450.72 The most common adverse effects associated with azole administration in birds are anorexia, vomiting, and alterations in liver function.71 , 72 Regular bile acid monitoring is recommended during treatment for early detection of hepatic adverse effects. Itraconazole is a first-generation triazole antifungal agent, commonly used in birds for treatment of aspergillosis.75 Voriconazole is a third-generation triazole antifungal agent.71 , 75 Voriconazole is increasingly used to treat invasive aspergillosis in birds, given the broad antifungal spectrum, which includes molds (fungicidal) and yeasts (fungistatic), and its rapid bioavailability.72 Acquired resistance of Aspergillus fumigatus strains to both itraconazole and voriconazole has been reported.73 , 76 Fluconazole is a water-soluble fungistatic agent that is rapidly absorbed with high bioavailability after oral administration.69 A blue-fronted Amazon parrot with Aspergillus keratomycosis was successfully treated with oral and topical fluconazole.77
Terbinafine is an allylamine, fungicidal agent with activity against several fungal species, including Aspergillus spp. 75 and Cryptococcus spp.78 Of note, the dose should be decreased in cases of impaired renal function.57
Studies have documented dose- and species-dependent variability, suggesting that different dosage regimens of antifungals may be required for different species of birds (Table 3 ).71 Caution should be applied when extrapolating a dose to a different avian species.75
Table 3.
Antifungal therapy in selected avian species
| Antifungal Agent | Active Against | Pharmacokinetic Studies | Recommended Doses | Adverse Effects | Comments |
|---|---|---|---|---|---|
| Amphotericin B | |||||
| C | A Cr |
Domestic turkey, broad-winged hawk, red-tailed hawk, great-horned owl74 | 1-1,5 mg/kg IV q8–12 h | Renal toxicity considered lower than in mammals because elimination phase faster in birds74 | |
| Itraconazole | |||||
| C ST |
A | Humboldt penguin82 | 8.5 mg/kg PO q12 h 20 mg/kg PO q24 h |
Anorexia, vomiting, and alterations in liver function are most common.71,72 | Itraconazole is better absorbed in an acidic gastric pH86; thus, antacid medications should not be administered concomitantly. |
| Blue-fronted Amazon parrot83 | 5 mg/kg PO q24 h | ||||
| Racing pigeons84 | 6–26 mg/kg PO q12 h | ||||
| Red-tailed hawk85 | 10 mg/kg PO q24 h | ||||
| African gray parrot | If itraconazole is used owing to monetary constraints, doses of 2.5 mg/kg PO q12–24 h have been used safely with frequent monitoring of plasma bile acid levels | Voriconazole is usually preferred over itraconazole owing to toxicity reports in African gray parrots | |||
| Voriconazole | |||||
| C (molds) ST (yeasts) |
Red-tailed hawk87,88 | 10–12.5 mg/kg q8–12h | Anorexia, vomiting, and alterations in liver function are most common.71,72 | Voriconazole induces its own metabolism via cytochrome P450 and doses should be increased over time.95 | |
| Timneh African gray parrot89 | 12–18 mg/kg PO q12 h | Owing to toxicity reports in African gray parrots, voriconazole is usually preferred over itraconazole in this species. | |||
| Hispaniolan Amazon parrot90 | 18 mg/kg PO q8 h | ||||
| Mallard duck91 | 20 mg/kg PO q8–12h | ||||
| Chicken92 | Poor bioavailability in chickens92 | ||||
| African penguins93 | |||||
| Falcon94 | |||||
| Fluconazole | |||||
| ST | Cockatiel | 5 mg/kg PO q24 h or 10 mg/kg PO q48 h or 100 mg/L in the drinking water | Fluconazole has the safest therapeutic index of the azoles. | Described doses resulted in plasma levels that exceeded human MIC for most strains of Candida albicans generally less effective against aspergillosis than itraconazole.69 | |
| Terbinafine | |||||
| C | A75 Cr78 |
Hispaniolan Amazon parrot79 | 60 mg/kg q24 h | Regurgitation in red-tailed hawk80 | Often combined with azoles71 Dose should be decreased in cases on impaired renal function57 |
| Red-tailed hawk80 | 22 mg/kg q24 h | ||||
| African penguin81 | 15 mg/kg q24 h | ||||
Cryptococcosis
Systemic cryptococcosis may also affect companion psittacine birds.78 , 96 Partial response to treatment with fluconazole (15 mg/kg by mouth every 12 hours) and terbinafine (15–20 mg/kg by mouth every 12 hours) was described in an African gray parrot (Psittacus erithacus) with renal cryptococcosis.96
Microsporidiosis
Renal microsporidiosis has been reported in lovebirds (Agapornis spp.),97 particularly in individuals positive for psittacine beak and feather disease,98 as well as budgerigar parakeets (Melopsittacus undulatus), eclectus (Eclectus roratus),99 red-bellied parrots (Poicephalus rufiventris),98 and other avian species.44 Treatment of renal microsporidiosis has not been reported in birds,100 but an umbrella cockatoo (Cacatua alba) with keratoconjunctivitis associated with microsporidia was successfully treated with albendazole (25 mg/kg by mouth every 24 hours) for 90 days.100 Clinicians should keep in mind potential toxicities associated with benzimidazoles in many birds.
Treatment of Intoxications Affecting Renal Function
Heavy metal intoxication
Lead and zinc toxicosis can cause renal nephrosis and acute tubular necrosis, respectively.101 , 102 Treatment of heavy metal toxicosis must begin with removal of metal from the gastrointestinal tract to halt further absorption.101 General supportive care is also important.101 Diets higher in calcium decreased morbidity and mortality in experimentally lead-poisoned ducks.103 Antioxidative therapies, such as supplemental vitamin C, may also be instituted, because lead induces free radicals.101
Various chelation agents have been used in avian species.101 Careful monitoring of renal parameters is important for the duration of chelation therapy. Elevated uric acid levels can be observed with heavy metal poisoning and improvement of hyperuricemia with therapy has been reported.102 , 104
Calcium disodium salt of EDTA (CaEDTA) is the main chelator for lead and zinc poisoning in avian species.101 CaEDTA must be administered parenterally because absorption from the gastrointestinal tract is poor.101 In a study conducted with children, nephrotoxicity and inducement of acute renal failure were reported as an adverse effect of CaEDTA.105 Although nephrotoxicity has not been reported in birds treated with CaEDTA, even at 40 mg/kg every 12 hours intramuscularly for 21 days,106 fluid therapy is still recommended to minimize the risk of renal adverse effects.102
Succimer (meso 2,3-dimercaptosuccinic acid or DMA) is an oral chelator, derived from British Anti-Lewisite capable of chelating lead from soft tissues, but not from bone.107 Succimer is less efficient in cases of zinc intoxication.101 Succimer has a narrow margin of safety, so accurate dosing is important. Doses as low as 15 mg/kg have been reported to be effective.101 In an experimental trial with induced lead intoxication in cockatiels, a dose of 40 mg/kg by mouth every 12 hours was found to be safe, whereas a dose of 80 mg/kg by mouth every 12 hours was associated with a high mortality rate.106
Treatment of intoxication by drugs or plants
Potentially nephrotoxic drugs include aminoglycosides,48 , 49 fenbendazole,108 and nonsteroidal anti-inflammatory drugs, such as diclofenac109 and flunixin meglumine.110 Ingestion of rhubarb leaves and other oxalic-acid rich plants can also cause kidney failure.102
Treatment options for intoxications include crop lavage111 or endoscopic removal of plant material when birds are presented within 1 to 6 hours of ingestion, depending on the avian species.112, 113, 114, 115 Fluid therapy and supportive care are also indicated. Some authors recommend the use of activated charcoal (1 g/kg or 1–3 mg/g body weight) as an adsorbent.102 This treatment is not recommended for acids or corrosive alkaloid agents because it will be useless and may complicate retrieval from the crop. For more information regarding treatment of avian intoxications, the reader should refer to the excellent review by Lightfoot and Yeager.116
Treatment of Nutritional Diseases Affecting Renal Function
Hypovitaminosis A
Vitamin A deficiency is commonly reported in companion parrots fed seed-based diets.20 Hypovitaminosis A can lead to squamous metaplasia of renal epithelium, ureteral mucosa, and collecting ducts leading to obstruction of the ureters and secondary hydronephrosis, hyperuricemia, and oliguric or anuric renal failure.44 Vitamin A may be supplemented at 2000 to 5000 IU/kg intramuscularly, then repeated every 1 to 3 weeks depending on patient condition and response. Of note, fat-soluble vitamin A is considered safer than water-soluble vitamin A.117 Vitamin A supplements are also available in powder form. Beta-carotenes and other provitamin A carotenoids can serve as a safer alternative to potentially toxic vitamin A in psittacine birds.20 Seeds and nuts are generally low in carotenoids, whereas some orange-colored fruits and vegetables, such as carrots, melon, and butternut squash, can provide large quantities thereof.14 A study in cockatiels demonstrated that vitamin A deficiency can be prevented with 4000 IU vitamin A/kg diet or 2.4 mg β-carotene/kg diet.118 Levels of less than 10,000 IU vitamin A/kg do not significantly influence plasma levels in cockatiels.118 Some avian species, such as recessive white canaries (Serinus canaria), are unable to convert ß-carotene to vitamin A and require 3 times as much vitamin A as colored canaries.119
Hypervitaminosis D
Excess vitamin D3 promotes metastatic mineralization of viscera, including the kidneys. Vitamin D3 is considered toxic at 4 to 10 times the recommended dose. Any bird species can potentially be susceptible to hypervitaminosis D16; however the dietary requirements for vitamin D vary among avian species, with optimum levels at 200 IU/kg in poultry, 900 IU/kg in turkey, and 1200 IU/kg in Japanese quail.14 In cases of hypervitaminosis D associated with hypercalcemia, fluid therapy and treatments stimulating calciuresis, such as bisphosphonates and corticosteroids, are recommended in dogs and cats.120 , 121 Unfortunately, the use of corticosteroids is controversial in birds owing to the risk of associated immunosuppression and safe doses of bisphosphonate have not been described in birds. Because metastatic calcifications are irreversible, prognosis is guarded.
Iron overload
Iron storage disease results from the accumulation of iron in various tissues, including the kidneys.122 High dietary iron has been implicated in the development of iron storage disease in susceptible species, such as hornbills, toucans, lories, and lorikeets, as well as mynahs and other Sturnidae.122 , 123 It is generally recommended that the iron content of commercial diets be maintained at less than 100 mg/kg.124 Iron-sensitive species require even lower amounts of iron, ranging from 19 to 25 mg/kg.122 The high vitamin C content of many fruits also enhances dietary iron uptake. Frugivorous species should be offered fruits low in vitamin C to minimize uptake of iron from commercial diets.122 Another common strategy is to soak commercial pellets in black tea (first discard the water after initial infusion to avoid caffeine administration, then add water again to the cup and let the pellets soak) to increase the amount of tannins in the food and thereby decrease iron absorption. Soaking should be done every other month to avoid causing other mineral deficiencies.
In case of renal hemochromatosis, treatments described in birds include therapeutic venipunctures to decrease hematocrit, oral deferiprone, or intramuscular deferoxamine injections (Table 4 ).
Table 4.
Treatments of hemochromatosis in selected avian species
| Therapeutic agent | Species | Doses | Action | Adverse effects | Comments |
|---|---|---|---|---|---|
| Deferiprone | Chickens and pigeons | 50 mg/kg PO q12 h | Significantly reduced iron concentration in liver and feces | Weight gain, decreased serum zinc levels, 30% mortality in chickens | Good gastrointestinal absorption at this dose |
| 70 mg/kg PO q24 h | Significantly reduced iron concentration in liver and feces | Weight gain, decreased serum zinc levels, 30% mortality in chickens | |||
| Hornbills (n = 3) | 75 mg/kg PO q24 h for 90 d | Significantly decreased hepatic iron concentration | |||
| Deferoxamine | Chestnut-fronted macaw (Ara severa) (n = 1) | 50 mg/kg IM q12 h for 14 d | Reduced hepatic iron concentration | Associated with a low-iron diet |
Treatments for Obstruction of Outflow
The underlying cause for urate concretions, such as a cloacolith or ureterolith, is rarely known.128 In rare instances, changes to digestive microbial flora may affect the cloacal environment and contribute to the formation of cloacoliths. A cloacolith composed of 100% uric acid was reported in a blue-fronted Amazon parrot fed a mixture of table food, seeds, and pellets.129 Cloacoliths can obstruct the ureteral opening and cause postrenal hyperuricemia. Cloacoliths can usually be disintegrated and removed with forceps via the cloaca with or without endoscopic assistance.
Ureteroliths have also been described in a double yellow-crowned Amazon parrot (Amazona ochrocephala), a chestnut-bellied seed finch (Oryzoborus angolensis), and in poultry.130, 131, 132 Imbalances in dietary calcium and phosphorus content and coronavirus infection are reported causes of urolithiasis in poultry.132 , 133 Treatment of ureteroliths requires a surgical approach130; lithotripsy may be an alternative treatment option.134
Treatment of Renal Neoplasia
Kidney neoplasms have been reported in several avian species44; however, budgerigar parakeets are overrepresented and renal neoplasms account for 17% to 20% of all neoplasms described in this species.135 Renal carcinoma is the most common renal neoplasm reported. Other renal neoplasms reported include renal adenoma, nephroblastoma, cystadenoma, and lymphoma.136
Nephrectomy is the treatment of choice for unilateral renal tumors in dogs.137 In birds, unless the renal neoplasm is contained and pedunculated, surgical removal is virtually impossible because of the kidney’s dorsal location, its intricate relationship with adjacent vessels and nerves,43 the limited access to the renal arteries, and the short distance between the renal artery and the aorta, which make ligation or hemostasis difficult if not impossible.44 Regional invasion by renal neoplasms into the synsacrum bone and sacral nerve plexus is also reported,138 precluding surgical excision.
No effective therapy for the management of renal tumors is recognized in birds.136 Palliative treatment is often selected, including the use of analgesics (see Pain management) and corticosteroids.43 , 44 Corticosteroids may predispose birds to opportunistic infections and should be used with caution.135 Prophylactic antibiotic and antifungal therapy are recommended whenever immunosuppressive drugs are used in avian species.139
In mammals, chemotherapy has not been shown to be effective against renal tumors other than lymphosarcoma.137 Chemotherapy has not been thoroughly evaluated for avian renal tumors. Carboplatin was used to treat renal adenocarcinoma in a budgerigar, resulting in a short-lived clinical improvement but the mass continued to grow.140 In this case, carboplatin was used at 5 mg/kg IV every 4 weeks without side effects.140 A few cases of lymphocytic leukemia affecting the kidneys and treated with chemotherapy have been described in psittacine birds.141 , 142
Radiation therapy for renal tumors has been rarely performed owing to questionable tolerance of adjacent tissues. In the case of a black swan (Cygnus atratus) presented with chronic T-cell lymphocytic leukemia affecting the kidneys, whole body radiation therapy with 2 Gy was performed over 31 days, in addition to chemotherapy with chlorambucil, followed by lomustine, l-asparaginase, and prednisone.143 The swan survived more than 1 year after treatment initiation and was euthanized owing to hyperviscosity syndrome associated with the leukemia. The white blood cell count decreased after radiation therapy and no adverse effects to radiation were detected clinically or at necropsy in this swan. The dose received was much lower than tolerable radiation doses evaluated in ring-necked parakeets (Psittacula krameri).144 Further studies are needed on the use of radiation therapy in birds for radiosensitive neoplasms.
Summary
The clinical management of bird with renal disease may prove challenging. Treatment choice is highly impacted by the cause and chronicity of the disease. The specific physiology of avian kidneys, and the large variety of species encountered in clinic implies that only a small part of the knowledge about mammalian therapeutics can be extrapolated to birds. More studies on renal disease treatments and their specific applications are warranted.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Grauer G.F. In: Manual of canine and feline nephrology and urology. 2nd edition. Elliott J., Grauer G.F., editors. BSAVA; Gloucester (England): 2007. Management of acute renal failure; pp. 215–222. [Google Scholar]
- 2.Brown S.A. In: Manual of canine and feline nephrology and urology. 2nd edition. Elliott J., Grauer G.F., editors. BSAVA; Gloucester (England): 2007. Management of chronic kidney disease; pp. 223–230. [Google Scholar]
- 3.Scope A., Schwendenwein I., Schauberger G. Plasma exogenous creatinine excretion for the assessment of renal function in avian medicine. Pharmacokinetic modeling in racing pigeons (Columba livia) J Avian Med Surg. 2013;27:173–179. doi: 10.1647/2012-015. [DOI] [PubMed] [Google Scholar]
- 4.Lierz M. Avian renal disease: pathogenesis, diagnosis, and therapy. Vet Clin North Am Exot Anim Pract. 2003;6:29–55. doi: 10.1016/s1094-9194(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 5.Langston C.E. In: Textbook of veterinary internal medicine. 8th edition. Ettinger S.J., Feldman E.C., Côté E., editors. Elsevier; Saint Louis (MO): 2017. Acute kidney injury; pp. 1919–1934. [Google Scholar]
- 6.Koutsos E.A., Smith J., Woods L.W., et al. Adult cockatiels (Nymphicus hollandicus) metabolically adapt to high protein diets. J Nutr. 2001;131:2014–2020. doi: 10.1093/jn/131.7.2014. [DOI] [PubMed] [Google Scholar]
- 7.Nechay B.R., Nechay L. Effects of probenecid, sodium salicylate, 2,4-dinitrophenol and pyrazinamide on renal secretion of uric acid in chickens. J Pharmacol Exp Ther. 1959;126:291–295. [PubMed] [Google Scholar]
- 8.Speer B. In: Avian medicine and surgery. Altman R.B., Clubb S.L., Dorrestein G.M., et al., editors. WB Saunders; Philadelphia: 1997. Diseases of the urogenital system; pp. 628–629. [Google Scholar]
- 9.Powers L.V. Common procedures in psittacines. Vet Clin North Am Exot Anim Pract. 2006;9:287–302. doi: 10.1016/j.cvex.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins J.R. Critical care of pet birds. Vet Clin North Am Exot Anim Pract. 2016;19:501–512. doi: 10.1016/j.cvex.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenberger M., Lennox A. In: Current therapy of avian medicine and surgery. 1st edition. Speer B.L., editor. Elsevier; Saint Louis (MO): 2016. Critical care; pp. 582–588. [Google Scholar]
- 12.Heard D. In: Current therapy of avian medicine and surgery. 1st edition. Speer B.L., editor. Elsevier; Saint Louis (MO): 2016. Anesthesia; pp. 601–615. [Google Scholar]
- 13.Kiang JH. Avian wing bones. UC San Diego Electronic Theses and Dissertations. 2013.
- 14.McDonald D. In: 2nd edition. Harrison G.J., Lightfoot T.L., editors. vol. 1. Spix Publishing Inc; Palm Beach (FL): 2005. Nutritional considerations: nutrition and dietary supplementation; pp. 86–107. (Clinical avian medicine). [Google Scholar]
- 15.Mayer J., Donnelly T.M. In: Clinical veterinary advisor. Birds and exotic pets. Mayer J., Donnelly T.M., editors. Elsevier; Saint Louis (MO): 2013. Renal disease; pp. 228–229. [Google Scholar]
- 16.Echols S. In: 2nd edition. Harrison G.J., Lightfoot T.L., editors. vol. 2. Spix Publishing Inc; Palm Beach (FL): 2005. Evaluating and treating the kidneys; pp. 451–492. (Clinical avian medicine). [Google Scholar]
- 17.Duncan M. In: Fowler's zoo and wild animal medicine. 8th edition. Miller R.E., Fowler M.E., editors. Elsevier; Saint Louis (MO): 2015. Gout in exotic animals; pp. 667–670. [Google Scholar]
- 18.Rupley A.E., Simone-Freilicher E. Psittacine wellness management and environmental enrichment. Vet Clin North Am Exot Anim Pract. 2015;18:197–211. doi: 10.1016/j.cvex.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Bartges J.W. In: Textbook of veterinary internal medicine. 8th edition. Ettinger S.J., Feldman E.C., Côté E., editors. Elsevier; Saint Louis (MO): 2017. Nutritional management of renal conditions; pp. 771–773. [Google Scholar]
- 20.Koutsos E., Gelis S., Echols M.S. In: Current therapy of avian medicine and surgery. 1st edition. Speer B.L., editor. Elsevier; Saint Louis (MO): 2016. Advancements in nutrition and nutritional therapy; pp. 142–176. [Google Scholar]
- 21.Nettleton J.A. Omega-3 fatty acids: comparison of plant and seafood sources in human nutrition. J Am Diet Assoc. 1991;91:331–337. [PubMed] [Google Scholar]
- 22.Heinze C.R., Hawkins M.G., Gillies L.A., et al. Effect of dietary omega-3 fatty acids on red blood cell lipid composition and plasma metabolites in the cockatiel, Nymphicus hollandicus. J Anim Sci. 2012;90:3068–3079. doi: 10.2527/jas.2011-4450. [DOI] [PubMed] [Google Scholar]
- 23.USDA Food Composition Databases [Internet] https://ndb.nal.usda.gov/ndb/ Available at: Accessed September 6, 2019.
- 24.Settle T., Carro M.D., Falkenstein E., et al. The effects of allopurinol, uric acid, and inosine administration on xanthine oxidoreductase activity and uric acid concentrations in broilers. Poult Sci. 2012;91:2895–2903. doi: 10.3382/ps.2012-02321. [DOI] [PubMed] [Google Scholar]
- 25.Carro M.D., Falkenstein E., Radke W.J., et al. Effects of allopurinol on uric acid concentrations, xanthine oxidoreductase activity and oxidative stress in broiler chickens. Comp Biochem Physiol C Toxicol Pharmacol. 2010;151:12–17. doi: 10.1016/j.cbpc.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Lumeij J.T., Sprang E.P., Redig P.T. Further studies on allopurinol-induced hyperuricaemia and visceral gout in red-tailed hawks (Buteo jamaicensis) Avian Pathol. 1998;27:390–393. doi: 10.1080/03079459808419356. [DOI] [PubMed] [Google Scholar]
- 27.Poffers J., Lumeij J.T., Timmermans-Sprang E.P.M., et al. Further studies on the use of allopurinol to reduce plasma uric acid concentrations in the red-tailed hawk (Buteo jamaicensis) hyperuricaemic model. Avian Pathol. 2002;31:567–572. doi: 10.1080/0307945021000024634. [DOI] [PubMed] [Google Scholar]
- 28.Siller W.G. Renal pathology of the fowl, a review. Avian Pathol. 1981;10:187–262. doi: 10.1080/03079458108418474. [DOI] [PubMed] [Google Scholar]
- 29.Poffers J., Lumeij J.T., Redig P.T. Investigations into the uricolytic properties of urate oxidase in a granivorous (Columba livia domestica) and in a carnivorous (Buteo jamaicensis) avian species. Avian Pathol. 2002;31:573–579. doi: 10.1080/0307945021000024616. [DOI] [PubMed] [Google Scholar]
- 30.Snoeyenbos G.H., Reynolds I.M., Tzianabos T. Articular gout in turkeys: a case report. Avian Dis. 1962;6:32–36. [Google Scholar]
- 31.Dudas P.L., Pelis R.M., Braun E.J., et al. Transepithelial urate transport by avian renal proximal tubule epithelium in primary culture. J Exp Biol. 2005;208:4305–4315. doi: 10.1242/jeb.01879. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Silvestre A. Treatment with allopurinol and probenecid for visceral gout in a Greek tortoise, Testudo graeca. Bulletin of the Association of Reptilians and Amphibian Veterinarians. 1997;7:4–5. [Google Scholar]
- 33.Creamer P., Hunt M., Dieppe P. Pain mechanisms in osteoarthritis of the knee: effect of intraarticular anesthetic. J Rheumatol. 1996;23:1031–1036. [PubMed] [Google Scholar]
- 34.Hocking P.M., Gentle M.J., Bernard R., et al. Evaluation of a protocol for determining the effectiveness of pretreatment with local analgesics for reducing experimentally induced articular pain in domestic fowl. Res Vet Sci. 1997;63:263–267. doi: 10.1016/s0034-5288(97)90031-x. [DOI] [PubMed] [Google Scholar]
- 35.Hawkins M.G., Paul-Murphy J., Sanchez-Migallon Guzman D. In: Current therapy of avian medicine and surgery. 1st edition. Speer B.L., editor. Elsevier; Saint Louis (MO): 2016. Advances in anesthesia, analgesia, and surgery; pp. 616–630. [Google Scholar]
- 36.Souza M.J., Gerhardt L., Cox S. Pharmacokinetics of repeated oral administration of tramadol hydrochloride in Hispaniolan Amazon parrots (Amazona ventralis) Am J Vet Res. 2013;74:957–962. doi: 10.2460/ajvr.74.7.957. [DOI] [PubMed] [Google Scholar]
- 37.Laniesse D., Sanchez-Migallon Guzman D., Knych H.K., et al. Pharmacokinetics of butorphanol tartrate in a long-acting poloxamer 407 gel formulation administered to Hispaniolan Amazon parrots (Amazona ventralis) Am J Vet Res. 2017;78:688–694. doi: 10.2460/ajvr.78.6.688. [DOI] [PubMed] [Google Scholar]
- 38.Baine K., Jones M.P., Cox S., et al. Pharmacokinetics of compounded intravenous and oral gabapentin in Hispaniolan Amazon Parrots (Amazona ventralis) J Avian Med Surg. 2015;29:165–174. doi: 10.1647/2014-025. [DOI] [PubMed] [Google Scholar]
- 39.Cole G.A., Paul-Murphy J., Krugner-Higby L., et al. Analgesic effects of intramuscular administration of meloxicam in Hispaniolan parrots (Amazona ventralis) with experimentally induced arthritis. Am J Vet Res. 2009;70:1471–1476. doi: 10.2460/ajvr.70.12.1471. [DOI] [PubMed] [Google Scholar]
- 40.Curtiss J.B., Leone A.M., Wellehan J.F.X., et al. Renal and cloacal cryptosporidiosis (Cryptosporidium avian genotype V) in a major Mitchell's cockatoo (Lophochroa leadbeateri) J Zoo Wildl Med. 2015;46:934–937. doi: 10.1638/2015-0025.1. [DOI] [PubMed] [Google Scholar]
- 41.Hinrichsen J.P., Neira M., Lopez C., et al. Omeprazole, a specific gastric secretion inhibitor on oxynticopeptic cells, reduces gizzard erosion in broiler chicks fed with toxic fish meals. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1997;117:267–273. doi: 10.1016/s0742-8413(97)00067-4. [DOI] [PubMed] [Google Scholar]
- 42.Harrison G.J., Lightfoot T.L., Flinchum G.B. In: 2nd edition. Harrison G.J., Lightfoot T.L., editors. vol. 1. Spix Publishing Inc; Palm Beach (FL): 2005. Emergency and critical care; pp. 213–232. (Clinical avian medicine). [Google Scholar]
- 43.Pollock C. Diagnosis and treatment of avian renal disease. Vet Clin North Am Exot Anim Pract. 2006;9:107–128. doi: 10.1016/j.cvex.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Johnson J.G., Brandao J., Perry S.M., et al. In: Current therapy of exotic pet practice. 1st edition. Mitchell M.A., Tully T.N., editors. Elsevier; Saint Louis (MO): 2016. Urinary system. [Google Scholar]
- 45.Mutalib A., Keirs R., Austin F. Erysipelas in quail and suspected erysipeloid in processing plant employees. Avian Dis. 1995;39:191–193. [PubMed] [Google Scholar]
- 46.Sato Y., Aoyagi T., Matsuura S., et al. An occurrence of avian tuberculosis in hooded merganser (Lophodytes cucullatus) Avian Dis. 1996;40:941–944. [PubMed] [Google Scholar]
- 47.Ackerman L.J., Benbrook S.C., Walton B.C. Mycobacterium tuberculosis infection in a parrot (Amazona farinosa) Am Rev Respir Dis. 1974;109:388–390. doi: 10.1164/arrd.1974.109.3.388. [DOI] [PubMed] [Google Scholar]
- 48.Junge R.E., MacCoy D.M. Amikacin therapy for Pseudomonas cellulitis in an Amazon parrot. J Am Vet Med Assoc. 1985;187:417–418. [PubMed] [Google Scholar]
- 49.Flammer K., Clark C.H., Drewes L.A., et al. Adverse effects of gentamicin in scarlet macaws and galahs. Am J Vet Res. 1990;51:404–407. [PubMed] [Google Scholar]
- 50.Leal de Araujo J., Rech R.R., Heatley J.J., et al. From nerves to brain to gastrointestinal tract: a time-based study of parrot bornavirus 2 (PaBV-2) pathogenesis in cockatiels (Nymphicus hollandicus) PLoS One. 2017;12:11. doi: 10.1371/journal.pone.0187797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Payne S., Shivaprasad H.L., Mirhosseini N., et al. Unusual and severe lesions of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) acting as healthy carriers of avian bornavirus (ABV) and subsequently infected with a virulent strain of ABV. Avian Pathol. 2011;40:15–22. doi: 10.1080/03079457.2010.536978. [DOI] [PubMed] [Google Scholar]
- 52.Palmieri C., Franca M., Uzal F., et al. Pathology and immunohistochemical findings of west Nile virus infection in psittaciformes. Vet Pathol. 2011;48:975–984. doi: 10.1177/0300985810391112. [DOI] [PubMed] [Google Scholar]
- 53.Gajadhar A.A., Cawthorn R.J., Wobeser G.A., et al. Prevalence of renal coccidia in wild waterfowl in Saskatchewan. Can J Zool. 1983;61:2631–2633. [Google Scholar]
- 54.Randall C.J. Renal and nasal cryptosporidiosis in a junglefowl (Gallus sonneratii) Vet Rec. 1986;119:130–131. doi: 10.1136/vr.119.6.130. [DOI] [PubMed] [Google Scholar]
- 55.Gardiner C.H., Imes G.D. Cryptosporidium sp in the kidneys of a black-throated finch. J Am Vet Med Assoc. 1984;185:1401–1402. [PubMed] [Google Scholar]
- 56.Baker K.C., Rettenmund C.L., Sander S.J., et al. Clinical effect of hemoparasite infections in snowy owls (Bubo Scandiacus) J Zoo Wildl Med. 2018;49:143–152. doi: 10.1638/2017-0042R.1. [DOI] [PubMed] [Google Scholar]
- 57.Plumb D.C. 9th edition. Wiley-Blackwell; Hoboken (NJ): 2018. Plumb’s veterinary drug handbook. [Google Scholar]
- 58.Forbes N.A., Fox M.T. Field trial of a Caryospora species vaccine for controlling clinical coccidiosis in falcons. Vet Rec. 2005;156:134–138. doi: 10.1136/vr.156.5.134. [DOI] [PubMed] [Google Scholar]
- 59.Carpenter J.W. 5th edition. Elsevier; Saint Louis (MO): 2018. Exotic animal formulary. [Google Scholar]
- 60.Mathis G.F., Froyman R., Irion T., et al. Coccidiosis control with toltrazuril in conjunction with anticoccidial medicated or nonmedicated feed. Avian Dis. 2003;47:463–469. doi: 10.1637/0005-2086(2003)047[0463:CCWTIC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 61.Sokól R., Galecki R. The resistance of Eimeria spp. to toltrazuril in black grouse (Lyrurus tetrix) kept in an aviary. Poult Sci. 2018;97(12):4193–4199. doi: 10.3382/ps/pey296. [DOI] [PubMed] [Google Scholar]
- 62.Stephen B., Rommel M., Daugschies A., et al. Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains. Vet Parasitol. 1997;69:19–29. doi: 10.1016/s0304-4017(96)01096-5. [DOI] [PubMed] [Google Scholar]
- 63.Harfoush M.A., Hegazy A.M., Soliman A.H., et al. Drug resistance evaluation of some commonly used anti-coccidial drugs in broiler chickens. J Egypt Soc Parasitol. 2010;40:337–348. [PubMed] [Google Scholar]
- 64.Lan L.H., Sun B.B., Zuo B.X.Z., et al. Prevalence and drug resistance of avian Eimeria species in broiler chicken farms of Zhejiang province, China. Poult Sci. 2017;96:2104–2109. doi: 10.3382/ps/pew499. [DOI] [PubMed] [Google Scholar]
- 65.Ahad S., Tanveer S., Nawchoo I.A., et al. Anticoccidial activity of Artemisia vestita (Anthemideae, Asteraceae), a traditional herb growing in the Western Himalayas, Kashmir, India. Microb Pathog. 2017;104:289–295. doi: 10.1016/j.micpath.2017.01.053. [DOI] [PubMed] [Google Scholar]
- 66.Hayajneh F.M.F., Jalal M., Zakaria H., et al. Anticoccidial effect of apple cider vinegar on broiler chicken: an organic treatment to measure anti-oxidant effect. Pol J Vet Sci. 2018;21:361–369. doi: 10.24425/122605. [DOI] [PubMed] [Google Scholar]
- 67.Malik T.A., Kamili A.N., Chishti M.Z., et al. Synergistic approach for treatment of chicken coccidiosis using berberine, a plant natural product. Microb Pathog. 2016;93:56–62. doi: 10.1016/j.micpath.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 68.Muthamilselvan T., Kuo T.F., Wu Y.C., et al. Herbal remedies for coccidiosis control: a review of plants, compounds, and anticoccidial actions. Evid Based Complement Alternat Med. 2016;2016:2657981. doi: 10.1155/2016/2657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dahlhausen R.D. In: 2nd edition. Harrison G.J., Lightfoot T.L., editors. vol. 2. Spix Publishing Inc; Palm Beach (FL): 2005. Implications of mycoses in clinical disorders; pp. 691–704. (Clinical avian medicine). [Google Scholar]
- 70.Greenacre C.B., Latimer K.S., Ritchie B.W. Leg paresis in a black palm cockatoo (Probosciger aterrimus) caused by aspergillosis. J Zoo Wildl Med. 1992;23:122–126. [Google Scholar]
- 71.Martel A., Wellehan J.F.X., Lierz M., et al. In: Current therapy of avian medicine and surgery. 1st edition. Speer B.L., editor. Elsevier; Saint Louis (MO): 2016. Infectious disease; pp. 22–106. [Google Scholar]
- 72.Antonissen G., Martel A. Antifungal therapy in birds: old drugs in a new jacket. Vet Clin North Am Exot Anim Pract. 2018;21:355–377. doi: 10.1016/j.cvex.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 73.Ziołkowska G., Tokarzewski S., Nowakiewicz A. Drug resistance of Aspergillus fumigatus strains isolated from flocks of domestic geese in Poland. Poult Sci. 2014;93:1106–1112. doi: 10.3382/ps.2013-03702. [DOI] [PubMed] [Google Scholar]
- 74.Redig P.T., Duke G.E. Comparative pharmacokinetics of antifungal drugs in domestic turkeys, red-tailed hawks, broad-winged hawks, and great-horned owls. Avian Dis. 1985;29:649–661. [PubMed] [Google Scholar]
- 75.Summa N.M., Sanchez-Migallon Guzman D. Evidence-based advances in avian medicine. Vet Clin North Am Exot Anim Pract. 2017;20:817–837. doi: 10.1016/j.cvex.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Beernaert L.A., Pasmans F., Van Waeyenberghe L., et al. Avian Aspergillus fumigatus strains resistant to both itraconazole and voriconazole. Antimicrob Agents Chemother. 2009;53:2199–2201. doi: 10.1128/AAC.01492-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoppes S., Gurfield N., Flammer K., et al. Mycotic keratitis in a blue-fronted Amazon Parrot (Amazona aestiva) J Avian Med Surg. 2000;14:185–190. [Google Scholar]
- 78.Maccolini É.O., Dufresne P.J., Aschenbroich S.A., et al. A disseminated Cryptococcus gattii VGIIa Infection in a citron-crested cockatoo (Cacatua sulphurea citrinocristata) in Québec, Canada. J Avian Med Surg. 2017;31:142–151. doi: 10.1647/2016-193. [DOI] [PubMed] [Google Scholar]
- 79.Evans E.E., Emery L.C., Cox S.K., et al. Pharmacokinetics of terbinafine after oral administration of a single dose to Hispaniolan Amazon parrots (Amazona ventralis) Am J Vet Res. 2013;74:835–838. doi: 10.2460/ajvr.74.6.835. [DOI] [PubMed] [Google Scholar]
- 80.Bechert U., Christensen J.M., Poppenga R., et al. Pharmacokinetics of terbinafine after single oral dose administration in red-tailed hawks (Buteo jamaicensis) J Avian Med Surg. 2010;24:122–130. doi: 10.1647/2008-052.1. [DOI] [PubMed] [Google Scholar]
- 81.Bechert U., Christensen J.M., Poppenga R., et al. Pharmacokinetics of orally administered terbinafine in African penguins (Spheniscus demersus) for potential treatment of aspergillosis. J Zoo Wildl Med. 2010;41:263–274. doi: 10.1638/2009-0211R.1. [DOI] [PubMed] [Google Scholar]
- 82.Bunting E.M., Abou-Madi N., Cox S., et al. Evaluation of oral itraconazole administration in captive Humboldt penguins (Spheniscus humboldti) J Zoo Wildl Med. 2009;40:508–518. doi: 10.1638/2009-0045.1. [DOI] [PubMed] [Google Scholar]
- 83.Orosz S.E., Frazier D.L., Schroeder E.C., et al. Pharmacokinetic properties of itraconazole in blue-fronted Amazon parrots (Amazona aestiva aestiva) J Avian Med Surg. 1996;10:168–173. [Google Scholar]
- 84.Lumeij J.T., Gorgevska D., Woestenborghs R. Plasma and tissue concentrations of itraconazole in racing pigeons (Columba livia domestica) J Avian Med Surg. 1995;9:32–35. [Google Scholar]
- 85.Jones M.P., Orosz S.E., Cox S.K., et al. Pharmacokinetic disposition of itraconazole in red-tailed hawks (Buteo jamaicensis) J Avian Med Surg. 2000;14:15–22. [Google Scholar]
- 86.Keller K.A. Itraconazole. J Exot Pet Med. 2011;20:156–160. [Google Scholar]
- 87.Parsley R.A., Tell L.A., Gehring R. Pharmacokinetics of a single dose of voriconazole administered orally with and without food to red-tailed hawks (Buteo jamaicensus) Am J Vet Res. 2017;78:433–439. doi: 10.2460/ajvr.78.4.433. [DOI] [PubMed] [Google Scholar]
- 88.Gentry J., Montgerard C., Crandall E., et al. Voriconazole disposition after single and multiple, oral doses in healthy, adult red-tailed hawks (Buteo jamaicensis) J Avian Med Surg. 2014;28:201–208. doi: 10.1647/20-077. [DOI] [PubMed] [Google Scholar]
- 89.Flammer K., Nettifee Osborne J.A., Webb D.J., et al. Pharmacokinetics of voriconazole after oral administration of single and multiple doses in African grey parrots (Psittacus erithacus timneh) Am J Vet Res. 2008;69:114–121. doi: 10.2460/ajvr.69.1.114. [DOI] [PubMed] [Google Scholar]
- 90.Sanchez-Migallon Guzman D., Flammer K., Papich M.G., et al. Pharmacokinetics of voriconazole after oral administration of single and multiple doses in Hispaniolan Amazon parrots (Amazona ventralis) Am J Vet Res. 2010;71:460–467. doi: 10.2460/ajvr.71.4.460. [DOI] [PubMed] [Google Scholar]
- 91.Kline Y., Clemons K.V., Woods L., et al. Pharmacokinetics of voriconazole in adult mallard ducks (Anas platyrhynchos) Med Mycol. 2011;49:500–512. doi: 10.3109/13693786.2010.542553. [DOI] [PubMed] [Google Scholar]
- 92.Burhenne J., Haefeli W.E., Hess M., et al. Pharmacokinetics, tissue concentrations, and safety of the antifungal agent voriconazole in chickens. J Avian Med Surg. 2008;22:199–207. doi: 10.1647/2007-003.1. [DOI] [PubMed] [Google Scholar]
- 93.Hyatt M.W., Wiederhold N.P., Hope W.W., et al. Pharmacokinetics of orally administered voriconazole in African penguins (Spheniscus demersus) after single and multiple doses. J Zoo Wildl Med. 2017;48:352–362. doi: 10.1638/2016-0160R2.1. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt V., Demiraj F., Somma A.D., et al. Plasma concentrations of voriconazole in falcons. Vet Rec. 2007;161:265–268. doi: 10.1136/vr.161.8.265. [DOI] [PubMed] [Google Scholar]
- 95.Sanchez-Migallon Guzman D. Advances in avian clinical therapeutics. J Exot Pet Med. 2014;23:6–20. [Google Scholar]
- 96.Schunk R.S.K., Sitinas N.E., Quesenberry K.E., et al. Multicentric cryptococcosis in a Congo African grey parrot (Psittacus erithacus erithacus) J Avian Med Surg. 2017;31:373–381. doi: 10.1647/2017-259. [DOI] [PubMed] [Google Scholar]
- 97.Randall C.J., Lees S., Higgins R.J., et al. Microsporidian infection in lovebirds (Agapornis spp.) Avian Pathol. 1986;15:223–231. doi: 10.1080/03079458608436283. [DOI] [PubMed] [Google Scholar]
- 98.Barton C.E., Phalen D.N., Snowden K.F. Prevalence of microsporidian spores shed by asymptomatic lovebirds: evidence for a potential emerging zoonosis. J Avian Med Surg. 2003;17:197–202. [Google Scholar]
- 99.Pulparampil N., Graham D., Phalen D., et al. Encephalitozoon hellem in two eclectus parrots (Eclectus roratus): identification from archival tissues. J Eukaryot Microbiol. 1998;45:651–655. doi: 10.1111/j.1550-7408.1998.tb04562.x. [DOI] [PubMed] [Google Scholar]
- 100.Phalen D.N., Logan K.S., Snowden K.F. Encephalitozoon hellem infection as the cause of a unilateral chronic keratoconjunctivitis in an umbrella cockatoo (Cacatua alba) Vet Ophthalmol. 2006;9:59–63. doi: 10.1111/j.1463-5224.2005.00434.x. [DOI] [PubMed] [Google Scholar]
- 101.Wismer T. In: Current therapy of avian medicine and surgery. 1st edition. Speer B.L., editor. Elsevier; Saint Louis (MO): 2016. Advancements in diagnosis and management of toxicologic problems; pp. 589–599. [Google Scholar]
- 102.Richardson J.A. In: 2nd edition. Harrison G.J., Lightfoot T.L., editors. vol. 2. Spix Publishing Inc; Palm Beach (FL): 2005. Implications of toxic substances in clinical disorders; pp. 711–720. (Clinical avian medicine). [Google Scholar]
- 103.Carlson B.L., Nielsen S.W. Influence of dietary calcium on lead poisoning in mallard ducks (Anas platyrynchos) Am J Vet Res. 1985;46:276–282. [PubMed] [Google Scholar]
- 104.Pikula J., Hajkova P., Bandouchova H., et al. Lead toxicosis of captive vultures: case description and responses to chelation therapy. BMC Vet Res. 2013;9:11. doi: 10.1186/1746-6148-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Samour J.H., Naldo J. Diagnosis and therapeutic management of lead toxicosis in falcons in Saudi Arabia. J Avian Med Surg. 2002;16:16–20. [Google Scholar]
- 106.Denver M.C., Tell L.A., Galey F.D., et al. Comparison of two heavy metal chelators for treatment of lead toxicosis in cockatiels. Am J Vet Res. 2000;61:935–940. doi: 10.2460/ajvr.2000.61.935. [DOI] [PubMed] [Google Scholar]
- 107.Andersen O. Principles and recent developments in chelation treatment of metal intoxication. Chem Rev. 1999;99:2683–2710. doi: 10.1021/cr980453a. [DOI] [PubMed] [Google Scholar]
- 108.Gozalo A.S., Schwiebert R.S., Lawson G.W. Mortality associated with fenbendazole administration in pigeons (Columba livia) J Am Assoc Lab Anim Sci. 2006;45:63–66. [PubMed] [Google Scholar]
- 109.Sharma A.K., Saini M., Singh S.D., et al. Diclofenac is toxic to the steppe eagle, Aquila nipalensis: widening the diversity of raptors threatened by NSAID misuse in South Asia. Bird Conserv Int. 2014;24:282–286. [Google Scholar]
- 110.Cuthbert R., Parry-Jones J., Green R.E., et al. NSAIDs and scavenging birds: potential impacts beyond Asia’s critically endangered vultures. Biol Lett. 2007;3:90–93. doi: 10.1098/rsbl.2006.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coutant T., Vergneau-Grosset C., Langlois I. Overview of drug delivery methods in exotics, including their anatomic and physiologic considerations. Vet Clin North Am Exot Anim Pract. 2018;21:215–259. doi: 10.1016/j.cvex.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 112.Kubiak M., Forbes N.A. Fluoroscopic evaluation of gastrointestinal transit time in African Grey parrots. Vet Rec. 2012;171:563. doi: 10.1136/vr.101145. [DOI] [PubMed] [Google Scholar]
- 113.Doss G.A., Williams J.M., Mans C. Determination of gastrointestinal transit times in barred owls (Strix varia) by contrast fluoroscopy. J Avian Med Surg. 2017;31:123–128. doi: 10.1647/2016-182. [DOI] [PubMed] [Google Scholar]
- 114.Bloch R.A., Cronin K., Hoover J.P., et al. Evaluation of gastrointestinal tract transit times using barium-impregnated polyethylene spheres and barium sulfate suspension in a domestic pigeon (Columba livia) model. J Avian Med Surg. 2010;24:1–8. doi: 10.1647/2008-043R.1. [DOI] [PubMed] [Google Scholar]
- 115.Vink-Nooteboom M., Lumeij J.T., Wolvekamp W.T.C. Radiography and image-intensified fluoroscopy of barium passage through the gastrointestinal tract in six healthy Amazon parrots (Amazona aestiva) Vet Radiol Ultrasound. 2003;44:43–48. doi: 10.1111/j.1740-8261.2003.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 116.Lightfoot T.L., Yeager J.M. Pet bird toxicity and related environmental concerns. Vet Clin North Am Exot Anim Pract. 2008;11:229–259. doi: 10.1016/j.cvex.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 117.Palmer D., Rubel A., Mettler F., et al. Experimentally induced skin changes in land tortoises by giving high doses of vitamin A parenterally. Zentralbl Veterinarmed A. 1984;31:625. [PubMed] [Google Scholar]
- 118.Koutsos E.A., Klasing K.C. Vitamin A nutrition of growing cockatiel chicks (Nymphicus hollandicus) J Anim Physiol Anim Nutr. 2005;89:379–387. doi: 10.1111/j.1439-0396.2005.00526.x. [DOI] [PubMed] [Google Scholar]
- 119.Wolf P., Bartels T., Sallmann H.P., et al. Vitamin A metabolism in recessive white canaries. Anim Welf. 2000;9:153–165. [Google Scholar]
- 120.Mellanby R.J., Mee A.P., Berry J.L., et al. Hypercalcaemia in two dogs caused by excessive dietary supplementation of vitamin D. J Small Anim Pract. 2005;46:334–338. doi: 10.1111/j.1748-5827.2005.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 121.Wehner A., Katzenberger J., Groth A., et al. Vitamin D intoxication caused by ingestion of commercial cat food in three kittens. J Feline Med Surg. 2013;15:730–736. doi: 10.1177/1098612X12472180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harrison G.J., McDonald D. In: 2nd edition. Harrison G.J., Lightfoot T.L., editors. vol. 1. Spix Publishing Inc; Palm Beach (FL): 2005. Nutritional considerations: nutritional disorders; pp. 108–140. (Clinical avian medicine). [Google Scholar]
- 123.Lowenstine L., Stasiak I.M. In: Fowler's zoo and wild animal medicine. 8th edition. Miller R.E., Fowler M.E., editors. Elsevier; Saint Louis (MO): 2015. Update on iron overload in zoologic species; pp. 674–680. [Google Scholar]
- 124.Johnston G.B. Iron storage disease (hemochromatosis) in softbilled birds. Journal of the American Federation of Aviculture. 1999;26:25–28. [Google Scholar]
- 125.Whiteside D.P., Barker I.K., Mehren K.G., et al. Clinical evaluation of the oral iron chelator deferiprone for the potential treatment of iron overload in bird species. J Zoo Wildl Med. 2004;35:40–49. doi: 10.1638/02-031. [DOI] [PubMed] [Google Scholar]
- 126.Sandmeier P., Clauss M., Donati O.F., et al. Use of deferiprone for the treatment of hepatic iron storage disease in three hornbills. J Am Vet Med Assoc. 2012;240:75–81. doi: 10.2460/javma.240.1.75. [DOI] [PubMed] [Google Scholar]
- 127.Gancz A.Y., Wellehan J.F.X., Boutette J., et al. Diabetes mellitus concurrent with hepatic haemosiderosis in two macaws (Ara severa, Ara militaris) Avian Pathol. 2007;36:331–336. doi: 10.1080/03079450701466093. [DOI] [PubMed] [Google Scholar]
- 128.Taylor W.M. In: Current therapy of avian medicine and surgery. 1st edition. Speer B.L., editor. Elsevier; Saint Louis (MO): 2016. Clinical significance of the avian cloaca; Interrelationships with the kidney and the hindgut; pp. 341–342. [Google Scholar]
- 129.Beaufrère H., Nevarez J., Tully T.N. Cloacolith in a blue-fronted amazon parrot (Amazona aestiva) J Avian Med Surg. 2010;24:142–145. doi: 10.1647/2009-013.1. [DOI] [PubMed] [Google Scholar]
- 130.Dennis P.M., Bennett R.A. Ureterotomy for removal of two ureteroliths in a parrot. J Am Vet Med Assoc. 2000;217:865–868. doi: 10.2460/javma.2000.217.865. [DOI] [PubMed] [Google Scholar]
- 131.Marietto-Gonçalves G.A., Salgado B.S. Post-mortem lesions of urolithiasis in a lesser seed finch (Sporophila angolensis) Acta Vet Bras. 2012;6:52–55. [Google Scholar]
- 132.Wideman R.F., Closser J.A., Roush W.B., et al. Urolithiasis in pullets and laying hens: role of dietary calcium and phosphorus. Poult Sci. 1985;64:2300–2307. doi: 10.3382/ps.0642300. [DOI] [PubMed] [Google Scholar]
- 133.Brown T.P., Glisson J.R., Rosales G., et al. Studies of avian urolithiasis associated with an infectious bronchitis virus. Avian Dis. 1987;31:629–636. [PubMed] [Google Scholar]
- 134.Machado C, Mihm F, Buckley DN. Disintegration of kidney stones by extracorporeal shockwave lithotripsy in a penguin. Proceeding of first international conference of zoological avian medicine. Oahu Hawaii, September 6–11, 1987. p. 343–9.
- 135.Simova-Curd S., Nitzl D., Mayer J., et al. Clinical approach to renal neoplasia in budgerigars (Melopsittacus undulatus) J Small Anim Pract. 2006;47:504–511. doi: 10.1111/j.1748-5827.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 136.Robat C.S., Ammersbach M., Mans C. Avian oncology: diseases, diagnostics, and therapeutics. Vet Clin North Am Exot Anim Pract. 2017;20:57–86. doi: 10.1016/j.cvex.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 137.Brown S., Sandersen S.L. In: The Merck veterinary manual. Non-infectious diseases of the urinary system in small animals. 9th edition. Kahn C.M., Line S., editors. John Wiley and Sons; Summerset, (South Dakota): 2005. Urinary system; pp. 1249–1288. [Google Scholar]
- 138.Freeman K.P., Hahn K.A., Jones M.P., et al. Right leg muscle atrophy and osteopenia caused by renal adenocarcinoma in a cockatiel (Melopsittacus, undulatus) Vet Radiol Ultrasound. 1999;40:144–147. doi: 10.1111/j.1740-8261.1999.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 139.Zehnder A., Graham J., Antonissen G. Update on cancer treatment in exotics. Vet Clin North Am Exot Anim Pract. 2018;21:465–509. doi: 10.1016/j.cvex.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 140.Macwhirter P., Pyke D., Wayne J. Use of carboplatin in the treatment of a renal adenocarcinoma in a budgerigar. Exotic DVM. 2002;4:11–12. [Google Scholar]
- 141.Hammond E.E., Sanchez-Migallon Guzman D., Garner M.M., et al. Long-term treatment of chronic lymphocytic leukemia in a green-winged macaw (Ara chloroptera) J Avian Med Surg. 2010;24:330–338. doi: 10.1647/2009-001.1. [DOI] [PubMed] [Google Scholar]
- 142.Osofsky A., Hawkins M.G., Foreman O., et al. T-cell chronic lymphocytic leukemia in a double yellow-headed Amazon parrot (Amazona ochrocephala oratrix) J Avian Med Surg. 2011;25:286–294. doi: 10.1647/2011-009.1. [DOI] [PubMed] [Google Scholar]
- 143.Sinclair K.M., Hawkins M.G., Wright L., et al. Chronic T-cell lymphocytic leukemia in a black swan (Cygnus atratus): diagnosis, treatment, and pathology. J Avian Med Surg. 2015;29:326–335. doi: 10.1647/2015-075. [DOI] [PubMed] [Google Scholar]
- 144.Barron H.W., Roberts R.E., Latimer K.S., et al. Tolerance doses of cutaneous and mucosal tissues in ring-necked parakeets (Psittacula krameri) for external beam megavoltage radiation. J Avian Med Surg. 2009;23:6–9. doi: 10.1647/2008-012R.1. [DOI] [PubMed] [Google Scholar]