Abstract
The non-structural protein 1 (NS1) of dengue virus is a useful target for diagnostics of dengue infection since the protein is abundantly circulating in blood during the acute phase of the disease. Prior work has established that secreted NS1 levels in plasma correlates with viremia levels and hence can also be used to diagnose patients at the risk for developing dengue hemorrhagic fever. Thus detection of non-structural dengue antigens may be of benefit for an early rapid diagnosis of dengue infection due to its long half life in the blood. Here we describe a simple and efficient method for the expression of NS1 in Escherichia coli, which could potentially be used to develop monoclonal and bispecific antibodies for point of care diagnostics. E. coli codon optimized synthetic full-length NS1 gene of dengue serotype 1 (DEN-1) was successfully cloned and expressed in very high-level as inclusion bodies. The NS1 protein was successfully affinity purified and refolded as a recombinant NS1 (rNS1) protein in E. coli and yield was 230–250 mg/L of bacterial culture. The rNS1 protein was used to immunize mice for hybridoma development. The polyclonal antiserum from animals immunized with this rNS1 protein was found to specifically recognize the rNS1, thus demonstrating the immunogenic nature of the protein. The rNS1 protein purified from E. coli could be useful for developing a sensitive serum diagnostic assay to monitor dengue outbreaks.
Keywords: Dengue, NS1, E. coli expression, IMAC, Refolding
Introduction
Dengue fever is an important mosquito-borne viral disease of humans. This has been a recurrent phenomenon throughout the tropics in the past decade. During 2002, more than 30 Latin American countries reported over a million dengue fever (DF) 1 cases with large number of dengue hemorrhagic fever (DHF). Annually, there are an estimated 100 million dengue virus infections worldwide [1]. Increasingly cases of the more severe and potentially lethal DHF and dengue shock syndrome (DSS) are reported with children bearing much of the disease burden. Dengue virus is endemic in at least 100 countries worldwide and causes more human cases than any other mosquito-borne virus. The mortality rate of DHF in most countries is 5%, primarily among children and young adults. In several Asian countries, this virus is the leading cause of hospitalization and death in children. Hence, there is an urgent need for diagnostic, prophylactic and therapeutic reagents to manage DHF.
The dengue virus non-structural NS1 protein is a 46–50 kDa glycoprotein expressed in infected mammalian cells. All non-structural proteins are intracellular proteins with the exception of dengue NS1 protein, which exists as secreted as well as a membrane-associated protein. Both forms are demonstrated to be immunogenic [2], [3], [4]. It was also reported that NS1 is one of 7 NS proteins produced during viral replication. It possesses not only group specific but also type specific determinants and has been recognized as an important antigen in dengue infection [2], [4], [5]. A high circulating level of NS1 was demonstrated in the acute phase of dengue by antigen capture ELISAs [2], [6]. The precise function of dengue NS1 protein remains unclear. However, antigen detection of non-structural dengue antigens may be of benefit for an early stage rapid diagnosis of infection due to its long half life in the blood.
The usefulness of this study was to clone and express of DEN-1 full-length NS1 gene in Escherichia coli, for future development of monoclonal antibodies exploiting hybridoma and quadroma technology for rapid point of care applications. In this study, we report the successful cloning and very high-level expression of the NS1 protein and purification from E. coli as inclusion bodies and subsequent refolding.
Materials and methods
Chemicals
Restriction endonucleases and modifying enzymes were purchased from New England Biolabs (Mississauga, Canada). The anti-His6 MAb was purchased from Novagen Inc. (Madison, USA). Prestained low range protein molecular weight markers, 40% acrylamide: bisacrylamide, glycine and protein assay reagents were purchased from Bio-Rad (Mississauga, Canada). ECL nitrocellulose membrane, X-ray film and Western blotting reagent were purchased from Amersham Pharmacia Biotech (BaiedUrfe, Quebec, Canada). Glutathione (reduced and oxidized), sodium deoxycholate, l-arginine, GAM-HRPO, urea and other general molecular biology grade reagents were purchased from Sigma (Oakville, Canada). Ni–NTA agarose, plasmid DNA isolation and gel extraction kits were obtained from Qiagen (Mississauga, Canada).
Construction of plasmid (pDS21NS1)
The NS1 full-length nucleotide sequence of dengue (DEN-1) was codon optimized for E. coli expression and chemically synthesized by GENEART Inc., Germany. The codon optimized NS1 gene containing plasmid obtained from GENEART Inc. and the expression vector pBM802 [7] were digested with NdeI and EcoRI, gel purified and ligated. The ligation mixtures were transformed in E. coli top 10 cells and bacterial colonies were analyzed by plasmid DNA isolation and restriction digestion fragment mapping [8].
Recombinant clones analysis
Single bacterial colonies were cultured in 2 ml TB medium [8] containing 5 μg/ml of tetracycline (Tet5) and were incubated overnight at 37 °C with shaking (250 rpm). The overnight culture was diluted to 1/100th volume in 10 ml fresh TB/Tet5 medium and grown at 37 °C. The bacterial culture was induced when the optical density (OD600nm) reached approximately 0.5–0.6 with arabinose [0.2% (w/v)] overnight (∼16 h) at 37 °C, where as in control sample arabinose was not added. The bacterial culture of test and control samples were harvested by centrifugation at 5000g for 10 min at 4 °C and the total cell lysate was prepared [8]. Total cell protein (TCP) was analyzed by SDS–PAGE using 10% polyacrylamide gel [9] with a Mini Protean III apparatus (Bio-Rad). The protein gel was stained with 0.25% (w/v) Coomassie Brilliant Blue R-250 in 10% acetic acid and 45% methanol and destained with 10% acetic acid and 30% methanol.
Expression optimization (inducer, temperature and time)
The expression of the NS1 protein was optimized for three different temperatures, time durations and inducer (arabinose) concentrations. Bacterial growth condition was similar to that described above. For arabinose dose optimization, the bacterial culture was induced with different concentrations of arabinose [2%, 0.2%, 0.02%, 0.002% and 0.0002% (w/v)] and allowed to grow overnight (∼16 h) at 30 °C. For temperature optimization, the bacterial culture was induced with arabinose [0.2% (w/v)] and allowed to grow overnight (∼16 h) at three different temperatures (37, 30 and 24 °C). For time optimization, the bacterial culture was induced with arabinose [0.2% (w/v)] and allowed to grow for 0 h, 2 h, 4 h, 6 h and overnight (∼16 h) at 30 °C. Total cell proteins from each optimization experiment were analyzed by SDS–PAGE and Western blot to select the ideal condition for optimum protein expression.
Medium scale expression and purification of rNS1 protein
A single bacterial colony was inoculated in 10 ml TB/Tet5 medium and allowed to grow overnight at 37 °C shaker. The overnight culture was diluted (1:100) in fresh 4 × 1 L TB/Tet5 medium and grown at 37 °C until an OD600nm of 0.5–0.6 was reached. Expression was done by optimized conditions as described in the previous section. Induction was initiated by adding 0.2% (w/v) arabinose and bacterial culture was incubated for 16 h with vigorous shaking at 30 °C. Bacterial culture was harvested by centrifugation at 5000g for 20 min at 4 °C and total cell protein (TCP) from induced and uninduced culture was analyzed by SDS–PAGE and Western blot probed with anti-His6 MAb.
Purification of inclusion bodies (IB)
The purification of inclusion bodies was done according to previously published method [10]. Briefly, 19.6 g of bacterial wet pellet from 4 L bacterial culture was suspended in 196 ml PBS (10 ml PBS per g of pellet) and completely lysed by passing through a French Press (20,000 psi). The total cell lysate was clarified by centrifugation at 27,000g for 30 min at 4 °C and supernatant was collected as total soluble protein. The pellet was resuspended in lysis buffer (Table 1 ) and then 2% sodium deoxycholate was added. The mixture was incubated at room temperature for 30 min with gentle shaking and centrifuged at 27,000g for 30 min at 4 °C. The pellet was resuspended in lysis buffer and washed thrice at 27,000g for 20 min at 4 °C to completely remove sodium deoxycholate.
Table 1.
Buffer used for the inclusion bodies purification and refolding.
| Buffer | Composition |
|---|---|
| Lysis buffer | 50 mM Tris, pH 8.0, 200 mM NaCl, 1 mM EDTA |
| Buffer B | 8 M urea, 100 mM NaH2PO4, 10 mM Tris–Cl, pH 8.0 |
| Buffer C | 8 M urea, 100 mM NaH2PO4, 10 mM Tris–Cl, pH 6.3 |
| Buffer D | 8 M urea, 100 mM NaH2PO4, 10 mM Tris–Cl, pH 5.9 |
| Buffer E | 8 M urea, 100 mM NaH2PO4, 10 mM Tris–Cl, pH 4.5 |
| TA buffer | 50 mM Tris, pH 8.0, 0.4 M l-arginine |
IB solubilization and immobilized metal affinity chromatography (IMAC) purification
Inclusion bodies were solubilized in denaturing buffer B (Table 1) for 1 h at room temperature with gentle shaking. Solubilized denatured rNS1 proteins from insoluble materials were separated by centrifugation at 27,000g for 30 min at 4 °C. Final yield of solubilized denatured protein was determined by protein assay using BSA as the standard protein [11]. A Ni–NTA column was prepared by loading the Ni–NTA agarose on a plastic column (Bio-Rad) and equilibrated with 10 bed volumes of buffer B. Twenty milligrams of solubilized denatured rNS1 protein was loaded on the column and the column was washed with 5–10 bed volumes with buffer C. After complete wash, bound protein was eluted with buffer D and buffer E. All the eluted fractions were analyzed by SDS–PAGE prior to refolding.
Refolding
Protein assay was done to quantitate the amount of protein eluted from the Ni–NTA column and it was estimated ∼0.4–0.6 mg/ml with a total amount of ∼12–14 mg. Refolding was done in three different concentrations to evaluate the best refolding condition. The eluted protein was adjusted to 100, 75 and 50 μg/ml with refolding TA buffer and refolding was done by dialysis in TA buffer in the presence of 1.0 mM GSH (glutathione, reduced), 0.1 mM GSSG (glutathione, oxidized) for 3 days with two changes at 4 °C. Final dialysis was done in PBS pH 7.4 at 4 °C.
Western blot analysis
TCP, inclusion bodies, IMAC eluted fractions or refolded rNS1 protein were electrophoresed on SDS–PAGE using 10% polyacrylamide gel and then electroblotted onto Hybond ECL nitrocellulose membranes [12]. The nitrocellulose membrane was blocked with 5% skim milk in PBST (0.1% Tween 20 in 1× PBS, pH 7.3) for overnight at 4 °C. The membrane was washed four times with PBST and incubated with anti-His6 MAb for 1 h. After washing, the membrane was incubated with HRPO labeled goat anti-mouse IgG (GAM-HRPO) for 1 h. Finally, the membrane was washed with PBS and enhanced chemiluminescence based detection was performed to visualize the binding.
In gel digestion
Protein identification was done at the Institute for Biomolecular Design, University of Alberta, Edmonton, Alberta, Canada as described earlier [13].
Results
NS1 gene cloning and small scale expression
The full-length codon optimized NS1 gene was cloned in the correct reading frame with the His6 tag at the C-terminal and designated as pDS21NS1 for high-level expression of proteins as inclusion bodies in E. coli [7]. The correct size recombinant clones were selected for protein expression. The plasmid containing full-length NS1 gene was isolated for expression. Expression results showed that all the NS1 clones selected were expressing the target protein of approximately 46 kDa at different levels when analyzed by SDS–PAGE where as in control sample (without arabinose) there was no expression of the target protein (Fig. 1 ). The expression of rNS1 protein was confirmed by Western blot probed with anti-His6 MAb (data not shown). The best NS1 clone was chosen for the expression optimization and further studies.
Fig. 1.
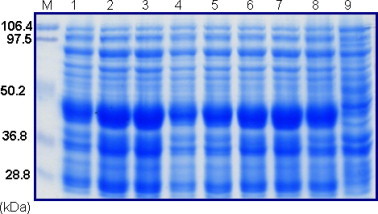
SDS–PAGE analysis of different NS1 clones expression in E. coli. Lane M: standard protein molecular weight markers, lanes 1–8: clone# 1, 3, 4, 5, 7, 9, 11, and 12, respectively, lane 9: control.
Expression optimization (inducer, temperature, and time)
The NS1 protein was successfully expressed as inclusion bodies in E. coli. The optimal conditions for rNS1 protein expression were 0.2% (w/v) arabinose concentrations (Fig. 2 A), 30 °C temperature (Fig. 2B) and 16 h induction time (Fig. 2C).
Fig. 2.
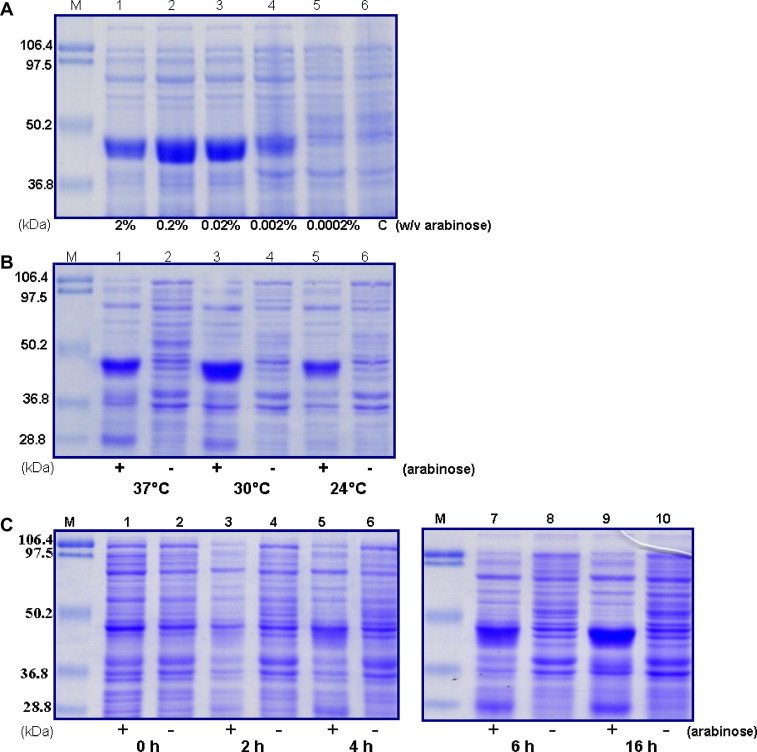
Expression optimization. (A) SDS–PAGE analysis of arabinose concentration dependent rNS1 protein expression. Bacterial cultures were induced with different concentrations of arabinose and grown at 30 °C for overnight. Lane M: standard protein molecular weight markers, lanes 1–5: bacterial cultures were induced with 2%, 0.2%, 0.02%, 0.002%, 0.0002% arabinose (w/v), respectively, lane 6: control. (B) SDS–PAGE analysis of temperature dependent rNS1 protein expression. Bacterial cultures were induced with 0.2% arabinose (w/v) and grown at different temperatures (37, 30 and 24 °C) for overnight. Lane M: standard protein molecular weight markers, lanes 1 and 2: 37 °C, lanes 3 and 4: 30 °C, lanes 5 and 6: 24 °C. “+” indicates arabinose was added, “−” indicates arabinose was not added (control). (C) SDS–PAGE analysis of time dependent rNS1 protein expression. Bacterial cultures were induced with 0.2% arabinose (w/v) and grown at 30 °C for different time period. Lane M: standard protein molecular weight markers, lanes 1 and 2: 0 h, lanes 3 and 4: 2 h, lanes 5 and 6: 4 h, lanes 7 and 8: 6 h, lanes 9 and 10: overnight (16 h). “+” indicates arabinose was added, “−” indicates arabinose was not added (control).
Medium scale expression of NS1 protein
The bacterial expression vector pBM802 was designed for high-level expression of recombinant protein in E. coli as inclusion bodies. The medium scale expression of the rNS1 was performed and there was high-level expression in E. coli when analyzed by SDS–PAGE (Fig. 3 ). Inclusion bodies were prepared from the bacterial pellet by a French Press. Following complete bacterial cell lysis, the insoluble inclusion bodies were separated from total soluble protein by centrifugation. The pellet was washed with sodium deoxycholate and subsequently washed with lysis buffer to remove any sodium deoxycholate. The final yield of denatured soluble inclusion bodies was estimated to be approximately 230–250 mg/L of initial bacterial culture. The purity of the inclusion bodies along with different washes was analyzed by SDS–PAGE (data not shown).
Fig. 3.
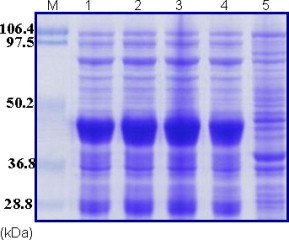
SDS–PAGE analysis of medium scale production of rNS1 protein. Lane M: standard protein molecular weight markers, lanes 1–4: bacterial culture flask 1–4 (f1–f4), respectively, lane 5: control.
NS1 protein purification and refolding
The rNS1 protein was predominantly expressed in E. coli as inclusion bodies. The rNS1 protein was isolated from inclusion bodies from bacterial shake flask culture and purified by IMAC chromatography under denaturing conditions (Fig. 4 A). The purity of the eluted rNS1 protein was analyzed by SDS–PAGE prior to refolding. Renaturing conditions, protein concentrations in the refolding buffer as well as suitable buffer compositions are important to simulate correct folding, formation of the proper disulfide bond and proper association of different domains. It has been reported that the presence of arginine in refolding buffer played an important role in solubilization, inhibiting the aggregation of refolding intermediates and thus increases the yield of the refolded protein [14]. It has also been clearly demonstrated in the literature that addition of GSH/GSSG into the refolding buffer facilitates disulfide bond formation and thus enhances renaturation of the protein [15]. The refolding step was done for 3 days by dialysis and any aggregate formed during refolding was removed by centrifugation. The supernatant was collected as refolded rNS1 for further use. Thus the in vitro refolding proved to be successful in recovering soluble protein expressed in E. coli as inclusion bodies. The purification steps and yield of rNS1 protein from 1 L of E. coli culture are summarized in Table 2 . The purity of the refolded rNS1 protein was evident from the SDS–PAGE (Fig. 4B) and Western blot (Fig. 4C) with a single band of approximate molecular weight of 46 kDa. Its purity was estimated to be greater than 90%.
Fig. 4.
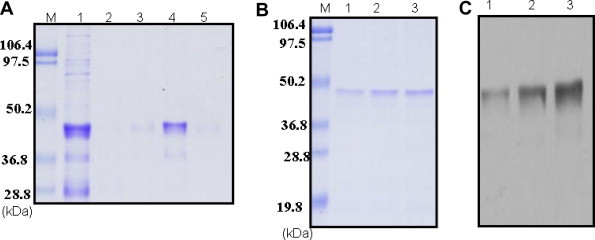
IMAC purification and refolding. (A) SDS–PAGE analysis of IMAC purified rNS1 protein. Lane M: standard protein molecular weight markers, lane 1: unbound protein, lanes 2 and 3: washes, lanes 4 and 5: elutions. (B) SDS–PAGE analysis of refolded rNS1 protein. Lane M: standard protein molecular weight markers, lanes 1–3: recovered rNS1 protein refolded at 50, 75, 100 μg/ml, respectively. (C) Western blot analysis of refolded rNS1 protein probed with anti-His6 MAb. Lanes 1–3: recovered rNS1 protein refolded at 50, 75, 100 μg/ml, respectively.
Table 2.
Purification of dengue rNS1 protein from E. coli in 1 L culture.
| Purification steps | Total protein (mg) | rNS1 (mg) | Recovery (%) |
|---|---|---|---|
| Inclusion bodies | 250 | 232 | 92.8 |
| IMAC elutionsa | 20 | 12 | 60 |
| Refolding | 12 | 12 | 100 |
In each batch of IMAC purification, 20 mg of protein was used.
In gel digestion
Protein identification from the generated LC/MS data was done by searching the NCBI (National Center for Biotechnology Information) non-redundant database [Database: NCBInr 20071130 (5678482 sequences; 1961803296 residues)] using Mascot Daemon search methodology (http://www.matrixscience.com). Mascot search results showed significant hits for the DEN-1 polyprotein which includes NS1 [gi|20135604 polyprotein (dengue virus type 1)].
Discussion
Rapid diagnostics of any infectious disease can lead to early therapeutic intervention of probable cases as well as suspect cases. In many viral diseases, virus shedding is greatest during the early symptomatic phase, i.e. around and immediately following the onset of symptoms. It has been reported that the NS1 antigen was found circulating from the first day after the onset of symptoms up to day 9. The NS1 levels ranged from 0.04 to 2 μg/ml in acute phase serum samples (from day 0 to 7), and the level for a convalescent phase serum (day 8 and later) was 0.04 μg/ml. In secondary phase infection, NS1 levels ranged from 0.01 to 2 μg/ml and were not detectable in convalescent phase sera. An antigen capture ELISA has been developed for detection of serum NS1 early in primary and secondary dengue infection [6]. According to these studies the presence of NS1 in human sera can be confirmed between days 0 and 9 [16], [17] and with a peak at days 6–10 [18].
Dengue NS1 antigen testing is suggested as a helpful tool for the early diagnosis of dengue infection after the onset of fever [19], [20]. Commercially available dengue NS1 antigen capture ELISA has been evaluated for the detection of NS1 from patient’s samples in different stages [17], [20], [21], [22]. It is therefore an important antigen for rapid viral diagnosis.
It has been reported in the literature that different expression systems have been exploited for NS1 expression and with a typical yield of 10–30 mg/L in bacteria [23], 25 mg/L of multi-epitope dengue protein in bacteria [24] and 70 mg/L in yeast [25]. Successful expression of NS1 protein has also been reported in both baculovirus and mammalian expression systems [26], [27], [28], [29].
Here, we have also exploited the bacterial expression system for the production of rNS1 protein. Bacterial expression is perhaps the most commonly employed expression system for the production of non-glycosylated recombinant proteins. The organism is relatively simple to manipulate and the small scale analysis of many different parameters can be optimized in a short period of time. This allows the rapid identification and optimization of several growth and induction conditions for medium scale production. Many eukaryotic genes cannot be expressed efficiently in the E. coli host due to the difference in codon preference as well as toxicity of foreign protein and mRNA instability for the expression of protein encoded by the gene. It is also a very well known fact that heterologously expressed eukaryotic proteins are not post-translationally modified when it is expressed in E. coli. It is also difficult to express as soluble protein or facilitate the secretion of expressed protein into the culture medium. In addition, proteins expressed in large amounts tend to precipitate, forming inclusion bodies [10], [30], [31] and present an advantage with respect to higher yield and especially the purification of expressed protein.
It has been demonstrated in the literature that genes can be codon optimized to the host translational system with the significantly higher expression level than native genes [7]. Based on this knowledge, we have obtained the codon optimized NS1 gene from GENEART Inc. for expression in E. coli. In the present study, we have cloned and purified the dengue NS1 protein in E. coli for the development of monoclonal antibody and subsequently bispecific antibodies for early diagnostic applications. NS1 gene was cloned under the control of the pBAD promoter for high-level expression of recombinant protein as inclusion bodies in the bacterial cytoplasm. The final yield of purified inclusion bodies was estimated by Bradford protein assay [11] to be approximately 230–250 mg/L of initial bacterial culture which is 10- to 25-fold higher compared to previous reports using the native NS1 gene sequence. We have expressed several viral antigens in E. coli exploiting codon optimized genes and the NS1 expression is the most robust yield we have achieved (Table 3 ). The bacterial cell lysis by French Press and washing steps with detergent were used successfully to purify inclusion bodies from soluble protein. SDS–PAGE analysis clearly demonstrated that lysis by French Press and several washings with lysis buffer increased the purity of the inclusion bodies since the bulk of the E. coli soluble proteins were separated. The purity of inclusion bodies was also judged by SDS–PAGE and Western blot (data not shown). The purification method exploits the immobilized metal affinity chromatography (IMAC) under denaturing conditions. The effectively adsorbed His-tagged protein could be purified to homogeneity [10]. IMAC purification under denaturing conditions yielded significant amount of pure rNS1 protein with a single band as judged by SDS–PAGE and with a typical yield of 60–80% of starting denatured inclusion bodies (Table 2). Most recombinant proteins are expressed in E. coli as inclusion bodies and different refolding methods have been reported to renature proteins from inclusion bodies [10], [32]. The IMAC eluted rNS1 protein was refolded in TA buffer in the presence of a redox pair (GSH/GSSG) with three different protein concentrations (Table 4 ). There are 12 cysteines conserved among flaviviruses that form disulfide bond (S–S) which suggesting their role in structure and function of the protein. The crystal structure of NS1 protein is yet not known but Wallis et al. has predicted 6 disulfide bonds present in the dengue NS1 protein [33]. Therefore the addition of GSH/GSSG into the refolding buffer which maintained the oxidizing environment enhances S–S bond formation and hence increases the solubility of the recombinant protein [15], [34]. The protein concentration plays a crucial role in refolding conditions and it was observed that when the concentration was above 100 μg/ml, aggregation was evident. The purity of the refolded protein was judged by SDS–PAGE (Fig. 4B) and Western blot (Fig. 4C) data indicated that anti-His6 MAb reacted with a single band of ∼46 kDa, suggesting that we had successfully purified rNS1. The identity of purified rNS1 protein was confirmed by in vitro gel digestion, mass spectrometry, and NCBI non-redundant database searching. The refolded rNS1 antigen is being used to immunize mice to develop monoclonal antibodies. The polyclonal antibodies from mouse serum were strongly reacting with recombinant antigen (Fig. 5 A) in Western blot (Fig. 5B). The rNS1 protein will be useful to select a pair of monoclonal antibodies with non-overlapping different specificities for antigen capture ELISA and point of care rapid assays [35]. In addition, the rNS1 antigen based ELISA could be the basis of developing a sensitive serum diagnostic to monitor dengue outbreaks by detecting the early human anti-dengue antibodies.
Table 3.
Expression yields of various codon optimized genes.
| Protein | Yield (mg/L) | Reference |
|---|---|---|
| SARS-CoV NP | 70 | [7] |
| Dengue NS1 | 230–250 | This report |
| H5N1 HA1 | 15–20 | Unpublished |
Table 4.
Different refolding conditions and yield.
| Protein | GSH:GSSG (mM) | Refolding TA buffer | Protein conc. in refolding buffer (μg/ml) | Yield (%) |
|---|---|---|---|---|
| rNS1 | 1:0.1 | 50 mM Tris–Cl, pH 8.0, 0.4 M l-arginine | 100 | No aggregation (100) |
| rNS1 | 1:0.1 | 50 mM Tris–Cl, pH 8.0, 0.4 M l-arginine | 75 | No aggregation (100) |
| rNS1 | 1:0.1 | 50 mM Tris–Cl, pH 8.0, 0.4 M l-arginine | 50 | No aggregation (100) |
Fig. 5.
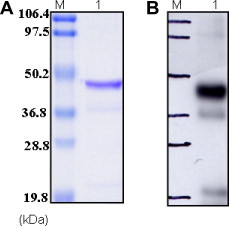
SDS–PAGE analysis of refolded rNS1 protein. (A) Lane M: standard protein molecular weight markers, lane 1: refolded rNS1 protein. (B) Western blot analysis of refolded rNS1 protein probed with mouse anti-NS1 polyclonal antibodies. Lane 1: refolded rNS1 protein.
Acknowledgments
This work was supported by research grant from The Natural Sciences and Engineering Research Council of Canada (NSERC-Strategic) and Cangene Inc. Thanks to Dr. Sandra Marcus for providing the French Press Facility, Department of Chemistry, University of Alberta, Edmonton, Alta., Canada.
Footnotes
Abbreviations used: DHF, dengue hemorrhagic fever; DSS, dengue shock syndrome; TB, 1.2% tryptone, 2.4% yeast extract, 0.4% (v/v) glycerol and 25 mM Hepes pH 7.2; Ni–NTA, nickel–nitrilotriacetic acid; MAb, monoclonal antibody; EDTA, ethylene diamine tetraacetic acid; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; h, hour; HRPO, horseradish peroxidase; ECL, enhanced chemiluminescence; BSA, bovine serum albumin.
References
- 1.Gubler D.J., Meltzer M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv. Virus Res. 1999;53:35–70. doi: 10.1016/s0065-3527(08)60342-5. [DOI] [PubMed] [Google Scholar]
- 2.Young P.R., Hilditch P.A., Bletchly C., Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 2000;38:1053–1057. doi: 10.1128/jcm.38.3.1053-1057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flamand M., Megret F., Mathieu M., Lepault J., Rey F.A., Deubel V. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J. Virol. 1999;73:6104–6110. doi: 10.1128/jvi.73.7.6104-6110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falconar A.K., Young P.R. Immunoaffinity purification of native dimer forms of the flavivirus non-structural glycoprotein, NS1. J. Virol. Methods. 1990;30:323–332. doi: 10.1016/0166-0934(90)90075-q. [DOI] [PubMed] [Google Scholar]
- 5.Henchal E.A., Henchal L.S., Thaisomboonsuk B.K. Topological mapping of unique epitopes on the dengue-2 virus NS1 protein using monoclonal antibodies. J. Gen. Virol. 1987;68(Pt. 3):845–851. doi: 10.1099/0022-1317-68-3-845. [DOI] [PubMed] [Google Scholar]
- 6.Alcon S., Talarmin A., Debruyne M., Falconar A., Deubel V., Flamand M. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 2002;40:376–381. doi: 10.1128/JCM.40.2.376-381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das D., Suresh M.R. Copious production of SARS-CoV nucleocapsid protein employing codon optimized synthetic gene. J. Virol. Methods. 2006;137:343–346. doi: 10.1016/j.jviromet.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sambrook J., Fritsch E.F., Maniatis T. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 9.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 10.Das D., Jacobs F., Feldmann H., Jones S.M., Suresh M.R. Differential expression of the Ebola virus GP(1,2) protein and its fragments in E. coli. Protein Expr. Purif. 2007;54:117–125. doi: 10.1016/j.pep.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das D., Allen T.M., Suresh M.R. Comparative evaluation of two purification methods of anti-CD19-c-myc-His6-Cys scFv. Protein Expr. Purif. 2005;39:199–208. doi: 10.1016/j.pep.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Tsumoto K., Umetsu M., Kumagai I., Ejima D., Philo J.S., Arakawa T. Role of arginine in protein refolding, solubilization, and purification. Biotechnol. Prog. 2004;20:1301–1308. doi: 10.1021/bp0498793. [DOI] [PubMed] [Google Scholar]
- 15.Wei C., Tang B., Zhang Y., Yang K. Oxidative refolding of recombinant prochymosin. Biochem. J. 1999;340(Pt. 1):345–351. [PMC free article] [PubMed] [Google Scholar]
- 16.Shu P.Y., Chen L.K., Chang S.F., Yueh Y.Y., Chow L., Chien L.J., Chin C., Yang H.H., Lin T.H., Huang J.H. Potential application of nonstructural protein NS1 serotype-specific immunoglobulin G enzyme-linked immunosorbent assay in the seroepidemiologic study of dengue virus infection: correlation of results with those of the plaque reduction neutralization test. J. Clin. Microbiol. 2002;40:1840–1844. doi: 10.1128/JCM.40.5.1840-1844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dussart P., Labeau B., Lagathu G., Louis P., Nunes M.R., Rodrigues S.G., Storck-Herrmann C., Cesaire R., Morvan J., Flamand M., Baril L. Evaluation of an enzyme immunoassay for detection of dengue virus NS1 antigen in human serum. Clin. Vaccine Immunol. 2006;13:1185–1189. doi: 10.1128/CVI.00229-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H., Di B., Pan Y.X., Qiu L.W., Wang Y.D., Hao W., He L.J., Yuen K.Y., Che X.Y. Serotype 1-specific monoclonal antibody-based antigen capture immunoassay for detection of circulating nonstructural protein NS1: implications for early diagnosis and serotyping of dengue virus infections. J. Clin. Microbiol. 2006;44:2872–2878. doi: 10.1128/JCM.00777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuansumrit A., Chaiyaratana W., Pongthanapisith V., Tangnararatchakit K., Lertwongrath S., Yoksan S. The use of dengue nonstructural protein 1 antigen for the early diagnosis during the febrile stage in patients with dengue infection. Pediatr. Infect. Dis. J. 2008;27:43–48. doi: 10.1097/INF.0b013e318150666d. [DOI] [PubMed] [Google Scholar]
- 20.Lapphra K., Sangcharaswichai A., Chokephaibulkit K., Tiengrim S., Piriyakarnsakul W., Chakorn T., Yoksan S., Wattanamongkolsil L., Thamlikitkul V. Evaluation of an NS1 antigen detection for diagnosis of acute dengue infection in patients with acute febrile illness. Diagn. Microbiol. Infect. Dis. 2008;60:387–391. doi: 10.1016/j.diagmicrobio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Kumarasamy V., Chua S.K., Hassan Z., Wahab A.H., Chem Y.K., Mohamad M., Chua K.B. Evaluating the sensitivity of a commercial dengue NS1 antigen-capture ELISA for early diagnosis of acute dengue virus infection. Singapore Med. J. 2007;48:669–673. [PubMed] [Google Scholar]
- 22.Kumarasamy V., Wahab A.H., Chua S.K., Hassan Z., Chem Y.K., Mohamad M., Chua K.B. Evaluation of a commercial dengue NS1 antigen-capture ELISA for laboratory diagnosis of acute dengue virus infection. J. Virol. Methods. 2007;140:75–79. doi: 10.1016/j.jviromet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Huang J.L., Huang J.H., Shyu R.H., Teng C.W., Lin Y.L., Kuo M.D., Yao C.W., Shaio M.F. High-level expression of recombinant dengue viral NS-1 protein and its potential use as a diagnostic antigen. J. Med. Virol. 2001;65:553–560. [PubMed] [Google Scholar]
- 24.AnandaRao R., Swaminathan S., Fernando S., Jana A.M., Khanna N. A custom-designed recombinant multiepitope protein as a dengue diagnostic reagent. Protein Expr. Purif. 2005;41:136–147. doi: 10.1016/j.pep.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J.M., Tang Y.X., Fang D.Y., Zhou J.J., Liang Y., Guo H.Y., Jiang L.F. Secreted expression and purification of dengue 2 virus full-length nonstructural glycoprotein NS1 in Pichia pastoris. Virus Genes. 2006;33:27–32. doi: 10.1007/s11262-005-0036-6. [DOI] [PubMed] [Google Scholar]
- 26.Parrish C.R., Woo W.S., Wright P.J. Expression of the NS1 gene of dengue virus type 2 using vaccinia virus. Dimerisation of the NS1 glycoprotein. Arch. Virol. 1991;117:279–286. doi: 10.1007/BF01310771. [DOI] [PubMed] [Google Scholar]
- 27.Qu X., Chen W., Maguire T., Austin F. Immunoreactivity and protective effects in mice of a recombinant dengue 2 Tonga virus NS1 protein produced in a baculovirus expression system. J. Gen. Virol. 1993;74(Pt. 1):89–97. doi: 10.1099/0022-1317-74-1-89. [DOI] [PubMed] [Google Scholar]
- 28.Pryor M.J., Gualano R.C., Lin B., Davidson A.D., Wright P.J. Growth restriction of dengue virus type 2 by site-specific mutagenesis of virus-encoded glycoproteins. J. Gen. Virol. 1998;79(Pt. 11):2631–2639. doi: 10.1099/0022-1317-79-11-2631. [DOI] [PubMed] [Google Scholar]
- 29.Leblois H., Young P.R. Maturation of the dengue-2 virus NS1 protein in insect cells: effects of downstream NS2A sequences on baculovirus-expressed gene constructs. J. Gen. Virol. 1995;76(Pt. 4):979–984. doi: 10.1099/0022-1317-76-4-979. [DOI] [PubMed] [Google Scholar]
- 30.Das D., Gares S.L., Nagata L.P., Suresh M.R. Evaluation of a western equine encephalitis recombinant E1 protein for protective immunity and diagnostics. Antiviral Res. 2004;64:85–92. doi: 10.1016/j.antiviral.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Kurucz I., Titus J.A., Jost C.R., Segal D.M. Correct disulfide pairing and efficient refolding of detergent-solubilized single-chain Fv proteins from bacterial inclusion bodies. Mol. Immunol. 1995;32:1443–1452. doi: 10.1016/0161-5890(95)00105-0. [DOI] [PubMed] [Google Scholar]
- 32.Das D., Kriangkum J., Nagata L.P., Fulton R.E., Suresh M.R. Development of a biotin mimic tagged ScFv antibody against western equine encephalitis virus: bacterial expression and refolding. J. Virol. Methods. 2004;117:169–177. doi: 10.1016/j.jviromet.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Wallis T.P., Huang C.Y., Nimkar S.B., Young P.R., Gorman J.J. Determination of the disulfide bond arrangement of dengue virus NS1 protein. J. Biol. Chem. 2004;279:20729–20741. doi: 10.1074/jbc.M312907200. [DOI] [PubMed] [Google Scholar]
- 34.Guo J.Q., You S.Y., Li L., Zhang Y.Z., Huang J.N., Zhang C.Y. Construction and high-level expression of a single-chain Fv antibody fragment specific for acidic isoferritin in Escherichia coli. J. Biotechnol. 2003;102:177–189. doi: 10.1016/s0168-1656(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 35.Kammila S., Das D., Bhatnagar P.K., Sunwoo H.H., Zayas-Zamora G., King M., Suresh M.R. A rapid point of care immunoswab assay for SARS-CoV detection. J. Virol. Methods. 2008;152:77–84. doi: 10.1016/j.jviromet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


