Abstract
For the past 30 years, my laboratory has concentrated its work on demonstrating that the epigenetic consequences of foreign DNA insertion into established mammalian genomes – de novo DNA methylation of the integrate and alterations of methylation patterns across the recipient genome – are essential elements in setting the stage towards oncogenic transformation. We have primarily studied human adenovirus type 12 (Ad12) which induces undifferentiated tumors in Syrian hamsters (Mesocricetus auratus) either at the site of subcutaneous Ad12 injection or intraperitoneally upon intramuscular injection. Up to 90% of the hamsters injected with Ad12 develop tumors within 3–6 weeks. Integration of foreign DNA, its de novo methylation, and the consequences of insertion on the cellular methylation and transcription profiles have been studied in detail. While viral infections are a frequent source of foreign genomes entering mammalian and other hosts and often their genomes, we have also pursued the fate of food-ingested foreign DNA in the mouse organism. The persistence of this DNA in the animals is transient and there is no evidence for the expression or germ line fixation of foreign DNA. Nevertheless, the occasional cell that carries integrated genomes from that foreign source deserves the oncologist's sustained interest.
Keywords: Viral oncogenesis, Human adenovirus type 12, Transgression of species barriers, Abortive infection and oncogenesis, Integration of Ad12 DNA, Adenovirus DNA integration a general phenomenon, De novo DNA methylation, Alterations of methylation patterns upon foreign DNA insertion
For a detailed molecular analysis of (epi)genetic alterations in viral oncology, I have chosen the human adenovirus type 12 (Ad12) – Syrian hamster tumor system [1] for the following reasons:
-
(i)
Upon the injection of about 109 plaque forming units of Ad12 into newborn Syrian hamsters (Mesocricetus auratus), undifferentiated tumors develop after 3–6 weeks in 70–90% of the animals that survive virus application. Subcutaneous injection elicits tumors at the site of Ad12 injection. Intramuscular delivery of Ad12 leads to a massive intraperitoneal tumor spread [2].
-
(ii)
Adenoviruses belong to the best molecularly characterized viruses, and many of the fundamental processes in molecular biology have been discovered in this system, e.g. RNA splicing [3], [4], trans-activation of genes [5], [6], mechanisms of mammalian DNA replication [7], long-term gene silencing by promoter methylation [8].
-
(iii)
My laboratory has studied the non-productive interaction of Ad12 with non-permissive Syrian hamster cells (for reviews [9], [10]).
-
(iv)
In Ad12- or Ad2-transformed hamster cells and Ad12-induced tumor cells, we have described for the first time an inverse correlation between promoter methylation and gene activity [11], [12]. Subsequently, we have in detail investigated (epi)genetic patterns and mechanisms in the adenovirus, iridovirus frog virus 3, and baculovirus systems, as well as in the human genome [13], [14]. This article will present a summary of work from this laboratory over the last 40 years.
1. Oncogenicity by Ad12 in Syrian hamsters
When viruses cross species barriers, they frequently undergo changes in their biological and/or pathogenetic potential. Prominent examples are the human immunodeficiency viruses (HIV1 and HIV2), SARS corona virus, H5N1 influenza virus, and – in this context- human Ad12. HIV1 and HIV2 having crossed from chimpanzees and the sooty mangabeys, respectively, to humans cause the acquired immunodeficiency syndrome (AIDS) [15]. SARS corona virus endemic in small canine species is the causative agent for the severe acute respiratory syndrome (SARS) in humans [16]. Avian influenza viruses, e.g. H5N1, occasionally afflict humans with a high mortality form of influenza [17].
Human Ad12 replicates with progressive cytophatic effects in human cell cultures killing all infected cells. In the non-permissive host M. auratus, human Ad12 becomes a highly efficient tumor virus. Ad12 cannot replicate its DNA in Syrian hamster cells and undergoes a completely abortive infection cycle (see Section 2). Ad12-infected hamster cells continue to grow, and transformed cells get a chance to survive and, when originating in newborn hamsters, to develop tumors. In contrast, human Ad2 replicates in Syrian hamster cells, but fails to induce tumors, presumably because the virus kills all infected cells.
When purified Ad12 virions are injected subcutaneously into newborn Syrian hamsters, tumors develop at the site of injection [1], [18], [19]. There are no metastases. Occasionally, tumor cells can be detected in the local lymphatic vessels. Upon the injection of Ad12 into the gluteal muscle of newborn hamsters, extensive tumorigenesis and widespread dissemination of up to 15 tumors throughout the entire peritoneal cavity are observed [2]. Independent of location, tumor histology revealed Homer-Wright rosette-like structures typical for highly undifferentiated neuroectodermal tumors (Fig. 1 ).
Fig. 1.
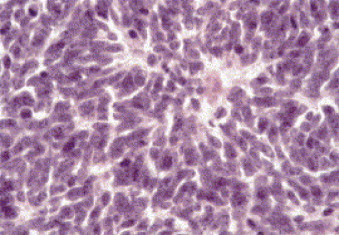
Histological section of an Ad12-induced hamster tumor stained with hematoxilin and eosin. From ref. [2].
.
2. The abortive interaction of Ad12 with Syrian hamster cells
The block for Ad12 replication in Syrian hamster cells lies before viral DNA replication [20], [21], [22]. There is minimal transcription of some of the early Ad12 functions [23], [24], and only traces of early viral proteins are detectable [24]. Late Ad12 genes are not transcribed in Ad12-infected hamster cells. In contrast, human Ad2 replicates in hamster cells [20], [25]. Several attempts to salvage Ad12 DNA replication or steps beyond it in Syrian hamster cells have, at best, led to limited Ad12 DNA replication, even to late RNA synthesis in Ad5-transformed BHK297-C131 cells [26] but not to replication of Ad12 virions in substituting systems [21], [22], [24], [27], [28], [29]. Even after introducing amounts of Ad12 DNA comparable to that found in productively infected human cells, Ad12 DNA was still unable to overcome the barrier in Syrian hamster cells (Fig. 2 ) [22]. The replication of Ad12 virions in hamster cells is deficient in several essential cellular and/or viral functions. Syrian hamsters therefore become susceptible to Ad12-induced tumorigenesis since the Ad12-infected hamster cells survive infection, and some of them can develop into tumor cells.
Fig. 2.
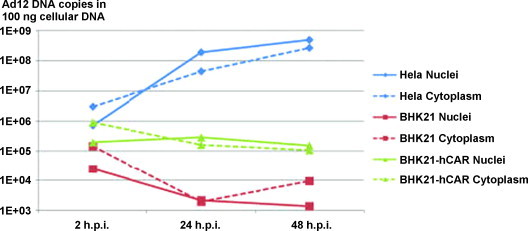
Quantitative time course analyses of Ad12 DNA in the nuclei or cytoplasm of Ad12-infected HeLa, BHK21, or BHK21-hCAR cells. From ref. [22].
3. Persistence of the Ad12 genome by chromosomal integration – clonal origin of Ad12-induced tumors
In 1966, I began to study the integration of parental Ad12 DNA into the hamster cell genome in abortively infected cells [30], [31]. Subsequently, my laboratory concentrated on the analyses of Ad12 or Ad2 genomes integrated into the genomes of adenovirus-transformed cells or Ad12-induced hamster tumor cells. The most important features of adenovirus DNA integration are as follows [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52].
Multiple copies of the viral genome persist in a chromosomally integrated state. Evidence for the persistence of free viral genomes was never obtained in any tumor or transformed cell analyzed. These conclusions were based on the results of Southern transfer and fluorescent in situ hybridization (FISH) experiments as well as of the cloning and sequencing of junction sites between adenoviral and cellular DNA sequences.
Ad12 oncogenesis in hamsters is a clonal event. In one tumor, all cells carry the Ad12 integrate at a singular chromosomal site. These sites are different from tumor to tumor.
The analyses of integration sites of adenoviral integrates in >100 different transformed and Ad12-induced hamster tumors and cell lines revealed no evidence for specific sites of viral DNA integration in the cellular genome.
Frequently, the complete Ad12 genome was co-linearly integrated with almost no loss of nucleotides at the viral DNA termini. In contrast, integrated Ad2 DNA in Ad2-premissive hamster cells transformed by Ad2 invariably showed deletions of viral DNA segments. At the integration sites of Ad12 DNA, cellular DNA segments were found to be deleted in some instances.
However, there are features that precondition a cellular site for integrative recombination with foreign (viral) transgenomes. Active transcriptional state, patchy homologies between the viral DNA termini and host cellular sequences, repetitive cellular sequences, seem to direct the invading foreign genomes to certain sites of the recipient genome. These concepts were derived both from the direct cloning and sequencing of integration sites and from the results of cell free in vitro recombination experiments between Ad12 DNA fragments and cellular DNA that had served as target for Ad12 DNA integration in an Ad12-induced tumor.
In adenovirus-transformed hamster cells or in Ad12-induced tumor cells, some of the early viral genes are transcribed, the late viral genes are almost completely silenced.
Integrated adenoviral genomes become extensively de novo methylated in specific profiles that are dependent on the transcriptional patterns of the integrated trans-genomes that in turn are determined by the selection for the oncogenic phenotype. For a transformed or tumor cell to arise and to survive under the given conditions, the transcription of specific sets of integrated viral genes is required. These genes have to escape de novo methylation. In contrast to integrated viral genomes, virion DNA or free adenoviral DNA in productively infected human cells or free Ad12 DNA in abortively infected hamster cells was never de novo methylated [53], [54]. De novo methylation is one of the functionally important consequences of adenoviral, probably of any foreign, DNA integration in mammalian genomes.
The de novo methylation reaction serves to silence foreign genomes in a specific manner and can be considered an ancient cellular defense mechanism to ascertain the undisturbed transcriptional profile of organisms that in nature are constantly exposed to foreign DNA influx [55], [56].
A second, perhaps even more generally relevant sequelae of foreign DNA integration is the alteration of cellular methylation and transcription patterns at sites remote from the integration loci (see Section 11).
4. On the mechanisms of foreign (Ad12) DNA integration
Patchy nucleotide sequence homologies between foreign Ad12 genomes and the recipient cellular genome, transcriptional activity of the cellular target sites and considerable flexibility in the selection of the insertion site are parameters observed in the mechanism of integrative recombination. The reaction is most likely akin to heterologous recombination which is the preponderant one in mammalian cells for the recombination between foreign and cellular DNAs. We have used a cell-free system to investigate junctions between viral and cellular DNAs during adenoviral DNA integration into the hamster cell genome [43], [44], [45], [57]. We have adduced evidence for the recombination of adenovirus DNA with cellular DNA also in productively infected human cells [37], [58]. Here the integrated state of the viral DNA is more difficult to recognize because human cells do not survive wildtype adenovirus infection (see Section 5).
5. Adenovirus DNA does integrate in human cells – a rebuttal of false claims to the contrary
A symmetric recombinant (SYREC) between Ad12 DNA and human KB cell DNA [37], [59] arose spontaneously during productive infection and was packaged into virions. The SYREC DNA molecule consisted of a long palindrome with a left-terminal 2081 bp fragment of Ad12 DNA including the viral packaging signal at either terminus and many kilo base pairs of cellular DNA on its inside. This palindrome could fold back upon itself to form a hairpin structure. These results provided definitive proof for the occurrence of recombinants between viral and cellular DNAs in human cells productively infected by Ad12 as had previously been shown [58], [60]. The cellular DNA incorporated into the SYREC molecule was not methylated, whereas the same DNA sequence in the cellular genome itself proved hypermethylated [37]. Hence, viral or cellular DNA, that was part of a free adenoviral genome and replicated in the nucleus of infected cells, escaped DNA methylation, although the same cellular DNA sequence as an integral part of the host genome remained subject to it. The relation of a DNA molecule to chromatin, not merely its presence in the nucleus, was required to maintain or establish de novo methylation. The discovery of the adenovirus SYREC molecule served as the model for the construction of the gutless adenovirus vector molecules [61].
The literature on adenovirus vectors is replete with false claims that adenovirus DNA did not integrate into the host genome. While this assertion can be easily refuted for the integrated state of Ad12 DNA in hamster tumor cells, it requires more detailed reasoning for adenovirus-infected human cells. In a productive adenovirus infection of human cells, the infected cells rapidly undergo apoptosis and eventually die. Hence it is difficult to study the integrated state of adenovirus DNA in this cell system. The adenovirus SYREC molecule was one example of how to demonstrate covalent linkage between Ad12 DNA and cellular DNA [37].
Moreover, with recombinant adenovirus vectors it was feasible to study the integrative potential of adenovirus genomes. The recombination of a high capacity (gutless) adenovirus vector with the host genome was quantified [62]. In primary human cells and in human cell lines, the frequency of homologous recombination ranged from 2 × 10−5 to 1.6 × 10−6, that of heterologous recombination between 5.5 × 10−3 and 1.1 × 10−4. Adenovirus vector DNA integrated via the termini mostly as intact molecules. Analysis of the junction sequences indicated vector integration without an obvious preference for particular chromosomal regions, but with a preference for integration into genes. Patchy homologies between vector termini and chromosomal DNA were found at many integration sites. These results confirmed many of the conclusions our laboratory had reached much earlier on the mechanism of adenovirus DNA integration in hamster cells [39], [47] These results [62] demonstrate uniformity of integration mechanisms in mammalian cells and also refute the unwarranted claim that adenoviral genomes did not integrate into the genomes of human host cells. These data re-open the important debate on the safety of adenoviral vectors for gene therapy.
6. Revertants of Ad12-induced tumor cells and of Ad12-transformed cells. Hit and run mechanism in adenoviral oncogenesis?
When Ad12-transformed cells or Ad12-induced hamster tumor cells were propagated in culture for several generations, fibroblastic revertants arose that differed morphologically from the more epitheloid Ad12-induced tumor cells. This observation was made repeatedly over the course of several decades in analyses of different Ad12-induced tumors. The revertants had lost most or all chromosomally integrated multiple Ad12 genome copies [19], [33], [63], [64], but nevertheless retained their oncogenic potential when re-injected into hamsters. Obviously, the persistence of integrated viral genomes was not required for the maintenance of the oncogenic potential. Thus, events set in motion by the integration of Ad12 DNA into the recipient genome initiated the oncogenic transformation of the hamster cells. Subsequently, the continuous presence of the viral genomes was no longer essential to maintain the oncogenic phenotype. Alterations of cellular DNA methylation patterns in hamster cells transformed by the integrated Ad12 genomes persisted in these revertants even in the absence of all viral genome copies [46]. The altered methylation patterns of cellular DNA are a significant indicator of profound genetic alterations in the structure and function of the hamster tumor cell genome.
The occurrence of revertants of Ad12-induced tumor cells with the loss of multiple copies of initially integrated Ad12 genomes and the preservation of their oncogenic potential are consistent with a hit and run mechanism of adenoviral oncogenesis. In human cancers, adenoviral gene sequences or gene products could never be identified in spite of intensive searches [65], [66], [67]. The existence of revertants of Ad12-induced hamster tumors, however, suggests caution in interpreting the absence of viral DNA sequences from human tumors as negative evidence for the possible oncogenic potential of adenoviruses in humans. Viral infections might still have initiated the transformation process and the viral genomes might then have been lost by an excision mechanism.
7. Consequences of foreign (Ad12) DNA insertion into the mammalian genome: de novo methylation of foreign DNA integrates
In early studies on the structure of integrated Ad12 genomes in hamster tumor cells, de novo methylation of foreign DNA integrates was discovered [11], [34]. Recently, we determined the epigenetic status of integrated Ad12 DNA in cell line TR12 which is a fibroblastic revertant of the Ad12-transformed epitheloid hamster cell line T637 with 15 copies of integrated Ad12 DNA and carries an integrate of one Ad12 DNA copy plus a 3.9-kbp fragment from an additional copy [52]. The cellular insertion site for the Ad12 integrate is identical in both cell lines and represents a >5.2-kbp inverted DNA repeat (Fig. 3 ). The Ad12 transgenome exhibits nucleosome configuration. The cellular nucleotide sequence at the Ad12-cell DNA junction is more sensitive to micrococcal nuclease digestion at Ad12-occupied than at unoccupied sites in non-Ad12 transgenic hamster cells. Bisulfite sequencing [52] revealed complete de novo methylation in most of the 1634 CpGs of the integrated viral DNA, except for sequences of the Ad12 E4 region that are located at its termini. Isolated unmethylated CpG dinucleotides extend over the entire Ad12 integrate (Fig. 4 ). Similar islands of unmethylated CpG dinucleotides are also observed in the fully methylated human FMR1 promoter in fragile X patients [68]. Islands of unmethylated CpG dinucleotides in a background of heavily methylated DNA sequences may be a landmark of de novo methylated DNA, as in an integrated Ad12 transgenome or a silenced FMR1 promoter in cells from individuals with the fragile X syndrome. The fully methylated Ad12 transgenome segments in cell line TR12 are characterized by promoter silencing and histone H3 and H4 hypoacetylation [52].
Fig. 3.
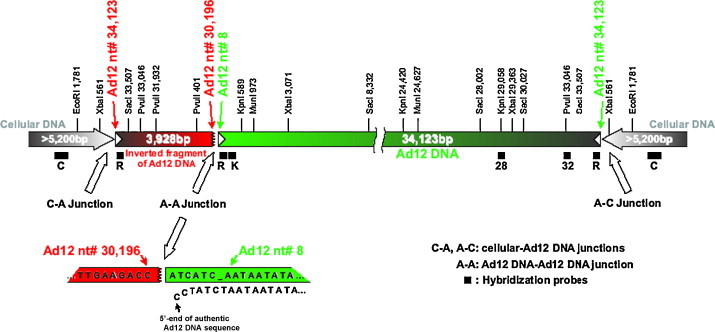
Scheme of the Ad12 transgenome and its integration site in cell line TR12. Filled bars beneath the map of the transgenome show the positions of hybridization probes. Probe C, for the cellular DNA flanking the integrated Ad12 genomes, was excised as a 1165-bp XbaI fragment from the plasmid subclone F7 [47]. Inverted terminal repeats of Ad12 DNA are depicted by white arrowheads. The green bar represents a full-length copy of Ad12 DNA, and the red bar is a truncated second, flip-flopped copy derived from the right terminus of Ad12 DNA. The cellular DNA segments of at least 5.2 kbp adjacent to the transgenome are palindromic and identical on both flanks of the Ad12 integrate. From ref. [52].
Fig. 4.

Methylation profile of the Ad12 transgenome in cell line TR12. The methylation pattern was investigated at single-CpG resolution using the bisulfite conversion of genomic DNA. Unmethylated CpGs are indicated by open squares and methylated ones by filled squares. CpG sites with variable methylation status are assigned diagonally half-filled symbols. Each horizontal array of symbols corresponds to one PCR clone. Individual CpGs are arranged vertically numbered; on top of these numbers Ad12 genome nucleotide positions of the PCR fragments are shown. Ad12 gene groups are indicated with gray bars. The Ad12 genome contains 1.500 CpG, the inverted right terminal fragment 134 CpG dinucleotides. From ref. [52].
8. Factors affecting the de novo methylation of transgenic inserts in mammalian cells
Integration of foreign DNA into an established host genome can lead to changes in methylation in both the inserted DNA and in host sequences and potentially alters transgene and cellular transcription patterns. We investigated factors that influenced de novo methylation. Homologous recombination was used to re-integrate the B lymphocyte kinase (BLK) gene into its authentic genome position in mouse embryonic stem (ES) cells [69]. The methylation patterns were analyzed for both homologously recombined and randomly integrated foreign DNA in the ES cell clones. Upon homologous reinsertion of the BLK gene into the mouse genome, its methylation patterns were reestablished as they existed in the non-manipulated mouse genome. In homologously recombined DNA, sequences carrying the weak adenovirus type 2 promoter were heavily methylated. In contrast, constructs with the much stronger SV40 promoter and an SV40 enhancer remained unmethylated or hypomethylated. Upon removal of the enhancer element, these inserted constructs also became heavily methylated. In addition, all randomly integrated constructs were heavily methylated independently of the promoter and enhancer element present in the construct. These results indicate that modes and sites of integration as well as the inserted nucleotide sequence, possibly promoter strength, are factors directing de novo methylation [69].
9. Reprise: long-term promoter silencing by sequence-specific promoter methylation
Our earlier studies on the de novo methylation of integrated Ad12 DNA and on the inverse correlation between DNA methylation and gene activity of integrated Ad12 genome segments [11], [12] set the stage for a series of studies on promoter methylation and regulation of gene activity that belonged to the first of their kind in the literature [8], [70], [71], [72], [73], [74], [75]. Remarkably, in frog virus 3 (FV3), promoter methylation was required for full gene activity [76], [77]. The presence of 5-mC in a promoter sequence has to be viewed as a modulating signal that, depending on the context of promoter sequence and the specific transcription factors involved, could either inactivate or activate the promoter. In most instances, however, sequence-specific promoter methylation serves as a genetic signal for long-term gene silencing [8], [78]. Many studies in a multitude of biological systems have proven the generality and applicability of our earlier conclusions.
10. Changes of cellular DNA methylation at the insertion site of foreign DNA
Methylation patterns in the genomes of mammalian cells are stable; however, occasional changes are observed. The analysis of the site of linkage between the left terminus of Ad12 DNA and unique hamster DNA in the Ad12-induced tumor T1111(2) revealed linkage of the Ad12 genome to cellular DNA in the vicinity of intracisternal A particle (IAP) genomes, a retro-transposon-like element, in hamster cells [79]. In normal hamster kidney and spleen DNA, on the non-occupied chromosome in the tumor cell line T1111(2), and in several Ad12-transformed hamster cell lines, this pre-insertion sequence is methylated at all 5′-CCGG-3′ (HpaII) and 5′-GCGC-3′ (HhaI) sequences. In contrast, the same sequence on the chromosome, that carries the integrated Ad12 DNA sequence in the tumor T1111(2), is unmethylated, as are the abutting Ad12 DNA sequences. Thus, the insertion of unmethylated foreign DNA can lead to the hypo-methylation, i.e. a significant change in DNA methylation in the flanking cellular target sequence [80]. Similar changes in cellular DNA methylation patterns have been reported for mouse cells after the insertion of retroviral genomes [81].
11. Alterations of patterns of DNA methylation in cellular genome segments at loci remote from the site of foreign DNA insertion
In an extension of these studies, Ad12-transformed hamster cells, Ad12-induced hamster tumor cells, or hamster cells carrying integrated DNA of bacteriophage lambda were investigated for changes of DNA methylation at genome sites remote from the insertion site of the foreign DNA. For several cellular DNA segments investigated, in particular for the intracisternal A particle retro-transposons, extensive increases in DNA methylation were found in comparison with the methylation patterns in BHK21 or primary Syrian hamster cells [46]. Interestingly, increased methylation of cellular genes was maintained in the hamster cell revertant TR3 that had originally carried integrated Ad12 DNA but subsequently lost all Ad12 DNA copies. Thus persistence of the foreign DNA was not required to preserve the changes in cellular DNA methylation patterns. By fluorescent in situ hybridization, the viral DNA integrate was located on only one hamster chromosome. The endogenous intracisternal A particle genomes showed striking distribution patterns on many hamster chromosomes, frequently on their short arms [82]. We attribute the alterations in cellular DNA methylation, at least in part, to the insertion of foreign DNA into the established hamster genome. Since alterations in DNA methylation patterns are likely to affect the transcription patterns of cellular genes, it is conceivable that these changes have played a role in the generation or in the maintenance of the Ad12-transformed phenotype.
Effects on the methylation profiles of cellular genomes might not be restricted to the insertion of adenoviral DNA but represent a general phenomenon in the wake of the insertion of any foreign DNA. Hence, clonal BHK21 hamster cell lines were generated that carried in their genomes bacteriophage lambda and/or plasmid pSV2neo DNA at a single chromosomal site [50]. In different cell lines, the loci of foreign DNA insertion were different. The inserted lambda DNA frequently became de novo methylated. In some of the thus generated hamster cell lines, the levels of DNA methylation in the retrotransposon genomes of the endogenous intracisternal A particles were increased in comparison to those in the non-transgenic BHK21 cell lines. The results of genomic bisulfite sequencing documented alterations in the patterns of DNA methylation in selected segments of the IAPI sequences [50]. Among the BHK21 cells used in these studies differences in methylation patterns did not existed prior to lambda DNA integration. The DNA in a total of 66 non-transgenic BHK21 cell clones showed identical IAPI methylation patterns [50]. Further control experiments [50] led to the conclusion that the observed changes in the DNA methylation patterns in BHK21 cells with integrated lambda DNA had not pre-existed and had not been caused by the transfection procedure. Our data support the interpretation that the insertion of foreign DNA into a pre-existing mammalian genome can profoundly change the cellular patterns of DNA methylation. The cellular sites affected by and the extent of these changes might depend on the site and size of foreign DNA insertions [50].
In a corollary study, a wide scope of cellular DNA segments and genes was analyzed for changes in methylation patterns as a consequence of foreign DNA insertion [51]. By applying the technique of methylation-sensitive representational difference analysis (MS-RDA) [83], we identified several cellular DNA segments that were indeed more heavily methylated in lambda DNA-transgenic as compared to non-transgenic hamster cell lines [51]. By using a suppressive subtractive hybridization technique to cDNA preparations from non-transgenic and Ad12-transformed or lambda DNA-transgenic hamster cells, several cellular genes with altered transcription patterns were cloned from the transgenic cells [51]. In control experiments, no differences in gene expression or DNA methylation patterns were detectable among individual non-transgenic BHK21 cell clones. We again conclude that the insertion of foreign DNA into mammalian genomes can lead to alterations in cellular DNA methylation and transcription patterns.
12. Transient persistence of food-ingested foreign DNA in the mouse organism
Foreign viral genomes can integrate their genomes into the host genome with long-term consequences for the host as exemplified in the preceding sections for Ad12-induced hamster tumors. Another source of foreign DNA entering mammalian organisms on a regular basis and in large amounts is the DNA ingested with the constant and inevitable food supply. We have therefore studied the fate of food ingested DNA in the mouse by several methods [56], [84]. Food-ingested foreign DNA was not completely degraded in the gastrointestinal tract of mice. Phage M13mp18 DNA as a test molecule was detected in the contents of the small intestine, the cecum, the large intestine, or the feces. In 254 animals, M13mp18 DNA fragments of up to 976 bp were found in blood 2–8 h after feeding. M13mp18 DNA fragments were traced by PCR in peripheral leukocytes and located by fluorescent in situ hybridization in about 1 of 1000 peripheral white blood cells between 2 and 8 h, and in spleen or liver cells up to 24 h after feeding. M13mp18 DNA could be traced by FISH in the columnar epithelial cells, in the leukocytes in Peyer's patches of the cecum wall, in liver cells, and in B cells, T cells, and macrophages from spleen. These findings suggested transport of foreign DNA through the intestinal wall and Peyer's patches to peripheral blood leukocytes and into several organs. Upon extended feeding, M13mp18 DNA could be re-cloned from total spleen DNA and, in one clone, was covalently linked to an 80 nt DNA segment with 70% homology to the mouse IgE receptor gene [56].
In a more natural scenario, soybean leaves were fed to mice [84]. The distribution of the plant-specific, nucleus-encoded ribulose-1,5-bisphosphate carboxylase (Rubisco) gene was then followed in the mouse. The Rubisco gene or fragments of it were recovered in the intestine from 2 up to 49 h after feeding, and in the cecum up to 121 h after ingestion. Rubisco gene-specific PCR products were also amplified from spleen and liver DNA. There was no evidence for the expression of orally administered genes, as assessed by the RT-PCR method.
Moreover, mice were continuously fed daily with the cloned gene for the green fluorescent protein for eight generations and were subsequently examined by PCR for the transgenic state. The results were uniformly negative and argued against the germ line transfer of orally administered DNA [84].
These data indicate that food-ingested DNA persists transiently not only in the GI tract but can become intimately associated with different cell systems in the mouse and may rarely be integrated into the host genome. Expression or germ line transmission of the foreign DNA was not found. It remains to be studied whether food-ingested DNA in rare instances can play a long-term important role in the organism.
13. Synopsis and working hypothesis on adenoviral oncogenesis
The salient features of this review on the events following Ad12 infection of hamster cells that can lead to viral oncogenesis are the following.
-
(i)
Ad12 is unable to replicate its DNA and to transcribe most of its genes in Syrian hamster cells.
-
(ii)
In a number of the Ad12-infected hamster cells, the viral genome becomes stably integrated into the host genome.
-
(iii)
Integration proceeds mainly by non-homologous recombination at a large number of possible cellular sites. There is no apparent specificity in the selection of the recombination site, except that short or patchy sequence homologies between the termini of the Ad12 genome and the recipient cellular DNA sequences or transcriptional activity can help target the integration reaction.
-
(iv)
In most instances, multiple copies of the Ad12 genome are integrated at a single chromosomal site. In 60 analyzed Ad12-induced tumors, only one had Ad12 genomes integrated at two different chromosomal locations. There are no free Ad12 DNA copies in Ad12-transformed or tumor cells. Ad12 oncogenesis is a clonal event.
-
(v)
Observations described for integrated Ad12 molecules also hold for integrated DNA of bacteriophage lambda, and probably for many types of foreign DNA when integrated into a mammalian host genome.
-
(vi)
There is ample evidence that adenovirus DNA can also be integrated into the genomes of permissive human cells. Integrates are found at many different sites in the human genome. Their existence has been proven in Ad12 SYREC molecules or upon the infection of human cells with gutless, high capacity adenovirus vectors that are unable to replicate in and therefore do not kill human host cells. These results clarify the integrative capacity of adenoviral DNA and present a caveat towards the application of adenovirus vectors in human gene therapy.
-
(vii)
Integrated foreign (Ad12) genomes become rapidly de novo methylated. Methylation starts regionally in the transgenomes. In a revertant of an Ad12-transformed hamster cell line that harbors only one copy of Ad12 DNA and a right terminal fragment of a second Ad12 genome copy, the epigenetic profile of the integrate both with respect to de novo methylation and chromatin modifications has been determined. Except for the two terminally located Ad12 E4 regions, the entire integrate has become methylated and the genes almost completely silenced. In a background of completely de novo methylated DNA, there remain a few clustered or single CpG dinucleotide isles that, for unknown reasons, have escaped de novo methylation. The occurrence of such isles of unmethylated CpGs in a background of hypermethylated DNA sequences appears to be a characteristic of de novo methylation.
-
(viii)
Adenovirion DNA, free viral DNA in the nuclei of productively or abortively infected cells does not become de novo methylated. Cellular DNA sequences that are methylated in the intact genome, lack methylation when inserted into a symmetric recombinant of Ad12 DNA.
-
(ix)
The insertion of foreign DNA into an established mammalian genome leads to changes in methylation and transcription patterns in the recipient cellular genome. It is unknown whether this phenomenon is general and to what extent the host genome can be affected by these alterations. Extent and regions affected by these alterations may depend on the location of the site of foreign DNA insertion [85], [86].
-
(x)
The finding of altered methylation patterns in cellular DNA segments upon foreign DNA insertion into the mammalian genome is of relevance for many fields of research in biology and medicine, e.g. in gene therapeutic regimens where the consequences on the structure and function of the rest of the genome have rarely been considered. Similarly, the frequent de novo methylation of inserted foreign DNA often limits the continued expression of the inserted foreign genes and even of reinserted authentic genes unless they become integrated at their authentic genome locations.
-
(xi)
As large amounts of foreign DNA constantly enter mammalian organisms through the food chain, it will be important to follow the fate of these DNA molecules in the recipient organisms. The persistence of a small fraction of this DNA in the animals is transient, and there is no evidence for the expression or germ line entry and fixation of foreign DNA in the host. Nevertheless, the occasional cell that genomically integrates fragments of food-ingested deserves the oncologist's interest.
Conflicts of interest
I declare that there is no conflict of interest.
Acknowledgments
Over the past 37 years, my laboratory was supported by the Deutsche Forschungsgemeinschaft (SFB74 and 274), the Federal Minister for Research and Technology, both in Bonn, the Bavarian Minister for Development and Protection of the Environment, München, the Fritz Thyssen Foundation, Köln, the Alexander von Humboldt Foundation, Bonn, the Wilhelm Sander Foundation, München, amaxa GmbH, Köln, the University of Cologne, and the Institute for Virology, Erlangen (W.D. Senior Research Group).
References
- 1.Trentin J.J., Yabe Y., Taylor G. The quest for human cancer viruses. Science. 1962:835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- 2.Hohlweg U., Hösel M., Dorn A., Webb D., Hilger-Eversheim K., Remus R. Intraperitoneal dissemination of Ad12-induced undifferentiated neuroectodermal hamster tumors: de novo methylation and transcription patterns of integrated viral and of cellular genes. Virus Res. 2003:45–56. doi: 10.1016/j.virusres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Berget S.M., Moore C., Sharp P.A. Spliced segments at the 5′terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci USA. 1977:3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow L.T., Gelinas R.E., Broker T.R., Roberts R.J. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell. 1977:1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- 5.Jones N., Shenk T An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci USA. 1979;76:3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nevins J.R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981:213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- 7.Nagata K., Guggenheimer R.A., Hurwitz J. Adenovirus DNA replication in vitro: synthesis of full-length DNA with purified proteins. Proc Natl Acad Sci USA. 1983:4266–4270. doi: 10.1073/pnas.80.14.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doerfler W. DNA methylation and gene activity. Ann Rev Biochem. 1983:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- 9.Hösel M., Webb D., Schröer J., Doerfler W. The abortive infection of Syrian hamster cells with human adenovirus type 12. Curr Top Microbiol Immunol. 2003:415–440. doi: 10.1007/978-3-662-05597-7_14. [DOI] [PubMed] [Google Scholar]
- 10.Doerfler W. Human adenovirus type 12: crossing species barriers to immortalize the viral genome. Methods Mol Med. 2007:197–211. doi: 10.1385/1-59745-277-7:197. [DOI] [PubMed] [Google Scholar]
- 11.Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci USA. 1980:253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vardimon L., Neumann R., Kuhlmann I., Sutter D., Doerfler W. DNA methylation and viral gene expression in adenovirus-transformed and -infected cells. Nucl Acids Res. 1980:2461–2473. doi: 10.1093/nar/8.11.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doerfler W. Wiley-VCH; Weinheim, New York, Chichester, Brisbane, Singapore, Toronto: 2000. Foreign DNA in mammalian systems. [Google Scholar]
- 14.Doerfler W. In pursuit of the first recognized epigenetic signal: DNA methylation. Epigenetics. 2008:125–133. doi: 10.4161/epi.3.3.6249. [DOI] [PubMed] [Google Scholar]
- 15.Hahn B.H., Shaw G.M., De Cock K.M., Sharp P.M. AIDS as a zoonosis: scientific and public health implications. Science. 2000:607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- 16.Holmes K.V. SARS coronavirus: a new challenge for prevention and therapy. J Clin Invest. 2003:1605–1609. doi: 10.1172/JCI18819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tumpey T.M., Basler C.F., Aguilar P.V., Zheng H., Solórzano A., Swayne D.E. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 18.Kuhlmann I., Doerfler W. Shifts in the extent and patterns of DNA methylation upon explantation and subcultivation of adenovirus type 12-induced hamster tumor cells. Virology. 1982:169–180. doi: 10.1016/0042-6822(82)90330-0. [DOI] [PubMed] [Google Scholar]
- 19.Kuhlmann I., Achten S., Rudolph R., Doerfler W. Tumor induction by human adenovirus type 12 in hamsters: loss of the viral genome from adenovirus type 12-induced tumor cells is compatible with tumor formation. EMBO J. 1982:79–86. doi: 10.1002/j.1460-2075.1982.tb01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969:587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- 21.Hösel M., Webb D., Schröer J., Schmitz B., Doerfler W. The overexpression of the adenovirus type 12 pTP or E1A gene facilitates Ad12 DNA replication in non-permissive BHK21 hamster cells. J Virol. 2001:16041–16053. doi: 10.1128/JVI.75.21.10041-10053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochstein N., Webb D., Hösel M., Seidel W., Auerochs S., Doerfler W. Human CAR gene expression in non-permissive hamster cells boosts entry of type 12 adenovirions and nuclear import of viral DNA. J Virol. 2008:4159–4163. doi: 10.1128/JVI.02657-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortin J., Doerfler W. Transcription of the genome of adenovirus type 12. I. Viral mRNA in abortively infected and transformed cells. J Virol. 1975:27–35. doi: 10.1128/jvi.15.1.27-35.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hösel M., Schröer J., Webb D., Jaroschevskaja E., Doerfler W. Cellular and early viral factors in the interaction of adenovirus type 12 with hamster cells: the abortive response. Virus Res. 2001:1–16. doi: 10.1016/s0168-1702(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 25.Strohl W.A. The response of BHK21 cells to infection with type 12 adenovirus. II. Relationship of virus-stimulated DNA synthesis to other viral functions. Virology. 1969:653–665. doi: 10.1016/0042-6822(69)90004-x. [DOI] [PubMed] [Google Scholar]
- 26.Visser L., van Maarschalkerweerd M.W., Rozijn T.H., Wassenaar A.D., Reemst A.M., Sussenbach J.S. Viral DNA sequences in adenovirus-transformed cells. Cold Spr Harbor Symp Quant Biol. 1980:541–550. doi: 10.1101/sqb.1980.044.01.056. [DOI] [PubMed] [Google Scholar]
- 27.Klimkait T., Doerfler W. Adenovirus types 2 and 5 functions elicit replication and late expression of adenovirus type 12 DNA in hamster cells. J Virol. 1985:466–474. doi: 10.1128/jvi.55.2.466-474.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klimkait T., Doerfler W. E1B functions of type C adenoviruses play a role in the complementation of blocked adenovirus type 12 DNA replication and late gene transcription in hamster cells. Virology. 1987:109–120. doi: 10.1016/0042-6822(87)90176-0. [DOI] [PubMed] [Google Scholar]
- 29.Schiedner G., Schmitz B., Doerfler W. Late transcripts of adenovirus type 12 DNA are not translated in hamster cells expressing the E1 region of adenovirus type 5. J Virol. 1994:5476–5482. doi: 10.1128/jvi.68.9.5476-5482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci USA. 1968:636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970:652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortin J., Scheidtmann K.H., Greenberg R., Westphal M., Doerfler W. Transcription of the genome of adenovirus type 12. III. Maps of stable RNA from productively infected human cells and abortively infected and transformed hamster cells. J Virol. 1976:355–372. doi: 10.1128/jvi.20.2.355-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groneberg J., Sutter D., Soboll H., Doerfler W. Morphological revertants of adenovirus type 12-transformed hamster cells. J Gen Virol. 1978:635–645. doi: 10.1099/0022-1317-40-3-635. [DOI] [PubMed] [Google Scholar]
- 34.Sutter D., Westphal M., Doerfler W. Patterns of integration of viral DNA sequences in the genomes of adenovirus type 12-transformed hamster cells. Cell. 1978:569–585. doi: 10.1016/0092-8674(78)90243-x. [DOI] [PubMed] [Google Scholar]
- 35.Stabel S., Doerfler W., Friis R.R. Integration sites of adenovirus type 12 DNA in transformed hamster cells and hamster tumor cells. J Virol. 1980:22–40. doi: 10.1128/jvi.36.1.22-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deuring R., Winterhoff U., Tamanoi F., Stabel S., Doerfler W. Site of linkage between adenovirus type 12 and cell DNAs in hamster tumour line CLAC3. Nature. 1981:81–84. doi: 10.1038/293081a0. [DOI] [PubMed] [Google Scholar]
- 37.Deuring R., Klotz G., Doerfler W. An unusual symmetric recombinant between adenovirus type 12 DNA and human cell DNA. Proc Natl Acad Sci USA. 1981:3142–3146. doi: 10.1073/pnas.78.5.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stabel S., Doerfler W. Nucleotide sequence at the site of junction between adenovirus type 12 DNA and repetitive hamster cell DNA in transformed cell line CLAC1. Nucl Acids Res. 1982:8007–8023. doi: 10.1093/nar/10.24.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doerfler W., Gahlmann R., Stabel S., Deuring R., Lichtenberg U., Schulz M. On the mechanism of recombination between adenoviral and cellular DNAs: the structure of junction sites. Curr Top Microbiol Immunol. 1983:193–228. doi: 10.1007/978-3-642-69460-8_9. [DOI] [PubMed] [Google Scholar]
- 40.Gahlmann R., Doerfler W. Integration of viral DNA into the genome of the adenovirus type 2-transformed hamster cell line HE5 without loss or alteration of cellular nucleotides. Nucl Acids Res. 1983:7347–7361. doi: 10.1093/nar/11.21.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gahlmann R., Schulz M., Doerfler W. Low molecular weight RNAs with homologies to cellular DNA at sites of adenovirus DNA insertion in hamster or mouse cells. EMBO J. 1984:3263–3269. doi: 10.1002/j.1460-2075.1984.tb02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz M., Freisem-Rabien U., Jessberger R., Doerfler W. Transcriptional activities of mammalian genomes at sites of recombination with foreign DNA. J Virol. 1987:344–353. doi: 10.1128/jvi.61.2.344-353.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jessberger R., Heuss D., Doerfler W. Recombination in hamster cell nuclear extracts between adenovirus type 12 DNA and two hamster preinsertion sequences. EMBO J. 1989:869–878. doi: 10.1002/j.1460-2075.1989.tb03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatzelt J., Scholz B., Fechteler K., Jessberger R., Doerfler W. Recombination between adenovirus type 12 DNA and a hamster preinsertion sequence in a cell-free system. Patch homologies and fractionation of nuclear extracts. J Mol Biol. 1992:117–126. doi: 10.1016/0022-2836(92)90128-7. [DOI] [PubMed] [Google Scholar]
- 45.Tatzelt J., Fechteler K., Langenbach P., Doerfler W. Fractionated nuclear extracts from hamster cells catalyze cell-free recombination at selective sequences between adenovirus DNA and a hamster preinsertion site. Proc Natl Acad Sci USA. 1993:7356–7360. doi: 10.1073/pnas.90.15.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heller H., Kämmer C., Wilgenbus P., Doerfler W. Chromosomal insertion of foreign (adenovirus type 12, plasmid, or bacteriophage lambda) DNA is associated with enhanced methylation of cellular DNA segments. Proc Natl Acad Sci USA. 1995:5515–5519. doi: 10.1073/pnas.92.12.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knoblauch M., Schröer J., Schmitz B., Doerfler W. The structure of adenovirus type 12 DNA integration sites in the hamster cell genome. J Virol. 1996:3788–3796. doi: 10.1128/jvi.70.6.3788-3796.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schröer J., Hölker I., Doerfler W. Adenovirus type 12 DNA firmly associates with mammalian chromosomes early after virus infection or after DNA transfer by the addition of DNA to the cell culture medium. J Virol. 1997:7923–7932. doi: 10.1128/jvi.71.10.7923-7932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hilger-Eversheim K., Doerfler W. Clonal origin of adenovirus type 12-induced hamster tumors: nonspecific chromosomal integration sites of viral DNA. Cancer Res. 1997:3001–3009. [PubMed] [Google Scholar]
- 50.Remus R., Kämmer C., Heller H., Schmitz B., Schell G., Doerfler W. Insertion of foreign DNA into an established mammalian genome can alter the methylation of cellular DNA sequences. J Virol. 1999:1010–1022. doi: 10.1128/jvi.73.2.1010-1022.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Müller K., Heller H., Doerfler W. Foreign DNA integration. Genome-wide perturbations of methylation and transcription in the recipient genomes. J Biol Chem. 2001:14271–14278. doi: 10.1074/jbc.M009380200. [DOI] [PubMed] [Google Scholar]
- 52.Hochstein N., Muiznieks I., Mangel L., Brondke H., Doerfler W. The epigenetic status of an adenovirus transgenome upon long-term cultivation in hamster cells. J Virol. 2007:5349–5361. doi: 10.1128/JVI.02624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Günthert U., Schweiger M., Stupp M., Doerfler W. DNA methylation in adenovirus, adenovirus-transformed cells, and host cells. Proc Natl Acad Sci USA. 1976:3923–3927. doi: 10.1073/pnas.73.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kämmer C., Doerfler W. Genomic sequencing reveals absence of DNA methylation in the major late promoter of adenovirus type 2 DNA in the virion and in productively infected cells. FEBS Lett. 1995:301–305. doi: 10.1016/0014-5793(95)00248-8. [DOI] [PubMed] [Google Scholar]
- 55.Doerfler W. Patterns of DNA methylation – evolutionary vestiges of foreign DNA inactivation as a host defense mechanism: A proposal. Biol Chem Hoppe-Seyler. 1991:557–564. [PubMed] [Google Scholar]
- 56.Schubbert R., Renz D., Schmitz B., Doerfler W. Foreign (M13) DNA ingested by mice reaches peripheral leukocytes, spleen and liver via the intestinal wall mucosa and can be covalently linked to mouse DNA. Proc Natl Acad Sci USA. 1997:961–966. doi: 10.1073/pnas.94.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wronka G., Fechteler K., Schmitz B., Doerfler W. Integrative recombination between adenovirus type 12 DNA and mammalian DNA in a cell-free system: joining by short sequence homologies. Virus Res. 2002:225–242. doi: 10.1016/s0168-1702(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 58.Schick J., Baczko K., Fanning E., Groneberg J., Burger H., Doerfler W. Intracellular forms of adenovirus DNA: Integrated form of adenovirus DNA appears early in productive infection. Proc Natl Acad Sci USA. 1976:1043–1047. doi: 10.1073/pnas.73.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deuring R., Doerfler W. Proof of recombination between viral and cellular genomes in human KB cells productively infected by adenovirus type 12: structure of the junction site in a symmetric recombinant (SYREC) Gene. 1983:283–289. doi: 10.1016/0378-1119(83)90198-1. [DOI] [PubMed] [Google Scholar]
- 60.Burger H., Doerfler W. Intracellular forms of adenovirus DNA. III. Integration of the DNA of adenovirus type 2 into host DNA in productively infected cells. J Virol. 1974:975–992. doi: 10.1128/jvi.13.5.975-992.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kochanek S., Clemens P.R., Mitani K., Chen H.H., Chan S., Caskey C.T. A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci USA. 1996:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stephen S.L., Sivanandam V.G., Kochanek S. Homologous and heterologous recombination between adenovirus vector DNA and chromosomal DNA. J Gene Med. 2008:1176–1189. doi: 10.1002/jgm.1246. [DOI] [PubMed] [Google Scholar]
- 63.Eick D., Doerfler W. Integrated adenovirus type 12 DNA in the transformed hamster cell line T637: sequence arrangements at the termini of viral DNA and mode of amplification. J Virol. 1982:317–321. doi: 10.1128/jvi.42.1.317-321.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfeffer A., Schubbert R., Orend G., Hilger-Eversheim K., Doerfler W. Integrated viral genomes can be lost from adenovirus type 12-induced hamster tumor cells in a clone-specific, multistep process with retention of the oncogenic phenotype. Virus Res. 1999:113–127. doi: 10.1016/s0168-1702(98)00131-2. [DOI] [PubMed] [Google Scholar]
- 65.Wold W.S., Mackey J.K., Rigden P., Green M. Analysis of human cancer DNA's for DNA sequence of human adenovirus serotypes 3, 7, 11, 14, 16, and 21 in group B1. Cancer Res. 1979:3479–3484. [PubMed] [Google Scholar]
- 66.Wold W.S., Green M. Historic milestones in cancer virology. Semin Oncol. 1979:461–478. [PubMed] [Google Scholar]
- 67.Mende Y., Schneider P.M., Baldus S.E., Doerfler W. PCR-screening of human esophageal and bronchial cancers reveals absence of adenoviral DNA sequences. Virus Res. 2004:81–85. doi: 10.1016/j.virusres.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 68.Naumann A, Hochstein N, Fanning E, Doerfler W. A distinct DNA methylation boundary in the 5′-upstream sequence of the FMR1 promoter binds nuclear proteins and is lost in fragile X syndrome, submitted for publication, 2009. [DOI] [PMC free article] [PubMed]
- 69.Hertz J., Schell G., Doerfler W. Factors affecting de novo methylation of foreign DNA in mouse embryonic stem cells. J Biol Chem. 1999:24232–24240. doi: 10.1074/jbc.274.34.24232. [DOI] [PubMed] [Google Scholar]
- 70.Vardimon L., Kressmann A., Cedar H., Maechler M., Doerfler W. Expression of a cloned adenovirus gene is inhibited by in vitro methylation. Proc Natl Acad Sci USA. 1982:1073–1077. doi: 10.1073/pnas.79.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kruczek I., Doerfler W. The unmethylated state of the promoter/leader and 5′-regions of integrated adenovirus genes correlates with gene expression. EMBO J. 1982:409–414. doi: 10.1002/j.1460-2075.1982.tb01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kruczek I., Doerfler W. Expression of the chloramphenicol acetyltransferase gene in mammalian cells under the control of adenovirus type 12 promoters: effect of promoter methylation on gene expression. Proc Natl Acad Sci USA. 1983:7586–7590. doi: 10.1073/pnas.80.24.7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langner K.D., Vardimon L., Renz D., Doerfler W. DNA methylation of three 5′C-C-G-G3′ sites in the promoter and 5′ region inactivates the E2a gene of adenovirus type 2. Proc Natl Acad Sci USA. 1984:2950–2954. doi: 10.1073/pnas.81.10.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Langner K.D., Weyer U., Doerfler W. Trans effect of the E1 region of adenoviruses on the expression of a prokaryotic gene in mammalian cells: resistance to 5′-CCGG-3′ methylation. Proc Natl Acad Sci USA. 1986:1598–1602. doi: 10.1073/pnas.83.6.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weisshaar B., Langner K.D., Jüttermann R., Müller U., Zock C., Klimkait T. Reactivation of the methylation-inactivated late E2A promoter of adenovirus type 2 by E1A (13S) functions. J Mol Biol. 1988:255–270. doi: 10.1016/0022-2836(88)90456-1. [DOI] [PubMed] [Google Scholar]
- 76.Willis D.B., Granoff A. Frog virus 3 DNA is heavily methylated at CpG sequences. Virology. 1980:250–257. doi: 10.1016/0042-6822(80)90290-1. [DOI] [PubMed] [Google Scholar]
- 77.Munnes M., Schetter C., Hölker I., Doerfler W. A fully 5′-CG-3′ but not a 5′-CCGG-3′ methylated late frog virus 3 promoter retains activity. J Virol. 1995:2240–2247. doi: 10.1128/jvi.69.4.2240-2247.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Munnes M., Doerfler W. DNA methylation in mammalian genomes: promoter activity and genetic imprinting. In: Dulbecco R., editor. vol. 3. Academic Press; San Diego, New York, Boston, London, Sydney, Tokyo, Toronto: 1997. pp. 435–446. (Encyclopedia of human biology). [Google Scholar]
- 79.Lichtenberg U., Zock C., Doerfler W. Insertion of adenovirus type 12 DNA in the vicinity of an intracisternal A particle genome in Syrian hamster tumor cells. J Virol. 1987:2719–2726. doi: 10.1128/jvi.61.9.2719-2726.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lichtenberg U., Zock C., Doerfler W. Integration of foreign DNA into mammalian genome can be associated with hypomethylation at site of insertion. Virus Res. 1988:335–342. doi: 10.1016/0168-1702(88)90006-8. [DOI] [PubMed] [Google Scholar]
- 81.Jähner D., Jaenisch R. Retrovirus-induced de novo methylation of flanking host sequences correlates with gene inactivity. Nature. 1985:594–597. doi: 10.1038/315594a0. [DOI] [PubMed] [Google Scholar]
- 82.Meyer zu Altenschildesche G., Heller H., Wilgenbus P., Tjia S.T., Doerfler W. Chromosomal distribution of the hamster intracisternal A-particle (IAP) retrotransposons. Chromosoma. 1996:341–344. doi: 10.1007/BF00337222. [DOI] [PubMed] [Google Scholar]
- 83.Ushijima T., Morimura K., Hosoya Y., Okonogi H., Tatematsu M., Sugimura T. Establishment of methylation-sensitive-representational difference analysis and isolation of hypo- and hypermethylated genomic fragments in mouse liver tumors. Proc Natl Acad Sci USA. 1997:2284–2289. doi: 10.1073/pnas.94.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hohlweg U., Doerfler W. On the fate of plant or other foreign genes upon the uptake in food or after intramuscular injection in mice. Mol Genet Genomics. 2001:225–233. doi: 10.1007/s004380100450. [DOI] [PubMed] [Google Scholar]
- 85.Doerfler W. Cohn W.E., Moldave K., editors. Adenoviral DNA integration and changes in DNA methylation patterns: a different view of insertional mutagenesisProgr Nucleic Acid Res Mol Biol. 1993;vol. 46:1–36. doi: 10.1016/s0079-6603(08)61016-8. [DOI] [PubMed] [Google Scholar]
- 86.Doerfler W., Hohlweg U., Müller K., Remus R., Heller H., Hertz J. Foreign DNA integration – perturbations of the genome – oncogenesis. Ann N Y Acad Sci. 2001;945:276–288. doi: 10.1111/j.1749-6632.2001.tb03896.x. [DOI] [PubMed] [Google Scholar]


