Abstract
Emerging evidence implicates that low levels of ATP in the extracellular space may contribute to the pathophysiology of major depressive disorder (MDD). The concentration of extracellular ATP is regulated by its hydrolase ectonucleotide tri(di)phosphohydrolase (ENTPD). However, the role of ENTPD in depression remains poorly understood. Here we examine the role of CD39 (known as ENTPD1) in mouse depression‐like behavior induced by chronic social defeat stress (CSDS). We demonstrate that CSDS enhances the expression and activity of CD39 in hippocampus. The CD39 functional analog apyrase also induces depression‐like behavior, which can be ameliorated by ATP replenishment. Pharmacological inhibition and genetic silencing of CD39 has an antidepressant‐like effect via increasing hippocampal extracellular ATP concentration, accompanied with an increase in hippocampal neurogenesis and dendritic spine numbers in defeated mice. These results suggest that hippocampal CD39 contributes to CSDS‐induced depression‐like behavior via hydrolyzing extracellular ATP, indicating that CD39 may be a promising new target for the treatment of depression.
Keywords: ATP, CD39, CSDS, neurogenesis, spine
Subject Categories: Neuroscience
The hippocampal ATP hydrolase CD39 contributes to chronic social defeat stress‐induced depression‐like behavior via hydrolyzing extracellular ATP, suggesting that CD39 may be a potential target for the treatment of depression.
Introduction
Major depressive disorder (MDD), a severe psychiatric disorder, affects hundreds of millions of individuals worldwide, but little is known about the cellular and molecular mechanisms of MDD. Although there are available antidepressants, only about one‐third of MDD patients achieve complete remission of their symptoms after their first antidepressant therapy 1. Depression is likely to be resulted from a complex combination of factors that include alterations in monoamines and non‐monoaminergic neurotransmitter systems. Increasing evidence has shown that altered glial function, inflammation, decreased hippocampal neurogenesis, and synaptogenesis are also involved in the pathophysiology of MDD 2, 3, 4, 5. Furthermore, the deficit of astrocytic adenosine triphosphate (ATP) release has been highlighted as the neurobiological mechanism of MDD 6, 7.
ATP can be released from neurons and astrocytes and is maintained at a high concentration in the brain. In addition, a large amount of ATP can be released from microglia, particularly during neuroinflammation 8. It acts as a fast excitatory neurotransmitter or a neuromodulator. Meanwhile, it mediates long‐term (trophic) purinergic signaling of cell proliferation, differentiation, and death in healthy individuals and patients with brain disorders 9, 10. However, after releasing to the extracellular space, ATP undergoes rapid enzymatic degradation by ectonucleotidases. For example, ectonucleoside tri(di)phosphohydrolase (ENTPD) converts ATP into 5′‐adenosine monophosphate (AMP), and then ecto‐5′‐nucleotidase (also known as CD73) dephosphorylates AMP into adenosine 11. Thus, the degradative activity of enzymes is most important for regulation of ATP function. Four families of ectonucleotidases that hydrolyze ATP have been identified. The ENTPD family comprises seven different isoforms (ENTPD1‐6 and ENTPD8), among which ENTPD1‐3 and ENTPD8 are known to hydrolyze ATP or adenosine diphosphate (ADP) to AMP with different substrate preferences 11. It has been reported that ENTPD1 to ENTPD3 are expressed in mammalian brain 12. ENTPD1 has been identified as ecto‐ATPase, ecto‐ATPDase, or CD39 previously 13, 14 and is now accepted as the major and rate‐limiting component for hydrolysis of extracellular ATP and ADP 15.
CD39 is involved in a series of physiological and pathological processes in multiple tissues, such as immunomodulation, cell–cell interactions, neurotransmission and neuronal activity, and thromboregulatory events in diabetes, atherosclerosis, hypoxia/ischemia, and cancer 15, 16, 17. For neurological disorders, CD39 is probably related to epilepsy by controlling neuronal activity 18. In addition, CD39 also regulates the ramification of microglial processes 19. However, as a key hydrolytic enzyme of ATP, the modulatory effects of CD39 on ATP abundance and depression‐like behaviors during chronic stress remain largely unknown.
In the present study, we used chronic social defeat stress (CSDS) to mimic depressive symptoms and investigated the role and the underlying mechanism of CD39 in depression‐like behaviors of mice. These findings might provide a novel therapeutic strategy for MDD.
Results
The expression and activity of hippocampal CD39 are selectively increased in susceptible mice after chronic social defeat stress (CSDS)
The CSDS model mimics several psychopathological dimensions of depression 20. As shown in Fig 1A, after 10‐day social defeat, ~56% (55/99) of test mice exhibited social avoidance with the social interaction (SI) ratio < 100 (Fig 1B), which were identified as susceptible mice. The mice with the ratio more than 100 were classified as resilient mice. In order to define depression‐like behaviors, sucrose preference test (SPT), tail suspended test (TST), and forced swim test (FST) were performed. It was found that susceptible but not resilient mice displayed a significant anhedonia accessing by sucrose preference (Fig 1C). Accordingly, the despair behaviors representing as the immobility time in TST and FST remarkably increased in susceptible mice (Fig 1D and E). In addition, chronic defeat stress also induced anxiety behaviors, as manifested by the elevated plus maze (EPM) test and open field test (OFT) (Fig 1F–H).
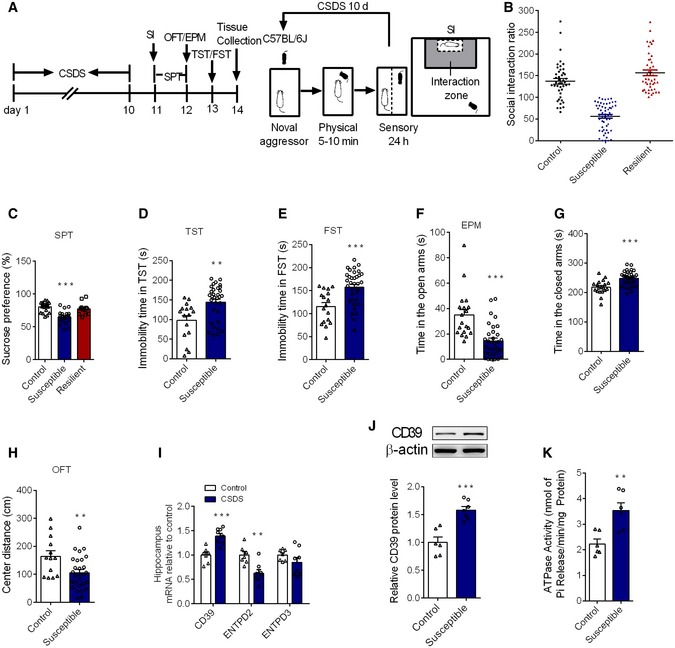
Figure 1. The expression of CD39 and ATPase activity is selectively upregulated in the hippocampus by chronic stress.
-
ATime‐line of experiment procedures in the chronic social defeat stress.
-
BSocial interaction ratio in control, susceptible, and resilient mice following the CSDS protocol (n = 44, 55, 44 mice).
-
CSucrose preference in control, susceptible, and resilient mice (n = 20, 20, 12 mice; Treatment F 2,49 = 16.05, P < 0.0001, one‐way ANOVA with Fisher's LSD test).
-
DThe immobility time in TST in susceptible mice, compared to control mice (n = 16, 32 mice; susceptible versus control, P = 0.002, Student's t‐test).
-
EThe immobility time in FST in susceptible mice, compared to control mice (n = 17, 35 mice; susceptible versus control, P = 0.0003, Student's t‐test).
-
F, GThe time in the open arms (F) and closed arms (G) in the EPM in susceptible mice, compared to control mice (n = 19, 33 mice; susceptible versus control, for open arms, P < 0.0001; for closed time, P < 0.0001, Student's t‐test).
-
HThe central distance in the OFT in susceptible mice, compared to control mice (n = 13, 28 mice; susceptible versus control, P = 0.0089, Student's t‐test).
-
IThe mRNA level of CD39, ENTPD2, and ENTPD3 in the hippocampus of susceptible mice after CSDS (n = 7, 8 mice; CSDS versus control, CD39 P = 0.0002, ENTPD2 P = 0.0025, Student's t‐test).
-
JThe CD39 protein expression in the hippocampus of susceptible mice induced by CSDS (n = 6, 7 mice; CSDS versus control, P = 0.0003, Student's t‐test).
-
KThe ATPase activity in the hippocampus of stressed animals (n = 6 mice/group; CSDS versus control, P = 0.0041, Student's t‐test).
Previous study has observed a lower abundance ATP in the medial prefrontal cortex (mPFC) of susceptible mice, which was proposed to be resulted from the deficiency of astrocytic ATP release 6. However, the extracellular concentration of ATP is not only determined by the amount of release, but also depended on the capacity of catabolic enzymes, especially the ectonucleotidases on cells 14. Therefore, we first investigated whether the mRNA level of CD39, ENTPD2, and ENTPD3 was altered in the hippocampus of susceptible mice. The qPCR results showed that the expression of CD39 gene was upregulated after CSDS; however, the expression of ENTPD2 mRNA was down‐regulated and the level of ENTPD3 mRNA kept unchanged in the hippocampus (Fig 1I). Western blotting analysis also revealed a significant increase of CD39 protein in the hippocampus of susceptible mice (Fig 1J). The results from analysis of ATPase activity showed that Pi releasing from hippocampal slices of susceptible mice was markedly enhanced, indicating augmentation of the ATPase activity for extracellular ATP phosphohydrolysis after CSDS (Fig 1K). Combination with the expression changes, the effect of CD39 should predominate among three ectonucleotidases, but not ENTPD2 or ENTPD3. We also detected the alteration of CD39 in resilient mice and found that the mRNA and protein expression of CD39, as well as the extracellular ATP levels, had no variation between control and resilient mice (Fig EV1A–C). The increase in the expression and activity of CD39 was significant in the hippocampus, but not in the mPFC of susceptible mice (Fig EV1D–F), indicating a region‐specific change.
Figure EV1. The CD39 expression and activity in hippocampus of stress resilient mice and in mPFC of stress susceptible mice.

- The CD39 mRNA level in the hippocampus of resilient mice exposure to CSDS (n = 5, 4 mice; resilient versus control, P = 0.1888, Student's t‐test).
- The expression of CD39 protein in the hippocampus of resilient mice exposure to CSDS (n = 4 mice/group; resilient versus control, P = 0.4403, Student's t‐test).
- The ATP level in the hippocampus of resilient mice exposure to CSDS (n = 4 mice/group; resilient versus control, P = 0.5084, Student's t‐test).
- The CD39 mRNA level in the mPFC of susceptible mice after CSDS (n = 8 mice/group; CSDS versus control, P = 0.8592, Student's t‐test).
- The expression of CD39 protein in the mPFC of susceptible mice induced by CSDS (n = 11 mice/group; CSDS versus control, P = 0.1194, Student's t‐test).
- The ATPase activity in the mPFC of stressed mice (n = 5, 6 mice; CSDS versus control, P = 0.2827, Student's t‐test).
In order to further clarify the alteration of CD39 in the hippocampal subregions, the fluorescence intensity of CD39 between control and susceptible mice was examined. The immunofluorescence staining showed that CD39 was expressed in the whole hippocampus, including CA1, CA3, and DG region, and the fluorescence intensity was strengthened in CA1, CA3, and DG subregions after CSDS (Appendix Fig S1A–C). These results indicate that chronic social defeat stress induces uniform increase in CD39 expression and activity in the hippocampus of mice.
CD39 analog apyrase induces depression‐like behaviors of mice
Given that high level of CD39 was involved in depression‐like behavior in mice, we asked whether apyrase, a functional analog of CD39, could induce depressive‐like behavior. The mice were daily infused with vehicle or apyrase (40, 80, or 160 U/ml) into lateral cerebral ventricle for 3 days, and the social interaction and sucrose preference tests were conducted 30 min following the last infusion (Fig 2A). As shown in Fig 2B, the social interaction time was significantly decreased by 80 U/ml apyrase compared to control mice, whereas low (40 U/ml) and high (160 U/ml) concentration of apyrase had no significant effects. Similarly, only 80 U/ml apyrase treatment produced a decrease in sucrose preference (Fig 2C). There were no differences among the total distance traveled in the OFT (Fig EV2A), suggesting that the apyrase treatment did not alter the locomotor activity of mice. Considering CSDS‐induced enhancement of CD39 activity appeared in the hippocampus, but not in mPFC, we then infused a low concentration of apyrase (40 U/ml) into the hippocampus (Fig 2D). It was found that apyrase treatment significantly reduced the social interaction time (Fig 2E) and sucrose preference (Fig 2F). In order to confirm the active apyrase that induced depressive behaviors, we used inactivated apyrase as a control, which was boiled 5 min at 95°C. The results showed that inactivated apyrase had no effects on social interaction time and sucrose preference (Fig EV2B and C). Consistently, apyrase but not inactivated apyrase treatment induced despair behaviors of mice in TST and FST (Fig EV2D and E). All above indicates that increased CD39 activity in the hippocampus contributes to the depression‐like behaviors.
Figure 2. Apyrase treatment induces depression‐like behaviors that can be rescued by ATP administration.
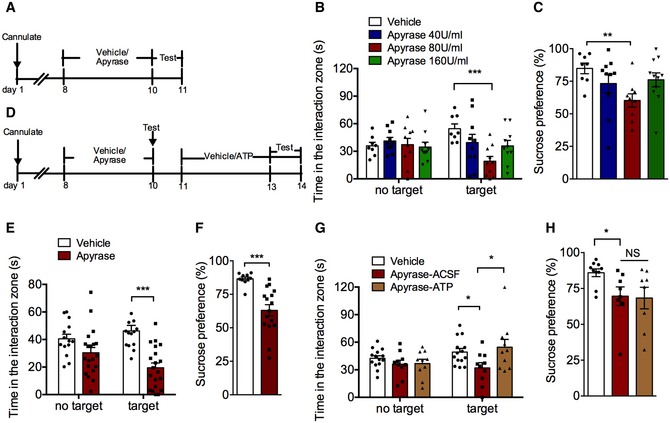
- Experimental timelines for apyrase administration and behavioral study.
- The effects of apyrase (40, 80, and 160 U/ml) infusion into cerebral ventricle on social interaction time (n = 8, 10, 10, 10 mice; Interaction F 3,34 = 3.917, P = 0.0167; Target F 1,34 = 0.0004, P = 0.9834; Drug F 3,34 = 2.295, P = 0.0954; for target, Apyrase 80 U/ml versus control, P = 0.0008, two‐way ANOVA with Tukey's post‐test).
- The effects of apyrase (40, 80, and 160 U/ml) infusion into cerebral ventricle on sucrose preference (n = 8, 10, 9, 11 mice; Treatment F 3,34 = 2.87, P = 0.0505; Apyrase 80 U/ml versus control, P = 0.007, one‐way ANOVA with Fisher's LSD test).
- Experimental timelines for apyrase/ATP administration and behavioral tests.
- The effect of apyrase (40 U/ml) infusion into hippocampal on the social interaction time (n = 14, 20 mice; Interaction F 1,32 = 7.0754, P = 0.0121; Target F 1,32 = 0.68447, P = 0.4142; Drug F 1,32 = 18.211, P = 0.0002; Apyrase versus control, P < 0.0001, Student's t‐test).
- The effect of apyrase (40 U/ml) infusion into hippocampal on the sucrose preference (n = 10, 16 mice; Apyrase versus control, P = 0.0003, Student's t‐test).
- The social avoidance behavior in mice with ATP intra‐hippocampal infusion after apyrase exposure (n = 14, 10, 10 mice; Interaction F 2,31 = 2.051, P = 0.1457; Target F 1,31 = 2.666, P = 0.1127; Drug F 2,31 = 3.711, P = 0.0359; for Target, Apyrase‐ACSF versus Vehicle, P = 0.0387; Apyrase‐ATP versus Apyrase‐ACSF, P = 0.0099, two‐way ANOVA with Tukey's post‐test).
- The sucrose preference in mice with ATP intra‐hippocampal infusion after apyrase exposure (n = 10, 8, 8 mice; Treatment F 2,23 = 3.32, P = 0.0540; Apyrase‐ACSF versus Vehicle, P = 0.0466, one‐way ANOVA with Fisher's LSD test).
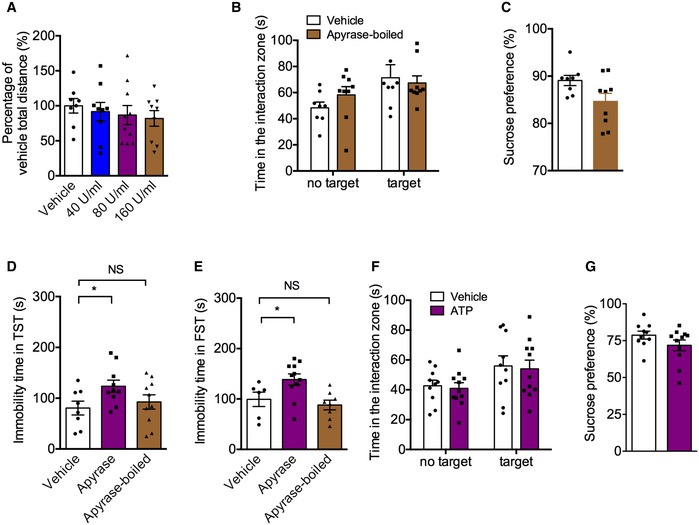
Figure EV2. The effects of apyrase or ATP on locomotor activity and depressive behaviors in normal mice.
-
AThe locomotor activity of mice with apyrase (40, 80, or 160 U/ml) infusion into lateral intracerebroventricular in the open field test (n = 8, 9, 10, 9 mice; Treatment F 3,32 = 0.37, P = 0.7757, one‐way ANOVA with Fisher's LSD test).
-
B, CSocial interaction time (B) and sucrose preference (C) of mice with inactivated apyrase infusion into hippocampus (n = 8, 9 mice; for SI, Interaction F 1,30 = 1.039, P = 0.3162; Drug F 1,30 = 5.591, P = 0.0247; Target F 1,30 = 0.1890, P = 0.6669, two‐way ANOVA with Tukey's post‐test; for SPT, P = 0.0517, Student's t‐test).
-
D, EImmobility time in the TST (D) and FST (E) of mice with apyrase and boiled‐apyrase (for TST, n = 8, 10, 10 mice; Treatment F 2,25 = 2.766, P = 0.0822; Apyrase versus vehicle, P = 0.0348; Apyrase‐boiled versus vehicle, P = 0.5386; for FST, n = 6, 11, 8 mice; Treatment F 2,22 = 5.92, P = 0.0088; Apyrase versus vehicle, P = 0.0307; Apyrase‐boiled versus vehicle, P = 0.5414, one‐way ANOVA with Fisher's LSD test).
-
F, GSocial interaction time (F) and sucrose preference (G) of normal mice with ATP (25 μM) infusion into hippocampus (n = 10, 11 mice; for SI, Interaction F 1,38 = 0.0005723, P = 0.9810; Drug F 1,38 = 6.520, P = 0.0148; Target F 1,38 = 0.1280, P = 0.7225, two‐way ANOVA with Tukey's post‐test; for SPT, P = 0.1484, Student's t‐test).
Next, we examined whether the changes in ATP level in the hippocampus mediated the apyrase‐induced depression‐like behaviors. The physiological concentration of ATP (25 μM) or vehicle was infused daily for 3 days into the hippocampus after apyrase (40 U/ml) treatment, and behavioral tests were performed 30 min following the last infusion. It was shown that ATP administration had no effect on social interaction time and sucrose preference (Fig EV2F and G) of normal mice, but it reversed social avoidance of mice induced by apyrase exposure (Fig 2G), without affecting the sucrose preference (Fig 2H). These results suggest that ATP can partially rescue the depression‐like behaviors induced by apyrase.
Pharmacological inhibition of CD39 alleviates stress‐induced depression‐like behaviors
We next investigated the effects of ARL67156, a nonspecific CD39 antagonist, on depression‐like behavior. First, the cannula was implanted into the right cerebral ventricle of control and defeated mice and then infused with vehicle or ARL67156 (100 μM) for 3 days 21, and the social interaction and sucrose preference tests were conducted 24 h after the last infusion (Fig 3A). It is unclear whether ARL67156 is specifically antagonized CD39 in vivo, so ATPase activity was measured here firstly. As expected, defeated mice with ARL67156 had lower ATPase activity (Fig 3B), confirming ARL67156 could be used as a CD39 antagonist. The susceptible mice exhibited significant reduction in the social interaction time after 3 days of vehicle treatment. In contrast, ARL67156 intracerebroventricular injection reversed the social avoidance of susceptible mice (Fig 3C). Similarly, ARL67156 infusion restored sucrose preference in susceptible mice (Fig 3D). There were no significant effects of ARL67156 on the total distance traveled by the susceptible mice, suggesting that the antidepressant effect of ARL67156 was not due to alteration of locomotor activity (Fig EV3A).
Figure 3. CD39 inhibition induces antidepressant‐like effects in adult mice.
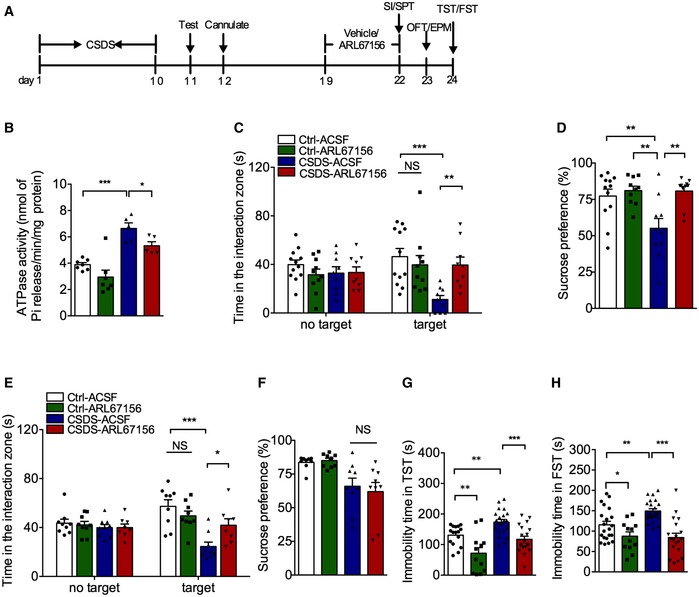
-
AExperimental timelines for CSDS, ARL67156 administration, and behavioral study.
- B
-
CThe social avoidance behavior of susceptible mice with intracerebroventricular infusion of ARL67156 (n = 12, 10, 9, 9 mice; Interaction F 3,36 = 5.119, P = 0.0047; Target F 1,36 = 0.005, P = 0.9437; Drug F 3,36 = 3.626, P = 0.022; for Target, CSDS – ACSF versus Ctrl ‐ACSF, P = 0.0001; CSDS – ARL67156 versus CSDS – ACSF, P = 0.0054, two‐way ANOVA with Tukey's post‐test).
-
DThe sucrose preference of susceptible mice with intracerebroventricular infusion of ARL67156 (n = 12, 10, 10, 10 mice; Interaction F 1,38 = 5.352, P = 0.0262; Group F 1,38 = 5.711, P = 0.0219; Drug F 1,38 = 9.703, P = 0.0035; CSDS – ACSF versus Ctrl – ACSF, P = 0.0083; Ctrl – ARL67156 versus CSDS – ACSF, P = 0.0027; CSDS – ARL67156 versus CSDS – ACSF, P = 0.0031, two‐way ANOVA with Tukey's post‐test).
-
E, FThe social avoidance behavior (E) and sucrose preference (F) in mice with intra‐hippocampal infusion of ARL67156 after CSDS (n = 9, 9, 9, 8 mice; for social behavior, Interaction F 3,31 = 5.579, P = 0.0035; Target F 1,31 = 0.5121, P = 0.4796; Drug F 3,31 = 8.367, P = 0.0003; CSDS – ACSF versus Ctrl – ACSF, P < 0.0001; CSDS – ARL67156 versus CSDS – ACSF, P = 0.0131; sucrose, Interaction F 1,30 = 0.3342, P = 0.5675; Group F 1,30 = 17.22, P = 0.0003; Drug F 1,30 = 0.05903, P = 0.8097; CSDS‐ ACSF versus Ctrl – ACSF, P = 0.0761, two‐way ANOVA with Tukey's post‐test).
-
GThe immobility time in the TST of mice with intra‐hippocampal infusion of ARL67156 after CSDS (n = 16, 12, 21, 19 mice; Treatment F 3,64 = 13.61, P < 0.0001; CSDS – ACSF versus Ctrl – ACSF, P = 0.0059; Ctrl – ARL67156 versus Ctrl – ACSF, P = 0.0011; CSDS – ARL67156 versus CSDS – ACSF, P = 0.0002, one‐way ANOVA with Fisher's LSD test).
-
HThe immobility time in the FST with intra‐hippocampal infusion of ARL67156 after CSDS (n = 21, 12, 23, 19 mice; Treatment F 3,71 = 12.94, P < 0.0001; CSDS – ACSF versus Ctrl – ACSF, P = 0.0036; Ctrl – ARL67156 versus Ctrl – ACSF, P = 0.0428; CSDS – ARL67156 versus CSDS – ACSF, P < 0.0001, one‐way ANOVA with Fisher's LSD test).
Figure EV3. The effects of ARL67156 on locomotor activity and anxiety behaviors of mice.
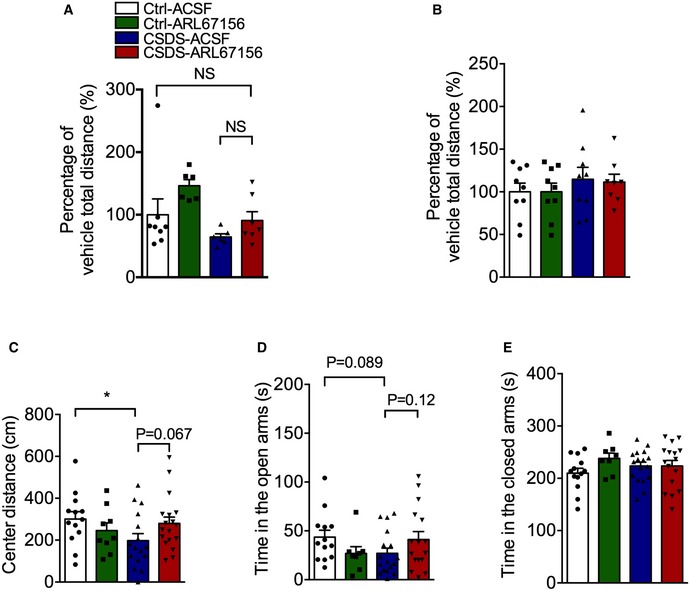
-
AThe locomotor activity of susceptible mice with ARL67156 (100 μM) infusion into the right cerebral ventricle in the open field test (n = 8, 6, 6, 7 mice; Interaction F 1,23 = 0.3174, P = 0.5786; Group F 1,23 = 6.619, P = 0.0170; Drug F 1,23 = 4.226, P = 0.0513, two‐way ANOVA with Tukey's post‐test).
-
BThe locomotor activity of mice with ARL67156 (100 μM) infusion into the hippocampus in the open field test (n = 9, 9, 9, 8 mice; Interaction F 1,31 = 0.1670, P = 0.6856; Group F 1,31 = 0.8477, P = 0.3643; Drug F 1,31 = 0.01314, P = 0.9095, two‐way ANOVA with Tukey's post‐test).
- C
-
D, EThe anxiety behaviors of stressed mice with ARL67156 (100 μM) accessing by the open arms (D) and the closed arms (E) in the EPM test (n = 13, 8, 17, 16 mice; for the open arms, Treatment F 3,50 = 1.569, P = 0.2086; CSDS – ACSF versus Ctrl – ACSF, P = 0.0891; CSDS – ARL67156 versus CSDS – ACSF, P = 0.1234; for the closed arms, Treatment F 3,50 = 1.063, P = 0.3731, one‐way ANOVA with Fisher's LSD test).
To determine whether hippocampus was involved in the mediating effect of ARL67156 on the behavioral response, ARL67156 was infused into the hippocampus of susceptible mice for 3 days after CSDS, and then, depressive and anxiety behaviors were performed sequentially (Fig 3A). It was shown that ARL67156 reversed the social avoidance but not the sucrose preference in susceptible mice (Fig 3E and F). Similarly, the prolonged immobility time in TST and FST was reduced by ARL67156 treatment irrespective of CSDS (Fig 3G and H), suggesting that CD39 inhibitor might have an inhibitory effect on acute stress. In addition, hippocampal ARL67156 treatment had no effect on locomotor activity (Fig EV3B), while it seemed to have mild anti‐anxiety actions with no statistical significance (Fig EV3C–E). Taken together, these findings suggest that inhibition of hippocampal CD39 activity produces antidepressant‐like effects.
Knockdown of CD39 in the hippocampus induces antidepressant‐like effects
To address the direct role of hippocampal CD39 in depression‐like behaviors, we constructed a siRNA for the specific knockdown of CD39 with lentivirus (LV‐siCD39) and then infused into hippocampus after 10‐day CSDS procedure (Fig 4A and B). The efficacy of LV‐siCD39 was verified in normal mice with the inhibition of CD39 expression about 50% (Fig 4C). Next, depression‐like behaviors of stressed mice were carried out after CD39 was knocked down. It was found that LV‐siCD39 had no effect on social interaction behaviors of normal mice (Fig EV4A); however, the social interaction time in susceptible mice with silencing CD39 was significantly longer than susceptible mice with only GFP (Fig 4D). Meanwhile, knockdown of CD39 alleviated CSDS‐induced despair behaviors in TST and FST (Fig 4E and F). Besides, CD39 knockdown in the hippocampus had no significant effect on locomotor activity (Fig EV4B) and anxiety‐like behaviors induced by CSDS (Fig EV4E–G). Thus, knockdown of CD39 in the hippocampus improves the depression‐like behaviors in stressed mice.
Figure 4. Knockdown of CD39 in the hippocampus improves the depression‐like behaviors in stressed mice.
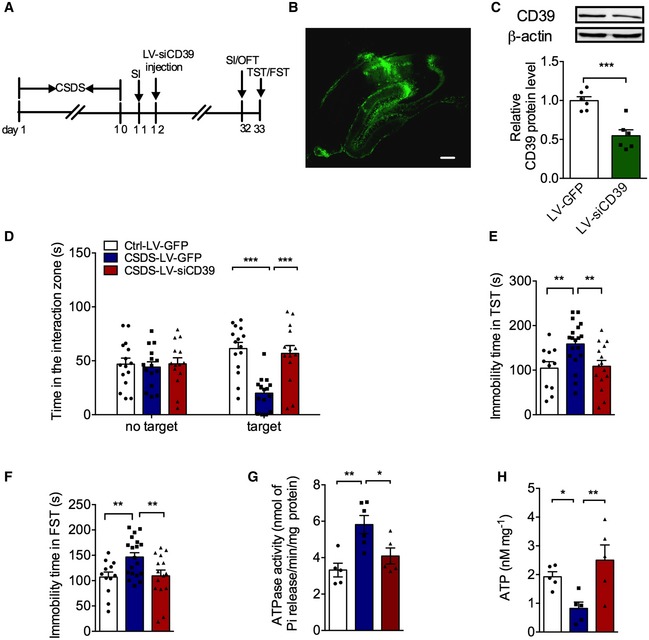
- Experimental timelines for CSDS, virus stereotaxic injections, and behavioral study.
- Imaging of GFP expression in the hippocampus 3 weeks after virus vector injection. Scale bar: 250 μm.
- Expression of CD39 in the hippocampus of mice injected with LV‐siCD39 (n = 6 mice/group; LV‐siCD39 versus Control, P = 0.0006, Student's t‐test).
- The social avoidance behavior for the stressed mice after knocking down CD39 (n = 15, 15, 14 mice; Interaction F 2,41 = 17.96, P < 0.0001; Target F 1,41 = 3.75e‐005, P = 0.9951; Drug F 2,41 = 6.099, P = 0.0048; for Target, CSDS – LV‐GFP versus Ctrl – LV‐GFP, P < 0.0001; CSDS – LV‐siCD39 versus CSDS – LV‐GFP, P < 0.0001, two‐way ANOVA with Tukey's post‐test).
- The immobility time in the TST of mice injected with LV‐ siCD39 (n = 11, 19, 15 mice; Treatment F 2,42 = 5.807, P = 0.0059; CSDS – LV‐GFP versus Ctrl – LV‐GFP, P = 0.0069; CSDS – LV‐siCD39 versus CSDS – LV‐GFP, P = 0.0065, one‐way ANOVA with Fisher's LSD test).
- The immobility time spent in the FST of mice treatment with LV‐siCD39 (n = 12, 19, 15 mice; Treatment F 2,43 = 5.237, P = 0.0092; CSDS – LV‐GFP versus Ctrl – LV‐GFP, P = 0.0095; CSDS – LV‐siCD39 versus CSDS – LV‐GFP, P = 0.0093, one‐way ANOVA with Fisher's LSD test).
- The ATPase hyperactivity of mice after knocking down CD39 in the hippocampal (n = 5, 6, 5 mice; Treatment F 2,13 = 8.45, P = 0.0044; CSDS – LV‐GFP versus Ctrl – LV‐GFP, P = 0.0016; CSDS – LV‐CD39 versus CSDS – LV‐GFP, P = 0.0166, one‐way ANOVA with Fisher's LSD test).
- The ATP level of mice after knocking down CD39 in the hippocampal (n = 5 mice/group; Treatment F 2,12 = 6.04, P = 0.0153; CSDS – LV‐GFP versus Ctrl – LV‐GFP, P = 0.0444; CSDS – LV‐CD39 versus CSDS – LV‐GFP, P = 0.0051, one‐way ANOVA with Fisher's LSD test).
Figure EV4. The effect of LV‐siCD39 on locomotor activity and anxiety behaviors of mice exposure to chronic stress.
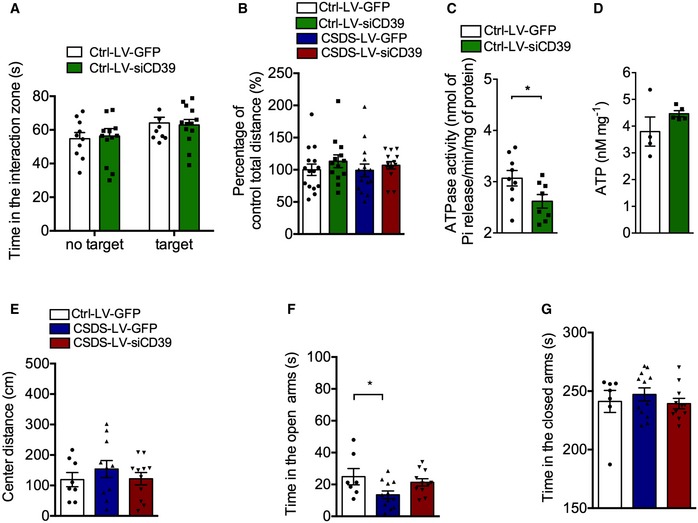
-
AThe social interaction time of control mice after knocking down CD39 (n = 10, 13 mice; Interaction F 1,42 = 0.1334, P = 0.7167; Group F 1,42 = 4.366, P = 0.0428; Drug F 1,42 = 0.002565, P = 0.9598, two‐way ANOVA with Tukey's post‐test).
-
BThe locomotor activity of mice with LV‐siCD39 infusion into hippocampus in the open field test (n = 15, 13, 15, 14 mice; Interaction F 1,53 = 0.09372, P = 0.7607; Group F 1,53 = 0.1669, P = 0.6845; Drug F 1,53 = 1.450, P = 0.2339, two‐way ANOVA with Tukey's post‐test).
-
CThe ATPase activity of control mice with LV‐ siCD39 intra‐hippocampal infusion (n = 9, 8 mice; P = 0.043, Student's t‐test).
-
DThe ATP level of control mice with LV‐siCD39 intra‐hippocampal infusion (n = 4, 5 mice; P = 0.2181, Student's t‐test).
-
EThe center distance of stressed mice with LV‐siCD39 in the OFT (n = 8, 11, 11 mice; Treatment, F 2,27 = 0.6317, P = 0.5394, one‐way ANOVA with Fisher's LSD test).
-
F, GTime spent in the open arms (F) and closed arms (G) of stressed mice with LV‐siCD39 in EPM test (n = 7, 12, 11 mice; for open arms, Treatment, F 2,27 = 3.650, P = 0.0395; CSDS – LV‐GFP versus Ctrl – LV‐GFP, P = 0.0183; for closed arms, Treatment, F 2,27 = 0.5164, P = 0.6024, one‐way ANOVA with Fisher's LSD test).
We then asked whether exogenous supplementation of CD39 could abolish the antidepressant actions of LV‐siCD39. As shown in Fig EV5A, LV‐siCD39 was infused into the hippocampus after CSDS, and 14 days later, recombinant mouse CD39 protein (CD39Fc) was injected into hippocampus. Firstly, we confirmed the efficiency of CD39Fc and found that it had about 50% overexpressed efficacy (Fig EV5B). The behavioral tests showed that CD39Fc treatment canceled the decreased social avoidance induced by knockdown of CD39 in the hippocampus (Fig EV5C and D). Consistent with the SI experiment, CD39Fc also recovered the reduced immobility time in the TST and FST (Fig EV5E and F) without influencing the locomotor activity in the OFT (Fig EV5G). Therefore, CD39 did mediate the depression‐like effects of mice.
Figure EV5. The effect of extracellular recombinant CD39 protein (CD39Fc) on depressive behaviors of stressed mice infusion with LV‐siCD39.
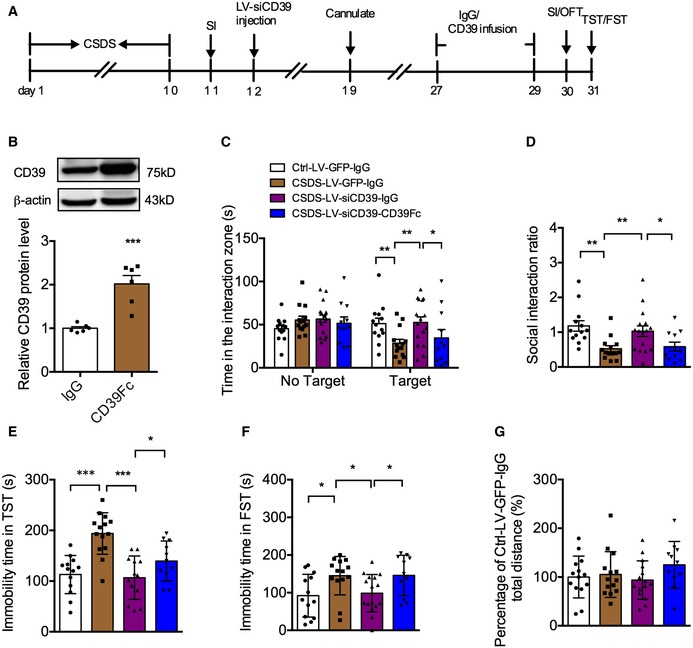
-
AExperimental timelines.
-
BExpression of CD39 in the hippocampus of mice injected with CD200Fc (n = 6 mice/group; CD39Fc versus IgG, P = 0.0004, Student's t‐test).
-
CTime in the interaction zone of mice infusion with CD39Fc after knockdown of CD39 in SI test (n = 13, 14, 16, 11 mice; Interaction, F 3,100 = 2.977, P = 0.0352; Target, F 1,100 = 6.175, P = 0.0146; Drug, F 3,100 = 2.028, P = 0.1148; for target, CSDS‐LV‐GFP‐IgG versus Ctrl‐LV‐GFP‐IgG, P = 0.0077; CSDS‐LV‐siCD39‐IgG versus CSDS‐LV‐GFP‐IgG, P = 0.0031; CSDS‐LV‐siCD39‐CD39Fc versus CSDS‐LV‐siCD39‐IgG, P = 0.0378, two‐way ANOVA with Tukey's post‐test).
-
DSocial interaction ratio of mice infusion with CD39Fc after knockdown of CD39 in SI test (n = 13, 14, 16, 11 mice; Treatment, F 3,50 = 5.690, P = 0.0020; CSDS‐LV‐GFP‐IgG versus Ctrl‐LV‐GFP‐IgG, P = 0.0012; CSDS‐LV‐siCD39‐IgG versus CSDS‐LV‐GFP‐IgG, P = 0075; CSDS‐LV‐siCD39‐CD39Fc versus CSDS‐LV‐siCD39‐IgG, P = 0.0263, one‐way ANOVA with Fisher's LSD test).
-
E, FInfusion with CD39Fc into hippocampus abolished the decreased immobility time of stressed mice injection with LV‐siCD39 in the TST (E) and FST (F) (for TST, n = 13, 14, 13, 11; Treatment, F 3,47 = 13.22, P < 0.0001; CSDS‐LV‐GFP‐IgG versus Ctrl‐LV‐GFP‐IgG, P < 0.0001; CSDS‐LV‐siCD39‐IgG versus CSDS‐LV‐GFP‐IgG, P < 0001; CSDS‐LV‐siCD39‐CD39Fc versus CSDS‐LV‐siCD39‐IgG, P = 0.05; for FST, n = 13, 14, 16, 11; Treatment, F 3,50 = 4.047, P = 0.0119; CSDS‐LV‐GFP‐IgG versus Ctrl‐LV‐GFP‐IgG, P = 0.0116; CSDS‐LV‐siCD39‐IgG versus CSDS‐LV‐GFP‐IgG, P = 0191; CSDS‐LV‐siCD39‐CD39Fc versus CSDS‐LV‐siCD39‐IgG, P = 0.0261, one‐way ANOVA with Fisher's LSD test).
-
GTotal distance of mice in the OFT (n = 14, 14, 15, 11 mice; Treatment, F 3,50 = 1.136, P = 0.3437, one‐way ANOVA with Fisher's LSD test).
To confirm that the antidepressant‐like effects of LV‐siCD39 were induced by reduced extracellular ATP hydrolysis, we measured hippocampal CD39 activity and extracellular ATP level after virus transfection. ATPase activity was significantly reduced (Fig EV4C), while the ATP level was correspondingly increased without statistical significance (Fig EV4D) in the hippocampus of normal mice with LV‐siCD39. However, LV‐siCD39 infusion significantly attenuated ATPase activity (Fig 4G) and increased extracellular ATP concentration (Fig 4H) in the hippocampus of susceptible mice. These results suggest that the antidepressant‐like effects of CD39 knockdown are mediated by elevating extracellular ATP level.
Inhibition and knockdown of CD39 promote hippocampal neurogenesis in susceptible mice
Earlier studies have demonstrated that the changes in hippocampal neurogenesis in the DG are correlated with depression‐like behavior 22, 23, 24, and the antidepressants increase the net magnitude of hippocampal neurogenesis 25, 26. To detect the hippocampal neurogenesis in depression, BrdU (100 mg/kg, i.p.) was administered to mice after 10‐day social defeat. Then, the mice were sacrificed for brain tissue collection 24 h later (Appendix Fig S2A). Dividing cells and immature neurons were identified with BrdU (blue) and DCX (green), respectively (Appendix Fig S2B). BrdU and DCX immunoreactive cells (BrdU+ DCX+ cells, cyan) were newborn immature neurons (Appendix Fig S2B). It was found that the numbers of BrdU+ and BrdU+ DCX+ cells were both significantly declined in DG of susceptible mice (Appendix Fig S2B–D). As a proliferative factor, ATP is required for astrocyte‐mediated proliferation of neural stem cells in the adult hippocampus 27. The subgranular zone (SGZ) of the hippocampus has high ectonucleotidase activity, with striking restriction to the region of dentate granule cell neurogenesis 28. We then asked whether CD39 inhibition could augment hippocampal neurogenesis. To address this issue, the mice were daily given ARL67156 or vehicle by intracerebroventricular (i.c.v.) injection for 3 days (Fig 5A). We found that the decreasing numbers of BrdU+ and BrdU+ DCX+ cells in DG of susceptible mice were completely blocked by infusion of ARL67156 (Fig 5B–D). Moreover, ARL67156 also increased the number of BrdU+ cells of normal mice (Appendix Fig S3A–C). To validate whether knockdown of CD39 could rescue neurogenesis in the DG region, we performed the experiment as shown in Fig 5E. Similarly, knockdown of CD39 also significantly upregulated the numbers of BrdU+ and BrdU+ DCX+ (purple) cells in the DG (Fig 5F–H). These results indicate that disruption of CD39 promotes DG neurogenesis of mice exposure to chronic stress.
Figure 5. Inhibition and knockdown of CD39 increase hippocampal neurogenesis in susceptible mice.
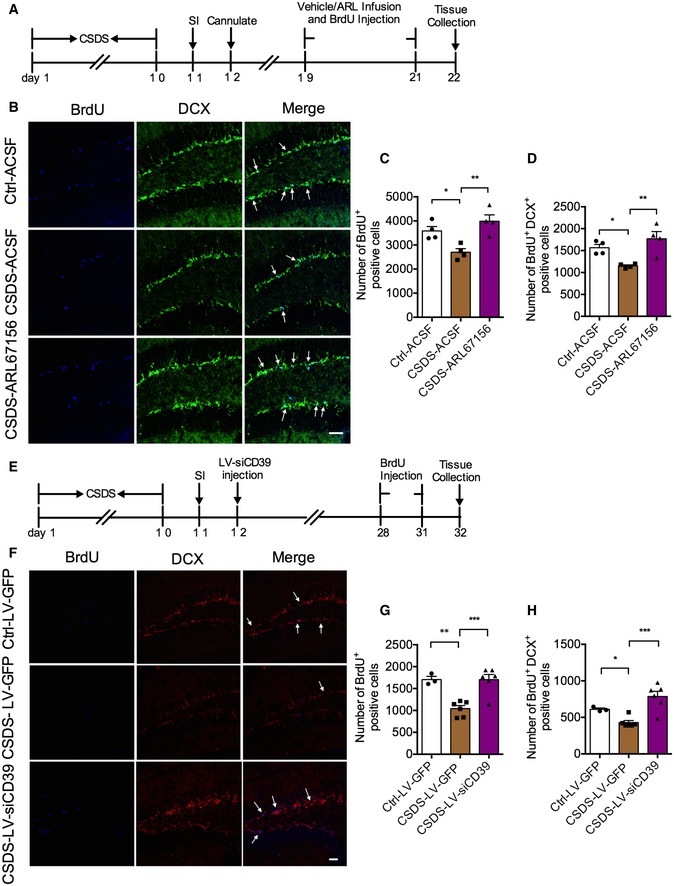
-
AExperimental timelines for CSDS, ARL67156 treatment, and immunostaining.
-
BImmunostaining for BrdU (blue) and DCX (green) in the DG region of control, susceptible, and ARL67156 treatment mice. Arrows indicate the BrdU+ DCX+ cells. Scale bar: 50 μm.
-
C, DQuantification of BrdU+ cells (C) and BrdU+ DCX+ cells (D) in the DG of susceptible mice with ARL67156 compared with vehicle treatment (n = 4 mice/group; for BrdU+ Treatment F 2,9 = 10.07, P = 0.0051; CSDS – ACSF versus Ctrl – ACSF, P = 0.0147; CSDS – ARL67156 versus CSDS – ACSF, P = 0.0018; BrdU+ DCX+ Treatment F 2,9 = 9.005, P = 0.0071; CSDS – ACSF versus Ctrl – ACSF, P = 0.0211; CSDS – ARL67156 versus CSDS – ACSF, P = 0.0024, one‐way ANOVA with Fisher's LSD test).
-
EExperimental timelines for neurogenesis examination with LV‐siCD39.
-
FConfocal images of BrdU (blue) and DCX (red) in mice treatment with LV‐siCD39 in the DG. Arrows indicate the BrdU+ DCX+ cells. Scale bar: 50 μm.
-
G, HQuantitative of BrdU+ (G) and BrdU+ DCX+ (H) cells in the DG of susceptible mice with LV‐siCD39 compared with LV‐GFP treatment (n = 3, 6, 6 mice; for BrdU+ Treatment F 2,12 = 15.79, P = 0.0004; CSDS – LV‐GFP versus Ctrl – LV‐GFP, P = 0.0012; CSDS – LV‐siCD39 versus CSDS – LV‐GFP, P = 0.0003; BrdU+ DCX+ Treatment F 2,12 = 12.79, P = 0.0011; CSDS – LV‐GFP versus Ctrl – LV‐GFP, P = 0.05; CSDS – LV‐siCD39 versus CSDS – LV‐GFP, P = 0.0003, one‐way ANOVA with Fisher's LSD test).
Inhibition and knockdown of CD39 in the hippocampus rescue CSDS‐induced loss in stubby spines
It has been reported that depression is associated with alterations in dendritic and synaptic structure and function 29, 30, and chronic stress leads to the reduction of dendritic complexity and spine density in DG, CA1, and CA3 31, 32. ATPase activity is also reported to be present in dendritic spines of cerebral cortex, which may be involved in the dynamics of cytoskeletal function, leading to the changes in dendritic spines and synapses 33. To understand the role of CD39 in CSDS‐induced dendritic remodeling of hippocampus, the dendritic segments in DG of mice injected with LV‐siCD39 or ARL67156 were observed by using automated three‐dimensional (3D) dendritic spine reconstruction (Fig 6A and F, 3D reconstruction in Appendix Fig S4A and C). It was found that LV‐siCD39 and ARL67156 had no effect on total spine numbers of normal mice (Appendix Fig S4B and D); however, they could ameliorate the decrease in the total spine density induced by chronic stress (Fig 6B and G). We then classified spines as stubby (immature) and thin (immature) or mushroom (mature) subtypes and found that only the density of stubby spines (white arrows) selectively decreased in the hippocampus of CSDS susceptible mice, which could be improved by knockdown or inhibition of CD39 (Fig 6C and H). The other two subtypes, mushroom (yellow arrows) or thin spines (blue arrows), had no changes (Fig 6D, E, I and J). These results indicate that CD39 disruption can prevent the reduction of stubby spines in DG elicited by chronic stress.
Figure 6. Inhibition and knockdown of CD39 in the hippocampus rescue stress‐induced stubby spine loss.

-
ARepresentative confocal z‐stack images of LV‐infected dendritic segments in the DG granule neurons from control and susceptible mice with different treatment. White arrows indicate stubby spines, yellow arrows indicate mushroom spines, and blue arrows indicate thin spines.
-
B–EQuantification of total (B), stubby (C), mushroom (D), and thin (E) spine density in DG from control and susceptible mice infected with LV‐GFP or LV‐siCD39 (n = 15 segments from 4 mice/group; for total density: Treatment F 2,42 = 3.12, P = 0.0544; Ctrl – LV‐GFP versus CSDS – LV‐siCD39, P = 0.0312; CSDS – LV‐siCD39 versus CSDS – LV‐GFP, P = 0.0425; stubby density: Treatment F 2,42 = 10.93, P = 0.0002; P = 0.0026; CSDS – LV‐CD39 versus CSDS – LV‐GFP, P < 0.0001; mushroom density: Treatment F 2,42 = 0.34, P = 0.7161; thin density: F 2,42 = 1.00, P = 0.3758, one‐way ANOVA with Fisher's LSD test).
-
FRepresentative confocal z‐stack images of LV‐infected dendritic segments in the DG granule neurons from control and susceptible mice with different treatment. White arrows indicate stubby spines, yellow arrows indicate mushroom spines, and blue arrows indicate thin spines.
-
G–JQuantification of total (G), stubby (H), mushroom (I), and thin (J) spine density in DG from control and susceptible mice treatment with ACSF or ARL67156 (n = 11, 13, 10 segments from 2, 4, 2 mice, respectively; for total density: Treatment F 2,31 = 4.474, P = 0.0196; CSDS – ASCF versus Ctrl – ACSF, P = 0.0366; CSDS – ARL67156 versus CSDS – ACSF, P = 0.0086; stubby density: Treatment F 2,31 = 3.327, P = 0.0491; CSDS – ASCF versus Ctrl – ACSF, P = 0.1026; CSDS – ARL67156 versus CSDS – ACSF, P = 0.0179; mushroom density: Treatment F 2,31 = 1.986, P = 0.1543; thin density: F 2,31 = 1.475, P = 0.2445, one‐way ANOVA with Fisher's LSD test).
Discussion
In the present study, we demonstrated that the elevated CD39 in the hippocampus mediated the depression‐like behavior of mice. Following CSDS, there was a significant increase in the expression and activity of CD39 in the hippocampus. Administration of a CD39 functional analog apyrase induced depression‐like behaviors, which could be improved by extracellular ATP infusion, whereas pharmacological inhibition and genetic silencing of CD39 produced an antidepressant‐like effect via increasing hippocampal extracellular ATP concentration. In addition, the impaired hippocampal neurogenesis and spine density in defeated mice were rescued by the inhibition or knockdown of CD39. Taken together, our results suggest that elevated CD39 results in excessive ATP hydrolysis, neurogenesis impairment, and spine loss and thus induced depression‐like behaviors.
Although our results confirmed the mediation of CD39 in depression‐like behaviors, anhedonia (decreased sucrose preference) in susceptible mice was only reversed by infusion of CD39 inhibitor in the right cerebral ventricle, but not in the hippocampus, indicating the possible participation of other brain regions in response to CD39 inhibitor. It took more than 3 weeks to achieve the effects of genetic deletion of CD39 with LV‐siRNA; however, the anhedonia cannot persist for 4 weeks in CSDS mice 20; and thus, it is difficult to evaluate the effect of LV‐siCD39 on anhedonia.
Previous study has shown that the dysfunction of ATP release is implicated in MDD pathophysiology, and ATP level specifically decreases in the PFC from mice subjected to 1‐day social defeat, while it decreases in both PFC and hippocampus of the mice that are susceptible to 10‐day social defeat 6. In the present study, we found that CD39 expression and activity increased in the hippocampus, but not in the mPFC from mice subjected to CSDS. Meanwhile, others found deficiencies in astrocytic ATP release from mPFC and hippocampus causing depressive‐like behaviors 6. The divergent results between the mPFC and hippocampus may be due to the different brain structure and cellular component, and the changes of ATP induced by stress have brain region specificity. Since CD39 is a major enzyme involved in the extracellular metabolism of ATP, we proposed that the depression‐like behaviors induced by activation of CD39 were resulted from the excessive hydrolysis of ATP with a consequent reduction of ATP in hippocampus.
Here, knockdown of CD39 in the hippocampus was only found to influence ATPase enzyme activity, but not ATP level in normal mice. The reason may be due to the self‐protection of ATP release, and increasing extracellular ATP level will induce the inflammatory response to restrict the excessive rise. We also noticed that CD39 analog apyrase in 80 U/ml induced depression‐like behaviors, while the higher concentration was not effective. This might be due to the two determinants of extracellular ATP level, ATPase hydrolyzing and direct cellular releasing 34. When there is a much higher concentration Apyrase to hydrolyze ATP, it may trigger cell releasing to compensate for the ATP loss 15. And then, the releasing ATP prevented the depression‐like behaviors. The self‐protective mechanism was broken after stress, we found that genetic deletion of CD39 restored the declined level of ATP in the hippocampus of mice subjected to 10‐d social defeat stress, and ATP infusion could reverse social avoidance induced by apyrase exposure. All of these suggest that the increase in CD39 quantity is a reason of the low ATP concentration in the hippocampus and is the primary effect of chronic stress. However, it has been reported that stimulating endogenous ATP release from astrocytes induced antidepressant‐like effects in mouse models of depression 6, and we could not exclude the role of a lower ATP release in chronic stress. Thus, all above results suggest that ATP hydrolysis is involved in the CD39‐induced depression, and ATP supplementation could partially alleviate depression‐like behaviors.
MDD is associated with the decline of hippocampal volume in humans 35, 36, 37 and reduction of hippocampal neurogenesis in animal models 38, 39. It has been reported that hippocampal neurogenesis is decreased by stress and increased by pharmacologic antidepressants as well as non‐pharmacological treatments such as exercise and deep brain stimulation 40, 41, which raises the possibility that incremental hippocampal neurogenesis may be a potential new strategy for treating depression. ENTPD‐mediated dephosphorylation of ATP produces AMP that is a weak agonist for P2Y receptors 28. Ectonucleotidase expressing in the SGZ of the hippocampus may serve as a brake on the proliferation of NSCs. Here, we observed that chronic stress down‐regulated hippocampal neurogenesis and CD39 disruption could alleviate this loss in susceptible mice. Considering that CD39 activity was enhanced in the hippocampus of CSDS mice, CD39‐mediated depressive‐like behaviors are likely due to excessive ATP hydrolysis, which caused deficiency of ATP and inhibit hippocampal neurogenesis.
ATP has been shown to prevent Aβ42‐mediated dendritic spine loss in primary cultured hippocampal neurons 42. In addition, 17β‐estradiol‐induced synaptic rearrangements in the hippocampus of male rat are accompanied by down‐regulation of CD39 and ENTPD2 and attenuation of adenine nucleotide hydrolysis 43. Consistent with both in vitro and in vivo evidence, our results also suggest that CD39 plays a key role in chronic stress‐elicited dendritic spine loss in the hippocampus, and inhibition and knockdown of CD39 could rescue the deficit of dendritic spines. It has been reported that chronic stress induces a loss of spine in the hippocampus 44, 45; however, the subtypes of deficit dendritic spine remain largely unknown. Here, we found that chronic stress generated a selective decrease in the density of stubby spines, but not mushroom or thin spines. Consistently, decreasing of stubby spines is also detected in the mPFC of patients with post‐traumatic stress disorder 46 and in the hippocampus of animals induced by social defeat stress 47.With regard to dendritic spines, increased synaptogenesis and functional synaptic plasticity are correlated with the size and shape of a dendritic spine 48. It is well known that stubby and thin spines (immature) may exhibit a higher turnover rate, whereas mushroom spines (mature) represent a more stable population 49, 50. Loss of stubby spines was the predominant driver of pruning during normal adolescent development 51. Stubby spines have greater actin motility than mushroom spines, thus contributing to enhanced plasticity during development 52. It has been reported decreased ATP caused dendritic spine loss in hippocampal neurons and supplement of ATP improved the neural plasticity and depression‐like behaviors of stressed mice 7. Meanwhile, stubby spines are thought to play an important role in coordinated and widespread Ca2+ transients in the parent dendrite, as well as coordinate Ca2+ signaling among adjacent spines 53, 54. Thus, the decrease in stubby spine density may lead to a deficit of synaptic information transfer, which may be associated with the depression‐like behaviors. Altogether, antidepressant actions of CD39 disruption may be related to enhancing synaptic transmission and neural plasticity. Our result that CD39 impairment made the stubby spine density increment in DG of susceptible mice was consistent with the fact that BDNF and antidepressant administration specifically upregulated the stubby spines of CA1 pyramidal neurons 55, 56.
In summary, our study demonstrated that the elevated CD39 in the hippocampus aggravated depressive‐like behavior of mice induced by chronic stress. The effect of CD39 on depression might be through two mechanisms: (i) ATP‐induced modulation of neural progenitor cell proliferation and (ii) ATP‐induced modulation of spine density on the pyramidal cells and molecular cells. These results highlight aberrant CD39 function as a potential molecular mechanism underlying MDD pathophysiology and suggest that CD39 may be a promising new target for the treatment of depression.
Materials and Methods
Animals
Eight‐week‐old C57BL/6J mice (Beijing Vital River Laboratory Animal Technology Co., Ltd. Beijing, China) were used for all experiments. For chronic social defeat stress (CSDS), 8‐month‐old retired CD‐1 breeders (Beijing Vital River Laboratory Animal Technology Co., Ltd. Beijing, China) were used as aggressors. One week before the start of all experiments, mice were group‐housed and maintained on a 12‐h light/dark cycle with ad libitum access to food and water. Behavioral assessments and tissue collection were performed from 9:00 AM to 6:00 PM concurrent with stated housing conditions. The research was conducted in accordance with the Guide for Care and Use of Laboratory Animals that adopted and promulgated by the National Institutes of Health. All experimental protocols were approved by the animal Welfare Committee of Huazhong University of Science and Technology.
Chronic social defeat stress
The CSDS protocol was described previously 57 and shown in Fig 1A. A single C57BL/6J intruder mouse was exposed to a different CD‐1 aggressor mouse (target mouse) for 5–10 min each day for a total of 10 days. Following 5–10 min of contact, the intruder mouse and the aggressor were separated by a perforated plexiglass divider. The divider was placed in the middle of the cage, and the intruder mouse was exposed to chronic stress in the form of a threat for the remainder of the 24‐h period. The control C57BL/6J mice were pair‐housed in equivalent cages with one mouse per side of the perforated divider, and they were rotated on a daily basis similar to the defeated mice.
Behavioral procedures
All behavioral tests were performed in adult C57BL/6J male mice. Before testing, mice were transferred to the testing room and adapted to the room conditions for at least 1 h. After every test session, the testing apparatus was cleaned with 75% alcohol to eliminate the odor of the previous mouse.
Social interaction (SI). SI test was carried out on day 11, in which the mice were placed in a novel area with a small animal cage at one end. Their movements were tracked for 2.5 min in the absence of the aggressor, followed by an additional 2.5 min in the presence of the caged aggressor. The duration of time that the mice spent in the interaction zone, as well as other measures, was automatically recorded by AniLab software (AniLab Software & Instruments, Ningbo, China). The apparatus was cleaned with ethanol to remove olfactory cues following each trial, and all of the behavioral tests were conducted under red‐light conditions in a room isolated from external sound sources. According to the time spent in the interaction zone, an interaction ratio of 100 (100 × time spent in the interaction zone with the presence of a target versus the absence of a target) was set as a cutoff: the mice with scores < 100 were considered susceptible and those with scores ≥ 100 were considered resilient.
Sucrose preference test (SPT). SPT was performed on days 11–12 using a two‐bottle choice procedure. Mice were habituated to the presence of two drinking bottles (50 ml, one containing 1% sucrose and the other water) for 2 days before 1 additional day of choice testing, and the position of the bottles was switched every 12 h to avoid a side bias. On the test day, consumption from the two bottles was measured for 12 h in the dark. The preference for sucrose over water was calculated as 100 × (sucrose/(water + sucrose)).
Tail suspension test (TST). TST was performed to evaluate despair or depression‐like behavior. Mice were suspended by the tail to the floor 17 cm with tape affixed 1.0 cm from the tip of the tail. ANY‐maze software recorded the immobility time of mice, and the duration of immobility within 6 min was quantified.
Forced swim test (FST). FST was also performed to evaluate despair or depression‐like behavior. In brief, mice were placed into a cylinder (25 cm height) filled with 20 cm water (25 ± 1°C). Mice were forced to swim for 6 min, and the duration time of immobility within the last 4 min of test period was examined using ANY‐maze software.
Open field test (OFT). OFT was used to examine the locomotor activity and anxiety behaviors. Mice were tested for 5 min at a plastic box (45 × 45 × 45 cm), which was divided into a 35 × 35 cm central area and surrounding area. The total distance in the box and distance travelled in the central area were recorded using ANY‐maze software.
Elevated plus maze (EPM). EPM was measured to assess anxiety behavior. The apparatus consisted of two opposite open arms and two opposite closed arms. The arms were 30 cm long and 5 cm wide, and connected with a 5 × 5 cm central platform. The closed arms were surrounded with 15 cm height black plastic. Mice were placed into the center of the apparatus and were allowed to explore for 5 min. The time spent in open arms and closed arms was recorded using ANY‐maze software.
Western blotting analysis
Western blots were performed according to a protocol that is routinely used in our laboratory 58. Briefly, mice were decapitated and the brain was quickly removed. Coronal brain slices (350 μm thick) containing the hippocampus or medial prefrontal cortex (mPFC) were cut with a vibratome (VT 1000S; Leica, Wetzlar, Germany) and then dissected. The tissues were washed twice with ice‐cold PBS and then lysed on ice in extraction buffer containing 50 mM Tris‐base (pH 7.4), 100 mM NaCl, 1% NP‐40, 10 mM EDTA, 20 mM NaF, 1 mM PMSF, 3 mM Na3VO4, and protease inhibitors. Protein samples were separated by 10% SDS‐polyacrylamide gel and then transferred to nitrocellulose membranes (millipore, Bedford, MA, USA). After blocking with 5% BSA in Tris‐buffered saline containing 0.1% Tween‐20 (TBST) for 1 h at room temperature, transferred membranes were incubated overnight at 4°C with different primary antibodies against β‐actin (1:3,000 dilution; Santa Cruz Biotechology, California, USA) and CD39 (1:12,000 dilution; Abcam, Cambridge, MA, USA). After horseradish peroxidase‐conjugated secondary antibodies incubation, bands were visualized using a Microchemi (DNR Bio‐Imaging Systems, Jerusalem, Israel).
Real‐time quantitative PCR (qPCR)
The samples were stored at −80°C until use. Total RNA was isolated from tissues using TriZol reagent (Invitrogen, Thermo Fisher Scientific Inc, Waltham, MA, USA) and was quantified spectrophotometrically. A volume of 500 ng of RNA was reverse transcribed to cDNA with RevertAid First Strand cDNA Synthesis Kit (Fermentas, Thermo Fisher Scientific Inc, Waltham, MA, USA). For qPCR, cDNA was diluted to 200 and 2 μl was used for each reaction. The reaction mixture consisted of Applied Biosystems SYBR Green (5 μl), Rox dye (0.2 μl), forward and reverse primers (0.2 μl each), water (2.4 μl), and cDNA template. Samples were then heated to 95°C for 10 s followed by 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. Analysis of gene expression was performed using the ΔΔCt method, and all samples were normalized to GAPDH. All primers are listed in Table EV1.
Drugs
Apyrase was purchased from Sigma‐Aldrich (St Louis, MO, USA) and dissolved in artificial cerebrospinal fluid (ACSF: 119 mM NaCl, 1.3 mM MgSO4, 3.5 mM KCl, 11 mM glucose, 26.2 mM NaHCO3, 1 mM NaH2PO4, 2.5 mM CaCl2, pH = 7.4) at 40, 80, or 160 U/ml. ARL67156 was purchased from Tocris Bioscience (Bristol, UK) and dissolved in ACSF at 100 μM. ATP was purchased from Sigma‐Aldrich and dissolved in ACSF at 25 μM. IgG protein was purchased from Abcam (Cambridge, USA). Recombinant Mouse CD39 protein was purchased from R&D systems (Minnesota, USA). The drugs were given by intracerebroventricular (i.c.v.) injection or intra‐hippocampal infusion with a volume of 4 or 2 μl per side. Bromodeoxyuridine (BrdU; Sigma, St Louis, MO, USA) was dissolved in PBS at 10 mg/ml and intraperitoneal injection at a dose of 100 mg/kg for 1 or 3 days. Twenty‐four hours after the last BrdU injection, the mice were perfused with 0.9% saline followed by 4% paraformaldehyde as described below.
Immunofluorescence
Mice were finally anaesthetized by intraperitoneal injection (i.p.) of sodium pentobarbital (40 mg/kg) and underwent intracardial perfusion with 0.9% saline followed by 4% paraformaldehyde. Brains were post‐fixed in paraformaldehyde (4%) for 24 h at 4°C and then cryoprotected in 30% sucrose. Frozen coronal sections (30 μm thick) containing the entire hippocampus were cut with a freezing microtome (CM1900, Leica Microsystems, Wetzlar, Germany).
Free floating sections were washed with PBS followed by permeabilization with 1% Triton X‐100 and 0.5% Tween‐20 in PBS for 10 min. For BrdU detection, sections were treated with 1 N HCl for 10 min and then 2 N HCl for 10 min at room temperature and additional 20 min at 37°C. Afterward, they were rinsed in 0.1 M boric acid (pH 8.5) for 10 min at room temperature. To reduce nonspecific binding, sections were incubated for 1 h with 5% BSA in TBS containing 0.3% Triton X‐100. Slices were incubated for 24–48 h at 4°C with primary antibodies as follows: 1:400 rabbit anti‐DCX (Cell Signaling Technologies, Denvars, MA, USA), 1:50 rabbit anti‐CD39 (Santa Cruz Biotechology, California, CA, USA), and 1:1,000 rat anti‐BrdU (abcam, Cambridge, MA, USA). Detection was with Alexa dye‐conjugated antibodies (1:600, Invitrogen, Thermo Fisher Scientific Inc, Waltham, MA, USA) or DyLight dye‐conjugated antibody (1:200, JacksonImmuno Research, West Grove, Pennsylvania, USA). Nuclei were visualized with DAPI (1:5,000; Biosharp, China). Images were acquired with a confocal microscope (FV1000, Olympus, Tokyo, Japan).
Quantification of BrdU and DCX immunoreactive cells
For counting the BrdU and DCX immunoreactive cells, the granular and subgranular layer of the dentate gyrus (DG) in every tenth section was examined, from which the total number of positive cells in both hemispheres was calculated. The total number of BrdU+ and BrdU+ DCX+ cells in the six sections was determined and multiplied by ten to obtain the total number of cells per DG.
Imaging and analysis of dendrite segments
For analysis of dendritic spine, dendritic segments (about 50–150 μm away from the soma) were randomly chosen from lentivirus (LV)‐infected cells that expressed green fluorescent protein (GFP) in DG. Dendritic segments were imaged using a 100 × lens with a zoom of 2.5 and were z‐sectioned at 0.39 μm increments. The number of dendritic spines and length were counted with three‐dimensional (3D) stacks using the measurement tool of the Imaris software (Bitplane, Zürich, Switzerland). The number of spines was normalized per micrometer of dendritic length. Imaris classified spines as thin, mushroom, or stubby based on the relationship between their length, minimum (min) diameter, and maximum (max) diameter as following parameters 59: stubby, in which the length was < 1 μm and the maxhead/minneck ratio was < 1.5; thin, in which the maxhead/minneck ratio was < 1.5 and the length ≥ 1 μm; and mushroom, in which the maxhead/minneck ratio was ≥ 1.5 (independent of their length).
Measurement of ATPase activity
ATPase activity was assayed with a Malachite Green Phosphate Assay Kit (BioAssay Systems, Hayward, CA, USA) as previously described 60. Briefly, two slices (350 μm) of hippocampus or mPFC per tube were preincubated for 10 min at 37°C with 200 μl incubation medium (115 mM NaCl, 3.0 mM KCl, 1.2 mM MgSO4, 25 mM NaHCO3, 10 mM glucose, 2.0 mM CaCl2, pH 7.4) and gassed directly with 95% O2 and 5% CO2. The reaction was started by adding ATP with a final concentration of 1.0 mM and incubated for 10 min at 37°C. Then, a 180 μl aliquot of the supernatant was collected and 20 μl of 10% trichloroacetic was added to stop the reaction. Non‐enzyme Pi released from nucleotides into assay medium without slices and Pi released from slices without nucleotide were subtracted from total Pi released during incubation. All assays were performed in triplicate. The enzyme activity was expressed as nmol of Pi released per minute per milligram of protein.
Extracellular ATP measurement
The concentration of extracellular ATP was quantified with a bioluminescent ATP assay kit (Promega, Leiden, The Netherlands) as described previously with some modifications 6. Luminescence was measured using a luminometer (Berthold Lumat LB 9507, Germany). A calibration curve was obtained from standard ATP samples, and the luminescence of normal culture medium was considered to be the background ATP level. For measurement of ATP release in acute isolated hippocampal slices, slices were incubated in a 600 μl oxygenized ACSF for 12 min. The ACSF was then collected, and ATP was assayed. For quantification, protein amounts were normalized to the total protein of each sample.
Stereotaxic microinjection
The mice were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and then placed on a stereotaxic apparatus. A stainless steel guide cannula was unilaterally implanted into the right cerebral ventricle (AP = −0.2 mm, ML = 0.8 mm, DV = 3.0 mm). Two brain cannula were bilaterally implanted into the hippocampus (AP = −2.2 mm, ML = ± 2.1 mm, DV = 1.8 mm). Seven days after implantation, vehicle, apyrase (40, 80, or 160 U/ml), ARL67156 (100 μM), boiled‐apyrase (40 U/ml), ATP (25 μM), IgG, or recombinant CD39 protein (2 nM) were microinjected into the right cerebral ventricle or hippocampus for 3 days. All compounds were dissolved in ACSF.
Stereotaxic virus injection
Lentiviral vector harboring siRNA sequence (GAACTGATACCAACATCCA) targeting the CD39 gene and expressing GFP (LV‐siCD39) was constructed by GenePharma Co. (Shanghai, China). The control lentiviral vector expressing GFP (LV‐GFP) was designed with a scramble sequence (TTCTCCGAACGTGTCACGT). For in vivo viral injection, viral vectors bilaterally targeted the hippocampus. Specifically, a Hamilton syringe fitted with glass capillaries with tip resistance of 5–10 MΩ was filled with 5 μl of virus. The needle was lowered into the hippocampus, and 2 μl of virus was delivered over 10 min. The injection needle was withdrawn 10 min after infusion. Behavioral testing was commenced 3 weeks after viral injection; then, mice were perfused transcardially; and serial brain sections were made. Effects of transfection in vivo could be observed by confocal microscopy and Western blotting.
Statistical analysis
Data are expressed as mean ± SEM and analyzed by employing the GraphPad 6.0 software (Graphpad, San Diego, CA, USA). The unpaired Student's t‐tests were used to compare differences between any given two groups throughout the study, unless otherwise specified. One‐way ANOVA followed by Fisher's LSD test was used in different treatment groups where appropriate. Significance of multiple comparisons involving more than one variable was tested using two‐way ANOVA followed by Tukey's post hoc test. A probability level of P < 0.05 was considered as statistically significant.
Author contributions
J‐GC, FW, and Z‐LH designed the experiment and revised the manuscript. Q‐QC and Y‐LH performed the experiment, wrote the manuscript and analyzed the data together. X‐JL and LM made CSDS models. XC and JW reconstructed 3D dendritic spines and analyzed the number of spines. MN implanted the cannula into the hippocampus.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Table EV1
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Acknowledgements
This work was supported by grants from the Foundation for Innovative Research Groups of NSFC (No. 81721005 to J.G.C. and F. W.), National Natural Science Foundation of China (No. 81473199 to Z.L.H, No. 81671438 to F.W., No. 81673414 to J.G.C.), PCSIRT (No. IRT13016) to J.G.C., the Program for HUST Academic Frontier Youth Team (2017QYTD17), and Integrated Innovative Team for Major Human Diseases Program of Tongji Medical College, HUST, to F. W.
EMBO Reports (2020) 21: e47857
See also: https://doi/org/10.15252/embr.201949921 (April 2020)
Contributor Information
Jian‐Guo Chen, Email: chenj@mails.tjmu.edu.cn.
Fang Wang, Email: wangfangtj0322@163.com.
References
- 1. Trivedi M, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ et al (2006) Evaluation of outcomes with citalopram for depression using measurement‐based care in STAR*D: implications for clinical practice. Am J Psychiatry 163: 28–40 [DOI] [PubMed] [Google Scholar]
- 2. Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, Ma S, Sang K, Tang S, Li Y et al (2018) Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554: 323–327 [DOI] [PubMed] [Google Scholar]
- 3. Nie X, Kitaoka S, Tanaka K, Segi‐Nishida E, Imoto Y, Ogawa A, Nakano F, Tomohiro A, Nakayama K, Taniguchi M et al (2018) The innate immune receptors TLR2/4 mediate repeated social defeat stress‐induced social avoidance through prefrontal microglial activation. Neuron 99: 464–479 [DOI] [PubMed] [Google Scholar]
- 4. Culig L, Surget A, Bourdey M, Khemissi W, Le Guisquet AM, Vogel E, Sahay A, Hen R, Belzung C (2017) Increasing adult hippocampal neurogenesis in mice after exposure to unpredictable chronic mild stress may counteract some of the effects of stress. Neuropharmacology 126: 179–189 [DOI] [PubMed] [Google Scholar]
- 5. Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Soltanzadeh Zarandi S, Sood A, Paddy MR et al (2018) Psychedelics promote structural and functional neural plasticity. Cell Rep 23: 3170–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC et al (2013) Astrocyte‐derived ATP modulates depressive‐like behaviors. Nat Med 19: 773–777 [DOI] [PubMed] [Google Scholar]
- 7. Jun M, Xiaolong Q, Chaojuan Y, Ruiyuan P, Shukun W, Junbing W, Li H, Hong C, Jinbo C, Rong W et al (2018) Calhm2 governs astrocytic ATP releasing in the development of depression‐like behaviors. Mol Psychiatry 23: 883–891 [DOI] [PubMed] [Google Scholar]
- 8. Beamer E, Goloncser F, Horvath G, Beko K, Otrokocsi L, Kovanyi B, Sperlagh B (2016) Purinergic mechanisms in neuroinflammation: an update from molecules to behavior. Neuropharmacology 104: 94–104 [DOI] [PubMed] [Google Scholar]
- 9. Zimmermann H (2006) Nucleotide signaling in nervous system development. Eur J Physiol 452: 573–588 [DOI] [PubMed] [Google Scholar]
- 10. Burnstock G, Krugel U, Abbracchio MP, Illes P (2011) Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol 95: 229–274 [DOI] [PubMed] [Google Scholar]
- 11. Junger WG (2011) Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11: 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia‐Esparcia P, Hernández‐Ortega K, Ansoleaga B, Carmona M, Ferrer I (2015) Purine metabolism gene deregulation in Parkinson's disease. Neuropathol Appl Neurobiol 41: 926–940 [DOI] [PubMed] [Google Scholar]
- 13. Robson SC, Sévigny J, Zimmermann H (2006) The E‐NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2: 409–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takenaka MC, Robson S, Quintana FJ (2016) Regulation of the T cell response by CD39. Trends Immunol 37: 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kishore BK, Robson SC, Dwyer KM (2018) CD39‐adenosinergic axis in renal pathophysiology and therapeutics. Purinergic Signal 14: 109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanthi Y, Hyman MC, Liao H, Baek AE, Visovatti SH, Sutton NR, Goonewardena SN, Neral MK, Jo H, Pinsky DJ (2015) Flow‐dependent expression of ectonucleotide tri(di)phosphohydrolase‐1 and suppression of atherosclerosis. J Clin Invest 125: 3027–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, Goodall CP, Blair TC, Fox BA, McDermott JE et al (2018) Co‐expression of CD39 and CD103 identifies tumor‐reactive CD8 T cells in human solid tumors. Nat Commun 9: 2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schetinger MRMV, Bonan CD, Wyse AT (2007) NTPDase and 5′‐nucleotidase activities in physiological and disease conditions: new perspectives for human health. BioFactors 31: 77–98 [DOI] [PubMed] [Google Scholar]
- 19. Matyash M, Zabiegalov O, Wendt S, Matyash V, Kettenmann H (2017) The adenosine generating enzymes CD39/CD73 control microglial processes ramification in the mouse brain. PLoS ONE 12: e0175012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC et al (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131: 391–404 [DOI] [PubMed] [Google Scholar]
- 21. Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Sévigny J (2007) Specificity of the ecto‐ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br J Pharmacol 152: 141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R (2018) Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559: 98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eisch AJPD (2012) Depression and hippocampal neurogenesis: a road to remission? Science 338: 72–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M (2011) In animal models, psychosocial stress‐induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry 35: 744–759 [DOI] [PubMed] [Google Scholar]
- 25. Brooker SM, Gobeske KT, Chen J, Peng CY, Kessler JA (2017) Hippocampal bone morphogenetic protein signaling mediates behavioral effects of antidepressant treatment. Mol Psychiatry 22: 910–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Bokhoven P, Oomen CA, Hoogendijk WJG, Smit AB, Lucassen PJ, Spijker S (2011) Reduction in hippocampal neurogenesis after social defeat is long‐lasting and responsive to late antidepressant treatment. Eur J Neurosci 33: 1833–1840 [DOI] [PubMed] [Google Scholar]
- 27. Cao X, Li LP, Qin XH, Li SJ, Zhang M, Wang Q, Hu HH, Fang YY, Gao YB, Li XW et al (2013) Astrocytic adenosine 5′‐triphosphate release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells 31: 1633–1643 [DOI] [PubMed] [Google Scholar]
- 28. Lin JH, Takano T, Arcuino G, Wang X, Hu F, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M (2007) Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev Biol 302: 356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duman RSAG (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338: 68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Licznerski P, Duman RS (2013) Remodeling of axo‐spinous synapses in the pathophysiology and treatment of depression. Neuroscience 251: 33–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maras PM, Molet J, Chen Y, Rice C, Ji SG, Solodkin A, Baram TZ (2014) Preferential loss of dorsal‐hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Mol Psychiatry 19: 811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abe‐Higuchi N, Uchida S, Yamagata H, Higuchi F, Hobara T, Hara K, Kobayashi A, Watanabe Y (2016) Hippocampal sirtuin 1 signaling mediates depression‐like behavior. Biol Psychiatry 80: 815–826 [DOI] [PubMed] [Google Scholar]
- 33. Cohen RSKV (1991) Localization of ATPase activity in dendritic spines of the cerebral cortex. J Neurocytol 20: 703–715 [DOI] [PubMed] [Google Scholar]
- 34. Buckley KA, Golding SL, Rice JM, Dillon JP, Gallagher JA (2003) Release and interconversion of P2 receptor agonists by human osteoblast‐like cells. FASEB J 17: 1401–1410 [DOI] [PubMed] [Google Scholar]
- 35. Schmaal L, Veltman DJ, van Erp TGM, Sämann PG, Frodl T, Jahanshad N, Loehrer E, Tiemeier H, Hofman A, Niessen WJ et al (2016) Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 21: 806–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campbell SMM, Nahmias C, MacQueen GM (2004) Lower hippocampal volume in patients suffering from depression: a meta‐analysis. Am J Psychiatry 161: 598–607 [DOI] [PubMed] [Google Scholar]
- 37. Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J (2013) Hippocampal granule neuron number and dentate gyrus volume in antidepressant‐treated and untreated major depression. Neuropsychopharmacology 38: 1068–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siopi E, Denizet M, Gabellec MM, de Chaumont F, Olivo‐Marin JC, Guilloux JP, Lledo PM, Lazarini F (2016) Anxiety‐ and depression‐like states lead to pronounced olfactory deficits and impaired adult neurogenesis in mice. J Neurosci 36: 518–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hill AS, Sahay A, Hen R (2015) Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression‐like behaviors. Neuropsychopharmacology 40: 2368–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kondo M, Nakamura Y, Ishida Y, Shimada S (2015) The 5‐HT3 receptor is essential for exercise‐induced hippocampal neurogenesis and antidepressant effects. Mol Psychiatry 20: 1428–1437 [DOI] [PubMed] [Google Scholar]
- 41. Schmuckermair C, Gaburro S, Sah A, Landgraf R, Sartori SB, Singewald N (2013) Behavioral and neurobiological effects of deep brain stimulation in a mouse model of high anxiety‐ and depression‐like behavior. Neuropsychopharmacology 38: 1234–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jung ES, An K, Hong HS, Kim JH, Mook‐Jung I (2012) Astrocyte‐originated ATP protects Abeta(1‐42)‐induced impairment of synaptic plasticity. J Neurosci 32: 3081–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitrovic N, Zaric M, Drakulic D, Martinovic J, Sevigny J, Stanojlovic M, Nedeljkovic N, Grkovic I (2017) 17beta‐estradiol‐induced synaptic rearrangements are accompanied by altered ectonucleotidase activities in male rat hippocampal synaptosomes. J Mol Neurosci 61: 412–422 [DOI] [PubMed] [Google Scholar]
- 44. Bai YY, Ruan CS, Yang CR, Li JY, Kang ZL, Zhou L, Liu D, Zeng YQ, Wang TH, Tian CF et al (2016) ProBDNF signaling regulates depression‐like behaviors in rodents under chronic stress. Neuropsychopharmacology 41: 2882–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pittenger C, Duman RS (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33: 88–109 [DOI] [PubMed] [Google Scholar]
- 46. Young KA, Thompson PM, Cruz DA, Williamson DE, Selemon LD (2015) BA11 FKBP5 expression levels correlate with dendritic spine density in postmortem PTSD and controls. Neurobiol Stress 2: 67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iniguez SD, Aubry A, Riggs LM, Alipio JB, Zanca RM, Flores‐Ramirez FJ, Hernandez MA, Nieto SJ, Musheyev D, Serrano PA (2016) Social defeat stress induces depression‐like behavior and alters spine morphology in the hippocampus of adolescent male C57BL/6 mice. Neurobiol Stress 5: 54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL et al (2013) Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med 19: 337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holtmaat A, Svoboda K (2009) Experience‐dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10: 647–658 [DOI] [PubMed] [Google Scholar]
- 50. Yoshihara Y, De Roo M, Muller D (2009) Dendritic spine formation and stabilization. Curr Opin Neurobiol 19: 146–153 [DOI] [PubMed] [Google Scholar]
- 51. Miller ML, Chadwick B, Dickstein DL, Purushothaman I, Egervari G, Rahman T, Tessereau C, Hof PR, Roussos P, Shen L et al (2018) Adolescent exposure to Δ9‐tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons. Mol Psychiatry 24: 588–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bourne J, Harris KM (2007) Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17: 381–386 [DOI] [PubMed] [Google Scholar]
- 53. Nimchinsky EA, Sabatini BL, Svoboda K (2003) Structure and function of dendritic spines. Annu Rev Physiol 64: 313–353 [DOI] [PubMed] [Google Scholar]
- 54. Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H (2003) Structure–stability–function relationships of dendritic spines. Trends Neurosci 26: 360–368 [DOI] [PubMed] [Google Scholar]
- 55. Tyler WJ, Pozzomiller L (2003) Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol 553: 497–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Marchetti C, Tafi E, Middei S, Rubinacci MA, Restivo L, Ammassariteule M, Marie H (2010) Synaptic adaptations of CA1 pyramidal neurons induced by a highly effective combinational antidepressant therapy. Biol Psychiat 67: 146–154 [DOI] [PubMed] [Google Scholar]
- 57. Li MX, Zheng HL, Luo Y, He JG, Wang W, Han J, Zhang L, Wang X, Ni L, Zhou HY et al (2018) Gene deficiency and pharmacological inhibition of caspase‐1 confers resilience to chronic social defeat stress via regulating the stability of surface AMPARs. Mol Psychiatry 23: 556–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li Y‐K, Wang F, Wang W, Luo Y, Wu P‐F, Xiao J‐L, Hu Z‐L, Jin Y, Hu G, Chen J‐G (2012) Aquaporin‐4 deficiency impairs synaptic plasticity and associative fear memory in the lateral amygdala: involvement of downregulation of glutamate transporter‐1 expression. Neuropsychopharmacology 37: 1867–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M (2005) The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci 25: 9989–9999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bruno AN, Bonan CD, Wofchuk ST, Sarkis JJ, Battastini AM (2002) ATP diphosphohydrolase (NTPDase 1) in rat hippocampal slices and effect of glutamate on the enzyme activity in different phases of development. Life Sci 71: 215–225 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Table EV1
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6


