Abstract
Human and animal cross-sectional studies have shown that maternal levels of the inflammatory cytokine interleukin-6 (IL-6) may compromise brain phenotypes assessed at single time points. However, how maternal IL-6 associates with the trajectory of brain development remains unclear. We investigated whether maternal IL-6 levels during pregnancy relate to offspring amygdala volume development and anxiety-like behavior in Japanese macaques. Magnetic resonance imaging (MRI) was administered to 39 Japanese macaque offspring (Female: 18), providing at least one or more time points at 4, 11, 21, and 36 months of age with a behavioral assessment at 11 months of age. Increased maternal third trimester plasma IL-6 levels were associated with offspring’s smaller left amygdala volume at 4 months, but with more rapid amygdala growth from 4 to 36 months. Maternal IL-6 predicted offspring anxiety-like behavior at 11 months, which was mediated by reduced amygdala volumes in the model’s intercept (i.e., 4 months). The results increase our understanding of the role of maternal inflammation in the development of neurobehavioral disorders by detailing the associations of a commonly examined inflammatory indicator, IL-6, on amygdala volume growth over time, and anxiety-like behavior.
Keywords: anxiety, inflammation, maternal environment, MRI, neurodevelopment
Introduction
Numerous studies in humans and animals have shown that variation in the in utero environment can affect fetal brain development and subsequently influence behavior (Rees and Harding 2004; Rees and Inder 2005; Sullivan et al. 2010; Piontkewitz et al. 2011; Mills et al. 2016). One factor receiving significant attention in this regard, in both animal and human studies, is maternal inflammation. Specifically, the inflammatory cytokine interleukin-6 (IL-6) plays a major role in fetal brain development (Smith et al. 2007; Hunter and Jones 2015; Glaus et al. 2017; Graham et al. 2017; Wu et al. 2017). Previous studies in human and animal models have linked maternal IL-6 to various behavioral outcomes and alterations in offspring brain function and structure (Smith et al. 2007; Bilbo and Schwarz 2009; Enayati et al. 2012; Kalmady et al. 2014; Graham et al. 2017; Wu et al. 2017; Gustafsson et al. 2018; Rasmussen et al. 2018; Rudolph et al. 2018). However, while neurodevelopmental trajectories have been discussed and investigated at the level of one or two time points, few studies have investigated the impact of maternal IL-6 on offspring brain development in sufficient density to estimate growth trajectories.
The amygdala may be a suitable exemplar for measuring the effect of maternal inflammation on brain growth trajectories. Levels of the maternal pro-inflammatory cytokine IL-6 during pregnancy have been associated with infant amygdala structure and function, and related behavioral outcomes across multiple studies (Smith et al. 2007; Enayati et al. 2012; Graham et al. 2017; Gustafsson et al. 2018; Rasmussen et al. 2018). In humans, we have demonstrated that higher levels of maternal gestational IL-6 are associated with increased bilateral amygdala connectivity and right-amygdala volumes, which mediated an effect on lower impulse control at 24 months of age (Graham et al. 2017). Other works have also recently demonstrated that infant functional connectivity between various brain networks are related to maternal IL-6 levels (Rudolph et al. 2018; Spann et al. 2018). Lastly, new data suggests that heightened maternal IL-6 levels relate to decreased integrity of structural connectivity between the amygdala and prefrontal cortex (uncinated fasciculus) in the neonatal time period and an increased rate of change in this structural connectivity from the neonatal period to 1-year-of-age (Rasmussen et al. 2018). In total, these human studies provide strong support for associations between maternal IL-6 levels during pregnancy and offspring amygdala development and emotionality. Here, this association is further investigated in a nonhuman primate (NHP) model, which offers several advantages for examining early developmental trajectories and isolating various factors of interest.
Though modern technologies have made studying brain-behavioral relationships possible in the human population, rodent and NHP models continue to be useful. Animal models, allow direct control over confounding variables such as socioeconomic status, diet, and other complex environmental influences that are often observed in human populations. For example, rodent models have been instrumental in pinpointing the initial causal relationships between maternal inflammation and brain and behavioral outcomes (Parker-Athill and Tan 2010; Wong and Hoeffer 2018; Wu et al. 2017). These types of studies set the stage for further investigation in a NHP model to offer a more accurate translational comparison. NHPs closely mirror the complex behaviors, brain structures, and functions present in humans (Orban et al. 2004; Hutchison and Everling 2012; Gottlieb and Capitanio 2013; Miranda-Dominguez, Mills, Carpenter et al. 2014a; Miranda-Dominguez, Mills, Grayson, et al. 2014b; Grayson et al. 2016; Casimo et al. 2017; Xu et al. 2018). Fetal NHP exposure to maternal inflammatory factors, nutrition, and secreted lipids is also closely comparable to humans due to similar placental structure and function. Since NHPs have a similar gestational and developmental timeline to humans with the majority of brain development occurring prenatally, NHPs are beneficial for examining the association of inflammatory cytokines secreted by the placenta (Sullivan and Kievit 2016). Finally, examining neurodevelopmental processes requires repeated assessments of the brain. In humans, repeated measures in brain imaging are challenging, particularly during early development. NHPs provide an opportunity for well-controlled repeated assessments, which allow for capturing neurodevelopmental processes as opposed to single snapshots of development.
Here, we investigate how maternal IL-6 levels during pregnancy relate to offspring amygdala structural development and behavior in a well-characterized cohort of Japanese macaque (Macaca fuscata) offspring (Sullivan et al. 2010, 2012; Thompson et al. 2017) We assessed how maternal IL-6 levels during pregnancy are associated with offspring amygdala development over the equivalent time frame of human infancy into puberty in a longitudinal Japanese macaque model. We further examined association pathways between maternal IL-6 offspring and anxiety-like behavior via alterations in amygdala structure.
Materials and Methods
Macaque Study Overview
We conducted this study using a set of primates comprising a well-defined NHP primate model of maternal Western-style diet (WSD) or control diet (CTR) (Sullivan et al. 2010, 2012; Thompson et al. 2017). Such a sample better reflects “real world” human populations in Western and developing countries (Thompson et al. 2018). Notably, IL-6 has been found to be similar between the two maternal diet groups, while still displaying large individual differences (Thompson et al. 2018). Rather than focusing on diet, the current study focused on differences in maternal IL-6 concentrations. Detailed characterizations of the maternal and offspring phenotypes have been described in earlier reports (McCurdy et al. 2009; Sullivan et al. 2010, 2012, 2017; Comstock et al. 2013; Thompson et al. 2017). All aspects of the study were approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee following National Institutes of Health guidelines on ethical use of animals.
Subjects
Mothers consumed either a WSD (TAD Primate Diet no. 5LOP, Test Diet, Purina Mills) or a CTR diet (Monkey Diet no. 5000; Purina Mills) for 1.2–8.5 years prior to offspring birth (age at offspring birth [mean {M} ± SEM]: CTR M = 9.44 ± 0.38 years; WSD M = 9.32 ± 0.37 years). Details on the maternal diet (Supplementary Table 1) and offspring rearing were recently described in a prior publication (Thompson et al. 2017). Briefly, offspring stayed with their mothers until weaning (~8 months of age), at which point they were housed in peer social groups of 6–10 juveniles and 1–2 unrelated female adults. Of the total subjects (n = 56; Female n = 26; CTR n = 23), the majority (n = 41; Female n = 19) consumed a CTR diet post-weaning; a subset consumed a WSD post-weaning (n = 15; Female n = 7) to account for potential effects of the postnatal diet. All of these factors were considered in subsequent analyses (see below).
Anxiety-like Behavior
Offspring underwent behavioral testing at the 11-month (M = 10.87 ± 0.03) time point (n = 44, Female n = 22; CTR n = 18). Subjects missing behavioral data do not systematically differ in sex (sample: M = 0.50, missing: M = 0.66, P = 0.31), maternal diet (sample: M = 0.59, missing: M = 0.58, P = 0.96), or Amygdala Intercept (sample: M = 208.13, missing: M = 211.01, P = 0.45), and slope (sample: M = 17.87, missing: M = 18.56, P = 0.73) (see Analysis Overview below for details on the modeling). Behavioral tests and procedures were performed as previously described (Thompson et al. 2017). In brief, animals underwent the human intruder and the novel object test. Typical and atypical stress responses on these tests were scored to form a single anxiety composite expressing the percent duration of total anxiety-like behaviors exhibited. More details are described in the supplementary materials.
Maternal IL-6 Concentrations
Plasma was collected from mothers during their third trimester (48.96 ± 1.06 days) before offspring birth (maternal age: 9.13 ± 0.39 months). These procedures have been previously described (Thompson et al. 2018) and are further explained in the supplemental materials of this manuscript. IL-6 values below the lower limit of quantification (LLOQ) of 1.23 pg/mL were excluded, resulting in a total of 46 subjects with usable IL-6 data (Female n = 21; CTR n = 16). Subjects missing IL-6 data do not systematically differ in sex (sample: M = 0.53, missing: M = 0.56, P = 0.90), maternal diet (sample: M = 0.64, missing: M = 0.33, P = 0.09), or Amygdala Intercept (sample: M = 207.99, missing: M = 210.30, P = 0.44), and slope (sample: M = 17.65, missing: M = 19.20, P = 0.32). For the current study, IL-6 levels were logarithmically transformed across all subjects in order to normalize the distribution and center the outliers closer to the mean.
MRI Acquisition
Offspring MRI scans were acquired at 4 (M = 4.37 ± 0.05), 11 (M = 11.09 ± 0.04), 21 (M = 21.11 ± 0.05) and 36 (M = 36.53 ± 0.09) months of age. MRI data were acquired on a Siemens TIM Trio 3 Tesla scanner using a 15-channel knee coil modified for scanning monkey heads. Prior to scanning, macaques were sedated with a single dose of ketamine (10–15 mg/kg) for intubation and maintained on <1.5% isoflurane anesthesia throughout the scan. Macaques were monitored throughout the session for abnormalities in heart rate, respiration, or peripheral oxygen saturation. For each macaque, we collected a total of four T1-weighted anatomical images (TE = 3.86 ms, TR = 2500 ms, TI = 1100 ms, flip angle = 12°, 0.5 mm isotropic voxel) and one T2-weighted anatomical image (TE = 95 ms, TR = 10 240 ms, flip angle = 150, 0.5 mm isotropic voxel). Other scans were collected at this time; however, they were not used for the present study.
MRI Preprocessing
The current study used a modified version of the Human Connectome Project (HCP) minimal preprocessing pipeline (Glasser et al. 2013) for use in macaques. Processing included the use of the FMRIB Software Library (FSL) (Smith et al. 2004; Woolrich et al. 2009; Jenkinson et al. 2012) and FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/) (Dale et al. 1999; Fischl et al. 1999). Structural scans were averaged. Study-specific templates were created for each age group from averaged T1-weighted (T1w) images using previously established methods (Scott et al. 2016) with Advanced Normalization Tools (ANTs) (version 1.9; http://stnava.github.io/ANTs/). For each subject, age-specific templates were registered and warped to the subject’s averaged T1w image using FSL and ANTs. Affine transformations and warps from this registration were then applied to the template mask and segmented in order to delineate white and grey matter structures and subcortical regions such as the amygdala. Subject automated segmented brain images (asegs) and masked structural images went through modified versions of the PreFreeSurfer, FreeSurfer, and PostFreeSurfer stages of the modified HCP pipeline (Glasser et al. 2013). Gradient distortion corrected T1w volumes were first aligned to the Yerkes19 (Donahue et al. 2016) AC-PC axis and then nonlinearly normalized to the Yerkes19 macaque surface-based atlas. AC-PC aligned T1w volumes are segmented using the recon-all FreeSurfer functions and previously defined asegs. The initial pial surface is calculated by finding voxels, which are beyond ±4 standard deviations from the grey matter mean. Next, the preliminary pial surface and white matter surface were used to define an initial cortical ribbon. The original T1w volume was smoothed with the ribbon using a Gaussian filter with a sigma of 2.5 mm. Then, the original T1w image was divided by the smoothed volume to account for low frequency spatial noise. This filtered volume was used to recalculate the pial surface, but now using ±2 (instead of ±4) standard deviations as the threshold to define the pial surface. These segmentations were then used to generate an individualized 3D surface rendering, using a number of surface features including subject curvature, sulcal depth, and myelination. These surfaces were registered to the Yerkes19 macaque surface-based atlas. This registration process allows all data types (cortical thickness, sulcal depth, function activity, functional connectivity, etc.) to be aligned directly within and between individuals (Xu et al. 2019). The pipelines follow our previous standards for human data and the ABCD project (available at: https://github.com/DCAN-Labs/ or https://github.com/ABCD-STUDY/abcd-hcp-pipeline), and are currently being prepped for a similar release.
A rigorous quality control assessment was conducted on the processed MRI data by quality control trained raters to determine the final MRI numbers used in the study (N = 48; Female n = 22; CTR n = 19). Rating was conducted on a 1–3 scale with 1 indicating a good quality registration and image, and 3 indicating a poor quality. An additional reviewer assessed the subjects, which received a score of 2 to determine if they were deemed usable or excluded from the study. Quality was based on artifacts such as poor surface delineations, ringing artifacts that result from movement in the scanner, abnormal warping of the brain, or excessive blurriness of the image. The amygdala volumes for this study were defined by the outputs from the Freesurfer stage of the pipeline, which were vetted by this quality control assessment (Examples detailing the quality of the structural outputs for all of the ages have been added as Supplementary Figs. 1–4). Of the subjects, which were determined good enough to use for analyses, a total of 30 subjects had scans for two or more different time points. Due to the nature of the longitudinal design, subject numbers varied for the 4 (n = 17; Female n = 6; CTR n = 8), 11 (n = 25; Female n = 11; CTR n = 11), 21 (n = 27; Female n = 13; CTR n = 10), and 36 (N = 31; Female n = 16; CTR n = 11) month time points. Subjects missing MRI data do not systematically differ in sex (sample: M = 0.54, missing: M = 0.5, P = 0.83), maternal diet (sample: M = 0.60, missing: M = 0.5, P = 0.59), or IL-6 level (sample: M = 8.55, missing: M = 6.19, P = 0.41). Of the initial 56 animals, 39 animals had both IL-6 and MRI data (Female n = 18; CTR n = 13). These 39 animals (4 month n = 15, 11 month n = 20, 21 month n = 25 and 36 month n = 24) were used for the analyses of this study. Missing data from different time points were later addressed in the analysis.
Analysis Overview
This study used latent growth curve models to investigate brain growth over time in relationship to maternal IL-6. Latent growth curve models derive from the structural equation modeling (SEM) framework, and allow for the estimation of a growth trajectory over time in relationship to other factors (McArdle and Epstein 1987; Meredith and Tisak 1990; Muthén 2002). This analysis framework allows one to first construct an unconditional model to identify the best fitting model of the typical growth trajectory. Once this is established, predictors and covariates can be added to create the conditional model. This conditional model can then be further refined to only include statistically relevant covariates by systematically reducing the covariates of the model to define the final model to use (Singer and Willett 2003; Lee and Thompson 2009; Curran et al. 2010; Muthén and Muthén, 1998-2017).
Data were analyzed in version 8 of Mplus (Muthén and Muthén, 1998-2017) to create the latent growth curve models using the robust maximum likelihood estimator to accommodate non-normal data, and the full information maximum likelihood method to handle missing data (Enders 2001). Extensive research has documented the utility of this method for estimating longitudinal parameters in studies with missing data at various time points (Enders 2001; Raykov 2005; Buhi 2008; Jeličić et al. 2009; Schlomer et al. 2010; Larsen 2011; Peyre et al. 2011; Gustavson et al. 2012). Model fit criteria for these analyses were based on a Comparative Fit Index (CFI) and a Tucker-Lewis index (TLI) above 0.90, and a Root Mean Square Error of Approximation (RMSEA) below 0.1 (Bentler 1990; Maccallum et al. 1996; Schumacker and Lomax 2004).
Establishing the Unconditional Model
We investigated left (LA) and right (RA) amygdala volumes separately (Fig. 1) to account for potential-lateralized effects, which may occur as a result of prenatal influences (Qiu et al. 2015). An important initial step when conducting latent growth models is to pinpoint the optimal functional form of the developmental trajectory of your data by testing different growth forms (Curran et al. 2010). To establish this best fitting unconditional models of typical amygdala volume development, we first tested a linear growth curve model; however, the model fit was poor (LA: χ2 (4) = 30.45, P < 0.01, CFI = 0.58, TLI = 0.48, RMSEA = 0.37, RA: χ2 (4) = 16.70, P = 0.01, CFI = 0.78, TLI = 0.73, RMSEA = 0.26). When adding a quadratic term, the model did not converge. Thus, we adjusted the parameters to a spline growth curve model as the mean amygdala volumes were neither quite linear nor quadratic across the 4 (M = 207.93), 11 (M = 231.28), 21 (M = 246.60), and 36-month (M = 266.03) time points. For the spline model, we freed the second and third time points, and suppressed the nonsignificant 36-month variance and slope with intercept variance to improve model fit. Spline models can be more accurate when describing biological growth systems, and are often used to substitute asymptotic models in data sets, which do not reach the asymptote (Aggrey 2002; Kahm et al. 2010). As the brain develops at different rates depending on the region (Ball and Seal 2019), spline models often best describe this nonlinear trajectory, as has previously been shown in a study in marmosets (Sawiak et al. 2018). Furthermore, a chi-square difference test indicated that the spline model significantly improved the model fit (LA: χ2 (1) = 23.41, P < 0.001, RA: χ2 (1) = 11.69, P < 0.001), which was used for the rest of the analysis (LA: χ2 (5) = 7.04, P = 0.22, CFI = 0.97, TLI = 0.97, RMSEA = 0.09, RA: χ2 (5) = 5.01, P = 0.29, CFI = 0.98, TLI = 0.98, RMSEA = 0.07).
Figure 1.
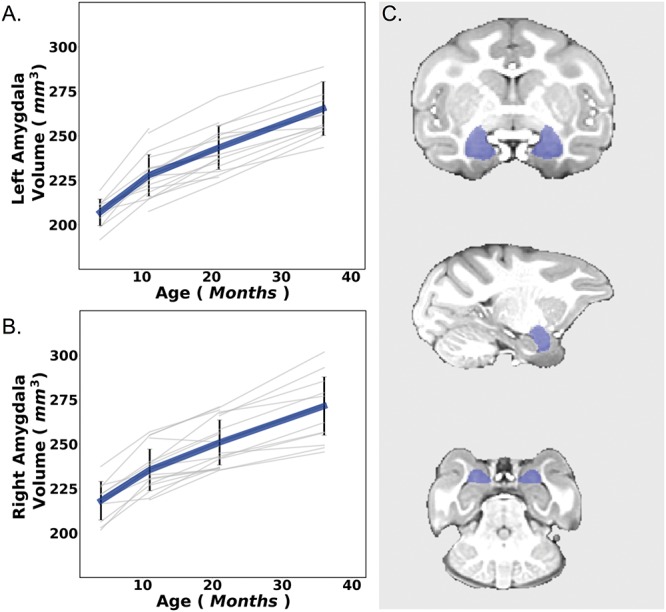
Here we depict raw left (A) and right (B) amygdala development over the four different time points. Error bars are depicted as SEM. Individual data points defined as non-significant outliers by the SPSS statistical software are seen above some of the bars as open circles. Macaque offspring amygdalae ROIs were defined using the modified version of the human connectome pipeline (C).
This model determined our two latent growth variables, the intercept (starting point) and slope (growth over time). The 4-month time point was coded as zero in our analysis to define the intercept. This is common practice in latent growth curve modeling. The number given to the intercept indicates the starting point for the model to identify growth over time (Muthén 2002; Muthén and Asparouhov 2002). Hence, the intercept mean growth factor parameter indicates the average of the outcome over individuals at the time point with the time score that is coded as zero (i.e., 4 months in this case). Additionally, the intercept variance parameter indicates the variance at our 4-month time point excluding the residual variance.
Establishing the Conditional Model
Having identified our best fitting unconditional model, we next introduced our predictor of interest (maternal IL-6) and potential covariates of interest. For this, we first defined which potential covariates to include in our final model. Additional models run to determine the covariates for our final left and right amygdala models are described in more detail in the supplemental materials. In brief, all covariates were initially added to the model; however, to improve the model fit and trustworthiness of our parameter estimates, we removed nonsignificant covariates for the left and right amygdala models (Supplementary Table 2).
As amygdala size is related to total brain volume (TBV) (O’Brien et al. 2011), we also modeled typical TBV unconditional growth trajectories and used TBV slope and intercept from these models as covariates in our amygdala volume analysis. Additional covariates with possible associations with offspring brain and behavioral development were introduced to the model to account for potential confounding variables. Covariates that were tested included: 1) maternal age at offspring birth, 2) maternal pre-pregnancy percent body fat, 3) number of prior pregnancies, 4) maternal diet, 5) offspring post-weaning diet, 6) offspring sex, and 7) offspring TBV slope and intercept. Offspring age at scan was not included as a time varying covariate as scans were scheduled to occur at the same age for all animals within each age group. Variation is measured in days (i.e., neither months nor years), were typically the result of scheduling conflicts or external complications, and are relatively small for each age (4-month mean age in days = 133.17, SD = 5.31, Min = 125, Max = 152, 11-month mean age in days =336.58, SD = 6.62, Min = 329, Max = 352, 21-month mean age in days = 643.84, SD = 6.74, Min = 630, Max = 669, and 36-month mean age in days = 1114.10, SD = 15.90, Min = 1083, Max = 1162) (further justification in supplemental materials).
Adding a Mediation to the Model
For the final refined conditional model, we planned to also examine the relevance of IL-6-amygdala associations for anxiety-like behavior by testing for statistical mediation. Specifically, we tested for the indirect effect of maternal IL-6 on anxiety-like behavior via amygdala volume using the “model indirect” command in Mplus. As anxiety-like behavior was only collected at the 11-month time point, we planned to test for this only in relation to 4-month amygdala volume using the intercept of our model.
Results
Amygdala Volume Increased Over Time and Varied Significantly Among Individuals
We first examined the development of LA and RA volumes (Fig. 1) to determine our unconditional model, before continuing to our final model that examined how IL-6 related to amygdala volume development and behavior via the amygdala. This unconditional model showed amygdala volume development with a significant positive intercept (LA: M = 208.43, P = 0.001, RA: M = 217.92, P < 0.001) and slope (LA: M = 17.94, P < 0.001, RA: M = 15.83, P < 0.001), which is indicative of the observed data in Fig. 1, Table 1 and Supplementary Table 2. Importantly, there was enough variance across subjects in both the slope and intercept terms to conduct the conditional models investigating whether amygdala trajectories were associated with maternal exposure to IL-6 and anxiety-like behavior. This finding was true for both the left (intercept: 95.17, P = 0.001; slope: 22.27, P = 0.001) and right (intercept: 86.18, P = 0.013; slope: 18.36, P = 0.001) amygdalae. These analyses defined our typical amygdala volume development and established an unconditional model to test our predictor of interest (Table 1 and Supplementary Table 2).
Table 1.
Model statistics for the right and left unconditional amygdala model.
| Unconditional left amygdala | Unconditionalright amygdala | |||
|---|---|---|---|---|
| Parameter | Estimate | S.E. | Estimate | S.E. |
| Intercept mean | ***208.47 | 1.946 | ***217.917 | 2.302 |
| Intercept variance | ***95.166 | 27.974 | *86.182 | 34.739 |
| Slope mean | ***17.939 | 0.834 | ***15.827 | 0.899 |
| Slope variance | ***22.267 | 5.948 | **18.364 | 5.657 |
| Intercept & slope covariance | Restricted | Restricted | ||
Establishing Our Final Model to Use for the Analysis
Once our amygdala volume trajectories were established via our unconditional models (Table 1) (also see LGM steps in Methods), we next determined the conditional model, which described how IL-6 related to amygdala volume development and also asked if early amygdala volumes at 4-month mediated the relationship between IL-6 and anxiety-like behavior at 11-month of age. Covariates included in the final left amygdala model were the TBV slope and intercept, and covariates included in the final right amygdala model were TBV, maternal diet, pre-pregnancy percent body fat, maternal age, and offspring sex.
Since changes in brain characteristics often drive behavioral differences, our final models included anxiety-like behavior and only the significantly relevant covariates for the left and right amygdala models (Table 2). We aimed to see if maternal IL-6 directly or indirectly associated with anxiety-like behaviors via the amygdala volume intercept of our model. Anxiety-like measures were only available at the 11-month time point; hence the mediation analysis only included the amygdala volume intercept, as the behavioral measures were not available across time.
Table 2.
Model statistics for the final right and left amygdala model
| Final left amygdala model | Final right amygdala model | |||
|---|---|---|---|---|
| Parameter | Estimate | S.E. | Estimate | S.E. |
| Intercept mean | ***18.672 | 5.075 | **17.776 | 5.140 |
| Intercept variance | *0.381 | 0.150 | ***0.688 | 0.148 |
| Slope mean | 1.106 | 0.721 | **2.394 | 0.811 |
| Slope variance | *0.305 | 0.141 | 0.121 | 0.074 |
| Intercept & slope covariance | Restricted | Restricted | ||
| Predictors of intercept | ||||
| IL-6 | ***-0.744 | 0.110 | −0.147 | 0.194 |
| TBV intercept | 0.255 | 0.144 | 0.295 | 0.255 |
| Maternal diet | N/A | N/A | 0.228 | 0.270 |
| Offspring sex | N/A | N/A | 0.352 | 0.243 |
| Pre-pregnancy % body fat | N/A | N/A | 0.397 | 0.312 |
| Maternal age at offspring birth | N/A | N/A | −0.443 | 0.227 |
| Predictors of Slope | ||||
| IL-6 | **0.451 | 0.157 | 0.034 | 0.109 |
| TBV slope | ***0.701 | 0.107 | ***0.820 | 0.066 |
| Maternal diet | N/A | N/A | −0.144 | 0.141 |
| Offspring sex | N/A | N/A | ***-0.379 | 0.185 |
| Pre-pregnancy % body fat | N/A | N/A | −0.348 | 0.185 |
| Maternal age at offspring birth | N/A | N/A | 0.034 | 0.174 |
| Mediation: (IL-6→amygdala intercept→anxiety-like behavior) | ||||
| Indirect | *0.580 | 0.281 | 0.024 | 0.047 |
| Direct | †− 0.574 | 0.307 | −0.020 | 0.169 |
| Intercept →anxiety | *-0.779 | 0.316 | −0.162 | 0.196 |
Note: †P < 0.10; *P < 0.05; **P < 0.01; ***P < 0.001. N/A indicates covariates not included in the final model.
Our final model for the left amygdala volumes resulted in a moderately well-fitting model (χ2 (18) = 23.68, P = 0.166, CFI = 0.94, TLI = 0.92, RMSEA = 0.09). Our final left amygdala volume model explained 62% (R2 = 0.62) of variance for the intercept and 70% (R2 = 0.70) for the slope. There was still a significant amount of variance in both the intercept and slope (see Table 2), which was not explained in the final model. This indicates that there are other factors, which we have not included in our study, which may play an important role in explaining individual differences in the trajectory of amygdala development across this time period.
For the right amygdala, we were not able to determine a usable model fit deeming the results for this hemisphere untrustworthy (χ2 (39) = 146.31, P < 0.001, CFI = 0.38, TLI = 0.30, RMSEA = 0.27). The poorly fitting right amygdala final model explained 31% (R2 = 0.31) of variance for the intercept and 88% (R2 = 0.88) for the slope. Regardless of model fit, IL-6 only showed a significant relationship with left but not right amygdala volume development. Hence, aside from reporting the findings in Table 1, all further investigations focused on the left amygdala volumes. Results from the final left amygdala model can be seen in Table 1 and Fig. 2.
Figure 2.
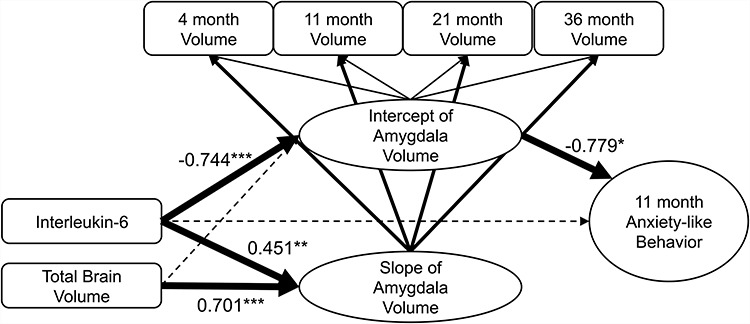
Spline latent growth curve models were used to model our results. 4 (n = 15), 11 (n = 20), 21 (n = 25) and 36 (n = 24) amygdala volumes were used to construct intercept and slope latent variables. Predictor (IL-6) and the covariate (Total Brain Volume) were introduced into the model to determine the extent to which they related to the latent variables. Finally, an indirect mediation effect was tested using only the intercept of this model to see how maternal IL-6 relates to anxiety-like behavior at 11-month of age via the amygdala intercept (not slope) at “4-months-of-age”. Thick solid lines indicate that the relationship was significant (P < 0.05) while dotted lines indicate insignificant relationships. Note: *P < 0.05;**P < 0.01;***P < 0.001.
IL-6 Levels Relate to Smaller Left Amygdala Volume at 4-Months and More Rapid Growth Over Time
In the context of the final model, maternal IL-6 levels were significantly associated with lower left amygdala volume intercept (B = -0.744, P < 0.001); the model also showed that maternal IL-6 exposure significantly predicted increased amygdala volume slope (B = 0.451, P = 0.004). Our TBV slope and intercept latent variable covariates also predicted an increase in amygdala volume slope but not intercept (Intercept: B = 0.255, P = 0.078, Slope: B = 0.701, P < 0.001). Table 1 and Fig. 2 depict these findings.
To facilitate visualization and interpretation purposes only, subjects were categorized into high and low IL-6 groups using a median split and compared at each time point to depict how the growth trajectories differ depending on the level of maternal IL-6 during pregnancy (Fig. 3A). Furthermore, to visualize the relationship between IL-6 and amygdala volumes independent of the TBV effect, TBV intercept and slope values were regressed out from amygdala volume intercept and slope, respectively (Fig. 3B,C). Having identified a link between maternal IL-6 and amygdala volumes, we next sought to examine whether these findings related to anxiety-like behaviors.
Figure 3.
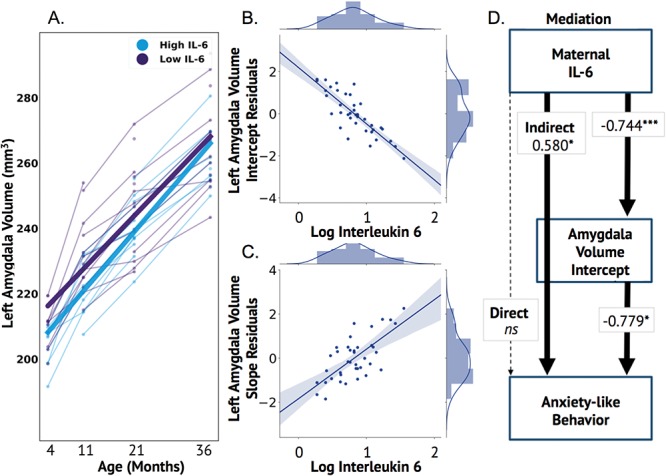
Maternal IL-6 associated with left but not right amygdala volumes and indirectly related to anxiety-like behavior via the amygdala intercept. Animals were median split into high and low IL-6 levels purely for visualization purposes in order to show that higher maternal IL-6 was associated with lower amygdala volumes at the intercept but an increased slope (A). Total brain volume values were regressed from amygdala volume values to more accurately visualize the association of the amygdala intercept (B) and slope (C) on IL-6 using these residuals. The mediation results are depicted in D and show a significant indirect, but not direct, effect of maternal IL-6 on anxiety-like behavior at 11-months via the amygdala intercept. Individual estimates are shown in text on the arrows. The total indirect effect on anxiety-like behavior is depicted in the continuous line from IL-6 to anxiety-like behavior through the amygdala volume intercept. The results of the individual connections (IL-6 to amygdala volume intercept & amygdala volume intercept to anxiety-like behavior) are illustrated in the lines between these variables. Note: *P < 0.05; **P < 0.01;***P < 0.001.
Larger Amygdala Volumes at 4-months Are Associated With Decreased Anxiety-like Behavior at 11-months and Mediate an Indirect Effect of IL-6 Levels on Anxiety-like Behavior
Results for the behavioral aspect of our final model indicated that left amygdala intercept significantly predicted anxiety-like behavior at 11-months of age (B = −0.779, P = 0.014). Higher amygdala volumes at “4-months-of-age” were associated with a lower anxiety at this time point. There was no significant direct effect of IL-6 on anxiety-like behavior (B = –0.574, P = 0.062). However, we observed a significant indirect effect of IL-6 on anxiety-like behavior at 11-months via the amygdala intercept (B = 0.580, P = 0.039) (Fig. 3D). Furthermore, adding an extra parameter for 11-month volumes as a “sensitivity analysis” did not change our results (Supplemental materials). Thus, higher maternal IL-6 during pregnancy was associated with elevated anxiety-like behavior at 11-months of age via differences in the “4-months” amygdala volume intercept of our model.
Discussion
The current study offers novel insights into our present understanding of how the maternal environment affects offspring brain development and behavior. These findings are the first to demonstrate that maternal IL-6 is associated with offspring macaque amygdala volume development and indirectly related to anxiety-like behavior through effects on the amygdala. We showed that heightened levels of IL-6 during pregnancy predict lower left amygdala volumes at 11-months-of-age and an increased rate of amygdala volume development. Furthermore, we showed that elevated maternal IL-6 levels predict higher anxiety-like behavior via the differences in amygdala volume at 4-months-of-age (statistical mediation). These findings are noteworthy as they corroborate several lines of work in humans and rodent models identifying an association between 1) maternal inflammatory cytokines and behavioral outcomes in NHPs, 2) maternal inflammatory cytokines and the newborn brain and rate of development in NHPs, and 3) the relationship between brain and behavioral outcomes.
Our findings showed a significant, positive, indirect relationship between maternal IL-6 and offspring anxiety-like behavior via amygdala volumes. This finding is relevant to the mental health literature, as several lines of evidence highlight negative valence systems as a core component dimension of several developmental psychopathologies (Schatz and Rostain 2006; Insel et al. 2010; Cuthbert 2014; Karalunas et al. 2014). Furthermore, negative valence behaviors, such as anxiety and depression symptomatology, have repeatedly been linked to heightened inflammation during pregnancy in rodent (Hava et al. 2006; Lucchina et al. 2010; Enayati et al. 2012) and human (Kiecolt-Glaser et al. 2015; Simanek and Meier 2015) literatures. Other disorders, such as, attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and schizophrenia have shown similar correspondence to maternal inflammation in human (Tohmi et al. 2004; Parker-Athill and Tan 2010; Bronson and Bale 2014; Wong and Hoeffer 2018) and rodent models (Patterson 2009; Estes and McAllister 2016). These findings add to the growing body of literature highlighting the effects of the immune system on long-term mental health outcomes during the earliest periods of brain development (Knuesel et al. 2014; Estes and McAllister 2015).
Interestingly, the findings in the present study were, in some ways, at odds with our previous report of higher maternal inflammation linked to larger amygdala volume in human infants (Graham et al. 2017). In the human infant study, larger right amygdala volumes at 4-weeks old were associated with higher maternal IL-6 during pregnancy. In contrast, here, we showed lower left amygdala volumes at the intercept in association with IL-6. Because these new findings incorporate the rate of change, while in the original human publication only one time point (the neonatal period) was examined, our interpretation of the original publication is refined. In the NHP model, maternal IL-6 concentrations predicted an increase in the rate of left amygdala volume development (slope); the human data was acquired at the “point” on the human developmental “curve” at which the NHP model could potentially predict an association between increased amygdala volumes and IL-6 after the increased growth rate. It was interesting to observe slightly larger right than left amygdala volumes in our study (Table 2). As we saw an increased rate of development associated with IL-6 in the left, it is possible that we would have seen a significant association with the right amygdala at an earlier time point before volumes increased at a greater growth rate (slope). Support for this hypothesis is further strengthened in a similar collaboration investigating the association of maternal IL-6 with human infant frontolimbic white matter tract integrity across two time points (4 weeks and 12 months of age) (Rasmussen et al. 2018). Similar to the current findings, this study found that IL-6 was associated with a decrease in fractional anisotropy (FA) of the uncinate fasciculus proximal to the amygdala at the first time point. However, IL-6 was also associated with a positive increase in rate of FA across the first year of life, comparable to the findings that are reported in our current study (Rasmussen et al. 2018).
Importantly, direct comparisons of NHP studies to human findings can often be complicated, as the brains develop at different rates. Indeed, while the human brain undergoes maximum growth right around birth, maximal brain growth happens approximately 60 days prior to birth in monkeys (Brambrink et al. 2010). The macaque brain is already above 50% of its full adult size at birth compared with the human infant brain, which is only approximately 35% of its full adult size. Because critical developmental processes such as myelination, synaptogenesis, and neurogenesis also occur at slightly different stages (Clancy et al. 2001; Workman et al. 2013), the impact of in utero environmental influences may vary across these two species and influence different stages of brain development.
Our finding that early amygdala volumes associate with later behavioral outcomes, and that the rate of amygdala growth changes across time is particularly significant because the majority of longitudinal human studies lack the dense sampling to examine this critical question. However, evidence from the available literature on human mood disorders is consistent with our overall findings, as smaller amygdala volumes have been linked to children with mood disorders, and the association either dissipates or is reversed into adulthood (Hajek et al. 2009; Warnell et al. 2017). Other neuropsychiatric disorders, such as ASD and ADHD, often share comorbidity with anxiety and are also associated with changes in amygdala volume development (Schatz and Rostain 2006; White et al. 2009). While children with ADHD have smaller amygdala volumes at early ages (Hoogman et al. 2017) (similar to our intercept findings), children with ASD (Nordahl et al. 2012) have larger and faster developing amygdala volumes (similar to our slope findings). Though amygdala volumes alone are unlikely to account for the entirety of sequela across these disorders, they may explain specific component behaviors such as anxiety (Amaral et al. 2003). Finally, the difference in findings from our previous human work (Graham et al. 2017) relating larger right amygdala volumes to impulse control behaviors and our current findings relating smaller left amygdala volumes with increased anxiety-like behaviors may further explain the complex nature of how the amygdala is associated with behavioral development.
While we believe these findings give promising novel insights into the association of maternal inflammation on offspring brain behavioral development, our study has some limitations, which we address here. This study utilized NHPs as part of an ongoing longitudinal study; however, behavioral data at the time of analysis were only cleaned and available for the 11-month time point. It is likely that a more complete longitudinal behavioral assessment would greatly benefit the overall interpretation of our findings. While “typical” amygdala development was assessed in our unconditional model, we used a spline model in place of a linear or quadratic model. A more complete data set would likely allow the models to converge in either a linear or quadratic fashion. With only seven NIH funded primate research centers in the United States, these types of data are particularly rare and difficult to acquire. Very few studies exist, which use infant monkeys scans, across multiple time points. Hence, future studies will greatly benefit from open access consortium studies such as the PRIME Data Exchange (Milham et al. 2017). With the current scarcity in available data, it is important to note that due to the nature of the longitudinal design and species, a substantial portion of the subjects had missing data, with the fewest data points at the 4-month time point. However, missing data is a common occurrence in longitudinal studies, and has been extensively addressed using the full information maximum likelihood estimator used in this study (Collins et al. 2001; Enders 2001; Graham 2003; Raykov 2005; Buhi 2008; Jeličić et al. 2009; Schlomer et al. 2010; Larsen 2011; Peyre et al. 2011; Gustavson et al. 2012). While our models were able to explain a large portion of the variance for the intercept and slope, significant variance still remained in our final model (Table 2). Other factors such as genetics, other cytokines, cortisol, or environmental stressors experiences post birth may also play an important role in explaining individual differences of amygdala volume trajectories (Graham, Pfeifer, Fisher, Carpenter, et al. 2015a; Graham, Pfeifer, Fisher, Lin, et al. 2015b; Graham et al. 2016, 2019; Buss et al. 2017). These other factors may also explain the difference in variance explained between the left (62%) and right (31%) intercept and our results only showing that IL-6 was significantly associated with the left amygdala volume. In addition, even though maternal and post-weaning diet did not significantly impact our model, having a more complete data set of this covariate, and a more complete picture of the inflammatory “milieu,” might offer a more comprehensive understanding of potential associations of diet on inflammation and subsequent brain development. It is important to note that other factors such as obesity, glucose/insulin homeostasis, and diet can all independently contribute to different aspects of offspring neurodevelopment and should be studied in concert for future experiments. Furthermore, this study used TBVs as covariates in the model. Though we consider the addition of this covariate as a strength, there is still debate in the field regarding how best to handle the effect of TBV, as different correction methods may lead to different results and interpretations. Complex familiar relationships between the mothers of this study could also be a potential confound of the study. As we did not have the power to address this problem using the batch analysis approach, our closest comparison to this measure was using the number of maternal pregnancies as a covariate, which was not found to be a significant confounder. Similarly, we did not control for age at scan, as we did not have the power to address this time varying covariate and obtain a trustworthy model. However, even when including this variable as a time varying covariate, it did not change the findings in the light of this untrustworthy model (more details on this in the Supplemental Materials and Methods section). Finally, previous research has shown that IL-6 concentrations can fluctuate over time during pregnancy; here, we measured IL-6 concentrations during the third trimester. However, this timing can also be considered a strength, as this critical window in development with regard to IL-6 has been shown to be highly influential to postnatal brain development (Rudolph et al. 2018) and, thus, may provide some specificity to our results. Nonetheless, our findings are likely to benefit from future work that characterizes the dynamics of maternal inflammation across pregnancy using multiple measurements.
The results from the present report increase our current understanding of how maternal inflammatory cytokines may impact brain and behavior relationships over time. Previous findings independently relating maternal inflammation with anxiety-like behaviors and amygdala volume differences are supported by our findings—indeed, these relationships are integrated together in the context of brain development over time. Though other risk factors, brain regions and behaviors undoubtedly contribute to these relationships, our findings suggest a promising avenue of study for future investigation. Finally, in light of rising obesity rates, stress, consumption of WSDs, and their effects on increased maternal inflammatory states, our findings are timely, relevant, and offer a deeper understanding of how the maternal immune system shapes long-term brain and behavioral development in offspring.
Funding
This work was supported by the National Institute of Health (R01 MH115357 to D.A.F. and J.T.N and R01 MH096773 to D.A.F., and P60 AA010760 to D.A.F. and T.P., and R01 MH107508 to E.L.S. and R01MH105538 to MPI: C. Buss, P. Wadhwa & D.A.F. and MHR3759107 to J.T.N. and R00 MH111805 to A.M.G and UL1GM118964, RL5GM118963, and TL4GM118965 to M.B.) and the National Library of Medicine (T15LM007088 to E.F.) and P51 OD011092 (ONPRC Core grant for support of the animals), and Bill & Melinda Gates Foundation to D.A.F., and DeStefano Family Foundation to D.A.F.
Notes
Conflict of Interest: None declared.
Supplementary Material
References
- Aggrey S. 2002. Comparison of three nonlinear and spline regression models for describing chicken growth curves. αPoult Sci. 81:1782–1788. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Mills SC. 2003. The amygdala and autism: implications from non-human primate studies. Genes, Brain Behav. 2:295–302. [DOI] [PubMed] [Google Scholar]
- Ball G, Seal ML. 2019. Individual variation in longitudinal postnatal development of the primate brain. Brain Struct Funct. 1–17. [DOI] [PubMed] [Google Scholar]
- Bentler PM. 1990. Comparative fit indexes in structural models. Psychol Bull. 107:238–246. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. 2009. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. 2010. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 112:834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, Bale TL. 2014. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 155:2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhi E. 2008. Out of Sight, Not Out of Mind: Strategies for Handling Missing Data. Am J Health Behav. 32. [DOI] [PubMed] [Google Scholar]
- Buss DC, Entringer DS, Moog MNK, Toepfer MP, Fair DDA, Simhan DHN, Heim DCM, Wadhwa DPD. 2017. Intergenerational transmission of maternal childhood maltreatment exposure: implications for fetal brain development. J Am Acad Child Adolesc Psychiatry. 56:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimo K, Levinson LH, Zanos S, Gkogkidis CA, Ball T, Fetz E, Weaver KE, Ojemann JG. 2017. An interspecies comparative study of invasive electrophysiological functional connectivity. Brain Behav. 7:e00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington R, Finlay B. 2001. Translating developmental time across mammalian species. Neuroscience. 105:7–17. [DOI] [PubMed] [Google Scholar]
- Collins LM, Schafer JL, Kam CM. 2001. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 6:330–351. [PubMed] [Google Scholar]
- Comstock SM, Pound LD, Bishop JM, Takahashi DL, Kostrba AM, Smith MS, Grove KL. 2013. High-fat diet consumption during pregnancy and the early post-natal period leads to decreased α cell plasticity in the nonhuman primate. Mol Metab. 2:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Obeidat K, Losardo D. 2010. Twelve frequently asked questions about growth curve modeling. J Cogn Dev. 11:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN. 2014. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 13:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis. Neuroimage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- Donahue CJ, Sotiropoulos SN, Jbabdi S, Hernandez-Fernandez M, Behrens TE, Dyrby TB, Coalson T, Kennedy H, Knoblauch K, Van Essen DC et al. . 2016. Using diffusion Tractography to predict cortical connection strength and distance: a quantitative comparison with tracers in the monkey. J Neurosci. 36:6758–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enayati M, Solati J, Hosseini M-H, Shahi H-R, Saki G, Salari A-A. 2012. Maternal infection during late pregnancy increases anxiety- and depression-like behaviors with increasing age in male offspring. Brain Res Bull. 87:295–302. [DOI] [PubMed] [Google Scholar]
- Enders CK. 2001. A primer on maximum likelihood algorithms available for use with missing data. Struct Equ Model A Multidiscip J. 8:128–141. [Google Scholar]
- Estes ML, McAllister AK. 2015. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci. 16:469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes ML, McAllister AK. 2016. Maternal immune activation: implications for neuropsychiatric disorders. Science. 353(6301):772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999. Cortical surface-based analysis. Neuroimage. 9:195–207. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR et al. . 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaus J, von Känel R, Lasserre AM, Strippoli M-PF, Vandeleur CL, Castelao E, Gholam-Rezaee M, Marangoni C, Wagner E-YN, Marques-Vidal P et al. . 2017. Mood disorders and circulating levels of inflammatory markers in a longitudinal population-based study. Psychol Med. 48(6):961–973. [DOI] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP. 2013. Latent variables affecting behavioral response to the human intruder test in infant rhesus macaques (Macaca mulatta). Am J Primatol. 75:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Buss C, Rasmussen JM, Rudolph MD, Demeter DV, Gilmore JH, Styner M, Entringer S, Wadhwa PD, Fair DA. 2016. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev Cogn Neurosci. 18:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Carpenter S, Fair DA. 2015a. Early life stress is associated with default system integrity and emotionality during infancy. J Child Psychol Psychiatry. 56:1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Lin W, Gao W, Fair DA. 2015b. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev Cogn Neurosci. 12:12–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Rasmussen JM, Entringer S, Ben Ward E, Rudolph MD, Gilmore JH, Styner M, Wadhwa PD, Fair DA, Buss C. 2019. Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol Psychiatry. 85:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, Potkin SG, Entringer S, Wadhwa PD, Fair DA et al. . 2017. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry. 83:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW. 2003. Adding missing-data-relevant variables to FIML-based structural equation models. Struct Equ Model A Multidiscip J. 10:80–100. [Google Scholar]
- Grayson DS, Bliss-Moreau E, Machado CJ, Bennett J, Shen K, Grant KA, Fair DA, Amaral DG. 2016. The rhesus monkey Connectome predicts disrupted functional networks resulting from pharmacogenetic inactivation of the amygdala. Neuron. 91:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson HC, Sullivan EL, Nousen EK, Sullivan CA, Huang E, Rincon M, Nigg JT, Loftis JM. 2018. Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain Behav Immun. 73:470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson K, von Soest T, Karevold E, Røysamb E. 2012. Attrition and generalizability in longitudinal studies: findings from a 15-year population-based study and a Monte Carlo simulation study. BMC Public Health. 12:918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek T, Kopecek M, Kozeny J, Gunde E, Alda M, Höschl C. 2009. Amygdala volumes in mood disorders--meta-analysis of magnetic resonance volumetry studies. J Affect Disord. 115:395–410. [DOI] [PubMed] [Google Scholar]
- Hava G, Vered L, Yael M, Mordechai H, Mahoud H. 2006. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev Psychobiol. 48:162–168. [DOI] [PubMed] [Google Scholar]
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, van Hulzen KJE, Medland SE, Shumskaya E, Jahanshad N et al. . 2017. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. The Lancet Psychiatry. 4:310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, Jones SA. 2015. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 16(5):448–457. [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Everling S. 2012. Monkey in the middle: why non-human primates are needed to bridge the gap in resting-state investigations. Front Neuroanat. 6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 167:748–751. [DOI] [PubMed] [Google Scholar]
- Jeličić H, Phelps E, Lerner RM. 2009. Use of missing data methods in longitudinal studies: the persistence of bad practices in developmental psychology. Dev Psychol. 45:1195–1199. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. 2012. FSL. Neuroimage. 62:782–790. [DOI] [PubMed] [Google Scholar]
- Kahm M, Hasenbrink RG, Lichtenberg-Fraté H, Ludwig J, Rheinahrcampus MK. 2010. Grofit: fitting biological growth curves with R. J Stat Softw. doi: 10.18637/jss.v033.i07. [DOI] [Google Scholar]
- Kalmady SV, Venkatasubramanian G, Shivakumar V, Gautham S, Subramaniam A, Jose DA, Maitra A, Ravi V, Gangadhar BN. 2014. Relationship between interleukin-6 gene polymorphism and hippocampal volume in antipsychotic-naïve schizophrenia: evidence for differential susceptibility? PLoS One. 9:e96021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, Nigg JT. 2014. Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: toward biologically based nosologic criteria. JAMA Psychiatry. 71:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kiecolt-Glaser JK, Derry HM, Fagundes CP. 2015. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 172:1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP. 2014. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 10:643–660. [DOI] [PubMed] [Google Scholar]
- Larsen R. 2011. Missing data imputation versus full information maximum likelihood with second-level dependencies. Struct Equ Model A Multidiscip J. 18:649–662. [Google Scholar]
- Lee BR, Thompson R. 2009. Examining externalizing behavior trajectories of youth in group homes: is there evidence for peer contagion? J Abnorm Child Psychol. 37:31–44. [DOI] [PubMed] [Google Scholar]
- Lucchina L, Carola V, Pitossi F, Depino AM. 2010. Evaluating the interaction between early postnatal inflammation and maternal care in the programming of adult anxiety and depression-related behaviors. Behav Brain Res. 213:56–65. [DOI] [PubMed] [Google Scholar]
- Maccallum RC, Browne MW, Sugawara HM. 1996. Power analysis and determination of sample size for covariance structure modeling. Psychol Methods. 1(2):130–149. [Google Scholar]
- McArdle JJ, Epstein D. 1987. Latent growth curves within developmental structural equation models. Child Dev. 58:110–133. [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. 2009. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 119:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith W, Tisak J. 1990. Latent curve analysis. Psychometrika. 55(1):107–122. [Google Scholar]
- Milham MP, Ai L, Koo B, Xu T, Balezeau F, Baxter MG, Croxson PL, Damatac CG, Harel N, Freiwald W et al. . 2017. An open resource for nonhuman primate imaging. Neuron. 100(1):61–74.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills BD, Pearce HL, Khan O, Jarrett BR, Fair DA, Lahvis GP. 2016. Prenatal domoic acid exposure disrupts mouse pro-social behavior and functional connectivity MRI. Behav Brain Res. 308:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Dominguez O, Mills BD, Carpenter SD, Grant KA, Kroenke CD, Nigg JT, Fair DA. 2014a. Connectotyping: model based fingerprinting of the functional Connectome. PLoS One. 9:e111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Dominguez O, Mills BD, Grayson D, Woodall A, Grant KA, Kroenke CD, Fair DA. 2014b. Bridging the gap between the human and macaque connectome: a quantitative comparison of global interspecies structure-function relationships and network topology. J Neurosci. 34:5552–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B, Asparouhov T. 2002. Latent Variable Analysis With Categorical Outcomes: Multiple-Group And Growth Modeling In Mplus. Mplus Web Notes: No. 4. www.statmodel.com.
- Muthén BO. 2002. Beyond SEM: General Latent Variable Modeling. Behaviormetrika. 29(1):81–117. [Google Scholar]
- Muthén LK, Muthén BO. 1998-2017. Mplus User’s Guide. Los Angeles (CA): Muthén & Muthén. [Google Scholar]
- Nordahl CW, Scholz R, Yang X, Buonocore MH, Simon T, Rogers S, Amaral DG. 2012. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Arch Gen Psychiatry. 69:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LM, Ziegler DA, Deutsch CK, Frazier JA, Herbert MR, Locascio JJ. 2011. Statistical adjustments for brain size in volumetric neuroimaging studies: some practical implications in methods. Psychiatry Res. 193:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. 2004. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 8:315–324. [DOI] [PubMed] [Google Scholar]
- Parker-Athill EC, Tan J. 2010. Maternal immune activation and autism spectrum disorder: interleukin-6 signaling as a key mechanistic pathway. Neurosignals. 18:113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. 2009. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 204:313–321. [DOI] [PubMed] [Google Scholar]
- Peyre H, Leplège A, Coste J. 2011. Missing data methods for dealing with missing items in quality of life questionnaires. A comparison by simulation of personal mean score, full information maximum likelihood, multiple imputation, and hot deck techniques applied to the SF-36 in the French 2003 decennial health survey. Qual Life Res. 20:287–300. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. 2011. Abnormal trajectories of neurodevelopment and behavior following in utero insult in the rat. Biol Psychiatry. 70:842–851. [DOI] [PubMed] [Google Scholar]
- Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BFP, Kwek K, Saw S-M, Chong Y-S, Gluckman PD et al. . 2015. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry. 5:e508–e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JM, Graham AM, Entringer S, Gilmore JH, Styner M, Fair DA, Wadhwa PD, Buss C. 2018. Maternal interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage. 185:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raykov T. 2005. Analysis of longitudinal studies with missing data using covariance structure modeling with full-information maximum likelihood. Struct Equ Model A Multidiscip J. 12:493–505. [Google Scholar]
- Rees S, Harding R. 2004. Brain development during fetal life: influences of the intra-uterine environment. Neurosci Lett. 361:111–114. [DOI] [PubMed] [Google Scholar]
- Rees S, Inder T. 2005. Fetal and neonatal origins of altered brain development. Early Hum Dev. 81:753–761. [DOI] [PubMed] [Google Scholar]
- Rudolph MD, Graham A, Feczko E, Miranda-Dominguez O, Rasmussen J, Nardos R, Entringer S, Wadhwa PD, Buss C, Fair DA. 2018. Maternal IL-6 during pregnancy can be estimated from the newborn brain connectivity and predicts future working memory performance in offspring. Nat Neurosci. 21(5):765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawiak SJ, Shiba Y, Oikonomidis L, Windle CP, Santangelo AM, Grydeland H, Cockcroft G, Bullmore ET, Roberts AC. 2018. Trajectories and milestones of cortical and subcortical development of the marmoset brain from infancy to adulthood. Cereb Cortex. 28:4440–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz DB, Rostain AL. 2006. ADHD with comorbid anxiety. J Atten Disord. 10:141–149. [DOI] [PubMed] [Google Scholar]
- Schlomer GL, Bauman S, Card NA. 2010. Best practices for missing data management in counseling psychology. J Couns Psychol. 57:1–10. [DOI] [PubMed] [Google Scholar]
- Schumacker R, Lomax R. 2004. A beginner’s guide to structural equation modeling.
- Scott JA, Grayson D, Fletcher E, Lee A, Bauman MD, Schumann CM, Buonocore MH, Amaral DG. 2016. Longitudinal analysis of the developing rhesus monkey brain using magnetic resonance imaging: birth to adulthood. Brain Struct Funct. 221:2847–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanek AM, Meier HCS. 2015. Association between prenatal exposure to maternal infection and offspring mood disorders: a review of the literature. Curr Probl Pediatr Adolesc Health Care. 45:325–364. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. 2003. Applied longitudinal data analysis: modeling change and event occurrence. New York (NY): Oxford University Press. [Google Scholar]
- Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. 2007. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 27:10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE et al. . 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Spann MN, Monk C, Scheinost D, Peterson BS. 2018. Maternal immune activation during the third trimester is associated with neonatal functional connectivity of the salience network and fetal to toddler behavior. J Neurosci. 38:2877–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Smith MS, Coleman K, Grove KL. 2010. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 30:3826–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Kievit P. 2016. The implications of maternal obesity on offspring physiology and behavior in the nonhuman primate In: Parental Obesity: Intergenerational Programming and Consequences. Springer New York: New York (NY), L.R. Green, R.L. Hester (eds.) pp. 201–234. [Google Scholar]
- Sullivan EL, Nousen EK, Chamlou KA, Grove KL. 2012. The impact of maternal high-fat diet consumption on neural development and behavior of offspring. Int J Obes Suppl. 2:S7–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EL, Rivera HM, True CA, Franco JG, Baquero K, Dean TA, Valleau JC, Takahashi DL, Frazee T, Hanna G et al. . 2017. Maternal and postnatal high-fat diet consumption programs energy balance and hypothalamic melanocortin signaling in nonhuman primate offspring. Am J Physiol Integr Comp Physiol. 313:R169–R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JR, Gustafsson HC, Decapo M, Takahashi DL. 2018. Maternal Diet, Metabolic State, and Inflammatory Response Exert Unique and Long-lasting Influences on Offspring Behavior in Non- human Primates. Front Endocrinol (Lausanne). 9:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JR, Valleau JC, Barling AN, Franco JG, DeCapo M, Bagley JL, Sullivan EL. 2017. Exposure to a high-fat diet during early development programs behavior and impairs the central serotonergic system in juvenile non-human Primates. Front Endocrinol (Lausanne). 8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohmi M, Tsuda N, Watanabe Y, Kakita A, Nawa H. 2004. Perinatal inflammatory cytokine challenge results in distinct neurobehavioral alterations in rats: implication in psychiatric disorders of developmental origin. Neurosci Res. 50:67–75. [DOI] [PubMed] [Google Scholar]
- Warnell KR, Pecukonis M, Redcay E. 2017. Developmental relations between amygdala volume and anxiety traits: effects of informant, sex, and age. Dev Psychopathol. 1–13. [DOI] [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, Scahill L. 2009. Anxiety in children and adolescents with autism spectrum disorders. Clin Psychol Rev. 29:216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H, Hoeffer C. 2018. Maternal IL-17A in autism. Exp Neurol. 299(Pt A):228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. 2009. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 45:S173–S186. [DOI] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. 2013. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 33:7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W-L, Hsiao EY, Yan Z, Mazmanian SK, Patterson PH. 2017. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain Behav Immun. 62:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Falchier A, Sullivan EL, Linn G, Ramirez JSB, Ross D, Feczko E, Opitz A, Bagley J, Sturgeon D et al. . 2018. Delineating the macroscale areal organization of the macaque cortex in vivo. Cell Rep. 23:429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Sturgeon D, Ramirez JSB, Froudist-Walsh S, Margulies DS, Schroeder CE, Fair DA, Milham MP. 2019. Inter-individual variability of functional connectivity in awake and anesthetized rhesus monkeys. Biol Psychiatry Cogn Neurosci Neuroimaging. 4(6):543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


