Abstract
Univariate analyses of structural neuroimaging data have produced heterogeneous results regarding anatomical sex- and gender-related differences. The current study aimed at delineating and cross-validating brain volumetric surrogates of sex and gender by comparing the structural magnetic resonance imaging data of cis- and transgender subjects using multivariate pattern analysis. Gray matter (GM) tissue maps of 29 transgender men, 23 transgender women, 35 cisgender women, and 34 cisgender men were created using voxel-based morphometry and analyzed using support vector classification. Generalizability of the models was estimated using repeated nested cross-validation. For external validation, significant models were applied to hormone-treated transgender subjects (n = 32) and individuals diagnosed with depression (n = 27). Sex was identified with a balanced accuracy (BAC) of 82.6% (false discovery rate [pFDR] < 0.001) in cisgender, but only with 67.5% (pFDR = 0.04) in transgender participants indicating differences in the neuroanatomical patterns associated with sex in transgender despite the major effect of sex on GM volume irrespective of the self-identification as a woman or man. Gender identity and gender incongruence could not be reliably identified (all pFDR > 0.05). The neuroanatomical signature of sex in cisgender did not interact with depressive features (BAC = 74.7%) but was affected by hormone therapy when applied in transgender women (P < 0.001).
Keywords: gender incongruence, gender identity, multivariate pattern analysis, sex differences, structural magnetic resonance imaging
Introduction
Sex differentiation of all somatic tissues, including the brain, is driven both by direct genetic influences and gonadal hormones with only minimal environmental effects (Bocklandt and Vilain 2007; Bao and Swaab 2011; Ngun et al. 2011; Lentini et al. 2013; Gooren et al. 2015). Specifically, sex determination or the commitment of an organism to develop toward a female or male phenotype (Bocklandt and Vilain 2007) is mediated via genetic factors, most importantly the presence or absence of the testis-determining gene Sry on chromosome Y (Arnold and Chen 2009). Morphological sex differences are reflected not only in sex-specific behavior in humans observed from early childhood onward (Alexander et al. 2009) but also in the sex-related susceptibility to neuropsychiatric disorders (Bao and Swaab 2010). Although there is a consensus of a significant relationship between brain structure and behavior in the context of sex differences, the underlying mechanisms are understood only in a few cases (de Vries and Södersten 2009). One example is the sexually dimorphic nucleus of the preoptic area in the human brain (Swaab and Fliers 1985), later also called the interstitial nucleus of the anterior hypothalamus-1 (INAH-1), which is associated with male sexual behavior (Oomura et al. 1983). At the neuroanatomical level, sex differences have been repeatedly observed, in particular mean lower total intracranial volume (TIV), but larger overall cortical volume relative to cerebrum size, in female as compared to male individuals (Goldstein et al. 2001; Cahill 2006; Ritchie et al. 2018). Additionally, the hippocampus, hypothalamus, amygdala, insula, and angular gyrus are repeatedly shown to be different for human male and female in postmortem and in vivo imaging studies (Swaab and Fliers 1985; Goldstein et al. 2001; Ruigrok et al. 2014; Lotze et al. 2019).
Understanding sex differences of the brain’s morphology further increases in complexity when introducing the necessary concept of gender identity (Money et al. 1955). While sex refers to the biological sex assigned at birth based on the anatomy of an individual’s reproductive system (male or female), gender (or gender identity) indicates the personal perception of oneself belonging to a certain gender (male/female/third gender). Hence, a cisgender person exhibits a matching gender identity and assigned sex, while a transgender individual experiences discrepancies between the expression of gender and biological sex. It has been hypothesized that the transgender identity emerges from a temporal mismatch between the sexual differentiation of the brain versus the rest of the body (Zhou et al. 1995; Swaab 2007). Specifically, while primary sexual characteristics develop very early in the womb (~6th week of pregnancy) based on the presence or absence of the Y chromosome, the sexual differentiation of the brain only starts in the second trimester of pregnancy and continues until puberty (Swaab 2007). Until recently, this mismatch was associated with the notion of gender identity disorder, which was relabeled as gender dysphoria by the diagnostic and statistical manual of mental disorders-5 (DSM-5) and refers to the distress experienced due to gender identity issues (Fraser et al. 2010). To fulfill diagnostic criteria, the marked incongruence between an individual’s experienced gender and assigned sex must be present after the onset of puberty for at least 6 months. Of note, gender dysphoria as used in DSM-5 will be replaced by the term gender incongruence in the upcoming International Classification of Diseases-11 (ICD-11) and moved out of the mental disorders into sexual healthy conditions in order to decrease the stigma for transgender people (Reed et al. 2016). ICD coding facilitates access to the significant health care needs associated with the condition even when not considered a mental disorder (WHO 2019). In compliance with this fundamental rethinking, we opted for the term gender incongruence to describe the condition throughout the manuscript. Gender incongruence is rare with prevalence rates ranging between 0.5% and 1.3% (Zucker 2017). Transgender populations are exposed to social distress (Valentine and Shipherd 2018), and gender incongruence has been associated with affective and anxiety disorder (Heylens et al. 2018), increased suicidality (Peterson et al. 2017), eating disorders (Feder et al. 2017), autism spectrum disorder (Øien et al. 2018), and substance abuse (Gonzalez et al. 2017) as compared to the cisgender groups. Therefore, an accurate biological characterization, early identification, and medical guidance of transgender individuals are of high clinical relevance.
The detection of structural brain patterns characterizing gender incongruence could inform these clinical applications. Several magnetic resonance imaging (MRI) studies comparing transgender with cisgender individuals described volumetric differences in biological men with female versus male gender identity, which were located in the putamen (Luders et al. 2009), the thalamus (Savic and Arver 2011), and the angular and the insular gyrus (Spizzirri et al. 2018). Moreover, several authors suggest a stronger resemblance of certain brain structures in persons sharing the same gender identity (Zhou et al. 1995; Garcia-Falgueras and Swaab 2008; Simon et al. 2013), although the greatest volumetric differences are still based on biological sex (Luders et al. 2009; Savic and Arver 2011; Simon et al. 2013). A comprehensive review concluded that the overall brain structure of transgender individuals was mostly similar to subjects sharing their biological sex while single regions seem to adjust to the structure of individuals sharing their gender identity (Smith et al. 2015). The available results are equivocal in terms of the sexually differing brain regions detected and particularly regarding the direction of the divergence between sex- and gender-specific neuroanatomical correlates.
Multivariate pattern analysis (MVPA) techniques provide major chances toward the implementation of imaging biomarkers in the field of psychiatry, for example, in schizophrenia (Kambeitz et al. 2015), major depression (Kambeitz et al. 2017) and dementia (Klöppel et al. 2008) (see also Lozupone et al. 2019). In contrast to classical univariate analysis, MVPA provides the opportunity to delineate neuroimaging patterns that are predictive or classificatory in nature, thus allowing to overcome the merely associative evidence levels of previous group-level paradigms (Dwyer et al. 2018). Furthermore, MVPA accounts for the intrinsic complex nature of the brain and the heterogeneity of psychiatric illness by evaluating the effect of different variables simultaneously (Rutledge et al. 2019). To the best of our knowledge, only one study has employed MVPA techniques on gray matter (GM) volumetric data to demonstrate that individuals with the same gender identity can be better differentiated than those sharing their natal sex based on spatially distributed neuroanatomical patterns (Hoekzema et al. 2015). Further studies need to be performed to fully understand the interaction between biological sex and gender incongruence.
Thus, we aimed at identifying neuroanatomical models of sex and gender identity by comparing 69 cisgender and 52 hormone-naïve transgender individuals using MVPA of structural MRI data. Based on current literature, we hypothesized that 1) sex differences in brain structure present in cisgender individuals will be less expressed in transgender individuals and 2) this pattern will distinguish sexes in independent patients with major depression at similar accuracy levels as in cisgender healthy controls. Additionally, we explored whether cross-sex hormonal treatment moderates the expression of this diverging brain pattern in a longitudinal design. Moreover, we hypothesized that distinct neuroanatomical signatures of male and female gender identity as well as gender incongruence exist beyond the brain classifier of biological sex.
Materials and Methods
Subjects
A total of 121 participants were included in the current study. The sample comprised 29 transgender men (TM, syn. female-to-male transgender, mean age ± SD = 27.17 ± 6.29), 23 transgender women (TW, syn. male-to-female transgender, mean age ± SD = 30.17 ± 8.24), 35 female cisgender (FC, mean age ± SD = 26.29 ± 5.90), and 34 male cisgender (MC, mean age ± SD = 27.09 ± 6.33). Transgender participants were recruited at the Department of Gynecology and Obstetrics, Unit for Gender Identity Disorder, Medical University of Vienna, Austria, while cisgender individuals were recruited via community advertisement. At the time of recruitment (2011–2015), transgender subjects met diagnostic criteria for gender identity disorder as assessed by experienced psychiatrists using the Structural Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID), fourth edition (DSM-IV) and the International Classification of Diseases, 10th revision, as well as several semistructured, sociodemographic, clinical, and psychiatric interviews based on the legal requirements for cross-sex hormonal treatment in Austria. Gender identity disorder in DSM-IV is described as a strong incongruence between a person’s experienced or expressed gender and their assigned gender based on their biological sexual characteristics, which persists over >6 months and causes clinically significant distress and daily life impairment. To avoid stigmatization and emphasize the emotional distress caused by the incongruence of belonging to- and the desire of being treated as a gender “other than the assigned gender” rather than the cross-sex identity issue per se, gender identity disorder was relabeled as gender dysphoria in DSM-5 and will be replaced by gender incongruence and moved out of the mental disorders in ICD-11. In the present sample, changes between DSM-IV and DSM-5 do not interfere with the interpretation of our results, since transgender participants explicitly reported feelings of belonging to the opposite gender to the one assigned and expressed the desire for sex reassignment. All transgender participants reported experiencing gender incongruence at a relatively early age (before or at puberty) and were hormone-naïve. All of them were free of current psychiatric comorbidities as assessed using SCID I (major mental disorders) and II (personality disorders), although some subjects reported a history of previous depressive symptoms (2 TW) and cannabis abuse (1 TW, 1 TM). One TM showed a previous history of eating and anxiety disorders. At the screening visit, all participants underwent standard medical examination, including physical examination, routine laboratory testing, and ECG, as well as a thorough anamnestic exploration. Exclusion criteria were the presence or history of any severe physical or neurological illness (and psychiatric disorders for control subjects), intake of psychotropic medication or hormones (including contraceptives), sexual development disorders (as assessed by an experienced gynecologist), pregnancy, breastfeeding, and any contraindications to magnetic resonance scanning. Sexual orientation and hormone levels were assessed for each participant (see Supplementary material). All subjects provided written informed consent and received financial reimbursement for participation. This study was approved by the Ethics Committee of the Medical University of Vienna and was performed according to the Declaration of Helsinki (internal ethics committee number 644/2010).
Study Design
The study was originally designed as a longitudinal monocentric study (Kranz et al. 2014) with transgender subjects undergoing structural and functional MRI at three time points: baseline, and then 4 weeks and 4 months after initiation of high-dose cross-sex hormone therapy administration (ClinicalTrials.gov Identifier: NCT01292785). Before baseline, participants had not undergone any hormonal treatment or sex-reassignment surgery. In parallel, sex- and age-matched control subjects were scanned at similar time points. Data of respective subsamples of subjects have been published in five previous cross-sectional (Kranz et al. 2014; Ganger et al. 2015; Hahn et al. 2015a, 2015b; Seiger et al. 2015) and five longitudinal investigations (Hahn et al. 2016; Seiger et al. 2016; Spies et al. 2016; Kranz et al. 2017; Kranz et al. 2018). The models created in the current study are based on the structural baseline scans in transgender and control subjects.
MRI Data Acquisition and Preprocessing
All participants underwent structural MRI on a 3T whole-body scanner (Siemens Tim Trio) at the Magnetic Resonance Centre of Excellence at the Medical University of Vienna, Austria. A 32-channel head coil and a T1-weighted magnetization-prepared rapid gradient echo sequence (160 slices, 256 × 240 matrix, voxel size 1.1 × 1 × 1 mm3, TE = 4.21 ms, TR = 2300 ms, TI = 900 ms; α = 9°; total acquisition time 7 min, 46 s) were used.
MRI preprocessing was performed using the CAT12 toolbox (Gaser and Dahnke 2009) for SPM12 v.6685 (Wellcome Trust Center for Neuroimaging, Statistical Parametric Mapping, version 12, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/, last accessed 19 July 2019) and MATLAB R2015a (The MathWorks, Inc, 2015, http://www.mathworks.com/, last accessed 19 July 2019). First, images were segmented into GM, white matter (WM), and cerebrospinal fluid probability maps. Then, GM maps were registered to Montreal Neurological Institute space and spatially normalized via the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra algorithm (Ashburner 2007). GM maps were modulated with the default option “modulated normalized” to analyze volumetric patterns differentiating between the target labels as described below. Finally, TIV was estimated. No spatial smoothing was applied as part of the MRI preprocessing because Gaussian smoothing was optimized between 0, 4, and 8 mm within the MVPA pipeline (see details in Supplementary material).
Multivariate Pattern Classification Analysis
Our open-source pattern recognition tool NeuroMiner (version 0.998; (Koutsouleris 2009) developed by N.K. was used to implement a fully automated machine learning pipeline to determine sets of predictive neuroanatomical features from the GM maps that best classify the study population according to sex and gender. More specifically, binary classification models were trained to predict: 1) sex in cisgender subjects (FC vs. MC, N = 69); 2) sex in transgender subjects (TM vs. TW, N = 52); 3) gender identity in all subjects (FC + TM vs. MC + TW, N = 121); 4) gender identity in biological females (FC vs. TM, N = 64); 5) biological males (MC vs. TW, N = 57); and 6) gender incongruence (FC + MC vs. TM + TW, N = 121). Model training and validation was performed using a repeated nested cross-validation (CV) framework as implemented in NeuroMiner, which strictly separated the training process from the evaluation of the predictors’ generalization capacity, as detailed previously (Borgwardt et al. 2013; Koutsouleris et al. 2015; Koutsouleris et al. 2018) and in the Supplementary material. This produced decision scores for each group comparison, which are arbitrary numbers between −1 and 1 measuring the neuroanatomical likeness of being either female versus male (FC vs. MC, TM vs. TW), cis- versus transgender (FC + MC vs. TM + TW) or exhibiting a female or male gender identity (FC vs. TM, MC vs. TW) of each single subject. Every participant obtaining a decision score >0 belongs to group A and decision scores < 0 belongs to group B. To test the model significance and generalizability, we determined whether the observed prediction performances for the sex and gender predictors significantly differed from a null distribution of the respective outcome labels by training and cross-validating SVM models on n = 1000 random label permutations (Koutsouleris et al. 2017). Model significance was defined at α = 0.05 as P = ∑n = 1000(balanced accuracy [BAC]observed < =BACpermuted)/n, where BAC = (sensitivity + specificity)/2. P values were corrected for multiple comparisons using the false discovery rate (pFDR) (Benjamini and Hochberg 1995).
To validate significant models further, we adopted two different approaches: using a crossover machine learning design, we tested whether the sex classifier trained on cisgender subjects could predict sex in transgender subjects and vice versa. Moreover, to determine the construct and discriminant validity of our models, we applied our cis- and transgender sex classifier on an independent data set of female and male medication-naïve patients suffering from major depression (N = 27). Furthermore, the cisgender sex classifier was applied on parts of our transgender sample (N = 32) for whom serial MRI scans were available after 4 weeks and 4 months of cross-sex hormonal treatment, respectively, to determine the influence of treatment on the individual expression of the discriminative pattern over time. The sample to which the respective classification model was applied was processed identically to the sample on which the models were created as described above. For further information regarding both validation samples see Supplementary material.
Additionally, we adopted a classical univariate approach with our data for comparison with our MVPA findings. Univariate data processing and results are detailed in the Supplementary material.
Statistical Analyses
Descriptive statistics and further analyses were performed in IBM SPSS v25, MATLAB R2015a, and R v3.
Results
Demographic Data
No statistically significant differences regarding age were observed between groups (F(3) = 1.71, P = 0.17, ANOVA, see Table 1). Regarding the distribution of sexual orientation and hormone levels, see Table 1 and Supplementary material.
Table 1.
Demographic data of the study sample
| Group | FC | TM | MC | TW | F/ch2 | P | |
|---|---|---|---|---|---|---|---|
| N | 121 | 35 | 29 | 34 | 23 | ||
| Age ± SD | 27.46 ± 6.66 | 26.29 ± 5.90 | 27.17 ± 6.29 | 27.09 ± 6.33 | 30.17 ± 8.24 | 1.72 | 0.17 |
| SexO | Hetero | 9 | 3 | 22 | 7 | 34.69 | <0.001 |
| Bi | 14 | 7 | 9 | 11 | |||
| Homo | 12 | 19 | 3 | 5 | |||
| E2 | 113.59 ± 12.70 | 110.03 ± 13.35 | 27.56 ± 12.70 | 25.55 ± 15.32 | 13.42 | <0.001 | |
| T | 0.32 ± 0.23 | 0.35 ± 0.25 | 5.12 ± 0.23 | 5.14 ± 0.28 | 126.67 | <0.001 |
Note: Demographic data of the study sample. FC, TM, (syn. female-to-male transgender); MC; TW (syn. male-to-female transgender); SexO, sexual orientation based on biological sex; Hetero, heterosexual; Bi, bisexual; Homo, homosexual; E2, estrogen in pg/ml; T, testosterone in ng/ml. Age did not differ between groups. Sexual orientation was not equally distributed across groups with mostly heterosexual men and homosexual women. E2 and T levels were significantly different across groups (ANOVA, F = 13.42 and F = 126.67, respectively, both P < 0.001). Post hoc t-tests corrected for multiple comparisons revealed differences in E2 and T levels between women and men irrespective of gender identity. The normal hormone ranges for E2 are 26.70–298.00 and 27.10–52.20 pg/mL and for T are 0.08–0.48 and 2.50–8.40 ng/mL for women and men, respectively (www.kimcl.at, last accessed 19 July 2019). Significant p values are marked in bold.
Neuroanatomical Classification of Biological Sex
The neuroanatomical sex classifier trained on the cisgender participants (FC vs. MC) correctly separated biological women from men with a cross-validated BAC of 82.6% (specificity, Spec = 82.4% and sensitivity, Sens = 82.9%; for more details, see Table 2). The model was highly significant (pFDR < 0.001 after permutation testing). In contrast, the sex classifier trained on the transgender sample (TM vs. TW) provided a cross-validated BAC of 67.5% (pFDR = 0.04, Spec = 52.2%, Sens = 82.8%), corresponding to a significantly lower classification performance than the cisgender sex classifier (χ2 = 10, P < 0.001, see Supplementary material).
Table 2.
Model performances
| Group comparisons | TP | FP | TN | FN | Spec (%) | Sens (%) | FPR | PPV | NPV | AUC | BAC | pFDR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trained models | Cisgender sex classifier FC versus MCa | 29 | 6 | 28 | 6 | 82.35 | 82.86 | 17.67 | 82.86 | 82.35 | 0.90 | 82.61 | <0.001 |
| Transgender sex classifier TM versus TWb | 24 | 11 | 12 | 5 | 52.17 | 82.76 | 47.83 | 68.57 | 70.59 | 0.73 | 67.47 | 0.04 | |
| Gender classifier in all FC + TW versus TM + MC | 19 | 25 | 38 | 39 | 60.32 | 32.76 | 39.68 | 43.18 | 49.35 | 0.49 | 46.54 | 0.77 | |
| Gender classifier in biological male MC versus TW | 24 | 15 | 8 | 10 | 34.78 | 70.59 | 65.22 | 61.54 | 44.44 | 0.57 | 52.69 | 0.43 | |
| Gender classifier in biological female FC versus TM | 24 | 17 | 12 | 11 | 41.38 | 68.57 | 58.62 | 58.54 | 52.17 | 0.53 | 54.98 | 0.38 | |
| Gender incongruence classifier FC + MC versus TM + TW | 50 | 30 | 22 | 19 | 42.31 | 72.46 | 57.69 | 62.50 | 53.66 | 0.58 | 57.39 | 0.11 | |
| Cross-validation | Cisgender sex classifiera in transgender TM versus TW | 24 | 7 | 16 | 5 | 69.57 | 82.76 | 30.43 | 77.42 | 76.19 | 0.82 | 76.16 | - |
| Transgender sex classifierb in cisgender FC versus MC | 29 | 13 | 21 | 6 | 61.76 | 82.86 | 38.24 | 69.05 | 77.78 | 0.85 | 72.31 | - | |
| Validation | Cisgender sex classifiera on depressed FDEP versus MDEP | 8 | 1 | 12 | 6 | 74.07 | 92.31 | 66.67 | 88.89 | 66.67 | 0.87 | 74.73 | - |
| Cisgender sex classifiera on transgender TM versus TW 4w | 15 | 3 | 9 | 5 | 75.00 | 75.00 | 25.00 | 83.33 | 64.29 | 0.81 | 75.00 | - | |
| Cisgender sex classifiera on transgender TM versus TW 4m | 15 | 3 | 9 | 5 | 75.00 | 75.00 | 25.00 | 83.33 | 64.29 | 0.80 | 75.00 | - | |
| Transgender sex classifierb on depressed FDEP versus MDEP | 11 | 9 | 4 | 3 | 30.77 | 78.57 | 69.23 | 55.00 | 57.14 | 0.72 | 54.67 | - |
Note: Model performances evaluated by means of Spec, Sens, false-positive rate (FPR), positive and negative predicted value (PPV and NPV), area under the receiver operating characteristic curve (AUC), and BAC. These measures were computed from the confusion matrix containing the number of true positives (TP), false positives (FP), true negatives (TN), and false negatives (FM). FC; MC; TM (syn. female-to-male transgender); TW (syn. male-to-female transgender); FDEP, female depressed; MDEP, male depressed; 4w, after 4 weeks of cross-sex hormonal treatment; 4m, after 4 months of cross-sex hormonal treatment. P values are based on a test where the observed prediction performances for each model were compared to a null distribution of the respective outcome labels by training and cross-validating support vector machine models on n = 1000 random label permutations. Model significance was defined at α = 0.05 as P = ∑n = 1000(BACobserved < BACpermuted)/n. P values were corrected for multiple comparisons using the pFDR. Significant p values and BAC are marked in bold.
aSex classifier trained in cisgender individuals
bSex classifier trained in transgender
The neuroanatomical patterns distinguishing between both sexes and chosen by 95% of the models of each classification, respectively, are displayed in Figure 1.
Figure 1.
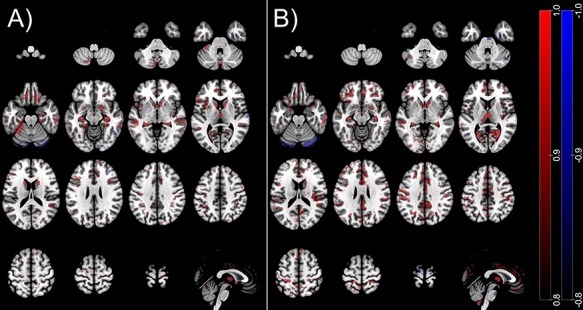
Voxel selection probability maps of cis- and transgender sex classifiers. Red/blue areas indicate volume increments/reductions in (A) cisgender female versus male (FC vs. MC) and (B) TM versus TW. Accordingly, red = F > M and blue = F < M. To visualize the average decision function, we used a method described previously (Koutsouleris et al. 2015). The colored areas shown represent voxels that contribute with a probability of 80% to the average neuroanatomical decision boundary in the respective model trained, overlaid on the single-subject MNI template using the software MRIcroGL (https://www.nitrc.org/projects/mricrogl, last accessed 19 July 2019).
In cisgender individuals, the neuroanatomical decision function involved less GM in men versus women covering the superior, medial, and inferior frontal cortex; the anterior and middle cingulate cortex; the middle temporal and superior occipital cortex; the lingual, fusiform, parahippocampal, and calcarine cortex; the post- and precentral cortex; the caudate, hippocampus, insula, and the thalamus as well as parts of the cerebellum. Less GM volume (GMV) in women versus men was more limited and localized in the middle frontal cortex, the inferior and superior parietal cortex, superior temporal and fusiform cortex, and the cerebellum. In transgender subjects, less GM in men versus women was observed in the inferior, superior, and middle frontal cortex; the anterior, middle, and posterior cingulate cortex; the insula; the thalamus; the precuneus and calcarine; the hippocampus and fusiform cortex; the caudate; the angular and supramarginal cortex; the pre- and postcentral cortex; and the precuneus and middle occipital cortex. Less GMV in women versus men was limited to the cerebellum (see Fig. 1). Although both sex classifier patterns partly overlap (caudate, hippocampus, and fusiform gyrus, see Fig. 2), the most striking differences can be observed in the insula, anterior cingulate cortex, thalamus, frontal gyrus, and precuneus regions appearing to depict a significantly more pronounced pattern in discriminating between women in men in transgender as compared to cisgender individuals (see Fig. 2).
Figure 2.
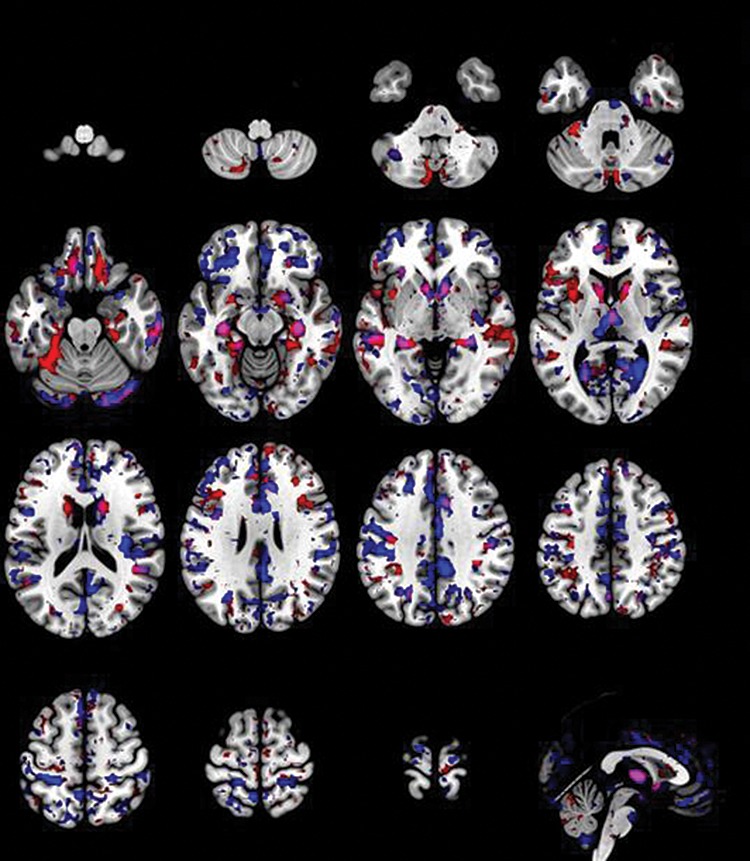
Overlay of voxel selection probability maps of cis- and transgender sex classifiers. Voxel selection probability maps shown in red represent the absolute values (positive and negative) of the voxels that reliably contributed to the classification of FC versus MC. The map in blue depicts the absolute values (positive and negative) of the relevant voxels for the classification between TM versus TW. The overlapping regions between the two classifiers are shown in magenta (bilateral caudate, hippocampus, fusiform gyrus).
Neuroanatomical Prediction Performance of Gender Identity and Gender Incongruence
The neuroanatomical gender identity classifier did not successfully separate individuals with a female gender identity (FC + TW) from individuals with a male gender identity (MC + TM; BAC = 46.5%, Spec = 60.3%, Sens = 32.8%, pFDR = 0.77, Table 2). Accordingly, no separation could be found when splitting the sample with respect to biological sex, namely FC versus TM (BAC = 55.0%, pFDR = 0.38, Spec = 41.4%, Sens = 68.6%) and MC versus TW (BAC = 52.7%, pFDR = 0.43, Spec = 34.8%, Sens = 70.6%). Similarly, no significant gender incongruence classifier could be trained on the whole sample separating cis- and transgender individuals (FC + MC vs. TM + TW, BAC = 57.4%, pFDR = 0.11, Spec = 42.3%, Sens = 72.5%, see Table 2).
Validation of the Sex Classifiers
First, using the crossover machine learning design, the cisgender sex classifier separated biological women and men in the transgender sample with a BAC of 76.2% (Spec = 69.6%, Sens = 82.8%, see Table 2) that was not significantly different from the classification performance in the cisgender sample (BAC = 82.6%, McNemar’s χ2 = 2.64, P = 0.10). Conversely, the transgender sex classifier applied to the cisgender individuals correctly differentiated both sexes with a BAC of 72.3% (Spec = 61.8%, Sens = 82.9%, see Table 2), which was also similar to the classification performance in the transgender sample (BAC = 67.5%, McNemar’s χ2 = 1.96, P = 0.20).
Secondly, the cisgender sex classifier model was tested on a subset of the transgender sample following 4 weeks and 4 months of cross-sex hormonal treatment. The cisgender sex classifier performed slightly but significantly worse at both time points with a BAC of 75.0% (Spec = 75.0%, Sens = 75.0%, McNemar’s χ2 = 12.64, P < 0.001) in the transgender sample after 4 weeks and a BAC of 75.0% (Spec = 75.0%, Sens = 75.0%, McNemar’s χ2 = 12.64 P < 0.001) after 4 months of treatment compared to baseline before treatment (BAC = 76.2%). Further details on this analysis are provided in the Supplementary material and Supplementary Figure 1A–D.
Finally, the cis- and transgender sex classifier models were adopted on an independent sample of medication-naïve patients suffering from major depression. When applying the cisgender sex classifier, depressed women and men were correctly classified with a BAC of 74.7% (Spec = 74.1%, Sens = 92.3%, similar to the performance in nondepressed cisgender subjects with McNemar’s χ2 = 1.96, P = 0.16). Conversely, the transgender sex classifier performed significantly worse (McNemar’s χ2 = 16.83, P < 0.001) in predicting sex in the sample of depressed patients (BAC = 54.7%, Spec = 30.8%, Sens = 78.6%, see also Supplementary Figure 1E).
Discussion
We investigated the sex- and gender-related neuroanatomical differences in cis- and transgender individuals by analyzing structural MRI data using machine learning. As initially hypothesized, the binary classification models trained in our sample of 121 subjects correctly classified women and men in cisgender subjects (FC vs. MC, N = 69) with a BAC of 83% and in transgender subjects (TM vs. TW, N = 52) with a BAC of 68%. In fact, this difference in BAC of 15% corresponds to a significantly lower sex classification performance in transgender subjects. The determined significant cis- and transgender sex classifier could be validated when applied to the transgender (BAC = 76%) and cisgender samples (BAC = 72%), respectively. When externally validating our classifiers in a sample of depressed patients, the cisgender classifier still produced a BAC of 75%, while the transgender sex classifier performed significantly worse (BAC = 55%, see Supplementary material). When applying the cisgender sex classifier to the subgroup of transgender participants receiving hormone treatment, a small yet significant change in prediction performance could be observed (baseline BAC = 76%, BAC after 4 weeks of treatment = 75%, BAC after 4 months of treatment = 75%, see also Supplementary material). We could not confirm our hypothesis that neuroanatomical classification would allow to distinguish male or female gender identity independently of the biological sex of a given person or the presence of gender incongruence.
Sex
To the best of our knowledge, this is the first study to show a significantly lower classification performance in transgender compared to cisgender individuals related to biological sex implying an interaction between biological sex and gender identity at the neuroanatomical level. The performance of our cisgender sex classifier replicates recent studies reporting on a single-person separability of the biological sex of 83% in a derivation sample and 77% in a replication sample by analyzing cortical thickness using machine learning (Sepehrband et al. 2018). Our findings are also in keeping with another study investigating sex differences in cis- and transgender youth (age < 19 years) using MVPA that showed a prediction accuracy of sex in cisgender subjects of 88%. Although a specific transgender sex classification (TM vs. TW) was not performed in this work, untreated TM and TW could be separated from MC (accuracy of 78%) and FC (accuracy of 86%), respectively, implying that sex can be predicted irrespective of gender identity (Hoekzema et al. 2015).
The widespread volumetric patterns underlying our sex classification models (see Fig. 1) primarily involved areas of increased GMV in female versus male participants except for the cerebellum that showed the opposite effect. Interestingly, a previous study on the same dataset described decreased intrahemispheric structural connections of subcortical/limbic to frontal and temporal areas in TM in comparison with TW and cisgender individuals (Hahn et al. 2015a). This might elucidate a balancing mechanism, in which brain volume is enlarged to compensate for less communication between areas in transgender individuals with female natal sex. As for the cerebellum, it appears to be consistently discriminating to biological sex regardless of gender identity. A study on 2-year cross-sex hormone-treated transgender showed that this structure is bilaterally larger in cisgender male than in cisgender female and TM even after at least 2 years of therapy (Mueller et al. 2017).
Even though our study was performed using structural data, it is of note that the most striking differences between the voxel probability maps of both sex classifiers were observed in regions belonging to the resting-state fMRI salience network (Menon 2015), namely the anterior cingulate cortex and the insula, and the default mode network (Raichle et al. 2001), namely the prefrontal, cingulate, precuneal, and hippocampal cortices. This finding is not surprising considering that both networks are thought to be responsible for connecting bodily perceptions and emotional states, self-reference, emotion of one’s self, theory of mind and social behavior, and self-awareness, respectively. The role of the insula acting as a key mediator of the subjective experience of emotions evoked by internal bodily sensations is especially noteworthy in this context (Ortigue et al. 2007; Uddin et al. 2017). Spizzirri et al. (2018) showed alterations in the insular cortex of untreated TW and speculated that these are related to the neural network of body perception and reflect the distress accompanying gender incongruence. It seems quite intuitive that the process of reflection about one’s self and the physical perception of one’s self is highly sex-specific and changed in persons experiencing an incongruent gender identity and natal sex (Nota et al. 2017).
The stability of our sex classifiers (cis- and transgender) was further underlined by the findings of our CV analysis, resulting in unchanged classification performance in both ways. However, as shown in Supplementary Figure 1B, the distribution of the decision scores produced by the cisgender sex classification model was less discriminative in transgender individuals. Specifically, the decision scores of TW subjects were shifted toward the positive range (=female) of the histogram implying a higher variability in sexually differing brain patterns in these subjects, in other words a less stable sex pattern in TW subject. This “feminization” of TW brain patterns is supported by several postmortems and structural MRI reports (Zhou et al. 1995; Kruijver et al. 2000; Garcia-Falgueras and Swaab 2008; Simon et al. 2013; Zubiaurre-Elorza et al. 2013); however, not all studies necessarily support this notion (Luders et al. 2009; Savic and Arver 2011; Guillamon et al. 2016). Additionally, we showed that the cisgender sex classifier was significantly less accurate when applied on the treated transgender sample across time (see Table 2). When looking in depth at the distribution of the decision scores (Supplementary Fig. 1C,D), again the TW scores were shifted in the “female” range, while TM scores remained largely unchanged over time. Numerically, the BAC decreased only by ~1% at both 4 weeks and 4 months of treatment compared to baseline; however, this decrease was significant and solely driven by effects of cross-sex hormone therapy in TW subjects. This is in line with previous findings by our group in the same sample showing significant volume decreases in the right hippocampus in TW subjects after 16 weeks of hormone administration compared to baseline, but no changes in TM subjects (Seiger et al., 2016). Effects in TM participants might be only visible after a more prolonged treatment time (Nguyen et al. 2019), as evident in a recent study in which the mean neuroanatomical volume for the amygdala, putamen, and corpus callosum differed between TM and FC after gender affirming surgery and at least 2 years of cross-sex hormone therapy (Mueller et al. 2017). The same group reported a correlation of androgens and local functional connectivity in long-term (>80 months) treated TM in the cerebellum and frontal regions, an association that was not detected in TW with estrogens (Mueller et al. 2016). Other authors have reported regional increases in cortical thickness associated with testosterone in TM subjects following 6 months of hormonal treatment. Estrogens and antiandrogens were associated with cortical thickness increments in TW (Zubiaurre-Elorza et al. 2014). Our findings imply a higher sensitivity of male brain structure to the influence of cross-sex hormonal treatment, mirrored in the decreased capacity of the cisgender sex classifier to separate female and male subjects in the treated transgender sample.
Finally, the strength of our cisgender sex classification model was confirmed when applied on depressed women and men (see Supplementary material), which was not true for our transgender sex classifier indicating a considerable spatial overlap between the sex differing brain regions selected by the transgender classifier and brain regions affected by brain pathology associated with depression, for instance, the insula, the amygdala, the anterior cingulate cortex, and the posterior cingulate cortex (Craddock et al. 2009; Lois and Wessa 2016).
Gender and Gender Incongruence
We did not detect a neuroanatomical pattern that separates individuals with a female and male gender identity (FC + TW vs. TM + MC, FC vs. TM, MC vs. TW). This is in agreement with the study performed by Hoekzema et al. (2015) who—using a similar methodology in adolescents—could not determine a gender identity signature on whole-brain voxel level separating FC from TM and MC from TW, respectively. On the other hand, this stands in contrast to previously published studies using univariate statistical analyses and gender incongruence as a model to show distinct regional differences between transgender and control subjects sharing the same biological sex (namely FC and TM, MC and TW) (Luders et al. 2009; Savic and Arver 2011; Simon et al. 2013; Spizzirri et al. 2018).
Furthermore, here, we were not able to detect a distinct neuroanatomical signature of gender incongruence (FC + MC vs. TM + TW). Previous studies comparing the brain structure of cis- versus untreated transgender individuals using MRI are limited and inconsistent in regard the direction of change and the brain regions associated with male and female gender identity (Smith et al. 2015). Simon et al. (2013) showed higher GMV in cisgender subjects in the cerebellum, left angular gyrus, and left inferior parietal lobule as compared to transgender subjects (TW and TM) independently from their biological sex. Savic and Arver (2011) reported decreased GMV in the thalamus and putamen and increased GMV and the right insular, inferior frontal, and right angular gyrus in TW compared to both cisgender women and men. Luders et al. (2009) showed that the brain structure of TW subjects more closely resembles individuals sharing their natal sex (MC) than their gender identity (FC) apart from the right putamen that was shown to be significantly larger in TW compared to MC. Consistently, gender incongruence was associated with brain anatomical findings as shown previously in postmortem studies for the INAH-1 (Garcia-Falgueras and Swaab 2008) and the size (Zhou et al. 1995) and neuron number (Kruijver et al. 2000) of the red nucleus of the stria terminalis.
In fact, the MVPA performed in this study was not designed to identify such region-specific and small differences but rather to detect widespread brain structural pattern carrying discriminative value. As such, even though our model was not able to define such patterns when investigating gender identity or gender incongruence, we cannot exclude that region-specific volume differences in male and female gender identity are present. Gender identity might exhibit—if any—a considerably more subtle and regional GMV signature, which is potentially masked by prominent sex effects. Potentially, gender identity might require a certain degree of smoothing in the data in order to be captured by the algorithm. On the other hand, such gender identity effects might be more strongly associated with WM patterns and structural and functional connectivity alterations, which can be measured using DTI and resting-state fMRI. This is backed by fMRI studies showing specific hormone-induced resting-state connectivity patterns that are different from the assigned and aspired sex in treated TW (Mueller et al. 2016; Clemens et al. 2017). Also, weaker structural and functional connectivity between the anterior cingulate-precuneus and the right occipito-parietal cortex was recently reported in TW and TM compared to cisgender controls (Manzouri and Savic 2018b). Additionally, using univariate methods and other MRI modalities, deviating WM fiber tracts (Kranz et al. 2014; Rametti et al. 2019), altered structural connectivity (Hahn et al. 2015a) and functional connectivity (Lin et al. 2014) have been described in transgender subjects, which supports further research in this direction.
To our knowledge, the only previously published MVPA of sex and gender identity, including both cis- and transgender subjects, did not compute a gender incongruence classification to enable a comparison with our data (Hoekzema et al. 2015).
Limitations
There are a few limitations to our analysis that need to be addressed. First of all, additional clinical and psychometric data to further characterize our transgender sample (e.g., education, quality of life, and depression scales for all participants including the cisgender sample) and disentangle neuroanatomical effects driven by gender incongruence or the associated psychological distress would be desirable. Future research should consider further clinical variables to account for potential subsyndromal depressive symptoms or other conditions related to gender incongruence. We would like to emphasize that at variance to previous reports we did include cis- and transgender individuals with divergent sexual orientation (hetero-, homo-, and bisexual) and provide data on hormonal levels that were considered in our analysis. At the same time, however, the inclusion of study populations with divergent sexual orientation might represent an additional bias. Although we made efforts to exclude a potential influence of sexual orientation (see Supplementary material), we cannot definitely rule out that the effects of gender incongruence on our GMV-based sex classifier (less-pronounced sexual dimorphism in transgender individuals) might be overlapping with effects driven by sexual orientation as homosexuality was shown to be associated with less cerebral sexual differentiation in WM tracts (Burke et al. 2017; Manzouri and Savic 2018a, 2018b). Future research in this direction should consider balancing the groups according to sexual orientation or including heterosexual cis- and transgender populations only.
Secondly, due to the limited sample size in our transgender sample, we were not able to perform an external validation of the gender incongruence classifier, which will be subject to future research. By increasing the sample size, also a better representation of the psychiatric comorbidities can be delineated, reaching better generalizability of our findings in daily clinical settings. Also, the external validation sample of depressed patients used here was measured on a different MR scanner. While we cannot rule out certain scanner effects, we assume that these do not explain the fact that the transgender sex classifier performed significantly worse than the cisgender sex classifier in predicting sex in the depressed sample as they would interfere with both models in a similar way.
Conclusions
A better understanding of neurobiological sex differences in terms of prevalence discrepancies, differing disease trajectories and outcomes in diverse psychiatric conditions is indispensable (Cahill 2006). Research dealing with gender issues in neuroscience claims that our brains are individual mosaics of female and male characteristics, thereby rejecting the simplistic idea of a “female” or “male” brain (Maney 2014; Joel et al. 2015). In light of this general rethinking, our findings support previously published evidence demonstrating that the brain structure of transgender people partially converges on an assumed sex continuum, although we cannot conclude from our findings that it resembles the morphology of the respective gender identity (Swaab 2007; Savic et al. 2010). Further studies using MVPA and other data modalities are needed to determine whether this entity is rather reflected in brain connectivity or WM. Generally, we can conclude from our analysis that sex has a major effect on GM irrespective of the self-perception of being a woman or a man. Also, in contrast to previous univariate analyses, structural brain changes in regard to sex are not limited to single areas but involve multiple regions and brain networks. Clinically, it would now be of major interest to assess whether transgender persons exhibit long-term brain structural change when receiving cross-sex hormonal treatment and thereby experience a shift to a risk profile for certain brain diseases in their gender identity. Ideally, further longitudinal studies using a pooled transgender sample and multiple imaging modalities should be envisaged to clarify the matter. Finally, in comparison with univariate analyses performed on the same datasets, our findings provide novel relevant insights into basic human biology in the context of brain morphological sex differences and gender identity reflected in brain structure.
Funding
Austrian Science Fund (FWF) (grant numbers P 23021 and KLI 504 to R.L.); Erasmus+ Staff Mobility grant (to P.B.-M. to complete her stay as a scientific assistant in the group of N.K. in Munich, Germany); Hochschuljubilaeumsstiftung, City of Vienna, Austria (to R. Seiger); DOC Fellowship of the Austrian Academy of Sciences at the Department of Psychiatry and Psychotherapy of the Medical University of Vienna (to M. Klöbl), PRONIA (for the NeuroMiner toolbox data analysis); European Union under the 7th Framework Programme (for a Collaborative Project, grant 602152 coordinated by N.K.).
Notes
We thank D. Winkler, A.S. Höflich, M. Spies, J. Unterholzner, and G.M. Godbersen for medical support. We also thank R. Sladky, L. Betz, and L. Antonucci for technical support. We are especially grateful to all transgender individuals for participating in this study. Preliminary findings of this study were presented at the 31th ECNP congress in Barcelona, Spain. Conflict of Interest: Without any relevance to this work, P. Baldinger-Melich declares that she has received travel grants and conference speaker honoraria from Austroplant. G.S. Kranz received travel grants and/or conference speaker honoraria from Orphan Pharmaceuticals AG, Pfizer, and Roche Austria GmbH. S. Kasper received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, Celegne GmbH, Eli Lilly, Janssen-Cilag Pharma GmbH, KRKA-Pharma, Lundbeck A/S, Mundipharma, Neuraxpharm, Pfizer, Sanofi, Schwabe, Servier, Shire, Sumitomo Dainippon Pharma Co. Ltd, and Takeda. R. L. received travel grants and/or conference speaker honoraria from Shire, AstraZeneca, Lundbeck A/S, Dr Willmar Schwabe GmbH, Orphan Pharmaceuticals AG, Janssen-Cilag Pharma GmbH, and Roche Austria GmbH. M.F. Urquijo Castro, R. Seiger, A. Ruef, D.B. Dwyer, M. Klöbl, J. Kambeitz, U. Kaufmann, C. Windischberger, P. Falkai, and N.K. declare that they have no conflict of interest to report.
Supplementary Material
References
- Alexander GM, Wilcox T, Woods R. 2009. Sex differences in infants’ visual interest in toys. Arch Sex Behav. 38(3):427–433. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Chen X. 2009. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 30(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. 2007. A fast diffeomorphic image registration algorithm. Neuroimage. 38(1):95–113. [DOI] [PubMed] [Google Scholar]
- Bao A-M, Swaab DF. 2010. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist. 16(5):550–565. [DOI] [PubMed] [Google Scholar]
- Bao A-M, Swaab DF. 2011. Sexual differentiation of the human brain: relation to gender identity, sexual orientation and neuropsychiatric disorders. Front Neuroendocrinol. 32(2):214–226. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 57(1):289–300. [Google Scholar]
- Bocklandt S, Vilain E. 2007. Sex differences in brain and behavior: hormones versus genes. Adv Genet. 59:245–266. [DOI] [PubMed] [Google Scholar]
- Borgwardt S, Koutsouleris N, Aston J, Studerus E, Smieskova R, Riecher-Rössler A, Meisenzahl EM. 2013. Distinguishing prodromal from First-episode psychosis using neuroanatomical single-subject pattern recognition. Schizophr Bull. 39(5):1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SM, Manzouri AH, Savic I. 2017. Structural connections in the brain in relation to gender identity and sexual orientation. Sci Rep. 7(1):17954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. 2006. Why sex matters for neuroscience. Nat Rev Neurosci. 7(6):477–484. [DOI] [PubMed] [Google Scholar]
- Clemens B, Junger J, Pauly K, Neulen J, Neuschaefer-Rube C, Frölich D, Mingoia G, Derntl B, Habel U. 2017. Male-to-female gender dysphoria: gender-specific differences in resting-state networks. Brain Behav. 7(5):e00691–e00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock RC, Holtzheimer PE, Hu XP, Mayberg HS. 2009. Disease state prediction from resting state functional connectivity. Magn Reson Med. 62(6):1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ, Södersten P. 2009. Sex differences in the brain: the relation between structure and function. Horm Behav. 55(5):589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DB, Falkai P, Koutsouleris N. 2018. Machine learning approaches for clinical psychology and psychiatry. Annu Rev Clin Psychol. 14(1):91–118. [DOI] [PubMed] [Google Scholar]
- Feder S, Isserlin L, Seale E, Hammond N, Norris ML. 2017. Exploring the association between eating disorders and gender dysphoria in youth. Eat Disord. 25(4):310–317. [DOI] [PubMed] [Google Scholar]
- Fraser L, Karasic DH, Meyer WJ, Wylie K. 2010. Recommendations for revision of the DSM diagnosis of gender identity disorder in adults. Int J Transgend. 12(2):80–85. [Google Scholar]
- Ganger S, Hahn A, Küblböck M, Kranz GS, Spies M, Vanicek T, Seiger R, Sladky R, Windischberger C, Kasper S et al. . 2015. Comparison of continuously acquired resting state and extracted analogues from active tasks. Hum Brain Mapp. 36(10):4053–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Swaab DF. 2008. A sex difference in the hypothalamic uncinate nucleus: relationship to gender identity. Brain. 131Pt 12:3132–3146. [DOI] [PubMed] [Google Scholar]
- Gaser C, Dahnke R.. 2009. A Computational Anatomy Toolbox for SPM (CAT12). http://www.neuro.uni-jena.de/cat/, last accessed 19 July 2019.
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness JVS, Faraone SV, Tsuang MT. 2001. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 11(6):490–497. [DOI] [PubMed] [Google Scholar]
- Gonzalez CA, Gallego JD, Bockting WO. 2017. Demographic characteristics, components of sexuality and gender, and minority stress and their associations to excessive alcohol, cannabis, and illicit (noncannabis) drug use among a large sample of transgender people in the United States. J Prim Prev. 38(4):419–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooren LJ, Kreukels B, Lapauw B, Giltay EJ. 2015. (Patho) physiology of cross-sex hormone administration to transsexual people: the potential impact of male–female genetic differences. Andrologia. 47(1):5–19. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Junque C, Gómez-Gil E. 2016. A review of the status of brain structure research in Transsexualism. Arch Sex Behav. 45(7):1615–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Kranz GS, Küblböck M, Kaufmann U, Ganger S, Hummer A, Seiger R, Spies M, Winkler D, Kasper S et al. . 2015a. Structural connectivity networks of transgender people. Cereb Cortex. 25(10):3527–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Kranz GS, Sladky R, Ganger S, Windischberger C, Kasper S, Lanzenberger R. 2015b. Individual diversity of functional brain network economy. Brain Connect. 5(3):156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Kranz GS, Sladky R, Kaufmann U, Ganger S, Hummer A, Seiger R, Spies M, Vanicek T, Winkler D et al. . 2016. Testosterone affects language areas of the adult human brain. Hum Brain Mapp. 37(5):1738–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heylens G, Elaut E, Kreukels BPC, Paap MCS, Cerwenka S, Richter-Appelt H, Cohen-Kettenis PT, Haraldsen IR, De Cuypere G. 2018. Psychiatric characteristics in transsexual individuals: multicentre study in four European countries. Br J Psychiatry. 204(2):151–156. [DOI] [PubMed] [Google Scholar]
- Hoekzema E, Schagen SEE, Kreukels BPC, Veltman DJ, Cohen-Kettenis PT, Delemarre-van de Waal H, Bakker J. 2015. Regional volumes and spatial volumetric distribution of gray matter in the gender dysphoric brain. Psychoneuroendocrinology. 55:59–71. [DOI] [PubMed] [Google Scholar]
- Joel D, Berman Z, Tavor I, Wexler N, Gaber O, Stein Y, Shefi N, Pool J, Urchs S, Margulies DS et al. . 2015. Sex beyond the genitalia: the human brain mosaic. Proc Natl Acad Sci USA. 112(50):15468–15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambeitz J, Cabral C, Sacchet MD, Gotlib IH, Zahn R, Serpa MH, Walter M, Falkai P, Koutsouleris N. 2017. Detecting neuroimaging biomarkers for depression: a meta-analysis of multivariate pattern recognition studies. Biol Psychiatry. 82(5):330–338. [DOI] [PubMed] [Google Scholar]
- Kambeitz J, Kambeitz-Ilankovic L, Leucht S, Wood S, Davatzikos C, Malchow B, Falkai P, Koutsouleris N. 2015. Detecting neuroimaging biomarkers for schizophrenia: a meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacology. 40:1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel S, Stonnington CM, Barnes J, Chen F, Chu C, Good CD, Mader I, Mitchell LA, Patel AC, Roberts CC et al. . 2008. Accuracy of dementia diagnosis—a direct comparison between radiologists and a computerized method. Brain. 131(11):2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N. 2009. NeuroMiner. https://www.pronia.eu/neurominer/, last accessed 19 July 2019.
- Koutsouleris N, Kambeitz-Ilankovic L, Ruhrmann S, Rosen M, Ruef A, Dwyer DB, Paolini M, Chisholm K, Kambeitz J, Haidl T et al. . 2018. Prediction models of functional outcomes for individuals in the clinical high-risk state for psychosis or with recent-onset depression: a multimodal, multisite machine learning AnalysisPrediction models of functional outcomes for the clinical high-risk state for psychosis or recent-onset DepressionPrediction models of functional outcomes for the clinical high-risk state for psychosis or recent-onset depression. JAMA Psychiat. 75(11):1156–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N, Meisenzahl EM, Borgwardt S, Riecher-Rössler A, Frodl T, Kambeitz J, Köhler Y, Falkai P, Möller H-J, Reiser M et al. . 2015. Individualized differential diagnosis of schizophrenia and mood disorders using neuroanatomical biomarkers. Brain. 138(7):2059–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N, Wobrock T, Guse B, Langguth B, Landgrebe M, Eichhammer P, Frank E, Cordes J, Wölwer W, Musso F et al. . 2017. Predicting response to repetitive Transcranial magnetic stimulation in patients with schizophrenia using structural magnetic resonance imaging: a multisite machine learning analysis. Schizophr Bull. sbx114–sbx114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Hahn A, Kaufmann U, Küblböck M, Hummer A, Ganger S, Seiger R, Winkler D, Swaab DF, Windischberger C et al. . 2014. White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. J Neurosci. 34(46):15466–15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Hahn A, Kaufmann U, Tik M, Ganger S, Seiger R, Hummer A, Windischberger C, Kasper S, Lanzenberger R. 2018. Effects of testosterone treatment on hypothalamic neuroplasticity in female-to-male transgender individuals. Brain Struct Funct. 223(1):321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz GS, Seiger R, Kaufmann U, Hummer A, Hahn A, Ganger S, Tik M, Windischberger C, Kasper S, Lanzenberger R. 2017. Effects of sex hormone treatment on white matter microstructure in individuals with gender dysphoria. Neuroimage. 150:60–67. [DOI] [PubMed] [Google Scholar]
- Kruijver FP, Zhou JN, Pool CW, Hofman MA, Gooren LJ, Swaab DF. 2000. Male-to-female transsexuals have female neuron numbers in a limbic nucleus. J Clin Endocrinol Metab. 85(5):2034–2041. [DOI] [PubMed] [Google Scholar]
- Lentini E, Kasahara M, Arver S, Savic I. 2013. Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cereb Cortex. 23(10):2322–2336. [DOI] [PubMed] [Google Scholar]
- Lin C-S, Ku H-L, Chao H-T, Tu P-C, Li C-T, Cheng C-M, Su T-P, Lee Y-C, Hsieh J-C. 2014. Neural network of body representation differs between transsexuals and Cissexuals. PLoS One. 9(1):e85914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois G, Wessa M. 2016. Differential association of default mode network connectivity and rumination in healthy individuals and remitted MDD patients. Soc Cogn Affect Neurosci. 11(11):1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Domin M, Gerlach FH, Gaser C, Lueders E, Schmidt CO, Neumann N. 2019. Novel findings from 2,838 adult brains on sex differences in Gray matter brain volume. Sci Rep. 9(1):1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone M, La Montagna M, D'Urso F, Daniele A, Greco A, Seripa D, Logroscino G, Bellomo A, Panza F. 2019. The role of biomarkers in psychiatry. Adv Exp Med Biol. 1118:135–162. [DOI] [PubMed] [Google Scholar]
- Luders E, Sánchez FJ, Gaser C, Toga AW, Narr KL, Hamilton LS, Vilain E. 2009. Regional gray matter variation in male-to-female transsexualism. Neuroimage. 46(4):904–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL. 2014. Just like a circus: the public consumption of sex differences In: Ethical issues in behavioral neuroscience. Berlin: Springer, pp. 279–296. [DOI] [PubMed] [Google Scholar]
- Manzouri A, Savic I. 2018a. Multimodal MRI suggests that male homosexuality may be linked to cerebral midline structures. PLoS One. 13(10):e0203189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzouri A, Savic I. 2018b. Possible neurobiological underpinnings of homosexuality and gender dysphoria. Cereb Cortex. bhy090–bhy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. 2015. Salience network A2 - Toga, Arthur W.Brain mapping. Waltham: Academic Press; p. 597–611. [Google Scholar]
- Money J, Hampson JG, Hampson JL. 1955. An examination of some basic sexual concepts: the evidence of human hermaphroditism. Bull Johns Hopkins Hosp. 97(4):301–319. [PubMed] [Google Scholar]
- Mueller SC, Landré L, Wierckx K, T'Sjoen G. 2017. A structural magnetic resonance imaging study in transgender persons on cross-sex hormone therapy. Neuroendocrinology. 105(2):123–130. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Wierckx K, Jackson K, T’Sjoen G. 2016. Circulating androgens correlate with resting-state MRI in transgender men. Psychoneuroendocrinology. 73:91–98. [DOI] [PubMed] [Google Scholar]
- Ngun TC, Ghahramani N, Sanchez FJ, Bocklandt S, Vilain E. 2011. The genetics of sex differences in brain and behavior. Front Neuroendocrinol. 32(2):227–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HB, Loughead J, Lipner E, Hantsoo L, Kornfield SL, Epperson CN. 2019. What has sex got to do with it? The role of hormones in the transgender brain. Neuropsychopharmacology. 44(1):22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nota NM, Kreukels BPC, den Heijer M, Veltman DJ, Cohen-Kettenis PT, Burke SM, Bakker J. 2017. Brain functional connectivity patterns in children and adolescents with gender dysphoria: sex-atypical or not? Psychoneuroendocrinology. 86:187–195. [DOI] [PubMed] [Google Scholar]
- Øien RA, Cicchetti DV, Nordahl-Hansen A. 2018. Gender Dysphoria, sexuality and autism Spectrum disorders: a systematic map review. J Autism Dev Disord. 48:4028–4037. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Yoshimatsu H, Aou S. 1983. Medial preoptic and hypothalamic neuronal activity during sexual behavior of the male monkey. Brain Res. 266(2):340–343. [DOI] [PubMed] [Google Scholar]
- Ortigue S, Grafton ST, Bianchi-Demicheli F. 2007. Correlation between insula activation and self-reported quality of orgasm in women. Neuroimage. 37(2):551–560. [DOI] [PubMed] [Google Scholar]
- Peterson CM, Matthews A, Copps-Smith E, Conard LA. 2017. Suicidality, self-harm, and body dissatisfaction in transgender adolescents and emerging adults with gender dysphoria. Suicide Life Threat Behav. 47(4):475–482. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci. 98(2):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rametti G, Carrillo B, Gómez-Gil E, Junque C, Segovia S, Gomez Á, Guillamon A. 2019. White matter microstructure in female to male transsexuals before cross-sex hormonal treatment. A diffusion tensor imaging study. J Psychiatr Res. 45(2):199–204. [DOI] [PubMed] [Google Scholar]
- Reed GM, Drescher J, Krueger RB, Atalla E, Cochran SD, First MB, Cohen-Kettenis PT, Arango-de Montis I, Parish SJ, Cottler S et al. . 2016. Disorders related to sexuality and gender identity in the ICD-11: revising the ICD-10 classification based on current scientific evidence, best clinical practices, and human rights considerations. World Psychiatry 15(3):205–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, Harris MA, Alderson HL, Hunter S, Neilson E et al. . 2018. Sex differences in the adult human brain: evidence from 5216 UK biobank participants. Cereb Cortex. 28(8):2959–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. 2014. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 39:34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge RB, Chekroud AM, Huys QJM. 2019. Machine learning and big data in psychiatry: toward clinical applications. Curr Opin Neurobiol. 55:152–159. [DOI] [PubMed] [Google Scholar]
- Savic I, Arver S. 2011. Sex dimorphism of the brain in male-to-female transsexuals. Cereb Cortex. 21(11):2525–2533. [DOI] [PubMed] [Google Scholar]
- Savic I, Garcia-Falgueras A, Swaab DF. 2010. Sexual differentiation of the human brain in relation to gender identity and sexual orientation. Prog Brain Res. 186:41–62. [DOI] [PubMed] [Google Scholar]
- Seiger R, Hahn A, Hummer A, Kranz GS, Ganger S, Küblböck M, Kraus C, Sladky R, Kasper S, Windischberger C et al. . 2015. Voxel-based morphometry at ultra-high fields. A comparison of 7T and 3T MRI data. Neuroimage. 113:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiger R, Hahn A, Hummer A, Kranz GS, Ganger S, Woletz M, Kraus C, Sladky R, Kautzky A, Kasper S et al. . 2016. Subcortical gray matter changes in transgender subjects after long-term cross-sex hormone administration. Psychoneuroendocrinology. 74:371–379. [DOI] [PubMed] [Google Scholar]
- Sepehrband F, Lynch KM, Cabeen RP, Gonzalez-Zacarias C, Zhao L, D'Arcy M, Kesselman C, Herting MM, Dinov ID, Toga AW et al. . 2018. Neuroanatomical morphometric characterization of sex differences in youth using statistical learning. Neuroimage. 172:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Kozák LR, Simon V, Czobor P, Unoka Z, Szabó Á, Csukly G. 2013. Regional Grey matter structure differences between transsexuals and healthy controls—a voxel based Morphometry study. PLoS One. 8(12):e83947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ES, Junger J, Derntl B, Habel U. 2015. The transsexual brain – a review of findings on the neural basis of transsexualism. Neurosci Biobehav Rev. 59:251–266. [DOI] [PubMed] [Google Scholar]
- Spies M, Hahn A, Kranz GS, Sladky R, Kaufmann U, Hummer A, Ganger S, Kraus C, Winkler D, Seiger R et al. . 2016. Gender transition affects neural correlates of empathy: a resting state functional connectivity study with ultra high-field 7T MR imaging. Neuroimage. 138:257–265. [DOI] [PubMed] [Google Scholar]
- Spizzirri G, Duran FLS, Chaim-Avancini TM, Serpa MH, Cavallet M, Pereira CMA, Santos PP, Squarzoni P, da Costa NA, Busatto GF et al. . 2018. Grey and white matter volumes either in treatment-naive or hormone-treated transgender women: a voxel-based morphometry study. Sci Rep. 8(1):736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF. 2007. Sexual differentiation of the brain and behavior. Best Pract Res Clin Endocrinol Metab. 21(3):431–444. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E. 1985. A sexually dimorphic nucleus in the human brain. Science. 228(4703):1112–1115. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. 2017. Structure and function of the human insula. J Clin Neurophysiol: Off Publ Am Electroencephalogr Soc. 34(4):300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine SE, Shipherd JC. 2018. A systematic review of social stress and mental health among transgender and gender non-conforming people in the United States. Clin Psychol Rev. 66:24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 2019. https://www.who.int/health-topics/international-classification-of-diseases, last accessed 19 July 2019.
- Zhou J-N, Hofman MA, Gooren LJG, Swaab DF. 1995. A sex difference in the human brain and its relation to transsexuality. Nature. 378:68–70. [DOI] [PubMed] [Google Scholar]
- Zubiaurre-Elorza L, Junque C, Gómez-Gil E, Guillamon A. 2014. Effects of cross-sex hormone treatment on cortical thickness in transsexual individuals. J Sex Med. 11(5):1248–1261. [DOI] [PubMed] [Google Scholar]
- Zubiaurre-Elorza L, Junque C, Gómez-Gil E, Segovia S, Carrillo B, Rametti G, Guillamon A. 2013. Cortical thickness in untreated transsexuals. Cereb Cortex. 23(12):2855–2862. [DOI] [PubMed] [Google Scholar]
- Zucker KJ. 2017. Epidemiology of gender dysphoria and transgender identity. Sex Health. 14(5):404–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


