Abstract
Mucus is a hydrogel that exhibits complex selective permeability, permitting the passage of some particles while restricting the passage of other particles including important therapeutics. In this review, we discuss biochemical mechanisms underlying mucus penetration and mucus binding, emphasizing the importance of steric, electrostatic, and hydrophobic interactions. We discuss emerging techniques for engineering nanoparticle surface chemistries for mucus penetration as well as recent advances in tuning mucus interactions with small molecule, peptide, or protein therapeutics. Finally, we highlight recent work suggesting that mucus permeability can serve as a biomarker for disease and physiological states such as pregnancy.
Graphical Abstract
Introduction
Mucus is a selectively permeable gel that covers all non-keratinized surfaces in the human body, including the respiratory, gastrointestinal and urogenital tracts [1]. The mucus barrier has critical functions in protecting tissues from attacks by pathogens and toxins, while permitting transport of beneficial particles such as nutrients and sperm. Mucus’s natural selectivity, while beneficial in normal contexts, also represents a core obstacle for engineers designing methods for drug delivery [1-6]. The selective permeability properties of mucus have important roles in health and disease, and unwanted changes in mucus permeability are associated with diseases such as cystic fibrosis (CF) [7], ulcerative colitis (UC) [8], and some forms of infertility [9].
Little is known about the detailed molecular properties that distinguish particles that penetrate, or are rejected by, a mucus barrier. Mucus is a complex mixture of water, salts, lipids, nucleic acids, and a variety of proteins, including high molecular weight glycoproteins called mucins [1-4,6]. Mucins are the main gel-forming polymers of mucus and contain threadlike core protein domains which contain large numbers of O-linked oligosaccharide chains that confer negative charge to the mucins through carboxyl and sulfate groups [10]. Moreover, mucins also contain hydrophobic domains which appear to mediate self-assembly of mucin polymer networks [11]. Mucins, along with other components such as lipids and DNA, create a plethora of binding sites for many incoming and secreted particles. Our inability to predict mucus passage of natural biological substrates, such as viruses, nutrients, or toxins, or synthetic particles such as small molecule drugs or nanoparticles, is related to our poor mechanistic and quantitative understanding of how substrates with complex charge and hydrophobic surface properties interact with the mucus barrier [1].
This review will provide an overview of what we know about transport selectivity in mucus, focusing on insights into molecular mechanisms from within the past two years, and highlighting in particular how properties beyond simple net charge or hydrophobicity modulate permeability. Progress has also been made on the design of nanoparticles with compelling mucus penetration properties, which we discuss along with exciting applications of these surface chemistries. Finally, we discuss changes in selective permeability associated with diseases related to mucosal surfaces, and how these changes can be offset to improve drug delivery or measured for diagnostics.
Mucus selectivity arises from its structure and biochemistry
Mucins are cross-linked both through reversible, hydrophobic interactions and disulfide bonds to form a polymer network (Figure 1A) with a mesh size ranging from 100-2000 nm, depending on the location in the body. The mesh size of mucus is typically highly heterogeneous, even within a given site [1]. For particles that are larger than the mesh size, mucus presents a geometrically constraining filter and hinders their passage regardless of surface chemistry (Figure 1B) [12]. Mesh size is not necessarily static, however. For example, increasing the mucus mesh size with the mucolytic N-acetylcysteine can enhance in vitro nanoparticle transport through mucus [13]. Similarly, synthetic nanoparticles coated with mucolytic proteases such as papain or bromelain can improve mucus penetration by degrading the mucin polymers [14-17].
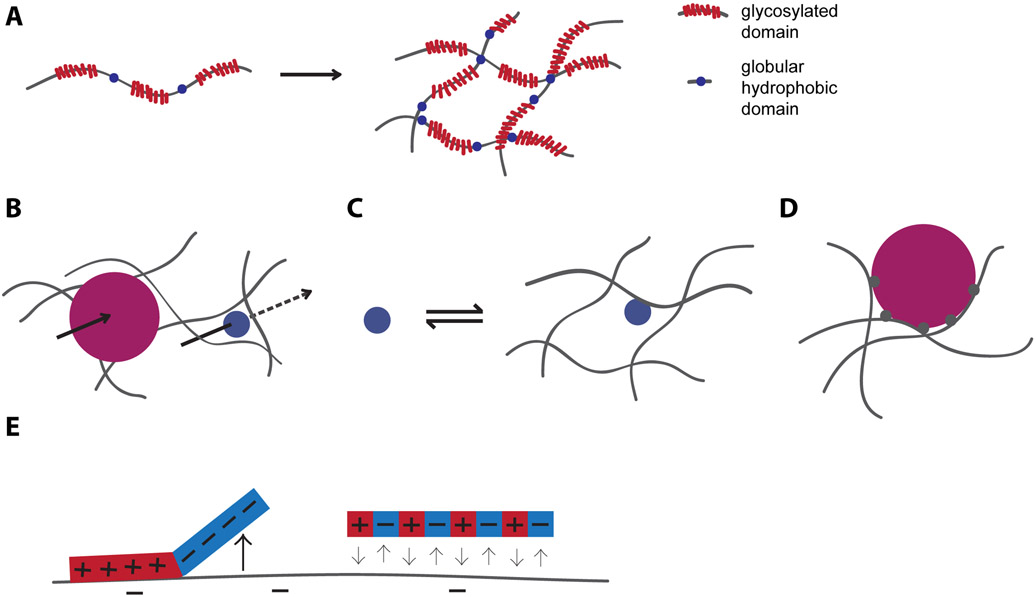
Figure 1.
Overview of mucus and mucus selectivity. (A) Mucins contain disordered, heavily glycosylated, polyanionic domains interspersed with globular hydrophobic domains. These polymers are chemically and physically cross-linked to form a polymer network. (B) Particles larger than the mesh size of mucus (magenta) have hindered passage, while particles smaller than the mesh size may pass through more freely. (C) Binding to polymeric mucin components slows diffusion. D) Polyvalent binding strongly hinders diffusion, even if each individual binding interaction is weak. E) Spatial arrangement of charge impacts the diffusion of particles in mucus.
Steric filtering has little to no effect on the movement of molecules smaller than the mesh size of mucus. However, mucus impacts the diffusion of particles of any size via interaction filtering, in which binding interactions with components inside the mucus arrest diffusion and thus inhibit transport [18]. Put plainly, the more time a molecule spends bound, the less time it has to freely diffuse (Figure 1C) [19]. A related, equally important effect is that the same binding that slows diffusion may reduce the concentration of free molecule and inhibit activity, even after enough time has elapsed for local equilibration. The polyanionic mucin polymers are major contributors to interaction filtering, so net positively charged particles are generally considered to bind with mucus. Mucin-associated lipids and proteins can modulate the detailed interaction capacity of the mucins [20,21]. Other polyanions in mucus, such as DNA and bacterial polysaccharides, which are particularly prevalent in CF, and shed epithelial cells, also contribute to interaction filtering [1,22].
The impact of interaction filtering on the diffusion of a particle depends on the specific biochemistry, but also, how many binding sites on the particle can interact with mucus. Specifically, mucus typically reduces the diffusivity of small molecules by no more than an order of magnitude [23]. However, for nanoparticles of ~100nm in size or larger, mucus can reduce the diffusivity by several thousand fold, even if the particle is still below the mesh size [24]. This dramatic effect on diffusion arises when a particle presents multiple binding sites for mucus that can engage in mucus binding simultaneously. This phenomenon, termed polyvalent binding, implies that even if each individual interaction is weak, the net effect is near-irreversible binding that traps the particles (Figure 1D) [25]. The concept of polyvalent interactions applies to virtually any particle that presents multiple binding sites, and a useful rule of thumb is that most nanoparticles present multiple binding sites and therefore, will be trapped in mucus unless they are designed, or have evolved, to escape retention by mucus.
Biochemical features that regulate interactions with mucus
While size and polyvalency have a strong impact on the effect of interactions, the biochemical mechanisms behind mucus binding are shared between small molecules and nanoparticles. We therefore discuss these mechanisms using examples from both small molecules and nanoparticles. Particularly well studied for small molecules are electrostatic interactions between mucus and cationic antibiotics including certain antimicrobial peptides, polymyxins, and aminoglycosides. For these drugs, binding inhibits diffusive penetration and reduces efficacy [26]. However, while aminoglycosides and polymyxins may have as many as five protonatable amines and so are capable of strong charge-based binding [26], most cationic drugs are only singly or doubly charged, hence, the effect of electrostatic interactions on their transport is weaker and sometimes seemingly non-existent. Unsurprisingly, positively charged nanoparticles, with many more positively charged moieties than aminoglycosides, are typically trapped in mucus. For example, adeno-associated virus serotype 2, a potential vector for inhaled gene therapy, is trapped in mucus, possibly due to electrostatic interactions [27].
While higher positive charge correlates with tighter binding in general, net charge is not necessarily a reliable predictor for mucus binding because different molecules with the same net charge may interact very differently with mucus. For example, Li et al. showed that a peptide with separated blocks of positive and negative charge interacted with mucin, while a peptide with the same sequence composition but alternating charges did not (Figure 1E). These experiments suggest that reducing large clusters of positive charge, such as by interspersing anionic groups, can ablate cation-mucin binding [28]. This is in line with results from antibody design and antifouling research, which show that nonspecific binding can be reduced by avoiding large surface patches of positive charge [29,30]. For mucosal applications, these results imply that balancing the charge of a molecule on the nanoscale may be an effective way to incorporate charge into a molecule while preventing undesired mucus binding. Antifouling appears to be particularly relevant in this context because just as reducing binding to chemically complex mucus components is one central goal of mucus research, hydrophilic antifouling coatings are designed to resist adsorption of proteins and other polymers with diverse binding chemistries [31].
Of note is that not all charged groups are necessarily created equal. While alternating glutamic acid-lysine (EK) peptide coatings are excellent for preventing non-specific binding for antifouling applications, alternating glutamic acid-arginine (ER) peptide coatings are less effective, implying that identity of the charged moiety is important to consider. The difference between EK and ER may arise because protonated arginine provides more hydrogen bond donors, is more weakly hydrated than protonated lysine, and possibly interacts with hydrophobic aromatic groups [29]. We expect that such detailed biochemical features will be relevant for regulating interactions with mucus as well.
The example of the ER peptide begins to illustrate the importance of hydrophobic interactions, which occur in mucus primarily with hydrophobic domains of mucins or mucus-associated lipids [32]. As with net charge, correlations between mucus binding and quantitative estimates of hydrophobicity of small molecules such as the octanol-water partition coefficient are substantial but far from perfect [32,33] and we expect that spatial arrangements of proximal charge and other parameters such as double bonds or aromaticity for π- π bonding come into play. For nanoparticles, any exposed hydrophobic surface typically means trapping: synthetic polystyrene [25] or metal oxide nanoparticles [34] and single-walled carbon nanotubes [34] are immobilized in mucus likely due to polyvalent hydrophobic interactions.
While hydrogen bonding is not clearly correlated with small molecule-mucus binding, it appears to be an important contributor for interactions between mucus and various polymers used as mucoadhesives for drug delivery, such as polyacrylic acid and alginate [35]. Hydrogen bond donors and acceptors on a diffusing particle may increase mucus binding via hydrogen bonding or decrease mucus binding by decreasing hydrophobicity. Antifouling coatings with hydrogen bond acceptors but not donors are better at reducing non-specific binding [36], and we anticipate this design principle may hold for mucopenetration.
Engineering strategies for tuning mucus interactions
Many strategies have been developed to regulate mucus binding for drug delivery, which have been summarized in recent excellent reviews [1-4,6,37]. The main molecular targets for modification are charge and hydrophobicity, and a common theme that emerges among these strategies is the neutralization, or physical shielding from mucus, of cationic or hydrophobic groups. Alternatively, positive charge may be strategically increased to enhance mucus interactions.
For small molecules, peptides, and proteins, a few studies have been reported for strategic designs to reduce drug binding to mucus. The Smyth group PEGylated the aminoglycoside tobramycin, which slightly reduced its net charge and potentially shielded it from electrostatic interactions. Likely due to enhanced diffusion, PEGylated tobramycin had better in vitro anti-P. aeruginosa biofilm activity than the unmodified variant[38] and this superior activity held up in a CF-like model combining mucus and a P. aeruginosa biofilm[39]. Another example is the cationic antimicrobial protein lysozyme, which is inhibited by polyanions in CF lung mucus [40,41]. An engineered charge-reduced lysozyme mutant reduces this inhibition while maintaining antimicrobial activity and has shown increased efficacy in mouse models of lung infection (Figure 2A) [42,43]. These two examples support the idea that the rational design of molecules may improve their mucosal function, and we argue that incorporating mucus binding into drug design will be an important future area, particularly for highly cationic drugs, but also potentially for hydrophobic drugs.
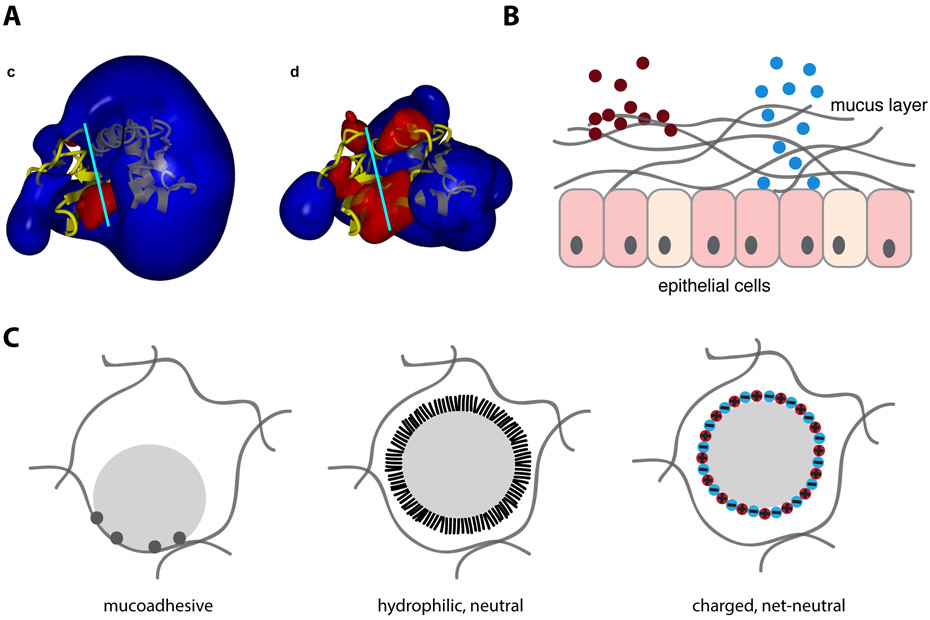
Figure 2.
Strategies for tuning mucus interactions. (A) Electrostatic potential fields of wild type lysozyme (left) and charge-reduced lysozyme (right), with blue representing positive potential and red negative potential. Charge-reduced version of lysozyme showed reduced polyanion inhibition. Reprinted with permission from Gill et al. (2011)[41] Copyright (2011) PLOS. Published under CC BY license https://creativecommons.org/licenses/by/4.0/legalcode (B) Strategies for tuning interactions between particles and mucus include, mucadhesion (left) in which a particle is designed bind mucus, and mucopenetration (right), in which a particle is designed to be mucoinert (C) Many nanoparticles have multiple binding sites with mucus and are therefore mucoadhesive (left). Mucopenetrating particles typically either have a hydrophilic and neutral polymer brush coating (middle), or highly charged, net-neutral surfaces (right).
Certain drug delivery applications take advantage of mucus attachment, or mucoadhesion (Figure 2B, left), for extended-release drug formulations in sites of the body where mucus is regenerated and shed slowly and when the encapsulated therapeutic can itself penetrate mucus [35]. These strategies involve the formulation of drug-loaded micro or nanoparticles composed of polymers that can engage in hydrogen bonding, hydrophobic, or electrostatic interactions with mucus, as well as physical entanglement or even the formation of disulfide bonds. However, mucoadhesion can become limiting in sites where mucus is shed rapidly, such as in the lungs and intestine [35].
For most applications, in particular for mucosal sites with rapid mucus turnover, strategies that prevent particle-mucus interactions and hence allow for free diffusion through mucus (mucopenetration) (Figure 2B, right), appear more effective [44-46]. Of note is that mucopenetrating particles may paradoxically be cleared from mucus more slowly than mucoadhesive particles because the inner layers of a mucus barrier, which these particles are designed to reach, are often cleared more slowly than outer layers [44-47].
The primary strategy for achieving mucopenetration is a hydrophilic but net-neutral surface that prevents both hydrophobic and electrostatic interactions. The most well-developed method for achieving such a surface is a dense brush coating of the neutral but hydrophilic polymer PEG (Figure 2C) [25,48,49]. Recent applications of this technology in mouse models include nanoparticle penetration through cervical mucus for anti-cancer drug delivery [50], inhaled gene therapy [7], and PEGylated nanoparticles for sustained anti-inflammatory drug release [44]. With the exception of current clinical trials for treatment of ocular diseases, however, PEGylated mucopenetrating particles have not yet reached the clinic.
Other recent experimental strategies to build hydrophilic but net-neutral surfaces include using zwitterionic coatings [51] and formulating nanoparticles from polycations complexed with polyanions (Figure 2C) [52,53]. The latter approach was further improved through combination with PEGylation [54]. Coating nanoparticles with neutral hydrophilic polymers other than PEG (Figure 2C) [55-58], has also been recently demonstrated to increase mucopenetration. For applications requiring nanoparticle uptake by cells, the Huang group has demonstrated that a balance of mucopenetrating properties and properties such as hydrophobicity and positive charge that assist in cell uptake may be possible to achieve [59-61]. Other approaches balancing the competing interests of mucopenetration and cell uptake are to include negative charges removable by intestinal alkaline phosphatase, allowing for a zeta potential increase following mucus penetration [62-64], and making the mucoinert coating dissociable [58].
Looking forward, we anticipate that strategies used to engineer antifouling surfaces [31] may be a direct source of inspiration for future strategies to achieve mucopenetration. This prediction is supported by experiments with surface coatings of PEG, poly(hydroxypropyl methacrylate), and poly(2-oxazoline)s, which have each been used with some success for both antifouling[31] and mucopenetration [49,56,58]. In particular, polyzwitterions such as poly(carboxybetaine) and poly(sulfobetaine) [65] and EK peptides are excellent antifouling coatings [29] and we predict they may be beneficial for mucopenetration as well.
While mucopenetrating nanoparticles can successfully avoid non-specific binding to mucus components, particle penetration may still be blocked by antibody response. For example, when specific to otherwise mucopenetrating particles such as PEG [66], HSV and even influenza [67,68], antibodies including IgG and IgM can block diffusion of these particles by polyvalently crosslinking them to mucins via weak antibody-mucin binding. This response may impede repeated administrations of therapeutic nanoparticles, but on the flip side antibody trapping is a promising new strategy to prevent viral penetration of mucus layers and subsequent infection [69].
Mucus permeability in health and disease
Understanding mucus permeability is important to predict or engineer transport through the mucus barrier, but it also presents cutting-edge applications with translational potential for using mucus as a non-invasive diagnostic for mucosal health. The rationale is that the physicochemical properties of mucus barriers are intricately related to health and disease, and a number of pulmonary, gastrointestinal and urogenital conditions are associated with mucus barrier alteration. For example, cervical mucus changes naturally during pregnancy to form a thickened plug, suggesting a strengthening of the mucus barrier toward microbial ascension, thereby maintaining a relatively sterile environment in the intrauterine cavity. Experimentally, cervical mucus from pregnant women is less permeable to both nanoparticles and small charged peptides than from non-pregnant women [70]. indicating an increase in the barrier function through a reduction in permeability. More broadly, mucin production is altered by a variety of microbial products and immunological factors [71]. While mucus permeability has not specifically been tracked as a function of these alterations to our knowledge, it is almost certainly changed by the altered mucus compositions.
While certain changes in mucus permeability are beneficial to health, its dysregulation is often associated with disease. In the lung diseases CF and chronic obstructive pulmonary disease, for example, lung mucus is thickened, resulting in decreased permeability [72-74]. Mucus is thickened due to the hyperconcentration of mucus components [75] (Figure 3A) including mucins, filamentous actin, bacterial DNA and polysaccharides [1], but also due to increased intermolecular disulfide cross-linking of mucins [76] and impaired secretion of bicarbonate, which sequesters calcium to prevent calcium-mediated mucin compaction [77]. Mucosal diseases can also be associated with increased mucus permeability. For example, an unstirred inner colonic mucus layer is thought to prevent contact between the colonic epithelium and bacteria. However, in UC, bacteria penetrate to the epithelium, which is likely mediated by a compromised mucin mesh (Figure 3B) [8]. Another example where increased permeability can be problematic is during pregnancy. Pregnant women at high risk for preterm birth have cervical mucus plugs that exhibit compromised barrier integrity compared to women undergoing healthy pregnancies (Figure 3C) [70]. The causes of increased permeability in these cases are not understood in molecular detail, but some possibilities include altered mucin glycosylation [78], mucin cleavage [79], pH changes [80], and microbiome composition [81].
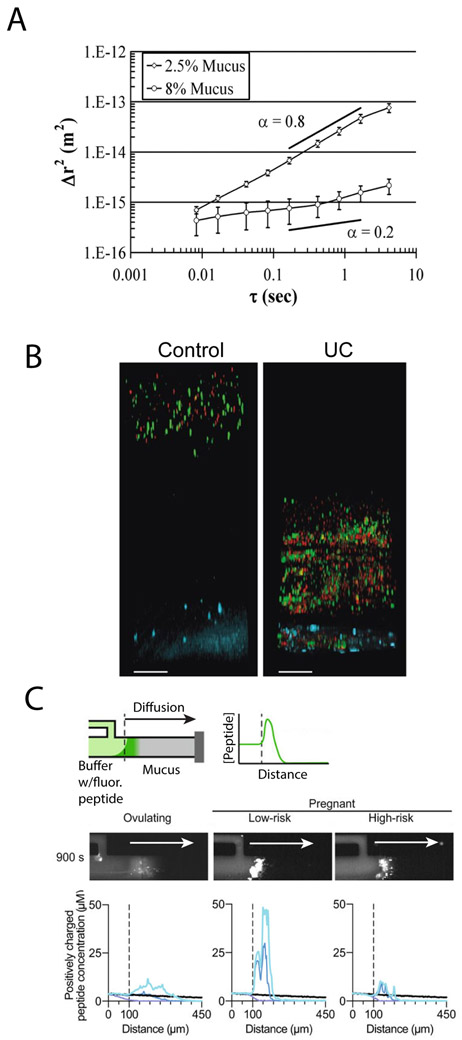
Figure 3:
Mucus permeability is a marker for disease. A) Mean squared displacement of 1μm carboxylated beads in normal (2.5% solids) and CF-like (8% solids) mucus. Hyperconcentrated mucus presents a greater barrier to diffusion. Reprinted from Matsui et al (2006)[74] Copyright (2006) National Academy of Sciences. (B) Penetration of 2μm (green) and 0.5μm (red) nanoparticles through colonic mucus layer to epithelium (blue) in healthy (control) patient and patient with UC. Penetration of beads in UC patient indicates increased permeability of inner colonic mucus layer. Reprinted with permission from Johansson et al. (2013)[85] BMJ Journals. Published under CC BY license https://creativecommons.org/licenses/by/4.0/legalcode. (C) Diffusion of positively charged peptides into native cervical mucus of ovulating patients and pregnant patients at low and high risk for preterm birth. Top: schematic of microfluidic device for measuring diffusion. Fluorescently labeled cationic peptides diffuse from buffer into mucus-filled channel, and the diffusion is then quantified and plotted. Middle: representative 900s diffusion timepoints for cervical mucus from women in each group. Bottom: averaged diffusion time courses over multiple patients from each group. Different colors on bottom schematic indicate different time points. Greater enrichment of peptides in low-risk mucus indicates greater adhesiveness to positively charged peptides. Reprinted with permission from Smith-Dupont et al. (2017) [70] NPG. Published under CC BY license https://creativecommons.org/licenses/by/4.0/legalcode.
Given that the permeability is a sensitive indicator for health and disease, we anticipate that mucus permeability will serve as a valuable biomarker for mucosal diseases throughout the body. It is striking that the same molecular defect which drives lung morbidity in CF – a dysfunctional anion channel called CFTR – also causes intestinal mucus to be thick, adherent, and static, with a concomitant reduction in permeability [82]. Another example of global mucus dysregulation may be Crohn’s disease and ulcerative colitis (UC), which are both also associated with periodontal disease [83] and thus potentially saliva dysregulation given saliva’s importance for maintenance of oral health [84]. The implications from this are twofold – first, that mucus dysregulation may be affected across the body and second, one could potentially use easily accessible mucus samples, such as saliva, to gather information about less accessible surfaces, such as the lungs, or the intestine. We anticipate that valuable insight can be gained by measuring permeability across the body, and that this could help us find new ways of predicting and diagnosing disease [85].
Understanding the biochemical mechanisms that underlie mucus permeability dysfunction can also direct the design of intervention strategies [86]. Thickened lung mucus in CF, for example, presents an even more challenging barrier to therapeutic delivery than healthy mucus. Some current strategies therefore focus on increasing mucus permeability by altering the organization of the mucus gel. For example, dissociating disulfide bonds with N-acetylcysteine [13], counteracting calcium-mediated mucin compaction with bicarbonate or other calcium chelators [82,87,88], disrupting hydrophobic crosslinking with surfactants [11], and diluting mucus using osmolytes such as hypertonic saline and mannitol to increase mucus liquid content [89] are all strategies that thin mucus and in some cases have improved in vitro nanoparticle transport. Overall, countering mucus’ increased tenacity in disease shows great promise for improving nanoparticle delivery. However, the same treatments that increase mucus permeability also typically increase clearance rate, thus reducing the time available for particles to penetrate to the epithelium, so it will be important to find the optimal balance between these two parameters. For the opposite situation, in which a thinned mucus barrier causes disease, it would be desirable to stimulate mucus secretion, but this area of research has received little attention thus far. Alternatively, therapeutically introducing mucus replacements may be a useful future direction of research.
Conclusions
Mucus is a selectively permeable hydrogel that acts as a barrier to particle diffusion across multiple size scales. Mucus slows diffusion of small particles via interaction filtering, while potentially fully blocking penetration of larger particles via steric filtering and interaction filtering. While some basic principles, such as the role of net charge or hydrophobicity, are understood, the effects of spatial variation of charge and hydrophobicity on mucus binding are only beginning to be unraveled. Finely engineering appropriate mucus interactions may have applications in drug or nanoparticle design, where uniform mucoinert surfaces may be limiting for physiological effect. Finally, mucus properties change in distinct states of health and disease, which manifests as altered permeability to cells, viruses, and nanotherapeutics. Understanding and addressing the molecular mechanisms of altered permeability could therefore advance treatment of mucus-associated diseases, and permeability may also be a valuable biomarker for disease.
Highlights.
Mucus filters particles based on size and surface chemistry
Simple descriptors like net charge are inadequate predictors for mucus interaction.
A variety of nanoparticle surface chemistries have been developed for mucus penetration.
Understanding mucus permeability may provide valuable biomarkers for disease.
Acknowledgements
This work was supported by the Department of Defense, Defense Threat Reduction Agency, under award HDTRA-13-1-0038, the National Institutes of Health under award NIH R01-EB017755, the National Science Foundation under award NSF Career PHY-1454673, the MRSEC Program of the National Science Foundation under award DMR – 0819762, and the Burroughs Wellcome Fund under award 1012566. J.W. and T.S. were supported in part by the National Science Foundation Graduate Research Fellowship under Grant No. 1122374. T.S was supported by the Siebel Scholarship and the MIT Collamore-Rogers Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- 1.Witten J, Ribbeck K: The particle in the spider’s web: transport through biological hydrogels. Nanoscale 2017, 9:8080–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu L, Shan W, Zhang Z, Huang Y: Engineering nanomaterials to overcome the mucosal barrier by modulating surface properties. Adv Drug Deliv Rev 2017, doi: 10.1016/j.addr.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Araújo F, Martins C, Azevedo C, Sarmento B: Chemical modification of drug molecules as strategy to reduce interactions with mucus. Adv Drug Deliv Rev 2017, doi: 10.1016/j.addr.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Murgia X, Loretz B, Hartwig O, Hittinger M, Lehr C-M: The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv Drug Deliv Rev 2017, doi: 10.1016/j.addr.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 5. *.Huckaby JT, Lai SK: PEGylation for enhancing nanoparticle diffusion in mucus. Adv Drug Deliv Rev 2017, doi: 10.1016/j.addr.2017.08.010.A review of applications of PEGylation to mucus transport. PEGylation is currently the most highly developed synthetic technique for mucus penetration.
- 6.Leal J, Smyth HDC, Ghosh D: Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int J Pharm 2017, 532:555–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastorakos P, da Silva AL, Chisholm J, Song E, Choi WK, Boyle MP, Morales MM, Hanes J, Suk JS: Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc Natl Acad Sci 2015, 112:8720–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. *.Johansson MEV, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjovall H, et al. : Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63:281–291.The authors show that the inner colonic mucus layer is permeable to bacteria and/or bacteria-sized beads in multiple mouse models of UC and human patients.
- 9.Curlin M, Bursac D: Cervical mucus: from biochemical structure to clinical implications. Front Biosci Sch Ed 2013, 5:507–515. [DOI] [PubMed] [Google Scholar]
- 10.Bansil R, Turner BS: Mucin structure, aggregation, physiological functions and biomedical applications. Curr Opin Colloid Interface Sci 2006, 11:164–170. [Google Scholar]
- 11.Wagner CE, Turner BS, Rubinstein M, McKinley GH, Ribbeck K: A Rheological Study of the Association and Dynamics of MUC5AC Gels. Biomacromolecules 2017, 18:3654–3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieleg O, Ribbeck K: Biological hydrogels as selective diffusion barriers. Trends Cell Biol 2011, 21:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suk JS, Lai SK, Boylan NJ, Dawson MR, Boyle MP, Hanes J: Rapid transport of mucoinert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomed 2011, 6:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. *.Menzel C, Bernkop-Schnürch A: Enzyme decorated drug carriers: Targeted swords to cleave and overcome the mucus barrier. Adv Drug Deliv Rev 2017, doi: 10.1016/j.addr.2017.10.004.A review of the applications, strengths, and limitations of including mucolytic enzymes in drug delivery systems to enhance mucopenetration.
- 15.Efiana NA, Phan TNQ, Wicaksono AJ, Bernkop-Schnürch A: Mucus permeating self-emulsifying drug delivery systems (SEDDS): About the impact of mucolytic enzymes. Colloids Surf B Biointerfaces 2018, 161:228–235. [DOI] [PubMed] [Google Scholar]
- 16.Leichner C, Menzel C, Laffleur F, Bernkop-Schnürch A: Development and in vitro characterization of a papain loaded mucolytic self-emulsifying drug delivery system (SEDDS). Int J Pharm 2017, 530:346–353. [DOI] [PubMed] [Google Scholar]
- 17.Mahmood A, Laffleur F, Leonaviciute G, Bernkop-Schnürch A: Protease-functionalized mucus penetrating microparticles: In-vivo evidence for their potential. Int J Pharm 2017, doi: 10.1016/j.ijpharm.2017.08.114. [DOI] [PubMed] [Google Scholar]
- 18.Lieleg O, Vladescu I, Ribbeck K: Characterization of particle translocation through mucin hydrogels. Biophys J 2010, 98:1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grodzinsky A: Forces, Fields and Flows in Biological Systems. Garland Science; 2011. [Google Scholar]
- 20.Radicioni G, Cao R, Carpenter J, Ford AA, Wang T, Li L, Kesimer M: The innate immune properties of airway mucosal surfaces are regulated by dynamic interactions between mucins and interacting proteins: the mucin interactome. Mucosal Immunol 2016, 9:1442–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larhed AW, Artursson P, Björk E: The influence of intestinal mucus components on the diffusion of drugs. Pharm Res 1998, 15:66–71. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y-Y, Schroeder HA, Nunn KL, Woods K, Anderson DJ, Lai SK, Cone RA: Diffusion of Immunoglobulin G in Shed Vaginal Epithelial Cells and in Cell-Free Regions of Human Cervicovaginal Mucus. PLOS ONE 2016, 11:e0158338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanvilkar K: Drug transfer through mucus. Adv Drug Deliv Rev 2001, 48:173–193. [DOI] [PubMed] [Google Scholar]
- 24. **.Xu Q, Ensign LM, Boylan NJ, Schön A, Gong X, Yang J-C, Lamb NW, Cai S, Yu T, Freire E, et al. : Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS Nano 2015, 9:9217–9227.Altering the density of nanoparticle PEGylation was shown to change diffusivity in cervicovaginal mucus by three orders of magnitude, a key reminder that “PEGylation” may not sufficient for mucus penetration if the PEG is not dense enough to form a dense brush coating.
- 25.Cone RA: Barrier properties of mucus. Adv Drug Deliv Rev 2009, 61:75–85. [DOI] [PubMed] [Google Scholar]
- 26.Huang JX, Blaskovich MA, Pelingon R, Ramu S, Kavanagh A, Elliott AG, Butler MS, Montgomery AB, Cooper MA: Mucin binding reduces colistin antimicrobial activity. Antimicrob Agents Chemother 2015, 59:5925–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuster BS, Kim AJ, Kays JC, Kanzawa MM, Guggino WB, Boyle MP, Rowe SM, Muzyczka N, Suk JS, Hanes J: Overcoming the Cystic Fibrosis Sputum Barrier to Leading Adeno-associated Virus Gene Therapy Vectors. Mol Ther 2014, 22:1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li LD, Crouzier T, Sarkar A, Dunphy L, Han J, Ribbeck K: Spatial configuration and composition of charge modulates transport into a mucin hydrogel barrier. Biophys J 2013,105:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Cao Z, Jiang S: Ultra-low fouling peptide surfaces derived from natural amino acids. Biomaterials 2009, 30:5892–5896. [DOI] [PubMed] [Google Scholar]
- 30.Datta-Mannan A, Thangaraju A, Leung D, Tang Y, Witcher DR, Lu J, Wroblewski VJ: Balancing charge in the complementarity-determining regions of humanized mAbs without affecting pI reduces non-specific binding and improves the pharmacokinetics. mAbs 2015, 7:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. *.Lowe S, O’Brien-Simpson NM, Connal LA: Antibiofouling polymer interfaces: poly(ethylene glycol) and other promising candidates. Polym Chem 2015, 6:198–212.A review describing numerous hydrophilic antifouling coatings, some of which have been found to be effective for mucopenetration and some of which have not yet been tested.
- 32.Murgia X, Loretz B, Hartwig O, Hittinger M, Lehr C-M: The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv Drug Deliv Rev 2017, doi: 10.1016/j.addr.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 33. *.Gargano AFG, Lämmerhofer M, Lönn H, Schoenmakers PJ, Leek T: Mucin-based stationary phases as tool for the characterization of drug-mucus interaction. J Chromatogr A 2014, 1351:70–81.Using mucin-based affinity chromatography, the authors measure mucin binding for 41 molecules, a substantially higher throughput than any other single small molecule-mucin binding study.
- 34.Jachak A, Lai SK, Hida K, Suk JS, Markovic N, Biswal S, Breysse PN, Hanes J: Transport of metal oxide nanoparticles and single-walled carbon nanotubes in human mucus. Nanotoxicology 2012, 6:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Netsomboon K, Bernkop-Schnürch A: Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur J Pharm Biopharm 2016, 98:76–89. [DOI] [PubMed] [Google Scholar]
- 36.Ostuni Emanuele, Chapman Robert G, Holmlin R Erik, Takayama Shuichi, and Whitesides GM: A Survey of Structure-Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir ACS J Surf Colloids 2001, 17:5605–5620. [Google Scholar]
- 37.Lock JY, Carlson TL, Carrier RL: Mucus models to evaluate the diffusion of drugs and particles. Adv Drug Deliv Rev 2017, doi: 10.1016/j.addr.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du J, Bandara HMHN, Du P, Huang H, Hoang K, Nguyen D, Mogarala SV, Smyth HDC: Improved Biofilm Antimicrobial Activity of Polyethylene Glycol Conjugated Tobramycin Compared to Tobramycin in Pseudomonas aeruginosa Biofilms. Mol Pharm 2015,12:1544–1553. [DOI] [PubMed] [Google Scholar]
- 39. **.Bahamondez-Canas TF, Zhang H, Tewes F, Leal J, Smyth HDC: PEGylation of Tobramycin Improves Mucus Penetration and Antimicrobial Activity against Pseudomonas aeruginosa Biofilms in Vitro. Mol Pharm 2018, doi: 10.1021/acs.molpharmaceut.8b00011.Tobramycin conjugated to a single 5kDa PEG chain was better than unmodified tobramycin at treating P. aeruginosa biofilms in an in vitro CF mucus-like environment.
- 40.Scanlon TC, Teneback CC, Gill A, Bement JL, Weiner JA, Lamppa JW, Leclair LW, Griswold KE: Enhanced antimicrobial activity of engineered human lysozyme. ACS Chem Biol 2010, 5:809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill A, Scanlon TC, Osipovitch DC, Madden DR, Griswold KE: Crystal structure of a charge engineered human lysozyme having enhanced bactericidal activity. PloS One 2011, 6:e16788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. *.Teneback CC, Scanlon TC, Wargo MJ, Bement JL, Griswold KE, Leclair LW: Bioengineered lysozyme reduces bacterial burden and inflammation in a murine model of mucoid Pseudomonas aeruginosa lung infection. Antimicrob Agents Chemother 2013, 57:5559–5564.Lysozyme engineered for reduced positive charge, and thus reduced mucus polyanion binding, outperforms wild-type lysozyme as a treatment for mucoid P. aeruginosa lung infection.
- 43.Griswold KE, Bement JL, Teneback CC, Scanlon TC, Wargo MJ, Leclair LW: Bioengineered lysozyme in combination therapies for Pseudomonas aeruginosa lung infections. Bioengineered 2014, 5:143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. *.Schneider CS, Xu Q, Boylan NJ, Chisholm J, Tang BC, Schuster BS, Henning A, Ensign LM, Lee E, Adstamongkonkul P, et al. : Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci Adv 2017, 3:e1601556.Mucopenetrating particles are shown to have a more uniform distribution and greater residence time in the airways than mucoadhesive particles, suggesting that mucopenetration is the more effective strategy for delivery of inhaled medication. The authors believe that this is because mucopenetrating particles penetrate to the more slowly cleared periciliary layer while mucoadhesive particles do not.
- 45.Maisel K, Ensign L, Reddy M, Cone R, Hanes J: Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. J Controlled Release 2015, 197:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ensign LM, Tang BC, Wang Y-Y, Tse TA, Hoen T, Cone R, Hanes J: Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci Transl Med 2012, 4:138ra79–138ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schopf L, Enlow E, Popov A, Bourassa J, Chen H: Ocular Pharmacokinetics of a Novel Loteprednol Etabonate 0.4% Ophthalmic Formulation. Ophthalmol Ther 2014, 3:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suk JS, Xu Q, Kim N, Hanes J, Ensign LM: PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 2016, 99:28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huckaby JT, Lai SK: PEGylation for enhancing nanoparticle diffusion in mucus. Adv Drug Deliv Rev 2017, doi: 10.1016/j.addr.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Yang M, Yu T, Wang Y-Y, Lai SK, Zeng Q, Miao B, Tang BC, Simons BW, Ensign LM, Liu G, et al. : Vaginal Delivery of Paclitaxel via Nanoparticles with Non-Mucoadhesive Surfaces Suppresses Cervical Tumor Growth. Adv Healthc Mater 2014, 3:1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. **.Shan W, Zhu X, Tao W, Cui Y, Liu M, Wu L, Li L, Zheng Y, Huang Y: Enhanced Oral Delivery of Protein Drugs Using Zwitterion-Functionalized Nanoparticles to Overcome both the Diffusion and Absorption Barriers. ACS Appl Mater Interfaces 2016, 8:25444–25453.Zwitterionic phospholipid-coated nanoparticles show a good combination of mucus penetration and cellular uptake.
- 52.Abdulkarim M, Agulló N, Cattoz B, Griffiths P, Bernkop-Schnürch A, Borros SG, Gumbleton M: Nanoparticle diffusion within intestinal mucus: Three-dimensional response analysis dissecting the impact of particle surface charge, size and heterogeneity across polyelectrolyte, pegylated and viral particles. Eur J Pharm Biopharm 2015, 97:230–238. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Cheng H, Dong W, Zhang M, Liu Q, Wang X, Guan J, Wu H, Mao S: Design and intestinal mucus penetration mechanism of core-shell nanocomplex. J Controlled Release 2018, 272:29–38. [DOI] [PubMed] [Google Scholar]
- 54.Pereira de Sousa I, Moser T, Steiner C, Fichtl B, Bernkop-Schnürch A: Insulin loaded mucus permeating nanoparticles: Addressing the surface characteristics as feature to improve mucus permeation. Int J Pharm 2016, 500:236–244. [DOI] [PubMed] [Google Scholar]
- 55. **.Khutoryanskiy VV: Beyond PEGylation: alternative surface-modification of nanoparticles with mucus-inert biomaterials. Adv Drug Deliv Rev 2017, doi: 10.1016/j.addr.2017.07.015.A comprehensive review of mucus penetrating strategies for nanoparticles besides PEGylation.
- 56.Mansfield EDH, de la Rosa VR, Kowalczyk RM, Grillo I, Hoogenboom R, Sillence K, Hole P, Williams AC, Khutoryanskiy VV: Side chain variations radically alter the diffusion of poly(2-alkyl-2-oxazoline) functionalised nanoparticles through a mucosal barrier. Biomater Sci 2016, 4:1318–1327. [DOI] [PubMed] [Google Scholar]
- 57.Popov A, Enlow E, Bourassa J, Chen H: Mucus-penetrating nanoparticles made with “mucoadhesive” poly(vinyl alcohol). Nanomed 2016, 12:1863–1871. [DOI] [PubMed] [Google Scholar]
- 58.Liu M, Zhang J, Zhu X, Shan W, Li L, Zhong J, Zhang Z, Huang Y: Efficient mucus permeation and tight junction opening by dissociable “mucus-inert” agent coated trimethyl chitosan nanoparticles for oral insulin delivery. J Controlled Release 2016, 222:67–77. [DOI] [PubMed] [Google Scholar]
- 59.Cui Y, Shan W, Liu M, Wu L, Huang Y: A strategy for developing effective orally-delivered nanoparticles through modulation of the surface “hydrophilicity/hydrophobicity balance.” J Mater Chem B 2017, 5:1302–1314. [DOI] [PubMed] [Google Scholar]
- 60. *.Zhu X, Wu J, Shan W, Zhou Z, Liu M, Huang Y: Sub-50 nm Nanoparticles with Biomimetic Surfaces to Sequentially Overcome the Mucosal Diffusion Barrier and the Epithelial Absorption Barrier. Adv Funct Mater 2016, 26:2728–2738.Nanoparticles with a surface containing PEG and polyarginine, a cationic cell-penetrating peptide, were examined. The authors found that by appropriately burying the polyarginine within a PEG brush, both mucopenetration and good cellular uptake could be achieved.
- 61.Wu L, Liu M, Shan W, Cui Y, Zhang Z, Huang Y: Lipid nanovehicles with adjustable surface properties for overcoming multiple barriers simultaneously in oral administration. Int J Pharm 2017, 520:216–227. [DOI] [PubMed] [Google Scholar]
- 62.Bonengel S, Prüfert F, Perera G, Schauer J, Bernkop-Schnürch A: Polyethylene imine-6-phosphogluconic acid nanoparticles – a novel zeta potential changing system. Int J Pharm 2015, 483:19–25. [DOI] [PubMed] [Google Scholar]
- 63.Suchaoin W, Pereira de Sousa I, Netsomboon K, Lam HT, Laffleur F, Bernkop-Schnürch A: Development and in vitro evaluation of zeta potential changing self-emulsifying drug delivery systems for enhanced mucus permeation. Int J Pharm 2016, 510:255–262. [DOI] [PubMed] [Google Scholar]
- 64. *.Griesser J, Burtscher S, Köllner S, Nardin I, Prüfert F, Bernkop-Schnürch A: Zeta potential changing self-emulsifying drug delivery systems containing phosphorylated polysaccharides. Eur J Pharm Biopharm 2017, 119:264–270.Nanoparticles containing intestinal alkaline phosphatase (IAP)-cleavable phosphates show good mucopenetration ability before cleavage, and then a shift to a positively charged surface (which is useful for cellular uptake) upon treatment with IAP.
- 65.Shao Q, Jiang S: Molecular understanding and design of zwitterionic materials. Adv Mater 2015, 27:15–26. [DOI] [PubMed] [Google Scholar]
- 66.Henry CE, Wang Y-Y, Yang Q, Hoang T, Chattopadhyay S, Hoen T, Ensign LM, Nunn KL, Schroeder H, McCallen J, et al. : Anti-PEG antibodies alter the mobility and biodistribution of densely PEGylated nanoparticles in mucus. Acta Biomater 2016, 43:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang YY, Kannan A, Nunn KL, Murphy MA, Subramani DB, Moench T, Cone R, Lai SK: IgG in cervicovaginal mucus traps HSV and prevents vaginal Herpes infections. Mucosal Immunol 2014, 7:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y-Y, Harit D, Subramani DB, Arora H, Kumar PA, Lai SK: Influenza-binding antibodies immobilise influenza viruses in fresh human airway mucus. Eur Respir J 2016, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen A, McKinley SA, Wang S, Shi F, Mucha PJ, Forest MG, Lai SK: Transient Antibody-Mucin Interactions Produce a Dynamic Molecular Shield against Viral Invasion. Biophys J 2014, 106:2028–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. *.Smith-Dupont KB, Wagner CE, Witten J, Conroy K, Rudoltz H, Pagidas K, Snegovskikh V, House M, Ribbeck K: Probing the potential of mucus permeability to signify preterm birth risk. Sci Rep 2017, 7:10302.Cervical mucus from pregnant patients at high risk for preterm birth is shown to be more permeable than cervical mucus from pregnant patients at low risk to charged peptides. Cervical mucus from pregnant patients as a whole is less permeable than cervical mucus from non-pregnant patients to charged peptides and nanoparticles.
- 71.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA: Mucins in the mucosal barrier to infection. Mucosal Immunol 2008, 1:183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fahy JV, Dickey BF: Airway Mucus Function and Dysfunction. N Engl J Med 2010, 363:2233–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, Doerschuk CM, Alexis NE, Anderson WH, Henderson AG, et al. : Airway Mucin Concentration as a Marker of Chronic Bronchitis. N Engl J Med 2017, 377:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsui H, Wagner VE, Hill DB, Schwab UE, Rogers TD, Button B, Taylor RM, Superfine R, Rubinstein M, Iglewski BH, et al. : A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci 2006, 103:18131–18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Button B, Anderson WH, Boucher RC: Mucus Hyperconcentration as a Unifying Aspect of the Chronic Bronchitic Phenotype. Ann Am Thorac Soc 2016, 13: S156–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan S, Hollinger M, Lachowicz-Scroggins ME, Kerr SC, Dunican EM, Daniel BM, Ghosh S, Erzurum SC, Willard B, Hazen SL, et al. : Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci Transl Med 2015, 7:276ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen EYT, Yang N, Quinton PM, Chin W-C: A new role for bicarbonate in mucus formation. Am J Physiol - Lung Cell Mol Physiol 2010, 299:L542–L549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bergstrom K, Liu X, Zhao Y, Gao N, Wu Q, Song K, Cui Y, Li Y, McDaniel JM, McGee S, et al. : Defective Intestinal Mucin-type O-glycosylation Causes Spontaneous Colitis-associated Cancer in Mice. Gastroenterology 2016, 151: 152–164.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lidell ME, Moncada DM, Chadee K, Hansson GC: Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci 2006, 103:9298–9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nunn KL, Wang Y-Y, Harit D, Humphrys MS, Ma B, Cone R, Ravel J, Lai SK: Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota. mBio 2015, 6:e01084–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jakobsson HE, Rodríguez-Piñeiro AM, Schütte A, Ermund A, Boysen P, Bemark M, Sommer F, Bäckhed F, Hansson GC, Johansson ME: The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep 2015, 16:164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gustafsson JK, Ermund A, Ambort D, Johansson MEV, Nilsson HE, Thorell K, Hebert H, Sjövall H, Hansson GC: Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 2012, 209:1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lira-Junior R, Figueredo CM: Periodontal and inflammatory bowel diseases: Is there evidence of complex pathogenic interactions? World J Gastroenterol 2016, 22:7963–7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frenkel ES, Ribbeck K: Salivary mucins in host defense and disease prevention. J Oral Microbiol 2015, 7:29759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brito F, de Barros FC, Zaltman C, Pugas Carvalho AT, De Vasconcellos Carneiro AJ, Fischer RG, Gustafsson A, De Silva Figueredo CM: Prevalence of periodontitis and DMFT index in patients with Crohn’s disease and ulcerative colitis. J Clin Periodontol 2008, 35:555–560. [DOI] [PubMed] [Google Scholar]
- 86.Nordgård CT, Draget KI: Co association of mucus modulating agents and nanoparticles for mucosal drug delivery. Adv Drug Deliv Rev 2018, doi: 10.1016/j.addr.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 87.Raynal BD, Hardingham TE, Sheehan JK, Thornton DJ: Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. J Biol Chem 2003, 278:28703–28710. [DOI] [PubMed] [Google Scholar]
- 88. *.Nordgård CT, Nonstad U, Olderøy MØ, Espevik T, Draget KI: Alterations in mucus barrier function and matrix structure induced by guluronate oligomers. Biomacromolecules 2014, 15:2294–2300.Treatment with a guluronate oligomer now in clinical trials as a CF treatment that disrupts mucus and enhances nanoparticle transport.
- 89.Ermund A, Meiss LN, Scholte BJ, Hansson GC: Hypertonic saline releases the attached small intestinal cystic fibrosis mucus. Clin Exp Pharmacol Physiol 2015, 42:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]