Abstract
Autosomal dominant polycystic kidney disease is characterized by progressive development and enlargement of kidney cysts, leading to ESKD. Because the kidneys are under high metabolic demand, it is not surprising that mounting evidence suggests that a metabolic defect exists in in vitro and animal models of autosomal dominant polycystic kidney disease, which likely contributes to cystic epithelial proliferation and subsequent cyst growth. Alterations include defective glucose metabolism (reprogramming to favor aerobic glycolysis), dysregulated lipid and amino acid metabolism, impaired autophagy, and mitochondrial dysfunction. Limited evidence supports that cellular kidney metabolism is also dysregulated in humans with autosomal dominant polycystic kidney disease. There are notable overlapping features and pathways among metabolism, obesity, and/or autosomal dominant polycystic kidney disease. Both dietary and pharmacologic-based strategies targeting metabolic abnormalities are being considered as therapies to slow autosomal dominant polycystic kidney disease progression and are attractive, particularly given the slowly progressive nature of the disease. Dietary strategies include daily caloric restriction, intermittent fasting, time-restricted feeding, a ketogenic diet, and 2-deoxy-glucose as well as alterations to nutrient availability. Pharmacologic-based strategies include AMP-activated kinase activators, sodium glucose cotransporter-2 inhibitors, niacinamide, and thiazolidenediones. The results from initial clinical trials targeting metabolism are upcoming and anxiously awaited within the scientific and polycystic kidney disease communities. There continues to be a need for additional mechanistic studies to better understand the role of dysregulated metabolism in autosomal dominant polycystic kidney disease and for subsequent translation to clinical trials. Beyond single-intervention trials focused on metabolic reprograming in autosomal dominant polycystic kidney disease, great potential also exists by combining metabolic-focused therapeutic approaches with compounds targeting other signaling cascades altered in autosomal dominant polycystic kidney disease, such as tolvaptan.
Keywords: diet, fasting, metabolism, polycystic kidney disease, animals, humans, autosomal dominant polycystic kidney, caloric restriction, deoxyglucose, glucose, tolvaptan, AMP-activated protein kinases, ketogenic diet, niacinamide, glycolysis, type 2 diabetes mellitus, sodium-glucose transporter 2 inhibitors, kidney, chronic kidney failure, obesity, cysts, autophagy, animal models, lipids, mitochondria
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by progressive development and enlargement of kidney cysts, leading to ESKD (1). Interestingly, although kidneys are only 0.5% of the human body mass, they consume 10% of the body’s oxygen, highlighting the organ’s high metabolic demand. Hence, it is not surprising that mounting evidence suggests that metabolic reprogramming is an important modulator of ADPKD pathology (Figure 1). This narrative review will summarize supporting data in humans with ADPKD; common features among metabolism, obesity, and/or ADPKD; and ongoing or potential dietary/pharmacologic-based strategies to correct metabolic abnormalities. Evidence of ADPKD metabolic reprogramming using in vitro or animal models has been excellently reviewed recently (2–4); hence, these findings will be only mentioned briefly.
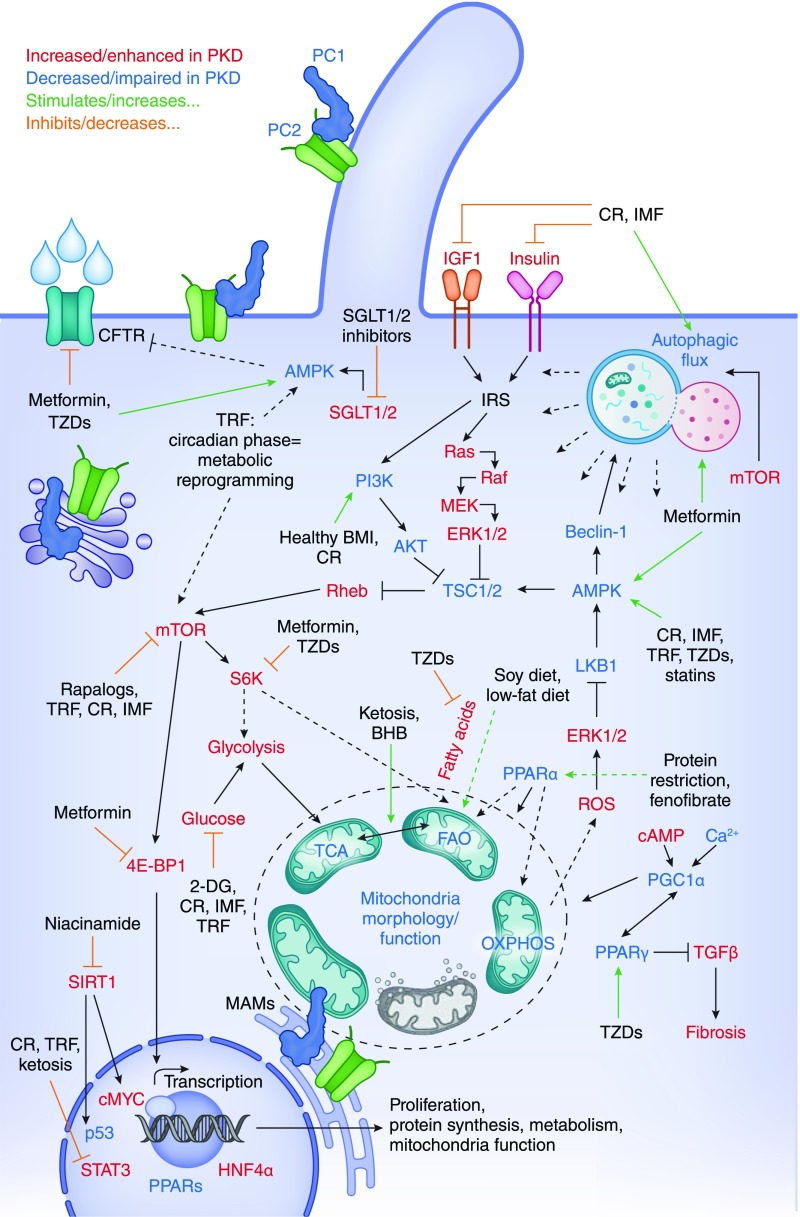
Figure 1.
Autosomal dominant polycystic kidney disease (ADPKD) and nutrient metabolisms have converging pathways. Central cellular processes that are known to be impaired in ADPKD pathology and are characteristic of metabolic reprogramming include autophagic flux, glycolysis, fatty acid oxidation, and mitochondrial function. Central signaling nodes that overlap between ADPKD and metabolic response include mammalian target of rapamycin (mTOR), AMP-activated kinase (AMPK), sirtuin-1 (SIRT-1), IGF-I, and peroxisome proliferator–activated receptor-α/γ (PPARα/γ). Alteration in diet intake or composition can affect many of these overlapping processes/pathways. Similarly, multiple pharmacologic approaches that are known to alter metabolic reprogramming target these central processes/signaling hubs. Collectively, this suggests that such interventions have high potential in alleviating ADPKD in humans. Dotted arrows indicate that signaling cascade is more complex than depicted. AKT, protein kinase B; BHB, β-hydroxybutyrate; BMI, body mass index; CFTR, cystic fibrosis transmembrane conductance regulator; cMYC, cellular mycelocytomatosis; CR, caloric restriction; 2-DG, 2-deoxyglucose; 4E-BP1, eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1; ERK1/2, extracellular signal–regulated kinase; FAO, fatty acid oxidation; HNF4α, hepatocyte nuclear factor 4α; IMF, intermittent fasting; IRS, insulin receptor substrate; MAM, mitochondria associated membranes; MEK, mitogen-activated protein kinase kinase; OXPHOS, oxidative phosphorylation; PC, polycystin; PI3K, phosphoinositide 3-kinase; Rheb, ras homolog enriched in brain; ROS, reactive oxygen species; S6K, ribosomal protein S6 kinase; SGLT1/2, sodium glucose cotransporter-1/2; STAT3, signal transducer and activator of transcription 3; TCA, tricarboxylic acid cycle; TRF, time-restricted feeding; TSC1/2, tuberous sclerosis 1/2; TZD, thiazolidinedione.
ADPKD, a Disease of Dysregulated Metabolism, as Established by In Vitro and Animal Models
Over the last decade, multiple in vitro and animal studies have shown that dysregulated metabolism is a key feature and disease modulator of ADPKD. For example, glucose metabolism is defective in ADPKD, which was initially established by studying Pkd1−/− mouse embryonic fibroblasts (5). These cells reprogram to favor aerobic glycolysis (i.e., Warburg effect), resulting in upregulation of mammalian target of rapamycin complex 1 (mTORC1); inhibition of AMP-activated kinase (AMPK); and subsequent increased proliferation, decreased apoptosis, and defective autophagy. Altered glucose metabolism was later reported in multiple in vivo models (5,6), evidenced by upregulation of key glycolytic genes in patient cystic epithelia and murine polycystic kidney disease (PKD) kidneys (5,6). Although glycolysis is not consistently observed to be altered in all PKD models (7,8), its inhibition with 2-deoxyglocose, a glucose analog that cannot be metabolized, reduces cell proliferation in human PKD cells and kidney cystogenesis in various murine models (5,6,9). Consistent with these results, higher glucose concentration increases kidney cyst growth (10), and hyperglycemia promotes cystogenesis, as well as kidney structural and functional damage in a nonorthologous rodent model of PKD (11).
Dysregulated lipid metabolism (i.e., reduced fatty acid oxidation) has also been identified as a key PKD feature via kidney transcriptomics, urine metabolomics, and lipidomics in different ADPKD models (7,12,13). Signaling via hepatocyte nuclear factor 4α (Hnf4α) or peroxisome proliferator–activated receptor-α (PPARα), two proteins key to multiple metabolic programming pathways, has been suggested to be central to the reduced fatty acid oxidation observed in PKD. Indeed, treatment with the PPARα agonist fenofibrate enhances fatty acid oxidation and reduces cystic disease in an orthologous ADPKD model (13). Similarly, loss of Hnf4α in the setting of PKD results in more severe cystic disease (12).
Furthermore, amino acid metabolism is also altered in PKD. For example, glutaminase 1 is upregulated in cyst-lining epithelial of human ADPKD kidneys and murine models (14). Correlatively, both Pkd1 mutant (but not wild-type) cells and primary ADPKD cyst lining (but not normal human kidney) cells require glutamine for growth, suggesting that PKD results in glutamine dependence (14,15). Consequently, inhibition of glutamine metabolism with the glutaminase inhibitor BPTES or CB839 slows cystogenesis in the Pkhd1-Cre;Pkd1flx/flx model; however, treatment with CB839 in the Aqp2-Cre;Pkd1flx/flx is ineffective (14,15). Additionally, kidney cystogenesis has also been proposed to be arginine dependent because arginosuccinate synthase 1 expression is reduced in human and murine ADPKD, and arginine depletion results in a dose-dependent increase of arginosuccinate synthase 1 and reduced cystogenesis ex vivo using metanephric organ culture (16).
Beyond the above-mentioned metabolic pathways, ADPKD is also associated with defects in two central players of metabolism—autophagy and mitochondria function. Autophagy helps maintain energy homeostasis and is activated when nutrients are lacking. Autophagy is impaired in PKD cells, nonorthologous murine models, and Pkd1 mutant zebrafish (5,17–19). The central defect seems to be fusion of the autophagosome with the lysosome (i.e., autophagic flux) (18,19). Correlatively, knockdown of the core autophagy protein Atg5 promotes cystogenesis, whereas treatment with the autophagy inducer Beclin-1 reduces cysts in a Pkd1 mutant zebrafish model (18). However, treatment with trehalose, a natural autophagy enhancer, is ineffective in ameliorating disease in a Pkd1 mutant model (17). Of note, mTORC1 is a known inhibitor of autophagy, and treatment with various rapalogs both enhances autophagy and reduces cystogenesis in PKD (5,18).
Finally, several lines of evidence support deregulated mitochondria, a central metabolic hub, in PKD. Mitochondrial dysfunction has been attributed in part to abnormalities in morphology and biogenesis. Observed phenotypes in kidney tissue from patients with ADPKD and murine models include mitochondrial fragmentation, swelling, nondirectional movement, and reduction in mitochondrial DNA copy number (2,20,21). Additionally, mitochondrial function is impaired in ADPKD models, including increased reactive oxygen species production, increased Ca2+ uptake, and decreased respiration (2,3). Interestingly, multiple studies highlight that both polycystin-1 and -2 may regulate mitochondria function directly (21,22).
Metabolic Reprogramming, a Concurrent Feature of Human ADPKD
Limited evidence supports that cellular kidney metabolism is also dysregulated in humans with ADPKD. Nontargeted metabolomics using plasma samples from patients with early-stage ADPKD who participated in the Halt Progression of PKD (HALT-PKD) Study A suggests an association between two long-chain triglycerides and height-adjusted total kidney volume (TKV) (23). Similarly, alterations in fatty acid metabolism, including the lipoxygenase pathways, have been observed using targeted analysis of bioactive lipid mediators in serum samples from HALT-PKD Study A participants (24). Notably, specific serum-detectable metabolomic alterations in ADPKD do appear to be unique compared with other etiologies of CKD, including glomerular disease (25). Among these changes are an increase in 16-hydroxyplamatite, consistent with the hypothesis of impaired fatty acid metabolism and mitochondrial β-oxidation (25).
Additional indirect evidence of dysregulated metabolism in humans with ADPKD includes an inverse association between HDL cholesterol levels and kidney growth, increased urinary acid excretion, and hypocitraturia, particularly in those with kidney stones (26). Limited evidence suggests that insulin resistance and/or impaired insulin secretion may also be a feature of ADPKD, although this observation is inconsistent (26). Individuals with type 2 diabetes mellitus in addition to ADPKD have significantly greater kidney volumes compared with nondiabetic individuals, suggesting that insulin resistance may play a mechanistic role in disease progression (27). Additionally, there is a significant association between ADPKD and risk of new-onset diabetes after kidney transplantation (28). Together, this literature not only strongly suggests that metabolic changes are important to the pathogenesis of PKD but also highlights the potential for multiple therapeutic strategies to ameliorate the disease.
Metabolic Pathways Altered in Overweight and Obesity That Parallel Metabolic Dysfunction in ADPKD
Similar to the general population, body mass index (BMI) has been increasing in patients with ADPKD over time (29), and approximately two thirds of adults with ADPKD are overweight or obese (30). Obesity is an independent risk factor for incident CKD and ESKD (31), and weight loss can prevent further decline in eGFR (32). However, the role of obesity in progression of ADPKD, surprisingly, had not been described until recently.
In the HALT-PKD Study A, overweight and particularly, obesity were strong independent predictors of more rapid kidney growth (30). Additionally, obesity was independently associated with greater decline in eGFR compared with normal-weight participants. Thus, weight loss in adults with ADPKD may slow ADPKD progression.
Overnutrition and obesity are known to activate mammalian target of rapamycin (mTOR) activity via PI3K/Akt, IGF-I, and AMPK (33), pathways commonly deregulated in PKD (Figure 1). Interestingly, mTOR overstimulation has been proposed to be critical in the development of diabetes (34). Similarly, AMPK activity is reduced in multiple rodent models of obesity, suggesting that AMPK activation, as in PKD, may serve as a therapeutic approach for this disease (35). Hence, these shared pathways could contribute to the observation that higher BMI is associated with PKD or highlight that PKD could drive obesity.
Dietary Strategies to Correct Metabolic Abnormalities
Multiple dietary strategies have the potential to slow ADPKD progression. These include daily caloric restriction (a more traditional approach consisting of reducing daily total caloric intake), intermittent fasting (either fasting or substantially reducing caloric intake 1–3 days per week), time-restricted feeding (limiting caloric intake to a narrow feeding interval), or alterations to nutrient availability (i.e., low-fat, low-protein, low-carbohydrate, or soy-based diets).
Dietary Approaches Using Non-PKD Rodent Models
Dietary interventions aimed to alter metabolic pathways have been extensively evaluated in non-PKD rodent models. Long-term daily caloric restriction with adequate nutrition extends lifespan in multiple animal models and improves most aging-related health aspects, including metabolic health (weight, lipids, and glucoregulatory function) (36). Interestingly, daily caloric restriction protects against kidney ischemic reperfusion injury in rodents through mechanisms that may include enhanced autophagy and stress resistance/preconditioning (37).
Numerous benefits of intermittent fasting are also observed in non-PKD rodent models, including reduced fasting glucose and insulin, improved insulin sensitivity, improved lipid profiles, and decreased BP and heart rate (38). Additionally, intermittent fasting increases glycogen and adipose tissue mobilization, promoting ketone body production and gluconeogenesis (38). Thus, intermittent fasting can promote metabolic reprogramming from carbohydrate to fat metabolism.
In a landmark study in Drosophila (39), time-restricted feeding slowed cardiac aging, improved sleep, and prevented weight gain, despite a lack of change in caloric intake or activity level. Benefits of time-restricted feeding have also been demonstrated in rodents, including prevention of weight gain from a high-fat/high-sucrose diet (40,41) as well as improved body composition (40). Similar to intermittent fasting, insulin sensitivity is also improved with time-restricted feeding (40). Notably, in mice being fed a high-fat diet, time-restricted feeding improves both mTOR and AMPK pathway functions compared with ad libitum feeding mice (41).
Caloric Restriction and Fasting Trials in Humans Free from ADPKD
To date, most daily caloric restriction clinical trials have focused on healthy populations with end points, including adherence, weight loss, and insulin sensitivity, rather than modifying clinical disease. Daily caloric restriction is the standard of care approach to weight loss in obesity; however, the safety and tolerability of 2 years of 25% daily caloric restriction have also been evaluated in healthy, nonobese adults in a multicenter, randomized, controlled trial (RCT; the Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy [CALERIE] trial) (42). Overall, in the CALERIE trial, sustained daily caloric restriction was feasible, safe, and well tolerated, although potential risks of bone loss and anemia were noted. Benefits were observed in cardiometabolic risk factors and inflammatory markers.
Trials conducted in healthy overweight and/or obese adults support that intermittent fasting is also feasible and safe (43–45). In addition to weight and body fat loss, intermittent fasting promotes reductions in oxidative stress, inflammation, and BP, as well as favorable changes in lipids (43–45).
Time-restricted feeding is a novel fasting regimen that may be an alternate strategy to promote weight loss or a more feasible approach than intermittent fasting in individuals of normal BMI to activate similar evolutionarily conserved pathways, acknowledging that timing of food intake can influence the metabolic response (46). RCTs on time-restricted feeding are far fewer than those for intermittent fasting, but a single 16-week pilot trial in overweight adults suggests feasibility (47). Here, total daily caloric intake was reduced by 20%, which resulted in modest weight loss, increased reported energy level, and better sleep all maintained at 1 year.
Caloric Restriction and Changes in Nutrient Availability in ADPKD
Mild to moderate daily caloric restriction (10%–40%) profoundly slows kidney growth and improves kidney function in multiple rodent models of ADPKD in as little as 2 months (8,48). These improvements are mediated in part by suppression of mTOR signaling, AMPK activation, and a reduction in IGF-I (8,48). Additionally, caloric restriction reverses the ADPKD-associated increase in hexokinase 2 expression, which is the rate-limiting step in glycolysis, thus supporting metabolic reprogramming (8). Improvements are also observed in kidney fibrosis, inflammatory markers, and evidence of injury in a dose-dependent manner (8). Remarkably, 40% daily caloric restriction initiated at advanced disease stage not only slows but reverses disease progression (8).
Furthermore, a beneficial effect of time-restricted feeding on ADPKD progression was recently demonstrated in a nonorthologous PKD model, the Han:SPRD rat (49). Ad libitum feeding limited to 8 hours within the rats’ wake/dark cycle significantly reduces kidney cystogenesis and fibrosis, improves kidney function, and corrects mTORC1 and STAT3 signaling without a reduction in caloric intake or overall body weight. Similar effects are observed with a ketogenic diet (low in carbohydrates) or supplementation of a normal diet with the natural ketone β-hydroxybutyrate. Ketone bodies may promote metabolic reprogramming by shifting fuel sources (decreasing glucose availability and increasing fatty acids). Additionally, protein restriction also ameliorates PKD in two nonorthologous PKD models (50), and a soy-based diet halts PKD via changes in lipid metabolism (51). Finally, lowering lipid intake also affects cyst growth in murine models (7).
To translate these findings to humans, the RCT NCT03342742 is evaluating the effects of both weight loss and periods of fasting in overweight/obese individuals with ADPKD. The trial will compare two weight loss interventions with the same weekly caloric reduction on the basis of either daily caloric restriction or intermittent fasting. The primary end points are feasibility of enrollment and adherence (weight loss). Safety/tolerability; quality of life; TKV; and markers of IGF-I, AMPK, and mTOR signaling will also be assessed.
Pharmacologic-Based Alternatives to Dietary Changes Modulating Metabolic Dysfunction in ADPKD
Dietary modifications can be challenging to implement and sustain over a long period, potentially limiting translation to clinical practice. Thus, pharmacologic agents targeting metabolic pathways may be attractive alternative candidates in slowing ADPKD progression (Figure 2).
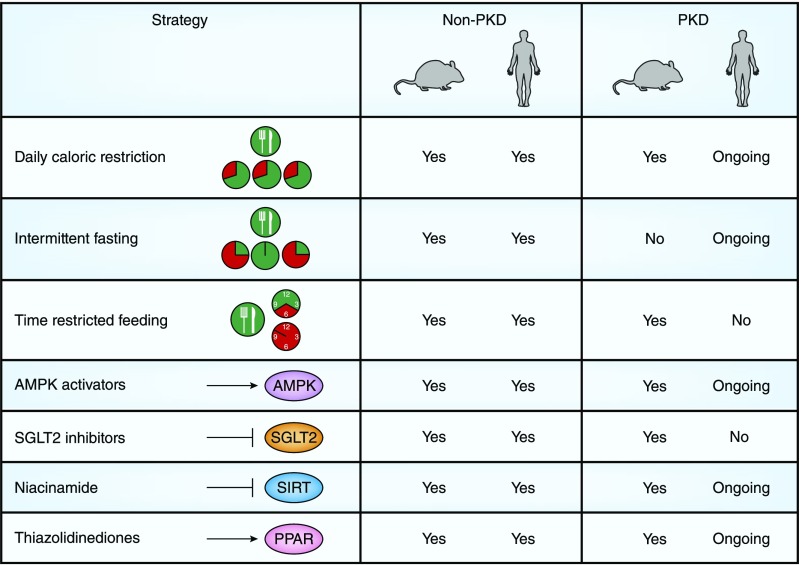
Figure 2.
Dietary and pharmacologic-based strategies may target dysregulated metabolism in ADPKD. A summary is provided regarding whether dietary and pharmacologic-based strategies to potentially target dysregulated metabolism in ADPKD have been evaluated in nonpolycystic kidney disease (non-PKD) models (rodents and humans) and in PKD models (rodents and humans).
AMPK Activators
Metformin is a pharmacologic activator of the AMPK pathway, and it is Food and Drug Administration (FDA) approved for the treatment of type 2 diabetes mellitus and polycystic ovarian syndrome. As evaluated in kidney epithelial cell culture and mouse/zebrafish models of PKD, metformin treatment inhibits mTOR and the cystic fibrosis transmembrane conductance regulator, represses subsequent epithelial secretion and proliferation, and slows cyst growth (52,53). Furthermore, statins are known to be AMPK activators and can decrease cyst formation, preserve kidney function, and alleviate intestinal inflammation/fibrosis in animal models (54). Two prospective RCTs are currently evaluating whether metformin may be an effective treatment strategy in adults with ADPKD. The Trial for Administration of Metformin to Tame PKD (NCT0256017) is a phase 2, double-blind, placebo-controlled RCT of 26 months in duration being conducted in 96 nondiabetic adults 18–60 years old with ADPKD and eGFR>50 ml/min per 1.73 m2. The primary outcomes are safety and tolerability. Secondary outcomes include change in TKV and liver volume, pain, quality of life, and targeted metabolomic biomarkers of the glycolytic and AMPK pathways.
A second RCT of 1-year duration is enrolling 50 nondiabetic adults 30–60 years of age with ADPKD and eGFR of 50–80 ml/min per 1.73 m2 (NCT02903511). Coprimary end points are (1) percentage of participants prescribed the full randomized dose according to the protocol at 12 months and (2) percentage of participants prescribed at least 50% of the randomized dose according to the protocol at 12 months. Secondary end points include change in TKV and eGFR. Of note, an additional RCT directly comparing metformin with tolvaptan in 150 adults 18–50 years old with eGFR of ≥45 ml/min per 1.73 m2 and a PKD1 truncating mutation is planned to begin in 2020 in Italy (NCT03764605).
Statins are another AMPK activator with FDA approval. Three years of treatment with pravastatin slowed kidney growth in an RCT conducted in children and young adults with ADPKD (55). An ongoing RCT is evaluating whether 2 years of treatment with pravastatin has similar benefit on change in TKV in adults with ADPKD and preserved kidney function (NCT03273413).
Sodium Glucose Cotransporter-2 Inhibitors
Sodium glucose cotransporter-2 (SGLT2) inhibitors are relatively new agents that promote glycosuria and thereby, lower blood glucose. The SGLT2 inhibitor canagliflozin was recently shown to reduce the risk of both kidney failure and cardiovascular events in an RCT of 4401 patients with type 2 diabetes mellitus and albuminuric CKD (56). Five weeks of treatment with an SGLT1 and SLGT2 inhibitor phlorizon inhibits cystogenesis in the Han:SPRD rat model of PKD (57). In contrast, the SGLT2 inhibitor dapaglofozin improves kidney function and albuminuria in the Han:SPRD rat, but it fails to slow cyst growth (58) and unexpectedly enhances cyst volume in the PCK rat model of ADPKD (59). Additionally, canagliflozin failed to reduce kidney disease severity in an orthologous ADPKD model (60). Thus, caution may be needed in considering SGLT2 inhibitors for the treatment of ADPKD.
Niacinamide/Nicotinamide
Niacinamide (also known as nicotinamide) is a derivative of nicotinic acid (niacin) and can act as an inhibitor of the NAD-dependent protein deacetylase sirtuin-1 (SIRT-1) in vitro. Sirtuins can act as metabolic energy sensors in response to caloric restriction. SIRT-1 expression is increased in the kidney of multiple rodent models of PKD (61). Niacinamide both slows cyst growth and improves kidney function in two orthologous mouse models of ADPKD, and it is suggested to specifically function through SIRT-1 inhibition (61). Notably, although caloric restriction is a known activator of SIRT-1 (36), food restriction does not seem to alter SIRT-1 expression in the PKD kidney (8).
A small phase 2 RCT with the dietary supplement niacinamide (30 mg/kg per day) or placebo administered to adults with ADPKD for 1 year is currently pending results (NCT02558595). The primary outcome is change in acetylated/total p53 (which is mediated by SIRT-1) in PBMCs. Other end points include change in height-corrected TKV, eGFR, and monocyte chemoattractant protein-1 levels.
Thiazolidinediones
Thiazolidinediones are synthetic ligands of PPARγ, a regulator of fatty acid storage and glucose metabolism, and they are FDA approved to control high blood glucose in type 2 diabetes mellitus. They can also inhibit cystic fibrosis transmembrane conductance regulator activity and subsequent chloride secretion in response to vasopressin (62). Importantly, the thiazolidinediones pioglitazone and rosiglitazone have each shown efficacy in halting cystogenesis in multiple animal models of PKD (63,64). A 1-year, phase 2, crossover trial to determine the safety and efficacy of pioglitazone in nondiabetic adults with ADPKD is ongoing; the primary outcome is safety, and TKV is a secondary end point (NCT02697617). Of note, the FDA recently issued a warning that pioglitazone may increase risk of bladder cancer; thus, caution should be exercised.
Conclusions, Limitations, and Future Directions
In this review, we discussed recent evidence that a metabolic defect exists in ADPKD, which likely contributes to cystic epithelial proliferation and subsequent cyst growth. We provided evidence of overlapping features and pathways among metabolism, obesity, and/or ADPKD. We also discussed both dietary and pharmacologic-based strategies targeting metabolic abnormalities that are being considered as therapies to slow ADPKD progression.
Interventions targeting dysregulated metabolism are attractive, particularly given the slowly progressive nature of ADPKD. The results from initial trials targeting metabolism are upcoming and anxiously awaited within the scientific and PKD communities. There continues to be a need for additional mechanistic studies to better understand the role of dysregulated metabolism in ADPKD and for subsequent translation to clinical trials. It is worth mentioning that current mechanistic studies have often not evaluated which specific cell type is displaying the observed metabolic defect. Although assumptions are being made that the cystic epithelium is likely the key culprit, metabolic defects in cells within the kidney microenvironment may also contribute to pathogenesis. Furthermore, it is unclear whether current interventions known to alleviate disease in humans or animal models (e.g., tolvaptan) may function at least in part via metabolic savings. Hence, advancing mechanistic research will be necessary to understand the true potential of targeting metabolic reprogramming in ADPKD and will likely provide novel therapeutic targets that warrant further testing.
Additionally, it is important to note that dietary strategies targeting metabolic abnormalities have inherent limitations that may impede widespread clinical implementation. Long-term adherence can be challenging, weight loss is not desirable in all individuals, and care is needed to ensure adequate nutrition. Because ongoing clinical trials that target metabolic reprogramming in ADPKD either via dietary or pharmacologic interventions are being conducted later in the course of disease (i.e., adults), it is unknown if such strategies would have adverse consequences during times of growth (i.e., childhood) and whether they could be used intermittently throughout life. Combining metabolic-focused therapeutic approaches with compounds targeting other signaling cascades altered in ADPKD, such as tolvaptan, may not only enhance the overall therapeutic benefit but also, allow a lower required dose of the pharmaceutical, which may decrease toxicity. Likewise, combination therapy may reduce the severity of dietary restriction that is required to achieve benefit, hence increasing translational feasibility. Continued research on this important and exciting topic will undoubtedly provide insight into such questions, moving toward the ultimate goal of slowing ADPKD progression.
Disclosures
Dr. Hopp and Dr. Nowak have nothing to disclose.
Funding
Dr. Hopp is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K01DK114164 and PKD Foundation research award 216G18a. Dr. Nowak is supported by National Institute of Diabetes and Digestive and Kidney Diseases grants K01DK103678 and R03DK118215.
Acknowledgments
Because of space limitations, not all contributions to the field could be discussed.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Menezes LF, Germino GG: The pathobiology of polycystic kidney disease from a metabolic viewpoint. Nat Rev Nephrol 15: 735–749, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Padovano V, Podrini C, Boletta A, Caplan MJ: Metabolism and mitochondria in polycystic kidney disease research and therapy. Nat Rev Nephrol 14: 678–687, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Weimbs T, Shillingford JM, Torres J, Kruger SL, Bourgeois BC: Emerging targeted strategies for the treatment of autosomal dominant polycystic kidney disease. Clin Kidney J 11[Suppl 1]: i27–i38, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, Pei Y, Musco G, Boletta A: Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19: 488–493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riwanto M, Kapoor S, Rodriguez D, Edenhofer I, Segerer S, Wüthrich RP: Inhibition of aerobic glycolysis attenuates disease progression in polycystic kidney disease. PLoS One 11: e0146654, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menezes LF, Lin CC, Zhou F, Germino GG: Fatty acid oxidation is impaired in an orthologous mouse model of autosomal dominant polycystic kidney disease. EBioMedicine 5: 183–192, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warner G, Hein KZ, Nin V, Edwards M, Chini CC, Hopp K, Harris PC, Torres VE, Chini EN: Food restriction ameliorates the development of polycystic kidney disease. J Am Soc Nephrol 27: 1437–1447, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiaravalli M, Rowe I, Mannella V, Quilici G, Canu T, Bianchi V, Gurgone A, Antunes S, D’Adamo P, Esposito A, Musco G, Boletta A: 2-Deoxy-d-glucose ameliorates PKD progression. J Am Soc Nephrol 27: 1958–1969, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraus A, Schley G, Kunzelmann K, Schreiber R, Peters DJ, Stadler R, Eckardt KU, Buchholz B: Glucose promotes secretion-dependent renal cyst growth. J Mol Med (Berl) 94: 107–117, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Sas KM, Yin H, Fitzgibbon WR, Baicu CF, Zile MR, Steele SL, Amria M, Saigusa T, Funk J, Bunni MA, Siegal GP, Siroky BJ, Bissler JJ, Bell PD: Hyperglycemia in the absence of cilia accelerates cystogenesis and induces renal damage. Am J Physiol Renal Physiol 309: F79–F87, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menezes LF, Zhou F, Patterson AD, Piontek KB, Krausz KW, Gonzalez FJ, Germino GG: Network analysis of a Pkd1-mouse model of autosomal dominant polycystic kidney disease identifies HNF4α as a disease modifier. PLoS Genet 8: e1003053, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakhia R, Yheskel M, Flaten A, Quittner-Strom EB, Holland WL, Patel V: PPARα agonist fenofibrate enhances fatty acid β-oxidation and attenuates polycystic kidney and liver disease in mice. Am J Physiol Renal Physiol 314: F122–F131, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soomro I, Sun Y, Li Z, Diggs L, Hatzivassiliou G, Thomas AG, Rais R, Slusher BS, Somlo S, Skolnik EY: Glutamine metabolism via glutaminase 1 in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant 33: 1343–1353, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flowers EM, Sudderth J, Zacharias L, Mernaugh G, Zent R, DeBerardinis RJ, Carroll TJ: Lkb1 deficiency confers glutamine dependency in polycystic kidney disease. Nat Commun 9: 814, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trott JF, Hwang VJ, Ishimaru T, Chmiel KJ, Zhou JX, Shim K, Stewart BJ, Mahjoub MR, Jen KY, Barupal DK, Li X, Weiss RH: Arginine reprogramming in ADPKD results in arginine-dependent cystogenesis. Am J Physiol Renal Physiol 315: F1855–F1868, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou LF, Cheng YL, Hsieh CY, Lin CY, Yang HY, Chen YC, Hung CC, Tian YC, Yang CW, Chang MY: Effect of trehalose supplementation on autophagy and cystogenesis in a mouse model of polycystic kidney disease. Nutrients 11: E42, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu P, Sieben CJ, Xu X, Harris PC, Lin X: Autophagy activators suppress cystogenesis in an autosomal dominant polycystic kidney disease model. Hum Mol Genet 26: 158–172, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belibi F, Zafar I, Ravichandran K, Segvic AB, Jani A, Ljubanovic DG, Edelstein CL: Hypoxia-inducible factor-1α (HIF-1α) and autophagy in polycystic kidney disease (PKD). Am J Physiol Renal Physiol 300: F1235–F1243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CC, Kurashige M, Liu Y, Terabayashi T, Ishimoto Y, Wang T, Choudhary V, Hobbs R, Liu LK, Lee PH, Outeda P, Zhou F, Restifo NP, Watnick T, Kawano H, Horie S, Prinz W, Xu H, Menezes LF, Germino GG: A cleavage product of Polycystin-1 is a mitochondrial matrix protein that affects mitochondria morphology and function when heterologously expressed. Sci Rep 8: 2743, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuo IY, Brill AL, Lemos FO, Jiang JY, Falcone JL, Kimmerling EP, Cai Y, Dong K, Kaplan DL, Wallace DP, Hofer AM, Ehrlich BE: Polycystin 2 regulates mitochondrial Ca2+ signaling, bioenergetics, and dynamics through mitofusin 2. Sci Signal 12: eaat7397, 2019. [DOI] [PMC free article] [PubMed]
- 22.Padovano V, Kuo IY, Stavola LK, Aerni HR, Flaherty BJ, Chapin HC, Ma M, Somlo S, Boletta A, Ehrlich BE, Rinehart J, Caplan MJ: The polycystins are modulated by cellular oxygen-sensing pathways and regulate mitochondrial function. Mol Biol Cell 28: 261–269, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim K, Trott JF, Gao G, Chapman A, Weiss RH: Plasma metabolites and lipids associate with kidney function and kidney volume in hypertensive ADPKD patients early in the disease course. BMC Nephrol 20: 66, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klawitter J, Klawitter J, McFann K, Pennington AT, Abebe KZ, Brosnahan G, Cadnapaphornchai MA, Chonchol M, Gitomer B, Christians U, Schrier RW: Bioactive lipid mediators in polycystic kidney disease. J Lipid Res 55: 1139–1149, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grams ME, Tin A, Rebholz CM, Shafi T, Köttgen A, Perrone RD, Sarnak MJ, Inker LA, Levey AS, Coresh J: Metabolomic alterations associated with cause of CKD. Clin J Am Soc Nephrol 12: 1787–1794, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao Z, Xie G, Ong AC: Metabolic abnormalities in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 30: 197–203, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Reed B, Helal I, McFann K, Wang W, Yan XD, Schrier RW: The impact of type II diabetes mellitus in patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 27: 2862–2865, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheungpasitporn W, Thongprayoon C, Vijayvargiya P, Anthanont P, Erickson SB: The risk for new-onset diabetes mellitus after kidney transplantation in patients with autosomal dominant polycystic kidney disease: A systematic review and meta-analysis. Can J Diabetes 40: 521–528, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Schrier RW, McFann KK, Johnson AM: Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int 63: 678–685, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Nowak KL, You Z, Gitomer B, Brosnahan G, Torres VE, Chapman AB, Perrone RD, Steinman TI, Abebe KZ, Rahbari-Oskoui FF, Yu ASL, Harris PC, Bae KT, Hogan M, Miskulin D, Chonchol M: Overweight and obesity are predictors of progression in early autosomal dominant polycystic kidney disease. J Am Soc Nephrol 29: 571–578, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ: Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int 73: 19–33, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Navaneethan SD, Yehnert H, Moustarah F, Schreiber MJ, Schauer PR, Beddhu S: Weight loss interventions in chronic kidney disease: A systematic review and meta-analysis. Clin J Am Soc Nephrol 4: 1565–1574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore T, Beltran L, Carbajal S, Strom S, Traag J, Hursting SD, DiGiovanni J: Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila) 1: 65–76, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Dann SG, Selvaraj A, Thomas G: mTOR Complex1-S6K1 signaling: At the crossroads of obesity, diabetes and cancer. Trends Mol Med 13: 252–259, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Steinberg GR, Kemp BE: AMPK in health and disease. Physiol Rev 89: 1025–1078, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Fontana L, Partridge L: Promoting health and longevity through diet: From model organisms to humans. Cell 161: 106–118, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lempiäinen J, Finckenberg P, Mervaala EE, Sankari S, Levijoki J, Mervaala EM: Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1α-eNOS pathway and enhanced autophagy. Acta Physiol (Oxf) 208: 410–421, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Longo VD, Mattson MP: Fasting: Molecular mechanisms and clinical applications. Cell Metab 19: 181–192, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gill S, Le HD, Melkani GC, Panda S: Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 347: 1265–1269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaix A, Zarrinpar A, Miu P, Panda S: Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20: 991–1005, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S: Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15: 848–860, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein RI, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Roberts SB; CALERIE Study Group: A 2-year Randomized controlled trial of human caloric restriction: Feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci 70: 1097–1104, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varady KA, Bhutani S, Church EC, Klempel MC: Short-term modified alternate-day fasting: A novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr 90: 1138–1143, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky A, Bhutani S, Varady KA: Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity (Silver Spring) 22: 2524–2531, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A: The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int J Obes 35: 714–727, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, Seyfried TN, Varady KA, Panda S: Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A 111: 16647–16653, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill S, Panda S: A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab 22: 789–798, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kipp KR, Rezaei M, Lin L, Dewey EC, Weimbs T: A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am J Physiol Renal Physiol 310: F726–F731, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres JA, Kruger SL, Broderick C, Amarlkhagva T, Agrawal S, Dodam JR, Mrug M, Lyons LA, Weimbs T: Ketosis ameliorates renal cyst growth in polycystic kidney disease. Cell Metab 30: 1007–1023.e5, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomobe K, Philbrick D, Aukema HM, Clark WF, Ogborn MR, Parbtani A, Takahashi H, Holub BJ: Early dietary protein restriction slows disease progression and lengthens survival in mice with polycystic kidney disease. J Am Soc Nephrol 5: 1355–1360, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Ogborn MR, Nitschmann E, Weiler HA, Bankovic-Calic N: Modification of polycystic kidney disease and fatty acid status by soy protein diet. Kidney Int 57: 159–166, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Takiar V, Nishio S, Seo-Mayer P, King JD Jr, Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ: Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci U S A 108: 2462–2467, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang MY, Ma TL, Hung CC, Tian YC, Chen YC, Yang CW, Cheng YC: Metformin inhibits cyst formation in a zebrafish model of polycystin-2 deficiency. Sci Rep 7: 7161, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gile RD, Cowley BD Jr, Gattone VH 2nd, O’Donnell MP, Swan SK, Grantham JJ: Effect of lovastatin on the development of polycystic kidney disease in the Han:SPRD rat. Am J Kidney Dis 26: 501–507, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Cadnapaphornchai MA, George DM, McFann K, Wang W, Gitomer B, Strain JD, Schrier RW: Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 9: 889–896, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Zhang S, Liu Y, Spichtig D, Kapoor S, Koepsell H, Mohebbi N, Segerer S, Serra AL, Rodriguez D, Devuyst O, Mei C, Wüthrich RP: Targeting of sodium-glucose cotransporters with phlorizin inhibits polycystic kidney disease progression in Han:SPRD rats. Kidney Int 84: 962–968, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez D, Kapoor S, Edenhofer I, Segerer S, Riwanto M, Kipar A, Yang M, Mei C, Wüthrich RP: Inhibition of sodium-glucose cotransporter 2 with dapagliflozin in Han: SPRD rats with polycystic kidney disease. Kidney Blood Press Res 40: 638–647, 2015 [DOI] [PubMed] [Google Scholar]
- 59.Kapoor S, Rodriguez D, Riwanto M, Edenhofer I, Segerer S, Mitchell K, Wüthrich RP: Effect of sodium-glucose cotransport inhibition on polycystic kidney disease progression in PCK rats. PLoS One 10: e0125603, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leonhard WN, Song X, Kanhai AA, Iliuta IA, Bozovic A, Steinberg GR, Peters DJM, Pei Y: Salsalate, but not metformin or canagliflozin, slows kidney cyst growth in an adult-onset mouse model of polycystic kidney disease. EBioMedicine 47: 436–445, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou X, Fan LX, Sweeney WE Jr, Denu JM, Avner ED, Li X: Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J Clin Invest 123: 3084–3098, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nofziger C, Brown KK, Smith CD, Harrington W, Murray D, Bisi J, Ashton TT, Maurio FP, Kalsi K, West TA, Baines D, Blazer-Yost BL: PPARgamma agonists inhibit vasopressin-mediated anion transport in the MDCK-C7 cell line. Am J Physiol Renal Physiol 297: F55–F62, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Dai B, Liu Y, Mei C, Fu L, Xiong X, Zhang Y, Shen X, Hua Z: Rosiglitazone attenuates development of polycystic kidney disease and prolongs survival in Han:SPRD rats. Clin Sci (Lond) 119: 323–333, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Muto S, Aiba A, Saito Y, Nakao K, Nakamura K, Tomita K, Kitamura T, Kurabayashi M, Nagai R, Higashihara E, Harris PC, Katsuki M, Horie S: Pioglitazone improves the phenotype and molecular defects of a targeted Pkd1 mutant. Hum Mol Genet 11: 1731–1742, 2002 [DOI] [PubMed] [Google Scholar]