Abstract
In this review we outline a rationale for identifying neuroprotectants aimed at inducing endogenous Klotho activity and expression, which is epigenetic action, by definition. Such an approach should promote remyelination and/or stimulate myelin repair by acting on mitochondrial function, thereby heralding a life-saving path forward for patients suffering from neuroinflammatory diseases. Disorders of myelin in the nervous system damage the transmission of signals, resulting in loss of vision, motion, sensation, and other functions depending on the affected nerves, currently with no effective treatment. Klotho genes and their single-pass transmembrane Klotho proteins are powerful governors of the threads of life and death, true to the origin of their name, Fates, in Greek mythology. Among its many important functions, Klotho is an obligatory co-receptor that binds, activates, and/or potentiates critical fibroblast growth factor activity. Since the discovery of Klotho a little over two decades ago, it has become ever more apparent that when Klotho pathways go awry, oxidative stress and mitochondrial dysfunction take over, and age-related chronic disorders are likely to follow. The physiological consequences can be wide ranging, potentially wreaking havoc on the brain, eye, kidney, muscle, and more. Central nervous system disorders, neurodegenerative in nature, and especially those affecting the myelin sheath, represent worthy targets for advancing therapies that act upon Klotho pathways. Current drugs for these diseases, even therapeutics that are disease modifying rather than treating only the symptoms, leave much room for improvement. It is thus no wonder that this topic has caught the attention of biomedical researchers around the world.
Keywords: amyotrophic lateral sclerosis, Klotho, mitochondria, multiple sclerosis, neurodegenerative disease
Background
The term “epigenetics” refers to changes resulting from modification of gene expression instead of alterations in the genetic code.1 We postulate that drugs aimed at inducing endogenous Klotho activity and expression—that is, therapeutics acting through epigenetic mechanisms—should promote remyelination and/or stimulate myelin repair by acting on mitochondrial function. As such, this approach may herald a life-saving path forward for patients suffering from neuroinflammatory diseases.
Klotho—a gene set of three members: α-Klotho, β-Klotho, and γ-Klotho2–4—is aptly named in the biological context of aging.5,6 According to Greek mythology, Klotho (or Clotho; Greek: Kλωθώ), the youngest of the Fates (Clotho, Lachesis: Λάχɛσις and Atropos: Άτροπος), is one of the three daughter deities (the spinner) of Zeus and Nyx (Nύξ, the goddess of night) or Themis (Θέμις, the goddess of law and order) who together spin out the thread of life, allot destiny, and choose the time of passing for both mortals and immortals.7 Thus, nothing could be more appropriate than Klotho serving as a longevity gene. Indeed, once Klotho fails to adequately express its proteins and variants,6,8–10 it is implicated in pathways that drive age-related chronic disorders such as kidney disease, tissue dysfunction, diabetic retinopathies, neurodegeneration, and impairments in mitochondrial function and muscle regeneration.4,8,11–16
α-Klotho is often referred to as an “anti-aging protein.”3,6,17,18 When overexpressed in mice, Klotho extends life (20–30%), reduces oxidative stress (OS), and demonstrates other prosurvival properties.19–25 The potential of extending these results to humans has captured pharmaceutical interest in developing Klotho-based therapeutics to hinder the degenerative illnesses of aging.5,6,8,26–28
Noticeably, a growing body of evidence asserts the therapeutic potential of Klotho in treating neurodegenerative diseases. As population aging is a global phenomenon,29 age-related neurodegenerative disorders are projected to surpass cancer as the foremost cause of death after cardiovascular disease in the developed world within 20 years.30 The late-onset sporadic form (LOAD) of Alzheimer's disease (AD)31–33 accounts for >90% of disease cases.31,34–36 Along with advanced aging,23,37–43 inheritance of the apolipoprotein E4 allele (also called APOE4 or APOEɛ4) remains the most significant known genetic risk factor for LOAD. The risk is higher and the age at onset of dementia is younger for individuals carrying multiple copies of APOE4, whereas other APOE alleles are considered protective.31,32,44 In a study of a gene variant of Klotho with respect to AD in at-risk but presymptomatic individuals, heterozygosity was found to reduce amyloid aggregation in an APOE4-associated manner.45 Of interest, in a research analysis that measured Klotho concentrations in the cerebrospinal fluid of AD subjects and in older versus younger adults, Klotho levels were found to be lower in women compared with men.46,47 Perhaps the latter observation may help to explain why women are more likely than men to have AD, although the reported difference may be the result of biological or social artifacts.48 In addition to AD, the most common neurodegenerative disease, Parkinson's disease (PD), the second most common neurodegenerative disease,49 has also been tied to Klotho pathways.50–52
Beyond AD and PD, age-related declines in Klotho8,13,17,24,53 are associated with a range of other deteriorating central nervous system (CNS) processes.17,24 For example, mounting evidence implicates dysregulation of Klotho in shared mechanistic pathological relationships linking iron and myelin in various common and rare brain diseases,54–56 including abnormalities in myelination and the maturation of oligodendrocytes that are central to the pathogenicity of diseases such as multiple sclerosis (MS)26,56,57 and amyotrophic lateral sclerosis (ALS).56,58
OS Demyelination and Mitochondrial Dysfunction
Mitochondrial dysfunction is a well-documented enabling factor in the pathophysiology of neurological conditions and disorders (Fig. 1).41,59–68 Although a principal role of mitochondria is to supply the bioenergy needed for cellular processes and maintenance,69–71 mitochondria also help regulate neurite branching and regeneration as well as synaptic strength, stability, and signaling in the CNS.72 In addition, myelin repair is intimately dependent on healthy mitochondrial function within the CNS in oligodendrocytes and neuronal cell bodies.63,64,73–77
FIG. 1.
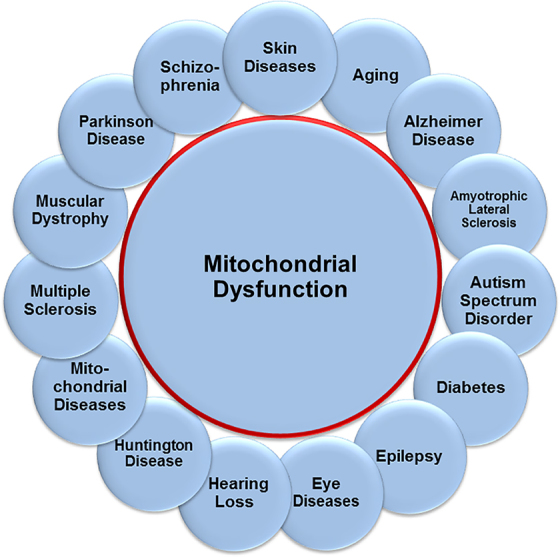
Sampling of neurologic conditions associated with mitochondrial dysfunction.
Dysfunctional mitochondria become sources of reactive oxygen species (ROS) that contribute to OS with deleterious effects on the cell's well-being.61,70,71,74,77–80 Manifestations of OS are hallmark symptoms in neurological disease, including cognitive deficits.52,54,65,76,79,81–88 In concert with the above, a correlation was found between OS in the CNS and demyelination, which results in the loss of integrity and proper maintenance of oligodendrocytes and their myelin sheaths, the latter being crucial for cognitive performance and higher brain function.57,89–91
Thus, inclusion of strategies for enhancing mitochondrial biogenesis, function, and protection68,80,92–96 that may also rely on pathways epigenetically induced by diet97–108 and/or exercise99,100,106–109 can be timely in the therapeutic protocols for treating myelination disorders.68,73–76,98,104,110–115
Dysregulated Myelination in Peripheral and CNS Diseases
Microglia are a distinct population of immune cells in the CNS.116–118 They execute fundamental tasks in brain development, physiology, and homeostasis and in influencing the pathological progression of brain diseases.117–122 There is evidence to suggest that microglia actively remove damaged myelin114,123 to recruit myelinating cells, oligodendrocytes in the CNS, and Schwann cells in the peripheral nervous system (PNS) to repair the injured myelin sheath.114,117,118,123–125 Dysregulated myelination is a characteristic feature of numerous heritable neurological diseases, such as the PNS hereditary disorder, Charcot–Marie–Tooth disease,126,127 X-linked adrenoleukodystrophy and metachromatic leukodystrophy,128 hereditary diffuse leukoencephalopathy with spheroids, Nasu–Hakola disease,114 and Huntington's disease,129,130 among others.55,131,132 A dysfunctional myelination apparatus is also evident in acquired demyelinating diseases such as diabetic peripheral neuropathy, drug-related peripheral neuropathies, leprosy, and peripheral neuropathies of inflammatory etiology.132
Most interestingly, converging evidence drawn from “Big Data” analytics in parallel with epigenetic, neuroimaging, and experimental model investigations seems to connect an adult-onset form of attention-deficit/hyperactivity disorder pathogenesis and persistence with dysregulated myelination.133,134 Many risk genes for CNS disorders such as AD, PD, schizophrenia, autism, and MS have been unveiled by genome-wide association studies to be expressed by microglia.117 Dysfunction of microglia is common in neurological diseases114 and recent studies have found that sex differences in microglial gene expression and functions seen in young adult mice tend to be increasingly pronounced in the aging brain.135
Klotho as an Obligatory Co-receptor
High concentrations of phosphate in the body are found in bone, teeth, and dental enamel as calcium phosphate crystals.136,137 Klotho regulates phosphorus and calcium homeostasis 5,6,18,23,138 and functions as an obligatory co-receptor that binds and activates its related endocrine fibroblast growth factor (FGF) receptors (FGFRs) to potentiate its biological activities.5,6,23,102,139–146 FGFs are exemplary pleiotropic hormones that play numerous roles in cellular and metabolic homeostasis.5,6,137,141,144–148 In particular, FGF23 is a bone-derived hormone that in conjunction with Klotho acts on the kidney to increase phosphate excretion and suppress biosynthesis of vitamin D.5,6,14,23,102,136,138,145,148,149 Vitamin D regulates epigenetic mechanisms that maintain the transcription of its target genes in regulatory networks, including the expression of Klotho and nuclear factor-erythroid-2-related factor 2 (Nrf2) to carry out many of its homoeostatic functions.17,97,150–153 Vitamin D is a modulator of the immune system,154,155 hence its mention here, and accumulating evidence suggests vitamin D deficiency is a risk factor for dysregulated Klotho-associated neurodegenerative diseases, the most noteworthy being MS.9,27,52,97,102,150,152,153,156–158
Multiple Sclerosis
MS is an insidious progressive neurodegenerative disease characterized by demyelinated lesions throughout the brain, spinal cord, and optic nerve resulting from immune-mediated attacks against myelin.159–165 It is the apotheosis of myelination disorders that affects ∼2.5 million people around the world166–168 and currently there are no definitive cures. The standard of chronic care, after using steroids for acute episodes, centers on the use of disease modifying therapies (DMTs) that modulate an overactive immune response, such as antibodies against interferon, interleukin, or related T cell targets.9,169–172 Unfortunately, although there is a growing armamentarium of DMTs for neurodegenerative diseases, they have to date had only a modest impact on disease progression173,174 and thus the demand for myelin repair-promoting therapies for MS remains a significant unmet medical need.159,175–178
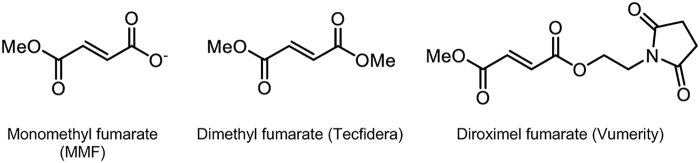
The discovery of new drugs is a daunting, lengthy, and costly endeavor. Drug repurposing—based on mechanism of action and/or biological activity, not uncommonly the result of serendipity—is a promising and cost-saving approach for the treatment of rare genetic diseases and diseases with limited therapeutic options.90,104,179,180 This approach has yielded derivatives of the simple organic chemical, fumarate, including Vumerity (diroximel fumarate), which is reported to be better tolerated than Tecfidera, with fewer gastrointestinal side effects and more favorable pharmacokinetic properties. Vumerity is a delayed release formulation of an inactive diester prodrug of monomethyl fumarate (Fig. 2). Both Vumerity and Tecfidera are converted into the same pharmacologically active drug, monomethyl fumarate in vivo.94,163 The medical potential of dimethyl fumarate was identified over 60 years ago and marketed for the treatment of psoriasis.181,182 MS therapeutics approved by the U.S. Food and Drug Administration (FDA) are given in Table 1.
FIG. 2.
Monomethyl fumarate, the pharmacologically active form of Tecfidera and Vumerity.
Table 1.
Food and Drug Administration-Approved Drugs for Multiple Sclerosis in Disease Modifying Therapies
| Older drugs, year approved | Recent approvals, year | Withdrawals, year |
|---|---|---|
| Betaseron (INF-β-1b), 1993 |
Lemtrada (alemtuzumab), 2014 |
Zinbryta (daclizumab), 2018 |
| Avonex (INF-β-1a), 1996 |
Plegridty (INF-β-1a), 2014 |
|
| Copaxone (glatiramer acetate), 1996 |
Glatopa (glatiramer acetate), 2015 |
|
| Rebif (INF-β-1a), 2002 |
Ocrevus (ocrelizumab), 2017 |
|
| Tysabri (natalizumab), 2004 |
Mavenclad (cladribine), 2019 |
|
| Extavia (INF-β-1b), 2009 |
Mayzent (siponimod), 2019 |
|
| Gilenya (fingolimod), 2010 |
Vumerity (diroximel fumarate), 2019 | |
| Aubagio (teriflunomide), 2012 | ||
| Tecfidera (dimethyl fumarate) 2013 |
Sources: FDA Drug Approvals and Databases (www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases). Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations (www.accessdata.fda.gov/scripts/cder/ob/index.cfm).
FDA, Food and Drug Administration.
Klotho Structure, Distribution, and Function in MS
Klotho is a single-pass transmembrane protein expressed in the brain (hippocampus and choroid plexus), kidney, eye (retina, optic nerve, lens) and parathyroid gland, and less so in other tissues.3,18,27,102,183,184 A soluble form of Klotho (sKlotho), primarily secreted from the kidney, circulates in blood, urine, and cerebrospinal fluid, exerting different biological effects in multiple tissues as a humoral factor.5,6,8,52,185–189
In the eye, Klotho protects against OS53,55,58,153,190,191 and is essential to the proper maintenance and function of the ocular system,12,26,192–195 being expressed throughout the retina, with the highest levels in retinal ganglion cells.196 The retinal pigment epithelium (RPE) is a highly specialized CNS tissue whose function is critical in preserving retinal homeostasis53,78 and an age-dependent decline of Klotho expression is said to contribute to RPE degeneration and retinal pathology.53 Apoptotic cells in models of retinal degeneration were found to exhibit high levels of Klotho,8 which is consistent with Klotho overexpression in its role as a protective protein that inhibits apoptosis.22,197,198 A recent study has shown that higher levels of circulating Klotho protein is protective in patients with diabetic retinopathy.199
Although the retina itself is a nonmyelinated tissue,200 optic neuritis, a disease affecting the myelinated part of retinal ganglion cell axons, is a serious and often difficult to assess manifestation of MS,201 particularly in the pediatric population.202 More than 70% of MS patients suffer vision loss as a secondary effect of optic neuritis disease progression.160,203,204 In recent studies, Klotho was shown to accelerate remyelination in a cuprizone-mediated demyelination mouse model.9,28 This important finding is refocusing attention on Klotho's role in neurodegeneration and research efforts are increasingly directed toward the development of MS treatments that promote remyelination and/or stimulate myelin repair.9,27,28,112,166,169,205–211 However, because Klotho does not cross the blood–brain barrier,10,212 a small molecule approach aimed at inducing endogenous Klotho activity and expression in the CNS is surfacing as a promising therapeutic strategy.27,81,90,143,213,214 Epigenetics10,90,100,169,215–219 and gene therapy-based methods are part of the emerging landscape under investigation.19,215,220,221
Amyotrophic Lateral Sclerosis
The global prevalence of ALS is estimated to be roughly two to four cases per 100,000 population222,223 compared with ∼30 cases per 100,000 population for MS.168 ALS (also referred to as progressive muscular atrophy or Lou Gehrig's disease) is a devastating neurodegenerative disease. It damages motor neurons in the brain and spinal cord leading to progressive muscle atrophy and paralysis that is fatal, usually within 3–5 years of diagnosis.58,224–227 Unfortunately, patients with ALS, at present, have limited therapeutic options (Table 2).96,173,228 Moreover, given the rapid and terminal progression of the disease postdiagnosis, there is a pressing need to develop new therapies and/or based on mechanism of action repurposing drugs already approved for other diseases.176,180 Recruiting ALS subjects into traditional clinical trials is challenging because of the low number of cases in the population. Trial-design protocols229 that rely on restrictive inclusion criteria, frequent study visits, use of a placebo control arm that denies patients early access to the therapy, and the comparatively long time it takes to document results relative to the rapid progression of the disease are additional impediments.230
Table 2.
Food and Drug Administration-Approved Drugs for Treating Amyotrophic Lateral Sclerosis
| Glutamate antagonist | Antioxidant | Other drugs |
|---|---|---|
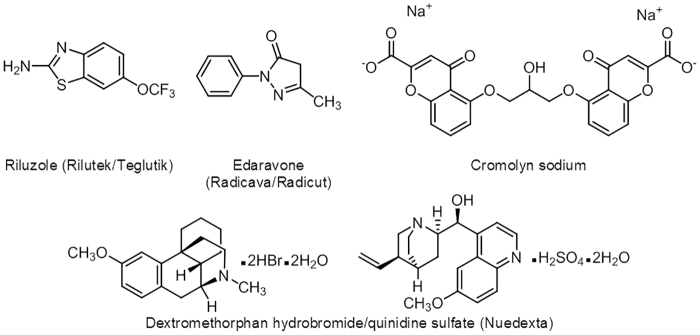
| Riluzole (Rilutek/Teglutik) | Edaravone (Radicava/Radicut) | Dextromethorphan hydrobromide/quinidine sulfate (Neudexta) for pseudobulbar affect |
Sources: FDA Drug Approvals and Databases (www.fda.gov/drugs/development-approval-process-drugs/drug-approvals-and-databases). Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations (www.accessdata.fda.gov/scripts/cder/ob/index.cfm).
Riluzole and edaravone, the principal therapeutics used in the treatment of ALS (Fig. 3), have a modest impact on disease progression, extending survival by ∼3 months.68,96,223,231 The combination of dextromethorphan and quinidine sulfate has shown positive results against pseudobulbar affect (emotional lability) and is FDA-approved for ALS and MS,232 although it is reported to be prescribed more to patients suffering from dementia or PD.233 Clearly, much more effective therapies are needed and a vigorous research effort has been underway for the past several years to screen for and develop new pharmaceuticals for treating neurodegenerative diseases including ALS.234,235 Cromolyn sodium (Fig. 3), an FDA-approved compound used to treat asthma and other conditions has recently emerged as a promising new therapeutic for ALS. In the SOD1G93A mouse model of ALS, treatment with cromolyn sodium delayed disease onset and showed neuroprotection by decreasing the inflammatory response.236 However, a focus on myelination may lead to more lasting and effective therapeutic outcomes. Klotho overexpression in the SOD1G93A mouse model was shown to suppress the production of proinflammatory cytokines, reduce the expression of neuroinflammatory markers, and prevent neuronal loss with a more profound effect in the spinal cord than in the motor cortex, thereby delaying the onset and progression of the disease.58 These results along with the positive effect Klotho has on the promyelinating properties of oligodendrocytes offer compelling evidence in support of developing Klotho-based therapeutic strategies for treating ALS.58
FIG. 3.
Chemical structures of FDA-approved therapeutics for ALS, including cromolyn sodium, a drug used to treat asthma and other conditions showing promising potential as a repurposed drug for ALS. ALS, amyotrophic lateral sclerosis; FDA, Food and Drug Administration.
Concluding Remarks
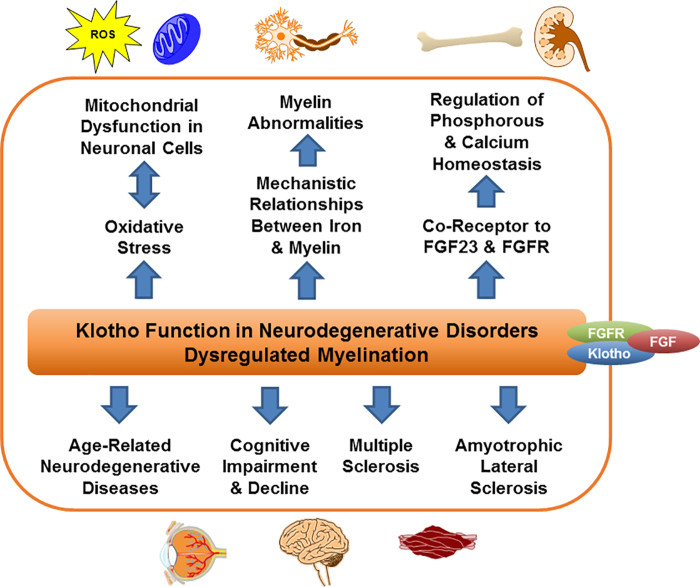
As outlined in the introduction, drugs aimed at inducing endogenous Klotho activity and expression—epigenetic action per se—should promote remyelination and/or stimulate myelin repair by acting on mitochondrial function. In the ensuing two decades since the serendipitous discovery of Klotho as an aging-suppressor gene, research has helped unmask many of its functional pathways in neurodegenerative disorders and/or dysregulated myelination (Fig. 4). Deficient levels of Klotho protein lead to excessive OS induction mainly from ROS produced in mitochondrial dysfunction. Myelin repair is intimately dependent on the energy made available by healthy mitochondria within the CNS in oligodendrocytes (Schwann cells in the PNS) and neuronal cell bodies. Thus, drugs aimed at inducing endogenous Klotho production may herald a life-saving path forward for patients suffering from neuroinflammatory diseases. In parallel, much as the old psoriasis drug, dimethyl fumarate, was repurposed to treat MS, more drug repurposing may find worthwhile paths here too. Will AD, PD, MS, or ALS yield to these approaches when coupled with drugs that attack such a powerful pathway as Klotho? As we kick off what we hope will be “the roaring 2020s” when it comes to the advancement of major new life-saving therapeutics, time and effort toward this goal will hopefully give us the answers.
FIG. 4.
Klotho function in neurodegenerative disorders and/or dysregulated myelination.
Acknowledgments
The authors gratefully acknowledge the generous financial support from the Foundation for a Cure for Mitochondrial Disease, Inc. (MitoCure). This article is dedicated to Dr. Brenda Milner on the occasion of her 102nd birthday.
Abbreviations Used
- AD
Alzheimer's disease
- ALS
amyotrophic lateral sclerosis
- CNS
central nervous system
- DMT
disease modifying therapy
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- LOAD
late-onset Alzheimer's disease
- MS
multiple sclerosis
- OS
oxidative stress
- PD
Parkinson's disease
- PNS
peripheral nervous system
- ROS
reactive oxygen species
Authors' Contribution
Authorship has been based on the principles of the International Committee of Medical Journal Editors: substantial contributions to the conception or design of the work or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author Disclosure Statement
W.H.M., in addition to academic and nonprofit roles, is employed by ShangPharma Innovation, is a managing director of Pandect Bioventures, receives royalties from Elsevier as a book author, is a consultant for Aduro Biotech, receives royalty and equity sharing benefits from SRI International, has stock or other financial interests in Aduro Biotech, Azkarra Therapeutics, Rigel Pharmaceuticals and Valitor, and serves on the boards of directors and/or scientific advisory boards of Aprinoia Therapeutics, Circle Pharma, Global Blood Therapeutics, Rigel Pharmaceuticals, ShangPharma Innovation and Valitor. D.V.F., in addition to academic and nonprofit roles, is employed by Viracta Therapeutics, Phoenicia Biosciences, and Takeda Pharmaceuticals and serves as a consultant to Briacell Therapeutics. Kosta Steliou, in addition to academic and nonprofit roles, is the founder and chief scientific officer of PhenoMatriX. K.K. consults with and/or serves as an executive or on the boards of various biotechnology and pharmaceutical companies from time to time, where he may receive compensation and/or stock options, and he is eligible to receive compensation from ShangPharma Innovation and Pandect Bioventures, health care venture incubator and venture capital firms.
Funding Information
The Foundation for a Cure for Mitochondrial Disease, Inc. (MitoCure).
Cite this article as: Moos WH, Faller DV, Glavas IP, Harpp DN, Kanara I, Mavrakis AN, Pernokas J, Pernokas M, Pinkert CA, Powers WR, Sampani K, Steliou K, Vavvas DG, Zamboni RJ, Kodukula K, Chen X (2020) Klotho pathways, myelination disorders, neurodegenerative diseases, and epigenetic drugs, BioResearch Open Access 9:1, 94–105, DOI: 10.1089/biores.2020.0004.
References
- 1. Noble D. Conrad Waddington and the origin of epigenetics. J Exp Biol. 2015;218:816–818 [DOI] [PubMed] [Google Scholar]
- 2. Jurado-Fasoli L, Amaro-Gahete FJ, De-La-O A, et al. Adherence to the Mediterranean diet, dietary factors, and S-Klotho plasma levels in sedentary middle-aged adults. Exp Gerontol. 2019;119:25–32 [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Wang L, Wu Z, et al. The expressions of Klotho family genes in human ocular tissues and in anterior lens capsules of age-related cataract. Curr Eye Res. 2017;42:871–875 [DOI] [PubMed] [Google Scholar]
- 4. Zou D, Wu W, He Y, et al. The role of klotho in chronic kidney disease. BMC Nephrol. 2018;19:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuro-o M. Molecular mechanisms underlying accelerated aging by defects in the FGF23-Klotho system. Int J Nephrol. 2018;2018:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuro-o M. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019;15:27–44 [DOI] [PubMed] [Google Scholar]
- 7. Grant M, Hazel J. Who's Who in Classical Mythology. Oxford University Press: New York, NY, 1993 [Google Scholar]
- 8. Cheikhi A, Barchowsky A, Sahu A, et al. Klotho: an elephant in aging research. J Gerontol A Biol Sci Med Sci. 2019;74:1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torbus-Paluszczak M, Bartman W, Adamczyk-Sowa M. Klotho protein in neurodegenerative disorders. Neurol Sci. 2018;39:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolf EJ, Morrison FG, Sullivan DR, et al. The goddess who spins the thread of life: Klotho, psychiatric stress, and accelerated aging. Brain Behav Immun. 2019;80:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kan W-C, Hwang J-Y, Chuang L-Y, et al. Effect of osthole on advanced glycation end products-induced renal tubular hypertrophy and role of klotho in its mechanism of action. Phytomedicine. 2019;53:205–212 [DOI] [PubMed] [Google Scholar]
- 12. Nusinovici S, Sabanayagam C, Teo BW, et al. Vision impairment in CKD patients: epidemiology, mechanisms, differential diagnoses, and prevention. Am J Kidney Dis. 2019;73:846–857 [DOI] [PubMed] [Google Scholar]
- 13. Sahu A, Mamiya H, Shinde SN, et al. Age-related declines in α-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat Commun. 2018;9:4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith ER, Holt SG, Hewitson TD. αKlotho–FGF23 interactions and their role in kidney disease: a molecular insight. Cell Mol Life Sci. 2019;76:4705–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wehling-Henricks M, Welc SS, Samengo G, et al. Macrophages escape Klotho gene silencing in the mdx mouse model of Duchenne muscular dystrophy and promote muscle growth and increase satellite cell numbers through a Klotho-mediated pathway. Hum Mol Genet. 2018;27:14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Welc SS, Wehling-Henricks M, Kuro-o M, et al. Modulation of Klotho expression in injured muscle perturbs Wnt signaling and influences the rate of muscle growth. Exp Physiol. 2020;105:132–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cararo-Lopes MM, Mazucanti CHY, Scavone C, et al. The relevance of α-KLOTHO to the central nervous system: some key questions. Ageing Res Rev. 2017;36:137–148 [DOI] [PubMed] [Google Scholar]
- 18. Jia G, Aroor AR, Jia C, et al. Endothelial cell senescence in aging-related vascular dysfunction. BBA Mol Basis Dis. 2019;1865:1802–1809 [DOI] [PubMed] [Google Scholar]
- 19. Chen C-D, Zeldich E, Li Y, et al. Activation of the anti-aging and cognition-enhancing gene Klotho by CRISPR-dCas9 transcriptional effector complex. J Mol Neurosci. 2018;64:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Isaev NK, Stelmashook EV, Genrikhs EE. Neurogenesis and brain aging. Rev Neurosci. 2019;30:573–580 [DOI] [PubMed] [Google Scholar]
- 21. Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mytych J, Wos I, Solek P, et al. Protective role of klotho protein on epithelial cells upon co-culture with activated or senescent monocytes. Exp Cell Res. 2017;350:358–367 [DOI] [PubMed] [Google Scholar]
- 23. Paroni G, Panza F, Cosmo SD, et al. Klotho at the edge of Alzheimer's disease and senile depression. Mol Neurobiol. 2018;56:1908–1920 [DOI] [PubMed] [Google Scholar]
- 24. Vo HT, Laszczyk AM, King GD. Klotho, the key to healthy brain aging? Brain Plast. 2018;3:183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone Klotho. J Biol Chem. 2005;280:38029–38034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C, Sloane JA, Li H, et al. The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS. J Neurosci. 2013;33:1927–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Göttle P, Förster M, Weyers V, et al. An unmet clinical need: roads to remyelination in MS. Neurol Res Pract. 2019;1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeldich E, Chen C-D, Avila R, et al. The anti-aging protein Klotho enhances remyelination following cuprizone-induced demyelination. J Mol Neurosci. 2015;57:185–196 [DOI] [PubMed] [Google Scholar]
- 29. United Nations, Department of Economic and Social Affairs, Population Division (2019). World Population Ageing 2019: Highlights (ST/ESA/SER.A/430)
- 30. Telling GC. Breakthroughs in antemortem diagnosis of neurodegenerative diseases. Proc Natl Acad Sci U S A. 2019;116:22894–22896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belloy ME, Napolioni V, Greicius MD. A quarter century of APOE and Alzheimer's disease: progress to date and the path forward. Neuron. 2019;101:820–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferri E, Gussago C, Casati M, et al. Apolipoprotein E gene in physiological and pathological aging. Mech Ageing Dev. 2019;178:41–45 [DOI] [PubMed] [Google Scholar]
- 33. Therriault J, Benedet AL, Pascoal TA, et al. Association of apolipoprotein E ɛ4 with medial temporal tau independent of amyloid-β. JAMA Neurol. 2020. [Epub ahead of print]; DOI: 10.1001/jamaneurol.2019.4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Durmaz A, Kumral E, Durmaz B, et al. Genetic factors associated with the predisposition to late onset Alzheimer's disease. Gene. 2019;707:212–215 [DOI] [PubMed] [Google Scholar]
- 35. Madmoli M, Modheji Y, Rafi A, et al. Diabetes and its predictive role in the incidence of Alzheimer's disease. Med Sci. 2019;23:30–34 [Google Scholar]
- 36. Raulin A, Kraft L, Al-Hilaly YK, et al. The molecular basis for apolipoprotein E4 as the major risk factor for late-onset Alzheimer's disease. J Mol Biol. 2019;431:2248–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Currais A, Huang L, Goldberg J, et al. Elevating acetyl-CoA levels reduces aspects of brain aging. eLife. 2019;8:e47866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dzau VJ, Inouye SK, Rowe JW, et al. Enabling healthful aging for all—The National Academy of Medicine Grand Challenge in Healthy Longevity. N Engl J Med. 2019;381:1699–1701 [DOI] [PubMed] [Google Scholar]
- 39. Hara Y, McKeehan N, Fillit HM. Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology. 2018;92:84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hou Y, Dan X, Babbar M, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–581 [DOI] [PubMed] [Google Scholar]
- 41. Irwin MH, Moos WH, Faller DV, et al. Epigenetic treatment of neurodegenerative disorders: alzheimer and Parkinson diseases. Drug Dev Res. 2016;77:109–123 [DOI] [PubMed] [Google Scholar]
- 42. Poddar J, Pradhan M, Ganguly G, et al. Biochemical deficits and cognitive decline in brain aging: intervention by dietary supplements. J Chem Neuroanat. 2019;95:70–80 [DOI] [PubMed] [Google Scholar]
- 43. Wegmann S, Bennett RE, Delorme L, et al. Experimental evidence for the age dependence of tau protein spread in the brain. Sci Adv. 2019;5:eaaw6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arboleda-Velasquez JF, Lopera F, et al. Resistance to autosomal dominant Alzheimer's disease in an APOE3 Christchurch homozygote: a case report. Nat Med. 2019;25:1680–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Erickson CM, Schultz SA, Oh JM, et al. KLOTHO heterozygosity attenuates APOE4-related amyloid burden in preclinical AD. Neurology. 2019;92:e1878–e1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dote-Montero M, Amaro-Gahete FJ, De-La-O A, et al. Study of the association of DHEAS, testosterone and cortisol with S-Klotho plasma levels in healthy sedentary middle-aged adults. Exp Gerontol. 2019;121:55–61 [DOI] [PubMed] [Google Scholar]
- 47. Semba RD, Moghekar AR, Hu J, et al. Klotho in the cerebrospinal fluid of adults with and without Alzheimer's disease. Neurosci Lett. 2014;558:37–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alzheimers Association. (2019). Alzheimer's disease facts and figures. Alzheimer's Dement. 2019;15:321–387 [Google Scholar]
- 49. Dawson VL, Dawson TM. Promising disease-modifying therapies for Parkinson's disease. Sci Transl Med. 2019;11:eaba1659. [DOI] [PubMed] [Google Scholar]
- 50. Baluchnejadmojarad T, Eftekhari S-M, Jamali-Raeufy N, et al. The anti-aging protein klotho alleviates injury of nigrostriatal dopaminergic pathway in 6-hydroxydopamine rat model of Parkinson's disease: involvement of PKA/CaMKII/CREB signaling. Exp Gerontol. 2017;100:70–76 [DOI] [PubMed] [Google Scholar]
- 51. Leon J, Moreno AJ, Garay BI, et al. Peripheral elevation of a Klotho fragment enhances brain function and resilience in young, aging, and α-synuclein transgenic mice. Cell Rep. 2017;20:1360–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pathare G, Shalia K. Klotho: an emerging factor in neurodegenerative diseases. Biomed Res J. 2019;6:1–6 [Google Scholar]
- 53. Kokkinaki M, Abu-Asab M, Gunawardena N, et al. Klotho regulates retinal pigment epithelial functions and protects against oxidative stress. J Neurosci. 2013;33:16346–16359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cioffi F, Adam RHI, Broersen K. Molecular mechanisms and genetics of oxidative stress in Alzheimer's disease. J Alzheimers Dis. 2019;72:981–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Heidari M, Gerami SH, Bassett B, et al. Pathological relationships involving iron and myelin may constitute a shared mechanism linking various rare and common brain diseases. Rare Dis. 2016;4:e1198458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wellman SM, Cambi F, Kozai TD. The role of oligodendrocytes and their progenitors on neural interface technology: a novel perspective on tissue regeneration and repair. Biomaterials. 2018;183:200–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Adamczyk B, Adamczyk-Sowa M. New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxid Med Cell Longev. 2016;2016:1973834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zeldich E, Chen C-D, Boden E, et al. Klotho is neuroprotective in the superoxide dismutase (SOD1G93A) mouse model of ALS. J Mol Neurosci. 2019;69:264–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carter C. Autism genes and the leukocyte transcriptome in autistic toddlers relate to pathogen interactomes, infection and the immune system. A role for excess neurotrophic sAPPα and reduced antimicrobial Aβ. Neurochem Int. 2019;126:36–58 [DOI] [PubMed] [Google Scholar]
- 60. Gautam M, Xie EF, Kocak N, et al. Mitoautophagy: a unique self-destructive path mitochondria of upper motor neurons with TDP-43 pathology take, very early in ALS. Front Cell Neurosci. 2019;13:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hoffman ME, Augsburger BN, Foradori CD, et al. Neuroprotective effects of carnitinoid compounds in rodent cellular and in vivo models of mitochondrial complex I dysfunction. Curr Anal Biotechnol. 2019;2:26–33 [Google Scholar]
- 62. Lou G, Palikaras K, Lautrup S, et al. Mitophagy and neuroprotection. Trends Mol Med. 2020;26:8–20 [DOI] [PubMed] [Google Scholar]
- 63. Madsen PM, Pinto M, Patel S, et al. Mitochondrial DNA double-strand breaks in oligodendrocytes cause demyelination, axonal injury, and CNS inflammation. J Neurosci. 2017;37:10185–10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McAvoy K, Kawamata H. Glial mitochondrial function and dysfunction in health and neurodegeneration. Mol Cell Neurosci. 2019;101:103417. [DOI] [PubMed] [Google Scholar]
- 65. Moos WH, Maneta E, Pinkert CA, et al. Epigenetic treatment of neuropsychiatric disorders: autism and schizophrenia. Drug Dev Res. 2016;77:53–72 [DOI] [PubMed] [Google Scholar]
- 66. Nellessen A, Nyamoya S, Zendedel A, et al. Nrf2 deficiency increases oligodendrocyte loss, demyelination, neuroinflammation and axonal damage in an MS animal model. Metab Brain Dis. 2020;35:353–362 [DOI] [PubMed] [Google Scholar]
- 67. van Horssen J, van Schaik P, Witte M. Inflammation and mitochondrial dysfunction: a vicious circle in neurodegenerative disorders? Neurosci Lett. 2019;710:132931. [DOI] [PubMed] [Google Scholar]
- 68. Wu Y, Chen M, Jiang J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion. 2019;49:35–45 [DOI] [PubMed] [Google Scholar]
- 69. Moos WH, Faller DV, Harpp DN, et al. Microbiota and neurological disorders: a gut feeling. BioRes Open Access. 2016;5:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Moos WH, Faller DV, Glavas IP, et al. Epigenetic treatment of neurodegenerative ophthalmic disorders: an eye toward the future. BioRes Open Access. 2017;6:169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Moos WH, Pinkert CA, Irwin MH, et al. Epigenetic treatment of persistent viral infections. Drug Dev Res. 2017;78:24–36 [DOI] [PubMed] [Google Scholar]
- 72. Fecher C, Trovò L, Müller SA, et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat Neurosci. 2019;22:1731–1742 [DOI] [PubMed] [Google Scholar]
- 73. Campbell G, Licht-Mayer S, Mahad D. Targeting mitochondria to protect axons in progressive MS. Neurosci Lett. 2019;710:134258. [DOI] [PubMed] [Google Scholar]
- 74. de Barcelos IP, Troxell RM, Graves JS. Mitochondrial dysfunction and multiple sclerosis. Biology (Basel). 2019;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Langley MR, Yoon H, Kim HN, et al. High fat diet consumption results in mitochondrial dysfunction, oxidative stress, and oligodendrocyte loss in the central nervous system. BBA Mol Basis Dis. 2020;1866:165630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tandon A, Singh SJ, Chaturvedi RK. Stem cells as potential targets of polyphenols in multiple sclerosis and Alzheimer's disease. Biomed Res Int. 2018;2018:1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vural G, Gümüşyayla Ş, Deniz O, et al. Relationship between thiol-disulphide homeostasis and visual evoked potentials in patients with multiple sclerosis. Neurol Sci. 2018;40:385–391 [DOI] [PubMed] [Google Scholar]
- 78. Ferrington DA, Fisher CR, Kowluru RA. Mitochondrial defects drive degenerative retinal diseases. Trends Mol Med. 2020;26:105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schmidlin CJ, Dodson MB, Madhavan L, et al. Redox regulation by NRF2 in aging and disease. Free Radic Biol Med. 2019;134:702–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weissig V. Drug development for the therapy of mitochondrial diseases. Trends Mol Med. 2020;26:40–57 [DOI] [PubMed] [Google Scholar]
- 81. Bahrami SA, Bakhtiari N. Ursolic acid regulates aging process through enhancing of metabolic sensor proteins level. Biomed Pharmacother. 2016;82:8–14 [DOI] [PubMed] [Google Scholar]
- 82. Brunoni AR, Supasitthumrong T, Teixeira AL, et al. Differences in the immune-inflammatory profiles of unipolar and bipolar depression. J Affect Disord. 2020;262:8–15 [DOI] [PubMed] [Google Scholar]
- 83. Buendia I, Michalska P, Navarro E, et al. Nrf2–ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84–104 [DOI] [PubMed] [Google Scholar]
- 84. Caprariello AV, Stys PK. Turned inside out: will myelin-protective therapies become the next-generation anti-inflammatories? DNA Cell Biol. 2019;38:219–222 [DOI] [PubMed] [Google Scholar]
- 85. Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lejri I, Agapouda A, Grimm A, et al. Mitochondria- and oxidative stress-targeting substances in cognitive decline-related disorders: from molecular mechanisms to clinical evidence. Oxid Med Cell Longev. 2019;2019:9695412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Waslo C, Bourdette D, Gray N, et al. Lipoic acid and other antioxidants as therapies for multiple sclerosis. Curr Treat Options Neurol. 2019;21:26. [DOI] [PubMed] [Google Scholar]
- 88. Zeldich E, Chen C-D, Colvin TA, et al. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J Biol Chem. 2014;289:24700–24715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen X, Wang F, Gan J, et al. Myelin deficits caused by olig2 deficiency lead to cognitive dysfunction and increase vulnerability to social withdrawal in adult mice. Neurosci Bull. 2020. [Epub ahead of print]; DOI: 10.1007/s12264-019-00449-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Reiche L, Küry P, Göttle P. Aberrant oligodendrogenesis in Down syndrome: shift in gliogenesis? Cells. 2019;8:1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shimizu T, Osanai Y, Ikenaka K. Oligodendrocyte–neuron interactions: impact on myelination and brain function. Neurochem Res. 2018;43:190–194 [DOI] [PubMed] [Google Scholar]
- 92. Blacher E, Bashiardes S, Shapiro H, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572:474–480 [DOI] [PubMed] [Google Scholar]
- 93. Belenguer P, Duarte JMN, Schuck PF, et al. Mitochondria and the brain: bioenergetics and beyond. Neurotox Res. 2019;36:219–238 [DOI] [PubMed] [Google Scholar]
- 94. Cuadrado A, Rojo AI, Wells G, et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. 2019;18:295–317 [DOI] [PubMed] [Google Scholar]
- 95. Morris G, Puri BK, Walder K, et al. The endoplasmic reticulum stress response in neuroprogressive diseases: emerging pathophysiological role and translational implications. Mol Neurobiol. 2018;55:8765–8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Smith EF, Shaw PJ, De Vos KJ. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci Lett. 2019;710:132933. [DOI] [PubMed] [Google Scholar]
- 97. Berridge MJ. Vitamin D cell signalling in health and disease. Biochem Biophys Res Commun. 2015;460:53–71 [DOI] [PubMed] [Google Scholar]
- 98. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381:2541–2551 [DOI] [PubMed] [Google Scholar]
- 99. Campisi J, Kapahi P, Lithgow GJ, et al. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Di Liegro CM, Schiera G, Proia P, et al. Physical activity and brain health. Genes. 2019;10:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Evans LW, Stratton MS, Ferguson BS. Dietary natural products as epigenetic modifiers in aging-associated inflammation and disease. Nat Prod Rep. 2020. [Epub ahead of print]; DOI: 10.1039/c9np00057g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Georgiou A, Lisacek-Kiosoglous A, Yiallouris A, et al. Klotho: the protein of faith. EC Neurol. 2017;7:189–223 [Google Scholar]
- 103. Huang C, Wu J, Chen D, et al. Effects of sulforaphane in the central nervous system. Eur J Pharmacol. 2019;853:153–168 [DOI] [PubMed] [Google Scholar]
- 104. Melchor GS, Khan T, Reger JF, et al. Remyelination pharmacotherapy investigations highlight diverse mechanisms underlying multiple sclerosis progression. ACS Pharmacol Transl Sci. 2019;2:372–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rani L, Mondal AC. Emerging concepts of mitochondrial dysfunction in Parkinson's disease progression: pathogenic and therapeutic implications. Mitochondrion. 2020;50:25–34 [DOI] [PubMed] [Google Scholar]
- 106. Rowin J. Integrative neuromuscular medicine: neuropathy and neuropathic pain: consider the alternatives. Muscle Nerve. 2019;60:124–136 [DOI] [PubMed] [Google Scholar]
- 107. Suomalainen A, Battersby BJ. Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat Rev Mol Cell Biol. 2018;19:77–92 [DOI] [PubMed] [Google Scholar]
- 108. Watson K, Nasca C, Aasly L, et al. Insulin resistance, an unmasked culprit in depressive disorders: promises for interventions. Neuropharmacology. 2018;136:327–334 [DOI] [PubMed] [Google Scholar]
- 109. Liu Y, Yan T, Chu JM-T, et al. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab Invest. 2019;99:943–957 [DOI] [PubMed] [Google Scholar]
- 110. Chu F, Shi M, Lang Y, et al. Gut microbiota in multiple sclerosis and experimental autoimmune encephalomyelitis: current applications and future perspectives. Mediators Inflamm. 2018;2018:8168717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Faiq MA, Wollstein G, Schuman JS, et al. Cholinergic nervous system and glaucoma: from basic science to clinical applications. Prog Retin Eye Res. 2019;72:100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Faissner S, Plemel JR, Gold R, et al. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov. 2019;18:905–922 [DOI] [PubMed] [Google Scholar]
- 113. Mehrabani G, Aminian S, Mehrabani G, et al. Dietetic plans within the multiple sclerosis community: a review. Int J Nutr Sci. 2019;4:14–22 [Google Scholar]
- 114. Stadelmann C, Timmler S, Barrantes-Freer A, et al. Myelin in the central nervous system: structure, function, and pathology. Physiol Rev. 2019;99:1381–1431 [DOI] [PubMed] [Google Scholar]
- 115. Zeng Q, Gong J, Liu X, et al. Gut dysbiosis and lack of short chain fatty acids in a Chinese cohort of patients with multiple sclerosis. Neurochem Int. 2019;129:104468. [DOI] [PubMed] [Google Scholar]
- 116. Cserép C, Pósfai B, Orsolits B, et al. Microglia monitor and protect neuronal function via specialized somatic purinergic junctions. Science. 2020;367:528–537 [DOI] [PubMed] [Google Scholar]
- 117. Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179:292–311 [DOI] [PubMed] [Google Scholar]
- 118. Wright-Jin EC, Gutmann DH. Microglia as dynamic cellular mediators of brain function. Trends Mol Med. 2019;25:967–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Divolis G, Stavropoulos A, Manioudaki M, et al. Activation of both transforming growth factor-β and bone morphogenetic protein signalling pathways upon traumatic brain injury restrains pro-inflammatory and boosts tissue reparatory responses of reactive astrocytes and microglia. Brain Commun. 2019;1:fcz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Paschalis EI, Lei F, Zhou C, et al. Permanent neuroglial remodeling of the retina following infiltration of CSF1R inhibition-resistant peripheral monocytes. Proc Natl Acad Sci U S A. 2018;115:E11359–E11368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Paschalis EI, Lei F, Zhou C, et al. Microglia regulate neuroglia remodeling in various ocular and retinal injuries. J Immunol. 2019;202:539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sierra A, Paolicelli RC, Kettenmann H. Cien años de microglía: milestones in a century of microglial research. Trends Neurosci. 2019;42:778–792 [DOI] [PubMed] [Google Scholar]
- 123. Kim MJ, Kang JH, Theotokis P, et al. Can we design a Nogo receptor-dependent cellular therapy to target MS? Cells. 2019;8:E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lin W, Stone S. Unfolded protein response in myelin disorders. Neural Regen Res. 2020;15:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shaimardanova AA, Solovyeva VV, Chulpanova DS, et al. Extracellular vesicles in the diagnosis and treatment of central nervous system diseases. Neural Regen Res. 2020;15:586–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cassereau J, Chevrollier A, Codron P, et al. Oxidative stress contributes differentially to the pathophysiology of Charcot-Marie-Tooth disease type 2K. Exp Neurol. 2020;323:113069. [DOI] [PubMed] [Google Scholar]
- 127. Wu X. Genome expression profiling predicts the molecular mechanism of peripheral myelination. Int J Mol Med. 2018;41:1500–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bergner CG, Meer F, Winkler A, et al. Microglia damage precedes major myelin breakdown in X-linked adrenoleukodystrophy and metachromatic leukodystrophy. Glia. 2019;67:1196–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Creus-Muncunill J, Ehrlich ME. Cell-autonomous and non-cell-autonomous pathogenic mechanisms in Huntington's disease: insights from in vitro and in vivo models. Neurotherapeutics. 2019;16:957–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Soloveva MV, Jamadar SD, Poudel G, et al. A critical review of brain and cognitive reserve in Huntington's disease. Neurosci Biobehav Rev. 2018;88:155–169 [DOI] [PubMed] [Google Scholar]
- 131. Duncan ID, Radcliff AB. Inherited and acquired disorders of myelin: the underlying myelin pathology. Exp Neurol. 2016;283:452–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tricaud N, Gautier B, Hameren GV, et al. Schwann cell demyelination is triggered by a transient mitochondrial calcium release through Voltage Dependent Anion Channel 1. bioRxiv. 2019;2019:581157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lesch K-P. Editorial: can dysregulated myelination be linked to ADHD pathogenesis and persistence? J Child Psychol Psychiatry. 2019;60:229–231 [DOI] [PubMed] [Google Scholar]
- 134. Taylor MJ, Larsson H, Gillberg C, et al. Investigating the childhood symptom profile of community-based individuals diagnosed with attention-deficit/hyperactivity disorder as adults. J Child Psychol Psychiatry. 2019;60:259–266 [DOI] [PubMed] [Google Scholar]
- 135. Kodama L, Guzman E, Etchegaray JI, et al. Microglial microRNAs mediate sex-specific responses to tau pathology. Nat Neurosci. 2020;23:167–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Bär L, Stournaras C, Lang F, et al. Regulation of fibroblast growth factor 23 (FGF 23) in health and disease. FEBS Lett. 2019;593:1879–1900 [DOI] [PubMed] [Google Scholar]
- 137. Brown RB. Dysregulated phosphate metabolism, periodontal disease, and cancer: possible global health implications. Dent J (Basel). 2019;7:E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Bacchetta J, Bardet C, Prié D. Physiology of FGF23 and overview of genetic diseases associated with renal phosphate wasting. Metabolism. 2020;103S:153865. [DOI] [PubMed] [Google Scholar]
- 139. Chen G, Liu Y, Goetz R, et al. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553:461–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jackson TC, Kochanek PM. A new vision for therapeutic hypothermia in the era of targeted temperature management: a speculative synthesis. Ther Hypothermia Temp Manag. 2019;9:13–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kim J-M, Lee W-S, Kim J. Therapeutic strategy for atherosclerosis based on bone-vascular axis hypothesis. Pharmacol Ther. 2020;206:107436. [DOI] [PubMed] [Google Scholar]
- 142. Klaus S, Ost M. Mitochondrial uncoupling and longevity – A role for mitokines? Exp Gerontol. 2020;130:110796. [DOI] [PubMed] [Google Scholar]
- 143. Kuroda M, Muramatsu R, Maedera N, et al. Peripherally derived FGF21 promotes remyelination in the central nervous system. J Clin Invest. 2017;127:3496–3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kuzina ES, Ung PM-U, Mohanty J, et al. Structures of ligand-occupied β-Klotho complexes reveal a molecular mechanism underlying endocrine FGF specificity and activity. Proc Natl Acad Sci U S A. 2019;116:7819–7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ye L, Wang X, Cai C, et al. FGF21 promotes functional recovery after hypoxic-ischemic brain injury in neonatal rats by activating the PI3K/Akt signaling pathway via FGFR1/β-klotho. Exp Neurol. 2019;317:34–50 [DOI] [PubMed] [Google Scholar]
- 147. Abolghasemi M, Yousefi T, Maniati M, et al. The interplay of Klotho with signaling pathway and microRNAs in cancers. J Cell Biochem. 2019;120:14306–14317 [DOI] [PubMed] [Google Scholar]
- 148. Li X. The FGF metabolic axis. Front Med. 2019;13:511–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Faye PA, Poumeaud F, Miressi F, et al. Focus on 1,25-dihydroxyvitamin D3 in the peripheral nervous system. Front Neurosci. 2019;13:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Berridge MJ. Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Berridge MJ. Vitamin D deficiency: infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia). Am J Physiol Cell Physiol. 2018;314:C135–C151 [DOI] [PubMed] [Google Scholar]
- 152. Chen L, Yang R, Qiao W, et al. 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell. 2019;18:e12951. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 153. Wimalawansa SJ. Vitamin D deficiency: effects on oxidative stress, epigenetics, gene regulation, and aging. Biology (Basel). 2019;8:E30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kaarniranta K, Pawlowska E, Szczepanska J, et al. Can vitamin D protect against age-related macular degeneration or slow its progression? Acta Biochim Pol. 2019;66:147–158, [DOI] [PubMed] [Google Scholar]
- 155. Murdaca G, Tonacci A, Negrini S, et al. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun Rev. 2019;18:102350. [DOI] [PubMed] [Google Scholar]
- 156. Guo Y, Zhuang X-D, Xian W-B, et al. Serum Klotho, vitamin D, and homocysteine in combination predict the outcomes of Chinese patients with multiple system atrophy. CNS Neurosci Ther. 2017;23:657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lang F, Ma K, Leibrock CB. 1,25(OH)2D3 in brain function and neuropsychiatric disease. Neurosignals. 2019;27:40–49 [DOI] [PubMed] [Google Scholar]
- 158. Vlot MC, Boekel L, Kragt J, et al. Multiple sclerosis patients show lower bioavailable 25(OH)D and 1,25(OH)2D, but no difference in ratio of 25(OH)D/24,25(OH)2D and FGF23 concentrations. Nutrients. 2019;11:E2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. de la Vega Gallardo N, Dittmer M, Dombrowski Y, et al. Regenerating CNS myelin: emerging roles of regulatory T cells and CCN proteins. Neurochem Int. 2019;130:104349. [DOI] [PubMed] [Google Scholar]
- 160. Filippi M, Bar-Or A, Piehl F, et al. Author Correction: multiple sclerosis. Nat Rev Dis Primers. 2018;4:49. [DOI] [PubMed] [Google Scholar]
- 161. Patel CH, Leone RD, Horton MR, et al. Targeting metabolism to regulate immune responses in autoimmunity and cancer. Nat Rev Drug Discov. 2019;18:669–688 [DOI] [PubMed] [Google Scholar]
- 162. Rommer PS, Sellner J. Repurposing multiple sclerosis drugs: a review of studies in neurological and psychiatric conditions. Drug Discov Today. 2019;24:1398–1404 [DOI] [PubMed] [Google Scholar]
- 163. Saidu NEB, Kavian N, Leroy K, et al. Dimethyl fumarate, a two-edged drug: current status and future directions. Med Res Rev. 2019;39:1923–1952 [DOI] [PubMed] [Google Scholar]
- 164. Vilariño-Güell C, Zimprich A, Martinelli-Boneschi F, et al. Exome sequencing in multiple sclerosis families identifies 12 candidate genes and nominates biological pathways for the genesis of disease. PLoS Genet. 2019;15:e1008180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yi W, Schlüter D, Wang X. Astrocytes in multiple sclerosis and experimental autoimmune encephalomyelitis: star-shaped cells illuminating the darkness of CNS autoimmunity. Brain Behav Immun. 2019;80:10–24 [DOI] [PubMed] [Google Scholar]
- 166. Duncan ID, Watters JJ. Remyelination and the gut−brain axis. Proc Natl Acad Sci U S A. 2019;116:24922–24924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Pahan S, Pahan K. Mode of action of aspirin in experimental autoimmune encephalomyelitis. DNA Cell Biol. 2019;38:593–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wallin MT, Culpepper WJ, Nichols E, et al. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:269–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Gholamzad M, Ebtekar M, Ardestani MS, et al. A comprehensive review on the treatment approaches of multiple sclerosis: currently and in the future. Inflamm Res. 2019;68:25–38 [DOI] [PubMed] [Google Scholar]
- 170. Kaplon H, Reichert JM. Antibodies to watch in 2018. MAbs. 2018;10:183–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24:96–120 [DOI] [PubMed] [Google Scholar]
- 172. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90:777–788 [DOI] [PubMed] [Google Scholar]
- 173. Feustel AC, Macpherson A, Fergusson DA, et al. Risks and benefits of unapproved disease-modifying treatments for neurodegenerative disease. Neurology. 2020;94:e1–e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Rezak M, Carvalho MD. Disease modification in neurodegenerative diseases. Not quite there yet. Neurology. 2020;94:12–13 [DOI] [PubMed] [Google Scholar]
- 175. Hooijmans CR, Hlavica M, Schuler FAF, et al. Remyelination promoting therapies in multiple sclerosis animal models: a systematic review and meta-analysis. Sci Rep. 2019;9:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kremer D, Göttle P, Flores-Rivera J, et al. Remyelination in multiple sclerosis: from concept to clinical trials. Curr Opin Neurol. 2019;32:378–384 [DOI] [PubMed] [Google Scholar]
- 177. Wooliscroft L, Silbermann E, Cameron M, et al. Approaches to remyelination therapies in multiple sclerosis. Curr Treat Options Neurol. 2019;21:34. [DOI] [PubMed] [Google Scholar]
- 178. Yu S, Liu M, Hu K. Natural products: potential therapeutic agents in multiple sclerosis. Int Immunopharmacol. 2019;67:87–97 [DOI] [PubMed] [Google Scholar]
- 179. Kumar R, Harilal S, Gupta SV, et al. Exploring the new horizons of drug repurposing: a vital tool for turning hard work into smart work. Eur J Med Chem. 2019;182:111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Mucke H. An answerable challenge. EBR. July 2019: pages 14–17. https://repo-trial.eu/wp-content/uploads/2019/07/Drug-Repurposing-for-Neurodegeneration-Mucke-EBR-July-2019.pdf
- 181. Moos WH, Faller DV, Glavas IP, et al. Epigenetic treatment of dermatologic disorders. Drug Dev Res. 2019;80:702–713 [Google Scholar]
- 182. Polamreddy P, Gattu N. The drug repurposing landscape from 2012 to 2017: evolution, challenges, and possible solutions. Drug Discov Today. 2019;24:789–795 [DOI] [PubMed] [Google Scholar]
- 183. Olauson H, Mencke R, Hillebrands J-L, et al. Tissue expression and source of circulating α-Klotho. Bone. 2017;100:19–35 [DOI] [PubMed] [Google Scholar]
- 184. Zhu L, Stein LR, Kim D, et al. Klotho controls the brain–immune system interface in the choroid plexus. Proc Natl Acad Sci U S A. 2018;115:E11388–E11396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Dalton GD, Xie J, An S-W, et al. New insights into the mechanism of action of soluble klotho. Front Endocrinol. 2017;8:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kawabata C, Komaba H, Ishida H, et al. Changes in fibroblast growth factor 23 and soluble klotho levels after hemodialysis initiation. Kidney Med. 2020;2:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Lim K, Halim A, Lu T-S, et al. Klotho: a major shareholder in vascular aging enterprises. Int J Mol Sci. 2019;20:E4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Mazucanti CH, Kawamoto EM, Mattson MP, et al. Activity-dependent neuronal Klotho enhances astrocytic aerobic glycolysis. J Cereb Blood Flow Metab. 2019;39:1544–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Tai NC, Kim S-A, Ahn S-G. Soluble klotho regulates the function of salivary glands by activating KLF4 pathways. Aging (Albany NY). 2019;11:8254–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Hu Z-J, Wang X-C, Zhu L-C, et al. Circulating Klotho is linked to prognosis of acute intracerebral hemorrhage. Clin Chim Acta. 2019;497:114–119 [DOI] [PubMed] [Google Scholar]
- 191. Ma X, Li H, Chen Y, et al. The transcription factor MITF in RPE function and dysfunction. Prog Retin Eye Res. 2019;73:100766. [DOI] [PubMed] [Google Scholar]
- 192. Alkozi HA, Franco R, Pintor JJ. Epigenetics in the eye: an overview of the most relevant ocular diseases. Front Genet. 2017;8:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Fan J, Lerner J, Wyatt MK, et al. The klotho-related protein KLPH (lctl) has preferred expression in lens and is essential for expression of clic5 and normal lens suture formation. Exp Eye Res. 2018;169:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Reish NJ, Maltare A, Mckeown AS, et al. The age-regulating protein Klotho is vital to sustain retinal function. Invest Ophthalmol Vis Sci. 2013;54:6675–6685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wong CW, Wong TY, et al. Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int. 2014;85:1290–1302 [DOI] [PubMed] [Google Scholar]
- 196. Yamamoto K, Sato K, Yukita M, et al. The neuroprotective effect of latanoprost acts via klotho-mediated suppression of calpain activation after optic nerve transection. J Neurochem. 2017;140:495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Oh HJ, Nam BY, Wu M, et al. Klotho plays a protective role against glomerular hypertrophy in a cell cycle-dependent manner in diabetic nephropathy. Am J Physiol Renal Physiol. 2018;315:F791–F805 [DOI] [PubMed] [Google Scholar]
- 198. Oh HJ, Oh H, Nam BY, et al. The protective effect of klotho against contrast-associated acute kidney injury via the antioxidative effect. Am J Physiol Renal Physiol. 2019;317:F881–F889 [DOI] [PubMed] [Google Scholar]
- 199. Ji B, Wei H, Ding Y, et al. Protective potential of klotho protein on diabetic retinopathy: evidence from clinical and in vitro studies. J Diabetes Invest. 2020;11:162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Skowronska-Krawczyk D, Budin I. Aging membranes: unexplored functions for lipids in the lifespan of the central nervous system. Exp Gerontol. 2020;131:110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Matiello M, Juliano AF, Bowley M, et al. Case 21–2019: a 31-year-old woman with vision loss. N Engl J Med. 2019;381:164–172 [DOI] [PubMed] [Google Scholar]
- 202. Waldman AT, Yeshokumar AK, Lavery A, et al. Validation of a symptom-based questionnaire for pediatric CNS demyelinating diseases. J AAPOS. 2019;23:157..e1–157.e7. [DOI] [PubMed] [Google Scholar]
- 203. Hashemian M, Ghasemi-Kasman M, Parsian H, et al. Fingolimod (FTY720) improves the functional recovery and myelin preservation of the optic pathway in focal demyelination model of rat optic chiasm. Brain Res Bull. 2019;153:109–121 [DOI] [PubMed] [Google Scholar]
- 204. Manogaran P, Samardzija M, Schad AN, et al. Retinal pathology in experimental optic neuritis is characterized by retrograde degeneration and gliosis. Acta Neuropathol Commun. 2019;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Andhavarapu S, Mubariz F, Arvas M, et al. Interplay between ER stress and autophagy: a possible mechanism in multiple sclerosis pathology. Exp Mol Pathol. 2019;108:183–190 [DOI] [PubMed] [Google Scholar]
- 206. Behrangi N, Fischbach F, Kipp M. Mechanism of siponimod: anti-inflammatory and neuroprotective mode of action. Cells. 2019;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Gruchot J, Weyers V, Göttle P, et al. The molecular basis for remyelination failure in multiple sclerosis. Cells. 2019;8:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Moura RP, Sarmento B. Therapeutic approaches toward multiple sclerosis: where do we stand and where are we headed? Adv Ther. 2019;2:1900070 [Google Scholar]
- 209. Nicaise AM, Wagstaff LJ, Willis CM, et al. Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc Natl Acad Sci U S A. 2019;116:9030–9039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Peshes-Yeloz N, Ungar L, Wohl A, et al. Role of Klotho protein in tumor genesis, cancer progression, and prognosis in patients with high-grade glioma. World Neurosurg. 2019;130:e324–e332 [DOI] [PubMed] [Google Scholar]
- 211. Robinson RR, Dietz AK, Maroof AM, et al. The role of glial–neuronal metabolic cooperation in modulating progression of multiple sclerosis and neuropathic pain. Immunotherapy. 2019;11:129–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Rao Z, Landry T, Li P, et al. Administration of alpha klotho reduces liver and adipose lipid accumulation in obese mice. Heliyon. 2019;5:e01494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Abraham CR, Chen C, Cuny GD, et al. Small-molecule Klotho enhancers as novel treatment of neurodegeneration. Future Med Chem. 4:1671–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Pérez-Hernández J, Zaldívar-Machorro VJ, Villanueva-Porras D, et al. A potential alternative against neurodegenerative diseases: phytodrugs. Oxid Med Cell Longev. 2016;2016:8378613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Liao H-K, Hatanaka F, Araoka T, et al. In vivo target gene activation via CRISPR/Cas9-mediated trans-epigenetic modulation. Cell. 2017;171:1495–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Lin W, Li Y, Chen F, et al. Klotho preservation via histone deacetylase inhibition attenuates chronic kidney disease-associated bone injury in mice. Sci Rep. 2017;7:46195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Lu G, Zhang M, Wang J, et al. Epigenetic regulation of myelination in health and disease. Eur J Neurosci 2019;49:1371–1387 [DOI] [PubMed] [Google Scholar]
- 218. Tomaselli D, Lucidi A, Rotili D, et al. Epigenetic polypharmacology: a new frontier for epi-drug discovery. Med Res Rev. 2020;40:190–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. VandenBosch LS, Reh TA. Epigenetics in neuronal regeneration. Semin Cell Dev Biol. 2020;97:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Davidsohn N, Pezone M, Vernet A, et al. A single combination gene therapy treats multiple age-related diseases. Proc Natl Acad Sci U S A. 2019;116:23505–23511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Vaiserman A, Falco ED, Koliada A, et al. Anti-ageing gene therapy: not so far away? Ageing Res Rev. 2019;56:100977. [DOI] [PubMed] [Google Scholar]
- 222. Xu L, Liu T, Liu L, et al. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol. 2020. [Epub ahead of print]; DOI: 10.1007/s00415-019-09652-y [DOI] [PubMed] [Google Scholar]
- 223. Volonté C, Apolloni S, Sabatelli M. Histamine beyond its effects on allergy: potential therapeutic benefits for the treatment of Amyotrophic Lateral Sclerosis (ALS). Pharmacol Ther. 2019;202:120–131 [DOI] [PubMed] [Google Scholar]
- 224. Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–172 [DOI] [PubMed] [Google Scholar]
- 225. Galvin M, Gaffney R, Corr B, et al. From first symptoms to diagnosis of amyotrophic lateral sclerosis: perspectives of an Irish informal caregiver cohort—a thematic analysis. BMJ Open. 2017;7:e014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Paganoni S, Macklin EA, Lee A, et al. Diagnostic timelines and delays in diagnosing amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:453–456. doi: 10.3109/21678421.2014.903974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Zwicker J, Qureshi D, Talarico R, et al. Dying of amyotrophic lateral sclerosis. Neurology. 2019;93:e2083–e2093 [DOI] [PubMed] [Google Scholar]
- 228. Swindell WR, Kruse CPS, List EO, et al. ALS blood expression profiling identifies new biomarkers, patient subgroups, and evidence for neutrophilia and hypoxia. J Transl Med. 2019;17:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. van den Berg LH, Sorenson E, Gronseth G, et al. Revised Airlie House consensus guidelines for design and implementation of ALS clinical trials. Neurology. 2019;92:e1610–e1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Bedlack R, Wicks P, Vaughan T, et al. Lunasin does not slow ALS progression: results of an open-label, single-center, hybrid-virtual 12-month trial. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:285–293 [DOI] [PubMed] [Google Scholar]
- 231. Wheeler RJ, Lee HO, Poser I, et al. Small molecules for modulating protein driven liquid-liquid phase separation in treating neurodegenerative disease. bioRxiv. 2019;2019:721001 [Google Scholar]
- 232. Dervishi I, Ozdinler PH. Incorporating upper motor neuron health in ALS drug discovery. Drug Discov Today. 2018;23:696–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Fralick M, Sacks CA, Kesselheim AS. Assessment of use of combined dextromethorphan and quinidine in patients with dementia or Parkinson disease after US Food and Drug Administration approval for pseudobulbar affect. JAMA Intern Med. 2019;179:224–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Brown DG, Shorter J, Wobst HJ. Emerging small-molecule therapeutic approaches for amyotrophic lateral sclerosis and frontotemporal dementia. Bioorg Med Chem Lett. 2020;30:126942. [DOI] [PubMed] [Google Scholar]
- 235. Brown DG, Wobst HJ. Opportunities and challenges in phenotypic screening for neurodegenerative disease research. J Med Chem. 2020;63:1823–1840 [DOI] [PubMed] [Google Scholar]
- 236. Granucci EJ, Griciuc A, Mueller KA, et al. Cromolyn sodium delays disease onset and is neuroprotective in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Sci Rep. 2019;9:17728. [DOI] [PMC free article] [PubMed] [Google Scholar]