Abstract
Adrenocortical carcinoma (ACC) is a rare, aggressive, and frequently deadly cancer. Up to 75% of all patients will eventually develop metastatic disease, and our current medical therapies for ACC provide limited – if any – survival benefit. These statistics highlight a crucial need for novel approaches. Recent studies performing comprehensive molecular profiling on ACC have illuminated that ACC is comprised of three clinically distinct molecular subtypes, bearing differential regulation of cell cycle, epigenetics, Wnt/β-catenin signaling, PKA signaling, steroidogenesis and immune cell biology. Furthermore, these studies have spurred the development of molecular subtype-based biomarkers, contextualized outcomes of recent clinical trials, and advanced our understanding of the underlying biology of adrenocortical homeostasis and cancer. In this review, we describe these findings and their implications for new strategies to apply targeted therapies to ACC.
Keywords: adrenocortical carcinoma, ACC, genomics, targeted therapy, biomarkers
1. Introduction
Adrenocortical carcinoma (ACC) is a rare cancer of the adrenal cortex with a global annual incidence of 0.5 to 2 individuals per million (1,2). Despite its rarity, outcomes for patients diagnosed with ACC remain dismal, with 5-year overall survival of ~35% (3). Furthermore, while 50% of patients are diagnosed with surgically resectable locoregional disease, ~75% of all patients will ultimately develop metastases (4). Treatment options for patients with metastatic disease are limited and often ineffective: patients receive the DDT-derived adrenolytic agent mitotane +/− cytotoxic chemotherapy, sometimes paired with palliative surgery for resectable lesions, but <10% of patients with metastatic disease survive 5 years on these agents (5,6). Taken together, these statistics highlight a critical need for novel therapeutic strategies to fight ACC, contingent on a deeper understanding of the molecular basis of this disease.
Recent advances in comprehensive molecular profiling, biomarker identification, clinical trials, and in vivo and in vitro modeling have now illuminated a spectrum of pharmacologically-targetable molecular programs essential for adrenocortical development and homeostasis and uniquely derailed in cancer, including: cell cycle, DNA damage response, epigenetics, Wnt signaling, PKA signaling, and steroidogenesis. In this review, we summarize these developments and describe their implications for the next generation of targeted therapies for ACC. Note that the scope of this review is largely restricted to adult ACC.
2. Methods
We selected literature for inclusion in this review by querying the NIH/NCBI PubMed database on or before June 23, 2019 for at least any of the following search terms: “adrenocortical carcinoma” (3490 items as of June 23, 2019); “adrenocortical carcinoma preclinical” (58 items); “adrenocortical carcinoma clinical trial” (103 items); “adrenocortical carcinoma trial” (133 items). We curated search results manually using the following criteria: relevance, impact, and recency
3. Results and Discussion
3.1. Multiplatform genomics reveal ACC is comprised of 3 distinct subtypes and provide pan-cancer contextualization
Our current understanding of the molecular basis of ACC is informed by two major studies utilizing multiplatform omics approaches to profile primary tumors – Assie, Letouze et al. (7) and The Cancer Genome Atlas Study on ACC (ACC-TCGA; (8)). These studies confirmed that 90% of ACC exhibit loss of heterozygosity of the IGF2 locus leading to upregulation of IGF2/IGF1R signaling (7,8). ACC also bear recurrent somatic alterations facilitating rapid cell cycling (TP53, CDKN2A, RB1, CDK4, CCNE1), telomere maintenance (TERT, TERF2), constitutive Wnt/β-catenin signaling (ZNRF3, CTNNB1), and constitutive PKA signaling (PRKAR1A); and involved in chromatin remodeling (MEN1, DAXX), transcription (MED12), and translation (RPL22) (7,8). While Assie, Letouze et al. observed ACC exhibit frequent copy number changes (7), ACC-TCGA identified three recurrent somatic copy number alteration (SCNA) signatures: “quiet” (rare; tumors possess a diploid genome), “chromosomal” (~2/3 of tumors possess loss of heterozygosity of entire chromosomes +/− hypodiploidy or whole genome doubling), and “noisy” (~1/3 of tumors possess frequent arm-level gains and losses throughout the genome +/− whole genome doubling) (8).
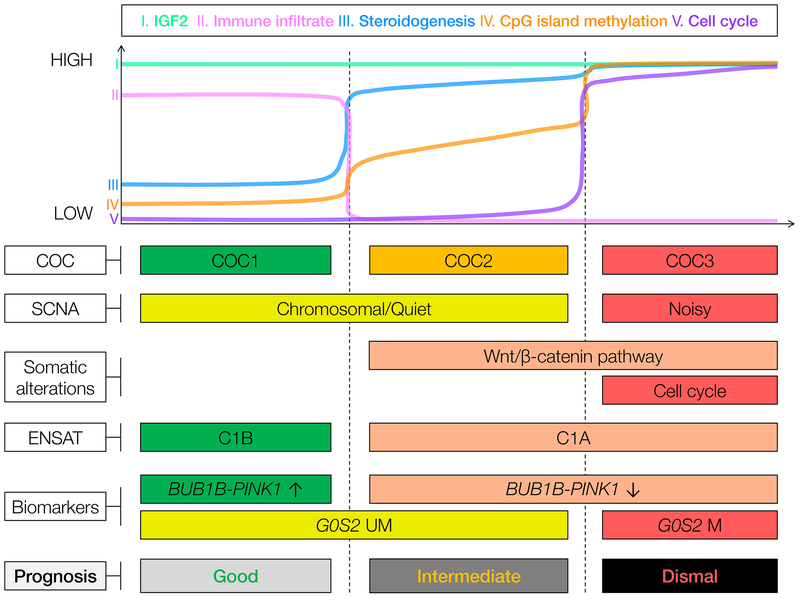
ACC-TCGA provided a novel molecular classification of ACC, identifying three comparably frequent and distinct molecular subtypes via a cluster of cluster (COC) analysis – COC1, COC2, and COC3 (Fig 1) (8). Good prognosis COC1 ACC have fewer somatic alterations, a quiet or chromosomal SCNA profile, a transcriptional signature characterized by immune infiltration and low expression of steroidogenic machinery (overlapping with a signature identified by Assie, Letouze et al. as “C1B”) (8). Intermediate prognosis COC2 and dismal prognosis COC3 ACC bear frequent Wnt/β-catenin pathway alterations (8). COC2 ACC bear a quiet or chromosomal SCNA profile and high expression of steroidogenic machinery (overlapping with Assie, Letouze et al. C1A). COC3 ACC bear frequent cell cycle alterations, a noisy SCNA profile, and high expression of steroidogenic and proliferative machinery (also overlapping with C1A) (8). Finally, on an epigenetic level, COC1 tumors bear low levels of genome-wide CpG island methylation (CIMP-low), COC2 bear intermediate levels (CIMP-intermediate), and COC3 bear high levels (CIMP-high) (8).
Figure 1. ACC is comprised of three distinct molecular subtypes amenable to targeted assessment.
As described in this review, ACC-TCGA (8) identified that ACC is comprised of three distinct molecular subtypes, COC1, COC2 and COC3. COC1 tumors have the best prognosis (longest progression-free survival), while COC2 tumors have intermediate prognosis and COC3 tumors have dismal prognosis. All subtypes have high expression of IGF2 (I). COC1 tumors bear the highest degree of immune infiltration (II), while COC2-3 tumors bear higher expression of steroidogenic machinery (III). COC3 tumors bear CpG island hypermethylation (IV) and high expression of cell cycling machinery (V). COC1-COC2 tumors bear a somatic copy number alteration (SCNA) profile termed chromosomal or quiet, while COC3 tumors bear an SCNA profile termed noisy. COC2-3 tumors bear a higher burden of somatic alterations leading to constitutive activation of the Wnt pathway, while COC3 tumors bear a higher burden of somatic alterations leading to constitutive cell cycling. ACC-TCGA also showed that COC1 tumors possess a transcriptional program identified by de Reynies, Assie and colleagues as C1B (17), while COC2-COC3 tumors possess a transcriptional program akin to C1A (17). Notably, these molecular subtypes can be captured using biomarkers, namely BUB1B-PINK1 score (17) and G0S2 methylation (22).
The completion of ACC-TCGA enabled the incorporation of ACC into pan-cancer analyses. These studies reveal that ACC has the lowest degree of immune infiltration of nearly all TCGA cancers (9,10) and a subset of ACC also exhibit a genomic signature suggestive of homologous recombination deficiency (11) . While primary ACC are notable for bearing a lower mutational burden than most TCGA cancers (10), metastatic ACC bear a mutational burden nearly 3-fold higher than that of primary tumors (12). A recent landmark study using ATAC-seq on TCGA samples revealed that the chromatin accessibility landscape of ACC is largely driven by critical transcription factor for adrenal organogenesis and steroidogenesis, SF1 (encoded by NR5A1), consistent with the hallmark steroidogenic transcriptional program active in most ACC (13).
3.2. Novel biomarkers stratify ACC into homogeneous classes
Currently, proliferation-based grade measured by Ki67 or mitotic counts on histologic sections of primary tumor samples is used to prognosticate ACC (14–16). However, the availability of high-throughput and multiplatform genomics data profiling ACC has enabled the discovery of several novel biomarkers that stratify ACC into molecular subtypes; such stratification is essential for the application of targeted therapies to specific subgroups of patients. The first of these molecular markers was developed by de Reynies, Assie, and colleagues, who demonstrated that cell cycle avid C1A and adenoma-like C1B tumors can be distinguished using a score derived from the mRNA expression of genes BUB1B and PINK1 (BUB1B-PINK1 score) (17). More recently, in line with ACC-TCGA, investigators have also shown that high mRNA expression of E2F target genes like EZH2 (18) or novel SF1 transcriptional targets like VAV2 are also associated with worse clinical outcomes (19,20).
Recent biomarkers take advantage of orthogonal approaches to capture the DNA hypermethylation signature characteristic of aggressive ACC (21,22). Our group recently demonstrated that uniform hypermethylation and silencing of the gene G0S2 accurately captures a subgroup of patients with homogeneously dismal disease course akin to patients with COC3/CIMP-high tumors in ACC-TCGA (22). Indeed, this signature can be combined with BUB1B-PINK1 to approximate the three molecular subtypes described by ACC-TCGA ((22); Fig 1).
While promising, most molecular biomarker studies use frozen primary tumor tissues, which are not available at all clinical centers. In an integrated study performing targeted assessment of somatic alterations, gene expression, and methylation in formalin-fixed paraffin-embedded tissues, Lippert et al. demonstrated that it is possible to molecularly prognosticate ACC using routinely available clinical samples (23). Other investigators have assessed less invasive approaches, demonstrating that benign and malignant lesions of the cortex can be distinguished by circulating steroids (24) or circulating microRNAs (25), and that it is possible to measure circulating tumor DNA from patients with ACC (26,27); such approaches may ultimately enable molecular subtype-directed neoadjuvant therapy and radiation-free tracking of ACC burden.
3.3. Clinical trials expose weaknesses of single pathway, “one size fits all” therapy
Molecular biomarkers are not currently used to direct therapies in ACC; patients with advanced and/or high-risk disease are uniformly directed to cytotoxic chemotherapy with or without mitotane (5,28,29). However, ongoing trials are evaluating the efficacy of prognostic grade in predicting therapeutic response to adjuvant mitotane alone or with combination cytotoxic chemotherapy (ADIUVO – NCT00777244, ADIUVO-2 – NCT03583710). While grade is certainly effective in pinpointing patients who are less likely to respond to standard of care, such an approach still falls short of rationally directing patients to therapy based on oncogenic pathways driving their specific type of ACC. Moreover, the therapeutic potential of mitotane is poorly understood in the context of ACC subtypes as recent studies suggest this drug may drive cytotoxic ER stress in responsive cells through SOAT-1 (30,31), the target of the investigational adrenolytic agent nevanimibe (ATR-101) (32,33). The molecular biomarker-directed application of novel or existing targeted agents to specific subtypes will be essential for advances in the care of this disease. However, previous evaluation of targeted therapies for ACC weaves a cautionary tale.
Overexpression of IGF2 in 90% of ACC (confirmed in (7,8)) and early observations that inhibition of IGF2/IGF1R signaling was efficacious in subcutaneous xenograft models (34) fueled phase I-III clinical trials evaluating IGF2/IGF1R inhibition by figitumumab, cixutumumab, or linsitinib in patients with advanced ACC (35–37). Shockingly, these studies revealed that only 3-5% of patients with refractory metastatic ACC responded to IGF2/IGF1R inhibition with longterm regression (37), suggesting downstream genetic events may confer resistance to IGF2/IGF1R monotherapy. ACC-TCGA suggests that patients with COC2-3 tumors will likely require additional therapies targeting the Wnt pathway and cell cycle ((8); Fig 1). More recently, the US Food and Drug Administration’s accelerated approval of PD-1/PD-L1 checkpoint therapy for patients with mismatch repair-deficient solid tumors has fueled studies demonstrating such therapies may be effective for some patients with ACC (38,39). However, the immunosuppressive effects of glucocorticoids (40) and the anti-correlation between steroidogenesis and immune infiltration in ACC-TCGA (8) suggests combination inhibition of steroidogenesis may be additionally required in mismatch repair-deficient, functional COC2-3 ACC. Taken together, these studies suggest that the application of targeted therapies to ACC likely requires multiple agents and a deeper understanding of the collaborative oncogenic pathways turned on in each tumor subtype.
3.4. Revisiting ACC at the bench
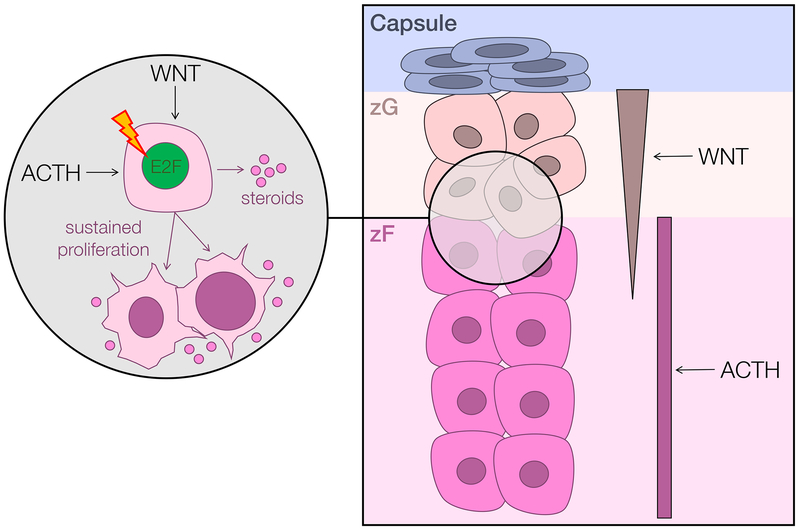
Advances in in vivo modeling of adrenocortical homeostasis and cancer have refined the understanding of oncogenic pathways derailed in ACC. Among the earliest of such studies was one by Heaton et al., who demonstrated that IGF2 overexpression likely requires collaboration with additional pathways (e.g. Wnt/β-catenin signaling) to promote adrenocortical tumorigenesis (41). More recent in vivo models point to one putative cell of origin of a subset of ACC, perhaps lying in the boundary between the mineralocorticoid-producing zona glomerulosa (zG) and glucocorticoid-producing zona fasciculata (zF) (Fig 2).
Figure 2. Murine models of adrenocortical homeostasis and cancer point to a putative cell of origin for Wnt-active ACC.
Schematized right is a portion of the capsular/cortical unit of the adrenal cortex. The upper layer of the adrenal cortex, the zona glomerulosa (zG), bears a gradient of active Wnt signaling and produces mineralocorticoids. The second layer of the adrenal cortex, the zona fasciculata (zF), proliferates and produces glucocorticoids in response to ACTH/PKA. Comprehensive molecular profiling studies identified recurrent mutations leading to constitutive activation of both ACTH/PKA and Wnt signaling ACC (7,8), and demonstrated that Wnt/β-catenin pathway alterations are significantly associated with clinical cortisol production (8). Recent mouse models of ACTH-driven zF regeneration (43), augmented Wnt/β-catenin signaling supported by ZNRF3 deficiency (44), and sustained proliferation triggered by adrenocortical expression of the SV40 large T-antigen (45) also demonstrate a unique interplay between Wnt/β-catenin and ACTH/PKA signaling in enabling proliferation of cells residing in the zG/zF boundary. Taken together, these studies support the existence of a small population of zG/zF boundary cells that are capable of rapidly proliferating in response to sustained Wnt/β-catenin and/or ACTH signaling. Prolonged cell cycle activation (schematized here by E2F) may render these cells susceptible to malignant transformation and ligand-independent growth.
It is well known that proliferation and differentiation of the adrenocortical zF is reliant on ACTH-dependent PKA signaling (42). We recently demonstrated that ACTH-dependent proliferation during zF regeneration also relies on intact Wnt/β-catenin signaling (43). This, combined with identification of recurrent mutations leading to constitutive activation of both pathways in ACC (7,8) and strong association between cortisol production and Wnt/β-catenin pathway alterations (8), suggests that components of the ACTH signaling pathway may contribute to Wnt/β-catenin-dependent adrenocortical carcinogenesis. This is strongly supported by a recent study from our group demonstrating that adrenocortical deletion of a negative regulator of ligand-dependent Wnt signaling, ZNRF3, leads to functional zF hyperplasia (44). Additionally, a transgenic mouse model of ACC driven by adrenocortical expression of the SV40 large T-antigen (which simultaneously inactivates pRb and p53) also demonstrated that glucocorticoid-producing, metastatic ACC emerge from dysplastic lesions lying in the zG/zF boundary that upregulate Wnt/β-catenin signaling (45). Intriguingly, these lesions also exhibited E2F-dependent upregulation of histone methyltransferase EZH2 (45), akin to cell cycle-avid ACC (18). The recent demonstration that adrenocortical deletion of EZH2 leads to zF aplasia and glucocorticoid insufficiency suggests that the integrity of ACTH signaling in the adrenal cortex is exquisitely reliant on this pharmacologically targetable epigenetic modifier (46).
New developments in xenograft and in vitro modeling of ACC also hold promise for enabling a deeper understanding of ACC biology. The steroidogenic NCI-H295R cell line has long been the classical, most established model of ACC (47), possessing high expression of SF1, constitutively active β-catenin (48), and inactivation of pRb (49,50) and p53 (51). Recently, several new adult ACC cell lines and xenograft models have emerged. Pinto et al. characterized the first pediatric xenograft model of ACC, SJ-ACC3, enabling the preclinical identification of topotecan as an efficacious medical therapy for a child with recurrent ACC (52). Hantel, Beuschlein and colleagues developed the adult ACC-derived, steroidogenic, and Wnt/β-catenin-active MUC-1 xenograft model and cell line which exhibited resistance to IGF-targeting therapy and recapitulated resistance to cytotoxic chemotherapy observed in the original patient (53–55). Kiseljak-Vassiliades and colleagues also developed two new adult ACC-derived cell lines and xenograft models, CU-ACC1 and CU-ACC2; CU-ACC1 is a cortisol-producing cell line bearing constitutively active β-catenin, whereas CU-ACC2 is a mismatch repair-deficient cell line bearing a mutation in TP53 (56). Comprehensive molecular profiling of the NCI-H295R cell line as well as these novel models will be essential for preclinical evaluation of ACC subtype-specific therapeutic approaches.
3.5. Implications for novel strategies to direct targeted therapies
The new molecular classification of ACC paired with biomarkers capturing molecular subtypes (Fig 1) and recent clinical and translational studies have clarified our understanding of ACC’s molecular basis and illuminated several novel therapeutic strategies. Notably, these studies have suggested that COC1, COC2, and COC3 ACC may be differentially responsive to therapies targeting the IGF2/IGF1R pathway, Wnt/β-catenin pathway, cell cycle, and immune system. The apparent reliance of most ACC on multiple oncogenic pathways may explain the observed broad resistance to IGF2/IGF1R monotherapy (37), suggesting that biomarker-based strategies to improve patient selection and new strategies incorporating combination therapy are paramount. Indeed, recently developed biomarkers that approximate ACC-TCGA molecular subtypes hold promise for enabling both prospective classification of ACC (Fig 1) and application of efficacious adjuvant therapies to patients likely to recur on standard of care (22). Advances in biomarker detection in archival material (23) and blood (24–27) will undoubtedly expand the patient population for which biomarker assessment is feasible.
The outlook for targeted therapies in ACC is promising. Currently, only a rare population of individuals with ACC respond to immunotherapy as a single agent (39); however, it is possible that combination therapy with inhibitors of steroidogenesis and/or cytotoxic agents (57) may enhance neoantigen presentation and immune clearance. The enrichment for Wnt/β-catenin pathway alterations in COC2-COC3 ACC suggests that individuals with these ACC types may be responsive to therapies targeting this pathway. Those tumors with ZNRF3 deficiency are likely reliant on Porcupine-dependent Wnt ligand secretion (44) and may be responsive to Porcupine inhibitors currently in phase I trials (e.g. NCT01351103). Tumors with mutations in CTNNB1 leading to constitutive stabilization of β-catenin may instead be responsive to therapies targeting the oncogenic β-catenin/CBP transcriptional program, which have recently completed phase I trials for solid tumors (e.g. NCT01302405, NCT01764477). Patients with COC3 ACC likely require additional therapies targeting the cell cycle, perhaps in combination with DNA demethylating agents (58,59). Well characterized in vitro and in vivo models of ACC will undoubtedly facilitate preclinical assessment of these approaches (47,52–56).
Finally, recent developments in murine modeling of adrenocortical homeostasis and cancer have implicated a role for collaboration between ACTH/PKA and Wnt/β-catenin signaling in enabling cell cycle activation in a proliferating population of cells residing in the zG/zF boundary (Fig 2). While it is yet unknown how targeting these cells will influence established ACC, the susceptibility of this population to hyperplasia and malignant transformation (44,45) suggests that targeting interplay between paracrine and endocrine signaling may ultimately be required to extinguish at least one type of ACC cell of origin.
4. Conclusions
ACC is a rare and often fatal cancer. In the last few years, our field has made numerous advances in the molecular understanding of ACC and adrenocortical biology. Moreover, the public availability of multiplatform data profiling ACC (particularly through ACC-TCGA (8)) has enabled the development of novel biomarkers and a deeper understanding of ACC from a pan-cancer perspective. The recent development of new scientific models of ACC and agents targeting pathways differentially upregulated in tumor subtypes supplies researchers and clinicians with a new set of therapeutic tools and renewed hope to fight this devastating disease.
Acknowledgments
Funding Sources
This work, A.M. Lerario and G.D. Hammer are supported by 2 R01 DK062027 (grant to G.D. Hammer). D.R. Mohan was/is supported by the University of Michigan Medical Scientist Training Program (5 T32 GM7863), the University of Michigan Doctoral Program in Cancer Biology, the University of Michigan Rogel Cancer Center (grant to G.D. Hammer, scholarship to D.R. Mohan), and The Drew O’Donoghue Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
D.R. Mohan, A.M. Lerario, and G.D. Hammer are co-inventors on a provisional patent application describing compositions and methods for characterizing cancer, owned by The Regents of the University of Michigan. G.D. Hammer is founder and advisor for Millendo Therapeutics. G. D. Hammer is also founder of Vasaragen.
References
- 1.Wajchenberg BL, Albergaria Pereira MA, Medonca BB, Latronico AC, Campos Carneiro P, Alves VA, et al. Adrenocortical carcinoma: clinical and laboratory observations. Cancer 2000;88(4):711–36. [PubMed] [Google Scholar]
- 2.Kerkhofs TM, Verhoeven RH, Van der Zwan JM, Dieleman J, Kerstens MN, Links TP, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer 2013;49(11):2579–86 doi: 10.1016/j.ejca.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, et al. Adrenocortical carcinoma. Endocr Rev 2014;35(2):282–326 doi: 10.1210/er.2013-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glenn JA, Else T, Hughes DT, Cohen MS, Jolly S, Giordano TJ, et al. Longitudinal patterns of recurrence in patients with adrenocortical carcinoma. Surgery 2019;165(1):186–95 doi: 10.1016/j.surg.2018.04.068. [DOI] [PubMed] [Google Scholar]
- 5.Lerario AM, Mohan DR, Lirov R, Else T, Hammer GD. 83. Adrenal Tumors In: DeVita VT, Lawrence TS, Rosenburg SA, editors. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. 11 ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2019. [Google Scholar]
- 6.Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab 2013;98(12):4551–64 doi: 10.1210/jc.2013-3020. [DOI] [PubMed] [Google Scholar]
- 7.Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, Barreau O, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet 2014;46(6):607–12 doi: 10.1038/ng.2953. [DOI] [PubMed] [Google Scholar]; * In the first integrated molecular profiling study of ACC, Assie, Letouze and colleagues introduced the concept that ACC may be comprised of distinct molecular subtypes and were the first to identify a negative regulator of the Wnt pathway, ZNRF3, as the most frequently altered gene in ACC.
- 8.Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA, et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell 2016;29(5):723–36 doi: 10.1016/j.ccell.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** As part of the National Institutes of Health’s The Cancer Genome Atlas (TCGA) series of projects, Zheng and colleagues performed the most comprehensive molecular profiling study on ACC to date, “ACC-TCGA.” ACC-TCGA identified 3 distinct molecular classes of ACC defined by distinct somatic alterations (mutations and copy number alteration patterns), transcriptional programs (controlling steroidogenesis and cell cycle), and methylation profiles (varying degress of promoter CpG island hypermethylation). Notably, these investigators identified that multiplatform ACC molecular subtypes are strongly predictive of clinical outcomes, suggesting that certain ACC subtypes are universally refractory to standard of care therapy and may be more susceptible to targeted agents. This study enabled the incorporation of ACC into pan-cancer studies, which have revealed that ACC as a group are exquisitely immune poor and uniquely defined by activation of an SF1-dependent transcriptional program.
- 9.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The Immune Landscape of Cancer. Immunity 2018;48(4):812–30.e14 doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018;173(2):291–304.e6 doi: 10.1016/j.cell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knijnenburg TA, Wang L, Zimmermann MT, Chambwe N, Gao GF, Cherniack AD, et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep 2018;23(1):239–54.e6 doi: 10.1016/j.celrep.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gara SK, Lack J, Zhang L, Harris E, Cam M, Kebebew E. Metastatic adrenocortical carcinoma displays higher mutation rate and tumor heterogeneity than primary tumors. Nat Commun 2018;9(1):4172 doi: 10.1038/s41467-018-06366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corces MR, Granja JM, Shams S, Louie BH, Seoane JA, Zhou W, et al. The chromatin accessibility landscape of primary human cancers. Science 2018;362(6413) doi: 10.1126/science.aav1898. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Also part of the TCGA series of projects, Corces and colleagues performed ATAC-seq to profile the chromatin accessiblity landscape of 410 tumor samples from TCGA (9 of which are primary ACC samples spanning different ACC-TCGA molecular subtypes). This tour de force demonstrated that ACC is uniquely defined by an SF1-driven chromatin accessibility landscape, and provides a wealth of data which may enable a deeper understanding of ACC chromatin biology.
- 14.Beuschlein F, Weigel J, Saeger W, Kroiss M, Wild V, Daffara F, et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab 2015;100(3):841–9 doi: 10.1210/jc.2014-3182. [DOI] [PubMed] [Google Scholar]
- 15.Giordano TJ. The argument for mitotic rate-based grading for the prognostication of adrenocortical carcinoma. Am J Surg Pathol 2011;35(4):471–3 doi: 10.1097/PAS.0b013e31820bcf21. [DOI] [PubMed] [Google Scholar]
- 16.Weiss LM, Medeiros LJ, Vickery AL Jr. Pathologic features of prognostic significance in adrenocortical carcinoma. Am J Surg Pathol 1989;13(3):202–6. [DOI] [PubMed] [Google Scholar]
- 17.de Reynies A, Assie G, Rickman DS, Tissier F, Groussin L, Rene-Corail F, et al. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol 2009;27(7):1108–15 doi: 10.1200/JCO.2008.18.5678. [DOI] [PubMed] [Google Scholar]
- 18.Drelon C, Berthon A, Mathieu M, Ragazzon B, Kuick R, Tabbal H, et al. EZH2 is overexpressed in adrenocortical carcinoma and is associated with disease progression. Hum Mol Genet 2016;25(13):2789–800 doi: 10.1093/hmg/ddw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggiero C, Doghman-Bouguerra M, Sbiera S, Sbiera I, Parsons M, Ragazzon B, et al. Dosage-dependent regulation of VAV2 expression by steroidogenic factor-1 drives adrenocortical carcinoma cell invasion. Sci Signal 2017;10(469) doi: 10.1126/scisignal.aal2464. [DOI] [PubMed] [Google Scholar]
- 20.Sbiera S, Sbiera I, Ruggiero C, Doghman-Bouguerra M, Korpershoek E, de Krijger RR, et al. Assessment of VAV2 Expression Refines Prognostic Prediction in Adrenocortical Carcinoma. J Clin Endocrinol Metab 2017;102(9):3491–8 doi 10.1210/jc.2017-00984. [DOI] [PubMed] [Google Scholar]
- 21.Jouinot A, Assie G, Libe R, Fassnacht M, Papathomas T, Barreau O, et al. DNA Methylation Is an Independent Prognostic Marker of Survival in Adrenocortical Cancer. J Clin Endocrinol Metab 2017;102(3):923–32 doi 10.1210/jc.2016-3205. [DOI] [PubMed] [Google Scholar]
- 22.Mohan DR, Lerario AM, Else T, Mukherjee B, Almeida MQ, Vinco M, et al. Targeted Assessment of G0S2 Methylation Identifies a Rapidly Recurrent, Routinely Fatal Molecular Subtype of Adrenocortical Carcinoma. Clin Cancer Res 2019. doi: 10.1158/1078-0432.CCR-18-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]; * We recently demonstrated that pathologic methylation and silencing of the gene G0S2 can accurately capture COC3/CIMP-high tumors from ACC-TCGA. We believe this strategy holds significant promise to translate ACC molecular subtypes to clinical care, especially if combined with other biomarkers like BUB1B-PINK1 and extended to newer approaches evaluating circulating tumor DNA.
- 23.Lippert J, Appenzeller S, Liang R, Sbiera S, Kircher S, Altieri B, et al. Targeted Molecular Analysis in Adrenocortical Carcinomas: A Strategy Toward Improved Personalized Prognostication. J Clin Endocrinol Metab 2018;103(12):4511–23 doi: 10.1210/jc.2018-01348. [DOI] [PubMed] [Google Scholar]
- 24.Schweitzer S, Kunz M, Kurlbaum M, Vey J, Kendl S, Deutschbein T, et al. Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma. Eur J Endocrinol 2019;180(2):117–25 doi 10.1530/EJE-18-0782. [DOI] [PubMed] [Google Scholar]
- 25.Decmann A, Bancos I, Khanna A, Thomas MA, Turai P, Perge P, et al. Comparison of plasma and urinary microRNA-483-5p for the diagnosis of adrenocortical malignancy. J Biotechnol 2019;297:49–53 doi 10.1016/j.jbiotec.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 26.McCabe MJ, Pinese M, Chan CL, Sheriff N, Thompson TJ, Grady J, et al. Genomic stratification and liquid biopsy in a rare adrenocortical carcinoma (ACC) case, with dual lung metastases. Cold Spring Harb Mol Case Stud 2019;5(2) doi 10.1101/mcs.a003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garinet S, Nectoux J, Neou M, Pasmant E, Jouinot A, Sibony M, et al. Detection and monitoring of circulating tumor DNA in adrenocortical carcinoma. Endocr Relat Cancer 2018;25(3):L13–L7 doi 10.1530/ERC-17-0467. [DOI] [PubMed] [Google Scholar]
- 28.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med 2012;366(23):2189–97 doi 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 29.Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2018;179(4):G1–G46 doi 10.1530/EJE-18-0608. [DOI] [PubMed] [Google Scholar]
- 30.Sbiera S, Leich E, Liebisch G, Sbiera I, Schirbel A, Wiemer L, et al. Mitotane Inhibits Sterol-O-Acyl Transferase 1 Triggering Lipid-Mediated Endoplasmic Reticulum Stress and Apoptosis in Adrenocortical Carcinoma Cells. Endocrinology 2015;156(11):3895–908 doi: 10.1210/en.2015-1367. [DOI] [PubMed] [Google Scholar]
- 31.Ruggiero C, Doghman-Bouguerra M, Ronco C, Benhida R, Rocchi S, Lalli E. The GRP78/BiP inhibitor HA15 synergizes with mitotane action against adrenocortical carcinoma cells through convergent activation of ER stress pathways. Mol Cell Endocrinol 2018;474:57–64 doi 10.1016/j.mce.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 32.LaPensee CR, Mann JE, Rainey WE, Crudo V, Hunt SW, Hammer GD. ATR-101, a Selective and Potent Inhibitor of Acyl-CoA Acyltransferase 1, Induces Apoptosis in H295R Adrenocortical Cells and in the Adrenal Cortex of Dogs. Endocrinology 2016;157(5):1775–88 doi: 10.1210/en.2015-2052. [DOI] [PubMed] [Google Scholar]
- 33.Langlois DK, Fritz MC, Schall WD, Bari Olivier N, Smedley RC, Pearson PG, et al. ATR-101, a selective ACAT1 inhibitor, decreases ACTH-stimulated cortisol concentrations in dogs with naturally occurring Cushing’s syndrome. BMC Endocr Disord 2018;18(1):24 doi: 10.1186/s12902-018-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, et al. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab 2009;94(1):204–12 doi 10.1210/jc.2008-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, et al. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol 2010;65(4):765–73 doi 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lerario AM, Worden FP, Ramm CA, Hesseltine EA, Hasseltine EA, Stadler WM, et al. The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: a multi-institutional NCI-sponsored trial. Horm Cancer 2014;5(4):232–9 doi: 10.1007/s12672-014-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol 2015;16(4):426–35 doi 10.1016/S1470-2045(15)70081-1. [DOI] [PubMed] [Google Scholar]; ** This phase 3, international, placebo-controlled trial evaluating therapy targeting the IGF2/IGF1R axis in patients with advanced ACC revealed that, while 3-5% patients had bona fide partial responses with long term disease regression, the vast majority of patients failed to respond to IGF2/IGF1R monotherapy. Though disappointing, this study highlights the critical importance of appropriate patient selection for targeted therapies and suggests that the presence of additional overactive oncogenic pathways in the majority of tumors (cell cycle, Wnt/β-catenin signaling) may confer therapeutic resistance. Post-hoc analysis of molecular data from this and future phase 3 studies will be essential to enable appropriate direction of targeted therapies.
- 38.Le Tourneau C, Hoimes C, Zarwan C, Wong DJ, Bauer S, Claus R, et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer 2018;6(1):111 doi: 10.1186/s40425-018-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mota JM, Sousa LG, Braghiroli MI, Siqueira LT, Neto JEB, Chapchap P, et al. Pembrolizumab for metastatic adrenocortical carcinoma with high mutational burden: Two case reports. Medicine (Baltimore) 2018;97(52):e13517 doi: 10.1097/MD.0000000000013517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiorentini C, Grisanti S, Cosentini D, Abate A, Rossini E, Berruti A, et al. Molecular Drivers of Potential Immunotherapy Failure in Adrenocortical Carcinoma. J Oncol 2019;2019:6072863 doi 10.1155/2019/6072863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heaton JH, Wood MA, Kim AC, Lima LO, Barlaskar FM, Almeida MQ, et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am J Pathol 2012;181(3):1017–33 doi 10.1016/j.ajpath.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xing Y, Lerario AM, Rainey W, Hammer GD. Development of adrenal cortex zonation. Endocrinol Metab Clin North Am 2015;44(2):243–74 doi 10.1016/j.ecl.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finco I, Lerario AM, Hammer GD. Sonic Hedgehog and WNT Signaling Promote Adrenal Gland Regeneration in Male Mice. Endocrinology 2018;159(2):579–96 doi: 10.1210/en.2017-03061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basham KJ, Rodriguez S, Turcu AF, Lerario AM, Logan CY, Rysztak MR, et al. A ZNRF3-dependent Wnt/β-catenin signaling gradient is required for adrenal homeostasis. Genes Dev 2019;33(3–4):209–20 doi 10.1101/gad.317412.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batisse-Lignier M, Sahut-Barnola I, Tissier F, Dumontet T, Mathieu M, Drelon C, et al. P53/Rb inhibition induces metastatic adrenocortical carcinomas in a preclinical transgenic model. Oncogene 2017;36(31):4445–56 doi 10.1038/onc.2017.54. [DOI] [PubMed] [Google Scholar]; * In this study, Batisse-Lignier and colleagues present a mouse model of ACC driven by adrenocortical expression of the SV40 large T-antigen which simultaneously inactivates pRb and p53. Intriguingly, these mice develop Wnt-active dysplastic lesions at the zG/zF boundary which invariably evolve to metastatic, glucocorticoid-producing ACC. This model highlights the susceptibility of proliferative cells lying in the zG/zF boundary to malignant transformation and provides a unique opportunity to evaluate secondary genetic events that lead to breaching of adrenal boundaries and disseminated metastasis.
- 46.Mathieu M, Drelon C, Rodriguez S, Tabbal H, Septier A, Damon-Soubeyrand C, et al. Steroidogenic differentiation and PKA signaling are programmed by histone methyltransferase EZH2 in the adrenal cortex. Proc Natl Acad Sci U S A 2018;115(52):E12265–E74 doi 10.1073/pnas.1809185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang T, Rainey WE. Human adrenocortical carcinoma cell lines. Mol Cell Endocrinol 2012;351(1):58–65 doi 10.1016/j.mce.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagnere AM, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res 2005;65(17):7622–7 doi 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 49.Hadjadj D, Kim SJ, Denecker T, Ben Driss L, Cadoret JC, Maric C, et al. A hypothesisdriven approach identifies CDK4 and CDK6 inhibitors as candidate drugs for treatments of adrenocortical carcinomas. Aging (Albany NY) 2017;9(12):2695–716 doi: 10.18632/aging.101356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ragazzon B, Libe R, Assie G, Tissier F, Barreau O, Houdayer C, et al. Mass-array screening of frequent mutations in cancers reveals RB1 alterations in aggressive adrenocortical carcinomas. Eur J Endocrinol 2014;170(3):385–91 doi 10.1530/EJE-13-0778. [DOI] [PubMed] [Google Scholar]
- 51.Cerquetti L, Bucci B, Marchese R, Misiti S, De Paula U, Miceli R, et al. Mitotane increases the radiotherapy inhibitory effect and induces G2-arrest in combined treatment on both H295R and SW13 adrenocortical cell lines. Endocr Relat Cancer 2008;15(2):623–34 doi: 10.1677/erc.1.1315. [DOI] [PubMed] [Google Scholar]
- 52.Pinto EM, Morton C, Rodriguez-Galindo C, McGregor L, Davidoff AM, Mercer K, et al. Establishment and characterization of the first pediatric adrenocortical carcinoma xenograft model identifies topotecan as a potential chemotherapeutic agent. Clin Cancer Res 2013;19(7):1740–7 doi 10.1158/1078-0432.CCR-12-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beuschlein F, Jakoby J, Mentz S, Zambetti G, Jung S, Reincke M, et al. IGF1-R inhibition and liposomal doxorubicin: Progress in preclinical evaluation for the treatment of adrenocortical carcinoma. Mol Cell Endocrinol 2016;428:82–8 doi: 10.1016/j.mce.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 54.Hantel C, Shapiro I, Poli G, Chiapponi C, Bidlingmaier M, Reincke M, et al. Targeting heterogeneity of adrenocortical carcinoma: Evaluation and extension of preclinical tumor models to improve clinical translation. Oncotarget 2016;7(48):79292–304 doi: 10.18632/oncotarget.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hantel C, Beuschlein F. Xenograft models for adrenocortical carcinoma. Mol Cell Endocrinol 2016;421:28–33 doi 10.1016/j.mce.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 56.Kiseljak-Vassiliades K, Zhang Y, Bagby SM, Kar A, Pozdeyev N, Xu M, et al. Development of new preclinical models to advance adrenocortical carcinoma research. Endocr Relat Cancer 2018;25(4):437–51 doi 10.1530/ERC-17-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Kiseljak-Vassiliades and colleagues contribute not one – but two! – new cell lines and xenograft models of ACC, bearing differences in genetic programs and steroid production. Additional characterization of these models may enable a deeper understanding of the susceptibility of different ACC molecular subtypes to targeted therapies.
- 57.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumuş M, Mazieres J, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379(21):2040–51 doi 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 58.Matei D, Fang F, Shen C, Schilder J, Arnold A, Zeng Y, et al. Epigenetic resensitization to platinum in ovarian cancer. Cancer Res 2012;72(9):2197–205 doi 10.1158/0008-5472.CAN-11-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azad N, Zahnow CA, Rudin CM, Baylin SB. The future of epigenetic therapy in solid tumours--lessons from the past. Nat Rev Clin Oncol 2013;10(5):256–66 doi: 10.1038/nrclinonc.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]