Abstract
Transcatheter aortic valve replacement (TAVR) has emerged as an effective therapy for the unmet clinical need of inoperable patients with severe aortic stenosis (AS). Current clinically-used tissue TAVR valves suffer from limited durability that hampers TAVR’s rapid expansion to younger, lower risk patients. Polymeric TAVR valves optimized for hemodynamic performance, hemocompatibility, extended durability, and resistance to calcific degeneration offer a viable solution to this challenge. We present extensive in-vitro durability and stability testing of a novel polymeric TAVR valve (PolyNova valve) using (i) accelerated wear testing (AWT, ISO 5840); (ii) calcification susceptibility (in the AWT)- compared to clinically-used tissue valves; and (iii) extended crimping stability (valves crimped to 16 Fr for 8 days). Hydrodynamic testing was performed every 50M cycles. The valves were also evaluated visually for structural integrity, and by Scanning Electron Microscopy for evaluation of surface damage in the micro-scale. Calcium and phosphorus deposition was evaluated using μCT and Inductive Coupled Plasma Spectroscopy. The valves passed 400M cycles in the AWT without failure. The effective orifice area kept stable at 1.8 cm2 with a desired gradual decrease in transvalvular pressure gradient and regurgitation (10.4 mmHg and 6.9%, respectively). Calcium and phosphorus deposition was significantly lower in the polymeric valve: down by a factor of 85 and 16, respectively- as compared to a tissue valve. Following the extended crimping testing, no tears nor surface damage were evident. The results of this study demonstrate the potential of a polymeric TAVR valve to be a viable alternative to tissue-based TAVR valves.
Keywords: TAVI, Aortic Stenosis, Heart Valve, Prosthetic Heart Valve, Valve durability, Medical Device, Calcific Degeneration, Accelerated Wear Testing, ISO 5840-3
1. Introduction
Transcatheter Aortic Valve Replacement (TAVR) has become an established technology that provides an alternative to open-heart Surgical Aortic Valve Replacement (SAVR) procedure for patients with severe Aortic Stenosis (AS).1 In this type of minimally invasive intervention, a stent with a mounted valve is delivered through the arterial tree, commonly in a transfemoral approach, and deployed through the stenotic native valve.2 All current FDA and CE-approved TAVR devices utilize bioprosthetic xenografts for their leaflets. While these have been significantly improved over the recent years, they have intrinsic limitations that are related to the tissue material, which might impede their expansion to new indications such as lower-risk surgical patients. Examples for such limitations include crimping and deployment-associated damage to the tissue leaflets,3–7 leaflet thrombosis,8–11 and calcific degeneration,9 which in-turn limit the durability and functionality of the valve.
Polymers have the potential to overcome these limitations, and there have been multiple attempts in the past for developing a polymeric prosthetic aortic valve.12–14 However, these past attempts have failed as none of the devices had met all the requirements for becoming a viable aortic valve substitute (must pass all): (a) hemocompatibility, (b) hydrodynamics, (c) low thrombogenicity, (d) high durability, and (e) low calcification susceptibility.12 For transcatheter valves the expected performance is even more challenging, as these valves are required to be crimped into a small bore delivery catheter (currently the smallest in use is 14 Fr, ~4.7 mm), and then deployed back to its nominal size, while avoiding damage to the valve components that might lead to premature failure; meaning (f) crimping stability. Despite previous attempts of older-generation polymeric valves to reach a viable clinical use, the motivation to develop alternative materials to xenografts seems now greater than ever with the success of TAVR. Other than better engineering freedom (both chemical and design), an important underlying benefit of synthetic materials over xenografts include the ability to manufacture at mass production with high reproducibility and lower overall manufacturing costs.
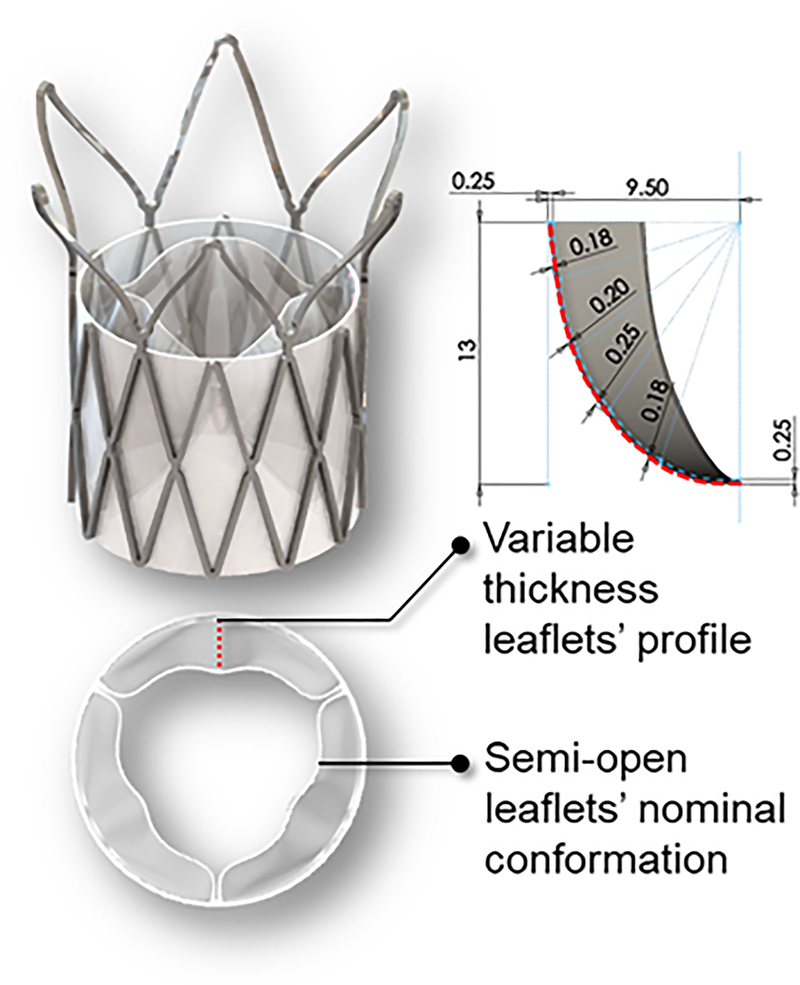
In recent years, novel polymeric valve technologies have emerged, demonstrating early promising results. A Hyaloronan-Polyethylene SAVR valve showed potential in terms of material properties, valve hydrodynamics, hydrophilicity, blood clotting, and platelet and leukocyte adhesion.15,16 The Triskele (UCL TAV™, University College London, London, UK) TAVR valve utilizes urethane (POSS-PCU) leaflets and skirt, demonstrated in in-vitro testing comparable hydrodynamic performance to other, clinically-used, tissue TAVR valves.17 Foldax Inc. (Salt Lake City, UT, USA) utilizes an elastomer in their prosthetic heart valve products,18,19 which include aortic and mitral surgical valves, and a transcatheter valve. Strait Access Technologies (SAT, Cape Town, South Africa) develops a heparinized polyurethane TAVR valve for rheumatic heart disease patients and recently passed over 600M cycles Accelerated wear testing (AWT) in-vitro.20 Recently we have introduced the PolyNova Valve (PolyNova Cardiovascular Inc., Stony Brook, NY), a novel xSIBS polymer-based valve for TAVR procedures (Figure 1).21 The PolyNova TAVR valve design was optimized based on the DTE methodology22,23 for minimization of thrombogenicity, and maximization of hydrodynamic performance and extended durability.24,25 In this process, which includes rigorous numerical analysis, the leaflets’ thickness profile was adjusted locally for minimizing the stresses during the cardiac cycle; regions of high flexural stresses were thickened, and vice versa. The feasibility of this optimization methodology was successfully demonstrated in-silico and in-vitro with our polymeric surgical prototypes.24–27 Moreover, the leaflets nominal confirmation (‘zero-stress’) was adjusted to semi-open for further reducing the accumulated stresses over the cardiac cycle (Figure 1). The valve demonstrated in-vitro superior hemodynamic performance compared to equivalent size bioprosthetic SAVR and TAVR valves.21 This study covered the aforementioned TAVR requirements (a)-(c) in-vitro.
Figure 1.
A – Illustration of the PolyNova TAVR valve and leaflets design, optimized for hemodynamics and durability: Variable thickness profile, and semi-open nominal conformation (‘zero-stress’). The top right illustration represents schematic of the leaflet thickness profile (red dashed line) at various locations, and dimensions, in mm. The shading in this schematic is out of plane section of the leaflet.
In the present study, we assess the durability and stability of the PolyNova valve (the three remaining requirements- after the valve has already met the other (a) to (c) criteria21,25–28): in-vitro (d) valve durability; (e) calcification susceptibility; and (f) crimping stability.
2. Materials and methods
2.1. Valve prototyping
The PolyNova TAVR valves, 20-mm size, were manufactured in-house using vacuum compression molding as previously described in Rotman et al.21 Briefly, the molding process included placing raw xSIBS pellets inside the mold, and then closing the mold under heat (220oC) and pressure (4 Tons) for 1 hour. The mold was designed to be connected to a vacuum line and hold vacuum throughout the molding process, therefore enhancing the density and quality of the molded polymer. The cured valves were then sutured to laser-cut nitinol stents (6–0 Silk black braided, Ethicon Inc., NJ, USA).
2.2. Durability
Testing the durability of the valves was performed by mounting the valves (n=4) in an Accelerated Wear Testing (AWT) machine (HiCycle, Vivitro Labs, Victoria, BC) according to ISO 5840; a defined target peak differential pressure of 100 mmHg across the closed valves was maintained for 95% or more of all the test cycles. Each test valve experienced a differential pressure equal to or greater than 100 mmHg for 5 % or more of the duration of each cycle. The cycling frequency was 10 Hz. The working fluid was saline (0.9% NaCl) at 37oC, and was replaced once a week. The valves were inspected daily for compliance with the ISO pressure gradient requirement, and valve kinematics evaluated visually using a stroboscope (Alaron Instruments Inc., Canada). The hydrodynamic performance of the valves was evaluated every ~50 million cycles as described below.
In order to assess the baseline durability of the valve design itself, the polymeric valves were tested in the AWT without the stent- mounted with a 3D-printed rigid sleeve as a support (Figure 2), excluding the effects of crimping, and oversized deployment. The endpoint for this test was defined as valve failure, which include significant decrease in hydrodynamic performance, with or without major tear in the leaflets.
Figure 2.
The stentless configuration of the polymeric valves tested in the accelerated durability testing (AWT) and the accelerated calcific susceptibility.
The valves’ hydrodynamics was tested in a pulse duplicator (Vivitro PD, Vivitro Labs, Victoria, BC) under the following conditions: average Cardiac Output (CO) of 5 l/min, Heart Rate (HR) of 70 bpm, systole-diastole ratio of 0.35–0.65, aortic pressure of 120/80 mmHg, and mean arterial pressure (MAP) of about 100 mmHg. Blood-analog fluid was used as the working fluid (50.3% Glycerol, 48.8% ddH2O, 0.9% NaCl, by weight; yielding dynamic viscosity μ = 3.5 mPa·s, and density ρ = 1,121 Kg/m3, at 37oC). Pressure (aortic and ventricular) and flow recordings were filtered using a 30 Hz analog low pass filter (LPF). In each test condition the system was allowed to stabilize, followed by 10 consecutive cycles of pressure and flow recordings. Hydrodynamic performance was evaluated by calculating the valves Effective Orifice Area (EOA, Eq.1 per ISO 5840), average systolic trans-valvular pressure drop (ΔP) and regurgitation flow.
| Eq.1 |
Where the EOA is calculated in cm2, QRMS is the root mean square forward flow (ml/s) during the positive differential pressure period (ΔP>0), ΔP [mmHg] is the mean pressure difference (measured during the positive differential pressure period), and ρ [g/cm3] is the density of the test fluid.
2.3. Calcification susceptibility
Calcification susceptibility is conservatively measured in-vivo in chronic animal preclinical studies, or only later on during clinical studies. In this study we have utilized an in-vitro protocol for accelerated testing of the valve’s calcification susceptibility.29,30 The valves were mounted in the AWT machine, similar to the conditions of the durability testing described above, except that the saline was replaced by Golomb and Wagner’s pro-calcific/phosphorus compound that resembles the ion concentrations in blood.30 This protocol allows testing the passive mechanism of prosthetic heart valve calcification, in which calcium ions can accumulate into defects in the leaflet,31 or via chemical bonding to the polymer surface.
The calcification compound was prepared by mixing equal volumes of 7.754mM CaCl2 and 4.642mM K2HPO4 in TRIS buffer (0.05M, pH 7.4), each, and then mixing together. The compound was replaced weekly, and the valves were tested for 50 million cycles. Three polymeric valves (n=3) were tested without the stent similar to the durability testing setup. 21-mm Carpentier-Edwards PERIMOUNT Magna Ease SAVR bioprosthetic valves (n=2) were tested as a clinically-used reference. Additional valve samples of both the PolyNova and the SAVR bioprosthetic valves (n=1, each) that have not been exposed to the AWT-calcification test were used as negative controls. It is important to note that the 50 million cycles duration of this protocol does not aim to evaluate durability of the valves. Rather, this duration was shown to be sufficient for evaluating the tendency of a heart valve to accumulate calcium and phosphorus in a comparative manner.
Following 50 million cycles, the valves were gently rinsed in saline, and then scanned in a μCT (Scanco Medical vivaCT 40, Scanco Medical, Brüttisellen, Switzerland). The polymeric valves were scanned under dry conditions. The tissue valves were first scanned in saline, then dried and rescanned under dry conditions for improved sensitivity. The scanning parameters were set as follows (images were acquired axially, from top to bottom): Resolution: 15 μm voxel size (15×15×15 μm); Acquisition Voltage= 70 kVp; Acquisition time: 250 ms; Current: 36 μAs. Analysis of μCT scans was performed using MATLAB (MathWorks, Inc., Natick, MA, USA), ITK-SNAP and ImageJ. Then, a single leaflet per valve was cut and scanned on both ventricular and aortic sides using Laser Ablation -Inductive Coupled Plasma Mass Spectroscopy (LA-ICP-MS) for measuring calcium (Ca) and phosphorus (P) concentrations of deposits on their surface. Samples were ablated with a NWR 213 UV laser and analyzed by an Agilent 7500cx ICP-MS. The data was then processed through Iolite software, which calculates downhole fractionation of the signal with time and accounts for drift in the mass spectrometer during the analysis period. Ten spots were randomly analyzed on each sample to give an unbiased average across the sample. National Institute of Standards and Technology glass standard 612 and a well-characterized internal carbonate standard from Walnut Canyon (New Mexico), were interspersed between every ten unknown analyses to account for machine drift and to calculate unknown Ca and P concentrations. The counts were then normalized by the average count in the control group to account for average accumulation of Ca and P. Overall, the μCT allowed us to identify mineral deposition within the bulk or on the surface of the leaflets, while the LA-ICP-MS scans confirmed and quantified the presence of calcium and phosphorous molecules. Statistical analysis was performed pairwise using the student T-test, intra-valve groups between the aortic and ventricular sides of the leaflets, and inter-valve groups. P value < 0.05 was considered significant.
2.4. Crimping stability
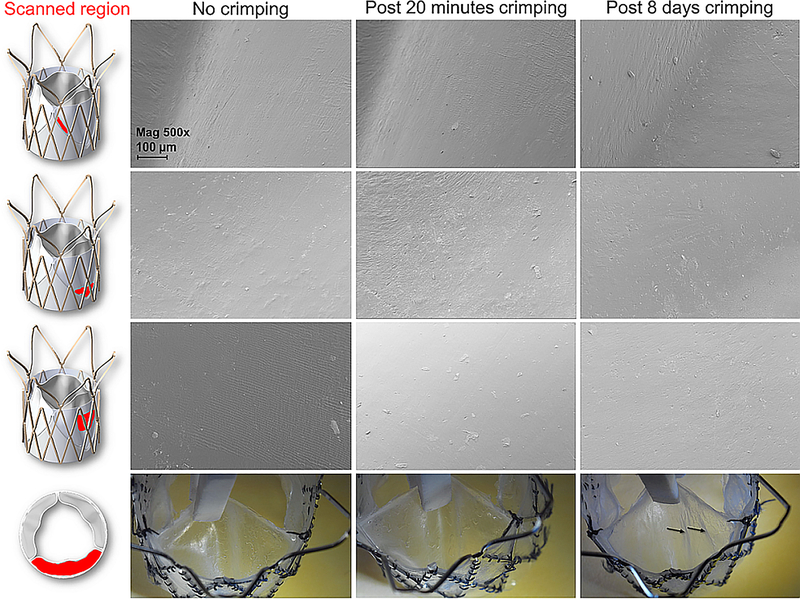
Stented valves were crimped to 16 Fr (~5.3 mm) using a stent crimper (Edwards Lifesciences, Irvine, CA) and then analyzed for damage. A 5 Fr catheter was placed concentrically within the valves to replicate crimping onto the delivery system (Figure 3). Three valves (n=3) were crimped for 20 minutes, similar to the clinical practice with the Edwards SAPIEN 3 tissue TAVR valve, while 3 additional valves (n=3) were crimped for 8 days, to test the valves under extended crimping, with implication toward factory crimping of the valves instead of the common clinical practice with tissue TAVR valve where crimping is performed just before the procedure in the cath-lab. At the end of the test the crimping was slowly released and the valves were self-expanded back to their nominal size. The valves were then inspected visually for evidence of damage using a 10x zoom camera. Then, a single leaflet was removed from each valve and analyzed in Scanning Electron Microscopy (SEM) for surface damage. The SEM scans focused on three wide regions of the leaflets, which cover the connection regions of the leaflets, as well as the belly of the leaflets. Those regions were chosen according to previous studies that performed strain analysis,24,25,27 or based on locations that we suspected to be more sensitive from the manufacturing point of view.
Figure 3.
Crimping of the valves.
3. Results
3.1. Durability
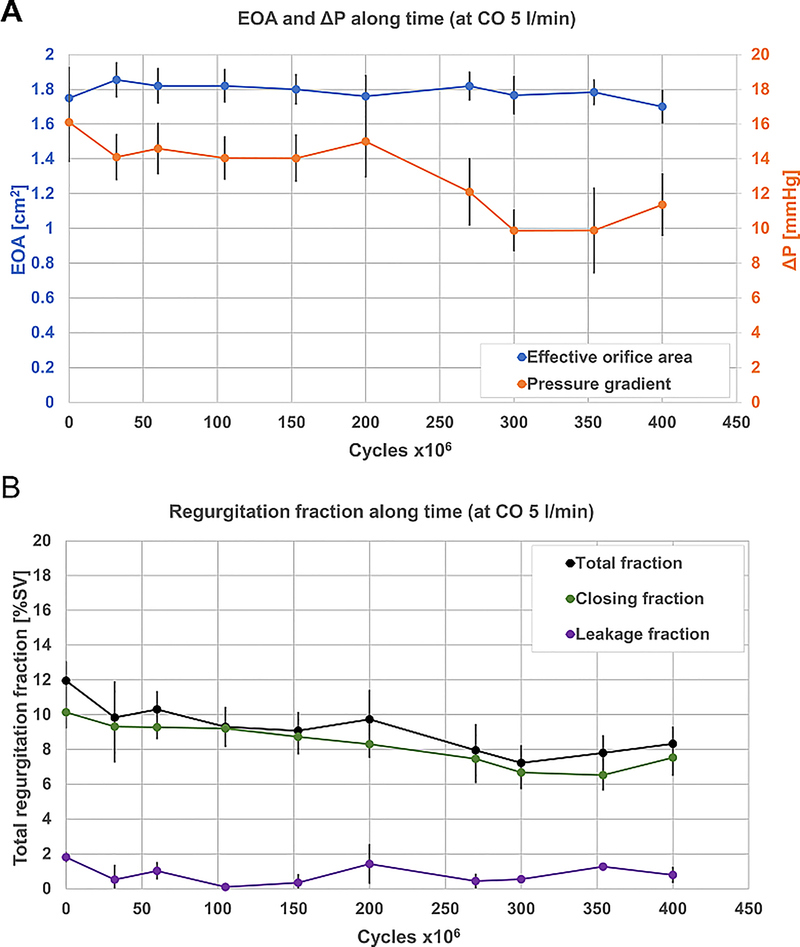
Four valves tested in the AWT at 10 Hz have so far surpassed 400 million cycles, with no damage apparent by visual inspection. The valves were tested for hydrodynamic performance every ~50 million cycles (Figure 4). After 400 million cycles, the EOA did not have any significantly noticeable changes, and was kept stable at an average of 1.8±0.04 cm2. The pressure gradient kept on a relatively stable value of 14.6±0.8 mmHg up to 200 million cycles, then started to decline gradually to a value of 10.4±0.9 mmHg. The regurgitation fraction in the stentless configuration was mostly affected by the closing volume, while the leakage volume remained stable throughout 400 million cycles (0.8±0.5%). In contrast, the closing regurgitation declined linearly with time in the first 350 million cycles (from 10.1±0.9% to 6.5±0.8%). Slight increase in closing regurgitation fraction was then measured at 400 million cycles (7.5±1.0%).
Figure 4.
Hydrodynamic performance of the polymeric valves along the AWT. A – EOA and pressure gradient; B – Regurgitation fraction (of SV), breakdown as total regurgitation, closing fraction, and leakage fraction. Dashed green line is represents the linear fit of the closing regurgitation fraction curve. AWT – Accelerated Wear Testing; CO - Cardiac Output; EOA – Effective Orifice Area; SV – Stroke Volume.
3.2. Calcification susceptibility
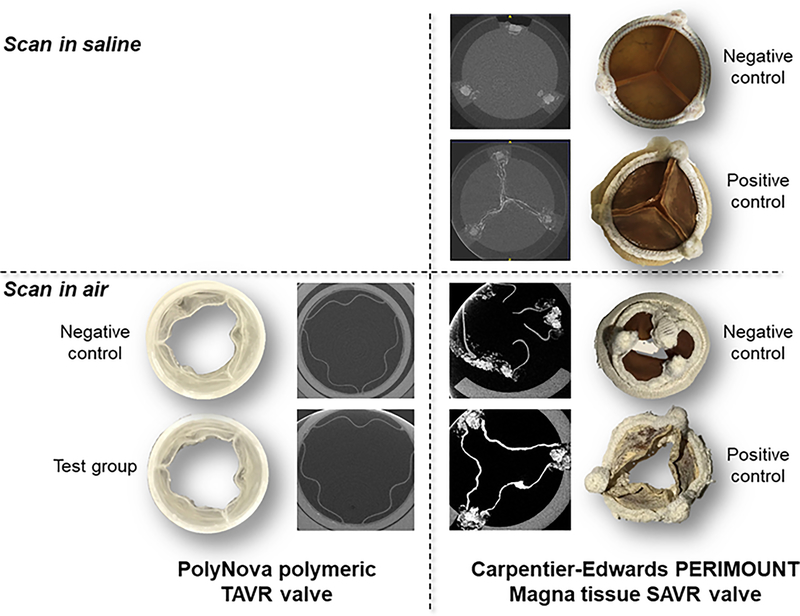
Following exposure to 50 million cycles in the AWT with pro-calcific compound, the valve samples have been removed and scanned in μCT. Both the polymeric valves and the tissue valves were scanned for comparison under dry conditions for getting enhanced contrast in the leaflets. Drying the tissue valves in air caused them to shrink and distort. It also revealed significant calcification all over the leaflets relative to the negative control sample, in contrast to the polymeric valves that did not show any mineralization to the naked eye (Figure 5). This qualitative observation was further supported by the high resolution μCT scan that clearly showed that the mineralization in the tissue valves penetrated into the bulk of the leaflets. In contrast, no difference was seen in the polymeric valves between the test samples and the negative control.
Figure 5.
The polymeric valves and tissue valves after 50M cycles in the pro-calcific compound. Top – saline wetted samples and matching μCT scans; Bottom – dried samples and matching μCT scans. The mineralization is clearer in the dried samples and spread all over the surface of the tissue leaflets, as well as in the bulk of the leaflet by the μCT. No evidence was seen in the polymeric valves.
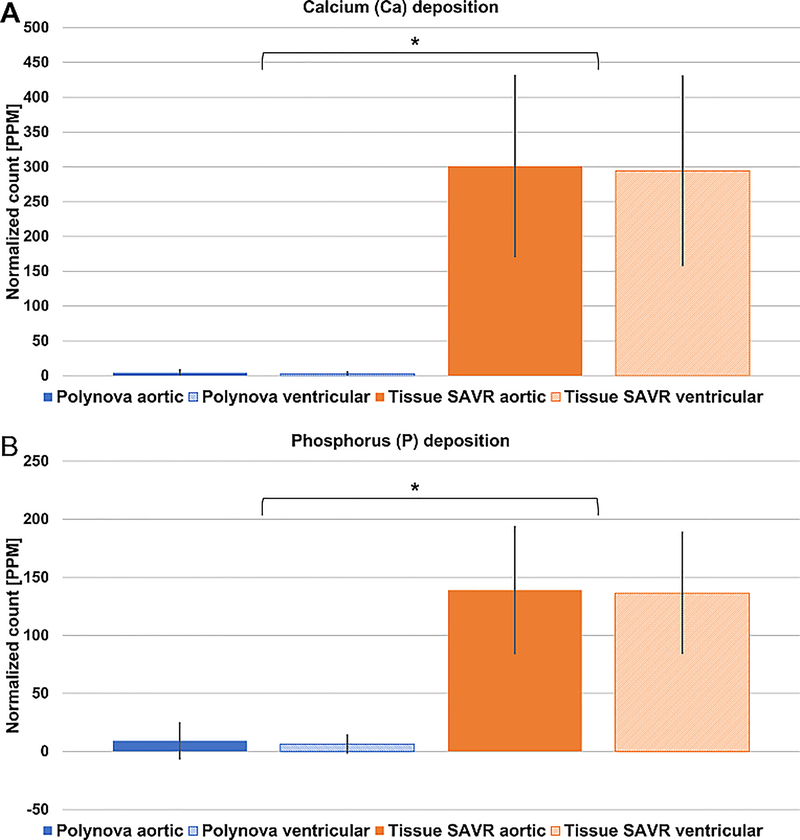
The Laser Ablation -Inductive Coupled Plasma Mass Spectroscopy (LA-ICP-MS) was used to confirm and quantify the accumulation of calcium and phosphorus on the leaflets’ surface (Figure 6). Per valve group, no difference was found between the ventricular and aortic sides of the leaflets, both for Calcium (Ca) or Phosphorus (P) (P>0.05). Between groups, the normalized accumulation of both Ca and P in the polymeric valves was negligible, and significantly lower than the tissue valves by a factor of 85 and 16, respectively (P<0.001).
Figure 6.
Laser Ablation -Inductive Coupled Plasma Mass Spectroscopy (LA-ICP-MS) quantification of (A) Calcium (Ca) and (B) Phosphorous (P) on the valves’ leaflets. The results were normalized by the counts of the negative controls of every valve group. Significant accumulation of Ca and P is seen in the tissue valves, while negligible levels accumulated onto the surface of the polymer valves. No difference was seen between the aortic and ventricular sides of the leaflets. * P<0.001.
3.3. Crimping stability
Stented polymeric valves were crimped to 16 Fr for 20 minutes or 8 days. Visual inspection of the valves after 20 minutes crimping, and additionally after 8 days of crimping, did not reveal any tear or damage to the valve- neither in the sleeve nor in the polymeric leaflets. Light longitudinal folding marks were evident in the valves crimped for 8 days. These were macro folding marks that were not evident in the SEM scans, nor were associated with any plastic deformation or superficial damage in the micro-scale (Figure 7).
Figure 7.
Top - SEM images of the polymeric valves leaflets before crimping, and after 20 minutes or 8 days of crimping to 16 Fr. On the left side, the scanned region of the leaflet is marked in red. Bottom – pictures the polymeric leaflets from the aortic side of the valve. The black arrows mark light folding marks that were evident in the leaflets after 8 days of crimping for 16 Fr. These marks were not visible in SEM in the micro-scale. SEM magnification is 500x, scale bar denotes 100 μm.
4. Discussion
In the present study, we performed stability testing of a novel polymeric TAVR valve. Despite the early promise of polymers to substitute tissue-based prosthetic aortic valves and their apparent advantages,14 no polymeric aortic valve to date was able to obtain approval for clinical use. Up until recently, only few polymeric aortic valves passed the 200M cycles durability requirement set by ISO 5840. This motivated us to complement the traditional AWT testing with two other specific in-vitro tests: calcification susceptibility, and crimping stability. The latter is a critical addition to transcatheter valve types as it may impair the long-term durability of the valve.
The PolyNova valves tested passed 400M cycles (double than the ISO requirement) without failure, and still running. The hydrodynamic performance remained stable throughout this accelerated wearability testing period, yet improvement was evident by reduction of the pressure gradient and the closing regurgitation fraction (Figure 4). These may indicate an improvement in the leaflet kinematics, e.g. faster opening and closing, which could possibly be attributed to priming of the leaflets. Importantly, while this positive priming effect may indicate a certain degree of fatigue, it was developing slow enough to easily pass the 200M cycles ISO 5840 durability standard set for TAVR valves, with no observable drop in the important cardiac parameters of EOA and regurgitation that may otherwise be attributed to a significant fatigue. Analyzing the trends of the hydrodynamic parameters over time reveals that the reduction of pressure gradient is attributed to the reduction in the regurgitation fraction; when the regurgitation volume becomes smaller (assume dV), the Stroke Volume (SV) needed for keeping a constant CO also becomes smaller by dV (see Supplementary Figure 1), hence the pressure gradient is practically measured over lower flow rate during systole. The EOA is positively dependent on the SV and negatively dependent on the pressure gradient (see Eq.1). Evidently, the reduction of both SV and the pressure gradient with time was balanced to keep the EOA stable.
For early indication on the valve’s calcification susceptibility, we adopted an in-vitro protocol29 that utilizes the AWT setup with a pro-calcific/phosphorus compound.30 While such in vitro testing cannot fully replicate calcification susceptibility in vivo, this is the only available in-vitro testing method and can clearly inform about calcification susceptibility of the valve. In-vivo animal tests could sometime reach endpoint of calcific degeneration, yet those are also subjected to some major limitations: (i) Chronic animal studies usually do not last more than a few months, while calcific degeneration in the clinical setting occurs after several years; (ii) The blood chemistry and immunologic activity of the animal (usually bovine or sheep) is different than that of humans; (iii) No AS animal model exist, meaning that the prosthetic valves are tested in healthy physiological environment. These differences may lead to a different process of calcification, which could be undetectable due to the relatively short duration of the test. The in-vitro protocol used in this study does not include active immunologic responses that could lead to calcification, yet does include the passive mechanisms of calcification, in which calcium minerals accumulate in cracks and accelerate valve failure, or deposition due to the chemistry of the polymer. Our results indicate on minimal deposition of calcium and phosphorus, which was significantly lower than that of clinically-used tissue-based valves. Importantly, based on the μCT scans the minor accumulation in the polymeric valves was only superficial, while in the tissue valves the calcific accumulation penetrated deep into the bulk of the leaflets. In that, the in-vitro calcification protocol indicates on clinically observed failure modes of TAVR and SAVR tissue valves, supporting the use of this in-vitro protocol.
It should be noted that all the valve samples were only lightly rinsed with saline after completion of the test, for keeping similarity in protocol between the tissue and polymeric samples. It is possible that the minor levels of Ca and P on the polymeric leaflets’ surface were not attributed to chemical bonding and that slightly stronger ‘cleaning’ of the valve samples would have reduced the counts to even a lower level.
Crimping is a major aspect of transcatheter valves deployment, which makes their design much more challenging than surgical valves. In recent years there is a growing evidence that bioprosthetic TAVR valves are subjected to irreversible mechanical damage during valve crimping3,4 and deployment.5,6 The damage to the leaflets is increased with lower crimping sizes,4 and with longer crimping durations.7 The concern of crimping duration restricts most TAVR devices today to be crimped onto the delivery catheter and immediately deployed at the procedure site (cath-lab) using trained personnel, with limited time between crimping to deployment (e.g. < 20 minute with the SAPIEN 3 at 14 Fr crimping size). Polymers, as an alternative material to tissue valves, may have better resistance to crimping damage.32 In this study we aimed to test the PolyNova polymeric TAVR valves’ resistance to crimping damage. We implemented this test using short-term crimping as currently performed in most bioprosthetic TAVR devices, additionally performing an extended long-term crimping (8 days), with consideration toward factory pre-crimping and pre-loading the valves in the delivery system. Post crimping, the valves were visually inspected using high magnification camera, and using SEM imaging. No tears were found in the polymer; not in the leaflets nor in the sleeve. No plastic deformations nor cracks/tears were found in the micro-scale by SEM. Light macro longitudinal folding marks were evident visually, yet those were not correlated with surface deformation or damage in the micro-scale. Not unlike wrinkled clothes, it is possible that these marks would fade away some time after deployment. This point was not verified in this study as the valves were cut right after deployment.
There are several limitations in this study that will require attention in future studies. The evaluation of the crimping stability cannot determine to what extent the crimping duration would affect the valve durability or hydrodynamic performance- it would necessitate an additional dedicated long term AWT comparative testing. We do not expect the sutures connecting the polymeric sleeve to the stent to affect the durability since those are not interfering with the leaflets. However, this aspect should be further verified in a future study. Following the successful testing of each of those tests independently, the next study should focus on the combined effect of crimping duration and durability (AWT).
The number of valve samples tested for durability evaluation in the AWT was relatively small. Therefore, conclusion about the valve durability should be carefully treated as proof of concept, that the PolyNova polymeric TAVR valve is able to surpass the 200M cycles minimum requirement set by ISO 5840. Larger sample size is required for reaching significant power.
The in-vitro calcification susceptibility testing performed under this study does not include all the calcification mechanisms that exist in-vivo. Chronic animal studies, as discussed above, also have significant limitations that might make calcific degeneration misleadingly go undetected. Nevertheless, the chronic animal studies are a critical step in prosthetic heart valve testing and should be performed to verify the findings of the present study, as well as other important aspects of efficacy and safety. If a strong correlation between in-vitro and in-vivo testing results will be established, such approach may at times obviate the need for animal studies- especially for devices that may qualify by the FDA as investigational devices under the humanitarian device exemption (HDE) category.
5. Conclusions
This in-vitro study shows very promising results of a novel polymeric valve and demonstrates its potential to be a viable substitute for tissue-based TAVR valves. Specifically, durability performance, calcification susceptibility, and long-term crimping capabilities were tested. Additional in-vitro and in-vivo studies are required for further assessing the valve’s performance under intended-use conditions.
Supplementary Material
Acknowledgements
This project was supported by NIH-NIBIB Quantum Award Phase II-C (DB), NHLBI STTR R41-HL134418 (DB), and the Center for Biotechnology: a New York State Center for Advanced Technology, New York State Department of Economic Development; and corporate support.
Key terms
- AS
Aortic stenosis
- AWT
Accelerate Wear Testing
- Ca
Calcium
- CO
Cardiac output
- EOA
Effective orifice area
- HR
Heart rate
- LA-ICP-MS
Laser Ablation -Inductive Coupled Plasma Mass Spectroscopy
- MAP
Mean arterial pressure
- P
Phosphorous
- PD
Pulse duplicator
- SEM
Scanning Electron Microscopy
- SV
Stroke Volume
- TAVR
Transcatheter aortic valve replacement
Footnotes
Conflict of interest: Author OMR is a consultant for PolyNova Cardiovascular Inc. Authors MJS and DB has stock ownership in PolyNova Cardiovascular Inc. Authors BK and MB declare that they have no conflicts of interest.
Disclaimer The device discussed in this study is investigational and has not yet been approved by the FDA for any purpose.
References
- 1.Cao C, Ang SC, Indraratna P, et al. : Systematic review and meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Annals of cardiothoracic surgery 2 (1): 10–23, 2013. doi: 10.3978/j.issn.2225-319X.2012.11.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones BM, Krishnaswamy A, Tuzcu EM, et al. : Matching patients with the ever-expanding range of TAVI devices. Nature reviews Cardiology 14 (10): 615–626, 2017. doi: 10.1038/nrcardio.2017.82. [DOI] [PubMed] [Google Scholar]
- 3.Khoffi F, Heim F: Mechanical degradation of biological heart valve tissue induced by low diameter crimping: an early assessment. J Mech Behav Biomed Mater 44: 71–5, 2015. doi: 10.1016/j.jmbbm.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Alavi SH, Groves EM, Kheradvar A: The effects of transcatheter valve crimping on pericardial leaflets. Ann Thorac Surg 97 (4): 1260–6, 2014. doi: 10.1016/j.athoracsur.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Convelbo C, El Hafci H, Petite H, Zegdi R: Traumatic leaflet injury: comparison of porcine leaflet self-expandable and bovine leaflet balloon-expandable prostheses. Eur J Cardiothorac Surg 53 (5): 1062–1067, 2018. doi: 10.1093/ejcts/ezx451. [DOI] [PubMed] [Google Scholar]
- 6.de Buhr W, Pfeifer S, Slotta-Huspenina J, Wintermantel E, Lutter G, Goetz WA: Impairment of pericardial leaflet structure from balloon-expanded valved stents. J Thorac Cardiovasc Surg 143 (6): 1417–21, 2012. doi: 10.1016/j.jtcvs.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Kiefer P, Gruenwald F, Kempfert J, et al. : Crimping may affect the durability of transcatheter valves: an experimental analysis. Ann Thorac Surg 92 (1): 155–60, 2011. doi: 10.1016/j.athoracsur.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Nakatani S: Subclinical leaflet thrombosis after transcatheter aortic valve implantation. Heart 103 (24): 1942–1946, 2017. doi: 10.1136/heartjnl-2017-311818. [DOI] [PubMed] [Google Scholar]
- 9.Arsalan M, Walther T: Durability of prostheses for transcatheter aortic valve implantation. Nature reviews Cardiology 13 (6): 360–7, 2016. doi: 10.1038/nrcardio.2016.43. [DOI] [PubMed] [Google Scholar]
- 10.Marwan M, Mekkhala N, Goller M, et al. : Leaflet thrombosis following transcatheter aortic valve implantation. J Cardiovasc Comput Tomogr 12 (1): 8–13, 2018. doi: 10.1016/j.jcct.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Vahidkhah K, Barakat M, Abbasi M, et al. : Valve thrombosis following transcatheter aortic valve replacement: significance of blood stasis on the leaflets. Eur J Cardiothorac Surg 51 (5): 927–935, 2017. doi: 10.1093/ejcts/ezw407. [DOI] [PubMed] [Google Scholar]
- 12.Kheradvar A, Groves EM, Dasi LP, et al. : Emerging trends in heart valve engineering: Part I. Solutions for future. Ann Biomed Eng 43 (4): 833–43, 2015. doi: 10.1007/s10439-014-1209-z. [DOI] [PubMed] [Google Scholar]
- 13.Bezuidenhout D, Williams DF, Zilla P: Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices. Biomaterials 36: 6–25, 2015. doi: 10.1016/j.biomaterials.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Claiborne TE, Slepian MJ, Hossainy S, Bluestein D: Polymeric trileaflet prosthetic heart valves: evolution and path to clinical reality. Expert Rev Med Devices 9 (6): 577–94, 2012. doi: 10.1586/erd.12.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prawel DA, Dean H, Forleo M, et al. : Hemocompatibility and Hemodynamics of Novel Hyaluronan-Polyethylene Materials for Flexible Heart Valve Leaflets. Cardiovasc Eng Technol 5 (1): 70–81, 2014. doi: 10.1007/s13239-013-0171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yousefi A, Bark DL, Dasi LP: Effect of Arched Leaflets and Stent Profile on the Hemodynamics of Tri-Leaflet Flexible Polymeric Heart Valves. Ann Biomed Eng, 2016. doi: 10.1007/s10439-016-1674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahmani B, Tzamtzis S, Sheridan R, et al. : In Vitro Hydrodynamic Assessment of a New Transcatheter Heart Valve Concept (the TRISKELE). J Cardiovasc Transl Res 10 (2): 104–115, 2017. doi: 10.1007/s12265-016-9722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dandeniyage LS, Adhikari R, Bown M, et al. : Morphology and surface properties of high strength siloxane poly(urethane-urea)s developed for heart valve application. J Biomed Mater Res B Appl Biomater, 2018. doi: 10.1002/jbm.b.34101. [DOI] [PubMed] [Google Scholar]
- 19.Dandeniyage LS, Gunatillake PA, Adhikari R, Bown M, Shanks R, Adhikari B: Development of high strength siloxane poly(urethane-urea) elastomers based on linked macrodiols for heart valve application. J Biomed Mater Res B Appl Biomater, 2017. doi: 10.1002/jbm.b.33970. [DOI] [PubMed] [Google Scholar]
- 20.Scherman J, Bezuidenhout D, Ofoegbu C, Williams DF, Zilla P: Tavi for low to middle income countries. European Heart Journal 38 (16): 1182–1184, 2017. doi: 10.1093/eurheartj/ehx169. [DOI] [Google Scholar]
- 21.Rotman OM, Kovarovic B, Chiu WC, et al. : Novel Polymeric Valve for Transcatheter Aortic Valve Replacement Applications: In Vitro Hemodynamic Study. Ann Biomed Eng, 2018. doi: 10.1007/s10439-018-02119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bluestein D, Einav S, Slepian MJ: Device thrombogenicity emulation: A novel methodology for optimizing the thromboresistance of cardiovascular devices. J Biomech, 2012. doi: S0021–9290(12)00685–9 [pii] 10.1016/j.jbiomech.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girdhar G, Xenos M, Alemu Y, et al. : Device Thrombogenicity Emulation: A Novel Method for Optimizing Mechanical Circulatory Support Device Thromboresistance. PLoS One 7 (3): e32463, 2012. doi: 10.1371/journal.pone.0032463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claiborne TE, Xenos M, Sheriff J, et al. : Toward optimization of a novel trileaflet polymeric prosthetic heart valve via device thrombogenicity emulation. ASAIO journal 59 (3): 275–83, 2013. doi: 10.1097/MAT.0b013e31828e4d80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh R, Marom G, Rotman O, et al. : Comparative Fluid-Structure Interaction Analysis of Polymeric Transcatheter and Surgical Aortic Valves’ Hemodynamics and Structural Mechanics. Journal of biomechanical engineering, 2018. doi: 10.1115/1.4040600. [DOI] [PubMed] [Google Scholar]
- 26.Claiborne TE, Sheriff J, Kuetting M, Steinseifer U, Slepian MJ, Bluestein D: In vitro evaluation of a novel hemodynamically optimized trileaflet polymeric prosthetic heart valve. Journal of biomechanical engineering 135 (2): 021021, 2013. doi: 10.1115/1.4023235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piatti F, Sturla F, Marom G, et al. : Hemodynamic and thrombogenic analysis of a trileaflet polymeric valve using a fluid-structure interaction approach. J Biomech, 2015. doi: 10.1016/j.jbiomech.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheriff J, Claiborne TE, Tran PL, et al. : Physical Characterization and Platelet Interactions under Shear Flows of a Novel Thermoset Polyisobutylene-based Co-polymer. ACS Appl Mater Interfaces 7 (39): 22058–66, 2015. doi: 10.1021/acsami.5b07254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boloori Zadeh P, Corbett SC, Nayeb-Hashemi H: In-vitro calcification study of polyurethane heart valves. Mater Sci Eng C Mater Biol Appl 35: 335–40, 2014. doi: 10.1016/j.msec.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Golomb G, Wagner D: Development of a new in vitro model for studying implantable polyurethane calcification. Biomaterials 12 (4): 397–405, 1991. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Gabella T, Voisine P, Puri R, Pibarot P, Rodes-Cabau J: Aortic Bioprosthetic Valve Durability: Incidence, Mechanisms, Predictors, and Management of Surgical and Transcatheter Valve Degeneration. J Am Coll Cardiol 70 (8): 1013–1028, 2017. doi: 10.1016/j.jacc.2017.07.715. [DOI] [PubMed] [Google Scholar]
- 32.Bianchi M, Ghosh RP, Marom G, Slepian MJ, Bluestein D: Simulation of Transcatheter Aortic Valve Replacement in patient-specific aortic roots: Effect of crimping and positioning on device performance, 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2015, pp 282–285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.