Abstract
Clinical findings show that a single subanesthetic dose of ketamine elicits rapid antidepressant effects. Accumulating data suggests that ketamine blocks the N-methyl-D-aspartate receptor and results in specific effects on intracellular signaling including increased brain-derived neurotrophic factor (BDNF) protein expression, which augments synaptic responses required for rapid antidepressant effects. To further investigate this potential mechanism for ketamine’s antidepressant action, we examined the effect of increasing ketamine doses on intracellular signaling, synaptic plasticity, and rapid antidepressant effects. Given that ketamine is often used at 2.5–10 mg/kg to examine antidepressant effects and 20–50 mg/kg to model schizophrenia, we compared effects at 5, 20 and 50 mg/kg. We found that intraperitoneal (i.p.) injection of low dose (5 mg/kg) ketamine produces rapid antidepressant effects, which were not observed at 20 or 50 mg/kg. At 5 mg/kg ketamine significantly increased the level of BDNF, a protein necessary for the rapid antidepressant effects, while 20 and 50 mg/kg ketamine did not alter BDNF levels in the hippocampus. Low concentration ketamine also evoked the highest synaptic potentiation in the hippocampal CA1, while higher concentrations significantly decreased the synaptic effects. Our results suggest low dose ketamine produces antidepressant effects and has independent behavioral and synaptic effects compared to higher doses of ketamine that are used to model schizophrenia. These findings strengthen our knowledge on specific signaling associated with ketamine’s rapid antidepressant effects.
Keywords: ketamine, dose-dependent, BDNF, synaptic plasticity, antidepressant, major depressive disorder
1. Introduction
Ketamine is a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist that is mainly used as an anesthetic but has other uses including for the treatment of depression [1]. Berman et al. demonstrated a single subanesthetic ketamine infusion (0.5 mg/kg) produces rapid antidepressant effects [2]. This result has been reproduced in subsequent clinical studies [3–9] and has triggered significant interest in the development of ketamine as a treatment for major depressive disorder.
Preclinical studies have postulated molecular mechanisms underlying the antidepressant effects of ketamine. The rapid antidepressant effects of ketamine are blocked by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonists, demonstrating a requirement for AMPA receptors [10–12]. Consistent with this finding, we have shown that ketamine produces synaptic potentiation in the CA1 hippocampal region by triggering the rapid translation of synaptic proteins, including brain-derived neurotrophic factor (BDNF), resulting in the insertion of AMPA receptors [11, 13]. Notably, the ketamine-induced potentiation and rapid antidepressant effects were not observed in mice in which Bdnf was deleted in broad forebrain areas [11, 13], suggesting BDNF is necessary to induce the augmented synaptic effects in the hippocampus and the rapid antidepressant effects. In separate work, the mammalian target of rapamycin (mTOR) and its downstream signaling molecule, p70S6 kinase (S6K), have also been indicated as target molecules for ketamine’s rapid antidepressant effects [14, 15]. Infusion into the prefrontal cortex of mice with rapamycin, an mTOR signaling inhibitor, prior to ketamine treatment was shown to prevent antidepressant effects suggesting a requirement for mTOR-dependent signaling changes [14, 15]. Inhibition of glycogen synthase kinase-3 (GSK3) has also been implicated in ketamine’s rapid antidepressant action. In these studies, ketamine increases phosphorylation at α (serine-21) and β subunits (serine-9) and, thereby, inhibits GSK3 activity [16, 17]. Moreover, the genetic ablation of these two phosphorylation sites abolished the ketamine-induced increase in AMPA receptor expression and rapid antidepressant responses, suggesting inhibition of GSK3 as another key target [16, 17].
A challenge to understand the mechanism of ketamine’s antidepressant action is that subanesthetic doses of ketamine also produce behavioral impairments that mimic symptoms of schizophrenic patients [18–20]. Subanesthetic ketamine is widely used to model schizophrenia in rodents [21, 22], although at a higher dose range (20–50 mg/kg) [23–27] than used for examining antidepressant effects (2.5–10 mg/kg) [10–12, 16, 28, 29]. A question that arises is whether the link between ketamine’s antidepressant action and the proposed mechanistic molecular and synaptic changes are also induced by these higher doses of ketamine that model symptoms of schizophrenia. We compared various subanesthetic doses of ketamine for antidepressant-like effects as well as for specific molecular and synaptic alterations. We found that low dose ketamine produces rapid antidepressant responses as well as key molecular and synaptic effects that were not observed at higher doses. These results suggest that low dose ketamine may have specific molecular and synaptic effects that are impaired by higher doses.
2. Materials and methods
2.1. Mice
C57BL/6J male adult mice (10–15 weeks old) were used for this study. Age-matched mice were randomly allocated for each dosage group. Animals were maintained on a 12h-light/12h-dark cycle, at ambient temperature (23 ± 3 °C) and humidity (50 ± 20%) with ad libitum access to chow pellets and water. All experiments were performed and scored by an observer that was blind to the drug treatment. All animal procedures were performed in accordance with the guidance for the care and use of laboratory animals and were approved by the institutional animal care and use committee at UT Southwestern Medical School and Vanderbilt University (APN No.2017–101831-G at UT Southwestern Medical Center, APN No. M1800164–00 at Vanderbilt University).
2.2. Ketamine treatment
For behavior and biochemistry experiments, ketamine-hydrochloride (Hospira or Zoetis) was freshly dissolved in 0.9% saline and intraperitoneally (i.p.) injected. For electrophysiology, ketamine-hydrochloride was diluted in artificial cerebrospinal fluid (ACSF, 124 mM NaCl, 5 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO3, 2 mM CaCl2, 2 mM MgCl2, and 10 mM D-glucose) and perfused into the recording chamber. All doses or concentrations of ketamine were calculated from the molecular weight of ketamine-hydrochloride. Different cohorts of mice were used for the respective analysis to avoid possible additive effects of multiple ketamine treatments.
2.3. Forced swim test (FST)
Prior to testing, mice were handled briefly each day for 2 weeks. On the day of testing, mice were acclimated to the behavioral room for 2 hrs and then received saline or a single dose of ketamine (i.p injection; 5, 20, or 50 mg/kg) 2 hrs prior to testing. Mice were placed in a 4 L Pyrex glass beaker containing 3 L of water (23–25°C) for a total of 6 min. Immobility was measured during the final 4 min of the test. All experiments were performed and scored by an observer that was blind to drug treatment. A mouse was considered immobile if it floated without deliberate motion in the water or showed only minor movement to balance its body or keep its head above water without struggling [30].
2.4. Brain preparation and western blot analysis
C57BL/6J mice were injected with saline or ketamine (5, 20, or 50 mg/kg, i.p.), sacrificed 30 minutes later, and hippocampal slices (~1 mm thick) were rapidly prepared using an ice-chilled matrix for the coronal dissection of the brain (Alto acrylic 1mm mouse brain coronal, AL-1175). The collected hippocampi were immediately snap-frozen and stored at −80°C. The collected hippocampi were lysed using RIPA buffer [50 mM Tris, pH 7.4, 1% Igepal® CA-630, 0.1% SDS, 0.5% Na deoxycholate, 4 mM EDTA, 150 mM NaCl, protease inhibitors (Roche), and phosphatase inhibitors (2 mM sodium orthovanadate, 50 mM sodium fluoride, and 10 mM sodium pyrophosphate)]. The total amount of protein was quantified by the bicinchoninic acid assay (Thermo Fisher Scientific). An equal amount of total protein was resolved by SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked for 1 hr at room temperature, washed three times with 0.1% TBS-Tween [0.1% (v/v) Tween 20, 500 mM NaCl, and 20 mM Tris-HCl with pH 7.5], and then incubated overnight at 4°C with the following primary antibodies: mature BDNF (mBDNF or BDNF, 1:1,500, Abcam, ab108319), phospho-S6K (Thr 389, 1:1000, Cell Signaling Technology, 9205S), S6K (1:5000, Cell Signaling Technology, 9202S), phospho-GSK3β (Ser 9, 1:5000, Cell Signaling Technology, 9323S), GSK3β (1:10,000, Cell Signaling Technology, 9315S), or GAPDH (1:100,000, Cell Signaling Technology, 2118S). After washing three more times with 0.1% TBS-Tween solution, blots were incubated with peroxidase-labeled secondary antibody (PI-1000, Vector Laboratories) at room temperature for 2 hrs. For blocking and incubating the blots, 5% skim milk in 0.1% TBS-Tween solution was used. The protein bands were detected using Clarity™ western ECL substrate (Biorad) and exposed to X-ray film.
2.5. Hippocampal slice field recording
Mice were briefly anesthetized for 2 min with isoflurane (Henry Schein Animal Health), brains were quickly removed, and hippocampal slices were prepared using a vibratome (VT 1000S, Leica) and placed in ice-cold dissection buffer (2.6 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO3, 0.5 mM CaCl2, 5 mM MgCl2, 212 mM sucrose, and 10 mM D-glucose). To record field excitatory postsynaptic potential (fEPSP) in the CA1 region, the CA3 region was surgically removed from each slice. The slices were transferred into a reservoir chamber filled with ACSF. Slices were incubated for 2–3 hrs at 30°C for recovery. The dissection buffer and ACSF were equilibrated with 95% O2 and 5% CO2.
For recording, slices were transferred to a submerged recording chamber that was maintained at 30°C and perfused continuously with ACSF at a rate of 3 ml/min. fEPSPs were recorded with extracellular recording electrodes filled with ACSF (resistance, 1–2 MΩ) and placed in the stratum radiatum of area CA1. fEPSPs were evoked by monophasic stimulation (duration, 200 μs) of Schaffer collateral/commissural afferents with a concentric bipolar microelectrode (FHC). Stable baseline responses were collected every 30 sec using a stimulus yielding 50–75% of the maximum peak. fEPSPs were filtered at 2 kHz and digitized at 10 kHz on a personal computer with a customized program (LabVIEW, National Instruments). The response was measured as the initial slope (10–40% of the rising phase) of the fEPSPs.
Ketamine-induced potentiation was measured as previously described with slight modifications [13]. After measuring the baseline response for 20 min, ketamine was infused at a rate of 18 ml/hr to a final concentration of 20, 50 or 100 μM for 30 min. After the ketamine infusion, a single stimulation was delivered and the response was recorded to document the initial ketamine-induced potentiation response. After a 50 min washout period, the stimulation was issued every 30 sec and the response recorded for 20 min to confirm the continuation of the ketamine potentiation. All responses were normalized to the baseline response. The magnitude of the potentiation was the averaged value of responses recorded during the last 20 min. The input-output (I-O) relationship was measured using stimulation intensities of 4, 8, 12, 16, 20, and 24 μA before and after ketamine treatment. To analyze the I-O curve, the initial slope of the obtained fEPSP was plotted as a function of presynaptic volley peak at a given stimulation intensity. Paired-pulse ratios (PPRs) were measured with interstimulus intervals (ISIs) of 20, 30, 50, 100, 200, 400, and 500 ms before and after ketamine treatment. PPR was calculated by the slope ratio of the second to the first response (P2/P1).
2.6. Statistics
Statistical analyses were performed using GraphPad Prism 8 software. Depending on experimental design and dataset, paired t-test, Wilcoxon test, or one-way analysis of variance (ANOVA) were performed. More specific statistical details are described in supplementary table. A p-value of < 0.05 was considered statistically significant in all experiments.
3. Results
3.1. Dose-dependent effects of ketamine on FST behavior
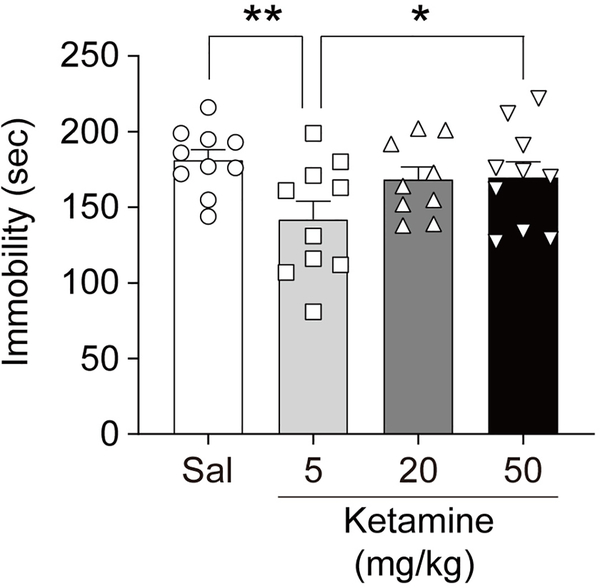
Based on previous studies [23–27, 29, 31, 32], we chose three subanesthetic dosages of ketamine, 5, 20 and 50 mg/kg. The 5 mg/kg ketamine is known to produce antidepressant-like drug effects in mice [29, 31, 32], while 20 and 50 mg/kg ketamine is used to model schizophrenia-related behaviors [23–27]. Ketamine is known to induce hyperlocomotion in a dose-dependent manner at subanesthetic doses, which usually dissipates within 2 hrs after i.p. injection [25, 28, 33]. Thus, to avoid any potential confounds of ketamine-induced hyperactivity, we tested the dose-dependent effects of ketamine 2 hrs after i.p. injection in the forced swim test (FST) (Figure 1). In agreement with previous data [29, 31, 32], 5 mg/kg ketamine treatment significantly reduced immobility suggestive of an antidepressant-like effect compared to saline treatment. In contrast, 20 and 50 mg/kg ketamine-treated groups did not alter immobility compared to the saline-treated control group.
Fig. 1: Dose-dependent effects of ketamine on antidepressant-like responses.
FST was performed 2 hr after ketamine treatment with three different dosages (5, 20, and 50 mg/kg). Ketamine significantly reduces immobility at 5 mg/kg, but not at 20 and 50 mg/kg, compared to saline-treated control (one-way ANOVA, F(3, 35) = 3.005, p = 0.0434, n = 9 ~ 10 per group). Graphs represent mean ± S.E.M., **, p < 0.05.
3.2. Ketamine doses differentially regulate intracellular signaling in the hippocampus
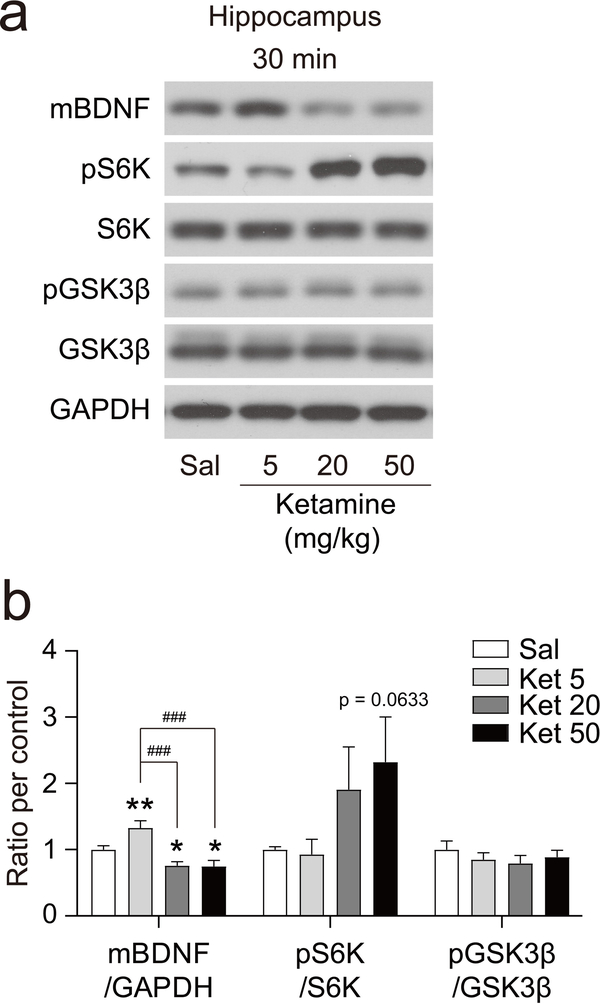
The rapid antidepressant effects of ketamine have been linked to several signaling molecules including BDNF [11, 33–36], phospho-S6K (pS6K, Thr389) [14, 15], and phospho-GSK3β (pGSK3β, Ser9) [16, 17]. To investigate whether the dose-dependent effect of ketamine on rapid antidepressant effects extends to intracellular signaling, we measured protein levels in the hippocampus, a brain region implicated in the antidepressant action of ketamine [11, 13, 16, 33]. Thirty minutes after i.p. injection of saline or ketamine, hippocampi were collected and protein levels were analyzed by Western blot analysis (Figure 2). BDNF protein expression was significantly increased by 5 mg/kg ketamine in agreement with previous data [11, 33]. By contrast, 20 and 50 mg/kg ketamine-treated groups did not increase BDNF protein levels but rather resulted in a trend towards decreased BDNF protein levels compared to the saline-treated group. Examination of phospho-S6K revealed no differences at 5 or 20 mg/kg ketamine compared to a saline-treated group, while 50 mg/kg ketamine showed a trend toward an increase that did not achieve statistical significance (p = 0.0633). The phospho-GSK3β levels also were not altered by 5, 20, or 50 mg/kg ketamine.
Fig. 2: Effect of different doses of ketamine on intracellular signaling.
Ketamine was i.p. injected into mice, 30 min later the animal was sacrificed and the hippocampus dissected out. (a) Representative Western blots. (b) Densitometric quantitation of blots. mBDNF was significantly increased at 5 mg/kg, while significantly decreased at 20 and 50 mg/kg of ketamine, compared to saline-treated control (one-way ANOVA, F(3, 27) = 10.67, p < 0.0001, n = 7 ~ 8 per group). pS6K/S6K showed a tendency toward an increase as a dose-dependent manner but did not achieve statistical significance compared to saline-treated mice (one-way ANOVA, F(3, 28) = 2.009, p = 0.1355, n = 8 per group). pGSK3β/GSK3β did not show any significant changes at 5, 20 or 50 mg/kg ketamine compared to saline-treated mice (one-way ANOVA, F(3, 28) = 0.5598, p = 0.6430, n = 8 per group). Graphs represent mean ± S.E.M., *, p < 0.05, ###, p < 0.001, * indicates comparison with saline control, # indicates comparison between indicated groups. Ket: ketamine
3.3. Concentration-dependent effects on ketamine-driven synaptic potentiation
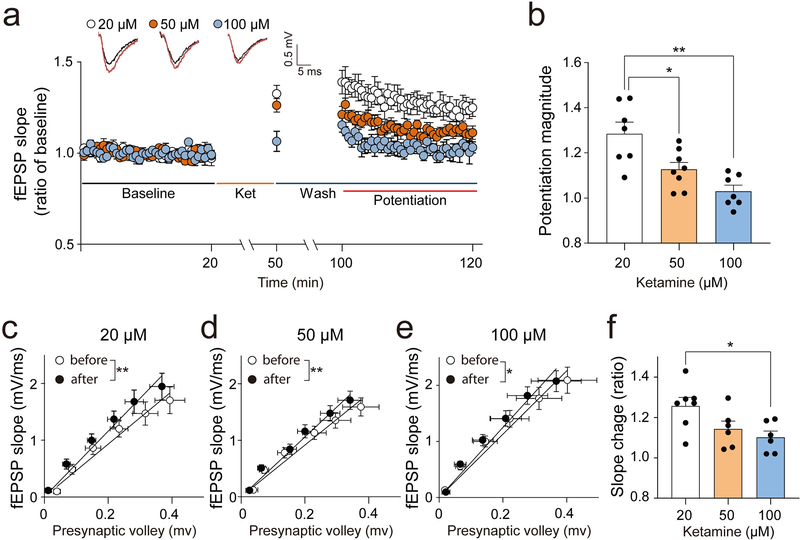
Our previous work identified ketamine-induced synaptic potentiation in the hippocampus, which is strongly correlated with antidepressant-like effects, as a possible mechanism for rapid antidepressant action [11, 13, 33]. We, therefore, examined whether ketamine-induced potentiation is dependent on the ketamine concentration (Figure 3). Ketamine blocks NMDA-receptor-induced fEPSP over 50% at 20 μM and abolishes it at 100 μM in the hippocampal CA1 region [37, 38]. In the CA1, ketamine blocks high-frequency- or theta-burst-stimulation-induced long-term-potentiation (LTP) mediated by activation of postsynaptic NMDA receptors with an IC50 value of 14.8 μM, and the LTP induction is totally blocked at 30 μM [39–41]. Based on these previous results, we chose 20, 50, and 100 μM of ketamine as 20 μM ketamine blocks about 60 −70% of postsynaptic NMDA receptors while 50 μM and 100 μM far exceed the concentration for blocking the postsynaptic NMDA receptors [37–41]. Low concentration (20 μM) ketamine perfused on hippocampal slices significantly increased synaptic responses in agreement with previous data [11, 13] (Figure 3a and b). The potentiation magnitude at 20 μM was the highest among the three concentrations examined (20, 50, and 100 μM). Moreover, the potentiation magnitudes at 50 and 100 μM ketamine were significantly lower than at 20 μM (Figure 3b). To examine the concentration-response relationship in basal neurotransmission, the slope change of the input-output (I-O) curve before and after ketamine-treatment was analyzed. Ketamine significantly increased the slope of the I-O curve at all tested concentrations (Figure 3c–e). However, 20 μM ketamine produced the biggest slope change while the ratio of slope change at 100 μM was significantly lower than at 20 μM (Figure 3f).
Fig. 3: Dose-dependent effects of ketamine-induced synaptic potentiation.
To examine the dose-response relationship in ketamine-driven postsynaptic potentiation, fEPSPs were recorded from the CA1 Schaffer-collateral pathway with three different concentrations of ketamine (20, 50 or 100 μM). After 20 min of stable baseline measurement, ketamine was applied for 30 min, then washed out for 50 min, followed by recordings of fEPSP responses for 20 min. (a, b) The highest potentiation magnitude was observed at 20 μM and significantly decreased at 50 and 100 μM (one-way ANOVA test, F(2, 17) = 9.721, p = 0.0015, n = 6 ~ 7 per group). (c-e) To confirm the change of basal neurotransmission by different concentrations of ketamine, the I-O curve relationship was measured before and after ketamine treatment. Ketamine significantly increased the slope of the IO-curve at all tested concentrations (paired t-test, 20 μM: t(6) = 5.359, p = 0.0017, 50 μM: t(5) = 4.505, p = 0.0064, 100 μM: t(5) = 3.049, p = 0.0285, n = 6 ~ 7 per group). (f) To confirm the dose-response relationship in the slope-change of the I-O curve, the ratio of the slope-change was analyzed. The biggest slope change was shown at 20 μM of ketamine. The ratio of slope change at 100 μM was significantly lower than at 20 μM of ketamine (one-way ANOVA test, F(2, 19) = 4.579, p = 0.0268, n = 6 ~ 7 per group). Graphs represent mean ± S.E.M., *, p < 0.05, **, p < 0.01
3.4. Ketamine does not affect presynaptic function
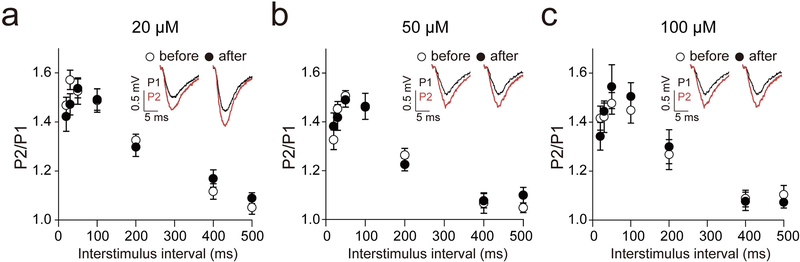
We previously showed that 20 μM ketamine does not affect presynaptic function [13]. However, it is unclear whether higher concentrations of ketamine impact presynaptic function. To test whether the suppressed potentiation observed with 50 and 100 μM ketamine is due to changes in presynaptic function, we measured PPRs before and after ketamine-treatment (Figure 4). We observed no differences in the PPRs at 20, 50 and 100 μM ketamine.
Fig. 4: Ketamine does not affect presynaptic functional change.
To examine the presynaptic functional change by ketamine, we measured PPRs before and after ketamine treatment. (a-c) Ketamine did not change the PPRs at 5, 20 or 50 mg/kg concentrations (paired t-test or Wilcoxon test, p > 0.05 in all tested interstimulus interval condition). Graphs represent mean ± S.E.M.
4. Discussion
In this study, we report that ketamine produces an antidepressant response at a low dose (5 mg/kg, i.p.) but not higher doses (20 and 50 mg/kg, i.p.). The 5 mg/kg ketamine also increased hippocampal BDNF expression, a critical component for ketamine action [11, 33–36], whereas 20 and 50 mg/kg ketamine did not. In the field recording experiment, 20 μM ketamine increased the magnitude of ketamine-induced potentiation and the slope change of the I-O curves greater than at higher concentrations (50 and 100 μM). Taken together, these results show that specific intracellular signaling and synaptic potentiation mechanisms associated with the rapid antidepressant response are activated by low dose ketamine but disrupted by higher dose ketamine used to model schizophrenia.
Numerous studies have shown that ketamine ranging from 2.5–10 mg/kg elicits a reproducible rapid antidepressant response in mice [10–12, 16, 28, 29, 31, 32] although the exact doses are variable depending on the strain, stress condition, and behavioral test [42]. Here, we observed that 5 mg/kg ketamine produced an antidepressant response in the FST. However, at higher doses, 20 or 50 mg/kg, there was no change in immobility. Our finding that higher doses of ketamine abolish antidepressant effects is in agreement with previous studies [14, 28, 43]. These data suggest that the rapid antidepressant effects of ketamine are mediated by mechanism activated within a specific dose range and interrupted by off-target effects at high doses. It is important to note that it is difficult to directly compare the ketamine doses used in preclinical studies with the clinically used dose range [0.5–1mg/kg intravenous infusion (i.v.) for 40 min [2, 3, 44–46]. However, given previous preclinical studies that BDNF is required for the rapid antidepressant effects of ketamine [11, 34, 36], which may have clinical relevance [35], it is plausible that this preclinical low dose range may have a connection to the clinical effects.
We found that hippocampal BDNF is increased by ketamine only at a low dose (5mg/kg) which produces antidepressant effects. We measured BDNF protein levels 30 min after ketamine treatment, a time point associated with rapid antidepressant effects. Previous work has shown the brain concentration of ketamine peaks at 10–15 min and then is rapidly eliminated within 2 hrs of ketamine injection in mice [28, 47]. In earlier work, we found that 3 mg/kg ketamine, in a different strain of mice, induced autophosphorylation of tropomyosin receptor kinase B (TrkB), the BDNF high-affinity receptor, in the hippocampus at 30 minutes after ketamine treatment suggesting that BDNF-TrkB signaling was engaged [11]. Another study reported that 10 mg/kg ketamine did not alter TrkB autophosphorylation in the medial prefrontal cortex, suggesting that BDNF-TrkB signaling may be differentially regulated by ketamine in specific brain regions [48]. In prior work, we demonstrated that the genetic deletion of BDNF or its receptor, TrkB, in postnatal forebrain regions resulted in an attenuated behavioral response to ketamine in the FST [11]. The abolished or attenuated antidepressant action of ketamine is also observed when intracellular-localization and activity-dependent release of BDNF is impaired by the BDNF val66met polymorphism [35, 36, 49]. These multiple lines of evidence strongly suggest that hippocampal BDNF is a critical mediator for rapid antidepressant effects.
In this study, we did not observe changes in pS6K expression at any of the ketamine doses examined. A number of papers suggested activation of mTOR and S6K as key regulators in rapid antidepressant action [14, 15, 50, 51]. One possible explanation for this discrepancy is that the importance of pS6K has been emphasized in the prefrontal cortex [14, 15], and may not be similar in the hippocampus. However, there have been several reports that have not observed ketamine-mediated changes in mTOR-S6K and its related pathway [11, 28, 52, 53] and have questioned the importance of mTOR-S6K pathway in ketamine action. A recent clinical study has shown that rapamycin does not prevent ketamine-mediated rapid antidepressant action, suggesting that activation of the mTOR-S6K pathway may not be important for the clinical effects [54]. Rapid spinogenesis and synaptogenesis via activation of the mTOR-S6K pathway have also been proposed as a key mechanism recapitulating the fast-acting antidepressant effects of ketamine [14]. However, a recent study demonstrated that ketamine’s antidepressant effects occur prior to new spine formation, suggesting structural changes in spines and synapses may not be a mediator for rapid antidepressant effects but for sustained antidepressant effects [55]. Collectively, these data suggest that activation of the mTOR-S6K pathway may not be critical in mediating rapid antidepressant effects.
In previous studies, pGSK3β was increased in the hippocampus following ketamine treatment [16, 17]. However, we did not see any significant change in the level of pGSK3β following any of the ketamine doses examined. A separate study reported increased pGSK3β levels following ketamine treatment but at 100 mg/kg [56], well outside the dose involved in the rapid antidepressant effects of ketamine. Co-treatment with lithium, a GSK3 inhibitor, potentiated rapid antidepressant effects by low dose ketamine (1 mg/kg) in rats [57], suggesting GSK3 signaling as a modulator rather than an inducer of rapid antidepressant effects. However, a recent clinical study reported that co-treatment with lithium does not affect the rapid efficacy and persistence of the antidepressant action of ketamine [58]. These results suggest a re-evaluation of the proposal that inhibition of GSK3 signaling is required for ketamine’s effects, although inhibition of GSK3 may be involved in the antidepressant effects of other drugs [59, 60].
In the current study, we did not see any changes in PPRs, suggesting presynaptic function in the hippocampal CA1 area is not affected by ketamine. Instead, we found ketamine-driven postsynaptic potentiation at 50 and 100 μM was significantly lower than at 20 μM, indicating that higher concentrations do not induce this synaptic augmentation. Previous studies have reproducibly demonstrated ketamine-driven synaptic potentiation at 20 μM ketamine in the CA1 region of control animals [11, 13, 61–63]. Given that 50 and 100 μM ketamine far exceed the concentration (30 μM) of blocking postsynaptic NMDA receptor activation in the CA1 region [39–41], these data suggest that the higher doses of ketamine may be triggering non-NMDA receptor-mediated effects that interfere with this postsynaptic NMDA receptor-driven potentiation [11, 13, 33, 63–65]. It is intriguing that previous work has shown a requirement for BDNF in ketamine-induced synaptic potentiation [11, 13] and that, in the current study, only the low dose of ketamine induces the rapid increase in BDNF protein and mediates the synaptic potentiation and rapid antidepressant effects. Of note, one study reported that 20 μM ketamine does not produce synaptic potentiation in the CA1 hippocampal field recording of rats prenatally exposed to dexamethasone [66]. However, prenatal exposure of dexamethasone may affect neural development, which may have complicated the experimental design in evaluating ketamine’s effect on synaptic potentiation.
Previous work from our laboratory has proposed that ketamine mediates rapid antidepressant effects by blocking the NMDA receptor at rest, resulting in inhibition of eukaryotic elongation factor 2 kinase (eEF2K), that desuppresses protein translation, including BDNF, resulting in the insertion of AMPA receptors and the synaptic potentiation in the hippocampal CA1 region [11, 13]. The results of the current study are consistent with the hypothesis and suggest that the doses of ketamine that mediate antidepressant effects exert specific signaling and synaptic effects that are not observed at higher doses of ketamine. Another proposed mechanism of ketamine action suggests that blockade of the NMDA receptor on inhibitory neurons in cortical regions leads to an increase in neuronal activity and glutamate release that produces antidepressant effects (also known as disinhibition) [14, 67–69]. However, one of the caveats of the disinhibition hypothesis is that the ketamine doses that increase glutamate release partly overlap with the doses producing schizophrenia-related symptoms. In preclinical studies, 10 to 30 mg/kg of ketamine increased extracellular glutamate levels [43, 68], and over 20 mg/kg produced schizophrenia-related symptoms [23–27], although results from the current study show doses over 20 mg/kg did not trigger the molecular, synaptic or behavioral effects associated with rapid antidepressant effects. Consistent with these preclinical findings, clinical studies have shown increased glutamate levels in the prefrontal cortex following ketamine treatment that correlates with schizophrenic symptoms [70, 71]. Additionally, pretreatment of lamotrigine, which suppresses glutamate release [72], did not block the antidepressant and mood-elevating effects of ketamine [73, 74]. Thus, these results suggest increased glutamate release may not be associated with the antidepressant effects of ketamine but rather with the schizophrenic symptoms as suggested previously [68, 69].
Clinically, it is unclear whether the antidepressant effects of ketamine are observed only within a specific dose range. The dose range of ketamine showing stable antidepressant effects is 0.5 mg/kg to 1 mg/kg, (i.v. infusion for 40 min) [2, 3, 44–46], while the dissociative symptoms are usually observed in the same dose range [44, 45], suggesting a possibile correlation between antidepressant and dissociative effects. However, some clinical studies have reported ketamine produces antidepressant effects at a lower dose range (0.1–0.2mg/kg, i.v., i.m., or s.c.) with minimal dissociative symptoms [75, 76]. Although some clinical studies have suggested dissociative symptoms predict antidepressant efficacy [77, 78], this correlation was not observed in other clinical studies [44, 79]. Of note, in a recent double-blind, placebo-controlled clinical study, several doses of ketamine (0.1, 0.2, 0.5, and 1mg/kg, i.v for 40 min) were tested and evaluated for a possible correlation between antidepressant effects and dissociative symptoms. However, the study did not find any significant correlation between these and argued that the dissociative symptoms may be an unblinding factor since patients can recognize when the drug has been administered [44]. Thus, these lines of evidence may indicate the antidepressant effects can be separated from dissociative effects in ketamine, although this possibility awaits further investigation.
5. Conclusion
Our findings show that low dose ketamine which triggers rapid antidepressant effects impacts key factors, rapidly increasing BDNF protein expression and inducing synaptic potentiation in the hippocampus, while these changes are not observed at higher doses. These results suggest the antidepressant effects of ketamine exert specific molecular and synaptic effects while higher doses impair this specificity. Further investigation of the pharmacological targets associated with hippocampal BDNF and synaptic potentiation will be fundamental to understanding the rapid antidepressant effects of ketamine.
Supplementary Material
Highlights.
Intraperitoneal injection (i.p.) of ketamine produces antidepressant effects at a low dose (5 mg/kg) but not higher doses (20 and 50 mg/kg).
Low dose (5 mg/kg, i.p.) ketamine rapidly increases BDNF expression in the hippocampus, while higher doses (20 and 50 mg/kg, i.p.) do not increase BDNF levels.
Perfusion of low concentration (20 μM) of ketamine on hippocampal slices induces postsynaptic potentiation in the hippocampal CA1 that is not observed with higher concentrations (50 and 100 μM).
Low dose ketamine which mediates rapid antidepressant responses may have specific molecular and synaptic effects that are impaired by higher doses.
Acknowledgments
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1A6A3A03008533, J.W.K) and a grant from the National Institute of Mental Health (MH070727, L.M.M.).
Footnotes
Declarations of interest: The authors declare no conflict of interest
References
- [1].Domino EF, Taming the ketamine tiger. 1965, Anesthesiology 113(3) (2010) 678–84. [DOI] [PubMed] [Google Scholar]
- [2].Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH, Antidepressant effects of ketamine in depressed patients, Biol Psychiatry 47(4) (2000) 351–4. [DOI] [PubMed] [Google Scholar]
- [3].Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression, Arch Gen Psychiatry 63(8) (2006) 856–64. [DOI] [PubMed] [Google Scholar]
- [4].Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr., A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression, Arch Gen Psychiatry 67(8) (2010) 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zarate CA Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA, Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial, Biol Psychiatry 71(11) (2012) 939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ, Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial, Am J Psychiatry 170(10) (2013) 1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV, Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression, Biol Psychiatry 74(4) (2013) 250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aan Het Rot M, Zarate CA Jr., Charney DS, Mathew SJ, Ketamine for depression: where do we go from here?, Biol Psychiatry 72(7) (2012) 537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, A.P.A.C.o.R.T.F.o.N. Biomarkers, Treatments, Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression, Am J Psychiatry 172(10) (2015) 950–66. [DOI] [PubMed] [Google Scholar]
- [10].Maeng S, Zarate CA Jr., Du J, Schloesser RJ, McCammon J, Chen G, Manji HK, Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors, Biol Psychiatry 63(4) (2008) 349–52. [DOI] [PubMed] [Google Scholar]
- [11].Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM, NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses, Nature 475(7354) (2011) 91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Koike H, Iijima M, Chaki S, Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression, Behav Brain Res 224(1) (2011) 107–11. [DOI] [PubMed] [Google Scholar]
- [13].Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET, Acute suppression of spontaneous neurotransmission drives synaptic potentiation, J Neurosci 33(16) (2013) 6990–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS, mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists, Science 329(5994) (2010) 959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dwyer JM, Maldonado-Aviles JG, Lepack AE, DiLeone RJ, Duman RS, Ribosomal protein S6 kinase 1 signaling in prefrontal cortex controls depressive behavior, Proc Natl Acad Sci U S A 112(19) (2015) 6188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Beurel E, Song L, Jope RS, Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice, Mol Psychiatry 16(11) (2011) 1068–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Beurel E, Grieco SF, Amadei C, Downey K, Jope RS, Ketamine-induced inhibition of glycogen synthase kinase-3 contributes to the augmentation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor signaling, Bipolar Disord 18(6) (2016) 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jentsch JD, Roth RH, The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia, Neuropsychopharmacology 20(3) (1999) 201–25. [DOI] [PubMed] [Google Scholar]
- [19].Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr., Charney DS, Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses, Arch Gen Psychiatry 51(3) (1994) 199–214. [DOI] [PubMed] [Google Scholar]
- [20].Mansbach RS, Geyer MA, Parametric determinants in pre-stimulus modification of acoustic startle: interaction with ketamine, Psychopharmacology (Berl) 105(2) (1991) 162–8. [DOI] [PubMed] [Google Scholar]
- [21].Javitt DC, Zukin SR, Recent advances in the phencyclidine model of schizophrenia, Am J Psychiatry 148(10) (1991) 1301–8. [DOI] [PubMed] [Google Scholar]
- [22].Olney JW, Newcomer JW, Farber NB, NMDA receptor hypofunction model of schizophrenia, J Psychiatr Res 33(6) (1999) 523–33. [DOI] [PubMed] [Google Scholar]
- [23].Goulart BK, de Lima MN, de Farias CB, Reolon GK, Almeida VR, Quevedo J, Kapczinski F, Schroder N, Roesler R, Ketamine impairs recognition memory consolidation and prevents learning-induced increase in hippocampal brain-derived neurotrophic factor levels, Neuroscience 167(4) (2010) 969–73. [DOI] [PubMed] [Google Scholar]
- [24].Kos T, Popik P, Pietraszek M, Schafer D, Danysz W, Dravolina O, Blokhina E, Galankin T, Bespalov AY, Effect of 5-HT3 receptor antagonist MDL 72222 on behaviors induced by ketamine in rats and mice, Eur Neuropsychopharmacol 16(4) (2006) 297–310. [DOI] [PubMed] [Google Scholar]
- [25].Irifune M, Shimizu T, Nomoto M, Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice, Pharmacol Biochem Behav 40(2) (1991) 399–407. [DOI] [PubMed] [Google Scholar]
- [26].Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ, Ketamine produces lasting disruptions in encoding of sensory stimuli, J Pharmacol Exp Ther 316(1) (2006) 315–24. [DOI] [PubMed] [Google Scholar]
- [27].Szlachta M, Pabian P, Kusmider M, Solich J, Kolasa M, Zurawek D, Dziedzicka-Wasylewska M, Faron-Gorecka A, Effect of clozapine on ketamine-induced deficits in attentional set shift task in mice, Psychopharmacology (Berl) 234(14) (2017) 2103–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., Gould TD, NMDAR inhibition-independent antidepressant actions of ketamine metabolites, Nature 533(7604) (2016) 481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM, Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress, Neuroscience 290 (2015) 49–60. [DOI] [PubMed] [Google Scholar]
- [30].Porsolt RD, Le Pichon M, Jalfre M, Depression: a new animal model sensitive to antidepressant treatments, Nature 266(5604) (1977) 730–2. [DOI] [PubMed] [Google Scholar]
- [31].Yang Y, Ju W, Zhang H, Sun L, Effect of Ketamine on LTP and NMDAR EPSC in Hippocampus of the Chronic Social Defeat Stress Mice Model of Depression, Front Behav Neurosci 12 (2018) 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Adaikkan C, Taha E, Barrera I, David O, Rosenblum K, Calcium/Calmodulin-Dependent Protein Kinase II and Eukaryotic Elongation Factor 2 Kinase Pathways Mediate the Antidepressant Action of Ketamine, Biol Psychiatry 84(1) (2018) 65–75. [DOI] [PubMed] [Google Scholar]
- [33].Gideons ES, Kavalali ET, Monteggia LM, Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses, Proc Natl Acad Sci U S A 111(23) (2014) 8649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS, BDNF release is required for the behavioral actions of ketamine, Int J Neuropsychopharmacol 18(1) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, Zarate C Jr., Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients, Biol Psychiatry 72(11) (2012) e27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK, Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex, Biol Psychiatry 71(11) (2012) 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Izumi Y, Zorumski CF, Metaplastic effects of subanesthetic ketamine on CA1 hippocampal function, Neuropharmacology 86 (2014) 273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kang H, Park P, Bortolotto ZA, Brandt SD, Colestock T, Wallach J, Collingridge GL, Lodge D, Ephenidine: A new psychoactive agent with ketamine-like NMDA receptor antagonist properties, Neuropharmacology 112(Pt A) (2017) 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ingram R, Kang H, Lightman S, Jane DE, Bortolotto ZA, Collingridge GL, Lodge D, Volianskis A, Some distorted thoughts about ketamine as a psychedelic and a novel hypothesis based on NMDA receptor-mediated synaptic plasticity, Neuropharmacology 142 (2018) 30–40. [DOI] [PubMed] [Google Scholar]
- [40].Ribeiro PO, Tome AR, Silva HB, Cunha RA, Antunes LM, Clinically relevant concentrations of ketamine mainly affect long-term potentiation rather than basal excitatory synaptic transmission and do not change paired-pulse facilitation in mouse hippocampal slices, Brain Res 1560 (2014) 10–7. [DOI] [PubMed] [Google Scholar]
- [41].Huang L, Yang XJ, Huang Y, Sun EY, Sun M, Ketamine Protects Gamma Oscillations by Inhibiting Hippocampal LTD, PLoS One 11(7) (2016) e0159192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Polis AJ, Fitzgerald PJ, Hale PJ, Watson BO, Rodent ketamine depression-related research: Finding patterns in a literature of variability, Behav Brain Res 376 (2019) 112153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, Bristow L, Schaeffer E, Duman RS, Rothman DL, Behar KL, Sanacora G, Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects, Mol Psychiatry 22(1) (2017) 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI, Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD), Mol Psychiatry (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr., Gould TD, Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms, Pharmacol Rev 70(3) (2018) 621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xu Y, Hackett M, Carter G, Loo C, Galvez V, Glozier N, Glue P, Lapidus K, McGirr A, Somogyi AA, Mitchell PB, Rodgers A, Effects of Low-Dose and Very Low-Dose Ketamine among Patients with Major Depression: a Systematic Review and Meta-Analysis, Int J Neuropsychopharmacol 19(4) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lord B, Wintmolders C, Langlois X, Nguyen L, Lovenberg T, Bonaventure P, Comparison of the ex vivo receptor occupancy profile of ketamine to several NMDA receptor antagonists in mouse hippocampus, Eur J Pharmacol 715(1–3) (2013) 21–5. [DOI] [PubMed] [Google Scholar]
- [48].Kohtala S, Theilmann W, Rosenholm M, Muller HK, Kiuru P, Wegener G, Yli-Kauhaluoma J, Rantamaki T, Ketamine-induced regulation of TrkB-GSK3beta signaling is accompanied by slow EEG oscillations and sedation but is independent of hydroxynorketamine metabolites, Neuropharmacology 157 (2019) 107684. [DOI] [PubMed] [Google Scholar]
- [49].Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR, The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function, Cell 112(2) (2003) 257–69. [DOI] [PubMed] [Google Scholar]
- [50].Harraz MM, Tyagi R, Cortes P, Snyder SH, Antidepressant action of ketamine via mTOR is mediated by inhibition of nitrergic Rheb degradation, Mol Psychiatry 21(3) (2016) 313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Duman RS, Aghajanian GK, Sanacora G, Krystal JH, Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants, Nat Med 22(3) (2016) 238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ma Z, Zang T, Birnbaum SG, Wang Z, Johnson JE, Zhang CL, Parada LF, TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response, Nat Commun 8(1) (2017) 1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Popp S, Behl B, Joshi J, Lanz T, Spedding M, Schenker E, Jay T, Svenningsson P, Caudal D, Cunningham J, Deaver D, Bespalov A, In search of the mechanisms of ketamine?s antidepressant effects: How robust is the evidence behind the mTor activation hypothesis [version 1; peer review: 1 approved, 1 approved with reservations], F1000Research 5(634) (2016). [Google Scholar]
- [54].Abdallah CG, Averill LA, Gueorguieva R, Goktas S, Purohit P, Ranganathan M, D’Souza DC, Formica R, Southwick SM, Duman RS, Sanacora G, Krystal JH, Rapamycin, an Immunosuppressant and mTORC1 Inhibitor, Triples the Antidepressant Response Rate of Ketamine at 2 Weeks Following Treatment: A double-blind, placebo-controlled, cross-over, randomized clinical trial, bioRxiv (2018) 500959. [Google Scholar]
- [55].Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, Witztum J, Shaver DC, Rosenthal DL, Alway EJ, Lopez K, Meng Y, Nellissen L, Grosenick L, Milner TA, Deisseroth K, Bito H, Kasai H, Liston C, Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation, Science 364(6436) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kohtala S, Theilmann W, Suomi T, Wigren HK, Porkka-Heiskanen T, Elo LL, Rokka A, Rantamaki T, Brief Isoflurane Anesthesia Produces Prominent Phosphoproteomic Changes in the Adult Mouse Hippocampus, ACS Chem Neurosci 7(6) (2016) 749–56. [DOI] [PubMed] [Google Scholar]
- [57].Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK, GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine, Neuropsychopharmacology 38(11) (2013) 2268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Costi S, Soleimani L, Glasgow A, Brallier J, Spivack J, Schwartz J, Levitch CF, Richards S, Hoch M, Wade E, Welch A, Collins KA, Feder A, Iosifescu DV, Charney DS, Murrough JW, Lithium continuation therapy following ketamine in patients with treatment resistant unipolar depression: a randomized controlled trial, Neuropsychopharmacology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Antila H, Ryazantseva M, Popova D, Sipila P, Guirado R, Kohtala S, Yalcin I, Lindholm J, Vesa L, Sato V, Cordeira J, Autio H, Kislin M, Rios M, Joca S, Casarotto P, Khiroug L, Lauri S, Taira T, Castren E, Rantamaki T, Isoflurane produces antidepressant effects and induces TrkB signaling in rodents, Sci Rep 7(1) (2017) 7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Quiroz JA, Machado-Vieira R, Zarate CA Jr., Manji HK, Novel insights into lithium’s mechanism of action: neurotrophic and neuroprotective effects, Neuropsychobiology 62(1) (2010) 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhang K, Xu T, Yuan Z, Wei Z, Yamaki VN, Huang M, Huganir RL, Cai X, Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine, Sci Signal 9(458) (2016) ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Crawford DC, Ramirez DM, Trauterman B, Monteggia LM, Kavalali ET, Selective molecular impairment of spontaneous neurotransmission modulates synaptic efficacy, Nat Commun 8 (2017) 14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nosyreva E, Autry AE, Kavalali ET, Monteggia LM, Age dependence of the rapid antidepressant and synaptic effects of acute NMDA receptor blockade, Front Mol Neurosci 7 (2014) 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM, Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis, Cell 125(4) (2006) 785–99. [DOI] [PubMed] [Google Scholar]
- [65].Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM, Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis, Neuron 55(4) (2007) 648–61. [DOI] [PubMed] [Google Scholar]
- [66].Michaelsson H, Andersson M, Svensson J, Karlsson L, Ehn J, Culley G, Engstrom A, Bergstrom N, Savvidi P, Kuhn HG, Hanse E, Seth H, The novel antidepressant ketamine enhances dentate gyrus proliferation with no effects on synaptic plasticity or hippocampal function in depressive-like rats, Acta Physiol (Oxf) 225(4) (2019) e13211. [DOI] [PubMed] [Google Scholar]
- [67].Duman RS, Li N, Liu RJ, Duric V, Aghajanian G, Signaling pathways underlying the rapid antidepressant actions of ketamine, Neuropharmacology 62(1) (2012) 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Moghaddam B, Adams B, Verma A, Daly D, Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex, J Neurosci 17(8) (1997) 2921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Homayoun H, Moghaddam B, NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons, J Neurosci 27(43) (2007) 11496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, Krystal JH, Nutt D, Barker GJ, Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology, Mol Psychiatry 17(7) (2012) 664–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Abdallah CG, De Feyter HM, Averill LA, Jiang L, Averill CL, Chowdhury GMI, Purohit P, de Graaf RA, Esterlis I, Juchem C, Pittman BP, Krystal JH, Rothman DL, Sanacora G, Mason GF, The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects, Neuropsychopharmacology 43(10) (2018) 2154–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Leach MJ, Marden CM, Miller AA, Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action, Epilepsia 27(5) (1986) 490–7. [DOI] [PubMed] [Google Scholar]
- [73].Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH, Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists, Arch Gen Psychiatry 57(3) (2000) 270–6. [DOI] [PubMed] [Google Scholar]
- [74].Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS, Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial, Int J Neuropsychopharmacol 13(1) (2010) 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lai R, Katalinic N, Glue P, Somogyi AA, Mitchell PB, Leyden J, Harper S, Loo CK, Pilot dose-response trial of i.v. ketamine in treatment-resistant depression, World J Biol Psychiatry 15(7) (2014) 579–84. [DOI] [PubMed] [Google Scholar]
- [76].Loo CK, Galvez V, O’Keefe E, Mitchell PB, Hadzi-Pavlovic D, Leyden J, Harper S, Somogyi AA, Lai R, Weickert CS, Glue P, Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression, Acta Psychiatr Scand 134(1) (2016) 48–56. [DOI] [PubMed] [Google Scholar]
- [77].Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA, Do the dissociative side effects of ketamine mediate its antidepressant effects?, J Affect Disord 159 (2014) 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Niciu MJ, Shovestul BJ, Jaso BA, Farmer C, Luckenbaugh DA, Brutsche NE, Park LT, Ballard ED, Zarate CA Jr., Features of dissociation differentially predict antidepressant response to ketamine in treatment-resistant depression, J Affect Disord 232 (2018) 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G, The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS, Psychiatry Res 191(2) (2011) 122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.