Abstract
Objective
To determine the association between traumatic brain injury (TBI) and any clinically diagnosed α-synucleinopathy including Parkinson disease (PD), dementia with Lewy bodies (DLB), PD dementia (PDD), and multiple system atrophy (MSA).
Methods
Using the medical records-linkage system of the Rochester Epidemiology Project, we identified incident cases of α-synucleinopathies in Olmsted County, Minnesota, from 1991 to 2010, matching by age (±1 year) at symptom onset and sex to controls. We reviewed records of cases and controls to detect TBI prior to clinical-motor onset of any α-synucleinopathies. We based severity (possible, probable, and definite) upon the Mayo Classification System for TBI Severity. Using conditional-logistic regression, we calculated the odds ratio (OR) of all α-synucleinopathies and type, adjusting for coffee intake and smoking.
Results
TBI frequency was lower among cases (7.0%) than controls (8.2%). No association was found between TBI and all α-synucleinopathies in multivariable analyses (OR 0.90, 95% confidence interval [CI] 0.54–1.52). No association presented when examining the number of TBIs, TBI severity, time between TBI exposure and index date, age at index date, or sex. When stratifying by each individual α-synucleinopathy, we did not identify any associations between TBI and PD, DLB, or PDD. Among the MSA group, 1 (6.4%) and 0 controls experienced a TBI (OR could not be estimated).
Conclusions
In this nested case-control population-based analysis, TBI was not associated with subsequent α-synucleinopathies in general or any individual α-synucleinopathy. This did not change based on the temporality or the severity of the TBI. Our findings may be limited by the study power.
Traumatic brain injury (TBI) is an acute event due to an external force, which may result in initial confusion, loss of consciousness, or coma. Several conflicting studies support and oppose the association between TBI and Parkinson disease (PD).1–11 One reason for the varied results could be that the cited studies gathered TBI data by interviewing individuals with established PD, risking recall bias and leading to an erroneous difference between cases and controls that inflate the odds ratios (ORs). In addition, these studies were not population-based, risking referral bias. Bower et al.,12 in contrast, utilized a population-based, case-control study to assess the association between TBI and PD in Olmsted County, Minnesota. By abstracting TBI data strictly from the medical records, Bower et al.12 demonstrated a significant association between TBI and the development of PD. Based on these findings, the authors proposed 2 hypotheses. (1) Head trauma may be associated with PD by reducing the number of neurons in the substantia nigra. (2) Head trauma could lead to inflammatory damage or overexpression of α-synuclein, which could initiate degeneration and, eventually, PD.
Based on the second hypothesis, we aimed to assess the association between α-synuclein and head trauma by also assessing other α-synucleinopathies including PD dementia (PDD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA). Although several studies have assessed the relationship between TBI and PD, fewer have examined the relationship between TBI and other α-synucleinopathies. Understanding the association between TBI and all the α-synucleinopathies may determine if the aforementioned hypothesis is true and provide additional insight into exposures that may make an individual prone to developing one α-synucleinopathy over another.
We utilized a population-based, age- and sex-matched nested case-control study to assess the association between TBI and the odds of developing any clinically diagnosed α-synucleinopathy, including PD, PDD, and the parkinsonian variants of dementia with Lewy bodies (DLB) and multiple system atrophy (MSA).
Methods
Our methods for case and control identification are described elsewhere.13 Briefly, we used the medical-records linkage system of the Rochester Epidemiology Project (REP) to identify residents of Olmsted County, Minnesota, who were diagnosed with parkinsonism due to α-synucleinopathy between 1991 and 2010. Details on case identification are reported elsewhere.13–15 The REP indexes all medical information of individuals residing and receiving care in Olmsted County, Minnesota.16–19 All medical diagnoses, surgical interventions, and procedures are indexed into the computerized system using the Hospital Adaptation of the ICD-8 (H-ICDA)20 and the ICD-9.21 Our diagnostic criteria for parkinsonism included 2 steps: defining parkinsonism as a syndrome and defining the type of parkinsonism within that syndrome. We defined parkinsonism as having at least 2 of the 4 following cardinal signs: rest tremor, bradykinesia, impaired postural reflexes, and rigidity.
We defined PD as parkinsonism that satisfied the 3 following criteria: (1) no other cause (e.g., repeated stroke with stepwise progression, repeated head injury [to exclude parkinsonism that was a side effect of multiple trauma rather than a direct symptom of PD], history of encephalitis, neuroleptic treatment within 6 months before onset, hydrocephalus, brain tumor); (2) no documented unresponsiveness to levodopa at doses of at least 1 g/d in combination with carbidopa (applied only to treated patients); (3) no prominent or early signs of extensive nervous system involvement (e.g., dysautonomia) otherwise unexplained.22 Of note, early extensive dysautonomia excluded individuals from being diagnosed with PD, but this did not exclude them from being diagnosed with any of the other α-synucleinopathies. Previously published consensus criteria were used to diagnose DLB, PDD, and MSA.14,15,23,24 Any individuals who had parkinsonism but did not meet the additional criteria for the other α-synucleinopathies were placed in other categories (e.g., drug-induced parkinsonism, vascular parkinsonism, corticobasal degeneration, progressive supranuclear palsy, other), which were not included in this study. All of the cases were reviewed and the clinical diagnoses were finalized by a movement disorders specialist (R.S.). The index date was defined as the year in which a cardinal sign was first described by the patient, family member, or care provider in the medical record. Our case-finding procedure has proven to be reliable.13,15 All cases of α-synucleinopathies in this study included patients PD, DLB, PDD, and MSA.
Controls
Cases were matched by sex and age (±1 year) to a referent living in Olmsted County, Minnesota, who was free of parkinsonism prior to the index date (year of onset of the parkinsonism). Matched controls with other neurologic diseases were not excluded from the study. The REP provided a list of all county residents from whom matched controls were randomly drawn. A nurse abstractor reviewed the potential controls and marked any who had a possible diagnosis of parkinsonism. These marked controls were reviewed by a neurologist (R.S.) to exclude the diagnosis of any type of parkinsonism before the index date of the matched case. Any individuals with a diagnosis on, before, or after the index date were included as a case and replaced with a randomly drawn referent individual from the general population.
Ascertainment of TBI
TBI was defined as a traumatically induced, physiologic disruption of brain function. Individuals were identified by a nurse abstractor if they had a diagnostic code in the medical records suggestive of a potential head injury. The diagnostic codes used in this study were recommended by the Centers for Disease Control and Prevention (CDC) (table 1) and Leibson et al.25 and Faul et al.26 A medical student (S.H.) blinded to the case/control assignment reviewed the medical records for the dates of the specific codes and determined whether a TBI occurred and its severity. The information was abstracted from the first available record up to the index date.
Table 1.
ICD-9-CM codes for traumatic brain injury–related emergency department visits and hospitalizations as recommended by Centers for Disease Control and Prevention21,25,26
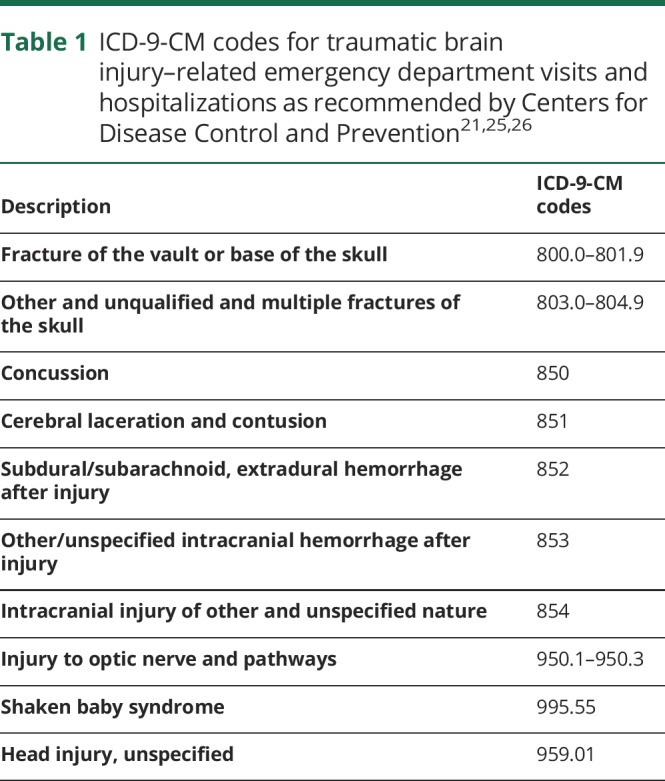
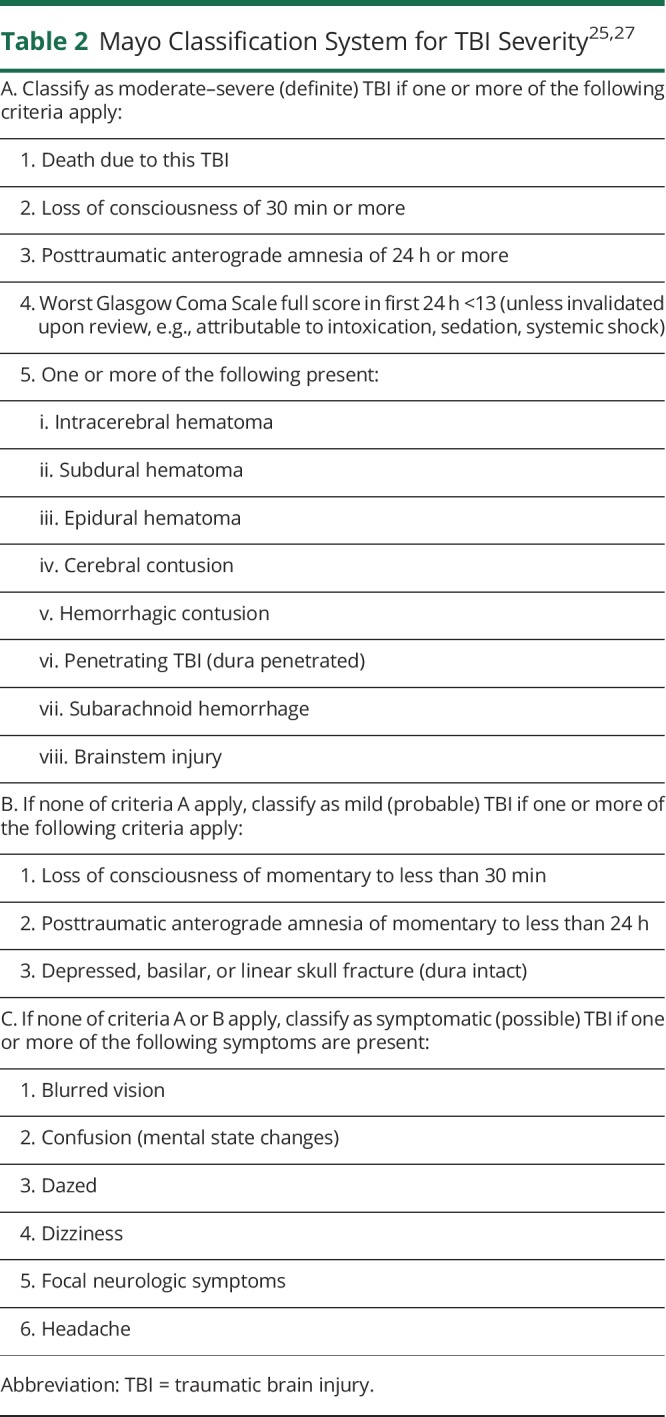
Each TBI was placed into one of the 3 categories of the Mayo Classification System for TBI Severity (definite, probable, and possible) (table 2).25,27 By this definition, definite had to have at least 1 of the following: loss of conscious of ≥30 minutes, posttraumatic anterograde amnesia of ≥24 hours, intracerebral hematoma, subdural hematoma, epidural hematoma, cerebral contusion, hemorrhagic contusion, penetrating TBI (dura penetrated), or subarachnoid hemorrhage; probable had to have at least 1 of the following: loss of consciousness of momentary to <30 minutes, posttraumatic anterograde amnesia of momentary to <24 hours, or depressed, basilar, or linear skull fracture; possible had to have at least 2 of the following: blurred vision, confusion (mental state changes), dazed, dizziness, focal neurologic symptoms, headache, or nausea. If none of the preceding criteria applied to an event, that event was not considered a TBI.
Table 2.
The mechanism of the TBI was classified into 6 categories: fall, motor vehicle accident, hit by an object, assault/gunshot, sports/recreation, and other.
Data analysis
We used conditional logistic regression to conduct matched-pairs analyses. We calculated the OR, 95% confidence interval (CI), and a p value (2-tailed test, α = 0.05) of having a TBI among the cases compared to the controls. We also separately analyzed for >1 TBI and severity of TBI (possible, probable, and definite). For TBI severity, individuals with no TBI served as the reference category. To account for the lag time between the TBI and index date, we compared the distribution (median and interquartile range [IQR]) of lag times between cases and controls using the Wilcoxon rank sum test. We also calculated the OR of experiencing a TBI within or more than 5 years from the index date. To account for age at the time of TBI, we calculated the OR of having a TBI above or below the median age at index date. We also stratified by sex and the type of α-synucleinopathy. For the multivariate model, we adjusted for smoking (ever vs never) and coffee consumption (ever vs never) as these have been shown to be associated with PD.28,29 Smoking and coffee consumption data were obtained manually from the available intake questionnaires that are distributed at each internal medicine visit, the first visit to Mayo Clinic, or wherever reported in the medical records. For the case and control characteristics, we calculated for any difference in distributions and proportions between cases and controls using the χ2 test as appropriate. All statistical analyses were performed using JMP version 14.0.0 and R version 3.4.2.
Standard protocol approvals, registration, and patient consent
The study was approved by the institutional review boards of Mayo Clinic and Olmsted Medical Center. Written informed consent was not required for passive medical record review, but all participants had provided consent for the use of their medical records for research.
Data availability
All the relevant data have been shared and published in this article; data regarding case ascertainment of parkinsonism and methodology on case identification have been published previously.15
Results
We identified 464 patients who developed parkinsonism clinically attributable to an α-synucleinopathy from 1991 to 2010, and 464 age-matched (±1 year) and sex-matched controls. However, 5 patients did not authorize the use of their medical records for research, and their corresponding controls could not be studied. Therefore, we included 459 individuals whose parkinsonism was presumably due to α-synucleinopathy between 1991 and 2010 and 459 age-matched (±1 year) and sex-matched controls.
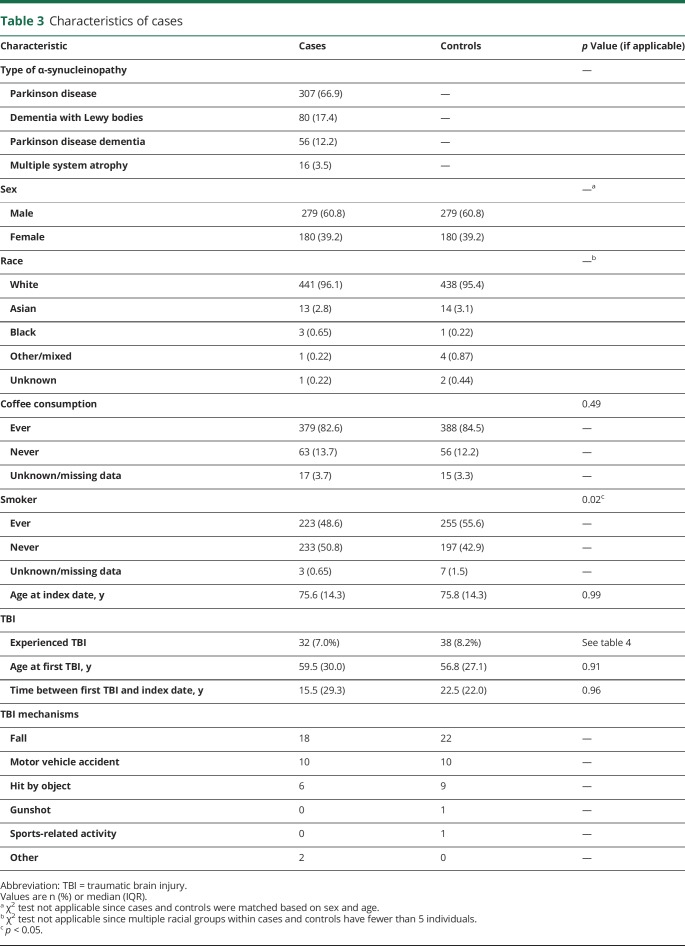
Table 3 demonstrates the characteristics of the cases and controls. Of the 459 cases, 307 (66.9%) had a diagnosis of PD, 80 (17.4%) had DLB, 56 (12.2%) had PDD, and 16 (3.5%) had MSA (table 3). Among the cases, 279 (60.8%) were men and 180 (39.2%) were women; 441 (96.1%) individuals were white, 13 (2.8%) were Asian, 3 (0.65%) were black, 1 (0.22%) was other/mixed, and 1 (0.22%) was unknown. Among the controls, 438 (95.4%) individuals were white, 14 (3.1%) were Asian, 1 (0.22%) was black, 4 (0.87%) were other/mixed, and 2 (0.44%) were unknown. The χ2 could not be calculated because certain groups were less than 5. Of note, 12 controls developed parkinsonism after their index date and were reassigned as cases. The median age at index date was 75.6 years (IQR 14.3) for cases and 75.8 (IQR 14.3) for controls. The median age at the first TBI was 59.5 years (IQR 30 years) for cases and 56.8 years (IQR 27.1 years) for controls. The difference was not statistically significant (p = 0.91).
Table 3.
Characteristics of cases
Because of the matching, the age and sex distribution were similar among cases and controls.
We recorded if the study participants ever drank coffee or smoked. Among cases, 379 (82.6%) individuals endorsed ever drinking coffee, 63 (13.7%) never drank coffee, and 17 (3.7%) were unknown or had missing data. Among controls, 388 (84.5%) individuals endorsed ever drinking coffee, 56 (12.2%) never drank coffee, and 15 (3.3%) were unknown or had missing data. The difference in coffee drinking among cases and controls was not statistically significant (p = 0.49). Among cases, 223 (48.6%) individuals endorsed ever smoking, 233 (50.8%) individuals never smoked, and 3 (0.65%) were unknown or had missing data. Among controls, 255 (55.6%) individuals endorsed ever smoking, 197 (42.9%) never smoked, and 7 (1.5%) were unknown or had missing data. The difference in the number of smokers among cases and controls was statistically significant (p = 0.02).
Overall, 70 individuals experienced at least 1 TBI (32 cases, 38 controls) before the index date. Of these, 34 experienced possible TBIs (12 cases, 22 controls), 27 experienced probable TBIs (15 cases, 12 controls), and 9 experienced definite TBIs (5 cases, 4 controls) (table 3). There were 79 total TBIs recorded in this population. The trauma mechanisms were falls (40; 18 cases, 22 controls), motor vehicle accidents (20; 10 cases, 10 controls), getting hit by an object (15; 6 cases, 9 controls), gunshot (1; 0 cases, 1 control), sports-related (1; 0 cases, 1 control), and miscellaneous mechanisms (2; 2 cases, 0 controls). Ten of these individuals (4 cases, 6 controls) experienced TBIs twice prior to the index date (table 3).
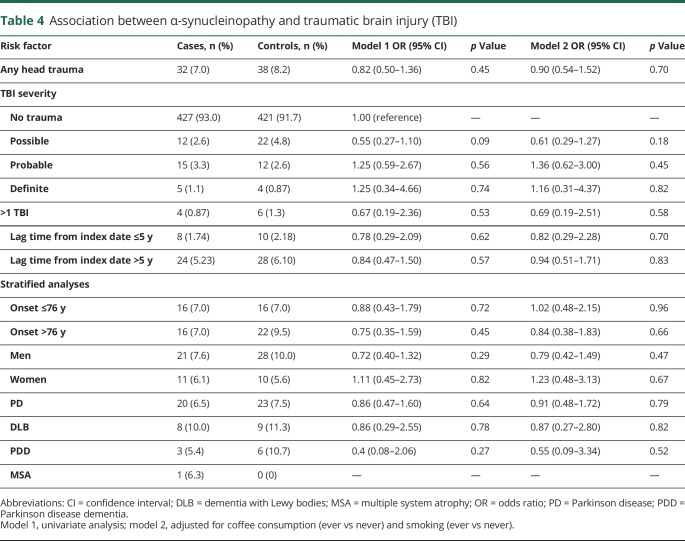
Table 4 summarizes our analyses of TBI and odds of any α-synucleinopathy. The frequency of TBI was lower among all α-synucleinopathy cases (7.0%) compared to controls (8.2%), equating to an insignificant OR of 0.82 (95% CI 0.50–1.36; p = 0.45). To account for the heterogeneity of TBIs, we also analyzed the association with α-synucleinopathies based on the severity of TBIs (possible, probable, and definite) and found no significant associations (table 4).
Table 4.
Association between α-synucleinopathy and traumatic brain injury (TBI)
We next determined whether the relationship between TBI and any α-synucleinopathy differed by age or sex. The association did not differ when stratifying by median age at index date (≥76 vs <76 years). Among men, TBI was less frequent among cases (7.6%) compared to controls (10.0%), but this difference was not significant (OR 0.72, 95% CI 0.39–1.32; p = 0.29). Among women, the frequency of TBI also did not differ between cases (6.1%) and controls (5.6%) (OR 1.11, 95% CI 0.45–2.73; p = 0.82). Adjusting for coffee and smoking in these models did not alter the associations. Experiencing more than 1 TBI prior to the index date also was not associated with developing any α-synucleinopathy (OR 0.67, 95% CI 0.19–2.36; p = 0.53).
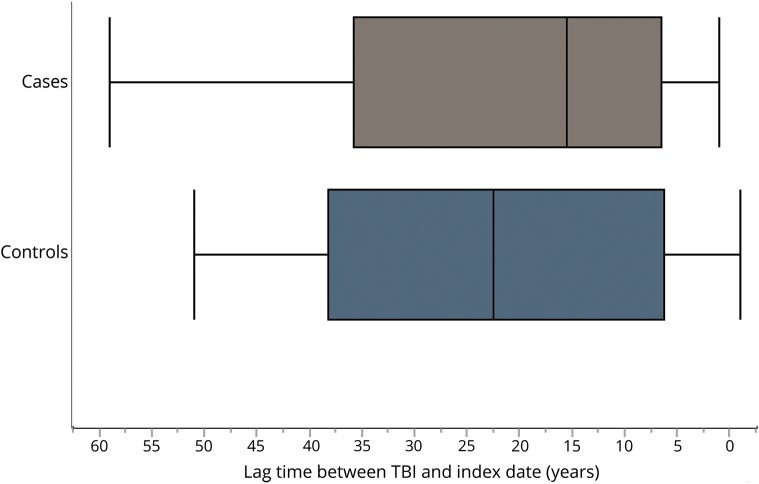
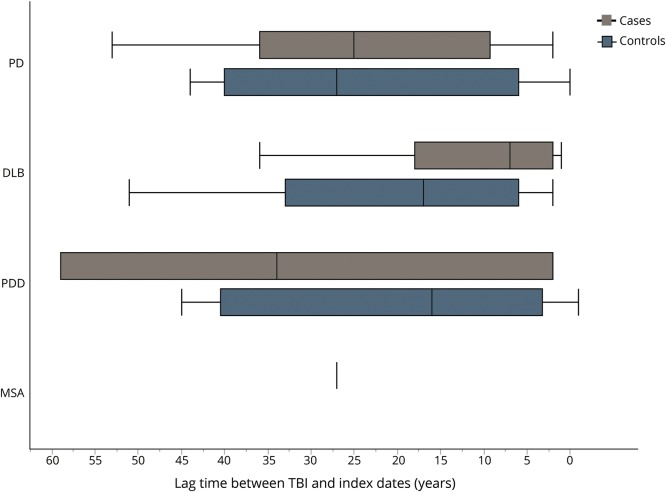
In addition, we examined the potential for reverse causation and for any differences in the lag times between the TBI exposure and index dates. No association was found between a TBI within 5 years of the index date and later development of α-synucleinopathy (OR 0.78, 95% CI 0.29–2.09; p = 0.62). Figure 1 represents the distribution of lag times between each TBI and index date among cases and controls. The lag time between TBI and index date among cases ranged from 0.10 to 59.1 years, with a median of 15.5 years (IQR 29.3 years). For controls, this ranged from 0.30 to 51.0 years, with a median of 22.5 years (IQR 22.0 years). The distribution of lag times was not statistically different (Wilcoxon rank sum test, p = 0.98). Figure 2 represents the distribution of lag times between a TBI and index date for each α-synucleinopathy. Among PD, the median lag time was 25.0 years (IQR 26.75 years) for cases and 27.0 years (IQR 35.0 years) for controls (p = 0.76). Among DLB, the median lag time was 7.0 years (IQR 16.0 years) for cases and 17.0 years (IQR 27.0 years) for controls (p = 0.20). Among PDD, the median lag time was 34 years (IQR 57 years) for cases and 16.0 years (IQR 37.25 years) for controls (p = 0.68). Interestingly, the PDD group was the only one in which the median lag time for cases was higher than for the controls. However, there were only 3 patients with PDD who had experienced TBI. The only case of MSA with a TBI occurred 27.0 years prior to the index date (could not perform Wilcoxon rank sum test). Similar to figure 1, the distribution of lag times for each α-synucleinopathy between cases and controls was not significantly different.
Figure 1. Lag time between traumatic brain injury (TBI) and index date among cases and controls.
Figure 2. Lag time between traumatic brain injury (TBI) and index date among α-synucleinopathies.
When stratifying by each individual α-synucleinopathy, we did not identify any significant associations between TBI and PD, DLB, or PDD. Among the MSA group, we observed only 1 patient who experienced a TBI before the index date. None of the MSA controls experienced a TBI. Therefore, we could not calculate the OR for this group of patients (table 4).
Discussion
The results of this population-based, case-control study suggest that TBI is not associated with the development of α-synucleinopathies. This was true for α-synucleinopathies as a whole, as well as for comparisons among individual α-synucleinopathies; that is, PD, PDD, DLB, and MSA. This association did not change when restricted to probable, possible, or definite TBI or after assessing for the temporality of the TBI.
There have been no studies we know of assessing TBI frequency among other α-synucleinopathies using a population-based study design. However, several studies have assessed the association between TBI and PD, per se, but the results have been conflicting.1–12,30–33 As mentioned previously, one reason for the conflicting results could be that several of these studies utilized questionnaires to gather data on TBI, risking recall bias.1–11,30 Another reason could be the way TBIs were defined. Certain studies may have required loss of consciousness or amnesia to qualify as a TBI.12,30,33 Our classification criteria, however, expanded upon that to also include injuries with several other symptoms, such as headache, dizziness, or confusion (table 2).
Of note, a similar population-based case-control study done within Olmsted County, Minnesota, between 1976 and 1995 reported a significant association between TBI and PD.12 The identification of cases and controls in our study and the study by Bower et al.12 were the same in that we defined the index date based on when parkinsonism began. However,in addition to the different study period, the way TBIs were abstracted differed.
Bower et al.12 required evidence of presumed brain involvement; that is, concussion with loss of consciousness, posttraumatic amnesia, neurologic signs of brain injury, or skull fracture. At a minimum, the criteria required some degree of loss of consciousness or posttraumatic amnesia. This would have only included the moderate to severe TBI cases based on the Mayo Classification System for TBI Severity criteria. In the current study, however, even when restricting to more severe cases, we did not find an association with any of the synucleinopathies. Another difference was that Bower et al.12 included 196 cases and controls of PD whereas this study included 307 cases and controls of PD. As a result, the differences in TBI incidences and samples sizes in this study and Bower et al.12 could have affected the study power. Additional discussion on the power of this study is elaborated in the limitations discussion.
These negative results do not completely exclude a relationship between early-life trauma and α-synucleinopathy. A positive association between head trauma and PD was not found overall but has been documented in individuals with a specific α-synuclein gene-promotor haplotype, Rep1, which previously was reported independently to increase PD risk.34,35
Another reason for conflicting results with other studies could be the way we defined TBIs. In the current study, we adopted the Mayo Classification System for TBI Severity to identify a wide spectrum of TBIs with higher sensitivity and specificity compared to the CDC recommendations and other single indicators used to classify severity (e.g., Glasgow Coma Scale score, posttraumatic amnesia, and loss of consciousness).27,36,37 The advantage of this classification system is that it uses information from the medical records rather than patient questionnaires. It also utilizes the presence of symptoms rather than absence to classify each level of severity. These criteria also include codes such as postconcussion syndrome (310.2), confusion (298.9), or late effects from injury (905.0, 907.0), in addition to the codes recommended by the CDC for identifying TBIs (table 1). Therefore, this classification scheme includes a wider spectrum of head injuries seen in both inpatient and outpatient settings. These criteria have 89% sensitivity and 98% specificity.27 Contrast this to the CDC codes for TBI, which do not account for other symptoms such as headache, nausea, visual symptoms, or visiting the outpatient setting after the event (table 2). The sensitivity and specificity for the CDC scheme is 46% and 98%, respectively.25,37 Thus, the CDC list may have limited ability to capture mild cases.25,26 The majority of TBIs in this study were mild and possible to capture using the Mayo Classification System for TBI Severity criteria.
Our study has multiple strengths. First, this study used the Mayo Classification System for TBI Severity criteria to include a wide spectrum of TBI. Second, this study utilized only medical records to identify TBIs prior to the index date, reducing recall bias. Third, this study used incident cases of α-synucleinopathies by marking the index date as the time of symptom onset rather than the time of clinical diagnosis. Reviewing the medical records allowed us to retro-date the exposure and reduced incidence–prevalence bias. Fourth, this study also included all incident cases of α-synucleinopathies within Olmsted County, Minnesota, therefore decreasing the risk of selection bias. Fifth, we adjusted for other factors associated with PD such as coffee consumption and smoking.29,38,39 Sixth, the control group consisted of randomly drawn residents without a confirmed α-synucleinopathy diagnosis from the general population of Olmsted County, thus reducing referral bias. Seventh, our study used a medical records-linkage system to access historical records with several decades of patient information, allowing us to abstract exposures that occurred during childhood. Eighth, we accounted for the temporality of TBIs prior to the index dates. This allowed us to consider reverse causation or if the timing of the TBI affected disease initiation. Our results did not support the hypothesis that early TBI initiates neurodegeneration through α-synucleinopathies nor did it support reverse causation. Finally, our study examined the association of the different types of clinically diagnosed α-synucleinopathies with TBI, providing insight into exposures that may make an individual prone to developing one α-synucleinopathy over another.
Our study also has limitations. First, the incidence of TBI was small, especially for severe cases, limiting the power to analyze the association with all α-synucleinopathies and each individual α-synucleinopathy. Based on the TBI incidences we recorded, we used JMP to calculate the sample size required to detect a statistically significant difference in the proportions of individuals with TBIs among cases and controls. The estimated sample size for 80% power, α = 0.05, and 1:1 matching was 1,918 cases and controls. Despite our population-based methods, our study size was smaller than this, which could explain the lack of statistical significance in our analyses. The sample sizes for our subgroup analyses were considerably smaller, limiting the power for those calculations as well. Second, the symptoms and mechanisms of these injuries were severe enough to warrant seeing a physician. This does not include incidents when individuals did not visit a doctor, but presumably such cases reflect less severe head trauma. Third, case identification was based on clinical, not pathologic, findings. Fourth, our MSA cases included individuals with the parkinsonian variant, not the cerebellar variant, based on our inclusion criteria. Fifth, Olmsted County is predominantly rural. Therefore, this may affect the spectrum of TBIs, including mechanisms of injury, seen by physicians. Sixth, as table 3 illustrates, the vast majority of our study population was white. This may limit the generalizability of our data to other populations within the United States and around the world. Finally, despite matching based on sex and age, we did not take into account the time each individual was in the medical record system, which could affect how far back one could go into an individual's records to gather TBI data. We could not take into account if an individual experienced a TBI prior to moving to Olmsted County. However, most of the participants in our study yielded at least 20 years of medical records, allowing us to collect exposures from early in life.
Our study did not demonstrate a significant association between TBI and the development of any α-synucleinopathy. This association did not change based on the severity of TBI, the timing of the TBI prior to the index date, or the specific type of α-synucleinopathy. Our findings for both initial and subgroup analyses may be limited by the study's power.
Acknowledgment
The authors thank Lea Dacy, AB, for proofreading and formatting assistance.
Glossary
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- DLB
dementia with Lewy bodies
- H-ICDA
Hospital Adaptation of the International Classification of Diseases, Eighth Revision
- ICD-9
International Classification of Diseases, Ninth Revision
- IQR
interquartile range
- MSA
multiple system atrophy
- OR
odds ratio
- PD
Parkinson disease
- PDD
Parkinson disease dementia
- REP
Rochester Epidemiology Project
- TBI
traumatic brain injury
Appendix. Authors
Footnotes
Editorial, page 335
Study funding
Study funded by CTSA grant number UL1 TR002377 from the National Center for Advancing Translational Sciences, a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. This study was also supported by grant R01 AG034676 from the National Institute on Aging of the NIH and by the Mayo Foundation for Medical Education and Research.
Disclosure
S. Hasan presented preliminary data from this project at the American Academy of Neurology annual meeting poster session, April 23, 2018, titled “Traumatic brain injury and erectile dysfunction preceding clinically diagnosed α-synucleinopathies: A case-control study in Olmsted County, MN (1991–2010).” M. Mielke consults for Eli Lilly and receives research support from the National Institute on Aging and unrestricted research grants from Biogen and Lundbeck. P. Turcano reports no disclosures relevant to the manuscript. J. Bower receives research support from AbbVie, Inc. E. Ahlskog receives author royalties from Oxford University Press. R. Savica receives research support from the National Institute on Aging, the National Institute of Neurologic Disorders and Stroke, and the Mayo Clinic Small Grants Program National Center for Advancing Translational Sciences and unrestricted research grants from Acadia Pharmaceutical, Inc. Go to Neurology.org/N for full disclosures.
References
- 1.Taylor CA, Saint-Hilaire MH, Cupples LA, et al. Environmental, medical, and family history risk factors for Parkinson's disease: a New England-based case control study. Am J Med Genet 1999;88:742–749. [PubMed] [Google Scholar]
- 2.Tanner CM, Chen B, Wang WZ, et al. Environmental factors in the etiology of Parkinson's disease. Can J Neurol Sci 1987;14:419–423. [DOI] [PubMed] [Google Scholar]
- 3.Stern M, Dulaney E, Gruber SB, et al. The epidemiology of Parkinson's disease: a case-control study of young-onset and old-onset patients. Arch Neurol 1991;48:903–907. [DOI] [PubMed] [Google Scholar]
- 4.Semchuk KM, Love EJ, Lee RG. Parkinson's disease: a test of the multifactorial etiologic hypothesis. Neurology 1993;43:1173–1180. [DOI] [PubMed] [Google Scholar]
- 5.Seidler A, Hellenbrand W, Robra BP, et al. Possible environmental, occupational, and other etiologic factors for Parkinson's disease: a case-control study in Germany. Neurology 1996;46:1275–1284. [DOI] [PubMed] [Google Scholar]
- 6.Morano A, Jimenez-Jimenez FJ, Molina JA, Antolin MA. Risk-factors for Parkinson's disease: case-control study in the province of Caceres, Spain. Acta Neurol Scand 1994;89:164–170. [DOI] [PubMed] [Google Scholar]
- 7.Hubble JP, Cao T, Hassanein RE, Neuberger JS, Koller WC. Risk factors for Parkinson's disease. Neurology 1993;43:1693–1697. [DOI] [PubMed] [Google Scholar]
- 8.Hofman A, Collette HJ, Bartelds AI. Incidence and risk factors of Parkinson's disease in the Netherlands. Neuroepidemiology 1989;8:296–299. [DOI] [PubMed] [Google Scholar]
- 9.Godwin-Austen RB, Lee PN, Marmot MG, Stern GM. Smoking and Parkinson's disease. J Neurol Neurosurg Psychiatry 1982;45:577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Factor SA, Weiner WJ. Prior history of head trauma in Parkinson's disease. Mov Disord 1991;6:225–229. [DOI] [PubMed] [Google Scholar]
- 11.De Michele G, Filla A, Volpe G, et al. Environmental and genetic risk factors in Parkinson's disease: a case-control study in southern Italy. Mov Disord 1996;11:17–23. [DOI] [PubMed] [Google Scholar]
- 12.Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA. Head trauma preceding PD: a case-control study. Neurology 2003;60:1610–1615. [DOI] [PubMed] [Google Scholar]
- 13.Savica R, Grossardt BR, Bower JH, et al. Survival and causes of death among people with clinically diagnosed synucleinopathies with parkinsonism: a population-based study. JAMA Neurol 2017;74:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol 2013;70:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol 2013;70:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ III, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ III. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic Proc 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics. H-ICDA: Hospital Adaptation of ICDA, 2nd ed. Ann Arbor: Commission on Professional and Hospital Activities, National Center for Health Statistics; 1973. [Google Scholar]
- 21.Manual of the International Classification of Diseases, Injuries, and Causes of Death, Based on the Recommendations of the Ninth Revision Conference, 1975, and Adopted by the Twenty-Ninth World Health Assembly. Geneva: World Health Organization; 1977. [Google Scholar]
- 22.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976-1990. Neurology 1999;52:1214–1220. [DOI] [PubMed] [Google Scholar]
- 23.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 24.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leibson CL, Brown AW, Ransom JE, et al. Incidence of traumatic brain injury across the full disease spectrum: a population-based medical record review study. Epidemiology 2011;22:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faul M, Xu L, Wald MW, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Bethesda: Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 27.Malec JF, Brown AW, Leibson CL, et al. The Mayo Classification System for Traumatic Brain Injury Severity. J Neurotrauma 2007;24:1417–1424. [DOI] [PubMed] [Google Scholar]
- 28.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Time trends in the incidence of Parkinson disease. JAMA Neurol 2016;73:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Risk factors for Parkinson's disease may differ in men and women: an exploratory study. Horm Behav 2013;63:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor KM, Saint-Hilaire MH, Sudarsky L, et al. Head injury at early ages is associated with risk of Parkinson's disease. Parkinsonism Relat Disord 2016;23:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson's disease after hospital contact for head injury: population based case-control study. BMJ 2008;337:a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang CH, Lin CW, Lee YC, et al. Is traumatic brain injury a risk factor for neurodegeneration? A meta-analysis of population-based studies. BMC Neurol 2018;18:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW. Head injury and Parkinson's disease risk in twins. Ann Neurol 2006;60:65–72. [DOI] [PubMed] [Google Scholar]
- 34.Maraganore DM, de Andrade M, Elbaz A, et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA 2006;296:661–670. [DOI] [PubMed] [Google Scholar]
- 35.Goldman SM, Kamel F, Ross GW, et al. Head injury, alpha-synuclein Rep1, and Parkinson's disease. Ann Neurol 2012;71:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma 2008;25:719–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazarian JJ, Veazie P, Mookerjee S, Lerner EB. Accuracy of mild traumatic brain injury case ascertainment using ICD-9 codes. Acad Emerg Med 2006;13:31–38. [DOI] [PubMed] [Google Scholar]
- 38.Palacios N, Gao X, McCullough ML, et al. Caffeine and risk of Parkinson's disease in a large cohort of men and women. Mov Disord 2012;27:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benedetti MD, Bower JH, Maraganore DM, et al. Smoking, alcohol, and coffee consumption preceding Parkinson's disease: a case-control study. Neurology 2000;55:1350–1358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant data have been shared and published in this article; data regarding case ascertainment of parkinsonism and methodology on case identification have been published previously.15