Abstract
Objective
To examine the association between prediagnostic plasma polyunsaturated fatty acids levels (PUFA) and amyotrophic lateral sclerosis (ALS).
Methods
We identified 275 individuals who developed ALS while enrolled in 5 US prospective cohorts, and randomly selected 2 controls, alive at the time of the case diagnosis, matched on cohort, birth year, sex, ethnicity, fasting status, and time of blood draw. We measured PUFA, expressed as percentages of total fatty acids, using gas liquid chromatography and used conditional logistic regression to estimate risk ratios (RR) and 95% confidence intervals (CI) for the association between PUFA and ALS.
Results
There was no association between total, n-3, and n-6 PUFA, eicosapentaenoic acid, or docosapentaenoic acid levels and ALS. Higher plasma α-linolenic acid (ALA) in men was associated with lower risk of ALS in age- and matching factor-adjusted analyses (top vs bottom quartile: RR = 0.21 [95% CI 0.07, 0.58], p for trend = 0.004). In women, higher plasma arachidonic acid was associated with higher risk (top vs bottom quartile: RR = 1.65 [95% CI 0.99, 2.76], p for trend = 0.052). Multivariable adjustment, including correlated PUFA, did not change the findings for ALA and arachidonic acid. In men and women combined, higher plasma docosahexaenoic acid (DHA) was associated with higher risk of ALS (top vs bottom quartile: RR = 1.56 [95% CI 1.01, 2.41], p for trend = 0.054), but in multivariable models the association was only evident in men.
Conclusions
The majority of individual PUFAs were not associated with ALS. In men, ALA was inversely and DHA was positively related to risk of ALS, while in women arachidonic acid was positively related. These findings warrant confirmation in future studies.
Mechanisms implicated in the etiology of amyotrophic lateral sclerosis (ALS) such as oxidative stress, excitotoxicity, and inflammation1 are potentially modulated by polyunsaturated fatty acids (PUFAs) in brain neural plasma membranes.2 Metabolites of long-chain fatty acids have different properties according to the number and position of double bonds in the parent fatty acid, but notably docosahexaenoic acid (DHA) (22:6 n-3), the most abundant PUFA in the brain, is metabolized to powerful anti-inflammatory and neuroprotective derivatives.3 Although expected to be protective, a diet with high-dose long-chain n-3 PUFA resulted in accelerated disease progression in presymptomatic familial ALS animal models,4,5 while diets supplemented with n-6 PUFA appeared to slow progression of ALS and be beneficial to survival.4
In a pooled prospective study comprising over 1 million participants, individuals with greater self-reported prediagnostic dietary intake of n-3 PUFAs had lower risk of ALS.6 Two case-control studies that examined the association between diet and ALS suggested that risk was lower among individuals with higher intake of PUFAs.7,8 While promising, it remains inconclusive which PUFAs, and in what quantities, are associated with risk of ALS. Dietary PUFAs may not fully capture the biologically relevant exposure, because both dietary intake and metabolism determine circulating levels of PUFAs. In addition, individuals with higher premorbid body mass index (BMI) have lower ALS risk,9–11 and metabolic dysregulation has been reported in association with ALS.12–14 We therefore examined the association between prediagnostic plasma PUFA levels and subsequent risk of ALS in a multicohort nested case-control study.15–17
Methods
Study population
Five large cohorts are included: Nurses’ Health Study (NHS), Health Professionals Follow-up Study (HPFS), Cancer Prevention Study II Nutrition Cohort (CPS-II Nutrition), Multiethnic Cohort Study (MEC), and Women's Health Initiative (WHI). Detailed descriptions of the individual cohorts have been published previously.18–22 We briefly described each cohort, case ascertainment, control selection, and biospecimen handling previously.15–17 The NHS enrolled 121,700 women in 1976 (30–55 years).18 A blood sample was collected from 32,826 women during 1989 and 1990, as described previously.23,24 Briefly, all nurses were invited to provide a sample and those who consented were mailed equipment to arrange a fasting blood draw to be returned on ice in heparin tubes. Ninety-seven percent were returned within 26 hours. Among the nurses who gave blood, 39 developed ALS between blood draw and end of follow-up. The HPFS enrolled 51,529 male health professionals (40–75 years) in 1986.19 Fasting blood samples were collected from 18,018 men between 1993 and 1995 in EDTA blood tubes, and returned on ice, with >95% of samples arriving within 24 hours, then centrifuged, aliquoted, and stored in −150°C liquid nitrogen. Among the men who provided blood, 26 developed ALS between blood draw and end of follow-up. The CPS-II Nutrition cohort, established in 1992, is a subgroup of the larger CPS-II cohort20 and includes 86,406 men and 97,788 women (50–74 years). A follow-up questionnaire was completed in 1997. Blood samples were collected from 39,380 participants living in urban and suburban areas, 58 of whom died of ALS during follow-up. The MEC enrolled 96,810 men and 118,441 women (45–75 years) in 1993 with the self-reported ethnic background of African American, Japanese American, Latino, Native Hawaiian, or white.21 Approximately 67,594 participants provided a blood sample between 2001 and 2006, 31 of whom died of ALS through the end of the follow-up. The WHI study encompassed an observational study and 3 clinical trials. During 1993 to 1998, 161,808 postmenopausal women (50–79 years) entered 1 or more trials (n = 68,132) or the observational study (n = 93,676).22 At baseline, all of the participants completed a questionnaire on lifestyle and disease history and provided a blood sample. During follow-up, 121 participants died of ALS. End of follow-up was December 31, 2010, for NHS, HPFS, and CPS-II Nutrition; September 30, 2012, for WHI; and December 31, 2012, for MEC.15–17
Standard protocol approvals, registrations, and patient consents
Each study included was reviewed and approved by the institutional review board representing the institution where it was conducted.
Endpoint definition
As we described previously,18–22 in CPS-II Nutrition, MEC, and WHI we identified ALS cases through the National Death Index. At the start of the current study, WHI self-reported ALS had not been adjudicated and therefore was not included. We considered an individual to have had ALS if code 335.2 (motor neuron disease) according to the ICD-9 was listed as the underlying or contributing cause of death. In a previous validation study, ALS was the primary diagnosis in 90% of the individuals with ICD-9 335.2 listed as the cause or contributory cause of death.25 We assigned the date of onset to 3 years before the date of death, based on median survival among patients with ALS.26
In NHS and HFPS, we identified incident ALS by self-report on the biennial questionnaire, as described previously.9 When ALS was self-reported, we requested permission from the participant to contact his or her neurologist with a questionnaire about the certainty of the diagnosis (definite, probable, or possible) and the clinical history and to obtain a copy of the medical records. Many participants had already died before we could request permission and then the request was sent to the closest family member. We included participants defined as having definite or probable ALS following a review of collected records by a neurologist with experience making ALS diagnosis. When we were unable to confirm the diagnosis of self-reported ALS, we relied on ALS being specifically listed on the death certificate.9
We selected 2 controls at random who were alive at the time of the case diagnosis and matched them to the patient on cohort, year of birth (±1 year), sex, ethnicity, fasting status, and the time of the blood draw.15–17
Assessment of PUFAs
In total, 319,627 participants across the cohorts provided blood samples that were stored at −70°C or below. Sample triplets were handled identically and assayed in the same analytic run. The plasma samples were ordered randomly within each case-control triplet. Each cohort was assayed at the same laboratory.
Fatty acids in plasma were extracted into isopropanol and hexane and subsequently all lipid species were transmethylated to fatty acid methyl esters (FAMEs) by methanolic sulfuric acid and heat. The resultant FAMEs were then evaporated and redissolved in isooctane. Gas chromatography was used to measure known peaks by comparison with accepted standards and quantified using the area under the peak. The percentage of total area under the peak was used to determine the concentration of each individual fatty acid and individual fatty acids are described as a percentage of total fatty acids measured.27 The variables n-3 PUFA, n-6 PUFA, and total PUFA are the sums of the relevant individual fatty acids expressed as percentages. In 1 patient and 2 controls, the assessment of PUFA failed. Except for arachidonic acid (average coefficient of variation across cohorts 18.2%), coefficients of variation were 7% or lower (average across cohorts: for α-linolenic acid [ALA], 3.2%; for eicosapentaenoic acid [EPA], 6.9%; for docosapentaenoic acid [DPA], 7.1%; for DHA, 6.2%; for linoleic acid, 2.1%; for γ-linolenic acid, 4.2%).
Assessment of covariates
Information on potential confounding variables, including smoking status, height, weight, educational level, physical activity, and diabetes status, was collected at baseline for all cohorts, every 2 years since baseline in HPFS and NHS, and every 2 years since 1997 for CPS-II Nutrition. We used data collected from the nearest questionnaire before or at the time of blood draw for covariates. Plasma urate, which was modestly associated with ALS in this population,15 and low-density lipoprotein (LDL)had been measured on all participants.
Statistical analysis
Conditional logistic regression estimated odds ratios (ORs) and 95% confidence intervals (CIs) for the association of PUFAs with ALS risk. The ORs estimate incidence rate ratios (RRs) because controls were matched using risk set sampling.28 We estimated Pearson correlation between PUFAs, urate, and BMI. Pooled analyses of individual and total PUFAs were modeled as sex-specific quartiles based on the distribution in controls. To test for linear trends across quartiles, the median value was assigned to each quartile and modeled as a continuous variable.
Multivariable models were used to adjust for possible confounders, including plasma urate, BMI, education (< high school, high school, > high school), and smoking status (never, past, current smoker). Further analyses were conducted by adjusting for LDL (quartiles) and by simultaneously adjusting the regression models for different correlated plasma PUFAs. A missing indicator was used when covariate data were missing (6.7% of participants had a missing covariate). Potential effect modification by sex, fasting status (<8 vs ≥8 hours since last meal), as well as time between blood draw and ALS onset (<5 vs ≥5 years) was explored by modeling their product with individual PUFAs. Significance was set at α = 0.05 and p values were not adjusted for multiple comparisons. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Data availability
The datasets analyzed in the current study are not publicly available because of restricted access, but further information about the datasets is available from the corresponding author on reasonable request.
Results
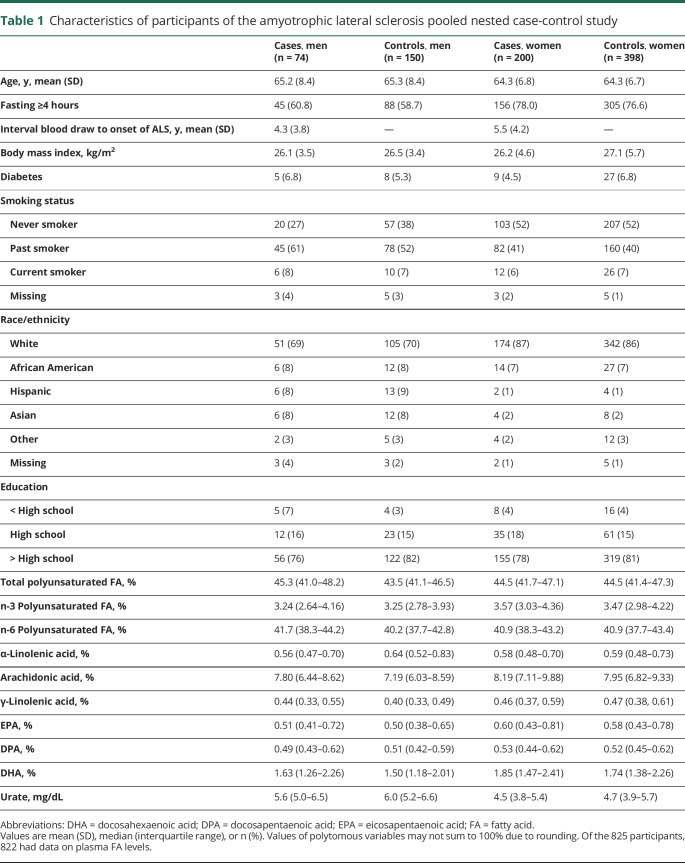
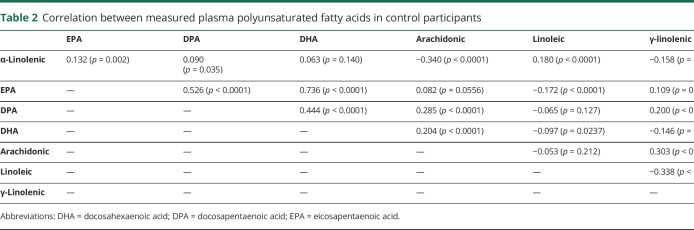
Among the 319,627 men and women who provided blood samples while enrolled in 5 cohorts, 275 incident cases of ALS were identified during follow-up. Selected characteristics of patients and controls are shown in table 1. The majority of the individual PUFAs were weakly intercorrelated (table 2). Plasma levels of EPA, DPA, and DHA were strongly correlated, while arachidonic acid and γ-linolenic acid were moderately correlated. ALA was moderately inversely correlated with arachidonic acid. Linoleic acid was inversely correlated with γ-linolenic acid.
Table 1.
Characteristics of participants of the amyotrophic lateral sclerosis pooled nested case-control study
Table 2.
Correlation between measured plasma polyunsaturated fatty acids in control participants
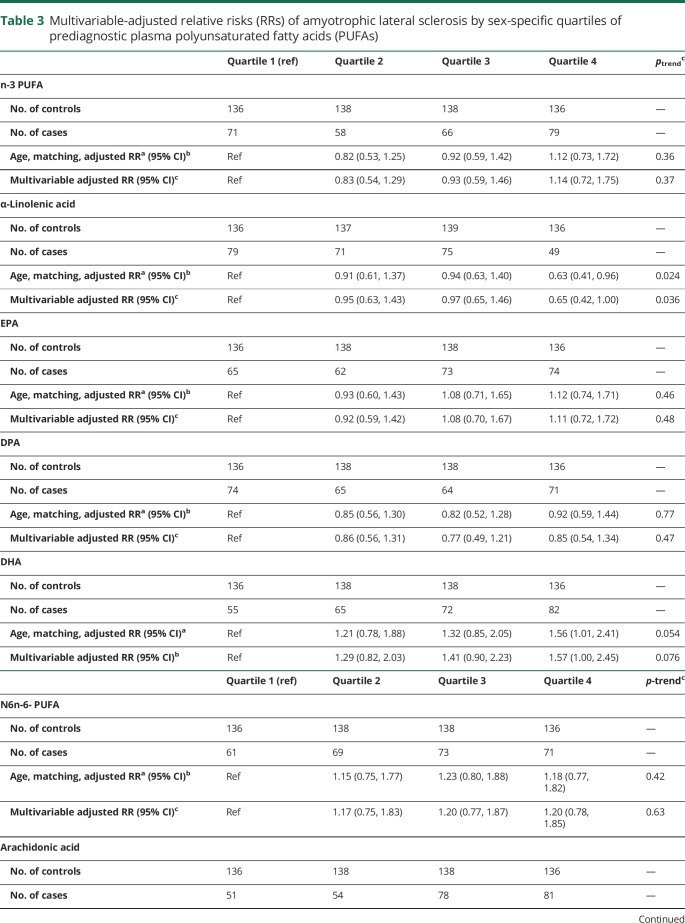
There was no association between total, n-3, or n-6 PUFA levels and ALS. Individuals with higher plasma ALA had lower risk of ALS in age- and matching factor-adjusted analyses (RR top vs bottom quartile: 0.63 [95% CI 0.41, 0.96]; p for trend = 0.02; table 3). Adjustment for BMI, smoking, education, and plasma urate and LDL did not materially change the findings. When men and women were considered separately, the association between ALA and ALS was present in men (RR top vs bottom quartile: RR = 0.21 [95% CI 0.07, 0.58], p for trend = 0.004; table e-1, e-2; doi:10.5061/dryad.cc13012) but not women (p for effect modification by sex = 0.022).
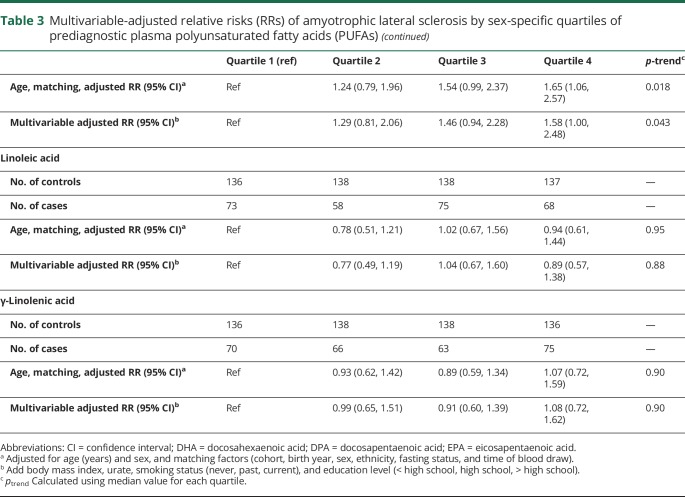
Table 3.
Multivariable-adjusted relative risks (RRs) of amyotrophic lateral sclerosis by sex-specific quartiles of prediagnostic plasma polyunsaturated fatty acids (PUFAs)
In multivariable-adjusted analyses, the RR of ALS did not vary significantly across quartiles of EPA, DPA, linoleic acid, or γ-linolenic acid (table 3). In contrast, increasing levels of DHA in men and arachidonic acid in women were associated with higher risk of ALS (table 3, table e-1, and e-2; doi:10.5061/dryad.cc13012).
Ratios of specific PUFA were used to estimate the activity of desaturase enzymes. Stearoyl-coenzyme A desaturase (using 16:1 n-7/16:0 and 18:1 n-9/18:0), δ-6 desaturase (using 18:3 n-6/18:2 n-6), and δ-5 desaturase (using 20:4 n-6/20:3 n-6) were not associated with ALS risk when adjusted for age and matching factors, or when further adjusted for BMI, smoking, education attained, and plasma urate. There was some evidence of an inverse association between stearoyl-coenzyme A desaturase activity measured using 18:1 n-9/18:0 ratio and ALS in men (p = 0.055) (table e-3, doi:10.5061/dryad.cc13012).
Mutual adjustment for PUFAs
Among men and women combined, adjustment for EPA and DPA derivatives did not appreciably change the association between ALA levels and ALS risk, but mutual adjustment for correlated PUFAs (EPA, arachidonic acid, linoleic acid, and γ-linolenic acid) modestly attenuated the overall findings (p for trend = 0.13). In contrast, the inverse association between ALA and ALS in men remained robust to simultaneous adjustment for correlated PUFA (RR top vs bottom quartile 0.10; 95% CI 0.02, 0.44; p = 0.002; p for trend = 0.001; table e2, doi:10.5061/dryad.cc13012). The increased ALS risk among women with higher plasma levels of arachidonic acid was robust to further adjustment for other PUFAs, including ALA (RR top vs bottom quartile 1.83; 95% CI 1.01, 3.33; p for trend = 0.054). The increased ALS risk among individuals, particularly men, with higher plasma levels of DHA was robust to further adjustment for correlated PUFA (men + women: RR top vs bottom quartile 1.81; 95% CI 1.03, 3.18; p for trend = 0.049; men: RR = 3.88; 95% CI 1.10,13.6; p for trend = 0.023); women: RR = 1.34 95% CI 0.69, 2.63; p for trend = 0.53)).
Time from blood draw to ALS onset and fasting status did not modify any associations (data not shown).
Discussion
In this pooled analysis of 5 large prospective cohorts, we observed no association between prediagnostic plasma levels of total, n-3, or n-6 PUFA, EPA, or DPA and ALS. We found a lower risk of ALS among men with higher levels of ALA. Higher DHA levels in men and higher arachidonic levels in women were associated with higher ALS risk.
There are a limited number of studies on PUFA as assessed by diet and ALS risk,6–8 and none examining prediagnostic plasma levels and disease risk. The results of previous studies on dietary fat and ALS risk have been inconsistent, but most used a case-control design,7,8 which is vulnerable to selection and recall biases and to reverse causation,29 which make the results difficult to interpret. The only exception is a prospective study conducted among >1 million participants in 5 cohorts (4 of which also contributed to this study).6 In that study, individuals with higher prediagnostic self-reported dietary intake of n-3 PUFA, including ALA, had significantly lower ALS risk. A significant trend across quintiles of dietary marine n-3 PUFAs was also observed. The results of the current study, which used plasma rather than self-reported PUFA, support the previous findings on ALA, but not those on marine n-3 PUFAs. This discrepancy was somewhat reduced, but not eliminated, when examining dietary intake in analyses restricted to the 95 ALS cases also included in the plasma study. In particular, although the association was attenuated, intake of marine n-3 PUFAs remained inversely associated with ALS risk, whereas in the present study there was a trend of increasing ALS risk with increasing plasma DHA, which is one of the major contributors to marine n-3 intake. Whether this discrepancy is due to chance or a real biological difference between intakes and plasma levels of marine n-3 fatty acids cannot be determined at this time. In interpreting these results, it should be noted that diet and biomarker data were obtained at different times (intervals ranging from 5 to 13 years), and the correlation between intakes and circulating levels were significant but weak, ranging from r = 0.10 to 0.22 for ALA, EPA, DHA, and linoleic acid. While the consistent ALA results in the dietary and the plasma studies adds weight to evidence of a role of ALA, we cannot rule out a role for other PUFAs.
Metabolism of individual PUFA depends on the relative presence of other fatty acids, and the resulting products may affect pathways relevant to pathogenesis of ALS. ALA has been reported to slow peroxidation activation of the binding of nuclear transcription factor κB and to reduce glutamate-mediated excitotoxic damage and oxidative stress, possibly preventing neuronal cell death.30–34 Our finding of an association between higher plasma levels of ALA and ALS is consistent with a protective effect of ALA on the development of the disease. The fact that this association was present only in men could be a chance occurrence, or it could reflect a true biological difference between men and women, as women have a higher capacity than men to convert ALA to EPA or DHA.35–37 Among the other PUFAs, DHA, the most abundant PUFA in the brain, is metabolized to powerful anti-inflammatory and neuroprotective derivatives.3 However, despite the antioxidant potential of DHA, its high oxidability means it could possibly increase the vulnerability of neurons and glial cells to oxidative stress. The potential for harm from DHA has been demonstrated in some animal models in which long-term high intake of fish oil increases oxidative stress and decreases lifespan.38,39 In addition, pretreatment with high doses of EPA accelerated disease progression in a mouse model of ALS.4,5 We found that higher plasma levels of DHA were associated with increased risk of ALS, which could be consistent with the results from these animal studies.
This study is the first to assess the possible role of δ-5 desaturase, δ-6 desaturase, and stearoyl-CoA desaturase-1 (SCD-1) activity prior to ALS onset. Δ-5 desaturase and δ-6 desaturase are sequentially required for the synthesis of n-6 and n-3 long-chain PUFAs. SCD-1 is an enzyme that introduces the first double bond in the δ-9 position of saturated fatty acyl-CoA substrates primarily converting palmitoyl-CoA (16:0) to palmitoleoyl-CoA (16:1 n-7) and stearoyl-CoA (18:0) to oleoyl-CoA (18:1 n-9). Experimental downregulation or knock-out of SCD-1 in mice results in a phenotype associated with augmented levels of β-oxidation, increased energy intake, and reduced lipogenesis.40 A similar hypermetabolic state has been observed in SOD1 mice, possibly due to reduced SCD-1 expression in muscle.41 In addition, SCD-1 downregulation was observed in relatively healthy deltoid muscle tissue of patients with ALS,41 suggesting that potential alterations in SCD-1 expression are an early event in ALS pathogenesis, a finding consistent with the evidence of hypermetabolism in individuals with sporadic ALS.14,42,43 However, our findings did not strongly support a role for SCD-1, δ-5 desaturase, or δ-6 desaturase activity prior to ALS onset.
Our study design has a number of strengths. The nested case-control design minimizes a number of the methodologic issues of the traditional case-control design, including selection bias and reverse causation.29 In our study, the controls were selected from the same source population (cohort) as the patients, and we assessed PUFAs in blood samples collected years before disease onset. Several important confounders were accounted for, including BMI, smoking, and plasma urate levels. Further, as we measured PUFA in plasma samples rather than relying on self-reported intake, we accounted for the inherent measurement error in self-reported dietary intake assessed by questionnaires and the endogenous elongation, desaturation, and other metabolic processes that can influence the actual circulating fatty acid exposure, which for some fatty acids is more relevant than the actual dietary intake of individual PUFA.
Our study has some limitations. Despite drawing participants from 5 large cohorts, our case number was only 275 due to the low incidence of ALS and statistical power may have been limited, particularly for the more tightly regulated PUFA (with modest between-person variation), and among men (27% of the population, by virtue of available cohorts with adequate numbers and prediagnostic blood samples). We relied on death certificates to capture ALS in CPS-II Nutrition, MEC, and WHI, which may lead to misclassification of ALS in some participants. Sensitivity for use of death certificates for ALS has been reported as approximately 85% in 2 studies while positive predictive value was 65% in one and 82% in another,44,45 which suggests that using death certificates for ALS is adequate in epidemiologic studies. PUFA were assessed from a single plasma measurement per individual, which may not reflect long-term exposure. However, a reproducibility study of plasma PUFA in women in NHS showed interclass correlations of 0.5–0.8 over 2–3 years in repeat samples,46 suggesting that a single measurement may reasonably reflect longer-term levels. It is unclear how well plasma PUFA levels correlate with brain levels. A few studies have considered the correlation of PUFA in blood and CSF. In small human studies, plasma ALA (r = 0.58) and DHA (r = 0.48) were correlated with CSF levels,47 and total n-3 PUFA was correlated with CSF DHA and DPA levels.48 We included 9 PUFA for analyses, which, while not highly likely, may result in significant findings due to multiple comparisons. In addition, simultaneous adjustment for collinear PUFAs could give rise to unstable effect estimates.
In this pooled analysis of 5 cohort studies of men and women, we did not observe an association between prediagnostic plasma total, n-3, and n-6 PUFA, EPA, or DPA and ALS risk. We found a significant inverse association between prediagnostic plasma levels of ALA and ALS. If replicated in future studies, our findings suggest that diets high in ALA may prevent or delay the onset of ALS.
Acknowledgment
The authors thank Prof. Lawrence Rand for help in obtaining funding for this project.
Glossary
- ALA
α-linolenic acid
- ALS
amyotrophic lateral sclerosis
- BMI
body mass index
- CI
confidence interval
- CPS-II Nutrition
Cancer Prevention Study II Nutrition Cohort
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
- FAME
fatty acid methyl ester
- HPFS
Health Professionals Follow-up Study
- ICD-9
International Classification of Diseases, Ninth Revision
- LDL
low-density lipoprotein
- MEC
Multiethnic Cohort Study
- NHS
Nurses’ Health Study
- OR
odds ratio
- PUFA
polyunsaturated fatty acid
- RR
rate ratio
- SCD-1
stearoyl-CoA desaturase-1
- WHI
Women's Health Initiative
Footnotes
Editorial, page 339
CME Course: NPub.org/cmelist
Author contributions
É.J. O'Reilly: drafting/revising the manuscript for content, analysis or interpretation of data, statistical analysis. K. Bjornevik: drafting/revising the manuscript for content, analysis or interpretation of data. J.D. Furtado: drafting/revising the manuscript for content, analysis or interpretation of data. M.L. McCullough: drafting/revising the manuscript for content, acquisition of data. V.L. Stevens: drafting/revising the manuscript for content, analysis or interpretation of data. L.N. Kolonel: drafting/revising the manuscript for content, acquisition of data. L.L. Marchand: drafting/revising the manuscript for content, acquisition of data. A.H. Shadyab: drafting/revising the manuscript for content, analysis or interpretation of data. L. Snetselaar: drafting/revising the manuscript for content, analysis or interpretation of data. J.E. Manson: drafting/revising the manuscript for content, acquisition of data. A. Ascherio: study concept or design, drafting/revising the manuscript for content, analysis or interpretation of data, acquisition of data, obtaining funding.
Study funding
This work was supported by research grants from The ALS Association, the Greater New York Chapter of the ALS Association, and the National Institutes of Neurological Diseases and Stroke (R01 NS045893) awarded to Alberto Ascherio, and by a contribution from Prof. Lawrence A. Rand. The NHS is funded by the NIH through grants UM1 CA186107 and R01 CA49449. The HPFS cohort is funded by the NIH through grant UM1 CA167552. The American Cancer Society funds the creation, maintenance, and updating of the CPS-II cohorts. The MEC cohort is funded by the NIH through U01 CA164973. The WHI program is funded by the National Heart, Lung, and Blood Institute, NIH, US Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Turner MR, Bowser R, Bruijn L, et al. Mechanisms, models and biomarkers in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2013;14(suppl 1):19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu JJ, Green P, John Mann J, Rapoport SI, Sublette ME. Pathways of polyunsaturated fatty acid utilization: implications for brain function in neuropsychiatric health and disease. Brain Res 2015;1597:220–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Li P, Hu X, Zhang F, Chen J, Gao Y. Omega-3 polyunsaturated fatty acids in the brain: metabolism and neuroprotection. Front Biosci 2011;16:2653–2670. [DOI] [PubMed] [Google Scholar]
- 4.Boumil EF, Vohnoutka RB, Liu Y, Lee S, Shea TB. Omega-3 hastens and omega-6 delays the progression of neuropathology in a murine model of familial ALS. Open Neurol J 2017;11:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yip PK, Pizzasegola C, Gladman S, et al. The omega-3 fatty acid eicosapentaenoic acid accelerates disease progression in a model of amyotrophic lateral sclerosis. PLoS One 2013;8:e61626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald KC, O'Reilly EJ, Falcone GJ, et al. Dietary omega-3 polyunsaturated fatty acid intake and risk for amyotrophic lateral sclerosis. JAMA Neurol 2014;71:1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto K, Kihira T, Kondo T, et al. Nutritional status and risk of amyotrophic lateral sclerosis in Japan. Amyotroph Lateral Scler 2007;8:300–304. [DOI] [PubMed] [Google Scholar]
- 8.Veldink JH, Kalmijn S, Groeneveld GJ, et al. Intake of polyunsaturated fatty acids and vitamin E reduces the risk of developing amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2007;784:367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Reilly ÉJ, Wang H, Weisskopf MG, et al. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve 2011;44:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Åberg M, Nyberg J, Robertson J, et al. Risk factors in Swedish young men for amyotrophic lateral sclerosis in adulthood. J Neurol 2018;265:460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariosa D, Hammar N, Malmström H, et al. Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: a more than 20-year follow-up of the Swedish AMORIS cohort. Ann Neurol 2017;81:718–728. [DOI] [PubMed] [Google Scholar]
- 13.Dupuis L, Corcia P, Fergani A, et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 2008;70:1004–1009. [DOI] [PubMed] [Google Scholar]
- 14.Dupuis L, Pradat PF, Ludolph AC, Loeffler JP. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol 2011;10:75–82. [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly EJ, Bjornevik K, Schwarzschild MA, et al. Pre-diagnostic plasma urate and the risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2018;19:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornevik K, O'Reilly EJ, Berry JD, et al. Prediagnostic plasma branched chain amino acids and the risk of amyotrophic lateral sclerosis. Neurology 2018;92:e2081–e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjornevik K, Zhang Z, O'Reilly EJ, et al. Prediagnostic plasma metabolomics and the risk of amyotrophic lateral sclerosis. Neurology 2019;92:e2089–e2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the three nurses' health studies. Am J Public Health 2016;106:1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464–468. [DOI] [PubMed] [Google Scholar]
- 20.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition cohort: rationale, study design, and baseline characteristics. Cancer 2002;94:2490–2501. [DOI] [PubMed] [Google Scholar]
- 21.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson GL, Manson J, Wallace R, et al. Implementation of the women's health initiative study design. Ann Epidemiol 2003;13:S5–S17. [DOI] [PubMed] [Google Scholar]
- 23.Townsend MK, Bao Y, Poole EM, et al. Impact of pre-analytic blood sample collection factors on metabolomics. Cancer Epidemiology Biomarkers Prev 2016;25:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hankinson SE, Willett WC, Manson JE, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst 1995;87:1297–1302. [DOI] [PubMed] [Google Scholar]
- 25.Weisskopf MG, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, Ascherio A. Prospective study of cigarette smoking and amyotrophic lateral sclerosis. Am J Epidemiol 2004;160:26–33. [DOI] [PubMed] [Google Scholar]
- 26.Traxinger K, Kelly C, Johnson BA, Lyles RH, Glass JD. Prognosis and epidemiology of amyotrophic lateral sclerosis: analysis of a clinic population, 1997-2011. Neurol Clin Pract 2013;3:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baylin A, Kim MK, Donovan-Palmer A, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol 2005;162:373–381. [DOI] [PubMed] [Google Scholar]
- 28.Knol MJ, Vandenbroucke JP, Scott P, Egger M. What do case-control studies estimate? Survey of methods and assumptions in published case-control research. Am J Epidemiol 2008;168:1073–1081. [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 30.Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Activation of the nuclear factor-kappaB is a key event in brain tolerance. J Neurosci 2001;21:4668–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heurteaux C, Laigle C, Blondeau N, Jarretou G, Lazdunski M. Alpha-linolenic acid and riluzole treatment confer cerebral protection and improve survival after focal brain ischemia. Neuroscience 2006;137:241–251. [DOI] [PubMed] [Google Scholar]
- 32.Lang-Lazdunski L, Blondeau N, Jarretou G, Lazdunski M, Heurteaux C. Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J Vasc Surg 2003;38:564–575. [DOI] [PubMed] [Google Scholar]
- 33.Pan H, Hu XZ, Jacobowitz DM, et al. Alpha-linolenic acid is a potent neuroprotective agent against soman-induced neuropathology. Neurotoxicology 2012;33:1219–1229. [DOI] [PubMed] [Google Scholar]
- 34.Zhu H, Fan C, Xu F, Tian C, Zhang F, Qi K. Dietary fish oil n-3 polyunsaturated fatty acids and alpha-linolenic acid differently affect brain accretion of docosahexaenoic acid and expression of desaturases and sterol regulatory element-binding protein 1 in mice. J Nutr Biochem 2010;21:954–960. [DOI] [PubMed] [Google Scholar]
- 35.Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br J Nutr 2002;88:355–363. [DOI] [PubMed] [Google Scholar]
- 36.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr 2002;88:411–420. [DOI] [PubMed] [Google Scholar]
- 37.Childs CE, Kew S, Finnegan YE, et al. Increased dietary alpha-linolenic acid has sex-specific effects upon eicosapentaenoic acid status in humans: re-examination of data from a randomised, placebo-controlled, parallel study. Nutr J 2014;13:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spindler SR, Mote PL, Flegal JM. Dietary supplementation with Lovaza and krill oil shortens the life span of long-lived F1 mice. Age 2014;36:9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuduki T, Honma T, Nakagawa K, Ikeda I, Miyazawa T. Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition 2011;27:334–337. [DOI] [PubMed] [Google Scholar]
- 40.Paton CM, Ntambi JM. Biochemical and physiological function of stearoyl-CoA desaturase. Am J Physiol Endocrinol Metab 2009;297:E28–E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hussain G, Schmitt F, Henriques A, et al. Systemic down-regulation of delta-9 desaturase promotes muscle oxidative metabolism and accelerates muscle function recovery following nerve injury. PLoS One 2013;8:e64525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model. Proc Natl Acad Sci USA 2004;101:11159–11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouteloup C, Desport JC, Clavelou P, et al. Hypermetabolism in ALS patients: an early and persistent phenomenon. J Neurol 2009;256:1236–1242. [DOI] [PubMed] [Google Scholar]
- 44.Stickler DE, Royer JA, Hardin JW. Accuracy and usefulness of ICD-10 death certificate coding for the identification of patients with ALS: results from the South Carolina ALS surveillance pilot project. Amyotroph Lateral Scler 2012;13:69–73. [DOI] [PubMed] [Google Scholar]
- 45.Kioumourtzoglou MA, Rotem RS, Seals RM, Gredal O, Hansen J, Weisskopf MG. Diabetes mellitus, obesity, and diagnosis of amyotrophic lateral sclerosis: a population-based study. JAMA Neurol 2015;72:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kotsopoulos J, Tworoger SS, Campos H, et al. Reproducibility of plasma and urine biomarkers among premenopausal and postmenopausal women from the nurses' health studies. Cancer Epidemiol Biomarkers Prev 2010;19:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jumpertz R, Guijarro A, Pratley RE, Mason CC, Piomelli D, Krakoff J. Associations of fatty acids in cerebrospinal fluid with peripheral glucose concentrations and energy metabolism. PLoS One 2012;7:e41503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guest J, Garg M, Bilgin A, Grant R. Relationship between central and peripheral fatty acids in humans. Lipids Health Dis 2013;12:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in the current study are not publicly available because of restricted access, but further information about the datasets is available from the corresponding author on reasonable request.