Abstract
The Sansha Yongle Blue Hole is the world’s deepest (301 m) underwater cave and has a sharp redox gradient, with oligotrophic, anoxic, and sulfidic bottom seawater. In order to discover the microbial communities and their special biogeochemical pathways in the blue hole, we analyzed the 16S ribosomal RNA amplicons and metagenomes of microbials from seawater depths with prominent physical, chemical, and biological features. Redundancy analysis showed that dissolved oxygen was the most important factor affecting the microbial assemblages of the blue hole and surrounding open sea waters, and significantly explained 44.7% of the total variation, followed by silicate, temperature, sulfide, ammonium, methane, nitrous oxide, nitrate, dissolved organic carbon, salinity, particulate organic carbon, and chlorophyll a. We identified a bloom of Alteromonas (34.9%) at the primary nitrite maximum occurring in close proximity to the chlorophyll a peak in the blue hole. Genomic potential for nitrate reduction of Alteromonas might contribute to this maximum under oxygen decrease. Genes that would allow for aerobic ammonium oxidation, complete denitrification, and sulfur-oxidization were enriched at nitrate/nitrite-sulfide transition zone (90 and 100 m) of the blue hole, but not anammox pathways. Moreover, γ-Proteobacterial clade SUP05, ε-Proteobacterial genera Sulfurimonas and Arcobacter, and Chlorobi harbored genes for sulfur-driven denitrification process that mediated nitrogen loss and sulfide removal. In the anoxic bottom seawater (100-300 m), high levels of sulfate reducers and dissimilatory sulfite reductase gene (dsrA) potentially created a sulfidic zone of ~200 m thickness. Our findings suggest that in the oligotrophic Sansha Yongle Blue Hole, O2 deficiency promotes nitrogen- and sulfur-cycling processes mediated by metabolically versatile microbials.
Subject terms: Water microbiology, Microbial ecology
Introduction
O2-deficient regions occur throughout global oceans1. Intermediate layers of the ocean develop O2-deficient water masses, referred to as oxygen minimum zones (OMZs), due to limitation in photosynthetic O2 production and high-level aerobic respiration during the degradation of surface-derived organics2. In these OMZs, such as the Eastern Tropical South Pacific (ETSP) and the Arabian Sea, O2 concentrations fall below sensor-specific detection limits3–5. In contrast, the Peru Upwelling Region, the Namibian Shelf, and the Indian Continental Shelf experience episodic plumes of hydrogen sulfide (H2S)6–8. These sulfidic environments are also found in enclosed or semi-enclosed basins, including the Black Sea Basin9–12, the Baltic Sea Basin13–15, the Cariaco Basin16,17, and submarine caves, such as the Bahamian blue holes18, the Belize Blue Hole19, and the Sansha Yongle Blue Hole20.
In O2-deficient regions, microbial reactions control key steps in carbon, nitrogen, and sulfur transformation under successional redox gradients extending throughout the water column21. NO3− is the most energetically favorable terminal electron acceptor for anaerobic respiration, prompting the development of a dynamic nitrogen cycle4,5,22. Much of the nitrogen loss in the ocean (30–50%) occurs in OMZs23. Heterotrophic denitrification and autotrophic anaerobic ammonium oxidation (anammox) are generally responsible for fixed nitrogen loss24–31. Dissimilatory NO3− reduction to NH4+ (DNRA) takes place under suboxic or anoxic conditions and has the potential to moderate fixed nitrogen loss and to regenerate redox couples (NO2− and NH4+) for anammox5. In OMZs, heterotrophic NO3− reducers supply significant amounts of both NH4+ (via organic matter decomposition) and NO2− (via NO3− reduction) to the anammox process, suggesting a possible link between anammox and denitrification29. The relative contributions of denitrification and anammox to nitrogen loss from OMZs might depend on organic matter input, as well as on the availability of fixed nitrogen29,30,32–34. Sulfur cycling also plays an essential role in O2-deficient waters, coupling the production and consumption of H2S4,6,7. The γ-Proteobacterial clade SUP05 couple water column H2S oxidation to NO3− reduction; these bacteria are widespread in the sulfidic waters at the bases of H2S/NO3− transition zones in OMZs, including the Arabian Sea35, the ETSP4,36,37, the Peru Upwelling Region6, the Eastern Tropical North Pacific (ETNP)38, the Bay of Bengal39, the Cariaco Basin40, as well as the Baltic and Black Seas41. Chemolithoautotrophic sulfur-oxidizing and denitrifying ε-Proteobacteria, such as the Sulfurimonas subgroup, are most abundant under higher sulfidic water conditions, such as the Namibian Shelf7, the Cariaco Basin40, and the Baltic and Black Seas41. Denitrification and H2S oxidation might create an upper limit on the escape of H2S from anoxic waters, as well as provide autotrophic organic carbon resources, namely dark primary production. Thus, in O2-deficient waters, metabolically versatile microorganisms create complex networks of carbon-, nitrogen-, and sulfur-transforming reactions, which remain to be determined.
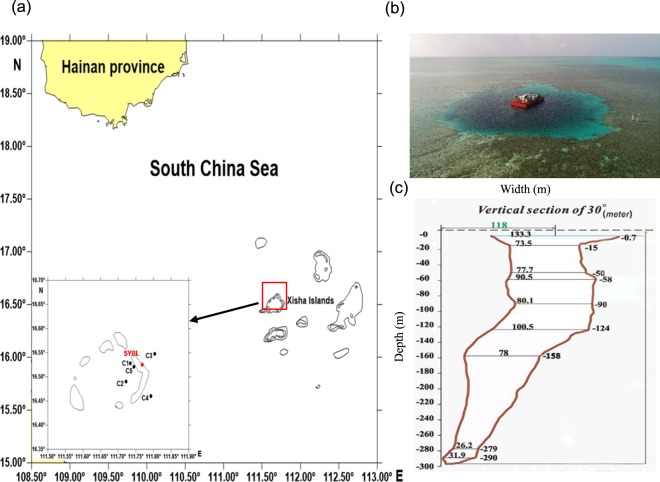
The Sansha Yongle Blue Hole is located in the Yongle Atoll of the Xisha Islands, South China Sea. The cave entrance is shaped like a comma and has an average width of 130 m (Fig. 1)42. The physiochemical characteristics are presented in detail in our parallel hydrochemical study20. Briefly, the blue hole has a sharp chemocline and sulfidic bottom waters. The O2 concentrations at surface layer in the blue hole were nearly equivalent to the maximum O2 concentrations in the euphotic layer of the surrounding open sea, as well as to the maximum O2 concentrations in the surface layers of global OMZs5–7,43. The O2 concentration declined below the detection limit (<1 μmol l−1) at 100 m using the Winkler method. The primary NO2− maximum (PNM, 0.4 µmol l−1) was identified at 30 m, and the secondary NO2− maximum (SNM, 0.2 µmol l−1) was identified at 90 m. The NH4+ concentration began to increase at 90 m, increasing to ~100 µmol l−1 at 150 m. This concentration was maintained throughout the bottom layer waters. Similar to NH4+, H2S concentration increased noticeably from ~10 µmol l−1 at 100 m, up to ~48 µmol l−1 in the deeper, euxinic waters (≥150 m) that was probably due to the much reduced ventilation. In the blue hole, a suboxic zone was identified at ~90 m, where NO3− (0.8 µmol l−1), NO2− (0.2 µmol l−1), O2 (13.4 µmol l−1), H2S (0.03 µmol l−1), and NH4+ (3.9 µmol l−1) co-existed. O2-free condition and trace amounts of NO3− and NO2− were observed between 100 and 300 m. These properties notably differed from other OMZs, including the ETNP, the OMZ off Chilean in the South Pacific Ocean, and the Arabian Sea, which are typically O2-free but NO2−-rich44. The H2S and NH4+ within the blue hole anoxic zones were several times higher than levels observed below the oxycline in the Cariaco Basin40, the Baltic and Black Seas41, and the OMZ off Peru in the South Pacific Ocean6, where trace amounts of NO3− were also detected. Particulate organic carbon (POC) concentration was low in the blue hole when compared to the Baltic sea, indicating that the blue hole had a poor nutrient input45. The blue hole is ~7 km and 70 km from Jinqing Island and Yongxing Island, respectively, and ~400 km south of Sanya42. Therefore, the anthropogenic activity has minimal influence. Together, the special geographical location and hydrochemical dynamics—in terms of the high levels of H2S and NH4+, as well as low levels of NO3−, NO2− and POC—of the blue hole might allow for distinct microbial community with diverse metabolic function to be sustained.
Figure 1.
(a) Location of sampling sites in the Sansha Yongle Blue Hole and the surrounding regions. (b) Arial view of the Sansha Yongle Blue Hole. (c) Vertical cross-section of the Sansha Yongle Blue Hole.
In this study, 16S rRNA amplicons and metagenomic analyses were utilized to determine the microbial composition and vertical distribution patterns throughout the chemical gradient profiles in the Sansha Yongle Blue Hole and the open sea. We also characterized the genomic capacity of carbon, nitrogen, and sulfur pathways, as well as linkages to physical, chemical, and biological distribution patterns. This multidisciplinary investigation will inform a new framework to explore the responses and plasticity of marine ecosystems to O2 deficiency, which is expanding, intensifying, and occurring at shallower depths due to climate change46,47.
Results and Discussion
Microbial community structures
Multiple sites were sampled across a range of depths for direct comparison: one blue hole site (SYBL), two ocean sites (C3 and C4), and three lagoon sites (C1, C2, and C5; Fig. 1; Table 1). We successfully sequenced 40 PCR samples, and high-quality sequence reads were generated for further analysis. Overall, 16, 596 operational taxonomic units (OTUs) were identified across all samples, with some samples having up to 3, 595 OTUs.
Table 1.
Details of the sampling sites in the Sansha Yongle Blue Hole and the surrounding regions.
| Site | Location | Depth(m) | Sampling depth (m) | Description |
|---|---|---|---|---|
| SYBL | 16.52°N, 111.77°E | 300 | 0, 10, 30, 50, 70, 80, 90, 100, 125, 150, 200, 300 | Sansha Yongle Blue Hole |
| C1 | 16.53°N, 111.73°E | 17 | 0, 10 | Lagoon |
| C2 | 16.49°N, 111.72°E | 40 | 0, 10, 30 | Lagoon |
| C5 | 16.52°N, 111.74°E | 30 | 0, 10, 30 | Lagoon |
| C3 | 16.55°N, 111.80°E | >600 | 0, 10, 30, 50, 75, 100, 150, 200, 300 | Open sea |
| C4 | 16.46°N, 111.79°E | >600 | 0, 10, 30, 50, 75, 100, 150, 200, 300 | Open sea |
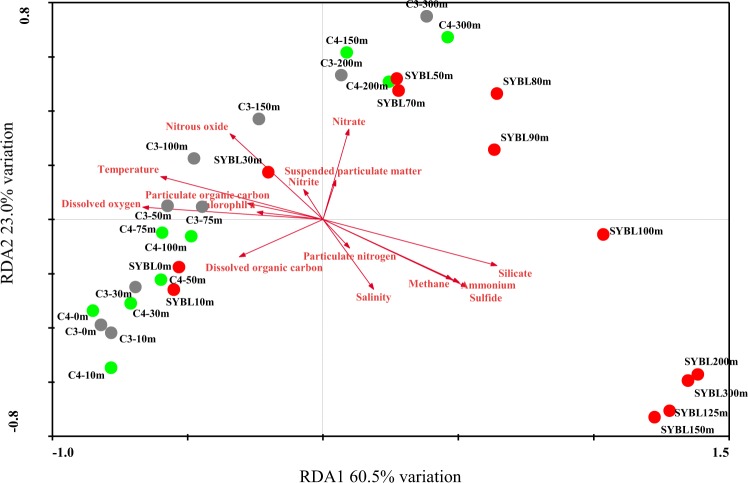
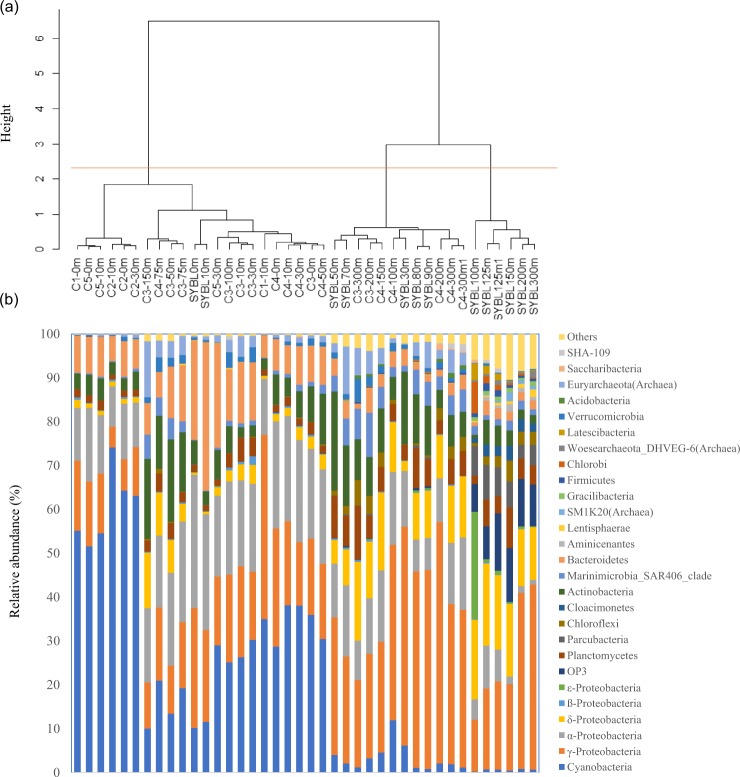
Based on redundancy analysis (RDA, Fig. 2), environmental data explained 83.5% of total community variation of the Sansha Yongle Blue Hole and surrounding open sea waters at the phylum level, where RDA1 explained 60.5% of the variation and RDA2 explained 23.0% of the variation. O2 was the most important factor affecting the microbial assemblages, and significantly explained 44.8% of the total variation, followed by silicate (44.2%), temperature (38.9%), H2S (34.8%), NH4+ (31.2%), CH4 (28.2%), nitrous oxide (N2O, 22.3%), NO3− (12.8%), dissolved organic carbon (DOC, 12.4%), salinity(11.7%), POC (9.2%), and chlorophyll a (7.3%) (P < 0.05). On the basis of the cluster analysis, all samples fell into three groups at the phylum level (Fig. 3a). The first group consisted of samples located between 0 m and 30 m at C5, 0 m and 10 m at C1, 0 m and 30 m at C2, 0 m and 10 m at SYBL, 0 m and 150 m at C3, and 0 m and 75 m at C4. The samples at SYBL, C3, and C4 within this group had high levels of O2, DOC, POC, and chlorophyll a (Fig. 2). High levels of cyanobacteria were also observed within this depth range (Fig. 3b). The theoretical euphotic layer (1% of surface irradiance) was 51.2 m at SYBL and 80.8 m at C4, suggesting the samples in the first group at SYBL and C4 were located above the euphotic layer. Thus, these samples were characterized by high primary productivity and O2 enrichment via a light-driven process. The second group included samples located between 200 m and 300 m at C3, 100 m and 300 m at C4, and 30 m and 90 m at SYBL. These samples were from the depths with lower O2 concentration and higher NO3− level, when compared with the first group, implying NO3− accumulation and transformation. Samples in the third group were distributed among the anoxic bottom layer of the blue hole (100–300 m) and were characterized by high levels of H2S, NH4+, and CH4, suggesting highly reductive. The oxic-to-suboxic zone (30–90 m) in the blue hole displayed a similar microbial composition with deep waters of the surrounding open sea when compared with the anoxic bottom layer. This result is consistent with the observation that the functional capability of microbial communities at the shallow Landsort Deep of the Baltic Sea was similar to those of two deep communities: the 6 km-depth of a trench off Puerto Rico and the 1 km-depth of the Marmara Sea47. The similarities were both likely due to the stagnant conditions and hypoxia that shifted towards the surface of the water column47. Therefore, biochemical processes in deep waters might occur in shallow waters under O2 deficiency.
Figure 2.
Redundancy analysis (RDA) of 70 microbial phyla from the Sansha Yongle Blue Hole and surrounding regions, based on 16S rRNA amplicon sequences.
Figure 3.
(a) Cluster analysis and (b) relative abundance of microbial phyla from the Sansha Yongle Blue Hole and surrounding regions, based on 16S rRNA amplicon sequences.
Samples were also recovered in three groups at the genus level, although some samples (C4-75 m, C3-50 m, and C3-150 m) were clustered with the deep-water open sea samples (Fig. S1). This suggested that the abundance and composition of the microbial genera shifted at the PNM (SYBL: 30 m; C3: 100–150 m; and C4: 75–100 m). Interestingly, the blue hole samples within each of the three branches formed separate sub-branches. This indicated that at high taxonomic level, microbial composition in the blue hole differed from that in the surrounding water. Microbial composition also varied throughout the water column, with distinct sub-divisions partitioned along the chemocline. Therefore, microorganisms occupied different niches in the blue hole that could be linked to different biogeochemical processes.
Microbial composition and distribution
Surface layer
Based on the 16S rRNA amplicons, Cyanobacteria (10.9%), α-Proteobacteria (28.4%), γ-Proteobacteria (24.3%), Bacteroidetes (28.1%), and Actinobacteria (4.2%) were dominant at 0 m and 10 m in the blue hole (Fig. 3b). These populations are typical in marine environments, including the oxic surface waters overlying OMZs46,48. Consistently, metagenomic sequences of Cyanobacteria (25.4%), α-Proteobacteria (26.1%), γ-Proteobacteria (28.5%), and Bacteroidetes (5.4%) were dominant at 10 m in the blue hole (Fig. S2). The relative abundance of Cyanobacteria in the surface layer was 10.2% and 11.5%, which is similar to C3-150 m and C4-100 m. The extinction coefficient of visible light in the blue hole was higher than in the open sea and this rapid attenuation of light might limit cyanobacterial growth. We detected sequences affiliated with α-Proteobacterial class Rhodobacteraceae (relative abundance, 22.9% and 20.7%). Rhodobacteraceae-affiliated sequences were also abundant in the oxic surface waters overlying OMZs, including the Saanich Inlet and the ETSP46. Many Rhodobacteraceae species are known for their close associations with algal blooms, as well with particles49–51, and preferentially use labile organic substrates51. Bacteroidetes are the most abundant phylum in the world ocean after Proteobacteria and Cyanobacteria. In the blue hole, Flavobacteriales sequences accounted for a majority of Bacteroidetes and were most abundant at 0 m (22.2%) and 10 m (33.3%). This is consistent with the abundance of Bacteroidetes in other coastal areas (10–30%)52. Flavobacteriales are often associated with marine snow and marine phytoplankton blooms50,53,54. These bacteria attach to phytoplankton aggregates and efficiently degrade and preferentially consume high-molecular-mass organic matter as primary carbon and energy sources51.
Intermediate layer
Between 30 m and 90 m, the blue hole exhibited a sharp oxycline: from oxic (30–70 m), to hypoxic (80 m), and then to suboxic (90 m). The prevalent 16S rRNA amplicons across this transition included those affiliated with the γ-Proteobacteria (24.4–49.9%), Actinobacteria (11.3–22.6%), α-Proteobacteria (7.3–16.3%), Planctomycetes (3.5–9.6%), Euryarchaeota (0.2–10.9%), SAR406 (1.3–6.1%), and Cyanobacteria (0.8–6.2%) (Fig. 3b). Metagenomic sequences of Cyanobacteria (8.1%, 1.2%), α-Proteobacteria (28.8%, 9.3%), γ-Proteobacteria (47.9%, 40.3%), Euryarchaeota (0.7%, 1.5%), and Actinobacteria (0.9%, 2.5%) were also dominant at 30 m and 90 m in the blue hole, respectively (Fig. S2). In the blue hole, γ-Proteobacterial genus Alteromonas 16S rRNA sequences were abundant throughout the water column, especially at 30 m, 80 m, and 90 m (23.3–34.9%). Alteromonas species are widespread in shallow and deep waters of global oceans, including the ETNP OMZ55–57. Alteromonas species are particle-associated microaerophilic bacteria. In addition to relying on phytoplankton-derived organic matter for survival, Alteromonas species can also use NO3− as a nitrogen source58. SAR406 might participate in sulfur cycling via dissimilatory polysulfide reduction or sulfide oxidation59. The abundance of 16S rRNA sequences affiliated with SAR406 was 5.4–6.1% at 70–90 m in the blue hole, equivalent to SAR406 abundances at 150 m and 300 m at C3 and C4 (4.9–10.1%). SAR406 sequences were also highly abundant in the global OMZs46,59. In addition, 16S rRNA sequences affiliated with the methane-oxidizing archaean Marine Group II (phylum Euryarchaeota, class Thermoplasmata) were highly abundant in the blue hole at 70 m and 90 m (10.9% and 6.0%, respectively). These levels were comparable to Marine Group II abundance at 150–300 m at C3 (5.2–12.7%). The nitrite-oxidizing autotrophic Nitrospina (phylum Nitrospirae) was abundant between 50 m and 90 m in the blue hole (3.3–7.2%). The greatest Nitrospina abundance was at 90 m in the blue hole (7.2%), at 300 m at C3 (8.6%), and at 300 m at C4 (4.2%), suggesting that this genus occupied a wide range of niches.
Anoxic bottom layer
In the anoxic deeper waters of the blue hole (100–300 m), O2 was <1.0 µmol l−1, concentrations of H2S, NH4+, SiO32−, PO43−, and CH4 increased with depth, and only trace amounts of NO2− and NO3− were detected20. The microbial composition in this water layer was distinct, with the most abundant 16S rRNA amplicon sequences affiliated with the γ-Proteobacteria (11.9–42.2%), δ-Proteobacteria (12.0–18.7%), Candidatus OP3 (6.3–13.1%), Planctomycetes (2.0–9.3%), and Candidatus Parcubacteria (3.0–7.8%) (Fig. 3b). Also, metagenomic sequences of γ-Proteobacteria (67.4%) were dominant at the bottom waters of the blue hole (Fig. S2). The 16S rRNA amplicon sequences associated with the δ-Proteobacteria primarily included SO42− reducers, such as species from Desulfarculaceae, Desulfobulbaceae, and Desulphobacteraceae. We also identified 16S rRNA amplicon sequences affiliated with heterotrophic γ-Proteobacterial Pseudoalteromonas (29.6% at 200 m, 21.9% at 300 m) and Alteromonas (15.3% at 300 m, ~11.0% at 125–150 m), ε-Proteobacterial sulfur oxidizer Arcobacter (24.1% at 100 m), and phototrophic Prosthecochloris (Chlorobi, 7.2% at 100 m).
The O2-deficient environments often display ecologically specialized microbial populations, potentially mediating organic carbon turnover and syntrophic interactions. In the bottom layer waters of the blue hole, the clades of syntrophic taxa identified could potentially degrade lignocellulosic plant material or algal-derived complex organic polymers in order to produce hydrogen (H2), including Fibrobacter succinogenes (phylum Fibrobacteres)60, Latescibacteria61, and Firmicutes. The syntrophic bacteria also included taxa that convert small molecular compounds, such as glucose, pyruvic acid, short chain fatty acids, and glycerol to acetate and H2 for CH4 production—e.g., Thermotogae, Spirochaetae, Sebaldella termitidis (Fusobacteria), Elusimicrobium minutum (Elusimicrobia), Cloacimonetes, Atribacteria, Candidatus Acetothermus autotrophicum (Acetothermia), and Candidatus Hydrogenedentes62. Therefore, the blue hole represented a great amount phylogenetic and functional diversity of microbial communities that could drive matter and energy transformation throughout the water column.
Nitrogen-based metabolic potential
NH4+ production
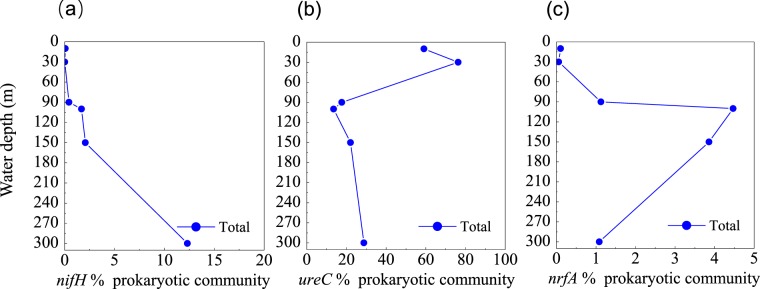
NH4+ is a central component of the marine nitrogen cycle. Sources of marine NH4+ include the degradation of organic nitrogen compounds, ammonification, N2 fixation, hydrolysis of urea, and DNRA63. We identified genes encoding molybdenum-iron nitrogenase (MoFe, nifHDK) in the blue hole, affiliated with Cyanobacteria, Chlorobi, Bacteroidetes, Proteobacteria, Firmicutes, and Verrucomicrobia. The gene of nifH increased with depth, indicating that the microbial fixation of N2 was more common in deep waters of the blue hole (Fig. 4a). We also identified ureABC genes, which encode urease, associated with Thermoplasmata, Thaumarchaeota, Cyanobacteria, Actinobacteria, and Proteobacteria. High abundance of ureC gene at 10 m and 30 m (59.1% and 76.3%) was associated with the clades of Cyanobacteria and Alteromonas australica (γ-Proteobacteria) (Fig. 4b). The gene of nrfA, encoding dissimilatory ammonia-forming nitrite reductase, peaked at 100 m (4.5%), and was primarily detected in γ-Proteobacteria and δ-Proteobacteria (Fig. 4c).
Figure 4.
Profile of the abundances of (a) nifH, (b) ureC, and (c) nrfA genes for NH4+ production, from the Sansha Yongle Blue Hole. The abundance of functional genes was shown relative to the putative single copy per organism of RNA polymerase subunit B (rpoB). Abundances per gene are normalized to gene length.
Denitrification
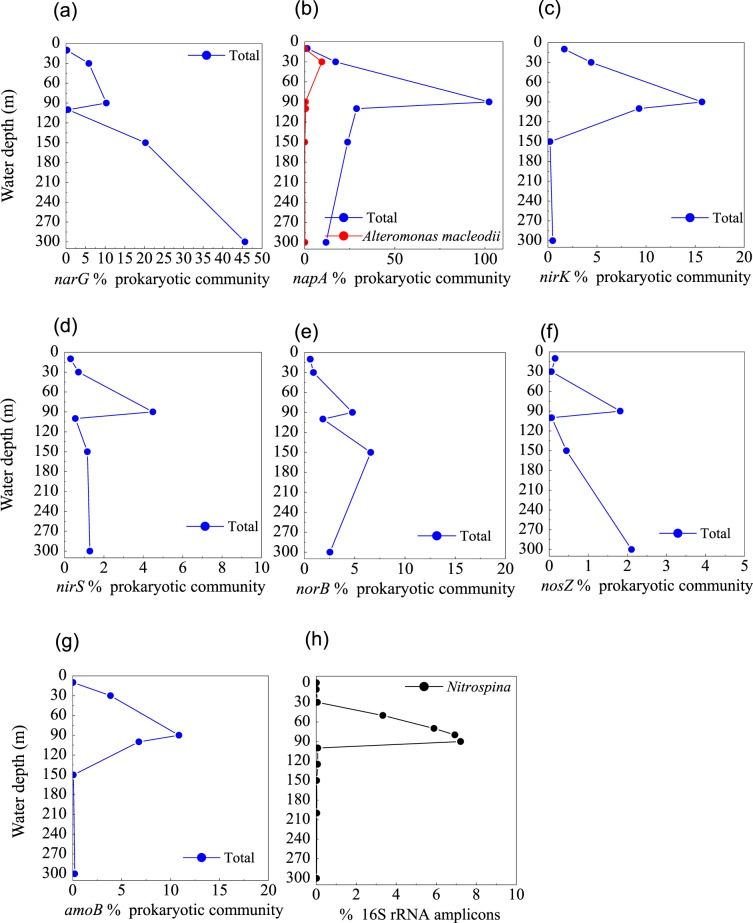
The first step in denitrification—NO3− reduction to NO2−—can be catalyzed by nitrate reductases. The metagenomes in the blue hole were enriched in the narG gene, which encodes respiratory nitrate reductase, and the napA gene, which encodes periplasmic nitrate reductase at 90 m, accounting for 10.3% and 102.3% of prokaryotic community, respectively (Fig. 5a,b). This corresponded well with a reduction in NO3− concentration and the SNM at 90 m (Fig. 6d,g), implying NO3− reduction activity. More than 100% of the prokaryotic community contained the napA gene, implying multiple copies per genome in some members. At 90 m, the narG sequences primarily matched α-Proteobacteria, as well as γ-Proteobacteria (Enterobacteriaceae and a thioautotrophic gill symbiont of Bathymodiolus septemdierum). The proportion of narG gene was much higher at 150 m and 300 m than at 90 m, Alteromonadales and unclassified bacteria contributed to the high abundance, however, the capacity for these populations to perform NO3− reduction under trace NO3− and NO2− conditions is unknown. The napA gene sequences primarily matched γ-Proteobacteria (Aeromonas hydrophila, Thiolapillus, endosymbionts from an unidentified scaly snail isolate Monju, and Candidatus Thioglobus sp. EF1), ß-Proteobacteria (Sulfuricella denitrificans and Burkholderia xenovorans), and ε-Proteobacterial genus Arcobacter. In addition, napA gene from Alteromonas macleodii accounted for up to half of all napA gene sequences at 30 m, suggesting that these species might be responsible for the PMN formation (Fig. 5b).
Figure 5.
Profile of the abundances of (a) narG, (b) napA, and (c) nirK, (d) nirS, (e) norB, and (f) nosZ genes for denitrification, (g) amoB gene for nitrification, (h) the relative abundance of Alteromonas based on 16S rRNA amplicon sequences from the Sansha Yongle Blue Hole.
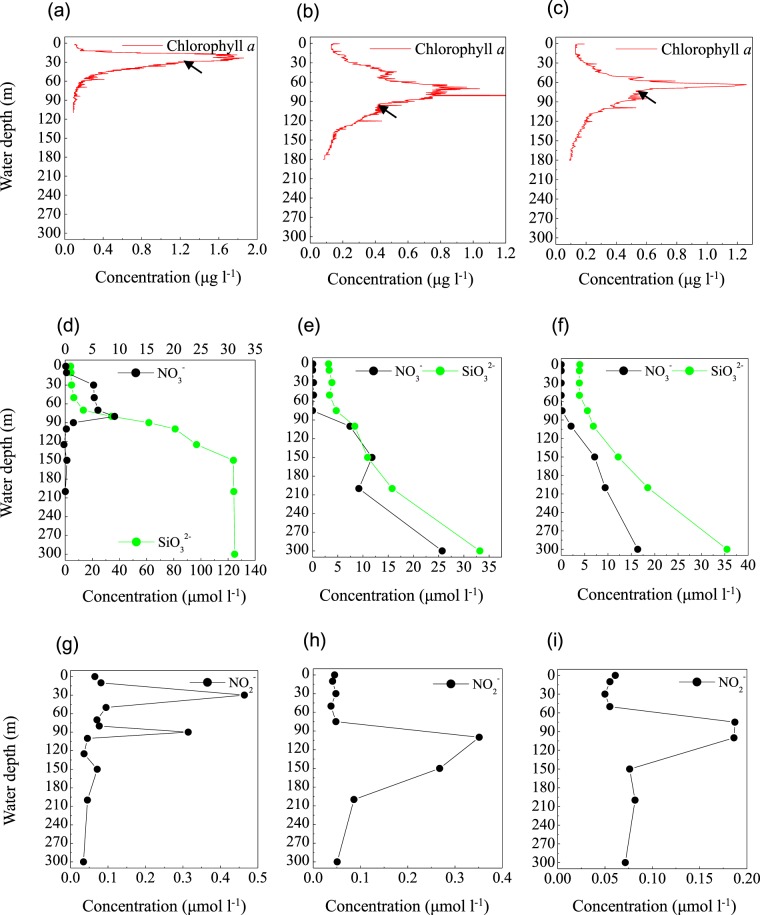
Figure 6.
Chlorophyll a concentration, NO3− and SiO32− concentrations; NO2− concentration from the Sansha Yongle Blue Hole (a,d,g); C3 (b,e,h); C4 (c,f,i). The arrows indicate the onset of PNM. (d–i) were based on our parallel hydrochemical study20.
Genes that could accomplish other steps of denitrification were identified from a consortium of diverse members, indicating a high genomic potential for complete denitrification to N2 in the blue hole. These denitrification genes encoded copper-containing nitrite reductase (nirK), iron-containing nitrite reductase (nirS), nitric oxide reductase (norB), and nitrous oxide reductase (nosZ), but were not detected at high frequencies in comparison to narG and napA. NO2− reduction to NO is mediated by nirK/nirS, and the greatest number of nirK gene was detected at 90 m, where it was present in ∼16% of the prokaryotic community (Fig. 5c). Marine Group I Thaumarchaeota was the dominant nirK-containing population. The nirS gene was present in a lower percentage of the community than nirK, but were also most abundant at 90 m (4.5%) (Fig. 5d). NO reduction to N2O is mediated by norB, which was affiliated with γ- and ε-Proteobacteria in this study, achieving two maxima at 90 m and 150 m, respectively (Fig. 5e). N2 production from N2O is mediated by nosZ gene, which peaked at 90 m and 300 m in abundance (Fig. 5f). The Rhodospirillaceae-related nosZ was abundant at 90 m, corresponding well to the upper nosZ maximum, and Flavobacteriaceae-related nosZ was abundant at 300 m. Flavobacterial nosZ was also detected in marine O2-deficient zones in the ETNP64. N2O produced in shallow water is likely to be released into the atmosphere65. However, the N2O concentration from the surface layer of the blue hole was similar to open sea surface water20, suggesting that a new balance may have been established.
Nitrification
Ammonia monooxygenase catalyzes NO2− production via NH4+ oxidation. The amoB gene encoding ammonia monooxygenase was primarily associated with Nitrosopumilus (Thaumarchaeota), reaching a maximum of 10.9% of the prokaryotic community at 90 m in the blue hole (Fig. 5g). This maximum in amoB corresponded well with the SNM and the onset of the NH4+ increase, implying that NO2− accumulation occurs via NH4+ oxidation. The relative abundance of Nitrospina based on 16S rRNA amplicons in the blue hole, increased with depth (0–90 m) and in parallel with NO3− concentration, indicating that the NO2−-oxidizing chemoautotroph might produce the observed NO3− (Figs. 5h and 6d). Nitrospina was also the main driver of NO2− oxidation in the upwelling areas of the Eastern South Pacific, increasing in abundance with depth66,67. However, the nxr gene (encoding a nitrite-oxidizing enzyme, nitrite oxidoreductase) could not be detected in the metagenomes from the blue hole water column, suggesting a low abundance of Nitrospina. The relative abundance of Nitrospina might be overestimated based on measured 16S rRNA amplicons.
Anammox
To date, Scalindua is the only genus of anammox bacteria found in marine environments68. Low abundance of 16S rRNA amplicons matching Scalindua was present at the water depth between 80 m and 100 m (0.01–0.02%) in the blue hole, where NH4+ and NO2− overlapped at 80 m and 90 m, and NO2− began to disappear at 100 m. However, Scalindua-related sequences were not recovered in the metagenomes from the blue hole. This suggested that denitrification could be the dominant pathway of N2 formation in the blue hole, considerably outpacing anammox. The NO2− depletion in the bottom waters could limit the anammox pathways, although high NH4+ concentration was detected. Moreover, H2S could also inhibit the anammox activity69. This phenomenon was also detected in the OMZ off Peru in association with a giant H2S plume6. In contrast, abundant anammox activity was detected in the suboxic zone of the Black Sea where high levels of NO3− and NO2− were present69.
The primary NO2− maximum (PNM)
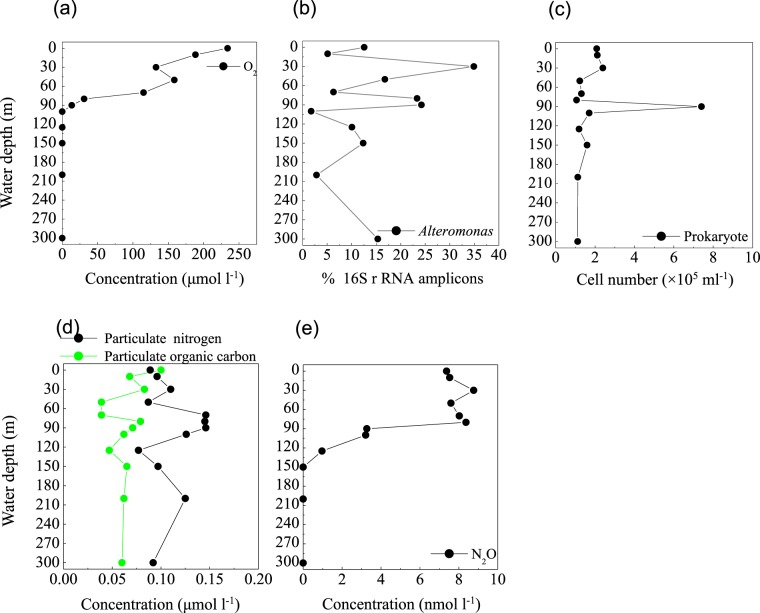
In the blue hole, based on the depth of the chlorophyll a peak base (~50 m) (Fig. 6a) and the onset of SiO32− accumulation (50 m) (Fig. 6d), we first hypothesized that the PNM would be located at ~50 m, consistent with the theoretical euphotic limit (51.2 m), and similar positions observed for the PNM in C3 (Fig. 6b,e,h) and C4 (Fig. 6c,f,i). Unexpectedly, the primary maxima of both NO2− and N2O were identified at 30 m, close to the depth of the chlorophyll a peak (Figs. 6g and 7e). At 30 m, we also identified peaks for a primary O2 minimum (~130 μmol l−1, Fig. 7a), a primary POC, and particulate nitrogen (PN) (Fig. 7d). However, the NO3− concentration peaked at the bottom of the PNM (80 m, Fig. 6d). These are all classic signals for denitrification. Indeed, Alteromonas species were maximally abundant (34.9%) at 30 m in the blue hole (Fig. 7b). Of these species, one particularly abundant species (32.1%) with a 99% identity to Alteromonas macleodii was identified (Fig. S3a). Moreover, the NapA gene from Alteromonas macleodii accounted for up to half of all napA gene sequences at 30 m (Fig. 5b). Therefore, we speculated that, at 30 m in the blue hole, photoautotrophs formed large amounts of POC, which fueled microbial growth and aerobic respiration, leading to O2 deficency. In addition, phytoplankton particles generate microscale oxyclines for suboxic or anoxic respiration in oxygenated waters57. Based on the formula of Stief et al.70, given an ambient O2 of 130 µmol l−1 at 14 °C, O2 concentration at the center of the diatom aggregate was ~40 µmol l−1, comparable to the value of 39 µmol l−1 that inhibits NO3− reduction71. Reasoning that the O2 solubility decreases with increasing temperature, at higher temperature of 25.6 °C at 30 m20, O2 concentration would be even lower within the organic aggregates. At such low O2 concentration, Alteromonas species might reduce NO3−, leading to the accumulation of NO2− in oxygenated waters. Experimental conditions have measured NO3− reduction at low O2 concentrations, which presumably matches to anoxic micro-environments71,72. Isolating and culturing an Alteromonas macleodii strain from 30 m in the blue hole revealed that this strain grew statically in diluted liquid 2216E marine medium (0.5 g yeast, 2.5 g tryptone, 1-L sea water) supplemented with 300 µmol l−1 NaNO3 for 3 d, and NO2− accumulation was evident (5.6 µmol l−1, unpublished data). This suggested that Alteromonas macleodii could perform NO3− reduction in the stagnant water. Altogether, in the O2-limited blue hole, a PNM at shallow water depth was identified and the denitrification activity of Alteromonas species might play important role in generating the PNM. Additionally, denitrification by aggregate-associated bacteria may shift the PNM towards the chlorophyll a peak in an O2-deficient marine system, which may previously have been overlooked. In addition, a primary NH4+ maximum was detected between 20 m and 80 m in the blue hole20. Low abundance of amoB gene sequences coupled with NH4+ substrate at 30 m could also partly contribute to NO2− accumulation (Fig. 5g).
Figure 7.
Profile of (a) O2 concentration, (b) the relative abundance of Alteromonas based on 16S rRNA amplicon sequences, (c) prokaryotic cell number, (d) Particulate nitrogen and particulate organic carbon concentrations, and (e) N2O concentration from the Sansha Yongle Blue Hole. (a,d,e) were based on our parallel hydrochemical study20.
The secondary NO2− maximum (SNM)
In low O2 environments, a SNM is often detected below the PNM73. NO2− in the SNM is mainly produced by dissimilatory NO3− reduction, an alternative respiratory mechanism that becomes favorable when O2 is limited. We observed a SNM in the blue hole at 90 m in close proximity to the base of the NO3− maximum (where O2 concentration had decreased to 13.4 µmol l−1) (Figs. 6g and 7a). The maximal POC and PN concentrations occurred in this layer, as well as highest abundance of prokaryotes (Fig. 7d,c). Both narG and napA genes present in heterotrophic Proteobacteria were also enriched at 90 m in the blue hole (Fig. 5a,b). Thus, at 90 m in the blue hole, O2-deficient condition and a high particle load might lead to an alternative respiration prevalent, with NO3− as an electron acceptor. In addition, at 90 m, populations containing NO3− reducing genes also harbored sulfur-oxidizing genes, including γ- Proteobacteria (thioautotrophic gill symbiont of Bathymodiolus septemdierum, and Candidatus Thioglobus), ε-Proteobacteria (Arcobacter and Sulfurimonas), and Chlorobiaceae. Therefore, the sulfur-driven chemolithotrophic denitrification could also be a crucial method for SNM formation. In addition, amoB gene reached a maximum of 10.9% of the community at 90 m, which might also be partly responsible for the NO2− accumulation (Fig. 5g).
Sulfur-based metabolic potential
Sulfate reduction
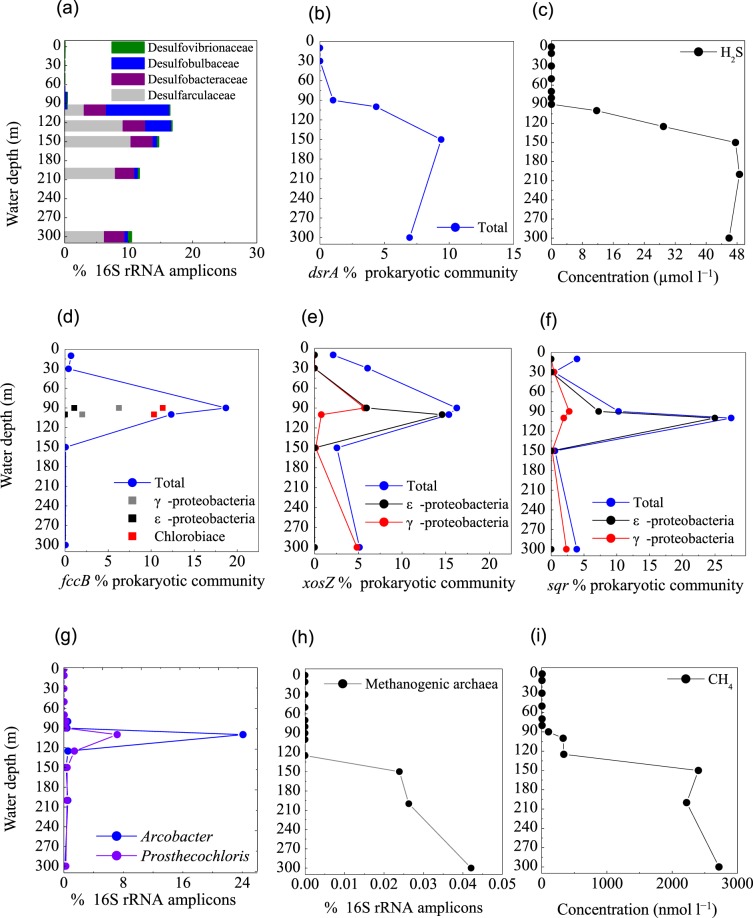
Under O2 depletion, both episodic plumes of H2S in continental shelf regions and permanent H2S under sulfidic conditions are produced by SO42–-reducing bacteria from SO42– 4,6,7. Based on 16S rRNA amplicons, diverse SO42−-reducing populations were detected at 90 m, accounting for 0.4% of total prokaryotes in the blue hole, which increased rapidly between 100 m and 300 m (10.6–16.7%). These SO42−reducers included Desulfatiglans (family Desulfarculaceae, 3.0–10.2%) and Desulfurivibrio (family Desulfobulbaceae, 0.1–2.6%). In addition, an unclassified genus in the Desulfobacteraceae (2.4–3.9%) and an unclassified genus in the Desulfobulbaceae (0.4–5.8%) were also identified. Among these taxa, Desulfococcus (0.04–0.21%) and Desulfovibrio (0.05–0.21%) were also detected in OMZ waters off the Chilean Coast4. The relative abundances of sequences associated with the Desulfovibrionaceae, Desulfarculaceae, Desulfobulbaceae, and Desulphobacteraceae were represented in Fig. 8a. In good agreement with this data, metagenomic results suggested that gene sequences encoding dissimilatory sulfite reductase (dsrA) were present in high proportions between 90 m and 300 m (1.0–9.3% of the community) (Fig. 8b). In contrast, SO42−-reducing population represented only ~0.04% between 0 m and 80 m in the blue hole, and 0.1–0.2% in the surrounding regions. The dsrA distribution was paralleled by SO42−-reducing populations and the H2S concentration (Fig. 7c) in the blue hole. Therefore, SO42− reduction in the water column is an important pathway, and might contribute to large volumes of H2S, creating a sulfidic zone as thick as ~200 m.
Figure 8.
Profile of the abundances of (a) representative SO42−-reducing populations, (b) dsrA gene for sulfate reduction, (c) H2S concentration, (d) fccB, (e) soxZ, and (f) sqr genes for sulfur oxidization, (g) representative sulfur-oxidizing denitrifiers, (h) Methanogenic archaea, (i) CH4 concentration, from the Sansha Yongle Blue Hole. (c,i) were based on our parallel hydrochemical study20.
Sulfur oxidization
Clades of sulfur-oxidizing bacteria are particularly enriched at the oxic–anoxic interfaces, where O2, NO3−, and metal oxides are available as electron acceptors6,7,19,74–76. At these interfaces, H2S can be oxidized using the sulfide: quinone oxidoreductase enzyme (Sqr) and flavocytochrome c/sulfide dehydrogenases (Fcc), forming SO3−. The SO3− can be further oxidized to SO42− by the adenylylsulfate reductase (Apr) and sulfate adenylyltransferase (Sat). Elemental sulfur and S2O32− are presumably oxidized to SO42− via the sulfur-oxidizing multienzyme complexes (Sox)77.
Mining the metagenomic data, we identified genes that could allow for dissimilatory sulfur oxidation, including genes that encode sulfide: quinone-oxidoreductase (sqr), flavocytochrome c/sulfide dehydrogenase (fccABC), sulfate adenylyltransferase (sat), adenylylsulfate reductase (apr) and sulfur-oxidizing multienzyme complexes (soxABCD and soxYZ) in the blue hole. These genes were detected in various combinations across diverse sulfur-utilizing taxa, primarily affiliated with γ-, ε-Proteobacteria, and Chlorobi. The greatest abundances of fccB and soxZ genes were present at the suboxic layer (90 m) (Fig. 8d,e), while sqr gene was present at the upper anoxic layer (100 m) (Fig. 8f), coinciding with a steep decline in H2S concentration20. This suggested that H2S oxidation may occur at the NO3−/NO2−-H2S transition in the blue hole. Further, fccB gene affiliated with Chlorobiaceae was comparable at 90 m and 100 m (~10% of community) (Fig. 8d). Genes encoding fccB, sqr, and soxZ affiliated with Candidatus Thioglobus (γ-Proteobacteria SUP05) were dominant at 90 m, while genes of sqr and soxZ affiliated with ε-Proteobacterial genera Sulfurimonas and Arcobacter were enriched at 100 m (Fig. 8d–f). Depth-specific patterns among different sulfur-metabolizing taxa might reflect differences in O2 sensitivity, as well as adaptations to varying energy substrates.
Chlorobiaceae species are phototrophic bacteria78. The high abundance of fccB gene(11.4% at 90 m and 10.3% at 100 m) and 16S rRNA amplicons (7.2% at 100 m), within the narrow layer might indicate that Chlorobiaceae members could potentially couple H2S oxidation to phototrophy, even at extremely low-light intensities (Fig. 8d,g). The Chlorobiaceae taxa contained sequences encoding nitrate reductase and nitrous reductase, as well as a RuBisCO-like protein for CO2 fixation. This suggested Chlorobiaceae could use NO3− as a potential terminal electron acceptor for H2S oxidation, linked to CO2 assimilation via Calvin cycle for dark primary production.
The metagenomic data suggested that sulfur oxidizing genes found in Candidatus Thioglobus (SUP05) were enriched in the suboxic and anoxic zones of the blue hole. Genes of sqr (0.7% at 90 m, 0.2% at 100 m), fccB (6% at 90 m, 1.4% at 100 m), soxZ (5.0% at 90 m) and napA (0.03% at 90 m, 0.01% at 100 m) were recovered, implying that Candidatus Thioglobus may prefer to reside within suboxic zone. These results support finding from recent surveys indicate that γ-Proteobacterial SUP05 can oxidize sulfur by denitrification, and is most abundant in slight to moderate redoxclines, thereby linking sulfur cycling to N-loss pathways4,12,37,38.
In contrast to SUP05-related sequences, ε-Proteobacteria preferentially colonized anoxic and highly sulfidic environments in the blue hole. The soxZ gene related to Sulfurimonas occupied 13.8% of the community at 100 m, although a minor component of the 16S rRNA amplicons (0.4%) affiliated with Sulfurimonas was detected. In addition, up to half of the norB sequences were related to Sulfurimonas species, further suggesting that the sulfur-oxidizing genus Sulfurimonas also supported reductive nitrogen metabolism. Sulfurimonas species are also widespread in the sulfidic anoxic waters of the Benguela system off Namibia7, as well as in the anoxic waters of the Baltic Sea, the Black Sea41, and the Cariaco Basin42. The sqr sequences were present in high abundance in Arcobacter (7.2% at 90 m and 25.0% at 100 m). Correspondingly, 16S rRNA amplicons affiliated with Arcobacter were also most abundant at 100 m (24.1%) (Fig. 8g). Based on the alignment of these 16S rRNA sequences with previously published sequences in GenBank, one Arcobacter-affiliated sequence from the blue hole (23.9%) were 99% identical to gill epibionts of hydrothermal vent gastropods, and Arcobacter clones from the Saanich Inlet, from the near-shore anoxic basin, and from the costal oxycline, respectively. Another Arcobacter-affiliated sequence also had 96% identity to Arcobacter nitrofigilis and 95% identity to Arcobacter sulfidicus (Fig. S3b). Arcobacter-associated sequences were also found in the OMZs off Peru6 and the sulfidic Benguela system off Namibia7, accounting for ~2–10% in abundance. These species were identified as key organisms in the chemolithotrophic oxidation of H2S with NO3− 7. The metagenomic data from our study indicated that Arcobacter species might perform denitrification (napA, nir, nor), as well as oxidizing HS–/S2– to S0 (sqr, fccB) and SO32− to SO42− (soxACD and soxYZ) for energy generation. Additionally, Arcobacter-affiliated sequences contained genes encoding clades of proteases, peptidases, and oligopeptidases, as well as enzymes critical for the oxidative tricarboxylic acid (TCA) cycle (citrate synthase). However, no glycerases were identified. This indicated that Arcobacter species used proteins, amino acids, propionates, and TCA cycle intermediates, but not carbohydrates79. We also identified gene sequences encoding key enzymes of the rTCA cycle for chemoautotrophic CO2 fixation, including citrate lyase (aclB), pyruvate flavodoxin oxidoreductase (porA), and 2-oxoglutarate-acceptor oxidoreductase (oorA). Therefore, Arcobacter species had the genomic capacity to grow chemolithoautotrophically via H2S or S2O32− oxidation that is linked to diverse steps of denitrification, as well as heterotrophically on various organic compounds. The metabolic versatility of Arcobacter might provide a competitive advantage in the energy-limited blue hole.
The microbial reduction of NOx coupling to sulfur oxidation pathways has been documented in diverse taxa from the H2S/NO3− transition zones in OMZs4,6,35–41. In the blue hole, sulfur-oxidizing denitrifiers—such as γ-, ε- Proteobacteria, and Chlorobiaceae—were enriched at 90 m and 100 m, supporting sulfur oxidation that is coupled to reductive nitrogen metabolism. It is obvious that sulfur-based denitrification occurs in this zone (90 m), where NO3−/NO2− and H2S overlapped. Meanwhile, amoB gene from Nitrosopumilus (Thaumarchaeota) was recovered at 100 m, indicating that NH4+ oxidation could provide the NO2− substrate necessary for denitrification, although this process is transient and cryptic, as trace NO3−/NO2− was detected at 100 m. This is in good agreement with a previous report on the anoxic water at Landsort Deep of the Baltic Sea47. We speculate that H2S produced by heterotrophic sulfur reducers could support sulfur-driven chemolithotrophic denitrification, which mediates both nitrogen loss and H2S removal from the blue hole.
CH4 cycle
In the blue hole, sequences associated with methanogens (phylum Euryarchaeota, order Methanomicrobiales and Methanosarcinales) were identified at 150–300 m, with a total abundance of 0.02–0.04% of total 16S rRNA amplicons (Fig. 8h). The total abundance of these taxa at 150–300 m was linear positively correlated with the concentration of CH4 (~2.4–2.7 µmol l−1, Fig. 8i, r = 0.838). Gene encoding methyl-coenzyme M reductase (mcrA), the best diagnostic enzyme for anaerobic methanogenesis, was not found in the metagenomic data. This could be explained by low levels of archaeal 16S rRNA amplicons. However, metagenomic and metatranscriptomic data in the 300 m surface sediment revealed a mcrA gene belonging to Methanosarcinales, suggesting active methanogenesis (unpublished data). Based on this study’s 16S rRNA amplicons and metagenomic sequences, coupled with recent published literatures, we propose three methanogenic pathways in the bottom waters of the blue hole. (1) Methanococcoides and Methermicoccus adopt methylotrophic pathways, including one-carbon compound pathways such as methanol conversion to CH480. Consistently, gene sequences for key enzymes were found, such as trimethylamine-corrinoid protein Co-methyltransferase and Methylated-thiol-coenzyme M methyltransferase. (2) Methanosaeta and Methanosarcina catalyze the acetoclastic pathway (acetate conversion to CH4)81,82. (3) The family Methanomicrobiaceae catalyzes the hydrogenotrophic pathway (H2 + CO2 → CH4)83. In terms of abundance, methylotrophic methanogenesis was the major pathway in the blue hole, in agreement with previous reports that some methanogens can survive in the presence of SO42- reducers by consuming noncompetitive methylated substrates84. In contrast, SO42− reduction processes could compete for these substrates, (e.g., H2 and acetate), potentially leading to a low proportion of sequences related to hydrogenotrophic and acetoclastic methanogenesis. Sequences affiliated with CH4-oxidizing archaeal Thermoplasmata displayed comparable abundance among the blue hole and the open sea waters, potentially explaining the low concentration of CH4 (<9 nmol l−1) in the oxic layers.
Conclusions
The O2 deficiency is ongoing in global oceans, and understanding the biogeochemical responses to deogxygenation in various marine ecosystems will help our adaptation to such changes. The Sansha Yongle Blue Hole can act as an indicator of how O2 loss might influence microbially mediated biochemical processes in oligotrophic marine ecosystems.
O2 plays the most important role in affecting the microbial assemblages of the blue hole and surrounding open sea waters (44.7% of the total variation). The microbial composition occurring in oxic-to-suboxic zone has characteristic of that in the deep waters of surrounding open sea. That means, biochemical processes (e.g. NO2− oxidation by Nitrospina and CH4 oxidation by archaean Marine Group II) in deep waters could occur in shallow waters when O2 is deficient. Moreover, heterotrophic aggregate-associated Alteromonas blooms and might enhance the NO3− reduction process under O2 decrease, shifting the PNM towards the chlorophyll a peak. These all might influence carbon- and nitrogen-transforming reactions in the marine ecosystems.
The NO3−/NO2−-H2S transition zone sunstains a diverse microbial community capable of sulfur oxidation by denitrification in the blue hole, such as γ-, ε-Proteobacteria, and Chlorobi. These are ubiquitous in diverse suboxic marine environments. The depth-specific patterns and metabolic versatilities enable to prevent the escape of H2S produced from the bottom layer waters. On the other hand, low level of NO2− and high level of H2S might limit anammox process, leading to NH4+ excessive.
Methods
Site locations, sampling, and biological analyses
Samples were collected in May 2017 aboard the R/V Changhe Ocean, a cargo ship, and an anchored working platform as previously described20. We established six sites in the Sansha Yongle Blue Hole and the surrounding waters: SYBL, C1, C2, C3, C4, and C5 (Fig. 1; Table 1). At each site, 5-L water samples were taken as described by Xie et al.20. All water samples were filtered through 0.22-µm acetate membranes using a vacuum pump while on board and then stored in liquid nitrogen for DNA extraction. A chlorophyll a fluorometer (Hydro-Bios Apparatebau GmbH, Kiel, Germany) was attached to a Conductivity Temperature Depth profiler (Sea-Bird SBE 911plus, Sea-Bird Electronics Inc., Bellevue, WA, USA) to measure chlorophyll a. Chlorophyll a was calculated from in vivo uncalibrated fluorescence. Prokaryotes were counted using a FACSCalibur flow cytometer (Becton Dickinson Biosciences, CA, USA) following the protocols of Marie et al.84.
We collected 40 water samples for DNA extraction across all six sites: SYBL0m, SYBL10m, SYBL30m, SYBL50m, SYBL70m, SYBL80m, SYBL90m, SYBL100m, SYBL125m, SYBL150m, SYBL200m, and SYBL300m; C3–0m, C3-10m, C3-30m, C3-50m, C3-75m, C3-100m, C3-150m, C3-200m, C3-300m; C4-0m, C4-10m, C4-30m, C4-50m, C4-75m, C4-100m, C4-150m, C4-200m, C4-300m; C1-0m, C1-10m; C2-0m, C2-10m, C2-30m; C5-0m, C5-10m, C5-30m. We sampled C4-300m (C4–300m-1) and SYBL125m (SYBL125m-1) repeatedly.
DNA extraction, 16S rRNA polymerase chain reaction (PCR) amplification, and sequencing
Total genomic DNA was extracted from each sample using a FastDNA Spin Kit for Soil (MP Biomedicals, Santa Ana, CA, USA) following the manufacturer’s instructions. The concentration and quality (A260/A280 ratio) of each DNA sample were measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA).
The V3–V4 region of the 16S ribosomal RNA gene was PCR amplified using primers 341 F (CCTACGGGNGGCWGCAG)85 and 806 R (GGACTACHVGGGTATCTAAT)86; an eight-base barcode unique to each sample was added to each sequence. PCRs were performed in triplicate. Each 50-µl PCR contained 5 μl of 10 × KOD Buffer, 5 μl of 2.5 mmol 1−1 dNTPs, 1.5 μl of each primer (5 μ mol 1−1), 1 μl of KOD polymerase, and 100 ng of template DNA. The amplification cycling program was an initial denaturation at 95 °C for 2 min, followed by 27 cycles of denaturation at 98 °C for 10 s, annealing at 62 °C for 30 s, and extension at 68 °C for 30 s, with a final extension at 68 °C for 10 min. PCR products were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, U.S.) following the manufacturer’s instructions. Equimolar volumes of purified amplicons were pooled and paired-end sequenced with an Illumina HiSeq. 2500 PE250 (Illumina, San Diego, CA, USA), following standard protocols, by GeneDenovo Biotechnology Co., Ltd. (Guangzhou, China).
Raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (accession numbers: SAMN1036346–SAMN10363471).
Bioinformatic analysis
Paired-end clean reads were merged as raw tags using FLSAH (v 1.2.11)87, with a minimum overlap of 10 bp and a mismatch error rate of 2%. We recovered 75044−109589 raw tags from each sample. Noisy sequences of raw tags were filtered using the QIIME (V1.9.1)88 pipeline with specific filtering conditions89 to obtain high-quality cleaned tags. All chimeric tags were removed. The remaining effective tags were clustered into OTUs with ≥ 97% similarity using the UPARSE pipeline90. The tag sequence with the highest abundance was selected as the representative sequence for each cluster. The representative sequences were assigned to organisms by a naive Bayesian model using the ribosomal database project classifier (Version 2.2)91, which is based on the SILVA database92. The abundances of major microbial divisions are shown as a percentage of total identifiable 16S rRNA gene sequences. Phylogenetic trees were constructed using the neighbor-joining algorithm implemented in MEGA493. Bootstrapping was performed by resampling 1000 times. Bootstrap values <50% are not shown. The scale bars represent estimated changes per nucleotide.
Metagenome sequencing and assembly
We used 1 μg DNA per sample (SYBL0m, SYBL30m, SYBL90m SYBL100m, SYBL150m, and SYBL300m) as input material for DNA library preparations. Sequencing libraries were generated using the NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA), following the manufacturer’s instructions. Index codes were added to attribute each sequence to a sample. The index-coded samples were clustered using a cBot Cluster Generation System (Illumina, San Diego, CA, USA), following the manufacturer’s instructions. After cluster generation, library preparations were sequenced on an Illumina HiSeq. 2500 platform (Illumina), and paired-end reads were generated. We recovered 66312588~87965958 clean reads from each sample. The Illumina sequencing data were assembled individually and by sample using MEGAHIT94 (the University of Hong Kong & L3 Bioinformatics Limited, Hong Kong, China; parameter:–k-min 21–k-max 81–k-step 20 -t 8). Overall, de novo assembly statistics were determined using BWA (Edition, 0.7.5a-r405)95, which calculated the percentage of paired or singleton reads realigned to the assembly. The unmapped reads from each sample were pooled and re-assembled using MEGAHIT to generate mixed assemblies. For each sample, the sample-derived and mixed assemblies were combined to obtain a final assembled contigs. A total of 2.1 Gb data were recovered from each sample.
Gene prediction and cataloging
We predicted the open reading frames (ORFs) of the final contigs (>500 bp) using MetaGeneMark96. All predicted ORFs ≥300 bp in length were pooled, and ORFs more than ≥95% identical present in ≥90% of all reads were combined with CD-HIT97 in order to reduce the number of redundant genes in the downstream assembly step. Reads were realigned to predicted genes, and read numbers were counted using BWA. The final gene catalogue included only non-redundant genes with gene read counts >2. All unique ORFs were annotated against the Kyoto Encyclopedia of Genes and Genomes using DIAMOND98. Reads were filtered, and taxonomic profiles were generated based on cleaned reads with MetaOthello99.
Abundances of metabolic function genes were calculated relative to the putative single copy per organism of RNA polymerase subunit B (rpoB). Abundances per gene were normalized to gene length4.
Statistical analysis
The similarity of the bacterial and archaeal composition across samples was analyzed by hierarchical clustering analysis in the “vegan” R package (R version 3.4.3)100. In this analysis, Hellinger distances for the relative abundances of phyla and genera among samples were calculated, coupled with the Ward linkage method. Statistically meaningful groups were then identified using fusion-level values and Mantle Pearson’s correlations in the “vegan” R package (R version 3.4.3)100. Redundancy analysis (RDA) was performed using Canoco 4.5 to assess the relationships between the biophysiochemical variables and microbial composition101. The significance of the variable was tested using Monte Carlo permutation tests with 499 unrestricted permutations (P < 0.05). Chlorophyll a and 14 physiochemical variables at the SYBL, C3 and C4 were standardized to Z–score values (zero mean, unit SD). These 14 physiochemical variables and methods were based on our parallel study20, and were shown in Table S1. The parameters included NO2−, NO3−, NH4+, SiO32−, H2S, N2O, suspended particulate matter (SPM), CH4, dissolved organic carbon (DOC), particulate organic carbon (POC), temperature, salinity, particulate nitrogen (PN), and dissolved oxygen (DO).The Hellinger distances among the relative abundances of phyla were calculated for all samples. Pearson’s correlation analyses were carried out with SPSS statistics 17.0 software to test relationships among relative abundances of different microbial groups and environmental variables.
Supplementary information
Acknowledgements
This study was supported by Basic Scientific Fund for National Public Research Institutes of China (GY0217Y02), Global Change and Air–Sea Interaction Program (GASI-01-01-01-23), Construction and Operation of Test and Technical Support System for Natural Resources Investigation and Evaluation, and the National Natural Science Foundation of China (41806099). We thank LetPub (www. LetPub. com) for its linguistic assistance during the preparation of this manuscript.
Author contributions
Peiqing He designed and performed experiment, analyzed data and wrote the manuscript. Linping Xie analyzed hydrochemical samples and data. Xuelei Zhang designed and participated in the scientific program at sea and contributed to the writing of the manuscript. Jiang Li and Xuezheng Lin participated in DNA preparation and data analysis. Xinming Pu conducted chlorophyll a fluorometer and data analysis. Chao Yuan conducted Calibur flow cytometer and data analysis. Ziwen Tian participated in sample analysis at sea. Jie Li took the photo of the arial view of the Sansha Yongle Blue Hole.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-62411-2.
References
- 1.Falkowski PG, et al. Ocean deoxygenation: past, present and future. Eos Transactions American Geophysical Union. 2011;92:409–420. doi: 10.1029/2011EO460001. [DOI] [Google Scholar]
- 2.Wyrtki K. Circulation and water masses in the eastern equatorial Pacific Ocean. Chinese Journal of Oceanology and Limnology. 1967;1:117–147. [Google Scholar]
- 3.Thamdrup B, Dalsgaard T, Revsbech NP. Wide spread functional anoxia in the oxygen minimum zone of the eastern South Pacific. Deep Sea Research Part I: Oceanographic Research Papers. 2012;65:36–45. doi: 10.1016/j.dsr.2012.03.001. [DOI] [Google Scholar]
- 4.Canfield DE, et al. A cryptic sulfur cycle in oxygen-minimum-zone waters off the Chilean Coast. Science. 2010;330:1375–1378. doi: 10.1126/science.1196889. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MM, et al. Intensive nitrogen loss over the Omani Shelf due to anammox coupled with dissimilatory nitrite reduction to ammonium. The ISME Journal. 2011;5:1660–1670. doi: 10.1038/ismej.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schunck H, et al. Giant hydrogen sulfide plume in the oxygen minimum zone off Peru supports chemolithoautotrophy. PLoS One. 2013;8:e68661. doi: 10.1371/journal.pone.0068661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavik G, et al. Detoxification of sulphidic African shelf waters by blooming chemolithotrophs. Nature. 2009;457:581–584. doi: 10.1038/nature07588. [DOI] [PubMed] [Google Scholar]
- 8.Naqvi SWA, et al. Increased marine production of N2O due to intensifying anoxia on the Indian continental shelf. Nature. 2000;408:346–349. doi: 10.1038/35042551. [DOI] [PubMed] [Google Scholar]
- 9.Jørgensen BB, Fossing H, Wirsen CO, Jannasch HW. Sulfide oxidation in the anoxic Black Sea chemocline. Deep Sea Research Part A. Oceanographic Research Papers. 1991;38:S1083–S1103. doi: 10.1016/S0198-0149(10)80025-1. [DOI] [Google Scholar]
- 10.Luther GW, Church TM, Powell D. Sulfur speciation and sulfide oxidation in the water column of the Black Sea. Deep Sea Research Part A. Oceanographic Research Papers. 1991;38:S1121–S1137. doi: 10.1016/S0198-0149(10)80027-5. [DOI] [Google Scholar]
- 11.Sorokin YI, Sorokin PY, Avdeev VA, Sorokin DY, Ilchenko SV. Biomass, production and activity of bacteria in the Black-Sea, with special reference to chemosynthesis and the sulfur cycle. Hydrobiologia. 1995;308:61–76. doi: 10.1007/bf00037788. [DOI] [Google Scholar]
- 12.Glaubitz S, Labrenz M, Jost G, Jürgens K. Diversity of active chemolithoautotrophic prokaryotes in the sulfidic zone of a Black Sea pelagic redoxcline as determined by rRNA-based stable isotope probing. FEMS Microbiology Ecology. 2010;74:32–41. doi: 10.1111/j.1574-6941.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 13.Glaubitz S, et al. 13C-isotope analyses reveal that chemolithoautotrophic Gamma- and Epsilonproteobacteria feed a microbial food web in a pelagic redoxcline of the central Baltic Sea. Environmental Microbiology. 2009;11:326–337. doi: 10.1111/j.1462-2920.2008.01770.x. [DOI] [PubMed] [Google Scholar]
- 14.Brettar I, Rheinheimer G. Denitrification in the Central Baltic: evidence for H2S-oxidation as motor of denitrification at the oxic-anoxic interface. Marine Ecology Progress Series. 1991;77:157–169. doi: 10.3354/meps077157. [DOI] [Google Scholar]
- 15.Brettar I, et al. Identification of a Thiomicrospira denitrificans-like Epsilonproteobacterium as a catalyst for autotrophic denitrification in the central Baltic Sea. Applied and Environmental Microbiology. 2006;72:1364–1372. doi: 10.1128/AEM.72.2.1364-1372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JZ, Millero FJ. The chemistry of the anoxic waters in the Cariaco Trench. Deep Sea Research Part I: Oceanographic Research Papers. 1993;40:1023–1041. doi: 10.1016/0967-0637(93)90088-K. [DOI] [Google Scholar]
- 17.Hayes, M. K., Taylor, G. T., Astor, Y. & Scranton, M. I. Vertical distributions of thiosulfate and sulfite in the Cariaco Basin. Limnology and Oceanography51, 280–287, 10.4319/lo.2006.51.1.0280 (2006).
- 18.Gonzalez BC, Iliffe TM, Macalady JL, Schaperdoth I, Kakuk B. Microbial hotspots in anchialine blue holes: initial discoveries from the Bahamas. Hydrobiologia. 2011;677:149–156. doi: 10.1007/s10750-011-0932-9. [DOI] [Google Scholar]
- 19.Gischler E, Anselmetti FS, Shinn EA. Seismic stratigraphy of the Blue Hole (Lighthouse Reef, Belize), a late Holocene climate and storm archive. Marine Geology. 2013;344:155–162. doi: 10.1016/j.margeo.2013.07.013. [DOI] [Google Scholar]
- 20.Xie L, et al. Hydrochemical properties and chemocline of the Sansha Yongle Blue Hole in the South China Sea. Science of the Total Environment. 2019;649:1281–1292. doi: 10.1016/j.scitotenv.2018.08.333. [DOI] [PubMed] [Google Scholar]
- 21.Orcutt BN, Sylvan JB, Knab NJ, Edwards KJ. Microbial ecology of the dark ocean above, at, and below the seafloor. Microbiology and Molecular Biology Reviews. 2011;75:361–422. doi: 10.1128/MMBR.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalvelage, T. et al. Oxygen sensitivity of anammox and coupled N-cycle processes in oxygen minimum zones. PLoS One6, e29299, 10.1371/journal.pone.0029299 (2011). [DOI] [PMC free article] [PubMed]
- 23.Codispoti L, et al. The oceanic fixed nitrogen and nitrous oxide budgets: moving targets as we enter the anthropocene? Scientia Marina. 2001;65:85–105. doi: 10.3989/scimar.2001.65s285. [DOI] [Google Scholar]
- 24.Lam P, Kuypers MMM. Microbial nitrogen cycling processes in oxygen minimum zones. Annual Review of Marine Science. 2011;3:317–345. doi: 10.1146/annurev-marine-120709-142814. [DOI] [PubMed] [Google Scholar]
- 25.Ward BB, et al. Denitrification as the dominant nitrogen loss process in the Arabian Sea. Nature. 2009;461:78–81. doi: 10.1038/nature08276. [DOI] [PubMed] [Google Scholar]
- 26.Bulow SE, Rich JJ, Naik HS, Pratihary AK, Ward BB. Denitrification exceeds anammox as a nitrogen loss pathway in the Arabian Sea oxygen minimum zone. Deep Sea Research Part I: Oceanographic Research Papers. 2010;57:384–393. doi: 10.1016/j.dsr.2009.10.014. [DOI] [Google Scholar]
- 27.Kuypers MMM, et al. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6478–6483. doi: 10.1073/pnas.0502088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamersley MR, et al. Anaerobic ammonium oxidation in the Peruvian oxygen minimum zone. Limnology and Oceanography. 2007;52:923–933. doi: 10.4319/lo.2007.52.3.0923. [DOI] [Google Scholar]
- 29.Lam P, et al. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4752–4757. doi: 10.1073/pnas.0812444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalvelage T, et al. Nitrogen cycling driven by organic matter export in the South Pacific oxygen minimum zone. Nature Geoscience. 2013;6:228–234. doi: 10.1038/ngeo1739. [DOI] [Google Scholar]
- 31.Thamdrup B, et al. Anaerobic ammonium oxidation in the oxygen-deficient waters off northern Chile. Limnology and Oceanography. 2006;51:2145–2156. doi: 10.4319/lo.2006.51.5.2145. [DOI] [Google Scholar]
- 32.Hannig M, et al. Shift from denitrification to anammox after inflow events in the central Baltic Sea. Limnology and Oceanography. 2007;52:1336–1345. doi: 10.4319/lo.2007.52.4.1336. [DOI] [Google Scholar]
- 33.Dalsgaard T, Thamdrup B, Farias L, Revsbech NP. Anammox and denitrification in the oxygen minimum zone of the eastern South Pacific. Limnology and Oceanography. 2012;57:1331–1346. doi: 10.4319/lo.2012.57.5.1331. [DOI] [Google Scholar]
- 34.De Brabandere L, et al. Vertical partitioning of nitrogen-loss processes across the oxic–anoxic interface of an oceanic oxygen minimum zone. Environmental Microbiology. 2014;16:3041–3054. doi: 10.1111/1462-2920.12255. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs BM, Woebken D, Zubkov MV, Burkill P, Amann R. Molecular identification of picoplankton populations in contrasting waters of the Arabian Sea. Aquatic Microbial Ecology. 2005;39:145–157. doi: 10.3354/ame039145. [DOI] [Google Scholar]
- 36.Stevens H, Ulloa O. Bacterial diversity in the oxygen minimum zone of the eastern tropical South Pacific. Environmental Microbiology. 2008;10:1244–1259. doi: 10.1111/j.1462-2920.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 37.Stewart FJ, Ulloa O, De Long EF. Microbial metatranscriptomics in a permanent marine oxygen minimum zone. Environmental Microbiology. 2012;14:23–40. doi: 10.1111/j.1462-2920.2010.02400.x. [DOI] [PubMed] [Google Scholar]
- 38.Carolan M, Beman JM. Transcriptomic evidence for microbial sulfur cycling in the eastern tropical North Pacific oxygen minimum zone. Frontiers in Microbiology. 2015;6:334. doi: 10.3389/fmicb.2015.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bristow LA, et al. N2 production rates limited by nitrite availability in the Bay of Bengal oxygen minimum zone. Nature Geoscience. 2017;10:24–29. doi: 10.1038/ngeo2847. [DOI] [Google Scholar]
- 40.Lin XJ, Scranton MI, Chistoserdov AY, Varela R, Taylor GT. Spatiotemporal dynamics of bacterial populations in the anoxic Cariaco Basin. Limnology and Oceanography. 2008;53:37–51. doi: 10.4319/lo.2008.53.1.0037. [DOI] [Google Scholar]
- 41.Grote J, Jost G, Labrenz M, Herndl GJ, Jürgens K. Epsilonproteobacteria represent the major portion of chemoautotrophic bacteria in sulfidic waters of pelagic redoxclines of the Baltic and Black Seas. Applied and Environmental Microbiology. 2008;74:7456–7551. doi: 10.1128/AEM.01186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li TG, et al. Three-dimensional (3D) morphology of Sansha Yongle Blue Hole in the South China Sea revealed by underwater remotely operated vehicle. Scientific Reports. 2018;8:17122. doi: 10.1038/s41598-018-35220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Füssel J, et al. Nitrite oxidation in the Namibian oxygen minimum zone. The ISME Journal. 2012;6:1200–1209. doi: 10.1038/ismej.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Robledo E, et al. Cryptic oxygen cycling in anoxic marine zones. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:8319–8324. doi: 10.1073/pnas.1619844114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Moigne FAC, Cisternas-Novoa C, Piontek J, Maßmig M, Engel A. On the effect of low oxygen concentrations on bacterial degradation of sinking particles. Scientific Reports. 2017;7:16722. doi: 10.1038/s41598-017-16903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright JJ, Konwar KM, Hallam SJ. Microbial ecology of expanding oxygen minimum zones. Nature Reviews Microbiology. 2012;10:381–394. doi: 10.1038/nrmicro2778. [DOI] [PubMed] [Google Scholar]
- 47.Thureborn P, et al. A metagenomics transect into the deepest point of the Baltic Sea reveals clear stratification of microbial functional capacities. PLoS One. 2013;8:e74983. doi: 10.1371/journal.pone.0074983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulloa O, Canfield DE, Delong EF, Letelier RM, Stewart FJ. Microbial oceanography of anoxic oxygen minimum zones. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15996–16003. doi: 10.1073/pnas.1205009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dang H, Lovell CR. Microbial surface colonization and biofilm development in marine environments. Microbiology & Molecular Biology Reviews. 2016;80:91–138. doi: 10.1128/MMBR.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinhassi J, et al. Changes in bacterioplankton composition under different phytoplankton regimens. Applied and Environmental Microbiology. 2004;70:6753–6766. doi: 10.1128/AEM.70.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cottrell MT, Kirchman DL. Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Applied and Environmental Microbiology. 2000;66:1692–1697. doi: 10.1128/aem.66.4.1692-1697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alonso-Sáez L, Gasol JM. Seasonal variations in the contributions of different bacterial groups to the uptake of low-molecular-weight compounds in northwestern Mediterranean coastal waters. Applied and Environmental Microbiology. 2007;73:3528–3535. doi: 10.1128/AEM.02627-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeLong EF, Franks DG, Alldredge AL. Phylogenetic diversity of aggregate-attached vs. free-living marine bacterial assemblages. Limnology and Oceanography. 1993;38:924–934. doi: 10.4319/lo.1993.38.5.0924. [DOI] [Google Scholar]
- 54.Teeling H, et al. Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science. 2012;336:608–611. doi: 10.1126/science.1218344. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Martinez J, Acinas SG, Massana R, Rodriguez-Valera F. Prevalence and microdiversity of Alteromonas macleodii-like microorganisms in different oceanic regions. Environmental Microbiology. 2002;4:42–50. doi: 10.1046/j.1462-2920.2002.00255.x. [DOI] [PubMed] [Google Scholar]
- 56.López-Pérez M, et al. Genomes of surface isolates of Alteromonas macleodii: the life of a widespread marine opportunistic copiotroph. Scientific Reports. 2012;2:696. doi: 10.1038/srep00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ganesh S, et al. Size-fraction partitioning of community gene transcription and nitrogen metabolism in a marine oxygen minimum zone. The ISME Journal. 2015;9:2682–2696. doi: 10.1038/ismej.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diner RE, Schwenck SM, Mc Crow JP, Zheng H, Allen AE. Genetic manipulation of competition for nitrate between heterotrophic bacteria and diatoms. Frontiers in Microbiology. 2016;7:880. doi: 10.3389/fmicb.2016.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wright JJ, et al. Genomic properties of Marine Group A bacteria indicate a role in the marine sulfur cycle. The ISME Journal. 2014;8:455–468. doi: 10.1038/ismej.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arntzen MØ, Várnai A, Mackie RI, Eijsink VGH, Pope PB. Outer membrane vesicles from Fibrobacter succinogenes S85 contain an array of carbohydrate-active enzymes with versatile polysaccharide-degrading capacity. Environmental Microbiology. 2017;19:2701–2714. doi: 10.1111/1462-2920.13770. [DOI] [PubMed] [Google Scholar]
- 61.Youssef NH, et al. In Silico analysis of the metabolic potential and niche specialization of candidate phylum “Latescibacteria” (WS3) PLoS One. 2015;10:e0127499. doi: 10.1371/journal.pone.0127499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nobu MK, et al. Microbial dark matter ecogenomics reveals complex synergistic networks in a methanogenic bioreactor. The ISME Journal. 2015;9:1710–22. doi: 10.1038/ismej.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuypers, M. M., Marchant, H. K. & Kartal, B. The microbial nitrogen-cycling network. Nature Reviews Microbiology16, 263–276, 10.1038/nrmicro.2018.9 (2018). [DOI] [PubMed]
- 64.Fuchsman CA, Devol AH, Saunders JK, McKay C, Rocap G. Niche partitioning of the N cycling microbial community of an offshore oxygen deficient zone. Frontiers in Microbiology. 2017;8:2384. doi: 10.3389/fmicb.2017.02384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Codispoti LA. Interesting times for marine N2O. Science. 2010;327:1339–1340. doi: 10.1126/science.1184945. [DOI] [PubMed] [Google Scholar]
- 66.Levipan HA, Molina V, Fernández C. Nitrospina-like bacteria are the main drivers of nitrite oxidation in the seasonal upwelling area of the Eastern South Pacific (central Chile ~36°S) Environmental Microbiology Reports. 2014;6:565–573. doi: 10.1111/1758-2229.12158. [DOI] [PubMed] [Google Scholar]
- 67.Mincer TJ, et al. Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environmental Microbiology. 2007;9:1162–1175. doi: 10.1111/j.1462-2920.2007.01239.x. [DOI] [PubMed] [Google Scholar]
- 68.van de Vossenberg J, et al. The metagenome of the marine anammox bacterium “Candidatus Scalindua profunda” illustrates the versatility of this globally important nitrogen cycle bacterium. Environmental Microbiology. 2013;15:1275–1289. doi: 10.1111/j.1462-2920.2012.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jensen MM, Kuypers MMM, Lavik G, Thamdrup B. Rates and regulation of anaerobic ammonium oxidation and denitrification in the Black Sea. Limnology and Oceanography. 2008;53:23–36. doi: 10.4319/lo.2008.53.1.0023. [DOI] [Google Scholar]
- 70.Stief P, Kamp A, Thamdrup B, Glud RN. Anaerobic nitrogen turnover by sinking diatom aggregates at varying ambient oxygen levels. Frontiers in Microbiology. 2016;7:98. doi: 10.3389/fmicb.2016.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oh J, Silverstein J. Acetate limitation and nitrite accumulation during denitrification. Journal of Environmental Engineering. 1999;125:234–242. doi: 10.1061/(ASCE)0733-9372(1999)125:3(234). [DOI] [Google Scholar]
- 72.Korner H, Zumft WG. Expression of denitrification enzymes in response to the dissolved oxygen levels and respiratory substrate in continuous cultures of Pseudomonas stutzeri. Applied and Environmental Microbiology. 1989;55:1670–1676. doi: 10.1128/AEM.55.7.1670-1676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brandhorst W. Nitrification and denitrification in the eastern tropical North Pacific. ICES Journal of Marine Science. 1959;25:3–20. doi: 10.1093/icesjms/25.1.3. [DOI] [Google Scholar]
- 74.Cameron M, et al. Oxygen minimum zone cryptic sulfur cycling sustained by offshore transport of key sulfur oxidizing bacteria. Nature Communications. 2018;9:1729. doi: 10.1038/s41467-018-04041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jost G, et al. Anaerobic sulfur oxidation in the absence of nitrate dominates microbial chemoautotrophy beneath the pelagic chemocline of the eastern Gotland Basin, Baltic Sea. FEMS Microbiology Ecology. 2010;71:226–236. doi: 10.1111/j.1574-6941.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 76.Inagaki F, Takai K, Kobayashi H, Nealson KH, Horikoshi K. Sulfurimonas autrotrophica gen. nov., sp. nov., a novel sulfur-oxidizing ε-proteobacterium isolated from hydrothermal sediments in the Mid-Okinawa Trough. International Journal Evolutionary Microbiology. 2003;53:1801–1805. doi: 10.1099/ijs.0.02682-0. [DOI] [PubMed] [Google Scholar]
- 77.Wasmund K, Mußmann M, Loy A. The life sulfuric: microbial ecology of sulfur cycling in marine sediments. Environmental Microbiology Reports. 2017;9:323–344. doi: 10.1111/1758-2229.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Findlay AJ, Bennett AJ, Hanson TE, Luther GW. Light-dependent sulfide oxidation in the anoxic zone of the Chesapeake Bay can be explained by small populations of phototrophic bacteria. Applied and Enviromental Microbiology. 2015;81:7560–7569. doi: 10.1128/AEM.02062-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gugliandolo C, Irrera GP, Lentini V, Maugeri TL. Pathogenic Vibrio, Aeromonas and Arcobacter spp. associated with copepods in the Straits of Messina (Italy) Marine Pollution Bulletin. 2008;56:600–606. doi: 10.1016/j.marpolbul.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Whitman WB, Ankwanda E, Wolfe RS. Nutrition and carbon metabolism of Methanococcus voltae. Journal of Bacteriology. 1982;149:852–863. doi: 10.1128/JB.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia JL. Taxonomy and ecology of methanogens. FEMS Microbiology Letters. 1990;87:297–308. doi: 10.1016/0378-1097(90)90470-B. [DOI] [Google Scholar]
- 82.Ferry JG. Enzymology of one-carbon metabolism in methanogenic pathways. FEMS Microbiology Reviews. 1999;23:13–38. doi: 10.1016/S0168-6445(98)00029-1. [DOI] [PubMed] [Google Scholar]
- 83.Leadbetter JR, Breznak JA. Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipe. Applied and Environmental Mircobiology. 1996;62:3620–3631. doi: 10.1128/AEM.62.10.3620-3631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marie D, Partensky F, Jacquet S, Vaulot D. Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Applied and Environmental Microbiology. 1997;63:186–193. doi: 10.1128/AEM.63.1.186-193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muyzer G, De Waal EC, Uitterlinden A. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and Environmental Mircobiology. 1993;59:695–700. doi: 10.1128/AEM.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caporaso JG, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bokulich NA, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nature methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 91.Wang Q, et al. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pruesse E, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Research. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 94.Li D, Liu CM, Luo R, Sadakane K, Lam TW. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- 95.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu W, Lomsadze A, Borodovsky M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Research. 2010;38:e132–e132. doi: 10.1093/nar/gkq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–1659. doi: 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 98.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nature Methods. 2015;12:59. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 99.Liu X, et al. A novel data structure to support ultra-fast taxonomic classification of metagenomic sequences with k-mer signatures. Bioinformatics. 2018;34:171–178. doi: 10.1093/bioinformatics/btx432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oksanen, J. et al. R package for community ecologists: popular ordination methods, ecological null models & diversity analysis (version 3.4.3). https://CRAN.R-project.org/package=vegan (2019).
- 101.ter Braak, C. T. F. & Smilauer, P. CANOCO reference manual and CanoDraw for windows user’s guide: software for canonical community ordination (version 4.5). (Microcomputer Power). Ithaca NY, USA: www.canoco.com (2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.