Abstract
Oxidative stress can trigger DNA damage response and activation of cellular senescence. Accumulating studies have demonstrated that senescent cells can produce senescence-associated secretory phenotype that leads to increased bone resorption and decreased bone formation. And elimination of senescent cells or inhibition of SASP secretion has been shown to prevent bone loss in mice. N-acetylcysteine (NAC) is a strong antioxidant. However, it is unclear whether reversed estrogen deficiency-induced bone loss by antioxidant NAC was associated with the inhibition of oxidative stress, DNA damage, osteocyte senescence and SASP. In this study, OVX mice were supplemented with/without E2 or NAC, and were compared with each other. Our results showed that oxidative stress, DNA damage, osteocyte senescence and the secretion of senescence-associated inflammatory cytokines were increased in OVX mice compared with sham-operated mice. However, these parameters were obviously rescued in OVX mice supplemented with E2 or NAC. Data from this study suggest that NAC can prevent OVX-induced bone loss by inhibiting oxidative stress, DNA damage, cell senescence and the secretion of the senescence-associated secretory phenotype.
Keywords: OVX-induced bone loss, N-acetylcysteine, oxidative stress, cell senescence
Introduction
Osteoporosis is a systemic skeletal disease characterized by decreased bone mass, poor bone strength and deterioration of bone microarchitectural changes, leading to an enhanced risk of fragility fractures, deformity and other complications that affect quality of life [1]. As the global population ages, the prevalence of osteoporosis and its consequences is increasing worldwide. Postmenopausal osteoporosis is a high prevalence form of primary osteoporosis where the pathogenesis is associated with decreased estrogen levels, which leads to enhanced bone remodelling, results in lower bone density and higher fracture rates [2,3]. Despite the intense research and the relevant progress achieved, the pathogenic mechanisms of how estrogen deficiency causes bone loss remain controversial.
In the last two decades, increasing oxidative stress has been identified as a vital pathogenic mechanism of bone loss caused by estrogen deficiency [4]. Oxidative stress occurs as a result of an overproduction of ROS not balanced by an adequate level of antioxidants. The excess of ROS can inhibit osteoblastic bone formation and promote osteoclastic bone resorption [5,6] while antioxidants contribute to activation of osteoblast differentiation, mineralization process and reduction of osteoclast activity [7,8]. Multiple human reports demonstrated an inverse and positive association between peripheral markers of oxidative stress and bone mass density in postmenopausal osteoporosis [9,10]. And numerous evidence from animal studies show that bilateral ovariectomy can induce a redox imbalance characterized by increased levels of lipoperoxidation markers, such as ROS and malonaldehyde (MDA), and decreased activity of antioxidant enzyme es like glutathione peroxidase (GSH-px), glutathione reductase (GSR) and superoxide dismutase (SOD) [11-13].
Oxidative stress is an important cause of cell senescence. Oxidative stress can cause DNA damage such as DNA double-strand breaks, activate ATM kinase activity, promote phosphorylation of γ-H2AX, up-regulate p53/p21 activity and trigger cellular senescence [14]. It is reported that accumulation of DNA damage and cell senescence has been proposed to play a causal role in deregulation of bone homeostasis [15]. Bone marrow stromal cells from old mice exhibit elevated expression of senescence-associated secretory phenotype (SASP) genes, such as TNF-α, IL-1α, MMP-13, CXCL12, which can be greatly attenuated by the senolytic drug ABT263 [16,17].
N-acetylcysteine (NAC) is one of the most commonly used antioxidant compounds frequently used to decrease the oxidative stress caused by excessive ROS. However, it is unclear whether reversed estrogen deficiency-induced bone loss by antioxidant NAC treatment was associated with the inhibition of DNA damage, cellular senescence and SASP secretion. To answer this question, we established a bilateral ovariectomy (OVX) mouse model which is a mature and widely used animal model in the study of postmenopausal osteoporosis and then supplementing the OVX mice with 17β-estradiol (E2) or NAC. The control mice underwent a sham operation and were fed normally. We examined the changes in bone microarchitecture, bone turnover, oxidative stress, DNA damage, cell senescence and SASP of these mice to investigate whether NAC can inhibit the estrogen deficiency-induced bone loss by inhibiting DNA damage, cell senescence and SASP secretion.
Materials and methods
Mice
Three-week-old female C57/BL6J mice were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). They were maintained in a virus- and parasite-free barrier facility and exposed to a 12-h light, 12-h dark cycle. All the mice were randomly divided into four groups: 1) sham surgery; 2) OVX group; 3) OVX group injected with 17β-estradiol (E2) at a dose of 10 µg/kg body weight subcutaneously from 8-week-old to 16-week-old; 4) OVX group supplemented with 1 mg/ml N-acetylcysteine (NAC) in the drinking water from 3-week-old to 16-week-old. Mice were anesthetized with chloral hydrate and bilaterally ovariectomized (OVX) when they are eight weeks old. Sham operations were independently performed. All mice were sacrificed at 16 weeks of age, 8 weeks after surgery. This study was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
X-ray and microcomputed tomography (micro-CT)
After sacrifice, the right femurs were removed and fixed in PLP fixative (2% paraformaldehyde containing 0.075 M lysine and 0.01 M sodium periodate). These samples were then scanned for X-ray and micro-CT. Radiographs were performed as described previously. Same samples were then scanned on a micro-CT scanner (Sky Scan 1072 Scanner) using energy of 100 kV, and 98 µA intensity. Three-dimensional (3D) images were generated using the 3D Creator software supplied with the instrument as described previously [18].
Histology
The right tibias were removed and dissected free of soft tissue, fixed with 2% PLP fixative at 4°C and processed histologically. Then the tibias were decalcified in EDTA glycerol solution for 5 to 7 days at 4°C. The decalcified tibias were dehydrated and embedded in paraffin, after which 5 μm sections were cut on a rotary microtome. The sections were stained histochemically for total collagen or tartrate resistant acid phosphatase (TRAP) activity as described previously [19] or immunohistochemically as described below.
Immunohistochemical staining
Immunohistochemical staining was carried out for phosphorylation of histone H2AX on Ser139 (γ-H2AX), β-galactosidase (β-gal), p16INK4a, Interleukin (IL)-1α, IL-1β and MMP13, using the avidin-biotin-peroxidase complex technique with affinity-purified goat anti-rabbit γ-H2AX (Cell Signaling Technology, MA, USA), β-gal (Santa Cruz Biotechnology, Santa Cruz, CA, USA), p16INK4a (Abcam, MA, USA), IL-1α (Abcam, MA, USA), IL-1β (Abcam, MA, USA) and MMP13 antibody (Abcam, MA, USA), following previously-described methods [20].
Detection of ROS levels
The bone marrow cells from long bones were converted into single cell suspensions with syringe, then washed with cold PBS, and resuspended in binding buffer, then 106 cells per sample were incubated with 5 µL 2’,7’-dichlorofluorescein diacetate (DCFH-DA, Sigma-Aldrich, St. Louis, MO, USA) for 30 mins in the dark followed by incubation with 10% FBS for 20 mins at 37°C. ROS levels were calculated from mean fluorescence intensity (MFI) measured using a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Western blotting
Proteins were extracted from the left femurs of each group of mice. Immunoblotting was carried out as previously described [21]. Primary antibodies against SOD2 (Novus Biological, Centennial, CO, USA), γ-H2AX (Cell Signaling Technology, MA, USA), 8-OHdG (Novus Biological, Centennial, CO, USA), p16INK4a (Abcam, MA, USA), TNF-α (Abcam, MA, USA) and MMP3 (Abcam, MA, USA) were used. And β-tubulin (Bioworld Technology, St. Louis Park MN, USA) were used as loading control. Immunoreactive bands were visualized with ECL chemiluminescence (Amersham Biosciences, Chalfont St. Giles, UK) and analyzed by Scion Image Beta 4.02 (Scion, National Institutes of Health, Bethesda, MD, USA).
RNA isolation and quantitative real-time RT-PCR
RNA was isolated from left tibias with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Reverse transcription reactions were performed with the SuperScript First-Strand Synthesis System (Invitrogen). To determine the relative expression of genes of interest, quantitative real-time RT-PCR was carried out in an Applied Biosystems Cycler with a SYBR Green PCR reagent kit. The PCR primers were used as described previously [20]. GAPDH was used as the internal control for each reaction. All primers were tested for their specificity by conventional PCR before being used for quantitative analysis by real-time RT-PCR. All PCRs were performed in triplicate. Results were analyzed with SDS 7300 software, and the relative amount of mRNA was calculated after normalization for GAPDH mRNA.
Computer-assisted image analysis
After histochemical or immunohistochemical staining of sections from mice of each group, images of micrographs from single sections were digitally recorded using a rectangular template, and recordings were processed and analysed using Northern Eclipse image analysis software as described previously [20].
Statistical analysis
Statistical analysis was performed using SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA). Measured data are presented as mean ± SEM. Statistical comparisons were performed using a one-way ANOVA of qualitative data to compare differences between groups. Values of P<0.05 were considered statistically significant.
Results
The effect of E2 or NAC on the bone mineral density and bone volume in ovariectomized mice
To examine the effect of E2 or NAC on the bone mineral density and bone mass in ovariectomized mice, eight-week-old mice were bilaterally ovariectomized (OVX) and supplemented with or without N-acetylcysteine (NAC) in the drinking water, or subcutaneously injected with 17β-estradiol (E2) at a dose of 10 μg/kg body weight. After 8 weeks, body weight and uterus weight were compared with each other. Results showed that, compared with sham-operated mice, the body weight was enhanced significantly in OVX mice which were rescued by NAC or E2 administration (Figure 1A). The uterus weight of the OVX mice was decreased compared to that of the sham-operated mice, and they were not altered by NAC supplementation. However, the supplementation of E2 significantly inhibited the reduction in uterus weight of OVX mice (Figure 1B). Then, we examined bone mineral density and bone volume parameters. We found that bone mineral density, total collagen positive area, bone volume, trabecular number and thickness (Figure 2A-I) were all decreased markedly while trabecular separation was increased in OVX mice. However, both exogenous E2 and NAC supplementation could normalize these parameters. These results indicate that the role of NAC supplementation in preventing bone loss of OVX mice is comparable to E2 supplementation.
Figure 1.
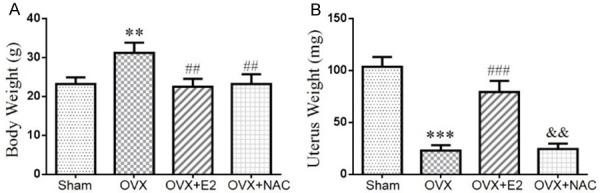
The effect of E2 or NAC on the body weight and uterus weight in ovariectomized mice. (A) Body weight and (B) uterus weight were measured before sacrificed at 16 weeks of age. Data are presented as the mean ± SEM of determinations, each data-point was the mean of five specimens. **P<0.01, ***P<0.001, versus sham mice. ##P<0.01, ###P<0.001 versus OVX mice. &&P<0.01 versus OVX+E2 mice.
Figure 2.
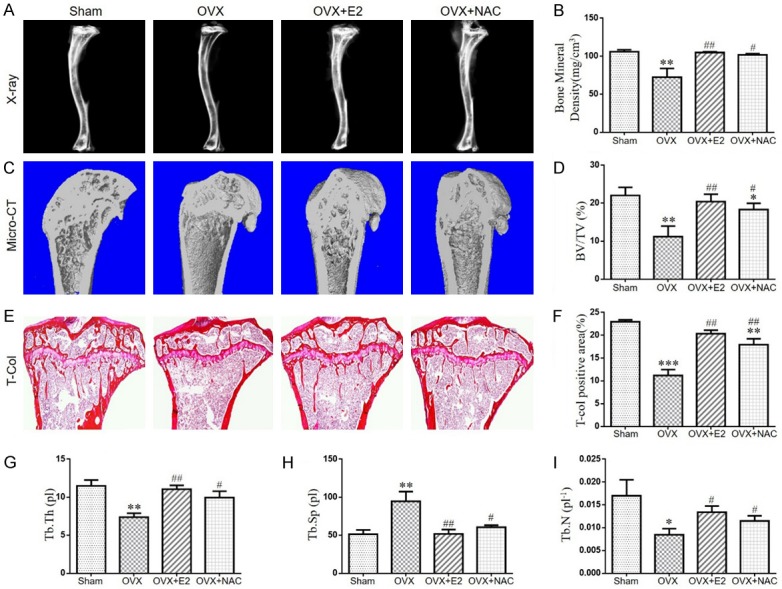
The effect of E2 or NAC on the bone mineral density and bone volume in ovariectomized mice. (A) Representative radiographs of tibias from 16-week-old mice of each group. (B) Bone mineral density analysis of the trabecular bone. (C) Representative Micro-CT-scanned and 3D reconstructed sections along the longitudinal direction of tibias. (D) Trabecular bone volume relative to tissue volume (BV/TV, %). (E) Representative micrographs of paraffin sections of tibias were stained histochemically for total collagen. (F) Total collagen positive area (%). (G) Thickness of trabecular bone (Tb.Th), (H) Trabecular separation (Tb.Sp), (I) Trabecular number (Tb.N) as analyzed with Micro-CT SkyscanCTAn software. Data are presented as the mean ± SEM of determinations, each data-point was the mean of five specimens. *P<0.05, **P<0.01, ***P<0.001, versus sham mice. #P<0.05, ##P<0.01 versus OVX mice. (E) Magnification, 50×.
The effect of E2 or NAC on the osteoclastic bone resorption in ovariectomized mice
To determine the potential role of osteoclasts in the bone loss after OVX operation and increased bone mineral density following E2 or NAC supplementation, the parameters of osteoclastic bone resorption were assessed by histochemical staining for TRAP, image analysis and real-time RT-PCR. The number of osteoclasts (Figure 3A, 3B), TRAP-positive osteoclast surface (Figure 3C) and RANKL/OPG ratio (Figure 3D) expression levels were increased in OVX mice compared with sham mice. However, these changes were largely rescued in OVX mice with E2 or NAC supplementation. Our data demonstrated that E2 or NAC supplementation could inhibit osteoclastic bone resorption in OVX mice.
Figure 3.
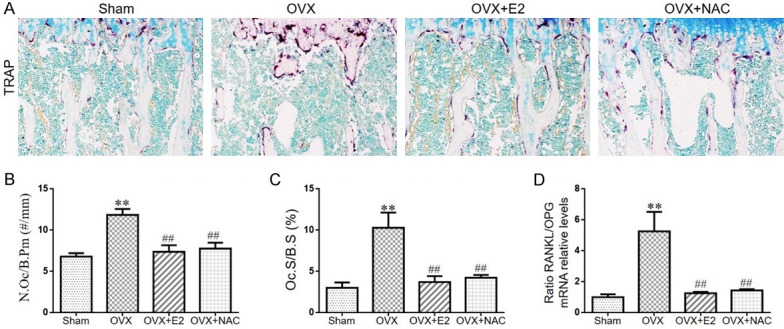
The effect of E2 or NAC on the osteoclastic bone resorption in ovariectomized mice. A. Representative photomicrographs of paraffin sections of tibias from 16-week-old mice of each group stained histochemically for TRAP. B. The number of TRAP-positive osteoclasts (N.Oc) per mm bone perimeter (B.Pm) was measured and presented. C. Osteoclast surface relative to bone surface (Oc.S/B.S, %). Real-time RT-PCR was performed on bone tissue extracts from 16-week-old mice of each group. D. Gene expression of RANKL/OPG ratio are shown. Messenger RNA expression, assessed by real-time RT-PCR analysis, was calculated as a ratio to the GAPDH mRNA level and expressed relative to levels in sham mice. Data are presented as the mean ± SEM of determinations, each data point is the mean of five specimens. **P<0.01, versus sham mice. ##P<0.01 versus OVX mice. A. Magnification, 200×.
The effect of E2 or NAC on redox balance in ovariectomized mice
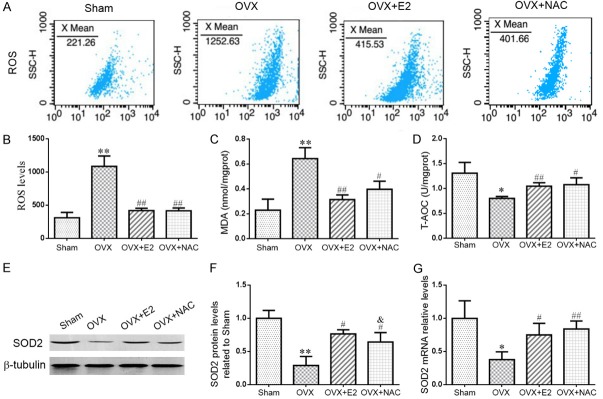
To determine whether the effect of E2 or NAC on OVX-induced bone loss was associated with oxidative stress, we examined the ROS levels, the antioxidant enzyme activity, MDA content, the protein and gene expression levels of antioxidant enzymes in long bone. We found that ROS levels (Figure 4A, 4B) and malondialdehyde (MDA) level (Figure 4C) were elevated significantly, while total antioxidant capacity (T-AOC) (Figure 4D), the protein (Figure 4E, 4F) and gene (Figure 4G) expression level of superoxide dismutase 2 (SOD2) were reduced markedly in OVX mice compared with sham mice. However, these changes were largely rescued in OVX mice supplemented with E2 or NAC. These data indicated that the role of E2 or NAC supplementation in the prevention of OVX-induced bone loss may be interrelated with decreased oxidative stress.
Figure 4.
The effect of E2 or NAC on redox balance in ovariectomized mice. (A) Representative flow cytometric analysis of ROS levels of bone marrow cells from sham, OVX, OVX+E2 and OVX+NAC mice. (B) Relative fluorescence intensity (RFI) of ROS was calculated and expressed relative to the sham mice. Biochemistry analysis of bone tissue extracts from sham, OVX, OVX+E2 and OVX+NAC mice for the (C) malonaldehyde (MDA) content and (D) total antioxidant capacity (T-AOC). (E) Representative western blots of bone tissue extracts showing expression of superoxide dismutase 2 (SOD2); β-tubulin was used as loading control for the western blots in the sham, OVX, OVX+E2 and OVX+NAC mice. (F) SOD2 protein levels relative to β-tubulin protein levels were assessed by densitometric analysis and expressed relative to levels in sham-operated mice. (G) Real-time RT-PCR was performed on long bone extracts from 16-week-old mice of each group. Gene expression of SOD2 were shown. Messenger RNA expression, assessed by real-time RT-PCR analysis, was calculated as a ratio to the GAPDH mRNA level and expressed relative to levels in sham-operated mice. Data are presented as the mean ± SEM of determinations, each data-point was the mean of five specimens. *P<0.05, **P<0.01 versus sham mice. #P<0.05, ##P<0.01 versus OVX mice. &P<0.05 versus OVX+E2 mice.
The effect of E2 or NAC on DNA damage in ovariectomized mice
To investigate whether increased oxidative stress could induce DNA damage in OVX mice, the DNA damage markers, phosphorylation of histone H2AX on Ser139 (γ-H2AX) and 8-hydroxy-2’-deoxyguanosine (8-OHdG) were examined by western blot, real time RT-PCR or immunohistochemical staining in paraffin-embedded sections of long bone. Results showed that the percentages of osteocytes positive for γ-H2AX (Figure 5A, 5B) and the protein (Figure 5C-E) and gene (Figure 5F, 5G) expression levels of γ-H2AX, 8-OHdG were higher in OVX mice than that in sham-operated mice. However, these changes were down-regulated dramatically in OVX mice supplemented with E2 or NAC. These results indicated that increased oxidative stress in OVX mice could induce DNA damage whereas E2 or NAC supplementation could inhibit DNA damage.
Figure 5.
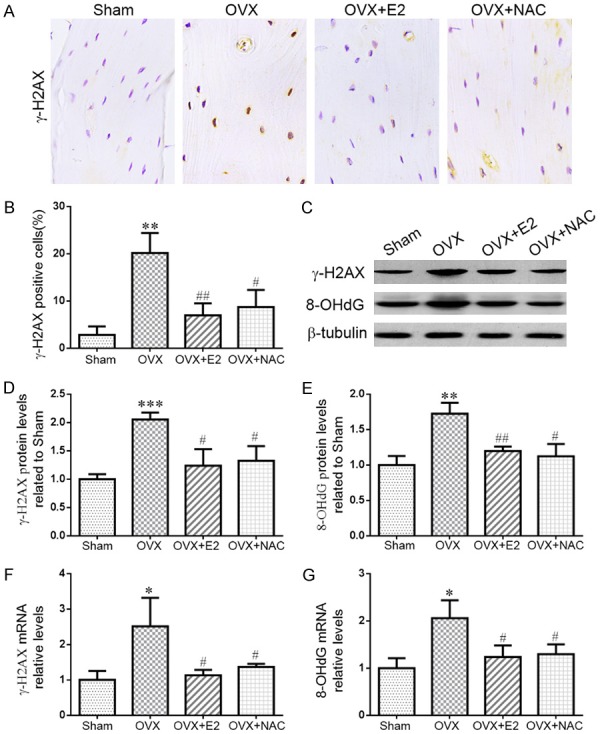
The effect of E2 or NAC on DNA damage in ovariectomized mice. (A) Representative micrographs of paraffin sections of tibias from 16-week-old mice of each group stained immunohistochemically for γ-H2AX. (B) The percentages of γ-H2AX-positive cells were determined by image analysis. (C) Representative western blots of bone tissue extracts showing expression of γ-H2AX and 8-OHdG; β-tubulin was used as loading control for the western blots in the sham, OVX, OVX+E2 and OVX+NAC groups. (D) γ-H2AX and (E) 8-OHdG protein levels relative to β-tubulin protein levels were assessed by densitometric analysis and expressed relative to levels in sham-operated mice. Real-time RT-PCR was performed on long bone extracts from 16-week-old mice of each group. Gene expression of (F) γ-H2AX and (G) 8-OHdG were shown. Messenger RNA expression, assessed by real-time RT-PCR analysis, was calculated as a ratio to the GAPDH mRNA level and expressed relative to levels in sham-operated mice. Data are presented as the mean ± SEM of determinations, each data-point was the mean of five specimens. **P<0.01, ***P<0.001 versus sham mice. #P<0.05, ##P<0.01 versus OVX mice. (A) Magnification, 400×.
The effect of E2 or NAC on osteocyte senescence in ovariectomized mice
DNA damage response can trigger the activation of cellular senescence. To determine the effect of E2 or NAC on osteocyte senescence in OVX mice, the cellular senescence markers, β-galactosidase (β-gal) and p16INK4a, were examined by Western blot or immunohistochemical staining in paraffin-embedded sections of long bone. We found that the percentages of osteocytes positive for p16INK4a (Figure 6A, 6B), β-gal (Figure 6C, 6D) and the protein expression levels of p16INK4a (Figure 6E, 6F) were elevated in OVX mice compared with sham-operated mice. However, these alterations were rescued markedly in OVX mice supplemented with E2 or NAC. Our results indicated that DNA damage could induce osteocyte senescence whereas E2 or NAC supplementation could prevent osteocyte senescence in OVX mice.
Figure 6.
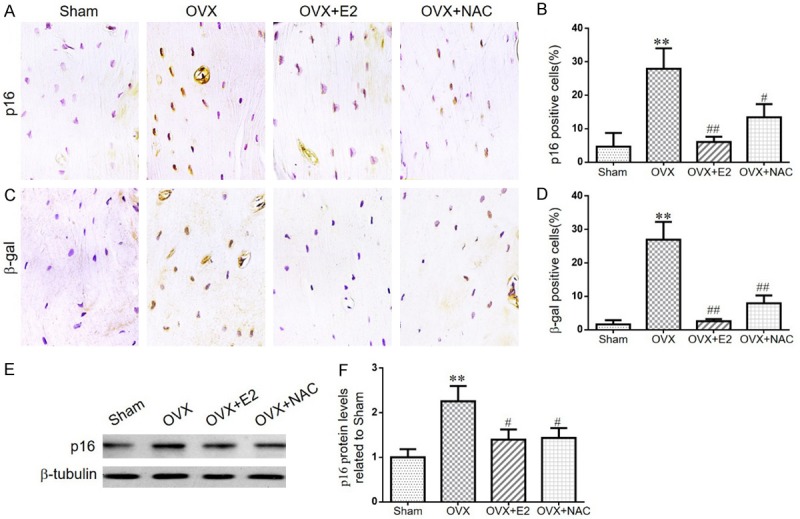
The effect of E2 or NAC on osteocyte senescence in ovariectomized mice. A. Representative micrographs of paraffin sections of tibias from 16-week-old mice of each group stained immunohistochemically for p16INK4a. B. The percentages of p16INK4a-positive cells were determined by image analysis. C. Representative micrographs of paraffin sections of tibias from 16-week-old mice of each group stained immunohistochemically for β-gal. D. The percentages of β-gal-positive cells were determined by image analysis. E. Representative western blots of bone tissue extracts showing expression of p16INK4a; β-tubulin was used as loading control for the western blots in the sham, OVX, OVX+E2 and OVX+NAC groups. F. p16INK4a protein levels relative to β-tubulin protein levels were assessed by densitometric analysis and expressed relative to levels in sham-operated mice. Data are presented as the mean ± SEM of determinations, each data-point was the mean of five specimens. **P<0.01 versus sham mice. #P<0.05, ##P<0.01 versus OVX mice. A, C. Magnification, 400×.
The effect of E2 or NAC on SASP in ovariectomized mice
Senescent cells can secrete a variety of cytokines, namely senescence-associated secretory phenotypes (SASP), including IL-1α, IL-1β, IL-6, IL-8, MMP-3, MMP-13, TNF-α and TGF-β. To verify if E2 or NAC supplementation could prevent SASP secretion in OVX mice, we examined the alterations of SASP by immunohistochemistry and western blotting. Compared with the sham-operated mice, the percentage of osteocytes positive for IL-1α (Figure 7A, 7B), IL-1β (Figure 7C, 7D), MMP13 (Figure 7E, 7F) and the protein expression levels of TNF-α, MMP3 (Figure 7G-I) were all increased dramatically in OVX mice. However, all the alterations were markedly rescued in OVX mice by E2 or NAC supplementation. These results indicated that E2 or NAC supplementation could inhibit the production of SASP.
Figure 7.
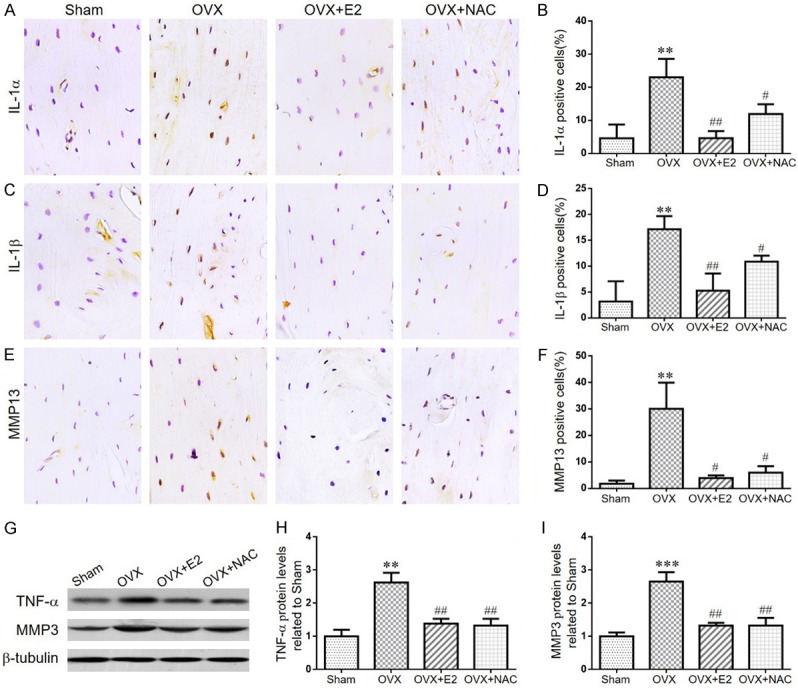
The effect of E2 or NAC on SASP in ovariectomized mice. (A) Representative micrographs of paraffin sections of tibias from 16-week-old mice of each group stained immunohistochemically for IL-1α. (B) The percentages of IL-1α-positive cells were determined by image analysis. (C) Representative micrographs of paraffin sections of tibias from 16-week-old mice of each group stained immunohistochemically for IL-1β. (D) The percentages of IL-1β-positive cells were determined by image analysis. (E) Representative micrographs of paraffin sections of tibias from 16-week-old mice of each group stained immunohistochemically for MMP13. (F) The percentages of MMP13-positive cells were determined by image analysis. (G) Representative western blots of bone tissue extracts showing expression of TNF-α, MMP3; β-tubulin was used as loading control for the western blots in the sham, OVX, OVX+E2 and OVX+NAC groups. (H) TNF-α and (I) MMP3 protein levels relative to β-tubulin protein levels were assessed by densitometric analysis and expressed relative to levels in sham-operated mice. Data are presented as the mean ± SEM of determinations, each data-point was the mean of five specimens. **P<0.01 versus sham mice. #P<0.05, ##P<0.01 versus OVX mice. (A, C, E) Magnification, 400×.
Discussion
Osteoporosis, characterized by a decrease in bone mineral density, deterioration of bone microstructure, the consequent increase in bone fragility and the risk of fracture, has become a global public health burden. Postmenopausal osteoporosis is a high prevalence form of osteoporosis, with an incidence of up to 75% [22]. In the absence of estrogen, upregulated formation and activation of osteoclasts increase bone resorption, resulting in a 3-5% reduction in bone mass per year after menopause. Drugs, such as bisphosphonates, parathyroid hormone-related peptides (PTH) and monoclonal antibodies against RANKL have been suggested for the treatment of postmenopausal osteoporosis to inhibit bone resorption or promote bone formation [23]. However, there are still many patients with osteoporosis who have not yet received appropriate treatment. Therefore, it is necessary to find better treatments for postmenopausal osteoporosis. In our current study, we used the OVX mouse model which is a mature and widely used animal model in the study of osteoporosis to investigate the possible mechanism of osteoporosis and the protective effect of NAC on the postmenopausal osteoporosis. Firstly, we showed that OVX mice exhibited estrogen deficiency, uterine atrophy and body weight gain while exogenous E2 supplementation can rescue serum E2 levels, uterine weight and body weight. At the same time, we found that antioxidant NAC supplementation limited the increase of the body weight and atrophy of the uterus, but did not alter serum E2 levels. Then, we followed the X-ray, Micro-CT scanning, three-dimensional (3D) reconstruction, total collagen staining and histomorphological parameters analysis to track changes in bone microarchitecture in each group of mice. We found that both E2 and NAC supplementation can save bone loss induced by OVX, which is consistent with previous studies.
Estrogen deficiency after menopause is recognized by increasing the formation of osteoclasts by providing a larger pool of osteoclast progenitors. The increased formation and activation of osteoclasts accelerates the area of resorption of the trabecular bone surface [24,25]. Then, we evaluated the effects of OVX, E2 or NAC supplementation on the role of osteoclasts. We found that OVX accelerated osteoclastic bone resorption, whereas E2 or NAC can significantly inhibit the bone resorption of osteoclasts. Our results demonstrated that the administration of E2 or NAC in OVX mice prevented the destruction of the bone architecture by suppressing the increase in the osteoclast activity.
Although significant progress has been made in the mechanism how estrogen deficiency induces osteoporosis, it has been found that the underlying pathogenesis is complex [26]. One of the most interesting hypotheses is that these sex hormones protect bones through antioxidants that act as antioxidants [10]. Experimental studies demonstrated that oxidative stress is an important factor in this disorder of bone homeostasis [27-29]. In vitro and animal experiments showed that the oxidative stress is produced due to insufficient defense against reactive oxygen species (ROS) by the endogenous antioxidant defense system, which in turn stimulates the formation and resorption activity of osteoclasts [30,31]. Then, we examined in our study whether osteoporosis caused by OVX was associated with the imbalance between ROS production and endogenous antioxidant defense capacity. Consistent with previous studies, our current study confirmed that OVX could increase ROS generation and reduce antioxidants levels, thereby supporting OVX-induced osteoporosis that may be related with oxidative stress. And NAC or E2 supplementation could effectively defend against oxidative stress in OVX mice, supporting that antioxidants prevented OVX-induced bone loss partially through defense against oxidative stress.
Excessive accumulation of intracellular ROS can cause oxidative damage to lipids, proteins and DNA, leading to the production of oxidized biomolecule products, such as MDA, protein carbonyl and 8-hydroxyguanine (8-OHG) [32,33]. It has been reported that the accumulation of oxidative DNA damage contributes to age-related disorders [34]. Genetic mutations encoding proteins required for DNA damage have been observed to induce dysregulation of bone homeostasis, confirming that DNA damage plays a crucial role in bone defects [15,35]. Our current results indicated that the DNA damage markers, γ-H2AX, and 8-OHdG, were increased in the osteocytes of OVX mice which suggested that increased oxidative stress can trigger DNA damage in the OVX mice whereas NAC could inhibit DNA damage in the estrogen deficiency-induced osteoporosis as effectively as the E2 supplementation.
Accumulation of DNA damage is the leading cause of senescence, which plays a fundamental role in the initiation of cellular senescence through the activation of cell cycle inhibitors, p16INK4a and p21WAF1/CIP [36]. Preclinical studies and supportive human data have shown an increase in senescent cells in the bone microenvironment with aging. Senescent cells can produce complex pro-inflammatory response called the senescence-associated secretory phenotype (SASP) that leads to increased bone resorption and decreased bone formation. Elimination of senescent cells or inhibition of SASP secretion has been shown to prevent age-related bone loss in mice [37-39]. Then, we asked if OVX-induced osteoporosis is associated with increased osteocyte senescence and SASP. To answer this question, the alterations of osteocyte senescence and SASP were examined. We demonstrated that OVX could induce osteocyte senescence and elevated SASP secretion as shown increased the expression levels of senescence marker, p16 and β-gal, and the secreted products of SASP, including IL-1α, IL-1β, MMP13, MMP3 and TNF-α, whereas E2 or NAC supplementation can efficiently suppress the expression of cell senescence markers and the development of the SASP. These results confirmed that E2 or NAC could exert an anti-osteoporosis role in OVX mice by inhibiting of senescent cells or SASP secretion.
Conclusively, the present work exhibited that supplementation of NAC may not only be useful to treat OVX-induced osteoporosis by simultaneously reducing osteoclastic bone resorption, oxidative stress and DNA damage, but also to concurrently prevent osteocyte senescence and SASP production by targeting a fundamental aging mechanism.
Acknowledgements
We thank International Science Editing for editing this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Tarantino U, Iolascon G, Cianferotti L, Masi L, Marcucci G, Giusti F, Marini F, Parri S, Feola M, Rao C, Piccirilli E, Zanetti EB, Cittadini N, Alvaro R, Moretti A, Calafiore D, Toro G, Gimigliano F, Resmini G, Brandi ML. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18:3–36. doi: 10.1007/s10195-017-0474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsson MK, Ahlborg HG, Svejme O, Nilsson JA, Rosengren BE. An increase in forearm cortical bone size after menopause may infl uence the estimated bone mineral loss--a 28-year prospective observational study. J Clin Densitom. 2016;19:174–179. doi: 10.1016/j.jocd.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 4.Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112:915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT. Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab. 2017;14:209–216. doi: 10.11138/ccmbm/2017.14.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romagnoli C, Marcucci G, Favilli F, Zonefrati R, Mavilia C, Galli G, Tanini A, Iantomasi T, Brandi ML, Vincenzini MT. Role of GSH/GSSG redox couple in osteogenic activity and osteoclastogenic markers of human osteoblast-like SaOS-2 cells. FEBS J. 2013;280:867–879. doi: 10.1111/febs.12075. [DOI] [PubMed] [Google Scholar]
- 8.Sendur OF, Turan Y, Tastaban E, Serter M. Antioxidant status in patients with osteoporosis: a controlled study. Joint Bone Spine. 2009;76:514–518. doi: 10.1016/j.jbspin.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Cervellati C, Bonaccorsi G, Cremonini E, Romani A, Fila E, Castaldini MC, Ferrazzini S, Giganti M, Massari L. Oxidative stress and bone resorption interplay as a possible trigger for postmenopausal osteoporosis. Biomed Res Int. 2014;2014:569563. doi: 10.1155/2014/569563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cervellati C, Bonaccorsi G, Cremonini E, Bergamini CM, Patella A, Castaldini C, Ferrazzini S, Capatti A, Picarelli V, Pansini FS, Massari L. Bone mass density selectively correlates with serum markers of oxidative damage in post-menopausal women. Clin Chem Lab Med. 2013;51:333–338. doi: 10.1515/cclm-2012-0095. [DOI] [PubMed] [Google Scholar]
- 11.Muthusami S, Ramachandran I, Muthusamy B, Vasudevan G, Prabhu V, Subramaniam V, Jagadeesan A, Narasimhan S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta. 2005;360:81–86. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Wang Q, Yang R, Zhang J, Li X, Zhou X, Miao D. BMI-1 mediates estrogen-deficiency-induced bone loss by inhibiting reactive oxygen species accumulation and T cell activation. J Bone Miner Res. 2017;32:962–973. doi: 10.1002/jbmr.3059. [DOI] [PubMed] [Google Scholar]
- 13.Shi C, Wu J, Yan Q, Wang R, Miao D. Bone marrow ablation demonstrates that estrogen plays an important role in osteogenesis and bone turnover via an antioxidative mechanism. Bone. 2015;79:94–104. doi: 10.1016/j.bone.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Rai P, Onder TT, Young JJ, McFaline JL, Pang B, Dedon PC, Weinberg RA. Continuous elimination of oxidized nucleotides is necessary to prevent rapid onset of cellular senescence. Proc Natl Acad Sci U S A. 2009;106:169–174. doi: 10.1073/pnas.0809834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Liu K, Robinson AR, Clauson CL, Blair HC, Robbins PD, Niedernhofer LJ, Ouyang H. DNA damage drives accelerated bone aging via an NF-kappaB-dependent mechanism. J Bone Miner Res. 2013;28:1214–1228. doi: 10.1002/jbmr.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HN, Chang J, Shao L, Han L, Iyer S, Manolagas SC, O’Brien CA, Jilka RL, Zhou D, Almeida M. DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell. 2017;16:693–703. doi: 10.1111/acel.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng Q, Gao H, Yang R, Guo K, Miao D. Pyrroloquinoline quinone prevents estrogen deficiency-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. Int J Biol Sci. 2019;15:58–68. doi: 10.7150/ijbs.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X, Li J, Zhang H, Wang H, Yin G, Miao D. Pyrroloquinoline quinone prevents testosterone deficiency-induced osteoporosis by stimulating osteoblastic bone formation and inhibiting osteoclastic bone resorption. Am J Transl Res. 2017;9:1230–1242. [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Wang G, Wang Q, Liu Q, Sun Q, Chen L. N-acetylcysteine prevents orchiectomy-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. Am J Transl Res. 2019;11:4337–4347. [PMC free article] [PubMed] [Google Scholar]
- 21.Qin R, Sun J, Wu J, Chen L. Pyrroloquinoline quinone prevents knee osteoarthritis by inhibiting oxidative stress and chondrocyte senescence. Am J Transl Res. 2019;11:1460–1472. [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders S, Geraci SA. Osteoporosis in postmenopausal women: considerations in prevention and treatment: (women’s health series) South Med J. 2013;106:698–706. doi: 10.1097/SMJ.0b013e3182a0df8b. [DOI] [PubMed] [Google Scholar]
- 23.Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016;374:254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 24.Miyauchi Y, Sato Y, Kobayashi T, Yoshida S, Mori T, Kanagawa H, Katsuyama E, Fujie A, Hao W, Miyamoto K, Tando T, Morioka H, Matsumoto M, Chambon P, Johnson RS, Kato S, Toyama Y, Miyamoto T. HIF1alpha is required for osteoclast activation by estrogen deficiency in postmenopausal osteoporosis. Proc Natl Acad Sci U S A. 2013;110:16568–16573. doi: 10.1073/pnas.1308755110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang YH, Kim KJ, Kim SJ, Mun SK, Hong SG, Son YJ, Yee ST. Suppression effect of astaxanthin on osteoclast formation in vitro and bone loss in vivo. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19030912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an infl ammatory tale. J Clin Invest. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rana K, Davey RA, Zajac JD. Human androgen deficiency: insights gained from androgen receptor knockout mouse models. Asian J Androl. 2014;16:169–177. doi: 10.4103/1008-682X.122590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q, Zhu L, Zhang D, Li N, Li Q, Dai P, Mao Y, Li X, Ma J, Huang S. Oxidative stress-related biomarkers in postmenopausal osteoporosis: a systematic review and meta-analyses. Dis Markers. 2016;2016:7067984. doi: 10.1155/2016/7067984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Wang G, Gong L, Sun G, Shi B, Bao H, Duan Y. Isopsoralen regulates PPAR gamma/WNT to inhibit oxidative stress in osteoporosis. Mol Med Rep. 2018;17:1125–1131. doi: 10.3892/mmr.2017.7954. [DOI] [PubMed] [Google Scholar]
- 30.Lean J, Kirstein B, Urry Z, Chambers T, Fuller K. Thioredoxin-1 mediates osteoclast stimulation by reactive oxygen species. Biochem Biophys Res Commun. 2004;321:845–850. doi: 10.1016/j.bbrc.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 31.Hodge JM, Collier FM, Pavlos NJ, Kirkland MA, Nicholson GC. M-CSF potently augments RANKL-induced resorption activation in mature human osteoclasts. PLoS One. 2011;6:e21462. doi: 10.1371/journal.pone.0021462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang S, Feskanich D, Willett WC, Eliassen AH, Wu T. Association between global biomarkers of oxidative stress and hip fracture in postmenopausal women: a prospective study. J Bone Miner Res. 2014;29:2577–2583. doi: 10.1002/jbmr.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labunskyy VM, Gladyshev VN. Role of reactive oxygen species-mediated signaling in aging. Antioxid Redox Signal. 2013;19:1362–1372. doi: 10.1089/ars.2012.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 35.Sijbers AM, van der Spek PJ, Odijk H, van den Berg J, van Duin M, Westerveld A, Jaspers NG, Bootsma D, Hoeijmakers JH. Mutational analysis of the human nucleotide excision repair gene ERCC1. Nucleic Acids Res. 1996;24:3370–3380. doi: 10.1093/nar/24.17.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandal PK, Blanpain C, Rossi DJ. DNA damage response in adult stem cells: pathways and consequences. Nat Rev Mol Cell Biol. 2011;12:198–202. doi: 10.1038/nrm3060. [DOI] [PubMed] [Google Scholar]
- 37.Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23:1072–1079. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112:E6301–6310. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]