Summary
In the USA, infectious diseases continue to exact a substantial toll on health and health-care resources. Endemic diseases such as chronic hepatitis, HIV, and other sexually transmitted infections affect millions of individuals and widen health disparities. Additional concerns include health-care-associated and foodborne infections—both of which have been targets of broad prevention efforts, with success in some areas, yet major challenges remain. Although substantial progress in reduction of the burden of vaccine-preventable diseases has been made, continued cases and outbreaks of these diseases persist, driven by various contributing factors. Worldwide, emerging and reemerging infections continue to challenge prevention and control strategies while the growing problem of antimicrobial resistance needs urgent action. An important priority for control of infectious disease is to ensure that scientific and technological advances in molecular diagnostics and bioinformatics are well integrated into public health. Broad and diverse partnerships across governments, health care, academia, and industry, and with the public, are essential to effectively reduce the burden of infectious diseases.
This is the second in a Series of five papers about the health of Americans
Introduction
Infectious disease prevention and control continues to improve for much of the world. During the past two decades, overall mortality from infectious causes decreased, with notable decreases recorded for lower respiratory tract infections and diarrhoeal diseases.1 Despite these successes, infectious diseases accounted for nearly a quarter of the estimated 52·8 million deaths in 2010.1 The effect of these diseases varies greatly by geographical area and populations, with low-income countries most affected.
In the USA, attention to and action against emerging infections and infectious diseases greatly increased after a 1992 landmark report by the Institute of Medicine (Emerging infections: microbial threats to health in the United States).2, 3 The report clearly outlined the increased risk of emerging infections in a highly connected world, highlighted the nation's crumbling public health infrastructure, and called for substantial improvements in the USA's capacity to address these mounting challenges. In response to this report, both the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC) developed research and prevention strategies to reduce emerging infectious diseases4, 5 and improve preparedness for outbreaks. At CDC, this plan led to the development and implementation of many new programmes designed to improve epidemiology and laboratory capacity at the national, state, and local levels. In the 1990s and early 2000s, substantial complementary investments were made to build similar, multilevel capacity against bioterrorism threats. During the past two decades, these networks, systems, and programmes have helped to forge strong links between clinical, research, and public health communities and strengthened US capacity to detect and respond to national and international outbreaks.
Our Series paper describes examples of recent and continuing challenges to control infectious diseases in the USA. We discuss vaccine-preventable diseases and the substantial successes and persistent challenges in their control; health-care-associated infections (HAIs), a leading preventable cause of death; emerging zoonotic and vector-borne diseases and their spread across a globalised world; foodborne infections, increasingly linked to new sources of contamination; and the intersecting epidemics of HIV, sexually transmitted infections (STIs), chronic viral hepatitis, and tuberculosis. Antimicrobial-resistant pathogens, several of which have become or are becoming resistant to all available drugs, are intensifying these threats. In addition to their effect on human health, these diseases cause substantial health-care, workplace, and other societal costs. We discuss priority actions needed to meet these challenges including modernisation of existing surveillance systems to better inform public health action; enhancement of public health laboratories to incorporate the latest molecular technologies for improved disease detection and outbreak response; and strengthening of the clinical and public health interface to facilitate information exchange and leverage changes in health care to improve prevention and control of infectious diseases.
Key messages.
-
•
Despite major advances towards their control, infectious diseases continue to present substantial challenges to health and health-care resources in the USA
-
•
Priority actions to meet these challenges include addressing high-burden diseases such as HIV and chronic hepatitis, foodborne diseases, and health-care-associated infections; targeting outbreaks of vaccine-preventable diseases; and controlling endemic and emerging vector-borne and zoonotic infections
-
•
Antimicrobial resistance has become a global health crisis that demands new multidisciplinary approaches and collaborations across local, state, regional, national, and international levels
-
•
Rapidly evolving molecular technologies and increases in microbial understanding offer new opportunities for treatment, prevention, and control of infectious diseases
-
•
Ongoing enhancements in communication capacities, including social media, are bringing new techniques to monitor infectious diseases and rapidly share prevention information
Vaccine-preventable diseases
The development and widespread delivery of safe and effective vaccines is one of the most beneficial and cost-saving means to control infectious diseases. For each US birth cohort receiving recommended childhood immunisations, around 20 million illnesses and more than 40 000 deaths are prevented, which results in about US$70 billion savings in direct and societal costs.6
Since 2000, new vaccines against pneumococcal disease, rotavirus, meningococcal disease, herpes zoster, and human papillomavirus (HPV) have been introduced and recommended for different populations as part of US public health policy. Immunisations against pneumococcal disease and rotavirus infection have had a substantial effect. Routine vaccinations for infants with a seven-valent pneumococcal conjugate vaccine (PCV7), which began in 2000, greatly decreased the incidence of invasive pneumococcal disease in young children, and indirectly in adults.7 From 1998–99 to 2007, overall incidence of invasive pneumococcal disease decreased by 45%;8 in children (aged <5 years) the incidence of invasive pneumococcal disease decreased from around 99 cases per 100 000 population during 1998–99 to 21 cases per 100 000 population in 2008.9 In 2010, a 13-valent vaccine (PCV13) was introduced that offered protection against an additional six serotypes of Streptococcus pneumoniae. By the end of 2011, rates of invasive pneumococcal disease caused by these six serotypes had decreased by nearly 90% in children younger than 5 years and by 45–64% in adults compared with pre-PCV13 introduction rates.10 Rotavirus vaccination, recommended for US infants from 2006, has had a substantial effect on the number of hospital admissions for diarrhoea in young children. Compared with pre-vaccine years, an estimated 77 000 fewer admissions for diarrhoea occurred in US children (aged <5 years) during 2008–09, which reduced hospital costs by around $242 million.11 Substantial and sustained decreases in rotavirus activity have occurred during every rotavirus season since the vaccine was introduced, with the number of positive rotavirus tests decreasing by 74–90% during 2010–11 and 2011–12 rotavirus seasons compared with pre-vaccine years.12 The success of these vaccines could mask the toll and dangers of these once prevalent infections; thus focused efforts are needed to communicate the continued importance of immunisations.
Vaccination against HPV, the most common STI in the USA,13 has been recommended for preteens and young adults since 2006 for girls14 and since 2011 for boys.15 HPV infections play a causal part in many cancers including cervical, other anogenital, and oropharyngeal cancers. Vaccination protects against the HPV types that cause 70% of cervical and most of the other HPV-attributable cancers. One of the two available vaccines also protects against genital warts. However, uptake of the three-dose HPV vaccination series has been disappointingly slow. In 2011, only 53% of girls aged 13–17 years had received at least one vaccine dose and only 35% had completed the series.16 Efforts to motivate health-care providers to offer the HPV vaccine and educate parents and young women of its cancer-preventing capacity are important components of this disease-reducing strategy.
US outbreaks of vaccine-preventable diseases—particularly measles, mumps, and pertussis—result from many factors including disease importation, parental decisions to delay or refuse immunisations, and waning vaccine-induced immunity. Although the USA was declared to have eliminated measles in 2000,17 around 50–100 imported cases occur every year. In 2011, however, more than 220 measles cases and 17 outbreaks were reported in the USA, mainly linked to large outbreaks in several European countries.18 This number was the highest yearly total of US reported cases of measles since the elimination of this disease until 2014, when ongoing outbreaks had resulted in almost 288 cases by May 23.19 These cases emphasise the importance of maintaining high vaccination coverage and ensuring appropriate messaging for travellers. Such cases can also present specific concerns for health-care settings. Because cases of measles are rare, health-care providers might not recognise signs or symptoms, which can facilitate further spread, including by health-care providers who do not have complete immunisations or immunity.20 Containment of measles outbreaks requires substantial public health resources. Estimated public health costs from two 2011 outbreaks in Utah affecting 13 people exceeded $330 000.21
In 2006, the largest US outbreak of mumps in two decades was reported, with 6584 cases—most from eight mid-western states and colleges.22 As with other US mumps outbreaks,23, 24 cases mainly included people living in close proximity such as dormitories and occurred despite high coverage rates of two-dose vaccine. Although the need for a third-dose of mumps-containing vaccine to be given during outbreaks and for the development of an improved vaccine has been discussed, the benefit of these additional strategies is unclear.
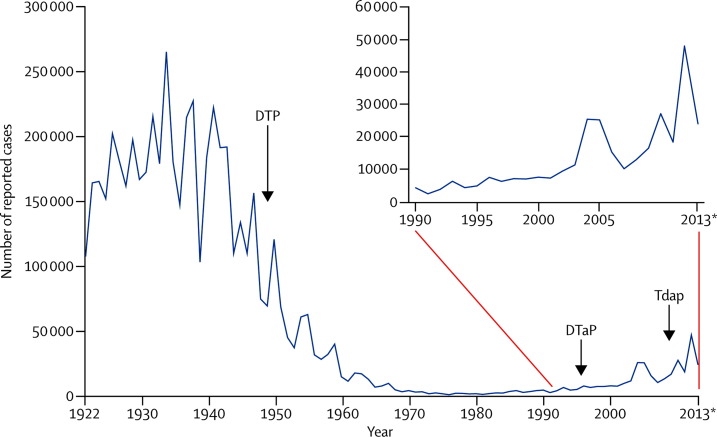
Although outbreaks of measles and mumps have been episodic in the USA, the country has had sustained increases in pertussis cases (figure 1 ). In the mid-1990s, pertussis vaccination shifted from whole-cell to an acellular vaccine to reduce adverse events. However, in the mid-2000s, illness in children and teens began to rise despite high vaccination rates, which prompted new recommendations for a pertussis booster (Tdap) in teenagers.25 Even with these new recommendations, pertussis rates have continued to increase, with substantial outbreaks in California in 2010,26 and in Washington State in 2011–12.27 A retrospective study28 in California showed that waning immunity from the acellular vaccines was the probable cause of disease resurgence. Research is in progress to improve definitions for predictors of immunity and to strengthen control efforts. Priority activities are focused on reduction of pertussis-related deaths, which occur mainly in young infants, prompting US recommendations for Tdap vaccination for pregnant women and people in close contact with infants.29
Figure 1.
US pertussis cases reported between 1922 and 2013*
DTP=diphtheria and tetanus toxoids and whole-cell pertussis vaccine. DtaP=diphtheria and tetanus toxoids and acellular pertussis vaccine, adsorbed. Tdap=tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine, adsorbed. *2013 data are provisional. Data source: Centers for Disease Control and Prevention, National Notifiable Diseases Surveillance System, Supplemental Pertussis Surveillance System 1922–49, passive reports to the Public Health Service (http://www.cdc.gov/nndss).
Health-care-associated infections
Every year, around one in five patients acquires an HAI in US hospitals.30 These infections result in about $26–33 billion in excess medical costs and around 99 000 deaths yearly.30, 31 However, these estimates do not include the substantial burden and consequences of HAIs in non-hospital settings such as long-term care facilities, dialysis settings, and outpatient clinics, where an increasing number of invasive practices are done. HAI reduction strategies largely focus on endemic problems such as catheter-associated urinary tract infections, central-line-associated bloodstream infections, and surgical-site infections, all of which can fuel antimicrobial resistance. Investigations of HAI-related outbreaks can provide important information for improvements in infection control.32 For example, infections due to unsafe injection practices,33 including reuse of syringes and inappropriate use of single-use vials, have led to targeted educational campaigns for both patients and health-care providers.34 Outbreaks have also occurred from contaminated products including those prepared in compounding pharmacies. In 2012–13, a large HAI outbreak in the USA—involving more than 14 000 patients who were potentially exposed and resulting in 751 reported cases of fungal infections, including 384 cases of meningitis and 64 deaths across 20 states35—was caused by injections of contaminated steroids produced by a single compounding facility.36
In the early to mid-2000s, several large-scale US regional and state-wide projects showed substantial decreases for central-line-associated bloodstream infections in intensive care units through focused efforts to increase adherence to a set of recommended practices for insertion and maintenance of intravascular catheters.37, 38 Momentum from these and other successes39, 40, 41 led to new federal, state, and local efforts to prevent HAIs. In 2009, a national action plan was released, setting goals and metrics for the prevention of HAIs.42 Additional efforts included state mandates for HAI reporting to improve accountability and financial incentives offered by the Centers for Medicare and Medicaid Services for adherence to CDC's infection control guidelines. All 50 states now have HAI prevention plans and state HAI coordinators, resulting in stronger links between health-care facilities, state health departments, state hospital associations, and quality-improvement organisations.
A major component of efforts to reduce HAI infections is CDC's National Healthcare Safety Network (NHSN), the most extensive and widely used HAI tracking system in the USA. With NHSN, data for HAIs and other related issues, including adherence rates of infection control, use of antibiotics, infections with multidrug-resistant organisms, and safety and vaccinations of health-care personnel, are obtained and made available to facilities, states, and regional and national organisations to help to identify and correct problems and to measure progress. Established as the National Nosocomial Infections Surveillance System in 1970, NHSN includes more than 12 000 facilities in all 50 states and extends beyond hospitals to include outpatient dialysis centres and nursing homes. Numbers of participating facilities and types of data obtained are expected to increase in the future. Reports of NHSN data, including national and state-specific reports of standardised infection ratios, have shown many successes in the reduction of the burden of certain HAIs, including a 41% decrease in central-line-associated bloodstream infections, 17% decrease in surgical-site infections, and 7% decrease in catheter-associated urinary tract infections in 2011 when compared with baseline (2008, 2009) rates.43
Despite these successes, additional efforts are needed, with intensified actions aimed at reduction of antibiotic-resistant threats.44 Comprehensive approaches are needed to prevent introduction and spread of infections as patients move between and within health-care facilities and into communities. A major concern is Clostridium difficile infection, a common and sometimes fatal infection that occurs mainly in association with health-care settings in patients receiving antibiotics. Transmitted by the faecal-oral route, C difficile infections rapidly spread in these settings because spores can survive on fomites and environmental surfaces for months. From 2000–09, discharge diagnoses of C difficile infection in patients in US hospitals more than doubled—from 139 000 to 336 600—and the number of patients with a primary C difficile infection diagnosis tripled, from 33 000 to 111 000, and only recently have these infections begun to plateau.45, 46 Data from three state-led programmes during 2008–11, showed that C difficile infections decreased by 20% in 71 hospitals that implemented specific, targeted prevention measures that focused on early detection, isolation, and enhanced environmental cleaning.46
Increases in drug-resistant Gram-negative bacterial infections, most notably carbapenem-resistant Enterobacteriaceae (CRE) are alarming. Among Enterobacteriaceae infections, several types are often transmitted in health-care settings including Escherichia coli, Klebsiella spp, and Enterobacter spp. The percentage of health-care-associated Enterobacteriaceae showing resistance to carbapenems increased from 1% to 4% during the past decade.47 CRE infections can be severe, with mortality rates more than 40%,48 and can easily spread from hospitals to other health-care settings in communities. CRE containing New Delhi metallo-β-lactamase (NDM) are of particular concern; NDM enzymes can be encoded on plasmids, which spreads multidrug-resistance to other Enterobacteriaceae and other bacteria. Identification of patients colonised by or infected with CRE and segregation of patients and staff can reduce these infections,49 but additional steps are needed. A crucial measure to control antibiotic resistance is effective implementation of antibiotic stewardship programmes to restrict the use of these drugs for appropriate indications. Additional efforts include enhanced surveillance and laboratory networks with advanced molecular diagnostic capacities to improve detection, characterisation, and control of these infections.
Zoonotic and vector-borne diseases
Zoonotic and vector-borne diseases represent most emerging infections in the USA and worldwide.50 In 2012, two notable outbreaks of zoonotic diseases in the USA were caused by Sin Nombre virus—the causative agent of hantavirus pulmonary syndrome—that was carried by deer mice infesting cabins in Yosemite National Park51 and by a variant swine influenza virus carried by pigs exhibited at agricultural fairs.52, 53 The variant swine influenza virus and avian influenza strains first reported in China (H5N154 and H7N955, 56) are closely monitored because of concerns about their potential to evolve into pandemic strains with the capacity for sustained human-to-human transmission. The influenza strain that caused the 2009–10 H1N1 pandemic, which was first reported in the USA, was a genetic reassortment of swine, avian, and human influenza viruses.57 Public health authorities are also carefully monitoring a novel coronavirus,58 termed the Middle East respiratory syndrome coronavirus (MERS-CoV), which belongs to the same viral species as the severe acute respiratory syndrome (SARS) coronavirus, a zoonotic virus linked to Chinese horseshoe bats.59 Associated with a 30% death rate, MERS-CoV has been focused in countries in the Arabian Peninsula, mainly Saudi Arabia, and has garnered global attention towards its control. Sporadic clusters of MERS-CoV cases had been reported since April, 2012, but in March, 2014, cases began to increase substantially, with 493 cases of the 699 laboratory-confirmed cases reported from March 27 to June 11, 2014.60 In May, 2014, two cases of MERS-CoV were reported in the USA (in Indiana and Florida) in travellers from Saudi Arabia.
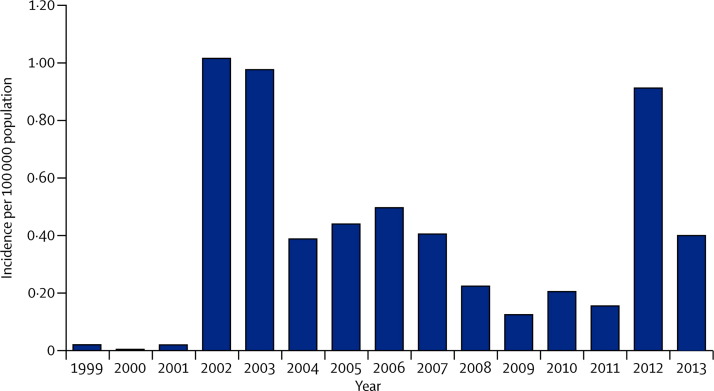
Mosquito-borne illnesses are particularly unpredictable and difficult to control.61 West Nile virus (WNV), which was first reported in in the USA in 1999,62 is now the main cause of arboviral disease in the country, affecting thousands of people every year.63, 64 Major outbreaks have occurred periodically (figure 2 ). In 2012, 5674 cases, including 2873 cases of WNV neuroinvasive disease and 286 deaths, were reported.65 Texas was at the epicentre of the 2012 outbreak66 with about a third of all reported cases.64 High proportions of WNV neuroinvasive disease suggest reporting bias, however, because severe cases are more likely than mild cases to be reported and asymptomatic infections would probably not be detected. Data from population-based surveys show that in all people who become infected with WNV (including people with asymptomatic infections) less than 1% will develop severe neuroinvasive disease.67
Figure 2.
West Nile virus neuroinvasive disease incidence in the USA, 1999–2013
Incidence reported to Centers for Disease Control and Prevention (CDC) every year. Data source: ArboNET, Arboviral Diseases Branch, CDC (http://www.cdc.gov/westnile/resources/pdfs/cummulative/99_2013_neuroinvasiveHumanCases.pdf).
In addition to WNV, dengue fever, the most prevalent vector-borne viral disease in the world, is resurging globally, affecting millions of people, including in Asia (70% of all cases, worldwide), Africa (16%), and the Americas (14%).68 Puerto Rico, which has had epidemics of dengue activity periodically since 1963, reported the largest dengue outbreak in its history in 2010, with more than 21 000 cases.69 In 2009 and 2010, Florida reported the first cases of dengue acquired in the continental USA outside of the Texas–Mexico border since 1945.70 The Pan American Health Organization (PAHO) and CDC are working to strengthen dengue control in the Americas and to ensure public heath preparedness for detection and control of exotic mosquito-borne diseases. One example is chikungunya fever,71 a dengue-like disease first detected in Tanganyika in 1953 that spread to parts of Asia and Europe,72 and has now emerged in the Americas.73 Between December, 2013, and June, 2014, cases of chikungunya infection have been reported in almost 20 countries in the Americas, mainly in the Caribbean.73
The incidence and geographical spread of tick-borne diseases is increasing in the USA. Lyme disease—the most commonly reported vector-borne illness in the USA—has spread from northeast to mid-Atlantic regions and the upper Midwest along with its insect vector (the black-legged tick, Ixodes scapularis); these insects can also carry parasites that cause babesiosis and bacteria that cause human granulocytic ehrlichiosis.74 Additional areas endemic for Lyme disease occur along the Pacific coast.75 Another endemic tick-borne disease, Rocky Mountain spotted fever—caused by the bacterium Rickettsia rickettsii—has become a particularly serious problem on Arizonan tribal lands in areas where the disease was not previously reported. These disease outbreaks represent the first time that Rhipicephalus sanguineus, the brown dog tick, has been confirmed as a vector for Rocky Mountain spotted fever in the USA.76, 77 This tick feeds on dogs at every stage of its life cycle and usually lives in and around homes. CDC, the Arizona Department of Health Services, the Indian Health Service, and tribal health departments are working to test a prevention and vector control strategy for Rocky Mountain spotted fever that includes homeowner education, use of acaricides, tick collaring, and control of the local dog population.78 Additionally, within the past 4 years, two previously unknown tick-borne pathogens whose epidemiology, transmission patterns, and clinical spectrum of illness remain unclear, have been identified in the USA: an Ehrlichia muris-like agent in Minnesota and Wisconsin79 and the Heartland phlebovirus in Missouri.80
Public health efforts to reduce illness and deaths from zoonotic and vector-borne diseases focus on improvements to disease surveillance, public health education, treatment, and prevention, and on actions to sustain and strengthen vector control programmes.81 These strategies also offer increased opportunities to improve collaborations for infectious disease control between human, animal, and environmental health experts—the concept of One Health.
Foodborne diseases
Every year, around 48 million foodborne illnesses occur in the USA, resulting in 128 000 admissions to hospital and 3000 deaths.82, 83 About 9·4 million of these illnesses can be attributed to major pathogens, mainly norovirus, non-typhoidal Salmonella spp, Clostridium perfringens, and Campylobacter spp.82 Although most foodborne illnesses do not occur as part of recognised outbreaks, around 1000 US foodborne disease outbreaks are reported every year, affecting tens of thousands of individuals.84 Most of these outbreaks are local; however, multistate outbreaks have been detected more often in recent years. In 2011, CDC investigated about 30 major multistate outbreaks. These increases in frequency, however, could partly be a result of improved detection, surveillance, and typing methods for foodborne infections.
Factors that might facilitate multistate foodborne disease outbreaks include centralised food production and processing, and subsequent broad distribution of products.2, 85 In the past few years, efforts to improve food safety in the USA through regulations and policies include the Egg Safety Rule, passed by the Food and Drug Administration (FDA) in 2010, to decrease Salmonella contamination of shell eggs, and the 2011 Food Safety Modernization Act, which expands FDA's authority to improve food safety and highlights CDC's efforts to improve foodborne disease surveillance and outbreak responses.
Since 1996, the Foodborne Diseases Active Surveillance Network (FoodNet)—a collaboration with CDC, ten state health departments, academic institutions, the US Department of Agriculture's Food Safety and Inspection Service (USDA-FSIS), and FDA—has done active, population-based surveillance of laboratory-confirmed infections caused by major foodborne pathogens. Although progress to address several key foodborne pathogens has been made,86 particularly with Shiga toxin-producing E coli serotype O157, in recent years progress has stalled.87, 88 The rate of Salmonella infections—the most commonly reported foodborne infection—has been largely unchanged.88 In the USA, Salmonella causes around 1 million infections82 and $365 million in direct medical costs every year.86
An important component in efforts to identify and stop foodborne disease outbreaks is PulseNet, a national network of public health and food regulatory agency laboratories that use pulsed-field gel electrophoresis (PFGE) patterns to identify clusters of foodborne illnesses that might signal an outbreak. In the USA, more than 80 state and federal laboratories participate in this surveillance system and submit PFGE patterns from six bacterial pathogens to a CDC database to identify similar cases of foodborne illnesses that might signal an outbreak. Over the past 15 years, this system has detected hundreds of outbreaks, enabling faster and more accurate responses. PulseNet-triggered investigations have resulted in the recall of more than 1·5 billion pounds of contaminated food and have prompted new actions by industry and food regulatory agencies to make food safer. Since 2006, data from foodborne disease investigations have identified more than a dozen newly implicated foods in foodborne disease outbreaks (panel ). Data from nearly 4600 outbreaks in 1998–2008 were used to help to establish the proportion of foodborne illnesses attributable to specific food commodities across 17 major food categories. Produce accounted for almost half of all foodborne illnesses from these outbreaks, with most deaths caused by meat and poultry.89
Panel. New**Not previously associated with foodborne disease outbreaks. Data sources: Foodborne Disease Outbreak Surveillance System, Centers for Disease Control and Prevention web postings (http://www.cdc.gov/Foodsafety/). food vehicles reported in USA multistate outbreaks since 2006.
-
•
Bagged spinach
-
•
Broccoli powder (on snack food)
-
•
Canned chilli sauce
-
•
Carrot juice
-
•
Cucumbers
-
•
Dog food
-
•
Hazelnuts
-
•
Hot peppers
-
•
Kosher broiled chicken livers
-
•
Peanut butter
-
•
Pepper
-
•
Pine nuts
-
•
Frozen pot pies and meals
-
•
Raw, prepackaged cookie dough
-
•
Raw cashew cheese
-
•
Scraped tuna product
-
•
Whole fresh papayas
Because many foodborne disease outbreaks occur across many states and countries and therefore could go undetected, efforts to ensure the continued capability of laboratory-based and cluster-detection surveillance systems such as PulseNet are crucial. Detection of bacterial pathogens is changing from culture to an increased use of point-of-care molecular tests.90 Although these tests have great potential for broader applications in detection and control of infectious diseases, the increased use of culture-independent diagnostics brings new challenges to public health laboratory-based surveillance systems that rely on culture and bacterial isolates for strain identification. Fundamental changes in these systems are needed to ensure their continued effectiveness.
HIV
Despite major advances in prevention, treatment, and care, HIV infection is a substantial challenge to public health worldwide. In the USA, around 1·1 million people are living with HIV.91 The epidemic is generally concentrated in urban areas and disproportionately affects African-Americans, Latinos, and men who have sex with men (MSM).91 Overall, incidence has remained stable for more than a decade,92 with an estimated 47 500 new infections in 2010.93 However, in young MSM, particularly young black MSM, rates of new infections have increased,93 prompting targeted campaigns to help to engage with these populations.94
Despite public health recommendations for routine HIV testing for people aged 13–64 years,95 around 16% of people infected are unaware of their infection,91 precluding access to life-prolonging treatment and increasing the risk of transmission to their sexual partners. With data showing that antiretroviral drugs reduce the risk of HIV transmission and acquisition,96, 97 prevention efforts include treatment for people living with HIV and pre-exposure prophylaxis (PrEP) for people who are HIV negative but at a substantial risk for HIV. Only 25% of the US population with HIV is virally suppressed, and improvements are needed at each stage of the continuum of care, with particular attention to disparities in race or ethnicity and age.98 For example, young people are less likely to have their infection diagnosed or have viral suppression compared with other age groups.98
To address the US epidemic, the National HIV/AIDS Strategy (NHAS) was released in 2010, with main goals to: reduce HIV incidence; increase access to care and improve health outcomes for people with HIV; and reduce HIV-related health disparities.99 CDC's efforts to advance the NHAS goals are focused on the combination of evidenced-based, cost-effective, and scalable prevention interventions for geographical areas and populations with the greatest burden of HIV. Expanded surveillance to monitor community CD4 cell counts and viral loads is also increasingly used to target high burden areas.100 As new prevention options such as PrEP emerge, operational research will guide implementation of interventions, whether biomedical, behavioural, or structural, to strengthen efforts to mitigate the US epidemic.101
Sexually transmitted infections
Around 20 million newly acquired STIs occur every year in the USA,13 resulting in about $16 billion in health-care costs.102 Nearly half these infections occur in young people (aged 15–24 years).13 Similar to HIV, racial and ethnic minorities are disproportionately affected by STIs, as shown in chlamydia and gonorrhoea national surveillance data.103 In 2012, chlamydia and gonorrhoea rates of infection for black people were around seven and 15 times higher, respectively, than those for white people.103 For syphilis, MSM (particularly young MSM) represent an increasingly high proportion of overall reported cases.103
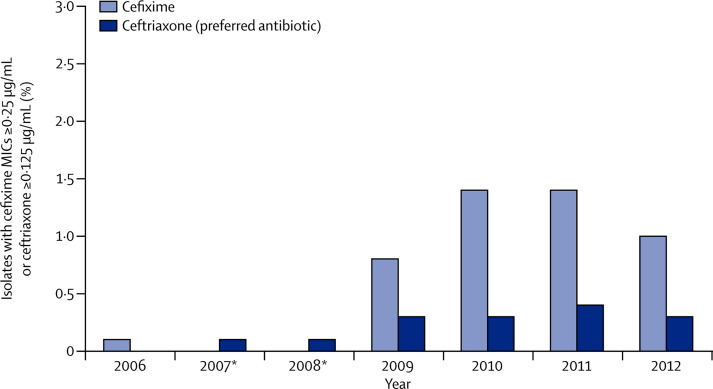
Another urgent challenge for prevention and control of STIs is the threat of emerging cephalosporin resistance, which could lead to untreatable gonorrhoea (figure 3 ).104, 105 Gonorrhoea is the second most commonly reported US notifiable disease,103 and if untreated can lead to severe health outcomes including pelvic inflammatory disease and subsequent infertility. To address this challenge requires enhanced surveillance of antibiotic susceptibility such as through the Gonococcal Isolate Surveillance Project; improved primary prevention, screening, and partner services; and development of new therapies.103, 105 Guidelines to treat gonorrhoea have been changed to preserve the last available effective treatment option for as long as possible.106
Figure 3.
Percentage of gonorrhoea specimens with reduced cephalosporin antibiotic susceptibility, 2006–12
MIC=minimum inhibitory concentrations. *Cefixime susceptibility not tested during 2007–08. Data source: Gonococcal Isolate Surveillance Project (http:/www.cdc.gov/STd/gisp/default.htm).
Chronic hepatitis
Although US rates of acute viral hepatitis infection have decreased overall during the past two decades,107 chronic hepatitis remains a substantial challenge. Around 800 000 to 1·4 million people in the USA are chronically infected with hepatitis B virus (HBV) and 2·7–3·9 million have chronic hepatitis C virus (HCV).107 Racial and ethnic minorities are disproportionately affected; HCV prevalence in non-Hispanic black people is about double that in non-Hispanic white people,108 and Asian and Pacific Islanders account for more than half of all Americans living with chronic HBV infection.109 Many people with chronic hepatitis do not know that they are infected, which increases their risk for cirrhosis or liver cancer and for unknowingly transmitting infection. A national action plan for viral hepatitis, released in 2011 and updated in 2014, provides steps to improve prevention, increase diagnosis and access to care for individuals infected, and improve coordination of federal and partner efforts.110
With improvements in treatment for HCV now available, CDC has issued revised HCV testing recommendations111 that augment risk-based guidelines112 and recommend one-time testing for people born between 1945 and 1965 to identify undiagnosed infections113 and link individuals with HCV to care. People in this designated baby boomer age cohort now account for around 75% of prevalent HCV infections and HCV-related mortality.114 Implementation of these revised testing recommendations could thus identify an estimated 800 000 additional cases and avert about 120 000 deaths.115
Tuberculosis
Although rates of tuberculosis cases in the USA are at record lows (less than 10 000 new cases were reported in 2012), the disease continues to disproportionately affect people born outside the USA and racial and ethnic minorities.116 Outbreaks also occur in hard-to-reach populations, such as drug users and the homeless.117, 118 Diagnosis and management of the 11 million people in the USA with latent tuberculosis infection is a challenge but is essential since most active disease results from such infections.119, 120 Key strategies include targeted testing and treatment and engagement of medical providers outside of public health departments.119 Although more pressing globally, management of tuberculosis and HIV co-infection and surveillance for drug resistance are important components of tuberculosis control efforts in the USA.116, 121 Multidrug-resistant (MDR) and extensively drug-resistant strains continue to challenge elimination goals for tuberculosis, and first-generation and second-generation antituberculosis drug shortages have occurred. The FDA accelerated approval of bedaquiline, a novel drug for the treatment of pulmonary MDR tuberculosis.122
Conclusions
For any country, effective control of infectious diseases is dependent on focused efforts to decrease endemic infections in at-risk and susceptible populations, combined with strong epidemiological and laboratory capacity to detect and stop emerging threats. Inherent in these efforts are well established links between clinical, public health, and research communities working with non-traditional partners such as industry, law enforcement, businesses, and the media. Although at their core these collaborations need to be established locally, such crucial partnerships have to extend across state, national, and international levels. Together, these alliances can form a global infrastructure and system to detect and control infectious diseases. With that aim, in February, 2014, the USA and other nations joined WHO, the Food and Agricultural Organization of the United Nations, and the World Organization for Animal Health in a new global health security agenda designed to enhance strategies to prevent, detect, and respond to threats of infectious disease.123 Partner countries will work together to meet a series of objectives covering crucial issues affecting control of infectious disease such as antimicrobial resistance, biosafety and biosecurity, global disease detection and response networks, and emergency responses.
Much emphasis has been placed on the aptly named perfect storm inherent in today's globalised world for rapid emergence and spread of infectious diseases. Despite this persistent challenge, extraordinary achievements in science and technology continue to bolster the capacity to recognise and respond to these threats. Scientifically, next-generation sequencing and state-of-the-art bioinformatics capabilities are transforming the practice of microbiology and the speed at which infectious pathogens can be detected and analysed.85, 124 These technologies provide a new level of information about infectious pathogens and therefore offer tremendous opportunities for public health efforts to detect and control outbreaks, target antimicrobial resistance, and develop and refine treatments and vaccines.125, 126, 127 Additionally, the increasing understanding of the role of human microbiomes in disease development and new links between infectious agents and chronic diseases opens new possibilities for disease prevention and treatment.128 Advances in electronic communications have also been essential and have enabled rapid sharing of health and safety information and expertise by health-care and public health workers, researchers, law enforcement, the media, and private individuals. These communication capacities can facilitate and enhance research and public health partnerships worldwide, generating broad sharing of research findings and health updates in near real time. The latest surge in health communications has come from social media tools, which are increasingly being used to extend the reach of public health information. To ensure that these rapidly changing scientific and technological advances are routinely used in public health is a crucial challenge and priority.
The remarkable ability of infectious agents to adapt to new environments and exploit the susceptibilities of their hosts will continue to complicate and obstruct efforts for their control. In the USA, the largest gains will be achieved through focused efforts to reduce high burden and high consequence diseases and to strengthen efforts to promote early detection of and rapid response to emerging infections. Priorities for these efforts include prevention measures with broad-reaching effects such as vaccines, multifaceted approaches to decrease antimicrobial resistance, and testing and education to reduce the further spread of preventable infections. In addition, new opportunities for control of infectious disease are envisioned through US health reforms, which are expanding access to care and improving health-care coordination for many underserved populations.
Contributors
We all contributed equally.
Declaration of interests
We declare no competing interests.
Footnotes
Not previously associated with foodborne disease outbreaks. Data sources: Foodborne Disease Outbreak Surveillance System, Centers for Disease Control and Prevention web postings (http://www.cdc.gov/Foodsafety/).
References
- 1.Lozano R, Naghavi M, Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Committee on Microbial Threats to Health. Institute of Medicine . Emerging infections; microbial threats to health in the United States. National Academies Press; Washington, DC: 1992. [PubMed] [Google Scholar]
- 3.Morens DM, Fauci AS. Emerging infectious diseases in 2012: 20 years after the Institute of Medicine report. mBio. 2012;3:e00494–e00512. doi: 10.1128/mBio.00494-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIAID. NIH . NIH/NIAID/DMID research agenda for emerging diseases. National Institute of Allergy and Infectious Diseases; Bethesda, MD: 1994. [Google Scholar]
- 5.Centers for Disease Control and Prevention . Preventing emerging infectious diseases: a strategy for the 21st century. US Department of Health and Human Services, Public Health Service; Atlanta: 1998. [Google Scholar]
- 6.Whitney CG, Zhou F, Singleton J, Schuchat A. Benefits from immunization during the vaccines for children program era—United States, 1994–2013. MMWR Morb Mortal Wkly Rep. 2014;63:352–355. [PMC free article] [PubMed] [Google Scholar]
- 7.Whitney CG, Pilishvili T, Farley MM. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–1502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 8.Pilishvili T, Lexau C, Farley MM. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 9.Nuorti JP, Whitney CG, Centers for Disease Control and Prevention Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59(RR–11):1–18. [PubMed] [Google Scholar]
- 10.Moore M, Link-Gelles R, Farley M. Impact of 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease, U.S, 2010–11. IDWeek 2012; San Diego, CA: Oct 19, 2012. Abstract number 1219. https://idsa.confex.com/idsa/2012/webprogram/Paper36569.html (accessed June 19, 2014).
- 11.Desai R, Curns AT, Steiner CA. All-cause gastroenteritis and rotavirus-coded hospitalizations among US children, 2000–2009. Clin Infect Dis. 2012;55:e28–e34. doi: 10.1093/cid/cis443. [DOI] [PubMed] [Google Scholar]
- 12.Tate J, Haynes A, Payne D. Trends in national rotavirus activity before and after introduction of rotavirus vaccine into the national immunization program in the United States, 2000–2012. Pediatr Infect Dis J. 2013;32:741–744. doi: 10.1097/INF.0b013e31828d639c. [DOI] [PubMed] [Google Scholar]
- 13.Satterwhite CL, Torrone E, Meites E. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 14.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER, Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR–02):1–24. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–1708. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention National and state vaccination coverage among adolescents aged 13–17 years–United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–677. [PubMed] [Google Scholar]
- 17.Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16–17 March 2000. J Infect Dis. 2004;189(suppl 1):S43–S47. doi: 10.1086/377696. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Measles–United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:253–257. [PubMed] [Google Scholar]
- 19.Gastañaduy PA, Redd SB, Parker Fiebelkorn A et al. Measles—United States, January1–May23, 2014. MMWR;63: 496–99. [PMC free article] [PubMed]
- 20.Chen SY, Anderson S, Kutty PK. Healthcare-associated measles outbreak in the United States after an importation: challenges and economic impact. J Infect Dis. 2011;203:1517–1525. doi: 10.1093/infdis/jir115. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Two measles outbreaks after importation—Utah, March–June 2011. MMWR Morb Mortal Wkly Rep. 2013;62:222–225. [PMC free article] [PubMed] [Google Scholar]
- 22.Dayan GH, Quinlisk MP, Parker AA. Recent resurgence of mumps in the United States. N Engl J Med. 2008;358:1580–1589. doi: 10.1056/NEJMoa0706589. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Mumps outbreak at a summer camp—New York, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:175–177. [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention Mumps outbreak on a university campus—California, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:986–989. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR–03):1–34. [PubMed] [Google Scholar]
- 26.Winter K, Harriman K, Zipprich J. California pertussis epidemic, 2010. J Pediatrics. 2012;161:1091–1096. doi: 10.1016/j.jpeds.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Pertussis Epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:517–522. [PubMed] [Google Scholar]
- 28.Misegades LK, Winter K, Harriman K. Association of childhood pertussis with receipt of 5 doses of pertussis vaccine by time since last vaccine dose, California, 2010. JAMA. 2012;308:2126–2132. doi: 10.1001/jama.2012.14939. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1424–1426. [PubMed] [Google Scholar]
- 30.Klevens RM, Edwards J, Richards C. Estimating health care–associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott RD. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Centers for Disease Control and Prevention; Atlanta: 2009. [Google Scholar]
- 32.Srinivasan A. Influential outbreaks of healthcare-associated infections in the past decade. Infect Control Hosp Epidemiol. 2010;31:S70–S72. doi: 10.1086/655987. [DOI] [PubMed] [Google Scholar]
- 33.Guh AY, Thompson NC, Schaefer MK, Patel PR, Perz JF. Patient notification for bloodborne pathogen testing due to unsafe injection practices in the US health care settings, 2001-2011. Med Care. 2012;50:785–791. doi: 10.1097/MLR.0b013e31825517d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.One & Only Campaign http://www.oneandonlycampaign.org/ (accessed June 18, 2014).
- 35.Centers for Disease Control and Prevention Multistate outbreak of fungal meningitis and other infections. Oct 23, 2013. http://www.cdc.gov/hai/outbreaks/meningitis.html (accessed June 19, 2014).
- 36.Smith RM, Schaefer MK, Kainer MA. Fungal infections associated with contaminated methylprednisolone injections–preliminary report. N Engl J Med. 2013;369:1598–1609. doi: 10.1056/NEJMoa1213978. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention Reductions in central line-associated bloodstream infections among patients in intensive care units—Pennsylvania, April 2001—March 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1013–1016. [PubMed] [Google Scholar]
- 38.Pronovost P, Needham D, Berenholtz S. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 39.Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301:727–736. doi: 10.1001/jama.2009.153. [DOI] [PubMed] [Google Scholar]
- 40.Kallen AJ, Mu Y, Bullen S, for the Active Bacterial Core surveillance (ABCs) MRSA Investigators of the Emerging Infections Program Health care-associated invasive MRSA infections, 2005–2008. JAMA. 2010;304:641–647. [Google Scholar]
- 41.Burton DC, Edwards JR, Srinivasan A, Fridkin SK, Gould CV. Trends in catheter-associated urinary tract infections in adult intensive care units—United States, 1990—2007. Infect Control Hosp Epidemiol. 2011;32:748–756. doi: 10.1086/660872. [DOI] [PubMed] [Google Scholar]
- 42.US Department of Health and Human Services Action plan to prevent healthcare-associated infections: road map to elimination. June, 2009. http://www.hhs.gov/ash/initiatives/hai/actionplan/hhs_hai_action_plan_final_06222009.pdf (accessed June 19, 2014).
- 43.Malpiedi PJ, Peterson KD, Soe MM. 2011 National and state healthcare-associated infection standardized infection ratio report: using data reported to the National Healthcare Safety Network as of September 4, 2012. Published Feb 11, 2013. http://www.cdc.gov/hai/pdfs/SIR/SIR-Report_02_07_2013.pdf (accessed June 19, 2014).
- 44.Centers for Disease Control and Prevention Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/index.html (accessed June 19, 2014).
- 45.Lucado J, Gould C, Elixhauser A. Clostridium difficile infections (CDI) in hospital stays, 2009. HCUP statistical brief number 124. Rockville, MD: US Department of Health and Human Services, Agency for Healthcare Research and Quality, 2011. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf (accessed June 19, 2014). [PubMed]
- 46.Centers for Disease Control and Prevention Vital signs: preventing Clostridium difficile infections. MMWR Morb Mortal Wkly Rep. 2012;61:157–162. [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 2013;62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 48.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumonia infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 49.Chitnis AS, Caruthers PS, Rao AK. Outbreak of carbapenem-resistant Enterobacteriaceae at a long-term acute care hospital: sustained reductions in transmissions through active surveillance and targeted interventions. Infect Control Hosp Epidemiol. 2012;33:984–992. doi: 10.1086/667738. [DOI] [PubMed] [Google Scholar]
- 50.Jones KE, Patel NG, Leva MA. Global trends in emerging infectious diseases. Nature. 2008;4512:990–994. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention Hantavirus pulmonary syndrome in visitors to a national park—Yosemite Valley, California, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:952. [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention Influenza A (H3N2) variant virus-related hospitalizations: Ohio, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:764–767. [PubMed] [Google Scholar]
- 53.Wong KK, Greenbaum A, Moll ME. Outbreak of influenza A (H3N2) variant virus infection among attendees of an agricultural fair, Pennsylvania, USA, 2011. Emerg Infect Dis. 2012;18:1937–1944. doi: 10.3201/eid1812.121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams S, Sandrock C. Avian influenza: update. Med Princ Pract. 2010;19:421–432. doi: 10.1159/000320299. [DOI] [PubMed] [Google Scholar]
- 55.Gao R, Cao B, Hu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 56.Wu S, Wu F, He J. Emerging risk of H7N9 influenza in China. Lancet. 2013;381:1539–1540. doi: 10.1016/S0140-6736(13)60767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jhung MA, Swerdlow D, Olsen SJ. Epidemiology of 2009 pandemic influenza A (H1N1) in the United States. Clin Infect Dis. 2011;52(suppl 1):S13–S26. doi: 10.1093/cid/ciq008. [DOI] [PubMed] [Google Scholar]
- 58.Khan G. A novel coronavirus capable of lethal human infections: an emerging picture. Virol J. 2013;10:66. doi: 10.1186/1743-422X-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lau SK, Woo PC, Li KS. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization Middle East respiratory syndrome coronavirus (MERS-CoV) summary and literature update—as of 11 June 2014 (update 16) http://www.who.int/csr/disease/coronavirus_infections/MERS-CoV_summary_update_20140611.pdf?ua=1 (accessed June 19, 2014).
- 61.Petersen LR, Fischer M. Unpredictable and difficult to control—the adolescence of West Nile virus. N Engl J Med. 2012;367:1281–1284. doi: 10.1056/NEJMp1210537. [DOI] [PubMed] [Google Scholar]
- 62.Nash D, Mostashari F, Fine A. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 63.Lindsey NP, Staples JE, Lehman JA, Fischer M. Centers for Disease Control and Prevention (CDC). Surveillance for human West Nile virus disease—United States, 1999–2008. MMWR Surveill Summ. 2010;59:1–17. [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention West Nile virus disease cases reported to CDC by state, 1999–2013. Data from ArboNET. http://www.cdc.gov/westnile/resources/pdfs/cummulative/99_2013_cummulativeHumanCases.pdf (accessed June 19, 2014).
- 65.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiol Infect. 2013;141:591–595. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roehr B. Texas records worst outbreak of West Nile Virus on record. BMJ. 2012;345:e6019. doi: 10.1136/bmj.e6019. [DOI] [PubMed] [Google Scholar]
- 67.Mostashari F, Bunning ML, Kitsutani PT. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 68.Bhatt S, Gething PW, Brady OJ. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention Notes from the field: dengue epidemic—Puerto Rico, January–July 2010. MMWR Morb Mortal Wkly Rep. 2010;59:878. [Google Scholar]
- 70.Centers for Disease Control and Prevention Locally acquired dengue—Key West, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:577–581. [PubMed] [Google Scholar]
- 71.Pan American Health Organization. Centers for Disease Control and Prevention . Preparedness and response for chikungunya virus. Introduction in the Americas. PAHO; Washington, DC: 2011. [Google Scholar]
- 72.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2012;379:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 73.Fischer M, Staples JE. Notes from the field: Chikungunya virus spreads in the Americas—Caribbean and South America, 2013–2014. MMWR;63: 500–01. [PMC free article] [PubMed]
- 74.Hoen AG, Margos G, Bent SJ. Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc Natl Acad Sci USA. 2009;106:15013–15018. doi: 10.1073/pnas.0903810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Centers for Disease Control and Prevention Summary of notifiable diseases—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;59:1–111. [PubMed] [Google Scholar]
- 76.Demma LJ, Traeger MS, Nicholson WL. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- 77.Demma LJ, Traeger M, Blau D. Serologic evidence for exposure to Rickettsia rickettsii in eastern Arizona and recent emergence of Rocky Mountain spotted fever in this region. Vector Borne Zoonotic Dis. 2006;6:423–429. doi: 10.1089/vbz.2006.6.423. [DOI] [PubMed] [Google Scholar]
- 78.Todd SR, Drexler NI, Brock A. Extreme Rodeo: results of a Rocky Mountain spotted fever intervention project—eastern Arizona, 2012. 62nd Epidemic Intelligence Service (EIS) conference; Atlanta, GA; April 22–26, 2013. Abstract, page 72. http://www.cdc.gov/eis/downloads/2013-EIS-Conference.pdf (accessed June 18, 2014).
- 79.Pritt BS, Sloan LM, Johnson DK. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med. 2011;365:422–429. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McMullan LK, Folk SM, Kelly AJ. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- 81.Centers for Disease Control and Prevention National capacity for surveillance, prevention, and control of West Nile virus and other arbovirus infections—United States, 2004 and 2012. MMWR Morb Mortal Wkly Rep. 2014;63:281–284. [PMC free article] [PubMed] [Google Scholar]
- 82.Scallan E, Hoekstra RM, Angulo FJ. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scallan E, Griffin PM, Angulo FJ, Tauxe RV, Hoekstra RM. Foodborne illness acquired in the United States—unspecified agents. Emerg Infect Dis. 2011;17:16–22. doi: 10.3201/eid1701.P21101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Centers for Disease Control and Prevention Surveillance for foodborne disease outbreaks—United States, 2007. MMWR Morb Mortal Wkly Rep. 2010;59:973–979. [PubMed] [Google Scholar]
- 85.Lipkin WI. The changing face of pathogen discovery and surveillance. Nat Rev. 2013;11:133–141. doi: 10.1038/nrmicro2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Centers for Disease Control and Prevention Vital signs: incidence and trends of infection with pathogens transmitted commonly through food: foodborne diseases active surveillance network, 10 US sites, 1996-2010. MMWR Morb Mortal Wkly Rep. 2011;60:749–755. [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention Incidence and trends of infection with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. sites, 1996–2012. MMWR Morb Mortal Wkly Rep. 2013;62:283–287. [PMC free article] [PubMed] [Google Scholar]
- 88.Centers for Disease Control and Prevention Incidence and trends of infection with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. sites, 2006–2013. MMWR Morb Mortal Wkly Rep. 2014;63:328–332. [PMC free article] [PubMed] [Google Scholar]
- 89.Painter JA, Hoekstra RM, Ayers T. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg Infect Dis. 2013;19:407–415. doi: 10.3201/eid1903.111866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cronquist AB, Mody RK, Atkinson R. Impacts of culture-independent diagnostic practices on public health surveillance for bacterial enteric pathogens. Clin Infect Dis. 2012;54(suppl 5):S432–S439. doi: 10.1093/cid/cis267. [DOI] [PubMed] [Google Scholar]
- 91.Centers for Disease Control and Prevention Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 U.S. dependent areas—2011. HIV Surveill SupplementalRep. 2013;18 [Google Scholar]
- 92.Hall HI, Song R, Rhodes P, for the HIV Incidence Surveillance Group Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Centers for Disease Control and Prevention Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Report. 2012;17 http://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf (accessed June 19, 2014). [Google Scholar]
- 94.Centers for Disease Control and Prevention Testing makes us stronger. http://hivtest.cdc.gov/stronger/index.html (accessed June 18, 2014).
- 95.Branson BM, Handsfield HH, Lampe MA. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR–14):1–17. [PubMed] [Google Scholar]
- 96.Cohen MS, Chen YQ, McCauley M. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baeten JM, Donnell D, Ndase P. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hall HI, Frazier EL, Rhodes P. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013;173:1337–1344. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 99.White House Office of National AIDS Policy . National HIV/AIDS strategy for the United States. The White House; Washington, DC: July 13, 2010. http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf (accessed June 19, 2014). [Google Scholar]
- 100.Centers for Disease Control and Prevention . High impact HIV prevention. CDC's approach to reducing HIV infections in the United States. Centers for Disease Control and Prevention; Atlanta, GA: 2011. http://www.cdc.gov/hiv/pdf/policies_NHPC_Booklet.pdf (accessed May 17, 2013). [Google Scholar]
- 101.Herbst JH, Glassman M, Carey JW. Operational research to improve HIV prevention in the United States. J Acquir Immune Defic Syndr. 2012;59:530–536. doi: 10.1097/QAI.0b013e3182479077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Owusu-Edusei K, Jr, Chesson HW, Gift TL. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis. 2013;40:197–201. doi: 10.1097/OLQ.0b013e318285c6d2. [DOI] [PubMed] [Google Scholar]
- 103.Centers for Disease Control and Prevention . Sexually transmitted disease surveillance 2012. US Department of Health and Human Services; Atlanta, GA: 2013. http://www.cdc.gov/std/stats12/Surv2012.pdf (accessed June 19, 2014). [Google Scholar]
- 104.Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med. 2012;66:485–487. doi: 10.1056/NEJMp1112456. [DOI] [PubMed] [Google Scholar]
- 105.Centers for Disease Control and Prevention CDC Grand Rounds: the growing threat of multidrug-resistant gonorrhea. MMWR Morb Mortal Wkly Rep. 2013;62:103–106. [PMC free article] [PubMed] [Google Scholar]
- 106.Centers for Disease Control and Prevention Update to CDC's sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep. 2012;61:590–594. [PubMed] [Google Scholar]
- 107.Centers for Disease Control and Prevention Disease burden from viral hepatitis A, B, and C in the United States, 2012. http://www.cdc.gov/hepatitis/pdfs/disease_burden.pdf (accessed June 19, 2014).
- 108.Tohme RA, Xing J, Liao Y, Holmberg SD. Hepatitis C testing, infection, and linkage to care among racial and ethnic minorities in the United States, 2009–2010. Am J Public Health. 2013;103:112–119. doi: 10.2105/AJPH.2012.300858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Centers for Disease Control and Prevention Viral hepatitis populations. Asian & Pacific Islanders. http://www.cdc.gov/hepatitis/Populations/api.htm (accessed June 19, 2014).
- 110.Department of Health and Human Services Action plan for the prevention, care and treatment of viral hepatitis 2014–2016. 2014. http://aids.gov/pdf/viral-hepatitis-action-plan.pdf (accessed June 18, 2014).
- 111.Smith BD, Morgan RL, Beckett GA. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR–04):1–32. [PubMed] [Google Scholar]
- 112.Centers for Disease Control and Prevention Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR–19):1–39. [PubMed] [Google Scholar]
- 113.Centers for Disease Control and Prevention Vital signs: evaluation of hepatitis C virus infection testing and reporting—eight U.S. Sites, 2005–2011. MMWR Morb Mortal Wkly Rep. 2013;62:357–361. [PMC free article] [PubMed] [Google Scholar]
- 114.Ward JW. The epidemiology of chronic hepatitis C and one-time hepatitis C virus testing of persons born during 1945 to 1965 in the United States. Clinics Liver Dis. 2013;17:1–11. doi: 10.1016/j.cld.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 115.Rein DB, Smith BD, Wittenborn JS. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156:263–270. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Centers for Disease Control and Prevention Trends in tuberculosis–United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:201–205. [PMC free article] [PubMed] [Google Scholar]
- 117.Centers for Disease Control and Prevention Tuberculosis outbreak associated with a homeless shelter–Kane County, Illinois, 2007–2011. MMWR Morb Mortal Wkly Rep. 2012;61:186–189. [PubMed] [Google Scholar]
- 118.Centers for Disease Control and Prevention Notes from the field: tuberculosis cluster associated with homelessness–Duval County, Florida, 2004–2012. MMWR Morb Mortal Wkly Rep. 2012;61:539–540. [PubMed] [Google Scholar]
- 119.Reves RR, Nolan CM. Tuberculosis elimination in the United States: an achievable goal or an illusion? Am J Respir Crit Care Med. 2012;186:i–iii. doi: 10.1164/rccm.201206-1039ED. [DOI] [PubMed] [Google Scholar]
- 120.Gordin FM, Masur H. Current approaches to tuberculosis in the United States. JAMA. 2012;308:283–289. doi: 10.1001/jama.2012.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Luetkemeyer AF. Current issues in the diagnosis and management of tuberculosis and HIV coinfection in the United States. Top HIV Med. 2010;18:143–148. [PubMed] [Google Scholar]
- 122.US Food Drug and Administration . FDA approves first drug to treat multi-drug resistant tuberculosis [press release] Food and Drug Administration; Dec 31; Silver Spring, MD: 2012. [Google Scholar]
- 123.Frieden TR, Tappero JW, Dowell SF, Hien NT, Guillaume FD, Aceng JR. Safer countries through global health security. Lancet. 2014;383:764–766. doi: 10.1016/S0140-6736(14)60189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Relman DA. Microbial genomics and infectious diseases. N Engl J Med. 2011;365:347–357. doi: 10.1056/NEJMra1003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Koser CU, Ellington MJ, Cartwright EJP. Routine use of microbial whole genome sequencing in diagnostic and public health microbiology. Plos Pathog. 2012;8:e1002824. doi: 10.1371/journal.ppat.1002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Snitkin ES, Zelazny AM, Thomas PJ. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoinae with whole-genome sequencing. Sci Transl Med. 2012;4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Relman DA. Metagenomics, infectious disease diagnostics, and outbreak investigations: sequence first, ask question later? JAMA. 2013;309:1531–1532. doi: 10.1001/jama.2013.3678. [DOI] [PubMed] [Google Scholar]
- 128.Lemon KP, Armitage GC, Relman DA, Fischback MA. Microbiota-targeted therapies: an ecological perspective. Sci Transl Med. 2012;4:137rv5. doi: 10.1126/scitranslmed.3004183. [DOI] [PMC free article] [PubMed] [Google Scholar]