Abstract
Metrodorea stipularis stem extracts were studied in the search for possible antichagastic, antimalarial, and antitumoral compounds using cruzain from Trypanosoma cruzi, Plasmodium falciparum, and cathepsins B and L, as molecular targets, respectively. Dihydrochalcones 1, 2, 3, and 4 showed significant inhibitory activity against all the targets. Compounds 1–4 displayed IC50 values ranging from 7.7 to 21.6 μM against cruzain; dihydrochalcones 2 and 4 inhibited the growth of three different strains of P. falciparum in low micromolar concentrations; and against cathepsins B and L these compounds presented good inhibitory activity with IC50 values ranging from 1.0 to 14.9 μM. The dihydrochalcones showed good selectivity in their inhibitory activities against the cysteine proteases.
Graphical Abstract
For thousands of years, plants have been used as medicines. They represent a source of structurally complex and unusual substances and are therefore an extremely attractive target for chemical and biological studies. One of the rational forms of study in the search for new potential drugs can be through screening, where a wide variety of extracts can be used for specific biological tests to obtain one or more metabolites with pharmacological potential.1
Metrodorea stipularis belongs to the family Rutaceae, a family that has a wide diversity of metabolites with large structural diversity that expresses different pharmacological properties. Only two species of the Metrodorea genus have been investigated for their chemical constituents: M. nigra and M. flavida. M. nigra is the only species shown to contain dihydrochalcones. In addition to Metrodorea, Esenbeckia is, as of yet, the only genus found to produce this class of compound in the Rutaceae family.2–4
Chagas disease affects 8 million people in Latin America, leading to an annual economic loss of approximately $18 billion. This disease is caused by the parasite Trypanosoma cruzi. There are only two drugs used for the treatment of this disease, nifurtimox and benznidazole. The former has been banned in some countries of South America, and both cause serious side effects. Cruzipain (cruzain) is an enzyme that participates in the pathway of proteolytic digestion of the parasite and plays an essential role in its life process; thus, it is an interesting biological target in the search for new compounds that can be used in the treatment of this disease.5–8
Plasmodium falciparum is the most malignant of the species causing malaria, and the progressive increase of resistant parasite strains to antimalarials has initiated studies aimed at developing new drugs that may help in the chemotherapy of this disease. Artemisinin and quinine used in the treatment of this disease are plant natural products.9
Cathepsins are cysteine proteases involved in several pathological situations, such as in the progression of malignant tumors and metastases, infectious disease, rheumatoid arthritis, osteoarthritis, osteoporosis, and atherosclerosis. The malignant tumors are mostly nonencapsulated and invasive, making it difficult for surgical resection. Evidence suggests that cathepsins B and L may facilitate the progression of tumors, and studies of the expression of these enzymes in different types of cancer have shown an increase in the expression of cathepsins in humans.10,11
RESULTS AND DISCUSSION
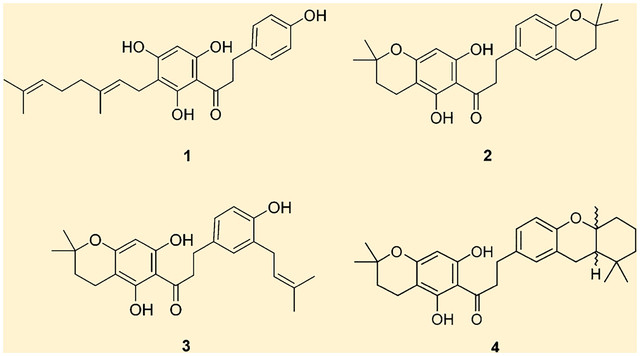
In a search for new antichagasic compounds, extracts from several Brazilian plants of the Rutaceae family were evaluated against cruzain. The dichloromethane fraction from liquid–liquid partitioning of the crude ethanol extract from the stems of M. stipularis showed the most promising results, with inhibition of 80% against cruzain at a concentration of 100 μg/mL. Phytochemical study of this fraction led to the active dihydrochalcones: 1-[3-(3,7-dimethylocta-2,6-dien-1-yl)-2,4,6-trihydroxyphenyl]-3-(4-hydroxyphenyl)propan-1-one (1),4 1-(5,7-dihydroxy-2,2-dimethylchroman-6-yl)-3-(2,2-dimethylchroman-6-yl)propan-1-one (2), 1-(5,7-dihydroxy-2,2-dimethylchroman-6-yl)-3-[4-hydroxy-3-(3-methylbut-2-en-1-yl)-phenyl]propan-1-one (3), and 1-(5,7-dihydroxy-2,2-dimethylchroman-6-yl)-3-(1,1,4a-trimethyl-2,3,4,4a,9a-hexahydro-1H-xanthen-7-yl)propan-1-one (4). This class of compounds was previously isolated from this genus.2
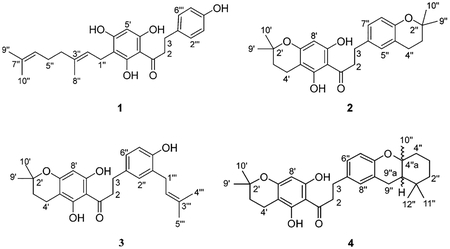
Compounds 2 and 3 were obtained as amorphous powders. The ESITOFMS data for both showed the molecular ion at m/z 410.2066 and 410.2040 (calculated 410.2094) for 2 and 3, respectively. The mass and 13C NMR data (Table 1) provided the same molecular formula of C25H30O5 for both compounds. The 1H NMR spectra for 2 and 3 showed one singlet at δ 5.92 attributed to H-8′, the only proton in ring A revealing the substitution observed for 1. However, for both 2 and 3 the presence of a 2,2-dimethyldihydropyran ring was characterized by signals at δ 1.76 (H-3′) and 2.53 (H-4′) and the methyl groups at δ 1.33 (H-9′ and H-10′). Further analysis revealed the presence of an ABX coupling pattern with signals at δ 6.91 (H-5″ and H-7″) and 6.56 (H-8″) for 2 and signals at δ 6.87 (H-2″), 6.83 (H-6″), and 6.64 (H-5″) for 3 assigned to the protons in the B ring. Compound 2 showed the presence of one 2,2-dimethyldihydropyran ring that was characterized by signals at δ 1.73 (H-3″) and 2.69 (H-4″) and the signals of the methyl groups at δ 1.22 (H-8″ and H-9″). Compound 3 displayed one prenyl group that was suggested from the characteristic signals of the two methyl groups at δ 1.68 and 1.69 (H-4‴ and H-5‴), one olefinic hydrogen at δ 5.28 (H-2‴), and the methylene signal at δ 3.25 (H-1‴). 13C NMR data were obtained from projections of the HSQC and HMBC spectra as in 1 and confirmed the structures of 2 and 3.
Table 1.
NMR Spectroscopic Data (400 MHz, Acetone-d6) for Compounds 2 and 3
| compound 2 | compound 3 | |||||
|---|---|---|---|---|---|---|
| position | δC,a type | δH (J in Hz) | HMBC | δC,a type | δH (J in Hz) | HMBC |
| 1 | 204.7, qC | 204.0, qC | ||||
| 2 | 45.5, CH2 | 3.30, t (7.2) | 6″, 1, 3 | 45.5, CH2 | 3.25, t | 1, 2″ |
| 3 | 29.7, CH2 | 2.83 | 5″, 7″, 1, 2 | 30.2, CH2 | 2.82, t (8.4) | 1″, 2″, 6″, 1, 2 |
| 2′ | 75.6, qC | 75.6, qC | ||||
| 3′ | 31.2, CH2 | 1.76, t (6.8) | 4′a, 2′, 4′, 9′ | 31.3, CH2 | 1.76, t (6.6) | 3″, 2′, 4′, 9′, 10′ |
| 4′ | 16.2, CH2 | 2.53, t (6.8) | 5′, 3′, 4′a | 15.9, CH2 | 2.55, t (6.6) | 2″, 3″, 4″, 3′ |
| 4′a | 104.8, qC | –, qC | ||||
| 5′ | 157.0, qC | 162.0, qC | ||||
| 6′ | 100.0, qC | 100.0, qC | ||||
| 7′ | 162.2, qC | 156.0, qC | ||||
| 8′ | 94.5, CH | 5.92, s | 6′, 7′, 4′a, 8′a | 94.1, CH | 5.87, s | 4′a, 7′ |
| 8′a | 165.5, qC | 164.0, qC | ||||
| 9′ | 25.5, CH3 | 1.22, s | 2′ | 25.0, CH3 | 1.33, s | 2′, 4′ |
| 10′ | 25.5, CH3 | 1.22, s | 2′ | 25.0, CH3 | 1.33, s | 2′, 4′ |
| 1″ | 132.2, qC | |||||
| 2″ | 73.2, qC | 128.7, CH | 6.87, d (1.7) | 6″, 4″ | ||
| 3″ | 32.4, CH2 | 2.69, t (6.8) | 2″, 4″, 5″, 9″ | 126.0, qC | ||
| 4″ | 22.1, CH2 | 2.69, t (6.8) | 4′, 1″a, 2″, 3″ | 152.0, qC | ||
| 4″a | 120.0, qC | |||||
| 5″ | 126.8, CH | 6.91, m | 114.3, CH | 6.64, d (8.0) | 1″ | |
| 6″ | 132.2, qC | 125.9, CH | 6.83, dd (1.7, 8.0) | |||
| 7″ | 128.1, CH | 6.93, m | ||||
| 8″ | 116.8, CH | 6.56, d (8.8) | 6″, 1″a | |||
| 8″a | 151.9, qC | |||||
| 9″ | 25.5, CH3 | 1.33, s | 2″ | |||
| 10″ | 25.5, CH3 | 1.33, s | 2″ | |||
| 1‴ | 27.7, qC | 3.25, brd | 3″, 2‴, 3‴ | |||
| 2‴ | 122.6, CH | 5.28, m | ||||
| 3‴ | 131.4, qC | |||||
| 4‴ | 16.4, CH3 | 1.68, s | 2‴, 3‴, 5‴ | |||
| 5‴ | 25.5, CH3 | 1.69, s | 2‴, 3‴, 4‴ | |||
13C NMR values obtained from projections of the HSQC and HMBC data (100 MHz, acetone-d6).
Compound 4 was obtained as an amorphous powder. The ESITOFMS data showed the molecular ion at m/z 478.2714 (calculated m/z 478.2720). The mass and 13C NMR data (Table 2) provided its molecular formula as C30H38O5. As mentioned for compounds 2 and 3, the 1H NMR spectrum showed the same substitution pattern for both A and B rings. The presence of a cyclized geranyl moiety yielding a hydroxanthene-type skeleton was suggested from the characteristic signals of the three methyl groups at δ 1.18, 0.93, and 1.01 and the methylene signals at δ 1.65, 2.16, 1.48, 1.63, 1.58, and 1.90. 13C NMR values were obtained from projections of the HSQC and HMBC data and confirmed the structure of 4.
Table 2.
NMR Spectroscopic Data (400 MHz, Methanol-d4) for Compound 4
| position | δC,a type | δh (J in Hz) | HMBC |
|---|---|---|---|
| 1 | 204.7, qC | ||
| 2 | 30.0, CH2 | 2.85, t (8.3) | 6″, 7″, 8″, 1, 2 |
| 3 | 45.4, CH2 | 3.28 | 7”, 1, 3 |
| 1′a | 162.2, qC | ||
| 2′ | 75.6, qC | ||
| 3′ | 31.2, CH2 | 1.77, t (6.8) | 4′a, 2′, 4′ 9′, 10′ |
| 4′ | 16.2, CH2 | 2.56, t (6.8) | 5′, 4′a, 8′a, 2′, 3′ |
| 4′a | 100.0, qC | ||
| 5′ | 157.0, qC | ||
| 6′ | 104.8, qC | ||
| 7′ | 164.5, qC | ||
| 8′ | 94.0, CH | 5.87, s | 6′, 4′a, 7′, 8′a |
| 9′ | 25.0, CH3 | 1.34, s | 2′, 10′, 3″ |
| 10′ | 41.0, CH3 | 1.34, s | 2′, 3′, 9′ |
| 1″ | 22.1, qC | ||
| 2″ | 39.5, CH2 | 1.58 and 1.90, m | 1″, 12″, 4‴a |
| 3″ | 19.5, CH2 | 1.63, m | 4″a, 1″ |
| 4″ | 41.2, CH2 | 1.48, m | |
| 4″a | 75.0, qC | ||
| 5″ | 116.0, CH | 6.57, d (8.5) | 7″, 5″a, 8″a |
| 5″a | 151.9, qC | ||
| 6″ | 129.4, CH | 6.89, m | 8″, 5″a |
| 7″ | 132.2, qC | ||
| 8″ | 126.6, CH | 6.89, m | 5″a, 6″, 9″ |
| 8″a | 120.0, qC | ||
| 9″ | 23.0, CH2 | 2.64, m | 8″, 5″a, 4′a, 4″a, 9″a |
| 9″a | 48.0, CH | 1.65, m | 4″a |
| 10″ | 18.5, CH3 | 1.18, s | 4″, 4″a, 9″a |
| 11″ | 33.1, CH3 | 0.93, s | 4″, 12″, 9″a |
| 12″ | 31.0, CH3 | 1.01, s | 4″, 3″, 1″, 11″, 9″a |
13C NMR values obtained from projections of the HSQC and HMBC data (100 MHz, methanol-d4).
The dihydrochalcones 1–4 showed inhibition activities against cruzain, with IC50 values ranging from 7.7 to 21.6 μM (Table 3). Although the dihydrochalcones have not been isolated from all the subfractions from the dichloromethane fraction, their presence was observed by 1H NMR spectra in practically all of the active subfractions. Thus, the observed enzyme inhibition is most likely due to the presence of this class of compounds. This is the first report of cruzain inhibitory activity for this class of compounds, as well as related to Chagas disease studies.
Table 3.
IC50 Values of Dihydrochalcones against Cruzain and Cathepsinsa
| compound | IC50 (μM) cruzain | IC50 (μM) cathepsin B | IC50 (μM) cathepsin L |
|---|---|---|---|
| 1 | 7.1 ± 0.2 | >100 | 1.0 ± 0.2 |
| 2 | 21.6 ± 2.5 | 14.9 ± 1.2 | >100 |
| 3 | 12.0 ± 0.1 | >100 | 3.3 ± 0.5 |
| 4 | 8.7 ± 2.0 | 8.5 ± 0.8 | >100 |
| E64b | 0.040 ± 0.004 | 0.030 ± 0.004 | |
| cystatinb | 0.0085 ± 0.025 |
All the assays were executed in triplicate.
Positive control used for enzymatic inhibition assays.
Dihydrochalcones showed inhibitory activity and selectivity for cathepsins B and L with IC50 values ranging from 1.0 to 14.9 μM (Table 4). The dihydrochalcones 2 and 4 displayed selectivity and inhibition of cathepsin B, while dihydrochalcones 1 and 3 showed inhibition of cathepsin L. This result is interesting and encourages more studies using these compounds, which may be implicated as prototypes for more effective treatments in cancer chemotherapy. Other dihydrochalcones have already been described as inhibitors of other cathepsins such as cathepsin K.12
Table 4.
EC50 Values of Compounds 2 and 4 against P. falciparum Growth
| strain | EC50 (μM)2 | EC50 (μM)4 |
|---|---|---|
| 3D7 | 14.4 ± 0.6 | 17.7 ± 1.4 |
| Dd2 | 11.7 ± 0.8 | 12.4 ± 0.6 |
| W2 | 6.7 ± 0.9 | 8.5 ± 0.7 |
The dihydrochalcones were also tested against Plasmodium falciparum growth using the 3D7 (wild type), Dd2, and W2 strains (both chloroquine resistant). Against P. falciparum the dihydrochalcones 2 and 4 showed significant growth inhibition of the parasite, and the EC50 values are collated in Table 4. The inhibitory activity was similar for the three strains, and the assays were run in quadruplicate. The positive control used was bromophycolide A, which presents a consistent EC50 value around 0.5 μM for all the strains.13 There is no previous report of activity against P. falciparum growth for this class of compounds.
Four compounds were tested against the cysteine proteases cruzain and cathepsins B and L and also against three strains of Plasmodium falciparum. The results described in this paper are significant, since this is the first report of dihydrochalcones with antiplasmodial activity and as inhibitors of these enzymes implied in Chagas disease and cancer. The compounds showed good activity against cruzain, and it is interesting that compounds 2 and 4 are active against cathepsin B, while compounds 1 and 3 are active against cathepsin L. Given the importance of finding new drug candidates against Chagas disease, malaria, and cancer, the dihydrochalcones represent a new class of compounds to be investigated in this context.
EXPERIMENTAL SECTION
General Experimental Procedures.
All commercially available reagents were purchased from Aldrich Chemical Co., and the solvents used for extract preparation and chromatographic fractionation were obtained from Vetec. The NMR spectra were recorded in acetone-d6 and methanol-d4, using TMS as the internal reference, on a Bruker Avance III 9.4 T spectrometer (1H: 400 MHz; 13C: 100 MHz) equipped with manual change of sample and probe of 5 mm BFO (smart probe ATMA). MS data were colledted on a Bruker Daltonics micrOTOF-Q II-ESITOF mass spectrometer. Analytical TLC was performed on precoated aluminum silica 60 F254 (Merck) and used to monitor isolation. Compounds were visualized by exposure under UV254/366 light and by spraying with H2SO4/vanillin solution. Chromatographic separations were performed on silica gel 60 (Merck, 230–700 mesh), Sephadex LH-20 (Amersham Pharmacia Biotech AB), and C18 (Luna).
Plant Materials.
The stems of Metrodorea stipularis were collected in July 2010 at the Agronomic Institute in Campinas, SP; the authentication was made by Luiz Carlos Bernacci. A voucher specimen (44005) was deposited at the same Institute.
Extraction and Isolation.
The stems were dried and macerated with EtOH for 3 days. The solvent was removed using reduced pressure at 40 °C, affording 30 g of a species. This extract was subjected to liquid–liquid partitioning to yield hexanes (2 g), CH2Cl2 (4 g), EtOAc (11 g), and hydroalcoholic (13 g) fractions. All were submitted to enzymatic assay against cruzain, and the CH2Cl2 fraction showed significant inhibition of the enzyme; thus it was chromatographed over silica gel 60 (φ = 5.0 cm × h = 22.0 cm) eluting with CH2Cl2/EtOAc (10:0, 15:5, 5:5, 0:10) to yield 24 fractions (MSD1–MSD24).
Fraction MSD15 (170 mg) was chromatographed over Sephadex LH-20 eluting with MeOH to give 10 subfractions (MSD15_1–MSD15_10). Subfraction MSD15_7 (10 mg) was chromatographed over Sephadex LH-20 (φ = 2.0 cm × h = 30.0 cm) eluting with MeOH to obtain 1 (2 mg).
Fraction MSD21 (400 mg) was chromatographed over silica gel 60 (φ = 3.0 cm × h = 23.0 cm) eluting with CH2Cl2/EtOAc (9:1) to give eight subfractions (MSD21_1–MSD21_8). Subfraction MSD21_8 (50 mg) was chromatographed over Sephadex LH-20 eluting with MeOH to give 20 subfractions (MSD21_8_1–MSD21_8_20). Subfraction MSD21_8_12 (20 mg) was chromatographed over silica gel 60 (φ = 2.0 cm × h = 25.0 cm) eluting with CH2Cl2 to obtain 2 (2 mg) and 3 (8 mg). Subfraction MSD21_8_19 (9 mg) was purified by reversed-phase preparative HPLC (C18 Luna, φ = 1.0 cm × h = 25.0 cm) using MeOH/ACN/H2O (4:1:5) as mobile phase and a flow of 3.5 mL/min to afford 4 (2.5 mg).
1-(5,7-Dihydroxy-2,2-dimethylchroman-6-yl)-3-(2,2-dimethylchroman-6-yl)propan-1-one (2): amorphous powder; NMR (400 MHz, acetone-d6) see Table 1; ESITOFMS m/z 410.2066 (calcd for C25H30O5 m/z 410.2094).
1-(5,7-Dihydroxy-2,2-dimethylchroman-6-yl)-3-[4-hydroxy-3-(3-methylbut-2-en-1-yl)phenyl]propan-1-one (3): amorphous powder; NMR (400 MHz, acetone-d6) see Table 1; ESITOFMS m/z 410.2040 (calcd for C25H30O5 m/z 410.2094).
1-(5,7-Dihydroxy-2,2-dimethylchroman-6-yl)-3-(1,1,4a-trimethyl-2,3,4,4a, 9a-hexahydro-1H-xanthen-7-yl)propan-1-one (4): amorphous powder; NMR (400 MHz, acetone-d6) see Table 2; ESITOFMS m/z 478.2714 (calcd for C30H38O5 m/z 478.2720).
Enzyme Inhibition Assays.
Cruzain.
All commercially available chemicals and reagents were purchased from Aldrich Chemical Co. and Sigma, and kinetic measurements were carried out in a Hitachi F2500 spectrofluorimeter. Inhibitory activity was measured by the hydrolysis of the synthetic substrate Z-Phe-Arg-AMC (benzyloxycarbonylphenylalanylarginine-4-methyl-7-coumarylamide). The enzyme activity was tracked for 5 min, with stirring, in phosphate buffer (pH 6.3) with 5 μL of DTT at 37 °C after addition of 2 μL of the substrate. The experiments were carried out in triplicate in quartz cuvettes, and the final volume of the reaction mixture was 1 mL (excitation 380 nm and emission 460 nm). Control assays were performed without inhibitor (negative control) and in the presence of the irreversible inhibitor for cysteine peptidase, E-64 (positive control). IC50 values were determined by rate measurements with five inhibitor concentrations. All kinetic parameters were determined by nonlinear regression employing the program GraFit 5.
Cathepsins.
All commercially available chemicals and reagents were purchased from Aldrich Chemical Co. and Sigma, and kinetic measurements were carried out in a Molecular Devices Spectra MAX M3 fluorimeter. Inhibitory activity was measured using the synthetic fluorometric substrate Z-Phe-Arg-AMC and a concentration of 185 μM for catB and 10 μM for catL. The enzyme was activated for 5 min with DTT and acetate buffer (pH 5.5) at 37 °C, and the reaction mixture was incubated for 5 min with the sample. The experiments were carried out in triplicate (in 96-well black plates), and the final volume of the reaction mixture was 200 μL, kept under stirring (excitation 355 nm and emission 460 nm). Control assays were performed without inhibitor (negative control) and in the presence of the irreversible inhibitor for cysteine peptidase, E-64 (positive control). IC50 values were determined by rate measurements with at least seven inhibitor concentrations. All kinetic parameters were determined by nonlinear regression employing the SigmaPlot 12.0.
Bioassay of Inhibition of Plasmodium falciparum growth.
The strains 3D7, W2, and Dd2 of P. falciparum malaria parasites (BEI Resources, MR4/ATCC, Manassas, VA, USA) were cultured in human type O+ erythrocytes in complete medium (RPMI 1640 (Cellgro), 0.043 mg/mL gentamicin (Gibco), 0.014 mg/mL hypoxanthine (Acros), 38.5 mM HEPES (Sigma), 0.18% NaHCO3 (Cellgro),0.20% Glucose (MP Biomedical), 0.003 mM NaOH (Sigma), 0.2% Albumax (Gibco), 5% human serum) as previously described.14 Briefly, cultures were maintained in 25 cm3 flasks (Corning) at a volume of 10 mL and were gassed for 30 s with an environment of 3% CO2, 1% O2, and 96% N2, then incubated at 37 °C.
The antimalarial activity was determined with a SYBR Green based parasite proliferation assay, adapted from Smilkstein15 and Bennett.16 Using this technique, the increase of parasite DNA contained in human red blood cells was measured after 72 h of incubation in the presence of serial dilutions of compounds.17 Relative fluorescence values were measured with a Molecular Devices SpectraMAX Gemini EM fluorimeter (excitation 495 nm and emission 525 nm). Data were analyzed using Microsoft Excel and plotted with SigmaPlot 10 (Systat).
Supplementary Material
ACKNOWLEDGMENTS
This research was financially supported by the State of São Paulo Research Foundation Proc. #2010/00496-8 (FAPESP, Fundação de Amparo à Pesquisa do Estado de São Paulo) and the National Council for Scientific and Technological Development (CNPq, Conselho Nacional de Pesquisa e Desenvolvimento).
Footnotes
Supporting Information
The 1H, HSQC, and HMBC NMR spectra of the compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- (1).Baker DD; Chu M; Oza U; Rajgarhia V Nat. Prod. Rep 2007, 24, 1225–1244. [DOI] [PubMed] [Google Scholar]
- (2).Baetas ACS; Arruda MSP; Muller AH; Arruda AC J. Braz. Chem. Soc 1999, 10, 181–183. [Google Scholar]
- (3).Muller AD; Vieira PC; Da Silva MF; Das GF; Fernandes JB Phytochemstry 1995, 40, 1797–1800. [Google Scholar]
- (4).Trani M; Carbonetti A; Monache GD; Monache DF Fitoterapia 2004, 75, 99–102. [DOI] [PubMed] [Google Scholar]
- (5).Gelb MH; Hol WG J. Science 2002, 297, 343–344. [DOI] [PubMed] [Google Scholar]
- (6).Said M; Roberson SA; Brinen LS; Mckerrow JH Adv. Exp. Med. Biol 2011, 712, 100–115. [DOI] [PubMed] [Google Scholar]
- (7).Fairlamb AH Medicina (Buenos Aires) 1999, 2, 179–182. [Google Scholar]
- (8).Urbina JC; Docampo R Trends Parasitol 2003, 19, 495–501. [DOI] [PubMed] [Google Scholar]
- (9).Braz filho R Quim. Nova 2010, 33, 229–239. [Google Scholar]
- (10).Lankelma JM; Voorend DM; Barwari T; Koetsveld J; Van Der Spek AH; De Porto AP; Van Rooijen G; Van Noorden CJ Life Sci 2010, 86, 225–233. [DOI] [PubMed] [Google Scholar]
- (11).Vasiljeva T; Reinheckel C; Peters D; Turk V; Turk B Curr. Pharm. Des 2007, 13, 387–403. [DOI] [PubMed] [Google Scholar]
- (12).Patil AD; Freyer AJ; Killmer L; Offen P; Taylor PB; Votta BJ; Johnson RK J. Nat. Prod 2002, 65, 624–627. [DOI] [PubMed] [Google Scholar]
- (13).Stout EP; Cervantes S; Prudhomme J; France S; La Clair JJ; Le Roch K; Kubanek J Chem. Med. Chem 2011, 6, 1572–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Le Roch KG; Zhou Y; Blair PL; Grainger M; Moch JK; Haynes JD; De La Vega P; Holder AA; Batalov S; Carucci DJ Science 2003, 301, 1503–1508. [DOI] [PubMed] [Google Scholar]
- (15).Smilkstein M; Sriwilaijaroen N; Kelly JX; Wilairat P; Riscoe M Antimicrob. Agents Chemother 2004, 48, 1803–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Bennett TN; Pagyio M; Gligorijevic B; Seucieu C; Kosar AD; Davison E; Roepe PD Antimicrob. Agents Chemother 2004, 48, 1807–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Prudhomme J; McDaniel E; Ponts N; Bertani S; Fenical W; Jensen P; Le Roch K PLoS One 2008, 3 (6), e2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


