Abstract
Objective:
To investigate the effects of levodopa on functional brain networks in Parkinson disease.
Methods:
We acquired resting state functional magnetic resonance imaging in 30 drug-naïve participants with Parkinson disease and 20 age-matched healthy controls. Each participant was studied following administration of a single oral dose of either levodopa or placebo, in a randomized, double-blind, crossover design.
Results:
The greatest observed differences in functional connectivity were between Parkinson disease vs. control participants, independent of pharmacologic intervention. By contrast, the effects of levodopa were much smaller and detectable only in the Parkinson disease group. Moreover, although levodopa administration in the Parkinson disease group measurably improved motor performance, it did not increase the similarity of functional connectivity in Parkinson disease to the control group.
Conclusions:
We found that a single, small dose of levodopa did not normalize functional connectivity in drug-naïve Parkinson disease.
Keywords: Parkinson disease, fMRI, resting state functional connectivity, levodopa, drug naive
Introduction
The dopamine precursor levodopa (L-3,4-dihydroxyphenylalanine; L-DOPA) is a highly effective pharmacologic treatment for many of the motor manifestations of Parkinson disease (PD). However, the precise neural mechanisms that account for its effects remain incompletely understood.
Functional connectivity (FC) within and between large-scale neural networks can be measured using resting state functional magnetic resonance imaging (rs-fMRI).1 Although the obtained results have been somewhat inconsistent,2 several recent rs-fMRI studies have reported that acute administration of L-DOPA or dopamine agonists “normalizes” FC abnormalities in chronically medicated PD (see Supplemental Table 1). However, chronic medication exposure may modify dopamine circuitry,3 thereby altering the L-DOPA response between drug naïve and chronically medicated PD.
We measured functional network organization in drug-naïve PD and matched healthy control (HC) participants with and without administration of L-DOPA using a within-subjects counterbalanced design with rigorous quality control measures. Statistical inference was computed using Object Oriented Data Analysis (OODA),4,5 which assesses omnibus differences between high-dimensional measures at the group level.
Methods
Participants
Participants with never medicated idiopathic PD (n = 30), based on modified United Kingdom PD Society Brain Bank clinical diagnostic criteria,6 and matched healthy control participants (n = 20) were recruited through the Washington University in St. Louis Movement Disorders Center. The university’s Institutional Review Board approved all procedures; all participants gave written informed consent. Clinical follow-up was available for 24 of these PD participants, with 23 followed for at least 3 years, who all had a diagnosis of levodopa-responsive idiopathic PD. Further details, including exclusion criteria, are presented in Appendix 1.
Procedure
Each participant completed two matched imaging sessions on separate days where they received either L-DOPA or placebo. Each participant received 200 mg carbidopa orally. Thirty minutes later, they received an oral dose of either carbidopa/levodopa 37.5mg/150mg or matched placebo. The first three PD participants inadvertently received carbidopa/levodopa 25mg/100mg. Drug or placebo administration was double blind, and the order was counterbalanced across individuals. Drug administration occurred approximately 1 hour prior to fMRI scanning. Before and after each scan, motor severity was rated using the Unified Parkinson Disease Rating Scale motor evaluation (UPDRS-III). A small battery of cognitive and mood assessments was also obtained. Blood samples were taken before and after MRI scanning, and concentrations of L-DOPA and dopamine were measured as described.7 See complete details in Appendix 1.
Statistical Analysis
Principal statistical results were obtained by comparing groups constituting the 2 × 2 design (PD vs. HC × L-DOPA vs. placebo) using object-oriented data analysis (OODA), a recently developed technique for contrasting connectomes (see Appendix 2). OODA was used to test whether correlation matrices systematically differed between conditions/groups, treating individual correlation matrices as single objects. This method allows the full matrices to be compared, rather than individual networks or connections, thereby obviating the loss of power from testing each connection separately. To determine which individual within- and between-network correlations differed significantly between groups and drug conditions, we used a previously described permutation approach.8
As a measure of the relative effect size between groups and drug conditions, we used Kullback’s symmetric divergence (J-divergence),9 an information theoretic measure of the dissimilarity between two distributions. The larger the J-divergence, the more dissimilar the two distributions, indicating a larger effect. See Appendix 2 for details.
We used multi-dimensional scaling to represent the similarity among matrices onto a lower dimensional space that maximally captures variance in the data. We examined whether certain FC variables (either the first dimension from multi-dimensional scaling or the average intra-network FC) related to motor severity (UPDRS-III total score or subscores) of the PD participants using Spearman’s correlations. Correlation of FC with behavioral measures was restricted to these measures to limit the number of multiple comparisons. See Appendix 1 for details.
Results
Participant Characteristics
PD and HC participants were well-matched, although PD participants endorsed more anxiety and depressive symptoms (Table 1). Mean baseline UPDRS-III (average of pre-drug ratings from both sessions) in the PD group was 22 ± 11 (mean ± SD). PD participant UPDRS-III improved after L-DOPA (mean difference = −2.7, V = 24, p < 0.001), and did not significantly improve following placebo (mean difference = −1.3, V = 88.5 p = 0.35). The paired difference between improvement in the L-DOPA versus placebo conditions was small (mean difference = −1.5, V = 71, p = 0.043). L-DOPA levels did not differ significantly between groups and dopamine levels were close to zero (Table 1).
Table 1.
Clinical characteristics
| Characteristic | PD | HC |
|---|---|---|
| Gender | 9 F, 21 M | 6 F, 14 M |
| Age (years) | 63 ± 11 | 63 ± 11 |
| Education (years) | 17 ± 2.8 | 17 ± 1.6 |
| MMSE | 29 ± 1.4 | 29 ± 1.2 |
| WTAR | 109 ± 13 | 111 ± 12 |
| STAI Trait | 31.5 ± 7.7 | 29.9 ± 5.8 |
| STAI State | 30.1 ± 7.6 | 25.6 ± 6.65* |
| BDI-II | 5.5 ± 4.2 | 2.1 ± 2.1** |
| Baseline UPDRS-III | 22 ± 11 | 14 ± 11*** |
| L-DOPA (ng/mL) | ||
| pre-scan | 1531 ± 1148 | 1474 ± 913 |
| post-scan | 814 ± 326 | 791 ± 373 |
| Dopamine (ng/mL) | ||
| pre-scan | 3.2 ± 13.6 | 4.3 ± 14.4 |
| post-scan | 0.6 ± 1.7 | 2.2 ± 8.5 |
| Mean FD (mm) | ||
| placebo | 0.118 ± 0.020 | 0.112 ± 0.019 |
| L-DOPA | 0.122 ± 0.021 | 0.116 ± 0.024 |
| Retained frames | ||
| placebo | 411 [182–539] | 424 [192–568] |
| L-DOPA | 396 [152–525] | 404 [162–540] |
Values represent group means ± standard deviations (SD) or means [minimum-maximum]. MMSE = Mini-Mental State Examination; WTAR = Weschler Test of Adult Reading; STAI = State-Trait Anxiety Inventory; BDI = Beck Depression Inventory, second edition; UPDRS-III = Unified Parkinson Disease Rating Scale subscale 3, FD = frame-wise displacement computed after censoring.
p < 0.05,
p < 0.001,
p-value < 10−10.
Functional Connectivity Differences
We measured large-scale functional networks in PD and HC across cortical, subcortical, and cerebellar regions representing the entire functional connectome (Supplemental Figure 3). Network organization was similar across groups and drug conditions (Figure 1A–D) and was consistent with prior network parcellations identified in young healthy controls.10–12 Direct comparisons revealed FC differences between PD and HC participants, involving cortical sensorimotor, subcortical, and association networks (Figure 1E–F). Differences between L-DOPA and placebo were much smaller in comparison (Figure 1G–H). We applied statistical analysis with OODA to determine whether whole-brain functional connectivity differed significantly between PD and HC and between L-DOPA and placebo. FC differed between PD and HC groups in both the placebo (p = 0.007) and L-DOPA (p = 0.013) conditions. There was a detectable difference between L-DOPA vs. placebo in the PD group (p = 0.026) but not the HC group (p = 0.234).
Figure 1.
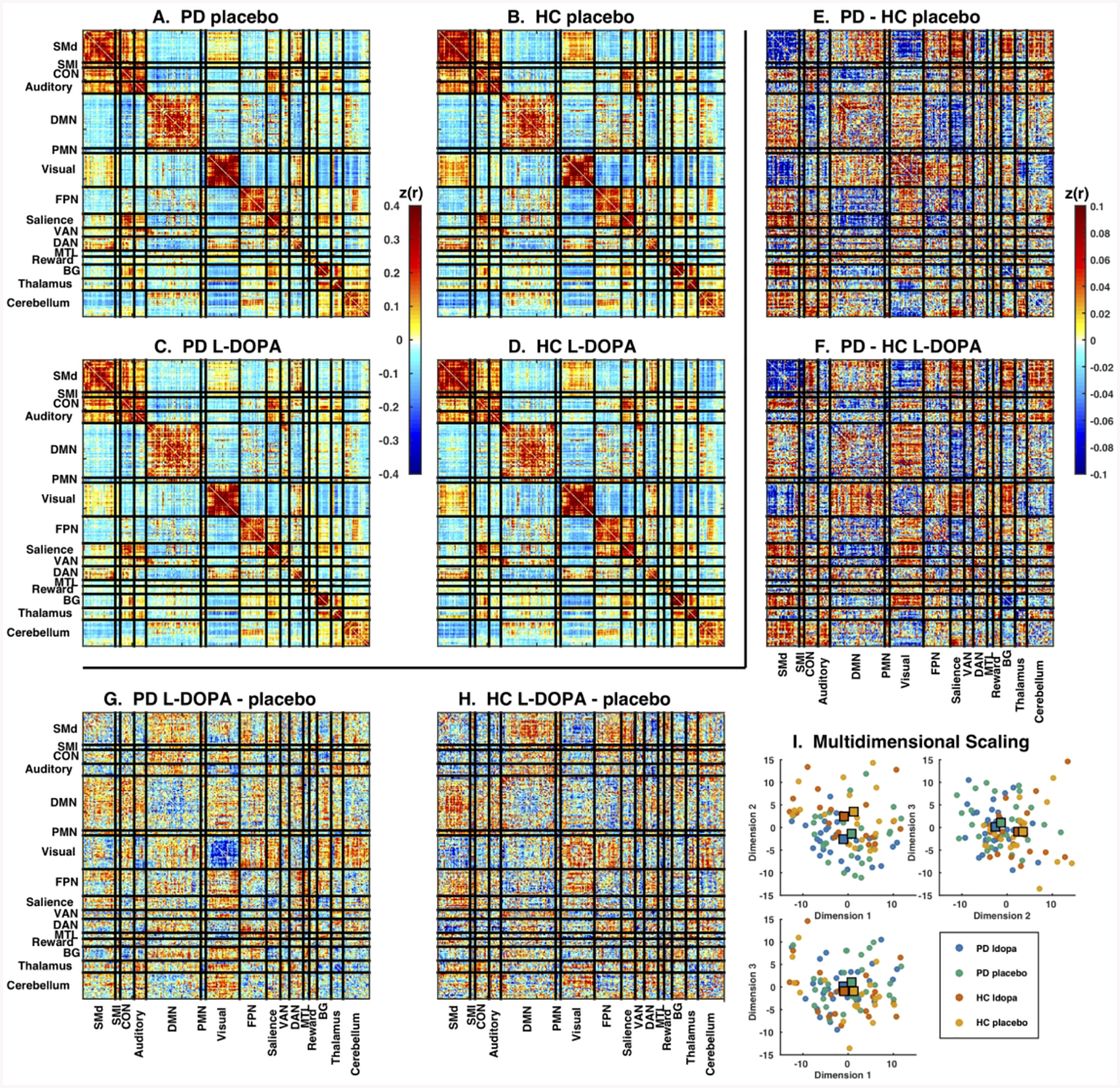
Large-scale networks in Parkinson disease and controls after L-DOPA or placebo. Mean correlation matrices in (A) PD placebo, (B) HC placebo, (C) PD L-DOPA and (D) HC L-DOPA conditions. All conditions showed strong network organization with well-defined block structure. Note generally strong positive correlations within each network (diagonal) and mixture of weaker positive and negative correlations between networks (off-diagonals). Direct comparison between groups (PD – HC) for the (E) L-DOPA and (F) placebo conditions, and between drug conditions (L-DOPA – placebo) for the (G) PD and (H) HC groups. There was a mixture of positive and negative differences. Note much larger differences between groups than between drug conditions. Color scales indicates magnitude of Z-transformed Pearson’s correlations (note different color scales for mean and difference matrices). (I) Each plot shows the relative positions of individual participants FC matrices in the first 3 dimensions from multi-dimensional scaling. Conditions are indicated by color: PD L-DOPA (blue), PD placebo (green), HC L-DOPA (red), and HC placebo (orange). Large squares represent the central tendency of each group. Note separation between PD and HC conditions, as well as lack of centroid shift with L-DOPA treatment.
To measure relative effect size, we calculated J-divergence, which quantifies the similarity between each pair of conditions. Values close to zero indicate close similarity, and larger values indicate more difference. We observed that J-divergences between drug conditions were small (L-DOPA vs. placebo: PD 1.273, p = 0.042; HC 1.161, p = 0.242), while J-divergences between participant groups were much larger (PD vs. HC: L-DOPA 2.562, p < 0.0001; placebo 2.584, p < 0.0001). We observed the largest J-divergence between PD L-DOPA and HC placebo (2.789, p = 0.002). If L-DOPA exerted a normalizing effect on FC, we would expect this distance to be smaller than the distance between PD placebo and HC placebo. No such effect was observed. Qualitatively similar results were observed with multidimensional scaling analysis (Figure 1I, Appendix 3).
We identified within- and between-network FC blocks with significant differences between groups. The only significant block was intra-network FC of the dorsal somatomotor network (SMd), which showed reduced FC in PD. See Appendix 3 for details.
To determine whether altered network-level FC relates to the clinical manifestations of PD, we examined the relationship between several FC measures and UPDRS-III ratings in the PD group (Appendix 3). The first dimension from our multi-dimensional scaling analysis correlated negatively with UPDRS-III ratings in the PD group (Supplemental Figure 6) in L-DOPA and placebo conditions, for both pre- and post-drug ratings. L-DOPA did not significantly alter this correlation (Supplemental Figure 7).
Discussion
We report a resting-state functional connectivity study comparing participants with drug-naïve, idiopathic PD to matched healthy controls following a single oral dose of either L-DOPA or placebo. Attributes of this study include rigorous quality assurance of the fMRI data based on effective nuisance regression, censoring of artifact-contaminated volumes, acquisition of multiple runs, and comprehensive sampling of cortical, subcortical and cerebellar regions. We found functional connectome-level differences between participant groups. In particular, FC within the somatomotor network was systematically lower in drug-naïve PD compared to controls. Differences between drug conditions were much smaller. The first multi-dimensional scaling dimension significantly correlated with UPDRS-III in the PD group, but L-DOPA did not significantly alter this correlation.
L-DOPA ameliorates most motor deficits in PD. Indeed, such an effect was observed here although the improvements were modest. The current rs-fMRI literature suggests that FC measure reflect the normalizing effect on motor system physiology in PD (Supplemental Table 1).2 However, this literature is inconsistent with regard to the direction of the FC effect of PD: both decreases and increases have been reported, including normalization (or hyper-normalization) following L-DOPA administration (increases or decreases, respectively). We detected a small but significant omnibus effect of L-DOPA in drug-naïve PD (OODA p = 0.026). Crucially, L-DOPA administration in PD did not shift FC towards the pattern seen in HC (Figure 1I). This outcome does not provide support for a normalizing effect of L-DOPA on FC in drug-naïve PD.
Only two prior studies have examined resting FC in response to L-DOPA in drug-naïve PD, one using separate treatment groups,13 and another using an un-blinded OFF-then-ON design.14 Esposito et al. reported decreased FC between SMA and the somatomotor network in drug naïve PD participants given placebo,13 consistent with the findings presented here. However, they found that this abnormal FC was partially normalized in a separate group of participants given L-DOPA, which we did not observe. We studied drug naïve PD participants, which could explain why we did not observe L-DOPA effects on FC as large as have been reported in chronically medicated participants. Chronic L-DOPA therapy may modify dopamine pathways,3 altering the L-DOPA response between naïve and chronically medicated PD.
Only a few other studies have investigated FC in drug-naïve PD, where alterations have been observed between various cortico-striatal15–17 cortico-cortical,16 or other subcortical18 connections. Here, we demonstrate significantly decreased somatomotor network FC in drug-naïve PD. This result suggests that rs-fMRI can detect changes in early PD, but these changes are downstream of the main sites of early pathology in PD.8,19,20
The main limitation of the current study is that motor improvements after a single dose of L-DOPA in our PD group were relatively small. The dose was only slightly lower (150 mg) than the dose used in most other rs-fMRI studies (200 mg), but the same dose that produced marked cerebral blood flow changes in drug-naïve PD.21,22 Thus, the lack of observed L-DOPA effects could have been due to insufficient stimulation of dopaminergic pathways. However, we detected clinically relevant plasma levels of L-DOPA23–27 Despite this limitation, the results of the current study argue against FC being a more sensitive indicator of acute L-DOPA response than clinical observation.
Our results highlight the importance of network effects that are distributed, but selective, even in early PD. We do not find evidence that a single dose of L-DOPA normalizes the characteristic FC abnormalities in PD at this stage.
Supplementary Material
Acknowledgements
We greatly appreciate the assistance of study coordinators Johanna Hartlein, Phil Lintzenich, Stacy Pratt, Yan Ling, and Thomas Belcher. We thank Ben Seitzman, Ashley Nielsen, and Deanna Greene for providing the ROI set. This study was funded by the St. Louis Chapter of the American Parkinson Disease Association.
Grants: NIH; American Parkinson Disease Association (APDA); Greater St. Louis Chapter of the APDA; Huntington Disease Society of America; Barnes-Jewish Hospital Foundation; CHDI Foundation; MJ Fox Foundation; U Michigan; Toronto Western University; Paula and Rodger Riney Foundation; Jo Oertli Foundation; Murphy Fund.
Honoraria: U Rochester; American Academy of Neurology; Movement Disorders Society; Emory U; Parkinson Foundation; St. Louis University; Harvard University; Stanford U; U Florida at Gainesville; Huntington Study Group; U Pennsylvania Consulting: Medical legal consultations
Footnotes
Full Financial Disclosures
Robert L. White
Employment: Department of Veteran’s Affairs; Washington University
Meghan C. Campbell
Employment: Washington University
Grants: NIH, Washington University
Dake Yang
Employment: BioRankings, LLC
William Shannon
Employment: BioRankings, LLC
Abraham Z. Snyder
Employment: Washington University
Grants: NIH; James S. McDonnell Foundation
Joel S. Perlmutter
Employment: Washington University
Financial Disclosures:
Robert L. White: no disclosures
Meghan C. Campbell: no disclosures
Dake Yang: employee at BioRankings, LLC
William Shannon: president and founder of BioRankings, LLC
Abraham Z. Snyder: no disclosures
Joel S. Perlmutter: no disclosures
Study funded by the Greater St. Louis Chapter of the American Parkinson Disease Association.
References
- 1.Raichle ME. The restless brain: how intrinsic activity organizes brain function. Philosophical Transactions of the Royal Society B: Biological Sciences. The Royal Society; 2015;370:20140172–20140172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tahmasian M, Bettray LM, van Eimeren T, et al. A systematic review on the applications of resting-state fMRI in Parkinson’s disease: Does dopamine replacement therapy play a role? Cortex. 2015;73:80–105. [DOI] [PubMed] [Google Scholar]
- 3.Gerfen CR. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000;23:S64–S70. [DOI] [PubMed] [Google Scholar]
- 4.La Rosa PS, Brooks TL, Deych E, et al. Gibbs distribution for statistical analysis of graphical data with a sample application to fcMRI brain images. Stat Med. 2016;35:566–580. [DOI] [PubMed] [Google Scholar]
- 5.La Rosa PS, Shands B, Deych E, et al. Statistical object data analysis of taxonomic trees from human microbiome data Hsiao CK, editor. PLoS ONE. Public Library of Science; 2012;7:e48996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatr. 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karimi M, Carl JL, Loftin S, Perlmutter JS. Modified high-performance liquid chromatography with electrochemical detection method for plasma measurement of levodopa, 3-O-methyldopa, dopamine, carbidopa and 3,4-dihydroxyphenyl acetic acid. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;836:120–123. [DOI] [PubMed] [Google Scholar]
- 8.Gratton C, Koller JM, shannon W, et al. Emergent Functional Network Effects in Parkinson Disease. Cereb Cortex. 2018;5:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kullback S. Information Theory and Statistics. Mineola, NY: Dover Publications, Inc; 1968. [Google Scholar]
- 10.Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex. 2016;26:288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene DJ, Laumann TO, Dubis JW, et al. Developmental changes in the organization of functional connections between the basal ganglia and cerebral cortex. J Neurosci. Society for Neuroscience; 2014;34:5842–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito F, Tessitore A, Giordano A, et al. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson’s disease by levodopa. Brain. 2013;136:710–725. [DOI] [PubMed] [Google Scholar]
- 14.Wu T, Wang J, Wang C, et al. Basal ganglia circuits changes in Parkinson’s disease patients. Neurosci Lett. 2012;524:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo C, Song W, Chen Q, et al. Reduced functional connectivity in early-stage drug-naive Parkinson’s disease: a resting-state fMRI study. Neurobiology of aging. 2014;35:431–441. [DOI] [PubMed] [Google Scholar]
- 16.Baik K, Cha J, Ham JH, et al. Dopaminergic modulation of resting-state functional connectivity in de novo patients with Parkinson’s disease. Hum Brain Mapp. Wiley-Blackwell; 2014;35:5431–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agosta F, Caso F, Stankovic I, et al. Cortico-striatal-thalamic network functional connectivity in hemiparkinsonism. Neurobiology of aging. 2014;35:2592–2602. [DOI] [PubMed] [Google Scholar]
- 18.Kurani AS, Seidler RD, Burciu RG, et al. Subthalamic nucleus--sensorimotor cortex functional connectivity in de novo and moderate Parkinson’s disease. Neurobiology of aging. 2015;36:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder AZ. Resting state functional connectivity of the striatum in Parkinson’s disease. Brain. 2012;135:3699–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell MC, Koller JM, Snyder AZ, Buddhala C, Kotzbauer PT, Perlmutter JS. CSF proteins and resting-state functional connectivity in Parkinson disease. Neurology. Lippincott Williams & Wilkins; 2015;84:2413–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hershey T, Black KJ, Carl JL, McGee-Minnich L, Snyder AZ, Perlmutter JS. Long term treatment and disease severity change brain responses to levodopa in Parkinson’s disease. J Neurol Neurosurg Psychiatr. BMJ Publishing Group; 2003;74:844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hershey T, Black KJ, Stambuk MK, Carl JL, McGee-Minnich LA, Perlmutter JS. Altered thalamic response to levodopa in Parkinson’s patients with dopa-induced dyskinesias. Proc Natl Acad Sci USA. 1998;95:12016–12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans MA, Triggs EJ, Broe GA, Saines N. Systemic activity of orally administered L-dopa in the elderly Parkinson patient. Eur J Clin Pharmacol. 1980;17:215–221. [DOI] [PubMed] [Google Scholar]
- 24.Nutt JG, Woodward WR, Hammerstad JP, Carter JH, Anderson JL. The “on-off” phenomenon in Parkinson’s disease. Relation to levodopa absorption and transport. N Engl J Med. Massachusetts Medical Society; 1984;310:483–488. [DOI] [PubMed] [Google Scholar]
- 25.Gancher ST, Nutt JG, Woodward WR. Peripheral pharmacokinetics of levodopa in untreated, stable, and fluctuating parkinsonian patients. Neurology. Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology; 1987;37:940–944. [DOI] [PubMed] [Google Scholar]
- 26.Contin M, Riva R, Martinelli P, Cortelli P, Albani F, Baruzzi A. Longitudinal monitoring of the levodopa concentration-effect relationship in Parkinson’s disease. Neurology. Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology; 1994;44:1287–1292. [DOI] [PubMed] [Google Scholar]
- 27.Contin M, Martinelli P. Pharmacokinetics of levodopa. J Neurol. Springer-Verlag; 2010;257:S253–S261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


