Abstract
Background
Nasolacrimal duct obstruction (NLDO) is a condition that results in the overflow of tears (epiphora) or infection of the nasolacrimal sac (dacryocystitis). The etiology of acquired NLDO is multifactorial and is not fully understood. Dacryocystorhinostomy (DCR) is the surgical correction of NLDO, which aims to establish a new drainage pathway between the lacrimal sac and the nose. The success of DCR is variable; the most common cause of failure is fibrosis and stenosis of the surgical ostium. Antimetabolites such as mitomycin‐C (MMC) and 5‐fluorouracil (5‐FU) have been shown to be safe and effective in reducing fibrosis and improving clinical outcomes in other ophthalmic surgery settings (e.g. glaucoma and cornea surgery). Application of antimetabolites at the time of DCR has been studied, but the utility of these treatments remains uncertain.
Objectives
Primary objective: To determine if adjuvant treatment with antimetabolites improves functional success in the setting of DCR compared to DCR alone.
Secondary objectives: To determine if anatomic success of DCR is increased with the use of antimetabolites, and if the surgical ostium is larger in participants treated with antimetabolites.
Search methods
We searched the Cochrane Register for Controlled Trials (CENTRAL) (which contains the Cochrane Eye and Vision Trials Register) (2019, Issue 9), Ovid MEDLINE, Embase.com, PubMed, LILACS (Latin American and Caribbean Health Sciences Literature database), ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We did not use any date or language restrictions in the electronic searches. We last searched the electronic databases on 6 September 2019.
Selection criteria
We only included randomized controlled trials. Eligible studies were those that compared the administration of antimetabolites of any dose and concentration versus placebo or another active treatment in participants with NLDO undergoing primary DCR and reoperation. We only included studies that had enrolled adults 18 years or older. We also included studies that used silicone intubation as part of the DCR procedure.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently screened the search results, assessed risk of bias, and extracted data from the included studies using an electronic data collection form.
Main results
We included 31 studies in the review, of which 23 (1309 participants) provided data relating to our primary and secondary outcomes. Many of the 23 studies evaluated functional success, while others also assessed our secondary outcomes of anatomic success or ostium size, or both.
Study characteristics
Participant characteristics varied across studies, with the age of participants ranging from 30 to 70 years. Participants were predominantly women. These demographics correspond to those most frequently affected by nasolacrimal duct obstruction. Almost all of the studies utilized MMC as the antimetabolite, with only one using 5‐FU. We assessed most trials as at unclear risk of bias for most domains. Conflicts of interest were not frequently reported, although the antimetabolites used are generic medications, and studies were not likely to be conducted for financial interest.
Findings
Twenty studies provided data on the primary outcome of functional success, of which 7 (356 participants) provided data at 6 months and 14 (909 participants) provided data beyond 6 months. At six months, the results showed no evidence of effect of antimetabolite on functional success (risk ratio (RR) 1.12, 95% confidence interval (CI) 0.98 to 1.29; low‐certainty evidence). Beyond six months, the results favored the antimetabolite group (RR 1.15, 95% CI 1.07 to 1.25; moderate‐certainty evidence).
Fourteen studies reported data on the secondary outcome of anatomic success, of which 4 (306 participants) reported data at 6 months and 12 (831 participants) provided data beyond 6 months. Results at six months showed no evidence of effect of antimetabolite on anatomic success (RR 1.02, 95% CI 0.95 to 1.11; low‐certainty evidence). Beyond six months, participants in the antimetabolite group were more likely to achieve anatomic success than those receiving DCR alone (RR 1.09, 95% CI 1.04 to 1.15; moderate‐certainty evidence).
At six months and beyond six months follow‐up, two studies reported mean change in ostium size. We did not conduct meta‐analysis for the various follow‐up periods due to clinical, methodological, and statistical heterogeneity. However, point estimates from these studies at six months consistently favored participants in the antimetabolite group (low‐certainty evidence). Beyond six months, while point estimates from one study favored participants in the antimetabolite group, estimates from another study showed no evidence of a difference between the two groups. The certainty of evidence at both time points was low.
Adverse events
Adverse events were rare. One study reported that one participant in the MMC group experienced delayed wound healing. Other studies reported no significant adverse events related to the application of antimetabolites.
Authors' conclusions
There is moderate‐certainty evidence that application of antimetabolites at the time of DCR increases functional and anatomic success of DCR when patients are followed for more than six months after surgery, but no evidence of a difference at six months, low‐certainty of evidence. There is low‐certainty evidence that combining antimetabolite with DCR increases the size of the lacrimal ostium at six months. However, beyond six months, the evidence remain uncertain. Adverse effects of the application of antimetabolites were minimal.
Plain language summary
Antimetabolites as an adjunct to dacryocystorhinostomy for nasolacrimal duct obstruction
What is the aim of the review? Dacryocystorhinostomy (DCR) is a type of surgery that creates a new tear drainage pathway between the eyelid and nose to relieve tearing symptoms (functional success), improve openness of the tear duct to irrigation (anatomic success), and increase the size of the opening into the nose (ostium size). Our aim was to assess whether antiscarring medications (antimetabolites) can increase the functional success, anatomic success, and ostium size of DCR.
Key results We found that antimetabolites may improve functional and anatomic success (relative to DCR alone) at a follow‐up time longer than six months. Antimetabolites may also improve ostium size at six months.
What was studied in the review? The lacrimal system of the eye produces tears, which nourish the eye surface and keep it moist. After passing along the eye surface, tears drain into the nose through the lacrimal drainage apparatus. Nasolacrimal duct obstruction (NLDO) is the blockage of this canal, which can cause an overflow of tears. NLDO is usually painless and can affect one or both eyes. NLDO can also lead to infection of the eye. NLDO is treated surgically with a procedure known as dacryocystorhinostomy (DCR), which establishes a new pathway by creating a pathway between the tear sac and the nose. Antimetabolites have been used to improve success rates of this procedure. We wanted to learn whether DCR in combination with antimetabolites can improve outcomes for functional success, anatomic success, and ostium size than DCR alone. We collected and analyzed all relevant randomized controlled trials to answer this question.
What are the main results of the review? We identified 31 relevant studies for inclusion, most of which originated in South and East Asia and involved predominantly women. These studies compared participants who underwent DCR with metabolites versus participants who underwent DCR alone. Twenty‐three of these studies (1309 participants) provided data on our outcomes of interest.
DCR with antimetabolites may improve functional and anatomic success when patients are followed more than six months after surgery; the certainty of this evidence was moderate. There was no difference in functional and anatomic success at six months among participants who underwent DCR with antimetabolites compared to participants who underwent DCR alone; the certainty of evidence is low.
At six months, participants who underwent DCR with antimetabolites may have increased ostium size compared to those receiving DCR alone. However, beyond six months, there is no evidence of a difference between participants who underwent DCR with antimetabolites compared to participants who underwent DCR alone. The certainty of the evidence was low due to substantial variability among the studies that assessed this outcome. Adverse effects of antimetabolites were minimal.
How up‐to‐date is this review? We reviewed studies published up to 6 September 2019.
Summary of findings
Summary of findings for the main comparison. Mitomycin C dacryocystorhinostomy compared to dacryocystorhinostomy alone for nasolacrimal duct obstruction.
| Mitomycin C dacryocystorhinostomy compared to dacryocystorhinostomy alone for nasolacrimal duct obstruction | ||||||
| Patient or population: nasolacrimal duct obstruction Setting: hospital Intervention: MMC DCR Comparison: DCR alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with DCR alone | Risk with MMC DCR | |||||
| Functional success, defined as the relief of epiphora Follow‐up: 6 months |
Study population | RR 1.12 (0.98 to 1.29) | 356 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 81 per 100 | 90 per 100 (79 to 100) | |||||
| Functional success, defined as the relief of epiphora Follow‐up: > 6 months | Study population | RR 1.15 (1.07 to 1.25) | 909 (14 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 73 per 100 | 84 per 100 (78 to 91) | |||||
| Anatomic success, defined as patency to lacrimal irrigation Follow‐up: 6 months |
Study population | RR 1.02 (0.95 to 1.11) | 306 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 87 per 100 | 89 per 100 (83 to 97) | |||||
| Anatomic success, defined as patency to lacrimal irrigation Follow‐up: > 6 months |
Study population | RR 1.09 (1.04 to 1.15) | 831 (12 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 82 per 100 | 89 per 100 (85 to 94) | |||||
| Ostium size on nasal endoscopy Follow‐up: 6 months |
The mean ostium size on nasal endoscopy ranged from 7 to 10 mm2. | Point estimates from two studies that reported mean change in ostium size at six months follow‐up. Both studies consistently show that participants treated with MMC are more likely to have larger ostium size in (mean difference (MD) 16.27, 95% CI 11.39 to 21.15; 1 study, 15 participants) and (MD 3.70, 95% CI 2.09 to 5.31; 1 study, 50 participants). | 65 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | As fewer than 10 studies assessed this outcome, publication bias could not be quantitatively assessed, however there may still be some but not very serious publication bias. We did not downgrade the certainty of evidence. | |
| Ostium size on nasal endoscopy at Follow‐up: > 6 months |
The mean ostium size on nasal endoscopy ranged from 2 to 13 mm2. | Beyond 6 months, one study found no evidence a difference in ostium size beyond six months follow up (MD 1.40, 95% CI 0.57 to 2.23; 1 study, 50 participants), and another found that participants who were treated with MMC may experience larger ostium size (MD 8.20, 95% CI 6.14 to 10.26; 1 study 50 participants) | 100 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | As fewer than 10 studies assessed this outcome, publication bias could not be quantitatively assessed, however there may still be some but not very serious publication bias. We did not downgrade the certainty of evidence. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DCR: dacryocystorhinostomy; MD: mean difference; MMC: mitomycin‐C; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded (‐1) due to risk of bias. 2Downgraded (‐1) due to imprecision. 3Downgraded (‐1) due to inconsistency.
Background
Description of the condition
The lacrimal system of the eye includes specialized glands that naturally produce tears. The tears nourish the ocular surface and keep the eye moist. After passing along the ocular surface, tears drain into the nose. The conduit for tears between the eye and the nose is known as the lacrimal drainage apparatus. This system includes a series of four key anatomic features: the puncta (opening on the surface of each eyelid), the canaliculi (small channels that connect the puncta with the sac), the nasolacrimal sac (where tears collect), and the nasolacrimal duct (the passage from the sac that leads into the nose). Disruption of any part of the lacrimal drainage apparatus can lead to an overflow of tears. Nasolacrimal duct obstruction (NLDO) refers to a blockage of the nasolacrimal duct.
NLDO is an important ophthalmic problem. One study found an annual incidence rate of 20.24 people with NLDO per 100,000 (Woog 2007). The demographics of NLDO include a higher incidence among older people and women. In Woog 2007, the male‐to‐female ratio was about 1:3 and the mean age 60 years. It is not known if the etiology of NLDO differs by race or socioeconomic status. NLDO may be partially due to anatomic changes in the diameter of the bony lacrimal canal (Janssen 2001), which occurs with aging. These bony changes appear to affect women more than men (because women have a smaller diameter lacrimal duct at baseline) and tend to progress with time.
NLDO is usually painless unless there is an associated infection. The condition can affect one or both eyes. People with NLDO commonly present with epiphora (watery eyes), which significantly impacts their quality of life (Shin 2015). The condition can also lead to dacryocystitis (infection of the lacrimal sac), which raises the risk of secondary infections such as endophthalmitis (infection inside the eye) after cataract surgery.
NLDO is diagnosed by assessing the patency of the lacrimal drainage system with lacrimal irrigation. Typically, a tube, known as a cannula, is placed into the puncta and canaliculi and saline is irrigated. Complete reflux from the other punctum of the same eye is diagnostic of NLDO.
NLDO can be divided into congenital and acquired. Congenital NLDO is primarily treated with probing, followed by balloon catheter dilation if probing fails (Casady 2006). Congenital NLDO that has not responded to probing or balloon catheter dilation may necessitate dacryocystorhinostomy (DCR). Acquired NLDO is primarily treated surgically with DCR.
The aim of DCR is to establish a new drainage pathway by creating a connection between the lacrimal sac and the nasal mucosa. This connection requires removal of maxillary and lacrimal bone that separates those tissues. DCR may be performed via either the traditional external approach (EX‐DCR), in which a surgical incision is made through the skin of the eyelid, or the endonasal approach (EN‐DCR), in which there is no skin incision and the osteotomy is made through a nasal mucosal incision site. An endoscope is typically used to visualize the operative site for the internal approach. The success of the DCR procedure ranges from 70% to 95% (Huang 2014). While successful DCR surgery results in improved quality of life for patients, unsuccessful DCR has a negative impact on patient health (Spielmann 2009). Adjuvant methods, such as silicone stents and antimetabolites, have been used to try to improve success rates. The authors of one systematic review have summarized the effects of these various interventions in EN‐DCR (Marcet 2014). The effectiveness of interventions for congenital NLDO is discussed in another Cochrane Review (Petris 2017).
Description of the intervention
Antimetabolites are adjunctive agents that alter the wound‐healing process by inhibiting postoperative fibrosis. Two common antimetabolites used in ocular surgical procedures are mitomycin C (MMC) and 5‐fluorouracil (5‐FU). MMC is a toxic natural product of certain bacteria that causes the cross‐linking of DNA. It is typically delivered to the eye in a 0.02% to 0.04% concentration. Antimetabolites may be applied topically or injected directly into the tissues. 5‐FU blocks DNA synthesis through its action as a thymidylate synthase inhibitor of collagen gene expression, which could play a role in altering scar formation (Wendling 2003). These actions prevent normal wound‐healing responses by inhibiting cellular proliferation and fibrosis.
Intraoperative MMC has proven useful for trabeculectomy in cases at high risk of bleb failure in glaucoma surgery. Its use is associated with a significantly lower intraocular pressure after five years' follow‐up in people who underwent glaucoma filtration surgery (Bindlish 2002; Wilkins 2005). Intraoperative MMC has also been shown to be more efficacious in reducing the rate of bleb failure from scarring compared with 5‐FU given postoperatively (Skuta 1992). However, 5‐FU has found a role in cases of bleb failure due to its antifibrotic effect in bleb needling (Kapasi 2009). A randomized controlled trial comparing conjunctival autograft with MMC to prevent recurrence after pterygium surgery demonstrated that the two methods were equivalent and reduced recurrence compared with bare sclera excision (Chen 1995).
The use of antimetabolites in eye surgery should be undertaken with caution as serious complications have been reported with their use (Rubinfeld 1992). Because of previous reports of vision‐threatening complications, the minimum amount of topical antimetabolite should be used (Rubinfeld 1992). Antimetabolites have been found to be useful in nasal applications, for example in the use of reduction of fibrosis in choanal atresia surgery (Prasad 2002). In DCR surgery, antimetabolites are applied intraoperatively to the surgical ostium to prevent postoperative closure of the opening. The concentration and length of application of the agents may vary.
How the intervention might work
In certain ophthalmology procedures (i.e. glaucoma filtration and pterygium surgeries), the development of scar tissue is associated with failure of the procedure. By reducing the development of fibrosis, MMC is thought to increase the success rates of these procedures. One of the key causes of failure with DCR is a blocked ostium due to membranous scarring (Hull 2013). MMC may reduce the scarring that often causes the drainage pathway created from DCR to decrease in size, a factor that presumably leads to DCR failure (Chan 2013).
Why it is important to do this review
A Cochrane Review showed that antimetabolites reduce surgical failures in glaucoma surgery, especially in high‐risk patients (Wilkins 2005). Antimetabolites reduce surgical failure in glaucoma surgery by preventing fibrosis that results in bleb failure. It is unclear if antimetabolites would also have the same biological mechanism and clinical benefit in participants undergoing DCR. While one randomized controlled trial showed a possible benefit to using antimetabolites as an adjunct to DCR, other studies have combined the use of antimetabolites with other interventions, such as silicone stents (Dogan 2013b; Mudhol 2013b), making it difficult to infer direct conclusions about the effects of MMC and 5‐FU. The comparative effectiveness and safety of antimetabolites in dacryocystorhinostomy for nasolacrimal duct obstruction is therefore unclear.
Objectives
Primary objective: To determine if adjuvant treatment with antimetabolites improves functional success in the setting of DCR compared to DCR alone.
Secondary objectives: To determine if anatomic success of DCR is increased with the use of antimetabolites, and if the surgical ostium is larger in participants treated with antimetabolites.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs). Eligible RCTs were those that compared the administration of antimetabolites versus placebo or other active treatments in participants undergoing DCR.
Types of participants
We included studies in which participants underwent primary DCR and reoperation for NLDO indication. We only included studies of adults 18 years or older.
Types of interventions
We included studies in which the use of antimetabolites (MMC or 5‐FU) at any concentration and dose was compared with placebo or another active treatment as an adjunct to either EN‐DCR or EX‐DCR. We also included studies that used silicone intubation.
Types of outcome measures
Primary outcomes
Functional success, defined as the relief of epiphora at six months postoperatively.
Secondary outcomes
Anatomic success, defined as patency to lacrimal irrigation at six months postoperatively.
Ostium size on nasal endoscopy at six months postoperatively.
Adverse events
We compared adverse events related to treatments, such as hemorrhage, infection, and scarring.
In addition to the primary time point of six months, we evaluated outcomes reported at follow‐up times greater than six months when data were available.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs. There were no restrictions on language or year of publication. We last searched the electronic databases on 6 September 2019.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 9) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 6 September 2019) (Appendix 1).
MEDLINE Ovid (1946 to 6 September 2019) (Appendix 2).
Embase.com (1947 to 6 September 2019) (Appendix 3).
PubMed (1948 to 6 September 2019) (Appendix 4).
LILACS (Latin American and Caribbean Health Sciences Literature database) (1982 to 6 September 2019) (Appendix 5).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 6 September 2019) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 6 September 2019) (Appendix 7).
Searching other resources
We searched the reference lists of included studies to identify additional studies. We used the Web of Science database to search for reports that have cited the studies in this review. We did not handsearch journals or conference proceedings for the specific purposes of this review.
Data collection and analysis
Selection of studies
Two review authors (PP and MM) independently reviewed the titles and abstracts identified by the electronic searches according to the Criteria for considering studies for this review, classifying each record as 'definitely relevant', 'possibly relevant', or 'definitely not relevant'. Any disagreements were resolved through discussion. We retrieved the full‐text reports for records classified as 'definitely relevant' or 'possibly relevant', and two review authors independently assessed each of these as 'include' or 'unsure'. We contacted the study investigators for those reports classified as 'unsure' for further information to determine eligibility as required. Any disagreements were resolved through discussion. We reported studies excluded after full‐text review and the reasons for their exclusion in the Characteristics of excluded studies table. We classified as 'ongoing' any included studies that met the eligibility criteria but have not yet been completed or for which the study results were not available.
Data extraction and management
Two review authors independently extracted and recorded study methods, participant characteristics, and outcome data using forms developed by Cochrane Eyes and Vision. One review author entered data into Review Manager 5 (Review Manager 2014), and a second review author verified all values. Any discrepancies were resolved through discussion.
Assessment of risk of bias in included studies
Two review authors (PP and MM) independently assessed the included studies for risk of potential bias according to the guidelines in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We evaluated each study for potential bias based on the following criteria: sequence generation and allocation concealment (selection bias), masking of participants and study personnel (performance bias), masking of outcome assessors (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. We reported the judgement for each study for each criterion as 'low risk of bias', 'high risk of bias', or ‘unclear' (information is insufficient to assess risk of bias). Any discrepancies were resolved through discussion. We contacted the study investigators for clarification as required after reviewing the study report. When the study investigators did not respond within two weeks, we based our 'Risk of bias' assessment on the available information. One review author entered data into the Characteristics of included studies table, and a second review author verified the data entry.
Measures of treatment effect
For dichotomous outcomes, we calculated risk ratios (RR) with 95% confidence intervals (CIs). Dichotomous outcomes for this review included functional success and anatomic success. We also considered the proportion of participants that had an adverse event as a dichotomous outcome. For continuous outcomes, we considered the normality of distributions and calculated mean differences (MDs) with 95% CIs when the measurements were considered normally distributed. We calculated standardized mean differences (SMDs) when continuous outcomes were measured using different scales. Continuous outcomes for this review included ostium size.
Unit of analysis issues
The unit of analysis was the participant (one eye per person). If two eyes were included per participant and received the same treatment, when possible we considered the unit of analysis to be the participant by calculating average values, or selecting one eye for analysis, per the guidelines in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When both eyes of the same participant were included, and one eye was assigned to one treatment group and the other eye was assigned to the second treatment group (i.e. paired‐eye design), we referred to Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions for guidelines regarding considerations of correlation between the two eyes of one person (Higgins 2011).
Dealing with missing data
We contacted the study investigators for incomplete or unclear information regarding study details, outcome data, and standard deviations for means. When the investigators did not respond within two weeks, we used the available information as reported in the study. We did not impute any data.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by examining potential variations in participant characteristics, interventions compared (EN‐DCR and EX‐DCR), and design features. We used the I2 statistic (%) to determine the proportion of variation due to statistical heterogeneity, considering a value above 50% as indicative of substantial statistical heterogeneity. We also examined the probabilities from Chi2 tests that suggested heterogeneity and the degree of overlap in CIs of effect estimates from the included studies. We considered poor overlap as indicating the presence of heterogeneity.
Assessment of reporting biases
We assessed selective outcome reporting by comparing the outcomes reported versus the outcomes listed in the study protocols or design articles, when these were available. We planned to assess small study‐effects using funnel plots for each meta‐analysis that included 10 or more trials and to examine the funnel plots for asymmetry. An asymmetric funnel plot may imply possible selection or publication bias, poor reporting of small trials, true heterogeneity, or chance.
Data synthesis
We performed a meta‐analysis when studies were clinically and methodologically comparable. We combined the outcomes from included studies in meta‐analysis using a random‐effects model, unless fewer than three studies were included, in which case we used a fixed‐effect model. When we found substantial statistical heterogeneity (I2 greater than 50%) and the direction of treatment effects was inconsistent across studies, we did not combine results in a meta‐analysis but instead presented a narrative summary.
Subgroup analysis and investigation of heterogeneity
We had planned subgroup analyses by agent used (MMC and 5‐FU) and by primary DCR and reoperation after failure. However, studies in these individual groups were insufficient to pursue a meaningful subgroup analysis. We had not planned subgroup analyses based on type of approach for DCR, but we decided post hoc to conduct subgroup analysis by stratifying data according to the approach used to visualize the operative site, either via the internal approach (EN‐DCR) or the external approach (EX‐DCR).
Sensitivity analysis
We had planned to performed sensitivity analyses to determine the impact of excluding studies at high risk of bias for incomplete outcome data and selective outcome reporting, but did not do this because many of the included studies had unclear risk of bias. We had also planned to perform sensitivity analyses by excluding studies funded by industry and those that were unpublished at the time of this review, but did not do this because no studies with these characteristics were included in the review.
Summary of findings
We summarized the main findings (see Table 1 table), including the strengths and limitations of evidence for all outcomes assessed in this review. We provided a summary of the effectiveness of the interventions and a general interpretation of the evidence in the context of other evidence, and implications for practice and future research. We used a 'Summary of findings' table according to the methods described in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a; Schünemann 2011b). Two review authors independently graded the overall certainty of the evidence for each outcome using the GRADE classification (www.gradeworkinggroup.org).
Results
Description of studies
Results of the search
The electronic search yielded 764 records (Figure 1). After removal of duplicates, we screened the remaining 520 records and excluded a further 448 records based on title and abstract review. We obtained the full‐text reports of 72 records for further investigation. We included 31 reports from 31 studies (see Characteristics of included studies table) and excluded 36 reports after full‐text screening (see Characteristics of excluded studies). We identified five ongoing studies that potentially meet the inclusion criteria, which we will assess when data become available (see Characteristics of ongoing studies).
1.
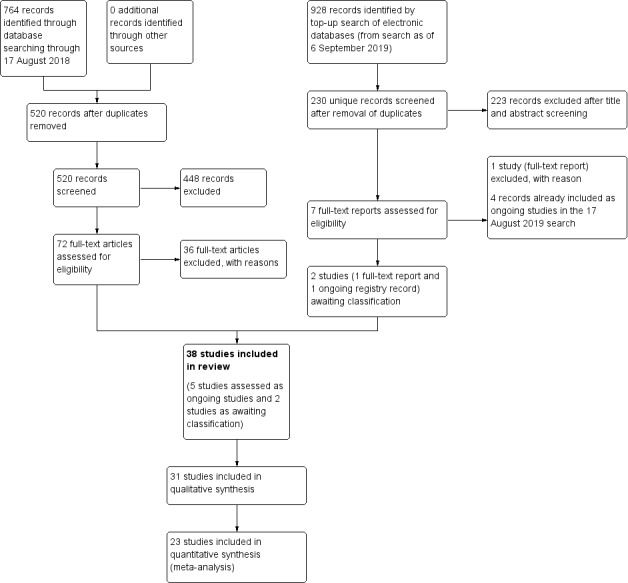
Study flow diagram.
In an additional top‐up search conducted on 6 September 2019 that yielded 928 records, we screened 230 titles and abstracts after removal of duplicates, of which 223 records were excluded. We excluded one report after full‐text review as well as four ongoing studies that were duplicates of ongoing studies identified in the 17 August 2019 search. We listed the remaining two records as studies awaiting classification.
Overall, we included 31 studies (31 reports), excluded 37 studies (37 reports), classified 5 studies (5 reports) as ongoing studies, and identified 2 records (1 full‐text and 1 ongoing study) from the top‐up search, which we assessed as awaiting classification (Figure 1).
Included studies
See Characteristics of included studies.
Types of studies
We included 31 studies in the systematic review (Ahmad 2002; Alañón 2006; Ari 2009; Bakri 2003; Cai 2003; Chavan 2018; Costa 2007; Dogan 2013a; Eshraghy 2012; Ghosh 2006; Gonzalvo 2000; Kao 1997; Kim 2002; Liao 2000; Mukhtar 2014; Ozkiris 2012; Park 2000; Penttilä 2011; Prasannaraj 2012; Qadir 2014; Qiu 2000; Ragab 2012; Roozitalab 2004; Shaikh 2015; Tirakunwichcha 2011; Wadhera 2013; Xie 2015; Yalaz 1999; Yan 2002; Yildirim 2007; You 2001). Most studies recruited participants in Asia: six in India, five in Turkey, five in China, two in Taiwan, two in South Korea, two in Iran, one in Thailand, one in Saudi Arabia, and one in Pakistan. Outside of Asia, two studies recruited participants in Spain, one in Finland, one in England, one in Egypt, and one in Brazil.
Of the 31 included studies, eight were not included in the meta‐analysis because they either did not report review specific primary or secondary outcome data or had a follow‐up duration of less than 6 months (Alañón 2006; Costa 2007; Qiu 2000; Shaikh 2015; Xie 2015; Yalaz 1999; Chavan 2018; Mukhtar 2014). We included 23 studies in the meta‐analyses of various outcomes (Ahmad 2002; Ari 2009; Bakri 2003; Cai 2003; Dogan 2013a; Eshraghy 2012; Ghosh 2006; Gonzalvo 2000; Kao 1997; Kim 2002; Liao 2000; Ozkiris 2012; Park 2000; Penttilä 2011; Prasannaraj 2012; Qadir 2014; Ragab 2012; Roozitalab 2004; Tirakunwichcha 2011; Wadhera 2013; Yan 2002; Yildirim 2007; You 2001).
The study with the earliest enrollment of participants from meta‐analysis began in 1994 (Kao 1997), and only two studies were published prior to 2000 (Kao 1997; Yalaz 1999). Study follow‐up time varied significantly, but all had at least 6 months of follow‐up, with the maximum follow‐up being 24 months (Dogan 2013a). None of the included studies declared any sources of funding or financial interests.
Type of participants
The 31 studies enrolled a total of 2299 participants (range from 15 to 200 participants per study). The youngest mean age was 30 years, in You 2001, and the oldest mean age was 70 years, in Penttilä 2011. Study participants were generally younger than expected in previous demographic studies of NLDO (Woog 2007). Among the 15 studies that reported information on gender (Ari 2009; Bakri 2003; Cai 2003; Eshraghy 2012; Gonzalvo 2000; Mukhtar 2014; Ozkiris 2012; Park 2000; Penttilä 2011; Qadir 2014; Roozitalab 2004; Shaikh 2015; Tirakunwichcha 2011; Wadhera 2013; You 2001), participants were predominantly female, except in three studies (Eshraghy 2012; Ozkiris 2012; Wadhera 2013). The diagnosis of NLDO varied among studies, with some studies including participants with primary acquired nasolacrimal duct obstruction and others those diagnosed with recurrent nasolacrimal duct obstruction. All studies excluded individuals with congenital NLDO.
Type of interventions
Of the 31 included studies, 11 compared EX‐DCR in combination with MMC to EX‐DCR alone (Ahmad 2002; Ari 2009; Ghosh 2006; Gonzalvo 2000; Kao 1997; Liao 2000; Mukhtar 2014; Qadir 2014; Roozitalab 2004; Shaikh 2015; Yildirim 2007). Ten studies compared treatment with EN‐DCR in combination with MMC to EN‐DCR alone (Chavan 2018; Kim 2002; Ozkiris 2012; Park 2000; Penttilä 2011; Prasannaraj 2012; Ragab 2012; Tirakunwichcha 2011; Wadhera 2013; Xie 2015). Five studies comparing treatment with DCR in combination with MMC, Cai 2003; Eshraghy 2012; Qiu 2000; Yan 2002, or 5‐FU, Costa 2007, did not specify what approach (EN‐DCR or EX‐DCR) was used. One study each compared treatment with EX‐DCR with different doses of MMC, You 2001, or treatment with EX‐DCR with different doses of MMC and 5‐FU, Yalaz 1999. The remaining studies compared endonasal and endocanalicular dacryocystorhinostomy with diode laser (TLA‐ELA DCR) in combination with MMC to TLA‐ELA DCR alone (Alañón 2006); or endonasal laser dacryocystorhinostomy (ELDCR) in combination with MMC to ELDCR alone (Bakri 2003); or endocanalicular dacryocystorhinostomy (ECL‐DCR) in combination with MMC to ECL‐DCR alone (Dogan 2013a).
Of the 23 studies included in the meta‐analyses, a subgroup of nine studies compared treatment with EN‐DCR in combination with MMC to EN‐DCR alone (Dogan 2013a; Kim 2002; Ozkiris 2012; Park 2000; Penttilä 2011; Prasannaraj 2012; Ragab 2012; Tirakunwichcha 2011; Wadhera 2013); one study utilized a laser in the EN‐DCR (Dogan 2013a). Another subgroup of 13 studies compared EX‐DCR in combination with MMC to EX‐DCR alone (Ahmad 2002; Ari 2009; Cai 2003; Eshraghy 2012; Ghosh 2006; Gonzalvo 2000; Kao 1997; Liao 2000; Qadir 2014; Roozitalab 2004; Yan 2002; Yildirim 2007; You 2001). One study compared endoscopic laser DCR with 5‐FU to endoscopic laser DCR alone (Bakri 2003).
Type of outcomes
Although 31 studies were included in the review, four studies did not provide analyzable outcomes data (Alañón 2006; Qiu 2000; Xie 2015; Yalaz 1999). A further four studies assessed outcomes at less than six months follow‐up (Chavan 2018; Costa 2007; Mukhtar 2014; Shaikh 2015). Twenty‐three of the 31 RCTs provided analyzable data on either primary or secondary outcomes, or both. At 6 months and beyond, 20 RCTs provided data on functional success of DCR, and 14 had data on anatomic success. Three studies reported on ostium size. Proportions of participants experiencing complications were variably reported among the included studies.
Excluded studies
Of the 81 full‐text articles assessed for eligibility, we excluded 39 with reasons: 20 were not RCTs; 14 did not evaluate the intervention of interest; four were duplicates; and one was conducted in a different patient population (see Characteristics of excluded studies). Four were duplicates of studies already identified in previous search and classified as ongoing studies (Figure 1).
Ongoing studies and studies awaiting classification
We identified five ongoing studies and two records from the top‐up search that we assessed as awaiting classification (see Characteristics of ongoing studies and Characteristics of studies awaiting classification).
Risk of bias in included studies
The risk of bias in the included trials is summarized in Figure 2 and Figure 3.
2.
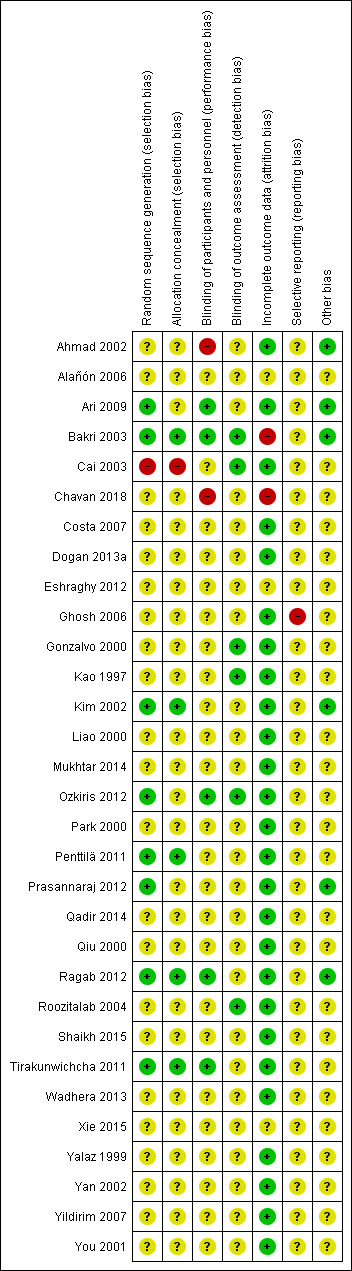
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
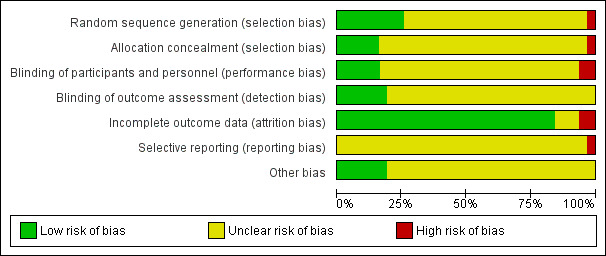
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Eight studies reported using a computer‐based random generator to generate the random allocation sequence, a method that we considered to be at low risk of bias (Ari 2009; Kim 2002; Mukhtar 2014; Ozkiris 2012; Penttilä 2011; Prasannaraj 2012; Ragab 2012; Tirakunwichcha 2011). We rated one study as having high risk of bias because the randomization was based on order of visitation (Cai 2003). The remaining 22 studies did not report the method of generating the allocation sequence and were assessed as at unclear risk of bias.
Allocation concealment
We assessed five studies that described the method used to conceal the treatment allocation sequence as at low risk of bias (Kim 2002; Penttilä 2011; Prasannaraj 2012; Ragab 2012; Tirakunwichcha 2011). One study used alternate allocation by order of visitation, therefore we determined that treatment allocation was not concealed de facto and assessed this study as at high risk of bias (Cai 2003). We assessed the remaining 25 studies as at unclear risk of bias.
Blinding
Five studies reported masking of participants (Ari 2009; Bakri 2003; Ozkiris 2012; Ragab 2012; Tirakunwichcha 2011), while five other studies reported masking of outcome assessors (Cai 2003; Gonzalvo 2000; Kao 1997; Ozkiris 2012; Roozitalab 2004); we assessed all of these studies as at low risk of bias. We assessed one study as at high risk of bias because participants and study personnel were not blinded (Ahmad 2002). We judged the remaining studies to be at unclear risk of bias due to lack of reporting of blinding of participants, study personnel, and outcome assessors.
Incomplete outcome data
We assessed 25 studies as at low risk of bias for incomplete outcome data because there were no missing data for the outcomes of our review (Ahmad 2002; Ari 2009; Cai 2003; Costa 2007; Dogan 2013a; Ghosh 2006; Gonzalvo 2000; Kao 1997; Kim 2002; Liao 2000; Mukhtar 2014; Ozkiris 2012; Park 2000; Penttilä 2011; Prasannaraj 2012; Qadir 2014; Qiu 2000; Ragab 2012; Roozitalab 2004; Shaikh 2015; Tirakunwichcha 2011; Wadhera 2013; Yalaz 1999; Yan 2002; Yildirim 2007; You 2001). We assessed two studies as at high risk of attrition bias because either they conducted analyses on as‐treated basis (Bakri 2003), or there were missing data that were not balanced across intervention arms, and reasons for missing data were not provided (Chavan 2018). We assessed the remaining three RCTs as at unclear risk of bias.
Selective reporting
We considered the risk of reporting bias as high in one study because syringing was performed, but there was no reporting of anatomic patency as a result (Ghosh 2006). The remaining studies had no study registration or published protocol available for comparison to ascertain selective outcome reporting and were therefore judged as at unclear risk of reporting bias.
Other potential sources of bias
We assessed six studies as free from other sources of bias (Ahmad 2002; Ari 2009; Bakri 2003; Kim 2002; Prasannaraj 2012; Ragab 2012). Information was insufficient to judge whether the remaining 25 studies were at low or high risk of other potential sources bias, therefore we assessed these studies as at unclear risk of bias.
Effects of interventions
See: Table 1
Functional success
Twenty studies reported data on functional success. Meta‐analysis of 7 studies (356 participants) suggests that antimetabolite had no evidence of benefit at 6 months (risk ratio (RR) 1.12, 95% confidence interval (CI) 0.98 to 1.29). There was moderate statistical heterogeneity (I2 = 44%) (Figure 4; Analysis 1.1). The certainty of the evidence was low, downgrading for risk of bias and imprecision. However, beyond six months, antimetabolite probably improves functional success as demonstrated in a meta‐analysis of 14 studies (909 participants) (RR 1.15, 95% CI 1.07 to 1.25). There was moderate statistical heterogeneity (I2 = 34%) (Figure 4; Analysis 1.1). Visual inspection of funnel plots for functional success outcomes at six months and beyond revealed no obvious funnel plot asymmetry (Figure 5). We assessed the certainty of the evidence as moderate, downgrading one level for risk of bias. The test for subgroup differences indicated no evidence of subgroup effect at six months (P = 0.72). However, the test for subgroup differences suggest evidence of a difference in subgroup effect (P=0.05) (Figure 6; Analysis 2.1, Figure 7; Analysis 2.2) suggesting that beyond six months, DCR approaches (EN‐DCR versus EX‐DCR) significantly modifies the effect of MMC DCR in comparison to DCR alone. The treatment effect beyond six months favors EX‐DCR over EN‐DCR.
4.
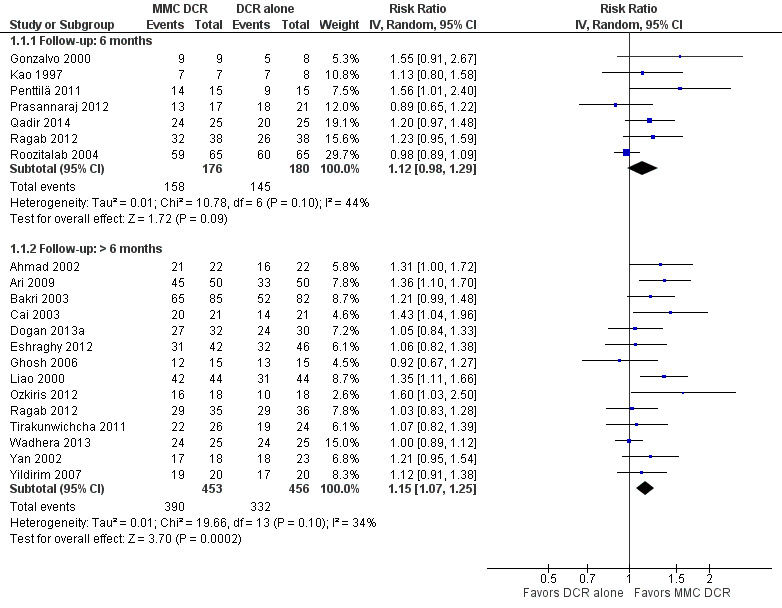
Forest plot of comparison: 1 Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, outcome: 1.1 Functional success, defined as the relief of epiphora.
1.1. Analysis.
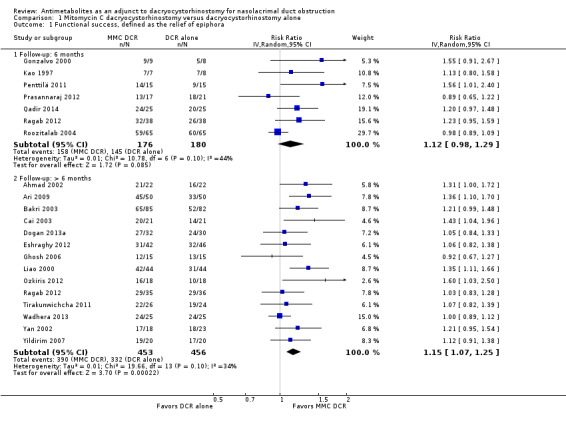
Comparison 1 Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, Outcome 1 Functional success, defined as the relief of epiphora.
5.
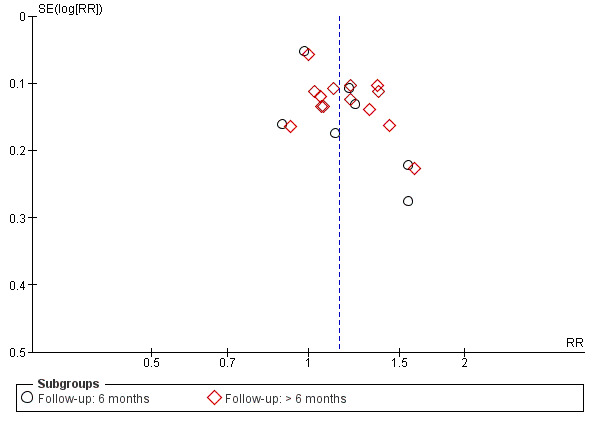
Funnel plot of comparison: 1 Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, outcome: 1.1 Functional success, defined as the relief of epiphora.
6.
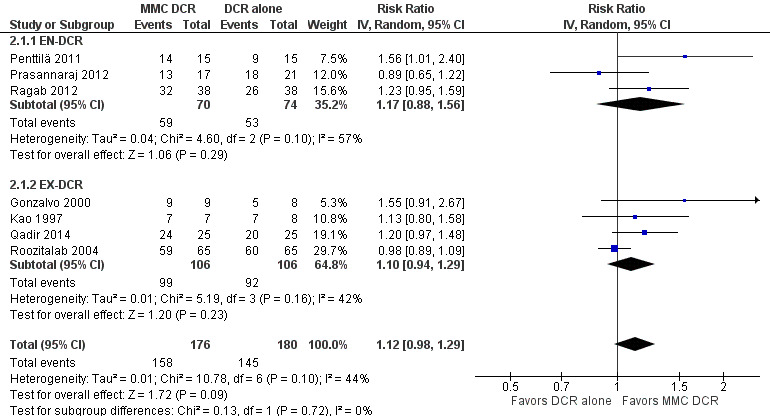
Forest plot of comparison: 2 Subgroup: Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, outcome: 2.1 Functional success, defined as the relief of epiphora at 6 months.
2.1. Analysis.
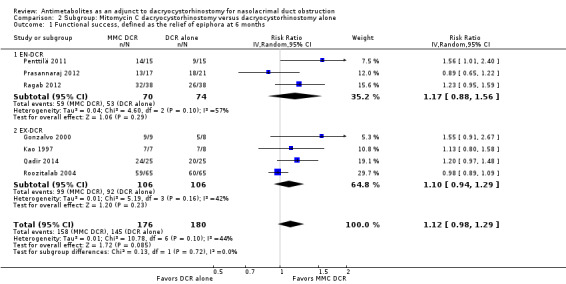
Comparison 2 Subgroup: Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, Outcome 1 Functional success, defined as the relief of epiphora at 6 months.
7.
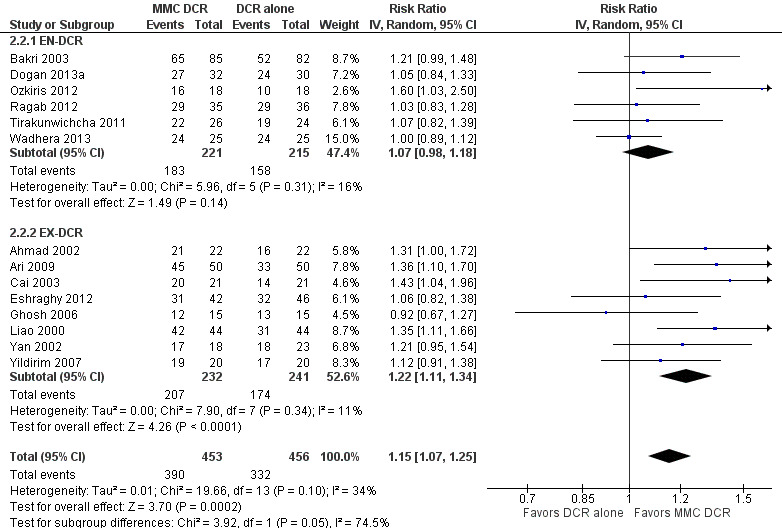
Forest plot of comparison: 2 Subgroup: Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, outcome: 2.2 Functional success, defined as the relief of epiphora at > 6 months.
2.2. Analysis.
Comparison 2 Subgroup: Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, Outcome 2 Functional success, defined as the relief of epiphora at > 6 months.
Anatomic success
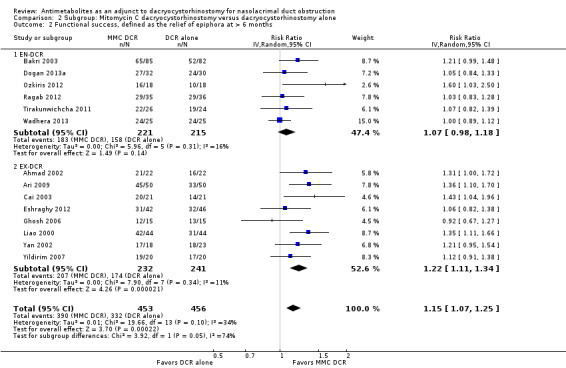
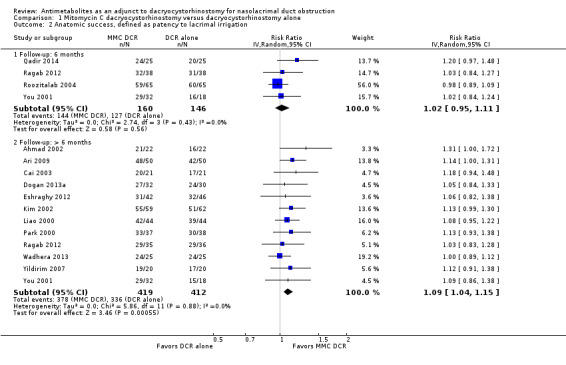
Fourteen studies reported data on anatomic success. Meta‐analysis of 4 RCTs (306 participants) indicated that antimetabolites had little or no effect on anatomic success at 6 months (RR 1.02, 95% CI 0.95 to 1.11) (Figure 8; Analysis 1.2). There were no concerns regarding statistical heterogeneity across the included studies (I2 = 0%). We assessed the certainty of the evidence as low, downgrading for risk of bias and imprecision. The beneficial effect was greater beyond 6 months of follow‐up, as observed in pooled analysis of 12 RCTs (831 participants) (RR 1.09, 95% CI 1.04 to 1.15), with low statistical heterogeneity (I2 = 0%). Visual inspection of funnel plots for anatomic success revealed no obvious funnel plot asymmetry, with the exception of anatomic success at six months, where a small study‐effect appeared to be present but was not serious enough to warrant a downgrade of the certainty of the evidence (Figure 9; Analysis 1.2). We rated the certainty of the evidence as moderate, downgrading one level for risk of bias. The test for subgroup differences indicated that there is no statistically significant subgroup effect at six months (P = 0.98) or beyond six months (P = 0.27) (Figure 10; Analysis 2.3, Figure 11; Analysis 2.4), suggesting that DCR approaches (EN‐DCR versus EX‐DCR) do not modify the effect of MMC DCR in comparison to DCR alone at both time points.
8.
Forest plot of comparison: 1 Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, outcome: 1.2 Anatomic success, defined as patency to lacrimal irrigation.
1.2. Analysis.
Comparison 1 Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, Outcome 2 Anatomic success, defined as patency to lacrimal irrigation.
9.
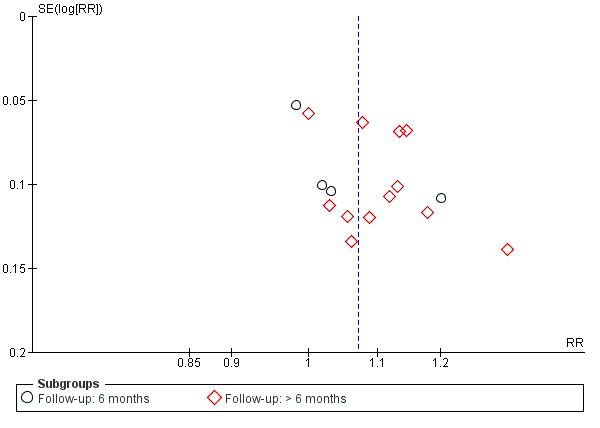
Funnel plot of comparison: 1 Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, outcome: 1.2 Anatomic success, defined as patency to lacrimal irrigation.
10.
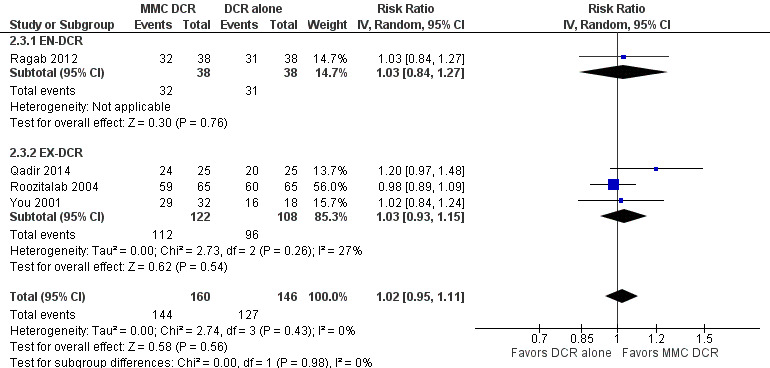
Forest plot of comparison: 2 Subgroup: Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, outcome: 2.3 Anatomic success, defined as patency to lacrimal irrigation at 6 months.
2.3. Analysis.
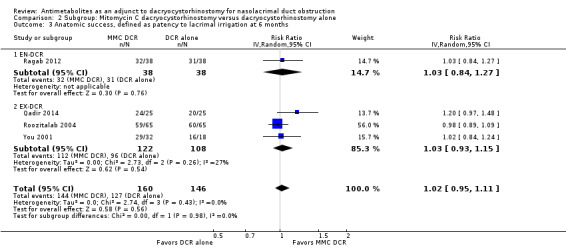
Comparison 2 Subgroup: Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, Outcome 3 Anatomic success, defined as patency to lacrimal irrigation at 6 months.
11.
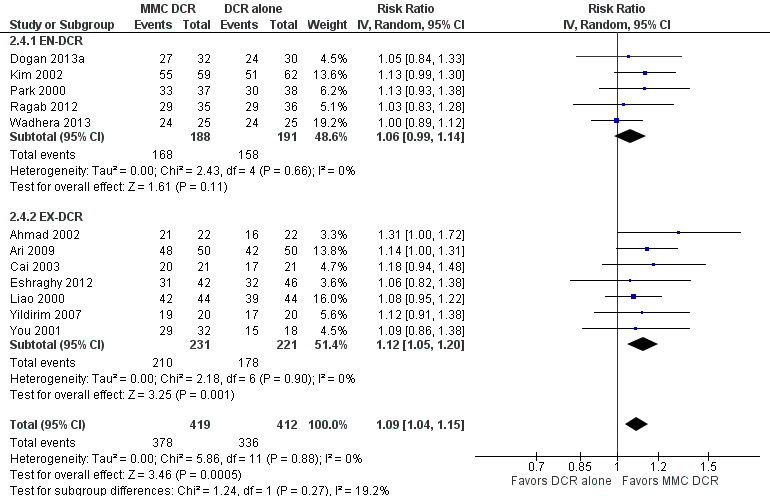
Forest plot of comparison: 2 Subgroup: Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, outcome: 2.4 Anatomic success, defined as patency to lacrimal irrigation at > 6 months.
2.4. Analysis.
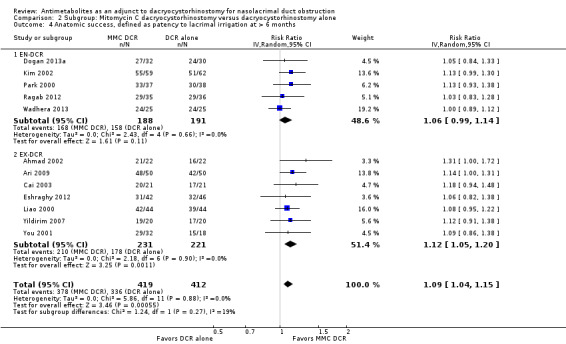
Comparison 2 Subgroup: Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, Outcome 4 Anatomic success, defined as patency to lacrimal irrigation at > 6 months.
Ostium size
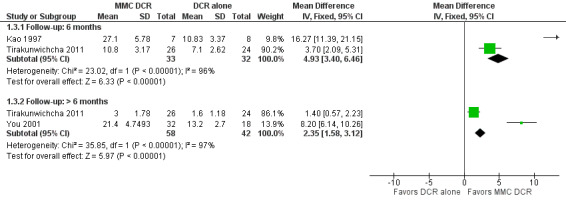
Two studies reported mean change in ostium size at six months follow‐up. At 6 months, Kao 1997 reported data on 15 participants which demonstrated significantly larger ostium size in participants treated with MMC (mean difference (MD) 16.27, 95% CI 11.39 to 21.15). The 50 participants in Tirakunwichcha 2011 similarly demonstrated significantly increased ostium size in participants treated with MMC (MD 3.70, 95% CI 2.09 to 5.31). However, we observed considerable heterogeneity (I2 = 96%) and therefore did not perform a meta‐analysis, but instead presented point estimates in a forest plot (Figure 12; Analysis 1.3). We graded the certainty of the evidence as low, downgrading one level each for risk of bias and inconsistency.
12.
Forest plot of comparison: 1 Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, outcome: 1.3 Ostium size on nasal endoscopy at 6 months postoperatively.
1.3. Analysis.
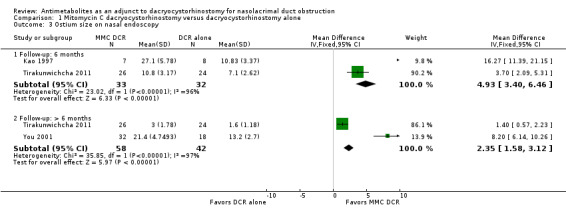
Comparison 1 Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone, Outcome 3 Ostium size on nasal endoscopy.
Beyond 6 months, two studies reported data on ostium size. Among the 50 participants in Tirakunwichcha 2011, those treated with MMC had no evidence of a difference in ostium size at follow up (MD 1.40, 95% CI 0.57 to 2.23). Among the 50 participants in You 2001, the ostium size of those treated with MMC was measured during the final follow up period between 23 and 42 months. In this study, the ostium size in participants who were treated with 0.2 mg/mL MMC (n = 16) vs. 0.5 mg/mL MMC (n =16) vs. external DCR alone (n = 18) were compared. The mean ostium size at the final follow‐up visit was 22.2 ± 5.0 mm2 in the group treated with 0.2 mg/mL MMC, 20.6 ± 5.0 mm2 in 0.5 mg/mL MMC group, and 13.2 ± 2.7 mm2 in the control group. Overall, investigators observed that those treated with MMC were likely to experience larger ostium size (MD 8.20, 95% CI 6.14 to 10.26) compared to those treated with DCR alone. Similarly, we did not conduct meta‐analysis for data reported beyond six months due to considerable heterogeneity (I2 = 97%), instead presenting point estimates in a forest plot (Figure 12; Analysis 1.3). We graded the certainty of the evidence as low, downgrading one level each for risk of bias and inconsistency.
Data were insufficient at both six months and beyond six months to perform subgroup analyses between EN‐DCR and EX‐DCR; if data by DCR approach become available in future updates of this review, we will include these subgroup analyses.
Adverse events
Adverse events were rare. One participant in the antimetabolites group experienced delayed wound healing due to what was thought to be wound disruption related to the accidental application of an MMC‐soaked sponge on the skin. The other studies reported no significant adverse events related to the application of antimetabolites.
Discussion
Summary of main results
We identified 31 studies that compared the adjuvant treatment of antimetabolites in the setting of DCR to DCR alone. After reviewing the available evidence we summarized our findings in Table 1 for the main comparison section. We evaluated 20 studies comparing treatment with antimetabolites in combination with DCR to DCR alone on functional success. Data from seven studies indicated that participants with NLDO randomized to antimetabolites showed no evidence of effect on functional success at six months post‐DCR. The certainty of the evidence was low, with moderate statistical heterogeneity. Fourteen studies assessed functional success beyond six months, results suggests that participants randomized to antimetabolites were 1.15 times more likely to experience improvement in functional success beyond six months post‐DCR. The certainty of the evidence was moderate with moderate statistical heterogeneity.
Fourteen included studies examined anatomic success. Data from four studies indicated that participants with NLDO randomized to antimetabolites showed no evidence of a difference in anatomic success at six months. The certainty of the evidence was low. Beyond six months, participants randomized to antimetabolites were likely to experience a small increase in anatomic success compared to the control group. The certainty of the evidence was moderate. However, the effect size was generally small, and as the majority of studies that contributed data to this outcome lacked trial registration, selective outcome reporting cannot be ruled out.
Additionally, in three studies examined ostium size a six months and beyond, point estimates consistently indicated that participants randomized to antimetabolites were more likely to experience improvement in mean ostium size six month post intervention. However, beyond six months, one study found no evidence of effect antimetabolites on ostium size and another observed a difference in favor of participants receiving antimetabolites. There was considerable statistical heterogeneity that rendered meta‐analysis inappropriate for both time points. The certainty of the evidence was low.
Overall completeness and applicability of evidence
We included only RCTs in this review. Our search strategy was comprehensive. We believe that we identified a high proportion if not all published studies on antimetabolite intervention in combination with DCR for the treatment of NLDO. Specific racial or ethnic groups may be underrepresented, since most randomized participants were from South and East Asia, so our conclusions may not translate to other populations. Treatment prior to DCR in the studies were varied, with participants undergoing a revision DCR in some cases. Additionally, the approach used for interventions was not the same (EN‐DCR versus EX‐DCR approach); however, we found no significant differences between the EN‐DCR and EX‐DCR subgroups on functional success at six months and anatomic success at both time points evaluated. Furthermore, none of the included studies reported any sources of funding or financial interests, and any undeclared financial interest or support from industry is likely to impact the level of certainty of the evidence (Guyatt 2011) .
Quality of the evidence
The certainty of the evidence was moderate for the functional and anatomic success outcomes of DCR participants who were followed beyond six months. We considered the certainty of the evidence for functional and anatomic success outcomes at six months and ostium size at six months and beyond as low. Most studies did not report how the random sequence was generated or the method of concealing treatment allocation. We assessed most trials as at unclear risk of detection bias because outcome assessors were not masked. None of the trials were registered or were CONSORT compliant. Most studies were at low risk of attrition bias. Additionally, considerable statistical heterogeneity among studies that examined ostium size precluded meta‐analysis.
Potential biases in the review process
We worked with an Information Specialist to conduct broad electronic searches of multiple databases including trial registries. Although visual inspection of funnel plots revealed no obvious funnel plot asymmetry, with the exception of anatomic success at six months (Figure 5; Figure 9), publication bias for studies that demonstrated an effect of antimetabolites could not be ruled out, as visual inspection of funnel plots alone may not be a reliable way to rule out publication bias (Terrin 2005). Two review authors independently completed all steps outlined in the methods section of this review in order to reduce bias during study selection, 'Risk of bias' assessment, and data extraction.
Agreements and disagreements with other studies or reviews
Our review is generally in agreement with Cheng 2013, the only other published review on this topic that we found, in which the authors observed that intraoperative combination of MMC and EN‐DCR is safe and could improve success rate after primary and revision EN‐DCR as well as reduce the closure rate of the ostium size after EN‐DCR (Cheng 2013). Cheng and colleagues reviewed 11 randomized and non‐randomized studies conducted mostly in Asia, which included 574 eyes and defined success as patency of the nasolacrimal canal and improvement of symptoms. They found higher success rates in favor of the MMC group compared with control group (RR 1.12, 95% CI 1.04 to 1.20; P = 0.004) (Cheng 2013). However, after excluding the two non‐randomized trials from their analysis, they observed little or no difference in success rates between the two groups (Cheng 2013). When analyzing a subgroup of primary and revision EN‐DCR, and EN‐DCR without silicone intubation, they observed higher success rates in favor of the MMC group compared with the control group, but no difference in the subgroup with silicone intubation (Cheng 2013). Similar to our review, the authors of Cheng 2013 also observed bigger ostium size at osteotomy site at 3 months (weighted mean difference (WMD) 7.65, 95% CI 0.33 to 14.98; P = 0.041) and 6 months (WMD 9.28, 95% CI 2.45 to 16.11; P = 0.008), but little or no difference at 12 months after surgery (WMD 11.63, 95% CI 21.04 to 24.29; P = 0.072) (Cheng 2013).
Authors' conclusions
Implications for practice.
We identified moderate‐ to low‐certainty evidence comparing treatment with antimetabolites in combination with dacryocystorhinostomy (DCR) to DCR alone in participants with nasolacrimal duct obstruction (NLDO). In the included studies, participants who received antimetabolites in addition to DCR experienced a small benefit from functional and anatomic success beyond six months post‐DCR intervention; however, the benefit at six months was questionable. The administration of antimetabolites to participants with NLDO undergoing DCR surgery seems to offer benefit in functional and anatomic success beyond six months. Given that only one included study assessed 5‐fluorouracil (5‐FU), and evidence of its beneficial effect as a stand‐alone treatment was not assessed, caution is advised in choosing it for use in NLDO patients. Additionally, the use of antimetabolite in combination with DCR for forms of NLDO other than primary acquired and recurrent NLDO, such as congenital NLDO, should be carefully considered since the current review did not cover this population. Furthermore, evidence was derived mainly from participants of Asian origin, rendering further the need for caution in the use of antimetabolites in other racial groups. Evidence from the five ongoing studies when completed may help clarify the value of antimetabolites in DCR. Use of the current evidence in clinical practice decisions should be based on provider judgement and patient preferences, taking the described limitations of the evidence into account.
Implications for research.
Given the large and increasing burden of NLDO and growing interest in minimally invasive lacrimal surgical procedures, future research should evaluate the effects of these interventions on outcomes that are meaningful both clinically and to patients and regulators. The effect of antimetabolites on health‐related quality of life and economic outcomes was not an objective of this review. Future reviews or updated reviews are expected to address these outcomes as well as outcomes that are important to patients, to better inform regulatory decision‐making, reimbursements, and other policy changes.
Acknowledgements
Cochrane Eyes and Vision (CEV) Information Specialists created and executed the electronic search strategies. The authors are grateful to the following peer reviewers for their time and comments: Dane Slentz (University of Michigan) and Bill Vaughan (National Committee to Preserve Social Security and Medicare), and also to the one peer reviewer who wishes to remain anonymous.
This review was managed by CEV@US and was signed off for publication by Tianjing Li and Richard Wormald.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Dacryocystorhinostomy] explode all trees #2 (dacryocystorhinostom* or dacryocystostom*) #3 DCR #4 ((probing or probe* or surg* or drain*) and (nasolacrimal or lacrimal or tear duct* or epiphor* or NLDO or NLO)) #5 MeSH descriptor: [Dacryocystitis] explode all trees and with qualifier(s): [Surgery ‐ SU] #6 MeSH descriptor: [Lacrimal Apparatus] explode all trees and with qualifier(s): [Surgery ‐ SU] #7 MeSH descriptor: [Lacrimal Duct Obstruction] explode all trees and with qualifier(s): [Surgery ‐ SU] #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Antimetabolites] this term only #10 MeSH descriptor: [Antimetabolites, Antineoplastic] this term only #11 MeSH descriptor: [Nucleic Acid Synthesis Inhibitors] this term only #12 (Antimetabolit* or anti‐metabolit*) #13 (Antifibrotic* or anti‐fibrotic*) #14 MeSH descriptor: [Fluorouracil] explode all trees #15 (5FU* or "5 FU" or Fluorouracil* or Fluoruracil* or "5 HU" or Adrucil or Carac or Efudix or "Fluoro Uracile" or "Fluoro Uracil" or Efudex or Fluoroplex or Flurodex or Fluracedyl or "Haemato fu" or Neofluor or Onkofluor or Ribofluor or "5 Fluorouracil" or "5 fluoro 2" or "4 pyrimidinedione" or accusite or "actino hermal" or effluderm or efurix or fivoflu or fluoroblastin or fluouracil or fluoxan or fluracil or fluracilium or fluril or "fluro uracil" or fluroblastin or ifacil or oncofu or uflahex or utoral or verrumal or "nsc 18913" or "nsc 19893" or nsc18913 or nsc19893 OR "ro 2 9757" or "ro2 9757" or "51‐21‐8") #16 MeSH descriptor: [Mitomycin] explode all trees #17 (Mitomycin* or "NSC 26980" or NSC26980 or Mutamycin or Ametycine or "Mitocin C" or MitocinC or mytomycin* or mitomicin* or mytomicin* or MMC or ameticine or ametycin or datisan or metomit or "mitocyn c" or mitocyna or "mitomicina c" or mitomycine or mitosol or mitozytrex or mixandex or mytocine or mytozytrex or vetio or "1404‐00‐8" or "50‐07‐7" or "74349‐48‐7") #18 MeSH descriptor: [Mitomycins] explode all trees #19 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 #20 #8 and #19
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp dacryocystorhinostomy/ 13. (dacryocystorhinostom* or dacryocystostom*).tw. 14. DCR.tw. 15. ((probing or probe* or surg* or drain*) and (nasolacrimal or lacrimal or tear duct* or epiphor* or NLDO or NLO)).tw. 16. exp Dacryocystitis/su 17. exp Lacrimal Apparatus/su 18. exp Lacrimal Duct Obstruction/su 19. or/12‐18 20. antimetabolites/ 21. Antimetabolites, Antineoplastic/ 22. Nucleic Acid Synthesis Inhibitors/ 23. (Antimetabolit* or anti‐metabolit*).tw. 24. (Antifibrotic* or anti‐fibrotic*).tw. 25. exp Fluorouracil/ 26. (5FU* or "5 FU" or Fluorouracil* or Fluoruracil* or "5 HU" or Adrucil or Carac or Efudix or "Fluoro Uracile" or "Fluoro Uracil" or Efudex or Fluoroplex or Flurodex or Fluracedyl or "Haemato fu" or Neofluor or Onkofluor or Ribofluor or "5 Fluorouracil" or "5 fluoro 2" or "4 pyrimidinedione" or accusite or "actino hermal" or effluderm or efurix or fivoflu or fluoroblastin or fluouracil or fluoxan or fluracil or fluracilium or fluril or "fluro uracil" or fluroblastin or ifacil or oncofu or uflahex or utoral or verrumal or "nsc 18913" or "nsc 19893" or nsc18913 or nsc19893 or "ro 2 9757" or "ro2 9757" or "51‐21‐8").tw. 27. "51‐21‐8".rn. 28. exp Mitomycin/ 29. (Mitomycin* or "NSC 26980" or NSC26980 or Mutamycin or Ametycine or "Mitocin C" or MitocinC or mytomycin* or mitomicin* or mytomicin* or MMC or ameticine or ametycin or datisan or metomit or "mitocyn c" or mitocyna or "mitomicina c" or mitomycine or mitosol or mitozytrex or mixandex or mytocine or mytozytrex or vetio or "1404‐00‐8" or "50‐07‐7" or "74349‐48‐7").tw. 30. ("1404‐00‐8" or "50‐07‐7" or "74349‐48‐7").rn. 31. exp Mitomycins/ 32. or/20‐31 33. 19 and 32 34. 11 and 33
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'dacryocystorhinostomy'/exp #34 dacryocystorhinostom*:ab,ti OR dacryocystostom*:ab,ti #35 dcr:ab,ti #36 probing:ab,ti OR probe*:ab,ti OR surg*:ab,ti OR drain*:ab,ti AND (nasolacrimal:ab,ti OR lacrimal:ab,ti OR 'tear duct*':ab,ti OR epiphor*:ab,ti OR nldo:ab,ti OR nlo:ab,ti) #37 'dacryocystitis'/exp/dm_su #38 #33 OR #34 OR #35 OR #36 OR #37 #39 'antimetabolite'/de #40 'antineoplastic antimetabolite'/de #41 'nucleic acid synthesis inhibitor'/de #42 antimetabolit*:tn,ab,ti OR (anti NEXT/1 metabolit*):tn,ab,ti #43 antifibrotic*:tn,ab,ti OR (anti NEXT/1 fibrotic*):tn,ab,ti #44 'fluorouracil'/exp #45 5fu*:tn,ab,ti OR '5 fu':tn,ab,ti OR fluorouracil*:tn,ab,ti OR fluoruracil*:tn,ab,ti OR '5 hu':tn,ab,ti OR adrucil:tn,ab,ti OR carac:tn,ab,ti OR efudix:tn,ab,ti OR 'fluoro uracile':tn,ab,ti OR 'fluoro uracil':tn,ab,ti OR efudex:tn,ab,ti OR fluoroplex:tn,ab,ti OR flurodex:tn,ab,ti OR fluracedyl:tn,ab,ti OR 'haemato fu':tn,ab,ti OR neofluor:tn,ab,ti OR onkofluor:tn,ab,ti OR ribofluor:tn,ab,ti OR '5 fluorouracil':tn,ab,ti OR '5 fluoro 2':tn,ab,ti OR '4 pyrimidinedione':tn,ab,ti OR accusite:tn,ab,ti OR 'actino hermal':tn,ab,ti OR effluderm:tn,ab,ti OR efurix:tn,ab,ti OR fivoflu:tn,ab,ti OR fluoroblastin:tn,ab,ti OR fluouracil:tn,ab,ti OR fluoxan:tn,ab,ti OR fluracil:tn,ab,ti OR fluracilium:tn,ab,ti OR fluril:tn,ab,ti OR 'fluro uracil':tn,ab,ti OR fluroblastin:tn,ab,ti OR ifacil:tn,ab,ti OR oncofu:tn,ab,ti OR uflahex:tn,ab,ti OR utoral:tn,ab,ti OR verrumal:tn,ab,ti OR 'nsc 18913':tn,ab,ti OR 'nsc 19893':tn,ab,ti OR nsc18913:tn,ab,ti OR nsc19893:tn,ab,ti OR 'ro 2 9757':tn,ab,ti OR 'ro2 9757':tn,ab,ti OR '51‐21‐8':tn,ab,ti #46 '51‐21‐8':rn #47 'mitomycin'/exp #48 mitomycin*:tn,ab,ti OR 'nsc 26980':tn,ab,ti OR nsc26980:tn,ab,ti OR mutamycin:tn,ab,ti OR ametycine:tn,ab,ti OR 'mitocin c':tn,ab,ti OR mitocinc:tn,ab,ti OR mytomycin*:tn,ab,ti OR mitomicin*:tn,ab,ti OR mytomicin*:tn,ab,ti OR mmc:tn,ab,ti OR ameticine:tn,ab,ti OR ametycin:tn,ab,ti OR datisan:tn,ab,ti OR metomit:tn,ab,ti OR 'mitocyn c':tn,ab,ti OR mitocyna:tn,ab,ti OR 'mitomicina c':tn,ab,ti OR mitomycine:tn,ab,ti OR mitosol:tn,ab,ti OR mitozytrex:tn,ab,ti OR mixandex:tn,ab,ti OR mytocine:tn,ab,ti OR mytozytrex:tn,ab,ti OR vetio:tn,ab,ti OR '1404‐00‐8':tn,ab,ti OR '50‐07‐7':tn,ab,ti OR '74349‐48‐7':tn,ab,ti #49 '1404‐00‐8':rn OR '50‐07‐7':rn OR '74349‐48‐7':rn #50 'mitomycin derivative'/exp #51 #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 #52 #38 AND #51 #53 #32 AND #52
Appendix 4. PubMed search strategy
1. (randomized controlled trial[pt] OR controlled clinical trial[pt] OR (randomised[tiab] OR randomized[tiab]) OR placebo[tiab] OR "drug therapy"[Subheading] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms]) 2. (dacryocystorhinostom*[tw] OR dacryocystostom*[tw] OR DCR[tw]) NOT Medline[sb] 3. ((probing[tw] OR probe*[tw] OR surg*[tw] OR drain*[tw]) AND (nasolacrimal[tw] OR lacrimal[tw] OR tear duct*[tw] OR epiphor*[tw] OR NLDO[tw] OR NLO[tw])) NOT Medline[sb] 4. #2 OR #3 5. (Antimetabolit*[tw] OR anti‐metabolit*[tw]) NOT Medline[sb] 6. (Antifibrotic*[tw] OR anti‐fibrotic*[tw]) NOT Medline[sb] 7. (5fu*[tw] OR '5 fu'[tw] OR fluorouracil*[tw] OR fluoruracil*[tw] OR '5 hu'[tw] OR adrucil[tw] OR carac[tw] OR efudix[tw] OR 'fluoro uracile'[tw] OR 'fluoro uracil'[tw] OR efudex[tw] OR fluoroplex[tw] OR flurodex[tw] OR fluracedyl[tw] OR 'haemato fu'[tw] OR neofluor[tw] OR onkofluor[tw] OR ribofluor[tw] OR '5 fluorouracil'[tw] OR '5 fluoro 2'[tw] OR '4 pyrimidinedione'[tw] OR accusite[tw] OR 'actino hermal'[tw] OR effluderm[tw] OR efurix[tw] OR fivoflu[tw] OR fluoroblastin[tw] OR fluouracil[tw] OR fluoxan[tw] OR fluracil[tw] OR fluracilium[tw] OR fluril[tw] OR 'fluro uracil'[tw] OR fluroblastin[tw] OR ifacil[tw] OR oncofu[tw] OR uflahex[tw] OR utoral[tw] OR verrumal[tw] OR "nsc 18913"[tw] OR "nsc 19893"[tw] OR nsc18913[tw] OR nsc19893[tw] OR "ro 2 9757"[tw] OR "ro2 9757"[tw] OR "51‐21‐8"[tw]) NOT Medline[sb] 8. (mitomycin*[tw] OR 'nsc 26980'[tw] OR nsc26980[tw] OR mutamycin[tw] OR ametycine[tw] OR 'mitocin c'[tw] OR mitocinc[tw] OR mytomycin*[tw] OR mitomicin*[tw] OR mytomicin*[tw] OR mmc[tw] OR ameticine[tw] OR ametycin[tw] OR datisan[tw] OR metomit[tw] OR 'mitocyn c'[tw] OR mitocyna[tw] OR 'mitomicina c'[tw] OR mitomycine[tw] OR mitosol[tw] OR mitozytrex[tw] OR mixandex[tw] OR mytocine[tw] OR mytozytrex[tw] OR vetio[tw] OR '1404‐00‐8'[tw] OR '50‐07‐7'[tw] OR '74349‐48‐7'[tw]) NOT Medline[sb] 9. #5 OR #6 OR #7 OR #8 10. #4 AND #9 11. #1 AND #10
Appendix 5. LILACS search strategy
(Dacryocystorhinostom$ OR Dacriocistorrinostom$ OR Dacriocistorinostom$ OR Dacryocystostom$ OR DCR OR MH:E04.540.255$ OR MH:E04.579.255$) AND (MH:D03.383.742.698.875.404$ OR Fluorouracil$ OR 5FU OR "5 FU" OR "5‐FU" OR Fluoruracil$ OR "5 HU" OR "5‐HU" OR Adrucil OR Carac OR Efudix OR "Fluoro Uracile" "Fluoro‐Uracile" OR "Fluoro Uracil" OR "Fluoro‐Uracil" OR Efudex OR Fluoroplex OR Flurodex OR Fluracedyl OR "Haemato fu" OR "Haemato‐fu" OR Neofluor OR Onkofluor OR Ribofluor OR "5 Fluorouracil" OR "5‐Fluorouracil" OR "5 fluoro 2" OR "4 pyrimidinedione" OR accusite OR "actino hermal" OR effluderm OR efurix OR fivoflu OR fluoroblastin OR fluouracil OR fluoxan OR fluracil OR fluracilium OR fluril OR "fluro uracil" OR fluroblastin OR ifacil OR oncofu OR uflahex OR utoral OR verrumal OR "nsc 18913" OR "nsc 19893" OR nsc18913 OR nsc19893 OR "ro 2 9757" OR "ro2 9757" OR "51‐21‐8" OR MH:D27.505.519.186 OR MH:D27.888.569.042 OR MH:D27.505.519.186.144$ OR MH:D27.505.954.248.144$ OR MH:D27.888.569.042.030$ OR MH:D27.505.519.389.675$ OR Antimetabolit$ OR anti‐metabolit$ OR Antifibrotic$ OR anti‐fibrotic$ OR MH:D02.806.400.249.350$ OR MH:D03.383.097.500.350$ OR MH:D03.438.473.412.249.350$ OR Mitomycin$ OR "NSC‐26980" OR "NSC 26980" OR NSC26980 OR Mutamycin OR Ametycine OR "Mitocin C" OR "Mitocin‐C" OR MitocinC OR mytomycin$ OR mitomicin$ OR mytomicin$ OR MMC OR ameticine OR ametycin OR datisan OR metomit OR "mitocyn c" OR "mitocyn‐c" OR mitocyna OR "mitomicina c" OR "mitomicina‐c" OR mitomycine OR mitosol OR mitozytrex OR mixandex OR mytocine OR mytozytrex OR vetio OR "1404‐00‐8" OR "50‐07‐7" OR "74349‐48‐7")
Appendix 6. ClinicalTrials.gov search strategy
dacryocystorhinostomy
Appendix 7. ICTRP search strategy
dacryocystorhinostomy
Data and analyses
Comparison 1. Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional success, defined as the relief of epiphora | 20 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Follow‐up: 6 months | 7 | 356 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.98, 1.29] |
| 1.2 Follow‐up: > 6 months | 14 | 909 | Risk Ratio (IV, Random, 95% CI) | 1.15 [1.07, 1.25] |
| 2 Anatomic success, defined as patency to lacrimal irrigation | 14 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Follow‐up: 6 months | 4 | 306 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.95, 1.11] |
| 2.2 Follow‐up: > 6 months | 12 | 831 | Risk Ratio (IV, Random, 95% CI) | 1.09 [1.04, 1.15] |
| 3 Ostium size on nasal endoscopy | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Follow‐up: 6 months | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 4.93 [3.40, 6.46] |
| 3.2 Follow‐up: > 6 months | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 2.35 [1.58, 3.12] |
Comparison 2. Subgroup: Mitomycin C dacryocystorhinostomy versus dacryocystorhinostomy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional success, defined as the relief of epiphora at 6 months | 7 | 356 | Risk Ratio (IV, Random, 95% CI) | 1.12 [0.98, 1.29] |
| 1.1 EN‐DCR | 3 | 144 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.88, 1.56] |
| 1.2 EX‐DCR | 4 | 212 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.94, 1.29] |
| 2 Functional success, defined as the relief of epiphora at > 6 months | 14 | 909 | Risk Ratio (IV, Random, 95% CI) | 1.15 [1.07, 1.25] |
| 2.1 EN‐DCR | 6 | 436 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.98, 1.18] |
| 2.2 EX‐DCR | 8 | 473 | Risk Ratio (IV, Random, 95% CI) | 1.22 [1.11, 1.34] |
| 3 Anatomic success, defined as patency to lacrimal irrigation at 6 months | 4 | 306 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.95, 1.11] |
| 3.1 EN‐DCR | 1 | 76 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.84, 1.27] |
| 3.2 EX‐DCR | 3 | 230 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.93, 1.15] |
| 4 Anatomic success, defined as patency to lacrimal irrigation at > 6 months | 12 | 831 | Risk Ratio (IV, Random, 95% CI) | 1.09 [1.04, 1.15] |
| 4.1 EN‐DCR | 5 | 379 | Risk Ratio (IV, Random, 95% CI) | 1.06 [0.99, 1.14] |
| 4.2 EX‐DCR | 7 | 452 | Risk Ratio (IV, Random, 95% CI) | 1.12 [1.05, 1.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmad 2002.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 44 total, 22 per group Number analyzed: 44 total, 22 per group Number of arms: 2 Enrollment start year: 1999 Length of follow‐up: more than 9 months Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: India Age (mean (SD)): 45.4 (NR) in the MMC group; 44.9 (NR) in the EX‐DCR alone group Females (n (%)): not reported Inclusion criteria: diagnosis of primary acquired nasolacrimal duct obstruction Exclusion criteria: not reported Study group differences: not reported |
|
| Interventions |
Intervention: EX‐DCR with application of 0.2 mg/mL MMC Comparison intervention: EX‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: fibrous tissue growth, scarring or granulation tissue formation, delayed wound healing |
|
| Identification |
Author name: Sheikh Sajjad Ahmad Institution: SKIMS Medical College Email: not reported |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Treatment allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although it seems participants were masked to treatment, the operating doctor knew the treatment group (using an applicator versus not using an applicator). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors state that: "All the examinations were done by the same physician with double blind control", but it is unclear whether this means outcome assessors were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition not reported, but participants were analyzed in the group to which they had been randomized. |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered, and no protocol available for comparison to ascertain selective outcome reporting. |
| Other bias | Low risk | Study appears to be free of other sources of bias. |
Alañón 2006.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 200 total, 150 in the MMC group, 50 in the endonasal and endocanalicular DCR by diode laser (TLA‐ELA DCR) group Number of arms: 2 Enrollment start year: 2002 Length of follow‐up: 6 months Sample size calculations: not reported Losses to follow‐up: not reported |
|
| Participants |
Country: Spain Age (mean (SD)): 59.51 (NR) in the TLA‐ELA DCR alone group, 62.33 (NR) in the MMC group Females (n (%)): 162 (88.5%) in total Inclusion criteria: not reported Exclusion criteria: not reported Study group differences: no statistically significant differences in age, sex, laterality, or follow‐up between groups |
|
| Interventions |
Intervention: TLA‐ELA DCR with application of 0.4 mg/mL MMC Comparison intervention: TLA‐ELA DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: excessive scarring of the nasal mucosa in the form of scabs, granulomas and synechia |
|
| Identification |
Author name: Miguel Ángel Alañón Fernández Institution: Instituto Internacional de Vías Nasolagrimales Email: miguelaaf@msn.com |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | How random sequence was generated is not described. |
| Allocation concealment (selection bias) | Unclear risk | No details are provided regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants or study personnel is not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors is not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data were not reported, unclear whether participants were analyzed in the group to which they were randomized. |
| Selective reporting (reporting bias) | Unclear risk | No mention of functional or anatomic success, and no prior registered trial to be used as comparison. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Ari 2009.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 100 total, 50 per group Number of arms: 2 Enrollment start year: 2005 Length of follow‐up: 1 year Sample size calculations: not reported Losses to follow‐up: not reported |
|
| Participants |
Country: Turkey Age (mean (SD)): 47.0 (7.6) in the MMC group; 46.6 (8.8) in the EX‐DCR alone group Females (n (%)): 53 (53) overall, 27 (54) in the MMC group, 26 (52) in the EX‐DCR alone group Inclusion criteria: diagnosis of primary acquired nasolacrimal duct obstruction Exclusion criteria: aged < 18 or > 70 years, previous nasolacrimal duct surgery, morphologic or functional palpebral disorders, or secondary causes of nasolacrimal duct obstruction Study group differences: no statistically significant between‐group differences |
|
| Interventions |
Intervention: application of 1 mL of 0.2 mg/mL mitomycin C during EX‐DCR surgery Comparison intervention: standard EX‐DCR surgery |
|
| Outcomes |
Measured outcomes:
Adverse events: none |
|
| Identification |
Author name: Seyhmus Ari Institution: Diyarbabr Devlet Hastanesi Email: sari@dicle.edu |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized to treatment using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | How treatment allocation was concealed is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patients and the researchers were masked to the treatment", but no mention of masking of surgeon. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "There was no contact between the surgeon and the researchers evaluating the study outcomes. The patients and the researchers were masked to the treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition not reported, but participants were analyzed in the group to which they had been randomized. |
| Selective reporting (reporting bias) | Unclear risk | Trial was not registered, and no protocol available for comparison to ascertain selective outcome reporting. |
| Other bias | Low risk | Study appears to be free of other sources of bias. |
Bakri 2003.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 201 total eyes, 103 in the fluorouracil group, 98 in the endonasal laser dacryocystorhinostomy (ELDCR) with isotonic saline group Number of arms: 2 Enrollment start year: not reported Length of follow‐up: 12 months or later Sample size calculations: not reported Losses to follow‐up: not reported |
|
| Participants |
Country: England Age (mean (SD)): 66 (NR) total Females (n (%)): 50 (63) in the ELDCR group, 51 (67) in the ELDCR with isotonic saline group Inclusion criteria: evidence of primary acquired nasolacrimal duct obstruction, symptoms severe enough to require surgery Exclusion criteria: not reported Study group differences: not reported |
|
| Interventions |
Intervention: ELDCR surgery with application of 0.5 mg/mL FU Comparison intervention: ELDCR surgery with application of isotonic sodium chloride solution |
|
| Outcomes |
Measured outcomes:
Adverse events: none |
|
| Identification |
Author name: Karim Bakri Institution: University Hospital Email: nick.jones@nottingham.ac.uk |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors do not specify how participants were randomized, only that "randomization was performed in the pharmacy department". |
| Allocation concealment (selection bias) | Low risk | "randomization was performed in the pharmacy department." "Surgeons and patients remained masked to the choice of treatment until the study and follow‐up had been completed", hence we determined that treatment allocation was concealed de facto. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Surgeons and patients remained masked to the choice of treatment until the study and follow‐up had been completed" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Article provides insufficient information regarding time points. Follow‐up time was 12 months or longer, but that could have been anywhere between 12 and 60 months (for the control) or 12 and 48 months (for the intervention). As‐treated analysis was conducted: "This figure may partly reflect a subgroup of patients who did not attend because their symptoms had been relieved. However... these patients were excluded from the statistical analysis". |
| Selective reporting (reporting bias) | Unclear risk | Study mentions a protocol, but none accessible for comparison with published study. The conclusions state that: "The study sought to determine whether the application of topical fluorouracil reduced scar formation and improved patency rates"; only patency rates (via epiphora) and postoperative levels of fluorouracil were reported in the results. |
| Other bias | Low risk | Study appears to be free of other sources of bias. |
Cai 2003.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 42 total, 21 per group Number of arms: 2 Enrollment start year: 2000 Length of follow‐up: 10 months Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: China Age (mean (SD, range)): not reported Females (n (%)): 32 (76) total, 18 (82) in the MMC group, 14 (70) in the DCR alone group Inclusion criteria: diagnosis of primary chronic dacryocystitis Exclusion criteria: not reported |
|
| Interventions |
Intervention: DCR with application of 0.2 mg/mL MMC Comparison intervention: DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: none |
|
| Identification |
Author name: S Cai Institution: Department of Ophthalmology, Kunshan No. 1 People's Hospital Email: not reported |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized by sequence, just by order of visitation. |
| Allocation concealment (selection bias) | High risk | Random sequence generation was by order of visitation, therefore treatment allocation was not concealed de facto. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants or study personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors mentioned that "all measurements were taken by the same physician in double‐blinded controlled fashion". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data reported. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Chavan 2018.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 150 total, 50 per group Number of arms: 3 Enrollment start year: 2014 Length of follow‐up: 150 days Sample size calculations: not reported Losses to follow‐up: 20 in Group 1, 5 in Group 2, 12 in Group 3 |
|
| Participants |
Country: India Age (mean (SD)): not reported Females (n (%)): 98 (65.3) total Inclusion criteria: aged 6 to 70 years, acquired nasolacrimal duct obstruction with or without mucopurulent discharge, delayed regurgitation with or without mucopurulent discharge from the opposite punctum on sac syringing examination Exclusion criteria: other causes of epiphora (e.g. eyelid malposition, entropion), sac syringing examination confirming common canalicular block, revision endonasal DCR, secondary nasolacrimal duct block due to nasolacrimal duct trauma or total maxillectomy Study group differences: not reported |
|
| Interventions |
Intervention 1: Group 1: endonasal DCR with MMC application at the stoma site Intervention 2: Group 2: endonasal DCR with silicon tubing to keep the stoma site patent for a period of 6 weeks Comparison intervention: conventional endonasal DCR leaving the wide neo‐ostium unchanged |
|
| Outcomes |
Measured outcomes:
Adverse events: immediate postoperative orbital emphysema, synechiae formation, granulation formation in a stoma site |
|
| Identification |
Author name: Shrinivas‐Shripatrao Chavan Institution: Grant Medical College And Sir Jj Group Of Hospitals Email: shrinivasc77@hotmail.com |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method was not reported. Although the authors state that "patients were randomly divided into three groups of 50 patients each based on a colored chit allocation", there is no indication of how participants were assigned colored chits. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment mechanism is unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study personnel were not masked to the intervention since they had to perform different surgical techniques. Unclear whether participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were masked to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data were not balanced across intervention arms. Reasons for missing data are not explained. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Costa 2007.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 50 in total, 13 in Group SS (saline solution), 17 in Group 5‐FU1 (5‐fluorouracil), 9 in Group 5‐FU3, 11 in Group C Number of arms: 4 Enrollment start year: not reported Length of follow‐up: 60 days Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: Brazil Age (mean (SD)): not reported Females (n (%)): not reported Inclusion criteria: patients with dacryocystitis Exclusion criteria: patients with nasal affections such as septal deviation, turbinate hypertrophy, nasal fractures, and other lacrimal system problems Study group differences: not reported |
|
| Interventions |
Intervention 1: Group 5‐FU1: DCR and a 0.50 mL injection of 5‐FU (1 mL of 5‐FU (250 mg/10 mL) added to 4 mL of 0.9% saline solution) during surgery Intervention 2: Group 5‐FU3: DCR and three 4 mL injections of 5‐FU (1 during surgery, 1 on the third postoperative day, and 1 on the fifth postoperative day) with the same concentration as that of Group 5‐FU1, for a total dose of 15 mg Intervention 3: Group SS: DCR and an injection of saline solution (4 mL of 0.9% saline) during the surgery, and 0.5 mL of the saline solution injected into the nasal mucosa at the end of the surgery Comparison intervention: Group C: DCR only |
|
| Outcomes |
Measured outcomes:
Adverse events: total ostium occlusion by the healing tissue, persistent epiphora |
|
| Identification |
Author name: Marilisa Nano Costa Institution: State University of Campinas Email: m.nano@uol.com.br |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not specified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment mechanism was not specified. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants was not specified. Study personnel would not have been blinded due to the differences in the surgical interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data; all participants who had been randomized were analyzed. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Dogan 2013a.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 80 in total, 30 in Group 1, 27 in Group 2, 23 in Group 3 Number of arms: 3 Enrollment start year: 2009 Length of follow‐up: 24 months Sample size calculations: not reported Losses to follow‐up: 3 in Group 1, 2 in Group 2, 3 in Group 3 |
|
| Participants |
Country: Turkey Age (mean (SD)): 62 (NR) in total, 63.4 (NR) in Group 1, 60.7 (NR) in Group 2, 61.8 (NR) in Group 3 Females (n (%)): 67 (83.7) in total Inclusion criteria: diagnosis of nasolacrimal canal obstruction Exclusion criteria: nasal pathologies (e.g. polyp, carcinoma, or advanced septal deviation), no previous lacrimal surgery, no history of naso‐orbital trauma Study group differences: not reported |
|
| Interventions |
Intervention 1: Group 1: endocanalicular DCR (ECL‐DCR) with 0.4 mg/mL MMC application during surgery and silicone intubation Intervention 2: Group 2: ECL‐DCR with silicone intubation Comparison intervention: Group 3: ECL‐DCR with 0.4 mg/mL MMC application during surgery |
|
| Outcomes |
Measured outcomes:
Adverse events: stenosis, premature tube loss, granulation, synechia, infection, hemorrhage |
|
| Identification |
Author name: Remi Zogan Institution: Bezmialem Vakif University Email: dr.remzidogan@gmail.com |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described in the article. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of study personnel and participants was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of study outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 failures in Group 1, 3 in Group 2, and 2 in Group 3 were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Eshraghy 2012.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 88 in total, 42 in Group A, 46 in Group B Number of arms: 2 Enrollment start year: 2008 Length of follow‐up: average of 10 months (range 6 to 15 months) Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: Iran Age (mean (SD)): 50.7 (8.9) in Group A, 49.5 (9.9) in Group B Females (n (%)): 41 (46.6) in total, 19 (45.2) in Group A, 22 (47.9) in Group B Inclusion criteria: history of dacryocystitis in past 3 months, inappropriate lacrimal sac or nasal mucosal sac Exclusion criteria: tearing secondary to identifiable and treatable causes (e.g. dry eye, trichiasis, entropion or ectropion, common canaliculus obstruction, fracture of the facial bones, tumor of the eyelid or the lacrimal sac), previously failed DCR surgery Study group differences: group differences in age were not statistically significant |
|
| Interventions |
Intervention: Group A: DCR with silicone intubation and application of MMC (0.02%) during surgery Comparison intervention: Group B: DCR with silicone intubation |
|
| Outcomes |
Measured outcomes:
Adverse events: none |
|
| Identification |
Author name: Firoozeh Raygan Institution: Farabi Eye Hospital Email: fraygan@gmail.com |
|
| Notes |
Funding source: not reported Declarations of interest: none Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization methods not reported. No sequence generation technique described in the text. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported whether participants were blinded. Personnel were not masked due to the nature of the procedure. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participant attrition rate is not reported. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Ghosh 2006.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 30 in total, 15 per group Number of arms: 2 Enrollment start year: 2003 Length of follow‐up: 12 months Sample size calculations: not reported Losses to follow‐up: not reported |
|
| Participants |
Country: India Age (mean (SD)): 32.5 (NR) in total Females (n (%)): 20 (67) in total Inclusion criteria: recurrent epiphora for more than 2 to 4 months not responding to medical therapy Exclusion criteria: acute dacryocystitis, recurrent abscesses, tumors of the lacrimal apparatus Study group differences: not reported |
|
| Interventions |
Intervention: EX‐DCR with application of 0.2 mg/mL MMC Comparison intervention: EX‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: stenosis of the stoma, synechia |
|
| Identification |
Author name: Soumitra Ghosh Institution: Ramakrishna Mission Seva Pratishthan, Vivekananda Institute of Medical Sciences Email: not reported |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how sequence generation was performed. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of masking of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants enrolled in the study were accounted for in the analysis. |
| Selective reporting (reporting bias) | High risk | Syringing performed, but no reporting of anatomic patency as a result. In addition, stoma and complaints of epiphora bound together as a combined outcome result. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Gonzalvo 2000.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 30 in total, 15 per group Number of arms: 2 Enrollment start year: 1996 Length of follow‐up: 6 months Sample size calculations: not reported Losses to follow‐up: not reported |
|
| Participants |
Country: Spain Age (mean (SD)): 48.11 (11.77) in total, 52.33 (4.82) in the MMC group, 43.5 (15.2) in the EX‐DCR alone group Females (n (%)): 11 (64) in total, 6 (75) in the MMC group, 5 (55) in the EX‐DCR alone group Inclusion criteria: patients with nasolacrimal duct obstruction after previous canaliculation Exclusion criteria: none Study group differences: not reported |
|
| Interventions |
Intervention: EX‐DCR with MMC (0.2 mg/mL) application during surgery Comparison intervention: EX‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: development of herpes zoster of cranial nerve V |
|
| Identification |
Author name: Francisco Jose Gonzalvo Ibanez Institution: Hospital Universitario Miguel Servet Email: not reported |
|
| Notes |
Funding source: not reported Declarations of interest: none Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The random component in the sequence generation process was not described. |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although participants and outcome assessors did not have knowledge of the groups to which participants had been assigned, masking of study personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The patient and the outcome assessor did not have knowledge of the group designated to each patient" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Kao 1997.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 15 eyes (of 14 participants), 7 eyes in the MMC group, 8 eyes in EX‐DCR alone group Number of arms: 2 Enrollment start year: 1994 Length of follow‐up: 6 months Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: Taiwan Age (mean (SD)): 55 (9.5) in the MMC group, 52 (14.7) in the EX‐DCR alone group Females (n (%)): not reported Inclusion criteria: patients with primary acquired nasolacrimal duct obstruction Exclusion criteria: not reported Study group differences: not reported |
|
| Interventions |
Intervention: EX‐DCR with 0.2 mg/mL MMC application during surgery Comparison intervention: EX‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: septo‐osteotomy adhesion |
|
| Identification |
Author name: Shine CS Kao Institution: National Taiwan University Hospital Email: not reported |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All the calculations were done by one of our staff members who did not know whether he was looking at a photograph of a mitomycin C group or a control group patient." Masking of other outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Kim 2002.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 100 participants in total, 50 participants (59 eyes) in Group A, 50 participants (62 eyes) in Group B Number of arms: 2 Enrollment start year: 1993 Length of follow‐up: average of 10.2 months (range 6 to 24 months) Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: South Korea Age (mean (SD)): 52 (NR) in total Females (n (%)): 89 (89) in total, 45 (90) in Group A, 44 (88) in Group B Inclusion criteria: diagnosis of nasolacrimal duct obstruction Exclusion criteria: not reported Study group differences: not reported |
|
| Interventions |
Intervention: Group A: endonasal DCR with 0.2 mg/mL MMC application during surgery Comparison intervention: Group B: endonasal DCR |
|
| Outcomes |
Measured outcomes:
Adverse events: granulation tissue, membranous obstruction, protrusion of silicone tube, prolapse of orbital fat, canaliculitis, nasal mucosal erosion |
|
| Identification |
Author name: Kim Yt Institution: Yeungnam University Hospital Email: chungwha@med.yu.ac.kr |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used 100 color cards (50 blue, 50 yellow) and randomly chose from a bag to assign participants. |
| Allocation concealment (selection bias) | Low risk | Color cards were used to conceal treatment allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and study personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Low risk | Study appears to be free of other sources of bias. |
Liao 2000.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 88 eyes in total, 44 per group Number of arms: 2 Enrollment start year: 1995 Length of follow‐up: 10 months or more Sample size calculations: not reported Losses to follow‐up: not reported |
|
| Participants |
Country: Taiwan Age (mean (SD)): 57.9 (7.4) in the MMC group, 57.4 (10.2) in the EX‐DCR alone group Females (n (%)): not reported Inclusion criteria: patients with nasolacrimal duct obstruction Exclusion criteria: not reported Study group differences: no significant differences with regard to age |
|
| Interventions |
Intervention: EX‐DCR with 0.2 mg/mL MMC application Comparison intervention: EX‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: wound disruption |
|
| Identification |
Author name: Shu Lang Liao Institution: Department of Ophthalmology National Taiwan University Hospital Email: lang89@ha.mc.ntu.edu.tw |
|
| Notes |
Funding source: not reported Declarations of interest: none Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported: "88 patients with a diagnosis of primary acquired nasolacrimal duct obstruction were randomly assigned into mitomycin C and conventional DCR groups". |
| Allocation concealment (selection bias) | Unclear risk | Method of treatment allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors state that "all the examinations were done by the same physician with double blind control"; however, details regarding masking and who was masked and how it was performed were not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors state that "all the examinations were done by the same physician with double blind control", but unclear whether this means outcome assessors were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Mukhtar 2014.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 160 in total, 80 per group Number of arms: 2 Enrollment start year: 2009 Length of follow‐up: 3 months Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: Pakistan Age (mean (SD)): 38.77 (10.96) in the MMC group, 40.96 (10.05) in the EX‐DCR alone group Females (n (%)): 94 (58.8) overall, 47 (58.8) in the MMC group, 47 (58.8) in the EX‐DCR alone group Inclusion criteria: aged 20 to 60 years, chronic dacryocystitis Exclusion criteria: previous dacryocystorhinostomy surgery or trauma, nasal and paranasal sinuses pathology Study group differences: not reported |
|
| Interventions |
Intervention: EX‐DCR with 0.2 mg/mL MMC application Comparison intervention: EX‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: not reported |
|
| Identification |
Author name: Sarfraz Ahmad Mukhtar
Institution: Department of Ophthalmology, Bahawal Victoria Hospital Email: ahmadzeeshandr@yahoo.com |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was done by "lottery method", but nature of method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No masking of participants or study personnel was described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants only followed for 3 months after surgery and were analyzed in the group to which they had been randomized. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Ozkiris 2012.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 36 in total, 18 per group Number of arms: 2 Enrollment start year: 2007 Length of follow‐up: mean of 11.5 months in the MMC group, mean of 12.7 months in the EN‐DCR alone group (overall range of 6 to 22 months) Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: Turkey Age (mean (SD)): 37.2 (10.2) in total Females (n (%)): 15 (41.7) in total, 7 (38.8) in the MMC group, 8 (44.4) in the EN‐DCR alone group Inclusion criteria: unilateral or bilateral nasolacrimal duct obstruction proven by nasolacrimal duct irrigation, aged > 18 years, previous history of DCR surgery, follow‐up of at least 6 months Exclusion criteria: distal canalicular or common canalicular obstruction on dacryocystography, patients with eyelid or sac abnormality, cases with suspicion of malignancy, previous radiation therapy, post‐traumatic lids/bony deformity, proximal obstruction, nasal structural abnormalities, severe atrophic rhinitis Study group differences: not reported |
|
| Interventions |
Intervention: EN‐DCR with 0.5 mg/mL MMC application and canalicular silicone intubation Comparison intervention: EN‐DCR with canalicular silicone intubation |
|
| Outcomes |
Measured outcomes:
Adverse events: none |
|
| Identification |
Author name: Mahmut Ozkiris Institution: Bozok University Medical Faculty Email: mozkiris@yahoo.com |
|
| Notes |
Funding source: not reported Declarations of interest: none Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by computer random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were masked to assigned group; although personnel were not (no placebo was used), this probably did not affect outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients were examined with an endoscope 2 days after surgery, after 1 week, and monthly thereafter for a minimum of 6 months by another surgeon (S.G.) who was blinded to the operation technique performed. Both subjective and objective assessments were performed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were analyzed in the group to which they had been randomized. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Park 2000.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 66 participants (75 eyes) in total, 37 in the MMC group, 38 in the EN‐DCR alone group Number of arms: 2 Enrollment start year: 1997 Length of follow‐up: mean of 6.8 months in the MMC group (range 4 to 16 months), mean of 7.2 months in the EN‐DCR alone group (range 4 to 19 months) Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: South Korea Age (mean (SD)): 54 (NR) in the MMC group, 52 (NR) in the EN‐DCR alone group Females (n (%)): 66 (88) in total, 35 (95) in the MMC group, 31 (82) in the EN‐DCR alone group Inclusion criteria: diagnosis of nasolacrimal duct obstruction Exclusion criteria: not reported Study group differences: no significant differences between groups |
|
| Interventions |
Intervention: EN‐DCR with application of 0.2 mg/mL MMC Comparison intervention: EN‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: orbital fat herniation, nasal septal wall injury, rebleeding, tube protrusion |
|
| Identification |
Author name: Mi Seon Kwak Institution: Taegu Fatima Hospital Email: not reported |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | 1 surgeon performed all surgeries; masking of participants not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition or missing data |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Penttilä 2011.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 30 in total, 15 per group Number of arms: 2 Enrollment start year: 2004 Length of follow‐up: 6 months Sample size calculations: not reported Losses to follow‐up: 2 (6.25%) lost to follow‐up (reasons and groups not reported) |
|
| Participants |
Country: Finland Age (mean (SD)): 65 (11) in the MMC group, 70 (10) in the EN‐DCR only group Females (n (%)): 27 (90) in total, 13 (86.7) in the MMC group, 14 (93.3) in the EN‐DCR only group Inclusion criteria: aged 18 years, American Society of Anesthesiologist physical status was I‐III, scheduled for revision lacrimal pathway surgery due to tearing or recurrent infection after failed EX‐DCR or EN‐DCR Exclusion criteria: presaccal obstruction; malignancy in the paranasal sinuses, nasal cavity, or lacrimal pathway; mental disability; pregnancy; breastfeeding Study group differences: not significant |
|
| Interventions |
Intervention: EN‐DCR with application of 0.4 mg/mL MMC Comparison intervention: EN‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: additional surgery for abnormalities |
|
| Identification |
Author name: Elina Penttila Institution: Department of Otorhinolaryngology at Kuopio University Hospital Email: grigori.smirnov@kuh.fi |
|
| Notes |
Funding source: not reported Declarations of interest: none Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation was computer‐generated and a sealed opaque envelope method was used to ensure blinding." |
| Allocation concealment (selection bias) | Low risk | "The allocation was computer‐generated and a sealed opaque envelope method was used to ensure blinding." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was not followed: 2/32 (6.25%) participants were withdrawn and not included in the final analysis, however we determined that this was unlikely to have impacted on the results. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Prasannaraj 2012.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participant Number randomized: 38 participants in total, 17 in the MMC group, 21 in the EN‐DCR alone group Number of arms: 2 Enrollment start year: 2003 Length of follow‐up: 6 months Sample size calculations: "A combined sample size of 38 patients was arrived at by using the power approach with a power of 90% and an assumed effect size of 35% between the mitomycin and control groups" Losses to follow‐up: none |
|
| Participants |
Country: India Age (mean (SD)): 33.6 (NR) in total Females (n (%)): 22 (57.9) in total Inclusion criteria: diagnosis of chronic dacryocystitis due to primary acquired postsaccal obstruction of the lacrimal apparatus Exclusion criteria: patients aged 15 years or younger, history of previous lacrimal sac surgery Study group differences: not reported |
|
| Interventions |
Intervention: EN‐DCR with application of 0.2 mg/mL MMC Comparison intervention: EN‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: granulations, synechiae, obliterative sclerosis |
|
| Identification |
Author name: Thomas Prasannaraj Institution: R.L. Jalappa Hospital and Research Centre Email: drtpr@yahoo.com |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random allocation of patients to the mitomycin group or the control group was done by allowing each patient to choose from a bunch of unbiased chits. This was done after counseling and before admission for surgery." |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described. Single‐blind study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Single‐blind" study, however details regarding masking were not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Low risk | Study appears to be free of other sources of bias. |
Qadir 2014.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 50 in total, 25 per group Number of arms: 2 Enrollment start year: not reported Length of follow‐up: 6 months Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: India Age (mean (SD)): 43 (12.6) in the MMC group, 47.3 (11.5) in the EX‐DCR alone group Females (n (%)): 36 (72) in total, 19 (76) in the MMC group, 17 (68) in the EX‐DCR alone group Inclusion criteria: patients with primary acquired nasolacrimal duct obstruction Exclusion criteria: presaccal obstructions, acute dacryocystitis, chronic granulomatous condition, longstanding chronic dacryocystitis with fibrosis of sac, chronic dacryocystitis with fistula, ectropion, entropion, nasal conditions like severe deviated nasal septum, atrophic rhinitis, previous failure of DCR Study group differences: no significant difference in ages; female preponderance in the study but no significant between‐group differences |
|
| Interventions |
Intervention: EX‐DCR with application of 0.2 mg/mL MMC Comparison intervention: EX‐DCR |
|
| Outcomes |
Measured outcomes:
Adverse events: injury to nasal mucosa, sac injury, severe bleeding, epistaxis, wound infection |
|
| Identification |
Author name: Andleeb Ahangar Institution: Department Of Ophthalmology, Government Medical College Srinagar Email: andleebali@gmail.com |
|
| Notes |
Funding source: not reported Declarations of interest: none Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Qiu 2000.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 92 in total, 48 to the MMC group, 44 to the DCR alone group Number of arms: 2 Enrollment start year: 1995 Length of follow‐up: 29 months Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: China Age (mean (SD)): 29.6 (NR) in total Females (n (%)): 82 (89) in total Inclusion criteria: patients with chronic dacryocystitis Exclusion criteria: patients with upper lacrimal duct or nasal disorders, other systemic disorders Study group differences: no difference |
|
| Interventions |
Intervention: DCR with application of 0.4 mg/mL MMC Comparison intervention: DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: anastomotic bleeding |
|
| Identification |
Author name: SK Qiu
Institution: Department of Ophthalmology, Tengzhou Central Hospital Email: not reported |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of treatment allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of study participants and investigators was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data, participants were analyzed in the group to which they had been randomized. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Ragab 2012.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 76 in total, 38 per group Number of arms: 2 Enrollment start year: 2004 Length of follow‐up: 12 months Sample size calculations: "A sample size of 70 procedures was calculated at the 5% level of significance to give the study a statistical power of 80%" Losses to follow‐up: at 12 months: 3 lost in the MMC group, 2 lost in the control group |
|
| Participants |
Country: Egypt Age (mean (SD)): 43.6 (10.4) in total Females (n (%)): 49 (64.5) in total Inclusion criteria: patent canaliculi, normal eyelid function, no suspected lacrimal sac neoplasia, no nasal pathology, recurrent acquired complete nasolacrimal obstruction after single endoscopic DCR, duration of persistent symptoms more than 1 year after the primary surgery Exclusion criteria: canalicular or common canalicular obstruction ascertained with probing, noticeable lower lid laxity, age younger than 18 years, Down’s syndrome, suspicion of malignancy, radiation therapy, post‐traumatic bony deformity, and bone diseases Study group differences: no significant difference in demographics |
|
| Interventions |
Intervention: revision EN‐DCR with application of 0.5 mg/mL MMC during surgery Comparison intervention: revision EN‐DCR |
|
| Outcomes |
Measured outcomes:
Adverse events: minor epistaxis, minimal synechia |
|
| Identification |
Author name: Sameh M Ragab Institution: Department of Otolaryngology, Tanta University Hospitals Email: sragab@doctors.org.uk |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done using random blocks. |
| Allocation concealment (selection bias) | Low risk | "The group assignment was placed in consecutively numbered envelopes, which were allocated to the successive cases in chronological order. The envelope was opened on the day of the operation. During the follow‐up period, the patient was assigned to a different investigator. The patient file was coded and linked to a study sheet. The study sheet summarized all the information related to the patient except the operative data. The sheet was copied and added to the patient file after each session, whereas the original sheet was kept in the study folder." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “At the time of randomization and during the follow‐up period, both the patient and the investigator were unaware of the group assignment. The group assignment was placed in consecutively numbered envelopes, which were allocated to the successive cases in chronological order. The envelope was opened on the day of the operation. During the follow‐up period, the patient was assigned to a different investigator. The patient file was coded and linked to a study sheet. The study sheet summarized all the information related to the patient except the operative data. The sheet was copied and added to the patient file after each session, whereas the original sheet was kept in the study folder.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data at 6 months (primary endpoint). At 12 months, 3 in the MMC group and 2 in the control group were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Low risk | Study appears to be free of other sources of bias. |
Roozitalab 2004.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 130 in total, 65 per group Number of arms: 2 Enrollment start year: 2001 Length of follow‐up: 6 months Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: Iran Age (mean (SD)): 40 (15) in the MMC group, 42 (16) in the EX‐DCR group Females (n (%)): 89 (68.5) in total, 49 (75) in the MMC group, 40 (62) in the EX‐DCR group Inclusion criteria: diagnosis of nasolacrimal duct obstruction (congenital and acquired) Exclusion criteria: not reported Study group differences: no significant difference in age |
|
| Interventions |
Intervention: EX‐DCR with application of 0.2 mg/mL MMC Comparison intervention: EX‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: none |
|
| Identification |
Author name: MR Namazi Institution: Shiraz University of Medical Sciences Email: Namazi_mr@yahoo.com |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of treatment allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the examinations were done by the second author, who was masked to the procedures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Shaikh 2015.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 200 in total, 100 per group Number of arms: 2 Enrollment start year: 2013 Length of follow‐up: 3 months Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: Saudi Arabia Age (mean (SD)): 37.77 (11.96) in the MMC group, 39.96 (9.05) in the EX‐DCR alone group Females (n (%)): 68 (68) in the MMC group, 76 (76) in the EX‐DCR alone group Inclusion criteria: patients who had gone through EX‐DCR, aged 20 to 70 years Exclusion criteria: gross nasal pathology, noticeable lid laxity, repeat DCR surgery for DCR failure, patients with post‐traumatic lids Study group differences: no difference |
|
| Interventions |
Intervention: EX‐DCR with application of MMC (dosage not reported) Comparison intervention: EX‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: not reported |
|
| Identification |
Author name: Rehan Moinuddin Shaikh Institution: King Fahad Armed Forces Hospital Email: drrehan@hotmail.com.au |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of treatment allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Tirakunwichcha 2011.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 50 in total, 26 in the MMC group, 24 in the EN‐DCR alone group Number of arms: 2 Enrollment start year: 2004 Length of follow‐up: 12 months Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: Thailand Age (mean (SD)): 44.6 (NR) in total, 44.3 (6.47) in the MMC group, 44.9 (6.87) in the EN‐DCR alone group Females (n (%)): 41 (82) in total, 22 (84.6) in the MMC group, 19 (79.2) in the EN‐DCR alone group Inclusion criteria: patients with primary acquired nasolacrimal duct obstruction Exclusion criteria: secondary causes of obstruction and canalicular obstructions Study group differences: no differences |
|
| Interventions |
Intervention: EN‐DCR with application of 0.5 mg/mL MMC Comparison intervention: EN‐DCR with application of placebo |
|
| Outcomes |
Measured outcomes:
Adverse events: none |
|
| Identification |
Author name: Suppapong Tirakunwichcha Institution: Chulalongkorn University Email: suppapong.t@chula.ac.th |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using a "block of four randomization, which was prepared in advance and concealed in 50 envelopes (in chronological order) by an independent ophthalmologist". |
| Allocation concealment (selection bias) | Low risk | "The patients were then allocated into the treatment group .... using the block of four randomization, which was prepared in advance and concealed in 50 envelopes (in chronological number) by another ophthalmologist (S.S.) who was not involved in the surgical process and outcome evaluation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐masked" study: "The 0.5 mg/ml mitomycin C solution and the placebo were prepared in the same color for each patient by the assigned scrub nurse who cooperated with the ophthalmologist (S.S.) who knew which group the patient was in, and the solution was served to the surgeon in the operating field. The endonasal endoscopic DCR was performed by the otolaryngologist (A.S.). The surgeon was masked to the intervention and only yielded to assess the outcomes." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Postoperative eye symptoms were assessed by the ophthalmologist (TS), and ostium sizes were measured by the otolaryngologist (AS). The collected data gathered by the ophthalmologist (SS) were disclosed after the 1‐year follow‐up visit. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Wadhera 2013.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 50 in total, 25 per group Number of arms: 2 Enrollment start year: 2004 Length of follow‐up: 1 year Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: India Age (mean (SD)): 32.4 (10.28) in the MMC group, 33.2 (9.3) in the EN‐DCR alone group Females (n (%)): 10 (25) in the MMC group, 8 (32) in the EN‐DCR alone group Inclusion criteria: aged 16 to 50 years, symptoms and signs suggestive of nasolacrimal duct blockage refractory to conventional medical treatment Exclusion criteria: marked deviation of nasal septum on same side, chronic sinusitis, nasal polyps, severe bony deformity of lacrimal sac fossa (post‐traumatic), bleeding disorders, nasal tumors, history of previous DCR Study group differences: no differences |
|
| Interventions |
Intervention: EN‐DCR with application of 0.5 mg/mL MMC Comparison intervention: EN‐DCR |
|
| Outcomes |
Measured outcomes:
Adverse events: mild postoperative bleeding |
|
| Identification |
Author name: Raman Wadhera Institution: Graduate Institute of Medical Sciences Email: dr.wadhera@yahoo.com |
|
| Notes |
Funding source: not reported Declarations of interest: none Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were assigned randomly into two groups of 25 patients each". Method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of treatment allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Xie 2015.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 62 in total, 31 per group Number of arms: 2 Enrollment start year: 2010 Length of follow‐up: 3 to 12 months Sample size calculations: not reported Losses to follow‐up: not reported |
|
| Participants |
Country: China Age (mean (SD)): 41.7 (0.6) in total Females (n (%)): 46 (74.2) in total Inclusion criteria: not reported Exclusion criteria: not reported Study group differences: not reported |
|
| Interventions |
Intervention: EN‐DCR with application of 0.2 g/L MMC Comparison intervention: EN‐DCR |
|
| Outcomes |
Measured outcomes:
Adverse events: not reported |
|
| Identification |
Author name: Ping Xie Institution: Jiujiang No. 1 People's Hospital Email: xieping1977@126.com |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of treatment allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and investigators was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Investigators assessed "Cure rate" and "effective rate" these terminologies may be different from functional or anatomic success. Additionally, attrition rate was not reported and it is unclear whether all participants were analyzed in the groups to which they were randomized |
| Selective reporting (reporting bias) | Unclear risk | Investigators assessed "Cure rate" and "effective rate" these terminologies may be different from functional or anatomic success, therefore selective outcome reporting could not be ruled out. Additionally, there were no protocols or trial registration with which to compare |
| Other bias | Unclear risk | Sources of funding and sample size estimation were not reported, there is also insufficient information to judge as to low or high risk of bias. |
Yalaz 1999.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 60 in total, 10 per group Number of arms: 5 Enrollment start year: 1995 Length of follow‐up: 12 months Sample size calculations: not reported Losses to follow‐up: none |
|
| Participants |
Country: Turkey Age (mean (SD)): 35 (13.81) in total Females (n (%)): 47 (78.3) in total Inclusion criteria: primary acquired idiopathic nasolacrimal duct obstruction Exclusion criteria: secondary nasolacrimal duct obstruction due to factors such as trauma, facial surgery, sinus disease, and revision DCR Study group differences: no difference |
|
| Interventions |
Intervention 1: EX‐DCR with application of 0.5 mg/mL MMC Intervention 2: EX‐DCR with application of 1 mg/mL MMC Intervention 3: EX‐DCR with application of 2.5 mg/mL 5‐FU Intervention 4: EX‐DCR with application of 5 mg/mL 5‐FU Comparison intervention: EX‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: none |
|
| Identification |
Author name: Müslime Yalaz Institution: Çukurova University Medical Faculty Email: not reported |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were randomly divided into three groups". Method of random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of treatment allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing outcome data at 12 months. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Yan 2002.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 41 in total, 18 in the MMC group, 23 in the DCR alone group Number of arms: 2 Enrollment start year: not reported Length of follow‐up: average 30 months Sample size calculations: not reported Losses to follow‐up: not reported |
|
| Participants |
Country: China Age (mean (SD)): 35.6 (NR) in total Females (n (%)): 31 (75.6) in total Inclusion criteria: not reported Exclusion criteria: not reported Study group differences: not reported |
|
| Interventions |
Intervention: DCR with application of 0.4 mg/mL MMC Comparison intervention: DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: not reported |
|
| Identification |
Author name: Yan Xiou Ju Institution: Second Affiliated Hospital, Chongqing University of Medical Sciences Email: not reported |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of treatment allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and investigators not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
Yildirim 2007.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: eyes Number randomized: 35 participants (40 eyes), 20 eyes per group Number of arms: 2 Enrollment start year: not reported Length of follow‐up: 19 months Sample size calculations: “The power calculation of the study was found to be 0.45 for the satisfaction rates and was 0.08 for success rates, both of which were underpowered." Losses to follow‐up: not reported |
|
| Participants |
Country: Turkey Age (mean (SD)): 41.2 (11.5) in the MMC group, 39 (7.5) in the EX‐DCR alone group Females (n (%)): not reported Inclusion criteria: diagnosis of primary acquired nasolacrimal duct obstruction Exclusion criteria: previous DCR surgery Study group differences: no significant differences |
|
| Interventions |
Intervention: EX‐DCR with application of 1 mL of 0.02 mg/mL MMC Comparison intervention: EX‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: none |
|
| Identification |
Author name: Cem Yildirim Institution: Pamukkale University Email: yildirimc@hotmail.com |
|
| Notes |
Funding source: not reported Declarations of interest: not reported Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | How the random sequence was generated is not described. |
| Allocation concealment (selection bias) | Unclear risk | How allocation was concealed is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Whether participants and personnel were masked is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The same physician, who did not know whether the participant had received MMC application during surgery, documented subjective symptoms and objective findings. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number randomized was analyzed. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
You 2001.
| Methods |
Study design: randomized controlled trial, parallel group Unit of analysis: participants Number randomized: 46 participants, 16 in the 0.2 mg/mL MMC group, 16 in the 0.5 mg/mL MMC group, 18 in the EX‐DCR alone group Number of arms: 3 Enrollment start year: 1996 Length of follow‐up: range 23 to 42 months Sample size calculations: not reported Losses to follow‐up: 4 |
|
| Participants |
Country: China Age (mean (SD)): 33.13 (13.17) in the 0.2 mg/mL MMC group, 30.18 (12.74) in the 0.5 mg/mL MMC group, 33.64 (11.89) in the EX‐DCR alone group Females (n (%)): 33 (72) in total, 11 (69) in the 0.2 mg/mL MMC group, 10 (62) in the 0.5 mg/mL MMC group, 12 (67) in the EX‐DCR alone group Inclusion criteria: primary nasolacrimal duct obstruction, duration of symptoms longer than 1 year Exclusion criteria: canalicular or common canalicular stenosis or obstruction ascertained by probing or dacryocystography, epiphora with a positive primary Jones dye test, acute dacryocystitis, tumor of the lacrimal sac, severe atrophic rhinitis Study group differences: not reported |
|
| Interventions |
Intervention 1: EX‐DCR with application of 0.2 mg/mL MMC Intervention 2: EX‐DCR with application of 0.5 mg/mL MMC Comparison intervention: EX‐DCR alone |
|
| Outcomes |
Measured outcomes:
Adverse events: mild postoperative hemorrhage |
|
| Identification |
Author name: Yi‐an You Institution: First Affiliated Hospital, Wenzhou Medical College Email: not reported |
|
| Notes |
Funding source: not reported Declarations of interest: none Trial registration number: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to treatment, method of randomization not reported. |
| Allocation concealment (selection bias) | Unclear risk | How allocation of participants to treatment was concealed is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Whether participants and personnel were masked is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whether outcome assessors were masked is not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was wide range of follow‐up: "Follow‐up time intervals ranged from 23 to 42 months (mean, 35.2 5.3 months)", and analysis appears not to be intention‐to‐treat, as 4 lost to follow‐up were not included in the analysis. The number lost to follow‐up was small and is unlikely to have impacted on results. |
| Selective reporting (reporting bias) | Unclear risk | No trial or protocol registration available for comparison to ascertain selective outcome reporting. |
| Other bias | Unclear risk | There was insufficient information to permit a judgement of 'low risk' or 'high risk'. |
DCR: dacryocystorhinostomy ECL‐DCR: endocanalicular dacryocystorhinostomy ELDCR: endonasal laser dacryocystorhinostomy EN‐DCR: endonasal dacryocystorhinostomy EX‐DCR: external dacryocystorhinostomy FU: fluorouracil MMC: mitomycin‐C NLDO: nasolacrimal duct obstruction NR: not reported SD: standard deviation TLA‐ELA DCR: endonasal and endocanalicular dacryocystorhinostomy with diode laser 5‐FU:5‐fluorouracil
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ali 2015 | Not an RCT |
| Altay 2015 | Not an RCT |
| Bakri 2002 | Duplicate study |
| Boboridis 2004 | Not an RCT |
| Camara 1999 | Not an RCT |
| Carifi 2014 | Not an RCT (letter to the editor) |
| ChiCTR‐INR‐16009702 | Not the intervention of interest |
| ChiCTR‐TRC‐09000721 | Not the intervention of interest |
| Costa 1992 | Duplicate study |
| CTRI/2014/09/004989 | Not an RCT |
| Deka 2006 | Not an RCT |
| Do 2016 | Not an RCT |
| Fan 2009 | Not an RCT |
| Farahani 2008 | Not the intervention of interest |
| IRCT201206216388N3 | Not the intervention of interest |
| IRCT201409166033N4 | Not the intervention of interest |
| Jawad 2015 | Not an RCT |
| Kim 2007 | Not an RCT |
| Leibovitch 2006 | Not an RCT |
| Li 2010 | Not the intervention of interest |
| Liao 2017 | Not the intervention of interest |
| Liu 2003 | Not the intervention of interest |
| Mudhol 2013a | Not the intervention of interest |
| NCT02636257 | Not the intervention of interest |
| NCT03780868 | Not the intervention of interest |
| Patel 2009 | Not an RCT |
| Piaton 2001 | Not an RCT |
| Qin 2010 | Not an RCT |
| Qiu 2016 | Not an RCT |
| Rathore 2009 | Not an RCT |
| Singh 2015 | Not an RCT |
| Tabatabaie 2007 | Not the intervention of interest |
| TCTR20161007003 | Not an RCT |
| Wang 2017 | Not the intervention of interest |
| Zeng 2008 | Not an RCT |
| Zhang 2006 | Not the population of interest |
| Zilelioglu 1998 | Not an RCT |
RCT: randomized controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
CTRI‐2014‐09‐004989.
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 440 adult patients (> 18 years), presence of regurgitation on pressure over lacrimal sac and/or regurgitation admixed with mucopurulent debris on syringing, patients who have a hard stop on probing, normal eyelid function Exclusion criteria: patients undergoing revision DCR, pediatric patients (< 18 years), NLDO secondary to trauma, presence of any canalicular obstruction/eyelid condition responsible for epiphora, anemia (hemoglobin < 7 gram%) or deranged coagulation profile, presence of significant nasal pathology like deviated nasal septum, nasal polyps |
| Interventions |
Intervention: mitomycin C injection (0.4 mg/mL): non‐endoscopic endonasal DCR with bicanalicular intubation with mitomycin C application at the ostium site Comparison intervention: no injection: non‐endoscopic endonasal DCR with bicanalicular intubation without mitomycin C application at the ostium site |
| Outcomes |
Primary outcome: anatomical patency on syringing (time point: 6 months) Secondary outcome: anatomical patency Maximum follow‐up: 1 year |
| Notes |
Start date: September 2014 Estimated end date: not reported |
Qian 2018.
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 39 adults with lacrimal duct obstruction Exclusion criteria: not reported |
| Interventions |
Intervention: drainage tube implantation combined with mitomycin C treatment Comparison intervention: drainage tube implantation alone |
| Outcomes |
Primary outcome: anatomical patency Secondary outcome: not reported |
| Notes |
DCR: dacryocystorhinostomy NLDO: nasolacrimal duct obstruction
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐INR‐16008616.
| Trial name or title | Mitomycin, Intubation vs No adjuvant In MUcosal‐preserving Mechanical endonasal dacryocystorhinostomy for primary acquired nasolacrimal duct obstruction (MINIMUM endonasal DCR for PANLDO) |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 340 adults ≥ 18 years old and there is no maximum age limit; primary acquired nasolacrimal duct obstruction (PANLDO) diagnosed by lacrimal irrigation and probing, confirmed intraoperatively after incision of lacrimal sac; informed consent for operation, randomization, and recording; compliance to follow‐up and treatment Exclusion criteria: pregnancy, lactation, allergy to mitomycin, cocaine, adrenaline, steroid, silicone material; contraindications of endonasal DCR or inability to undergo nasal endoscopy; acute (< 3 months) or non‐bacterial dacryocystitis, e.g. tuberculosis, fungal, or parasitic; ipsilateral canalicular disorder, e.g. obstruction, canaliculitis, canaliculocele, diverticulum; ipsilateral recurrent NLDO or any prior lacrimal intervention except punctoplasty; ipsilateral facial paralysis despite apparent clinical recovery; ipsilateral conditions affecting bony nasolacrimal outflow, e.g. midfacial trauma/fracture, osteoma, fibrous dysplasia and other skull‐base disorders; ipsilateral suspected or confirmed nasolacrimal or sino‐orbital neoplasm; ipsilateral severe ocular surface disorders, e.g. ocular cicatricial pemphigoid, chemical burns, Steven Johnson syndrome, toxic epidermal necrolysis; conditions affecting mucosa of the nose or nasolacrimal system, e.g. rhinosinusitis, Wegener’s granulomatosis, sarcoidosis, radioactive iodide, head and neck radiotherapy, ipsilateral maxillectomy, systemic chemotherapy (5‐fluorouracil, docetaxel); ipsilateral topical antiglaucomatous or chemotherapy drops (e.g. timolol, mitomycin C); intraoperative false passage of Bowman probe or metal part of silicone stent; dacryolith or intrasaccular mass |
| Interventions |
Intervention: topical mitomycin C Comparison intervention: normal saline |
| Outcomes |
Primary outcome: anatomical patency, functional patency, ostium morphologies, additional procedure(s), and trial‐related complication(s) Secondary outcome: preoperative (demographic), intraoperative (endonasal, lacrimal sac), and postoperative (ostial) features associated with poor outcomes Maximum follow‐up: 12 months |
| Starting date | Not reported Estimated end date: not reported |
| Contact information | www.chictr.org.cn/showprojen.aspx?proj=14599 |
| Notes |
CTRI/2013/02/003352.
| Trial name or title | Effect of a drug Mitomycin C in repair of tear ducts |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 90 adults aged 18 years and above with primary acquired nasolacrimal duct obstruction Exclusion criteria: secondary causes like deviated nasal septum, nasal polyps, atrophic rhinitis, revision DCR/stents, renal failure or immunosuppression, pregnancy and lactation, out‐station patients, and patients not willing to consent |
| Interventions |
Intervention: mitomycin C Comparison intervention: saline solution |
| Outcomes |
Primary outcome: success rates of EX‐DCR with and without intraoperative mitomycin C Secondary outcome: none Maximum follow‐up: 3 months |
| Starting date | February 2013 Estimated end date: not reported |
| Contact information | ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=5663&EncHid=&modid=&compid=%27,%275663det%27 |
| Notes |
IRCT2014010816136N1.
| Trial name or title | Effect of mitomycine C on DCR (dacryosystorhinostomy) with endonasal endoscopic guidance in lacrimal duct stenosis referring to medical center affiliated to Kashan university of medical sciences |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 92 adults aged between 35 and 65 years old and tearing along with (recurrent acute dacryocystitis); increase in lacrimal minisc; have a reflux when the pressure on the lacrimal sac; confirmed the diagnosis of nasolacrimal lavage selected Exclusion criteria: patients with history of tearing at birth, trauma to face and nose, nasal and sinus surgery, mucosal lacrimal sac, canalicole obstruction, coagulation disorders, hemophilia, other external nasal disease, lacrimal sac neoplasm, corneal ulcers or foreign body on cornea, abnormality in ponctum position |
| Interventions |
Intervention: 0.02% mitomycin C Comparison intervention: placebo |
| Outcomes |
Primary outcome: tearing Secondary outcome: tearing reflex Maximum follow‐up: 6 months |
| Starting date | May 2014 Estimated end date: December 2014 |
| Contact information | en.irct.ir/trial/15192 |
| Notes |
ISRCTN15566163.
| Trial name or title | Use of mitomycin C to improve endonasal dacryocystorhinostomy (DCR) success rates |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 40 participants, no other inclusion criteria provided Exclusion criteria: not reported |
| Interventions |
Intervention: mitomycin C Comparison intervention: standard practice |
| Outcomes |
Primary outcome: symptom free or patency to saline irrigation, or both Secondary outcome: not reported Maximum follow‐up: not reported |
| Starting date | January 2002 Estimated end date: June 2004 |
| Contact information | www.isrctn.com/ISRCTN15566163 |
| Notes | Consider classifying as awaiting classification as study no longer recruiting |
NCT00571129.
| Trial name or title | Endoscopic dacryocystorhinostomy prospective research |
| Methods | Randomized parallel‐group design |
| Participants |
Inclusion criteria: 80 adults aged > 18 years, ASAI‐III, scheduled for primary or revision lacrimal pathway surgery due to recurrent or chronic watering eyes or conjunctival discharge. Patients were excluded if they had undergone previous nasolacrimal surgery; malignancy in the paranasal sinuses, nasal cavity, or lacrimal pathway; presaccal obstruction; pregnancy or lactation; or mental disability. Exclusion criteria: malignancy in the paranasal sinuses, nasal cavity, or lacrimal pathway, presaccal obstruction, pregnancy or lactation, or mental disability |
| Interventions |
Intervention: mitomycin C Comparison intervention: standard practice |
| Outcomes |
Primary outcome: success rate after primary DCR with and without silicone tubes; success rate after revision DCR with and without mitomycin C Secondary outcome: influence of EN‐DCR on participant subjective symptoms and quality of life before and after operation Maximum follow‐up: 5 years |
| Starting date | September 2004 Estimated end date: December 2019 |
| Contact information | clinicaltrials.gov/ct2/show/nct00571129 |
| Notes |
ASAI‐III: American society of anesthesiologist class III DCR: dacryocystorhinostomy EN‐DCR: endonasal dacryocystorhinostomy EX‐DCR: external dacryocystorhinostomy NLDO: nasolacrimal duct obstruction
Differences between protocol and review
In the protocol for this review, we had not planned to perform subgroup analyses based on type of approach for dacryocystorhinostomy, but decided post hoc to conduct subgroup analysis by stratifying data according to the approach used to visualize the operative site, either via the internal approach (EN‐DCR) or the external approach (EX‐DCR).
Contributions of authors
MMM and POP conceived the review. MMM and POP designed and wrote the review. MMM, POP, and SAA screened studies for inclusion and extracted data from studies. BJC and DS contributed to revision of the review. MMM, POP, and SAA drafted the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Eye Institute, National Institutes of Health, USA.
Methodological support provided by the Cochrane Eyes and Vision Group US Project, funded by grant 1 U01 EY020522
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
POP: none known. SAA: none known. BJC: for projects unrelated to this review, BJC received research funding from MedImmune Inc and Sanofi Pasteur, and consulted for Crucell NV, for influenza research. DS: none known. MMM: none known.
New
References
References to studies included in this review
Ahmad 2002 {published data only}
- Ahmad SS, Untoo RA. Results of intraoperative mitomycin C application in dacryocystorhinostomy. JK Science 2002;4(1):27‐31. [Google Scholar]
Alañón 2006 {published data only}
- Alañón FM, Alañón FF, Martínez FA, Cárdenas LM. Results of the application of mitomycin during endonasal and endocanalicular dacryocystorhinostomy by diode laser. Acta Otorrinolaringológica Española 2006;57(8):355‐8. [DOI] [PubMed] [Google Scholar]
Ari 2009 {published data only}
- Ari S, Gun R, Surmeli S, Atay AE, Caca I. Use of adjunctive mitomycin C in external dacryocystorhinostomy surgery compared with surgery alone in patients with nasolacrimal duct obstruction: a prospective, double‐masked, randomized, controlled trial. Current Therapeutic Research ‐ Clinical and Experimental 2009;70(4):267‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakri 2003 {published data only}
- Bakri K, Jones NS, Downes R, Sadiq SA. Intraoperative fluorouracil in endonasal laser dacryocystorhinostomy. Archives of Otolaryngology ‐ Head & Neck Surgery 2003;129(2):233‐5. [DOI] [PubMed] [Google Scholar]
Cai 2003 {published data only}
- Cai SJ, Wu YZ, Hu LM. Use of mitomycin C in dacryocystorhinostomy—a clinical study. Ophthalmology in China 2003;12(6):353‐6. [Google Scholar]
Chavan 2018 {published data only}
- Chavan SS, Kale VD, Rao KN, Rengaraja D, Hekare A. Comparison of application of mitomycin C vs silicon stenting vs conventional method in endonasal dacryocystorhinostomy: a randomized controlled trial of 150 patients. Iranian Journal of Otorhinolaryngology 2018;30(1):11‐8. [PMC free article] [PubMed] [Google Scholar]
Costa 2007 {published data only}
- Costa MN, Marcondes AM, Sakano E, Kara‐José N. Endoscopic study of the intranasal ostium in external dacryocystorhinostomy postoperative. Influence of saline solution and 5‐fluorouracil. Clinics 2007;62(1):41‐6. [DOI] [PubMed] [Google Scholar]
Dogan 2013a {published data only}
- Dogan R, Meric A, Ozsütcü M, Yenigun A. Diode laser‐assisted endoscopic dacryocystorhinostomy: a comparison of three different combinations of adjunctive procedures. European Archives of Oto‐Rhino‐Laryngology 2013;270(8):2255‐61. [DOI] [PubMed] [Google Scholar]
Eshraghy 2012 {published data only}
- Eshraghy B, Raygan F, Tabatabaie SZ, Tari AS, Kasaee A, Rajabi MT. Effect of mitomycin C on success rate in dacryocystorhinostomy with silicone tube intubation and improper flaps. European Journal of Ophthalmology 2012;22(3):326‐9. [DOI] [PubMed] [Google Scholar]
Ghosh 2006 {published data only}
- Ghosh S, Roychoudhury A, Roychaudhuri BK. Use of mitomycin C in endo‐DCR. Indian Journal of Otolaryngology and Head & Neck Surgery 2006;58(4):368‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalvo 2000 {published data only}
- Gonzalvo IFJ, Fuertes FI, Fernández TFJ, Hernández DG, Rabinal AF, Honrubia LFM. External dacryocystorhinostomy with mitomycin C. Clinical and anatomical evaluation with helical computed tomography. Archivos de la Sociedad Espanola de Oftalmologia 2000;75(9):611‐7. [PubMed] [Google Scholar]
Kao 1997 {published data only}
- Kao SC, Liao CL, Tseng JH, Chen MS, Hou PK. Dacryocystorhinostomy with intraoperative mitomycin C. Ophthalmology 1997;104(1):86‐91. [DOI] [PubMed] [Google Scholar]
Kim 2002 {published data only}
- Kim YT, Chung WS. The effect of mitomycin C in endonasal dacryocystorhinostomy. Journal of the Korean Ophthalmological Society 2002;43(4):728‐32. [Google Scholar]
Liao 2000 {published data only}
- Liao SL, Kao SC, Tseng JH, Chen MS, Hou PK. Results of intraoperative mitomycin C application in dacryocystorhinostomy. British Journal of Ophthalmology 2000;84(8):903‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mukhtar 2014 {published data only}
- Mukhtar SA, Jamil AZ, Ali Z. Efficacy of external dacryocystorhinostomy (DCR) with and without mitomycin‐C in chronic dacryocystitis. Journal of the College of Physicians & Surgeons Pakistan 2014;24(10):732‐5. [DOI] [PubMed] [Google Scholar]
Ozkiris 2012 {published data only}
- Ozkiris M, Ozkiris A, Goktas S. Effect of mitomycin C on revision endoscopic dacryocystorhinostomy. Journal of Craniofacial Surgery 2012;23(6):e608‐10. [DOI] [PubMed] [Google Scholar]
Park 2000 {published data only}
- Park DJ, Kwak MS. The effect of mitomycin C on the success rate of endoscopic dacryocystorhinostomy. Journal of the Korean Ophthalmological Society 2000;41(8):1674‐9. [Google Scholar]
Penttilä 2011 {published data only}
- Penttilä E, Smirnov G, Seppä J, Kaarniranta K, Tuomilehto H. Mitomycin C in revision endoscopic dacryocystorhinostomy: a prospective randomized study. American Journal of Rhinology & Allergy 2011;25(6):425‐8. [DOI] [PubMed] [Google Scholar]
Prasannaraj 2012 {published data only}
- Prasannaraj T, Kumar BY, Narasimhan I, Shivaprakash KV. Significance of adjunctive mitomycin C in endoscopic dacryocystorhinostomy. American Journal of Otolaryngology 2012;33(1):47‐50. [DOI] [PubMed] [Google Scholar]
Qadir 2014 {published data only}
- Qadir M, Ahangar A, Dar MA, Hamid S, Keng MQ. Comparative study of dacryocystorhinostomy with and without intraoperative application of mitomycin C. Saudi Journal of Ophthalmology 2014;28(1):44‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qiu 2000 {published data only}
- Qiu SK, Qing J, Wang HM, Jiang KL. Application of mitomycin C in dacryocystorhinostomy. Ophthalmology 2000;9(6):351‐3. [Google Scholar]
Ragab 2012 {published data only}
- Ragab SM, Elsherif HS, Shehata EM, Younes A, Gamea AM. Mitomycin C‐enhanced revision endoscopic dacryocystorhinostomy: a prospective randomized controlled trial. Otolaryngology Head and Neck Surgery 2012;147(5):937‐42. [DOI] [PubMed] [Google Scholar]
Roozitalab 2004 {published data only}
- Roozitalab MH, Amirahmadi M, Namazi MR. Results of the application of intraoperative mitomycin C in dacryocystorhinostomy. European Journal of Ophthalmology 2004;14(6):461‐3. [DOI] [PubMed] [Google Scholar]
Shaikh 2015 {published data only}
- Shaikh RM, Hadrawi MT, Danish EY. Comparison of success rate of external dacryocystorhinostomy with and without mitomycin‐c in patients of chronic dacryocystitis. Pakistan Journal of Medical and Health Sciences 2015;9(3):934‐6. [Google Scholar]
Tirakunwichcha 2011 {published data only}
- Tirakunwichcha S, Aeumjaturapat S, Sinprajakphon S. Efficacy of mitomycin C in endonasal endoscopic dacryocystorhinostomy. Laryngoscope 2011;121(2):433‐6. [DOI] [PubMed] [Google Scholar]
Wadhera 2013 {published data only}
- Wadhera R, Gulati SP, Khurana AK, Sharma H, Kalra V, Ghai A. A comparative study of endoscopic endonasal dacryocystorhinostomy with and without intraoperative mitomycin‐C application. Clinical Rhinology 2013;6(1):5‐9. [Google Scholar]
Xie 2015 {published data only}
- Xie P, Ouyang J, He J. Clinical observation of endoscopic dacryocystorhinostomy on the treatment of recurrent chronic dacryocystitis. International Eye Science 2015;15(10):1828‐9. [Google Scholar]
Yalaz 1999 {published data only}
- Yalaz M, Firinciogullari E, Zeren H. Use of mitomycin C and 5‐fluorouracil in external dacryocystorhinostomy. Orbit 1999;18(4):239‐45. [DOI] [PubMed] [Google Scholar]
Yan 2002 {published data only}
- Yan XJ, Wu CF. Effect of mitomycin C therapy on dacryocystorhinostomy. Chongqing Medical Journal 2002;31(10):954‐5. [Google Scholar]
Yildirim 2007 {published data only}
- Yildirim C, Yaylali V, Esme A, Ozden S. Long‐term results of adjunctive use of mitomycin C in external dacryocystorhinostomy. International Ophthalmology 2007;27(1):31‐5. [DOI] [PubMed] [Google Scholar]
You 2001 {published data only}
- You YA, Fang CT. Intraoperative mitomycin C in dacryocystorhinostomy. Ophthalmic Plastic and Reconstructive Surgery 2001;17(2):115‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ali 2015 {published data only}
- Ali MJ, Baig F, Lakshman M, Naik MN. Electron microscopic features of nasal mucosa treated with topical and circumostial injection of mitomycin C: implications in dacryocystorhinostomy. Ophthalmic Plastic & Reconstructive Surgery 2015;31(2):103‐7. [DOI] [PubMed] [Google Scholar]
Altay 2015 {published data only}
- Altay Y, Bicakci H. The success rate of endocanalicular laser dacryocystorhinostomy with two different mitomycin‐c application. Journal of Clinical and Analytical Medicine 2015;6(1):25‐8. [Google Scholar]
Bakri 2002 {published data only}
- Bakri K, Jones NS, Downes R, Sadiq A. Intraoperative 5‐fluorouracil in endonasal laser dacryocystorhinostomy. Investigative Ophthalmology & Visual Science 2002;43:ARVO E‐abstract 3022. [Google Scholar]
Boboridis 2004 {published data only}
- Boboridis K, Ziakas N, Georgiadis N, Liu D, Bosley TM. Nasolacrimal intubation with mitomycin C. Ophthalmology 2004;111(2):416‐7. [DOI] [PubMed] [Google Scholar]
Camara 1999 {published data only}
- Camara JG, Santiago MDD, Hartikainen J, Seppa H, Grenman R. Success rate of endoscopic laser‐assisted dacryocystorhinostomy. Ophthalmology 1999;106(3):441‐2. [DOI] [PubMed] [Google Scholar]
Carifi 2014 {published data only}
- Carifi M, Morandi M, Carifi G. Re: "Use of mitomycin C in dacryocystorhinostomies". Ophthalmic Plastic & Reconstructive Surgery 2014;30(5):441. [DOI] [PubMed] [Google Scholar]
ChiCTR‐INR‐16009702 {published data only}
- ChiCTR‐INR‐16009702. Clinical trial of modified nasal endoscopic dacryocystorhinostomy. www.chictr.org.cn/showprojen.aspx?proj=16560 (first received 1 November 2016).
ChiCTR‐TRC‐09000721 {published data only}
- ChiCTR‐TRC‐09000721. Prospective randomized study of silicone stent intubation in endoscopic dacryocystorhinostomy for acquired nasolacrimal duct obstruction—SEND study. https://www2.ccrb.cuhk.edu.hk/registry/public/107 (first received 24 April 2008).
Costa 1992 {published data only}
- Costa MN. Endoscopic study of the intranasal ostium in external dacryocystorhinostomy postoperative. Influence of saline solution and 5‐fluorouracil. bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&src=google&base=LILACS&lang=p&nextAction=lnk&exprSearch=114753&indexSearch=ID 1992;1:51. [DOI] [PubMed] [Google Scholar]
CTRI/2014/09/004989 {published data only}
- CTRI/2014/09/004989. Anti‐fibrotic agent in endonasal lacrimal sac surgery. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2014/09/004989 (first received 8 September 2014).
Deka 2006 {published data only}
- Deka A, Bhattacharjee K, Bhuyan SK, Barua CK, Bhattacharjee H, Khaund G. Effect of mitomycin C on ostium in dacryocystorhinostomy. Clinical & Experimental Ophthalmology 2006;34(6):557‐61. [DOI] [PubMed] [Google Scholar]
Do 2016 {published data only}
- Do JR, Lee H, Baek S, Lee TS, Chang M. Efficacy of postoperative mitomycin‐C eye drops on the clinical outcome in endoscopic dacryocystorhinostomy. Graefes Archive for Clinical & Experimental Ophthalmology 2016;254(4):785‐90. [DOI] [PubMed] [Google Scholar]
Fan 2009 {published data only}
- Fan JH, Li XX, Pan DP. Effect of suspending the valve combined with placing drainage‐tube in dacryocystorhinostomy. International Journal of Ophthalmology 2009;9(8):1630‐1. [Google Scholar]
Farahani 2008 {published data only}
- Farahani F, Ramezani A. Effect of intraoperative mitomycin C application on recurrence of endoscopic dacryocystorhinostomy. Saudi Medical Journal 2008;29(9):1354‐6. [PubMed] [Google Scholar]
IRCT201206216388N3 {published data only}
- IRCT201206216388N3. Treatment of nasolacrimal duct obstruction. en.irct.ir/trial/6824 (first received 27 June 2012).
IRCT201409166033N4 {published data only}
- IRCT201409166033N4. Treatment of nasolacrimal duct obstruction. en.irct.ir/trial/6497?revision=6497 (first received 26 September 2014).
Jawad 2015 {published data only}
- Jawad M, Ali Z, Tariq S, Qayum I, Aftab H. Effect of intraoperative mitomycin‐C application in outcome of external dacryocystorhinostomy. Journal of Ayub Medical College 2015;27(3):598‐600. [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim KR, Song HY, Shin JH, Kim JH, Choi EK, Lee YJ. Efficacy of mitomycin C irrigation after removal of an occluded nasolacrimal stent. Journal of Vascular & Interventional Radiology 2007;18(4):519‐25. [DOI] [PubMed] [Google Scholar]
Leibovitch 2006 {published data only}
- Leibovitch I, Selva D. Mitomycin C in dacryocystorhinostomy. Clinical & Experimental Ophthalmology 2006;34(6):511‐2. [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li LC, Ouyang L, Li DJ, Huang Q. Ring silicone tube implantation combined with mitomycin C for the treatment of upper lacrimal duct obstruction. International Journal of Ophthalmology 2010;10(1):187‐8. [Google Scholar]
Liao 2017 {published data only}
- Liao RB, Cai SH, Zhang SH, Li N. Clinical research on modified endoscopic dacryocystorhinostomy for chronic dacryocystitis. International Eye Science 2017;17(2):359‐61. [Google Scholar]
Liu 2003 {published data only}
- Liu D, Bosley TM. Silicone nasolacrimal intubation with mitomycin‐C: a prospective, randomized, double‐masked study. Ophthalmology 2003;110(2):306‐10. [DOI] [PubMed] [Google Scholar]
Mudhol 2013a {published data only}
- Mudhol RR, Zingade ND, Mudhol RS, Harugop AS, Das AT. Prospective randomized comparison of mitomycin C application in endoscopic and external dacryocystorhinostomy. Indian Journal of Otolaryngology and Head & Neck Surgery 2013;65(Suppl 2):255‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02636257 {published data only}
- NCT02636257. A comparative study of two endoscopic operations for lacrimal duct obstruction. clinicaltrials.gov/ct2/show/NCT02636257 (first received 21 December 2015).
NCT03780868 {published data only}
- NCT03780868. External DCR versus canalicular SI with MMC in NLDO. clinicaltrials.gov/ct2/show/NCT03780868 (first received 19 December 2018).
Patel 2009 {published data only}
- Patel BC. Management of acquired nasolacrimal duct obstruction: external and endonasal dacryocystorhinostomy. Is there a third way?. British Journal of Ophthalmology 2009;93(11):1416‐9. [DOI] [PubMed] [Google Scholar]
Piaton 2001 {published data only}
- Piaton JM, Keller P, Limon S, Quenot S. Revision of failed dacryocystorhinostomies using the transcanalicular approach. Results of 118 procedures. Journal Francais d'Ophtalmologie 2001;24(3):265‐73. [PubMed] [Google Scholar]
Qin 2010 {published data only}
- Qin ZY, Lu ZM, Liang ZJ. Application of mitomycin C in nasal endoscopic dacryocystorhinostomy. International Journal of Ophthalmology 2010;10(8):1569‐71. [Google Scholar]
Qiu 2016 {published data only}
- Qiu SQ. Clinical observation of mitomycin C used in nasal endoscopic dacryocystorhinostomy. International Eye Science 2016;16(1):166‐8. [Google Scholar]
Rathore 2009 {published data only}
- Rathore PK, Kumari Sodhi P, Pandey RM. Topical mitomycin C as a postoperative adjunct to endonasal dacryocystorhinostomy in patients with anatomical endonasal variants. Orbit 2009;28(5):297‐302. [DOI] [PubMed] [Google Scholar]
Singh 2015 {published data only}
- Singh M, Ali MJ, Naik MN. Long‐term outcomes of circumostial injection of mitomycin C (COS‐MMC) in dacryocystorhinostomy. Ophthalmic Plastic & Reconstructive Surgery 2015;31(5):423‐4. [DOI] [PubMed] [Google Scholar]
Tabatabaie 2007 {published data only}
- Tabatabaie SZ, Heirati A, Rajabi MT, Kasaee A. Silicone intubation with intraoperative mitomycin C for nasolacrimal duct obstruction in adults: a prospective, randomized, double‐masked study. Ophthalmic Plastic and Reconstructive Surgery 2007;23(6):455‐8. [DOI] [PubMed] [Google Scholar]
TCTR20161007003 {published data only}
- TCTR20161007003. Factors affecting the success of external dacryocystorhinostomy. www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=2095 (first received 1 October 2016).
Wang 2017 {published data only}
- Wang Y, Zhang YY, Zhang HG, Wang H, Shen XJ. Efficiency and complications of single lacrimal duct intubation versus annular lacrimal duct intubation combined with drugs injection in lacrimal duct obstruction treatment. International Eye Science 2017;17(6):1191‐3. [Google Scholar]
Zeng 2008 {published data only}
- Zeng XS, Peng YY. Clinical effects of improved dacryocystorhinostomy with mitomycin C for chronic dacryocystitis. International Journal of Ophthalmology 2008;8(2):424‐5. [Google Scholar]
Zhang 2006 {published data only}
- Zhang K, Zhang MN. Effect of Nd:YAG laser combine with mitomycin C for punctal stenosis. International Journal of Ophthalmology 2006;6(3):690‐1. [Google Scholar]
Zilelioglu 1998 {published data only}
- Zilelioglu G, Ugurbas SH, Anadolu Y, Akiner M, Akturk T. Adjunctive use of mitomycin C on endoscopic lacrimal surgery. British Journal of Ophthalmology 1998;82(1):63‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
CTRI‐2014‐09‐004989 {published and unpublished data}
- CTRI‐2014‐09‐004989. A randomized controlled trial comparing efficacy of mitomycin C (0.4mg/ml) versus no injection at the ostium in non‐endoscopic endonasal dacryocystorhinostomy (DCR) with bicanalicular intubation in primary acquired nasolacrimal duct obstruction in adults. ctri.nic.in/Clinicaltrials/showallp.php?mid1=8437&EncHid=&userName=2014/09/00498 (first received 8 September 2014).
Qian 2018 {published and unpublished data}
- Qian X. Mitomycin C combined with anterograde lacrimal drainage tube implantation for the treatment of upper lacrimal duct obstruction. International Eye Science 2018;18(12):2275‐7. [Google Scholar]
References to ongoing studies
ChiCTR‐INR‐16008616 {published data only}
- ChiCTR‐INR‐16008616. Mitomycin, Intubation vs No adjuvant In MUcosal‐preserving Mechanical endonasal dacryocystorhinostomy for primary acquired nasolacrimal duct obstruction (MINIMUM endonasal DCR for PANLDO). www2.ccrb.cuhk.edu.hk/registry/public/377 (first received 6 August 2016).
CTRI/2013/02/003352 {published data only}
- CTRI/2013/02/003352. Effect of a drug mitomycin C in repair of tear ducts. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=5663&EncHid=&modid=&compid=%27,%275663det%27 (first received 6 February 2013 ).
IRCT2014010816136N1 {published data only}
- IRCT2014010816136N1. Effect of mitomycine C on DCR (dacryosystorhinostomy) with endonasal endoscopic guidance in lacrimal duct stenosis referring to medical center affiliated to Kashan university of medical sciences. en.irct.ir/trial/15192 (first received 18 May 2014).
ISRCTN15566163 {published data only}
- ISRCTN15566163. Use of mitomycin C to improve endonasal dacryocystorhinostomy (DCR) success rates. isrctn.com/ISRCTN15566163 (first received 30 September 2004).
NCT00571129 {published data only}
- ISRCTN15566163. Endoscopic dacryocystorhinostomy prospective research. clinicaltrials.gov/show/nct00571129 (first received 11 December 2007).
Additional references
Bindlish 2002
- Bindlish R, Condon GP, Schlosser JD, D'Antonio J, Lauer KB, Lehrer R. Efficacy and safety of mitomycin‐C in primary trabeculectomy: five‐year follow‐up. Ophthalmology 2002;109(7):1336‐41. [DOI] [PubMed] [Google Scholar]
Casady 2006
- Casady DR, Meyer DR, Simon JW, Stasior GO, Zobal‐Ratner JL. Stepwise treatment paradigm for congenital nasolacrimal duct obstruction. Ophthalmic Plastic and Reconstructive Surgery 2006;22(4):243‐7. [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan W, Selva D. Ostium shrinkage after endoscopic dacryocystorhinostomy. Ophthalmology 2013;120(8):1693‐6. [DOI] [PubMed] [Google Scholar]
Chen 1995
- Chen PP, Ariyasu RG, Kaza V, LaBree LD, McDonnell PJ. A randomized trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. American Journal of Ophthalmology 1995;120(2):151‐60. [DOI] [PubMed] [Google Scholar]
Cheng 2013
- Cheng SM, Feng YF, Xu L, Li Y, Huang JH. Efficacy of mitomycin C in endoscopic dacryocystorhinostomy: a systematic review and meta‐analysis. PLOS ONE 2013;8(5):e62737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dogan 2013b
- Dogan R, Meric A, Ozsütcü M, Yenigun A. Diode laser‐assisted endoscopic dacryocystorhinostomy: a comparison of three different combinations of adjunctive procedures. European Archives of Oto‐Rhino‐Laryngology 2013;270(8):2255‐61. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Huang 2014
- Huang J, Malek J, Chin D, Snidvongs K, Wilcsek G, Tumuluri K, et al. Systematic review and meta‐analysis on outcomes for endoscopic versus external dacryocystorhinostomy. Orbit 2014;33(2):81‐90. [DOI] [PubMed] [Google Scholar]
Hull 2013
- Hull S, Lalchan SA, Olver JM. Success rates in powered endonasal revision surgery for failed dacryocystorhinostomy in a tertiary referral center. Ophthalmic Plastic and Reconstructive Surgery 2013;29(4):267‐71. [DOI] [PubMed] [Google Scholar]
Janssen 2001
- Janssen AG, Mansour K, Bos JJ, Castelijns JA. Diameter of the bony lacrimal canal: normal values and values related to nasolacrimal duct obstruction: assessment with CT. AJNR: American Journal of Neuroradiology 2001;22(5):845‐50. [PMC free article] [PubMed] [Google Scholar]
Kapasi 2009
- Kapasi MS, Birt CM. The efficacy of 5‐fluorouracil bleb needling performed 1 year or more posttrabeculectomy: a retrospective study. Journal of Glaucoma 2009;18(2):144‐8. [DOI] [PubMed] [Google Scholar]
Mudhol 2013b
- Mudhol RR, Zingade ND, Mudhol RS, Harugop AS, Das AT. Prospective randomized comparison of mitomycin C application in endoscopic and external dacryocystorhinostomy. Indian Journal of Otolaryngology and Head & Neck Surgery 2013;65(Suppl 2):255‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petris 2017
- Petris C, Liu D. Probing for congenital nasolacrimal duct obstruction. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD011109.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prasad 2002
- Prasad M, Ward RF, April MM, Bent JP, Froehlich P. Topical mitomycin as an adjunct to choanal atresia repair. Archives of Otolaryngology ‐ Head & Neck Surgery 2002;128(4):398‐400. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rubinfeld 1992
- Rubinfeld RS, Pfister RR, Stein RM, Foster CS, Martin NF, Stoleru S, et al. Serious complications of topical mitomycin‐C after pterygium surgery. Ophthalmology 1992;99(11):1647‐54. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shin 2015
- Shin JH, Kim YD, Woo KI, Korean Society of Ophthalmic Plastic and Reconstructive Surgery (KSOPRS). Impact of epiphora on vision‐related quality of life. BMC Ophthalmology 2015;15(6):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skuta 1992
- Skuta GL, Beeson CC, Higginbotham EJ, Lichter PR, Musch DC, Bergstrom TJ, et al. Intraoperative mitomycin versus postoperative 5‐fluorouracil in high‐risk glaucoma filtering surgery. Ophthalmology 1992;99(3):438‐44. [DOI] [PubMed] [Google Scholar]
Spielmann 2009
- Spielmann PM, Hathorn I, Ahsan F, Cain AJ, White PS. The impact of endonasal dacryocystorhinostomy (DCR) on patient health status as assessed by the Glasgow benefit inventory. Rhinology 2009;47(1):48‐50. [PubMed] [Google Scholar]
Terrin 2005
- Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. Journal of Clinical Epidemiology 2005;58(9):894‐901. [DOI] [PubMed] [Google Scholar]
Wendling 2003
- Wendling J, Marchand A, Mauviel A, Verrecchia F. 5‐fluorouracil blocks transforming growth factor‐beta‐induced alpha 2 type I collagen gene (COL1A2) expression in human fibroblasts via c‐Jun NH2‐terminal kinase/activator protein‐1 activation. Molecular Pharmacology 2003;64(3):707‐13. [DOI] [PubMed] [Google Scholar]
Wilkins 2005
- Wilkins M, Indar A, Wormald R. Intra‐operative mitomycin C for glaucoma surgery. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD002897.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woog 2007
- Woog JJ. The incidence of symptomatic acquired lacrimal outflow obstruction among residents of Olmsted County, Minnesota, 1976‐2000 (an American Ophthalmological Society thesis). Transactions of the American Ophthalmological Society 2007;105:649‐66. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Marcet 2016
- Marcet MM, Phelps PO, Cowling BJ, Selva D. Antimetabolites as an adjunct to dacryocystorhinostomy for nasolacrimal duct obstruction. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012309] [DOI] [PMC free article] [PubMed] [Google Scholar]


