ABSTRACT
Background
Observational studies have linked low vitamin D status to unfavorable cardiometabolic risk markers, but double-blinded vitamin D intervention studies in children are scarce.
Objectives
The aim was to evaluate the effect of different doses of a vitamin D supplement on cardiometabolic risk markers in young healthy Swedish children with fair and dark skin.
Methods
Cardiometabolic risk markers were analyzed as secondary outcomes of a double-blind, randomized, milk-based vitamin D intervention trial conducted during late fall and winter in 2 areas of Sweden (latitude 63°N and 55°N, respectively) in both fair- and dark-skinned 5- to 7-y-old children. During the 3-mo intervention, 206 children were randomly assigned to a daily milk-based vitamin D3 supplement of either 10 or 25 µg or placebo (2 µg; only at 55°N). Anthropometric measures, blood pressure, serum 25-hydroxyvitamin D [25(OH)D], total cholesterol, HDL cholesterol, apoA-I, apoB, and C-reactive protein (CRP) were analyzed and non–HDL cholesterol calculated at baseline and after the intervention.
Results
At baseline, serum 25(OH)D was negatively associated with systolic and diastolic blood pressure (β = −0.194; 95% CI: −0.153, −0.013; and β = −0.187; 95% CI: −0.150, −0.011, respectively). At follow-up, there was no statistically significant difference in any of the cardiometabolic markers between groups.
Conclusions
We could not confirm any effect of vitamin D supplementation on serum lipids, blood pressure, or CRP in healthy 5- to 7-y-old children. The study was registered at clinicaltrials.gov (NCT01741324).
Keywords: serum lipids, blood pressure, latitude, skin color, vitamin D supplement
Introduction
Low vitamin D status has been associated with the metabolic syndrome and with cardiovascular diseases (CVDs) in adults (1), and higher concentrations of serum 25-hydroxyvitamin D [25(OH)D] have been associated with a substantial reduction in CVD (2, 3). Cardiometabolic risk markers for disease in adulthood (e.g., unfavorable lipid profile, high blood pressure, and overweight) may originate already in early life (4–6).
Few studies, mostly cross-sectional or observational, have examined associations between serum 25(OH)D and cardiometabolic risk markers in children and adolescents (7–13). In a Danish observational study in healthy children aged 8–11 y (13), higher 25(OH)D was associated with lower diastolic blood pressure, lower total cholesterol (TC), and LDL cholesterol. Similarly, in the Canadian TARGets Kids study (11), higher 25(OH)D was associated with a more favorable lipoprotein profile—in particular, non–HDL cholesterol. However none of these results were in accordance with a recent randomized controlled trial (RCT) in healthy Danish children aged 4–8 y (14). A large intervention study on vitamin D supplements in adolescent girls in Iran resulted in improved 25(OH)D, diastolic blood pressure, LDL cholesterol, and TC, but not systolic blood pressure, triglycerides, or HDL cholesterol (15). On the contrary, neither in healthy adolescents in the United Kingdom (16) nor in obese adolescents in the United States (17) did supplementation with vitamin D affect markers of cardiometabolic risk and the Cardiovascular Risk in Young Finns Study found associations between subclinical atherosclerosis in adults and low concentrations of 25(OH)D in childhood but not in adulthood (18).
Even if some findings (11, 13, 18) point towards associations between cardiometabolic risk factors and vitamin D status, the causal relation remains unclear. One systematic review revealed a weak association between higher concentrations of 25(OH)D and a more favorable lipid profile, but those conclusions were based on both contradictory and nonsignificant associations (19). Another review suggested that low vitamin D status may be a marker of illness rather than a cause of disease. The authors emphasized that many studies had a follow-up time that was too short, as well as vitamin D supplementation that was too low dose or of too short a duration to reveal a difference and thus concluded that better-designed trials are needed to draw firm conclusions (20).
Insufficient vitamin D status in preschool children and adolescents in an area at high latitude (21, 22), and controversy about recommended intakes of vitamin D (23, 24), prompted us to perform a double-blind RCT in young Swedish children. Vitamin D supplements of 10 or 25 µg/d achieved the primary outcome of 25(OH)D concentrations ≥50 nmol/L in almost all children (25, 26). In that trial, data on serum lipids, serum C-reactive protein (CRP), blood pressure, and anthropometric measures were also collected as secondary outcomes. This gave us an opportunity to study relations between vitamin D supplementation and cardiometabolic risk markers. We hypothesized that a milk-based vitamin D3 supplement provided to Swedish children aged 5–7 y, resulting in increased 25(OH)D concentrations >50 nmol/L, might affect cardiometabolic risk markers. The objective of this article was to examine baseline associations between vitamin D status and cardiometabolic risk markers, and to evaluate the effect of milk-based vitamin D3 supplements on these markers in young Swedish children after a 3-mo intervention.
Methods
Study cohort and data collection
This trial used secondary outcomes from a double-blind, randomized, milk-based vitamin D intervention trial (registered at clinicaltrials.gov; identifier: NCT01741324) reported in detail elsewhere (25–27). Briefly, the study was conducted in the northern (Umeå, latitude 63°N) and southern (Malmö, latitude 55°N) areas of Sweden in 206 children aged 5–7 y, half with fair and half with dark skin (Figure 1). The skin color of each child was visually classified by the parents together with a research nurse as “fair skin” or “dark skin” according to Fitzpatrick (28). The children were randomized by a computer-generated scheme prepared by one of the authors and assigned by nurses to a milk-based vitamin D3 supplement of either 10 or 25 µg (both sites) or the standard fortified milk as placebo (2 µg; in Malmö only). Due to earlier vitamin D studies in Umeå, parents were expected to condition their participation on active vitamin D supplements for their child. The milk was lactose-free, low-fat (0.5 g/100 mL), ultra-high-temperature treated, in packages of 200 mL for consumption of 1 dose daily for 3 mo (90 packages). The placebo milk was the regular milk from Valio Ltd containing 2 μg/package, whereas in Sweden, low-fat milk but not full-fat milk was fortified at that time. The children drank the study milk instead of their ordinary milk once per day. Total intake of vitamin D from the study product and from the diet was calculated. Packages were labeled A, B, and C, and research staff and parents were blinded until the intervention was completed. Inclusion criteria were healthy children aged 5–7 y, with no known gastrointestinal disease or cow-milk allergy. The intervention was conducted during late fall and winter (November 2012 to March 2013), when cutaneous synthesis of vitamin D is negligible in Sweden.
FIGURE 1.
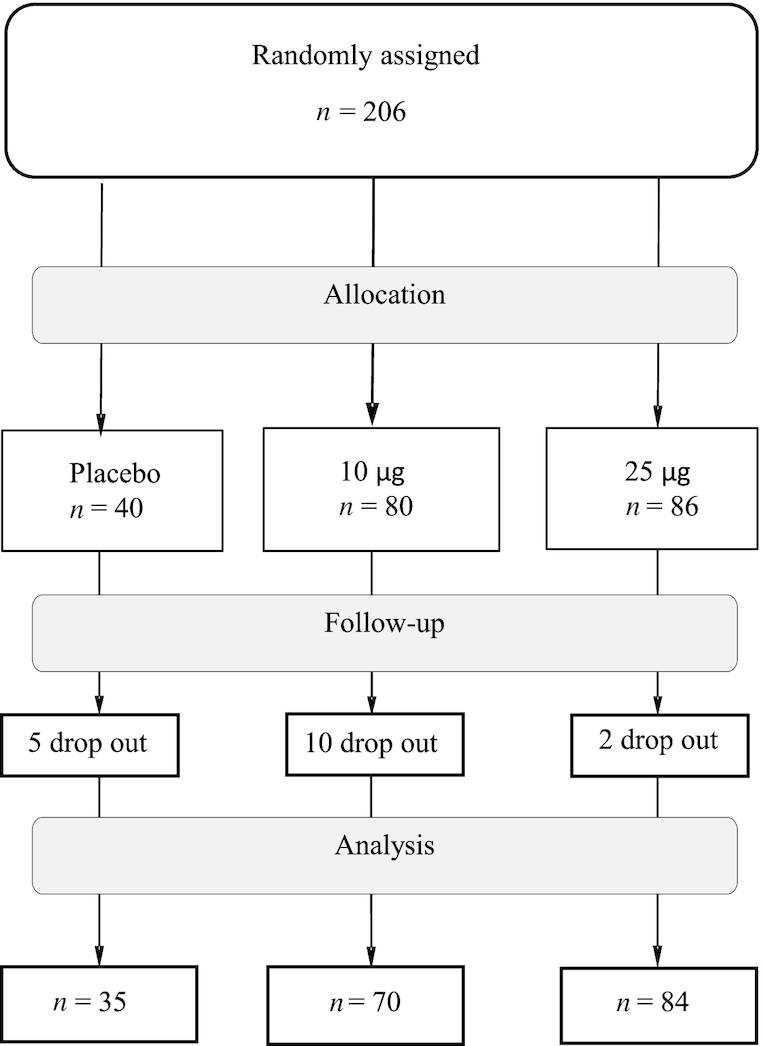
Flow diagram of Swedish 5- to 7-y-old study children, randomly assigned to milk-based vitamin D3 supplements [10 or 25 µg or placebo (2 µg)] during 3 winter months, followed from baseline until analysis.
Of the 206 included children, 189 completed the blood sampling at the follow-up visit. Sixteen of the 17 dropouts were not willing or not able to participate in the follow-up blood sampling, or disliked the study product from the study start, whereas 1 child was unable to carry the study products when travelling abroad (25, 26).
Questionnaire
A questionnaire, described earlier (25, 26), regarding the child's skin type, foreign travel to sunnier destinations, use of sunscreens, time spent outdoors, general health, and socioeconomic data, was used. The questionnaire included a validated short FFQ to describe dietary intake of vitamin D (27) at baseline and follow-up. Parents not fluent in Swedish were assisted by an interpreter at the study visits.
Anthropometric measures and biochemical analyses
Clinical examinations and blood sampling were conducted at baseline and follow-up. Height was measured to the nearest millimeter using a Seca 264 digital scale or a Seca 213 portable stadiometer (Seca) in Umeå and in Malmö, respectively. Weight was measured to the nearest 100 g using a Tanita BWB-800MA and a Seca 878 calibrated digital floor scale in Umeå and Malmö, respectively. BMI (kg/m2) was calculated and converted to BMI z score (BMIz) using the AnthroPlus-programme (available at http://www.who.int/growthref/en/) for children and adolescents aged 5–19 y based on the WHO reference dataset 2007 (29). Waist circumference was measured to the nearest millimeter with a plastic, nonstretchable measuring tape. Systolic and diastolic blood pressure was measured with an automated oscillometric sphygmomanometer (Dinamap V 100; GE Healthcare) in Umeå and with an Omron M6W automatic blood pressure monitor in Malmö.
Anesthetic cream (EMLA; AstraZeneca) was used before blood sampling. Venous blood samples were collected in a nonfasting state but ≥2 h after a meal. Samples were immediately protected from light, centrifuged, and serum and plasma were stored at −80°C until analyses.
Serum 25(OH)D2 (CV: 5–6%) and 25(OH)D3 (CV: 4–6%) were analyzed by MS on an API 4000 LC/MS/MS system (AB Sciex). Serum lipids (TC, HDL cholesterol, apoA-I, apoB) were assessed on Cobas 6000/8000 analyzers (Roche Diagnostics Ltd; CV: 3–5%), all according to the laboratory routines at the Department of Clinical Chemistry, Malmö University Hospital. Non–HDL cholesterol was calculated as TC minus HDL cholesterol.
CRP was analyzed with a commercial high-sensitivity kit (Quantikine ELISA kit; R&D Systems) at the Umeå University Hospital Pediatric Research Laboratory. The assay was performed according to the instructions of the manufacturer. The difference of absorbance at 570 nm and 450 nm was measured using the SpectraMax 340 Microplate Reader (Molecular Devices) and calculated as mean value of duplicate analyses with SoftMax Pro 2.6.1 software using a 4-parameter logistic equation. Samples with a CV (%CV) >10% were reanalyzed. A control serum was analyzed for intra- and interassay variability, resulting in 4% and 7% variability, respectively.
Statistical analyses and calculations
Statistical analyses were performed using SPSS version 24 (SPSS, Inc.) and R 3.4.2 (30). Descriptive data are presented as means (SDs), medians and IQRs, and frequency (%).
At baseline, differences between sex and between fair- and dark-skinned children were assessed by t test. Baseline relations between 25(OH)D and cardiometabolic risk markers (i.e., serum TC, HDL cholesterol, calculated non–HDL cholesterol, apoA-I, apoB, CRP, blood pressure, and BMIz) were investigated using univariate and adjusted regression analyses. In the adjusted model, sex, skin color, study site, and mothers' education were included as potential confounders.
The effect of the intervention was evaluated with respect to the following outcome variables: TC, HDL cholesterol, non–HDL cholesterol, apoA-I, apoB, CRP, and systolic and diastolic blood pressure. For each outcome, an ANCOVA model was fitted with the follow-up measurement as a dependent variable, the corresponding baseline measurement as covariate, and group (intervention or control) as an explanatory variable. The models were also adjusted for sex and study site (Malmö or Umeå), as we considered these 2 factors important predictors with the potential to increase statistical power and precision in effect size estimates. Model assumptions of equality of variance across groups and normality of residuals were assessed and verified from graphical inspections. A markedly non-normal distribution was found for CRP, and this variable was therefore logarithmically transformed, for both baseline and follow-up measurements; thereafter, model assumptions were deemed acceptable. Pairwise comparisons of model-estimated follow-up concentrations between the 3 groups were performed using Tukey's honestly significant difference test. Additionally, explorative post hoc analyses were performed when also including baseline 25(OH)D as a covariate in the ANCOVAs.
As a sensitivity analysis, analyses were also done under an intention-to-treat (ITT) principle, using multiple imputation of missing data from dropouts or other causes, with 100 replications. The analyses were conducted using the R function aregImpute from the Hmisc package. As a further explorative analysis, we included a group × skin color interaction in each model to investigate potential heterogeneity in treatment response.
Sample size calculations for the initial vitamin D intervention trial (26) was based on a previous prevalence study in Swedish children (21). Assuming a modest effect (effect size of 0.25), a total of 160 children was needed (power >87%, α = 0.05) to identify a group difference in 25(OH)D of 3.75 nmol/L. In addition, a control group with an additional 40 children was included in southern Sweden. Anticipating a 10% drop-out rate, 220 participants were needed. The level of significance was set at P ≤ 0.05.
Ethics
The study was approved by the Ethics Committee of the Medical Faculty of Umeå University (Dnr 2012-158-31M) and registered at clinicaltrials.gov (NCT01741324).
Results
Baseline characteristics
As reported in detail elsewhere (25), most children were born in Sweden, but >50% of their parents were born elsewhere. Approximately 50% of the parents had a higher educational level (≥12 y). During weekdays, nearly all children were at daycare centers, preschool, or school. In addition, during the intervention period, 5 children traveled abroad 1–2 wk, 3 from the northern and 2 from the southern study site, all of whom were dark skinned. They all used high-factor sun protection and their change in 25(OH)D did not differ compared with other children given the same supplement.
The mean ± SD dietary vitamin D intake at baseline was 5.9 ± 2.5 µg/d, with small variations between the study groups (Table 1), and 41% of the children had insufficient concentrations of serum 25(OH)D (i.e., ≤50 nmol/L), whereas deficiency, defined as concentrations <30 nmol/L, was found in 10%, particularly in those with dark skin (25).
TABLE 1.
Baseline characteristics of 5- to 7-y-old children with fair and dark skin divided by placebo and vitamin D intervention groups1
| Vitamin D supplement | |||
|---|---|---|---|
| Placebo: 2 µg | 10 µg | 25 µg | |
| n | 40 | 80 | 86 |
| Age, mo | 76.8 [72.9, 78.3] | 75.30 [70.8, 82.5] | 75.6 [70.0, 80.9] |
| Sex, boys/girls | 19 (47.5)/21 (52.5) | 42 (52.5)/38 (47.5) | 34 (39.5)/52 (60.5) |
| Skin type, fair/dark | 22 (55.0)/18 (45.0) | 41 (51.2)/39 (48.8) | 45 (52.3)/41 (47.7) |
| Weight (z score, WHO) | 0.31 [−0.46, 0.97] | 0.44 [−0.04, 1.16] | 0.50 [−0.18, 1.33] |
| Height (z score, WHO) | 0.60 [−0.12, 1.38] | 0.74 [−0.05, 1.58] | 0.68 [0.15, 1.27] |
| Waist circumference, cm | 52.0 [49.0, 55.0] | 53.0 [51.0, 55.6] | 54.0 [51.5, 57.0] |
| BMI (z score, WHO) | −0.22 [−0.79, 0.79] | 0.19 [−0.44, 0.79] | 0.34 [−0.56, 0.97] |
| Dietary vitamin D, µg/d | 5.2 ± 2.2 | 6.2 ± 2.6 | 6.0 ± 2.4 |
| Serum 25(OH)D, nmol/L | 49 [34, 65] | 57 [42, 69] | 59 [41, 71] |
| TC, mmol/L | 3.85 [3.58, 4.40] | 4.20 [3.80, 4.60] | 4.40 [4.00, 4.90] |
| Non–HDL cholesterol, mmol/L | 2.44 [2.10, 2.85] | 2.54 [2.28, 3.09] | 2.81 [2.50, 3.30] |
| HDL cholesterol, mmol/L | 1.46 [1.30, 1.61] | 1.50 [1.23, 1.77] | 1.50 [1.24, 1.80] |
| apoA-I, g/L | 1.34 [1.23, 1.45] | 1.40 [1.23, 1.53] | 1.45 [1.25, 1.59] |
| apoB, g/L | 0.67 [0.63, 0.73] | 0.68 [0.61, 0.80] | 0.75 [0.64, 0.83] |
| CRP, ng/mL | 432.5 [185.1, 830.4] | 274.3 [137.1, 851.3] | 237.8 [110.8, 938.9] |
| Systolic blood pressure, mm Hg | 106 [99, 112] | 105 [101. 110] | 106 [99, 111] |
| Diastolic blood pressure, mm Hg | 65 [56, 68] | 63 [59, 68] | 65 [59, 69] |
1Values are shown as medians [IQRs], frequency (%), and means ± SDs. BMIz, BMI z score by WHO reference dataset 2007; CRP, C-reactive protein; TC, total cholesterol; 25(OH)D, 25-hydroxyvitamin D.
Dyslipidemia, defined as TC ≥5.2 mmol/L (31), was found in 6.6% of children, and overweight and obesity, defined by Cole et al. (32), in 14.4% and 4.4% of children, respectively. Neither BMI nor waist circumference were associated with measured serum lipids, or with 25(OH)D (data not shown).
Girls had higher mean ± SD concentrations than boys of non–HDL cholesterol (2.8 ± 0.55 mmol/L vs. 2.6 ± 0.58 mmol/L; t test, P = 0.015) and apoB (0.73 ± 0.12 g/L vs. 0.69 ± 0.14 g/L; t test, P = 0.047), whereas no differences in anthropometric measures related to sex or skin color were noticed. Children with dark skin had lower mean ± SD 25(OH)D than those with fair skin (47 ± 17 nmol/L vs. 63 ± 17 nmol/L; P ≤ 0.001) and higher mean ± SD diastolic blood pressure (65 ± 9 mm Hg vs. 63 ± 7 mm Hg; P = 0.05) but with no differences in serum lipid concentrations (data not shown).
Baseline and postintervention associations with 25(OH)D
In the univariate regression analysis of baseline data, 25(OH)D was positively associated with serum lipids (i.e., TC, non–HDL cholesterol, and apoB), but negatively with CRP and diastolic blood pressure (Table 2). Adjusting for sex, skin color, study site, and mothers' education, only blood pressure remained negatively associated with 25(OH)D.
TABLE 2.
Baseline associations between serum 25(OH)D and metabolic markers in Swedish 5- to 7-y-old children assessed by linear unadjusted and adjusted regression analysis1
| Unadjusted | Adjusted2 | |||||
|---|---|---|---|---|---|---|
| β | 95% CI3 | P | β | 95% CI3 | P | |
| TC, mmol/L | 0.170 | 0.001, 0.010 | 0.017 | 0.125 | −0.001, 0.001 | 0.135 |
| Non–HDL cholesterol, mmol/L | 0.145 | 0.000, 0.009 | 0.042 | 0.062 | −0.003, 0.007 | 0.454 |
| HDL cholesterol, mmol/L | 0.074 | −0.001, 0.004 | 0.302 | 0.130 | −0.001, 0.005 | 0.122 |
| apoA-I, g/L | 0.103 | 0.000, 0.003 | 0.150 | 0.084 | −0.001, 0.003 | 0.325 |
| apoB, g/L | 0.178 | 0.000, 0.002 | 0.013 | 0.120 | 0.000, 0.003 | 0.153 |
| Systolic blood pressure, mm Hg | −0.134 | −0.116, −0.002 | 0.057 | −0.194 | −0.153, −0.013 | 0.021 |
| Diastolic blood pressure, mm Hg | −0.202 | −0.145, −0.028 | 0.004 | −0.187 | −0.150, −0.011 | 0.023 |
| Log CRP, ng/mL | −0.210 | −0.027, −0.006 | 0.003 | −0.113 | −0.022, 0.044 | 0.167 |
| BMIz | 0.037 | −0.006, 0.011 | 0.603 | −0.026 | −0.011, 0.008 | 0.754 |
| Waist circumference, cm | 0.022 | −0.032, 0.044 | 0.756 | 0.006 | −0.043, 0.046 | 0.917 |
1 n = 206. BMIz, BMI z score by WHO reference dataset 2007; Log CRP, logarithmically transformed C-reactive protein; TC, total cholesterol; 25(OH)D, 25-hydroxyvitamin D.
2All variables were adjusted for sex, skin color, study site, and mothers' education.
95% CI at baseline.
Impact of intervention
As previously reported (26), intervention with a daily vitamin D supplement of either 10 µg or 25 µg resulted in a substantial increase (∆) in 25(OH)D concentration [∆ = 11 (95% CI: 10, 12) µg and ∆ = 25 (95% CI: 21, 28) µg, respectively], and particularly among those with lower baseline concentrations, but with no change (∆ = 0.1; 95% CI: −3, 3 µg) in the placebo group. After the intervention, insufficient concentrations of 25(OH)D (≤50 nmol/L) remained in 16.4% of the children and deficiency (<30 nmol/L) remained in 2.6%, mainly in the placebo group.
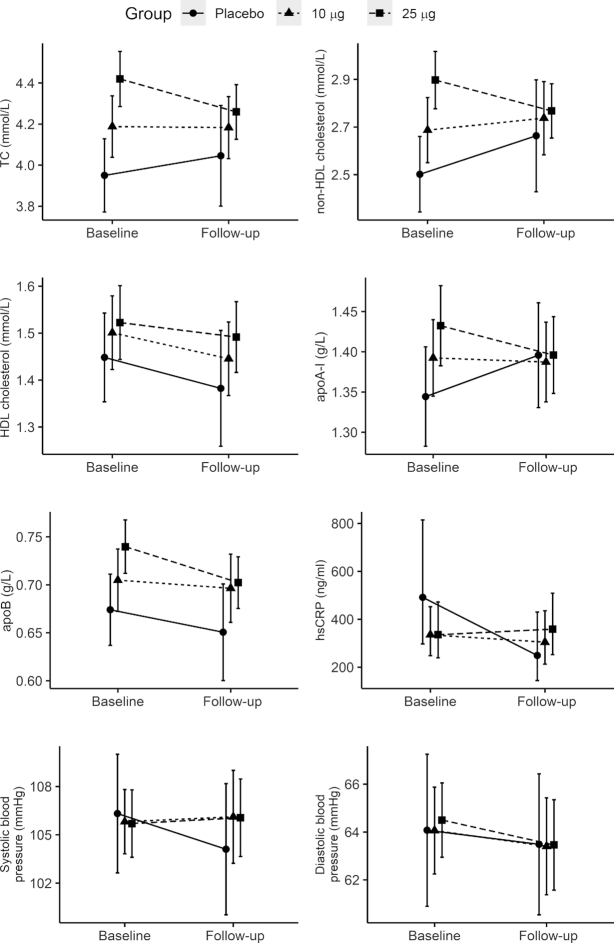
Differences in cardiometabolic markers between the intervention and placebo groups after the intervention, as estimated from the ANCOVA models, are presented in Table 3. The concentration of apoB (g/L) was higher in the 25-µg group compared with placebo, but other differences in serum lipids at follow-up were small and nonsignificant. However, when reanalyzing the ANCOVAs under the ITT principle using multiple imputation, apoB was no longer statistically significant (P = 0.41). No other changes compared with the per-protocol analysis were observed. Cardiometabolic markers (mean, 95% CI) at baseline and follow-up in all groups are shown in Figure 2. Note that the randomization resulted in substantial group differences at baseline for several outcome variables (Figure 2). Results from unadjusted analyses (i.e., not adjusted for sex and residence region) are presented in Supplemental Table 1.
TABLE 3.
Differences in serum lipid concentrations, CRP, and blood pressure after the intervention between placebo and vitamin D intervention groups (10 µg and 25 µg) in children aged 5–7 y (n = 189), adjusted for baseline measurements1
| 10 µg vitamin D vs. placebo | 25 µg vitamin D vs. placebo | 25 µg vs. 10 µg vitamin D | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Diff | 95% CI | P 2 | Diff | 95% CI | P 2 | Diff | 95% CI | P 2 | |
| TC, mmol/L | 0.15 | −0.09, 0.38 | 0.32 | 0.21 | −0.02, 0.44 | 0.08 | 0.07 | −0.12, 0.25 | 0.70 |
| Non–HDL cholesterol, mmol/L | 0.08 | −0.14, 0.30 | 0.66 | 0.10 | −0.11, 0.32 | 0.50 | 0.02 | −0.16, 0.20 | 0.96 |
| HDL cholesterol, mmol/L | 0.06 | −0.06, 0.19 | 0.47 | 0.11 | −0.02, 0.23 | 0.11 | 0.04 | −0.06, 0.15 | 0.57 |
| apoA-I, g/L | 0.00 | 0.08, 0.08 | 0.99 | 0.00 | 0.08, 0.08 | 1.00 | 0.00 | −0.06, 0.07 | 0.98 |
| apoB, g/L | 0.05 | 0.00, 0.09 | 0.06 | 0.05 | 0.01, 0.10 | 0.02 | 0.01 | −0.03, 0.05 | 0.88 |
| CRP, ng/mL | 0.23 | −0.51, 0.98 | 0.74 | 0.36 | −0.35, 1.08 | 0.45 | 0.13 | −0.44, 0.71 | 0.85 |
| Systolic blood pressure, mm Hg | 0.01 | −4.01, 4.04 | 1.00 | −0.03 | −3.93, 3.88 | 1.00 | −0.04 | −3.18, 3.10 | 1.00 |
| Diastolic blood pressure, mm Hg | 1.60 | −2.58, 5.78 | 0.64 | 1.63 | −2.43, 5.68 | 0.61 | 0.03 | −3.23, 3.29 | 1.00 |
P values and 95% CIs were adjusted for multiple pairwise comparisons between the 3 groups using Tukey's honestly significant difference test. Numbers of children were 35, 70, and 84 in the placebo, 10-µg vitamin D, and 25-µg vitamin D groups, respectively. CRP, serum C-reactive protein; Diff, difference; TC, total cholesterol.
Between-group differences assessed by ANCOVA.
FIGURE 2.
Profile plot of observed mean values of cardiometabolic markers at baseline and follow-up for placebo (n = 35), the 10-µg group (n = 70), and the 25-µg group (n = 84), with corresponding 95% CIs. Note that the randomization solely by chance resulted in spurious group differences that may appear as dose–response relations already at baseline for TC, non–HDL cholesterol, HDL cholesterol, apoA-I, and apoB. hsCRP, high-sensitivity C-reactive protein; TC, total cholesterol.
Discussion
In the present study, all differences in cardiometabolic markers at follow-up were small and statistically nonsignificant. No effect of clinical importance on cardiometabolic markers in healthy children with fair and dark skin was found after a 3-mo vitamin D supplementation period, despite a significant increase (∆) in 25(OH)D, both within and between the intervention groups from baseline to follow-up (25).
These results are in accordance with a recent RCT in 4- to 8-y-old healthy children in Denmark (14) and a similar RCT in healthy adolescents aged 14–18 y in the United Kingdom (16), but with some differences. First, the Swedish study groups comprised both fair- and dark-skinned children. Second, the increase in 25(OH)D after the vitamin D intervention was higher in Swedish than in Danish fair-skinned children and UK adolescents, and highest in those Swedish children with dark skin. Contrary to the Danish and UK studies, no decrease in 25(OH)D occurred in the Swedish placebo group. Moreover, daily dietary vitamin D intake, excluding the daily vitamin D supplement, was higher in the Swedish than in the Danish children and UK adolescents, which is probably explained by the fortification of many Swedish foods with vitamin D. Neither the present study nor the Danish or UK RCTs (14) could confirm results from observational studies in which adverse cardiometabolic risk markers have been suggested to be associated with lower concentrations of 25(OH)D (9–13).
Adjusting for baseline values in the ANCOVA was particularly important in the present analyses due to the group differences at baseline in some of the outcome variables, generated by the randomization (33) (Figure 2). All differences at follow-up were small and statistically nonsignificant, with 1 exception: apoB remained higher in the 25-µg group compared with the placebo group. However, since this analysis was based on secondary outcomes from a previously published RCT, adjustments for multiple comparisons were only performed to compensate for post hoc between-group comparisons within each outcome, and not to compensate for an increased familywise error rate due to tests performed on 8 outcomes. This calls for caution when interpreting results from single statistical tests. In fact, using ITT analysis revealed no statistically significant difference in apoB between groups, and hence observed differences in the per-protocol analysis might have been affected by unbalanced drop-out rates. apoB is an important marker of cardiovascular risk and may be a favorable alternative to LDL cholesterol (34). Vitamin D interventions in Argentinian children (35) and Saudi females resulted in decreased concentrations of apoB (36), but this could not be confirmed by us.
In our baseline analysis, 25(OH)D was negatively associated with CRP and systolic and diastolic blood pressure, consistent with a Danish observational study (13), but not associated with BMIz or waist circumference. However, at follow-up, the association between 25(OH)D and blood pressure was no longer present and we could not confirm any significant effect of vitamin D supplements on blood pressure, in accordance with a large meta-analysis (37). In addition, and contrary to our baseline results, the Danish observational study also reported a negative association between vitamin D status and BMIz, waist circumference, TC, and LDL cholesterol (13), whereas the Canadian TARGets Kids study (11) found higher 25(OH)D to be associated with lower non–HDL cholesterol in young children.
Diverse, or opposite, results may be caused by differences in concentrations of 25(OH)D and cardiometabolic markers. A recent systematic review on cardiometabolic risk score in children revealed a weak association between higher concentrations of 25(OH)D and more favorable lipid profiles in children, albeit partly based on contradictory and nonsignificant associations (19). Even though meta-analyses (20) of observational studies and randomized trials found some suggestive relations between vitamin D and CVD, hypertension, high BMI, and the metabolic syndrome, only significant associations of vitamin D with birth weight and dental caries for children could be confirmed. In addition, a systematic review of 13 observational studies and 18 trials in healthy adults could not confirm any significant effect on cardiometabolic outcomes (38). In fact, it has been hypothesized that low vitamin D status may be a consequence of ill health rather than its cause (39). Note that most meta-analyses of published RCTs are from studies in adult populations with sufficient 25(OH)D concentrations and most of them showed no beneficial effect on cardiometabolic markers. The hypothesis suggested by observational findings on adverse health outcomes of low vitamin D status could therefore not be rejected (40).
In the present study, there were 18.8% of children with overweight and obesity (i.e., comparable to young Swedish children in general) (41). Obesity is a cardiovascular risk factor in young children (42) and has been associated with low concentrations of 25(OH)D, and as mentioned, the latter has been associated with cardiometabolic risk factors (9–13). We earlier reported higher BMI to be associated with higher concentrations of 25(OH)D, which was explained by higher dietary vitamin D intake (21). In the present RCT, BMIz was not associated with 25(OH)D, in contrast to a recent Danish study in obese children and adolescents (43), and vitamin D supplementation did not affect serum lipids in accordance with an RCT in obese adolescents supplemented with a daily dose of 50 µg vitamin D (17).
Reasons for the comorbidity between obesity, cardiovascular risk markers, and 25(OH)D have been suggested to be multifactorial, including behavioral differences regarding quality of diet, extent of outdoor activities, genetics, and adipose tissue metabolism (44–46).
The strength of this study is its prospective, double-blind, randomized controlled design within a relatively large cohort, conducted during the winter season without interference of sun exposure on vitamin D status. Furthermore, our study comprised both fair- and dark-skinned children, adding an additional aspect to the impact of vitamin D supplements. In addition, a daily log for adherence to the study product was filled out and the food-frequency questionnaire was validated (27), together allowing us to calculate total vitamin D intake.
One limitation is the difference in baseline concentrations of serum lipids, with significantly higher baseline concentrations of TC in the study group randomly assigned to 25 µg, and another is that markers of insulin or glycated hemoglobin not were included. Moreover, no placebo group was recruited from Umeå. We do not know if a longer period of vitamin D supplementation would have changed our results, but we can speculate that a longer duration of supplementation may have had a larger impact on serum lipids, although studies in adults could not find any additional benefit after intervention periods of 1–5 y (40). Furthermore, as the outcomes of the present study are secondary outcomes, they were not taken into account in the sample size estimation of the power analysis.
The present study, as well as the Danish double-blind, placebo-controlled trial with similar results, both comprised healthy children with no known cardiovascular risk factors. Thus, our finding that vitamin D supplementation did not seem to affect normal concentrations of serum lipids does not exclude that additional vitamin D would be beneficial to a risk-group population of children (i.e., with the metabolic syndrome or with a high risk of CVDs, particularly if having insufficient vitamin D status at baseline). This remains to be investigated in future trials.
In conclusion, in this clinical trial, no effect of vitamin D supplementation on serum lipids, blood pressure, or CRP in healthy 5- to 7-y-old children could be confirmed despite a substantial increase in 25(OH)D concentration in the intervention groups, particularly among those with insufficient baseline vitamin D status.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to the staff in Umeå and Malmö; research nurses Carina Forslund, Birgitta Isaksson, Åsa Sundström, Margareta Bäckman in Umeå; research nurses Ulrika Lööf, and Ingrid Sandborgh in Malmö; the dietitians Ann-Kristine Sandström and Kristin Jansen in Umeå and Malmö respectively; and the interpreter Lul Ali Hassan in Malmö. We received invaluable help with handling the blood samples from the biomedical technician Carina Lagerqvist at the pediatric research laboratory at Umeå University Hospital.
The authors’ responsibilities were as follows—IÖ, TL, OH, SAS, and PKÅ: contributed to design, conception, and development of overall research plan and study oversight of the project; IÖ and PKÅ: were primary investigators at the respective sites (hands-on conduct of data collection); IÖ: analyzed data and performed statistical analysis together with TL and PL and drafted the manuscript; and all authors: reviewed and participated in writing and read and approved the final manuscript. OH is a previous member of Valio Baby Food Scientific Advisory Board. The other authors report no conflicts of interest.
Notes
Financial support was provided through the regional agreement between Umeå University and Västerbotten County Council on cooperation in the field of Medicine, Odontology, and Health (ALF); the Oskar Foundation; the Jerring Foundation; the Per Håkansson Foundation; the Fanny Ekdahl Foundation; and the Samariten Foundation. Valio Ltd (Helsinki, Finland) contributed the vitamin D–supplemented and placebo milks used for the intervention. None of the funders had any influence on the design of the study nor on the analyses, interpretation, or implementation of the data.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: BMIz, BMI z score; CVD, cardiovascular disease; CRP, C-reactive protein; ITT, intention-to-treat; RCT, randomized controlled trial; TC, total cholesterol; 25(OH)D, 25-hydroxyvitamin D.
References
- 1. Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res. 2011;50:303–12. [DOI] [PubMed] [Google Scholar]
- 2. Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala NB, Clarke A, Franco OH. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 2010;65:225–36. [DOI] [PubMed] [Google Scholar]
- 3. Khaw KT, Luben R, Wareham N. Serum 25-hydroxyvitamin D, mortality, and incident cardiovascular disease, respiratory disease, cancers, and fractures: a 13-y prospective population study. Am J Clin Nutr. 2014;100:1361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Öhlund I, Hernell O, Hörnell A, Lind T. Serum lipid and apolipoprotein levels in 4-year-old children are associated with parental levels and track over time. Eur J Clin Nutr. 2011;65:463–9. [DOI] [PubMed] [Google Scholar]
- 5. Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, Lehtimaki T, Akerblom HK, Pietikainen M, Laitinen T. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159:584–90. [DOI] [PubMed] [Google Scholar]
- 6. Berenson GS. Prevention of heart disease beginning in childhood through comprehensive school health: the Heart Smart Program. Prev Med. 1993;22(4):507–12. [DOI] [PubMed] [Google Scholar]
- 7. Ekbom K, Marcus C. Vitamin D deficiency is associated with prediabetes in obese Swedish children. Acta Paediatr. 2016;105(10):1192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee SH, Kim SM, Park HS, Choi KM, Cho GJ, Ko BJ, Kim JH. Serum 25-hydroxyvitamin D levels, obesity and the metabolic syndrome among Korean children. Nutr Metab Cardiovasc Dis. 2013;23:785–91. [DOI] [PubMed] [Google Scholar]
- 9. Pacifico L, Anania C, Osborn JF, Ferraro F, Bonci E, Olivero E, Chiesa C. Low 25(OH)D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur J Endocrinol. 2011;165:603–11. [DOI] [PubMed] [Google Scholar]
- 10. Ganji V, Zhang X, Shaikh N, Tangpricha V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001–2006. Am J Clin Nutr. 2011;94:225–33. [DOI] [PubMed] [Google Scholar]
- 11. Birken CS, Lebovic G, Anderson LN, McCrindle BW, Mamdani M, Kandasamy S, Khovratovich M, Parkin PC, Maguire JL,TARGet Kids! collaboration. Association between vitamin D and circulating lipids in early childhood. PLoS One. 2015;10:e0131938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnberg K, Ostergard M, Madsen AL, Krarup H, Michaelsen KF, Molgaard C. Associations between vitamin D status in infants and blood lipids, body mass index and waist circumference. Acta Paediatr. 2011;100:1244–8. [DOI] [PubMed] [Google Scholar]
- 13. Petersen RA, Dalskov SM, Sorensen LB, Hjorth MF, Andersen R, Tetens I, Krarup H, Ritz C, Astrup A, Michaelsen KF. Vitamin D status is associated with cardiometabolic markers in 8-11-year-old children, independently of body fat and physical activity. Br J Nutr. 2015;114:1647–55. [DOI] [PubMed] [Google Scholar]
- 14. Hauger H, Molgaard C, Mortensen C, Ritz C, Frokiaer H, Smith TJ, Hart K, Lanham-New SA, Damsgaard CT. Winter cholecalciferol supplementation at 55 degrees N has no effect on markers of cardiometabolic risk in healthy children aged 4–8 years. J Nutr. 2018;148:1261–8. [DOI] [PubMed] [Google Scholar]
- 15. Khayyatzadeh SS, Mirmoosavi SJ, Fazeli M, Abasalti Z, Avan A, Javandoost A, Rahmani F, Tayefi M, Hanachi P, Ferns GA. High-dose vitamin D supplementation is associated with an improvement in several cardio-metabolic risk factors in adolescent girls: a nine-week follow-up study. Ann Clin Biochem. 2018;55:227–35. [DOI] [PubMed] [Google Scholar]
- 16. Smith TJ, Tripkovic L, Hauger H, Damsgaard CT, Molgaard C, Lanham-New SA, Hart KH. Winter cholecalciferol supplementation at 51 degrees N has no effect on markers of cardiometabolic risk in healthy adolescents aged 14–18 years. J Nutr. 2018;148:1269–75. [DOI] [PubMed] [Google Scholar]
- 17. Nader NS, Aguirre Castaneda R, Wallace J, Singh R, Weaver A, Kumar S. Effect of vitamin D3 supplementation on serum 25(OH)D, lipids and markers of insulin resistance in obese adolescents: a prospective, randomized, placebo-controlled pilot trial. Horm Res Paediatr. 2014;82:107–12. [DOI] [PubMed] [Google Scholar]
- 18. Juonala M, Voipio A, Pahkala K, Viikari JS, Mikkila V, Kahonen M, Hutri-Kahonen N, Jula A, Burgner D, Sabin MA. Childhood 25-OH vitamin D levels and carotid intima-media thickness in adulthood: the cardiovascular risk in young Finns study. J Clin Endocrinol Metab. 2015;100:1469–76. [DOI] [PubMed] [Google Scholar]
- 19. Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89. [DOI] [PubMed] [Google Scholar]
- 20. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Öhlund I, Silfverdal SA, Hernell O, Lind T. Serum 25-hydroxyvitamin D levels in preschool-age children in northern Sweden are inadequate after summer and diminish further during winter. J Pediatr Gastroenterol Nutr. 2013;56:551–5. [DOI] [PubMed] [Google Scholar]
- 22. Persson K, Öhlund I, Nordström L, Winberg A, Rönmark E, West CE. Vitamin D deficiency at the Arctic Circle—a study in food-allergic adolescents and controls. Acta Paediatr. 2013;102:644–9. [DOI] [PubMed] [Google Scholar]
- 23. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G. The 2011 report on Dietary Reference Intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holick MF. The D-batable Institute of Medicine report: a D-lightful perspective. Endocr Pract. 2011;17:143–9. [DOI] [PubMed] [Google Scholar]
- 25. Åkeson PK, Lind T, Hernell O, Silfverdal SA, Öhlund I. Serum vitamin D depends less on latitude than on skin color and dietary intake during early winter in Northern Europe. J Pediatr Gastroenterol Nutr. 2016;62:643–9. [DOI] [PubMed] [Google Scholar]
- 26. Öhlund I, Lind T, Hernell O, Silfverdal SA, Karlsland Åkeson P, Increased vitamin D intake differentiated according to skin color is needed to meet requirements in young Swedish children during winter: a double-blind randomized clinical trial. Am J Clin Nutr. 2017;106:105–12. [DOI] [PubMed] [Google Scholar]
- 27. Söderberg L, Lind T, Karlsland Akeson P, Sandstrom AK, Hernell O, Ohlund I. A validation study of an interviewer-administered short food frequency questionnaire in assessing dietary vitamin D and calcium intake in Swedish children. Nutrients. 2017;9, doi: 10.3390/nu9070682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–71. [DOI] [PubMed] [Google Scholar]
- 29. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: Foundation for Statistical Computing; 2017. Available from: https://www.R-project.org/. [Google Scholar]
- 31. Nielsen TRH, Lausten-Thomsen U, Fonvig CE, Bojsoe C, Pedersen L, Bratholm PS, Hansen T, Pedersen O, Holm JC. Dyslipidemia and reference values for fasting plasma lipid concentrations in Danish/North-European white children and adolescents. BMC Pediatr. 2017;17:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Breukelen GJ. ANCOVA versus change from baseline: more power in randomized studies, more bias in nonrando. mized studies [corrected]. J Clin Epidemiol. 2006;59:920–5., Erratum in J Clin Epidemiol 2006 Dec;59:1334. [DOI] [PubMed] [Google Scholar]
- 34. Zhu YM, Verma S, Fung M, McQueen MJ, Anderson TJ, Lonn EM. Association of apolipoproteins B and A-1 with markers of vascular health or cardiovascular events. Can J Cardiol. 2017;33:1305–11. [DOI] [PubMed] [Google Scholar]
- 35. Hirschler V, Maccallini G, Tamborenea MI, Gonzalez C, Sanchez M, Molinari C, San Antonio de Los Cobres Study Group, Castano L, Colque G, Hidalgo M. Improvement in lipid profile after vitamin D supplementation in indigenous argentine school children. Cardiovasc Hematol Agents Med Chem. 2014;12:42–9. [PubMed] [Google Scholar]
- 36. Al-Daghri NM, Wani K, Sabico S, Garbis SD, Chrousos GP, Amer OE, Ansari MGA, Al-Saleh Y, Aljohani NJ, Al-Attas OS. Sex-specific expression of apolipoprotein levels following replenishment of vitamin D. J Steroid Biochem Mol Biol. 2018;180:129–36. [DOI] [PubMed] [Google Scholar]
- 37. Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, Alvarez JA, Boxer RS, Dalbeni A, Gepner AD. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175(5):745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kelishadi R, Farajzadegan Z, Bahreynian M. Association between vitamin D status and lipid profile in children and adolescents: a systematic review and meta-analysis. Int J Food Sci Nutr. 2014;65:404–10. [DOI] [PubMed] [Google Scholar]
- 40. Rejnmark L, Bislev LS, Cashman KD, Eiriksdottir G, Gaksch M, Grubler M, Grimnes G, Gudnason V, Lips SP. Non-skeletal health effects of vitamin D supplementation: a systematic review on findings from meta-analyses summarizing trial data. PLoS One. 2017;12:e0180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bergström E, Blomquist HK. Is the prevalence of overweight and obesity declining among 4-year-old Swedish children?. Acta Paediatr. 2009;98:1956–8. [DOI] [PubMed] [Google Scholar]
- 42. Kamel M, Smith BT, Wahi G, Carsley S, Birken CS, Anderson LN. Continuous cardiometabolic risk score definitions in early childhood: a scoping review. Obes Rev. 2018;19:1688–99. [DOI] [PubMed] [Google Scholar]
- 43. Plesner JL, Dahl M, Fonvig CE, Nielsen TRH, Kloppenborg JT, Pedersen O, Hansen T, Holm JC. Obesity is associated with vitamin D deficiency in Danish children and adolescents. J Pediatr Endocrinol Metab. 2018;31:53–61. [DOI] [PubMed] [Google Scholar]
- 44. Mark S, Lambert M, Delvin EE, O'Loughlin J, Tremblay A, Gray-Donald K. Higher vitamin D intake is needed to achieve serum 25(OH)D levels greater than 50 nmol/l in Quebec youth at high risk of obesity. Eur J Clin Nutr. 2011;65:486–92. [DOI] [PubMed] [Google Scholar]
- 45. Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66:S153–64. [DOI] [PubMed] [Google Scholar]
- 46. Mortensen C, Molgaard C, Hauger H, Kristensen M, Damsgaard CT. Winter vitamin D3 supplementation does not increase muscle strength, but modulates the IGF-axis in young children. Eur J Nutr. 2019;58:1183–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.