Abstract
Di(2-ethylhexyl) phthalate (DEHP) is a known endocrine disruptor and diisononyl phthalate (DiNP) is a common DEHP replacement chemical. However, little is known about late-life consequences due to DEHP or DiNP exposure during adulthood. Thus, this study tested the hypothesis that adult exposure to DEHP or DiNP affects female reproductive parameters during late-life in female mice. Female CD-1 mice (age 39–40 days) were dosed with either vehicle control, DEHP (20 μg/kg/day-200 mg/kg/day), or DiNP (20 μg/kg/day-200 mg/kg/day) for 10 days and breeding trials were conducted at 12 and 15 months post-dosing. Further, ovaries and sera were collected at 12, 15, and 18 months post-dosing. DEHP and DiNP disrupted estrous cyclicity, increased pregnancy loss, decreased fertility, altered the sex ratio of pups, altered ovarian follicle populations, and disrupted hormone levels. Collectively, these data show that short-term exposure to DEHP or DiNP during adulthood has long-term consequences in late-life.
Keywords: DEHP, DiNP, reproductive aging, ovary, hormones, fertility, cyclicity
INTRODUCTION
Female mammals are born with a finite number of oocytes to use throughout their reproductive lives [1]. Once this reserve of oocytes has been depleted, the female enters reproductive senescence. During years of fertility, females undergo reproductive cycles in which species-specific numbers of oocytes are ovulated for fertilization in coordination with a consistent and predictable fluctuation in hormones. The primary hormones that take on a cyclic nature within females are the sex steroid hormones estradiol, progesterone, and testosterone as well as peptide hormones such as follicle stimulating hormone (FSH), luteinizing hormone (LH), and inhibin A and B [2, 3]. Because the sex steroid hormones are primarily produced by structures only present in actively cycling females, namely the antral follicle and the corpus luteum (CL), high levels of sex steroid hormones are characteristic of fertile and actively cycling females. Additionally, some sex steroid hormones share antagonistic relationships with the peptide hormones [3]. Thus, as antral follicles, corpora lutea, and subsequently, sex steroid hormones decline with age, levels of some peptide hormones such as FSH increase as the negative feedback exerted by the sex steroid hormones is alleviated [4]. Additionally, inhibin B levels decline as the number of small growing follicles that are responsible for its production drop. Thus, the hormone profile of a normal, cycling female will have higher levels of sex steroid hormones and inhibin B and lower levels of FSH, and inverse characteristics will be present for acyclic females who have entered reproductive senescence. Taken together, data on levels of multiple hormones can be used as a metric for assessing female reproductive health and reproductive aging [4].
To prevent early entry into reproductive senescence, proper regulation of the activation and use of the ovarian reserve is imperative. Aberrant regulation can lead to early depletion of the ovarian reserve and, subsequently, early reproductive senescence [4]. In women, early menopause is associated with an increased risk of osteoporosis, stroke, cardiovascular disease, and increased mortality [5–9]. Concerningly, some studies have found that some environmental contaminants can lead to dysregulation of the ovarian reserve, and some studies have found markers of reproductive aging are altered by these chemicals as well [10–12]. One class of chemicals that has been shown to have endocrine disrupting capabilities is phthalates [13, 14].
Phthalates are a family of chemicals used widely for their plasticizing and stabilizing properties. Lower molecular weight phthalates can commonly be found in personal care products because they are used to maintain and stabilize fragrances and colors [15, 16]. Higher molecular weight phthalates are more often used for their plasticizing capabilities, resulting in their high use in plastic products such as polyvinyl chloride (PVC) goods and other consumer goods such as clothing and food packaging [16].
Di(2-ethylhexyl) phthalate (DEHP) is a high molecular weight phthalate and is often found in products such as plastic children’s toys, upholstery, construction materials such as floor tiles, and medical equipment such as blood bags and tubing [17]. Millions of pounds of DEHP are produced every year within the U.S., and this vast production and use has led to DEHP becoming a top environmental contaminant that humans are exposed to daily [17, 18]. The average person is estimated to be exposed to between 3–30 μg/kg/day [17], and some populations can experience even higher levels of exposure to DEHP due to occupation or medical exposure [19, 20]. This is a concern because DEHP has endocrine disrupting capabilities and has been associated with a variety of reproductive disorders in women in addition to affecting oocyte development, ovulation, and steroidogenesis [13, 14]. However, few studies have investigated how exposure to DEHP during adulthood affects reproductive aging in females.
Another phthalate of concern is diisononyl phthalate (DiNP), a phthalate often used as a DEHP replacement because it is thought to be less anti-androgenic than DEHP. Because of the increased use of DiNP as a DEHP replacement chemical, a steep increase in the levels of DiNP metabolites has been detected in human urine samples from the National Health and Nutrition Examination Survey (NHANES) [21]. This is concerning because of a paucity of information on the effects of DiNP on reproductive aging in females, especially in the context of exposure during adulthood. Further, studies from our own research group have found similar reproductive toxicity when comparing DEHP to DiNP [22]. Subsequently, this begs the question of whether DiNP is an adequate replacement for DEHP if the goal of substitution is decreased toxicity and increased safety for the consumer.
This study aims to fill the gap in knowledge concerning the effects of exposure to DEHP or DiNP during adulthood on reproductive aging in the female mouse. Previously, our laboratory has shown that 10 days of exposure during adulthood to either DEHP or DiNP is adequate to disrupt aspects of female fertility up to 9 months following completion of dosing [11, 22]. However, data on how DEHP and DiNP affect parameters of female fertility in late-life are still scarce. Thus, this study tested the hypothesis that short-term exposure to DEHP and/or DiNP during adulthood alters markers of reproductive aging, including cyclicity, ovarian hormone populations, overall fertility, and circulating hormone levels.
METHODS
Chemicals
DEHP and DiNP were purchased from Sigma-Aldrich (St. Louis, MO). Corn oil (vehicle control) was purchased from MP Biomedicals (Solon, OH). Dosing stock solutions were made via serial dilutions from the highest concentration dose. Stock solutions were stored at room temperature away from exposure to direct light for up to a month. If a month elapsed from the time the stock solutions were made, then a new batch of stock solutions was made.
Animals and Dosing
Female CD-1 mice were purchased from Charles River (Wilmington, MA) and housed in the animal facility at the College of Veterinary Medicine at the University of Illinois at Urbana-Champaign (Urbana, IL). Animals were allowed to acclimate to the facilities for a minimum of 5 days before the dosing period began. Facility temperature was maintained at 21.1 ± 2.2°C and humidity was maintained at 50 ± 20%. Mice were given ad libitum access to chow (Teklad Rodent Diet 8604) and reverse osmosis-treated water. Light-dark cycles were 12 hours light and 12 hours dark. All animal handling procedures were approved by the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee (Protocol No.: 17079).
Mice were dosed at age 39–40 days (young adulthood) orally via insertion of a pipette tip into the mouth. Treatments consisted of vehicle control (corn oil), DEHP (20 μg/kg/day, 200 μg/kg/day, 20 mg/kg/day, 200 mg/kg/day), or DiNP (20 μg/kg/day, 100 μg/kg/day, 20 mg/kg/day, 200 mg/kg/day) for 10 days. The 20 μg/kg/day and 200 μg/kg/day doses of DEHP were chosen because they represent average daily exposure and occupational exposure, respectively [17, 23]. The 20 and 200 mg/kg/day doses of DEHP were chosen because they have been used in previous studies and also allow investigation of a wide range of doses for DEHP [11, 24]. The 20 μg/kg/day DiNP dose falls within the estimated range of occupational exposure to DiNP [25]. The 100 μg/kg/day DiNP dose falls within the range of estimated daily exposure for infants who are exposed through chewing on plastic toys [26]. Lastly, the 20 and 200 mg/kg/day doses for DiNP were chosen because they are the same doses used for DEHP, thus allowing for direct comparison of the toxicity of DEHP and DiNP at these doses. To determine dose volume, mice were weighed daily immediately before dosing. Mice were housed 3 to a cage with all mice within one cage receiving the same dose to minimize contamination.
Experimental Design
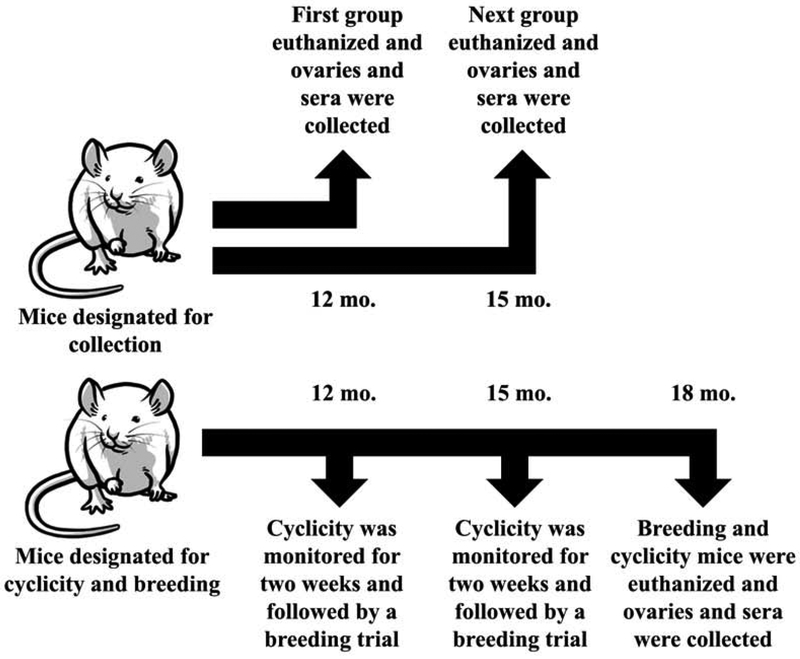
To determine how DEHP and DiNP affect fertility parameters, hormones, and ovarian follicle populations in late-life, groups of animals were dosed and data were taken at pre-determined time points (Fig. 1). One group of mice was designated for cyclicity monitoring and breeding, and cyclicity was monitored at time points 12 and 15 months post-dosing. Breeding trials for this same group of mice commenced following completion of the cyclicity monitoring period. These mice were euthanized via CO2 asphyxiation during diestrus at 18 months post-dosing, and blood and ovaries were collected.
Figure 1.
Experimental Design. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). Following completion of dosing, mice were designated either for collection or for cyclicity and breeding. One group of mice designated for collection waited 12 months following completion of dosing before being euthanized for collection of ovaries and sera. An additional group of mice designated for collection waited 15 months following completion of dosing before being euthanized for collection of ovaries and sera. Mice that were designated for cyclicity and breeding waited 12 months following completion of dosing before they were monitored for cyclicity followed by a breeding trial. These same mice then waited an additional 3 months (totaling 15 months post-dosing) before they were again monitored for cyclicity and underwent another breeding trial. These same mice then waited an additional 3 months (totaling 18 months post-dosing) before they were euthanized for collection of ovaries and sera.
Additional groups of mice that were not used for cyclicity monitoring or breeding were dosed and euthanized via CO2 during diestrus at 12 months post-dosing and 15 months post-dosing, and blood and ovaries were collected. Collectively, this generated breeding and cyclicity data for 12 and 15 months post-dosing as well as hormone data and ovarian follicle population data for 12, 15, and 18 months post-dosing.
Estrous Cyclicity Monitoring
At time points 12 and 15 months post-dosing, mice were vaginally lavaged via pipette with 90μL of 1X phosphate-buffered saline for 14 consecutive days, and the lavage samples were analyzed under a light microscope to assess stage of the estrous cycle using previously defined criteria [27]. Lavaging was conducted at 2 hours after the beginning of the light cycle. Lavage samples were analyzed no later than 3 days following collection, and samples were kept at 4 °C until analysis. Mice were housed in groups of 3 animals per cage. For statistical analysis, the metestrous and diestrous stages were combined into a single category because they are similar in mice [27]. Percent times spent in proestrus, estrus, and metestrus/diestrus were calculated by dividing the number of days spent in each stage of the cycle by 14 and multiplying by 100.
Histological Evaluation of Ovarian Tissues
Following euthanasia, ovaries were removed and fixed overnight in Dietrich’s solution (250 mL 40% formaldehyde, 712.5 mL 100% ethanol, 1537.5 mL water, 50 mL glacial acetic acid). Following fixation, ovaries were transferred to 70% ethanol and then embedded in paraffin wax. Ovaries were sectioned serially on a microtome at 8 μm in width and mounted onto microscope slides. Slides were baked overnight at 50 °C and were stained the following day with hematoxylin and eosin. Every tenth section of the ovary was used to assess follicle populations as described previously [10]. In addition to normal follicle types, the percent of unhealthy follicle types, including abnormal and atretic follicles were assessed. Abnormal follicles were designated as follicles with either a fragmented oocyte, a multinucleated oocyte, or multiple oocytes. The nucleus must have been present in all follicle types to be counted to avoid double counting between sections, except for primordial and primary follicles which are too small to span more than 10 sections. To blind counters to treatment groups and avoid bias, ovaries were given a unique histological ID with no relation to treatment group. Data that are reported as follicle numbers represent unaltered, raw number of follicles in each category. Data that are reported as percent of follicles were determined by dividing the raw number of follicles in each category by the total number of follicles within the ovary and multiplying by 100 to achieve a percentage. The total number of follicles was determined by summing all follicle types together, including unhealthy follicles.
Breeding and Fertility Indices
Following completion of the cyclicity monitoring periods, females were paired with an untreated CD-1 male mouse (age 7 weeks) in a new cage. Breeding pairs were kept in a harem fashion with 2 females and a single male. Following introduction of the male, females were checked every morning and afternoon for the presence of a copulatory plug, which indicated successful mating with the male. Females remained housed with the male until a copulatory plug was observed or if 14 days had passed. If a copulatory plug was noted or the female had been with the male for 14 days, the female was removed and single-housed in a new cage. To calculate time to mating, day one was considered the morning immediately after the introduction of the male. To calculate gestation length, the day the copulatory plug was noted was designated gestational day 0.5. Females were weighed twice a week starting from the introduction of the male until the litter was born. If a female gained 4 g or more in weight, she was considered pregnant. Around the time of litter bearing, females were checked in the morning and afternoon for presence of pups. Live pups on postnatal day (PND) 0 were weighed together and the weights were averaged, and live and deceased pups were counted and sexed, if possible. Percent of female pups was measured by dividing the number of female pups by the total number of pups within the litter and multiplying by 100. If sex or number of pups could not be determined due to cannibalism by the dam, those data were not used in the statistical analysis.
Breeding trial indices were used to assess fertility success at multiple stages of the breeding trial. The fertility index measured the percent of females who successfully achieved pregnancy from a single, confirmed mating by dividing the number of pregnant females by the number of females with a copulatory plug and multiplying by 100. The gestational index measured the percent of females who carried their pregnancy to term and gave birth to pups by dividing the number of females who gave birth by the number of females that achieved pregnancy and multiplying by 100. The percent of females who gave birth was calculated by dividing the number of females who gave birth to pups by the total number of females within the treatment group and multiplying by 100.
Steroid and Peptide Hormone Assays
Groups of animals were euthanized via CO2 asphyxiation at different periods following completion of dosing; 12 months post-dosing, 15 months post-dosing, and 18 months post-dosing. All animals were euthanized in the diestrous stage of the estrous cycle. Immediately after euthanasia, blood was collected in a heparinized needle and allowed to clot in an Eppendorf® tube at room temperature for at least 15 minutes. Blood samples were then put on ice for at least 15 minutes. Following 15 minutes on ice, samples were centrifuged in a 4 °C room at 14,000 RPM for 15 minutes. Sera were collected and stored at −80 °C until use in enzyme-linked immunosorbent assays (ELISAs) or radioimmunoassays. ELISA kits for testosterone, progesterone, and estradiol were purchased from DRG®. The limits of detection (LODs) for testosterone, progesterone, and estradiol were 0.083 ng/mL, 0.045 ng/mL, and 10.60 pg/mL, respectively. The inter- and intra-assay %CVs were ≤ 9.94 and ≤ 4.16, ≤ 9.96 and ≤ 6.99, and ≤ 14.91 and ≤ 9.23 for testosterone, progesterone, and estradiol, respectively. Lypocheck® from Bio-Rad Laboratories was used as a control with known values in all ELISAs in this study. Further, high and low controls present in estradiol kits purchased from DRG® were run in every estradiol ELISA to provide additional quality control of estradiol results. If control values were outside of the designated acceptable range, samples were assayed again using a new kit. Aliquots of sera were shipped to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. The facility used radioimmunoassays to measure levels of FSH and inhibin B. The LODs for FSH and inhibin B were 1.6 ng/mL and 35 pg/mL, respectively. Intra- and inter-assay %CVs for FSH were 7.2 and 8.5, respectively, and intra- and inter-assay %CVs for inhibin B were 1.8 and 6.6, respectively (https://med.virginia.edu/research-in-reproduction/wp-content/uploads/sites/311/2019/04/2019-INTRA-INTER-ASSAY-CVs_030419.pdf). Samples with values below the limit of detection (LOD) were statistically analyzed using the LOD value specific for each assay divided by the square root of 2.
Statistical Analysis
Data that were normally distributed were assessed for outliers using Grubb’s Test. If outliers were detected, they were not used in the following analysis, and all data presented do not include outliers. Removal of outliers was rare and only occurred for 7 data points throughout the data presented in this study. In two cases, statistical outcome was affected by the removal of outliers. Inclusion of the outlier in the 100 μg/kg/day DiNP treatment group for percent of antral follicles at 15 months post-dosing results in p = 0.069. Inclusion of the outlier in the 200 mg/kg/day DiNP treatment group for percent antral follicles at 18 months post-dosing results in p >0.10. Data that were normally distributed and met the assumption of homogeneity of variance were analyzed via one-way analysis of variance (ANOVA). Dunnett’s 2-sided test was used post hoc. If data were percentages or did not meet the assumption of normality and/or the assumption of homogeneity of variance, the Mann-Whitney U test was used. Statistical significance was set at p ≤ 0.05.
RESULTS
Effects of DEHP and DiNP on Estrous Cyclicity
12 months post-dosing
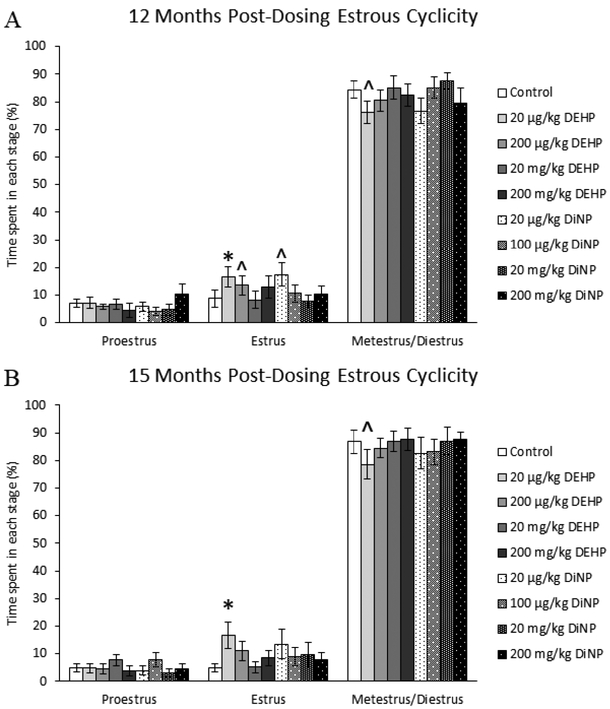
At 12 months post-dosing, 20 μg/kg/day DEHP-treated mice spent significantly more time in estrus (Fig. 2A, n = 9–22 mice/group, p ≤ 0.05) and borderline decreased time in metestrus/diestrus (Fig. 2A, n = 9–22 mice/group, p = 0.09) when compared to control. Females treated with 20 μg/kg/day of DiNP had borderline increased time spent in estrus when compared to control (Fig. 2A, n = 9–22 mice/group, p = 0.06).
Figure 2.
Effects of DEHP and DiNP on estrous cyclicity at 12 and 15 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). Females were vaginally lavaged daily for 14 days to assess cyclicity at 12 months post-dosing (control n = 22 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 10–12 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 9–12 mice/group) and 15 months post-dosing (control n = 20 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 9–11 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 8–11 mice/group). Statistical significance (p ≤ 0.05) is denoted with an asterisk (*). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
15 months post-dosing
Following 15 months after dosing, females in the 20 μg/kg/day DEHP treatment group spent significantly more time in estrus (Fig. 2B, n = 8–20 mice/group, p ≤ 0.05) and borderline decreased time in metestrus/diestrus (Fig. 2B, n = 8–20 mice/group, p = 0.09) when compared to control. No other treatments affected estrous cyclicity when compared to control.
Effects of DEHP and DiNP on Ovarian Follicle Populations
12 months post-dosing
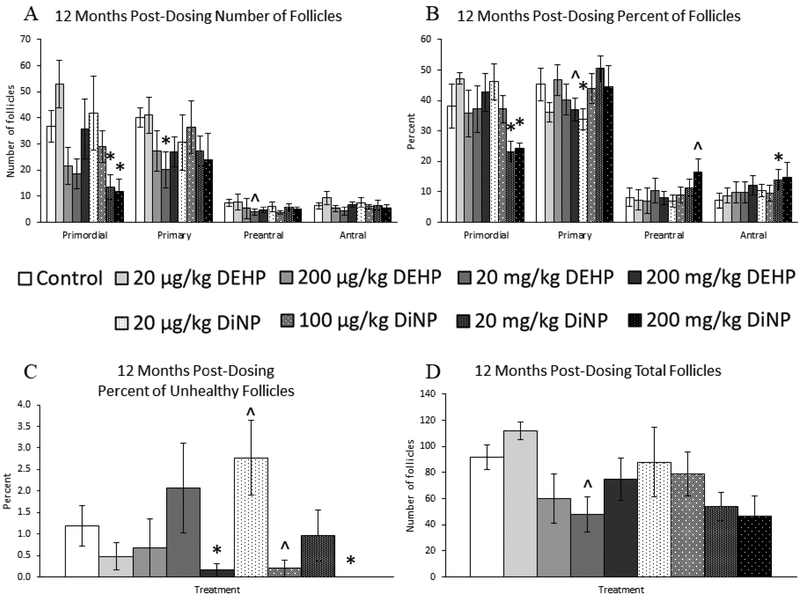
At 12 months post-dosing, treatment with 20 and 200 mg/kg/day DiNP decreased numbers of primordial follicles when compared to control (Fig. 3A, n = 3–12 mice/group, p ≤ 0.05). Further, 20 mg/kg/day of DEHP significantly decreased numbers of primary follicles (Fig. 3A, n = 3–12 mice/group, p ≤ 0.05) and borderline decreased numbers of preantral follicles when compared to control (Fig. 3A, n = 3–12 mice/group, p = 0.10). Further, 20 mg/kg/day of DEHP borderline decreased the total number of follicles within the ovary when compared to control (Fig. 3D, n = 312 mice/group, p = 0.06).
Figure 3.
Effects of DEHP and DiNP on ovarian follicle populations at 12 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 12 months following completion of the dosing period, females were euthanized and ovaries were collected and histologically evaluated for raw follicle numbers (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 3–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 5 mice/group) (A), percent of follicles (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 3–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 5 mice/group) (B), percent of unhealthy follicles (n = 3–12 mice/group) (C), and total number of follicles (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 3–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 5 mice/group) (D). Statistical significance (p ≤ 0.05) is denoted with an asterisk (*). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
When follicle populations were analyzed as percentages, treatment with 20 and 200 mg/kg/day of DiNP significantly decreased the percent of primordial follicles when compared to control (Fig. 3B, n = 3–12 mice/group, p < 0.05). Further, treatment with 200 mg/kg/day of DEHP (Fig. 3B, n = 3–12 mice/group, p = 0.07) and 20 μg/kg/day of DiNP (Fig. 3B, n = 3–12 mice/group, p ≤ 0.05) decreased the percent of primary follicles when compared to control. Additionally, 200 mg/kg/day of DiNP borderline increased the percent of preantral follicles when compared to control (Fig. 3B, n = 3–12 mice/group, p = 0.08), and 20 mg/kg/day of DiNP significantly increased the percent of antral follicles when compared to control (Fig. 3B, n = 3–12 mice/group, p ≤ 0.05).
Interestingly, we observed various effects on the percent of unhealthy follicles. Treatment with 200 mg/kg/day of DEHP (Fig. 3C, n = 3–12 mice/group, p ≤ 0.05), 100 μg/kg/day DiNP (Fig. 3C, n = 3–12 mice/group, p = 0.10), and 200 mg/kg/day DiNP (Fig. 3C, n = 3–12 mice/group, p ≤ 0.05) decreased percent of unhealthy follicles when compared to control. In contrast, 20 μg/kg/day of DiNP increased the percent of unhealthy follicles when compared to control (Fig. 3C, n = 3–12 mice/group, p = 0.10).
15 months post-dosing
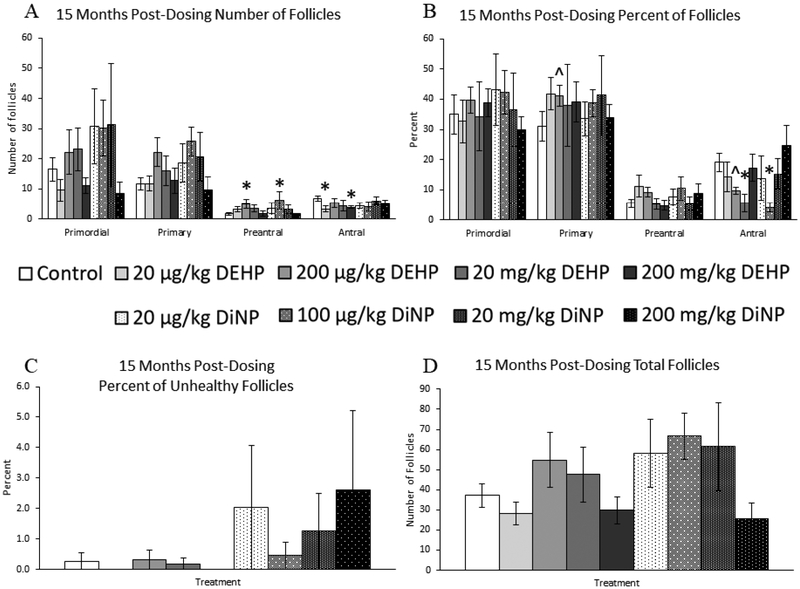
At 15 months post-dosing, 200 μg/kg/day of DEHP and 100 μg/kg/day of DiNP both increased the number of preantral follicles when compared to control (Fig. 4A, n = 4–11 mice/group, p ≤ 0.05). Additionally, 20 μg/kg/day and 200 mg/kg/day of DEHP decreased the number of antral follicles when compared to control (Fig. 4A, n = 4–11 mice/group, p ≤ 0.05). Conversely, no doses of DEHP or DiNP affected the total number of follicles when compared to control (Fig. 4D, n = 4–11 mice/group).
Figure 4.
Effects of DEHP and DiNP on ovarian follicle populations at 15 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 15 months following completion of the dosing period, females were euthanized and ovaries were collected and histologically evaluated for raw follicle numbers (control n = 11 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 4–5 mice/group) (A), percent of follicles (control n =11 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 4–5 mice/group) (B), percent of unhealthy follicles (control n = 11 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 4–5 mice/group) (C), and total number of follicles (n = 4–11 mice/group) (D). Statistical significance (p ≤ 0.05) is denoted with an asterisk (*). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
When follicles were analyzed as percentages, 200 μg/kg/day of DEHP borderline increased the percent of primary follicles when compared to control (Fig. 4B, n = 4–11 mice/group, p = 0.10) and borderline decreased the percent of antral follicles when compared to control (Fig. 4B, n = 4–11 mice/group, p = 0.10). Similarly, 20 mg/kg/day of DEHP and 100 μg/kg/day of DiNP significantly decreased the percent of antral follicles when compared to control (Fig. 4B, n = 4–11 mice/group, p < 0.05). No doses of DEHP or DiNP affected the percent of unhealthy follicles when compared to control (Fig. 4C, n = 4–11 mice/group).
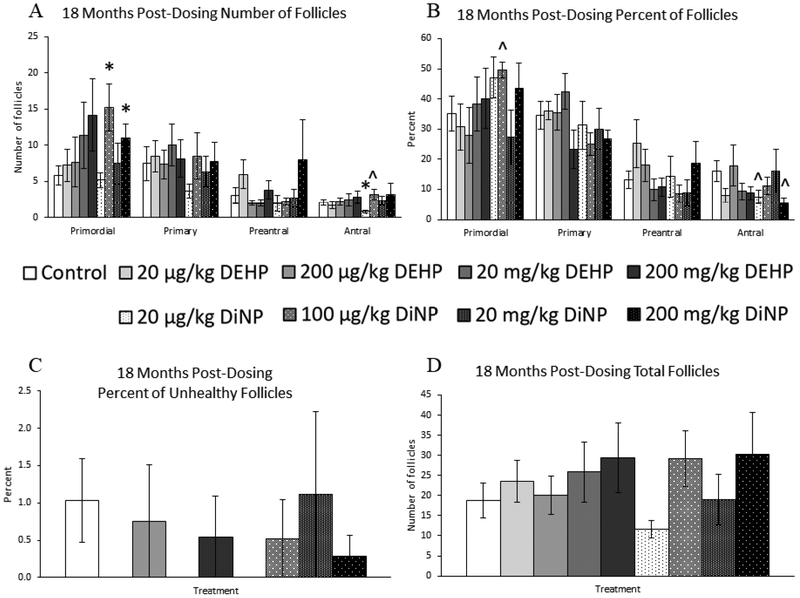
18 months post-dosing
At 18 months post-dosing, treatment with 100 μg/kg/day and 200mg/kg/day of DiNP significantly increased the number of primordial follicles when compared to control (Fig. 5A, n = 4–12 mice/group, p ≤ 0.05). Further, 20 μg/kg/day of DiNP significantly decreased the number of antral follicles (Fig. 5A, n = 4–12 mice/group, p < 0.05), and in contrast, 100 μg/kg/day of DiNP borderline increased the number of antral follicles (Fig. 5A, n – 4–12 mice/group, p = 0.10). However, no doses of DEHP or DiNP affected total follicle numbers when compared to control at this time point (Fig. 5D, n = 4–12 mice/group).
Figure 5.
Effects of DEHP and DiNP on ovarian follicle populations at 18 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 18 months following completion of the dosing period, females were euthanized and ovaries were collected and histologically evaluated for raw follicle numbers (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 4–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 5–6 mice/group) (A), percent of follicles (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 4–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 5–6 mice/group) (B), percent of unhealthy follicles (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 4–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 5–6 mice/group) (C), and total number of follicles (n = 4–12 mice/group) (D). Statistical significance (p ≤ 0.05) is denoted with an asterisk (*). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
When follicle populations were analyzed as percentages, 100 μg/kg/day of DiNP borderline increased the percent of primordial follicles when compared to control (Fig. 5B, n = 4–12 mice/group, p = 0.06). Further, both 20 μg/kg/day of DiNP (Fig. 5B, n = 4–12 mice/group, p = 0.08) and 200 mg/kg/day of DiNP (Fig. 5B, n = 4–12 mice/group, p = 0.10) decreased the percent of antral follicles when compared to control. In contrast, DEHP and DiNP did not affect the percent of unhealthy follicles when compared to control (Fig. 5C, n = 4–12 mice/group).
Effects of DEHP and DiNP on Fertility
12 months post-dosing
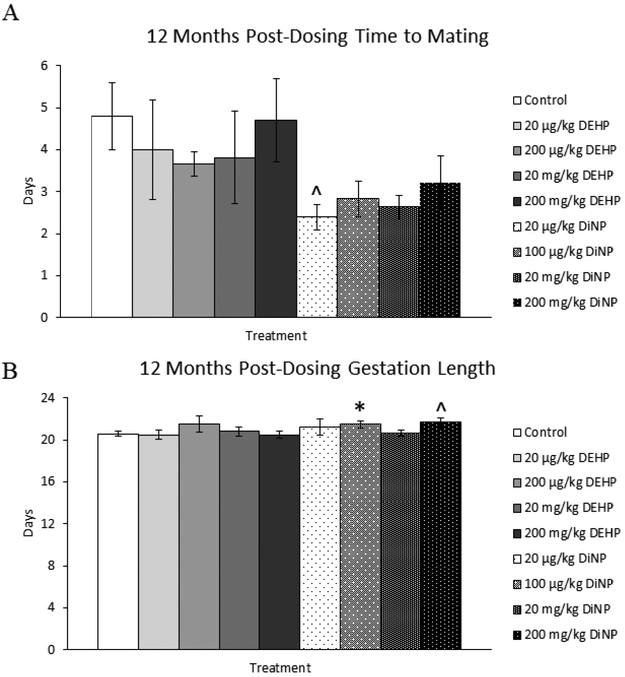
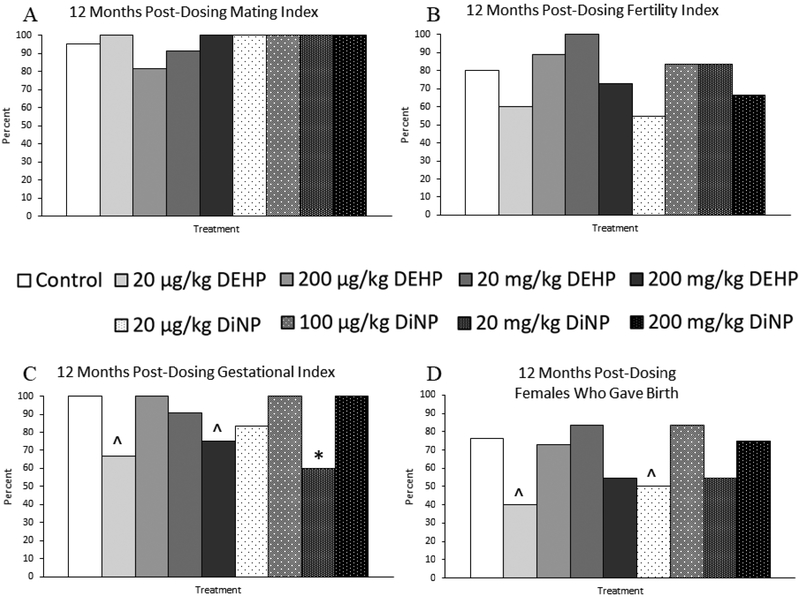
Treatment with 20 μg/kg/day of DiNP resulted in borderline decreased time to mating when compared to control (Fig. 6A, n = 9–19 mice/group, p = 0.06). Treatment with 100 μg/kg/day of DiNP (Fig. 6B, n = 4–13 mice/group, p < 0.05) and 200 mg/kg/day of DiNP (Fig. 6B, n = 4–13 mice/group, p = 0.06) increased the length of gestation when compared to control. DEHP and DiNP did not affect the mating index (Fig. 7A, n = 9–21 mice/group) or the fertility index (Fig. 7B, n = 8–20 mice/group) at 12 months post-dosing, indicating that treated females were able to mate and conceive pregnancies at rates comparable to control. However, treatment with 20 μg/kg/day (Fig. 7C, n = 6–16 mice/group, p = 0.07) and 200 mg/kg/day of DEHP (Fig. 7C, n = 6–16 mice/group, p = 0.10) borderline decreased the gestational index of females in these groups when compared to control. Additionally, females in the 20 mg/kg/day treatment group of DiNP had a significantly reduced gestational index when compared to control (Fig. 7C, n = 6–16 mice/group, p ≤ 0.05), indicating these females were less able to carry their pregnancy to term and give birth to pups when compared to control. Further, the overall ability of females to produce pups as measured by the percent of females in each group who gave birth was borderline reduced in the 20 μg/kg/day treatment group for both DEHP (Fig. 7D, n = 8–21 mice/group, p = 0.06) and DiNP (Fig. 7D, n = 8–21 mice/group, p = 0.08) when compared to control.
Figure 6.
Effects of DEHP and DiNP on time to mating and gestation length at 12 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 12 months post-dosing, females were paired with an untreated male mouse directly after completion of the estrous cyclicity monitoring period. Females were checked every morning and afternoon for the presence of a copulatory plug. If a copulatory plug was observed or if 14 days had elapsed, the female was put into a new clean cage. If a female presented with a copulatory plug the day immediately after introduction of the male, the time to mating was considered 1 day (control n = 19 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 9–11 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 9–12 mice/group) (A). The day the copulatory plug was observed was considered day 0.5 of gestation (control n = 13 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 4–10 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 4–10 mice/group) (B). Females were checked morning and afternoon starting a week before the expected delivery date of the litter. Statistical significance (p ≤ 0.05) is denoted with an asterisk (*). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
Figure 7.
Effects of DEHP and DiNP on fertility indices at 12 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 12 months following the completion of the dosing period, breeding trials were conducted by pairing females with untreated male mice. The mating index (control n = 21 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 10–12 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 9–12 mice/group) (A) is the number of females who presented with a copulatory plug divided by the total number of females in the group and multiplied by 100. The fertility index (control n = 20 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 9–11 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 8–12 mice/group) (B) is the number of females who became pregnant divided by the number of females who presented with a copulatory plug and multiplied by 100. The gestational index (control n = 16 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 6–11 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 6–10 mice/group) (C) is the number of females who gave birth divided by the number of females who became pregnant and multiplied by 100. Females who gave birth (control n = 21 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 10–12 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 8–12 mice/group) (D) is the number of females who gave birth divided by the total number of females in the group and multiplied by 100. Statistical significance (p ≤ 0.05) is denoted with an asterisk (*). Data are represented as percentages. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
15 months post-dosing
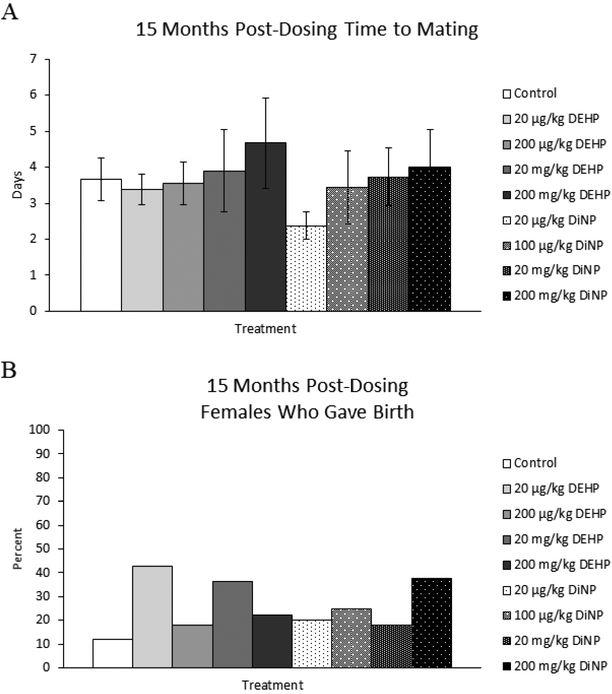
Treatment with DEHP and DiNP did not affect time to mating at 15 months post-dosing (Fig. 8A, n = 8–15 mice/group). At this time point, the natural and steep decline in fertility of CD-1 mice at this age made it difficult to collect data and perform statistical analysis for this breeding trial. However, the percent of females who produced pups was able to be statistically analyzed, and treatment with DEHP and DiNP did not affect the overall ability of females to produce pups when compared to control (Fig. 8B, n = 7–17 mice/group).
Figure 8.
Effects of DEHP and DiNP on time to mating and overall fertility at 15 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 15 months post-dosing, females were paired with an untreated male mouse directly after completion of the estrous cyclicity monitoring period. Females were checked every morning and afternoon for the presence of a copulatory plug. If a copulatory plug was observed or if 14 days had elapsed, the female was put into a new clean cage. If a female presented with a copulatory plug the day immediately after introduction of the male, the time to mating was considered 1 day (control n = 15 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 8–11 mice/group, DiNP 20μg/kg/day – 200 mg/kg/day n = 8–11 mice/group) (A). Females who gave birth (control n = 17 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 7–11 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 8–1 mice/group) (B) was calculated as the number of females who gave birth divided by the total number of females in the group and multiplied by 100. Statistical significance (p ≤ 0.05) is denoted with an asterisk (*). Data are represented as means ± standard error (A) and percentages (B). Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
Effects of DEHP and DiNP on Litter Outcomes
12 months post-dosing
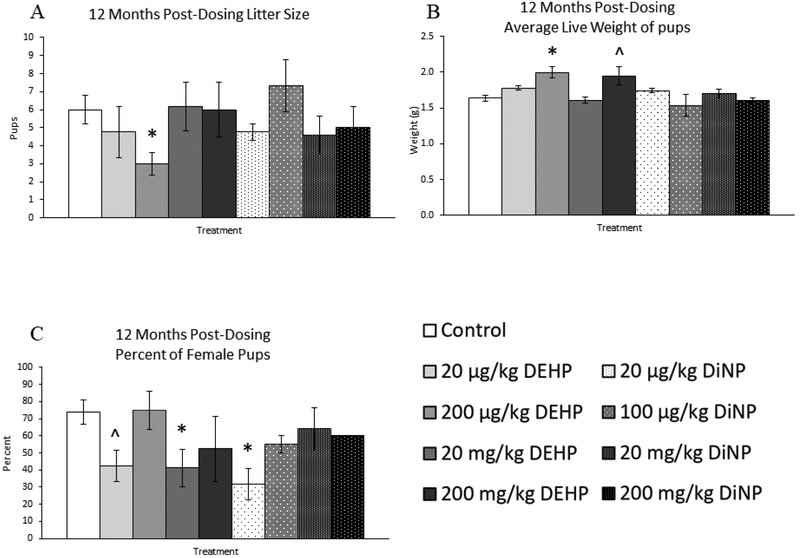
At 12 months post-dosing, 200 μg/kg/day of DEHP significantly decreased litter size in treated females when compared to control (Fig. 9A, n = 3–8 mice/group, p ≤ 0.05). Further, average live weight of pups on PND 0 was significantly increased by 200 μg/kg/day DEHP treatment (Fig. 9B, n = 3–9 mice/group, p ≤ 0.05) and borderline increased by 200 mg/kg/day of DEHP when compared to control (Fig. 9B, n = 3–9 mice/group, p = 0.07). However, too few litters were born to the 200 mg/kg/day DiNP treatment group that statistical analysis of average live weight of pups on PND 0 could not be performed (n = 2 mice). Interestingly, percent of female pups was borderline decreased in the 20 μg/kg/day group DEHP (Fig. 9C, n = 3–7 mice/group, p = 0.07) and significantly decreased in the 20 mg/kg/day DEHP and 20 μg/kg/day DiNP treatment groups when compared to control (Fig. 9C, n = 3–7 mice/group, p ≤ 0.05). Because of the reduced number of litters born at this time point in addition to the inability to confidently sex pups due to cannibalization, sample size was too low for statistical analysis of the percent of female pups for the 200 mg/kg/day DEHP (n = 2 mice), 100 μg/kg/day DiNP (n = 2 mice), and 200 mg/kg/day DiNP groups (n = 1 mouse).
Figure 9.
Effects of DEHP and DiNP on litter outcomes at 12 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 12 months following completion of dosing, females were paired with untreated male CD-1 mice for breeding trials. If females delivered pups, litter size on PND 0 was determined (control n = 8 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 3–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 3–5 mice/group) (A), average weight of all live pups on PND 0 was measured (control n = 9 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 3–5 mice/group, DiNP 20 μg/kg/day – 20 mg/kg/day n = 4–5 mice/group, 200 mg/kg/day DiNP n = 2 mice) (B), and the percent of female pups was measured (control n = 7 mice/group, DEHP 20μg/kg/day – 20 mg/kg/day n = 4–5 mice/group, 200 mg/kg/day DEHP n = 2 mice, 20 μg/kg/day DiNP n = 3 mice, 100 μg/kg/day DiNP n = 2 mice, 20 mg/kg/day DiNP n = 5 mice, 200 mg/kg/day DiNP n = 1 mouse) (C). However, some groups lacked proper sample size to analyze outcomes, including average live weight for 200 mg/kg/day DiNP (n = 2 mice) and percent female pups for 200 mg/kg/day DEHP (n = 2 mice), 100 μg/kg/day DiNP (n = 2 mice), and 200 mg/kg/day DiNP (n=1 mouse). Statistical significance (p ≤ 0.05) is denoted with an asterisk (*). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
15 months post-dosing
Because the fertility of the CD-1 mouse naturally takes a sharp decline around this time in the life of the mouse, very few litters were born to both treatment and control. Thus, we were not able to assess whether treatment affected litter outcomes for the breeding trial conducted at this time point.
Effects of DEHP and DiNP on Hormone Levels
12 months post-dosing
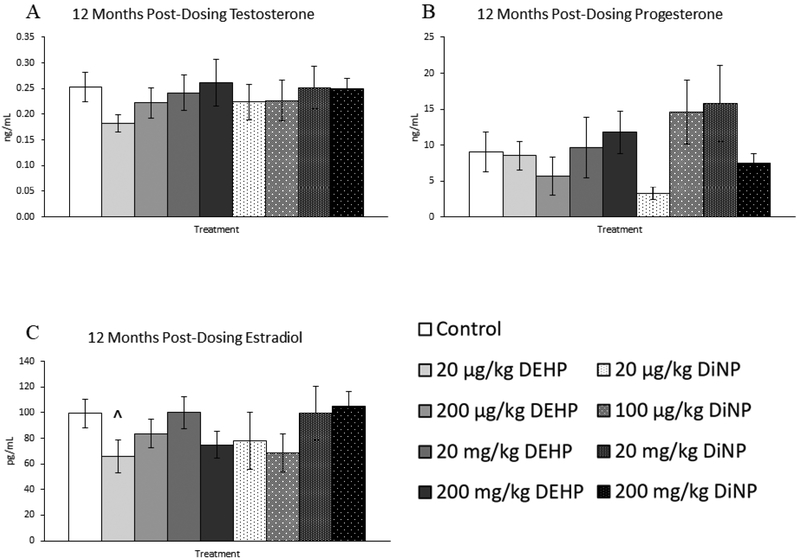
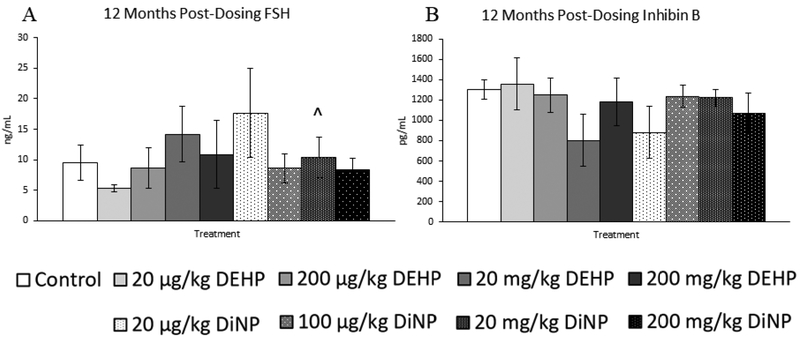
Treatment with DEHP and DiNP did not affect testosterone and progesterone levels at 12 months post-dosing (Fig. 10A and 10B, n = 5–12 mice/group). However, 20 |μg/kg/day of DEHP borderline decreased levels of estradiol when compared to control (Fig. 10C, n = 5–12 mice/group, p = 0.06), and 20 mg/kg/day of DiNP borderline increased levels of FSH when compared to control (Fig. 11A, n = 5–12 mice/group, p = 0.10). No doses of DEHP or DiNP affected levels of inhibin B when compared to control at this time point (Fig. 11B, n = 4–10 mice/group).
Figure 10.
Effects of DEHP and DiNP on levels of sex steroid hormones at 12 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 12 months following completion of dosing, mice were euthanized in the diestrous stage and sera were collected for analysis of testosterone (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 5–6 mice/group) (A), progesterone (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 5–6 mice/group) (B), and estradiol (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 5–6 mice/group) (C) via enzyme-linked immunosorbent assay (ELISA). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
Figure 11.
Effects of DEHP and DiNP on levels of follicle stimulating hormone and inhibin B at 12 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 12 months following completion of dosing, mice were euthanized in the diestrous stage and sera were collected. Sera samples were shipped to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for analysis of follicle stimulating hormone (FSH) (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 5–6 mice/group) (A) and inhibin B (control n = 10 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 4–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 5–6 mice/group) (B) via radioimmunoassay (RIA). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
15 months post-dosing
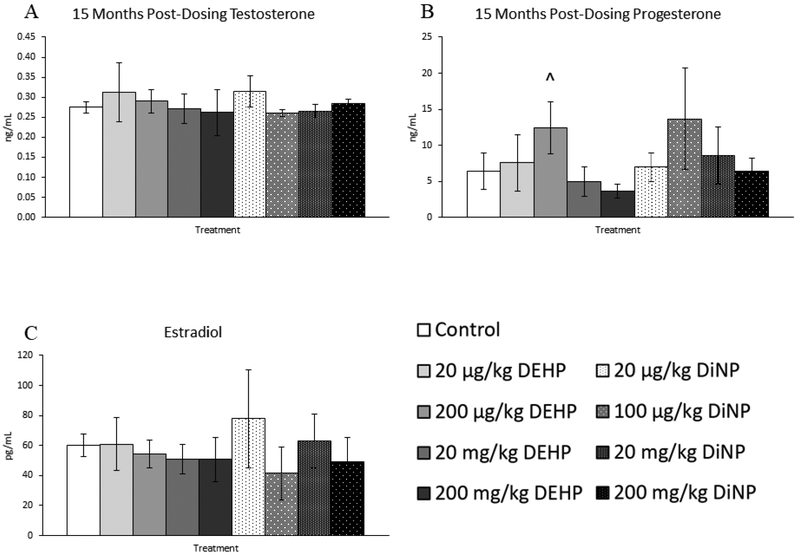
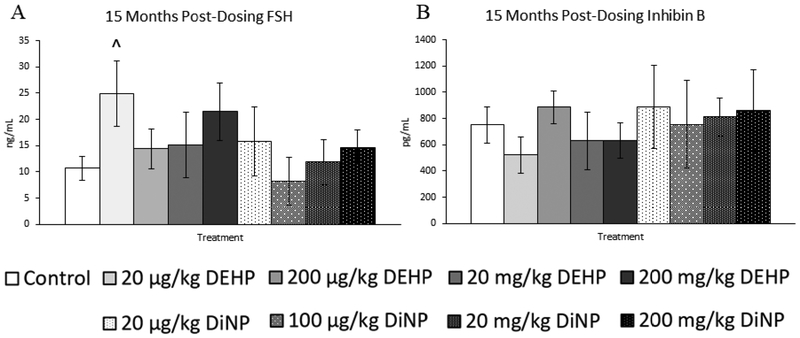
Treatment with DEHP and DiNP did not affect levels of testosterone and estradiol at 15 months post-dosing when compared to control (Fig. 12A and 12C, n = 4–12 mice/group). In contrast, 200 μg/kg/day of DEHP borderline increased levels of progesterone in treated mice (Fig. 12B, n = 4–12 mice/group, p = 0.07). Further, 20 μg/kg/day of DEHP borderline increased levels of FSH when compared to control (Fig. 13A, n = 4–12, p = 0.09). No doses of DEHP or DiNP affected levels of inhibin B at 15 months post-dosing when compared to control (Fig. 13B, n = 4–10 mice/group).
Figure 12.
Effects of DEHP and DiNP on levels of sex steroid hormones at 15 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 15 months following completion of dosing, mice were euthanized in the diestrous stage and sera were collected for analysis of testosterone (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 4–6 mice/group) (A), progesterone (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 4–6 mice/group) B), and estradiol (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 4–6 mice/group) (C) via enzyme-linked immunosorbent assay (ELISA). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
Figure 13.
Effects of DEHP and DiNP on levels of follicle stimulating hormone and inhibin B at 15 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 15 months following completion of dosing, mice were euthanized in the diestrous stage and sera were collected. Sera samples were shipped to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for analysis of follicle stimulating hormone (FSH) (control n = 12 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 4–6 mice/group) (A) and inhibin B (control n = 10 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 5–6 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 4–6 mice/group) (B) via radioimmunoassay (RIA). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
18 months post-dosing
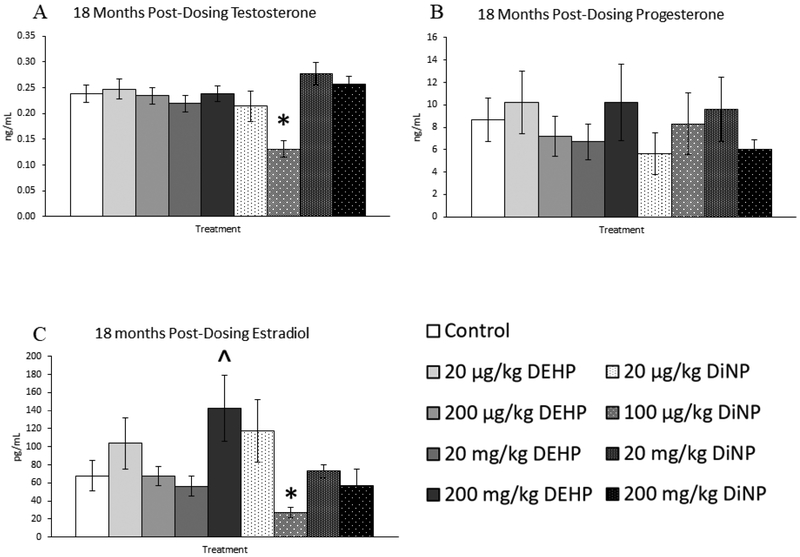
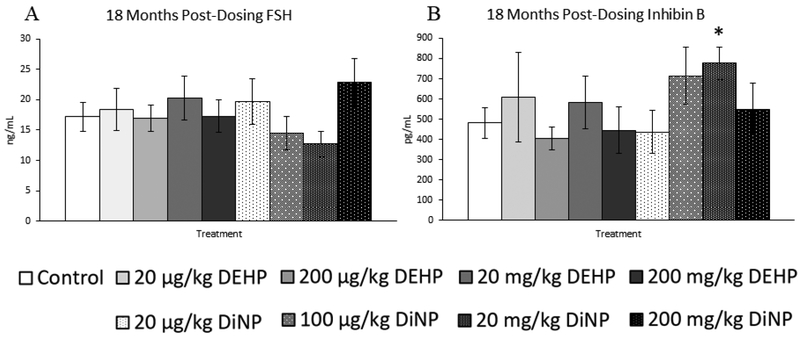
Treatment with DEHP and DiNP did not affect progesterone levels when compared to control at this time point (Fig. 14B, n = 7–16 mice/group). In contrast, treatment with 200 mg/kg/day of DEHP borderline increased levels of estradiol when compared to control (Fig. 14C, n = 7–16 mice/group, p = 0.07), and 100 |μg/kg/day of DiNP significantly decreased levels of estradiol when compared to control (Fig. 14C, n = 7–16 mice/group, p ≤ 0.05). Further, 100 μg/kg/day of DiNP also decreased testosterone levels when compared to control (Fig. 14A, n = 7–16 mice/group, p ≤ 0.05). No effects of treatment were observed on levels of FSH when compared to control at this time point (Fig. 15A, n = 7–16 mice/group). In contrast, the 20 mg/kg/day DiNP treatment group displayed increased levels of inhibin B when compared to control (Fig. 15B, n = 7–16 mice/group, p < 0.05).
Figure 14.
Effects of DEHP and DiNP on levels of sex steroid hormones at 18 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 18 months following completion of dosing, mice were euthanized in the diestrous stage and sera were collected for analysis of testosterone (control n = 16 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 7–11 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 7–11 mice/group) (A), progesterone (control n = 16 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 7–11 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 7–11 mice/group) (B), and estradiol (control n = 16 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 7–11 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 8–11 mice/group) (C) via enzyme-linked immunosorbent assay (ELISA). Statistical significance (p ≤ 0.05) is denoted with an asterisk (*). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
Figure 15.
Effects of DEHP and DiNP on levels of follicle stimulating hormone and inhibin B at 18 months post-dosing. Female CD-1 mice were orally dosed at age 39–40 days for 10 days with either vehicle control (corn oil), DEHP (20μg/kg/day – 200 mg/kg/day), or DiNP (20μg/kg/day – 200 mg/kg/day). At 18 months following completion of dosing, mice were euthanized in the diestrous stage and sera were collected. Sera samples were shipped to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for analysis of follicle stimulating hormone (FSH) (control n = 16 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 7–11 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 8–11 mice/group) (A) and inhibin B (control n = 16 mice/group, DEHP 20μg/kg/day – 200 mg/kg/day n = 7–11 mice/group, DiNP 20 μg/kg/day – 200 mg/kg/day n = 8–11 mice/group) (B) via radioimmunoassay (RIA). Statistical significance (p ≤ 0.05) is denoted with an asterisk (*). Data are represented as means ± standard error. Borderline statistical significance (0.05 < p ≤ 0.10) is denoted with a caret (^).
DISCUSSION
This study tested the hypothesis that short-term exposure to DEHP and DiNP during adulthood has long lasting consequences on fertility, estrous cyclicity, follicle populations, and circulating levels of hormones at time points just before, during, and after the transition into reproductive senescence in mice. Previously, we have shown that short-term exposure to DEHP and DiNP can have negative consequences on fertility up to 9 months post-dosing [11, 22]. The current study builds upon existing knowledge by investigating the effects of short-term exposure to DEHP and DiNP during adulthood on time points that have not yet been studied, including those encompassing reproductive senescence in the mouse.
We observed that DEHP and DiNP disrupted estrous cyclicity at 12 and 15 months post-dosing wherein females spent more time in estrus and less time in metestrus/diestrus compared to control. Increased time in spent in estrus has been shown to occur in the aging rodent [28, 29]. Thus, this could be indicative of accelerated reproductive aging in the treatment groups displaying increased time spent in estrus. Previous studies in our laboratory have shown that both DEHP and DiNP have long-lasting effects on cyclicity up to 9 months post-dosing [11, 22]. In contrast, a study investigating exposure to DEHP during adulthood in mice reported that DEHP increased time spent outside of the estrous phase [30]. Further, a different study found that rats that were perinatally exposed to DiNP did not display disrupted estrous cycles [31]. However, the differences observed between these studies and the present study are likely due to differences in age at cyclicity monitoring and window of exposure.
At 12 months post-dosing, we found that DiNP decreased the number and the percent of primordial follicles and percent of primary follicles as well as increased percent of preantral and antral follicles. Further, DEHP decreased the number and percent of primary follicles and decreased the number of preantral follicles. Of note is the trend that both DEHP and DiNP reduced populations of more immature follicle types, such as primordial and primary follicles, and increased percent of more mature follicle types. Skewing the follicle populations from more immature follicle types to more mature follicle types could be indicative of accelerated folliculogenesis, a phenomenon observed in a previous study conducted in our laboratory using the same dosing paradigm [10]. If not corrected, accelerated folliculogenesis can lead to early reproductive senescence.
We also observed reduced total follicle numbers in the mice treated with 20 mg/kg/day of DEHP in addition to reduced percent of unhealthy follicle types in both DEHP- and DiNP-treated groups. In contrast, 20 μg/kg/day of DiNP-treated mice displayed an increased percent of unhealthy follicle types. When comparing the total follicle numbers to the numbers of each follicle type, the reduction in total follicles observed in the 20 mg/kg/day DEHP treatment group can largely be attributed to the lower numbers of primordial and primary follicles when compared to control. Further, we found the reduction in unhealthy follicle types very interesting, and we suspect that treatment with DEHP and DiNP has upregulated clearance of unhealthy or dying tissues, giving an appearance of a reduction in these types of follicles. This hypothesis could also explain the increase in unhealthy follicles observed in the lower treatment group of DiNP, which may not have been a high enough dose to induce this clearance.
At 15 months post-dosing, we primarily observed treatment-induced disruptions in preantral and antral numbers and percentages. This suggests that DEHP and DiNP altered the rate at which these follicles progress from the preantral to antral stage. The transition from the preantral to antral stage is regulated by a variety of hormones such as FSH and genes including Sma- and Mad-related proteins (SMAD) family member 3 (Smad3) and members of the forkhead transcription factors (Foxo) family [32]. Thus, it is possible that treatment with DEHP and DiNP caused aberrant regulation of the genes or receptors for hormones involved in this transition.
At 18 months post-dosing, only some doses of DiNP affected follicle numbers and percentages. We observed DiNP-induced increases in the number and percent of primordial follicles and disruption of antral follicle numbers and percentages. Interestingly, we observed contrasting effects between the 20 μg/kg/day and 100 μg/kg/day doses of DiNP on antral follicle numbers, with 20 μg/kg/day decreasing and 100 μg/kg/day increasing antral follicle numbers. This non-monotonic dose response is common with endocrine disrupting chemicals [33] and highlights the importance of the investigation of the mechanisms through which these chemicals act. The DiNP-induced increase in primordial follicles is particularly perplexing because previous in vitro and in vivo studies in our laboratory have shown that phthalates can accelerate primordial follicle recruitment [10, 34]. However, a previous study conducted in our laboratory has shown that exposure to a phthalate mixture can reduce atresia in antral follicles cultured in vitro [35]. Because females are born with a finite number of primordial follicles, an increase in primordial follicle numbers can only be explained by less atresia or less recruitment to the next stages of growth. The lack of increased numbers or percentages of primary follicles suggests that the increase observed in primordial follicle numbers is likely due to less atresia in the primordial follicle pool as opposed to increased primordial follicle recruitment. It is possible that DiNP is acting through a similar mechanism as seen in this previous study that observed reduced atresia. Thus, future studies should investigate the mechanisms through which DiNP may regulate follicular atresia at different stages of growth.
The variability of treatment-induced effects on follicle numbers and follicle percentages between time points was unexpected. It is possible that some of these changes may be due to compensatory action. At 12 months post-dosing, we observed treatment-induced decreases in more immature follicle types such as the primordial and primary follicles, and at 18 months we observed the inverse and found that some doses increased the number of primordial follicles compared to control. Thus, it is possible that the rate of folliculogenesis was altered due to the initial treatment-induced decrease in particular follicle types. It is also possible that the dysregulation of the hypothalamic-pituitary-ovarian (HPO) axis that occurs at the onset of reproductive senescence in combination with treatment can trigger further aberrant regulation of folliculogenesis [4]. Thus, reproductive senescent status may be another variable between time points that account for the different effects observed due to treatment.
At 12 months post-dosing, we observed that DEHP and DiNP increased pregnancy loss and overall decreased the ability of treated females to produce offspring in multiple treatment groups when compared to control. The reductions in the fertility of the 20 μg/kg/day treatment groups for both DEHP and DiNP observed in the present study have been observed in a previous study in our laboratory that examined fertility at 3 months post-dosing [22], indicating these effects can arise at multiple time points throughout the life of the exposed individual. Further, these treatment groups both exhibited increased time spent in estrus is a marker of reproductive aging [29]. Thus, the combination of reduced fertility and increased time spent in estrus suggest the onset of reproductive senescence is occurring earlier in these treatment groups. Although few studies have investigated the late-life consequences of exposure to DEHP and DiNP during adulthood, previous studies have found that DEHP exposure during adulthood or through transgenerational exposure can impact markers of reproductive aging in mice [11, 12, 36]. Further, epidemiological evidence shows associations between phthalate exposure and early age at menopause [37]. Additionally, phthalate exposure has also been associated with increased pregnancy loss in women [38], and some animal studies have found that DEHP exposure disrupts placental growth and development, possibly providing insufficient support for the fetus [39, 40].
In terms of pup outcomes, we found that at 12 months post-dosing, DEHP and DiNP decreased the percent of female pups and that DEHP alone decreased litter size and increased average live weight of pups when compared to control. One study has shown that DEHP can interfere with receptivity of the uterine lining to implantation [41], which could explain the reduced litter size observed in the present study. The increase in average live weight at birth is a result we have observed previously at earlier time points closer to the exposure window [22]. Although the decrease in litter size in the 200 μg/kg/day DEHP group may have led to decreased intrauterine growth restriction and subsequently, larger pups, this does not explain the increase in average live weight in the 200 mg/kg/day DEHP group. DEHP has been shown to have obesogenic properties, potentially through activation of peroxisome proliferator-activated receptor (PPAR) activation [42, 43]. Thus, it is possible DEHP increased the adiposity of the pups, leading to increased weight at birth. These results contrast with some epidemiological findings that phthalate exposure is associated with reduced weight at birth [44, 45], but some studies have found inverse results [46].
The alteration in percent of female pups born was surprising due to the pre-conception dosing paradigm of the study. However, sex ratio was altered in a previous study using the same dosing paradigm conducted in our laboratory at 9 months post-dosing [22]. In that previous study, we postulated that the difference in sex ratio may be due to treatment affecting hormones and, subsequently, sperm selection as the sperm traversed the female reproductive tract. However, we did not observe correlations between hormones and alterations in sex ratio in the current study, suggesting this is not the mechanism through which DEHP and DiNP alter sex ratio. Previous studies have shown that maternal age and condition can affect sex ratio of offspring [47–49]; thus, it is possible that treatment may lead to altered sex ratios by altering aspects not detectable through methods used in this study.
Overall, the effects of treatment on hormone levels were limited. However, alterations in hormones were present throughout the study through the last time point at 18 months post-dosing. This indicates that hormones may be affected throughout the life of the exposed individual. Indeed, the most striking effects on hormones observed in this study were observed at 18 months post-dosing, wherein the 100 μg/kg/day DiNP treatment group exhibited reduced levels of both estradiol and testosterone. Interestingly, this same treatment group had increased number of antral follicles, indicating that reducing the antral follicle population is not necessary to reduce the levels of sex steroid hormones. It is likely that either the function of the antral follicles has been disrupted or that metabolism of these hormones is occurring at a faster rate than production when compared to control. The other effect seen in hormones at 18 months post-dosing was an increase in inhibin B levels in the 20 mg/kg/day DiNP treatment group when compared to control. We find this result surprising because inhibin B is a marker of ovarian reserve, and we observed no changes in any follicle types for this group at this time point. Thus, we suspect that this increase in inhibin B is not due to an increase in the ovarian reserve and that it may reflect disruptions in the metabolism or production of inhibin B itself.
We find the lack of correlation between the breeding outcomes and hormonal and follicle count data perplexing because a lack of fecundity can often be explained by disruptions to follicular populations or hormones. It is possible that the observed disruptions in fertility may be based in the uterus and not in the ovary or the production of ovarian hormones. Thus, future studies should investigate the effects of DEHP and DiNP on uterine function, especially as it concerns the support of pregnancy in light of the increased pregnancy loss observed in this study.
We also find the contrast between the hormone and follicle population data interesting. The lack of correlation between antral follicle numbers and changes in hormones at most time points suggests that the answer may lie in alterations of metabolism or production of these hormones and not the absolute follicle numbers. Previous studies in our research group have shown that phthalates can alter expression of a variety of steroidogenic enzymes present within the ovary [35, 50]. Thus, it is possible that DEHP and DiNP cause altered expression or activity of the steroidogenic enzymes present in the ovary.
In conclusion, this study found that short-term exposure during adulthood has long-lasting consequences on estrous cyclicity, ovarian follicle populations, fertility, and hormones in the female mouse. The effects of DEHP and DiNP observed in this study occurred a year or longer after cessation of exposure, thus, future studies should investigate the mechanisms through which DEHP and DiNP may be imparting these long-lasting effects during such a short-term window of exposure.
Highlights.
DEHP and DiNP increased pregnancy loss one year following exposure
DEHP and DiNP reduced overall fertility one year following exposure
Preconception exposure to DEHP and DiNP alters sex ratio of pups
DiNP alters ovarian follicle populations up to 18 months post-dosing
DEHP and DiNP alter hormone levels up to 18 months post-dosing
ACKNOWLEDGMENTS
We would like to thank the members of the Flaws laboratory group for their technical assistance. We would also like to thank the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core that is supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934 for their work in assaying serum samples for FSH and inhibin B. This work was supported by NIH R56 ES 025147, R01 ES 028661, T32 ES 007326, and a Billie Field Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors do not have any conflicts of interest or potential conflicts of interest to disclose.
REFERENCES
- 1.Cohen PE and Holloway JK, Chapter 1 - Mammalian Meiosis, in Knobil and Neill’s Physiology of Reproduction (Fourth Edition), Plant TM and Zeleznik AJ, Editors. 2015, Academic Press: San Diego: p. 5–57. [Google Scholar]
- 2.Auchus RJ, Chapter 8 - Human Steroid Biosynthesis, in Knobil and Neill’s Physiology of Reproduction (Fourth Edition), Plant TM and Zeleznik AJ, Editors. 2015, Academic Press: San Diego: p. 295–312. [Google Scholar]
- 3.McArdle CA and Roberson MS, Chapter 10 - Gonadotropes and Gonadotropin-Releasing Hormone Signaling, in Knobil and Neill’s Physiology of Reproduction (Fourth Edition), Plant TM and Zeleznik AJ, Editors. 2015, Academic Press: San Diego: p. 335–397. [Google Scholar]
- 4.Gore AC, Hall JE, and Hayes FJ, Chapter 37 - Aging and Reproduction, in Knobil and Neill’s Physiology of Reproduction (Fourth Edition), Plant TM and Zeleznik AJ, Editors. 2015, Academic Press: San Diego: p. 1661–1693. [Google Scholar]
- 5.van der Schouw YT, et al. , Age at menopause as a risk factor for cardiovascular mortality. Lancet, 1996. 347(9003): p. 714–8. [DOI] [PubMed] [Google Scholar]
- 6.Cooper GS and Sandler DP, Age at natural menopause and mortality. Ann Epidemiol, 1998. 8(4): p. 229–35. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, et al. , Age at natural menopause and risk of cardiovascular disease. Arch Intern Med, 1999. 159(10): p. 1061–6. [DOI] [PubMed] [Google Scholar]
- 8.Rocca WA, et al. , Premature menopause or early menopause and risk of ischemic stroke. Menopause, 2012. 19(3): p. 272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher JC, Effect of early menopause on bone mineral density and fractures. Menopause, 2007. 14(3 Pt 2): p. 567–71. [DOI] [PubMed] [Google Scholar]
- 10.Hannon PR, Peretz J, and Flaws JA, Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod, 2014. 90(6): p. 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannon PR, Niermann S, and Flaws JA, Acute Exposure to Di(2-Ethylhexyl) Phthalate in Adulthood Causes Adverse Reproductive Outcomes Later in Life and Accelerates Reproductive Aging in Female Mice. Toxicol Sci, 2016. 150(1): p. 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brehm E, et al. , Prenatal Exposure to Di(2-Ethylhexyl) Phthalate Causes Long-Term Transgenerational Effects on Female Reproduction in Mice. Endocrinology, 2018. 159(2): p. 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannon PR and Flaws JA, The effects of phthalates on the ovary. Front Endocrinol (Lausanne), 2015. 6: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang C, Mahalingam S, and Flaws JA, Environmental Contaminants Affecting Fertility and Somatic Health. Semin Reprod Med, 2017. 35(3): p. 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Api AM, Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients. Food Chem Toxicol, 2001. 39(2): p. 97–108. [DOI] [PubMed] [Google Scholar]
- 16.Lyche JL, Chapter 48 - Phthalates, in Reproductive and Developmental Toxicology, Gupta RC, Editor. 2011, Academic Press: San Diego: p. 637–655. [Google Scholar]
- 17.U.S. Department of Health and Human Services, P.H.S., Agency for Toxic Substances and Disease Registry, TOXICOLOGICAL PROFILE FOR DI(2-ETHYLHEXYL)PHTHALATE. 2002. [PubMed]
- 18.NTP, N.T.P., Di(2-ethylhexyl) phthalate. Rep Carcinog, 2016. 14. [Google Scholar]
- 19.Hines CJ, et al. , Urinary phthalate metabolite concentrations among workers in selected industries: a pilot biomonitoring study. Ann Occup Hyg, 2009. 53(1): p. 1–17. [DOI] [PubMed] [Google Scholar]
- 20.Faouzi MA, et al. , Exposure of hemodialysis patients to di-2-ethylhexyl phthalate. Int J Pharm, 1999. 180(1): p. 113–21. [DOI] [PubMed] [Google Scholar]
- 21.Zota AR, Calafat AM, and Woodruff TJ, Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect, 2014. 122(3): p. 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang C and Flaws JA, Subchronic Exposure to Di(2-ethylhexyl) Phthalate and Diisononyl Phthalate During Adulthood Has Immediate and Long-Term Reproductive Consequences in Female Mice. Toxicol Sci, 2019. 168(2): p. 620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kavlock R, et al. , NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol, 2002. 16(5): p. 529–653. [DOI] [PubMed] [Google Scholar]
- 24.Niermann S, et al. , Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod Toxicol, 2015. 53: p. 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hines CJ, et al. , Occupational exposure to diisononyl phthalate (DiNP) in polyvinyl chloride processing operations. Int Arch Occup Environ Health, 2012. 85(3): p. 317–25. [DOI] [PubMed] [Google Scholar]
- 26.CPSC, U.S.C.P.S.C., Chronic Hazard Advisory Panel On Diisononyl Phthalate (DiNP). 2001.
- 27.Hartman CG, Some New Observations on the Vaginal Smear of the Rat. Yale J Biol Med, 1944. 17(1): p. 99–112. [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson JF, et al. , Longitudinal studies of estrous cyclicity in C57BL/6J mice: III. Dietary modulation declines during aging. Mech Ageing Dev, 1989. 48(1): p. 73–84. [DOI] [PubMed] [Google Scholar]
- 29.LeFevre J and McClintock MK, Reproductive senescence in female rats: a longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod, 1988. 38(4): p. 780–9. [DOI] [PubMed] [Google Scholar]
- 30.Li N, et al. , Di-(2-ethylhcxyl) phthalate reduces progesterone levels and induces apoptosis of ovarian granulosa cell in adult female ICR mice. Environ Toxicol Pharmacol, 2012. 34(3): p. 869–75. [DOI] [PubMed] [Google Scholar]
- 31.Lee HC, Yamanouchi K, and Nishihara M, Effects of perinatal exposure to phthalate/adipate esters on hypothalamic gene expression and sexual behavior in rats. J Reprod Dev, 2006. 52(3): p. 343–52. [DOI] [PubMed] [Google Scholar]
- 32.Pangas SA and Rajkovic A, Chapter 21 - Follicular Development: Mouse, Sheep, and Human Models, in Knobil and Neill’s Physiology of Reproduction (Fourth Edition), Plant TM and Zeleznik AJ, Editors. 2015, Academic Press: San Diego: p. 947–995. [Google Scholar]
- 33.Vandenberg LN, et al. , Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev, 2012. 33(3): p. 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hannon PR, et al. , Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol Reprod, 2015. 92(5): p. 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou C and Flaws JA, Effects of an Environmentally Relevant Phthalate Mixture on Cultured Mouse Antral Follicles. Toxicol Sci, 2017. 156(1): p. 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barakat R, et al. , Prenatal Exposure to DEHP Induces Premature Reproductive Senescence in Male Mice. Toxicol Sci, 2017. 156(1): p. 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grindler NM, et al. , Persistent organic pollutants and early menopause in U.S. women. PLoS One, 2015. 10(1): p. e0116057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao H, et al. , Urinary concentrations of phthalate metabolites in early pregnancy associated with clinical pregnancy loss in Chinese women. Sci Rep, 2017. 7(1): p. 6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zong T, et al. , Maternal exposure to di-(2-ethylhexyl) phthalate disrupts placental growth and development in pregnant mice. J Hazard Mater, 2015. 297: p. 25–33. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, et al. , [Effect of di-(2-ethylhexyl) phthalate exposure on placental development in pregnant mice]. Nan Fang Yi Ke Da Xue Xue Bao, 2016. 36(4): p. 467–71. [PubMed] [Google Scholar]
- 41.Li R, et al. , Effects of DEHP on endometrial receptivity and embryo implantation in pregnant mice. J Hazard Mater, 2012. 241–242: p. 231–40. [DOI] [PubMed] [Google Scholar]
- 42.Wassenaar PNH and Legler J, Systematic review and meta-analysis of early life exposure to di(2-ethylhexyl) phthalate and obesity related outcomes in rodents. Chemosphere, 2017. 188: p. 174–181. [DOI] [PubMed] [Google Scholar]
- 43.Hao C, et al. , Perinatal exposure to diethyl-hexyl-phthalate induces obesity in mice. Front Biosci (Elite Ed), 2013. 5: p. 725–33. [DOI] [PubMed] [Google Scholar]
- 44.Song Q, et al. , Evaluating effects of prenatal exposure to phthalates on neonatal birth weight: Structural equation model approaches. Chemosphere, 2018. 205: p. 674–681. [DOI] [PubMed] [Google Scholar]
- 45.Strommen K, et al. , Increased levels of phthalates in very low birth weight infants with septicemia and bronchopulmonary dysplasia. Environ Int, 2016. 89–90: p. 228–34. [DOI] [PubMed] [Google Scholar]
- 46.Zhu Y, et al. , Relationship between maternal phthalate exposure and offspring size at birth. Sci Total Environ, 2018. 612: p. 1072–1078. [DOI] [PubMed] [Google Scholar]
- 47.Santos MM, et al. , Sex ratio of equine offspring is affected by the ages of the mare and stallion. Theriogenology, 2015. 84(7): p. 1238–1245. [DOI] [PubMed] [Google Scholar]
- 48.Trivers RL and Willard DE, Natural selection of parental ability to vary the sex ratio of offspring. Science, 1973. 179(4068): p. 90–2. [DOI] [PubMed] [Google Scholar]
- 49.Roche JR, Lee JM, and Berry DP, Pre-conception energy balance and secondary sex ratio--partial support for the Trivers-Willard hypothesis in dairy cows. J Dairy Sci, 2006. 89(6): p. 2119–25. [DOI] [PubMed] [Google Scholar]
- 50.Hannon PR, et al. , Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol Appl Pharmacol, 2015. 284(1): p. 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]