Abstract
Background:
Whether soy products confer health benefits related to coronary heart disease (CHD) remains controversial due to inconsistent evidence.
Methods:
A total of 74,241 women from the Nurses’ Health Study (NHS; 1984–2012), 94,233 women from the NHSII (1991–2013), and 42,226 men from the Health Professionals Follow-Up Study (1986–2012), who were free of cardiovascular disease and cancer at baseline, were included in the current analysis. Dietary data were updated every 2–4 y using a validated food frequency questionnaire. Nonfatal myocardial infarction and CHD deaths were adjudicated through reviewing medical records, death certificates, and other medical documents.
Results:
In these cohorts 8,359 incident CHD cases were documented during 4,826,122 person-years of follow-up. In multivariable-adjusted analyses, isoflavone intake was inversely associated with CHD (pooled hazard ratio [HR] comparing the extreme quintiles: 0.87; 95%CI: 0.81, 0.94; P=0.008). Consumption of tofu, but not soy milk, was inversely associated with the risk of CHD, with pooled HRs (95% CIs) of 0.82 (0.70, 0.95; P=0.005) and 0.87 (0.69, 1.10; P=0.41), respectively, comparing ≥1 serving/week with <1 serving/month. Further analyses showed that in women the favorable association of tofu was primarily driven by stronger inverse association of tofu intake observed in younger women before menopause and postmenopausal women without hormone use (Pinteraction=0.002).
Conclusions:
Higher intake of isoflavones and tofu was associated with a moderately lower risk of developing CHD, and in women the favorable association of tofu were more pronounced in young women or postmenopausal women without hormone use.
Keywords: coronary heart disease, isoflavone, soy, tofu, diet
Introduction
The role of soy products and isoflavones in heart health has become a controversial topic. The US Food and Drug Administration (FDA) approved a health claim for soy products as being protective against coronary heart disease (CHD) in 1999.1 However, based on mixed results from clinical trials and epidemiological studies thereafter, the FDA is reconsidering this health claim specific to soy intake and heart health.2 In addition, updated nutritional guidelines by the American Heart Association concluded that the evidence for the cardiovascular health offered by isoflavones was minimal, and that the health benefits of soy products may be attributed to its higher contents of polyunsaturated fats, fiber, vitamins, and minerals and lower contents of saturated fat.3
Nevertheless, multiple cardioprotective benefits have been attributed to isoflavones including reducing the low-density lipoprotein (LDL) cholesterol, inhibiting pro-inflammatory cytokines, cell adhesion proteins and platelet aggregation, inducing nitric oxide (NO) production, and improving vascular reactivity.4, 5 Multiple prospective observational studies have been conducted to examine the associations of intake of isoflavones and soy products with CHD incidence, and some, but not all, reported inverse associations between isoflavones and cardiovascular risk factors and CHD prevention.6, 7 Limitations common in these previous investigations, such as small sample size, relatively short duration of follow-up, different food sources of isoflavones considered, and, more importantly, lack of repeated assessments of diet during follow-up, may contribute to the inconsistent findings. In addition, given the heterogeneity in nutrients and other constituents across individual isoflavone-rich food sources, it is important to investigate specific food sources of isoflavones. Such evidence can be particularly informative for establishing food-based dietary guidelines. Although soy products are an important source of plant proteins, especially for vegetarians or vegans, epidemiological studies pertaining to individual soy products in relation to CHD risk are scarce. Another critical aspect in isoflavone research is potential, synergistic effects between isoflavones and estrogens. Isoflavones are structurally similar to estradiol and may exert estrogenic effects through binding estrogen receptors (ERs).8 It is thus plausible that the associations of isoflavones and soy foods may depend on menopausal status and use of hormone therapy (HT), although such interactions have not been extensively examined in previous studies.
To provide further evidence, the hypotheses that intake of isoflavones and soy foods is prospectively associated with a lower CHD risk and that these relations may differ by menopausal status and hormone use were evaluated in the current analysis.
Methods
The data, analytical methods, and study materials will be made available to other researchers from the corresponding authors upon reasonable request for the purposes of reproducing the results or replicating the procedure through existing data-sharing guidelines implemented in the cohort studies.
Study population
The Nurses’ Health Study (NHS) was initiated in 1976 and consisted of 121,700 female registered nurses aged 30 to 55 years enrolled from 11 states.9 The NHSII is a parallel cohort of younger female registered nurses and consisted of 116,671 women aged 25–42 years at enrollment in 1989.10 The Health Professionals Follow-Up Study (HPFS) cohort was established in 1986 with an enrollment of 51,529 US male health professionals, 40–75 y of age, from all 50 states.11 In these three cohorts, information on participants’ lifestyle and medical history was updated every 2 years with the use of self-administered questionnaires. The cumulative response rate exceeded 90% in the three cohorts. In the current investigation, participants were excluded if they had physician-diagnosed cancer, or cardiovascular disease (angina, stroke, CHD, or coronary revascularization) at baseline (1984 for NHS, 1991 for NHSII, and 1986 for HPFS), had incomplete information of dietary data, had missing information on isoflavone intake, or reported implausible total energy intake (<2,510 [2nd percentile] or >14,644 [99th percentile] kJ/day for women, and <3,347 [2nd percentile] or >17,573 [99th percentile] kJ/day for men). After exclusions, a total of 210,700 participants (74,241 women in the NHS, 94,233 women in the NHSII, and 42,226 men in the HPFS) remained in the analysis (Supplemental Figure 1).
The study was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health. The completion of the self-administered questionnaire was considered to imply informed consent.
Assessment of isoflavones and soy food consumption
In 1984, 1986, and every 4 y thereafter, validated food frequency questionnaires (FFQs) including approximate 130 food items were mailed to the NHS participants to assess and update information on their usual intake of foods and beverages. The FFQs have been sent every 4 y to the NHSII participants since 1991 and to the HPFS participants since 1986. In all FFQs, the average consumption frequency of food items in the previous year was inquired with a prespecified portion size for each food item. Nine response options were provided, ranging from “less than once per month” to “6 or more times per day.” Since 1998 in the NHS, 1999 in the NHSII, and 2002 in the HPFS, consumption of soy milk was added to the FFQs. The average daily intake of isoflavones was calculated by multiplying the frequency of consumption of each food item that contains isoflavones by isoflavone content and then summing across from all foods. The food composition database used to calculate isoflavone values was based primarily on the US Department of Agriculture Nutrient Database for the Isoflavone Content of Selected Foods (Supplemental Table 1).12 The validity and reproducibility of the FFQs have been described in detail elsewhere. The correlation coefficient for tofu consumption between FFQs and diet records was 0.56 in a validation study conducted in HPFS.13 In 47 NHSII women,14 Spearman correlation coefficients (rs) between self-reported intake of tofu and soymilk and urinary excretion of isoflavone metabolites measured in two 24-hr urine samples16 were calculated. The rs ranged from 0.18 (daidzein) to 0.28 (dihydrodaidzein) for tofu intake or 0.29 (dihydrodaidzein) to 0.33 (O-desmethylangolensin) for soy milk consumption.
Assessment of Covariates
In the follow-up questionnaires administered every two years, information on anthropometric and lifestyle factors, such as body weight, cigarette smoking, physical activity, medication or supplement use was updated. Menopausal status and postmenopausal HT use were ascertained in women. Alcohol intake was assessed and updated every 4 years using the FFQ. Information on age, race, partner’s education (NHS and NHSII), self-rated socioeconomic status or social standing (NHS and NHSII), family history of myocardial infarction (MI) (defined as maternal history of MI before age 65y or paternal history of MI before age 55y), as well as a history of diabetes, hypertension and hypercholesterolemia was also collected. The alternative healthy eating index (AHEI) score was used to assess overall diet quality. The AHEI score summarizes the intake of 11 foods or nutrients (including consumption of vegetables, fruits, whole grains, sugar sweetened beverages and fruit juice, nuts and legumes, red and processed meat, trans fat, long chain n-3 fats, polyunsaturated fats, sodium, and alcohol).15 In the current analysis, tofu and soy milk were excluded from the calculation of the AHEI score.
Ascertainment of endpoint
The primary endpoint for the current analysis was incident CHD (defined as nonfatal MI and fatal CHD). Participants reporting a nonfatal MI in follow-up questionnaires were requested for permission of access their medical records, which were then reviewed by study physicians who were blinded to the participants’ exposure status. Nonfatal MI was confirmed using the World Health Organization criteria of typical symptoms plus either elevated cardiac enzyme levels or diagnostic electrocardiogram abnormality.16 Fatal CHD was identified by reports from next of kin, the postal authorities, or by searching the National Death Index. Fatal CHD was confirmed by reviewing hospital record or autopsy report, if CHD was listed as the cause of death on the death certificate and there was prior evidence of CHD in the medical records. For cases with CHD as the underlying cause on the death certificate but medical records concerning the death were unavailable and no prior knowledge of CHD was indicated, such cases were designated as probable fatal CHD cases. Both confirmed (n = 5,626) and probable (n = 2,733) CHD cases were included in this analysis to maximize statistical power.
Statistical analysis
Person-years of follow-up for each individual were calculated from the return of the baseline questionnaires to the date of diagnosis of CHD, death, or the end of follow-up (30 June 2012 in NHS, 30 June 2013 in NHSII, and 31 January 2012 in HPFS), whichever came first. To minimize random within-person variation and to best represent long-term diet, the cumulative averages of dietary variables from all FFQs were calculated.17 These averages were derived as the means of all intake assessments and used to represent diet for next 4-year follow-up. Missing values in each follow-up FFQ were replaced with the immediately-preceding cumulative averages. Because participants might change their diet after the diagnosis of a major illness, diet was not updated if participants reported having a diagnosis of diabetes, cancer, angina, or coronary bypass surgery. Time-dependent Cox proportional hazards model was used to estimate the hazard ratios (HRs) for CHD associated with isoflavones and soy foods. In multivariable analyses, in addition to age and calendar time, ethnicity, smoking status, alcohol intake, physical activity, multivitamin use, aspirin use, history of hypertension and hypercholesterolemia, family history of MI, body mass index (BMI), total energy intake, and AHEI score were adjusted. For women, self-rated socioeconomic status, partner’s education, oral contraceptive use (NHSII only), menopausal status, and postmenopausal hormone use were also adjusted for. Of note, time-varying covariates were used, except for ethnicity, self-rated socioeconomic status, and partner’s education. The HRs from each cohort were pooled to obtain a summary risk estimate using the fixed-effects model. P values for heterogeneity of associations among the cohorts were calculated using the Cochran Q test. The significance of linear trends was evaluated by assigning the median values of each quintile of dietary intake to a continuous variable and then examining the significance of this variable. In addition, restricted cubic spline regression with 4 knots were used to examine dose-response relationship between isoflavone intake and risk of CHD. Given the estrogenic effects of isoflavones, potential effect modifications by menopausal status and HT were examined. Interactions with some major risk factors of CHD, including race, age, BMI, modified AHEI score, physical activity, smoking status, or alcohol consumption, were also explored. The likelihood ratio test of the cross-product terms between isoflavones/soy foods and effect modifiers was used to test the significance of interactions. Proportional hazards assumption was tested by evaluating the significance of the interaction term between isoflavone consumption and follow-up time, and no evidence of violation of the assumption was found. Secondary analyses were also performed to evaluate the associations of 3 individual isoflavones on risk of CHD. The statistical analysis was performed with the SAS statistical package (version 9.2, SAS Institute, Cary, NC). P values < 0.05 were considered to indicate statistical significance.
Results
During 4,826,122 person-years of follow-up, 8,359 incident CHD cases (NHS: 3,508 CHD cases during 1,869,575 person-years; NHSII: 665 CHD cases during 2,029,811 person-years; and HPFS: 4,186 CHD cases during 926,736 person-years) were documented. For most participants (inner 90 percentile), isoflavone consumption was between 0.11 and 4.24 mg/day. At the midpoint of follow-up, the median isoflavone intake was 0.34 mg/day in NHS (1998), 0.36 mg/day in NHSII (1999), and 0.43 mg/day in HPFS (1998) mg/day. Table 1 describes the distribution of characteristics of study participants according to intake of isoflavones at the midpoint of follow-up (NHS 1998, NHSII 1999, HPFS 1998). In all three cohorts, individuals who had higher isoflavone intake exercised more. A higher isoflavone intake was also associated with a higher vegetable intake and AHEI score. At the midpoint of follow-up, 4.1% of participants consumed tofu >1 serving/week. Participants who frequently ate tofu were more likely to be physically active, had a higher AHEI score, and tended to consume more fruits and vegetables and less red meat or trans fat (Supplemental Table 2).
Table 1.
Age-standardized characteristics of participants according to quintiles of isoflavone intake at the midpoint of follow-up (NHS 1998, NHSII 1999, HPFS 1998)*
| NHS | NHSII | HPFS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Participants, n | 13791 | 13681 | 13742 | 18547 | 18505 | 18614 | 7202 | 7196 | 7203 |
| Isoflavone intake, mg/d | 0.15† | 0.36 | 3.14 | 0.11 | 0.34 | 4.24 | 0.17 | 0.43 | 3.83 |
| Age‡, years | 63.8 | 63.9 | 63.7 | 45.9 | 46.4 | 46.4 | 65.8 | 66.0 | 64.3 |
| Caucasians, % | 98 | 99 | 95 | 97 | 97 | 93 | 97 | 97 | 89 |
| Asian, % | 0 | 0 | 3 | 1 | 1 | 5 | 0 | 0 | 7 |
| Level education of partner, % of college and above | 73 | 73 | 72 | 63 | 67 | 71 | - | - | - |
| Self-rated socioeconomic status, % of Top 30% | 35 | 35 | 39 | 28 | 31 | 37 | - | - | - |
| Current smoker, % | 6 | 11 | 11 | 6 | 10 | 7 | 3 | 5 | 3 |
| Alcohol intake, g/day | 3.7 | 6.2 | 4.7 | 2.3 | 4.6 | 4.4 | 8.7 | 12.7 | 9.9 |
| Physical activity, MET/week | 16.0 | 16.4 | 19.5 | 17.8 | 19.3 | 26.2 | 32.3 | 32.2 | 36.3 |
| BMI, kg/m2 | 26.6 | 26.6 | 26.4 | 28.0 | 26.8 | 25.5 | 25.9 | 26.5 | 26.0 |
| Family history of myocardial infarction, % | 39 | 38 | 38 | 34 | 32 | 31 | 31 | 31 | 31 |
| Multivitamin use, % | 54 | 53 | 59 | 43 | 45 | 54 | 52 | 51 | 56 |
| Current use of aspirin, % | 45 | 49 | 48 | 88 | 88 | 88 | 39 | 42 | 39 |
| Hypertension, % | 47 | 44 | 42 | 26 | 18 | 10 | 40 | 40 | 39 |
| Hypercholesterolemia, % | 58 | 56 | 55 | 45 | 31 | 17 | 49 | 48 | 49 |
| Postmenopausal women, % | 94 | 94 | 94 | 42 | 39 | 35 | - | - | - |
| Ever menopausal hormone use, % | 65 | 64 | 65 | 26 | 24 | 23 | - | - | - |
| Current use of oral-contraceptive, % | - | - | - | 7 | 7 | 8 | - | - | - |
| Total energy intake, kcal/day | 1733 | 1774 | 1729 | 1784 | 1859 | 1796 | 1956 | 2016 | 1965 |
| Modified AHEI score | 44.4 | 45.0 | 49.0 | 40.8 | 43.3 | 49.9 | 46.7 | 47.3 | 52.5 |
| Trans fat intake, % energy | 1.7 | 1.7 | 1.5 | 1.7 | 1.6 | 1.4 | 1.4 | 1.5 | 1.2 |
| Polyunsaturated fat-to-saturated fat ratio | 0.6 | 0.6 | 0.6 | 0.5 | 0.5 | 0.6 | 0.6 | 0.6 | 0.7 |
| Total fruits intake, servings/day | 2.3 | 2.3 | 2.5 | 1.1 | 1.2 | 1.5 | 2.4 | 2.3 | 2.8 |
| Total vegetables intake, servings/day | 3.0 | 3.1 | 3.5 | 2.7 | 3.3 | 4.0 | 2.9 | 3.1 | 3.8 |
| Red meats intake, servings/day | 0.8 | 1.0 | 0.8 | 0.8 | 0.9 | 0.6 | 1.0 | 1.2 | 0.8 |
AHEI, alternative health eating index; BMI, body mass index; HPFS, the Health Professionals Follow-Up Study; MET, metabolic equivalents of task; NHS, Nurses’ Health Study.
Values were standardized to the age distribution of the study population.
Data are mean unless otherwise indicated.
Values were not age adjusted.
In the age-adjusted models, isoflavone intake was inversely associated with CHD risk, and the pooled HR (95% CI) was 0.79 (0.73, 0.85), comparing the highest with the lowest quintiles (Table 2). After multivariable adjustment for potential confounding factors, the inverse association was moderately attenuated (pooled HR comparing extreme categories: 0.81; 95% CI: 0.76, 0.87). In the fully-adjusted model with further adjustment of the AHEI score, the pooled HRs (95% CIs) across increasing quintiles of isoflavones were 0.92 (0.86, 0.98), 0.89 (0.83, 0.95), 0.90 (0.84, 0.96), and 0.87 (0.81, 0.94; Ptrend= 0.008). Spline regression models showed a linear inverse relationship of isoflavone intake with CHD risk (P linearity<0.001; Figure 1). In a sensitivity analysis, the statistically significant inverse association for isoflavones and CHD did not change after adjustment for polyunsaturated fats, fiber, vitamins (vitamins C and E), minerals (magnesium, potassium, and calcium), saturated fat, and other flavonoids (pooled HR: 0.87; 95% CI: 0.80, 0.94; Ptrend = 0.008). Intake of major individual isoflavones—daidzein (HR: 0.87, 95% CI: 0.81, 0.93); genistein (HR: 0.89, 95% CI: 0.83, 0.96); and glycitein (HR: 0.83, 95% CI: 0.74, 0.93)—was inversely associated with CHD risk, comparing extreme quintiles (Supplemental Table 3).
Table 2.
Hazard ratio (95% CI) of coronary heart disease according to quintiles of isoflavone intake
| Quintile of isoflavone intake | P trend | |||||
|---|---|---|---|---|---|---|
| 1 (low) | 2 | 3 | 4 | 5 (high) | ||
| NHS | ||||||
| Median intake, mg/d | 0.16 | 0.26 | 0.37 | 0.52 | 1.43 | |
| No. of case/person years | 773/373857 | 713/373776 | 650/372196 | 744/374260 | 628/375486 | |
| Rate per 100,000 person-years | 207 | 191 | 175 | 199 | 167 | |
| Age-adjusted model* | 1 | 0.92 (0.83, 1.02) | 0.85 (0.77, 0.95) | 1.00 (0.90, 1.11) | 0.85 (0.76, 0.94) | 0.02 |
| Multivariable-adjusted model† | 1 | 0.91 (0.82, 1.01) | 0.81 (0.73, 0.90) | 0.87 (0.79, 0.97) | 0.84 (0.75, 0.93) | 0.02 |
| Fully-adjusted model‡ | 1 | 0.91 (0.83, 1.01) | 0.82 (0.73, 0.91) | 0.88 (0.79, 0.97) | 0.88 (0.79, 0.98) | 0.22 |
| NHS II | ||||||
| Median intake, mg/d | 0.11 | 0.20 | 0.33 | 0.52 | 2.31 | |
| No. of case/person years | 158/405480 | 136/408747 | 155/404086 | 127/404120 | 89/407379 | |
| Rate per 100,000 person-years | 39 | 33 | 38 | 31 | 22 | |
| Age-adjusted model* | 1 | 0.87 (0.69, 1.09) | 0.93 (0.75, 1.17) | 0.75 (0.59, 0.95) | 0.53 (0.41, 0.69) | <0.001 |
| Multivariable-adjusted model† | 1 | 0.92 (0.73, 1.16) | 0.93 (0.74, 1.17) | 0.68 (0.53, 0.86) | 0.62 (0.47, 0.81) | <0.001 |
| Fully-adjusted model‡ | 1 | 0.94 (0.74, 1.18) | 0.97 (0.77, 1.22) | 0.72 (0.56, 0.91) | 0.70 (0.53, 0.92) | 0.01 |
| HPFS | ||||||
| Median intake, mg/d | 0.18 | 0.29 | 0.43 | 0.66 | 2.22 | |
| No. of case/person years | 956/184211 | 883/186621 | 870/184926 | 829/184812 | 648/186166 | |
| Rate per 100,000 person-years | 519 | 473 | 470 | 449 | 348 | |
| Age-adjusted model* | 1 | 0.91 (0.83, 1.00) | 0.93 (0.85, 1.03) | 0.96 (0.87, 1.05) | 0.78 (0.71, 0.86) | <0.001 |
| Multivariable-adjusted model† | 1 | 0.91 (0.83, 1.00) | 0.93 (0.84, 1.02) | 0.93 (0.85, 1.03) | 0.82 (0.74, 0.91) | 0.001 |
| Fully-adjusted model‡ | 1 | 0.92 (0.84, 1.02) | 0.94 (0.85, 1.03) | 0.95 (0.86, 1.04) | 0.89 (0.80, 0.99) | 0.12 |
| Pooled§ | ||||||
| Age-adjusted model* | 1 | 0.91 (0.85, 0.97) | 0.90 (0.84, 0.96) | 0.96 (0.90, 1.02) | 0.79 (0.73, 0.85) | <0.001 |
| Multivariable-adjusted model† | 1 | 0.91 (0.85, 0.98) | 0.88 (0.82, 0.94) | 0.88 (0.83, 0.95) | 0.81 (0.76, 0.87) | <0.001 |
| Fully-adjusted model‡ | 1 | 0.92 (0.86, 0.98) | 0.89 (0.83, 0.95) | 0.90 (0.84, 0.96) | 0.87 (0.81, 0.94) | 0.008 |
CI, confidence interval; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study.
Cumulative average energy-adjusted intake from the 1984 questionnaire for NHS, the 1991 questionnaire for NHS II, and 1986 questionnaire for HPFS.
Estimates are calculated in Cox proportional hazards models. Age-adjusted model, adjusted for age (years);
Multivariable-adjusted model, further adjusted for ethnicity (Caucasian, African American, Asian, or other ethnicity), self-rated socioeconomic status (top 30%, median 40%, or bottom 30%, for women), partner’s education (<high school, high school, or above college, for women), smoking status (never, former, current [1–14, 15–24, or ≥25 cigarettes/day], or missing), alcohol intake (0, 0.1–4.9, 5.0–14.9, and ≥15.0 g/day for women, 0, 0.1–4.9, 5.0–29.9, and ≥30.0 g/day for men, or missing), physical activity (metabolic equivalents of tasks-hr/week), multivitamin use (yes/no), aspirin use (yes/no), history of hypertension (yes/no) and hypercholesterolemia (yes/no), family history of myocardial infarction (yes/no), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause [never, former, or current hormone use], or missing, for women), oral contraceptive use (yes, no, or missing, NHSII only), body mass index (kg/m2), and total energy intake (kcal/day) based on age-adjusted model.
Fully-adjusted model, further adjusted for modified alternative health eating index score, based on multivariable-adjusted model.
Results from each cohort were pooled using fixed-effects model.
Figure 1. Restricted cubic spline analysis of the association between isoflavone intake and coronary heart disease.
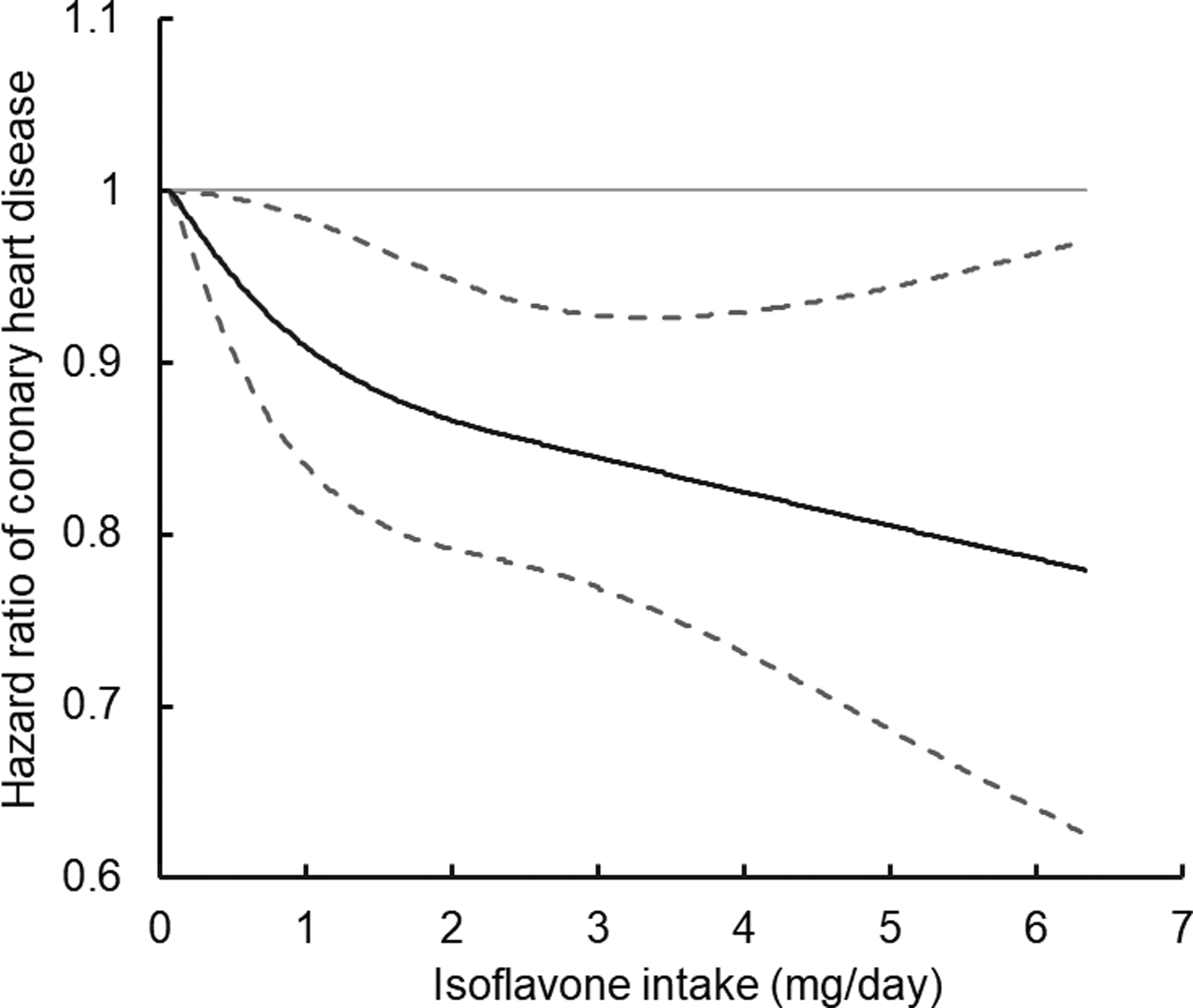
Estimates are calculated in Cox proportional hazards models. Adjusted for ethnicity (Caucasian, African American, Asian, and other ethnicity), self-rated socioeconomic status (top 30%, median 40%, and bottom 30% for women), partner’s education (<high school, high school, and above college for women), smoking status (never, former, current [1–14, 15–24, or ≥25 cigarettes/day], or missing), alcohol intake (0, 0.1–4.9, 5.0–14.9, and ≥15.0 g/day for women, 0, 0.1–4.9, 5.0–29.9, and ≥30.0 g/day for men, or missing), physical activity (metabolic equivalents of tasks-hr/week), multivitamin use (yes/no), aspirin use (yes/no), history of hypertension (yes/no) and hypercholesterolemia (yes/no), family history of myocardial infarction (yes/no), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause [never, former, or current hormone use], or missing), oral contraceptive use (yes, no, or missing, Nurses’ Health Study II only), body mass index (kg/m2), total energy intake (kcal/day), and the modified alternate healthy eating index score. Solid line is point estimate, and dashed lines are 95% confidence intervals.
Table 3 shows the HRs of incident CHD by intake of tofu and soy milk. Tofu intake was inversely associated with CHD; the fully-adjusted pooled HR (95% CI) was 0.82 (0.70, 0.95; Ptrend=0.005), comparing participants who consumed ≥1 serving/week of tofu with those who rarely consumed tofu. The pooled HR (95% CI) for every 1 serving/week increase in consumption of tofu was 0.91 (0.86, 0.97). To examine whether the observed association of tofu to CHD risk could be explained by intake of the abovementioned fats, vitamins, and minerals that exist in soy products, these nutrients were further controlled for in multivariable analysis, and the results did not change appreciably (pooled HR: 0.79; 95% CI: 0.68, 0.93; Ptrend =0.002). Additional adjustment of other flavonoids also did not change the association of tofu (data not shown). A higher consumption of soy milk was also associated with a lower CHD risk, although this association was not statistically significant (pooled HR: 0.87; 95% CI: 0.69, 1.10; Ptrend=0.41).
Table 3.
Hazard ratio (95% CI) of coronary heart disease according to consumption levels of tofu and soy milk
| Consumption level | Every 1 serving/week | P trend | |||
|---|---|---|---|---|---|
| <1 serving/month | <1 serving/week | ≥1 serving/week | |||
| Tofu | |||||
| NHS | |||||
| No. of case/person years | 3081/1574855 | 342/230210 | 85/64510 | ||
| Rate per 100,000 person-years | 196 | 149 | 132 | ||
| Fully-adjusted HR* | 1 | 0.92 (0.83, 1.03) | 0.93 (0.71, 1.23) | 0.93 (0.83, 1.04) | 0.29 |
| NHS II | |||||
| No. of case/person years | 611/16942923 | 41/252080 | 13/83435 | ||
| Rate per 100,000 person-years | 36 | 16 | 16 | ||
| Fully-adjusted HR* | 1 | 0.56 (0.40, 0.78) | 0.55 (0.31, 0.97) | 0.75 (0.57, 0.99) | 0.007 |
| HPFS | |||||
| No. of case/person years | 3230/733682 | 651/181594 | 121/44792 | ||
| Rate per 100,000 person-years | 440 | 358 | 270 | ||
| Fully-adjusted HR* | 1 | 0.96 (0.87, 1.04) | 0.80 (0.66, 0.97) | 0.92 (0.85, 0.99) | 0.03 |
| Pooled results† | 1 | 0.92 (0.86, 0.99) | 0.82 (0.70, 0.95) | 0.91 (0.86, 0.97) | 0.005 |
| Soy milk | |||||
| NHS | |||||
| No. of case/person years | 1398/1341046 | 7/9664 | 15/24942 | ||
| Rate per 100,000 person-years | 104 | 72 | 60 | ||
| Fully-adjusted HR* | 1 | 1.04 (0.49, 2.20) | 0.83 (0.50, 1.39) | 0.95 (0.86, 1.04) | 0.49 |
| NHS II | |||||
| No. of case/person years | 516/1499079 | 13/66670 | 14/107356 | ||
| Rate per 100,000 person-years | 34 | 20 | 13 | ||
| Fully-adjusted HR* | 1 | 0.73 (0.42, 1.27) | 0.52 (0.30, 0.90) | 0.87 (0.76, 1.00) | 0.02 |
| HPFS | |||||
| No. of case/person years | 716/470129 | 11/14399 | 48/39368 | ||
| Rate per 100,000 person-years | 152 | 76 | 122 | ||
| Fully-adjusted HR* | 1 | 0.67 (0.36, 1.22) | 1.04 (0.76, 1.41) | 1.02 (0.98, 1.06) | 0.80 |
| Pooled results† | 1 | 0.77 (0.53, 1.10) | 0.87 (0.69, 1.10) | 1.00 (0.96, 1.03) | 0.41 |
CI, confidence interval; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; NHS, Nurses’ Health Study.
Estimates are calculated in Cox proportional hazards models. Adjusted for ethnicity (Caucasian, African American, Asian, or other ethnicity), self-rated socioeconomic status (top 30%, median 40%, or bottom 30% for women), partner’s education (<high school, high school, or above college for women), smoking status (never, former, current [1–14, 15–24, or ≥25 cigarettes/day], or missing), alcohol intake (0, 0.1–4.9, 5.0–14.9, and ≥15.0 g/day for women, 0, 0.1–4.9, 5.0–29.9, and ≥30.0 g/day for men, or missing), physical activity (metabolic equivalents of tasks-hr/week), multivitamin use (yes/no), aspirin use (yes/no), history of hypertension (yes/no) and hypercholesterolemia (yes/no), family history of myocardial infarction (yes/no), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause [never, former, or current hormone use], or missing), oral contraceptive use (yes, no, or missing, Nurses’ Health Study II only), body mass index (kg/m2), total energy intake (kcal/day), and the modified alternate healthy eating index score.
Results from each cohort were pooled using fixed-effects model.
Menopausal status and HT significantly modulated the association of tofu intake with CHD risk (Table 4). The HRs (95% CIs) comparing participants who consumed ≥1 serving/week of tofu with those who rarely consumed tofu were 0.45 (0.16, 1.23; Ptrend = 0.02) among premenopausal women, 0.51 (0.26, 0.99; Ptrend = 0.03) among postmenopausal women without hormone use, and 1.04 (0.76, 1.42; Ptrend = 0.87) among postmenopausal women receiving HT (Pinteraction=0.002). When premenopausal women and postmenopausal women without HT were further pooled to achieve better statistical power, comparing the extreme categories of tofu intake, the HR (95% CI) was 0.50 (0.29, 0.87; Ptrend =0.002) in these women (Pinteraction= 0.005). In addition, the inverse association between isoflavone intake and CHD risk tended to be stronger among premenopausal women (HR: 0.64, 95% CI: 0.45, 0.93), although the test for interaction test did not achieve statistical significance (Pinteraction= 0.09, Table 4).
Table 4.
The association of isoflavone/tofu intake and coronary heart disease among women by menopausal status and postmenopausal hormone use
| Consumption levels | P trend | P interaction† | |||||
|---|---|---|---|---|---|---|---|
| Isoflavones | Q1 of intake | Q2 of intake | Q3 of intake | Q4 of intake | Q5 of intake | 0.09 | |
| Premenopause | |||||||
| No. of case/person years | 74/296580 | 70/306544 | 81/296294 | 71/298524 | 52/311546 | ||
| Rate per 100,000 person-years | 25 | 23 | 27 | 24 | 17 | ||
| Fully-adjusted HR* | 1 | 0.96 (0.69, 1.33) | 1.06 (0.77, 1.45) | 0.77 (0.55, 1.07) | 0.64 (0.45, 0.93) | 0.01 | |
| Postmenopausal, never used hormone | |||||||
| No. of case/person years | 214/107333 | 208/114726 | 209/120824 | 252/127021 | 193/118253 | ||
| Rate per 100,000 person-years | 199 | 181 | 173 | 198 | 163 | ||
| Fully-adjusted HR* | 1 | 0.88 (0.73, 1.07) | 0.81 (0.67, 0.98) | 0.88 (0.73, 1.07) | 0.85 (0.70, 1.04) | 0.28 | |
| Postmenopausal, hormone use | |||||||
| No. of case/person years | 452/278805 | 439/275381 | 387/280382 | 393/277644 | 379/285889 | ||
| Rate per 100,000 person-years | 162 | 159 | 138 | 142 | 133 | ||
| Fully-adjusted HR* | 1 | 0.95 (0.83, 1.08) | 0.81 (0.71, 0.93) | 0.81 (0.71, 0.93) | 0.90 (0.78, 1.04) | 0.28 | |
| Tofu | Non-consumer | <1 serving/week | ≥1 serving/week | 0.002 | |||
| Premenopause | |||||||
| No. of case/person years | 331/1292926 | 13/162637 | 4/53924 | ||||
| Rate per 100,000 person-years | 26 | 8 | 7 | ||||
| Fully-adjusted HR* | 1 | 0.40 (0.23, 0.71) | 0.45 (0.16, 1.23) | 0.02 | |||
| Postmenopausal, never used hormone | |||||||
| No. of case/person years | 971/493336 | 90/72316 | 15/22505 | ||||
| Rate per 100,000 person-years | 197 | 124 | 67 | ||||
| Fully-adjusted HR* | 1 | 0.91 (0.73, 1.12) | 0.51 (0.26, 0.99) | 0.03 | |||
| Postmenopausal, hormone use | |||||||
| No. of case/person years | 1753/1137658 | 234/204859 | 63/55584 | ||||
| Rate per 100,000 person-years | 154 | 114 | 113 | ||||
| Fully-adjusted HR* | 1 | 0.91 (0.80, 1.05) | 1.04 (0.76, 1.42) | 0.87 | |||
HR, hazard ratio; Q, Quintile
Estimates are calculated in Cox proportional hazards models. Adjusted for ethnicity (Caucasian, African American, Asian, or other ethnicity), self-rated socioeconomic status (top 30%, median 40%, or bottom 30% for women), partner’s education (<high school, high school, or above college for women), smoking status (never, former, current [1–14, 15–24, or ≥25 cigarettes/day], or missing), alcohol intake (0, 0.1–4.9, 5.0–14.9, and ≥15.0 g/day for women, 0, 0.1–4.9, 5.0–29.9, and ≥30.0 g/day for men, or missing), physical activity (metabolic equivalents of tasks-hr/week), multivitamin use (yes/no), aspirin use (yes/no), history of hypertension (yes/no) and hypercholesterolemia (yes/no), family history of myocardial infarction (yes/no), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause [never, former, or current hormone use], or missing), oral contraceptive use (yes, no, or missing, Nurses’ Health Study II only), body mass index (kg/m2),total energy intake (kcal/day), and the modified alternate healthy eating index score.
Pinteraction was calculated using likelihood-ratio test.
Race, sex, BMI, modified AHEI score, physical activity, smoking status, or alcohol consumption did not modulate these associations of interest (all Pinteraction> 0.05; Supplemental Table 4 for isoflavones and Supplemental Table 5 for tofu intake). In the sensitivity analyses in which only baseline dietary isoflavone intake was used, the association was attenuated to the null (Supplemental Table 6). Adjustment for other major dietary variables instead of AHEI score did not materially alter the association between isoflavones and CHD risk, and the HR (95% CI) comparing the extreme quintiles was 0.83 (0.78, 0.90). The results did not change when a 4-year lag (HR comparing extreme quintiles: 0.86; 95% CI: 0.80, 0.93) or 8-year lag (HR comparing the extreme quintiles: 0.88; 95% CI: 0.81, 0.95) were added between isoflavone intake assessments and each follow-up period. Results were similar after excluding Asian participants (HR comparing the extreme quintiles: 0.88; 95% CI: 0.82, 0.94). The exclusion of probable CHD events also did not alter the results (HR comparing the extreme isoflavone quintiles: 0.87; 95% CI: 0.80, 0.95). Excluding participants who reported the use of soy supplements (2.2% of total population) did not materially change the results (HR comparing the extreme isoflavone quintiles: 0.88; 95% CI: 0.82, 0.94). These results also remained robust in several similar sensitivity analyses for the associations of tofu intake (Supplemental Table 6).
Discussion
In these three prospective cohorts of US men and women with over two decades of follow-up, a higher isoflavone intake was associated with a moderately lower risk of CHD. Consumption of tofu was also significantly, inversely associated with the risk of CHD. In women, the inverse association of tofu was more pronounced in younger women before menopause and postmenopausal women receiving HT, whereas a null association was observed among postmenopausal women who used HT.
A few prospective observational studies have been conducted to examine associations between isoflavone intake and cardiovascular disease (CVD) risk. In a cohort of 40,462 Japanese men and women, a significant inverse association between isoflavone intake and risk of MI was observed in women but not in men.6 In studies conducted in western populations whose isoflavone intake was much lower than that in Asian populations, this association was largely null. For example, in the Iowa Women’s Study consisting of 34,489 postmenopausal women (37% reporting ever use of hormone), higher isoflavone intake was not associated with CHD mortality during 16y of follow-up.7 In the Cancer Prevention Study II Nutrition Cohort, there was a modest, inverse association observed for women but not for men.18 Lastly, Ponzo et al. followed 1,658 individuals for 12 years and observed that isoflavone consumption was associated with a non-significantly lower risk of CVD and CVD mortality.19 Of note, the magnitude of associations of interest observed in these studies was similar to that in the current analyses, although the current investigation was based on a much larger sample size, which rendered more statistical power for detecting modest associations. In addition, the present study accounted for potential changes in diet during extended follow-up by using cumulative averages of repeated assessments of diet to characterize long-term diet. In a sensitivity analysis in which only baseline isoflavone intake was used to predict CHD risk, the association was attenuated.
Several biological mechanisms can be proposed to explain the putative beneficial effects of isoflavones on CHD. Isoflavones have a spatial configuration similar to that of estrogens, preferably bind to ER, and lead to subsequent ER-mediated gene transcription.8, 20 The higher binding affinity of isoflavones is stronger for ERβ, compared with ERα.21 ERβ is highly expressed in coronary vessels,22 and the activation of membrane ERβ initiates a cascade of intracellular mechanisms, including changes in membrane permeability, ion concentration, expression of endothelial NO-synthase, and rapid vasodilatation of blood vessels.23–25 Through stimulating endothelial NO synthesis and inducing activation of nuclear factor erythroid 2-related factor 2, soy isoflavones counteracted oxidative stress and related inflammation in vascular cells in vitro.26 In vivo, isoflavones improved endothelium-dependent vasodilation in ovariectomized rats fed high soy diet for four weeks.27 Similar effects were observed in postmenopausal women after 6 months of supplementation of isoflavones.28 Isoflavones may also exert hypocholesterolemic effects by altering hepatic metabolism with augmented removal of LDL and very low-density lipoprotein by hepatocytes.29 A study in ApoE (−/−) mice demonstrated the effect of isoflavones on alleviating hypercholesterolemia via restoration of altered cholesterol metabolism.30 Isoflavones may also reverse ox-LDL-induced oxidative damage in vitro.31 In humans, accumulating evidence from randomized controlled trials also suggested beneficial effects of isoflavone consumption on reducing LDL oxidation in postmenopausal women and patients with type 2 diabetes.32 Lastly, Isoflavones may interact with microbiota and produce bioactive compounds that exert beneficial effects on human health. For example, isoflavone supplementation among postmenopausal women led to the enrichment of Faecalibacterium prausnitzii, Bifidobacteria, and other bacteria in human gut that have anti-inflammatory properties.33, 34 Individual isoflavones such as daidzein, genistein and glycitein, can be converted by gut bacteria into different metabolites, such as equol, O-desmethylangolensin, dihydrogenistein, and dihydrodaidzein.35 In particular, equol, the main metabolite of daidzein, exhibits superior antioxidant activity compared with other isoflavone metabolite,36 and may account for the anti-inflammatory activities of isoflavone intake,37 although in western populations the proportion of equol producers is low.38, 39 In the current study, similar associations for all three individual isoflavones were observed, suggesting that the metabolic differences among the individual isoflavones are probably not critical for determining the associations with CHD in the current analysis.
The findings of significant, favorable associations of tofu intake were consistent with results for total isoflavones. In addition to isoflavones, tofu also contains several bioactive components such as polyunsaturated fats, fiber, and minerals that may act synergistically with isoflavones through various pathways to modulate CHD risk,40 although the inverse association for tofu persisted after adjustment for these nutrients in the current study. No significant association was observed for soy milk, potentially because of its lower consumption levels and the shorter duration of follow-up in the current analysis. Differences in the nutritional profiles between tofu and soy milk could also lead to differential associations with CHD risk. Unlike tofu, soy milk may contain added sugar, emulsifiers, and other constituents, which may mask the protective effects of isoflavones on CHD risk to certain extent.12, 41
Another interesting finding is that in women the inverse association of tofu was primarily observed in younger women before menopause and postmenopausal women who did not use postmenopausal hormone. Similar pattern of associations was observed for isoflavone intake, although the test for interaction did not achieve statistical significance. Of note, a previous analysis in the NHS and NHSII also found that urinary excretion of daidzein was more strongly associated with a lower risk of diabetes among postmenopausal women who did not use hormones.42 One possible mechanism underlying the divergent associations on CHD by menopausal status and hormone use is perhaps that upon menopause, isoflavones may function as estrogens when circulating levels of estrogen are low and subsequently exert estrogen-like effects.43 It is possible that among postmenopausal women who use hormones, the health effects of isoflavones are likely to be masked by hormone use, which is in accordance with the previous findings that the synthetic hormones have stronger affinities and estrogenic effects than isoflavones.44 Another mechanism is probably pertinent to the higher expression level of estrogen receptors in the vasculature before menopause,45, 46 and thus the beneficial effects of isoflavones are stronger among younger women before menopause. Nonetheless, more mechanistic studies are needed to elucidate the complex relationships between isoflavones, menopause, hormone use, and CHD risk.
The strengths of the current study include large sample size, high follow-up rate, long follow-up period, repeated assessments of dietary assessments, and the availability of comprehensive information on dietary and non-dietary covariates to facilitate control of confounding by these factors. There are also several limitations that are worth discussing. First, it is possible that the observed associations are ascribed to potential additive or synergistic effects between isoflavones and other nutrients and non-nutrient constituents in soy foods, although the examination of these interactions requires much larger sample size and is beyond the scope of this study. Second, measurement errors in dietary assessments are inevitable, especially for tofu and soy milk that are not widely consumed in western populations. Because of the prospective study design, the measurement errors were independent of the disease outcome ascertainment and thus were more likely to bias the true associations toward the null. Use of repeated measurements of diet to calculate cumulative means for dietary intakes reduces random measurement error caused by within-person variation and also represent long-term dietary habits. Third, other types of soy foods (e.g., tempeh, soy yoghurt, and soy cheese) or cooking/preparation methods for soy foods were not inquired about in the FFQ. In intervention trials conducted among free-living individuals, urinary isoflavone excretion did not differ after consumption of different types of soy foods (texturized vegetable protein, tofu, cooked soybeans, or tempeh), suggesting that the intake or bioavailability of isoflavones was not appreciably influenced by the preparation methods or types of soy foods.47, 48 Fourth, the cohort participants were mainly comprised of health professionals of European ancestry; thus, the generalization of the current findings to other demographic or ethnic groups may be limited. Meanwhile, the homogeneity of socioeconomic status helps reduce potential confounding by this factor. Fifth, tofu intake may be just a marker of health consciousness or an overall plant-based diet. Although socioeconomic status, diet quality, and other dietary and lifestyle risk factors were adjusted for, residual or unmeasured confounding may still exist and thus a causal inference may be difficult to establish in this observational study. As such, the current findings warrant replication in other populations and intervention studies on cardiovascular risk factors to establish causal relationship. Sixth, the role of chance in some of the findings cannot be ruled out, especially for the interactions between tofu intake and menopausal status/hormone use. Lastly, the strength of associations of interest varied among the three cohorts. In particular, in women stronger association was observed in the NHSII than NHS, probably because of lower intake of isoflavones and tofu intake in the NHS and differential proportions of menopausal status and hormone use in these two female cohorts.
Conclusions
In three US cohorts of men and women, higher intake of isoflavones and tofu was associated with a moderately lower risk of developing CHD risk. In women, the inverse association of tofu intake was primarily observed in premenopausal women and postmenopausal women who did not use hormones. While these associations warrant replications in other populations, as well as in intervention studies on CVD risk factors, the present study overall implied that tofu and other soy products could be incorporated into overall healthy plant-base diets to facilitate the prevention of CHD.
Supplementary Material
Clinical Perspective.
What Is New?
Intake of isoflavones and tofu was associated with a lower CHD risk in three large prospective cohorts of US men and women.
The inverse association of tofu was primarily observed among younger women before menopause or postmenopausal women who did not use hormone replacement therapy.
What Are the Clinical Implications?
Increased intake of isoflavone-rich foods, such as tofu, may confer benefits for reducing CHD risk.
Soy products such as tofu can be integrated into healthy plant-based diets as an important source of plant proteins and aid in the prevention of CHD.
Acknowledgments
We would like to thank the participants, the Channing Division of Network Medicine, as well as staff of the NHS and the HPFS for their valuable contributions. QS and LM participated in project conception and development of research methods; QS, FBH, WCW, EBR, EBR, and JEM obtained funding and provided oversight; LM, GL, MD, GZ and QS analyzed data and performed analysis; LM and QS drafted the manuscript.
Sources of Funding
This study was funded by the National Institutes of Health (CA186107, HL034594, CA176726, CA167552, HL60712, HL035464, and DK120870). The study sponsor had no role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Non-standard Abbreviations and Acronyms
- AHEI
alternative healthy eating index
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- ER
estrogen receptor
- FDA
Food and Drug Administration
- FFQ
food frequency questionnaire
- HPFS
Health Professionals Follow-Up Study
- HR
hazard ratio
- HT
hormone therapy
- LDL
low-density lipoprotein
- MI
myocardial infarction
- NHS
Nurses’ Health Study
- NO
nitric oxide
- Q
quintile
Footnotes
Disclosures
All authors have no conflict of interest to disclose.
Reference List
- 1.Food labeling: health claims; soy protein and coronary heart disease. Food and Drug Administration, HHS. Final rule. Fed Regist. 1999;64:57700–33 [PubMed] [Google Scholar]
- 2.Petersen KS. The Dilemma With the Soy Protein Health Claim. J Am Heart Assoc. 2019;8:e013202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P and Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–44 [DOI] [PubMed] [Google Scholar]
- 4.Zahradka P, Wright B, Weighell W, Blewett H, Baldwin A, O K, Guzman RP and Taylor CG. Daily non-soy legume consumption reverses vascular impairment due to peripheral artery disease. Atherosclerosis. 2013;230:310–4 [DOI] [PubMed] [Google Scholar]
- 5.Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA and Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care. 2012;35:226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S and Group JS. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation. 2007;116:2553–2562 [DOI] [PubMed] [Google Scholar]
- 7.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong C-P, Nettleton JA and Jacobs DR Jr., Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909 [DOI] [PubMed] [Google Scholar]
- 8.Jiang Q, Payton-Stewart F, Elliott S, Driver J, Rhodes LV, Zhang Q, Zheng S, Bhatnagar D, Boue SM, Collins-Burow BM, Sridhar J, Stevens C, McLachlan JA, Wiese TE, Burow ME and Wang G. Effects of 7-O substitutions on estrogenic and anti-estrogenic activities of daidzein analogues in MCF-7 breast cancer cells. J Med Chem. 2010;53:6153–6163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belanger CF, Hennekens CH, Rosner B and Speizer FE. The nurses’ health study. Am J Nurs. 1978;78:1039–1040 [PubMed] [Google Scholar]
- 10.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, Stampfer MJ, Speizer FE, Spiegelman D and Manson JE. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278:1078–1083 [PubMed] [Google Scholar]
- 11.Grobbee DE, Rimm EB, Giovannucci E, Colditz G, Stampfer M and Willett W. Coffee, caffeine, and cardiovascular disease in men. N Engl J Med. 1990;323:1026–1032 [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Agriculture, Agricultural Research Service. USDA Database for the Isoflavone Content of Selected Foods, Release 2.1. Nutrient Data Laboratory Home Page: http://www.ars.usda.gov/nutrientdata/isoflav. Accessed Dec 29, 2019 2015. [Google Scholar]
- 13.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB and Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796 [DOI] [PubMed] [Google Scholar]
- 14.Sun Q, Bertrand KA, Franke AA, Rosner B, Curhan GC and Willett WC. Reproducibility of urinary biomarkers in multiple 24-h urine samples. Am J Clin Nutr. 2017;105:159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ and Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose GA, Blackburn H, Gillum R and Prineas R. Cardiovascular survey methods: Geneva, Switzerland; WHO; 1982. [Google Scholar]
- 17.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D and Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540 [DOI] [PubMed] [Google Scholar]
- 18.McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R and Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012;95:454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponzo V, Goitre I, Fadda M, Gambino R, De Francesco A, Soldati L, Gentile L, Magistroni P, Cassader M and Bo S. Dietary flavonoid intake and cardiovascular risk: a population-based cohort study. J Transl Med. 2015;13:218–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katchy A, Pinto C, Jonsson P, Nguyen-Vu T, Pandelova M, Riu A, Schramm K-W, Samarov D, Gustafsson J-Å, Bondesson M and Williams C. Coexposure to phytoestrogens and bisphenol a mimics estrogenic effects in an additive manner. Toxicol Sci. 2014;138:21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y, Gong P, Madak-Erdogan Z, Martin T, Jeyakumar M, Carlson K, Khan I, Smillie TJ, Chittiboyina AG, Rotte SCK, Helferich WG, Katzenellenbogen JA and Katzenellenbogen BS. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013;27:4406–4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christian RC, Liu PY, Harrington S, Ruan M, Miller VM and Fitzpatrick LA. Intimal Estrogen Receptor (ER)β, But Not ERα Expression, Is Correlated with Coronary Calcification and Atherosclerosis in Pre- and Postmenopausal Women. The Journal of Clinical Endocrinology & Metabolism. 2006;91:2713–2720 [DOI] [PubMed] [Google Scholar]
- 23.Siow RCM and Mann GE. Dietary isoflavones and vascular protection: activation of cellular antioxidant defenses by SERMs or hormesis? Mol Aspects Med. 2010;31:468–477 [DOI] [PubMed] [Google Scholar]
- 24.Siow RCM, Li FYL, Rowlands DJ, de Winter P and Mann GE. Cardiovascular targets for estrogens and phytoestrogens: transcriptional regulation of nitric oxide synthase and antioxidant defense genes. Free Radic Biol Med. 2007;42:909–925 [DOI] [PubMed] [Google Scholar]
- 25.Menazza S and Murphy E. The Expanding Complexity of Estrogen Receptor Signaling in the Cardiovascular System. Circ Res. 2016;118:994–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann GE, Rowlands DJ, Li FYL, de Winter P and Siow RCM. Activation of endothelial nitric oxide synthase by dietary isoflavones: role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc Res. 2007;75:261–274 [DOI] [PubMed] [Google Scholar]
- 27.Schreihofer DA, Deutsch C, Lovekamp-Swan T, Sullivan JC and Dorrance AM. Effect of high soy diet on the cerebrovasculature and endothelial nitric oxide synthase in the ovariectomized rat. Vascul Pharmacol. 2010;52:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irace C, Marini H, Bitto A, Altavilla D, Polito F, Adamo EB, Arcoraci V, Minutoli L, Di Benedetto A, Di Vieste G, de Gregorio C, Gnasso A, Corrao S, Licata G and Squadrito F. Genistein and endothelial function in postmenopausal women with metabolic syndrome. Eur J Clin Invest. 2013;43:1025–1031 [DOI] [PubMed] [Google Scholar]
- 29.Yan L-P, Chan S-W, Chan AS-C, Chen S-L, Ma X-J and Xu H-X. Puerarin decreases serum total cholesterol and enhances thoracic aorta endothelial nitric oxide synthase expression in diet-induced hypercholesterolemic rats. Life Sci. 2006;79:324–330 [DOI] [PubMed] [Google Scholar]
- 30.Jeon S, Park Y-J and Kwon YH. Genistein alleviates the development of nonalcoholic steatohepatitis in ApoE(−/−) mice fed a high-fat diet. Mol Nutr Food Res. 2014;58:830–841 [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Zhao Z, Pang X, Yang J, Yu H, Zhang Y, Zhou H and Zhao J. MiR-34a/sirtuin-1/foxo3a is involved in genistein protecting against ox-LDL-induced oxidative damage in HUVECs. Toxicol Lett. 2017;277:115–122 [DOI] [PubMed] [Google Scholar]
- 32.Clerici C, Nardi E, Battezzati PM, Asciutti S, Castellani D, Corazzi N, Giuliano V, Gizzi S, Perriello G, Di Matteo G, Galli F and Setchell KDR. Novel soy germ pasta improves endothelial function, blood pressure, and oxidative stress in patients with type 2 diabetes. Diabetes Care. 2011;34:1946–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clavel T, Fallani M, Lepage P, Levenez F, Mathey J, Rochet V, Sérézat M, Sutren M, Henderson G, Bennetau-Pelissero C, Tondu F, Blaut M, Doré J and Coxam V. Isoflavones and functional foods alter the dominant intestinal microbiota in postmenopausal women. J Nutr. 2005;135:2786–2792 [DOI] [PubMed] [Google Scholar]
- 34.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux J-J, Blugeon S, Bridonneau C, Furet J-P, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P and Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–16736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selma MV, Espín JC and Tomás-Barberán FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57:6485–6501 [DOI] [PubMed] [Google Scholar]
- 36.Hwang J, Wang J, Morazzoni P, Hodis HN and Sevanian A. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: an antioxidant mechanism for cell-mediated LDL modification. Free Radic Biol Med. 2003;34:1271–1282 [DOI] [PubMed] [Google Scholar]
- 37.Yum MK, Jung MY, Cho D and Kim TS. Suppression of dendritic cells’ maturation and functions by daidzein, a phytoestrogen. Toxicol Appl Pharmacol. 2011;257:174–181 [DOI] [PubMed] [Google Scholar]
- 38.Setchell KDR, Brown NM and Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584 [DOI] [PubMed] [Google Scholar]
- 39.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H and Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32 [DOI] [PubMed] [Google Scholar]
- 40.Setchell KDR, Brown NM, Summer S, King EC, Heubi JE, Cole S, Guy T and Hokin B. Dietary factors influence production of the soy isoflavone metabolite s-(−)equol in healthy adults. J Nutr. 2013;143:1950–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE and Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding M, Franke AA, Rosner BA, Giovannucci E, van Dam RM, Tworoger SS, Hu FB and Sun Q. Urinary isoflavonoids and risk of type 2 diabetes: a prospective investigation in US women. Br J Nutr. 2015;114:1694–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messina MJ and Wood CE. Soy isoflavones, estrogen therapy, and breast cancer risk: analysis and commentary. Nutr J. 2008;7:17–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG and Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–1567 [DOI] [PubMed] [Google Scholar]
- 45.Mendelsohn ME and Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587 [DOI] [PubMed] [Google Scholar]
- 46.Gavin KM, Seals DR, Silver AE and Moreau KL. Vascular Endothelial Estrogen Receptor α Is Modulated by Estrogen Status and Related to Endothelial Function and Endothelial Nitric Oxide Synthase in Healthy Women. The Journal of Clinical Endocrinology & Metabolism. 2009;94:3513–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tew BY, Xu X, Wang HJ, Murphy PA and Hendrich S. A diet high in wheat fiber decreases the bioavailability of soybean isoflavones in a single meal fed to women. J Nutr. 1996;126:871–877 [DOI] [PubMed] [Google Scholar]
- 48.Xu X, Wang HJ, Murphy PA and Hendrich S. Neither background diet nor type of soy food affects short-term isoflavone bioavailability in women. J Nutr. 2000;130:798–801 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


