Abstract
Objective:
This study compared the effects of alternate day fasting (ADF) to daily calorie restriction (CR) on body weight and glucoregulatory factors in adults with overweight/obesity and insulin resistance.
Methods:
This secondary analysis examined the data of insulin resistant subjects (n = 43) who participated in a 12-month study comparing ADF (25% energy needs on “fast days”; 125% needs on alternating “feast days”) to CR (75% energy needs every day) and a control group.
Results:
In insulin resistant subjects, weight loss was not different between ADF (−8 ± 2%) and CR (−6 ± 1%) by month 12, relative to controls (P < 0.0001). Fat mass and BMI decreased (P < 0.05) similarly by ADF and CR. ADF produced greater decreases (P < 0.05) in fasting insulin (−52 ± 9%) and insulin resistance (HOMA-IR) (−53 ± 9%) when compared to CR (−14 ± 9%; −17 ± 11%) and controls by month 12. Lean mass, visceral fat mass, LDL cholesterol, HDL cholesterol, triglycerides, blood pressure, CRP, TNF-α, and IL-6 remained unchanged.
Conclusions:
These findings suggest that ADF may produce greater reductions in fasting insulin and insulin resistance versus CR, in insulin resistant subjects, despite similar decreases in body weight.
Keywords: Insulin resistance, fasting, calorie restriction, metabolic disease, adults with overweight and obesity
Introduction
Current guidelines for the treatment of obesity recommend moderate calorie restriction (CR; 20–30% reduction in energy needs daily) (1, 2). Another form of dietary restriction that has gained attention in recent years is alternate day fasting (ADF) (3, 4). ADF regimens generally consist of an ad libitum “feast day” alternated with a 25% energy intake “fast day”. Accumulating evidence suggests that ADF and CR produce similar weight loss (6–8%) over 2–12 months in healthy adults with overweight and obesity (5, 6, 7, 8). Research also indicates that both diets are safe and well tolerated (5, 6, 9).
Whether these diets produce comparable changes in glucoregulatory factors, however, is less clear. In a study by Harvie et al (7), women with overweight participated in an intermittent fasting (two ~650 kcal fast days per week) or daily CR regimen (~1500 kcal daily) for 6 months. By the end of the study, both groups lost similar amounts of weight, but the fasting subjects observed greater decreases in fasting insulin and insulin resistance when compared to CR. In line with these findings, Hutchison et al (10), demonstrated superior reductions in fasting glucose and insulin resistance by ADF (three 0 kcal fast days per week) versus CR (~1500 kcal daily), after 2 months of treatment in women with overweight. In contrast, Catenacci et al (6), observed no change in fasting insulin or insulin sensitivity by ADF (three 0 kcal fast days per week) or CR (~1600 kcal daily) after 2 months in men and women with overweight and obesity.
While these studies offer valuable preliminary data (6, 7, 10), they are limited in that they enrolled only metabolically healthy individuals. A key question that remains unknown is whether ADF produces superior changes in glucoregulatory factors versus CR, in populations at risk for developing diabetes (i.e. insulin resistant subjects with overweight and obesity). Accordingly, the goal of this study was to compare the effects of ADF to daily CR on body weight and glucoregulatory factors in subjects with insulin resistance and overweight/obesity. We hypothesized that ADF would produce greater reductions in insulin resistance versus CR in this sample, despite similar weight loss.
Methods
Subject selection
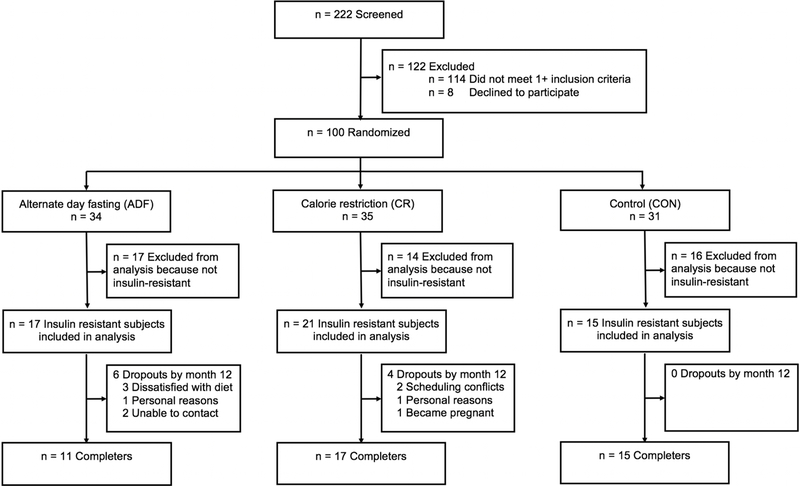
This is a secondary analysis of a study that compared the effects of ADF versus CR on body weight (5). Independently living subjects were recruited from the University of Illinois, Chicago campus by flyers. Participants were included if they were 18–65 years old; BMI 25.0–39.9 kg/m2; and previously inactive (<60 minutes/week of light activity for the 3 months prior to the study). Individuals were excluded if they had a history of type 1 or type 2 diabetes, cardiovascular disease, were taking weight loss medications, were not weight stable for 3 months prior to the beginning of the study (> 4 kg weight loss or gain), were peri-menopausal, pregnant, or smokers. Subjects (n = 100) were randomized by a stratified random sample (based on age, sex, and BMI) to 1 of 3 groups for 12-months: 1) ADF (n = 34); 2) CR (n = 35); or 3) control (n = 31) (Figure 1). All participants provided informed consent to participate in this study. The protocol was approved by the Office for the Protection of Research Subjects at the University of Illinois, Chicago.
Figure 1.
Study flow chart
Alternate day fasting (ADF) and calorie restriction (CR) group protocols
The 12-month trial included a 6-month weight loss phase followed by a 6-month weight maintenance phase (5). Baseline total energy expenditure was measured using doubly labeled water (DLW) (11). DLW was used to estimate energy needs for the energy intake prescriptions. During the 6-month weight loss phase, ADF and CR subjects reduced their net energy intake by approximately 25% per day. To achieve this, ADF participants consumed 25% of baseline energy needs as a lunch (between 12:00 pm and 2:00 pm) on fast days, and 125% of baseline energy needs over 3 meals on alternating feast days. CR participants consumed 75% of baseline energy needs over 3 meals every day. From months 0 to 3, ADF and CR subjects were provided with all meals. The meals were picked up from the research center on weekly basis, and transported via a rolling insulated cooler. All of the provided meals were consumed outside of the research center. The provided meals had the following macronutrient distribution: 30% of energy as fat, 55% of energy as carbohydrate, and 15% of energy as protein. From months 4 to 6, when food was no longer provided, intervention participants met one-on-one each week with a nutritionist to learn how to continue with their diets on their own. During the 6-month weight maintenance phase, participants were instructed to maintain their body weight. ADF subjects consumed 50% of energy needs as a lunch on fast days and 150% of energy needs over 3 meals on alternating feast days. CR subjects consumed 100% of energy needs over 3 meals every day. During the maintenance phase, ADF and CR participants met one-on-one each month with a nutritionist to learn cognitive behavioral strategies to prevent weight regain (12).
Control group protocol
Controls were asked to maintain their body weight during the 12-month trial by not changing their usual eating and activity habits. Control subjects did not receive any food or dietary counselling, but visited the research center at the same frequency as the intervention groups to control for any investigator-interaction bias. During their visits, the study coordinator measured their body weight and blood pressure, and also asked each control if they had made any changes to their eating or activity habits.
Adherence, dietary intake and physical activity assessment
Dietary intake and adherence were measured using a 7-day food record, and analyzed with Nutritionist Pro software (Axxya Systems, Stafford, TX). Adherence to the ADF and CR interventions was assessed by comparing actual versus prescribed energy intakes at month 6 and 12. All subjects were instructed to maintain their level of physical activity during the 12-month study. Activity was assessed over a 7-d period at baseline, month 6 and 12 by a validated (13) pattern recognition monitor (Sense Wear Mini, Bodymedia, Pittsburg, PA).
Body weight and body composition assessment
Body weight was measured monthly after an overnight fast (after a feast day) in a hospital gown by a digital scale (HBF-500, Omron, Bannockburn, IL). Fat mass and lean mass were assessed in the fasted state at baseline, month 6 and 12 (after a feast day) by dual energy X-ray absorptiometry (DXA; QDR 4500W, Hologic, Arlington, MA). Visceral fat mass was measured at baseline, month 6 and 12 with a 1.5-tesla magnet (Siemens, Erlangen, Germany), as described previously (14). Subjects were instructed to lie in the magnet in a supine position with arms extended above the head. Contiguous slices were obtained every 1 cm from the ninth thoracic vertebra to the first sacral vertebra. This resulted in a range of 21 to 35 MRI axial images per person, depending on the subject’s height. Images were analyzed using validated software (14).
Metabolic disease risk factor quantification
Blood samples were obtained following a 12-hour fast at baseline, month 6 and 12 (after a feast day in the ADF group). All participants were asked to avoid exercise, alcohol, and coffee for 12 h before each visit. Samples were centrifuged for 10 min at 1000 ☓ g at 4°C to separate plasma from red cells and were stored at −80°C until analyzed. Plasma total cholesterol, direct LDL cholesterol, HDL-cholesterol, and triglyceride concentrations were measured in duplicate using enzymatic kits (Roche Diagnostics, Indianapolis, IN). Glucose was quantified using the glucose oxidase procedure (Beckman Autoanalyser II, Beckman Coulter Inc, Fullerton, CA). Insulin was quantified using an electrochemiluminescence assay (Pacific Biomarkers, Seattle, WA). Insulin resistance was calculated by applying the Homeostatic Model Assessment-Insulin Resistance (HOMA-IR) formula = insulin (μIU/mL) x glucose (mg/dL)/405. Participants with HOMA-IR values > 2.73 were considered insulin resistant (15, 16). Blood pressure and heart rate was assessed at baseline, month 6 and 12 after the weigh-in following a 10 min rest. C-reactive protein (CRP) was measured by Immulite 1000 High Sensitivity CRP kits (Diagnostic Products Corporation, Los Angeles, CA). Plasma tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were measured by ELISA (R&D Systems, Minneapolis, MN).
Statistical analysis
Results are presented as means ± standard error of the mean (SEM). Normality was assessed by the Kolmogorov-Smirnov test, and no variables were found to be skewed. One-way ANOVA with a Tukey post hoc test was used to test the differences among groups at baseline, and percent change differences from baseline to month 6 or 12. ANCOVA with baseline as a covariate was used to test the differences between groups at month 6 and month 12. Bonferroni post-hoc tests were used for these comparisons. A two-tailed P-value of less than 0.05 was used for statistical significance. Data were analyzed by using SPSS software for Mac (v.25, SPSS Inc, Chicago, IL).
Results
Baseline characteristics of insulin resistant subjects
For the present study, only subjects with insulin resistance who completed the entire 12-month study were included in the analysis (insulin resistance defined as a HOMA-IR value > 2.73 (15, 16)). The total number of completers with insulin resistance in each intervention group was as follows: ADF n = 11, CR n = 17, control n = 15 (Figure 1). Baseline characteristics of the insulin resistant subjects were comparable between the ADF, CR, and control groups (Table 1).
Table 1.
Baseline characteristics of insulin resistant subjects 1
| Characteristic | Alternate Day Fasting | Calorie Restriction | Control | P-value 2 |
|---|---|---|---|---|
| Age – yr | 43 ± 3 | 42 ± 3 | 41 ± 3 | 0.96 |
| Sex – no. | ||||
| Female | 9 | 13 | 11 | 0.64 |
| Male | 2 | 4 | 4 | |
| Race or ethnic group – no. (%) | 0.76 | |||
| White | 5 | 7 | 6 | |
| Black | 4 | 9 | 9 | |
| Asian | 1 | 0 | 0 | |
| Hispanic | 1 | 1 | 0 | |
| Other | 0 | 0 | 0 | |
| Height – cm | 166 ± 2 | 167 ± 3 | 165 ± 2 | 0.71 |
| Weight – kg | 95 ± 5 | 101 ± 4 | 94 ± 3 | 0.38 |
| Body-mass index – kg/m2 | 34 ± 1 | 36 ± 1 | 35 ± 1 | 0.49 |
| Fasting glucose (mg/dl) | 99 ± 2 | 96 ± 2 | 95 ± 3 | 0.89 |
| Fasting insulin (μIU/mL) | 23 ± 4 | 22 ± 4 | 20 ± 4 | 0.52 |
| HOMA-IR | 5.6 ± 0.8 | 5.2 ± 1.0 | 4.7 ± 1.0 | 0.58 |
Values are expressed as means ± SEM. HOMA-IR: Homeostatic Model Assessment-Insulin Resistance.
Only subjects with insulin resistance who completed the full 12-month trial were included in the analysis (ADF n = 11, CR n = 17, control n = 15).
P-value: One-way ANOVA. No significant difference between groups for any variable at baseline.
Adherence, dietary intake and physical activity in insulin resistant subjects
Energy intake, macronutrient intake, and physical activity are shown in Table 2. ADF subjects consumed less energy than prescribed on the feast day at month 6 and 12, and more energy than prescribed on the fast day at month 6 and 12. CR subjects consumed more energy than prescribed at month 6, and less energy than prescribed at month 12. Percent energy intake from protein, carbohydrates, and fat, did not change over the course of the trial in any group. Cholesterol and fiber intake also remained unchanged. Physical activity (measured as steps/d) did not change significantly by month 6 or 12 in the intervention or control groups.
Table 2.
Dietary intake and physical activity after 6 and 12 months of alternate day fasting versus daily calorie restriction in insulin resistant subjects 1
| Variable | Alternate Day Fasting | Calorie Restriction | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Month 0 | Month 6 Feast day | Month 12 Feast day | Month 6 Fast day | Month 12 Fast day | Month 0 | Month 6 | Month 12 | Month 0 | Month 6 | Month 12 | |
| Actual energy (kcal) | 1565 ± 156 | 1409 ± 128 | 1763 ± 314 | 1049 ± 169 | 1175 ± 149 | 2122 ± 211 | 1755 ± 161 | 1761 ± 145 | 1711 ± 130 | 1625 ± 217 | 1892 ± 241 |
| Prescribed energy (kcal) | -- | 1956 ± 195 | 2347 ± 234 | 399 ± 43 | 799 ± 85 | -- | 1603 ± 168 | 1967 ± 195 | -- | -- | -- |
| Difference (kcal) (Actual – Prescribed) |
-- | −547 ± 166 | −584 ± 229 | 650 ± 143 | 376 ± 93 | -- | 152 ± 170 | −206 ± 233 | -- | -- | -- |
| Protein (% kcal) | 17 ± 1 | 18 ± 1 | 18 ± 1 | 20 ± 2 | 17 ± 1 | 17 ± 1 | 18 ± 1 | 18 ± 1 | 17 ± 1 | 18 ± 1 | 18 ± 1 |
| Carbohydrates (% kcal) | 47 ± 2 | 46 ± 2 | 45 ± 2 | 45 ± 2 | 45 ± 4 | 48 ± 1 | 47 ± 1 | 47 ± 2 | 47 ± 2 | 46 ± 2 | 45 ± 2 |
| Fat (% kcal) | 36 ± 1 | 36 ± 2 | 37 ± 2 | 35 ± 4 | 38 ± 5 | 35 ± 1 | 35 ± 1 | 35 ± 2 | 36 ± 1 | 36 ± 1 | 37 ± 2 |
| Cholesterol (mg) | 197 ± 28 | 185 ± 29 | 275 ± 59 | 177 ± 40 | 134 ± 21 | 303 ± 36 | 264 ± 36 | 276 ± 25 | 288 ± 28 | 242 ± 20 | 244 ± 25 |
| Fiber (g) | 13 ± 2 | 13 ± 2 | 16 ± 2 | 12 ± 2 | 12 ± 1 | 16 ± 1 | 17 ± 2 | 19 ± 2 | 16 ± 2 | 14 ± 2 | 20 ± 4 |
| Steps/d | 6873 ± 851 | -- | -- | 8140 ± 377 | 9417 ± 1106 | 7364 ± 652 | 8872 ± 1105 | 8275 ± 997 | 7549 ± 1122 | 7154 ± 837 | 7900 ± 1013 |
Values are expressed as means ± SEM.
Only subjects with insulin resistance who completed the full 12-month trial were included in the analysis (ADF n = 11, CR n = 17, control n = 15).
Changes in body weight and body composition in insulin resistant subjects
Changes in body weight are displayed in Figure 2 and Table 3. In insulin resistant subjects, ADF and CR produced comparable body weight reductions (P < 0.0001) by month 6 (ADF: −10 ± 1%; CR: −8 ± 1%) and by month 12 (ADF: −8 ± 2%; CR: −6 ± 1%), relative to controls. Fat mass and BMI also decreased (P < 0.05) similarly by ADF and CR at month 6 and 12 versus controls (Table 3). Changes in lean mass, and visceral fat mass by ADF and CR were not significantly different from controls at month 6 or 12 (Table 3).
Figure 2.
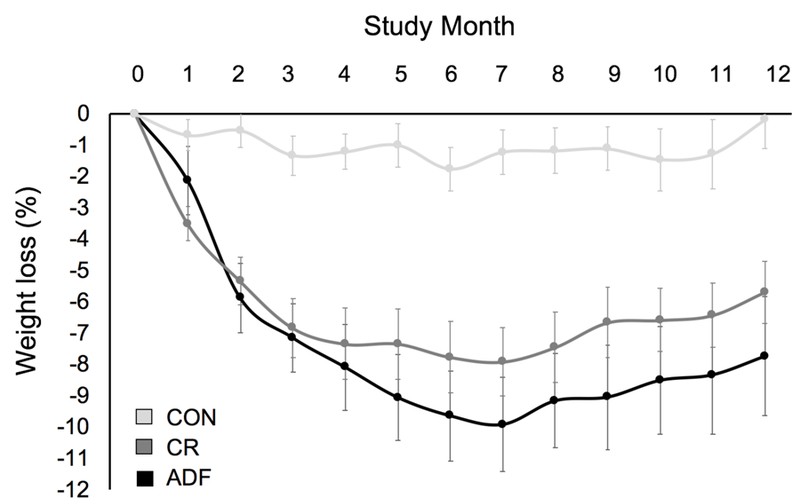
Weight loss during 12 months of alternate day fasting versus daily calorie restriction in insulin resistant subjects.
Table 3.
Metabolic disease risk factors after 6 and 12 months of alternate day fasting versus daily calorie restriction in insulin resistant subjects1,2
| Variable | Alternate Day Fasting | Calorie Restriction | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month 0 | Month 6 | Month 12 | Δ 3 | Month 0 | Month 6 | Month 12 | Δ 3 | Month 0 | Month 6 | Month 12 | Δ 3 | |
| Body weight (kg) | 95 ± 5 | 86 ± 5 a | 87 ± 4 a | −8 ± 2 | 101 ± 4 | 93 ± 4 a | 96 ± 4 a | −5 ± 1 | 94 ± 4 | 92 ± 4 b | 94 ± 4 b | 0 ± 1 |
| BMI (kg/m2) | 34 ± 1 | 33 ± 1 a | 32 ± 1 a | −2 ± 0 | 36 ± 1 | 35 ± 1 a | 34 ± 1 a | −2 ± 0 | 35 ± 1 | 34 ± 1 b | 35 ± 1 b | 0 ± 0 |
| Fat mass (kg) | 37 ± 2 | 31 ± 2 a | 31 ± 2 a | −6 ± 1 | 40 ± 2 | 34 ± 2 a | 35 ± 2 a | −5 ± 1 | 35 ± 2 | 34 ± 3 b | 35 ± 3 b | 0 ± 0 |
| Lean mass (kg) | 55 ± 3 | 53 ± 4 | 54 ± 3 | −1 ± 1 | 59 ± 4 | 58 ± 3 | 58 ± 3 | −1 ± 1 | 56 ± 3 | 56 ± 3 | 56 ± 3 | 0 ± 1 |
| Visceral fat mass (kg) | 2.2 ± 0.4 | 1.6 ± 0.3 | 1.7 ± 0.3 | −0.5 ± 0.2 | 2.4 ± 0.3 | 1.9 ± 0.2 | 2.1 ± 0.3 | −0.3 ± 0.2 | 2.5 ± 0.3 | 2.1 ± 0.3 | 2.2 ± 0.3 | −0.3 ± 0.3 |
| Glucose (mg/dl) | 99 ± 2 | 91 ± 2 | 96 ± 1 | −3 ± 3 | 96 ± 2 | 99 ± 3 | 92 ± 2 | −4 ± 2 | 95 ± 3 | 99 ± 4 | 99 ± 5 | 4 ± 4 |
| Insulin (μIU/mL) | 23 ± 4 | 13 ± 2 a | 11 ± 2 a | −12 ± 4 | 22 ± 4 | 17 ± 3 b | 19 ± 3 b | −1 ± 3 | 20 ± 4 | 17 ± 3 b | 17 ± 4 b | −3 ± 5 |
| HOMA-IR | 5.6 ± 0.8 | 2.9 ± 0.5 a | 2.6 ± 0.5 a | −3.0 ± 0.8 | 5.2 ± 1.0 | 4.2 ± 0.8 b | 4.3 ± 0.7 b | −0.9 ± 0.8 | 4.7 ± 1.0 | 4.2 ± 0.8 b | 4.2 ± 0.8 b | −0.5 ± 0.9 |
| Total chol. (mg/dl) | 180 ± 12 | 174 ± 12 | 184 ± 13 | 4 ± 6 | 183 ± 6 | 172 ± 7 | 177 ± 9 | −6 ± 6 | 185 ± 9 | 177 ± 9 | 184 ± 10 | −1 ± 4 |
| LDL chol. (mg/dl) | 102 ± 10 | 99 ± 9 | 109 ± 11 | 7 ± 4 | 114 ± 5 | 101 ± 6 | 108 ± 7 | −6 ± 4 | 111 ± 10 | 105 ± 7 | 111 ± 10 | 0 ± 4 |
| HDL chol. (mg/dl) | 53 ± 5 | 55 ± 6 | 56 ± 5 | 3 ± 3 | 49 ± 3 | 52 ± 3 | 51 ± 3 | 2 ± 2 | 53 ± 3 | 53 ± 3 | 55 ± 3 | 2 ± 2 |
| Triglycerides (mg/dl) | 124 ± 23 | 95 ± 12 | 97 ± 10 | −27 ± 18 | 96 ± 5 | 94 ± 11 | 90 ± 8 | −6 ± 7 | 102 ± 9 | 96 ± 15 | 94 ± 8 | −8 ± 5 |
| Systolic BP (mm Hg) | 128 ± 5 | 119 ± 5 | 119 ± 5 | −9 ± 7 | 124 ± 5 | 112 ± 3 | 117 ± 4 | −7 ± 5 | 123 ± 5 | 121 ± 3 | 122 ± 3 | −1 ± 3 |
| Diastolic BP (mm Hg) | 85 ± 4 | 75 ± 4 | 80 ± 4 | −5 ± 5 | 81 ± 3 | 75 ± 2 | 79 ± 3 | −2 ± 3 | 83 ± 3 | 80 ± 2 | 80 ± 2 | −3 ± 2 |
| Heart rate (bpm) | 78 ± 3 | 64 ± 2 | 71 ± 3 | −7 ± 4 | 75 ± 2 | 76 ± 3 | 74 ± 3 | −1 ± 2 | 75 ± 2 | 74 ± 3 | 69 ± 2 | −6 ± 2 |
| HS CRP | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | −0.1 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0 ± 0.1 |
| TNF-α | 1.3 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 0.2 ± 0.2 | 1.8 ± 0.2 | 1.8 ± 0.3 | 1.6 ± 0.2 | −0.2 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.3 | 2.0 ± 0.3 | 0.4 ± 0.3 |
| IL-6 | 1.9 ± 0.5 | 1.8 ± 0.2 | 1.4 ± 0.3 | −0.5 ± 0.5 | 2.3 ± 0.2 | 2.0 ± 0.2 | 1.9 ± 0.2 | −0.4 ± 0.2 | 2.5 ± 0.4 | 3.1 ± 0.6 | 2.4 ± 0.4 | −0.1 ± 0.4 |
Means ± SEM. BP: Blood pressure. HS CRP: High sensitivity C-reactive protein, HOMA-IR: Homeostatic Model Assessment-Insulin Resistance, TNF-α: tumor necrosis factor-α, IL-6: Interleukin-6
Only subjects with insulin resistance who completed the full 12-month trial were included in the analysis (ADF n = 11, CR n = 17, control n = 15).
Means not sharing a common letter are significantly different (P < 0.05) between groups based on ANCOVA with baseline as a covariate at month 6 or 12.
Absolute change from baseline to month 12.
Changes in fasting glucose, insulin, and HOMA-IR in insulin resistant subjects
Changes in fasting glucose, insulin and HOMA-IR (marker of insulin resistance) are reported in Figure 3 and Table 3. Fasting glucose levels were not significantly different between the ADF, CR, and control groups by month 6 or 12. ADF produced greater decreases (P < 0.05) in fasting insulin by month 6 (−44 ± 6%) and 12 (−52 ± 9%) when compared to CR and controls. ADF also achieved greater reductions (P < 0.05) in HOMA-IR by month 6 (−48 ± 6%) and month 12 (−53 ± 9%) when compared to CR and controls.
Figure 3.
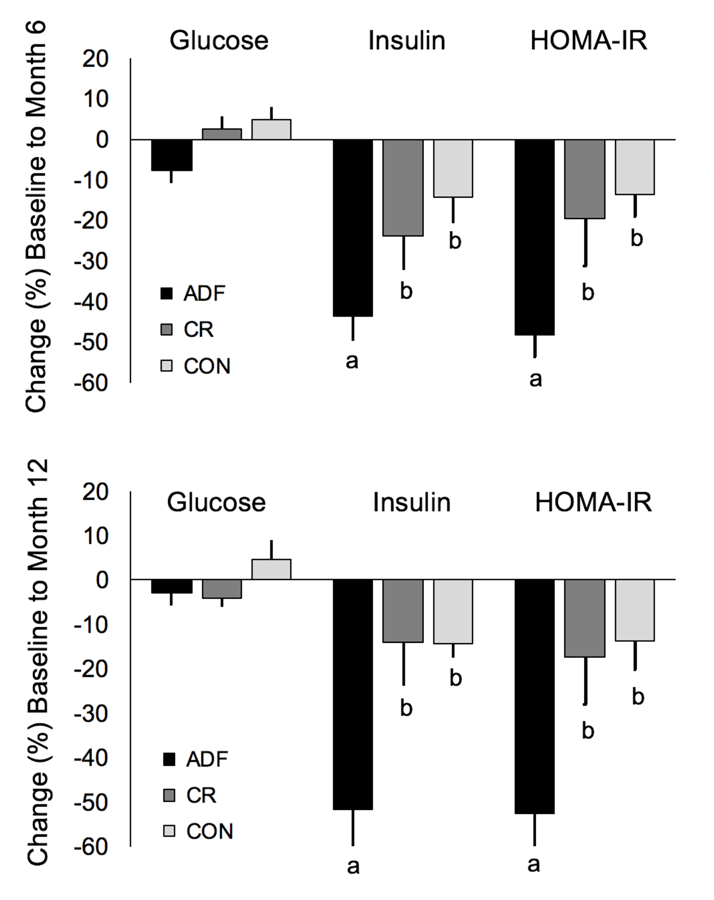
Fasting glucose, insulin, and HOMA-IR after 6 and 12 months of alternate day fasting versus daily calorie restriction in insulin resistant subjects.
Changes in plasma lipids, blood pressure, and inflammatory markers in insulin resistant subjects
Plasma lipids, blood pressure and inflammatory markers are displayed in Table 3. Total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, blood pressure, and heart rate did not change by month 6 or 12 in any group. Inflammatory mediators (CRP, TNF-α, and IL-6) also remained unchanged at month 6 and 12 in the ADF and CR groups, relative to controls.
Discussion
This study is the first to show that ADF produces superior reductions in HOMA-IR (a marker of insulin resistance) versus daily CR, in a cohort of subjects at risk for developing diabetes. Interestingly, these improvements were noted despite similar weight and fat mass loss in the ADF and CR groups. Other metabolic disease risk factors, such plasma lipids, blood pressure, and inflammatory mediators, were not preferentially altered by one diet versus the other.
The impact of intermittent fasting versus daily calorie restriction on glucoregulatory factors has been tested in a handful of clinical trials (5, 6, 7, 10). Studies performed in metabolically healthy individuals generally demonstrate either superior improvements by fasting versus daily restriction (7, 10), or no effect (5, 6). For instance, Harvie et al (7) and Hutchison et al (10) noted greater decreases in insulin resistance (~25–35% reductions) by intermittent fasting versus daily restriction (~15% reductions) after 2–6 months of intervention in healthy subjects with overweight and obesity. In both of these studies (7, 10), the fasting and daily restriction groups achieved the same degree of weight loss (~5–6%). In contrast, in a study by Trepanowski et al (5), HOMA-IR, fasting glucose and insulin remained unchanged after 12 months of ADF and CR with ~5% weight loss. Likewise, Catenacci et al (6) observed no changes in glucoregulatory parameters after 2 months of ADF or CR in metabolically healthy individuals with obesity despite ~6–8% reductions in body weight. To our knowledge, only one study (17) has examined the effects of fasting on glucoregulatory factors in subjects with obesity and marked insulin resistance. In this pilot study by Hoddy et al (17), subjects participating in a 2-month ADF regimen were divided into tertiles according to degree of insulin resistance based on HOMA-IR. Results from this study reveal that ADF decreased fasting insulin (~25% reduction) and HOMA-IR (~30% reduction) only in subjects in the highest tertile of HOMA-IR (i.e. >3.7) (17). Participants who were not insulin resistant at baseline, experienced no such improvements (17). The degree of weight loss was similar for each tertile (~4%). This study is limited, however, in that it did not involve a CR comparison group or a no-intervention control group. Taken together, these findings suggest that fasting generally produces superior reductions in insulin resistance versus daily CR, despite similar weight loss. Moreover, it’s likely that these effects may be more pronounced in participants who display higher levels of insulin resistance at baseline.
Our findings also show that neither ADF nor CR had any effect on other metabolic disease risk factors, such as plasma lipids, blood pressure, and heart rate, in this sub-analysis of insulin resistant subjects. It should be noted, however, that these participants were not hypercholesterolemic or hypertensive at baseline. Subjects in all of the groups had LDL cholesterol, HDL cholesterol, triglyceride levels and blood pressure within the clinically healthy range. Thus, it’s not surprising that plasma lipid levels and blood pressure were not further improved. Our results differ somewhat from other trials that compared intermittent fasting to daily calorie restriction (7, 10). In the 6-month study by Harvie et al (7), women with obesity experienced reductions in LDL cholesterol, triglycerides, systolic and diastolic blood pressure by fasting, even though these participants were not hypercholesterolemic or hypertensive at baseline. Similarly, Hutchison et al (10) reported greater reductions in LDL cholesterol by ADF versus daily CR after 2 months of diet in normocholesterolemic women with overweight. The reason why our findings differ from that of Harvie et al (7) and Hutchison et al (10) is uncertain. It is possible, however, that our subjects were not as responsive to the fasting intervention because their level of adherence was suboptimal. More specifically, the ADF subjects were consuming ~400–600 kcal/d more than prescribed on the fast day, and ~600 kcal/d less than their prescribed on the feast day. In comparison, in the studies by Harvie et al (7) and Hutchison et al (10), adherence to the fasting interventions was higher as subjects were much more compliant with their energy prescriptions. Thus, the suboptimal adherence to the ADF intervention in the present study could partly explain why our findings differ from that of previous fasting trials.
Circulating concentrations of CRP, TNF-α and IL-6 are generally elevated in subjects with insulin resistance and obesity (18, 19, 20). Weight loss has been shown to decrease concentrations of these pro-inflammatory factors and improve insulin sensitivity (21, 22, 23). Very few studies have examined the impact of intermittent fasting versus daily calorie restriction on inflammatory mediators (5, 7, 8). Harvie et al (7) observed potent reductions in plasma CRP by intermittent fasting and CR, in women with obesity (and elevated baseline CRP) after 6 months of intervention. In contrast, Trepanowski et al (5) and Hutchison et al (10) saw no change in circulating CRP levels by ADF or CR in metabolically healthy individuals with overweight and obesity. In the present study, we observed no effect of ADF or CR on circulating levels of CRP, TNF-α, and IL-6. However, plasma levels of these inflammatory mediators were not elevated at baseline, which could explain why no improvement was observed. Future trials in this field should examine the effect of fasting on other mediators of inflammation, such as interleukin-1β (IL-1β), and chemokines such as monocyte chemoattractant protein-1 (MCP-1). IL-1β is a pro-inflammatory mediator produced by monocytes and macrophages that contributes to the development of type 2 diabetes by destroying beta-cell mass and suppressing beta-cell function (24, 25). MCP-1 is a chemokine that has been shown to impair insulin signalling in skeletal muscle (26, 27). Expression of MCP-1 in adipose tissue is increased with obesity (28, 29). Whether intermittent fasting can improve circulating levels of IL-1β and MCP-1 in a way that protects against the development of diabetes, is of great interest.
Key strengths of this study are the inclusion of a no-intervention control group and the 12-month trial duration. However, this study does have some limitations. First, we used HOMA-IR to assess insulin resistance. While HOMA-IR is an adequate surrogate marker of insulin resistance (30), our findings need to be validated by more robust techniques such as the hyperinsulinemic-euglycemic clamp. Second, we were not able to assess the number of prediabetic individuals in our sample, as we did not measure HbA1c or perform oral glucose tolerance testing at baseline in our original study (5). Third, there is a high degree of variability in the threshold of HOMA-IR (i.e. 1.6 to 3.8 (15)) used to define insulin resistance. We chose a cut-off of 2.73 based on a United States NHANES population study (15). However, this higher cut-off value may introduce bias in our study, as part of the effect may be due to higher baseline HOMA-IR values. Fourth, we used 7-day food records to measure dietary intake over the course of the trial. It is well known that individuals with obesity underreport food intake by 20–40% in diet records (31, 32), thus our estimates of energy intake are most likely inaccurate. Fifth, we did not analyze when (i.e. time of day) the extra fast day calories were consumed and how this affected the total fasting duration. Sixth, the residual acute effects of fasting need to be considered when interpreting these data. In a recent study by Antoni et al (33), intermittent fasting and CR produced similar reductions in postprandial insulin concentrations, following matched weight loss (5%). In the present study, measurements were taken within 48 h of the fast day. Therefore, the residual acute effects of fasting may have contributed to the improvements observed here.
The suboptimal adherence to the ADF protocol is also a major limitation to the study. Subjects consistently struggled to stick to their fast day calorie goals, which puts into question the sustainability and tolerability of ADF long-term. On the other hand, it’s interesting that ADF subjects consumed almost twice as many calories on fast days, but still observed greater metabolic effects when compared to CR. This suggests that simply reducing energy intake by ~1000 kcal/d a few days per week may have significant metabolic benefits.
In summary, these preliminary findings show that intermittent fasting may be more effective than daily calorie restriction to lower insulin resistance in adults at risk for developing diabetes. While these results offer promise for the use of fasting in this population, they still require confirmation by a large randomized controlled trial performed in individuals with prediabetes that implements hyperinsulinemic-euglycemic clamp to assess insulin resistance and sensitivity.
What is already known about this subject?
Alternate day fasting and daily calorie restriction produce similar weight loss, but have little effect on glucoregulatory factors in metabolically healthy individuals with overweight and obesity.
What does your study add?
A key question that remains unknown is whether alternate day fasting produces superior changes in glucoregulatory factors versus daily restriction, in populations at risk for developing diabetes (i.e. insulin resistant subjects with overweight and obesity).
Findings from this 12-month randomized controlled trial demonstrate that alternate day fasting produces greater reductions in fasting insulin and HOMA-IR (a maker of insulin resistance) versus daily calorie restriction, in insulin resistant subjects, despite similar decreases in body weight.
Acknowledgments
Funding: National Institutes of Health (NHLBI) R01HL106228
Footnotes
Disclosure: Krista Varady received author fees from Hachette Book Group for the book “The Every Other Day Diet”. The other authors have no conflicts of interest to disclose.
Data sharing statement
Will individual deidentified participant data be available (including data dictionaries)? No What other documents will be available (e.g., study protocol, statistical analysis plan)? The study protocol has been previously published in JAMA Internal Medicine (5) With whom will data be shared, for what types of analyses, and by what mechanism? Data will not be shared with other parties.
Trial registration: Clinicaltrials.gov, NCT00960505.
References
- 1.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014;129: S102–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes A. 8. Obesity Management for the Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes care 2019;42: S81–S89. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev 2017;39: 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr 2017;37: 371–393. [DOI] [PubMed] [Google Scholar]
- 5.Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern Med 2017;177: 930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016;24: 1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvie MNPM, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A,, A H. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. International journal of obesity 2011;35: 714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutchison AT LB, Wood RE, Vincent AD, Thompson CH, O’Callaghan NJ, Wittert GAHL Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity 2019;27: 50–58. [DOI] [PubMed] [Google Scholar]
- 9.Hoddy KK, Kroeger CM, Trepanowski JF, Barnosky AR, Bhutani S, Varady KA. Safety of alternate day fasting and effect on disordered eating behaviors. Nutrition journal 2015;14: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchison AT, Heilbronn LK. Metabolic impacts of altering meal frequency and timing - Does when we eat matter? Biochimie 2015. [DOI] [PubMed]
- 11.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. The journals of gerontology Series A, Biological sciences and medical sciences 2015;70: 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laliberte M, McCabe R, Taylor V The Cognitive Behavioral Workbook for Weight Management: A Step-by-Step Program New Harbinger Publications, 2009. [Google Scholar]
- 13.Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC, Welk GJ. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Medicine and science in sports and exercise 2010;42: 2134–2140. [DOI] [PubMed] [Google Scholar]
- 14.Demerath EW, Ritter KJ, Couch WA, Rogers NL, Moreno GM, Choh A, et al. Validity of a new automated software program for visceral adipose tissue estimation. International journal of obesity 2007;31: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis 2008;196: 696–703. [DOI] [PubMed] [Google Scholar]
- 16.Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvarez MX, Gude F, Garcia F, De Francisco A, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC endocrine disorders 2013;13: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoddy KK, Bhutani S, Phillips SA, Varady KA. Effects of different degrees of insulin resistance on endothelial function in obese adults undergoing alternate day fasting. Nutr Healthy Aging 2016;4: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116: 1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Chen R, Wang H, Liang F. Mechanisms Linking Inflammation to Insulin Resistance. Int J Endocrinol 2015;2015: 508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trakhtenbroit MA, Leichman JG, Algahim MF, Miller CC 3rd, Moody FG, Lux TR, et al. Body weight, insulin resistance, and serum adipokine levels 2 years after 2 types of bariatric surgery. Am J Med 2009;122: 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol 2003;148: 535–542. [DOI] [PubMed] [Google Scholar]
- 23.Rosc D, Adamczyk P, Boinska J, Szafkowski R, Ponikowska I, Stankowska K, et al. CRP, but not TNF-alpha or IL-6, decreases after weight loss in patients with morbid obesity exposed to intensive weight reduction and balneological treatment. J Zhejiang Univ Sci B 2015;16: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 2010;17: 314–321. [DOI] [PubMed] [Google Scholar]
- 25.Donath MY, Dalmas E, Sauter NS, Boni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell metabolism 2013;17: 860–872. [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Kitade H, Ni Y, Ota T. Roles of Chemokines and Chemokine Receptors in Obesity-Associated Insulin Resistance and Nonalcoholic Fatty Liver Disease. Biomolecules 2015;5: 1563–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009;29: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen A, Mumick S, Zhang C, Lamb J, Dai H, Weingarth D, et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res 2005;13: 1311–1320. [DOI] [PubMed] [Google Scholar]
- 29.Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. International journal of obesity 2006;30: 1347–1355. [DOI] [PubMed] [Google Scholar]
- 30.Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab 2015;19: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goris AH, Westerterp-Plantenga MS, Westerterp KR. Undereating and underrecording of habitual food intake in obese men: selective underreporting of fat intake. Am J Clin Nutr 2000;71: 130–134. [DOI] [PubMed] [Google Scholar]
- 32.Kretsch MJ, Fong AK, Green MW. Behavioral and body size correlates of energy intake underreporting by obese and normal-weight women. Journal of the American Dietetic Association 1999;99: 300–306; quiz 307–308. [DOI] [PubMed] [Google Scholar]
- 33.Antoni RJK, Collins AL, Robertson MD. Intermittent v. continuous energy restriction: differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. British Journal of Nutrition 2018;119: 507–516. [DOI] [PubMed] [Google Scholar]