Abstract
Eucalyptus is a worldwide hard-wood species which increasingly focused on. To adapt to various biotic and abiotic stresses, Eucalyptus have evolved complex mechanisms, increasing the cellular concentration of reactive oxygen species (ROS) by numerous ROS controlling enzymes. To better analyse the ROS gene network and discuss the differences between four Eucalyptus species, ROS gene network including 11 proteins families (1CysPrx, 2CysPrx, APx, APx-R, CIII Prx, Diox, GPx, Kat, PrxII, PrxQ and Rboh) were annotated and compared in an expert and exhaustive manner from the genomic data available from E. camaldulensis, E. globulus, E. grandis, and E. gunnii. In addition, a specific sequencing strategy was performed in order to determine if the missed sequences in at least one organism are the results of gain/loss events or only sequencing gaps. We observed that the automatic annotation applied to multigenic families is the source of miss-annotation. Base on the family size, the 11 families can be categorized into duplicated gene families (CIII Prx, Kat, 1CysPrx, and GPx), which contain a lot of gene duplication events and non-duplicated families (APx, APx-R, Rboh, DiOx, 2CysPrx, PrxII, and PrxQ). The gene family sizes are much larger in Eucalyptus than most of other angiosperms due to recent gene duplications, which could give higher adaptability to environmental changes and stresses. The cross-species comparative analysis shows gene gain and loss events during the evolutionary process. The 11 families possess different expression patterns, while in the Eucalyptus genus, the ROS families present similar expression patterns. Overall, the comparative analysis might be a good criterion to evaluate the adaptation of different species with different characters, but only if data mining is as exhaustive as possible. It is also a good indicator to explore the evolutionary process.
Keywords: reactive oxygen species, Eucalyptus, expert annotation, divergence time, peroxidases, evolutionary rate
1. Introduction
Due to their fast growth rate, valuable wood and fibre properties, and wide adaptabilities, Eucalyptus species with a haploid chromosome number of 11 have been rapidly introduced from Australia to other countries such as France, Brazil, Portugal, and China. Over 700 species constitute the Eucalyptus genus which have different growth conditions and phenotypes [1]. In the past 15 years, several studies have led to a better understanding of the Eucalyptus genome and the development of an important set of genetic/genomic tools, which can be used to enhance future breeding efforts. Along with the genomic sequencing projects [2] and the expansion of the expressed sequence tag (EST) libraries for some species such as E. camaldulensis, E. globulus, E. grandis, and E. gunnii, expert annotation and comparative analysis have become possible. These four species originated from different environments, with different morphologies and genotypes. E. camadulensis is a species widely represented throughout Australia, very tolerant to salt stress and drought, and a variable tolerance to cold depending on the origin. The trees are most often of medium size and they are highly cultivated. E. globulus is mainly located in south Australia and in Tasmania island, is among the tallest tree in the world. E. grandis grows in the east coast and sub-coast. The adult trees are medium to large. E. gunnii is an endemic species of Tasmania island, found in mountainous regions and tolerant up to –18 ° C. The adult tree is medium size with many branches and round leaves. Eucalyptus species are very reactive after stress, explaining the competitiveness of eucalyptus trees for the occupation of space.
To adapt to various biotic and abiotic stresses, plants have evolved very complex regulatory mechanisms that can increase the cellular concentration of reactive oxygen species (ROS), including singlet oxygen (1O2), superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (HO•). A high level of ROS is highly toxic and will lead to oxidative cell damage. When their concentrations are controlled, they participate in some positive biological processes such as cell growth [3], programmed cell death [4], and signalling [5]. The level of ROS in cells is determined by interplay between ROS producing pathways and scavenging mechanisms. During the evolution processes of plants, efficient ROS scavenging mechanisms have been developed allowing a tight regulation of the ROS homeostasis. Proteins able to regulate the ROS homeostasis (production and scavenging) are part of the ROS gene network [6]. In this study, genes, proteins, and families belonging to this network will be named ROS genes, ROS proteins, and ROS families respectively.
Among this network, peroxidases are enzymes able to reduce H2O2 by oxidizing various substrates such as lignin subunits, lipid membranes, and some amino acid side chains or regulate the ROS homeostasis [7,8]. These enzymes are present in all kingdoms and play very important roles in plants from germination to senescence, as well as during defence mechanisms, immune responses and pathogeny [8,9,10]. They can be divided into heme and non-heme peroxidases according to the presence or absence of a protoporphyrin IX and Fe (III) complex. In plants, the ROS gene network comprise 10 multigenic families of peroxidases: ascorbate peroxidase (APx), ascorbate peroxidase related (APx-R), catalase (Kat), glutathione peroxidase (GPx), alpha-dioxygenase (DiOx), Class III peroxidases (CIII Prx), and four small and highly conserved families, namely 1-Cysteine peroxiredoxin (1CysPrx), Typical 2-Cysteine peroxiredoxin (2CysPrx), Atypical 2-Cysteine peroxiredoxin type II (PrxII), and type Q (PrxQ) which belong to the peroxiredoxin superfamily [11]. In addition, the plant respiratory burst oxidase homologues (Rboh), also known as NADPH oxidases, belonging to the ROS gene network even if not members of peroxidase families, have been analysed. Present in all kingdoms, these proteins are responsible for the superoxide generation [12]. Genes belonging to multigenic families are often subjected to gene gain or loss events [13]. Gene duplications are a major source of differences between species during the process of evolution and this is more evident when the species are subject to selection pressures and restrictive growth conditions [9]. Therefore, the study of the relationships between heterogeneous data such as genome structure, gene structure, gene gain/loss and function across different species or strains is necessary for the large scale evolution and adaptability analyses [14]. The explosion of sequencing projects resulted in the production of a large amount of data obtained from automatic annotation procedures. However, the quality of the automatic annotation needs to be improved and completed with manual curation in order to obtain a more accurate and global analysis [15]. Finally, a complementary annotation procedure with experimental detection and EST mining would be necessary to overcome the risks associated with assembly and annotation biases [9,15]. The gene conservation and variation should be a crucial criterion to evaluate the capabilities of some organisms to adapt to different environmental variations. However, no direct evidence has yet demonstrated that the gain/loss events of some genes between the four Eucalyptus species could be responsible of the morphological and physiological characteristics differences. Comparative analysis of the molecular sequence data is essential for reconstructing the evolutionary history of species and inferring the nature and extent of selective forces shaping the evolution of genes and species [16].
The ratio between non-synonymous (Ka) and synonymous (Ks) nucleotide substitution is an indicator of selective pressures on genes and can be used to identify protein coding genes that may have changed function [17]. A ratio significantly greater than 1 indicates positive selective evolutionary pressures while a ratio less than 1 indicates the negative selective pressures which could conserve sequence with few mutations during the process of evolution [18,19,20]. For several decades, the well-known hypothesis called “molecular clock” has been used. This hypothesis proposes a roughly proportional relationship between the amino acid substitutions and the separation time between compared species. Substitution rate can be used for divergence analysis which is very useful for the evolutionary research [21]. Estimating the divergence time is generally more difficult than reconstructing of a phylogenetic tree, because genes do not evolve at a constant rate. For this reason, authors have used many independently evolving genes to estimate divergence times in the hope of reducing the effect of rate variation [22,23]. In recent years, a large number of authors have investigated the evolutionary relationships of plants using both molecular and paleontological data [24,25]. BEAST (Bayesian evolutionary analysis sampling trees) is a powerful and flexible evolutionary analysis bioinformatics package for molecular sequence variation. It also provides a resource for the further development of new models and statistical methods of evolutionary analysis [26].
Comparative analysis of DNA sequences from multiple species is a powerful approach for identifying coding and functional non-coding sequences, as well as sequences that are unique of a given organism [27]. In this study, 11 families belonging to the ROS gene network have been annotated from genomic sequences available from four Eucalyptus species. Then, the distribution and the duplication of the ROS genes were analysed, followed by a global comparative analysis including gene gain and loss, family conservation, and expression study based on publicly available EST libraries and RNA-seq (RNA Sequencing) data. The evolutionary rate and the divergence time among species have also been studied. Comparative genomic analysis can show differences between the genomes of very closely related species. These differences could then shed light on the phenotypic differences between the four species. Genes that differ between species can be studied deeply to determine their roles in phenotypic differences. To our knowledge, this is the first time that a large-scale and expert annotation and comparative analysis have been performed on four Eucalyptus species, allowing to analyse the duplication events in the process of evolution and the divergence between different organisms.
2. Materials and Methods
2.1. Source of Genomic and Protein Sequences
The genome and the proteome of E. camaldulensis and E. grandis were downloaded respectively from Kazusa Database (http://www.kazusa.or.jp/eucaly/index.html) and Phytozome (http://www.phytozome.net/Eucalyptus.php) a joint project of the Department of Energy’s Joint Genome Institute (JGI) and the Centre for Integrative Genomics (CIG). Sequencing program of E. globulus clone X46 and E. gunnii clone FCBA #634 genomes (Cagire Azura, protected under UPOV number 20070559) were obtain from JGI and Tree for Joule respectively. The annotated peroxidase sequences from the four Eucalyptus species, Arabidopsis thaliana, Vitis vinifera, Populus trichocarpa, and Medicago truncatula can be found in the RedoxiBase database (http://peroxibase.toulouse.inra.fr) [28,29].
2.2. Data Mining and Expert Annotation
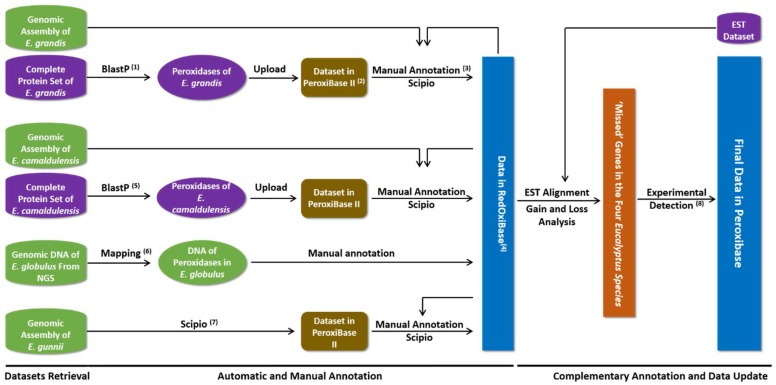
An expert strategy was used for data mining and annotation (Figure 1). To obtain the automatically predicted peroxidases, the protein sequences of P. trichocarpa (retrieved from RedxiBase) were used as a query to search the proteome of E. grandis and obtain an initial set of automatically annotated proteins corresponding to the 11 families, followed by an expert process to discard prediction errors. In this process, alternative transcript variants and redundant sequences were discarded to prevent artefact during phylogenetic analysis. Partial gene models were verified based on gene structure, presence of conserved domains, and EST supports. The corrected set of protein sequences were used for genome homology prediction using Scipio [30] to obtain the corresponding chromosomal positions, gene structures, DNA, and CDS sequences. New paralogs have been obtained and added to the initial protein set. Each gene has been named as following: Egr, followed by the protein family abbreviation and by a number which represents the position order on the chromosomes. The annotation protocol for E. camaldulensis was performed using E. grandis sequences previously annotated, following a similar three-step process.
Figure 1.
Workflow of the comprehensive annotation process of Eucalyptus ROS genes in four Eucalyptus species. The comprehensive annotation, starting from the acquisition of data to the storage in a database, consists of automatic annotation, manual annotation and experimental detection as a complimentary annotation. The experimental detection for the missed sequences would only be possible when the relationship between target organisms is very close. (1) The query data for this BlastP is the protein sequence set of P. trichocarpa. (2) The ReroxiBase II is an assistant database of RedOxiBase, which is used for keeping some private temporary data during the annotation process. (3) The Scipio program takes the manually annotated protein sequences as the query. (4) The data stored in RedOxiBase contain protein, DNA, CDS sequences and the gene structure information for most of the records. Chromosomal positions are also included for the E. grandis genes. (5) The query for the BlastP similarity search is the protein set of E. grandis. (6) Mapping program takes the DNA set of E. grandis and E. camaldulensis as the query to obtain the peroxidase DNA sequences of E. globulus. (7) The protein set containing peroxidases of E. grandis, E. camaldulensis and E. globulus is used as the query for the Scipio program to obtain the peroxidase data. (8) PCR was performed for the “missing” genes among the organisms.
The short-read of E. globulus genomic DNA was assembled with mapping method [31]. The reads were assembled using E. grandis peroxidase DNA sequences as reference, building a sequence that is similar but not necessarily identical to the base sequence, visualized with software Tablet version 1.12 [32] and manually annotated. A similar strategy has been used for E. gunnii annotation using the proteins detected in the three other Eucalyptus species as a query set. The sequences of E. camaldulensis, E. globulus, and E. gunnii were named according to the E. grandis orthologous sequences.
2.3. Pairwise Comparison and Search for Missed Peroxidase Sequences
Because closely related species have similar genomes, the sequenced genes of one species can be used as a template to design primers to search the missed related sequences or to complete partial sequences or to modify the pseudogenes. A gene gain/loss analysis has been performed according to the % of identities between protein sequences of these four species.
In order to search for putative missed sequences, 90 pairs of PCR primers have been designed (Table S1) based on the DNA, promoter and terminator sequences available for at least one organism. The genomic DNA of the four Eucalyptus species was extracted from leaves with the cetyl-trimethyl-ammonium bromide (CTAB) method. For each pair of primers, three contrasted annealing temperatures (53 °C, 58°C and 63 °C) were used coupled with an elongation temperature of 72 °C and a pre- and denaturation temperature of 94 °C. In the reaction of each pair of primers, at least one positive control has been used with the DNA containing the target gene as the template. The PCR products of the newly found genes were sequenced, assembled, annotated and deposited in RedOxiBase. The annotation process containing automatic annotation, manual annotation, and experiments for ‘missed’ genes can be considered as the comprehensive annotation processes (Figure 1). The Venn diagrams of the ROS genes in the four Eucalyptus species constructed by NetVenn [33].
2.4. Analysis of Phylogeny, Chromosomal Localization and Duplication Events
The phylogenetic analysis was conducted with all the complete protein sequences of E. grandis aligned using MAFFT with default parameters [34] and further inspected and visually adjusted using BioEdit version 7.2 [35]. The phylogenetic trees were reconstructed with the Maximum-likelihood (ML) method using PhyML-aLRT 3.0 [36] and edited with Mega 6 [37]. The graphical presentation of gene localisation of the 11 families on E. grandis chromosomes and the duplication linkage between genes were produced using MapChart V2.1 [38]. The duplication events of E. grandis including whole genome duplication (WGD), segmental duplication (SD) and tandem duplication (TD) [9] were analysed based on comparative phylogeny between A. thaliana and E. grandis.
2.5. Expression Analysis Based on ESTs and RNA-Seq Data
To analyse the expression patterns of ROS gene families, data were retrieved from EST libraries and RNA sequencing projects. The expression data of the whole set of annotated proteins have been analysed using an alignment (tBlastN) against the EST libraries of the four Eucalyptus species available on NCBI, while the EST data of A. thaliana were obtained from The Arabidopsis Information Resource (TAIR) (https://www.arabidopsis.org/). To analyse the relationship between gene duplication and gene expression profiles, the RNA-seq data, available from six tissues (root phloem, immature root xylem, roots, mature leaf, young leaf, shoot tip and flower) of E. grandis [2], have been visualised for the ROS genes by heatmap using software Expander V6 [39]. The average EST or RNA numbers of each family in the five organisms were calculated from the EST and RNA-seq data and the expression level of each family in each organism were defined with the following formula: average numbers of ESTs of a family / total EST numbers of ROS gene network.
2.6. Analysis of Evolutionary Rate and Divergence Time
The coding DNA sequences (CDS) from the complete sequences were used for the Ka and Ks analysis. The consensus parts of the CDS sequences were used for the analysis with the DNAsp 5.0 software [40]. The tree was produced by Beast v1.8.0 [26] with the chimeric CDS sequences, annotated with TreeAnnotator v1.8.0 and finally visualized with FigTree v1.4.0.
3. Results and Discussion
3.1. Data Retrieval, Semi-Automatic Annotation and Statistics
Thanks to the semi-automatic and manual protocol used for the annotation, 884 genes from 11 families have been annotated from the four genomes of Eucalyptus species including complete, partial and pseudogenes sequences (Table 1). Here, 229 genes of E. grandis including 64 pseudogenes have been identified. Only 92 proteins were correctly predicted by Phytozome using homology-based FgenesH and GenomeScan prediction programs [2], while 40 genes were incorrectly predicted. The remaining 97 sequences, not automatically predicted by Phytozome, have been finally annotated manually from the genomic assembly and the support of EST libraries available on NCBI (Table S2). In E. camaldulensis, 82 of the 214 sequences were correctly predicted by Kazusa combining several gene prediction programs (GeneMark.hmm, GeneScan, NetGene2 and Splicepredictor) [41], while 124 genes were incorrectly predicted, meaning that only 8 not predicted sequences have been manually annotated (Table S3). In E. globulus and E. gunnii, since no predictions were available, the annotation was performed using E. grandis proteins as a template. 232 and 209 genes were respectively annotated, including 70 and 62 pseudogenes respectively (Tables S4 and S5). The qualities and completeness of the four genomic data sets were variable. Indeed, 112, 114, 70 and 78 incomplete genes have been obtained from E. camaldulensis, E. globulus, E. grandis and E. gunnii respectively. These incomplete sequences (partial genes and pseudogenes) are related to undetermined nucleotide acids or frame shifts probably due to the sequencing quality, low coverage, and mis-assembly or the genomic pseudogenisation. Among these four sequencing and assembly projects, E. globulus showed much better outputs compared with the other three organisms due to the sequencing coverage. The elevated level of incorrect or not predicted genes detected in this study is mostly due to the complexity of multigenic family annotation.
Table 1.
ROS gene numbers of four Eucalyptus species annotated from various database sources and from experimental detection performed in this study.
| Organisms | E. camaldulensis | E. globulus | E. grandis | E. gunnii | ||||
|---|---|---|---|---|---|---|---|---|
| Data Sources | From Databases* | From PCR | From Databases | From PCR | From Databases | From PCR | From Databases | From PCR |
| 1CysPrx | 3 (2+0+1) | 1 (0+0+1) | 4 (0+2+2) | 0 | 3 (1+0+2) | 0 | 3 (1+0+2) | 1 (0+0+1) |
| 2CysPrx | 1 (0+1+0) | 0 | 1 (1+0+0) | 0 | 1 (1+0+0) | 0 | 1 (1+0+0) | 0 |
| APx | 10 (3+5+2) | 0 | 10 (7+0+3) | 1 (0+0+1) | 11 (7+0+4) | 0 | 10 (7+0+3) | 0 |
| Apx-R | 2 (1+0+1) | 0 | 1 (1+0+0) | 0 | 2 (1+0+1) | 0 | 2 (1+0+1) | 0 |
| CIII Prx | 163 (84+39+40) | 16 (0+6+10) | 180 (93+32+55) | 3 (0+2+1) | 179 (126+2+51) | 12 (2+5+5) | 159 (100+11+48) | 17 (1+8+8) |
| DiOx | 1 (1+0+0) | 0 | 1 (1+0+0) | 0 | 1 (1+0+0) | 0 | 1 (1+0+0) | 0 |
| GPx | 11 (3+5+3) | 0 | 10 (5+3+2) | 0 | 9 (9+0+0) | 1 (0+0+1) | 9 (7+1+1) | 1 (0+0+1) |
| Kat | 12 (1+4+7) | 2 (0+1+1) | 14 (3+3+8) | 0 | 12 (2+4+6) | 2 (0+1+1) | 13 (4+2+7) | 1 (0+1+0) |
| PrxII | 3 (3+0+0) | 0 | 3 (2+1+0) | 0 | 3 (3+0+0) | 0 | 3 (2+1+0) | 0 |
| PrxQ | 1 (1+0+0) | 0 | 1 (0+1+0) | 0 | 1 (1+0+0) | 0 | 1 (1+0+0) | 0 |
| Rboh | 7 (3+4+0) | 0 | 7 (5+2+0) | 0 | 7 (7+0+0) | 0 | 7 (6+1+0) | 0 |
| Total | 214 (102+58+54) | 19 (0+7+12) | 232 (118+44+70) | 4 (0+2+2) | 229 (159+6+64) | 15 (2+6+7) | 209 (131+16+62) | 19 (1+9+9) |
| Automatic correct prediction | 82 (35.19%) | na | 92 (37.70%) | na | ||||
| Coverage of Genomic Data | 91.8% | 98.3% | 93.9% | 91.7% | ||||
*: The data from the four Eucalyptus species were obtained after the annotation of available genomic and EST data. The detail of total number found per organism and per family is written in bracket: including the numbers of complete, partial sequences and theoretical translation or pseudogenes detected separated by plus symbol. The coverage of genomic data corresponds to the following formula:.Genomic coverage = Number of genes from database / (Total number of genes from database + PCR detection). na: no automatic prediction available.
Although the quality of the annotations of new genomes has been improved thanks to new tools for assembly and annotation, the percentage of incorrect or missed annotations remains high [15]. Results obtained from E. grandis and E. camaldulensis confirmed the bias of automatic annotation process and particularly in the case of large multigenic families. Some genes are still not or not correctly predicted and annotated.
3.2. Necessary and Effective Detection of Missed Genes
The comparative analysis of the gene sets belonging to ROS gene network found in the four Eucalyptus species allows identifying the putative genomic “missed” sequences in one or several organisms (Table S6). Even if the studied families were subjected to many duplications and gene number variations, the differences detected between the four Eucalyptus species appeared to be higher than expected.
In order to determine if the missed sequences are due to gene pseudogenisation, gain/loss events or partial genomic sequencing coverage, 90 pairs of primers were designed to clone and sequence the putative missed sequences. 57 new sequences have been identified (19, 4, 15, and 19 in E. camaldulensis, E. globulus, E. grandis, and E. gunnii respectively, Table 1 and Table S6). Most of the newly found genes are CIII Prxs (82.5%). The percentages of new genes detected by PCR in the total gene number are 8.2%, 1.7%, 6.1%, and 8.3% from E. camaldulensis, E. globulus, E. grandis, and E. gunnii respectively. These values can inform on the genome coverage and need to be added to the non-annotated or mis-annotated sequences in order to estimate the quality of the genomic sequencing and assembly.
3.3. Phylogeny and Chromosomal Localisation of ROS Genes Network
The exhaustive in silico and experimental mining allowed to draw of a global phylogenetic overview of the 11 gene families in E. grandis (Figure S1). Each family is well defined and the superfamily membership respected.
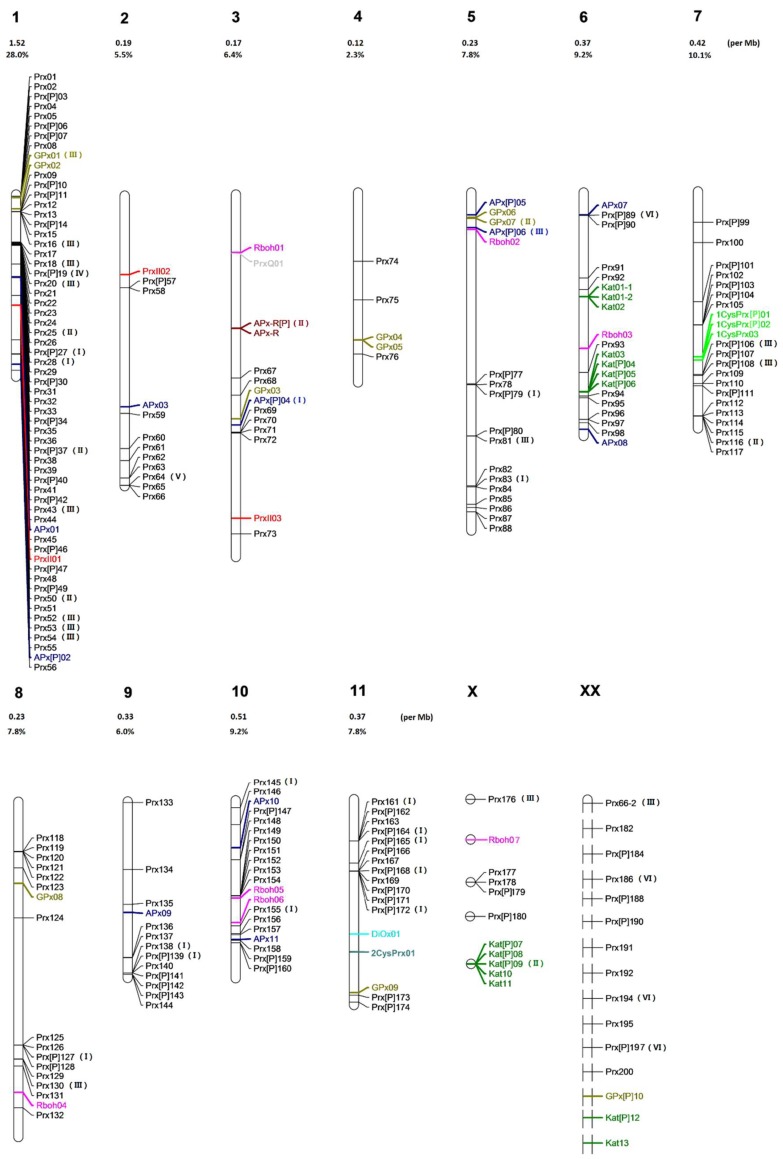
Chromosomal localisation of 218 genes part of the ROS gene network allowed a global distribution analysis. 61 of 218 sequences (28%) were located on chromosome 1 among which there were 56 CIII Prxs (32.2% of 174 CIII Prxs), while only five genes (2.3%) have been found on chromosome 4 (Figure 2, Table S7). The highest concentration of genes is on chromosome 1 (1.52 per Mb) while the lowest is on chromosome 4 (0.12 per Mb). Most of the genes detected on chromosome 1 are located in clusters such as Prx1–08, Prx09–16, Prx17–25, Prx30–41, and Prx50–55 with intergenic distances shorter than 15 Kb (average intergenic distance calculated for the whole genome) which support the theory of hot spots of duplication events already detected for other superfamilies [9]. This can suggest a hot spot of duplications related with high functional priority.
Figure 2.
Genomic localization of the ROS gene families from E. grandis. All the predicted ROS genes annotated from the genomic program including complete sequences, partial sequences and pseudogenes which can be located on the 11 chromosomes are presented. This synthetic chromosomal localization was displayed by MapChart 2.1. New sequences obtained from cloning strategies are not localized. Different colors represent different families. (I) E. grandis specific genes compared to E. camaldulensis. (II) E. grandis specific genes compared to E. globulus. (III) E. grandis specific genes compared to E. gunnii. (IV) E. grandis specific genes compared to E. camaldulensis, E. globulus and E. gunnii. (V) E. grandis specific genes compared to E. camaldulensis and E. gunnii. (VI) E. grandis specific genes compared to E. globulus and E. gunnii. The 11 chromosomes are labelled with 1–11 above each chart. The genes on small scaffolds are visualized on ‘chromosome’ X. The newly found genes without position information are visualized on ‘chromosome’ XX. The ROS gene concentration (ROS gene number per Mb chromosome) and the % of ROS genes on each chromosome (ROS gene number on the chromosome / total ROS gene number in E. grandis) are written above the chart without considering the genes on ‘chromosome’ X and ‘chromosome’ XX. The concentration is calculated as formula: gene number / size of the chromosome.
3.4. Gene Gain and Loss Events during the Evolutionary Process
The speciation process was accompanied with the birth of organism specific genes [42]. This process is also detected between close species and could be enhanced in multigenic families. Gene gain and loss events have been found between the four species (Figure S2). For example, E. globulus contains two specific CIII Prxs orthologous, 11 missed, and 181 common sequences compared with CIII Prxs family of E. grandis. Concerning E. gunnii, 16 CIII Prxs were lost and no gene were gained compared to E. grandis, 11 are lost and 6 isoforms were gained compared to E. globulus, and 14 are lost and 10 genes were gained compared to E. camaldulensis.
Based on the chromosomal location and the phylogenetic analysis, part of the missed genes were members of identified clusters or belong to the pseudogene group (missed genes in one organism are all pseudogenes in other organisms) which support recent evolution events (Figure 2, Table S6) [9]. For example, 1CysPrx03-2, missed in E. grandis genome, is a duplication of 1CysPrx03-1 (Table 2). Prx183, Prx189 and GPx11 missed in E. grandis are pseudogene in the three other Eucalyptus species. In this case, these gene loss events might not have such an effect on plant biology due to the gene redundancy or to the mis-functionality of pseudogenes. In contrast, missed genes which are singletons in other organisms could directly introduce different features for the different Eucalyptus species. The loss of functional genes might be the crucial reason why the four organisms possess different biological characteristics and adaptabilities.
Table 2.
Missed sequences from the genomes of the four Eucalyptus species.
| Types of Missed Genes | E. camaldulensis | E. globulus | E. grandis | E. gunnii |
|---|---|---|---|---|
| Missed genes in clusters 1 | Prx19, Prx37, Prx64, Prx79, Prx83, Prx116, Prx127, Prx138, Prx139, Prx161, Prx164 | Apx-R[P], Prx19, Prx23, Prx37, Prx39, Prx50, Prx116, Prx129-2, GPx07 | 1CysPrx03-2, Prx129-2, Prx188 | Prx16, Prx18, Prx19, Prx20, Prx52, Prx53, Prx54, Prx64, Prx66-2, Prx106, Prx129-2, Prx130, Prx176, Prx188, GPx01 |
| Singletons 2 | APx04, Prx27, Prx28, Prx145, Prx155, Prx165, Prx168 | Prx89, Prx189, Prx194, Prx195, Prx197, Prx198 | Prx183, Prx189, Prx198, GPx11 | APx06, Prx81, Prx89, Prx108, Prx183, Prx189, Prx194 |
1 Genes missed in one organism are members of clusters in other organisms; 2 Genes missed in one organism are singletons in other organisms; Pink Genes missed in one organism are all pseudogenes in other organisms; Green Genes missed in one organism are not pseudogenes in all other organisms. Other missed genes without colour are not all pseudogene and not all partial or complete in other organisms.
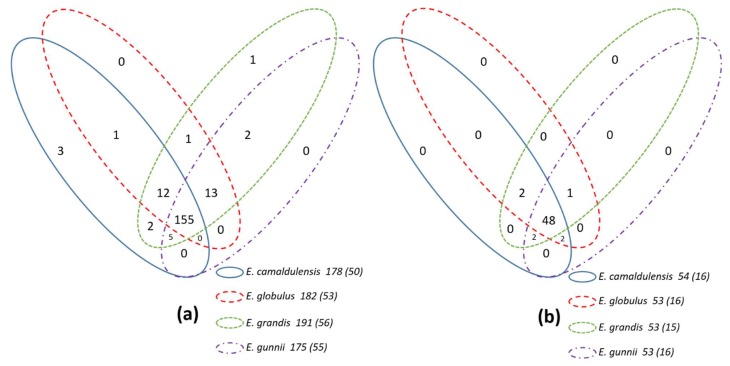
Among the CIII Prxs, 155 sequences are common to the four Eucalyptus species. One sequence has been found to be specific to E. grandis and 3 to E. camaldulensis. However, in E. globulus and E. gunnii no specific CIII Prx genes were found (Figure 3, Table S6). Regarding the other genetic families, the four Eucalyptus species contain similar gene numbers (54, 53, 53, and 53 respectively in E. camaldulensis, E. globulus, E. grandis, and E. gunnii). 48 sequences are common to the 4 organisms. No organism specific gene has been detected in the four Eucalyptus species. However, it is still difficult to determine if this organism specific gene resulted from a gene gain event or a gene lost event in other organisms. Anyway, the pairwise comparison should be still considered as a useful method for the selection of candidate genes that have potentials of organism specific functions like EguPrx171 with no paralogs in the other three organisms. The number of gene absents from E. gunnii (23) is higher than the other species which indicate the possibility that there were fewer ROS genes especially CIII Prx genes. This might be the reason why the ROS gene network size of E. gunnii is smaller than other three organisms.
Figure 3.
Venn diagrams of the ROS genes in the four Eucalyptus species. The Venn diagrams show the numbers of peroxidases gene shared between the four Eucalyptus species: E. gunnii, E. camaldulensis, E. grandis and E. globulus. (a) Venn diagram of CIII Prx family; (b) Venn diagram of other 10 ROS gene families. The total gene numbers of each organism, represented by the ovals, were written on the left of each specific name with the numbers of pseudogenes enclosed in brackets. The area of every intersection region and the gene number are not to scale.
3.5. ROS Gene Families Possess Different Features of Conservation
Phylogenetic analysis and the chromosomal localisation, allow identifying various duplication events such as TD, SD, and WGD events. Based on the duplication events, the 11 families have been be categorized into duplicated gene families such as CIII Prx, Kat, 1CysPrx, and GPx and non-duplicated families such as APx, APx-R, Rboh, DiOx, 2CysPrx, PrxII, and PrxQ.
In order to determine if the observations made for the four Eucalyptus species are also valid for more distant species, compositions of the ROS genes network have been analysed for four other dicotyledon organisms: A. thaliana, V. vinifera, M. truncatula, and P. trichocarpa (Table 3). Non-duplicated families (APx, APx-R, Rboh, DiOx, 2CysPrx, PrxII, GPx, and PrxQ) present a stable isoform number between the four more distant dicotyledon organisms (Table 3). However, CIII Prx, 1CysPrx and Kat which are families subjected to size variation and duplications between Eucalyptus species, are also variable between the selected more distant dicotyledon organisms with a large increase of gene numbers in Eucalyptus species compared to the other one.
Table 3.
Isoform numbers found in 8 organisms.
| Multigenic Families | A. thaliana | E. camaldulensis | E. globulus | E. grandis | E. gunnii | M. truncatula | P. trichocarpa | V. vinifera |
|---|---|---|---|---|---|---|---|---|
| 1CysPrx | 1 (0) | 4 (2) | 4 (2) | 3 (2) | 4 (3) | 1 (0) | 1 (0) | 2 (1) |
| 2CysPrx | 2 (0) | 1 (0) | 1 (0) | 1 (0) | 1 (0) | 2 (0) | 2 (0) | 1 (0) |
| APx | 8 (1) | 10 (2) | 11 (4) | 11 (4) | 10 (3) | 8 (1) | 10 (1) | 9 (2) |
| APx-R | 1 (0) | 2 (1) | 1 (0) | 2 (1) | 2 (1) | 1 (0) | 1 (0) | 1 (0) |
| CIII Prx | 75 (2) | 179 (50) | 183 (56) | 191 (56) | 176 (56) | 106 (8) | 101 (12) | 97 (10) |
| DiOx | 2 (0) | 1 (0) | 1 (0) | 1 (0) | 1 (0) | 2 (0) | 2 (0) | 3 (0) |
| GPx | 8 (0) | 11 (3) | 10 (2) | 10 (1) | 10 (2) | 7 (0) | 8 (2) | 5 (0) |
| Kat | 3 (0) | 14 (8) | 14 (8) | 14 (7) | 14 (7) | 1 (0) | 4 (1) | 2 (0) |
| PrxII | 6 (1) | 3 (0) | 3 (0) | 3 (0) | 3 (0) | 4 (0) | 5 (1) | 4 (0) |
| PrxQ | 1 (0) | 1 (0) | 1 (0) | 1 (0) | 1 (0) | 1 (0) | 2 (0) | 1 (0) |
| Rboh | 10 (0) | 7 (0) | 7 (0) | 7 (0) | 7 (0) | 10 (0) | 10 (0) | 9 (0) |
| Total | 117 (4) | 233 (66) | 236 (72) | 244 (71) | 228 (71) | 143 (9) | 146 (17) | 134 (13) |
The data from the four Eucalyptus species were obtained from the annotation and the PCR detection performed in this study while the data of A. thaliana, V. vinifera (Grape), P. trichocarpa and M. truncatula were directly retrieved from RedOxiBase. The number of theoretical translation or pseudogenes is notified in brackets. Each value consists of genes from genomic data, EST data, experimental detection and other sources. Based on the sufficient quantity of genomic and EST data, the values of A. thaliana and M. truncatula should be able to represent the actual gene numbers even though no experimental detection by PCR were made in this study.
Special attention was paid to detect and identify the partial sequences and pseudogenes for global analysis. Indeed, the presence of pseudogenes in some species can reflect recent duplication events that are disappearing. However, the conservation of these duplications can be necessary to adapt to the contrasted and extreme environments. Taking this into account and according to the research of Chen [43], the gene families with conserved size and little pseudogenes such as APx-R, 2CysPrx, DiOx, PrxQ, APx, GPx, Rboh, and PrxII could be essential genes and subjected to less function variations. On the other hand, gene families with large size variation and pseudogenes, such as CIII Prx, 1CysPrx, and Kat, could contain functional redundancy but spatio-temporal specificity necessary for rapid adaptation. For a further research, most genes belonging to families with conserved size should be potential candidates for simple but crucial functions.
3.6. Families with Size Variation Contain a Lot of Gene Duplication Events
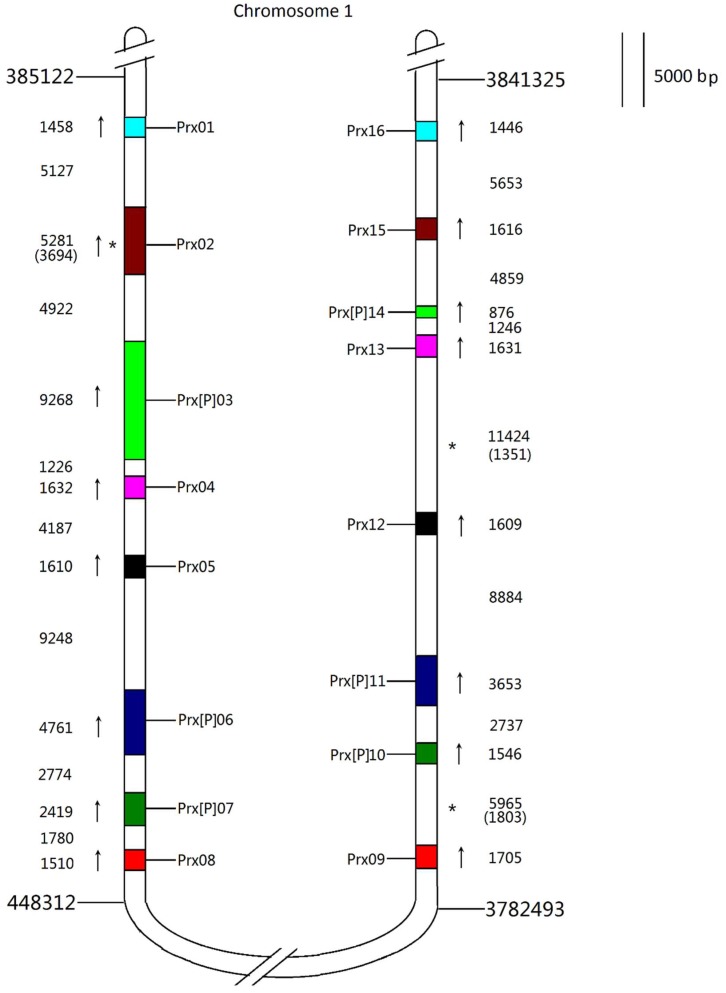
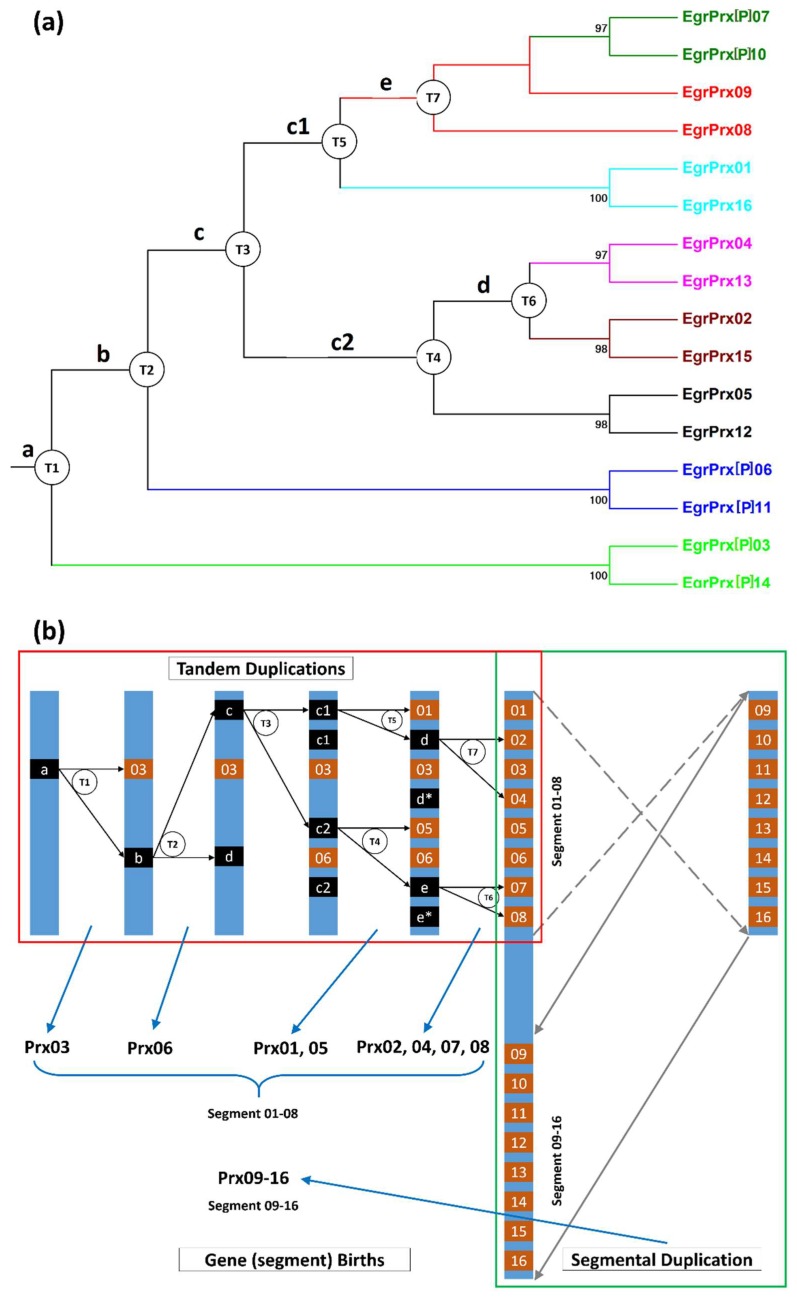
CIII Prx, 1CysPrx, and Kat families present size variations between close or more distant species. These differences are mainly due to the different levels of duplications. From our recent research 80 TDs, eight SDs, and 10 WGDs have been found for CIII Prx family of E. grandis. For example, one large and recent SD can be found on chromosome 1 including EgrPrx01–08 and EgrPrx09–16 (Figure 4). These two regions result from a recent SD with eight genes obtained from seven older and successive TDs (Figure 5A). Genes derived from a duplication event have a very similar coding region from the sequences and the gene structures point of view. One ancestral gene was duplicated seven times and formed the EgrPrx01–08 segment. Then this segment was duplicated and reversely inserted into chromosome 1 to form the EgrPrx09–16 segment (Figure 5B). The paralogs of these 16 genes can be detected in the other three Eucalyptus species (except the paralog of EgrPrx16 missed in E. gunnii) but not in the other organisms such as A. thaliana, P. trichocarpa and M. truncatula. Hence, the duplication events described above should have occur after the divergence of the Eucalyptus genus and before the divergences of the four Eucalyptus species. Similarly, recent duplication events have been observed for both Kat and 1CysPrx families. Indeed, 9 TDs (including Kat01-1, 01-2, 02 and Kat03, 04, 05 and 06) and 1 TD (including 1CysPrx01, 02) can also be detected for these two families.
Figure 4.
A large duplicated CIII Prx gene cluster on chromosome 1. The sizes of genes and segments are to scale. The start and stop positions of the duplicated segments were written on the top and the bottom of the segments. The colorful regions represent the CIII Prxs in the segments on chromosome 1 and the gene orientations were shown with the arrows on the left or right. The sizes of genes and intervals are noted on the side of each sequence with the numbers of undetermined nucleotide acids in brackets. The homologous genes were displayed with the same color on the two duplicated segments. [P]: the gene has been annotated as pseudogene. *: there are undetermined nucleotide acids (NNN…) in the DNA sequences. The visualization of the chromosomal localization was built by Mapchart 2.1. Arrows represent the gene orientations on chromosomes.
Figure 5.
Phylogenetic relationships and hypothetical evolutionary histories of two duplicated segments. (a) Phylogenetic tree of the 16 genes. Genes were given different colors according to the colors in Figure 5. The percentage of trees in which the associated taxa clustered together is shown next to the branches. The alignment was performed by MAFFT and the tree was visualized by Mega 6. Cycled letters T with following numbers represent the TD events; (b) Schematic diagram of the evolutionary histories of the 16 genes. The putative TD process was visualized in the red rectangle while the SD process in green rectangle. Genes shown with black rectangles (named a, b, c, d and e) represent the ancestors of some CIII Prx genes appeared in the evolutionary process. Asterisk (*) represents the alternative localization of the ancestral gene named with the same letter. T1–T7 represent the tandem duplication events. 1–16 represent Prx01–16. The dotted arrow (grey) in B shows the reversing process of gene segment while the grey arrow represents the genomic insertion event. Cycled letters T with following numbers represent the TD events. The order of gene apparition (genes Prx01–16) was displayed on the lower left corner. Gene sizes are not up to scale.
3.7. Expression Profiles of ROS Gene Families within and among Species
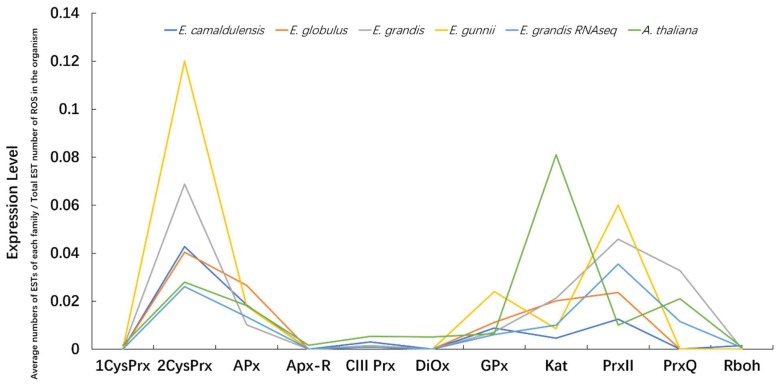
Expression profiles of members of the 11 gene families within and among species show similar expression levels for highly expressed orthologs such as PrxII03, 2CysPrx01 and APx03 (Table S2–S5, S8–S10). However, some orthologs can present different expression profiles. For example, Prx113 is only highly expressed in E. camaldulensis and E. globulus, Kat03 is highly expressed in E. camaldulensis but lowly expressed in the other three organisms. Orthologs with different expression profiles might be related to the different properties of the four species. The expression of the 11 ROS gene families shows similar profiles for the four Eucalyptus species but different from A. thaliana. Between families, the expression levels of 2CysPrx and PrxII are absolutely higher than the other families while the expression of 1CysPrxI, APx-R, DiOx, and Rboh are very low (Figure 6) going against Chen’s conclusion that the families with size conservation have higher level of gene expression [44]. In contrast with the expression levels observed in Eucalyptus species, the Kat family shows the highest expression and PrxII family the lowest in A. thaliana.
Figure 6.
Expression levels of ROS gene families in the four Eucalyptus species and A. thaliana. Expression levels were calculated by the formula: average EST (or RNA seq reads) numbers of each family / Total EST (or RNA seq reads) number of genes in the organism. EST data were obtained from EST libraries of NCBI.
Within the Eucalyptus genus, the ROS families present similar expression patterns even after the genus being splitted in different species. It can be explained by the fact that species evolving under the natural selection keep some common properties of Eucalyptus genus by having a similar expression level of some genes, while along with a long evolution process after the split of the Eucalyptus and Arabidopsis genera, expression statuses of ROS network families became more and more different from each other.
3.8. Duplicated Genes Possess Different Expression Profiles
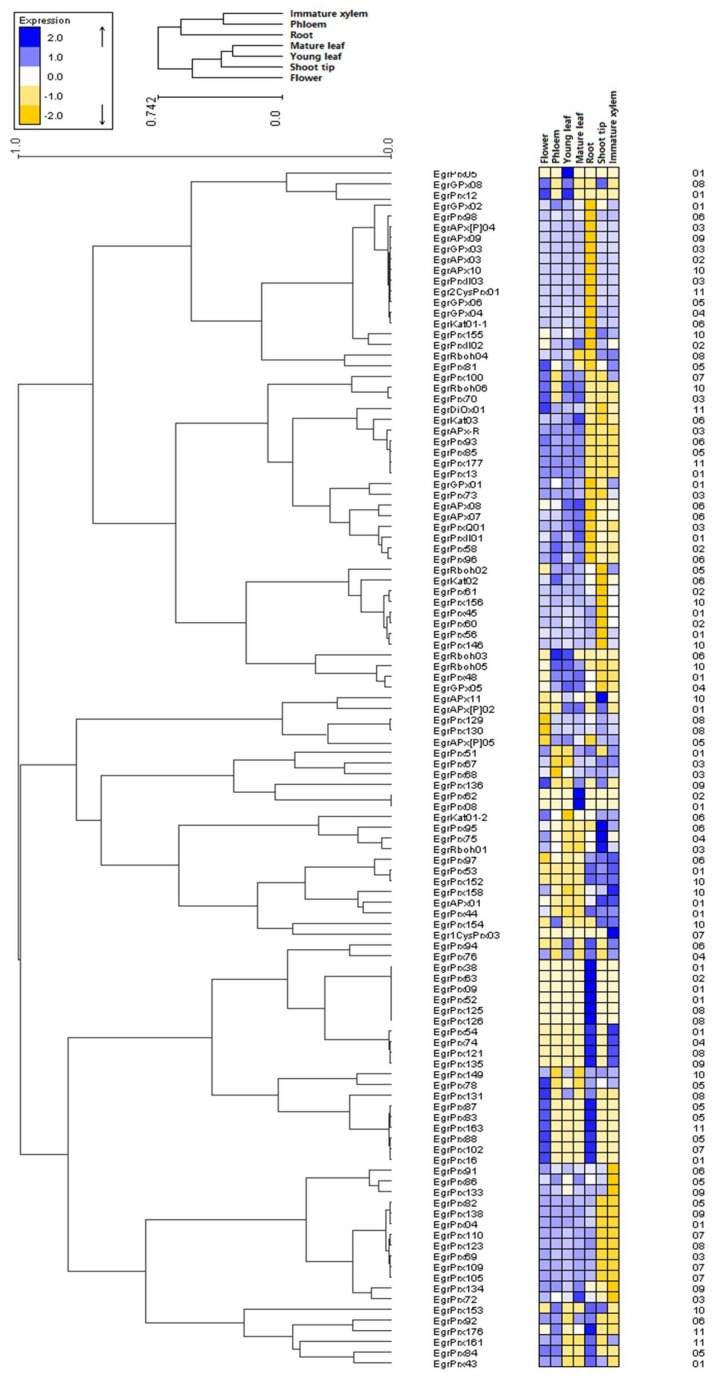
Regarding the heat map of ROS gene network in E. grandis, some groups or sub-groups can be determined based on the expression profiles (Figure 7). No unique relationship has been found between the expression profiles and the duplicated genes. Genes included in SDs, which resulted from large segments containing the coding and regulatory sequences, might have the same expression profile such as SD EgrPrx67, 68 and SD EgrPrx83, 87–88. In contrast, TDs obtained mainly from duplications of coding region only present different expression profiles. For example, EgrPrx62 and EgrPrx63 are highly expressed in mature leaf and root respectively or EgrPrx118–122, among which only EgrPrx121 can be detected, is highly expressed in roots and immature xylem while others are not expressed. In addition, during the evolution process, the duplicated genes can evolve differentially and obtained specific spatio-temporal expression. Even though the question why redundant duplicated genes are found widely has not been answered, the duplication events giving birth to more genes will accumulate mutations faster than a functional single-copy gene, making it possible for one of the two copies to develop new or different functions over generations.
Figure 7.
Heatmap of the expression of the ROS genes from E. grandis in different tissues. Each line represents a gene and each column represents a tissue. Mature leaf, young leaf, shoot tip, phloem, immature xylem, flower and root have been sampled and tested. The chromosome of each gene is written on the right.
3.9. Divergence Dates of the Four Eucalyptus Species
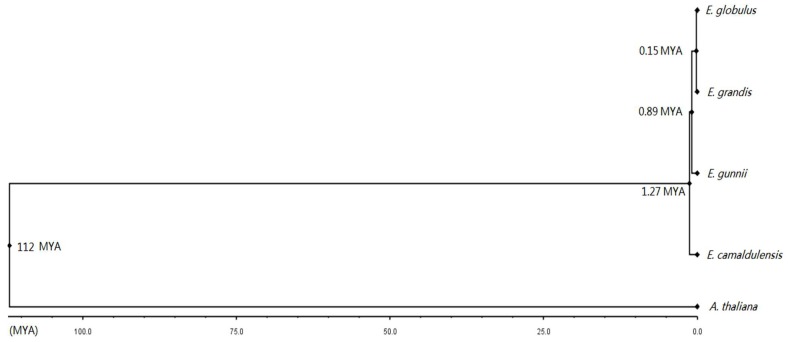
Expert and exhaustive annotation of the four Eucalyptus species and A. thaliana allowed generating high-quality sequence batch for divergence time analysis. The known divergence between A. thaliana and Eucalyptus species (112 million years ago (MYA)) has been used to calibrate the time tree and to get the divergence date between the four Eucalyptus species [44]. E. camaldulensis diverged firstly 1.27 MYA, following by E. gunnii 0.89 MYA, and more recently E. grandis and E. globulus diverged 0.15 MYA (Figure 8). According to the evolutionary rate (Ka/Ks) most ROS genes evolved under negative selection (Table S11). Interestingly, the CIII Prxs evolved faster than other families based on the higher evolutionary rate. The faster evolution and the high duplication rate could be correlated and due to the intrinsic properties of the CIII Prx family.
Figure 8.
Divergence time of the four Eucalyptus species. For each organism the chimerical gene of 49 ROS genes was used in this study. The divergence time between Eucalyptus species was written beside the nodes. Million years ago (MYA).
4. Discussion
In this study, we performed exhaustive and expert annotation and compared 11 gene families part of ROS genes network in four Eucalyptus species with a useful and necessary complementary process. This complementary annotation process included manual annotation and correction, and confirmation with EST libraries checks and PCR detection. This provided more expert and exhaustive data in order to reduce the errors produced by automatic assembly and annotation and to obtain the more complete and accurate results. Of course, this method was easier to apply for annotations of closely-related genomes and cannot be applied with far-related ones. Even if the coverage of genomic sequencing is high (more than 90%), we have demonstrated that the bias of automatic annotation is still elevated with over 60% of sequences not or not correctly predicted. Then, the conclusions made from automatic annotation are partial and erroneous. The 11 families studied presented different features of conservation, duplication and expression. During the process of evolution, gene gain and loss occurred between the species. The number of Eucalyptus genes belonging to the ROS gene network is much larger compared with some other angiosperms mainly due to recent gene duplications in CIII Prx family during the evolution.
This large number of ROS genes copies can be easily linked to the Eucalyptus species particularities such as its very rapid growth, the persistence of its foliage throughout the year, its relative resistance to drought and to temperatures below freezing. Then this large battery of proteins allows a rapid response to biotic and abiotic stresses and the micro differences observed between the four Eucalyptus species may be associated to their environment specificities.
Indeed, the analysis of ROS genes between Eucalytpus species demonstrated recent gain and lost events which confirm that Eucalytpus species genomes are very dynamic, as previously demonstrated. These analyses also provided a method for the selection of candidate gene for next functional research. The families of ROS genes possess different expression levels between the different organisms, but similar profiles among Eucalytpus species. The explosion and the conservation of the ROS encoding genes numbers may be associated with organ diversification, climatic changes and the constant appearance of new pathogens. Nevertheless, there is still question about the family-explosion: why are some families subjected to numerous duplication events while other protein families have kept a similar gene number after speciation?
Large set of complete sequences collected from the four Eucalyptus species allowed determining the divergence time. They diverged from 0.15 MYA to 1.27 MYA. After the divergence of Eucalyptus species, most sequences have been maintained by negative selection in the evolutionary process despite speciation, to conserve the main characters of the Eucalyptus genus. Our results will help better understand the genetic differences between closely related species, and stimulate additional studies on the mechanisms that underlie speciation and biodiversification.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/9/3/257/s1, Table S1. List of the primers used in the exploration of the “missed” genes. Table S2. List of genes part of ROS gene network in E. grandis. Table S3. List of genes part of ROS gene network in E. camaldulensis. Table S4. List of genes part of ROS gene network in E. globulus. Table S5. List of genes part of ROS gene network in E. gunnii. Table S6. Corresponding gene by gene between the four E. species and A. thaliana. Table S7. Distribution and concentration of genes part of ROS gene network on the chromosomes. Table S8. List of ROS genes in A. thaliana. Table S9. RNA-seq data of ROS network in E. grandis. Table S10. EST number of each family in some organisms. Table S11. Evolutionary rate (Ka/Ks) of genes of CIII Prx and other families. Figure S1. Phylogenetic presentation (uncompressed) of all the ROS genes in E. grandis. Figure S2. Gene gain and loss during the evolutionary process.
Author Contributions
Conceptualization, C.D. and Q.L.; Methodology, Q.L. and H.S.C.; Software, C.D.; Formal Analysis, Q.L., Y.H. and Y.F.; Writing – Original Draft Preparation, Q.L. and C.D.; Writing – Review & Editing, C.D.; Project Administration, C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National key Research and Development Program of China (2018YFD1000306), Guangxi Science and Technology Key Project (GuiKeAA18118046-6), Basic Research and Frontier Exploration Project of Chongqing Science and Technology Bureau (cstc2019jcyj-msxmX0014), by Paul Sabatier Toulouse 3 University and by the Centre National de la Recherche Scientifique (CNRS). The authors especially thank all the contributors of the RedOxiBase. The authors especially thank Luc Harvengt and all the contributors of the genomic sequencing (FCBA). The E. gunnii plant material was propagated using Xylobiotech platform funded by Xyloforest (ANR-10-EQPX-16) and its sequencing was funded by Tree for joules (ANR-2010-KBBE-007).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tournier V., Grat S., Marque C., El Kayal W., Penchel R., de Andrade G., Boudet A.M., Teulières C. An efficient procedure to stably introduce genes into an economically important pulp tree (Eucalyptus grandis × Eucalyptus urophylla) Transgenic Res. 2003;12:403–411. doi: 10.1023/A:1024217910354. [DOI] [PubMed] [Google Scholar]
- 2.Myburg A.A., Grattapaglia D., Tuskan G.A., Hellsten U., Hayes R.D., Grimwood J., Jenkins J., Lindquist E., Tice H., Bauer D., et al. The genome of Eucalyptus grandis. Nature. 2014;509:356–362. doi: 10.1038/nature13308. [DOI] [PubMed] [Google Scholar]
- 3.Foreman J., Demidchik V., Bothwell J.H., Mylona P., Miedema H., Torres M.A., Linstead P., Costa S., Brownlee C., Jones J.D., et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 4.Overmyer K., Brosché M., Kangasjärvi J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003;8:335–342. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- 5.Neill S., Desikan R., Hancock J. Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 2002;5:388–395. doi: 10.1016/S1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 6.Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Lazzarotto F., Teixeira F.K., Rosa S.B., Dunand C., Fernandes C.L., Fontenele A.V., Silveira J.A., Verli H., Margis R., Margis-Pinheiro M. Ascorbate peroxidase-related (APx-R) is a new heme-containing protein functionally associated with ascorbate peroxidase but evolutionarily divergent. New Phytol. 2011;191:234–250. doi: 10.1111/j.1469-8137.2011.03659.x. [DOI] [PubMed] [Google Scholar]
- 8.Passardi F., Longet D., Penel C., Dunand C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry. 2004;65:1879–1893. doi: 10.1016/j.phytochem.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Li Q., Yu H., Cao P.B., Fawal N., Mathé C., Azar S., Cassan-Wang H., Myburg A.A., Grima-Pettenati J., Marque C., et al. Explosive Tandem and Segmental Duplications of Multigenic Families in Eucalyptus grandis. Genome Biol. Evol. 2015;7:1068–1081. doi: 10.1093/gbe/evv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q., Dou W., Qi J., Qin X., Chen S., He Y. Genomewide analysis of the CIII peroxidase family in sweet orange (Citrus sinensis ) and expression profiles induced by Xanthomonas citri subsp. citri and hormones. J. Genet. 2020;99:10. doi: 10.1007/s12041-019-1163-5. [DOI] [PubMed] [Google Scholar]
- 11.Koua D., Cerutti L., Falquet L., Sigrist C.J., Theiler G., Hulo N., Dunand C. PeroxiBase: A database with new tools for peroxidase family classification. Nucleic Acids Res. 2009;37:D261–D266. doi: 10.1093/nar/gkn680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki N., Miller G., Morales J., Shulaev V., Torres M.A., Mittler R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011;14:691–699. doi: 10.1016/j.pbi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Kettler G.C., Martiny A.C., Huang K., Zucker J., Coleman M.L., Rodrigue S., Chen F., Lapidus A., Ferriera S., Johnson J., et al. Patterns and implications of gene gain and loss in the evolution of Prochlorococcus. PLoS Genet. 2007;3:e231. doi: 10.1371/journal.pgen.0030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filipski E., Innominato P.F., Wu M., Li X.M., Iacobelli S., Xian L.J., Lévi F. Effects of light and food schedules on liver and tumor molecular clocks in mice. J. Natl. Cancer Inst. 2005;97:507–517. doi: 10.1093/jnci/dji083. [DOI] [PubMed] [Google Scholar]
- 15.Fawal N., Li Q., Mathé C., Dunand C. Automatic multigenic family annotation: Risks and solutions. Trends Genet. 2014;30:325. doi: 10.1016/j.tig.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z., Swanson W., Vacquier V. Maximum-likelihood analysis of molecular adaptation in abalone sperm lysin reveals variable selective pressures among lineages and sites. Mol. Biol. Evol. 2000;17:1446–1455. doi: 10.1093/oxfordjournals.molbev.a026245. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z., Nielsen R., Goldman N., Pedersen A.M. Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics. 2000;155:431–449. doi: 10.1093/genetics/155.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z., Li J., Zhao X.Q., Wang J., Wong G.K., Yu J. KaKs_Calculator: Calculating Ka and Ks through model selection and model averaging. Genom. Proteom. Bioinform. 2006;4:259–263. doi: 10.1016/S1672-0229(07)60007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Easteal S., Herbert G. Molecular evidence from the nuclear genome for the time frame of human evolution. J. Mol. Evol. 1997;44(Suppl. 1):S121–S132. doi: 10.1007/PL00000066. [DOI] [PubMed] [Google Scholar]
- 21.Nei M., Xu P., Glazko G. Estimation of divergence times from multiprotein sequences for a few mammalian species and several distantly related organisms. Proc. Natl. Acad. Sci. USA. 2001;98:2497–2502. doi: 10.1073/pnas.051611498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doolittle R.F., Feng D.F., Tsang S., Cho G., Little E. Determining divergence times of the major kingdoms of living organisms with a protein clock. Science. 1996;271:470–477. doi: 10.1126/science.271.5248.470. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S., Hedges S.B. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 24.dos Reis M., Yang Z. Approximate likelihood calculation on a phylogeny for Bayesian estimation of divergence times. Mol. Biol. Evol. 2011;28:2161–2172. doi: 10.1093/molbev/msr045. [DOI] [PubMed] [Google Scholar]
- 25.Wang X.Q., Tank D.C., Sang T. Phylogeny and divergence times in Pinaceae: Evidence from three genomes. Mol. Biol. Evol. 2000;17:773–781. doi: 10.1093/oxfordjournals.molbev.a026356. [DOI] [PubMed] [Google Scholar]
- 26.Drummond A.J., Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frazer K.A., Chen X., Hinds D.A., Pant P.V., Patil N., Cox D.R. Genomic DNA insertions and deletions occur frequently between humans and nonhuman primates. Genome Res. 2003;13:341–346. doi: 10.1101/gr.554603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fawal N., Li Q., Savelli B., Brette M., Passaia G., Fabre M., Mathé C., Dunand C. PeroxiBase: A database for large-scale evolutionary analysis of peroxidases. Nucleic Acids Res. 2013;41:D441–D444. doi: 10.1093/nar/gks1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savelli B., Li Q., Webber M., Jemmat A.M., Robitaille A., Zamocky M., Mathé C., Dunand C. RedoxiBase: A database for ROS homeostasis regulated proteins. Redox Biol. 2019;26:101247. doi: 10.1016/j.redox.2019.101247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keller O., Odronitz F., Stanke M., Kollmar M., Waack S. Scipio: Using protein sequences to determine the precise exon/intron structures of genes and their orthologs in closely related species. BMC Bioinform. 2008;9:278. doi: 10.1186/1471-2105-9-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai I.J., Otto T.D., Berriman M. Improving draft assemblies by iterative mapping and assembly of short reads to eliminate gaps. Genome Biol. 2010;11:R41. doi: 10.1186/gb-2010-11-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milne I., Bayer M., Cardle L., Shaw P., Stephen G., Wright F., Marshall D. Tablet--next generation sequence assembly visualization. Bioinformatics. 2010;26:401–402. doi: 10.1093/bioinformatics/btp666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Thilmony R., Gu Y.Q. NetVenn: An integrated network analysis web platform for gene lists. Nucleic Acids Res. 2014;42:W161–W166. doi: 10.1093/nar/gku331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tippmann H.F. Analysis for free: Comparing programs for sequence analysis. Brief Bioinform. 2004;5:82–87. doi: 10.1093/bib/5.1.82. [DOI] [PubMed] [Google Scholar]
- 36.Guindon S., Dufayard J.F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 37.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voorrips R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002;93:77–78. doi: 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]
- 39.Ulitsky I., Maron-Katz A., Shavit S., Sagir D., Linhart C., Elkon R., Tanay A., Sharan R., Shiloh Y., Shamir R. Expander: From expression microarrays to networks and functions. Nat. Protoc. 2010;5:303–322. doi: 10.1038/nprot.2009.230. [DOI] [PubMed] [Google Scholar]
- 40.Rozas J., Rozas R. DnaSP, DNA sequence polymorphism: An interactive program for estimating population genetics parameters from DNA sequence data. Comput. Appl. Biosci. 1995;11:621–625. doi: 10.1093/bioinformatics/11.6.621. [DOI] [PubMed] [Google Scholar]
- 41.Hirakawa H., Nakamura Y., Kaneko T., Isobe S., Sakai H., Kato T., Hibino T., Sasamoto S., Watanabe A., Yamada M., et al. Survey of the genetic information carried in the genome of Eucalyptus camaldulensis. Plant Biotechnol. 2011;28:471–480. doi: 10.5511/plantbiotechnology.11.1027b. [DOI] [Google Scholar]
- 42.Nei M., Nozawa M. Roles of Mutation and Selection in Speciation: From Hugo de Vries to the Modern Genomic Era. Genome Biol. Evol. 2011;3:812–829. doi: 10.1093/gbe/evr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen F.C., Chen C.J., Li W.H., Chuang T.J. Gene family size conservation is a good indicator of evolutionary rates. Mol. Biol. Evol. 2010;27:1750–1758. doi: 10.1093/molbev/msq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hedges S.B., Dudley J., Kumar S. TimeTree: A public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22:2971–2972. doi: 10.1093/bioinformatics/btl505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.