Abstract
Background: Delayed cerebral vasospasm (DCVS) due to aneurysmal subarachnoid hemorrhage (aSAH) and its sequela, delayed cerebral ischemia (DCI), are associated with poor functional outcome. Endothelin-1 (ET-1) is known to play a major role in mediating cerebral vasoconstriction. Angiotensin-II-type-1-receptor antagonists such as Sartans may have a beneficial effect after aSAH by reducing DCVS due to crosstalk with the endothelin system. In this review, we discuss the role of Sartans in the treatment of stroke and their potential impact in aSAH. Methods: We conducted a literature research of the MEDLINE PubMed database in accordance with PRISMA criteria on articles published between 1980 to 2019 reviewing: “Sartans AND ischemic stroke”. Of 227 studies, 64 preclinical and 19 clinical trials fulfilled the eligibility criteria. Results: There was a positive effect of Sartans on ischemic stroke in both preclinical and clinical settings (attenuating ischemic brain damage, reducing cerebral inflammation and infarct size, increasing cerebral blood flow). In addition, Sartans reduced DCVS after aSAH in animal models by diminishing the effect of ET-1 mediated vasoconstriction (including cerebral inflammation and cerebral epileptogenic activity reduction, cerebral blood flow autoregulation restoration as well as pressure-dependent cerebral vasoconstriction). Conclusion: Thus, Sartans might play a key role in the treatment of patients with aSAH.
Keywords: aneurysmal subarachnoid hemorrhage, delayed cerebral vasospasm, ischemic stroke, Sartans, therapeutic interventions
1. Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) induces delayed cerebral vasospasm (DCVS) [1], cerebral inflammation [2,3], early brain injury [4], cortical spreading depression [5], delayed cerebral ischemia (DCI) [6], and lack of cerebral autoregulation [7] contributing to poor functional patients’ outcome. DCVS remains a major cause of patient’s morbidity and mortality by inducing delayed cerebral ischemia [8].
Multiple studies showed that endothelin-1 (ET-1), a most potent vasoconstrictor [9,10,11], plays a key role in the development of DCVS [12,13,14,15,16,17,18,19]. Although endothelin-A-receptor (ETA-R) antagonists in the treatment of DCVS in animal models are effective [10,20], clinical studies did not show beneficial effects [21,22]. It has been reported that the polypeptide angiotensin-II acts through two specific receptors, in essence the angiotensin-II-type-1- and angiotensin-II-type-2-receptor (AT2-1-R and AT2-2-R). Important to note is that activation of the AT2-1-R results in vasoconstriction while binding of angiotensin-II to the AT2-2-R causes vasorelaxation [23]. In line with this notion, preclinical as well as clinical trials showed promising results of Sartans, which are AT2-1-R antagonists, in ischemic stroke. Hence, Sartans may have a positive effect after aSAH by reducing DCVS due to crosstalk with the endothelin system. Thus, we aimed to analyze the potential role of Sartans in the treatment of aSAH.
2. Materials and Methods
We conducted a systematic literature research of the MEDLINE PubMed database in accordance with PRISMA guidelines on preclinical studies on the one and on clinical studies on the other hand published between 1980 to 2019 reviewing: “Sartans and ischemic stroke” [24]. Only articles in English were chosen for review. Search items with “Sartans” (n = 19,064) and “ischemic stroke” (n = 89,465) were extracted. For “Sartans AND ischemic stroke”, 227 publications met the inclusion criteria by excluding studies with commentary only, any duplicates, or results not commenting on cerebral effects of Sartans.
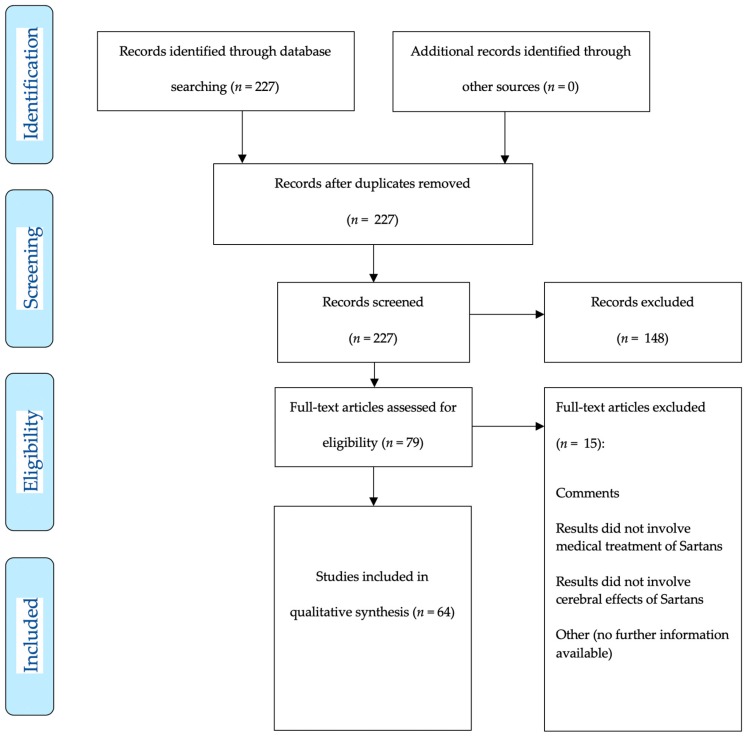
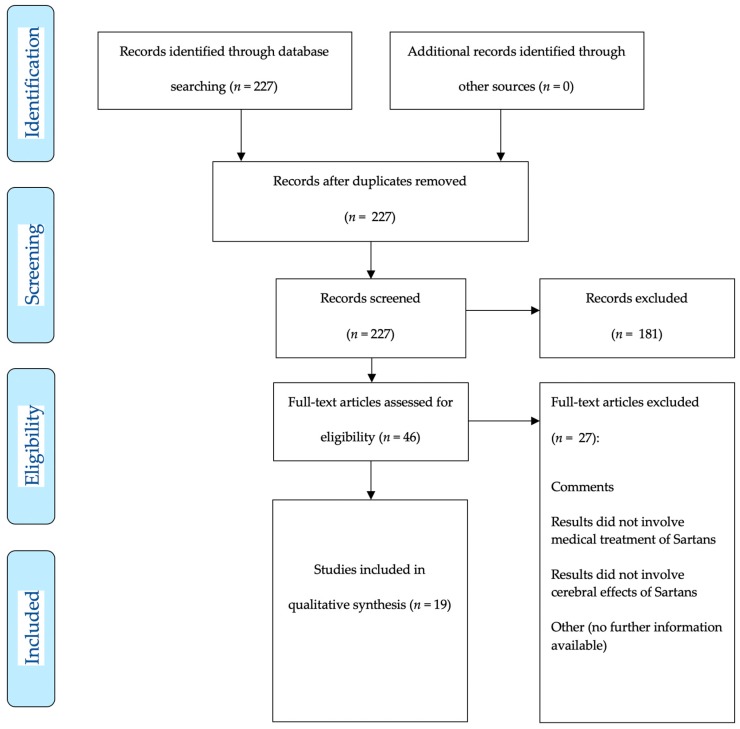
Two hundred and twenty-seven studies were assessed for eligibility, 83 met inclusion criteria for systematic review and qualitative analysis with 64 preclinical studies (Figure 1 demonstrates the inclusion pathway for basic research studies selected via MEDLINE PubMed search) and 19 clinical studies (Figure 2 shows the inclusion pathway for clinical research studies selected via MEDLINE PubMed search).
Figure 1.
Two hundred and twenty-seven articles (published 01-01-1980–01-07-2019) were detected for preclinical and clinical research articles. After manual abstract screening for preclinical research articles only, 79 articles remained for further analysis. Each of the 79 articles was explicitly screened for potential drug applications after ischemic stroke. Finally, 64 articles were included for qualitative analysis.
Figure 2.
Two hundred and twenty-seven articles (published 01-01-1980–01-07-2019) were detected for preclinical and clinical research articles. After manual abstract screening for clinical research articles only, 46 articles remained for further analysis. Each of the 46 articles was explicitly screened for potential drug applications after ischemic stroke. Finally, 19 articles were included for final analysis.
Of the articles included in the final analysis, a systematic review on the beneficial and non-beneficial effect in the preclinical and clinical settings was performed. Summary measures are reported as outcome measures (i.e., infarct size, neurocognition, inflammation).
3. Results
3.1. Preclinical Studies on Sartans in Animal Models of Ischemic Stroke
The search finally yielded 64 preclinical studies on “Sartans AND ischemic stroke”, eligible for systematic review (Table 1).
Table 1.
Tabular listing of different preclinical studies showing various effects of Sartan administration (Abbreviations: Angiotensin-II-type-1-receptor (AT2-1-R); Angiotensin-II-type-2-receptor (AT2-2-R); common carotid artery occlusion (CCAO); chemokine receptor 2 (CCR2); cluster of differentiation (CD); candesartan (CS); desoxy ribonucleic acid (DNA); endothelial nitric oxide synthase (eNOS); endothelin-A-receptor (ETA-R); hours (h); inducible nitric oxide synthase (iNOS); irbesartan (IS); kilogram (kg); losartan (LS); middle cerebral artery occlusion (MCAO); matrix-metallo-proteinase (MMP); messenger ribonucleic acid (mRNA); milligram (mg); minutes (min); n-acetyl-glucosamine oligomer (NAGO); nlr family pyrin domain containing 3 (NLRP3); oxygen glucose deprivation (OGD); olmesartan (OMS); stroke-resistant spontaneously hypertensive rats (SR-SHR); telmisartan (TMS); tumor necrosis factor alpha (TNFα); ribonucleic acid (RNA); vascular endothelial growth factor (VEGF); valsartan (VS)).
| Drug | Model | Outcome | Beneficial Effect | Special Remarks |
|---|---|---|---|---|
| TMS [25] | Global ischemic mice model | Cerebral perfusion | Restored cerebral blood flow | - |
| TMS [26] | MCAO mice | Neuroscore, infarct size | Improved neuroscore and decreased infarct size, increased cerebral blood flow, reduced superoxide production and inflammatory cytokine expression | - |
| TMS [27] | Murine model of transient and permanent focal ischemia | Infarct size, reperfusion injury | Reduced stroke volume 72 h after transient ischemia, likewise pro-inflammatory adhesion molecules and infiltration of inflammatory cells in the ischemic region | No reduction in stroke volume 72 h after permanent ischemia |
| TMS [28] | MCAO mice | Focal brain ischemia, atherosclerotic lesions | Attenuated ischemic brain damage, neurological deficits and superoxide production in ischemic area; attenuated reduction of cerebral blood flow in the penumbra without significantly changing blood pressure | Anti-atherosclerotic effects |
| TMS [29] | MCAO rat | Cerebral perfusion | Improved cerebral blood flow, enhanced vascular density (CD31 immunofluorescence staining), antiapoptotic effects | - |
| TMS [30] | MCAO rat | Cognitive function, level of matrix metalloproteinases | Improved spatial memory ability, decreased expression levels of MMP-2 and MMP-9 | - |
| TMS [31] | MCAO rat | Behavior alterations, neuroprotective effects on secondary reperfusion phase | Normalized behavioral alterations comparable to pre-ischemic treatment (protected neurons from ischemic reperfusion injury), attenuated excitatory amino acid release in secondary reperfusion phase | In combination with nimodipine. Drug treatments immediately after reperfusion, effects compared with pretreatment |
| TMS [32] | MCAO rat | Effects on neurovascular unit and neuroinflammation | Reduced decrease of NAGO-positive endothelium, similar increase of MMP-9 positive neurons and NLRP3-positive inflammasome in the cerebral cortex | Low dose TMS improved changes without lowering blood pressure, high dose TMS further improved changes with lowering blood pressure |
| TMS [33] | Open skull preparation rat | Cerebral arteriolar pressure, cerebral blood flow, internal vessel diameter | Normalization of arteriolar pressure and lower limit of cerebral autoregulation | Combined with Ramipril |
| TMS [34] | MCAO rats | Metabolic related post-ischemic changes | Ameliorated metabolic related post-ischemic changes | - |
| TMS [35] | MCAO rats | Neurological outcome, infarct volume, inflammation | Improved outcome, reduced infarct volume and inflammation | Subcutaneous TMS application 5 days prior to MCAO with reperfusion |
| TMS [36] | MCAO rats | Infarct volume, immunohistochemical parameters | Significantly reduced infarct volume, reduced neurotoxic cytosolic phospholipase A2, ameliorates ischemic changes of neurons in the peri-infarct area | Pretreatment for 7 days |
| TMS [37] | Collagenase infusion or autologous blood injection to induce intracerebral hemorrhage in rats | Hemorrhage volume, functional recovery | Reduced hemorrhage volume, brain edema, inflammatory/apoptotic cells in perihematomal area; induced endothelial nitric-oxide-synthase, decreased oxidative stress, apoptotic signals, and TNFα | - |
| TMS [38] | Stroke-resistant spontaneously hypertensive rats | Oxidative stress | Reduced advanced glycation end product, 4-hydroxy-2-nonenal- and phosphorylated a-synuclein-positive cells in the cerebral cortex and hippocampus | - |
| CS [39] | MCAO mice | Ischemic brain damage | Reduced ischemic brain area and neurological deficits in non-hypotensive doses; improved reduction of brain surface blood flow and inhibited superoxide production in the cortex and brain arterial wall at non-hypotensive and hypotensive doses; AT2-2-R expression in the ischemic area was increased by prior pretreatment with CS | - |
| CS [40] | MCAO mice | Antioxidant enzyme activity | Restored superoxide dismutase activity and cerebral blood flow | - |
| CS [41] | MCAO rats | Neurobehavioral outcome, infarct size, vascular density | Improved neurobehavioral outcome, reduced infarct size and vascular density | In vitro vascular density was assessed using human brain endothelial cells |
| CS [42] | MCAO rats | Infarct size, neurological outcome | Improved neurobehavioral and motor functions, decreased infarct size | Intravenous CS administration |
| CS [43] | MCAO rats | Neurological outcome | Improved recovery from ischemic stroke | Only 0.3 mg/kg CS with neuroprotective function |
| CS [44] | MCAO rats | Neurological outcome, oxidative enzymes | Improved motor function and reduced endoplasmatic reticulum stress markers | Only early beneficial effect after 24 h |
| CS [45] | MCAO rats | Neurological outcome, vascular density/synaptogenesis | Improved functional outcome, increased vascular density/synaptogenesis only in the control group | Intracerebroventricular injection of short hairpin RNA lentiviral particles to knock down brain-derived neurotrophic factor or nontargeting control vector |
| CS [46] | MCAO rats | Angiogenesis | Induced prolonged proangiogenic effect and upregulation of VEGF-A and VEGF-B; stabilized hypoxia-inducible factor-1a and preserves angiopoetin-1 | - |
| CS [47] | Spontaneously hypertensive rats | Angiogenesis | Exerted proangiogenic effects on brain microvascular endothelial cells | - |
| CS [48] | In vitro monolayer model using rat brain capillary endothelial cells | Stability of blood brain barrier | Improved cell function and viability of brain capillary endothelial cells under OGD | Normoxia versus 6 h OGD |
| CS [49] | MCAO rats | Neurological outcome, infarct size | Improved neurological function, significantly reduced blood brain barrier disruption/edema/infarct volume | - |
| CS [50] | MCAO rats | Infarct size, functional recovery, neuroplasticity | Significantly reduced infarct size, ameliorated functional recovery and increased neuroplasticity markers | - |
| CS [51] | MCAO rats | Infarct size, neurological outcome | Decreased infarct size and improved neurological outcome | - |
| CS [52] | MCAO rats | Mortality, infarct size | Significantly reduced mortality and infarct size | - |
| CS [53] | MCAO rats | Infarct size | Reduced infarct size | Oral administration |
| CS [54] | MCAO rats | Infarct size, edema, neurological outcome | Reduced infarct size, edema formation and improves neurological outcome | - |
| CS [55] | MCAO rats | Infarct size, neurological outcome | Significantly reduced stroke volume and improved neurological outcome | - |
| CS [56] | MCAO rats | Infarct size, edema | Reduced infarct size and edema, improved neurologic function | - |
| CS [57] | MCAO rats | Infarct volume, neurological deficit | Reduced infarct size and improved neurologic outcome | - |
| CS [58] | MCAO rats | Infarct volume, neurological deficits | Reduced infarct size, improved neurological outcome, reduced lipid peroxidation | Subcutaneous infusion for 14 days |
| CS [59] | MCAO rats | Infarct volume, neurological deficits | Reduced infarct size/edema and improved neurological outcome | Long-term blockade (subcutaneous injection twice daily 5 days before ischemia), not short-term administration (intravenous once 4 h prior to ischemia), improves neurological outcome |
| CS [60] | MCAO rats | Infarct volume, brain edema | Significantly reduced cortical infarct volume and brain edema | - |
| CS [61] | Bilateral CCAO rats | Neurological outcome, oxidative damage | Attenuated neurobehavioral alterations, oxidative damage and restored mitochondrial enzyme dysfunction | Occlusion for 30 min, followed by 24 h reperfusion; CS pretreatment for 7 days |
| CS [62] | MCAO rats | Infarct size | Reduced infarct area | - |
| CS [63] | MCAO rats | Infarct size, neurological outcome | Pretreatment reduced infarct area and improved neurological outcome | - |
| CS [64] | MCAO rats | Infarct size, neurological outcome | Reduced infarct size and neurological deficits; significantly reduced mRNA expression of inflammatory markers | - |
| CS [65] | Spontaneously hypertensive rats | AT2-1-R expression | Increased AT2-2-R expression in spontaneously hypertensive rats | CS application via subcutaneous osmotic minipumps for 4 weeks |
| CS [66] | MCAO rats | Neurological outcome, vascular density | Improved neurological outcome and increased vascular density | - |
| CS [67] | Embolic stroke model | Mortality, neurological outcome, infarct size | Significantly decreased mortality, neurological deficits, and infarct size | Injection of calibrated microspheres |
| CS [68] | MCAO rat | Infarct size, neurological outcome | Reduced infarct size and improved neurological outcome | Combined treatment with ETA-R antagonist |
| CS [69] | MCAO rats | Contractile response to angiontensin II | Abolished the enhanced responses to angiotensin II | - |
| CS [70] | MCAO rats | Infarct volume, neurological outcome | Reduced infarct size with low but not high dose of CS, improved neurological outcome | Subcutaneous CS administration |
| CS [71] | MCAO rats | Infarct size, neuroscores, cerebral blood flow | Reduced infarct size and increased cerebral blood flow | Intravenous CS administration |
| CS [72] | Spontaneously hypertensive rats | Vascular remodeling, expression of eNOS/iNOS | Reversed negative vascular remodeling and alterations in eNOS/iNOS expression | - |
| OMS [73] | Bilateral CCAO mice | Cognitive impairment | Ameliorated cognitive impairment | - |
| OMS [74] | Single carotid ligation stroke model gerbil | Survival | Significantly increased survival at day 30 | - |
| OMS [75] | MCAO rats | Neurological outcome, infarct size, cell death | Significantly improved functional scores, reduced infarct size and cell death | Only continuous administration of OMS before and after stroke reduced oxidative stress levels |
| OMS [76] | MCAO rats | Infarct volume | Reduced infarct volume 48 h after transient focal brain ischemia | OMS administration via drinking water |
| OMS [77] | MCAO rats | Stroke index score, infarct volume, quantity of MMPs | Improved stroke index score, infarct volume, reduced cerebral edema and upregulation of MMPs | - |
| VS [78] | MCAO mice | Infarct volume, DNA damage, superoxide production | Significantly reduced infarct size, DNA damage, superoxide production, mRNA levels of monocyte chemoattractant protein-1, increases cerebral blood flow, increased eNOS activation and nitric oxide production | - |
| VS [79] | MCAO mice | Infarct volume, neurological outcome | Significantly reduced infarct volume and improved neurological outcome | - |
| VS [80] | MCAO mice | Infarct volume, neurological outcome | Significantly reduced ischemic area, neurological deficits, reduction of cerebral blood flow and superoxide production | - |
| VS [81] | High salt loaded SR-SHR | Brain injury | Enhanced protective effects against brain injury, white matter lesions and glial activation | Combined with amlodipine |
| IS [82] | MCAO rats | Infarct size, neurological outcome | Reduced infarct size and number of apoptotic cells in the peri-infarct cortex on day 3, attenuated invasion of microglia and macrophages on day 3 and 7 after ischemia | - |
| IS [83] | MCAO rats | Neurological outcome | Significantly improved neurological outcome | Administration of IS intracerebroventricularly over 5 days |
| IS [84] | MCAO rats | Infarct size | Reduced infarct volume | Coadministration of propagermanium (CCR2 antagonist) |
| LS [85] | Single carotid ligation stroke model gerbil | Mortality | Did not increase mortality after unilateral carotid ligation in gerbils | - |
| LS [86] | MCAO mice | OGD-induced cell injury | Abolished OGD-induced exaggeration of cell injury in mice overexpressing renin and angiotensinogen animals | - |
| LS [87] | MCAO rats | Gene expression levels of pro-apoptotic genes | Significant reduced gene expression of pro-apoptotic genes | - |
| LS [88] | Cerebral focal ischemia by cauterization of cortical surface vessels rats | Cessation of blood flow, infarct size | Maintained angiogenesis, vascular delivery, and significantly decreased infarct size | Administration of LS in drinking water 2 weeks before inducing ischemia |
Telmisartan (TMS), a selective AT2-1-R antagonist, displayed the capacity to increase cerebral blood flow (CBF) in global cerebral ischemia [25]. It ameliorated reduction of CBF in the penumbra (0.3 mg/kg/day) without significant changes in blood pressure (BP) [28]. Following middle cerebral artery occlusion (MCAO), TMS decreased ischemic infarct area, reduced superoxide production and expression of inflammatory cytokines, infiltration of inflammatory cells, improved neurological scores, and increased CBF [26,27]. Angiogenesis in ischemic areas after MCAO was enhanced by TMS, as well as neuroregeneration by downregulating caspase activation [29]. A combination of TMS with nimodipine (2.5–5 mg/kg) in a transient MCAO rat model revealed beneficial influences affecting the attenuation of excitatory amino acids in different brain regions nine days after MCAO with neurobehavioural outcomes normalized seven days after MCAO [31]. Low doses of TMS (0.3–3 mg/kg/d) after MCAO in a model of stroke-resistant spontaneously hypertensive rats (SR-SHR) reduced progressive decrease of N-acetylglucosamine oligomer and increase of MMP-9 positive neurons without reducing BP [32]. Likewise combination therapies with ramipril (0.8 mg/kg per day TMS + 0.1 mg/kg per day ramipril or 0.5 mg/kg per day TMS + 0.25 mg/kg per day ramipril) normalized BP as well as maintained cerebral blood flow autoregulation [33]. Deguchi et al. demonstrated that TMS dose-dependently (0.3 mg/kg/day or 3 mg/kg/day) ameliorated metabolic syndrome related changes in the post stroke brain of SR-SHR with direct neuroprotective effects [34]. Moreover, incidence of stroke was reduced along with prolonged survival and improved neurological outcome following TMS application (0.5 mg/kg once daily) [35]. Pretreatment of rats with TMS (1 mg/kg) seven days before inducing cerebral ischemia also showed significant reduced infarct size and histopathologically normal appearance of neurons in the periinfarct cortical regions [36].
Candesartan (CS), another AT2-1-R antagonist, reduced ischemic brain damage following MCAO occlusion [39]. CS and curcumin together significantly restored superoxide dismutase activity and blood flow compared with the untreated group [40]. Further, CS upregulated vascular endothelial growth factor (VEGF) B after induction of focal cerebral ischemia using a MCAO model. In contrast to saline-treatment after reperfusion, CS further improved neurobehavioral and motor functions and decreased infarct size [41]. VEGF-B silencing was shown to diminish CS (1 mg/kg) protective effects [42]. CS (0.3 mg/kg) was able to improve recovery from ischemic stroke in low doses by maintaining blood pressure during reperfusion [43]. CS induced early protective effects with improvement in motor function, upregulated brain-derived neutrotrophic factor (BDNF), and also reduced endoplasmatic reticulum stress markers [44]. In a MCAO BDNF, knock-out model rats received CS or saline at reperfusion for 14 days, revealing better functional outcomes, increased vascular density, and synaptogenesis in the CS (1 mg/kg) group [45,46]. In addition, CS (0.16 μM) significantly increased BDNF production [47]. Furthermore, CS (10 nM) improved cell function and viability of brain capillary endothelial cells under oxygen glucose deprivation, providing protective blood–brain-barrier (BBB) effects [48]. In other transient MCAO rat models, CS (0.1 mg/kg; 0.3 mg/kg; 1.5 or 10 mg/kg per day; 0.1, 1 and 10 mg/kg; 0.1, 0.3 or 1 mg/kg; 0.1 mg/kg twice daily; 1 mg/kg; 0.3 or 3 mg/kg per day; 0.5 mg/kg per day for 14 days; 0.1 or 0.3 mg/kg; 0.5 mg/kg per day for 3 to 14 days) showed improved neurological function with significant reduction in BBB disruption, in cerebral ischemia, and in edema [39,49,50,51,52,53,54,55,56,57,58,59,60]. In a bilateral common carotid artery occlusion (CCAO) model in rats, pretreatment with CS (0.1 and 0.3 mg/kg) and atorvastatin significantly attenuated neurobehavioral alterations, oxidative damage, and restored mitochondrial enzyme dysfunction compared to the control group [61,62]. AT2-1-R administration prior to ET-1 induced MCAO provides neuroprotective effects, with CS (0.2 mg/kg per day for seven days) pretreatment attenuating infarct size and neurological deficits without altering systemic BP [63]. Pretreatment with CS for five days significantly decreased mortality, neurological deficits, and infarct size [67]. A combined inhibition of AT2-1- (0.05 mg/kg per day) and ETA-receptors decreased brain damage as well; additionally, an upregulation of AT2-1-R in ischemic middle cerebral artery smooth muscle cells (SMCs) was found [68,69]. Also, early (3 h) and delayed (24 h) effects of CS treatment (0.3 and 3 mg/kg) continued for seven days after onset of MCAO with reperfusion in normotensive rats involved a reduction of the infarct volume by low doses of CS [70]. CBF in CS (0.5 mg/kg) pretreated animals at 0.5 h after MCAO was significantly increased compared to the control group [71]. Other groups additionally showed a four-week CS-pretreatment (0.3 mg/kg per day) before MCAO clearly associated with complete reversal of a decreased lumen diameter and increased media thickness as well as decreased endothelial nitric oxide synthase (eNOS) and increased inducible nitric oxide synthase (iNOS) protein and mRNA in SR-SHR and in a normotensive control group [72].
Olmesartan (OMS), an AT2-1-R antagonist, has been evaluated in a bilateral CCAO model in mice, revealing improved cognitive outcome, neuroprotective effects, attenuation of oxidative hippocampal stress, and suppression of BBB disruption compared to control groups [73]. A single carotid ligation stroke model in gerbils showed that OMS (10 mg/kg per day started 36 h after stroke) was associated with an increased survival [74]. Other studies demonstrated that OMS (10 mg/kg per day for 14 days after infarct; 10 mg/kg per day for 7 days before and 14 days after infarct; 10 mg/kg per day for 7 days before infarct) treatment in a rat MCAO model showed significantly better functional scores and reduced infarct size and cell death [75]. OMS (0.01 or 0.1 μmol/kg per hour for seven days) reduced brain angiotensin II, MMP-2 and MMP-9 upregulation following brain ischemia [77].
Valsartan (VS), a selective AT2-1-R antagonist, reduced ischemic brain area and improved the neurological deficit after MCAO with restoration of cerebral blood flow [78]. VS significantly reduced infarct volume and improved the neurological deficit scores. VS at nonhypotensive doses significantly diminished ischemic area, neurological deficits, and reduction of cerebral blood flow as well as superoxide production [27,78,80].
Irbesartan (IS), a selective AT2-1-R antagonist improved motor functions, reduced infarct size and decreased the number of apoptotic cells particularly in the periinfarct area by attenuated invasion of activated microglia likewise macrophages [82,83,84].
Losartan (LS), a clinical established selective AT2-1-R antagonist, did not increase mortality in acute cerebral ischemia [85]. Also, LS (20 μmol/L) abolished ischemic exaggeration of cell injury [26,86]. Expression levels of pro-apoptotic genes were significant reduced by LS treatment [87]. Further LS administration initiates cerebral angiogenic response with a significantly larger vessel surface area, and administration before initiation of cerebral focal ischemia (50 mg/day for 2 weeks) markedly reduces infarct size [88].
3.2. Clinical Studies on Sartans in Ischemic Stroke
The search yielded 19 clinical studies on “Sartans AND ischemic stroke”, eligible for systematic review (Table 2). Beneficial aspects of using AT2-1-R antagonists before the onset of ischemic stroke have already been elucidated in a retrospective analysis of 151 patients [89].
Table 2.
Tabular listing of different clinical studies showing various effects of Sartan administration (Abbreviations: Candesartan (CS); hours (h); losartan (LS); milligram (mg); minutes (min); μmol (micromolar); modified ranking Scale (mRS); mol (molar); nmol (nanomolar); telmisartan (TMS); valsartan (VS)).
| Drug | Outcome | Beneficial Effect | Special Remarks |
|---|---|---|---|
| CS [90] | Vascular event (vascular death, nonfatal stroke or nonfatal myocardial infarction) over 6 months and mRS | No overall effect on vascular events in ischemic and/or hemorrhagic stroke, adjusted odds ratio for vascular events of patients treated within 6 h reached significance | Administration at least within 30 h of ischemic or hemorrhagic stroke. CS treatment for 7 days, increasing from 4 mg on day 1 to 16 mg on day 3 to 7 |
| CS [91] | Barthel index and level of care assessed after 6 months | No significant effects on Barthel Index or level of care at 6 months | Administration at least within 30 h of ischemic or hemorrhagic stroke. CS treatment for 7 days, increasing from 4 mg on day 1 to 16 mg on day 3 to 7 |
| CS [92] | Vascular death, myocardial infarction, stroke during first 6 months and functional outcome at 6 months | Significant trend towards a better effect of CS in patients with larger infarcts; no differences in treatment effect for composite vascular end point | CS treatment for 7 days, increasing from 4 mg on day 1 to 16 mg on day 3 to 7 |
| CS [93] | Vascular death, myocardial infarction, stroke during first 6 months and functional outcome at 6 months | After 6 months the risk of the composite vascular endpoint did not differ between treatment groups | CS treatment for 7 days, increasing from 4 mg on day 1 to 16 mg on day 3 to 7 |
| CS [94] | Safety of modest blood pressure reduction by CS cilexetil in the early treatment of stroke | The cumulative 12 months mortality and the number of vascular events differed significantly in favor of the CS cilexetil group | CS treatment with 4 mg on day 1; dosage increased to 8 mg on day 2 or 16 mg if blood pressure exceeded 160 mmHg systolic or 100 mmHg diastolic |
| CS [95] | Short-term safety of blood pressure reduction in hypertensive patients with acute ischemic stroke | CS treatment safely reduces blood pressure in hypertensive patients with acute ischemic stroke | 4 mg/day for 14 days |
| CS [96] | Adhesion of neutrophils to human endothelial cells in acute ischemic stroke | CS inhibited the adhesion of neutrophils to vascular endothelium in ischemic stroke patients (not in chronic stroke patients or healthy volunteers) | Incubation with 10−9 mol for 30 min |
| CS [97] | Effect of blood pressure lowering in patients with acute ischemic stroke and carotid artery stenosis (Vascular death, stroke, myocardial infarction, and functional outcome at 6 months) | No evidence that CS effect is qualitatively different in patients with carotid artery stenosis | CS treatment for 7 days, increasing from 4 mg on day 1 to 16 mg on day 3 to 7 |
| VS [98] | Safety of modest blood pressure reduction within 48 h of acute ischemic stroke | After 90 days the mRS as well the rate of major vascular events differed not significantly between both groups | 80 mg/day (dose was modified in the subsequent six-days of treatment if the target systolic blood pressure was not achieved) |
| VS [99] | Effect of vs. on human platelet aggregation | VS exhibited significant inhibition of human platelets and therefore might be able to reduce vascular ischemic events | 10 nmol to 100 μmol |
| TMS [100] | Time to first recurrent stroke | Low glomerular filtration rate (<60 mL/min) is independently associated with a higher risk of recurrent stroke, TMS not able to mitigate this risk | TMS dosage not reported |
| TMS [101] | Recurrent stroke of any type | Similar rates of recurrent strokes comparing aspirin plus extended-release dipyridamole with clopidogrel and TMS | 80 mg/day |
| TMS [102] | Prevention of cerebral white matter lesions | TMS on top of existing antihypertensive medication did not prevent the progression of white matter lesions | 80 mg/day. Analysis limited by the relatively short follow-up |
| TMS [103] | Functional outcome at 30 days (primary outcome), death, recurrence, and hemodynamic measures up to 90 days (secondary outcomes) | TMS treatment appears to be safe with no excess in adverse events and not associated with a significant effect on functional dependency, death, or stroke recurrence | 80 mg/day |
| TMS [104] | Recurrent stroke | TMS initiated soon after ischemic stroke and continued for 2.5 years did not significantly lower the rate of recurrent stroke, major cardiovascular events, or diabetes | 80 mg/day |
| LS [105] | Global change of cerebral blood flow | LS treatment increases the global cerebral blood flow despite blood pressure lowering | 50–100 mg/day for 4 weeks |
| LS [106] | Effect on stroke in patients with isolated systolic hypertension and left ventricular hypertrophy | Incidence of any stroke (40% risk reduction), fatal stroke (70% risk reduction), and atherothrombotic stroke (45% risk reduction) was significantly lower in the LS treated group compared to atenolol treated patients | Mean LS dose of 79 mg |
| LS [107] | Effect on global and focal cerebral blood flow in hypertensive patients 2–7 days after stroke | No neurological deterioration in the LS group | 25–50 mg/day |
| LS [108] | Spontaneous platelet aggregation and P-selectin levels (in patients with hypertension and chronic ischemic stroke) | Spontaneous platelet aggregation was not, P-selectin levels significantly reduced after LS treatment. This suggests that standard doses of LS display antiplatelet effect | 50 mg/day |
CS has been evaluated in the Scandinavian Candesartan Acute Stroke Trial (SCAST). Within 30 h of ischemic or hemorrhagic stroke, 2029 patients either received CS- or placebo-treatment. The modified ranking Scale (mRS) was used for outcome analysis. CS showed no overall effect on vascular events in ischemic and/or hemorrhagic stroke, and the adjusted odds ratio for vascular events of patients treated within 6 h reached significance [90]. At six months, activities of daily living and level of care were assessed. In more than 1800 patients, over 1500 suffered ischemic and almost 250 hemorrhagic strokes. No statistically significant effects of CS on Barthel index or level of care could be identified [91]. Furthermore, the SCAST group evaluated whether the effect of CS treatment varies in subtypes of over 1700 ischemic strokes. Concerning functional outcomes, a trend towards a beneficial effect of CS was observed in patients with larger infarcts (total anterior circulation or partial anterior circulation) than in patients with smaller lacunar infarcts [92]. Further on, over 2000 SCAST patients were randomly allocated to placebo or CS treatment for seven days with increasing doses from 4 mg (starting day 1) to 16 mg (from day 3 to 7). After six months’ follow-up, the risk of the composite vascular endpoint did not differ between the placebo and CS treatment group [93]. Also, the Acute Candesartan Cilexetil Therapy in Stroke Survivors study confirmed that administration of CS in the acute phase of stroke in 339 patients confers long-term benefits in patients who sustained acute ischemic stroke [94]. VS has been evaluated in a multicenter trial concerning efficacy and safety of modest blood pressure reduction within 48 h in more than 370 patients with acute ischemic stroke, considering the primary outcome death or dependency. The VS-treated group showed 46 of 187 patients with a 90-day mRS of 3–6, compared with 42 of 185 patients in the control group. The rate of major vascular events did not differ significantly between both groups [98]. TMS has also been evaluated concerning beneficial effects after stroke treatment. A multicenter trial, involving more than 18,500 patients with ischemic stroke, had a follow-up of 2.5 years. The primary outcome parameter was time to first recurrent stroke. Only short-term add-on TMS (80 mg/day) treatment did not mitigate this risk [100,101]. Treatment with TMS (80 mg/day) did not prevent progression of white matter lesions in patients with recent ischemic stroke [102]. Another study group enrolled 20,332 patients and analyzed 1360 patients within 72 h of ischemic stroke onset (TMS vs. placebo) concerning functional outcome after 30 days as primary outcome. Combined death or dependency did not differ between the treatment groups, showing treatment with TMS (80 mg/day) in patients with acute mild ischemic stroke and mildly elevated BP safe with no excess in adverse events [103]. Also, effects of TMS (80 mg/day) initiation early after stroke have been analyzed. From 20,332 patients with recent ischemic stroke, 10,146 patients were randomly assigned in the TMS group and 10,186 in the placebo group; 8.7% in the TMS group and 9.2% in the placebo group suffered from subsequent stroke, showing no significant reduction of recurrent stroke after early initiation [104]. LS has also been analyzed in recent clinical stroke trials. In a double-blinded multi-center trial, 196 hypertensive patients with previous ischemic stroke were randomized to cilnidipine- or LS-treatment (50–100 mg per day for four weeks) once daily for four weeks. Both treatments, however, increased global CBF despite BP lowering [105]. Additionally, the effect of long-term therapy with LS regarding cognitive function in 6206 essential hypertonic patients with additional cerebrovascular risk factors was investigated. The LS-based antihypertensive treatment increased the proportion of patients with normal cognitive function [109]. Also, the Losartan Intervention for Endpoint reduction in hypertension study group reported cardioprotective effects of a LS-based antihypertensive regimen. The incidence of any stroke, fatal stroke, and atherothrombotic stroke was significantly lower in LS-treated compared to the atenolol-treated isolated systolic hypertensive patients [106]. Other groups assessed the effect of LS treatment on mean arterial blood pressure, global, and focal CBF in 24 hypertensive patients without occlusive carotid disease 2–7 days after ischemic stroke and/or transient ischemic attack. LS (25–50 mg per day) was generally well tolerated and none of the patients suffered neurological deterioration. No changes occurred in internal carotid artery flow or cortical as well as hemispheric CBF [107].
3.3. Therapeutic Interventions After aSAH
Poor patients’ outcome after aSAH is owed a multifactorial process (early brain injury, DCVS, DCI, cerebral inflammation, cortical spreading depression, loss of pressure dependent cerebral autoregulation) [4,5,7,9,110,111,112,113]. DCVS is treated with moderate hypertensive, normovolemic, hemodilution, and in cases of therapy-refractory, DCVS with intra-arterial spasmolysis or balloon dilatation [114,115]. Research to improve poor functional outcome in patients suffering from aSAH and related DCVS is pivotal [1,5,21,116,117]. Multiple preclinical and clinical trials showed the effect of ET-1 in mediating DCVS after aSAH. CONSCIOUS-1, a randomized, double-blind, placebo-controlled study assessed the efficacy of intravenous clazosentan (ETA-R antagonist) in preventing vasospasm following aSAH. It significantly decreased angiographic DCVS with a trend for reduction in vasospasm-related morbidity/mortality [118]. CONSCIOUS-2 assigned patients with aSAH and clip ligation to clazosentan- or placebo. Thereby, clazosentan showed no significant difference in the mortality and vasospasm-related morbidity [119]. CONSCIOUS-3 assessed whether clazosentan reduced DCVS-related morbidity and mortality after aSAH and endovascular coiling. Pulmonary complications and anemia were more common in patients with clazosentan administration than in the placebo group, and mortality rates after 12 weeks were the same, respectively [120]. The REVERSE-study, infusing clazosentan intravenously in patients developing moderate to severe angiographic vasospasm after aSAH, showed a clear pharmacodynamic dilating effect on DCVS 24 h in most patients suffering aSAH, being able to reverse established angiographic vasospasm [22].
Antihypertensive agents are usually discontinued to maintain a sufficient mean arterial cerebral perfusion pressure considering the prolonged phase of DCVS between days 5 to 14 after the ictus [114]. In contrast, nimodipine, a calcium-channel antagonist, is administered for risk reduction of DCVS, yet rather its neuroprotective effects have been discussed in its beneficial role in aSAH [8,121].
3.4. Effects of Losartan Following aSAH
LS, an already well-established antihypertensive drug in daily clinical practice and well examined in preclinical and clinical settings of ischemic stroke, shows promising results by attenuating cerebral inflammation and restoring cerebral autoregulation [64,105,122,123,124,125]. Facing preclinical aSAH research, beneficial effects of Sartans have been shown. Under already physiological conditions, LS diminished cerebral inflammation and associated DCVS [126] as well as ET-1 mediated vasoconstriction. Targeted ETB1- and ETA-R-antagonism under LS administration revealed a direct modulatory ETB1-R dependent effect via inducing upregulation of the NO-pathway with a significantly increased relaxation accompanied with enhanced sensitivity of the ETB1-R [23]. After induction of aSAH, ET-1-induced vasoconstriction was likewise decreased by LS preincubation, abolished after pretreatment with an ETB1-R antagonist. In precontracted vessels with LS and ETA-R-antagonism, ET-1 induced a higher vasorelaxation compared to the control group without, clearly demonstrating a modulatory and functional restoring effect of LS on the normally after aSAH impaired ETB1-R function [127].
Beneficial effects of LS on ET-1- and PGF2α-mediated DCVS after aSAH in a rat model have been reported, too [23,127]. An ET-1 mediated vasoconstriction was diminished, and ETB1-R mediated vasorelaxation under selective ETA-R blockade was restored [126,127]. In addition, PGF2α-elicited vasoconstriction of a basilar artery was markedly diminished [23,126,127]. Interestingly, several work groups could also verify positive vasomodulating effects of LS on the cerebral vessel wall, especially affecting SMCs [128,129]. Furthermore, aneurysm rupture was prevented in mice under LS treatment [129]. As already mentioned, after aSAH, increased synthesis of ET-1 triggers enhanced cerebral vasoconstriction; loss of the ETB1-R mediated vasorelaxation contributes to this effect, too [127]. Furthermore, upregulated AT2-1-R and PGF2α-synthesis contribute in enhancing and maintaining cerebral vasocontraction [7,130,131,132,133]. LS showed promising aspects in preclinical aSAH studies and therefore might have an effect in the treatment of patients with aSAH.
4. Discussions
This systematic review demonstrated Sartan administration after ischemic stroke clearly associated with beneficial effects on preclinical models as well regarding clinical trials. Clear evidence of which doses in preclinical and clinical settings for treatment of ischemic stroke with Sartans exactly might be useful are heterogenous and therefore not consistent yet. In a preclinical setting, Sartans significantly reduced infarct volume and edema, augmented CBF, diminished superoxide production, inflammatory processes, and disruption of the BBB. In clinical studies, clear trends towards a better functional outcome and neurocognitive function after stroke with Sartan use have been reported. Thus, the question arises whether Sartans might provide positive effects on DCVS or DCI after aSAH. In summary, LS provided in a preclinical physiological and pathophysiological setup after aSAH beneficial aspects in reducing ET-1- and PGF2α mediated cerebral vasoconstriction [126,130]. Vasoconstriction was notably reduced and the vasorelaxant properties of the ETB1-R were restored. Furthermore, clear evidence exists, that after aSAH, AT2-1-R are upregulated in experimental settings [132]. Here, an additive direct antagonism on these receptors could reduce the sensitivity to an AT2-1-R-mediated vasocontraction to angiotensin II, too [125,134]. LS possesses beneficial aspects on cerebral epileptogenicity, which could be applied to the issue of reducing cortical spreading depression post aSAH [135,136,137,138]. Also, it is able to restore post-ischemic cerebral autoregulation after hemorrhagic stroke [134].
Considering these neuroprotective effects of LS, the ethical question arises of whether the philosophy of strictly discontinuing all antihypertensive agents after aSAH (except of new administration of nimodipine), especially of LS, should stay state of the art. Next to beneficial influences on DCVS after aSAH in rats as mentioned above, AT2-1-R antagonists clearly possess beneficial effects after stroke regarding cerebral inflammation, the areal of infarction, cortical spreading depression, cerebral microcirculation, and maintenance of pressure-dependent cerebral vasoconstriction [23,64,71,105,127,134,139,140,141]. Appreciating these facts, a systemic LS administration over and above the phase of DCVS, could be a promising approach in preventing these effects; particularly because LS seems to not influence the global CBF in essential hypertonic patients, which can be set equivalent to a needed-hypertonia after aSAH [142]. Here, LS could be an interesting approach, because it increases global CBF despite lowering blood pressure [105], and is therefore capable to reduce DCI [92]. Also, considering the positive vasomodulatory influences of LS, the question arises whether after aSAH this medication should be established as secondary prophylaxis to avoid a de-novo-aneurysm genesis, ergo, if aneurysms under LS are anyway arising [143].
4.1. Translational Aspects
Both abovementioned questions after aSAH are difficult to adapt to the affected patient group, because common sense to date stays in discontinuing all antihypertensive agents after the initial bleeding event. Also, it is vague to postulate that a LS effect persists after discharging this medication on admission over the phase of DCVS for 14 days. Furthermore, the numbers of patients with LS as standard antihypertonic medication receiving follow-up angiographys are too scarce to testify a valid statement concerning case-control studies of aneurysm-growth/-development, as reviewed in our own patient series in 2009–2015. Nevertheless, LS seems to be an underrated neuroprotective drug, reducing cerebral inflammation and epileptogenicity, DCVS, and infarct size after ischemic stroke. These results of preclinical ischemic stroke and aSAH research as well as clinical ischemic stroke research could be applied in a prospective clinical setting of patients suffering aSAH. Also, the question of a de-novo-aneurysm-genesis in further cranial control imaging could be addressed.
4.2. Synopsis and Forecast
LS, a selective AT2-1-R antagonist, was shown to directly antagonize and ameliorate the impaired ETB1-R vasodilatory function. Given that in most clinical centers, antihypertensive agents are discontinued during the period of DCVS, LS, although an antihypertensive drug, may have a role in preventing delayed DCVS after aneurysm rupture given the effects shown in ischemia. Following aSAH, immediate therapy with LS might antagonize the vasoconstrictive AT2-1-R without affecting the dilatory AT2-2-R effect [132,144,145,146,147,148,149,150,151]. Furthermore, AT2 interestingly increases endothelin production in non-cerebral vessels (an increased ET-1 concentration in rat aortas could be inhibited through LS administration [140]) and thus indirectly enhances ET-1-mediated DCVS [123,152,153,154,155,156]. All these aspects might suggest a crosstalk between both peptidergic systems extra- and intracranially [71,157].
5. Conclusions
There is a promising effect on LS in the treatment of ischemic stroke both in preclinical and clinical studies as well as in preclinical studies on aSAH. LS has shown to reduce ET-1-mediated vasocontraction, cerebral inflammation, and restores vasodilatory function of the ETB1-R [26,27,28]. Thus, LS may decrease the incidence of symptomatic vasospasm and improve functional outcome in aSAH patients. Large, randomized, double-blinded clinical trials are necessary to determine its benefit in aSAH.
Author Contributions
S.W. and L.A.: Design of the review, collection and analysis of data, drafting the manuscript; B.E.G., S.D.S., H.R.W., S.M., and J.F.: Critical revision of the first draft; All of the authors: Critical revision of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vatter H., Konczalla J., Weidauer S., Preibisch C., Zimmermann M., Raabe A., Seifert V. Effect of delayed cerebral vasospasm on cerebrovascular endothelin A receptor expression and function. J. Neurosurg. 2007;107:121–127. doi: 10.3171/JNS-07/07/0121. [DOI] [PubMed] [Google Scholar]
- 2.Brandt L., Ljunggren B., Andersson K.E., Hindfelt B., Uski T. Prostaglandin metabolism and prostacyclin in cerebral vasospasm. Gen. Pharmacol. 1983;14:141–143. doi: 10.1016/0306-3623(83)90085-X. [DOI] [PubMed] [Google Scholar]
- 3.Egg D., Herold M., Rumpl E., Gunther R. Prostaglandin F2 alpha levels in human cerebrospinal fluid in normal and pathological conditions. J. Neurol. 1980;222:239–248. doi: 10.1007/BF00313153. [DOI] [PubMed] [Google Scholar]
- 4.Fujii M., Yan J., Rolland W.B., Soejima Y., Caner B., Zhang J.H. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl. Stroke Res. 2013;4:432–446. doi: 10.1007/s12975-013-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreier J.P., Drenckhahn C., Woitzik J., Major S., Offenhauser N., Weber-Carstens S., Wolf S., Strong A.J., Vajkoczy P., Hartings J.A., et al. Spreading ischemia after aneurysmal subarachnoid hemorrhage. Acta Neurochir. Suppl. 2013;115:125–129. doi: 10.1007/978-3-7091-1192-5_26. [DOI] [PubMed] [Google Scholar]
- 6.Vergouwen M.D., Vermeulen M., van Gijn J., Rinkel G.J., Wijdicks E.F., Muizelaar J.P., Mendelow A.D., Juvela S., Yonas H., Terbrugge K.G., et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke. 2010;41:2391–2395. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- 7.Budohoski K.P., Czosnyka M., Smielewski P., Kasprowicz M., Helmy A., Bulters D., Pickard J.D., Kirkpatrick P.J. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: A prospective observational study. Stroke. 2012;43:3230–3237. doi: 10.1161/STROKEAHA.112.669788. [DOI] [PubMed] [Google Scholar]
- 8.Bederson J.B., Connolly E.S., Batjer H.H., Jr., Dacey R.G., Dion J.E., Diringer M.N., Duldner J.E., Jr., Harbaugh R.E., Patel A.B., Rosenwasser R.H., et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 9.Salom J.B., Torregrosa G., Alborch E. Endothelins and the cerebral circulation. Cerebrovasc. Brain Metab. Rev. 1995;7:131–152. [PubMed] [Google Scholar]
- 10.Vatter H., Zimmermann M., Seifert V., Schilling L. Experimental approaches to evaluate endothelin-A receptor antagonists. Methods Find. Exp. Clin. Pharmacol. 2004;26:277–286. [PubMed] [Google Scholar]
- 11.Zimmermann M., Seifert V. Endothelin and subarachnoid hemorrhage: An overview. Neurosurgery. 1998;43:863–875. doi: 10.1097/00006123-199810000-00083. [DOI] [PubMed] [Google Scholar]
- 12.Neuschmelting V., Marbacher S., Fathi A.R., Jakob S.M., Fandino J. Elevated level of endothelin-1 in cerebrospinal fluid and lack of nitric oxide in basilar arterial plasma associated with cerebral vasospasm after subarachnoid haemorrhage in rabbits. Acta Neurochir. 2009;151:795–801. doi: 10.1007/s00701-009-0350-1. [DOI] [PubMed] [Google Scholar]
- 13.Josko J., Hendryk S., Jedrzejowska-Szypulka H., Slowinski J., Gwozdz B., Lange D., Harabin-Slowinska M. Effect of endothelin-1 receptor antagonist BQ-123 on basilar artery diameter after subarachnoid hemorrhage (SAH) in rats. J. Physiol. Pharmacol. 2000;51:241–249. [PubMed] [Google Scholar]
- 14.Nishizawa S., Chen D., Yokoyama T., Yokota N., Otha S. Endothelin-1 initiates the development of vasospasm after subarachnoid haemorrhage through protein kinase C activation, but does not contribute to prolonged vasospasm. Acta Neurochir. 2000;142:1409–1415. doi: 10.1007/s007010070013. [DOI] [PubMed] [Google Scholar]
- 15.Hansen-Schwartz J., Hoel N.L., Zhou M., Xu C.B., Svendgaard N.A., Edvinsson L. Subarachnoid hemorrhage enhances endothelin receptor expression and function in rat cerebral arteries. Neurosurgery. 2003;52:1188–1194. [PubMed] [Google Scholar]
- 16.Lei Q., Li S., Zheng R., Xu K., Li S. Endothelin-1 expression and alterations of cerebral microcirculation after experimental subarachnoid hemorrhage. Neuroradiology. 2015;57:63–70. doi: 10.1007/s00234-014-1435-y. [DOI] [PubMed] [Google Scholar]
- 17.Josko J., Hendryk S., Jedrzejowska-Szypulka H., Slowinski J., Gwozdz B., Lange D., Snietura M., Zwirska-Korczala K., Jochem J. Cerebral angiogenesis after subarachnoid hemorrhage (SAH) and endothelin receptor blockage with BQ-123 antagonist in rats. J. Physiol. Pharmacol. 2001;52:237–248. [PubMed] [Google Scholar]
- 18.Xie A., Aihara Y., Bouryi V.A., Nikitina E., Jahromi B.S., Zhang Z.D., Takahashi M., Macdonald R.L. Novel mechanism of endothelin-1-induced vasospasm after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2007;27:1692–1701. doi: 10.1038/sj.jcbfm.9600471. [DOI] [PubMed] [Google Scholar]
- 19.Kim C.Y., Paek S.H., Seo B.G., Kim J.H., Han D.H. Changes in vascular responses of the basilar artery to acetylcholine and endothelin-1 in an experimental rabbit vasospasm model. Acta Neurochir. 2003;145:571–577. doi: 10.1007/s00701-003-0024-3. [DOI] [PubMed] [Google Scholar]
- 20.Chow M., Dumont A.S., Kassell N.F. Endothelin receptor antagonists and cerebral vasospasm: An update. Neurosurgery. 2002;51:1333–1341. doi: 10.1097/00006123-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald R.L., Higashida R.T., Keller E., Mayer S.A., Molyneux A., Raabe A., Vajkoczy P., Wanke I., Bach D., Frey A., et al. Randomised trial of clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping (CONSCIOUS-2) Acta Neurochir. Suppl. 2013;115:27–31. doi: 10.1007/978-3-7091-1192-5_7. [DOI] [PubMed] [Google Scholar]
- 22.Higashida R.T., Bruder N., Gupta R., Guzman R., Hmissi A., Marr A., Mayer S.A., Roux S., Weidauer S., Aldrich E.F. Reversal of Vasospasm with Clazosentan After Aneurysmal Subarachnoid Hemorrhage: A Pilot Study. World Neurosurg. 2019;128:e639–e648. doi: 10.1016/j.wneu.2019.04.222. [DOI] [PubMed] [Google Scholar]
- 23.Konczalla J., Wanderer S., Mrosek J., Schuss P., Platz J., Güresir E., Seifert V., Vatter H. Crosstalk between the angiotensin and endothelin-system in the cerebrovasculature. Curr. Neurovasc. Res. 2013;10:335–345. doi: 10.2174/15672026113109990030. [DOI] [PubMed] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Justin A., Divakar S., Ramanathan M. Cerebral ischemia induced inflammatory response and altered glutaminergic function mediated through brain AT1 and not AT2 receptor. Biomed. Pharmacother. 2018;102:947–958. doi: 10.1016/j.biopha.2018.03.164. [DOI] [PubMed] [Google Scholar]
- 26.Iwanami J., Mogi M., Tsukuda K., Min L.J., Sakata A., Jing F., Iwai M., Horiuchi M. Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator-activated receptor-gamma activation in diabetic mice. J. Hypertens. 2010;28:1730–1737. doi: 10.1097/HJH.0b013e32833a551a. [DOI] [PubMed] [Google Scholar]
- 27.Kasahara Y., Taguchi A., Uno H., Nakano A., Nakagomi T., Hirose H., Stern D.M., Matsuyama T. Telmisartan suppresses cerebral injury in a murine model of transient focal ischemia. Brain Res. 2010;1340:70–80. doi: 10.1016/j.brainres.2010.03.101. [DOI] [PubMed] [Google Scholar]
- 28.Iwai M., Inaba S., Tomono Y., Kanno H., Iwanami J., Mogi M., Horiuchi M. Attenuation of focal brain ischemia by telmisartan, an angiotensin II type 1 receptor blocker, in atherosclerotic apolipoprotein E-deficient mice. Hypertens. Res. 2008;31:161–168. doi: 10.1291/hypres.31.161. [DOI] [PubMed] [Google Scholar]
- 29.Li T., Zhang Y., Zhu B., Wu C., Chen Y. Telmisartan regulates the development of cerebral ischemia by alleviating endoplasmic reticulum stress. Pharmazie. 2018;73:585–588. doi: 10.1691/ph.2018.8592. [DOI] [PubMed] [Google Scholar]
- 30.Gao Y., Li W., Liu Y., Wang Y., Zhang J., Li M., Bu M. Effect of Telmisartan on Preventing Learning and Memory Deficits Via Peroxisome Proliferator-Activated Receptor-gamma in Vascular Dementia Spontaneously Hypertensive Rats. J. Stroke Cerebrovasc. Dis. 2018;27:277–285. doi: 10.1016/j.jstrokecerebrovasdis.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 31.Ramanathan M., Justin A., Sudheer A., Shanthakumari S. Comparison of pre- and post-ischemic treatment of telmisartan and nimodipine combination in experimentally induced cerebral ischemia. Indian J. Exp. Biol. 2016;54:560–568. [PubMed] [Google Scholar]
- 32.Kono S., Kurata T., Sato K., Omote Y., Hishikawa N., Yamashita T., Deguchi K., Abe K. Neurovascular protection by telmisartan via reducing neuroinflammation in stroke-resistant spontaneously hypertensive rat brain after ischemic stroke. J. Stroke Cerebrovasc. Dis. 2015;24:537–547. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 33.Dupuis F., Vincent J.M., Liminana P., Chillon J.M., Capdeville-Atkinson C., Atkinson J. Effects of suboptimal doses of the AT1 receptor blocker, telmisartan, with the angiotensin-converting enzyme inhibitor, ramipril, on cerebral arterioles in spontaneously hypertensive rat. J. Hypertens. 2010;28:1566–1573. doi: 10.1097/HJH.0b013e328339f1f3. [DOI] [PubMed] [Google Scholar]
- 34.Deguchi K., Kurata T., Fukui Y., Liu W., Yun Z., Omote Y., Sato K., Kono S., Hishikawa N., Yamashita T., et al. Long-term amelioration of telmisartan on metabolic syndrome-related molecules in stroke-resistant spontaneously hypertensive rat after transient middle cerebral artery occlusion. J. Stroke Cerebrovasc. Dis. 2014;23:2646–2653. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Thoene-Reineke C., Rumschussel K., Schmerbach K., Krikov M., Wengenmayer C., Godes M., Mueller S., Villringer A., Steckelings U., Namsolleck P., et al. Prevention and intervention studies with telmisartan, ramipril and their combination in different rat stroke models. PLoS ONE. 2011;6:e23646. doi: 10.1371/journal.pone.0023646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi T., Kawamata T., Shibata N., Okada Y., Kobayashi M., Hori T. Angiotensin II type 1 receptor blocker telmisartan reduces cerebral infarct volume and peri-infarct cytosolic phospholipase A(2) level in experimental stroke. J. Neurotrauma. 2009;26:2355–2364. doi: 10.1089/neu.2009.0965. [DOI] [PubMed] [Google Scholar]
- 37.Jung K.H., Chu K., Lee S.T., Kim S.J., Song E.C., Kim E.H., Park D.K., Sinn D.I., Kim J.M., Kim M. Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J. Pharmacol. Exp. Ther. 2007;322:1051–1058. doi: 10.1124/jpet.107.120097. [DOI] [PubMed] [Google Scholar]
- 38.Fukui Y., Yamashita T., Kurata T., Sato K., Lukic V., Hishikawa N., Deguchi K., Abe K. Protective effect of telmisartan against progressive oxidative brain damage and synuclein phosphorylation in stroke-resistant spontaneously hypertensive rats. J. Stroke Cerebrovasc. Dis. 2014;23:1545–1553. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 39.Hamai M., Iwai M., Ide A., Tomochika H., Tomono Y., Mogi M., Horiuchi M. Comparison of inhibitory action of candesartan and enalapril on brain ischemia through inhibition of oxidative stress. Neuropharmacology. 2006;51:822–828. doi: 10.1016/j.neuropharm.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 40.Awad A.S. Effect of combined treatment with curcumin and candesartan on ischemic brain damage in mice. J. Stroke Cerebrovasc. Dis. 2011;20:541–548. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Soliman S., Ishrat T., Fouda A.Y., Patel A., Pillai B., Fagan S.C. Sequential Therapy with Minocycline and Candesartan Improves Long-Term Recovery After Experimental Stroke. Transl. Stroke Res. 2015;6:309–322. doi: 10.1007/s12975-015-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishrat T., Soliman S., Eldahshan W., Pillai B., Ergul A., Fagan S.C. Silencing VEGF-B Diminishes the Neuroprotective Effect of Candesartan Treatment After Experimental Focal Cerebral Ischemia. Neurochem. Res. 2018;43:1869–1878. doi: 10.1007/s11064-018-2604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Culman J., Jacob T., Schuster S.O., Brolund-Spaether K., Brolund L., Cascorbi I., Zhao Y., Gohlke P. Neuroprotective effects of AT1 receptor antagonists after experimental ischemic stroke: What is important? Naunyn. Schmiedebergs Arch. Pharmacol. 2017;390:949–959. doi: 10.1007/s00210-017-1395-y. [DOI] [PubMed] [Google Scholar]
- 44.Alhusban A., Kozak A., Pillai B., Ahmed H., Sayed M.A., Johnson M.H., Ishrat T., Ergul A., Fagan S.C. Mechanisms of acute neurovascular protection with AT1 blockade after stroke: Effect of prestroke hypertension. PLoS ONE. 2017;12:e0178867. doi: 10.1371/journal.pone.0178867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fouda A.Y., Alhusban A., Ishrat T., Pillai B., Eldahshan W., Waller J.L., Ergul A., Fagan S.C. Brain-Derived Neurotrophic Factor Knockdown Blocks the Angiogenic and Protective Effects of Angiotensin Modulation After Experimental Stroke. Mol. Neurobiol. 2017;54:661–670. doi: 10.1007/s12035-015-9675-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soliman S., Ishrat T., Pillai A., Somanath P.R., Ergul A., El-Remessy A.B., Fagan S.C. Candesartan induces a prolonged proangiogenic effect and augments endothelium-mediated neuroprotection after oxygen and glucose deprivation: Role of vascular endothelial growth factors A and B. J. Pharmacol. Exp. Ther. 2014;349:444–457. doi: 10.1124/jpet.113.212613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alhusban A., Kozak A., Ergul A., Fagan S.C. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J. Pharmacol. Exp. Ther. 2013;344:348–359. doi: 10.1124/jpet.112.197483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.So G., Nakagawa S., Morofuji Y., Hiu T., Hayashi K., Tanaka K., Suyama K., Deli M.A., Nagata I., Matsuo T., et al. Candesartan improves ischemia-induced impairment of the blood-brain barrier in vitro. Cell Mol. Neurobiol. 2015;35:563–572. doi: 10.1007/s10571-014-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panahpour H., Nekooeian A.A., Dehghani G.A. Candesartan attenuates ischemic brain edema and protects the blood-brain barrier integrity from ischemia/reperfusion injury in rats. Iran. Biomed. J. 2014;18:232–238. doi: 10.6091/ibj.13672.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishrat T., Pillai B., Soliman S., Fouda A.Y., Kozak A., Johnson M.H., Ergul A., Fagan S.C. Low-dose candesartan enhances molecular mediators of neuroplasticity and subsequent functional recovery after ischemic stroke in rats. Mol. Neurobiol. 2015;51:1542–1553. doi: 10.1007/s12035-014-8830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guan W., Kozak A., Fagan S.C. Drug repurposing for vascular protection after acute ischemic stroke. Acta Neurochir. Suppl. 2011;111:295–298. doi: 10.1007/978-3-7091-0693-8_49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmerbach K., Pfab T., Zhao Y., Culman J., Mueller S., Villringer A., Muller D.N., Hocher B., Unger T., Thoene-Reineke C. Effects of aliskiren on stroke in rats expressing human renin and angiotensinogen genes. PLoS ONE. 2010;5:e15052. doi: 10.1371/journal.pone.0015052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Omura-Matsuoka E., Yagita Y., Sasaki T., Terasaki Y., Oyama N., Sugiyama Y., Okazaki S., Ssakoda S., Kitagawa K. Postischemic administration of angiotensin II type 1 receptor blocker reduces cerebral infarction size in hypertensive rats. Hypertens. Res. 2009;32:548–553. doi: 10.1038/hr.2009.69. [DOI] [PubMed] [Google Scholar]
- 54.Kozak W., Kozak A., Johnson M.H., Elewa H.F., Fagan S.C. Vascular protection with candesartan after experimental acute stroke in hypertensive rats: A dose-response study. J. Pharmacol. Exp. Ther. 2008;326:773–782. doi: 10.1124/jpet.108.139618. [DOI] [PubMed] [Google Scholar]
- 55.Krikov M., Thone-Reineke C., Muller S., Villringer A., Unger T. Candesartan but not ramipril pretreatment improves outcome after stroke and stimulates neurotrophin BNDF/TrkB system in rats. J. Hypertens. 2008;26:544–552. doi: 10.1097/HJH.0b013e3282f2dac9. [DOI] [PubMed] [Google Scholar]
- 56.Fagan S.C., Kozak A., Hill W.D., Pollock D.M., Xu L., Johnson M.H., Ergul A., Hess D.C. Hypertension after experimental cerebral ischemia: Candesartan provides neurovascular protection. J. Hypertens. 2006;24:535–539. doi: 10.1097/01.hjh.0000209990.41304.43. [DOI] [PubMed] [Google Scholar]
- 57.Lu Q., Zhu Y.Z., Wong P.T. Neuroprotective effects of candesartan against cerebral ischemia in spontaneously hypertensive rats. Neuroreport. 2005;16:1963–1967. doi: 10.1097/01.wnr.0000187636.13147.cd. [DOI] [PubMed] [Google Scholar]
- 58.Kusaka I., Kusaka G., Zhou C., Ishikawa M., Nanda A., Granger D.N., Zhang J.H., Tang J. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H2442–H2451. doi: 10.1152/ajpheart.01169.2003. [DOI] [PubMed] [Google Scholar]
- 59.Groth W., Blume A., Gohlke P., Unger T., Culman J. Chronic pretreatment with candesartan improves recovery from focal cerebral ischaemia in rats. J. Hypertens. 2003;21:2175–2182. doi: 10.1097/00004872-200311000-00028. [DOI] [PubMed] [Google Scholar]
- 60.Nishimura Y., Ito T., Saavedra J.M. Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke. 2000;31:2478–2486. doi: 10.1161/01.STR.31.10.2478. [DOI] [PubMed] [Google Scholar]
- 61.Gaur V., Kumar A. Neuroprotective potentials of candesartan, atorvastatin and their combination against stroke induced motor dysfunction. Inflammopharmacology. 2011;19:205–214. doi: 10.1007/s10787-010-0068-y. [DOI] [PubMed] [Google Scholar]
- 62.Engelhorn T., Doerfler A., Heusch G., Schulz R. Reduction of cerebral infarct size by the AT1-receptor blocker candesartan, the HMG-CoA reductase inhibitor rosuvastatin and their combination. An experimental study in rats. Neurosci. Lett. 2006;406:92–96. doi: 10.1016/j.neulet.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 63.Mecca A.P., O’Connor T.E., Katovich M.J., Sumners C. Candesartan pretreatment is cerebroprotective in a rat model of endothelin-1-induced middle cerebral artery occlusion. Exp. Physiol. 2009;94:937–946. doi: 10.1113/expphysiol.2009.047936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmerbach K., Schefe J.H., Krikov M., Müller S., Villringer A., Kintscher U., Unger T., Thoene-Reineke C. Comparison between single and combined treatment with candesartan and pioglitazone following transient focal ischemia in rat brain. Brain Res. 2008;1208:225–233. doi: 10.1016/j.brainres.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 65.Zhou J., Pavel J., Macova M., Yu Z.X., Imboden H., Ge L., Nishioku T., Dou J., Delgiacco E., Saavedra J.M. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke. 2006;37:1271–1276. doi: 10.1161/01.STR.0000217404.64352.d7. [DOI] [PubMed] [Google Scholar]
- 66.Kozak A., Ergul A., El-Remessy A.B., Johnson M.H., Machado L.S., Elewa H.F., Abdelsaid M., Wiley D.C., Fagan S.C. Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke. 2009;40:1870–1876. doi: 10.1161/STROKEAHA.108.537225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faure S., Bureau A., Oudart N., Javellaud J., Fournier A., Achard J.M. Protective effect of candesartan in experimental ischemic stroke in the rat mediated by AT2 and AT4 receptors. J. Hypertens. 2008;26:2008–2015. doi: 10.1097/HJH.0b013e32830dd5ee. [DOI] [PubMed] [Google Scholar]
- 68.Stenman E., Jamali R., Henriksson M., Maddahi A., Edvinsson L. Cooperative effect of angiotensin AT(1) and endothelin ET(A) receptor antagonism limits the brain damage after ischemic stroke in rat. Eur. J. Pharmacol. 2007;570:142–148. doi: 10.1016/j.ejphar.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 69.Stenman E., Edvinsson L. Cerebral ischemia enhances vascular angiotensin AT1 receptor-mediated contraction in rats. Stroke. 2004;35:970–974. doi: 10.1161/01.STR.0000121642.53822.58. [DOI] [PubMed] [Google Scholar]
- 70.Brdon J., Kaiser S., Hagemann F., Zhao Y., Culman J., Gohlke P. Comparison between early and delayed systemic treatment with candesartan of rats after ischaemic stroke. J. Hypertens. 2007;25:187–196. doi: 10.1097/01.hjh.0000254376.80864.d3. [DOI] [PubMed] [Google Scholar]
- 71.Engelhorn T., Goerike S., Doerfler A., Okorn C., Forsting M., Heusch G., Schulz R. The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2004;24:467–474. doi: 10.1097/00004647-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 72.Yamakawa H., Jezova M., Ando H., Saavedra J.M. Normalization of endothelial and inducible nitric oxide synthase expression in brain microvessels of spontaneously hypertensive rats by angiotensin II AT1 receptor inhibition. J. Cereb. Blood Flow Metab. 2003;23:371–380. doi: 10.1097/01.WCB.0000047369.05600.03. [DOI] [PubMed] [Google Scholar]
- 73.Nakagawa T., Hasegawa Y., Uekawa K., Senju S., Nakagata N., Matsui K., Kim-Mitsuyama S. Transient Mild Cerebral Ischemia Significantly Deteriorated Cognitive Impairment in a Mouse Model of Alzheimer’s Disease via Angiotensin AT1 Receptor. Am. J. Hypertens. 2017;30:141–150. doi: 10.1093/ajh/hpw099. [DOI] [PubMed] [Google Scholar]
- 74.Faure S., Oudart N., Javellaud J., Fournier A., Warnock D.G., Achard J.M. Synergistic protective effects of erythropoietin and olmesartan on ischemic stroke survival and post-stroke memory dysfunctions in the gerbil. J. Hypertens. 2006;24:2255–2261. doi: 10.1097/01.hjh.0000249704.34607.4c. [DOI] [PubMed] [Google Scholar]
- 75.Gutierrez-Fernandez M., Fuentes B., Rodriguez-Frutos B., Ramos-Cejudo J., Otero-Ortega L., Diez-Tejedor E. Different protective and reparative effects of olmesartan in stroke according to time of administration and withdrawal. J. Neurosci. Res. 2015;93:806–814. doi: 10.1002/jnr.23532. [DOI] [PubMed] [Google Scholar]
- 76.Oyama N., Yagita Y., Sasaki T., Omura-Matsuoka E., Terasaki Y., Sugiyama Y., Sakoda S., Kitagawa K. An angiotensin II type 1 receptor blocker can preserve endothelial function and attenuate brain ischemic damage in spontaneously hypertensive rats. J. Neurosci. Res. 2010;88:2889–2898. doi: 10.1002/jnr.22441. [DOI] [PubMed] [Google Scholar]
- 77.Hosomi N., Nishiyama A., Ban C.R., Naya T., Takahashi T., Kohno M., Koziol J.A. Angiotensin type 1 receptor blockage improves ischemic injury following transient focal cerebral ischemia. Neuroscience. 2005;134:225–231. doi: 10.1016/j.neuroscience.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 78.Li J.M., Mogi M., Iwanami J., Min L.J., Tsukuda K., Sakata A., Fujita T., Iwai M., Horiuchi M. Temporary pretreatment with the angiotensin II type 1 receptor blocker, valsartan, prevents ischemic brain damage through an increase in capillary density. Stroke. 2008;39:2029–2036. doi: 10.1161/STROKEAHA.107.503458. [DOI] [PubMed] [Google Scholar]
- 79.Miyamoto N., Zhang N., Tanaka R., Liu M., Hattori N., Urabe T. Neuroprotective role of angiotensin II type 2 receptor after transient focal ischemia in mice brain. Neurosci. Res. 2008;61:249–256. doi: 10.1016/j.neures.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 80.Iwai M., Liu H.W., Chen R., Ide A., Okamoto S., Hata R., Sakanaka M., Shiuchi T., Horiuchi M. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation. 2004;110:843–848. doi: 10.1161/01.CIR.0000138848.58269.80. [DOI] [PubMed] [Google Scholar]
- 81.Dong Y.F., Kataoka K., Tokutomi Y., Nako H., Nakamura T., Toyama K., Sueta D., Koibuchi N., Yamamoto E., Ogawa H., et al. Beneficial effects of combination of valsartan and amlodipine on salt-induced brain injury in hypertensive rats. J. Pharmacol. Exp. Ther. 2011;339:358–366. doi: 10.1124/jpet.111.182576. [DOI] [PubMed] [Google Scholar]
- 82.Lou M., Blume A., Zhao Y., Gohlke P., Deuschl G., Herdegen T., Culman J. Sustained blockade of brain AT1 receptors before and after focal cerebral ischemia alleviates neurologic deficits and reduces neuronal injury, apoptosis, and inflammatory responses in the rat. J. Cereb. Blood Flow Metab. 2004;24:536–547. doi: 10.1097/00004647-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 83.Dai W.J., Funk A., Herdegen T., Unger T., Culman J. Blockade of central angiotensin AT(1) receptors improves neurological outcome and reduces expression of AP-1 transcription factors after focal brain ischemia in rats. Stroke. 1999;30:2391–2398. doi: 10.1161/01.STR.30.11.2391. [DOI] [PubMed] [Google Scholar]
- 84.Tsukuda K., Mogi M., Iwanami J., Min L.J., Jing F., Oshima K., Horiuchi M. Irbesartan attenuates ischemic brain damage by inhibition of MCP-1/CCR2 signaling pathway beyond AT(1) receptor blockade. Biochem. Biophys. Res. Commun. 2011;409:275–279. doi: 10.1016/j.bbrc.2011.04.142. [DOI] [PubMed] [Google Scholar]
- 85.Dalmay F., Mazouz H., Allard J., Pesteil F., Achard J.M., Fournier A. Non-AT(1)-receptor-mediated protective effect of angiotensin against acute ischaemic stroke in the gerbil. J. Renin Angiotensin Aldosterone Syst. 2001;2:103–106. doi: 10.3317/jraas.2001.009. [DOI] [PubMed] [Google Scholar]
- 86.Chen S., Li G., Zhang W., Sigmund C.D., Olson J.E., Chen Y. Ischemia-induced brain damage is enhanced in human renin and angiotensinogen double-transgenic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R1526–R1531. doi: 10.1152/ajpregu.91040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loh K.P., Low L.S., Wong W.H., Zhou S., Huang S.H., De Silva R., Duan W., Chou W.H., Zhu Y.Z. A comparison study of cerebral protection using Ginkgo biloba extract and Losartan on stroked rats. Neurosci. Lett. 2006;398:28–33. doi: 10.1016/j.neulet.2005.12.083. [DOI] [PubMed] [Google Scholar]
- 88.Forder J.P., Munzenmaier D.H., Greene A.S. Angiogenic protection from focal ischemia with angiotensin II type 1 receptor blockade in the rat. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1989–H1996. doi: 10.1152/ajpheart.00839.2004. [DOI] [PubMed] [Google Scholar]
- 89.Miyamoto N., Tanaka Y., Ueno Y., Tanaka R., Hattori N., Urabe T. Benefits of prestroke use of angiotensin type 1 receptor blockers on ischemic stroke severity. J. Stroke Cerebrovasc. Dis. 2012;21:363–368. doi: 10.1016/j.jstrokecerebrovasdis.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 90.Jusufovic M., Sandset E.C., Bath P.M., Berge E., Scandinavian Candesartan Acute Stroke Trial Study Group Early blood pressure lowering treatment in acute stroke. Ordinal analysis of vascular events in the Scandinavian Candesartan Acute Stroke Trial (SCAST) J. Hypertens. 2016;34:1594–1598. doi: 10.1097/HJH.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 91.Hornslien A.G., Sandset E.C., Wyller T.B., Berge E., Scandinavian Candesartan Acute Stroke Trial Study Group Effects of candesartan in acute stroke on activities of daily living and level of care at 6 months. J. Hypertens. 2015;33:1487–1491. doi: 10.1097/HJH.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 92.Sandset E.C., Jusufovic M., Sandset P.M., Bath P.M., Berge E., Group S.S. Effects of blood pressure-lowering treatment in different subtypes of acute ischemic stroke. Stroke. 2015;46:877–879. doi: 10.1161/STROKEAHA.114.008512. [DOI] [PubMed] [Google Scholar]
- 93.Sandset E.C., Bath P.M., Boysen G., Jatuzis D., Korv J., Lüders S., Murray G.D., Richter P.S., Roine R.O., Terent A., et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): A randomised, placebo-controlled, double-blind trial. Lancet. 2011;377:741–750. doi: 10.1016/S0140-6736(11)60104-9. [DOI] [PubMed] [Google Scholar]
- 94.Schrader J., Luders S., Kulschewski A., Berger J., Zidek W., Treib J., Einhäupl K., Diener H.C., Dominiak P., Acute Candesartan Cilexetil Therapy in Stroke Survivors Study Group The ACCESS Study: Evaluation of Acute Candesartan Cilexetil Therapy in Stroke Survivors. Stroke. 2003;34:1699–1703. doi: 10.1161/01.STR.0000075777.18006.89. [DOI] [PubMed] [Google Scholar]
- 95.Nakamura T., Tsutsumi Y., Shimizu Y., Uchiyama S. Renin-angiotensin system blockade safely reduces blood pressure in patients with minor ischemic stroke during the acute phase. J. Stroke Cerebrovasc. Dis. 2010;19:435–440. doi: 10.1016/j.jstrokecerebrovasdis.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 96.Hallevi H., Hazan-Halevy I., Paran E. Modification of neutrophil adhesion to human endothelial cell line in acute ischemic stroke by dipyridamole and candesartan. Eur. J. Neurol. 2007;14:1002–1007. doi: 10.1111/j.1468-1331.2007.01847.x. [DOI] [PubMed] [Google Scholar]
- 97.Jusufovic M., Sandset E.C., Bath P.M., Karlson B.W., Berge E., Scandinavian Candesartan Acute Stroke Trial Study Group Effects of blood pressure lowering in patients with acute ischemic stroke and carotid artery stenosis. Int. J. Stroke. 2015;10:354–359. doi: 10.1111/ijs.12418. [DOI] [PubMed] [Google Scholar]
- 98.Oh M.S., Yu K.H., Hong K.S., Kang D.W., Park J.M., Bae H.J., Koo J., Lee J., Lee B.C., Valsartan Efficacy oN modesT blood pressUre Reduction in acute ischemic stroke (VENTURE) study group Modest blood pressure reduction with valsartan in acute ischemic stroke: A prospective, randomized, open-label, blinded-end-point trial. Int. J. Stroke. 2015;10:745–751. doi: 10.1111/ijs.12446. [DOI] [PubMed] [Google Scholar]
- 99.Serebruany V.L., Malinin A.I., Lowry D.R., Sane D.C., Webb R.L., Gottlieb S.O., O’Connor C.M., Hennekens C.H. Effects of valsartan and valeryl 4-hydroxy valsartan on human platelets: A possible additional mechanism for clinical benefits. J. Cardiovasc. Pharmacol. 2004;43:677–684. doi: 10.1097/00005344-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 100.Ovbiagele B., Bath P.M., Cotton D., Sha N., Diener H.C., Investigators P.R. Low glomerular filtration rate, recurrent stroke risk, and effect of renin-angiotensin system modulation. Stroke. 2013;44:3223–3225. doi: 10.1161/STROKEAHA.113.002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wadiwala M.F., Kamal A.K. What is better antiplatelet agent to prevent recurrent stroke? J. Pak. Med. Assoc. 2012;62:976–977. [PMC free article] [PubMed] [Google Scholar]
- 102.Weber R., Weimar C., Blatchford J., Hermansson K., Wanke I., Möller-Hartmann C., Gizweksi E.R., Forsting M., Demchuck A.M., Sacco R.L., et al. Telmisartan on top of antihypertensive treatment does not prevent progression of cerebral white matter lesions in the prevention regimen for effectively avoiding second strokes (PRoFESS) MRI substudy. Stroke. 2012;43:2336–2342. doi: 10.1161/STROKEAHA.111.648576. [DOI] [PubMed] [Google Scholar]
- 103.Bath P.M., Martin R.H., Palesch Y., Cotton D., Yusuf S., Saccor R., Diener H.C., Toni D., Estol C., Roberts R., et al. Effect of telmisartan on functional outcome, recurrence, and blood pressure in patients with acute mild ischemic stroke: A PRoFESS subgroup analysis. Stroke. 2009;40:3541–3546. doi: 10.1161/STROKEAHA.109.555623. [DOI] [PubMed] [Google Scholar]
- 104.Yusuf S., Diener H.C., Sacco R.L., Cotton D., Ounpuu S., Lawton W.A., Palesch Y., Martin R.H., Albers G.W., Bath P., et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N. Engl. J. Med. 2008;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hong K.S., Kang D.W., Bae H.J., Kim Y.K., Han M.K., Park J.M., Rha J.H., Lee Y.S., Koo J.S., Cho Y.J., et al. Effect of cilnidipine vs. losartan on cerebral blood flow in hypertensive patients with a history of ischemic stroke: A randomized controlled trial. Acta Neurol. Scand. 2010;121:51–57. doi: 10.1111/j.1600-0404.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- 106.Kjeldsen S.E., Lyle P.A., Kizer J.R., Dahlhöf B., Devereux R.B., Julius S., Beevers G., de Faire U., Fyhrquist F., Ibsen H., et al. The effects of losartan compared to atenolol on stroke in patients with isolated systolic hypertension and left ventricular hypertrophy. The LIFE study. J. Clin. Hypertens. 2005;7:152–158. doi: 10.1111/j.1524-6175.2005.04254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nazir F.S., Overell J.R., Bolster A., Hilditch T.E., Reid J.L., Lees K.R. The effect of losartan on global and focal cerebral perfusion and on renal function in hypertensives in mild early ischaemic stroke. J. Hypertens. 2004;22:989–995. doi: 10.1097/00004872-200405000-00022. [DOI] [PubMed] [Google Scholar]
- 108.Yamada K., Hirayama T., Hasegawa Y. Antiplatelet effect of losartan and telmisartan in patients with ischemic stroke. J. Stroke Cerebrovasc. Dis. 2007;16:225–231. doi: 10.1016/j.jstrokecerebrovasdis.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 109.Van Ginneken V., Engel P., Fiebach J.B., Audebert H.J., Nolte C.H., Rocco A. Prior antiplatelet therapy is not associated with larger hematoma volume or hematoma growth in intracerebral hemorrhage. Neurol. Sci. 2018;39:745–748. doi: 10.1007/s10072-018-3255-z. [DOI] [PubMed] [Google Scholar]
- 110.Vatter H., Konczalla J., Seifert V. Endothelin related pathophysiology in cerebral vasospasm: What happens to the cerebral vessels? Acta Neurochir. Suppl. 2011;110:177–180. doi: 10.1007/978-3-7091-0353-1_31. [DOI] [PubMed] [Google Scholar]
- 111.Provencio J.J. Inflammation in subarachnoid hemorrhage and delayed deterioration associated with vasospasm: A review. Acta Neurochir. Suppl. 2013;115:233–238. doi: 10.1007/978-3-7091-1192-5_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Larysz-Brysz M., Lewin-Kowalik J., Czuba Z., Kotulska K., Olakowska E., Marcol W., Liskiewicz A., Jedrzejowska-Szypulka H. Interleukin-1beta increases release of endothelin-1 and tumor necrosis factor as well as reactive oxygen species by peripheral leukocytes during experimental subarachnoid hemorrhage. Curr. Neurovasc. Res. 2012;9:159–166. doi: 10.2174/156720212801619045. [DOI] [PubMed] [Google Scholar]
- 113.Paczkowska E., Golab-Janowska M., Bajer-Czajkowska A., Machalinska A., Ustianowski P., Rybicka M., Klos P., Dziedziejko V., Safranow K., Nowacki P. Increased circulating endothelial progenitor cells in patients with haemorrhagic and ischaemic stroke: The role of endothelin-1. J. Neurol. Sci. 2013;325:90–99. doi: 10.1016/j.jns.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 114.Andereggen L., Beck J., Z’Graggen W.J., Schroth G., Andres R.H., Murek M., Haenggi M., Reinert M., Raabe A., Gralla J. Feasibility and Safety of Repeat Instant Endovascular Interventions in Patients with Refractory Cerebral Vasospasms. AJNR Am. J. Neuroradiol. 2017;38:561–567. doi: 10.3174/ajnr.A5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hosmann A., Rauscher S., Wang W.T., Dodier P., Bavinzski G., Knosp E., Gruber A. Intra-Arterial Papaverine-Hydrochloride and Transluminal Balloon Angioplasty for Neurointerventional Management of Delayed-Onset Post-Aneurysmal Subarachnoid Hemorrhage Vasospasm. World Neurosurg. 2018;119:e301–e312. doi: 10.1016/j.wneu.2018.07.138. [DOI] [PubMed] [Google Scholar]
- 116.Macdonald R.L., Higashida R.T., Keller E., Mayer S.A., Molyneux A., Raabe A., Vajkoczy P., Wanke I., Frey A., Marr A., et al. Preventing vasospasm improves outcome after aneurysmal subarachnoid hemorrhage: Rationale and design of CONSCIOUS-2 and CONSCIOUS-3 trials. Neurocrit. Care. 2010;13:416–424. doi: 10.1007/s12028-010-9433-3. [DOI] [PubMed] [Google Scholar]
- 117.Raabe A., Beck J., Keller M., Vatter H., Zimmermann M., Seifert V. Relative importance of hypertension compared with hypervolemia for increasing cerebral oxygenation in patients with cerebral vasospasm after subarachnoid hemorrhage. J. Neurosurg. 2005;103:974–981. doi: 10.3171/jns.2005.103.6.0974. [DOI] [PubMed] [Google Scholar]
- 118.Macdonald R.L., Kassell N.F., Mayer S., Ruefenacht D., Schmiedek P., Weidauer S., Frey A., Roux S., Pasqualin A., CONSCIOUS-1 Investigators Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): Randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- 119.Macdonald R.L., Higashida R.T., Keller E., Mayer S.A., Molyneux A., Raabe A., Vajkoczy P., Wanke I., Bach D., Frey A., et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2) Lancet Neurol. 2011;10:618–625. doi: 10.1016/S1474-4422(11)70108-9. [DOI] [PubMed] [Google Scholar]
- 120.Macdonald R.L., Higashida R.T., Keller E., Mayer S.A., Molyneux A., Raabe A., Vajkoczy P., Wanke I., Bach D., Frey A., et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke. 2012;43:1463–1469. doi: 10.1161/STROKEAHA.111.648980. [DOI] [PubMed] [Google Scholar]
- 121.Loch Macdonald R. Management of cerebral vasospasm. Neurosurg. Rev. 2006;29:179–193. doi: 10.1007/s10143-005-0013-5. [DOI] [PubMed] [Google Scholar]
- 122.Rodrigues S.F., Granger D.N. Cerebral microvascular inflammation in DOCA salt-induced hypertension: Role of angiotensin II and mitochondrial superoxide. J. Cereb. Blood Flow Metab. 2012;32:368–375. doi: 10.1038/jcbfm.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang R., Witkowski S., Fu Q., Claassen J.A., Levine B.D. Cerebral hemodynamics after short- and long-term reduction in blood pressure in mild and moderate hypertension. Hypertension. 2007;49:1149–1155. doi: 10.1161/HYPERTENSIONAHA.106.084939. [DOI] [PubMed] [Google Scholar]
- 124.Andereggen L., Neuschmelting V., von Gunten M., Widmer H.R., Fandino J., Marbacher S. The role of microclot formation in an acute subarachnoid hemorrhage model in the rabbit. Biomed. Res. Int. 2014;2014:161702. doi: 10.1155/2014/161702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bar-Klein G., Cacheaux L.P., Kamintsky L., Prager O., Weissberg I., Schoknecht K., Cheng P., Kim S.Y., Wood L., Heinemann U., et al. Losartan prevents acquired epilepsy via TGF-beta signaling suppression. Ann. Neurol. 2014;75:864–875. doi: 10.1002/ana.24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wanderer S., Mrosek J., Gessler F., Seifert V., Konczalla J. Vasomodulatory effects of the angiotensin II type 1 receptor antagonist losartan on experimentally induced cerebral vasospasm after subarachnoid haemorrhage. Acta Neurochir. 2018;160:277–284. doi: 10.1007/s00701-017-3419-2. [DOI] [PubMed] [Google Scholar]
- 127.Wanderer S., Mrosek J., Vatter H., Seifert V., Konczalla J. Crosstalk between the angiotensin and endothelin system in the cerebrovasculature after experimental induced subarachnoid hemorrhage. Neurosurg. Rev. 2018;41:539–548. doi: 10.1007/s10143-017-0887-z. [DOI] [PubMed] [Google Scholar]
- 128.Gomez-Garre D., Martin-Ventura J.L., Granados R., Sancho T., Torres R., Ruano M., Garcia-Puig J., Egido J. Losartan improves resistance artery lesions and prevents CTGF and TGF-beta production in mild hypertensive patients. Kidney Int. 2006;69:1237–1244. doi: 10.1038/sj.ki.5000034. [DOI] [PubMed] [Google Scholar]
- 129.Tada Y., Wada K., Shimada K., Makino H., Liang E.I., Murakami S., Kudo M., Kitazato K.T., Nagahiro S., Hashimoto T. Roles of hypertension in the rupture of intracranial aneurysms. Stroke. 2014;45:579–586. doi: 10.1161/STROKEAHA.113.003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Asaeda M., Sakamoto M., Kurosaki M., Tabuchi S., Kamitani H., Yokota M., Watanabe T. A non-enzymatic derived arachidonyl peroxide, 8-iso-prostaglandin F2 alpha, in cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage participates in the pathogenesis of delayed cerebral vasospasm. Neurosci. Lett. 2005;373:222–225. doi: 10.1016/j.neulet.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 131.Konczalla J., Vatter H., Weidauer S., Raabe A., Seifert V. Alteration of the cerebrovascular function of endothelin B receptor after subarachnoidal hemorrhage in the rat. Exp. Biol. Med. 2006;231:1064–1068. [PubMed] [Google Scholar]
- 132.Ansar S., Vikman P., Nielsen M., Edvinsson L. Cerebrovascular ETB, 5-HT1B, and AT1 receptor upregulation correlates with reduction in regional CBF after subarachnoid hemorrhage. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3750–H3758. doi: 10.1152/ajpheart.00857.2007. [DOI] [PubMed] [Google Scholar]
- 133.Sakamoto M., Takaki E., Yamashita K., Watanabe K., Tabuchi S., Watanabe T., Satoh K. Nonenzymatic derived lipid peroxide, 8-iso-PGF2 alpha, participates in the pathogenesis of delayed cerebral vasospasm in a canine SAH model. Neurol. Res. 2002;24:301–306. doi: 10.1179/016164102101199783. [DOI] [PubMed] [Google Scholar]
- 134.Smeda J.S., Daneshtalab N. The effects of poststroke captopril and losartan treatment on cerebral blood flow autoregulation in SHRsp with hemorrhagic stroke. J. Cereb. Blood Flow Metab. 2011;31:476–485. doi: 10.1038/jcbfm.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tchekalarova J.D., Ivanova N.M., Pechlivanova D.M., Atanasovo D., Lazarov N., Kortenska L., Mitreva R., Lozanov V., Stoynev A. Antiepileptogenic and neuroprotective effects of losartan in kainate model of temporal lobe epilepsy. Pharmacol. Biochem. Behav. 2014;127:27–36. doi: 10.1016/j.pbb.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 136.Sun H., Wu H., Yu X., Zhang G., Zhang R., Zhan S., Wang H., Bu N., Ma X., Li Y. Angiotensin II and its receptor in activated microglia enhanced neuronal loss and cognitive impairment following pilocarpine-induced status epilepticus. Mol. Cell Neurosci. 2015;65:58–67. doi: 10.1016/j.mcn.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 137.Tchekalarova J.D., Ivanova N., Atanasova D., Pechlivanova D.M., Lazarov N., Kortenska L., Mitreva R., Lozanov V., Stoynev A. Long-Term Treatment with Losartan Attenuates Seizure Activity and Neuronal Damage Without Affecting Behavioral Changes in a Model of Co-morbid Hypertension and Epilepsy. Cell Mol. Neurobiol. 2016;36:927–941. doi: 10.1007/s10571-015-0278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nozaki T., Ura H., Takumi I., Kobayashi S., Maru E., Morita A. The angiotensin II type I receptor antagonist losartan retards amygdala kindling-induced epileptogenesis. Brain Res. 2018;1694:121–128. doi: 10.1016/j.brainres.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 139.Biancardi V.C., Stranahan A.M., Krause E.G., de Kloet A.D., Stern J.E. Cross talk between AT1 receptors and Toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H404–H415. doi: 10.1152/ajpheart.00247.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maeso R., Rodrigo E., Munoz-Garcia R., Navarro-Cid J., Ruilope L.M., Lahera V., Cachofeiro V. Losartan reduces constrictor responses to endothelin-1 and the thromboxane A2 analogue in aortic rings from spontaneously hypertensive rats: Role of nitric oxide. J. Hypertens. 1997;15:1677–1684. doi: 10.1097/00004872-199715120-00072. [DOI] [PubMed] [Google Scholar]
- 141.Guan W., Kozak A., El-Remessy A.B., Johnson M.H., Pillai B.A., Fagan S.C. Acute treatment with candesartan reduces early injury after permanent middle cerebral artery occlusion. Transl. Stroke Res. 2011;2:179–185. doi: 10.1007/s12975-010-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oku N., Kitagawa K., Imaizumi M., Takasawa M., Piao R., Kimura Y., Kajimoto K., Matsumoto M., Hori M., Hatazawa J. Hemodynamic influences of losartan on the brain in hypertensive patients. Hypertens. Res. 2005;28:43–49. doi: 10.1291/hypres.28.43. [DOI] [PubMed] [Google Scholar]
- 143.Habashi J.P., Judge D.P., Holm T.M., Cohn R.D., Loeys B.L., Cooper T.K., Myers L., Klein E.C., Liu G., Calvi C. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Povlsen G.K., Waldsee R., Ahnstedt H., Kristiansen K.A., Johansen F.F., Edvinsson L. In vivo experimental stroke and in vitro organ culture induce similar changes in vasoconstrictor receptors and intracellular calcium handling in rat cerebral arteries. Exp. Brain Res. 2012;219:507–520. doi: 10.1007/s00221-012-3108-6. [DOI] [PubMed] [Google Scholar]
- 145.Vikman P., Beg S., Khurana T.S., Hansen-Schwartz J., Edvinsson L. Gene expression and molecular changes in cerebral arteries following subarachnoid hemorrhage in the rat. J. Neurosurg. 2006;105:438–444. doi: 10.3171/jns.2006.105.3.438. [DOI] [PubMed] [Google Scholar]
- 146.Dimitropoulou C., Chatterjee A., McCloud L., Yetik-Anacak G., Catravas J.D. Handbook of Experimental Pharmacology. Volume 176. Springer; New York, NY, USA: 2006. Angiotensin, bradykinin and the endothelium; pp. 255–294. [DOI] [PubMed] [Google Scholar]
- 147.Tirapelli C.R., Bonaventura D., Tirapelli L.F., de Oliveira A.M. Mechanisms underlying the vascular actions of endothelin 1, angiotensin II and bradykinin in the rat carotid. Pharmacology. 2009;84:111–126. doi: 10.1159/000231974. [DOI] [PubMed] [Google Scholar]
- 148.Mehta P.K., Griendling K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 149.Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 150.Toda N., Miyazaki M. Angiotensin-induced relaxation in isolated dog renal and cerebral arteries. Am. J. Physiol. 1981;240:H247–H254. doi: 10.1152/ajpheart.1981.240.2.H247. [DOI] [PubMed] [Google Scholar]
- 151.Tsutsumi Y., Matsubara H., Masaki H., Kurihara H., Murasawa S., Takai S., Miyazaki M., Nozawa Y., Ozono R., Nakagawa K., et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J. Clin. Investig. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abassi Z.A., Klein H., Golomb E., Keiser H.R. Regulation of the urinary excretion of endothelin in the rat. Am. J. Hypertens. 1993;6:453–457. doi: 10.1093/ajh/6.6.453. [DOI] [PubMed] [Google Scholar]
- 153.Brunner F., Kukovetz W.R. Postischemic antiarrhythmic effects of angiotensin-converting enzyme inhibitors. Role of suppression of endogenous endothelin secretion. Circulation. 1996;94:1752–1761. doi: 10.1161/01.CIR.94.7.1752. [DOI] [PubMed] [Google Scholar]
- 154.Chua B.H., Chua C.C., Diglio C.A., Siu B.B. Regulation of endothelin-1 mRNA by angiotensin II in rat heart endothelial cells. Biochim. Biophys. Acta. 1993;1178:201–206. doi: 10.1016/0167-4889(93)90010-M. [DOI] [PubMed] [Google Scholar]
- 155.Dohi Y., Hahn A.W., Boulanger C.M., Buhler F.R., Luscher T.F. Endothelin stimulated by angiotensin II augments contractility of spontaneously hypertensive rat resistance arteries. Hypertension. 1992;19:131–137. doi: 10.1161/01.HYP.19.2.131. [DOI] [PubMed] [Google Scholar]
- 156.Kohno M., Horio T., Ikeda M., Yokokawa K., Fukui T., Yasunari K., Kurihara N., Takeda T. Angiotensin II stimulates endothelin-1 secretion in cultured rat mesangial cells. Kidney Int. 1992;42:860–866. doi: 10.1038/ki.1992.361. [DOI] [PubMed] [Google Scholar]
- 157.Saavedra J.M., Benicky J., Zhou J. Mechanisms of the Anti-Ischemic Effect of Angiotensin II AT(1) Receptor Antagonists in the Brain. Cell Mol. Neurobiol. 2006;26:1099–1111. doi: 10.1007/s10571-006-9009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]